- 1Joint Surgery and Sport Medicine Department, Hunan Provincial People's Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, Hunan, China
- 2Nursing Department, Hunan Provincial People's Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, Hunan, China
- 3Endocrinology Department, Hunan Provincial People's Hospital (The First Affiliated Hospital of Hunan Normal University), Changsha, Hunan, China
Background: Globally, type 2 diabetes mellitus (T2DM) accounts for approximately 90% of diabetes cases. Resistance training (RT) is frequently employed to diminish Glycated Hemoglobin (HbA1c) and Fast Blood Glucose (FBG) levels in T2DM patients. Yet, the specific dose-response relationships between RT variables such as training duration, frequency, and intensity for T2DM remain under-researched.
Objectives: This meta-analysis aimed to elucidate the overarching effects of RT on HbA1c and FBG metrics and to provide dose-response relationships of RT variables. This was achieved by examining randomized controlled trials (RCTs) that reported reductions in HbA1c and FBG among T2DM patients.
Methods: Comprehensive literature searches were conducted up to 25th February 2023 across databases including EMBASE, Pubmed, Cochrane, CENTRAL, Web of Science, CNKI, Wanfang Data, VIP Database for Chinese Technical Periodicals, and the Chinese Biomedical Database. The Physical Therapy Evidence Database (PEDro) was leveraged to appraise the quality of selected studies based on predefined inclusion and exclusion criteria. The meta-analysis was conducted using Stata 16.
Results: 26 studies that include 1336 participants met the criteria for inclusion. RT significantly reduced HbA1c and FBG levels in comparison to control groups (P<0.05). Meta-regression analyses revealed that the number of repetitions per set (p=0.034) was a significant predictor of RT’s efficacy on HbA1c. Subgroup analyses indicated that the most pronounced reductions in HbA1c and FBG occurred with a training duration of 12-16 weeks, intensities of 70-80% of 1 RM, training frequencies of 2-3 times per week, 3 sets per session, 8-10 repetitions per set, and less than a 60-second rest interval.
Conclusion: The beneficial impact of RT on HbA1c and FBG in T2DM patients is affirmed by this systematic review and meta-analysis. Moreover, the critical training parameters identified in this study are pivotal in enhancing HbA1c and FBG reductions, providing a reference for clinical staff to formulate RT exercise regiments for T2DM patients.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42023414616.
1 Introduction
Diabetes ranks as the third most prevalent chronic disease globally, trailing only cardiovascular diseases and tumors. An estimated 10.5% of the global population aged between 20 and 79 years suffers from diabetes, and this prevalence is forecasted to escalate to 12.2% (or 783.2 million individuals) by 2045 (1). Notably, T2DM patients constitute over 90% of the overall diabetic population (2). Diabetes can precipitate a myriad of complications, including coronary heart disease, cerebrovascular disease, kidney disease, and blindness, leading to significant health detriments and straining socioeconomic and medical infrastructures (3). In 2021, the global expenditure associated with diabetes-related ailments was pegged at 966 billion USD, with projections indicating an increase to 1054 billion USD by 2045 (1).Exercise elicits numerous metabolic adaptations in the body, prominently including enhanced insulin sensitivity and superior blood glucose regulation (4). Historically, Aerobic training (AT) has been lauded as the quintessential strategy for T2DM management (5). However, a growing body of evidence now underscores the unique advantages of RT in glycemic control (6). A series of investigations (6–8) has accentuated the role of resistance exercise in efficaciously managing type 2 diabetes. The American College of Sports Medicine (ACSM) posits that T2DM patient exercise regimens should incorporate resistance exercises, viewing them as both safe and efficacious modalities (9).
Glycated hemoglobin (HbA1c) serves as an indicator of a patient’s blood glucose trajectory over the preceding two to three months (10). It’s routinely harnessed for diagnosing diabetes, supervising blood glucose consistency, and directing T2DM patient care – gaining esteem as a longstanding “gold standard” for gauging blood glucose oversight (11). Both FBG and HbA1c have been advocated as pivotal markers for diabetic glycemic management (12). Yun et al. (13) deduced from their investigation, which surveyed the interplay between resistance exercise and HbA1c levels among Korean diabetic subjects, that RT augments HbA1c level regulation. A recent meta-analysis (6) corroborated the premise that RT efficaciously diminishes HbA1c in adult T2DM individuals, strongly bolstering reductions in both HbA1c and FBG. Yet, one encompassing meta-analysis comprising 24 trials revealed high-intensity RT to outperform moderate-intensity RT in lowering HbA1c levels within T2DM cohorts (14). Conversely, moderate-intensity RT spanning 12 weeks manifested a palpable drop in FBG for T2DM patients (15). However, high-intensity RT (≥70% 1RM) failed to showcase discernible declines in FBG levels 26 weeks post-training among diabetic individuals (16). Venturing to pinpoint the optimal resistance training regimen, Ishiguro et al. (17) discerned that intervention duration bore no influence on RT’s impact on HbA1c, suggesting potential variability based on specific RT parameters (intensity, frequency, sets). The endeavor to elucidate the ideal quantification of exercise-associated variables during RT for optimal HbA1c reduction and FBG control remains ongoing. Moreover, considering the methodological caveats in extant meta-analyses, which incorporate non-randomized controlled trials (RCTs) (4) and exclusively hinge on direct group comparisons (e.g., high-intensity vs. low-intensity) (14), a pressing need emerges to evaluate exercise variables via dose-response relationships rooted in comprehensive systematic reviews and meta-analyses.
Thus, the objective of this meta-analysis was to ascertain the overarching impact of RT on HbA1c and FBG metrics. We employed meta-regression to scrutinize how particular training parameters (e.g., duration, frequency, and intensity) influence HbA1c and FBG values. Furthermore, we delineated dose-response associations for pivotal RT variables by analyzing RCTs that demonstrated reductions in HbA1c and FBG among T2DM patients.
2 Methods
In alignment with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (18), we conducted our study. Our research protocol was registered with PROSPERO (CRD42023414616). The PRISMA checklist can be found in the Supplementary Table S1.
2.1 Strategy of searching
Searches were conducted on databases including EMBASE, PubMed, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), Wanfang Data, CNKI, VIP Database for Chinese Technical Periodicals, and the Chinese Biomedical Database. The search spanned until February 25, 2023, guided by predefined search strategies utilizing medical subject headings (MeSH) or their synonyms. We imposed no constraints on publication date, study design, or language. Results were imported into the Endnote bibliographic management tool. The specific search strategies employed in Embase are detailed in the Supplementary Table S2.
2.2 Selection criteria
The PICOS principle (19) shaped our selection process: (a) Population: Inclusion criteria entailed patients diagnosed with type 2 diabetes aged >18 years. (b) Intervention: RT that detailed at least one training variable (e.g., training intensity). (c) Comparator: Control groups that lacked physical intervention (e.g., health education, no intervention). (d) Outcome: Must report both pre- and post-intervention values for at least HbA1c or FBG. (e) Study Design: RCTs.
Exclusion criteria included: (a) Patients diagnosed with gestational diabetes. (b) Interventions not exclusive to RT (e.g., RT combined with aerobic exercise). (c) Previously published or duplicate literature. (d) Document types like “case reports”, “reviews”, “meta-analysis”, and “letters”.
