- 1Department of Endocrinology, Imperial College Healthcare NHS Trust, London, United Kingdom
- 2Department of Surgery & Cancer, Imperial College London, London, London, United Kingdom
- 3Operative Research Unit of Medical Oncology, Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy
- 4Department of Imaging, Imperial College Healthcare NHS Trust, London, United Kingdom
- 5Department of Medical Oncology, Imperial College Healthcare NHS Trust, London, United Kingdom
- 6Division of Oncology, Department of Translational Medicine, University of Piemonte Orientale, Novara, Italy
- 7Department of Pharmacy, Imperial College Healthcare NHS Trust, London, United Kingdom
- 8Section of Endocrinology and Investigative Medicine, Division of Diabetes, Endocrinology and Metabolism, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, United Kingdom
Objectives: Immune checkpoint inhibitors (ICIs) are associated with immune-related adverse events (irAEs), of which endocrinopathies are common. We characterized endocrine and non-endocrine irAEs in cancer patients receiving ICIs, identified risk factors for their development and established whether endocrine and non-endocrine irAEs were differentially associated with improved cancer prognosis.
Design and methods: Single-center, retrospective cohort study of patients with advanced or metastatic solid tumors receiving at least one ICI treatment cycle (242 men, 151 women, median age 65 years). Main outcome measures were incidence of any irAE during the study period, overall survival and time to treatment failure.
Results: Non-endocrine irAEs occurred in 32% and endocrine irAEs in 12% of patients. Primary thyroid dysfunction was the most common endocrine irAE (9.5%) and the majority of endocrinopathies required permanent hormone replacement. Women had an increased risk of developing endocrine irAEs (p = 0.017). The biggest survival advantage occurred in patients who developed both endocrine and non-endocrine irAEs (overall survival: HR 0.16, CI 0.09-0.28). Time to treatment failure was also significantly improved in patients who developed endocrine irAEs (HR 0.49, CI 0.34 – 0.71) or both (HR 0.41, CI 0.25 – 0.64) but not in those who only developed non-endocrine irAEs.
Conclusions: Women may have increased risk of endocrine irAEs secondary to ICI treatment. This is the first study to compare the effects of endocrine irAEs with non-endocrine irAEs on survival. Development of endocrine irAEs may confer survival benefit in ICI treatment and future, prospective studies are needed to elucidate this.
Introduction
Immune checkpoint inhibitors (ICIs) upregulate the adaptive immune system by promoting T-lymphocyte mediated cytotoxic cell-death. In doing so, ICIs have revolutionised cancer care with excellent response rates and improved overall survival in multiple cancer types (1). More recently, it has been proposed that ICIs may also have a potential therapeutic role in more rare tumor subtypes such as phaeochromocytoma and paraganglioma (2).
ICIs are classified into three groups: cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors (1). Ipilimumab was the first ICI to be approved and remains a commonly used CTLA-4 inhibitor, targeting CTLA-4 on CD4 positive and CD8 positive T lymphocytes. This was swiftly followed by anti-PD-1 therapies, including nivolumab and pembrolizumab, targeting PD-1 on CD8 positive T lymphocytes. More novel anti-PD-L1 therapies, including atezolizumab, avelumab and durvalumab, bind directly to PD-L1 expressed on the tumor itself (3).
While activation of the immune system by ICIs provides significant therapeutic benefits, these drugs are also associated with immune-related adverse events (irAEs) which can be challenging to predict, diagnose and treat (3). IrAEs typically occur in a dose-dependent fashion with regimens containing CTLA-4 inhibitors, but are less predictable for PD-1 and PD-L1 inhibitors administered as monotherapies (4, 5). IrAEs can occur in any organ system, but more frequently occur in those with extensive environmental interfaces (e.g., skin, lungs, liver and gastrointestinal tract) or those with increased predisposition to autoimmunity (e.g., thyroid and joints). In general, irAEs occur most often during the first three months of treatment but can arise at any time during therapy and after treatment cessation (6).
Endocrine irAEs are among the most commonly encountered irAEs in patients receiving ICI therapy with a reported incidence of 15 – 40% depending on ICI type (5, 7). The most common endocrine irAEs encompass thyroid dysfunction, primary adrenal insufficiency, hypophysitis and immune-mediated diabetes mellitus (5). As with other irAEs, onset is more common during the first few months of treatment, but can also occur at any time during and after treatment (5). Endocrine irAEs often remain undiagnosed for some time due to the non-specific manifestations of endocrinopathies which can overlap with cancer-related or therapy-related complications (e.g., fatigue, loss of libido, depression, nausea) (5). Although often considered mild compared to other irAEs, endocrine irAEs can present as emergencies and fatalities have been reported, highlighting the need for prompt recognition and treatment (8, 9).
A growing body of evidence suggests that development of irAEs is associated with improvements in progression-free survival, overall survival and overall disease response rate (10–13). Studies have demonstrated shared T cell receptor sequences and/or upregulated organ-specific transcripts between tumors and non-malignant tissues affected by irAEs, which suggest that irAEs and the anti-tumor immune response to ICIs could be mechanistically linked (14). Prior studies investigating survival outcomes and irAEs have been focussed on clinical trial data and although valuable, can be limited by small numbers, single agent use, or disease-site specific studies, and may not reflect standard clinical care (15–17). Real-world studies evaluating the incidence and management of endocrine irAEs and assessing their effects on survival, will contribute to establishing best practice within every day clinical care.
In this study, we characterise endocrine and non-endocrine irAEs in cancer patients receiving ICIs within the standard clinical practice of a single tertiary center. We aimed to identify risk factors for development of endocrine irAEs and to establish whether development of endocrine and/or non-endocrine irAEs are associated with improved cancer prognosis.
Methods
Study design and participants
A single-center retrospective cohort study was conducted at Imperial College Healthcare NHS Trust (ICHNT) with advanced or metastatic solid tumors receiving one of the following ICIs: atezolizumab, avelumab, durvalumab, nivolumab, pembrolizumab or combination therapy with ipilimumab and nivolumab. Using a pharmacy prescribing database, all adult patients (>18y) diagnosed with a cancer eligible for ICI treatment who had received a minimum of one treatment cycle between February 2017 and September 2021 at our center were identified for inclusion in this study. Demographic and clinical information (including a previous medical history of autoimmune disease) were extracted from electronic patient records. Anonymised data were recorded until treatment ended, death or the datalock of 1st March 2022. Patients were excluded from subsequent analysis only if there were insufficient data at follow up, if ICI treatment was not initiated or if patients died prior to starting ICI treatment.
During the organisation of this audit of non-identifiable data from routine clinical practice, the Health Research Authority online decision tool http://www.hra-decisiontools.org.uk/research/ (18) was used which confirmed that regulatory approval by an NHS Research Ethics Committee (REC) was not required. This study was registered as an audit with our institution (audit number END_023).
