- 1First School of Clinical Medicine, Henan University of Chinese Medicine, Zhengzhou, China
- 2Department of Endocrinology, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
- 3Department of Geriatrics, First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Aims: The aim of this meta-analysis is to evaluate the potential correlation between obesity and overweight, and the vulnerability to urinary incontinence (UI) in women aged middle-aged and above.
Methods: We searched PubMed, Cochrane Library, and Embase for observational studies published between the inception of the databases and April 25, 2023. A fixed-effects model was used when the P>0.1 and the I2 ≤ 50%. In cases where I2 ≥ 50% (indicating significant heterogeneity), a random-effects model was applied. For the purpose of evaluating publication bias, a funnel plot and Egger’s test were used. Stata 14.0 was used for all statistical analyses.
Findings: This meta-analysis includes 16 observational studies, covering29,618 individuals. The pooled analysis shows that being overweight(25 kg/m2≤BMI<30kg/m2) in middle-aged and elderly women is more likely to develop UI (OR=1.27; 95% CI: 1.17-1.37; I2 = 51.8%, P=0.013). Middle-aged and elderly women with obesity(30 kg/m2≤BMI<35 kg/m2) are significantly more likely to develop UI (OR=1.60; 95% CI: 1.42-1.81; I2 = 71.8%, P=0.000). In addition, the results indicated a higher probability of UI in middle-aged and older women with obesity class II (BMI≥35 kg/m2) (OR=1.85; 95% CI: 1.59-2.16; I2 = 48.1%, P=0.103). In subgroup analysis, there is no direct relationship between the obesity in middle-aged and elderly women and an increased risk of stress urinary incontinence (SUI) (OR=1.31; 95% CI: 0.99-1.74; I2 = 63.7%, P=0.011). In middle-aged and elderly women with obesity are more likely to develop urgent urinary incontinence (UUI) (OR=2.11; 95% CI: 1.54-2.89; I2 = 80.2%, P=0.000).
Conclusion: In this meta-analysis, overweight and obesity are associated with an increased risk of UI in middle-aged and elderly women. Obesity and overweight are independent risk factors for UI, as demonstrated by this study.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023421986.
1 Introduction
Urinary incontinence (UI) refers to the involuntary loss of urine from the urethra, which is not controlled by the individual’s will (1). It is a common yet often neglected condition, with an estimated incidence rate ranging from 10% to 40% (2). There are three types of UI: stress urinary incontinence (SUI), urgency urinary incontinence (UUI) and mixed urinary incontinence (MUI) (3).
It is widely believed that the mode and frequency of delivery are commonly considered the main causes of female UI (4). However, the prevalence rate of UI in women of all ages is expanding, particularly with the emergence of social aging (5, 6). The issue is more pronounced in middle-aged and elderly women (7). Although UI affects a majority of women, it is often a private issue that prevents them from actively seeking timely treatment, resulting in potential long-term consequences. Therefore, early identification of risk factors linked to UI could aid in preventing its onset and progression.
Recent studies have demonstrated that overweight and obesity pose a heightened risk for UI in women (8–10). The prevalence of obesity globally is highest in the age range of 60 to 74 years, with roughly 48% of women in the 50-54 age group being overweight and approximately 19% being obese (11). Notably, severe obesity (defined as BMI ≥ 35 kg/m2) is markedly more common in women compared to men (12). Additionally, overweight and obesity have been identified as robust predictors of UI, with the risk being significantly elevated in cases of obesity. Nevertheless, no published meta-analysis currently exists that comprehensively examines the relationship between overweight/obesity and UI risk in middle-aged and elderly women. The purpose of this study is to (i) examine the association between overweight, obesity, and UI in middle-aged and elderly women, and (ii) obesity is investigated in relation to UI subtypes.
2 Methods
This investigation adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13) and the protocol was preregistered on the PROSPERO platform (CRD42023421986), exhibiting its commitment to transparent and thorough methodology.
2.1 Data sources
A systematic search of relevant databases, including PubMed, Cochrane Library, and EMBASE, was conducted up until 25 April 2023. Medical subject headings (MeSHs) and keywords were combined in the search strategy, with no language restrictions. The keywords used were “obesity”, “obese”, “urinary incontinence”, “uroclepsia”, and “uracratia”, along with their variants (Appendix, Text S1). The reference lists of included studies and relevant systematic reviews were also manually searched to identify potentially eligible studies. Our search approach was aimed at ensuring an exhaustive identification of relevant study.
2.2 Eligibility criteria
The eligible studies were required to meet the following criteria (1): case-control, cross-sectional, or cohort study design; (2) investigation of the association between obesity, overweight, and the risk of UI; (3) use of adjusted OR and corresponding 95% CI to present the risk of UI as the outcome. In this study, ‘overweight’ was defined as ‘25 kg/m2≤BMI ≤ 30kg/m2’, ‘obesity’ was defined as ‘BMI ≥30kg/m2’and ‘obesity classⅡ’ was defined as ‘BMI ≥35kg/m2’.
The analysis excluded trials that did not report an OR estimate along with its 95% CI, in situations where multiple studies presented data from the same cohort, the study with the longest follow-up period or the largest number of participants was selected to ensure the inclusion of the most comprehensive and reliable information in our analysis. In addition, conference abstracts, study protocols, duplicate publications, and studies that did not report outcomes of interest were excluded.
2.3 Study selection
Based on predefined eligibility and exclusion criteria, two independent reviewers (X Shang and XQ Jin) carefully screened the literature. Initially, duplicate and irrelevant articles were eliminated based on their titles and abstracts. After obtaining the complete texts of the potentially eligible articles, their eligibility was assessed thoroughly. In case of any discrepancies, discussions were held with SX Yan to arrive at a consensus. The selection of studies was thus meticulously carried out to ensure the reliability and validity of the final results.
2.4 Data extraction
X Shang and XQ Jin independently extracted the pertinent data, including the first author, publication year, country or region of origin, study design, sample size, duration of follow-up, study period, age range, diagnostic criteria utilized, and confounder adjustments. The extracted data were subsequently reviewed and verified by SX Yan, who engaged in discussions with the former two researchers in cases where discrepancies arose.
2.5 Risk of bias
The quality of cohort studies was assessed using the Newcastle-Ottawa Scale (NOS) (14), which consists of stars ranging from 0 to 9 for selecting participants and measuring exposure, 2 stars for comparing results and 3 stars to the assessment of outcomes and adequacy of follow-up. A higher score indicates a higher quality study. Scores 0-3 indicate low quality, 4-6 indicate moderate quality, and 7-9 indicate high quality. To evaluate the quality of a cross-sectional study, we adopted the American Agency for Health Care Quality and Research’s (AHRQ) (15) quality evaluation items for cross-sectional studies and scored each item as ‘1’ for a ‘YES’ response, or ‘0’ for an ‘UNCLEAR’ or ‘NO’ response. The resulting scores were categorized into low quality (0–3), moderate quality (4–7), and high quality (8–11).