2.3 Data extraction
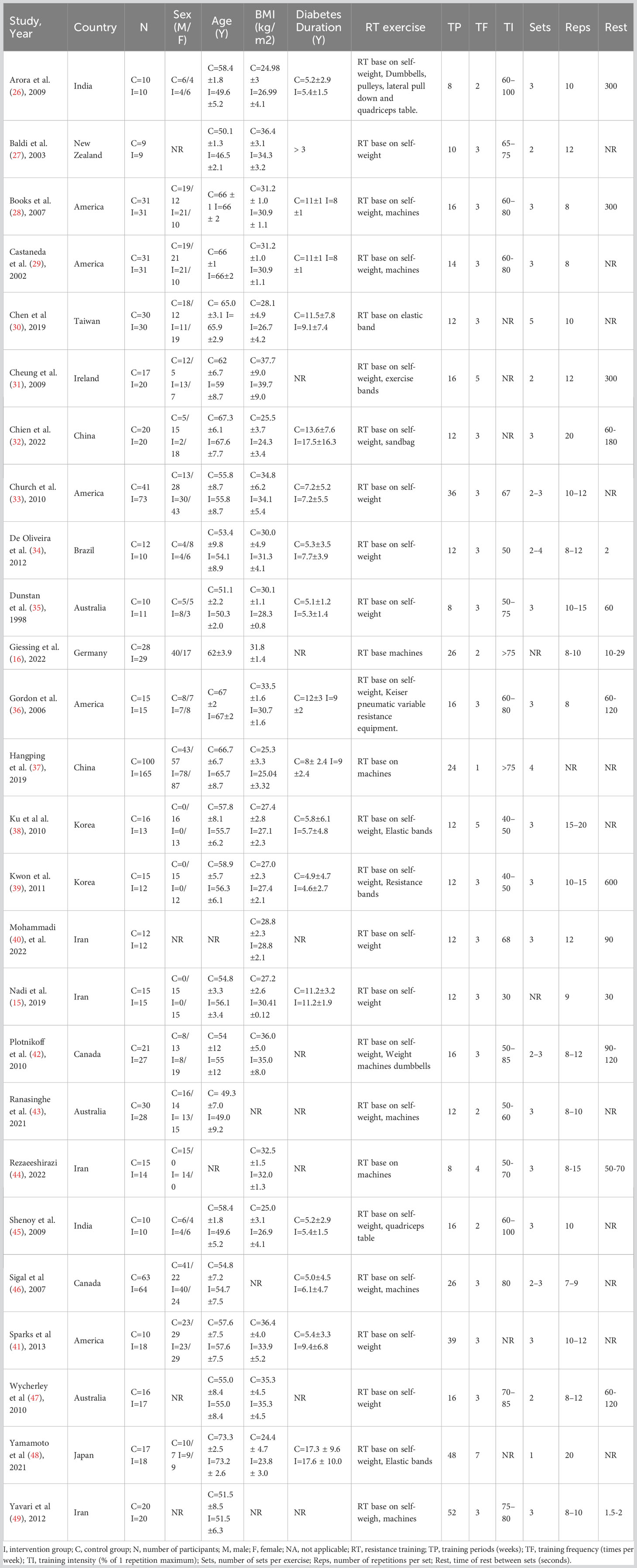
Two researchers (SW, TK) independently screened titles and abstracts based on our selection criteria, further examining papers that initially met the criteria. Any discrepancies between their selections were addressed through discussion or, if necessary, mediation by a third researcher (ML). Extracted data covered details such as authors, country, sample size, gender, age, body mass index (BMI), disease duration, and RT training variables (e.g., period, intensity, sets, repetitions). RT groups were categorized by training intensity: high-intensity (≥70% 1RM), moderate-intensity (51%-69% 1RM), and low-intensity (≤50% 1RM) (20). After extraction, the two primary researchers cross-checked their findings. In instances where critical data was absent or unclear in the studies, we reached out to the authors for clarification.
2.4 Assessment of methodological study quality
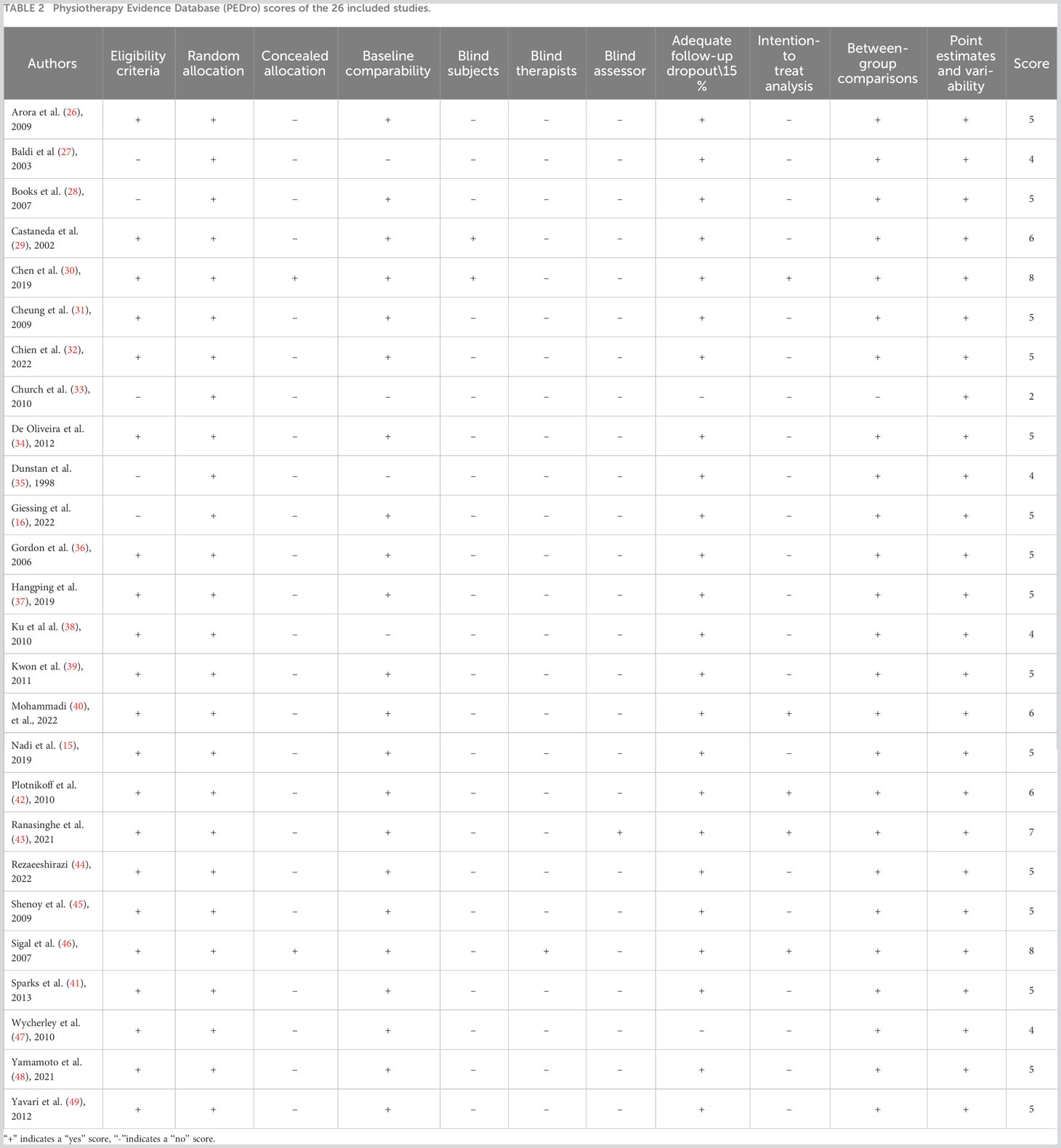
Two independent reviewers utilized the Physical Therapy Evidence Database (PEDro) scale (21) to gauge the methodological quality of the studies. The PEDro scale, encompassing 11 criteria, integrates three from the Jadad scale (22) and nine from the Delphi list (23). RCTs are scored between 0-10 (from low to high quality) on the PEDro scale, with scores ≥6 indicating high-quality studies (21). The scale’s first item, specifying eligibility criteria, is geared towards establishing external validity and isn’t factored into the cumulative score. Previous evaluations by Maher et al. (21) confirmed an inter-rater reliability intraclass correlation coefficient of 0.68, based on consensus ratings from two or three independent raters.
2.5 Statistical analyses
To evaluate the overall effect of RT on HbA1c and FBG and establish a dose-response relationship in patients with type 2 diabetes, the standardized mean differences (SMD) was calculated using the formula: formula: = (24). Here, SMDi represents the standardized difference between reported measures, where m1i and m2i represent the means of the intervention and control groups, respectively, while si is the pooled standard deviation. This formula was adjusted for sample size by Hedges and Olkin as: g =( 1- ) (24), where Ni denotes the combined sample size of both groups. SMD signifies the difference between post-test averages of the treatment and control groups divided by the combined group’s standard deviation and is accompanied by 95% confidence intervals (CIs). Due to significant heterogeneity among studies, stemming from factors such as varying muscle groups, a random-effects model was utilized to estimate the effect of RT interventions (19). Our categorical variable meta-analysis was executed using Stata 16 software. Cohen categorized effect sizes as: 0.00 to ≤0.49 for small, 0.50 to ≤0.79 for moderate, and ≥0.80 for large effects (25). For clarity, a positive SMD was reported when RT exhibited superiority over the control group. The I² statistic assessed heterogeneity. Additionally, meta-analytic regression (metareg) investigated if the combined values of diverse training variables could predict RT’s influence on HbA1c and FBG. Subcategories were established to identify pivotal training variables, encompassing training duration, frequency, repetitions per session, repetitions per set, intensity, and rest intervals. Each subcategory was examined using a random-effects meta-regression model to discern potential enhancements in HbA1c and FBG measurements.
