- 1Department of Endocrinology and Internal Medicine, Medical University of Gdańsk, Gdańsk, Poland
- 2First Department of Cardiology, Medical University of Gdańsk, Gdańsk, Poland
Background: Catestatin (Cts) is a peptide derived from proteolytic cleavage of chromogranin A, which exhibits cardioprotective and anti-inflammatory properties. Cts has been proposed as a potential biomarker for cardiovascular (CV) disease.
Objectives: examining Cts in patients with incidentally discovered adrenocortical adenomas (AI), and its associations with CV risk factors and blood pressure (BP).
Materials and methods: In this cross-sectional study, 64 AI patients without overt CV disease other than primary hypertension were recruited along with 24 age-, sex-, and body-mass-index (BMI)-matched controls with normal adrenal morphology. Laboratory, 24-h ambulatory BP monitoring, echocardiography, and common carotid artery sonography examinations were performed.
Results: Unadjusted Cts was higher in AI patients (median 6.5, interquartile range: 4.9-37 ng/ml) versus controls (4.5 (3.5 – 28)), p=0.048, however, the difference was insignificant after adjusting for confounding variables. Cts was lower in subjects with metabolic syndrome than in those without it (5.2 (3.9- 6.9) vs. 25.7 (5.8-115) ng/ml, p<0.01), and in men compared to women (4.9 (4-7.4) ng/ml vs. 7 (4.8-100), p=0.015). AI patients in the lower half of Cts levels compared to those in the upper had a higher prevalence of hypertension (OR 0.15, 95% CI: 0.041-0.5, p<0.001) and metabolic syndrome (OR 0.15, 95% CI 0.041-0.5, p<0.001). In AI patients Cts correlated positively with high-density lipoprotein cholesterol (Spearman’s r=0.31), negatively with BMI (r=-0.31), and 10-year atherosclerotic CV disease risk (r=-0.42).
Conclusions: Our data indicate associations between CV risk factors and Cts. More clinical research is needed to apply serum Cts as a biomarker.
1 Introduction
Risk factors for atherosclerotic cardiovascular disease (ASCVD) can be divided into nonmodifiable (e.g. age or sex) and modifiable (smoking, elevated blood pressure (BP), dyslipidemia, diabetes (DM), and obesity). Apart from established risk factors, new are sought (e.g. uric acid (UA) (1) and high-sensitivity C-reactive protein [hs-CRP) (2)]) to help distinguish persons at higher risk of developing cardiovascular disease (CVD), who would benefit more from medical interventions such as low-density lipoprotein cholesterol (LDL-C) reduction (3).
The sympathetic nervous system plays a pivotal role in CVD development. Chromogranin A (CgA) is co-stored and co-released with catecholaminec from sympathetic neuronal vesicles and the adrenall medulla. One of CgA's proteolytic cleavage products is catestatin (Cts), a cardioprotective, anti-hypertensive, and anti-inflammatory peptide (4, 5). In vitro, Cts was shown to bind to nicotinic acetylcholine receptors, which inhibits membrane depolarization and blocks calcium influx, and, consequently, suppresses catecholamine release and activation of the sympathetic nervous system (6). Studies with animal models demonstrated Cts exerts anti-inflammatory effects, cardioprotection, and reduces obesity and insulin resistance (7–9). Clinical studies indicate Cts is involved in the course of hypertension (HT), coronary artery diseases (CAD), and heart failure (HF) (10–12). Adolescents with metabolic syndrome (MetS) had decreased Cts, which was postulated as a novel CVD risk factor (12–14).
In the current study we aimed at 1) determining Cts levels in patients with an incidentally-discovered adrenocortical adenoma/hyperplasia (AI) and without overt CVD other than HT, as well as 2) investigating associations between Cts and ASCVD risk modifiers, and asymptomatic HT-mediated organ damage (15). The presence of an AI per se, and particularly mild autonomous cortisol secretion (MACS) in its course, have been associated with metabolic disorders, elevated CV risk and mortality (16). So far, Cts has not been investigated in this patient population.
2 Subjects and methods
2.1 Study population
Study participants with an AI were recruited among 376 consecutive adult patients hospitalized in the Department of Endocrinology and Internal Medicine of the University Clinical Center of the Medical University of Gdańsk between November 2018 through February 2020 due to an adrenal lesion. We included 64 patients with radiological features of an adrenal adenoma/hyperplasia revealed by computed tomography (CT) or magnetic resonance (MR), who agreed to participate in the study, and met none of the following exclusion criteria: 1) age over 75 or under 40; 2) obesity grade III (BMI >40 kg/m2); 3) premenopausal period; 4) adrenal hormone excess other than MACS, i.e. cortisolemia between 50 and 138 nmol/l in the overnight 1-mg dexamethasone suppression test (DST) and no phenotypic features of Cushing’s syndrome (17); 5) kidney disease with eGFR<60 ml/min/1.73m2 and/or proteinuria >0.25 g/24h); 6) treatment with a mineralocorticoid receptor antagonist; 7) established and/or overt CVD other than primary HT, including: a) ASCVD (CAD, stroke, transient ischemic attack, peripheral artery disease), b) significant cardiac disease (e.g. pathological arrhythmia, severe valvular heart disease, cardiac tamponade, cardiomyopathy, congenital heart disease, HF), c) vascular diseases (among others venous thromboembolism and vasculitis); 8) active malignancy; 9) decompensated autoimmune disease or immune disease associated with CV and/or renal complications; 10) infectious diseases; 11) current or past addiction to alcohol and/or illicit drugs. Study participants were recruited based on anamnesis, physical examination, additional examinations available for review prior to enrollment and performed in the course of the study. Initially, 73 patients were included, however, three withdrew their consent to participate due to the COVID-19 pandemic, in four patients transthoracic echocardiography (TTE) revealed cardiac post-ischemic lesions, and two were diagnosed with primary aldosteronism.
Based on medical records of our hospital, which included examinations ordered in outpatient clinics and the emergency department, we identified 153 persons with normal adrenal morphology in a CT/MRI scan performed within five years preceding this study. There were 129 who met at least one of the above-listed exclusion criteria, declined participation or were unreachable, therefore, 24 subjects without an AI were enrolled as controls.
The research complied with the Declaration of Helsinki and was approved by the Independent Bioethics Committee for Research of our University. Informed consent for inclusion in the study was obtained in writing from each participant.
2.2 Study design
Both AI patients and controls underwent the following evaluation: 1) medical interview; 2) physical examination; 3) antecubital venous blood sampling for laboratory analyses; 4) resting 12-lead electrocardiography (ECG); 5) TTE; 6) common carotid artery (CCA) ultrasonography (USG) including CIMT determination, 7) 24-hour ambulatory blood pressure monitoring (ABPM).
Body-mass-index (BMI) was calculated by dividing body weight (W) in kg by the square of height (H) in meters. 2009 International Diabetes Federation criteria were used to diagnose MetS (18). Subjects with HT received hypotensive medications at the time of enrollment or were diagnosed by ABPM based on mean systolic and diastolic BP (SBP and DBP, respectively) of at least 135/85 mmHg for daytime, 120/75 mmHg for nighttime, and/or 130/80 mmHg for the 24-h period (15). Atherogenic dyslipidemia was defined as triglycerides (TGL) ≥150 mg/dL and serum high-density lipoprotein cholesterol (HDL-C) <40 mg/dL for men and <45 mg/dL for women.
In all study participants, 10-year ACSVD risk was estimated using the 2018 calculator provided online by the American Heart Association and the American College of Cardiology based on Framingham Heart Study (FHS-ASCVD Risk) (19, 20). The calculator estimates 10-year risk of developing ASCVD including coronary death, myocardial infarction, coronary insufficiency, angina, ischemic stroke, hemorrhagic stroke, transient ischemic attack, peripheral artery disease, and HF for individuals aged 30 to 74 and without CVD at baseline based on the following predictors: age, type 2 DM (DMt2), smoking, treated and untreated SBP, total cholesterol (TC), HDL-C, and LDL-C.
For nondiabetic subjects, 10-year CVD risk was also calculated using Systematic Coronary Risk Estimation 2 (SCORE2) for subjects aged 40-69 and SCORE2-Older People (SCORE2-OP) for those aged 70-75 for high CV risk countries, which include Poland (21). Predictors used in SCORE2/-OP are: age, sex, smoking, SBP, and non-HDL-C. We are aware SCORE2/-OP was developed to estimate risk in treatment-naïve persons, and that a significant portion of our subjects received lipid- and BP-lowering therapy. Nevertheless, we concluded applying this estimation tool along with FHS-ASCVD Risk calculation is of value.
2.3 Laboratory examinations
Blood was drawn between 8 and 10 a.m. after a fasting period of at least 8 hours from an antecubital vein, and used for regular examinations in the laboratory of our hospital apart from samples preserved for the determination of plasma Cts in all subjects, serum aldosterone and plasma direct renin concentration (DRC) in controls. These were centrifuged at 2,000 rpms for 20 minutes at 4 degrees C, aliquoted and stored at -80 degrees C until analysis.