Non-endocrine and endocrine irAEs were recorded after the first ICI treatment cycle and at each clinical visit, scheduled prior to each treatment cycle. Due to the reported frequency of ICI-induced thyroid dysfunction, all patients had thyroid function measured before ICI treatment initiation and before each treatment cycle. Patients were monitored for the development of all irAEs (non-endocrine and endocrine) at each clinical visit and these were recorded in the electronic patient records which were subsequently carefully reviewed for this study. Specific endocrine tests (i.e., in addition to thyroid function) e.g., pituitary profile, HbA1c, were performed according to symptoms.
Definitions
Thyroid irAEs were defined as new thyroid dysfunction developing after ICI treatment and were further subcategorised into primary hypothyroidism, subclinical hypothyroidism, isolated hyperthyroidism or biphasic thyroiditis using specific criteria (19, 20).
• Primary hypothyroidism - Thyroid Stimulating Hormone (TSH) concentration above 10mU/L, irrespective of fT4 concentration.
• Subclinical hypothyroidism – TSH concentration above 4mU/L but below 10mU/L, with associated fT4 within the reference range.
• Isolated hyperthyroidism - TSH concentration below 0.5mU/L with associated fT4 above the upper limit of the reference range that is not followed by a hypothyroid phase.
• Biphasic thyroiditis - Transient thyrotoxicosis defined by a TSH concentration below 0.5mU/L (irrespective of fT4 concentration) followed by a hypothyroid phase defined by a high TSH concentration exceeding 4mU/L.
Reference ranges in our institution are: fT4 9-23 pmol/L, fT3 2.4-6 pmol/L and TSH 0.30-4.20 mu/L.
Hypophysitis was defined as new onset deficiency of one or more pituitary hormones and/or evidence of pituitary gland or stalk enlargement on brain or pituitary MRI without prior glucocorticoid treatment (20, 21). All available neuroimaging studies for each patient were reported by a neuroradiologist at the time of acquisition as part of routine clinical care. For this study, a second, independent neuroradiologist (E.A.L.) re-reviewed this imaging and specifically focused on the presence of radiological features of hypophysitis and associated complications including compression of the anterior visual pathways, features suggestive of an alternative cause for a primary/secondary hypophysitis and exclusion of other structural adenomatous/non-adenomatous pituitary and infundibular abnormalities.
Primary adrenal insufficiency was defined as random cortisol concentration < 100 nmol/L or cortisol concentration < 350 nmol/L on short synACTHen testing (250mcg tetracosactide) with baseline ACTH at least twice the upper limit of the reference range (ng/L) without prior glucocorticoid treatment (21).
Diabetes mellitus was diagnosed in patients presenting with new onset hyperglycaemia with subsequent characterisation including measurement of blood ketones, C-peptide, HbA1c and anti-glutamic acid decarboxylase antibodies (anti-GAD) (22).
Assay characteristics
Laboratory measurement of serum sodium was via indirect ion-selective electrode methodology (Abbott Architect and Alinity-C). Measurement of prolactin, Thyroid Stimulating Hormone (TSH), free triiodothyronine (free T3) and free thyroxine (free T4) used a two-step immunoassay (IA) using chemiluminescent microparticle IA (CMIA) technology (Abbott Architect and Alinity-C). Measurement of serum cortisol involved a one-step IA using CMIA technology (Abbott Architect and Alinity-C). Measurement of adrenocorticotrophic hormone (ACTH) was performed by a solid-phase, two site sequential chemiluminescent immunometric assay (Immulite 2000, Siemens). A delayed one-step IA using CMIA technology was used to measure oestradiol and testosterone in serum (Abbott Architect and Alinity-C). Serum follicle stimulating hormone (FSH) and luteinising hormone (LH) was measured by a two-step IA using CMIA technology (Abbott Architect and Alinity-C).
C-peptide measurement was carried out using a two-step IA using CMIA technology (Alinity-I analyser, Abbott). Glycated haemoglobin (HbA1c) was measured by ion exchange high performance liquid chromatography (HPLC) (HLC-723G11 analyser, Tosoh) and reported as mmol per mol of unglycated HbA in line with the International Federation for Clinical Chemistry (IFCC) standardisation. Glucose was measured via enzymatic measurement (Hexokinase/Glucose-6-phosphate Dehydrogenase) (Abbott Architect and Alinity-C). Enzyme-linked immunosorbent assay (ELISA) was used to measure thyroid stimulating hormone receptor (TSHR) autoantibody (ElisaRSR TRAb 3rd Generation Kit) as well as glutamic acid decarboxylase (GAD) Autoantibody (ElisaRSR GAdAb Kit), which were tested manually and read using the MRX Dynatec software. The assay for autoantibodies against tyrosine phosphatase-related islet antigen 2 (IA2) was manual ELISA (Euroimmun Kit) and read using the MRX Dynatec software.
Statistical analysis
Baseline patient characteristics were reported with descriptive statistics as appropriate. The χ2 test was used to compare categorical variables. The Kruskal-Wallis test was used to compare continuous variables.
Oncological outcomes of interest included overall survival (OS), defined as the time from treatment initiation to patient death or loss to follow-up and Time to Treatment Failure (TTF), defined as the time from treatment initiation to its interruption for any cause including death. For OS and TTF, patients without events were considered as censored at the time of the last follow-up. Median OS and TTF were evaluated and compared using the Kaplan-Meier method and the log-rank test according to the experience of endocrine and non-endocrine irAEs. Patients who did not develop any irAEs were set as the control group. Duration of follow-up was calculated according to the reverse Kaplan-Meier method.
A fixed multivariable Cox proportional hazards regression including all the available baseline characteristics (age, sex, primary tumor) was used to estimate the risks of treatment interruption and death, and to compute the hazard ratios (HR) with 95% confidence intervals (CIs). Acknowledging the intrinsic association between the experience of irAEs and treatment exposure, time-adjusted Cox multivariable regression models for the risks of treatment interruption and death were also used.
All P-values were two-sided and confidence intervals set at the 95% level, with significance pre-defined to be at <0.05. Analyses were performed using the MedCalc® Statistical Software version 20 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021).
Results
Study cohort
452 eligible patients were identified. 54 were excluded (n = 26 insufficient data, n = 19 ICI treatment subsequently contraindicated, n = 9 died prior to starting ICI treatment), leaving 398 patients for inclusion in the study.
Out of the 398 patients included, treatment details were: anti-PD-1 inhibitors: nivolumab (n = 57) or pembrolizumab (n = 201), anti-PD-L1 inhibitors: ateluzumab (n = 85), durvalumab (n = 6) or avelumab (n = 12), combination anti-PD-1 and anti-CTLA4 inhibitors: nivolumab plus ipilimumab (n = 37). For patients receiving concomitant chemotherapy: pembrolizumab; 45 received pemetrexed plus carboplatin, 4 received pemetrexed plus cisplatin, 1 received pemetrexed plus cisoplatin/carboplatin, 8 received paclitaxel plus carboplatin, ateluzumab; 14 received etoposide plus carboplatin, 4 received bevacizumab, carboplatin and paclitaxel, 14 received bevacizumab, 4 received paclitaxel albumin, avelumab; 9 received axitinib.