3 Results
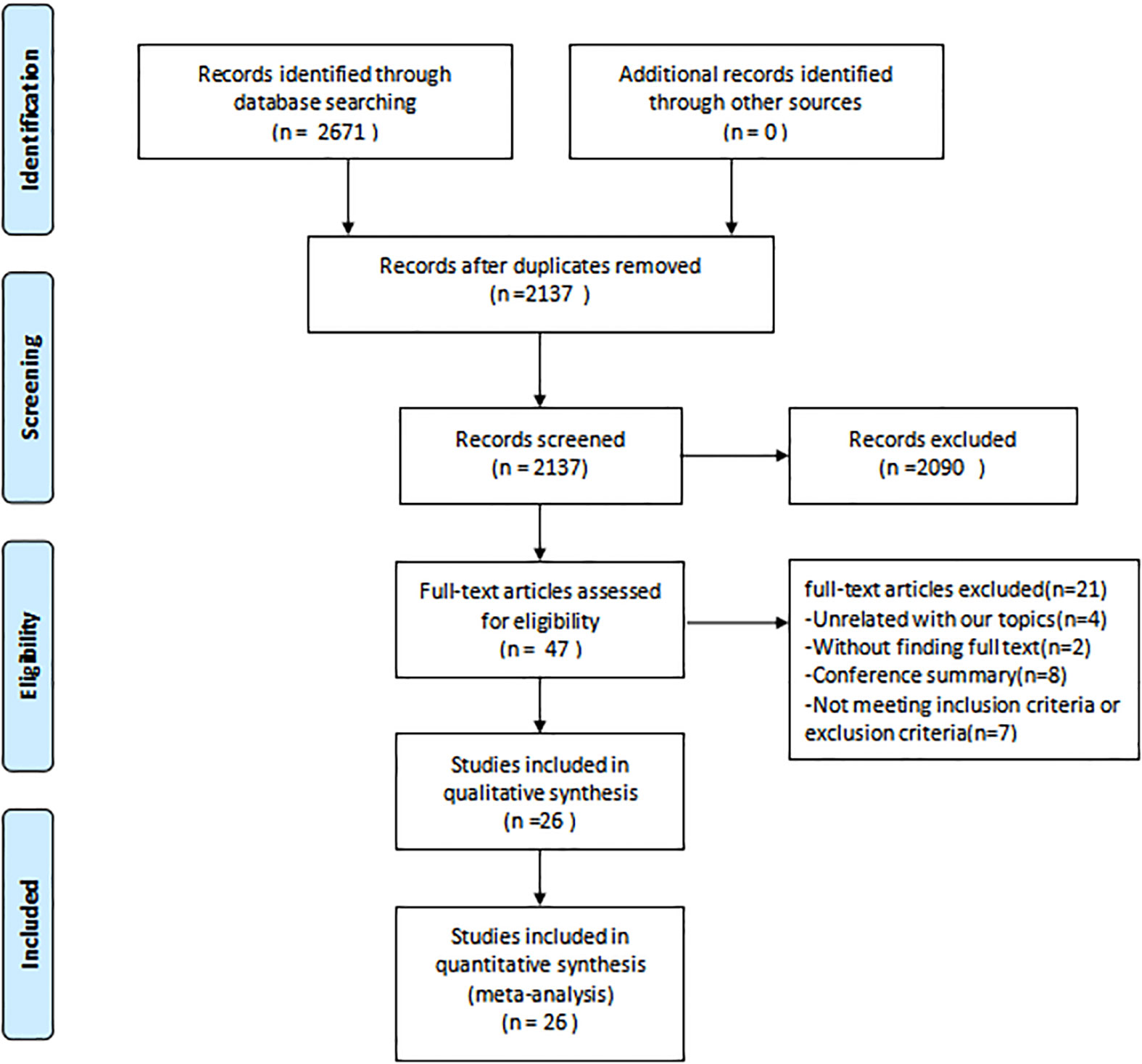
Figure 1 depicts the PRISMA flowchart detailing the literature search process. An initial search yielded 2671 potential studies. After the exclusion of duplicates, 2137 studies were retained. A screening based on titles and abstracts resulted in the removal of 2090 studies. Of the 47 articles that remained, 21 were excluded due to irrelevance to the subject, absence of full text, or unavailability of conference abstracts. Thus, the quantitative synthesis incorporated 26 studies (15, 16, 26–49) comprising 1336 participants; the characteristics of these studies are presented in Table 1. The intervention groups in these studies had sample sizes ranging from 9 to 165 participants, with ages spanning 47 to 73 years and diabetes duration from 1 to 18 years. RT interventions lasted between 8 and 52 weeks, with training frequencies of 1 to 7 sessions weekly, encompassing 1-5 sets per session, 8-20 repetitions per set, and rest intervals of 30-600 seconds between sets. The reviewed literature indicated training intensities between 30% and 80% of a single repetition maximum (RM). Concerning RT modalities, 8 studies utilized body weight, 1 employed machines, 7 combined machines and body weight, 1 used elastic bands, 5 incorporated both elastic bands and body weight, 1 employed body weight and sandbags, 1 integrated machines, elastic bands, and body weight, 1 combined body weight, machines, and dumbbells, while 2 studies implemented a complex training approach.