Samples were analyzed in Central Clinical Laboratory in Gdańsk using standard laboratory methods (with a Siemens IMMULITE 1000 Immunoassay System for most biochemical tests, and an Abbott Architect analyzer, which applies the spectrophotometric method). Serum Cts was determined by an enzyme-linked immunosorbent assay (ELISA) by using a commercially-available diagnostic kit (SunRedBio, catalogue no: 201-12-8276; sensitivity: 0.268 ng/mL; assay range: 0.3-90 ng/mL). Cts concentrations above 90 ng/ml (n=15) were extrapolated based on ELISA standard curve.
Serum Cts, creatinine, sodium, potassium, aldosterone, renin, lipid profile (TC, HDL-C, LDL-C, and TGL), UA, hs-CRP, 24-h urinary protein and albumin excretion were determined both in AI patients and controls. Morning serum cortisol, dehydroepiandrosterone sulphate (DHEA-S), overnight 1 mg-DST cortisol and 24-h urinary cortisol were determined in AI patients. In most (n=50) AI patients, 24-h urinary meta- and normetanephrine excretion was determined, in others it had been performed prior to hospitalization. Screening for primary hyperaldosteronism based on aldosterone-to-renin ratio (ADRR) was performed without modifying antihypertensive medications in both AI patients and controls; there were no study participants with both HT and an ADRR above 2 ng/dL:μIU/mL.
2.4 Ambulatory blood pressure monitoring
24h ABPM was conducted using a Spacelabs Ontrak 90227 monitor on the non-dominant arm. During the day BP was recorded every 20 minutes, while during nighttime rest every 30 minutes. ABPM was repeated or not considered in the analysis if more than 30% of measurements were invalid. Normal results were adopted according to the European Society of Cardiology/European Society of Hypertension 2018 guideline: <130/80 mmHg for the 24-h period, <135/85 mmHg for daytime, and <120/70 mmHg for nighttime (22). Patients were classified as ‘non-dippers’ if their mean diurnal SBP and DBP were not at least 10% higher than nocturnal (22).
2.5 Transthoracic echocardiography
All measurements were performed in accordance with the recommendations endorsed by the American Society of Echocardiography and the European Association of Cardiovascular Imaging (23). Three on-site cardiology consultants with an expertise in ultrasonography performed TTE using the GE Vivid E9/E95 ultrasound system.
Measurements included left-ventricular (LV) internal dimension in diastole (LVIDd) and systole (LVIDs), LV ejection fraction (LVEF) according to modified Simpson’s rule (24), posterior LV wall thickness (LVPWd), and interventricular septal thickness (IVSd). LV mass (LVM) was calculated with the cube formula: LVM (g)=0.8×1.04 ×[(LVEDd+IVSd+LVPWd)3−LVEDd3]+0.6. LVM was indexed to body surface area (BSA) calculated using the DuBois formula (BSA=0.007184×H0.725×W0.425): LVM index (LVMI)=LVM/BSA. Relative wall thickness (RWT) was calculated with the formula: RWT=(2×LVPWd)/LVEDd. Left ventricular hypertrophy (LVH) was defined as LVMI >95 g/m2 for females and >115 g/m2 for males. RWT was used to further classify LVH as either concentric (RWT >0.42) or eccentric (RWT ≤ 0.42).
Disk summation technique from apical four and two-chamber views was used to determine left atrial volume (LAV), which was indexed to BSA: LAV index (LAVI) (ml/m2) = LAV/BSA (15). Apical four-chamber view was used to record peak blood flow velocity from LV relaxation in early diastole (E) and peak velocity flow in late diastole (A). Since LVEF was normal in all study participants, four criteria were applied to assess diastolic function: (1) LAVI ≥34 ml/m2, (2) tricuspid regurgitation velocity (TR) ≥2.8 m/s, (3) ratio of E to average early mitral annular velocity (e’) ≥14, (4) septal e’<7 cm/s or lateral e’<10 cm/s. Indeterminate diastolic function was stated if two criteria were met, and dysfunction if three or four (15).
2.6 Common carotid artery USG
Maximum carotid intima-media thickness (CIMT) measurements were recorded using echo-tracking technology on the distal wall of the right carotid artery, 1 to 3 cm below the carotid artery bifurcation. The presence of atherosclerotic plaques (ASP) defined as a CIMT ≥1.5 mm, or by a focal increase in thickness of 0.5 mm or 50% of the surrounding CIMT value was also recorded.
2.7 Statistical analysis
Data were analyzed using R-studio. Discrete variables were presented as number (n) or n (percentage). Continuous quantitative data with a normal distribution were presented as mean ± standard deviation (SD), and in the case of a non-normal distribution as median (interquartile range, IQR). We used the Shapiro-Wilk test to determine if a data set was well-modeled by a normal distribution. To compare differences between two independent groups Welch’s t-test was used when variables were normally distributed or the Mann-Whitney U test in the case of non-normal distribution. One-way ANOVA and Tukey’s honestly significant difference (HSD) tests were used to compare three or more independent groups. Simple (bivariate) correlations were computed with the non-parametric Spearman rank-order method (correlation coefficient r is given). Associations between dichotomous categorical variables were examined with Fisher’s exact test, and Benjamini-Hochberg Method was applied to correct for multiple testing.
Multiple regression models were applied to adjust for differences in Cts concentrations depending on potential confounding variables including gender, age, BMI, smoking status, comorbidities (HT, DMt2, MetS), and medications (ACEI/ARB, CCB, BB, diuretics, statins, and PPIs). An exhaustive search method was used to select factors that had the strongest relationship with Cts, i.e.: 1) gender, presence of 2) MetS, 3) HT, therapy with 4) statins, and 5) PPIs. The final multivariate model had a R-squared of 0.1831. Out of five variables included in the model only the presence of MetS (ß=-30, p=0.005) was significantly and negatively associated with Cts. For dichotomous dependent variables (Cts halves, HT, DMt2, and MetS) binary logistic regression was used to adjust for gender, age, and BMI. Significance was set at 0.05.
3 Results
3.1 Comparison of examined parameters between AI patients and controls
To assess whether the presence of an AI affected Cts levels, verification of matching between AI patients and controls was undertaken. These groups did not differ in regard to age, sex, BMI, smoking status, and comorbidities (incidence of HT, DM t.2, ASP, and dyslipidemia), see Table 1. Concerning subjects with HT, the number of patients on mono-, dual- and triple-drug therapy (including betablockers) was also comparable (Supplementary Table 1).
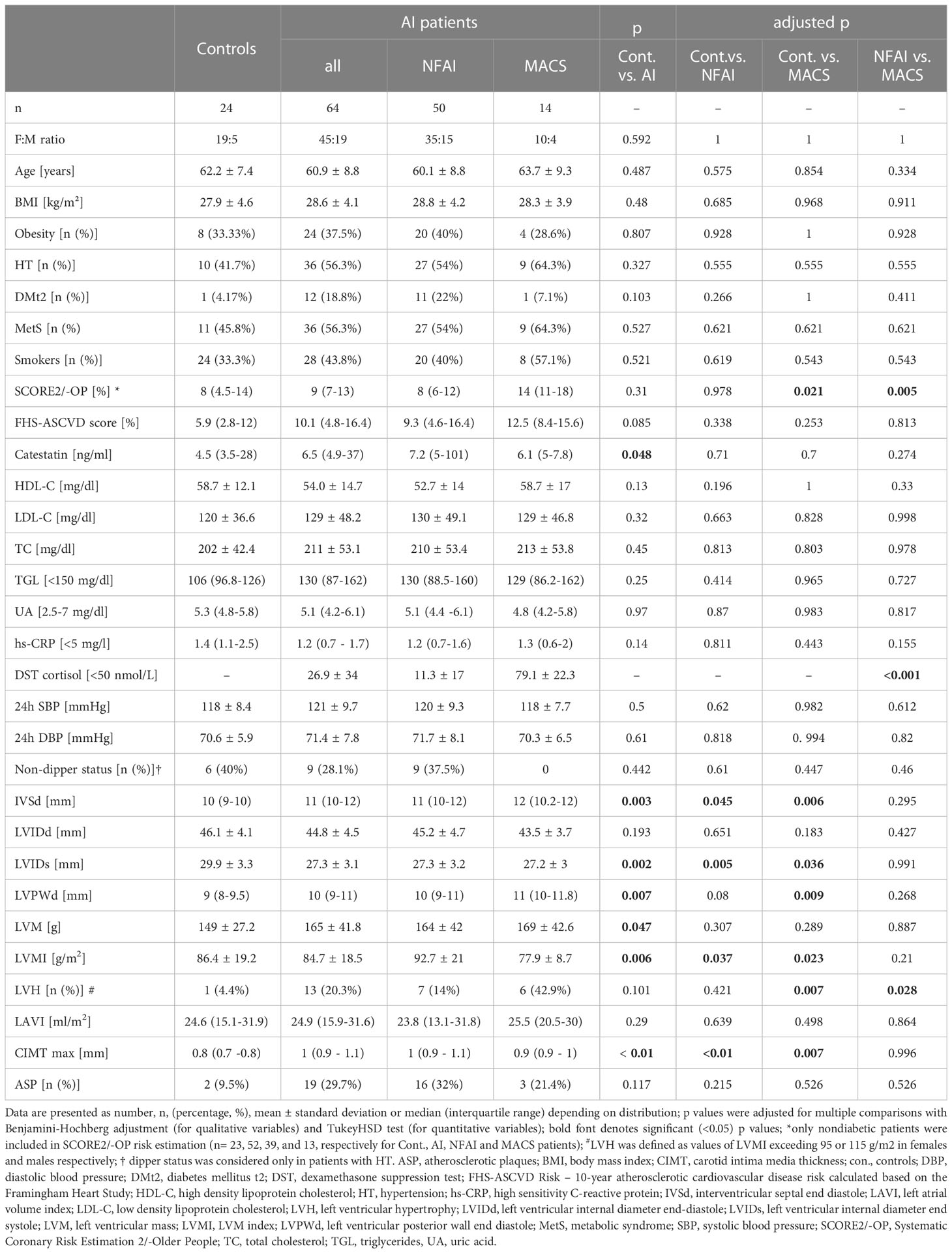
Table 1 Clinical, laboratory, ABPM, echocardiographic, and CCA sonography parameters in AI patients and controls.
