- Department of Clinical Pharmacy, School of Pharmacy, Fudan University, Shanghai, China
Background: As a popular antidiabetic drug, teneligliptin has been used for over 10 years, but its efficacy and safety have rarely been systematically evaluated. Therefore, a Bayesian network meta-analysis was conducted to evaluate the efficacy and safety of teneligliptin in patients with type 2 diabetes mellitus (T2DM).
Methods: We systematically searched PubMed, Web of Science, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov. Randomized controlled trials (RCTs) comparing teneligliptin with placebo or active comparators in T2DM patients for at least 12 weeks were included in the study. Data analysis was performed using R 4.2.3 and Stata 17.0 software. Each outcome was presented as a mean difference (MD) or an odds ratio (OR) along with 95% confidence interval (CI) and the surface under the cumulative ranking curve value (SUCRA).
Results: A total of 18 RCTs with 3,290 participants with T2DM were included in this study. Generally, compared to placebo, sitagliptin, vildagliptin, metformin, and bromocriptine, 20 mg of teneligliptin showed better efficacy in reducing HbA1c (MD [95% CI], −0.78 [−0.86 to −0.70], −0.08 [−0.36 to 0.19], −0.04 [−0.72 to 0.60], −0.12 [−0.65 to 0.42], and −0.50 [−0.74 to −0.26], respectively) and fasting plasma glucose (FPG) (MD [95% CI], −18.02 [−20.64 to −15.13], 1.17 [−9.39 to 11.70], −8.06 [−30.95 to 14.35], −2.75 [−18.89 to 13.01], and −34.23 [−45.93 to −22.96], respectively), and 40 mg of teneligliptin also showed better efficacy in reducing HbA1c (MD [95% CI], −0.84 [−1.03 to −0.65], −0.15 [−0.49 to 0.19], −0.10 [−0.81 to 0.57], −0.18 [−0.76 to 0.39], and −0.56 [−0.88 to −0.26], respectively) and FPG (MD [95% CI], −20.40 [−26.07 to −14.57], −1.20 [−13.21 to 10.38], −10.43 [−34.16 to 12.65], −5.13 [−22.21 to 11.66], and −36.61 [−49.33 to −24.01], respectively). Compared to placebo, 20 mg of teneligliptin showed no significant difference in incidences of hypoglycemia and gastrointestinal adverse events (OR [95% CI], 1.30 [0.70 to 2.19] and 1.48 [0.78 to 2.98], respectively), and 40 mg of teneligliptin showed no significant difference in incidence of hypoglycemia (OR [95% CI], 2.63 [0.46 to 8.10]). Generally, antidiabetic effect and hypoglycemia risk of teneligliptin gradually increased as its dose increased from 5 mg to 40 mg. Compared to 20 mg of teneligliptin, 40 mg of teneligliptin showed superior efficacy and no-inferior safety, which was considered as the best option in reducing HbA1c, FPG, and 2h PPG and increasing proportion of the patients achieving HbA1c < 7% (SUCRA, 85.51%, 84.24%, 79.06%, and 85.81%, respectively) among all the included interventions.
Conclusion: Compared to sitagliptin, vildagliptin, metformin, bromocriptine, and placebo, teneligliptin displayed favorable efficacy and acceptable safety in treating T2DM. Twenty milligrams or 40 mg per day was the optimal dosage regimen of teneligliptin. The results of this study will provide important evidence-based basis for rational use of teneligliptin and clinical decision-making of T2DM medication.
1 Introduction
Diabetes mellitus (DM) is a chronic metabolic disease mainly characterized by hyperglycemia, which seriously endangers human life and health. It is estimated that 537 million adults suffer from DM in the world at present, and this number is projected to increase to 783 million by 2045 (1, 2). Prevalence of DM is high, but its treatment rate and cure rate are low (3). Type 2 diabetes mellitus (T2DM) accounts for nearly 90% of DM in the world (4). The primary pathophysiology of T2DM is characterized by defective insulin secretion and insulin resistance (5). T2DM is mainly caused by a combination of genetic, metabolic, and environmental factors (6, 7). It is associated with increased risk of cardiovascular and renal outcomes under the long-term suboptimal glycemic control (8–10).
In recent years, some new antidiabetic drugs, such as dipeptidyl peptidase-4 inhibitors (DPP-4is), sodium-glucose cotransporter-2 inhibitors (SGLT-2is), and glucagon-like peptide-1 receptor agonists (GLP-1RAs), have been widely used in the treatment of T2DM (11). Of them, DPP-4is universally increase insulin secretion, and decrease levels of intact glucagon in patients with diabetes via potentiation of GLP-1 action (12). DPP-4is are widely welcomed by T2DM patients because of their excellent efficacy and safety.
As an oral DPP-4i launched in recent years, teneligliptin was approved as a treatment option for T2DM patients who have failed to control the blood glucose level under diet and exercise treatment in Japan (2012), Korea (2016), Thailand (2020), and China (2021) (13). It can significantly decrease the glycated hemoglobin A1c (HbA1c) and fasting plasma glucose (FPG) levels, and had a slight influence on body weight (BW). Twenty milligrams once daily is the current recommended dosage regimen for teneligliptin. In Japan, it is also being practiced with close monitoring to increase the dose of teneligliptin to 40 mg per day (14). Therefore, it needs to be further evaluated whether 40 mg of teneligliptin for T2DM patients is effective and safe or not.
It is crucial for rational application of antidiabetic drugs (e.g., teneligliptin) and precise drug treatment of DM by evaluating their efficacy and safety using some scientific methods. Network meta-analysis is a popular method to evaluate multiple treatments or interventions, and has usually been performed by the Bayesian approach (15, 16). Bayesian network meta-analysis is an effective method to simultaneously compare multiple treatments by combining the direct and indirect evidence, and it can provide results of relative rankings of different interventions (17, 18). The aim of this study was to evaluate the efficacy and safety of the DPP-4i teneligliptin in patients with T2DM by Bayesian network meta-analysis. The results of this study will provide important evidence-based basis for rational use of teneligliptin and clinical decision-making of T2DM medication.
2 Materials and methods
The Bayesian network meta-analysis was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and its extension for Network Meta-Analysis (19, 20).
2.1 Search strategy
The literature search was conducted in PubMed, Web of Science, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov from their inception to 22 March 2023, without language restriction. The databases were searched with the following MeSH (Medical Subject Headings) terms or keywords: (1) “Teneligliptin” OR “MP-513”; AND (2) “diabetes mellitus, type 2” OR “diabetes mellitus, type II” OR “noninsulin dependent diabetes” OR “non-insulin dependent diabetes” OR “NIDDM” OR “type II diabetes” OR “type 2 diabetes” OR “T2DM” OR “mature onset diabetes” OR “late onset diabetes” OR “adult onset diabetes”. Furthermore, the reference lists of identified trials were screened to further identify eligible trials.
2.2 Study selection
The concrete inclusion criteria were as follows: (1) Patients: any ethnicity, either gender, aged 18 years or older, and HbA1c ≥ 6.5%. (2) Interventions: any dose of teneligliptin used as monotherapy or combination therapy with duration of at least 12 weeks. (3) Comparison: placebo or active comparators with or without background therapy. (4) Outcomes: at least one of the following indicators was reported: HbA1c, the patients achieving HbA1c < 7%, FPG, 2 h postprandial plasma glucose (2h PPG), BW, body mass index (BMI), hypoglycemia, and gastrointestinal adverse events (GIAEs). (5) Study design: randomized controlled trials (RCTs) published without language restrictions.
The studies were excluded if they included patients of age over 75 years, with HbA1c>10%, and with a history of renal, hepatic failures, dyslipidemia, or cardiovascular disorders. Moreover, phase I studies and secondary analyses were excluded.
2.3 Data extraction
The following information from the included studies were extracted: study information, baseline characteristics of the patient, intervention measures, and prespecified outcomes. For all the outcomes, we extracted data for the intention-to-treat (ITT) population, which comprised all the randomly assigned patients who received at least one dose of the study medication.
2.4 Risk-of-bias assessment
Assessment of the risk of bias was conducted by Cochrane Review Manager (RevMan), which included selection, performance, detection, attrition, and reporting bias (21). The scores for each aspect of eligible studies were recorded as high, low, or unclear risk.
The study search and selection, data extraction, and risk of bias assessment were conducted independently by two reviewers (MZ and RFG). Any differences were resolved through discussion or consultation with a third independent reviewer (GM).
2.5 Statistical analysis
A network meta-analysis with Bayesian approach was performed using R version 4.2.3 (http://www.r-project.org/) with the packages GEMTC, RJAGS, EXPORT, and BUGSnet and Stata MP 17.0 (StataCrop LLC) in this study (22, 23).
2.5.1 Synthesis of treatments and outcomes
For each prespecified outcome, a network plot of all the interventions was made to identify possible direct and indirect comparisons. The width of the lines in the network plot is proportional to the number of studies, and the node sizes correspond to number of the participants achieving a certain treatment in the comparisons. Effect estimates included odds ratio (OR) for categorical outcomes and mean difference (MD) for continuous outcomes. If the standard deviation (SD) was not reported, it was calculated from the standard error (SE), probability (p) value, confidence interval (CI), or MD according to the guidance from the Cochrane Handbook for Systematic Reviews of Interventions (24).
2.5.2 Model fitting and consistency evaluation
The Markov chain Monte Carlo algorithm was used for each outcome based on 20,000 simulation iterations and 5,000 adaptation iterations. A thinning interval of 1 was applied, which collected one sample every one iteration. The selection between fixed and random model and the evaluation of model fit goodness were realized through deviance information criteria (DIC), and the model with a lower value was chosen. The difference value of DIC between inconsistency and consistency models under 3 indicates a good consistency of network meta-analysis.