3.1 Methodological quality of the study
As presented in Table 2, the studies exhibited an average quality score of 5.23 ± 1.31 points, with scores ranging from 3 to 8. Notably, six studies (29, 30, 40, 42, 43, 46) secured a score of 6 or above.
3.2 Overall findings
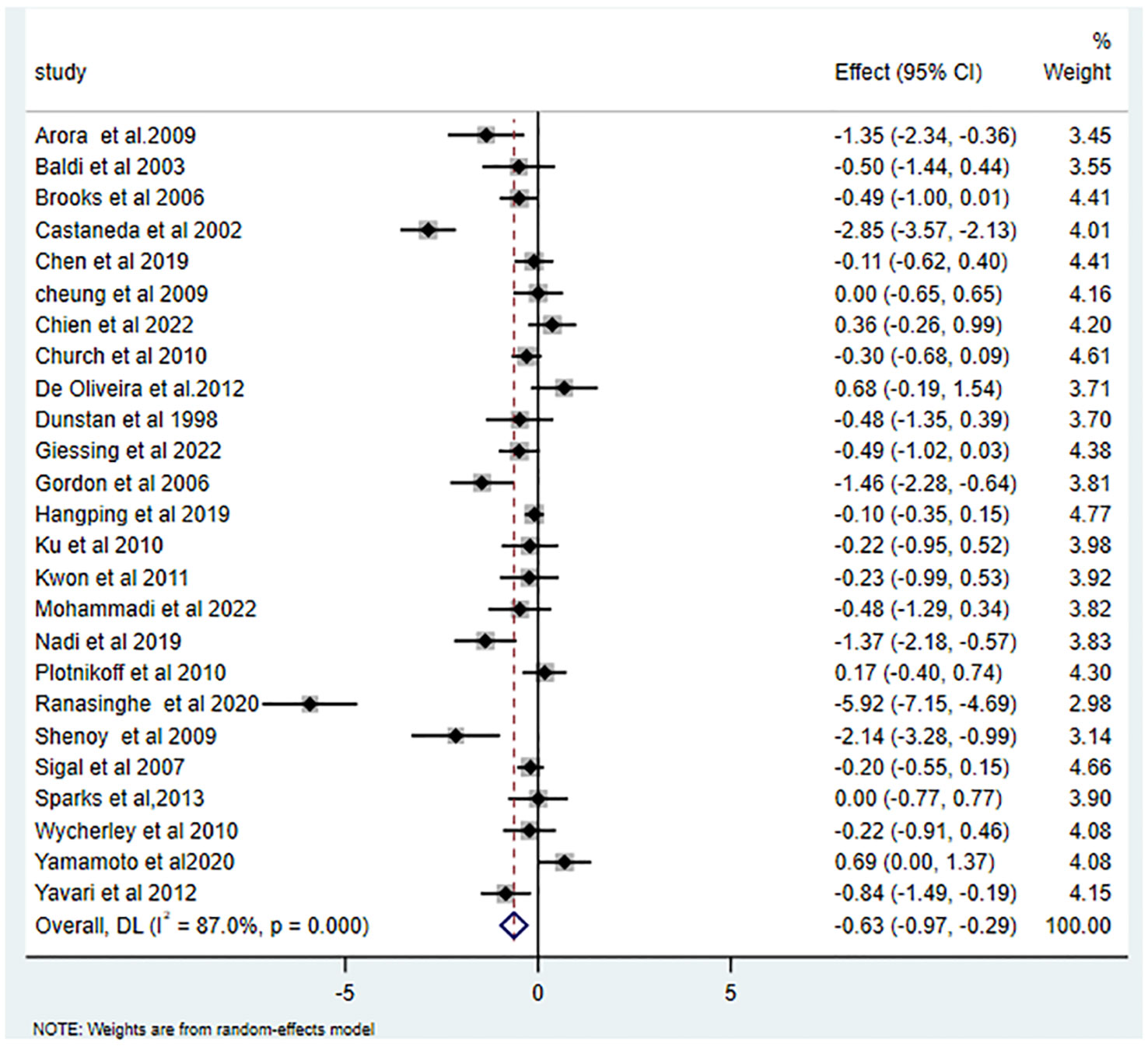
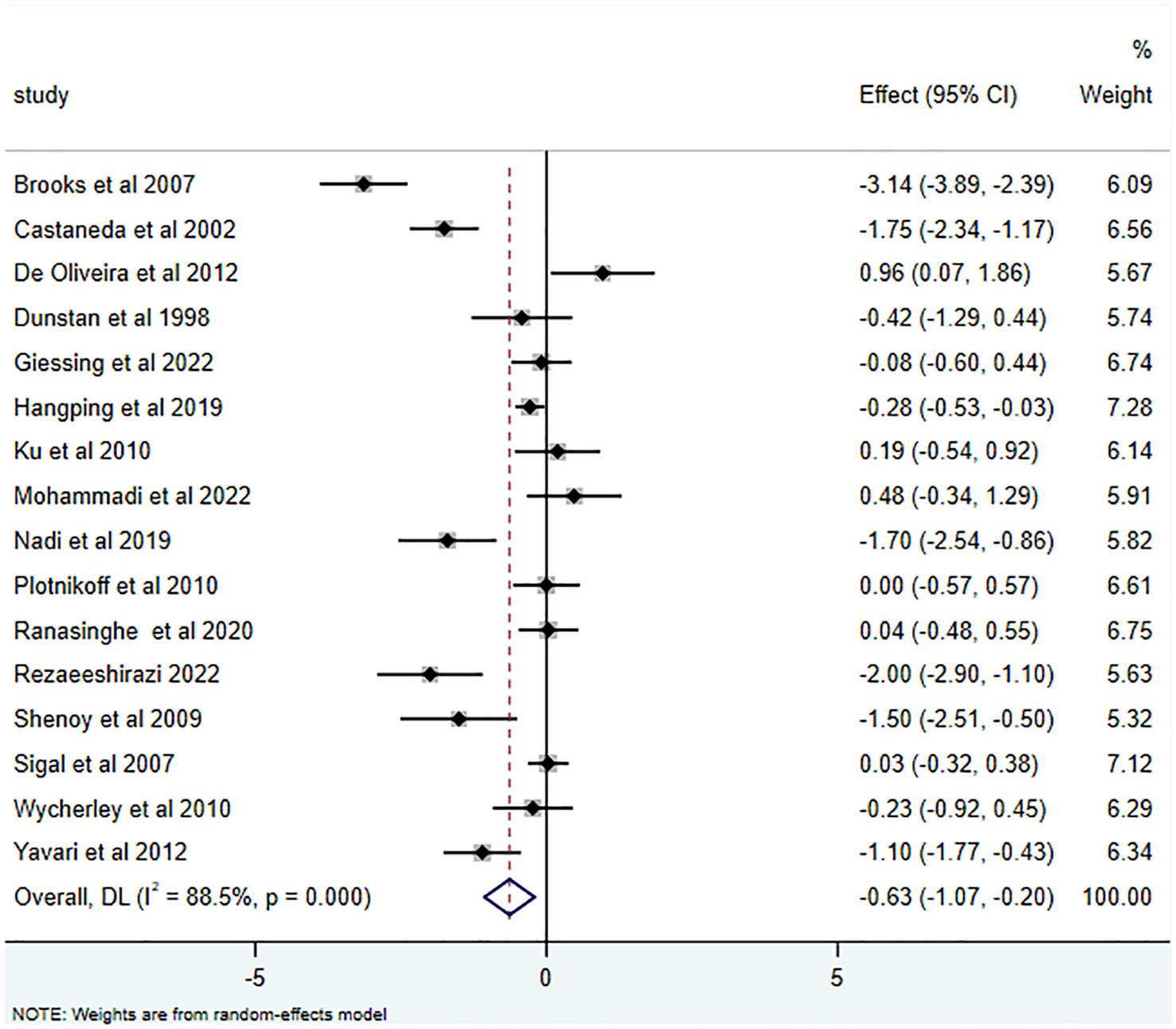
25 studies (15, 16, 26–43, 45–49) reported the effects of RT on HbA1c, yielding an SMD of -0.63 (95% CI: -0.97 to -0.29; I2 = 87.0%, p<0.001). Relative to the control group, RT substantially decreased HbA1c levels in T2DM patients, as illustrated in Figure 2. Of the 16 studies (15, 16, 28, 29, 34, 35, 37, 38, 40, 42–47, 49) that examined RT’s influence on FBG, the average SMD was -0.63 (95% CI: -1.07 to -0.20; I2 = 88.5%, p<0.001). This is depicted in Figure 3 and highlights the significant effect.
3.3 Meta-regression analysis of training variables
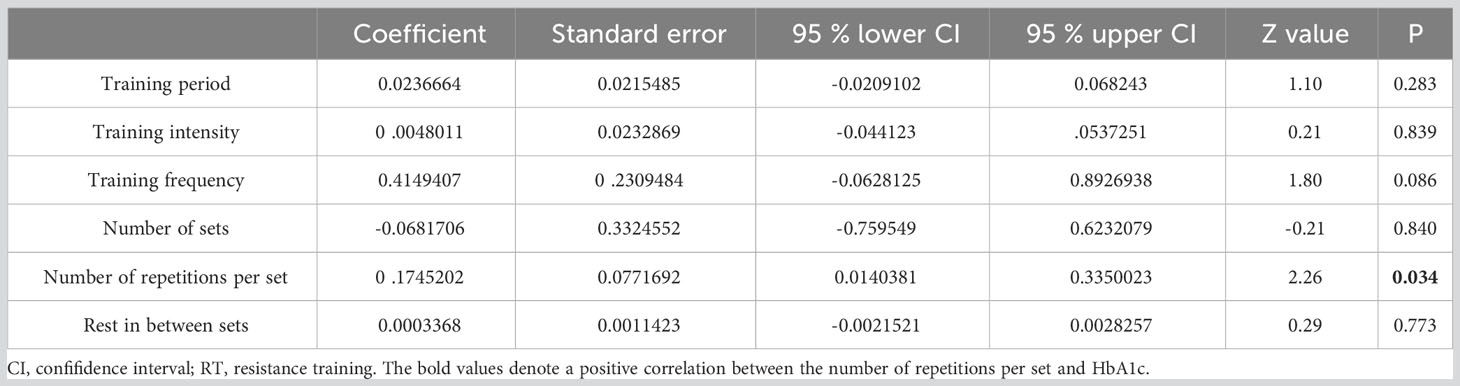
Meta-regression results pinpointed the number of repetitions per set (p=0.034) as a predictor of RT’s impact on HbA1c (refer to Table 3). Regression analysis, however, revealed that no specific training parameters—including duration, intensity, frequency, number of sets, and number of repetitions—significantly affected FBG measurements (see Table 4).