2.6 Statistical analysis
Software Stata 14.0 was used for the analysis, whereby adjusted OR and their 95% CI were extracted from the included studies to assess the association between obesity and UI risk. Heterogeneity was evaluated using the χ2-test and I2-values (16). In such high-quality research (17), it is mentioned that I2 ≤ 50% and P > 0.1, we used a fixed-effects model. Conversely, a random-effects model was adopted if I2 exceeded 50%, indicating significant heterogeneity (18). To ensure the validity of the overall effects, sensitivity analysis was performed. Additionally, funnel plots and Egger’s regression test were employed to evaluate for publication biases (19).
3 Results
3.1 Literature selection
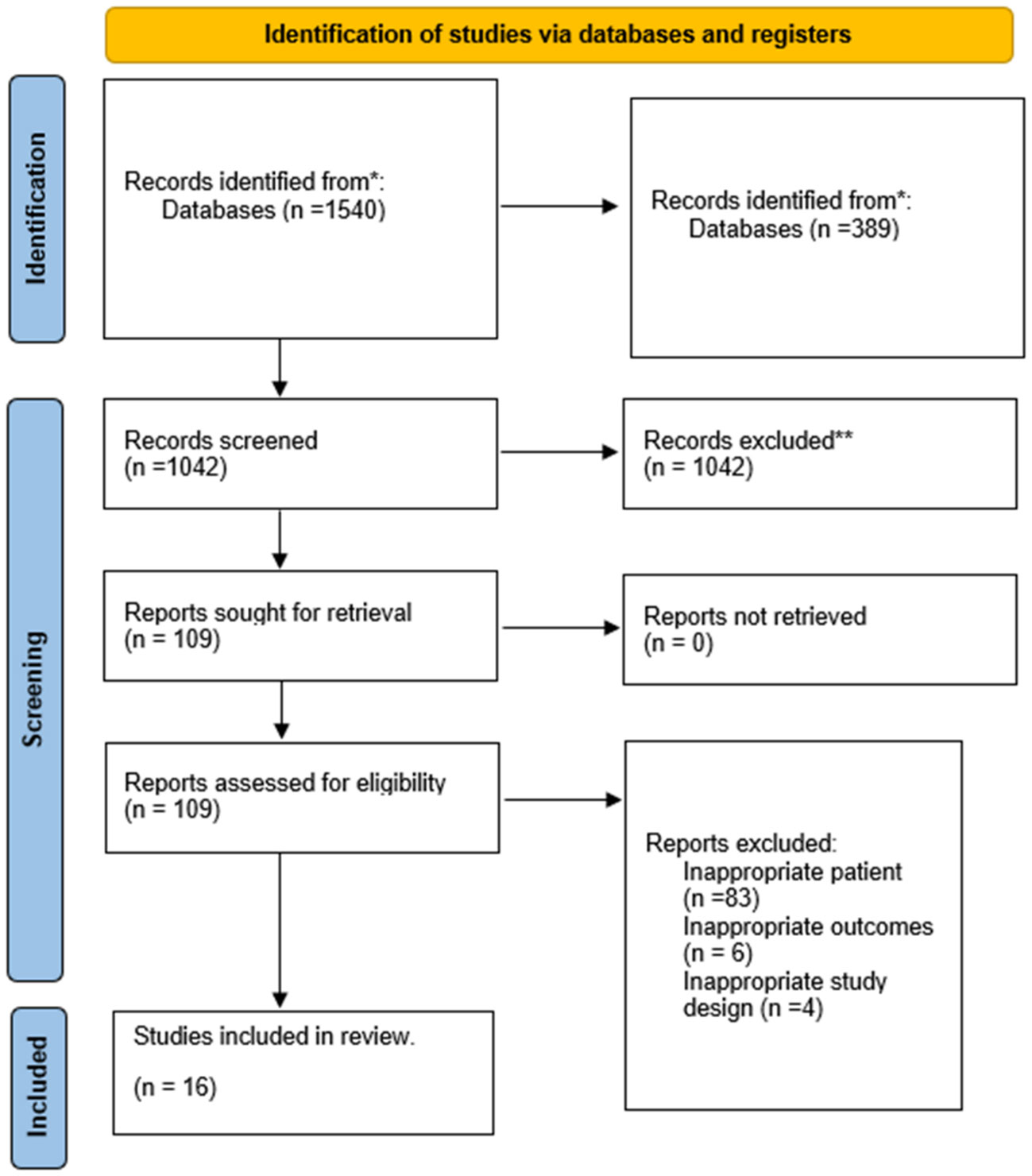
A systematic search was conducted for observational studies published before April 25, 2023, yielding a total of 1,540 results. Subsequently, 389 duplicate articles were removed, and 1,042 articles were excluded following the screening of titles and abstracts. After reading the full-text, 109 articles were further excluded, with 92 identified as inappropriate due to patient population, three due to inappropriate outcomes, and four due to inappropriate study design. Ultimately, a total of 16 studies were included in this systematic review. Details of the selection process are presented in Figure 1.
3.2 Study characteristics
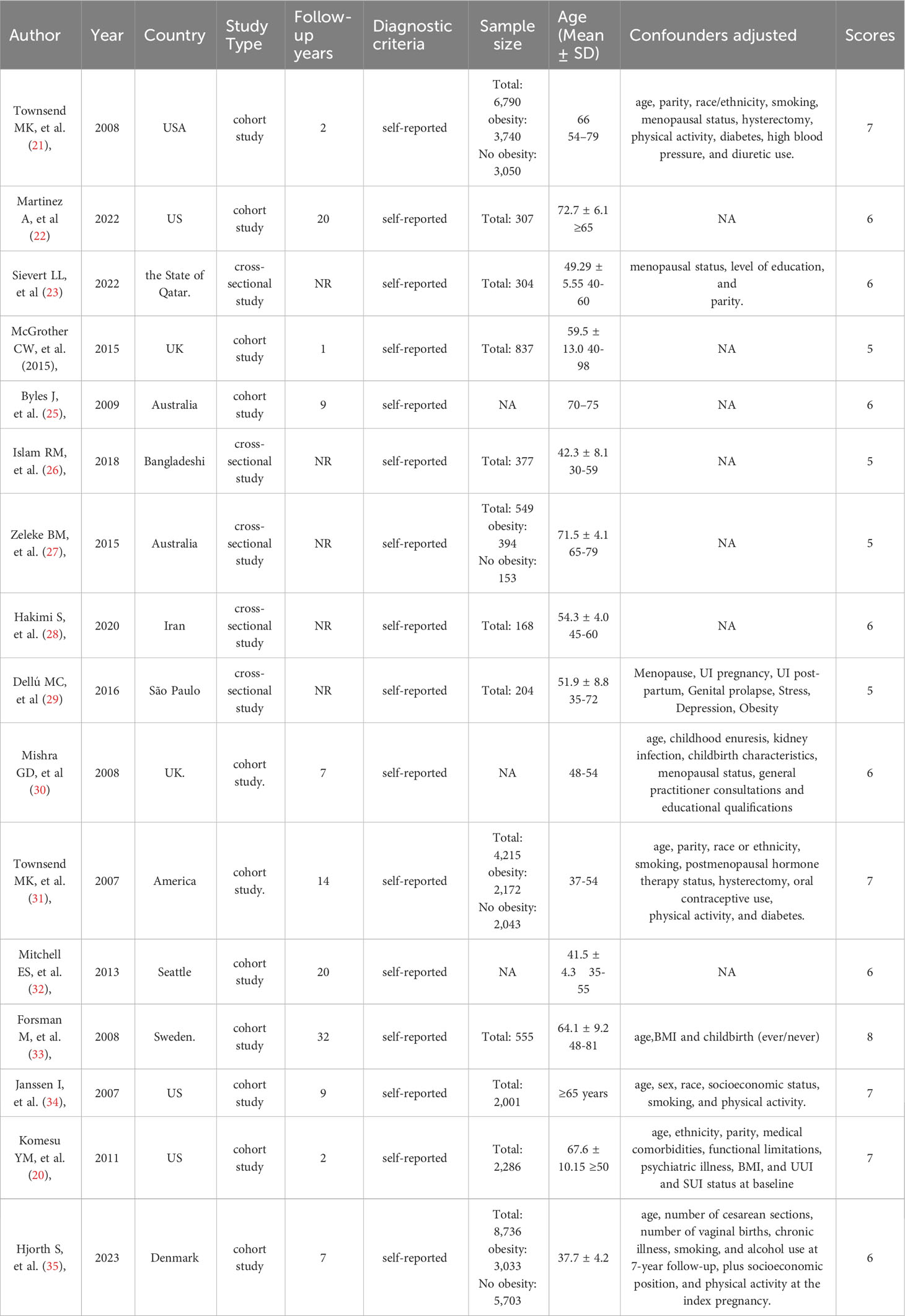