Among AI patients, there were 14 with MACS and 31 classified as NFAI; analyses for AI patients and these two subgroups were performed separately.
Ten-year FHS-ASCVD Risk was comparable between patients with an AI/NFAI/MACS and controls, while in nondiabetic subjects, SCORE2/-OP was significantly higher in patients with MACS than in controls and patients with NFAI: 14% (11-18) vs. 8% (4.5-14), p = 0.021, and 8% (6-12), p = 0.005, respectively (Table 1). Still, this CVD risk index was comparable between controls and all AI patients (p=0.31), which illustrates effective matching between these groups.
Cts distribution was bimodal both in AI patients and controls. Unadjusted Cts was slightly higher in AI patients: 6.45 (4.9-37) vs. 4.5 (3.5-28) ng/ml, p=0.047 (Figure 1). However, after adjusting for potential confounding variables (gender, age, and BMI), solely BMI and male gender were significantly (negatively) associated with Cts (ß=-28.3, p=0.01 and ß=-2.3, p=0.04, respectively) but not the presence of an AI (ß=-8.1, p=0.44).
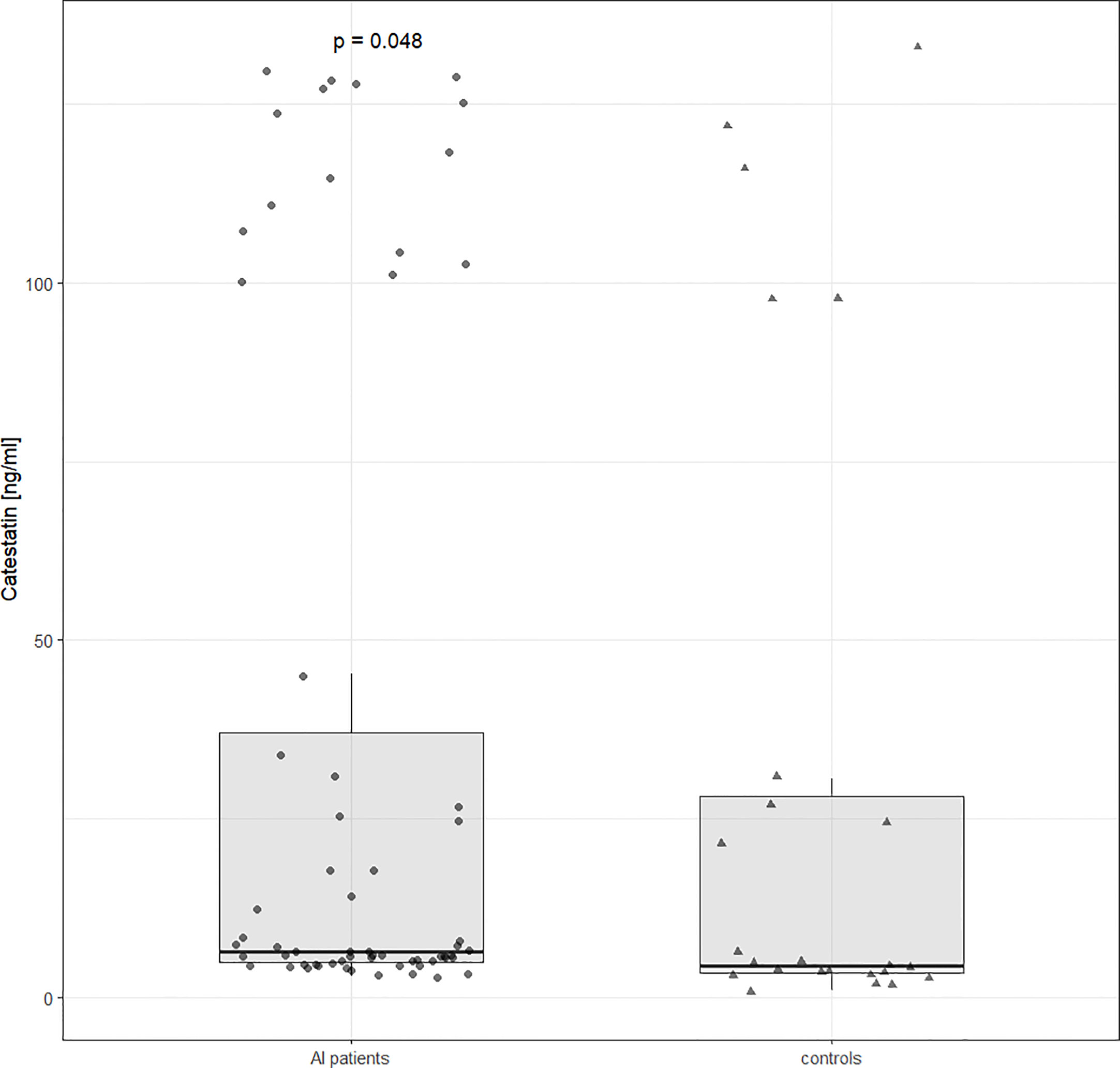
Figure 1 Catestatin distribution in controls and AI patients. Boxplot and data distribution with dots (AI patients) and triangles (controls) indicating individual datapoints. Unadjusted p value was determined using the Mann Whitney U test. AI, adrenal incidentaloma.
Lipid profile, hs-CRP, as well as UA were comparable between controls and AI patients, be it with a NFAI or MACS. Proteinuria and albuminuria were normal in all study participants (respectively below 150 and 30 mg/24h). ABPM parameters (SBP, DBP, and pulse rate, PR) were comparable between AI patients and controls (Table 1 and Supplementary Table 1).
Concerning TTE, there were significant differences in IVSd, LVPWd, and LVMI between AI patients and controls (respectively 11 (10-12) vs. 10 (9-10) mm, p=0.003; 10 (9-11) vs. 9 (8-9.5) mm, p=0.007; 86.4 ± 119.2 vs. 84.7 ± 18.5 g/m2, p=0.001). Moreover, LVH was more prevalent in MACS patients than controls (42.9% vs. 4.4%, p=0.007) and NFAI patients (42.9% vs. 14%, p=0.028).
Maximum CIMT was higher in patients with an AI, be it with a NFAI or MACS, than in controls: 1 (0.9-1.1) vs. 0.8 (0.8-0.9) mm, p<0.01. However, there were no differences in maximum CIMT between patients with a NFAI and MACS (Table 1). A trend toward a higher prevalence of an ASP in AI, NFAI, and MACS patients (29.7%, 32%, 21.43%, respectively) than controls (9.5%) could be observed (p=0.12) (Table 1).
3.2 Catestatin in clinically-specified patient groups
Upon comparing Cts levels between controls with normal adrenal morphology and AI patients, peptide’s levels were tested in different patient groups. Cts was higher in women than in men: 7 (4.8-100) vs. 4.9 (4-7.4) ng/ml, p=0.015, and the difference between sexes was significant in both AI patients (7.3 (5.5-103) vs. 6 (4.26-7.6) ng/ml, p=0.03) and controls (5.1 (3.8 - 62.6) vs. 2.8 (1.7 - 3.5) ng/ml, p = 0.043), see Figure 2.