3.4 Subcategories analysis of training variables
3.4.1 Subcategories analysis of training vtariables on HbA1c
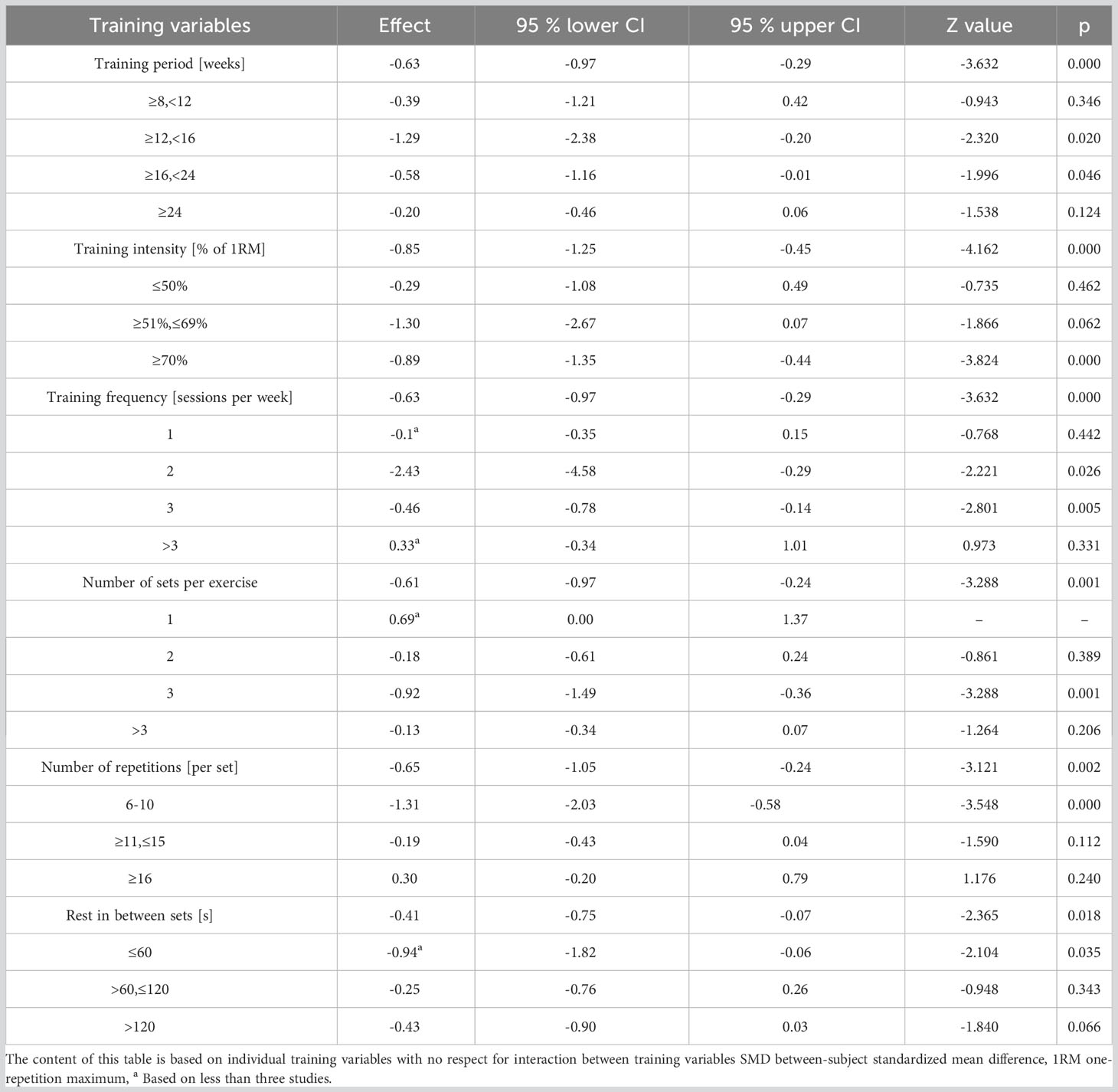
Effect sizes for each training parameter were independently computed to discern their influence on HbA1c (as detailed in Table 5). Analysis inferred that a training duration of 12-16 weeks, intensities equating to 70-80% of 1RM, 2-3 weekly training sessions, 3 sets per activity, 8 to 10 repetitions within each set, and inter-set rest periods under 60 seconds yield the optimal reduction in HbA1c.
3.4.2 Subcategories analysis for training variables on FBG
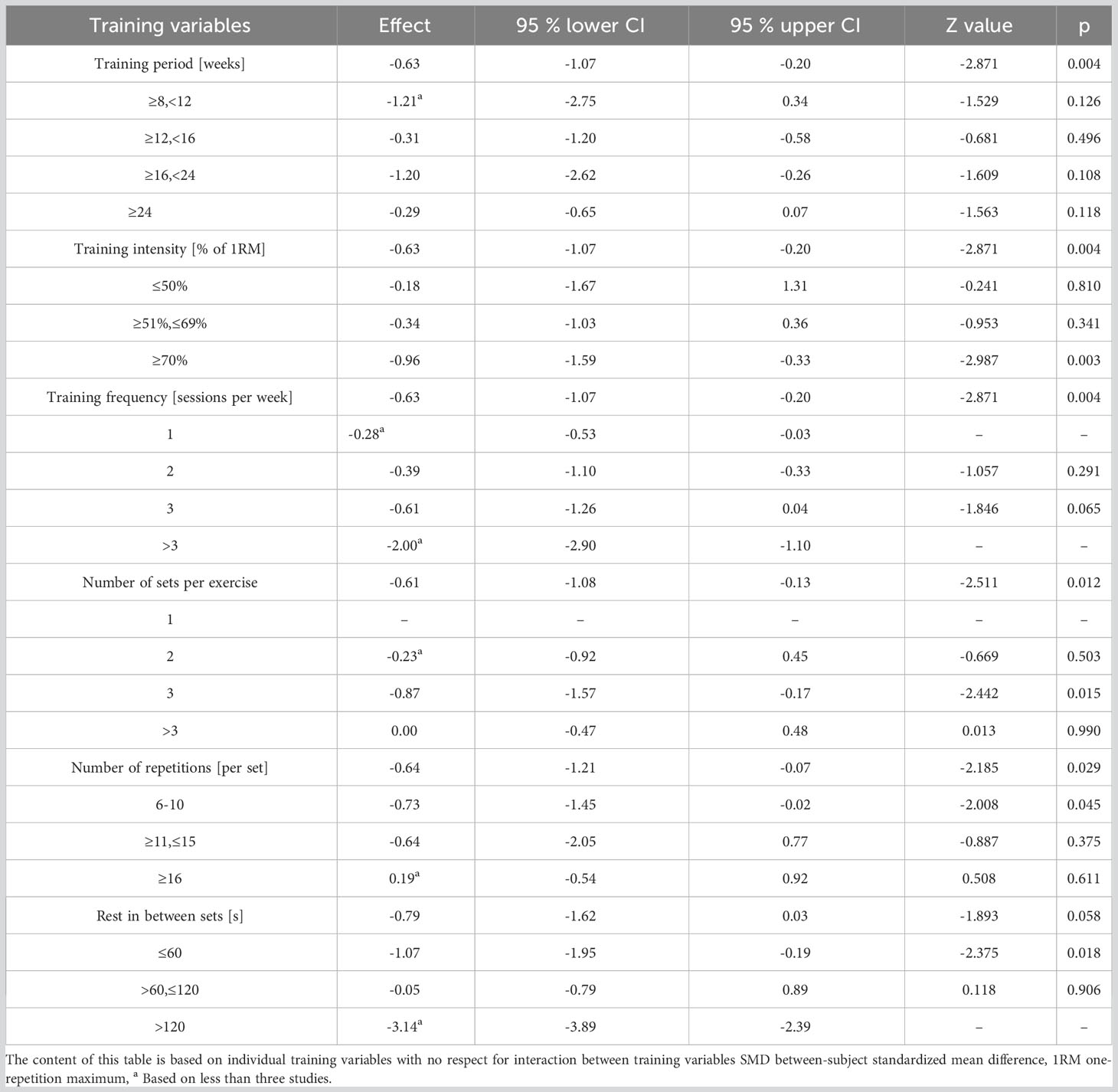
Effect sizes for each training parameter, in the context of FBG, were separately evaluated (refer to Table 6). It emerged that 70-80% of 1RM intensities, 3 weekly training sessions, 3 sets per session, 8 to 10 repetitions per set, and rest intervals shorter than 60 seconds between sets are most conducive to FBG reduction.