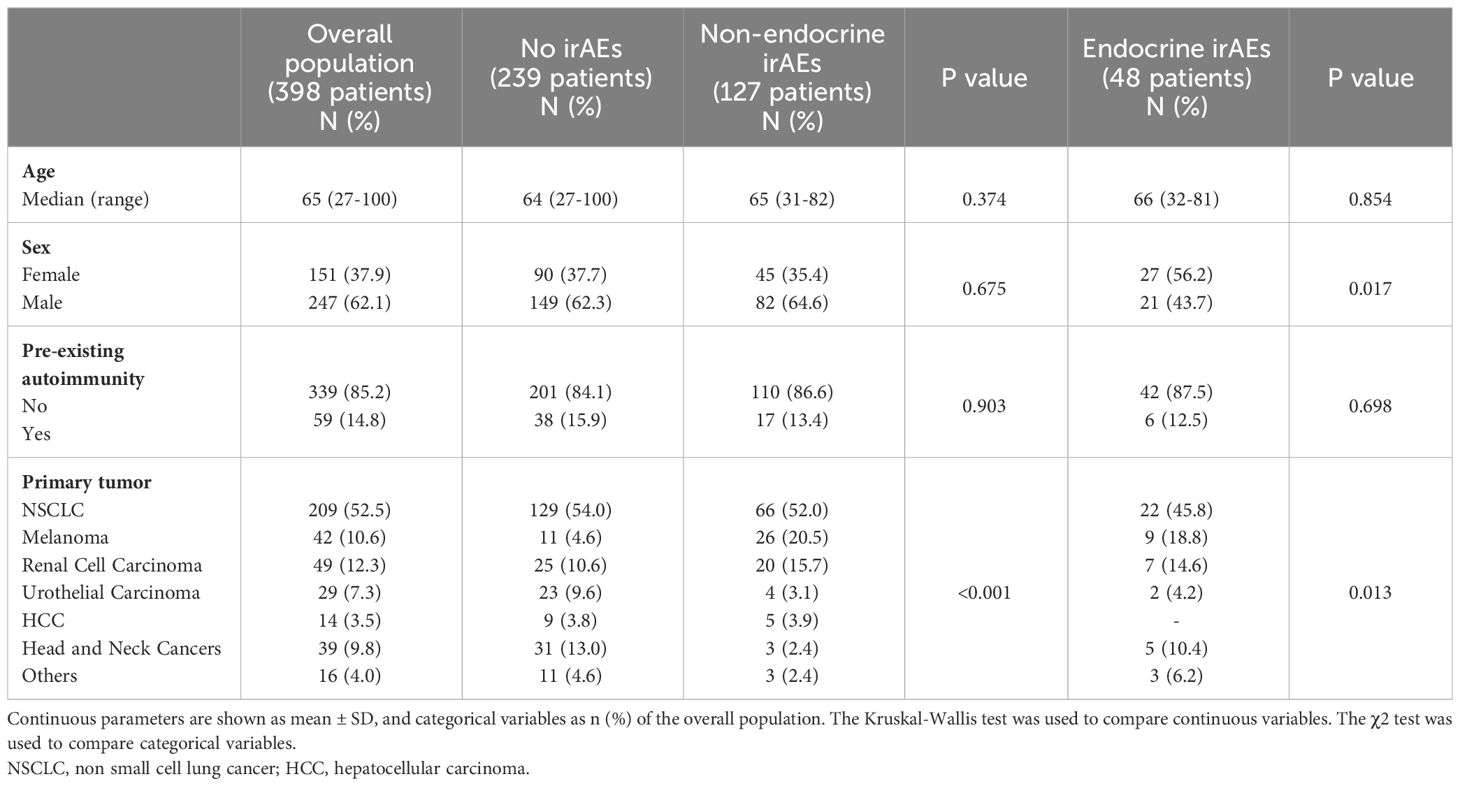
Baseline patient characteristics are presented in Table 1. The study population had a median age of 65 years, 14.8% had a prior history of autoimmunity, and 37.9% were females. Median follow-up was 26.2 months (95%CI: 21.1-30.4). The majority of patients receiving ICI treatment were diagnosed with non small cell lung cancer (NSCLC) (52.5%). Overall, non-endocrine irAEs occurred in 32% (n = 127) and endocrine irAEs occurred in 12% (n = 48) of patients treated with ICIs. 4.0% (n = 16) developed both non-endocrine and endocrine irAEs during ICI treatment.
Non-endocrine irAEs
The most common non-endocrine irAE was dermatitis occurring in 10.3% (n = 41) of patients. This was followed by hepatitis which was found in 9.6% (n = 38). Other non-endocrine irAEs encountered in this cohort included inflammatory arthritis, mucositis, pneumonitis, colitis, nephritis and peripheral neuropathy. Rare irAEs (less than two cases) included discitis, glossitis, myositis, sinusitis and vulvitis.