This meta-analysis encompasses a total of 16 studies (20–35),which aimed at investigating an overall sample size of 27,389 individuals. Out of the 16 studies, eleven were cohort studies (20–22, 24, 25, 30–35), while the remaining five were cross-sectional studies16,19-22 (23, 26–29). The studies included in this analysis were published between 2007 and 2023. The sample size of the studies ranged from 168 to 8,736 participants. The majority of the studies presented adjusted estimates, however, some studies did not, including seven studies (22, 24–28, 32).The adjusted confounders used in these studies varied slightly. Detailed information of the characteristics of these 16 studies are provided in Table 1.
3.3 Quality assessment
We assessed the quality of the eighteen studies via the NOS and AHRQ, and the scores are presented in Table 1. Five studies (20, 21, 31, 33, 34) were scored as ≥7 (high quality) and six studies (22, 24, 25, 30, 32, 35) were scored as 6 and 5 (moderate quality) according to the NOS criteria. An average score of 6.5 was obtained from these thirteen studies, representing an overall moderate quality. Five studies (23, 26–29) were scored as 6 and 5 (moderate quality) according to the AHRQ criteria. The average score of these five studies was 5.4, representing an overall moderate quality.
3.4 Overweight and risk of UI
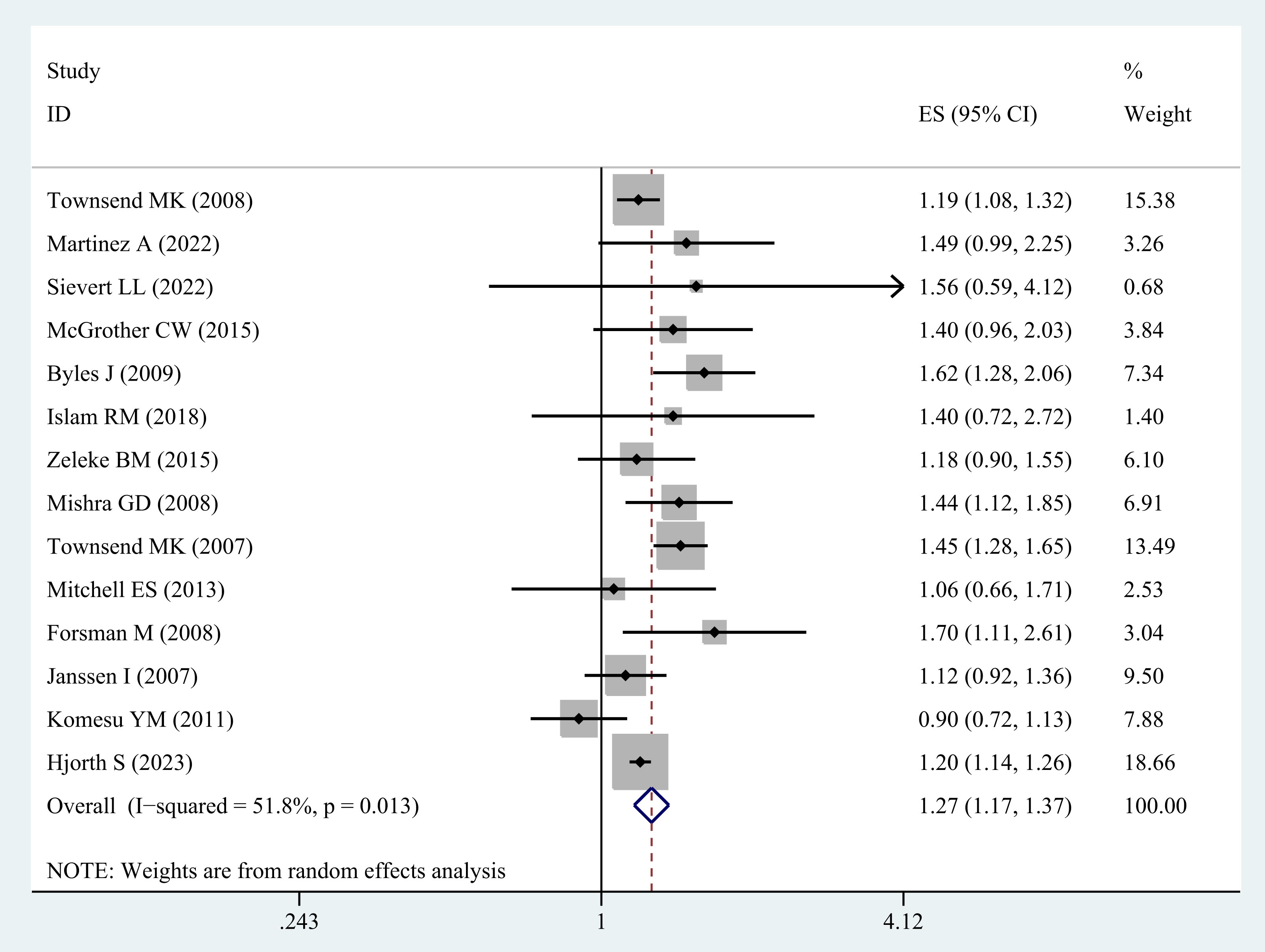
A total of 14 studies (20–27, 30–35) examined the relationship between overweight and UI in middle-aged and older women. The synthesis of these studies reveals that overweight is significantly associated with a heightened risk of UI (OR= 1.27; 95% CI: 1.17-1.37; I2 = 51.8%, P=0.013; Figure 2).