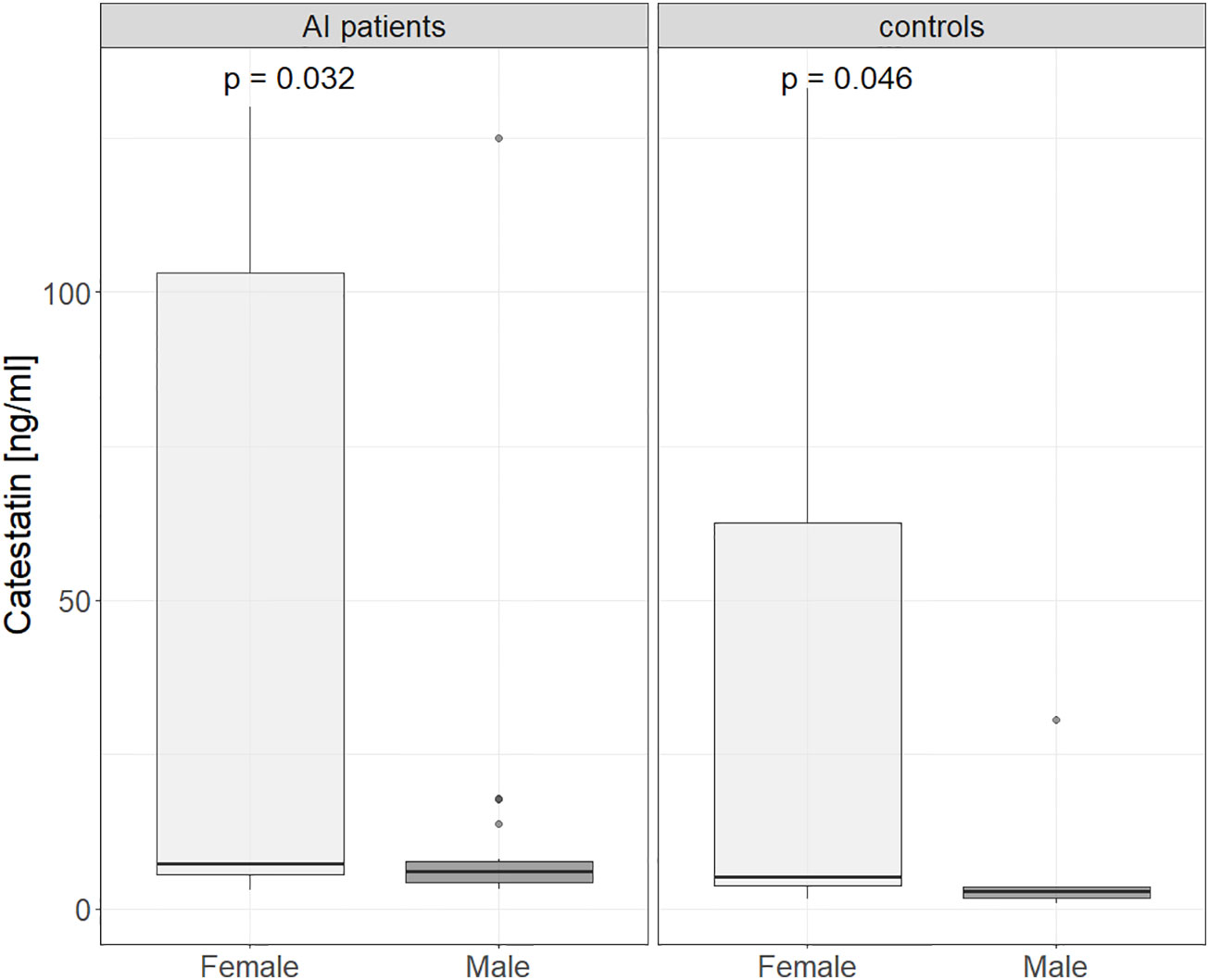
Figure 2 Catestatin in male and female controls and AI patients. Boxplot chart. P-value was determined using the Mann-Whitney U test. AI, adrenal incidentaloma.
Further, in AI patients and controls analyzed together Cts was lower in hyper- versus normotensive subjects: 5.6 (4-7.1) vs. 15.8 (5.2-103) ng/ml, p=0.003, which was also found for AI patients alone: 5.6 (4.36-6.82) vs. 21.7 (6.85-107), p < 0.001) (Figure 3). Cts was also significantly lower in subjects with MetsS than in those without it: 25.7 (5.8-115) vs. 5.2 (3.9- 6.9) ng/ml, p<0.01 (Figure 4), regardless of potential confounders (gender, age, BMI, presence of an AI and/or HT, statin and PPI use). We confirmed these differences (normo- versus hypertensive subjects as well as those without and with MetS) in women but not men (probably due to their low number). Cts in hypertensive AI females was lower than in normotensive ones: 5.6 (4.7 – 11.6) vs. 45.2 (8.2 – 118) ng/ml, p <0.01, and also lower in those with MetS than without it, both among AI patients: 5.6 (4.8-6.9) vs. 34.3 (7.7 - 121) ng/ml, p= <0.01 and controls: 3.8 (3.7-5.1) vs. 61.2 (8.4-112) ng/ml, p = 0.025 (Supplementary Figures 1, 2).

Figure 3 Catestatin in normotensive and hypertensive in controls and AI patients. Boxplot chart. P-values were determined using the Mann-Whitney U test. AI, adrenal incidentaloma.
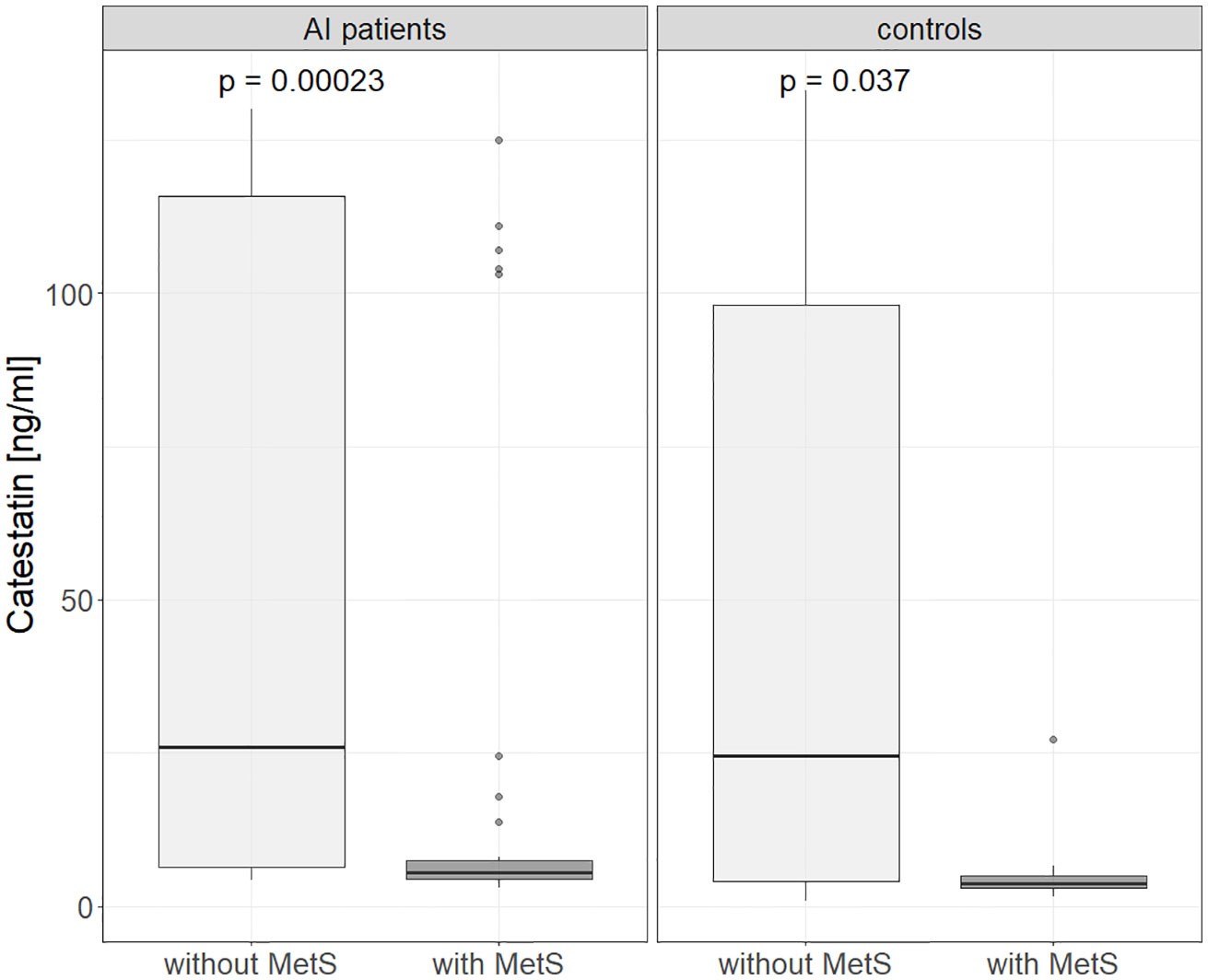
Figure 4 Catestatin in subjects without and with metabolic syndrome in controls and AI patients. AI, adrenal incidentaloma; MetS, metabolic syndrome. P-values determined using the Mann-Whitney U test.
There were no differences in Cts between obese and non-obese subjects, smokers and non-smokers, or, among subjects with HT, ‘dippers’ and ‘non-dippers’.