2.5.3 Effectiveness evaluation
The difference between the comparisons was considered statistically significant when the 95% CI did not contain 0.00 for continuous outcomes or 1.00 for categorical outcomes. The point estimates with 95% CI for each treatment comparison were presented in a league table. The surface under the cumulative ranking curve (SUCRA), showing each intervention ranking with respective ranking possibility, was calculated to rank the efficacy of each treatment. The SUCRA values ranged from 0% to 100%. The higher value indicates that a particular treatment is more possible to be in a top rank; similarly, the lower value indicates that a particular treatment is more possible to be in a bottom rank (25).
2.5.4 Heterogeneity evaluation and publication bias
I2 statistic was used to assess the heterogeneity arising from differences between the studies within each direct comparison of treatments, and the value of >50% indicated significant heterogeneity between the studies. Publication bias was evaluated using funnel plots.
3 Results
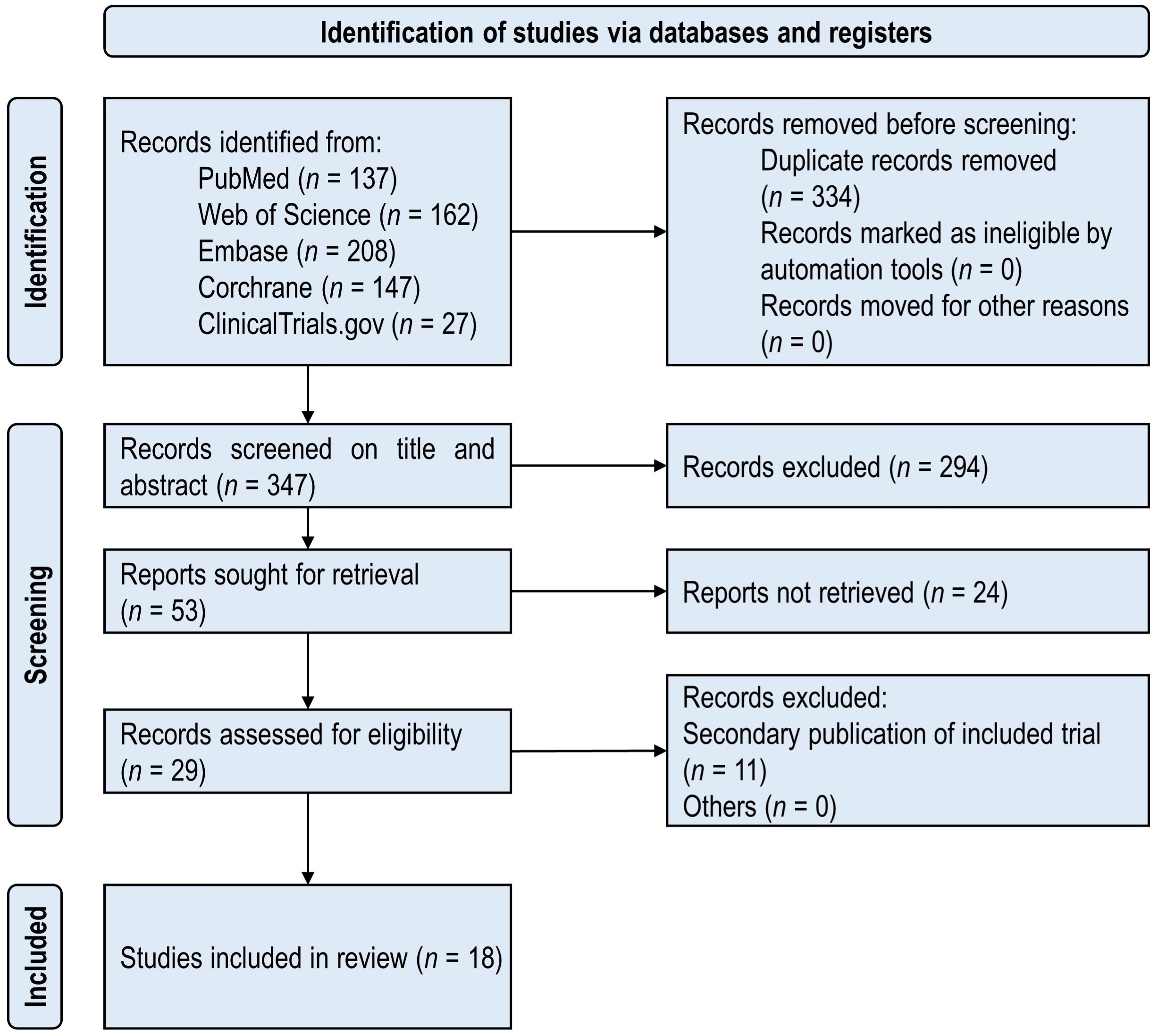
3.1 Study selection and characteristics
The PRISMA flowchart of the included studies is shown in Figure 1. There were 681 publications initially identified in five databases. A total of 18 unique RCTs (26–43) met the inclusion criteria and were considered for the proposed study. The included studies were published from 2013 to 2023, which consisted of 3,290 participants with T2DM in total. The minimum number of participants was 40, and the maximum number was 447 in the 18 included RCTs.
The detailed characteristics of the included studies are provided in Table S1. The weighted means of age, BW, BMI, baseline HbA1c, and baseline FPG were 56.6 years, 73.5 kg, 26.7 kg/m2, 8.0%, and 156.0 mg/dL, respectively. Compared with the others, only one study (33) stood out in terms of BW and BMI (weighted means were 92.8 kg and 32.3 kg/m2, respectively). These differences did not have a significant impact on similarity of baseline characteristics of the included studies. The duration of treatment ranged from 12 to 24 weeks.
Included in the meta-analysis were the following doses: 5 mg, 10 mg, 20 mg, and 40 mg of teneligliptin (qd), 100 mg of sitagliptin (qd), 50 mg of vildagliptin (bid), 500 mg of metformin (qd), and 0.8 mg of bromocriptine (qd). Efficacy outcomes contained mean changes of HbA1c, FPG, 2h PPG, BW, and BMI as well as proportion of the patients achieving HbA1c < 7%. Safety outcomes included incidences of hypoglycemia and GIAEs. Outcomes of HbA1c and FPG were reported in all the 18 included studies. The other six outcomes were only reported in a part of the 18 included studies (Table S1). Network plots of all the efficacy and safety outcomes are illustrated in Figure 2.
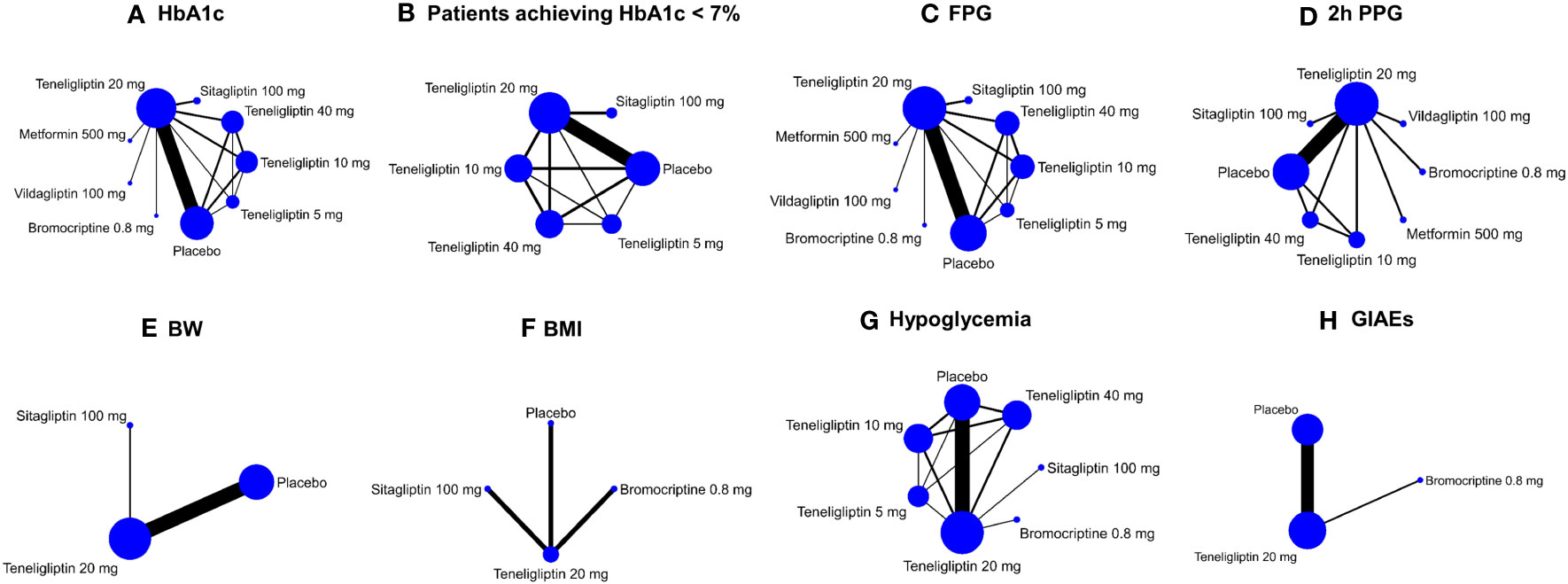
Figure 2 Network plots of efficacy and safety outcomes. These network plots show comparisons of teneligliptin, sitagliptin, vildagliptin, metformin, bromocriptine, and placebo in the RCTs with respect to the sample sizes and number of studies according to eight different outcome measures. The doses of all the antidiabetic drugs are daily dose. Each node represents a certain intervention, and its size represents number of the participants given a certain intervention in the comparisons. The width of the lines represents the number of studies comparing every pair of interventions. HbA1c, glycated hemoglobin A1c; FPG, fasting plasma glucose; 2h PPG, 2 h postprandial plasma glucose; BW, body weight; BMI, body mass index; GIAEs, gastrointestinal adverse events. (A) HbA1c; (B) Patients achieving HbA1c < 7%; (C) FPG; (D) 2h PPG; (E) BW; (F) BMI; (G) Hypoglycemia; (H) GIAEs.