Furthermore, the sensitivity analysis of the individual studies did not demonstrate a reversal of the pooled-effect size, indicating the robustness of the results (Supplementary Figure A).
3.5 Obesity and risk of UI
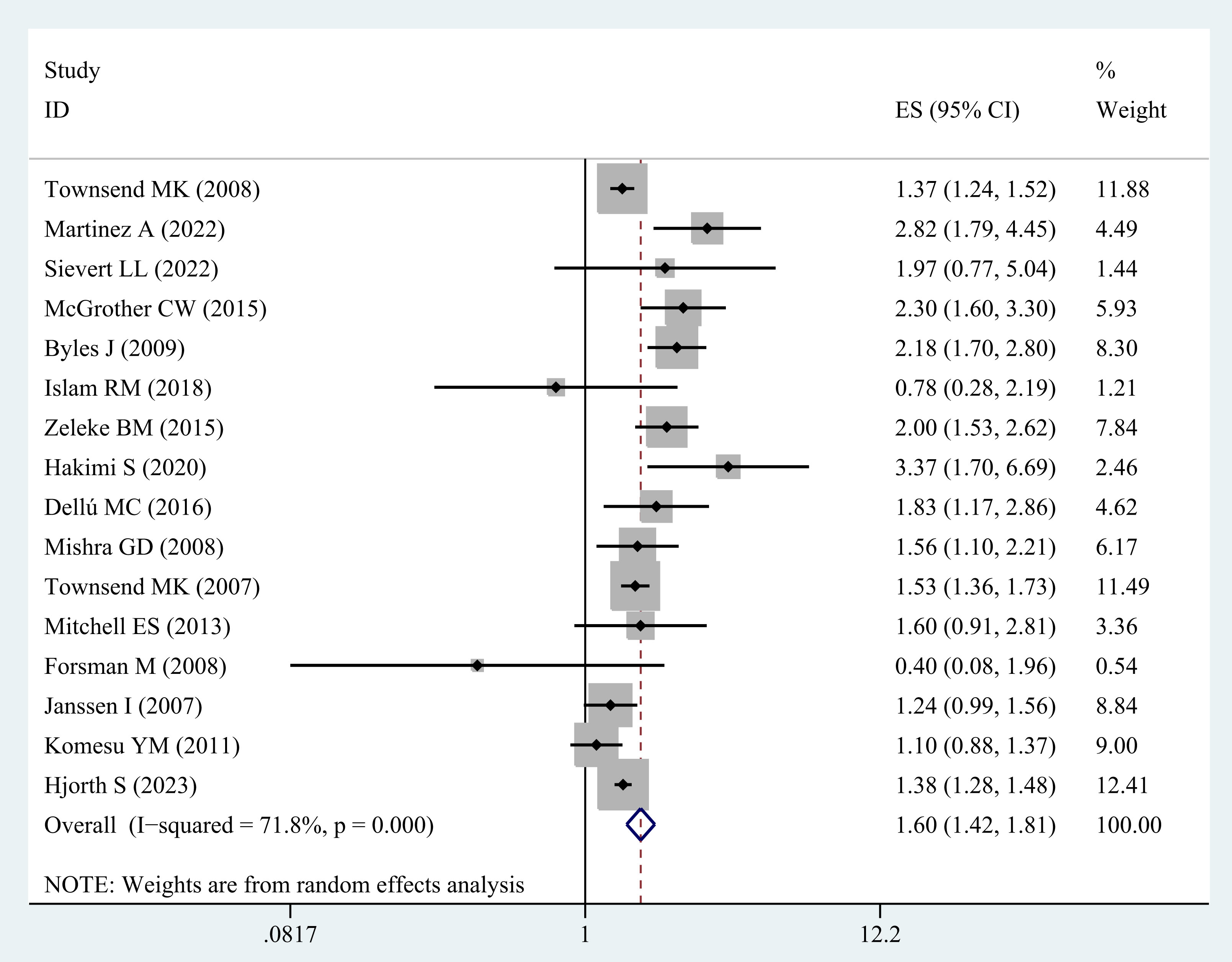
Sixteen studies (20–35) were included in the analysis evaluating the correlation between obesity and the risk of UI among middle-aged and elderly women. There is a significant association between obesity and UI in this group of women (OR = 1.60; 95% CI: 1.42-1.81; I2 = 71.8%, p=0.000; Figure 3).
Moreover, the sensitivity analysis corroborated the robustness of the results, as none of the individual studies changed the pooled effect size (Supplementary Figure B).
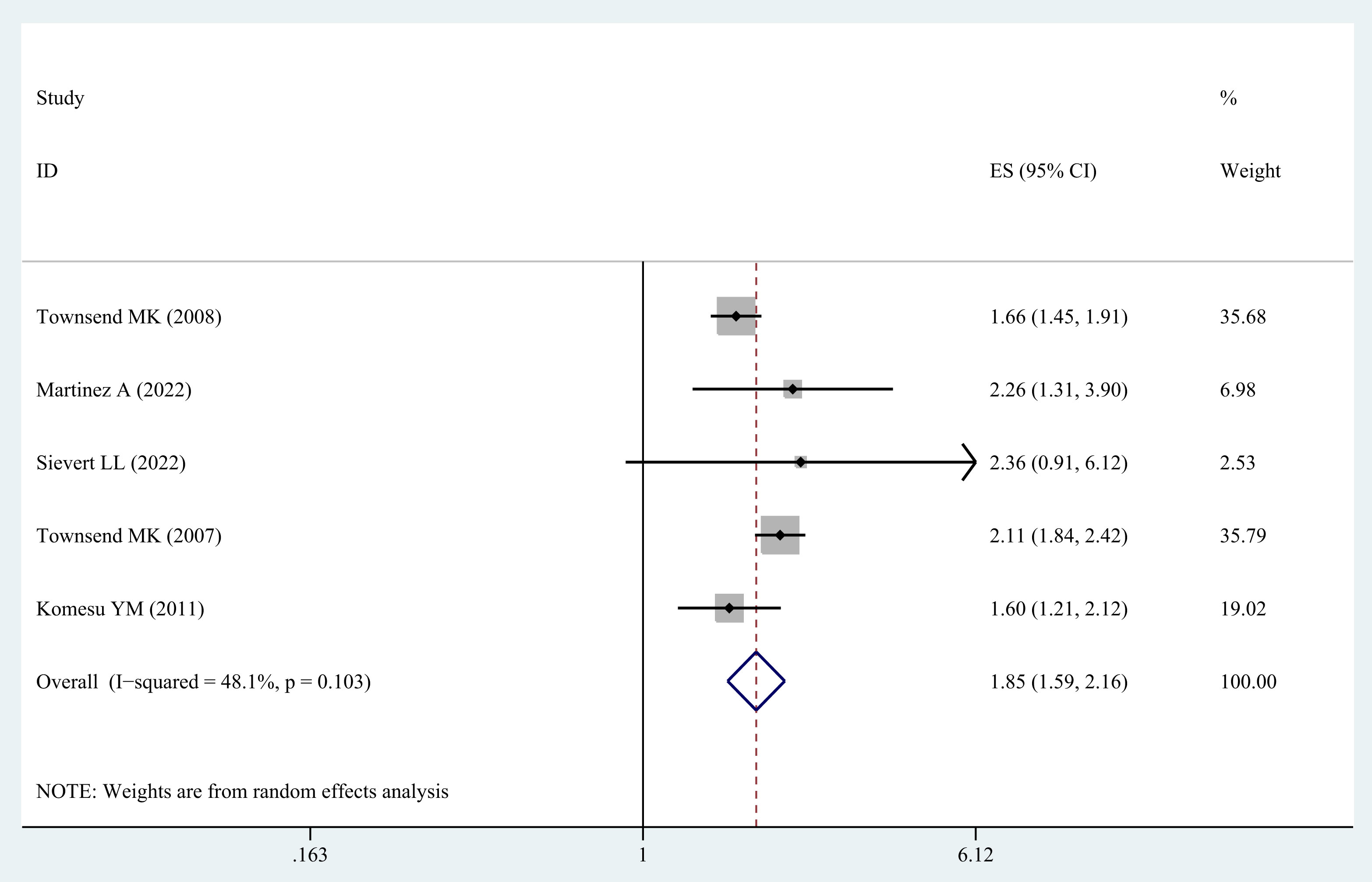
3.6 Obesity class II and risk of UI
Five studies (20–23, 31) were incorporated in order to examine the correlation between obesity class II in middle-aged and older women and the possibility of UI. In general, the results indicated a higher probability of UI in middle-aged and older women with obesity class II (OR=1.85; 95% CI: 1.59-2.16; I2 = 48.1%, p=0.103; Figure 4).