Endocrine irAEs
Primary thyroid dysfunction
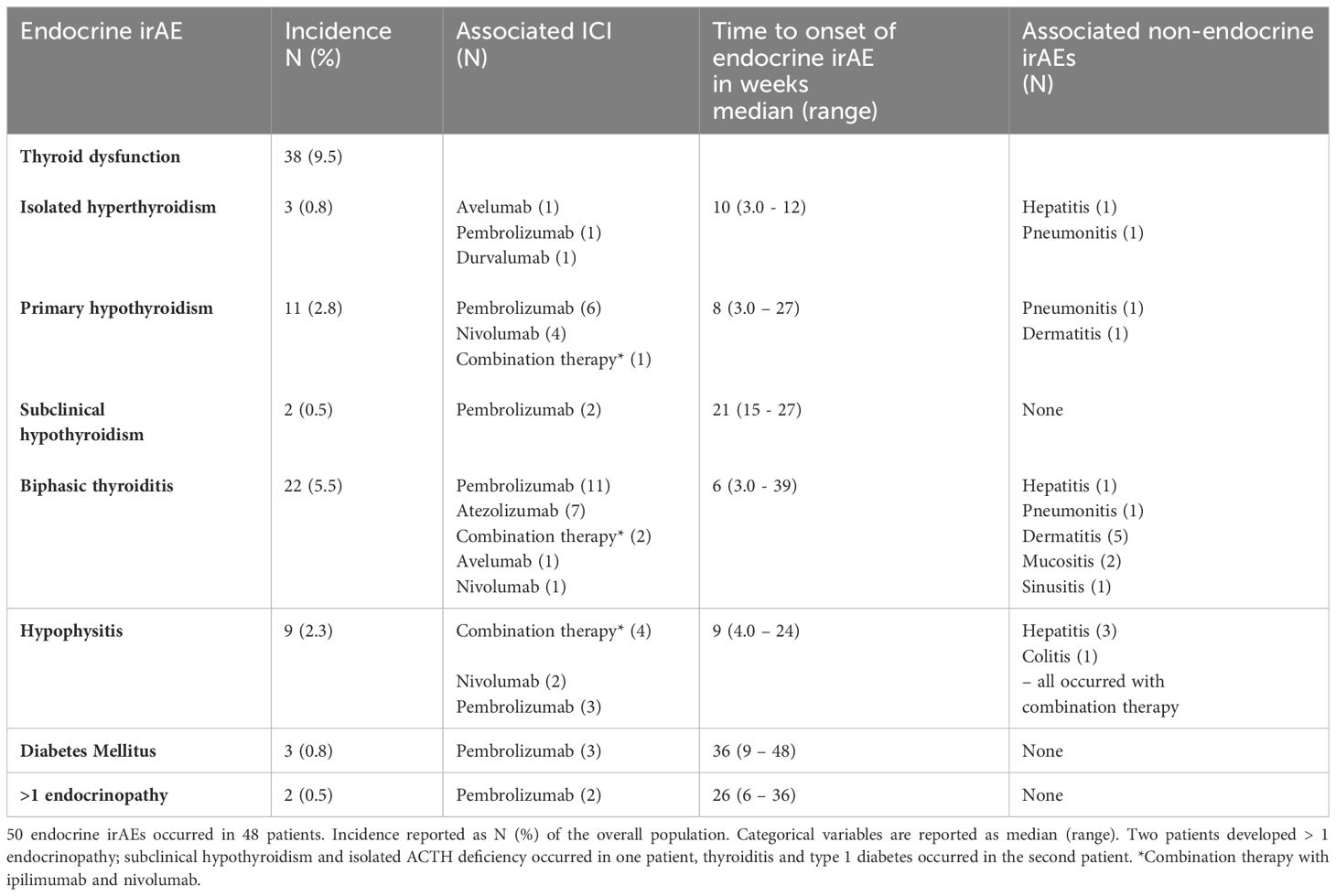
Primary thyroid dysfunction was the most frequently encountered endocrinopathy, comprising two thirds of all endocrine irAEs (incidence 9.5%) (Table 2). Thyroiditis was the most common cause of primary thyroid dysfunction (22 of 38 cases), occurred earliest (median onset six weeks) and was associated with all classes of ICI, although 50% of cases occurred with the PD-1 inhibitor pembrolizumab. Primary hypothyroidism was associated with anti-PD-1 inhibitors; pembrolizumab and nivolumab and with combination anti-PD-1 and CTLA4 inhibitor therapy (nivolumab plus ipilimumab). Isolated hyperthyroidism (i.e., without subsequent hypothyroidism) presented later than biphasic thyroiditis and primary hypothyroidism, and was associated with anti-PD-1 and anti-PDL1 inhibitors (avelumab, pembrolizumab and durvalumab) but not with combination therapy. All patients who developed biphasic thyroiditis and primary hypothyroidism required long-term treatment with levothyroxine.
Hypophysitis
Hypophysitis occurred in nine patients (incidence 2.3%), with a median onset of nine weeks (Table 2). Two patients presented with headache accompanied by changes consistent with hypophysitis on imaging and multiple pituitary hormone deficiencies both of which received combination anti-PD-1 and CTLA4 inhibitor therapy (nivolumab plus ipilimumab). These multiple pituitary hormone deficiencies were secondary hypoadrenalism (both patients, permanent), secondary hypogonadism (in one patient, transient) and secondary hypothyroidism (both patients, permanent in one, transient in one). Seven patients had isolated ACTH deficiency (three received pembrolizumab, two received nivolumab, two received nivolumab plus ipilimumab), with 5/7 having no appearance of hypophysitis on imaging (two patients did not have imaging). In all nine cases, ACTH deficiency was permanent. AVP deficiency did not occur in any patient.
Primary adrenal insufficiency
There were no cases of primary adrenal insufficiency identified in this cohort.
Diabetes mellitus
Diabetes mellitus occurred in three patients (incidence 0.8%), who all received the anti-PD-1 inhibitor pembrolizumab, and presented later than other endocrinopathies (median onset 36 weeks) (Table 2). All patients presented with diabetic ketoacidosis and required lifelong insulin therapy. C-peptide levels were low in all three cases (< 27 – 81 pmol/L). Anti-GAD and anti-islet cell antibodies were checked, with only one patient showing positive antibodies.
Factors associated with development of irAEs
There was no significant difference in age or previous history of autoimmunity between the patients who developed non-endocrine irAEs, endocrine irAEs and those who developed no irAEs. Women had an increased risk of developing endocrine irAEs (p = 0.017) (Table 1).
Overall survival and time to treatment failure
The development of non-endocrine irAEs or endocrine irAEs had positive effects on overall survival and time to treatment failure (Figures 1 and 2). Since the majority of endocrine irAEs in this study comprised primary thyroid dysfunction, the survival benefits of thyroid irAEs alone were similar to those observed in the endocrine irAEs group. Comparing overall survival between groups (Figure 3), this was significantly improved in patients who developed non-endocrine irAEs (HR 0.66, CI 0.49 – 0.89) compared with no irAEs. This was further improved in patients who developed endocrine irAEs (HR 0.54, CI 0.34 – 0.85), with the biggest survival advantage in those who developed both non-endocrine irAEs and endocrine irAEs (HR 0.16, CI 0.09-0.28). Consistent with this, time to treatment failure (Figure 4) was significantly improved in patients who developed endocrine irAEs (HR 0.49, CI 0.34 – 0.71) and in those who developed both non-endocrine and endocrine irAEs (HR 0.41, CI 0.25 – 0.64) but not in those who developed non-endocrine irAEs only (HR 0.93, CI 0.71 – 1.21), when compared with the no irAEs group. Overall survival according to cancer subtype is shown in Supplementary Figure 1 (NSCLC) and Supplementary Table 1, showing a survival benefit in the NSCLC subgroup associated with endocrine irAEs and thyroid irAEs, but not non-endocrine irAEs. Supplementary Table 2 shows overall survival in patients developing non-endocrine irAEs, endocrine irAEs and thyroid irAEs according to type of immune checkpoint inhibitor.