3.2 Risk-of-bias analysis
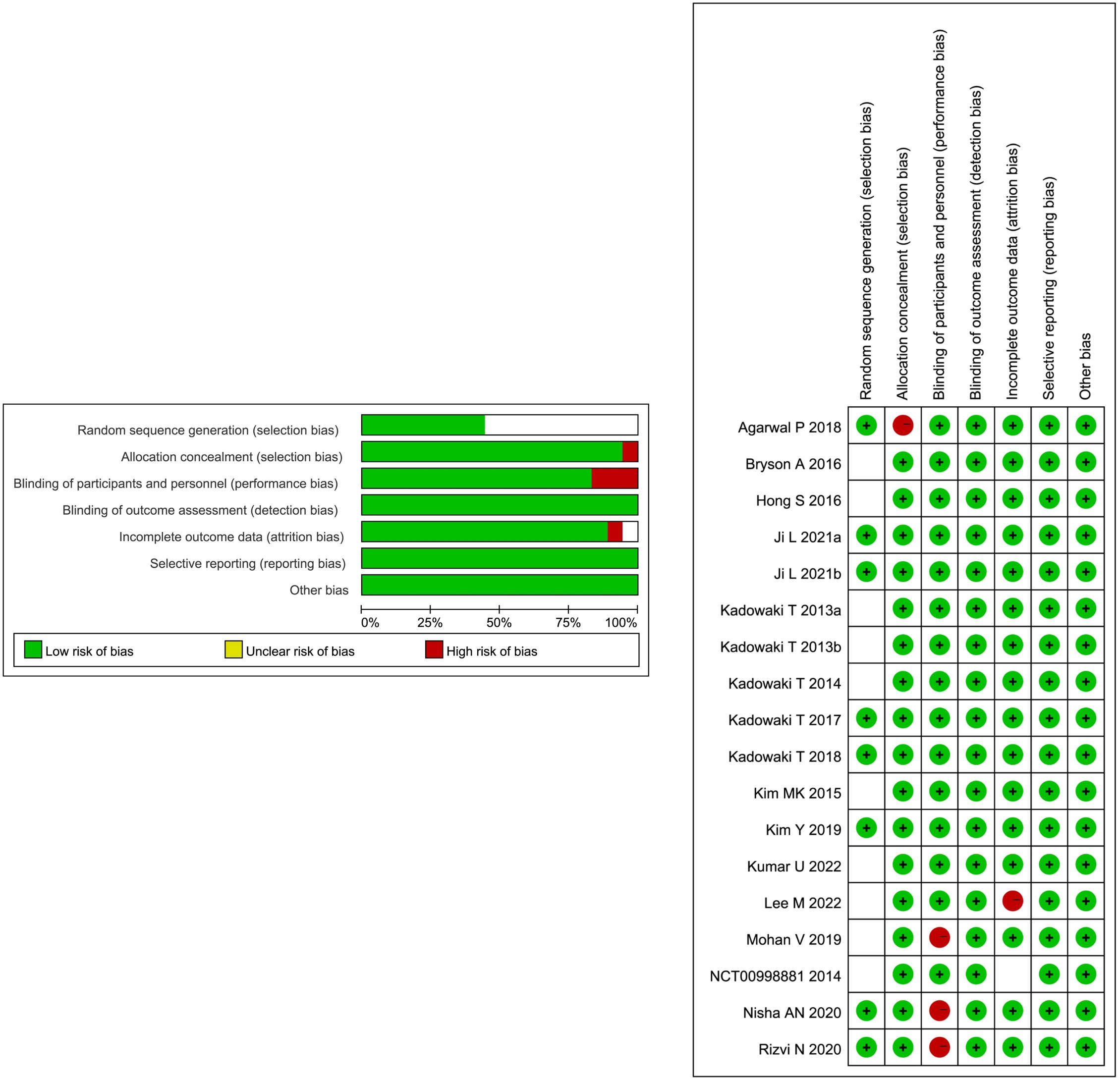
The risk of bias in the 18 included RCTs is summarized in Figure 3. Among the 18 RCTs, all of them had low risk for bias of blinding of outcome assessment, incomplete outcome data, and selective reporting, 8 RCTs had low risk for bias in random sequence generation, and the other 10 RCTs had unclear risk of bias, 17 RCTs had low risk for bias in allocation concealment, 15 RCTs had low risk for bias in blinding of participants and personnel, 16 RCTs had low risk for bias in incomplete outcome data, and 1 RCT had unclear risk. Overall, these studies had a low or moderate level of risk.
3.3 Network meta-analysis
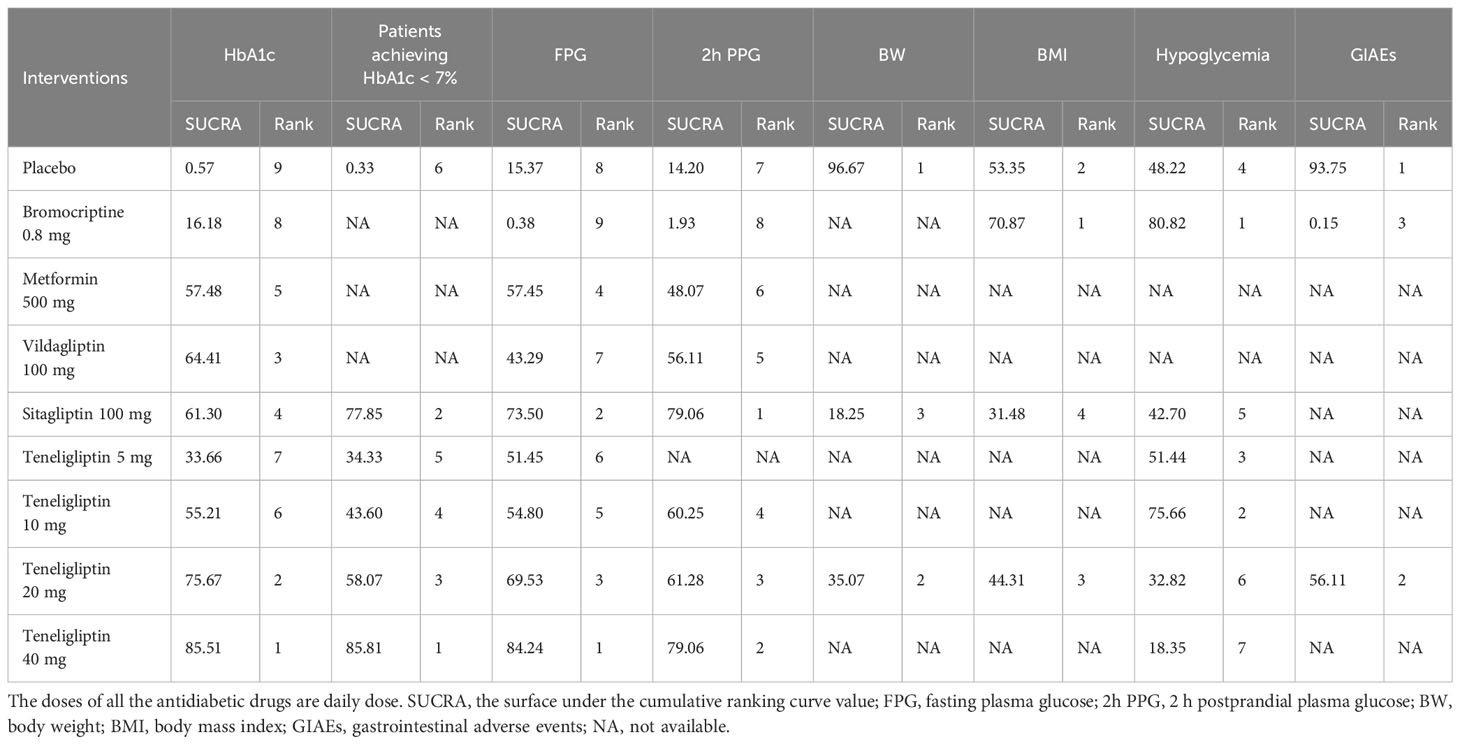
The statistical analysis of all indicators was performed using a random-effects model. SUCRA values of all the interventions and outcomes are shown in Figure 4 and Table 1. League tables of the efficacy outcomes (HbA1c, proportion of the patients achieving HbA1c < 7%, FPG, 2h PPG, BW, and BMI) are shown in Tables 2–4. League table of the safety outcomes (hypoglycemia, GIAEs) is shown in Table 5.