According to the sensitivity analysis, none of the included studies significantly reversed the pooled effect size when analyzed individually. This indicates that the results obtained from the pooled analysis are robust and reliable (Supplementary Figure C).
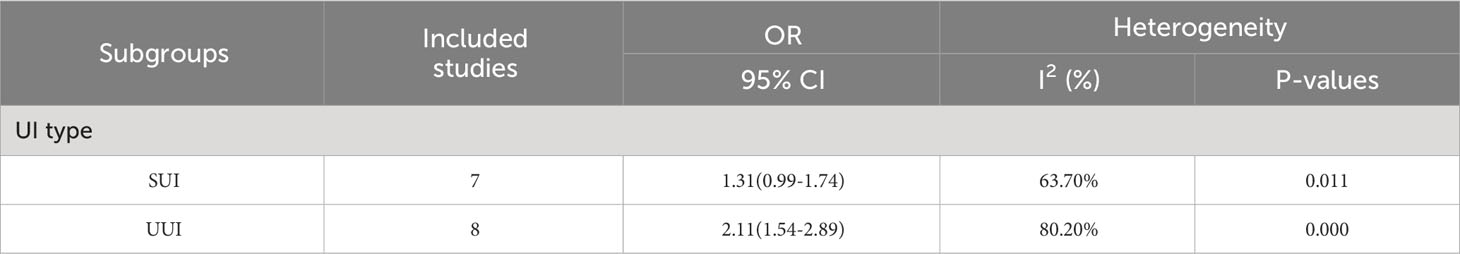
3.7 Subgroup analysis
We performed a subgroup analysis on the various types of UI in our study. The results indicated no significant association between obesity and stress urinary incontinence (SUI) in middle-aged and elderly women (OR=1.31; 95% CI: 0.99-1.74; I2 = 63.7%, P=0.011). Our analysis, however, revealed that obesity is significantly associated with urge urinary incontinence (UUI) (OR=2.11; 95% CI: 1.54-2.89; I2 = 80.2%, P=0.000) (Table 2).
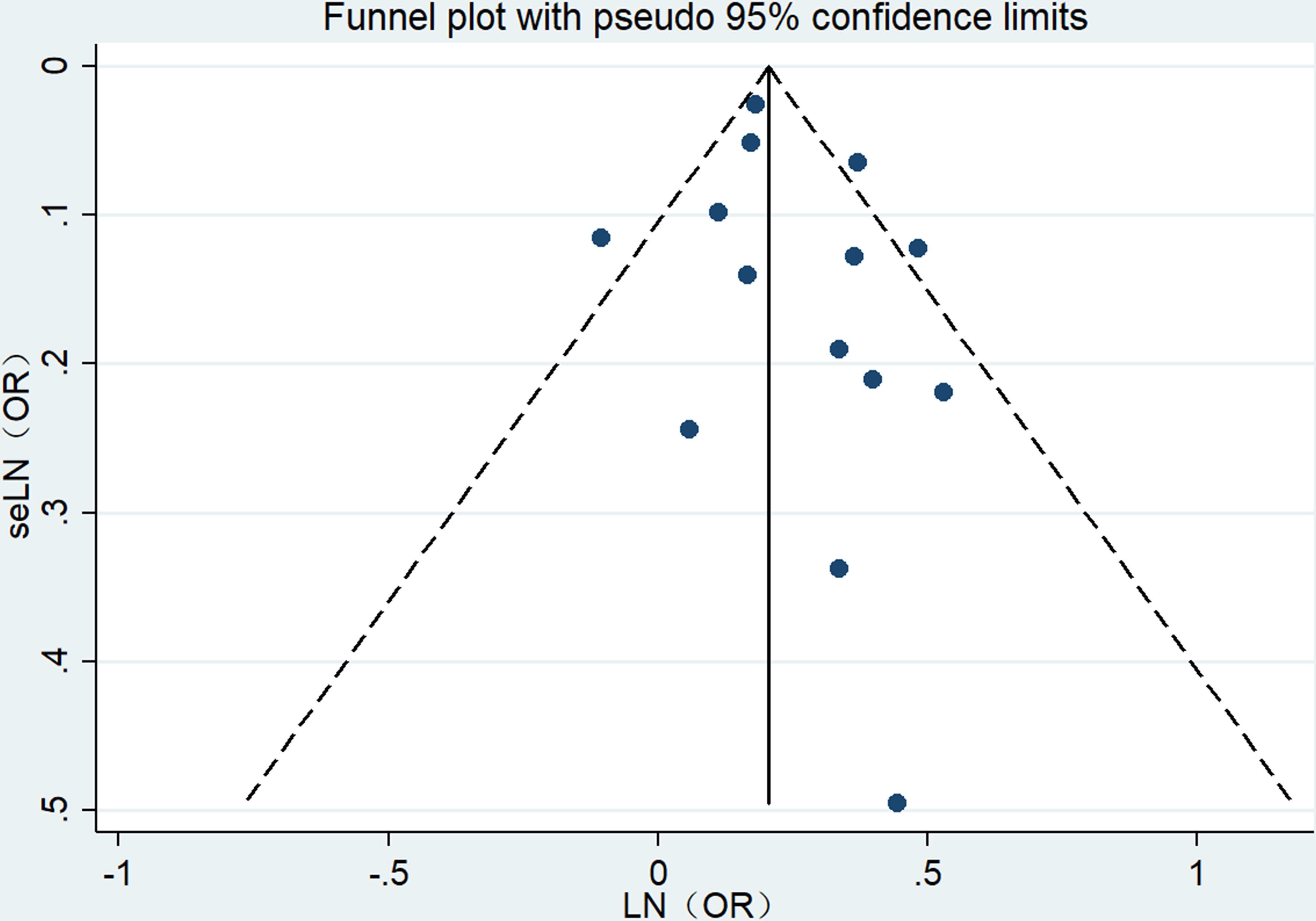
3.8 Publication bias
The visual inspection of the funnel plot in our meta-analysis revealed a notable absence of any significant publication bias concerning the relationship between obesity in individuals of middle and advanced age and the risk of UI (Figure 5). Moreover, Egger’s regression test (P = 0.294) confirmed that our meta-analysis did not contain any publication bias.