4 Discussion
In this study, we examined the impact of RT on HbA1c and FBG levels in T2DM patients. Our meta-regression sought to identify the most influential training variables on these outcomes after RT. We further analyzed the dose-response relationships for each training variable. Key findings include: (1). RT effectively decreases both HbA1c and FBG in T2DM patients. (2). The number of repetitions per set emerged as a predictor of RT’s effect on HbA1c. (3). The optimal RT regimen to ameliorate HbA1c and FBG in T2DM patients encompasses a training duration of 12-16 weeks, an intensity of 70-80% of 1 RM, frequency of 2-3 times per week, and performing 3 sets of 8-10 repetitions for each exercise with less than a 60-second rest interval.
4.1 RT’s effect on HbA1c and FBG in T2DM patients
Our data suggests a substantial benefit of RT on HbA1c levels in T2DM patients (-0.63 (95% CI -0.97- -0.29; I2 = 87.0%, p=0.000)), aligning with previous studies (14, 17, 50). Effective HbA1c management can attenuate diabetes-related complications and mortality (51). Maintaining glucose levels at 7% or below can curtail long-term complications by up to 76% (49). A 1% decline in HbA1c can potentially decrease diabetes-related myocardial infarction risk by 14% and overall mortality by 21% (52). Our results are in line with Kelly, who reported an approximate 0.8% decrease in HbA1c through exercise, optimizing glycemic control (53). The therapeutic potential of RT may arise from augmented glucose transporter presence, increased muscle mass, and enhanced insulin receptor activity in muscle cells (54).
Furthermore, RT has been acknowledged for its efficacy in diminishing FBG in T2DM patients (-0.63 (95% CI -1.07- -0.20; I2 = 88.5%, p=0.000)). This is consistent with Yaping et al., who posited that RT exerts a more potent influence on FBG enhancement (55). Brooks et al. (28), Castaneda et al. (29), and Yavari et al. (49) reported analogous findings. Mechanisms underlying these improvements include increased muscle strength, augmented GLUT-4 receptor activity, rapid glucose transportation, and refined insulin resistance and sensitivity (56). Rezaeeshirazi et al. (44) also reported increased insulin sensitivity in elderly T2DM patients’ post-resistance exercise, leading to substantial FBG reduction.
4.2 Dose-response correlations of RT to reduce HbA1c
Numerous studies have established that various training variables, such as duration, frequency, and intensity, within sports training can optimize the hypoglycemic effect (14, 57). Consequently, discerning which variable correlates with a more potent positive impact of exercise on T2DM patients becomes crucial. Our analysis, encompassing 25 studies (15, 16, 26–43, 45–49), revealed that the duration of RT had no significant association with HbA1c reduction. Training durations ranging from 12-16 weeks seem to be the most effective in decreasing HbA1c. This may be attributed to the notion that a noticeable exercise effect necessitates a more extended training duration. For instance, Dunstan et al. (35) observed no marked improvement in HbA1c following 8 weeks of moderate-intensity RT. The lack of significant change in HbA1c could be due to brief training durations, specifically, 4-6 weeks (58). Conversely, even with prolonged RT cycles, some patients might not achieve the desired results, possibly due to inadequate exercise intensity or compliance. Church et al. (33) found no significant amelioration in HbA1c after 36 weeks of moderate-intensity RT, and Yamamoto et al. (48) reported 27 participants dropping out of their study. Hence, intervention duration alone doesn’t dictate HbA1c reduction but is influenced by both training intensity and adherence.
Our meta-analysis, which incorporated 20 studies (15, 16, 26–29, 33–40, 42, 43, 45–47, 49), recorded a 0.89% and 0.29% decrease in HbA1c with high and low intensity RT, respectively. This aligns with another meta-analysis (encompassing 24 trials) which suggested that high-intensity RT is superior in reducing HbA1c in T2DM patients as compared to low-to-moderate intensity RT (48). Contrarily, a separate Australian study observed that 3 months of moderate-intensity RT led to a significant decline in HbA1c levels (by 5.92%) in T2DM patients (43). In our research, it was evident that when the mean baseline HbA1c exceeded 7.5%, participants displayed greater HbA1c improvement post-RT, a finding corroborated by studies from Segal et al. (Canada) (46) and Kadoglou et al. (Greece) (59). However, the intensity of RT didn’t exhibit a direct correlation with its effect on HbA1c reduction in diabetic patients.
Moreover, our analysis indicated that conducting 2-3 sets per week and 3 sets per exercise session were most effective in HbA1c reduction. Conventionally, the advised frequency for RT is two to three times weekly, although it’s recommended to be integrated with AT (60). Excessive training frequency could lead to fatigue; hence, the number of sets might not be the primary determinant for HbA1c reduction in T2DM patients. Meta-regression analysis underscored the pivotal role of repetition count in HbA1c decline. T2DM patients witnessed the most significant drop in HbA1c with 6-10 repetitions per set (mean SMD= 1.31). While the aggregate repetitions in an RT set might induce a substantial physiological stimulus for enhanced strength and subsequent HbA1c reduction, fewer repetitions might be more potent (17). This is because repetition count is often equated with training intensity. Historically, emphasis was on “training intensity” rather than “number of repetitions”, leading to the inference that fewer repetitions correspond to augmented training intensity (61). Lastly, no discernible correlation existed between RT rest periods and HbA1c reduction. Rest intervals under 60 seconds appeared most effective in diminishing HbA1c in our study. A potential rationale is that shorter rest intervals induce more fatigue, thus offering a stimulus augmenting muscular strength and subsequently lowering HbA1c (62).
4.3 Dose-response correlations of RT to reduce FBG
Sixteen studies (15, 16, 28, 29, 34, 35, 37, 38, 40, 42–47, 49) were incorporated into this analysis. Meta-regression identified no significant association between training variables and FBG. Nonetheless, the optimal RT program for enhancing FBG in T2DM patients is characterized by a training intensity of 70-80% of 1RM, conducted thrice weekly, encompassing 3 sets during each session, with 8-10 repetitions, and inter-set rest intervals of less than 60 seconds.
Evidence from randomized controlled trials indicates distinct glycemic responses in T2DM patients undergoing RT. Notably, a 26-week high-intensity RT (70% 1RM) regimen yielded minimal alterations in blood glucose levels (16). Conversely, a 12-week moderate-intensity RT regimen precipitated notable reductions in fasting blood glucose (15). However, a separate 12-week moderate-intensity RT program (50-60% 1RM) failed to elicit significant glucose reductions (43). A recent meta-analysis posited that older T2DM patients should prioritize RT intensity over duration and frequency to ensure optimal glycemic management (50). Collectively, the impact of RT on glycemic fluctuations appears to be heterogenous, and the standalone effect of RT intensity on glucose levels in diabetics remains nuanced.
Subgroup analysis from our research reveals that a training intensity of 70-80% of 1RM, executed thrice weekly in 3 sets, with 8-10 repetitions per set, and rest periods of less than 60 seconds between sets, is most conducive for FBG reduction. These observations align with prior studies (28, 29, 47). Ferriolli et al. (63) suggested an RT protocol for elderly diabetic patients, advocating for sessions on at least 2 days per week at a moderate-to-high intensity of 1RM. Similarly, Liu et al.’s meta-analysis (14) endorsed an RT regimen with an average high intensity (75-100% of 1RM), performed in 2-3 sets per week and 8-10 repetitions per set, as efficacious in reducing FBG among T2DM patients. Furthermore, in scenarios devoid of contraindications, Pan et al. (64) recommended diabetic patients to train thrice weekly, targeting all major muscle groups over 3 sets of 8-10 repetitions, but never exceeding 10 repetitions. These recommendations resonate with the insights of our meta-analysis. Despite these insights, it remains imperative to further probe the efficacy of resistance exercise interventions on FBG.
4.4 Limitations of the meta-analysis
A notable limitation of our study is the inability to conduct a thorough analysis of the interrelationships among the suggested training variables. Our research draws from several studies that employed diverse training parameter combinations, such as intensity, training frequency, and number of sets. Given the variability in these parameters, it remains inconclusive whether the outcomes would remain optimal if each element of the RT method were tailored based on the prevailing dose-response relationship. Hence, further investigations are imperative to devise an analytical framework that provides insights into the interplay between these training parameters. One approach to overcome this limitation is through the systematic modeling of training variables, wherein one RT variable remains constant while another is varied. While our study evaluated the influence of radiation on HbA1c and FBG, the assessment did not extend to hypertrophy (specifically, alterations in lean tissue) due to the paucity of studies documenting such changes. Consequently, the direct link between exercise and augmented muscle mass remains speculative. We advocate for future studies examining the relationship between hypertrophy and HbA1c.