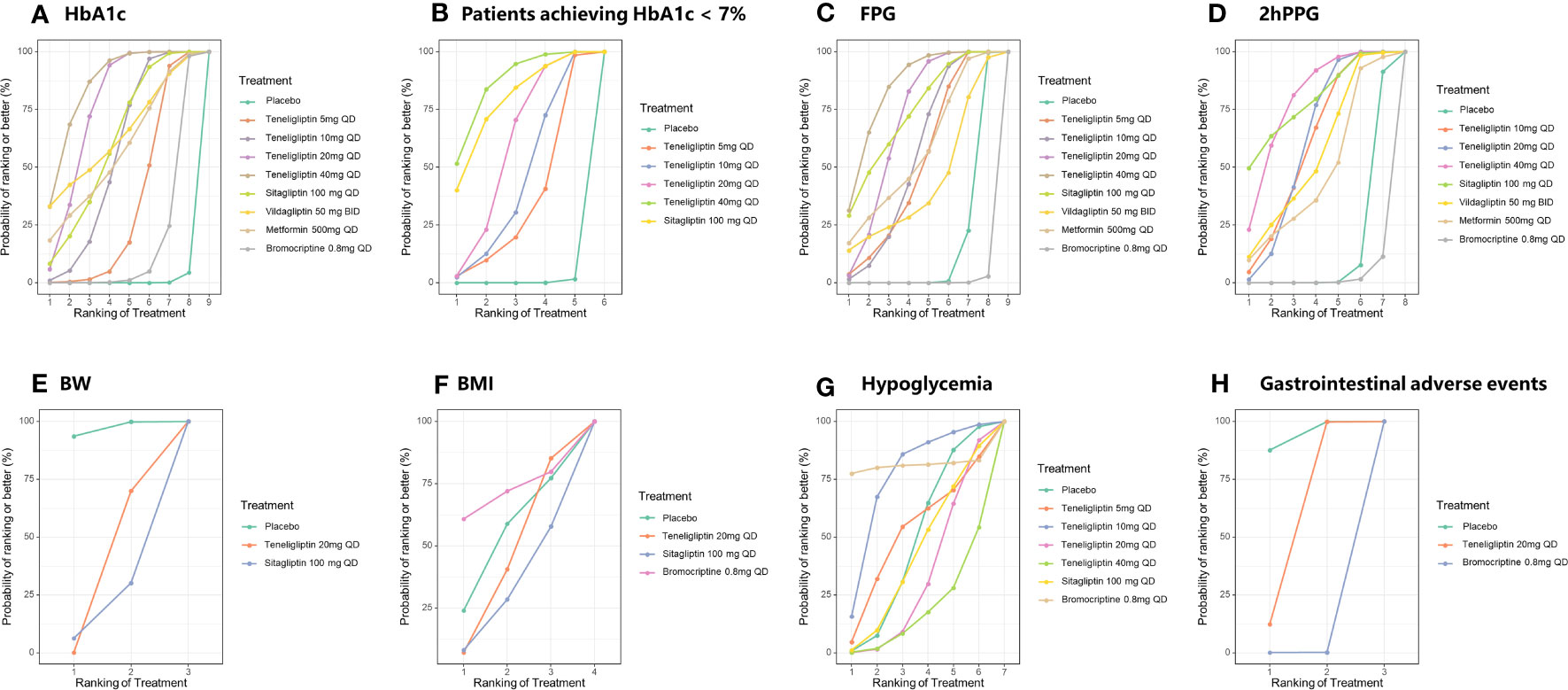
Figure 4 SUCRA plots of efficacy and safety outcomes. SUCRA, the surface under the cumulative ranking curve value; HbA1c, glycated hemoglobin A1c; FPG, fasting plasma glucose; 2h PPG, 2 h postprandial plasma glucose; BW, body weight; BMI, body mass index; GIAEs, gastrointestinal adverse events. (A) HbA1c; (B) Patients achieving HbA1c < 7%; (C) FPG; (D) 2h PPG; (E) BW; (F) BMI; (G) Hypoglycemia; (H) GIAEs.
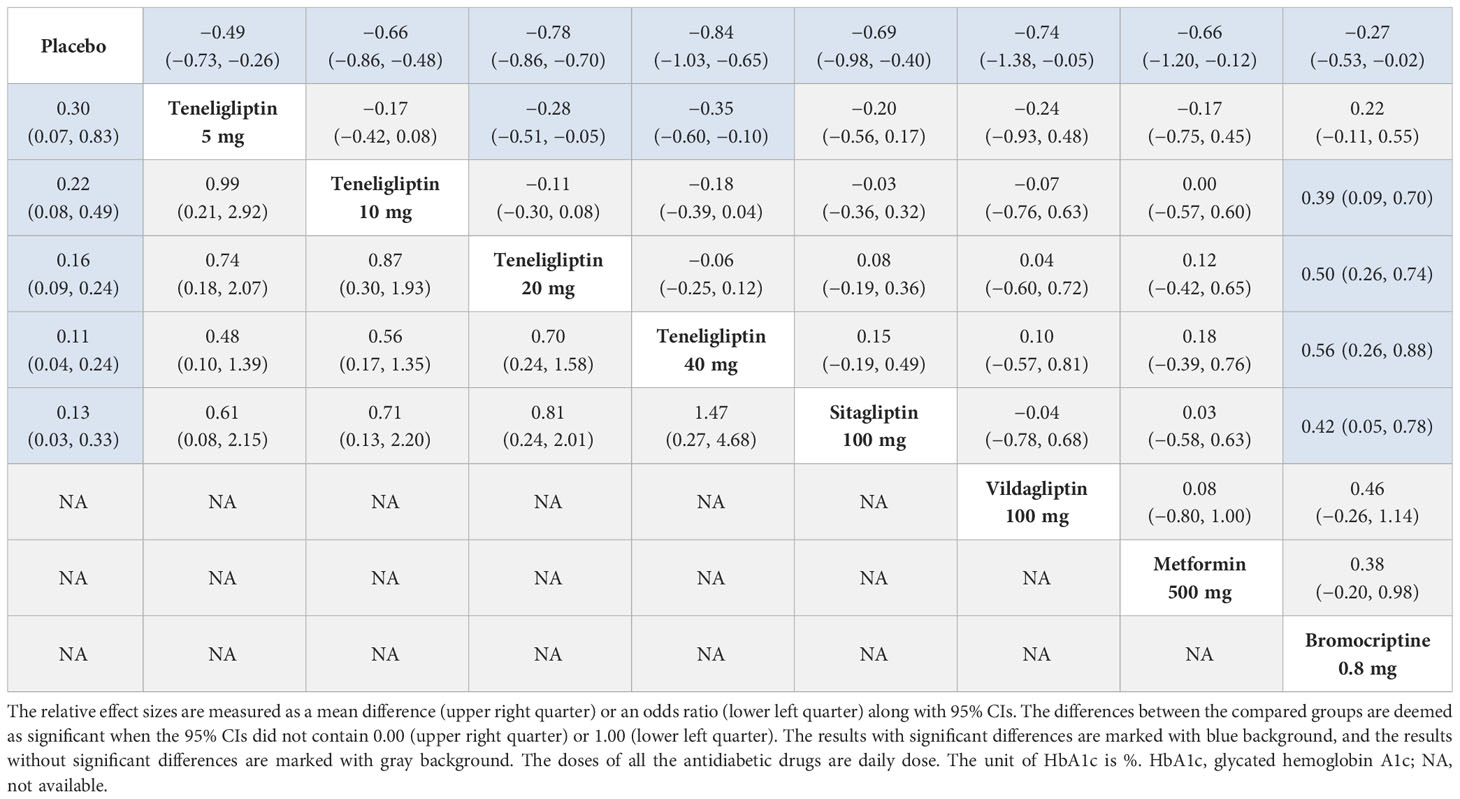
Table 2 League table of HbA1c (upper right quarter) and proportion of the patients achieving HbA1c < 7% (lower left quarter).
3.3.1 HbA1c
As shown in Table 2, compared to placebo, 5 mg, 10 mg, 20 mg, and 40 mg of teneligliptin showed significant efficacy in reducing HbA1c (MD [95% CI], −0.49 [−0.73 to −0.26], −0.66 [−0.86 to −0.48], −0.78 [−0.86 to −0.70], and −0.84 [−1.03 to −0.65], respectively). Compared to sitagliptin, vildagliptin, and metformin, 20 mg and 40 mg of teneligliptin showed better efficacy in reducing HbA1c (MD [95% CI], 20 mg: −0.08 [−0.36 to 0.19], −0.04 [−0.72 to 0.60], and −0.12 [−0.65 to 0.42]; 40 mg: −0.15 [−0.49 to 0.19], −0.10 [−0.81 to 0.57], and −0.18 [−0.76 to 0.39], respectively). Compared to sitagliptin, vildagliptin, and metformin, 5 mg and 10 mg of teneligliptin showed weaker efficacy in reducing HbA1c (MD [95% CI], 5 mg: 0.20 [−0.17 to 0.54], 0.24 [−0.48 to 0.93], and 0.17 [−0.45 to 0.75]; 10 mg: 0.03 [−0.32 to 0.36], 0.07 [−0.63 to 0.76], and 0.00 [−0.60 to 0.57], respectively). Compared to bromocriptine, 10 mg, 20 mg, and 40 mg of teneligliptin showed significant efficacy in reducing HbA1c (MD [95% CI], −0.39 [−0.70 to −0.09], −0.50 [−0.74 to −0.26], and −0.56 [−0.88 to −0.26], respectively).
The results of SUCRA indicated that 40 mg of teneligliptin was the best option in reducing HbA1c (85.51%), followed by 20 mg of teneligliptin (75.67%), 100 mg of vildagliptin (64.41%), 100 mg of sitagliptin (61.3%), 500 mg of metformin (57.48%), 10 mg of teneligliptin (55.21%), 5 mg of teneligliptin (33.66%), 0.8 mg of bromocriptine (16.18%), and placebo (0.57%) (Figure 4A and Table 1).
3.3.2 Proportion of the patients achieving HbA1c < 7%
Compared to placebo, 5 mg, 10 mg, 20 mg, and 40 mg of teneligliptin showed significant efficacy in increasing the proportion of the patients achieving HbA1c < 7% (OR [95% CI], 4.95 [1.20 to 14.09], 5.74 [2.03 to 13.22], 6.81 [4.13 to 11.03], and 11.97 [4.19 to 28.48], respectively). Compared to sitagliptin, 40 mg of teneligliptin showed better efficacy in increasing the proportion of the patients achieving HbA1c < 7% (OR [95% CI], 1.47 [0.27 to 4.68]). Compared to sitagliptin, 5 mg, 10 mg, and 20 mg of teneligliptin showed weaker efficacy (OR [95% CI], 0.61 [0.08 to 2.15], 0.71 [0.13 to 2.20], and 0.81 [0.24 to 2.01], respectively) (Table 2).