4 Discussion
4.1 Main findings
The present meta-analysis involved a total of 16 studies that encompassed a vast sample of 27,389 individuals, and thoroughly assessed the association between overweight, obesity, and the likelihood of UI in middle-aged and elderly women. Our systematic analysis revealed that the risk of developing UI in overweight and obese middle-aged and elderly women was significantly higher, with an overall risk increase of 1.27-fold, 1.60-fold, and 1.85-fold, respectively, compared to the non-obese control group. These results suggest that both overweight and obesity may potentiate the risk of UI as independent contributory factors.
4.2 Interpretation of findings
It is noteworthy that a previous review has explored the correlation between overweight and obesity and UI in young to middle-aged women, confirming them as risk factors for UI (8). However, the health concerns of middle-aged and elderly women have become increasingly important with a growing aging population. Therefore, our study aims to examine the association between overweight and obesity in middle-aged and elderly women and UI, providing more robust evidence through a greater number of cohort and cross-sectional studies. We also conducted a subgroup analysis to examine the impact of overweight and obesity on different types of UI, enhancing our understanding of their specific effects. Our study results highlight overweight and obesity as significant risk factors for UI in middle-aged and elderly women, emphasizing the need for their consideration in clinical practice.
An alternative investigation examined a meta-analysis pertaining to BMI, abdominal obesity, weight gain, and their association with UI risk (10). The findings demonstrated that the total RR for each 5 kg/m2 increase in BMI was 1.20 (95% CI: 1.16-1.25, I2 = 58%, n=13), whereas the RR for every 10 kg weight gain was 1.34 (95% CI: 1.11-1.62, I2 = 90%, n=2). Notably, no correlation was established between high BMI and UI risk in middle-aged and elderly women. Hence, we sought to re-examine and analyze the link between overweight/obesity and UI risk in middle-aged and elderly women. Our study revealed that both overweight and obesity augment UI risk in this demographic.
The global prevalence of UI has reached 8.7%. Studies indicate that approximately 50% of adult women may experience UI, which is twice the incidence rate of male UI (36, 37). The likelihood of developing UI increases with age and it is a common condition among the elderly. This condition has a considerable impact on quality of life, as it can hinder normal social activities and physical health (38). Although UI is strongly associated with age, recent research suggests that obesity plays a significant role in both the prevalence and 5-year incidence rate of UI (39). Overweight and obesity have been identified as risk factors for UI in women, with obese women being more susceptible to this condition than their normal-weight counterparts. However, limited research is available on the pathophysiological mechanisms underlying the association between obesity and UI.
Research has revealed that middle-aged and elderly women face a significantly higher risk of UI as they age (35, 40). Many women attribute UI to age and underestimate the impact of overweight and obesity on their symptoms, presuming it to be a natural occurrence. Due to several factors, women often conceal their UI medical history, and even when diagnosed, few seek active treatment (41, 42). However, the impact of obesity on the UI of middle-aged and elderly women should be given more consideration. Obesity has also been linked to UI surgery in certain studies. Weight loss interventions are beneficial in improving UI, and procedures like weight loss surgery can ameliorate the quality of life of UI patients (43, 44).
The female urinary system has a unique structure that increases the susceptibility to risk factors such as pregnancy, childbirth, and hysterectomy, which might damage pelvic floor muscle and connective tissues. In postmenopausal women, with decreased estrogen levels, there is a reduction in the capacity to maintain the health of the bladder lining and urethra, leading to tissue degradation and exacerbation of UI development. Furthermore, with increasing age, the muscles of the bladder and urethra become weak and lose their contractility. decreased bladder capacity and feeling of fullness, decreased rate of detrusor muscle contraction, decreased pelvic floor muscle resistance and increased residual urine volume. Dysfunction of the detrusor and sphincter reduces bladder capacity, thereby increasing the risk of involuntary urination (45). Overweight and obesity can impair normal urination and elevate the incidence of UI. Being overweight places more stress on the bladder and surrounding muscles, leading to a decrease in their strength and urine leakage during coughing or sneezing. Central adipose tissue weight may result in a chronic increase in abdominal pressure, causing tension in the urethral support, increasing the risk of UI development. Apart from these reasons, an increase in BMI might trigger UI development via other mechanisms. Overweight and obesity result in oxidative strain and chronic insulin resistance, leading to pelvic floor vascular damage and sclerosis of detrusor and sphincter. Current research conducted in high-income countries suggests that SUI is the most prevalent type of UI (6, 46). Our research also supports the argument that BMI is an independent predictor of SUI.
The development of UI may pose a series of associated challenges, including persistent skin moisture leading to skin problems such as rashes, infections, and sores, an increased risk of recurrent urinary tract infections, as well as physical and psychological discomfort that can disrupt personal lives. Recent studies indicate that a longer period of being overweight or obese heightens the risk of UI, thus calling for targeted interventions aimed at enhancing physical health among middle-aged and elderly women to prevent UI. Our findings reveal a strong association between high BMI, abdominal pressure, and bladder pressure, lending credence to the proposition that avoiding high BMI may help alleviate the burden of UI reported among middle-aged and elderly women.
4.3 Implications and limitations
This meta-analysis provides a comprehensive overview of the current evidence concerning the association between overweight/obesity in middle-aged and elderly populations and the risk of UI. Our findings highlight the necessity of increased attention towards the risk of UI in obese patients during these life stages, facilitating the early identification of high-risk populations for UI.
It is important to note, however, that our study has certain limitations. First and foremost, only 16 related studies were eligible for inclusion due to the complexity of grouping types. Moreover, subgroup analysis was solely performed on two common subtypes of SUI and UUI, covariate analysis was not conducted. The adjustment factors incorporated into the study exhibit variability, with certain studies omitting any form of adjustment. This divergence in adjustment practices could potentially impact the precision of the findings. Nonetheless, most of the included studies mentioned adjusted confounding factors, effectively controlling for confounding bias. Therefore, our results are convincing with considerable clinical practicality.