3.3 Correlations between catestatin and laboratory, TTE, and CCA USG parameters
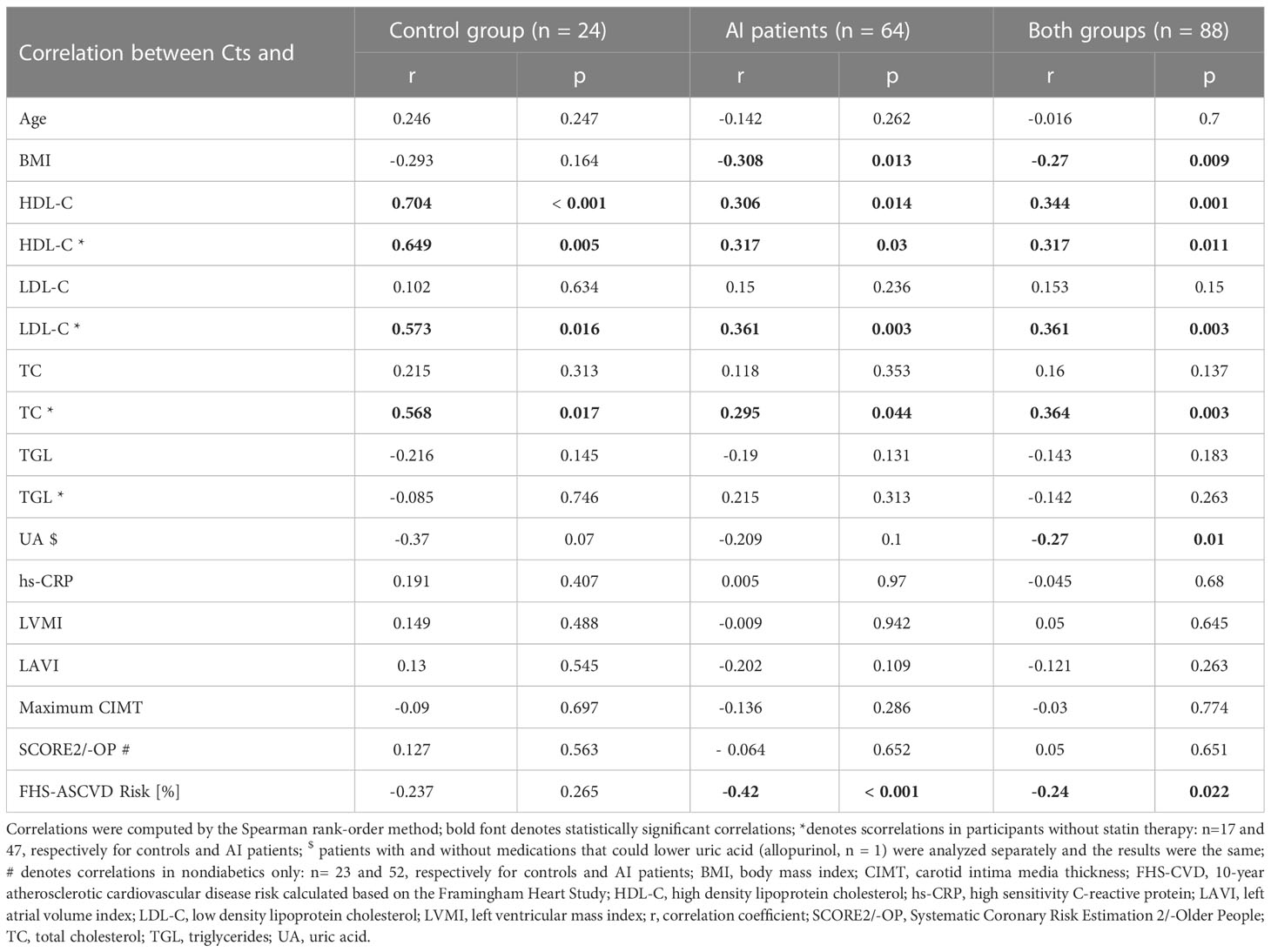
To further investigate associations between Cts and CVD risk, correlations were tested between peptide’s levels and other parameters. In AI patients, weak correlations were found between Cts and: BMI (r=-0.31) (Figure 5A), FHS-ASCVD Risk (r=-0.42) (Figure 5B), and HDL-C (r=0.32) regardless of statin therapy (Figure 5C). Interestingly, among participants without it, there were also positive correlations between Cts and: TC and LDL-C (r=0.36 for both) (Table 2 and Supplementary Figures 3, 4). In AI patients and controls analyzed as a whole a negative correlation between Cts and UA was also observed (r=-0.27, p=0.01), while for each group analyzed separately, significance has not been reached probably due to sample size.
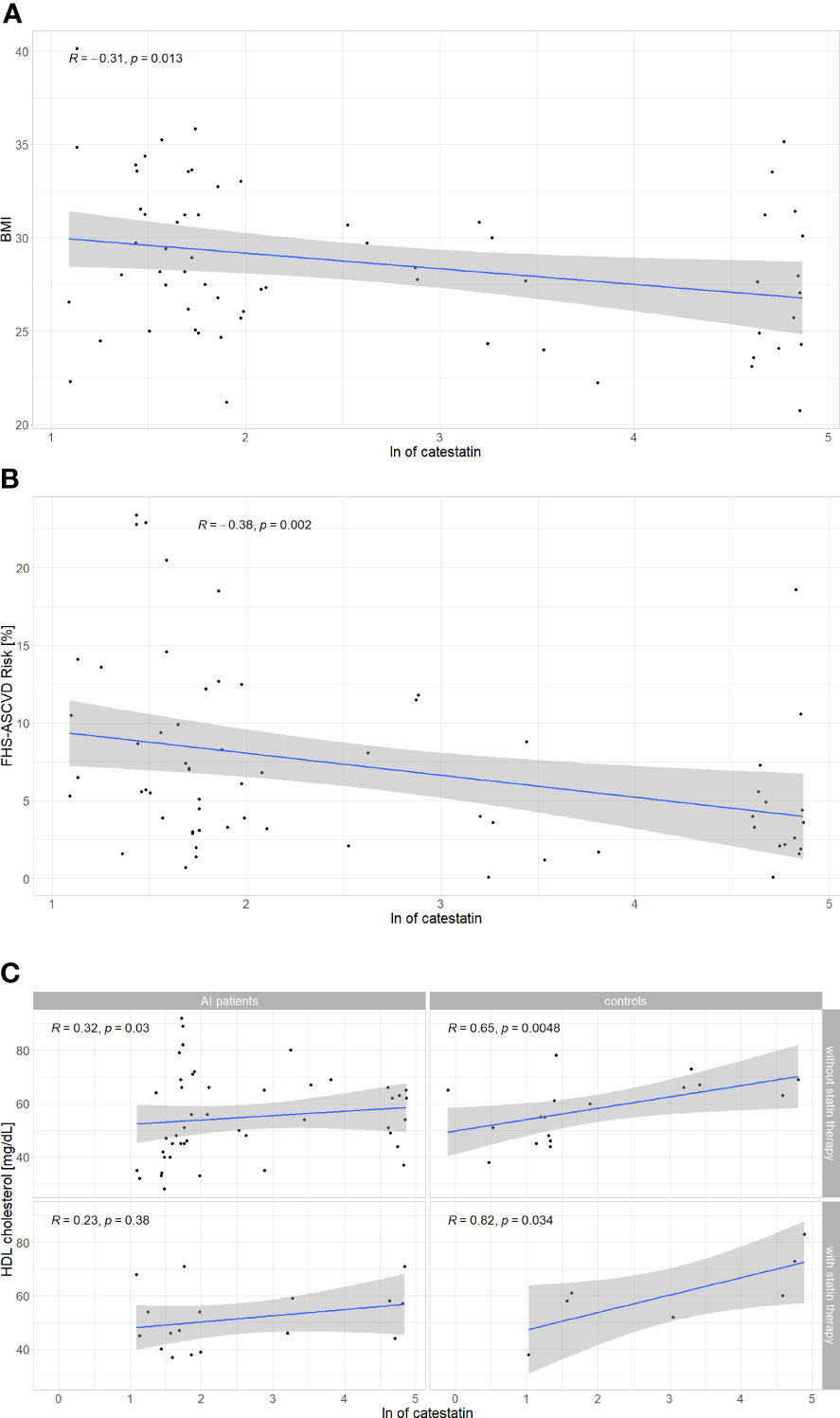
Figure 5 Correlations between catestatin and: (A) BMI in AI patients; (B) FHS-ASCVD Risk in AI patients; (C) HDL-C in AI patients and controls. Correlations were computed by the Spearman rank-order method. For HDL-C, correlations were tested separately in subjects with and without statin therapy. AI, adrenal incidentaloma, BMI, body mass index, FHS-ASCVD Risk - 10-year atherosclerotic cardiovascular disease risk calculated based on the Framingham Heart Study; HDL-C, high density lipoprotein cholesterol, ln, natural logarithm.
Analyses in subjects of each sex revealed Cts correlated with HDL (r=0.31, p=0.014), BMI (r=-0.29, p=0.019), and UA (r=-0.27, p=0.031) in women, but not in men (respectively r=0.13, p=0.53; r=-0.06, p=0.78; r=-0.12, p=0.56). A negative correlation between Cts and FHS-ASCVD Risk was also recorded in women with an AI (r=-0.3, p=0.049) but not in female controls (r =0.025, p=0.92), nor men with an AI (n=19, r=- 0.37, p=0.12). Correlations for male controls were not tested due to a low number of these subjects (n=5).
Concerning hormonal tests, only a weak correlation between Cts and DRC was observed, but not with aldosterone, nor ADRR (Supplementary Table 2). Cts did not correlate with ABPM, CCA USG, nor TTE parameters (LVPWd, IVSd, LVMI, LAVI) (Table 2 and Supplementary Table 2).