The results of SUCRA indicated that, superiority of increasing proportion of the patients achieving HbA1c < 7% ranked as follows: 40 mg of teneligliptin (85.81%), 100 mg of sitagliptin (77.85%), 20 mg of teneligliptin (58.07%), 10 mg of teneligliptin (43.6%), 5 mg of teneligliptin (34.33%), and placebo (0.33%) (Figure 4B and Table 1).
3.3.3 FPG
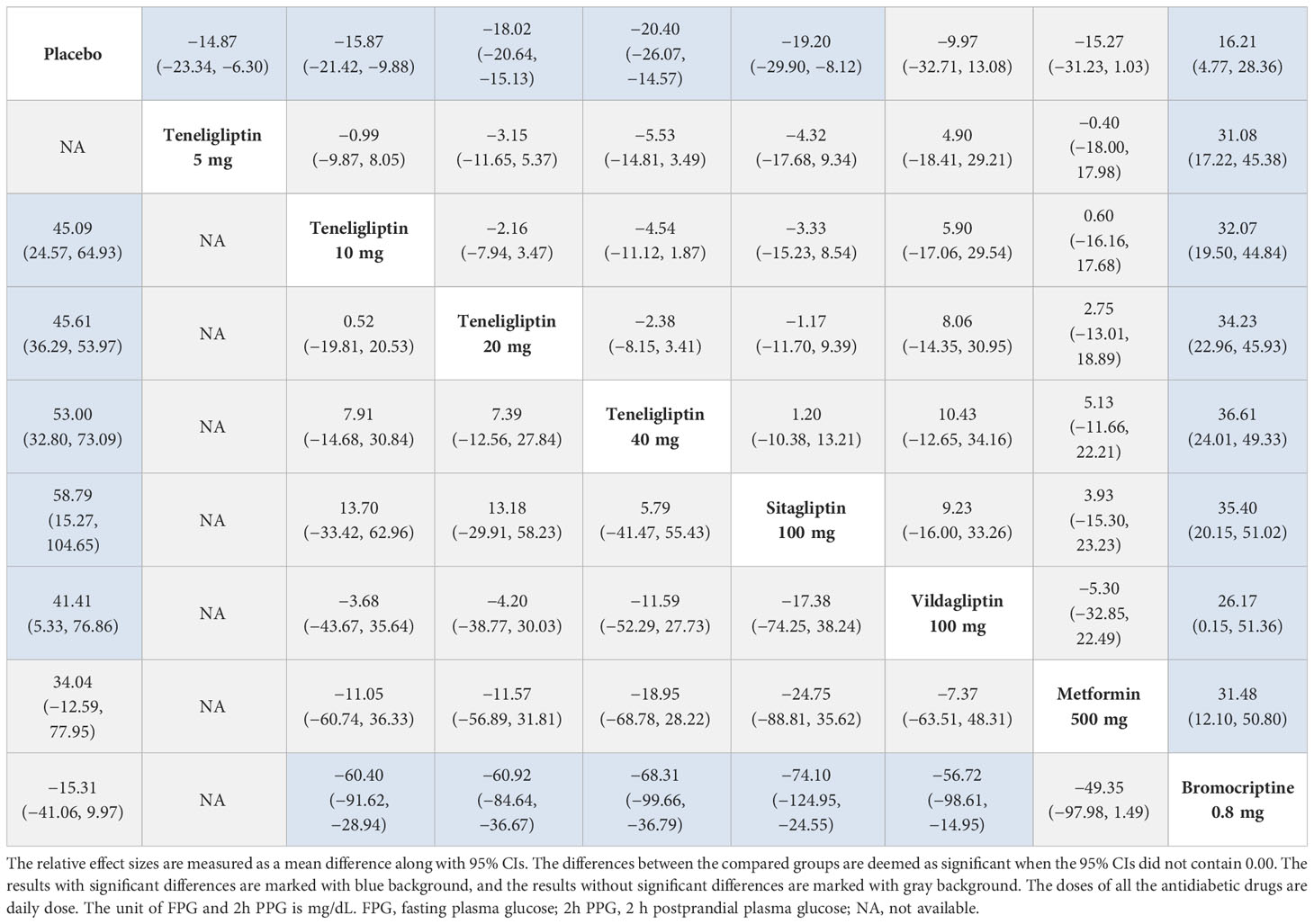
As shown in Table 3, compared to placebo, 5 mg, 10 mg, 20 mg, and 40 mg of teneligliptin showed significant efficacy in reducing FPG (MD [95% CI], −14.87 [−23.34 to −6.30], −15.87 [−21.42 to −9.88], −18.02 [−20.64 to −15.13], and −20.40 [−26.07 to −14.57], respectively). Compared to sitagliptin, 40 mg of teneligliptin showed better efficacy in reducing FPG (MD [95% CI], −1.20 [−13.21 to 10.38]). Compared to sitagliptin, 5 mg, 10 mg, and 20 mg of teneligliptin showed weaker efficacy (MD [95% CI], 4.32 [−9.34 to 17.68], 3.33 [−8.54 to 15.23], and 1.17 [−9.39 to 11.70], respectively). Compared to vildagliptin, 5 mg, 10 mg, 20 mg, and 40 mg of teneligliptin showed better efficacy in reducing FPG (MD [95% CI], −4.90 [−29.21 to 18.41], −5.90 [−29.54 to 17.06], −8.06 [−30.95 to 14.35], and −10.43 [−34.16 to 12.65], respectively). Compared to metformin, 10 mg, 20 mg, and 40 mg of teneligliptin showed better efficacy in reducing FPG (MD [95% CI], −0.60 [−17.68 to 16.16], −2.75 [−18.89 to 13.01], and −5.13 [−22.21 to 11.66], respectively). Compared to metformin, 5 mg of teneligliptin showed weaker efficacy (MD [95% CI], 0.40 [−17.98 to 18.00]). Moreover, compared to bromocriptine, 5 mg, 10 mg, 20 mg, and 40 mg of teneligliptin showed significant efficacy in reducing FPG (MD [95% CI], −31.08 [−45.38 to −17.22], −32.07 [−44.84 to −19.50], −34.23 [−45.93 to −22.96], and −36.61 [−49.33 to −24.01], respectively).
According to the SUCRA (Figure 4C and Table 1), 40 mg of teneligliptin (84.24%) seemed to be the most effective option in reducing FPG, followed by 100 mg of sitagliptin (73.5%), 20 mg of teneligliptin (69.53%), 500 mg of metformin (57.45%), 10 mg of teneligliptin (54.8%), 5 mg of teneligliptin (51.45%), 100 mg of vildagliptin (43.29%), placebo (15.37%), and 0.8 mg of bromocriptine (0.38%).
3.3.4 2h PPG
Compared to placebo, 10 mg, 20 mg and 40 mg of teneligliptin showed significant efficacy in reducing 2h PPG (MD [95% CI], −45.09 [−64.93 to −24.57], −45.61 [−53.97 to −36.29], and −53.00 [−73.09 to −32.80], respectively). Compared to sitagliptin, 10 mg, 20 mg, and 40 mg of teneligliptin showed weaker efficacy in reducing 2h PPG (MD [95% CI], 13.70 [−33.42 to 62.96], 13.18 [−29.91 to 58.23], and 5.79 [−41.47 to 55.43], respectively). Compared to vildagliptin, 10 mg, 20 mg, and 40 mg of teneligliptin showed better efficacy in reducing 2h PPG (MD [95% CI], −3.68 [−43.67 to 35.64], −4.20 [−38.77 to 30.03], and −11.59 [−52.29 to 27.73], respectively). Compared to metformin, 10 mg, 20 mg, and 40 mg of teneligliptin also showed better efficacy in reducing 2h PPG (MD [95% CI], −11.05 [−60.74 to 36.33], −11.57 [−56.89 to 31.81], and −18.95 [−68.78 to 28.22], respectively). Compared to bromocriptine, 10 mg, 20 mg, and 40 mg of teneligliptin showed significant efficacy in reducing 2h PPG (MD [95% CI], −60.40 [−91.62 to −28.94], −60.92 [−84.64 to −36.67], and −68.31 [−99.66 to −36.79], respectively) (Table 3).
According to the SUCRA (Figure 4D and Table 1), both 100 mg of sitagliptin and 40 mg of teneligliptin seemed to be the best intervention in reducing 2h PPG (79.06%), followed by 20 mg of teneligliptin (61.28%), 10 mg of teneligliptin (60.25%), 100 mg of vildagliptin (56.11%), 500 mg of metformin (48.07%), placebo (14.2%), and 0.8 mg of bromocriptine (1.93%).
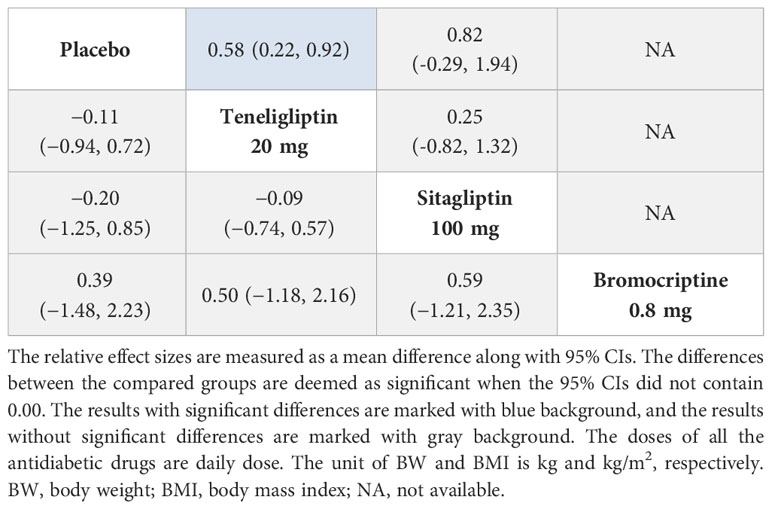
3.3.5 BW
As shown in Table 4, 20 mg of teneligliptin showed better efficacy in reducing BW than sitagliptin (MD [95% CI], −0.25 [−1.32 to 0.82]). However, 20 mg of teneligliptin showed significantly weaker efficacy than placebo (MD [95% CI], 0.58 [0.22 to 0.92]). According to the SUCRA (Figure 4E and Table 1), 20 mg of teneligliptin (35.07%) showed a better effect in reducing BW than sitagliptin (18.25%).