Due to the absence of standardized diagnostic criteria, we have synthesized several definitions of UI in our analysis. The diagnostic criteria for UI vary across different questionnaires and can be broadly categorized into three groups. First, UI is defined based on the criterion of experiencing leakage at least once a month or less frequently, with enough fluid discharge to wet the inner clothing. Second, definitions are established according to the frequency of UI occurrences within the past year. Third, some questionnaires specifically highlight the origin of their criteria, employing established tools like Questionnaire for Urinary Incontinence Diagnosis (QUID) or International Consultation on Incontinence Questionnaire (ICIQ) to delineate UI. Moreover, SUI is defined as instances of urinary leakage during activities such as coughing, sneezing, exercising, or laughing.
Lastly, all included studies relied on questionnaires and self-reports to ascertain UI diagnosis, which may lead to high-risk respondent bias in observational studies and an increased rate of misdiagnosis. The results obtained from self-reported questionnaires are subjective and cannot be considered equivalent to clinical diagnoses. The inherent inaccuracies of self-reporting measurements can compromise the accuracy of our risk assessment. While these findings might align with those of other studies, it is essential to conduct further research involving larger and more diverse populations across various age groups. It is worth noting that previous studies have validated the reliability and effectiveness of self-reporting UI, indicating that it is not a significant limitation (47). Self-reported UI displays an 83% concordance rate with clinician assessments (48). Thus, clinicians should focus on the thorough examination, diagnosis, and treatment of UI patients.
5 Conclusions
Among women in middle and old age who experience UI, this is the primary meta-analysis to examine the correlation between overweight and obesity. Overweight and obesity increase UI risk during this stage of life. Furthermore, the risk for various subtypes of UI persists and magnifies in women with a BMI exceeding 30. Nevertheless, A deeper understanding of the pathophysiology of this phenomenon is required. Therefore, healthcare professionals and researchers should contemplate preventing weight gain as a prophylactic measure against UI, and develop incontinence management strategies for obese women in middle and old age.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XS and SY conceived the study. XS and XJ collected the data and drafted the manuscript. SY revised the manuscript and language. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Special Research Project of Traditional Chinese Medicine in Henan Province (2018JDZX018), Henan Traditional Chinese Medicine Inheritance and Innovation Talent Project (Zhongjing Project) Chinese Medicine Top Talent Project (CZ0237-02), Zhengzhou Science and Technology Benefiting People Plan (2021KJHM0017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1220551/full#supplementary-material
References
1. Peate I. Urinary incontinence in women: treatment recommendations. Br J Nursing. (2019) 28(22):1486–8. doi: 10.12968/bjon.2019.28.22.1486
2. Troko J, Bach F, Toozs-Hobson P. Predicting urinary incontinence in women in later life: A systematic review. Maturitas (2016) 94:110–6. doi: 10.1016/j.maturitas.2016.09.006
3. Vaughan CP, Markland AD. Urinary incontinence in women. Ann Internal Med (2020) 172(3):ITC17–32. doi: 10.7326/AITC202002040
4. Quiboeuf E, Saurel-Cubizolles MJ, Fritel X., EDEN Mother-Child Cohort Study Group. Trends in urinary incontinence in women between 4 and 24 months postpartum in the EDEN cohort. BJOG (2016) 123(7):1222–8. doi: 10.1111/1471-0528.13545
5. Luo R, Dai W, Tay LH, Ng FC, Koh LT. Urinary incontinence in female outpatients in Singapore. Int Urogynecology J (2018) 29(4):579–84. doi: 10.1007/s00192-017-3488-z
6. Masenga GG, Shayo BC, Msuya S, Rasch V. Urinary incontinence and its relation to delivery circumstances: A population-based study from rural Kilimanjaro, Tanzania. PloS One (2019) 14(1):e0208733. doi: 10.1371/journal.pone.0208733
7. Milsom I, Gyhagen M. The prevalence of urinary incontinence. Climacteric (2019) 22(3):217–22. doi: 10.1080/13697137.2018.1543263
8. Lamerton TJ, Torquati L, Brown WJ. Overweight and obesity as major, modifiable risk factors for urinary incontinence in young to mid-aged women: a systematic review and meta-analysis. Obes Rev (2018) 19(12):1735–45. doi: 10.1111/obr.12756
9. Batmani S, Jalali R, Mohammadi M, Bokaee S. Prevalence and factors related to urinary incontinence in older adults women worldwide: a comprehensive systematic review and meta-analysis of observational studies. BMC Geriatr (2021) 21(1):212. doi: 10.1186/s12877-021-02135-8
10. Aune D, Mahamat-Saleh Y, Norat T, Riboli E. Body mass index, abdominal fatness, weight gain and the risk of urinary incontinence: a systematic review and dose–response meta-analysis of prospective studies. BJOG (2019) 126(12):1424–33. doi: 10.1111/1471-0528.15897
11. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (2014) 384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8
12. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet (2016) 387(10026):1377–96. doi: 10.1016/S0140-6736(16)30054-X
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
15. Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, Garritty C, et al. Celiac disease. Evid Rep Technol Assess (Summ) (2004) (104):1–6.
16. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
17. Qu H, Yang S, Yao Z, Sun X, Chen H. Association of headache disorders and the risk of dementia: meta-analysis of cohort studies. Front Aging Neurosci (2022) 14:804341. doi: 10.3389/fnagi.2022.804341
18. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
20. Komesu YM, Schrader RM, Rogers RG, Ketai LH. Urgency urinary incontinence in women 50 years or older: incidence, remission, and predictors of change. Female Pelvic Med Reconstr Surg (2011) 17(1):17–23. doi: 10.1097/SPV.0b013e31820446e6
21. Townsend MK, Curhan GC, Resnick NM, Grodstein F. BMI, waist circumference, and incident urinary incontinence in older women. Obesity (Silver Spring). (2008) 16(4):881–6. doi: 10.1038/oby.2008.14
22. Martinez A, Rodriguez MA, Al Snih S. Factors associated with urgency urinary incontinence among older mexican american women aged 65 years and older. Gerontol Geriatr Med (2022) 8:23337214221119061. doi: 10.1177/23337214221119061
23. Sievert LL, Whitcomb BW, Verjee MA, Gerber LM. Limited evidence of a threshold effect for increasing adiposity on risk of symptoms at midlife. Menopause (2022) 29(12):1381–7. doi: 10.1097/GME.0000000000002074