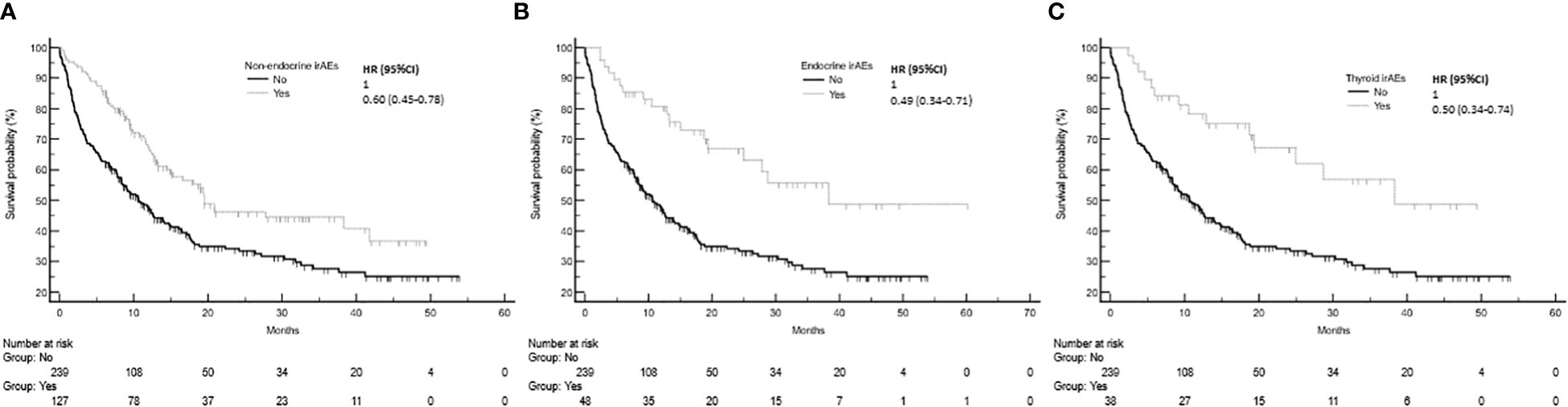
Figure 1 Overall survival in patients with and without non-endocrine, endocrine or thyroid immune related adverse events (irAEs). (A) Overall survival among patients who experienced non-endocrine irAEs was 19.5 months (95%CI: 15.1-41.7, 60 events), that of patients who did not experienced irAEs was 10.6 months (95%CI: 8.3-13.9, 152 events), Log-rank p value = 0.001. (B) Overall survival of patients who experienced endocrine irAEs was 38.3 months (95%CI: 24.9-38.3, 19 events). Log-rank p value = 0.001 (comparison with no irAEs). (C) Overall survival of patients who experienced thyroid irAEs was 38.3 months.
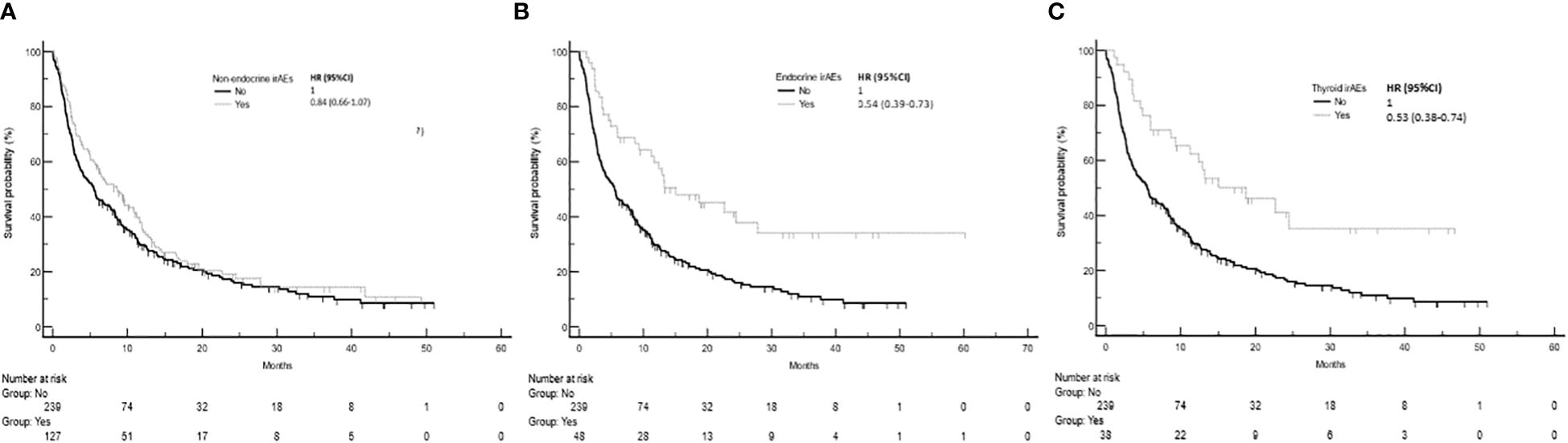
Figure 2 Time to treatment failure in patients with and without non-endocrine, endocrine or thyroid immune related adverse events (irAEs). (A) Time to treatment failure among patients who experienced non-endocrine irAEs was 8.6 months (95%CI: 5.7-11.1, 100 events), while that of patients who did not experience irAEs was 5.5 months (95%CI: 3.9-7.5, 192 events), Log-rank p value = 0.154. (B) Time to treatment failure of patients who experienced endocrine irAEs was 13.3 months (95%CI: 9.3-24.5, 31 events). Log-rank p value = 0.001 (comparison with no irAEs). (C) Time to treatment failure of patients who experienced thyroid irAEs was 15.0 months (95%CI: 9.3-24.4, 23 events). Log-rank p values = 0.001 (comparison with no irAEs).