3.3.6 BMI
Compared to placebo, 20 mg of teneligliptin and 100 mg of sitagliptin showed weaker efficacy in reducing BMI (MD [95% CI], 0.11 [−0.72 to 0.94] and 0.20 [−0.85 to 1.25], respectively). Compared to placebo, 0.8 mg of bromocriptine showed better efficacy (MD [95% CI], −0.39 [−2.23 to 1.48]) (Table 4). According to the SUCRA, 0.8 mg of bromocriptine was the best option in reducing BMI (70.87%), followed by placebo (53.35%), 20 mg of teneligliptin (44.31%), and 100 mg of sitagliptin (31.48%) (Figure 4F and Table 1).
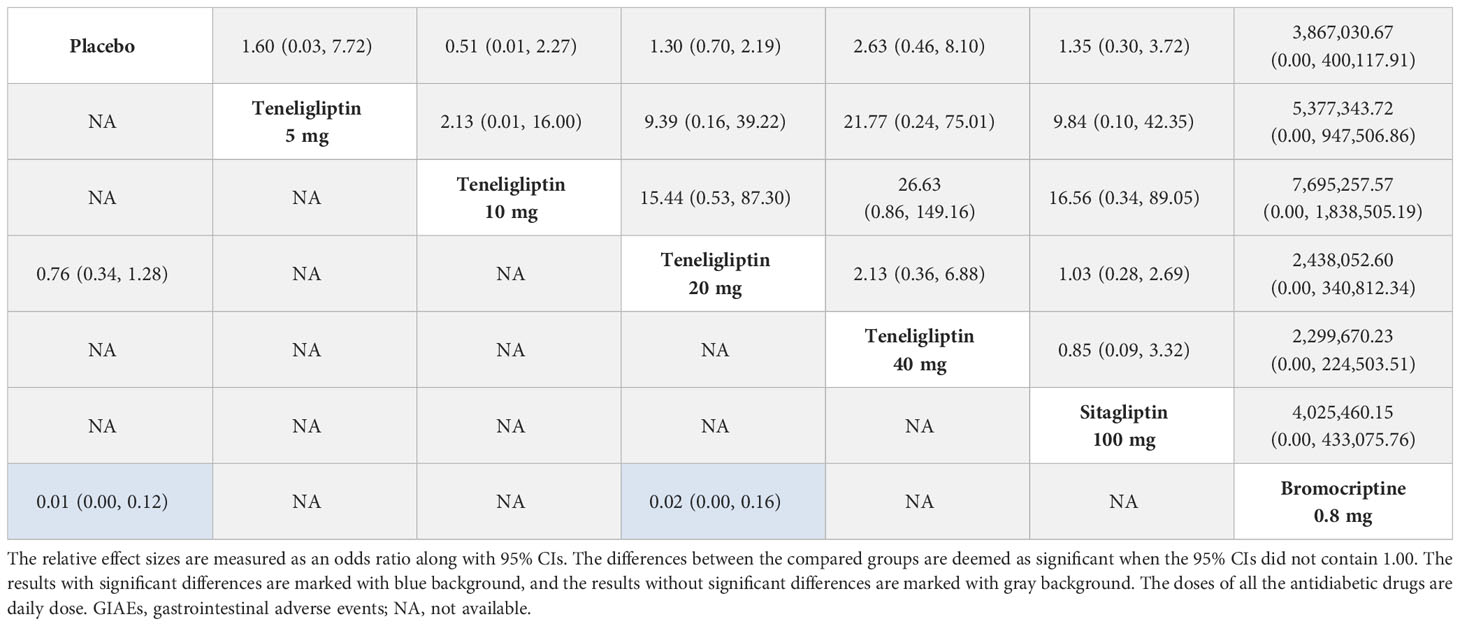
3.3.7 Hypoglycemia
As shown in Table 5, compared to placebo, 5 mg, 10 mg, 20 mg, and 40 mg of teneligliptin showed no-inferior risk of hypoglycemia (OR [95% CI], 1.60 [0.03 to 7.72], 0.51 [0.01 to 2.27], 1.30 [0.70 to 2.19], and 2.63 [0.46 to 8.10], respectively). In addition, compared to placebo, sitagliptin and bromocriptine also showed no significant difference in incidence of hypoglycemia. According to the SUCRA (Figure 4G and Table 1), 0.8 mg of bromocriptine (80.82%) was considered as the best intervention in avoiding hypoglycemia, followed by 10 mg of teneligliptin (75.66%), 5 mg of teneligliptin (51.44%), placebo (48.22%), 100 mg of sitagliptin (42.70%), 20 mg of teneligliptin (32.82%), and 40 mg of teneligliptin (18.35%).
3.3.8 GIAEs
Compared to placebo, 20 mg of teneligliptin showed no significant difference in incidence of GIAEs (OR [95% CI], 1.48 [0.78 to 2.98]). Compared to bromocriptine, 20 mg of teneligliptin had a significantly lower risk of GIAEs (OR [95% CI], 0.02 [0.00 to 0.16]) (Table 5). According to the SUCRA (Figure 4H and Table 1), 20 mg of teneligliptin (56.11%) had a lower incidence of GIAEs than 0.8 mg of bromocriptine (0.15%).
3.3.9 Consistency and heterogeneity tests
Difference value of DIC between inconsistency and consistency models for each outcome was less than three, indicating that inconsistency of this network analysis was not significant. For most outcome measures of this study, I2 statistic value was under 50%, and heterogeneity was not obvious. Only I2 values of two outcomes (i.e., BW and BMI) were over 50%. According to the sensitivity analysis (Table S2), the I2 value of network analysis of BW outcome decreased to 42.62% (<50%) after eliminating one (35) of the included studies. However, for network analysis of BMI outcome, the heterogeneity was obvious with a I2 value > 50% after eliminating one of the included studies (36, 38, 43) in turn.
3.3.10 Publication bias
The comparison-adjusted funnel plots for assessment of publication bias are shown in Figure 5. Visual inspections indicated that, distribution of the included studies was not asymmetric, and there was some angle between the adjusted auxiliary line and the horizontal zero line, suggesting that some publication bias may exist.
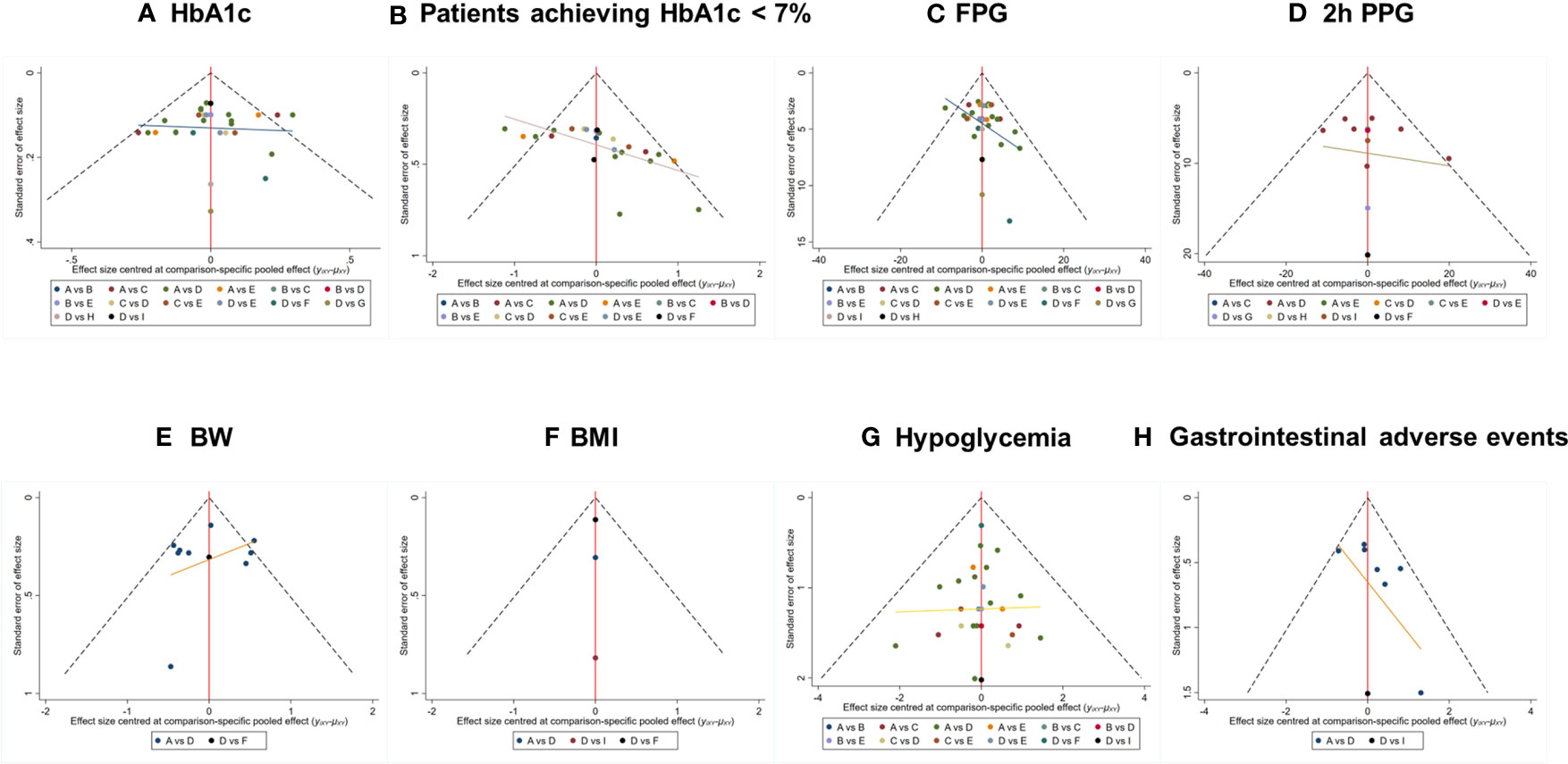
Figure 5 Comparison-adjusted funnel plot for outcomes. The funnel plots displayed publication bias of respective outcomes: (A) HbA1c, (B) Patients achieving HbA1c < 7%, (C) FPG, (D) 2h PPG, (E) BW, (F) BMI, (G) Hypoglycemia, (H) GIAEs. The different colored nodes in the plots represent certain paired comparisons of respective interventions: (A) Placebo, (B) 5 mg of teneligliptin, (C) 10 mg of teneligliptin, (D) 20 mg of teneligliptin, (E) 40 mg of teneligliptin, (F) 100 mg of sitagliptin, (G) 100 mg of vildagliptin, (H) 500mg of metformin, (I) 0.8 mg of bromocriptine. HbA1c, glycated hemoglobin A1c; FPG, fasting plasma glucose; 2h PPG, 2 h postprandial plasma glucose; BW, body weight; BMI, body mass index; GIAEs, gastrointestinal adverse events.