3.4 Clinical, laboratory, and TTE parameters according to catestatin categories
Further analyses were performed among AI patients based on Cts categories. First, adjustment for gender, age and BMI in binary logistic regression analysis revealed AI patients in the lower half of Cts concentrations (median 6.5, IQR 4.9-37 ng/ml) compared to those in the upper had a higher prevalence of HT (OR 0.17, CI 0.05-5.37, p=0.003), and MetS (OR 0.21, CI 0.06-7.51, p=0.018). Moreover, BMI, 24-SBP, and FHS-ASCVD Risk were also higher in the former (respectively 30.1 ± 4 vs. 27.2 ± 3.6 kg/m2, p=0.004; 123 ± 7.4 vs. 117 ± 9.5 mmHg, p=0.022; 13.2%(8.9-19.2) vs. 6.3%(4.2-10.8), p=0.002), as summarized in Table 3.
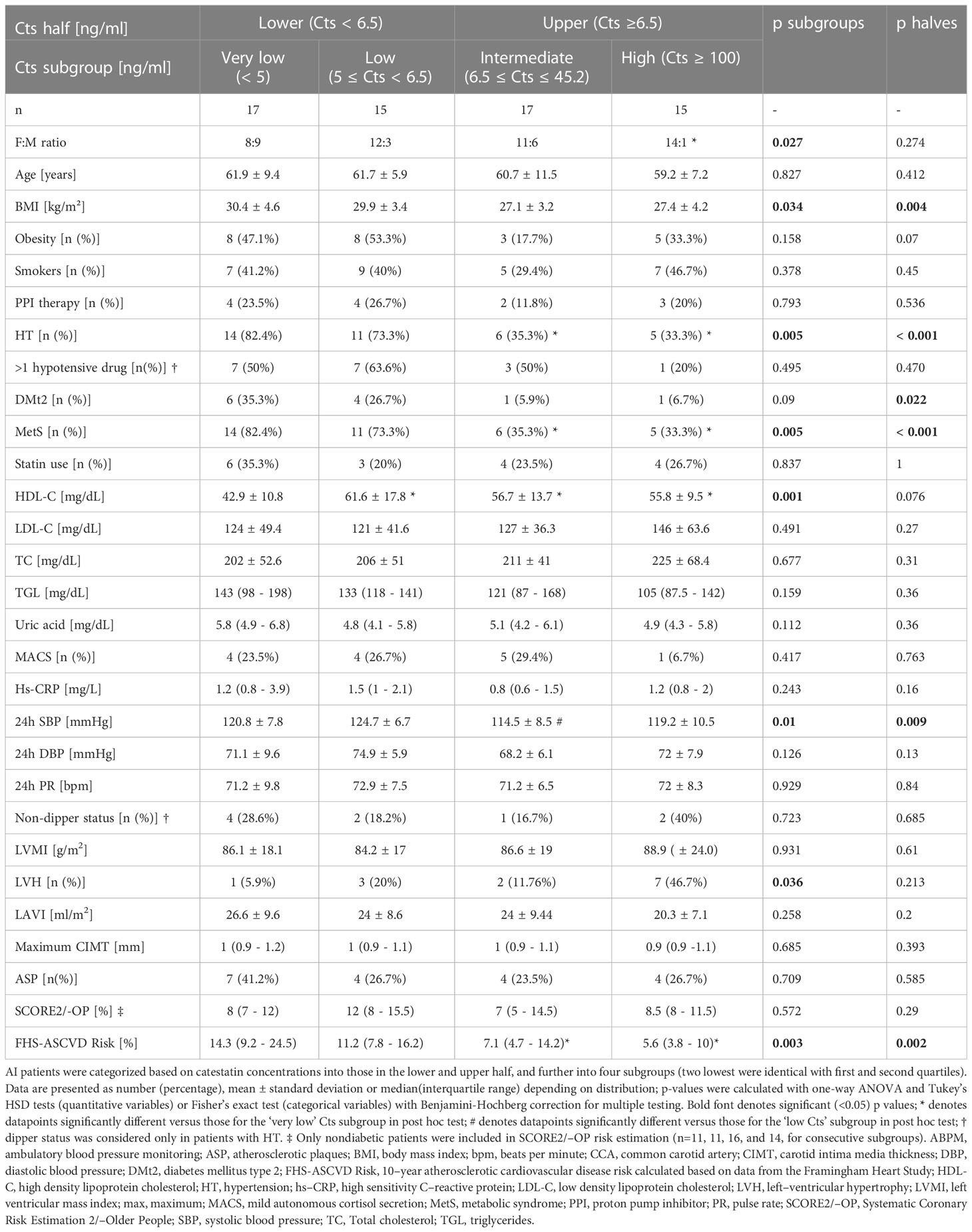
Table 3 Clinical, laboratory, ABPM, echocardiographic, and CCA sonography parameters in AI patients according to catestatin category.
Second, based on Cts distribution, we divided AI participants into four subgroups, i.e. with: ‘very low’ (Cts <4.9 ng/ml, n=17), ‘low’ (≥4.9 and <6.5 ng/ml, n=15), ‘intermediate’ (≥6.5 and ≤45.2 ng/ml, n=17), and ‘high’ (≥100 ng/ml, n=15) Cts levels (Table 3). The first two comprised subjects from two lower quarters, while the ‘high Cts’ subgroup corresponded to almost all patients in the fourth quarter (15 instead of 16 patients were included since there were none in the 45.2 - 100 ng/ml range, see Figure 1).
These four Cts subgroups differed significantly in male-to-female ratio, prevalence of HT and MetS, mean/median BMI, HDL-C, 24h SBP, and FHS-ASCVD Risk (Table 3). Post hoc analysis revealed male gender was more prevalent in the ‘very low’ versus ‘high’ Cts subgroup (53% vs. 6.7%), while HT and MetS in the ‘very low’ versus ‘intermediate’ (82.4% vs. 35.3%, p=0.04 for both) and ‘high’ Cts subgroups (82.4% vs. 33.3%, p=0.04 for both). HDL-C was lower in the ‘very low’ than in the three remaining Cts subgroups (42.9 ± 42.9 vs. 61.6 ± 17.8, 56.7 ± 13.7, and 55.8 ± 9.5 mg/dL, adjusted p=0.001, 0.01, and 0.03, respectively). What is more, 24h SBP in the ‘low’ Cts subgroup was higher than in the ‘intermediate’ (124.7 ± 6.7 vs. 114.5 ± 8.5 mmHg, adj. p=0.008).
Ten-year FHS-ASCVD Risk in the ‘very low’ Cts subgroup was higher than in the ‘intermediate’ and ‘high’: 14.3% (19.2-24.5) vs. 7.1% (4.7-14.2) and 5.6% (3.8-9.8), respective adjusted p=0.014 and 0.005, in line with differences in gender proportions, prevalence of metabolic disorders and HT between the subgroups.
Fisher’s exact test revealed differences in LVH prevalence between four Cts subgroups, yet, without significance in pairwise comparisons with correction for multiple testing (Holm–Bonferroni method). No other significant differences were recorded between Cts halves and subgroups in TTE and CCA USG parameters (Table 3). Finally, clinical, laboratory and TTE parameters were also analyzed in the same Cts subgroups for females only (Supplementary Table 3). Significant differences were recorded for: HT and MetS prevalence, HDL-C and hs-CRP concentrations, mean 24h DBP, and FHS-ASCVD Risk. Calculations for male AI subjects were not performed due to their low number.
4 Discussion
Clinical research on Cts is scarce, even though it deserves attention due to its protective effects on the CV system demonstrated in vitro and in vivo. To our knowledge, our study is the first to examine Cts in patients with an AI, and to show lower Cts in adult patients with MetS than those without it, as well as correlations between Cts and FHS ASCVD risk index.
A thorough assessment was undertaken to investigate associations between Cts and CV risk factors. It must be highlighted that Cts changes dynamically in response to sympathetic nervous system activation in a negative feedback mechanism (25). Also, multiple diseases and drugs lead to CgA secretion, which may affect the concentration of its derivatives, including Cts. For these reasons, we excluded patients with established CVD, stage 3-5 chronic kidney disease, cancer, etc., and controlled the use of PPIs (10, 12, 26, 27). Limitations of our study include a small, heterogeneous patient sample, lack of CgA determination (Cts : CgA ratios may have provided further insights) and hormonal work-up in controls.
Since CgA is not expressed in the adrenocortical adenoma tissue, Cts levels are unlikely to differ between subjects with and without an adrenal adenoma (28, 29), which is what we found here for AI patients compared to age-, sex-, and BMI-matched controls after adjusting for confounding factors. Other researchers showed higher plasma CgA in patients with an adrenal adenoma than in subjects without one, which may underlie a slightly higher unadjusted Cts in our AI patients than controls (24, 25). What should be noticed is a similar distribution of Cts levels in controls and AI patients, including the proportion of subjects with high (>97 ng/ml) Cts: 21% in the former and 23% in the latter. This further suggests that the presence of an AI does not affect Cts. Regarding hormonal activity, we found no differences in Cts between patients with MACS and NFAI, nor correlations between Cts and UFC or 1-mg DST cortisol, possibly due to small sample size.