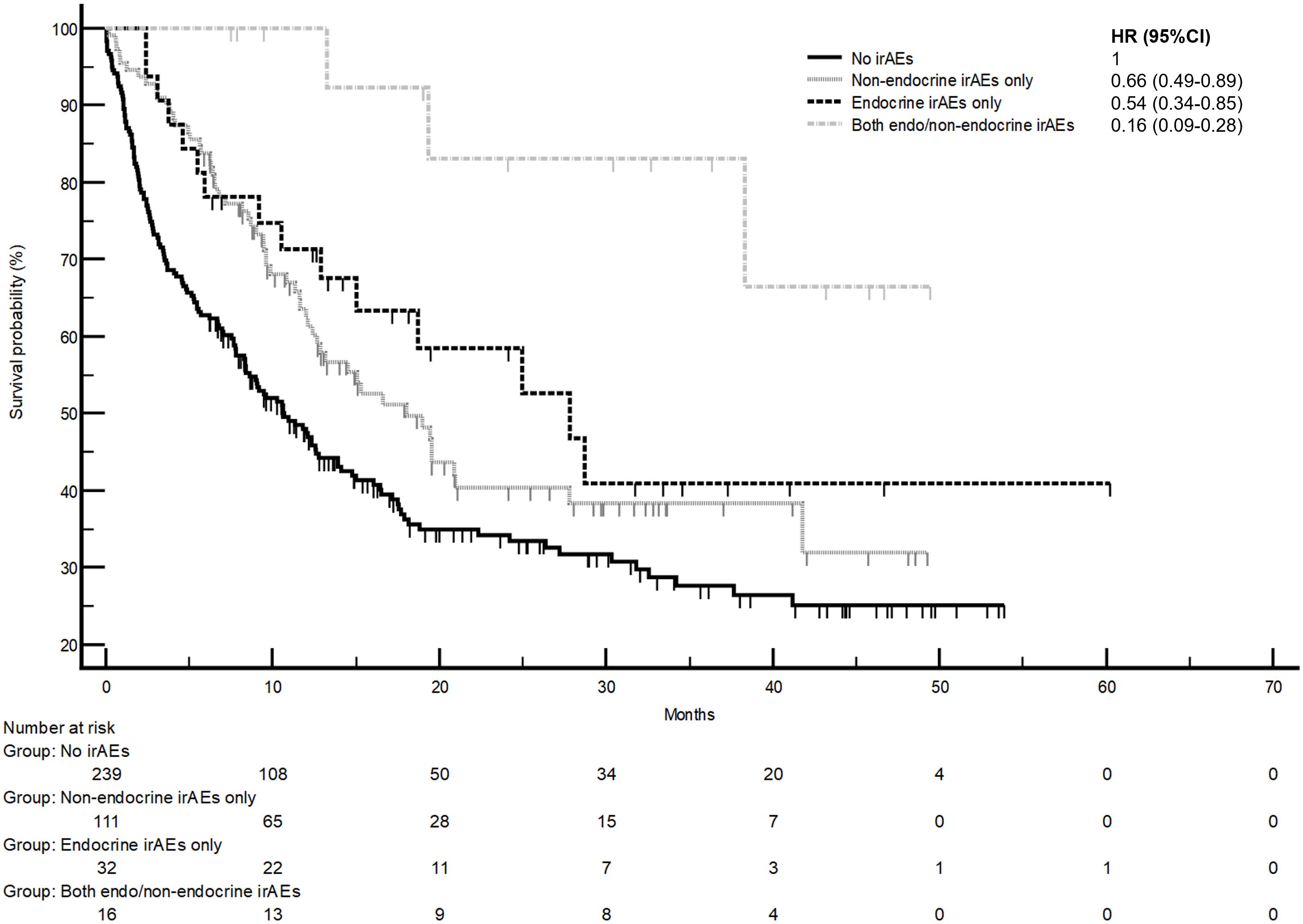
Figure 3 Comparison of overall survival in patients with and without endocrine and/or non-endocrine immune related adverse events (irAEs). Overall survival of patients who experienced non-endocrine irAEs only was 18.0 months (95%CI: 12.4-27.8, 57 events), that of patients who experienced endocrine irAEs only was 27.8 months (95%CI: 12.9-27.7, 15 events), that of patients who experienced both non-endocrine and endocrine irAEs was not reached (three events). Log-rank p values = 0.001 (comparison with no irAEs for hazard ratio).
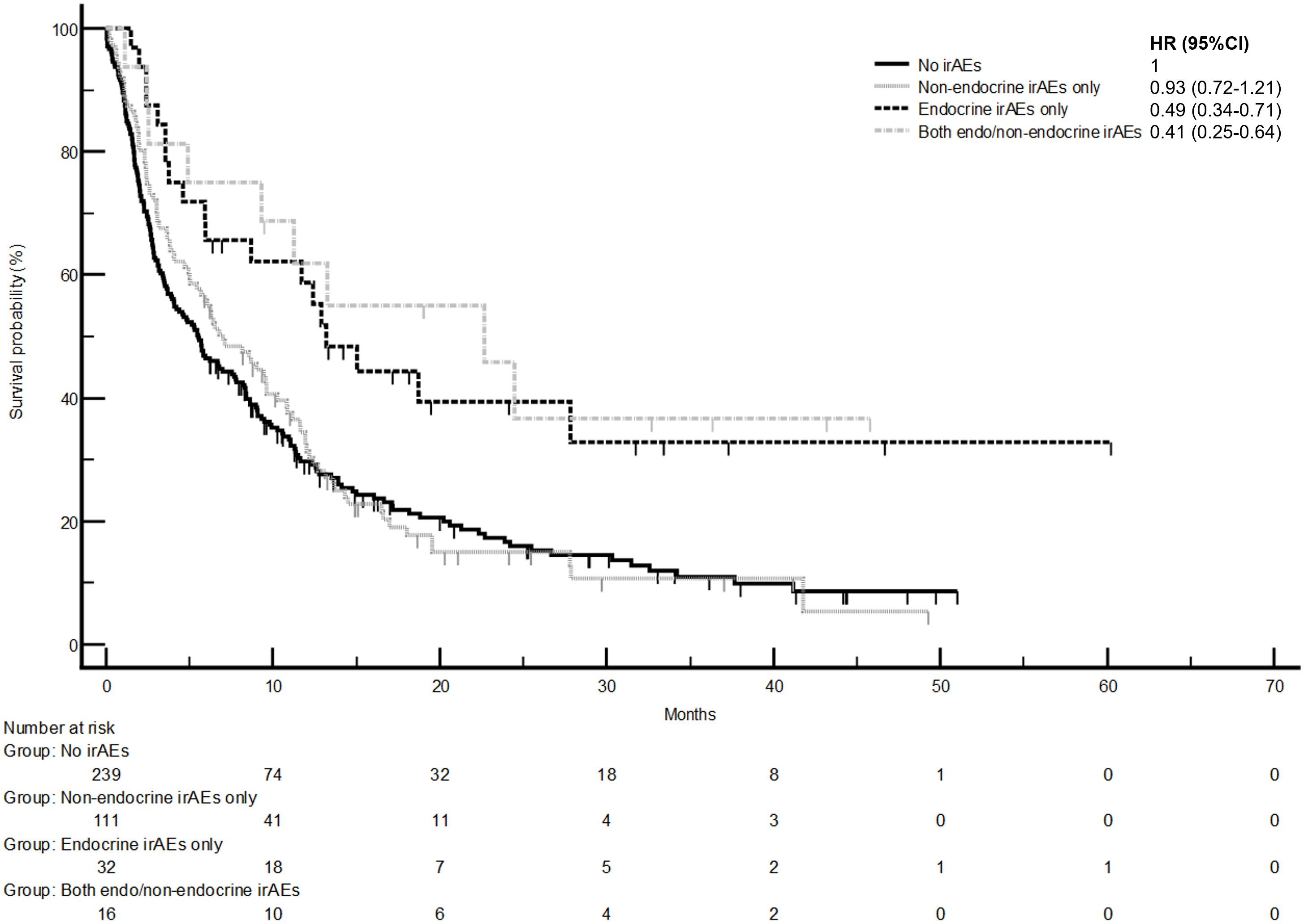
Figure 4 Time to treatment failure in patients with and without endocrine and/or non-endocrine immune related adverse events (irAEs). Time to treatment failure of patients who experienced non-endocrine irAEs only was 7.0 months (95%CI: 5.0-9.6, 91 events), that of patients who experienced endocrine irAEs only was 13.2 months (95%CI: 5.9-27.9, 19 events), that of patients who experienced both non-endo and endo irAEs was 22.7 months (95%CI: 4.9-24.5, 9 events). Log-rank p values = 0.001 (comparison with no irAEs for hazard ratio).