Moreover, the majority of the studies reviewed did not provide details on medication adjustments throughout the intervention. If the control group received a more potent medication regimen compared to the intervention group, the impact of RT on glycemic control could have been underestimated. This was particularly evident in studies with combined medication interventions. Another challenge encountered in our literature review and meta-analysis was the pronounced bias inclination of the included studies (only 6 out of 26 studies attained a PEDro score of 6), the marked heterogeneity across the studies (i.e., I^2 values ranging from 59.5% to 88.5%), and the uneven SMD reported for specific training parameters.
5 Conclusion
This literature review and meta-analysis substantiate that RT effectively reduces HbA1c and FBG levels in T2DM patients. An optimal training regimen encompasses a duration of 12-16 weeks, an intensity set at 70-80% of 1 RM, a frequency of 2-3 sessions per week, and a structure of 3 sets with 8-10 repetitions for each exercise. Moreover, allowing a rest interval of less than 60 seconds between sets has been demonstrated to be efficacious in lowering HbA1c and FBG levels. Such a regimen can provide reference for clinical researchers to develop exercise training programs tailored for T2DM patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
WS: Conceptualization, data curation, formal analysis, methodology, software, writing original draft, writing review and editing, and translate. KT, FX, and MT: translate, validation, supervision, writing review and editing, data curation, and methodology. LM and XD: validation, software, and investigation. YQ: conceptualization, project administration, resources, validation, supervision, and writing review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Health Commission of Hunan Province of China (No. 202114021174) and Changsha Science and Technology Bureau Hunan Province of China(No. kq1907068).
Acknowledgments
We thank Dr. Yang for comments on the methodological approach in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1224161/full#supplementary-material
Abbreviations
RT, Resistance training; T2DM, Type 2 diabetes mellitus; HbA1c, Glycated hemoglobin; FBG, Fast Blood Glucose (FBG); RCTs, Randomized controlled trials; PEDro, Physical Therapy Evidence Database; SMD, Standardized mean difference.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol (2018) 14:88–98. doi: 10.1038/nrendo.2017.151
3. Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care (2011) 34:1249–57. doi: 10.2337/dc11-0442
4. Jiahao L, Jiajin L, Yifan L. Effects of resistance training on insulin sensitivity in the elderly: a meta-analysis of randomized controlled trials. J Exerc Sci Fit (2021) 19:241–51. doi: 10.1016/j.jesf.2021.08.002
5. Aschner P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res Clin Pract (2017) 132:169–70. doi: 10.1016/j.diabres.2017.09.002
6. Jansson AK, Chan LX, Lubans DR, Duncan MJ, Plotnikoff RC. Effect of resistance training on HbA1c in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: a systematic review and meta-analysis. BMJ Open Diabetes Res Care (2022) 10(2):e002595. doi: 10.1136/bmjdrc-2021-002595
7. Yang X, Liu L, Yang L, Li W, Zhang J. Effect of resistance exercise on peripheral neuropathy in Type 2 diabetes mellitus. Zhong Nan Da Xue Xue Bao Yi Xue Ban (2020) 45:1185–92. doi: 10.11817/j.issn.1672-7347.2020.190505
8. Brown EC, Franklin BA, Regensteiner JG, Stewart KJ. Effects of single bout resistance exercise on glucose levels, insulin action, and cardiovascular risk in type 2 diabetes: a narrative review. J Diabetes Complications (2020) 34:107610. doi: 10.1016/j.jdiacomp.2020.107610
9. Chodzko-Zajko WJ, Proctor DN, Fiatarone SM, Minson CT, Nigg CR, Salem GJ, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc (2009) 41:1510–30. doi: 10.1249/MSS.0b013e3181a0c95c
10. Nitin S. HbA1c and factors other than diabetes mellitus affecting it. Singapore Med J (2010) 51:616–22.
11. Gomez-Peralta F, Choudhary P, Cosson E, Irace C, Rami-Merhar B, Seibold A. Understanding the clinical implications of differences between glucose management indicator and glycated haemoglobin. Diabetes Obes Metab (2022) 24:599–608. doi: 10.1111/dom.14638
12. Lee CL, Sheu WH, Lee IT, Lin SY, Liang WM, Wang JS, et al. Trajectories of fasting plasma glucose variability and mortality in type 2 diabetes. Diabetes Metab (2018) 44:121–28. doi: 10.1016/j.diabet.2017.09.001
13. Yun I, Joo HJ, Park YS, Park EC. Association between physical exercise and glycated hemoglobin levels in korean patients diagnosed with diabetes. Int J Environ Res Public Health (2022) 19(6):3280. doi: 10.3390/ijerph19063280
14. Liu Y, Ye W, Chen Q, Zhang Y, Kuo CH, Korivi M. Resistance exercise intensity is correlated with attenuation of HbA1c and insulin in patients with type 2 diabetes: a systematic review and meta-analysis. Int J Environ Res Public Health (2019) 16(1):140. doi: 10.3390/ijerph16010140
15. Nadi M, Bambaeichi E, Marandi SM. Comparison of the effect of two therapeutic exercises on the inflammatory and physiological conditions and complications of diabetic neuropathy in female patients. Diabetes Metab Syndr Obes (2019) 12:1493–501. doi: 10.2147/DMSO.S206454
16. Giessing J, Eichmann B, Kemmler W, Westcott WL, Winett R, Busuttil K, et al. The effects of adding high-intensity of effort resistance training to routine care in persons with type II diabetes: an exploratory randomized parallel-group time-series study. Physiol Behav (2022) 245:113677. doi: 10.1016/j.physbeh.2021.113677
17. Ishiguro H, Kodama S, Horikawa C, Fujihara K, Hirose AS, Hirasawa R, et al. In search of the ideal resistance training program to improve glycemic control and its indication for patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Sports Med (2016) 46:67–77. doi: 10.1007/s40279-015-0379-7
18. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Bmj (2021) 372:n160. doi: 10.1136/bmj.n160
19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
20. Raymond MJ, Bramley-Tzerefos RE, Jeffs KJ, Winter A, Holland AE. Systematic review of high-intensity progressive resistance strength training of the lower limb compared with other intensities of strength training in older adults. Arch Phys Med Rehabil (2013) 94:1458–72. doi: 10.1016/j.apmr.2013.02.022
21. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther (2003) 83:713–21.
22. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
23. Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol (1998) 51:1235–41. doi: 10.1016/s0895-4356(98)00131-0
24. Trikalinos TA, Salanti G, Zintzaras E, Ioannidis JP. Meta-analysis methods. Adv Genet (2008) 60:311–34. doi: 10.1016/S0065-2660(07)00413-0
25. Parker RI, Hagan-Burke S. Useful effect size interpretations for single case research. Behav Ther (2007) 38:95–105. doi: 10.1016/j.beth.2006.05.002
26. Arora E, Shenoy S, Sandhu JS. Effects of resistance training on metabolic profile of adults with type 2 diabetes. Indian J Med Res (2009) 129:515–19.