Despite comparable age, BMI, male-to-female ratio, smoking status and comorbidities, IVSd, LVPWd, LVM, and LVMI were higher in AI patients than controls, which was driven by values recorded in subjects with MACS. Iacobellis et al. reported similar results (higher LVM in AI subjects versus controls, the difference depended on patients with MACS) (30). In our study, SCORE2/-OP was higher in MACS compared to NFAI patients and controls, which indicates increased CV risk associated with subclinical hypercortisolemia. The data support the hypothesis that chronic mild elevation of cortisol levels in AI patients adversely affect the CV system rather than the presence of an adrenal adenoma per se. Low Cts was associated here with a higher prevalence of male gender, HT, MetS, as well as BMI, 24h SBP, UA and lower HDL-C. Consequently, an association between Cts and ASCVD risk was recorded (Figure 6). In line with our results, Cts was lower in obese children with MetS than in those without it, and in normal-weight controls (13); O’Connor et al. showed Cts correlated negatively with BMI (12), and Durakoğlugil et al. reported a positive correlation between Cts and HDL-C (14). In the latter study, a negative correlation between plasma Cts and TGL concentration was also observed (14), which was not confirmed here. Surprisingly, among participants without statins, we recorded weak positive correlations between Cts and TC as well as LDL-C. The former may be connected with a positive correlation between Cts and HDL-C, however, the latter is difficult to explain, and requires further clarification.
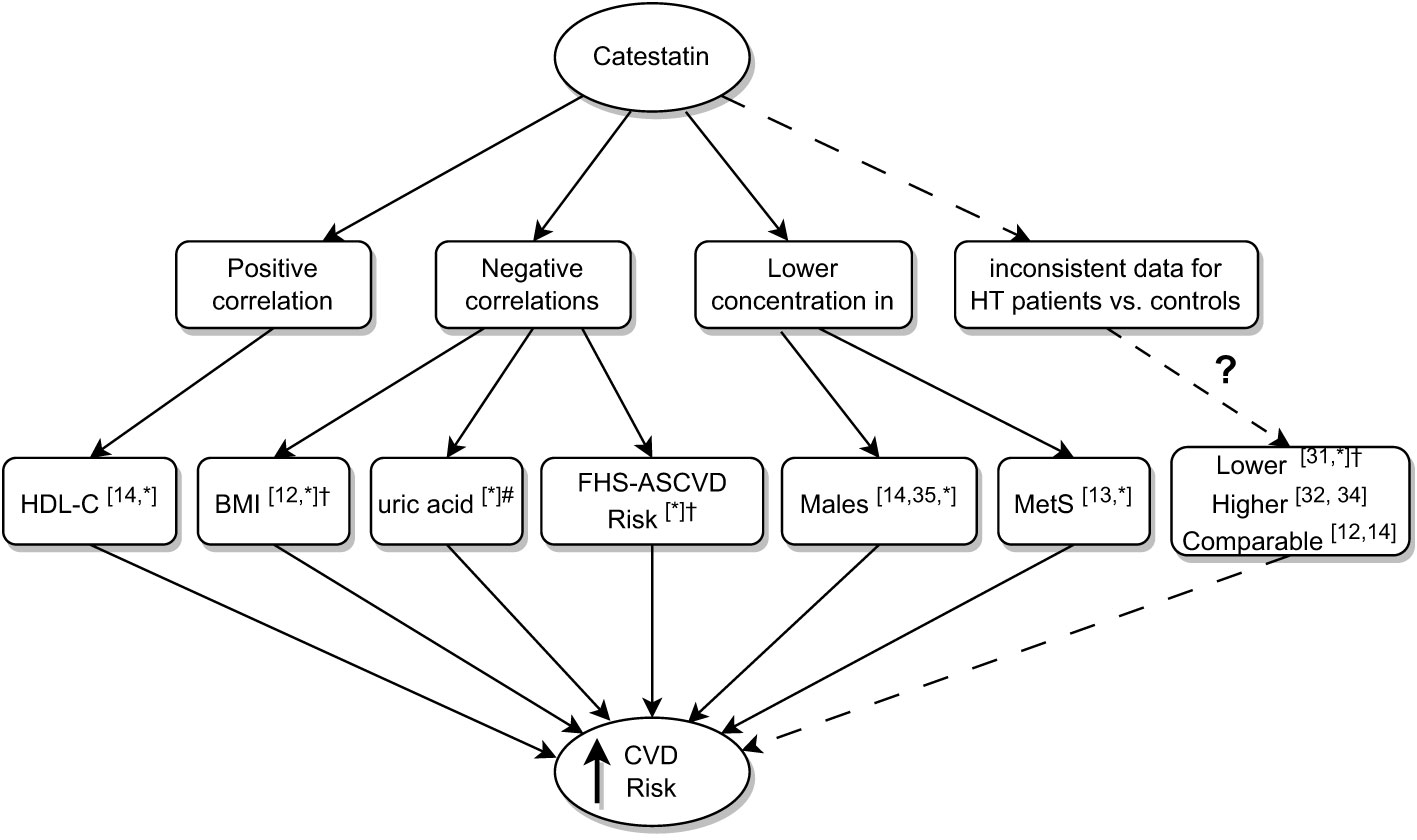
Figure 6 Summary of research on associations between low catestatin and cardiovascular risk. Superscript numbers indicate references to previous studies; *indicate results of the current study; #correlation between uric acid and Cts did not reach significance in AI patients analyzed here, it did upon analyzing AI patients and controls together; †significance achieved in AI patients. AI, adrenal incidentaloma, BMI, body mass index; Cts, catestatin; CVD, cardiovascular disease; FHS-ASCVD Risk, 10-year atherosclerotic cardiovascular disease risk calculated based on the Framingham Heart Study; HDL-C, high density lipoprotein cholesterol; HT, hypertension; MetS, Metabolic Syndrome.
To date, there are controversies regarding Cts in HT; lower Cts levels have been associated with this disease (12, 14, 31–35) (Figure 6). Here, Cts in AI subjects with HT was lower than in normotensive ones, however, the difference was not significant in the controls and we recorded no correlations between Cts and ABPM results. Our data add important facts to the discussion: low Cts levels are more common in HT, however, some patients do exhibit intermediate and high concentrations of the peptide. This was observed in individuals with effective hypotensive treatment revealed by 24-h monitoring.
Further, no significant associations were recorded here between Cts and TTE as well as CCA USG parameters. Small sample size clearly limits conclusions that can be drawn from these data. More sensitive methods (e.g. global longitudinal strain and microvasculature assessment) may have yielded different results.
Possibly, the most intriguing question is the clinical significance of high versus very low/low Cts in individuals with similar established CV risk factors. For instance, non-smoking females aged ca. 60, with overweight and HT (FHS-ASCVD Risk between 10 and 20%) were recorded both in the first half and highest quarter of Cts levels among AI patients. Longitudinal assessment of much larger populations is required to determine whether Cts provides protection against CVD. If so, determining therapeutic strategies that stimulate Cts would be beneficial.
5 Conclusions
We are the first to report that among persons without overt CVD other than primary HT, plasma Cts concentrations in patients with an AI are comparable to those of matched controls with normal adrenal morphology. Correlations between Cts and: HDL-C (positive) as well as BMI, UA and FHS-ASCVD Risk (negative) point at cardioprotective effects of the peptide. Data from ABPM, TTE and CCA intima-media assessment did not yield associations between Cts and BP or HT-mediated organ damage. It must be highlighted that many factors influence Cts, and further research is necessary to apply it as a biomarker.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Independent Bioethics Committee for Scientific Research at Medical University of Gdańsk (NKBB/659/2019). The patients/participants provided their written informed consent to participate in this study.
Author contributions
EZ secured ethical approval for the study, analyzed the data, and reviewed the literature. EZ and PK collected the data and wrote the manuscript. JS and AK performed echocardiography and ultrasound examination of the common carotid artery. PK and KS carried out critical interpretations. All authors contributed to the article, approved the submitted version, and are accountable for the content of the work.
Funding
The study was supported by the project POWR.03.05.00-00-z082/18 co-financed by the European Union through the European Social Fund under the Operational Programme Knowledge Education Development 2014–2020 and by the Medical University of Gdansk (grant MB 2022: 01-10022 and MN 01-350/08/126).
Acknowledgments
The authors would like to thank Anna Koelmer and Peter Grešner from Center for Biostatistics and Bioinformatics of Medical University of Gdańsk and Piotr Wiśniewski for support in data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1198911/full#supplementary-material
Supplementary Figure 1 | Catestatin in normotensive and hypertensive AI patients and controls - analyses in subjects of each sex. Boxplot chart. P-values determined using the Mann-Whitney U test. AI – adrenal incidentaloma.
Supplementary Figure 2 | Catestatin in subjects without and with metabolic syndrome in controls and AI patients - analyses in subjects of each sex. Boxplot chart. P-values determined using the Mann-Whitney U test. AI – adrenal incidentaloma; MetS – metabolic syndrome.
Supplementary Figure 3 | Correlations between catestatin and LDL-C level in AI patients and controls. Correlations were computed by the Spearman rank-order method and were tested separately in subjects with and without statin therapy. AI – adrenal incidentaloma, LDL-C – low density lipoprotein cholesterol, ln – natural logarithm.