4 Discussion
Compared to placebo, 5 mg, 10 mg, 20 mg, and 40 mg of teneligliptin were better in most efficacy outcomes except for reducing BW and BMI. This indicated that teneligliptin showed observable glucose-lowering and poor weight-loss effect. In all safety outcomes, there was no significant difference among placebo and the four doses of teneligliptin. This implied that teneligliptin showed acceptable safety. It should be noted that the heterogeneity in the network meta-analyses of BW and BMI were obvious, so their reliability was lower than the other outcome measures.
Efficacy of teneligliptin increased with its dose from 5 mg to 40 mg, but the risk of hypoglycemia also increased. In particular, 10 mg of teneligliptin showed the lowest risk of hypoglycemia among the four doses. Compared to 20 mg of teneligliptin, 40 mg of teneligliptin showed superior glucose-lowering efficacy and no-inferior safety. Therefore, it is a favorable option to increase the dose of teneligliptin from 20 mg to 40 mg per day when its antidiabetic effect is not satisfactory. It should be noted that the limitation of this study was that 40 mg of teneligliptin was only presented in two RCTs with 169 patients in this meta-analysis, and more clinical trials with more patients should be conducted to further confirm this result in the future.
Compared to the single dose of sitagliptin and vildagliptin, four doses of teneligliptin showed a different antidiabetic effect. Among these included DPP-4is, their antidiabetic effects ranked as follows: 40 mg of teneligliptin, 20 mg of teneligliptin, 100 mg of sitagliptin, 100 mg of vildagliptin, 10 mg of teneligliptin, and 5 mg of teneligliptin. It should be noted that a lower dose of teneligliptin showed better antidiabetic effect than sitagliptin and vildagliptin. Additionally, there was no significant difference among the four doses of teneligliptin and sitagliptin in all safety outcomes. The results can provide reference for the rational selection among these DPP-4is.
Compared to 500 mg of metformin, 20 mg and 40 mg of teneligliptin showed better antidiabetic effect, but 5 mg and 10 mg of teneligliptin showed poorer efficacy. Metformin (500–2,500 mg) is the first-line medication for treatment of T2DM (44). If the therapeutic effect of metformin is not ideal (HbA1c ≥ 7%), teneligliptin combined with metformin can achieve synergistic effect. As a note, the number of participants treated with metformin was limited (n = 35) in the included RCTs, which may lead to deviations for this study.
Bromocriptine was approved for the treatment of T2DM as an adjunct to diet and exercise to improve glycemic control by FDA in 2009 (45). It does not belong to popular antidiabetic drugs. Compared to four doses of teneligliptin, bromocriptine was poorer in most efficacy outcomes and better in reducing BMI, and showed a lower risk of hypoglycemia and a higher risk of GIAEs. As a note, limitation of the number of participants treated with bromocriptine (n = 25) may lead to deviation of the results.
We conducted a literature search and review about the meta-analyses containing teneligliptin, as summarized in Table S3. In previous studies (46, 47), efficacy and safety of teneligliptin were evaluated by very limited systematic reviews and traditional meta-analyses, which only included 10 (47) or 13 (46) RCTs. In the present study, 18 RCTs enrolling 3,290 patients with different interventions were included. There were also two network meta-analyses (48, 49) involving teneligliptin. However, teneligliptin was not the main evaluation object in the two network meta-analyses, and the outcome measure of evaluation of teneligliptin was very limited, which only included HbA1c (48) or incidence of GIAEs (49). Teneligliptin was the main evaluation object in the present Bayesian network meta-analysis, and eight outcome measures were included to systematically evaluate the efficacy and safety of teneligliptin.
In the present study, a serious search comprehensively covered the latest research findings, and independent study identification, selection, and data extraction were performed by two reviewers. Nevertheless, the heterogeneity and publication bias of included studies were not avoided. As a note, our inference was based on currently available data from a limited number of RCTs, and more large-scale, high-quality, and long-term clinical trials are needed to assess efficacy and safety of teneligliptin in the future.
5 Conclusion
In summary, efficacy and safety of teneligliptin in patients with T2DM were evaluated by Bayesian network meta-analysis of 18 RCTs in this study. Compared to sitagliptin, vildagliptin, metformin, bromocriptine, and placebo, teneligliptin displayed favorable efficacy and acceptable safety in the treatment of T2DM. Twenty milligrams or 40 mg per day could be chosen as the optimal dosage regimen for teneligliptin.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MZ: Investigation, Methodology, Writing – original draft, Data curation, Validation. RG: Data curation, Investigation, Methodology, Validation, Writing – original draft. GM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Writing – review & editing, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grants 82374133, 82074109, 81873078, and 81374051).
Acknowledgments
We would like to thank the researchers and study participants for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1282584/full#supplementary-material
Abbreviations
DM, diabetes mellitus; T2DM, type 2 diabetes mellitus; DPP-4i, dipeptidyl peptidase-4 inhibitor; SGLT-2i, sodium-glucose cotransporter-2 inhibitor; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin A1c; FPG, fasting plasma glucose; 2h PPG, 2 h postprandial plasma glucose; BW, body weight; BMI, body mass index; GIAEs, gastrointestinal adverse events; RCT, randomized controlled trial; ITT, intention-to-treat; MD, mean difference; OR, odds ratio; 95% CI, 95% confidence interval; SD, standard deviation; SE, standard error; DIC, deviance information criteria; SUCRA, the surface under the cumulative ranking curve value.
References
1. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Kaabi JAl. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Global Health (2020) 10(1):107–15. doi: 10.2991/jegh.k.191028.001
2. Magliano DJ, Boyko EJ, IDF Diabetes Atlas 10th edition scientific committee. IDF DIABETES ATLAS. 10th ed. IDF Diabetes Atlas. Brussels: International Diabetes Federation (2021). Available at: http://www.ncbi.nlm.nih.gov/books/NBK581934/.
3. Ogurtsova K, Guariguata L, Barengo NoëlC, Ruiz PL-D, Sacre JW, Karuranga S, et al. IDF diabetes atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract (2022) 183(January):109118. doi: 10.1016/j.diabres.2021.109118
4. Ahmad E, Soo L, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet (London England) (2022) 400(10365):1803–205. doi: 10.1016/S0140-6736(22)01655-5
5. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci (2020) 21(17):62755. doi: 10.3390/ijms21176275
6. Borse SP, Chhipa AS, Sharma V, Singh DP, Nivsarkar M. Management of type 2 diabetes: current strategies, unfocussed aspects, challenges, and alternatives. Med Principles Practice: Int J Kuwait University Health Sci Centre (2021) 30(2):109–215. doi: 10.1159/000511002
7. Papadopoulou A, Papadopoulos KI. Successful lifestyle modifications may underlie umbilical cord-mesenchymal stromal cell effects in type 2 diabetes mellitus. World J Diabetes (2023) 14(3):347–515. doi: 10.4239/wjd.v14.i3.347
8. Scott ES, Januszewski AS, O’Connell R, Fulcher G, Scott R, Kesaniemi A, et al. Long-term glycemic variability and vascular complications in type 2 diabetes: post hoc analysis of the FIELD study. J Clin Endocrinol Metab (2020) 105(10):dgaa361. doi: 10.1210/clinem/dgaa361
9. Heo GaY, Koh HB, Kim HW, Park JT, Yoo T-H, Kang S-W, et al. Glycemic control and adverse clinical outcomes in patients with chronic kidney disease and type 2 diabetes mellitus: results from KNOW-CKD. Diabetes Metab J (2023) 47(4):535–46. doi: 10.4093/dmj.2022.0112
10. Niu J, Zhang X, Li M, Wu S, Zheng R, Chen Li, et al. Risk of cardiovascular disease, death, and renal progression in diabetes according to albuminuria and estimated glomerular filtration rate. Diabetes Metab (2023) 49(2):101420. doi: 10.1016/j.diabet.2023.101420
11. Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, et al. Management of hyperglycaemia in type 2 diabetes 2022. A consensus report by the american diabetes association (ADA) and the european association for the study of diabetes (EASD). Diabetologia (2022) 65(12):1925–66. doi: 10.1007/s00125-022-05787-2
12. Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocrine Rev (2014) 35(6):992–10195. doi: 10.1210/er.2014-1035
13. Singh H, Singh J, Bhangu RK, Singla M, Singh J, Javid F. Potential approaches using teneligliptin for the treatment of type 2 diabetes mellitus: current status and future prospects. Expert Rev Clin Pharmacol (2023) 16(1):49–595. doi: 10.1080/17512433.2023.2163386
14. Sharma SK, Panneerselvam A, Singh KP, Parmar G, Gadge P, Swami OC. Teneligliptin in management of type 2 diabetes mellitus. Diabetes Metab Syndrome Obesity-Targets Ther (2016) 9:251–60. doi: 10.2147/DMSO.S106133
15. Watt J, Del Giovane C. Network meta-analysis. Methods Mol Biol (Clifton N.J.) (2022) 2345:187–201. doi: 10.1007/978-1-0716-1566-9_12
16. Beis G, Papasotiriou I. Is network meta-analysis a revolutionary statistical tool for improving the reliability of clinical trial results? A brief overview and emerging issues arising. In Vivo (2023) 37(3):972–845. doi: 10.21873/invivo.13171
17. Ahn E, Kang H. Concepts and emerging issues of network meta-analysis. Korean J Anesthesiol (2021) 74(5):371–825. doi: 10.4097/kja.21358
18. Seitidis G, Nikolakopoulos S, Hennessy EA, Tanner-Smith EE, Mavridis D. Network meta-analysis techniques for synthesizing prevention science evidence. Prev Science: Off J Soc Prev Res (2022) 23(3):415–24. doi: 10.1007/s11121-021-01289-6
19. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Reprint—Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther (2009) 89(9):873–05. doi: 10.1093/ptj/89.9.873
20. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Internal Med (2015) 162(11):777–84. doi: 10.7326/M14-2385
21. Higgins JPT, Altman DG, Gotzsche PC, Jueni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj-British Med J (2011) 343(October):d5928. doi: 10.1136/bmj.d5928
22. Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS One (2013) 8(10):e766545. doi: 10.1371/journal.pone.0076654
23. Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. BUGSnet: an R package to facilitate the conduct and reporting of bayesian network meta-analyses. BMC Med Res Method (2019) 19(1):1965. doi: 10.1186/s12874-019-0829-2
24. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (2022). Available at: https://www.training.cochrane.org/handbook.
25. Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Systematic Rev (2017) 6(1):795. doi: 10.1186/s13643-017-0473-z
26. Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Invest (2013) 4(6):576–845. doi: 10.1111/jdi.12092
27. Kadowaki T, Kondo K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab (2013) 15(9):810–18. doi: 10.1111/dom.12092
28. Kadowaki T, Kondo K. Efficacy and safety of teneligliptin added to glimepiride in Japanese patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study with an open-label, long-term extension. Diabetes Obes Metab (2014) 16(5):418–25. doi: 10.1111/dom.12235
29. NCT00998881. Monotherapy study of MP-513 in patients with type 2 diabetes (2014). Available at: https://clinicaltrials.gov/ct2/show/NCT00998881.
30. Kim MK, Rhee E-J, Han KA, Woo AC, Lee M-K, Ku BJ, et al. Efficacy and safety of teneligliptin, a dipeptidyl peptidase-4 inhibitor, combined with metformin in korean patients with type 2 diabetes mellitus: A 16-week, randomized, double-blind, placebo-controlled phase III trial. Diabetes Obes Metab (2015) 17(3):309–12. doi: 10.1111/dom.12424
31. Bryson A, Jennings PE, Deak L, Paveliu FS, Lawson M. The efficacy and safety of teneligliptin added to ongoing metformin monotherapy in patients with type 2 diabetes: A randomized study with open label extension. Expert Opin Pharmacother (2016) 17(10):1309–165. doi: 10.1080/14656566.2016.1190334
32. Hong S, Park C-Y, Han KA, Chung CH, Ku BJ, Jang HC, et al. Efficacy and safety of teneligliptin, a novel dipeptidyl peptidase-4 inhibitor, in Korean patients with type 2 diabetes mellitus: A 24-week multicentre, randomized, double-blind, placebo-controlled phase III trial. Diabetes Obes Metab (2016) 18(5):528–32. doi: 10.1111/dom.12631
33. Kadowaki T, Kondo K, Sasaki N, Miyayama K, Yokota S, Terata R, et al. Efficacy and safety of teneligliptin add-on to insulin monotherapy in Japanese patients with type 2 diabetes mellitus: A 16-week, randomized, double-blind, placebo-controlled trial with an open-label period. Expert Opin Pharmacother (2017) 18(13):1291–13005. doi: 10.1080/14656566.2017.1359259
34. Agarwal P, Chhavi J, Vinayak S. Efficacy and safety of teneligliptin in Indian patients with inadequately controlled type 2 diabetes mellitus: A randomized, double-blind study. Indian J Endocrinol Metab (2018) 22(1):41–465. doi: 10.4103/ijem.IJEM_97_16
35. Kadowaki T, Inagaki N, Kondo K, Nishimura K, Kaneko G, Maruyama N, et al. Efficacy and safety of teneligliptin added to canagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: A multicentre, randomized, double-blind, placebo-controlled, parallel-group comparative study. Diabetes Obes Metab (2018) 20(2):453–575. doi: 10.1111/dom.13079
36. Kim Y, Kang ES, Jang HC, Kim DJ, Oh T, Kim ES, et al. “Teneligliptin versus sitagliptin in Korean patients with type 2 diabetes inadequately controlled with metformin and glimepiride: A randomized, double-blind, non-inferiority trial.” Diabetes Obes Metab (2019) 21(3):631–39. doi: 10.1111/dom.13566
37. Mohan V, Ramu M, Poongothai S, Kasthuri S. A prospective, open-label, randomized study comparing efficacy and safety of teneligliptin versus sitagliptin in Indian patients with inadequately controlled type 2 diabetes mellitus: INSITES study. J Assoc Physicians India (2019) 67(10):14–9. doi: 10.13005/bpj/1886
38. Nisha A, Ruckmani A, Rajasekaran D, Abinaya E, Lella T, Arunkumar R. Assessment of safety and efficacy of bromocriptine in comparison with teneligliptin in newly diagnosed type 2 diabetes mellitus. Biomed Pharmacol J (2020) 13(1):269–80. doi: 10.13005/bpj/1886
39. Rizvi N, Ahmad J, Ahmad F, Siddiqui SS, Khan BH. Efficacy and safety of vildagliptin and teneligliptin in patients of type 2 diabets mellitus inadequately controlled on stable doses of metformin. Int J Pharm Sci Res (2020) 11(4):1629–34. doi: 10.13040/IJPSR.0975-8232.11(4).1629-34
40. Ji L, Li L, Ma J, Li X, Li D, Meng B, et al. Efficacy and safety of teneligliptin added to metformin in Chinese patients with type 2 diabetes mellitus inadequately controlled with metformin: A phase 3, randomized, double-blind, placebo-controlled study. Endocrinol Diabetes Metab (2021) 4(2):e00222. doi: 10.1002/edm2.222
41. Ji L, Ma J, Lu W, Liu J, Zeng Jiao’e, Yang J, et al. Phase III, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of teneligliptin monotherapy in chinese patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. J Diabetes Invest (2021) 12(4):537–45. doi: 10.1111/jdi.13389
42. Kumar U, Ranjan A, Ranjan P, Kumar D, Prasad K. Assessment of effect of teneligliptin vs metformin on glycemic control in patients with type 2 diabetes mellitus at a tertiary centre in Bihar. Int J Pharm Clin Res (2022) 14(12):145–50. doi: 10.24926/iip.v11i4.3355
43. Lee M, Lee W-J, Kim JH, Lee B-W. Effectiveness and safety of teneligliptin added to patients with type 2 diabetes inadequately controlled by oral triple combination therapy: A multicentre, randomized, double-blind, and placebo-controlled study. Diabetes Obes Metab (2022) 24(6):1105–135. doi: 10.1111/dom.14679
44. Chappell L, Brown SA, Wensel TM. Evaluation of vitamin B12 monitoring in patients on concomitant metformin and proton pump inhibitors. Innov Pharm (2020) 11(4):10.24926/iip.v11i4.3355. doi: 10.24926/iip.v11i4.3355
45. Ozery M, Wadhwa R. Bromocriptine. In: StatPearls. Treasure Island (FL: StatPearls Publishing (2023). Available at: http://www.ncbi.nlm.nih.gov/books/NBK555948/.
46. Li X, Huang X, Bai C, Qin D, Cao S, Mei Q, et al. Efficacy and safety of teneligliptin in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Front Pharmacol (2018) 9:449. doi: 10.3389/fphar.2018.00449
47. Pelluri R, Kongara S, Nagasubramanian VR, Mahadevan S, Chimakurthy J. Systematic review and meta-analysis of teneligliptin for treatment of type 2 diabetes. J Endocrinological Invest (2023) 46(5):855–67. doi: 10.1007/s40618-023-02003-9
48. Ayers D, Kanters S, Goldgrub R, Hughes M, Kato R, Kragh N. Network meta-analysis of liraglutide versus dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes in Japanese patients. Curr Med Res Opin (2017) 33(9):1653–615. doi: 10.1080/03007995.2017.1345730
Keywords: teneligliptin, type 2 diabetes mellitus, systematic review, bayesian network meta-analysis, efficacy, safety
Citation: Zhu M, Guan R and Ma G (2023) Efficacy and safety of teneligliptin in patients with type 2 diabetes mellitus: a Bayesian network meta-analysis. Front. Endocrinol. 14:1282584. doi: 10.3389/fendo.2023.1282584
Received: 26 August 2023; Accepted: 09 November 2023;
Published: 18 December 2023.
Edited by:
Spyridon N. Karras, Aristotle University of Thessaloniki, GreeceReviewed by:
Elettra Mancuso, University Magna Graecia of Catanzaro, ItalyRaffaele Carraro, La Princesa University Hospital, Spain
Copyright © 2023 Zhu, Guan and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo Ma, bWcwMzI4QGZ1ZGFuLmVkdS5jbg==
 Miao Zhu
Miao Zhu Ruifang Guan
Ruifang Guan Guo Ma
Guo Ma