Fixed multivariable analysis including primary tumor type, age and sex (Supplementary Table 3 and Supplementary Table 4), showed showed that both endocrine irAEs (HR 0.50,95%CI: 0.30-0.82) and non-endocrine irAEs (HR 0.66, 95%CI: 0.48-0.90) were significantly associated with a decreased risk of death in comparison to the non-irAEs group, whilst only endocrine irAEs were confirmed to be associated with a decreased risk of treatment failure (HR 0.48, 95%CI: 0.32-0.73).
After adjustment for time/exposure, significance was lost for the risk of death/treatment failure for both non-endocrine irAEs and endocrine irAEs groups, with only endocrine irAEs maintaining a trend towards improved survival outcomes (Supplementary Table 1 and Supplementary Table 2).
Discussion
This is the first single-center, real-world study describing the occurrence of endocrine and non-endocrine irAEs following use of CTLA4, PD-1 and PD-L1 inhibitors in a broad range of solid tumor malignancies and to investigate their association with cancer survival.
Consistent with previous findings, we report a 12% incidence of endocrine irAEs across all solid tumor malignancies treated with CTLA4, PD-1 and PD-L1 inhibitors in our cohort (5, 7, 23). Primary thyroid dysfunction was the most common endocrine irAE, accounting for 79% of all endocrine irAE cases and particularly associated with the anti-PD-1 inhibitor pembrolizumab, which is in agreement with previous studies (5, 7). Biphasic thyroiditis, a T lymphocyte mediated process, was the most frequently observed thyroid dysfunction in our study and is most commonly associated with anti PD-1 and anti-PDL1 treatment (24, 25). Previous studies have specifically identified that thyroiditis following anti-PD1 blockade may confer survival benefit in patients with advanced non-small-cell lung cancer (NSCLC) (26, 27). In accordance with this, NSCLC was the largest cancer subgroup in our study and improved overall survival was observed in NSCLC patients who developed thyroid irAEs.
Immune-related hypophysitis is most frequently associated with the CTLA-4 inhibitor ipilimumab and although hypophysitis has also been reported in patients receiving anti-PD1/PD-L1 therapy, this occurs with a much lower incidence (28). Interestingly, hypophysitis secondary to ipilimumab either alone or in combination with nivolumab has a distinct clinical phenotype to hypophysitis induced by anti-PD1 monotherapy (28–32). Hypophysitis associated with ipilimumab-based regimens typically presents with headache, loss of multiple anterior pituitary hormone axes, most frequently ACTH deficiency, and pituitary enlargement on MRI. Animal studies have found the pituitary gland expresses CTLA-4 in a subset of cells, which are targets for CTLA-4 antibody binding and this may explain the predisposition towards hypophysitis with anti-CTLA-4 therapy (33). In contrast, PD-1 and PD-L1 inhibitors often cause an isolated ACTH deficiency without headache or radiological changes. In our study, only two patients with ICI-associated hypophysitis presented with headache, multiple anterior pituitary hormone deficiencies and abnormalities on imaging, both of which had received anti-CTLA-4 inhibitors as combination therapy.
There were no reported incidences of ICI-associated primary adrenal insufficiency in our cohort. This is consistent with this being described as a rare complication of ICI treatment (32, 34).
DM developed much later than other endocrine irAEs in this study (median onset 36 weeks) and all cases occurred with the anti-PD-1 inhibitor pembrolizumab, which is in agreement with previously published data (22). DM is a less common endocrine irAE associated with ICI treatment, with a reported incidence of 0.2-1.4% (3). Consistent with this, the incidence of DM was only 0.8% in our population (22). All three patients presented with diabetic ketoacidosis and had low C-peptide levels, however, only one patient had positive anti-GAD and anti-islet cell autoantibodies. Approximately 25% of ICI-treated patients who do not develop DM show positive anti-GAD antibodies (35). ICI-induced DM can be categorised into early-onset and late-onset disease, with higher rates of anti-GAD positivity seen in early-onset DM (36). All the cases of DM in our study were late onset (range 9 – 48 weeks).
Exacerbation of pre-existing autoimmune disease is a well-recognised phenomenon of ICI inhibitor treatment, and this is often an exclusion criteria for clinical trials of ICI therapy (37, 38). However, pre-existing autoimmune disease was not an exclusion criterion for ICI therapy within the standard clinical practice of our center. In our cohort, 14.8% of patients had pre-existing autoimmune disease. There was no statistical difference between those developing non-endocrine irAEs, endocrine irAEs and those with no irAEs, which is comparable with previous reports (39). There was a significantly higher proportion of women who developed endocrine irAEs (56.2%) compared to the overall study population (37.9% female). This likely reflects the higher overall incidence of endocrinopathies in women (40), yet there is currently no consensus regarding whether there are any sex differences in immune responses to ICI treatment (41–43). Notably, the majority of endocrine irAEs reported in the current study are related to primary thyroid dysfunction and women have an increased baseline risk of autoimmune thyroid disease (44). Interestingly, a large meta-analysis of the sex differences in the risk of developing irAEs following ICI treatment showed an increased risk of symptomatic irAEs in women, but not specifically endocrine irAEs (45). Future studies are needed focusing on delineating the relationship between subtype of endocrine irAEs and sex.
Our current clinical protocol does not include measuring thyroid autoantibodies prior to ICI initiation. This is a limitation that a future, prospective study could address. Anti-thyroid peroxidase and anti-thyroglobulin antibodies may predict the development of thyroid irAEs with ICI treatment (46). Furthermore, the risk of developing non-thyroid endocrine irAEs such as hypophysitis, may be increased in patients who are positive for anti-thyroid peroxidase antibodies at baseline (21, 47). Pre-existing anti thyroid peroxidase antibodies are associated with improved prognosis in patients with advanced NSCLC receiving the PD-1 inhibitors nivolumab or pembrolizumab (48). Therefore, future, prospective studies including baseline thyroid autoantibody status would be important to further investigate associations between autoimmunity and endocrine irAEs following ICI treatment.
This study is limited by its retrospective observational design. We have not recorded data regarding whether ICIs were discontinued according to the development of irAEs. Such suspension or discontinuation of ICI treatment may shorten exposure to treatment, which may impact survival outcomes and may reduce the likelihood of patients reporting more than one ICI associated irAE. Similarly, we did not record details of the specific stage and grade of disease, histological features, location and number of metastases, and ECOG Performance Status Scale, all which may impact on cancer survival outcomes. Furthermore, face-to-face clinic appointments were reduced during the study period due to the COVID-19 pandemic, affecting patient monitoring. Since data were collected during standard clinical care, patients only had regular biochemical monitoring for thyroid dysfunction during follow up. Other endocrine irAEs were identified following clinical assessment and subsequent investigation rather than via regular biochemical monitoring. Both these approaches are consistent with recent recommendations for management of immune-related endocrinopathies in patients receiving ICI treatment for cancer (22). Nevertheless, endocrine irAEs may have developed earlier than reported or may have been missed if transient.