24. McGrother CW, Donaldson MM, Hayward T, Matthews R, Dallosso HM, Hyde C, et al. Urinary storage symptoms and comorbidities: a prospective population cohort study in middle-aged and older women. Age Ageing (2006) 35(1):16–24. doi: 10.1093/ageing/afi205
25. Byles J, Millar CJ, Sibbritt DW, Chiarelli P. Living with urinary incontinence: a longitudinal study of older women. Age Ageing (2009) 38(3):333–8. doi: 10.1093/ageing/afp013
26. Islam RM, Bell RJ, Hossain MB, Davis SR. Types of urinary incontinence in Bangladeshi women at midlife: Prevalence and risk factors. Maturitas (2018) 116:18–23. doi: 10.1016/j.maturitas.2018.07.012
27. Zeleke BM, Bell RJ, Billah B, Davis SR. Symptomatic pelvic floor disorders in community-dwelling older Australian women. Maturitas (2016) 85:34–41. doi: 10.1016/j.maturitas.2015.12.012
28. Demir N, Yuruyen M, Atay K, Yavuzer H, Hatemi I, Doventas A, et al. Prevalence of fecal incontinence and associated risk factors in elderly outpatients: a cross-sectional study. Aging Clin Exp Res (2017) 29(6):1165–71. doi: 10.1007/s40520-017-0723-x
29. Dellu MC, Schmitt AC, Cardoso MR, Pereira WM, Pereira EC, Vasconcelos Éda S, et al. Prevalence and factors associated with urinary incontinence in climacteric. Rev Assoc Med Bras (1992). (2016) 62(5):441–6. doi: 10.1590/1806-9282.62.05.441
30. Mishra GD, Hardy R, Cardozo L, Kuh D. Body weight through adult life and risk of urinary incontinence in middle-aged women: results from a British prospective cohort. Int J Obes (Lond) (2008) 32(9):1415–22. doi: 10.1038/ijo.2008.107
31. Townsend MK, Danforth KN, Rosner B, Curhan GC, Resnick NM, Grodstein F. Body mass index, weight gain, and incident urinary incontinence in middle-aged women. Obstetr Gynecol (2007) 110(2 Pt 1):346–53. doi: 10.1097/01.Aog.0000270121.15510.57
32. Mitchell ES, Woods NF. Correlates of urinary incontinence during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. Climacteric (2013) 16(6):653–62. doi: 10.3109/13697137.2013.777038
33. Forsman M, Iliadou A, Magnusson P, Falconer C, Altman D. Diabetes and obesity-related risks for pelvic reconstructive surgery in a cohort of Swedish twins. Diabetes Care (2008) 31(10):1997–9. doi: 10.2337/dc08-0988
34. Janssen I. Morbidity and mortality risk associated with an overweight BMI in older men and women. Obesity (2007) 15(7):1827–40. doi: 10.1038/oby.2007.217
35. Hjorth S, Axelsen SM, Gommesen D, Kjeldsen ACM, Taastrøm KA, Nohr EA. Body mass index, waist circumference, and urinary incontinence in midlife: A follow-up of mothers in the Danish National Birth Cohort. Neurourol Urodyn (2023) 42(5):1111–21. doi: 10.1002/nau.25175
36. Minassian VA, Stewart WF, Wood GC. Urinary incontinence in women: variation in prevalence estimates and risk factors. Obstetrics Gynecology. (2008) 111(2 Part 1):324–31. doi: 10.1097/01.Aog.0000267220.48987.17
37. Abrams P, Andersson KE, Apostolidis A, Birder L, Bliss D, Brubaker L, et al. 6th international consultation on incontinence. Recommendations of the international scientific committee: EVALUATION AND TREATMENT OF URINARY INCONTINENCE, PELVIC ORGAN PROLAPSE AND FAECAL INCONTINENCE. Neurourol Urodyn (2018) 37(7):2271–2. doi: 10.1002/nau.23551
38. Tang DH, Colayco DC, Khalaf KM, Piercy J, Patel V, Globe D, et al. Impact of urinary incontinence on healthcare resource utilization, health-related quality of life and productivity in patients with overactive bladder. BJU Int (2014) 113(3):484–91. doi: 10.1111/bju.12505
39. Al-Mukhtar Othman J, Åkervall S, Milsom I, Gyhagen M. Urinary incontinence in nulliparous women aged 25-64 years: a national survey. Am J Obstetr Gynecol (2017) 216(2):149.e1–.e11. doi: 10.1016/j.ajog.2016.09.104
40. Khan S, Ansari MA, Vasenwala S, Mohsin Z. The influence of menopause on urinary incontinence in the women of the community: a cross-sectional study from North India. Int J Reprod. Contracept. Obstet. Gynecol (2017) 6:911. doi: 10.18203/2320-1770.ijrcog20170555
41. Perera J, Kirthinanda DS, Wijeratne S, Wickramarachchi TK. Descriptive cross sectional study on prevalence, perceptions, predisposing factors and health seeking behaviour of women with stress urinary incontinence. BMC Women's Health (2014) 14(1):78. doi: 10.1186/1472-6874-14-78
42. Elbiss HM, Osman N, Hammad FT. Social impact and healthcare-seeking behavior among women with urinary incontinence in the United Arab Emirates. Int J Gynecology Obstetrics. (2013) 122(2):136–9. doi: 10.1016/j.ijgo.2013.03.023
43. Purwar B, Cartwright R, Cavalcanti G, Digesu GA, Fernando R, Khullar V. The impact of bariatric surgery on urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J (2019) 30(8):1225–37. doi: 10.1007/s00192-018-03865-x
44. Sheridan W, Da Silva AS, Leca BM, Ostarijas E, Patel AG, Aylwin SJ, et al. Weight loss with bariatric surgery or behaviour modification and the impact on female obesity-related urine incontinence: A comprehensive systematic review and meta-analysis. Clin Obes (2021) 11(4):e12450. doi: 10.1111/cob.12450
45. Samuels JB, Pezzella A, Berenholz J, Alinsod R. Safety and efficacy of a non-invasive high-intensity focused electromagnetic field (HIFEM) device for treatment of urinary incontinence and enhancement of quality of life. Lasers Surg Med (2019) 51(9):760–6. doi: 10.1002/lsm.23106
46. Schreiber Pedersen L, Lose G, Høybye MT, Elsner S, Waldmann A, Rudnicki M. Prevalence of urinary incontinence among women and analysis of potential risk factors in Germany and Denmark. Acta Obstet Gynecol Scand (2017) 96(8):939–48. doi: 10.1111/aogs.13149
47. Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses' Health Study. Am J Obstet Gynecol (2003) 189(2):428–34. doi: 10.1067/S0002-9378(03)00361-2
Keywords: overweight, obesity, urinary Incontinence, meta-analysis summary, systematic review
Citation: Shang X, Fu Y, Jin X, Wang C, Wang P, Guo P, Wang Y and Yan S (2023) Association of overweight, obesity and risk of urinary incontinence in middle-aged and older women: a meta epidemiology study. Front. Endocrinol. 14:1220551. doi: 10.3389/fendo.2023.1220551
Received: 10 May 2023; Accepted: 21 September 2023;
Published: 10 October 2023.
Edited by:
Jin Young Huh, Sogang University, Republic of KoreaReviewed by:
Jens Djurhuus, Aarhus University, DenmarkFerdinando Antonio Gulino, Azienda di Rilievo Nazionale e di Alta Specializzazione (ARNAS) Garibaldi, Italy
Copyright © 2023 Shang, Fu, Jin, Wang, Wang, Guo, Wang and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuxun Yan, eXN4OTgyMDAxQDE2My5jb20=; Ying Wang, d2FuZ3lpbmc2NjYyMDAxQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Xin Shang
Xin Shang Yu Fu
Yu Fu Xiaoqin Jin2
Xiaoqin Jin2 Shuxun Yan
Shuxun Yan