Supplementary Figure 4 | Correlations between catestatin and total cholesterol level in AI patients and controls. Correlations were computed by the Spearman rank-order method and were tested separately in subjects with and without statin therapy. AI – adrenal incidentaloma, ln – natural logarithm.
References
1. Yu W, Cheng JD. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharmacol (2020) 11:582680. doi: 10.3389/fphar.2020.582680
2. Koosha P, Roohafza H, Sarrafzadegan N, Vakhshoori M, Talaei M, Sheikhbahaei E, et al. High sensitivity c-reactive protein predictive value for cardiovascular disease: a nested case control from isfahan cohort study (ICS). Glob Heart (2020) 15(1):1–13. doi: 10.5334/GH.367
3. Mozaffarian D, Wilson PWF, Kannel WB. Beyond established and novel risk factors lifestyle risk factors for cardiovascular disease. Circulation (2008) 117(23):3031–8. doi: 10.1161/CIRCULATIONAHA.107.738732
4. Mahata SK, Corti A. Chromogranin a and its fragments in cardiovascular, immunometabolic, and cancer regulation. Ann N Y Acad Sci (2019) 1455(1):34–58. doi: 10.1111/nyas.14249
5. Zalewska E, Kmieć P, Sworczak K. Role of catestatin in the cardiovascular system and metabolic disorders. Front Cardiovasc Med (2022) 9:909480. doi: 10.3389/fcvm.2022.909480
6. Mahata SK, Mahapatra NR, Mahata M, Wang TC, Kennedy BP, Ziegler MG, et al. Catecholamine secretory vesicle stimulus-transcription coupling in vivo. demonstration by a novel transgenic promoter/photoprotein reporter and inhibition of secretion and transcription by the chromogranin a fragment catestatin. J Biol Chem (2003) 278(34):32058–67. doi: 10.1074/jbc.M305545200
7. Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, et al. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology (2008) 149(10):4780–93. doi: 10.1210/en.2008-0318
8. Bandyopadhyay GK, Vu CU, Gentile S, Lee H, Biswas N, Chi NW, et al. Catestatin (Chromogranin A352-372) and novel effects on mobilization of fat from adipose tissue through regulation of adrenergic and leptin signaling. J Biol Chem (2012) 287(27):23141–51. doi: 10.1074/jbc.M111.335877
9. Bandyopadhyay G, Tang K, Webster NJG, van den Bogaart G, Mahata SK. Catestatin induces glycogenesis by stimulating the phosphoinositide 3-kinase-AKT pathway. Acta Physiol (Oxf) (2022) 235(1):e13775. doi: 10.1111/apha.13775
10. Chen Y, Wang X, Yang C, Su X, Yang W, Dai Y, et al. Decreased circulating catestatin levels are associated with coronary artery disease: the emerging anti-inflammatory role. Atherosclerosis (2019) 281:78–88. doi: 10.1016/j.atherosclerosis.2018.12.025
11. Wang X, Xu S, Liang Y, Zhu D, Mi L, Wang G, et al. Dramatic changes in catestatin are associated with hemodynamics in acute myocardial infarction. Biomarkers (2011) 16(4):372–7. doi: 10.3109/1354750X.2011.578260
12. O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens (2002) 20(7):1335–45. doi: 10.1097/00004872-200207000-00020
13. Simunovic M, Supe-Domic D, Karin Z, Degoricija M, Paradzik M, Bozic J, et al. Serum catestatin concentrations are decreased in obese children and adolescents. Pediatr Diabetes (2019) 20(5):549–55. doi: 10.1111/pedi.12825
14. Durakoğlugil ME, Ayaz T, Kocaman SA, Kırbaş A, Durakoğlugil T, Erdoğan T, et al. The relationship of plasma catestatin concentrations with metabolic and vascular parameters in untreated hypertensive patients: influence on high-density lipoprotein cholesterol. Anatol J Cardiol (2015) 15(7):577–85. doi: 10.5152/akd.2014.5536
15. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology (ESC) and the European society of hypertension (ESH). Eur Heart J (2018) 39(33):3021–104. doi: 10.1093/eurheartj/ehy339
16. Yağiz B, Akalin A, Yorulmaz G, Macunluoğlu AC, Yağiz O. Can non-functional adrenal incidentaloma be ranked among cardiovascular risk factors? Eur Res J (2022) 8(6):747–54. doi: 10.18621/eurj.872835
17. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol (2016) 175(2):G1–34. doi: 10.1530/EJE-16-0467
18. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international. Circulation (2009) 120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
19. D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the framingham heart study. Circulation (2008) 117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579
20. American Heart Association. (n.d.). 2018 Prevention Guidelines Tool CV Risk Calculator. Available at: https://static.heart.org/riskcalc/app/index.html#!/baseline-risk/.
21. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J (2021) 42(34):3227–337. doi: 10.1093/eurheartj/ehab484
22. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J (2018) 39(33):3021–104. doi: 10.1093/eurheartj/ehy339
23. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging (2015) 16(3):233–71. doi: 10.1093/ehjci/jev014
24. Starling MR, Walsh RA. Accuracy of biplane axial oblique and oblique cineangiographic left ventricular cast volume determinations using a modification of simpson’s rule algorithm. Am Heart J (1985) 110(6):1219–25. doi: 10.1016/0002-8703(85)90016-x
25. Mahata SK, Mahata M, Yoo SH, Taupenot L, Wu H, Aroda VR, et al. A novel, catecholamine release-inhibitory peptide from chromogranin a: autocrine control of nicotinic cholinergic-stimulated exocytosis. Adv Pharmacol (1998) 42(1):260–4. doi: 10.1016/S1054-3589(08)60743-7
26. Zhu D, Wang F, Yu H, Mi L, Gao W. Catestatin is useful in detecting patients with stage b heart failure. Biomarkers (2011) 16(8):691–7. doi: 10.3109/1354750X.2011.629058
27. Glinicki P, Jeske W. Chromogranin a (CgA) - the influence of various factors in vivo and in vitro, and existing disorders on it’s concentration in blood. Endokrynol Pol (2010) 61(4):384–7.
28. Bernini G, Moretti A, Fontana V, Orlandini C, Miccoli P, Berti P, et al. Plasma chromogranin a in incidental non-functioning, benign, solid adrenocortical tumors. Eur J Endocrinol (2004) 151(2):215–22. doi: 10.1530/eje.0.1510215
29. Bernini GP, Moretti A, Borgioli M, Bardini M, Miccoli P, Berti P, et al. Plasma and tissue chromogranin in patients with adrenocrtical adenomas. J Endocrinol Invest (2004) 27(9):821–5. doi: 10.1007/BF03346275
30. Iacobellis G, Petramala L, Barbaro G, Kargi AY, Serra V, Zinnamosca L, et al. Epicardial fat thickness and left ventricular mass in subjects with adrenal incidentaloma. Endocrine (2013) 44(2):532–6. doi: 10.1007/s12020-013-9902-5
31. O’Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, et al. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin a: implications for secretion and blood pressure. Circulation (2008) 118(3):247–57. doi: 10.1161/CIRCULATIONAHA.107.709105
32. Meng L, Ye XJ, Ding WH, Yang Y, Di BB, Liu L, et al. Plasma catecholamine release-inhibitory peptide catestatin in patients with essential hypertension. J Cardiovasc Med (2011) 12(9):643–7. doi: 10.2459/JCM.0b013e328346c142
33. Tüten N, Güralp O, Gök K, Hamzaoglu K, Oner YO, Makul M, et al. Serum catestatin level is increased in women with preeclampsia. J Obstet Gynaecol (Lahore) (2022) 42(1):55–60. doi: 10.1080/01443615.2021.1873922
34. Kumric M, Vrdoljak J, Dujic G, Supe-Domic D, Ticinovic Kurir T, Dujic Z, et al. Serum catestatin levels correlate with ambulatory blood pressure and indices of arterial stiffness in patients with primary hypertension. Biomolecules (2022) 12(9). doi: 10.3390/biom12091204
Keywords: catestatin, adrenal incidentaloma (AI), cardiovascular disease(s), risk predictor, metabolic syndrome
Citation: Zalewska E, Kmieć P, Sobolewski J, Koprowski A and Sworczak K (2023) Low catestatin as a risk factor for cardiovascular disease – assessment in patients with adrenal incidentalomas. Front. Endocrinol. 14:1198911. doi: 10.3389/fendo.2023.1198911
Received: 02 April 2023; Accepted: 20 June 2023;
Published: 14 July 2023.
Edited by:
Carmine Izzo, University of Salerno, ItalyReviewed by:
Elke Marjolein Muntjewerff, Uppsala University, SwedenYalin Wu, University of Alabama at Birmingham, United States
Copyright © 2023 Zalewska, Kmieć, Sobolewski, Koprowski and Sworczak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piotr Kmieć, cGlvdHJrbWllY0BndW1lZC5lZHUucGw=
 Ewa Zalewska
Ewa Zalewska Piotr Kmieć
Piotr Kmieć Jakub Sobolewski
Jakub Sobolewski Andrzej Koprowski2
Andrzej Koprowski2