27. Baldi JC, Snowling N. Resistance training improves glycaemic control in obese type 2 diabetic men. Int J Sports Med (2003) 24:419–23. doi: 10.1055/s-2003-41173
28. Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci (2006) 4:19–27. doi: 10.7150/ijms.4.19
29. Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care (2002) 25:2335–41. doi: 10.2337/diacare.25.12.2335
30. Chen SM, Shen FC, Chen JF, Chang WD, Chang NJ. Effects of resistance exercise on glycated hemoglobin and functional performance in older patients with comorbid diabetes mellitus and knee osteoarthritis: a randomized trial. Int J Environ Res Public Health (2019) 17(1):224. doi: 10.3390/ijerph17010224
31. Cheung NW, Cinnadaio N, Russo M, Marek S. A pilot randomised controlled trial of resistance exercise bands in the management of sedentary subjects with type 2 diabetes. Diabetes Res Clin Pract (2009) 83:e68–71. doi: 10.1016/j.diabres.2008.12.009
32. Chien YH, Tsai CJ, Wang DC, Chuang PH, Lin HT. Effects of 12-week progressive sandbag exercise training on glycemic control and muscle strength in patients with type 2 diabetes mellitus combined with possible sarcopenia. Int J Environ Res Public Health (2022) 19(22):15009. doi: 10.3390/ijerph192215009
33. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. Jama (2010) 304:2253–62. doi: 10.1001/jama.2010.1710
34. de Oliveira VN, Bessa A, Jorge ML, Oliveira RJ, de Mello MT, De Agostini GG, et al. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl Physiol Nutr Metab (2012) 37:334–44. doi: 10.1139/h2012-004
35. Dunstan DW, Puddey IB, Beilin LJ, Burke V, Morton AR, Stanton KG. Effects of a short-term circuit weight training program on glycaemic control in NIDDM. Diabetes Res Clin Pract (1998) 40:53–61. doi: 10.1016/s0168-8227(98)00027-8
36. Gordon PL, Vannier E, Hamada K, Layne J, Hurley BF, Roubenoff R, et al. Resistance training alters cytokine gene expression in skeletal muscle of adults with type 2 diabetes. Int J Immunopathol Pharmacol (2006) 19:739–49. doi: 10.1177/039463200601900404
37. Hangping Z, Xiaona Q, Qi Z, Qingchun L, Na Y, Lijin J, et al. The impact on glycemic control through progressive resistance training with bioDensity(TM) in Chinese elderly patients with type 2 diabetes: The PReTTy2 (Progressive Resistance Training in Type 2 Diabetes) Trial. Diabetes Res Clin Pract (2019) 150:64–71. doi: 10.1016/j.diabres.2019.02.011
38. Ku YH, Han KA, Ahn H, Kwon H, Koo BK, Kim HC, et al. Resistance exercise did not alter intramuscular adipose tissue but reduced retinol-binding protein-4 concentration in individuals with type 2 diabetes mellitus. J Int Med Res (2010) 38:782–91. doi: 10.1177/147323001003800305
39. Kwon HR, Min KW, Ahn HJ, Seok HG, Lee JH, Park GS, et al. Effects of aerobic exercise vs. Resistance training on endothelial function in women with type 2 diabetes mellitus. Diabetes Metab J (2011) 35:364–73. doi: 10.4093/dmj.2011.35.4.364
40. Mohammadi A, Bijeh N, Moazzami M, Kazem K, Rahimi N. Effect of exercise training on spexin level, appetite, lipid accumulation product, visceral adiposity index, and body composition in adults with type 2 diabetes. Biol Res Nurs (2022) 24:152–62. doi: 10.1177/10998004211050596
41. Sparks LM, Johannsen NM, Church TS, Earnest CP, Moonen-Kornips E, Moro C, et al. Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab (2013) 98:1694–702. doi: 10.1210/jc.2012-3874
42. Plotnikoff RC, Eves N, Jung M, Sigal RJ, Padwal R, Karunamuni N. Multicomponent, home-based resistance training for obese adults with type 2 diabetes: a randomized controlled trial. Int J Obes (Lond) (2010) 34:1733–41. doi: 10.1038/ijo.2010.109
43. Ranasinghe C, Devage S, Constantine GR, Katulanda P, Hills AP, King NA. Glycemic and cardiometabolic effects of exercise in South Asian Sri Lankans with type 2 diabetes mellitus: a randomized controlled trial Sri Lanka diabetes aerobic and resistance training study (SL-DARTS). Diabetes Metab Syndr (2021) 15:77–85. doi: 10.1016/j.dsx.2020.12.011
44. Rezaeeshirazi R. Aerobic versus resistance training: leptin and metabolic parameters improvement in type 2 diabetes obese men. Res Q Exerc Sport (2022) 93:537–47. doi: 10.1080/02701367.2021.1875111
45. Shenoy S, Arora E, Sandhu JS. Effects of progressive resistance training and aerobic exercise on type 2 diabetics in Indian population. Int J Diabetes Metab (2009) 17:27–30.
46. Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud'Homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med (2007) 147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005
47. Wycherley TP, Noakes M, Clifton PM, Cleanthous X, Keogh JB, Brinkworth GD. A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care (2010) 33:969–76. doi: 10.2337/dc09-1974
48. Yamamoto Y, Nagai Y, Kawanabe S, Hishida Y, Hiraki K, Sone M, et al. Effects of resistance training using elastic bands on muscle strength with or without a leucine supplement for 48 weeks in elderly patients with type 2 diabetes. Endocr J (2021) 68:291–98. doi: 10.1507/endocrj.EJ20-0550
49. Yavari A, Najafipoor F, Aliasgarzadeh A, Niafar M, Mobasseri M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardiovascular risk factors in patients with type 2 diabetes. Biol Sport (2012) 29:135–43. doi: 10.5604/20831862.990466
50. Lee J, Kim D, Kim C. Resistance training for glycemic control, muscular strength, and lean body mass in old type 2 diabetic patients: a meta-analysis. Diabetes Ther (2017) 8:459–73. doi: 10.1007/s13300-017-0258-3
51. Luo M, Lim WY, Tan CS, Ning Y, Chia KS, van Dam RM, et al. Longitudinal trends in HbA1c and associations with comorbidity and all-cause mortality in Asian patients with type 2 diabetes: a cohort study. Diabetes Res Clin Pract (2017) 133:69–77. doi: 10.1016/j.diabres.2017.08.013
52. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet (1998) 352:837–53.
53. Kelley GA, Kelley KS. Effects of aerobic exercise on lipids and lipoproteins in adults with type 2 diabetes: a meta-analysis of randomized-controlled trials. Public Health (2007) 121:643–55. doi: 10.1016/j.puhe.2007.02.014
54. Cauza E, Hanusch-Enserer U, Strasser B, Ludvik B, Metz-Schimmerl S, Pacini G, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil (2005) 86:1527–33. doi: 10.1016/j.apmr.2005.01.007
55. Yaping X, Huifen Z, Chunhong L, Fengfeng H, Huibin H, Meijing Z. A meta-analysis of the effects of resistance training on blood sugar and pregnancy outcomes. Midwifery (2020) 91:102839. doi: 10.1016/j.midw.2020.102839
56. Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JF, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes (2004) 53:294–305. doi: 10.2337/diabetes.53.2.294
57. Collins KA, Ross LM, Slentz CA, Huffman KM, Kraus WE. Differential effects of amount, intensity, and mode of exercise training on insulin sensitivity and glucose homeostasis: a narrative review. Sports Med Open (2022) 8:90. doi: 10.1186/s40798-022-00480-5
58. Ishii T, Yamakita T, Sato T, Tanaka S, Fujii S. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care (1998) 21:1353–55. doi: 10.2337/diacare.21.8.1353
59. Kadoglou NP, Fotiadis G, Athanasiadou Z, Vitta I, Lampropoulos S, Vrabas IS. The effects of resistance training on ApoB/ApoA-I ratio, Lp(a) and inflammatory markers in patients with type 2 diabetes. Endocrine (2012) 42:561–69. doi: 10.1007/s12020-012-9650-y
60. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care (2010) 33:e147–67. doi: 10.2337/dc10-9990
61. Walker S, Hulmi JJ, Wernbom M, Nyman K, Kraemer WJ, Ahtiainen JP, et al. Variable resistance training promotes greater fatigue resistance but not hypertrophy versus constant resistance training. Eur J Appl Physiol (2013) 113:2233–44. doi: 10.1007/s00421-013-2653-4
62. Borde R, Hortobágyi T, Granacher U. Dose-response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med (2015) 45:1693–720. doi: 10.1007/s40279-015-0385-9
63. Ferriolli E, Pessanha FP, Marchesi JC. Diabetes and exercise in the elderly. Med Sport Sci (2014) 60:122–29. doi: 10.1159/000357342
Keywords: resistance training, diabetes, glycated hemoglobin, blood glucose, meta-analysis
Citation: Su W, Tao M, Ma L, Tang K, Xiong F, Dai X and Qin Y (2023) Dose-response relationships of resistance training in Type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Front. Endocrinol. 14:1224161. doi: 10.3389/fendo.2023.1224161
Received: 17 May 2023; Accepted: 28 August 2023;
Published: 25 September 2023.
Edited by:
João Soares Felício, Federal University of Pará, BrazilReviewed by:
Teresa Bento, Polytechnic Institute of Santarém, PortugalSamuel Acquah, University of Cape Coast, Ghana
Lubia Velázquez López, Unidad de Investigación Biómedica. Hospital Carlos Mac Gregor Sánchez Navarro. Instituto Mexicano del Seguro Social, Mexico
Copyright © 2023 Su, Tao, Ma, Tang, Xiong, Dai and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuelan Qin, cXlsMTM4MDczMTg3NjJAMTI2LmNvbQ==
 Wanying Su
Wanying Su Meiyi Tao2
Meiyi Tao2 Yuelan Qin
Yuelan Qin