There are reports of rarer endocrinopathies associated with ICI treatment, including diabetes insipidus, hypoparathyroidism and autoimmune polyglandular syndromes (9, 49). However, we focussed on endocrine irAEs most likely to be encountered in clinical practice (8, 9).
Typically, irAEs associated with ICI treatment are graded using the five point Common Terminology Criteria for Adverse Events (CTCAE) system. However, the CTCAE has limitations for reporting of endocrine irAEs (32, 50) and in our institution, we do not routinely grade endocrine irAEs according to CTCAE criteria. However, future studies of the relationship between ICI treatment, the grade of severity of endocrine and non-endocrine irAEs and survival outcomes could be interesting.
Our survival analyses are in keeping with prior evidence highlighting an association between irAEs and improved outcomes in patients receiving ICI treatment (15–17). Earlier studies investigating survival outcomes and ICI-associated irAEs have been limited to clinical trials of the effects of specific ICI treatments in patients with advanced cancers of a single tumor type (15–17). Interestingly, our findings are also consistent with recent studies of ICI-associated endocrine irAEs and their positive effects on survival in patients with metastatic solid organ malignancy of different types (21, 23). Building on work by Paschou et al., our larger study included patients treated with anti-CTLA4 inhibitors and also determined risk factors for developing endocrine irAEs (23). Although several of these prior studies included similar classes of ICI to those in our study (21), both had a shorter follow up period. The current study is the first to compare the effects of endocrine irAEs with non-endocrine irAEs on survival. Therefore, our work adds to the limited literature comprising real-world clinical data on development of irAEs and survival benefit with ICI treatment.
Many studies of the relationship between endocrine and non-endocrine irAEs associated with ICI treatment and survival are not adjusted for immortal time bias (ITB). ITB may be introduced when patients remain in a study for a shorter time period if they develop disease progression or die earlier compared to those patients who remain in the study for a longer period if they remain free of disease progression, hence potentially having an increased risk of developing a clinical outcome. Time-adjusted analyses acknowledge a change in treatment exposure status over time. In the current study, adjustment for time showed the survival benefit of endocrine irAEs was lost but the trend remained. Interestingly, a recent comprehensive meta-analysis assessing the relationship between thyroid irAEs with ICI treatment and cancer survival outcomes showed positive benefits of thyroid irAEs on overall survival persisted when adjusting for ITB (51). In contrast, the differences in survival outcome associated with the development of hypophysitis from PD-1 blockade or CLTA-4 inhibitors are less clear when adjusting for ITB (52, 53). The positive trend restricted towards endocrine irAEs indicates this class of adverse events as a potential pharmacodynamic marker of ICI activity, with possible T-cell cross-reactivity between the tumor and endocrine irAE tissues as the most likely underlying mechanistic link (54).
There is a need for further studies in this field as endocrinopathies are associated with considerable morbidity. This is especially relevant in younger patients achieving cancer remission following ICI treatment, but in whom permanent endocrine dysfunction may cause a significant negative impact on quality of life. This study and others are retrospective. Future, larger prospective studies are needed to further investigate the risk factors for irAEs, with the aim to develop a personalised approach to the selection of ICI agent(s) and surveillance strategy based on individualised risk for developing irAEs.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: This is anonymised clinical data. This will not be accessible to any individuals outside of our hospital.
Ethics statement
During the organization of this audit of nonidentifiable data from routine clinical practice, the Health Research Authority online decision tool http://www.hra-decisiontools.org.uk/research/ was used which confirmed that regulatory approval by an NHS Research Ethics Committee (REC) was not required. This study was registered as an audit with our institution (audit number END_023). The studies were conducted in accordance with the local legislation and institutional requirements. This was a retrospective, observational study of clinical data. These data were anonymized and all patient identifiable details removed. Hence, no individual patient consent was required.
Author contributions
LY: Writing – review & editing, Writing – original draft, Investigation, Formal Analysis, Data curation. SMu: Writing – review & editing, Writing – original draft, Investigation, Data curation. AC: Validation, Software, Formal Analysis, Writing – review & editing, Writing – original draft, Data curation. EL: Methodology, Investigation, Writing – review & editing, Writing – original draft, Formal Analysis. MG: Resources, Conceptualization, Writing – review & editing, Writing – original draft. DP: Formal Analysis, Data curation, Writing – review & editing, Writing – original draft. MA: Methodology, Writing – review & editing, Writing – original draft. SMa: Resources, Writing – review & editing, Writing – original draft. NM: Visualization, Validation, Supervision, Software, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization, Writing – review & editing, Writing – original draft, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institute for Health Research (NIHR), the NIHR Imperial Clinical Research Facility, and NIHR Imperial Biomedical Research Centre at Imperial College Healthcare NHS Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. The Section of Endocrinology and Investigative Medicine is funded by grants from the UK Medical Research Council (MRC), Biotechnology and Biological Sciences Research Council, and NIHR. SM was supported by a Society for Endocrinology BES Student Registration Grant 2022. DJP is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416), the Associazione Italiana per la Ricerca sul Cancro (AIRC MFAG 25697) and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT) and The Foundation for Liver Research.
Conflict of interest
AC declares grants for consultancies/advisory boards from BMS, MSD, OncoC4, IQVIA, AstraZeneca. He also declares speaker fees from AstraZeneca, EISAI and Pierre-Fabre. DJP reports grants from GSK, BMS, and MSD (institutions) for supporting academic trials in oncology; personal payment from Wiley for Editor in Chief role unrelated to the topic of this manuscript; consulting fees from Mina Therapeutics, EISAI, H3B, Astra Zeneca, DaVolterra, Avamune, BMS, Roche for supporting research in oncology unrelated to topic of the manuscript; speaker's fees from Roche, BMS, EISAI, and Ewopharma unrelated to the topic of the manuscript; travel support from MSD and Bayer; and participation on advisory board for Astra Zeneca, EISAI, H3B.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1369268/full#supplementary-material
References
1. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. (2018) 175:313–26. doi: 10.1016/J.CELL.2018.09.035
2. Fanciulli G, Di Molfetta S, Dotto A, Florio T, Feola T, Rubino M, et al. Emerging therapies in pheochromocytoma and paraganglioma: immune checkpoint inhibitors in the starting blocks. J Clin Med. (2020) 10:88. doi: 10.3390/JCM10010088
3. Cardona Z, Sosman JA, Chandra S, Huang W. Endocrine side effects of immune checkpoint inhibitors. Front Endocrinol (Lausanne). (2023) 14:1157805/BIBTEX. doi: 10.3389/fendo.2023.1157805
4. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J-J, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2017) 377:1345–56. doi: 10.1056/NEJMOA1709684
5. Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. (2021) 17:389–99. doi: 10.1038/S41574-021-00484-3
6. Weber JS, Hodi FS, Wolchok JD, Topalian SL, SChadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol. (2017) 35:785–92. doi: 10.1200/JCO.2015.66.1389
7. De Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. (2019) 51:145–56. doi: 10.1055/A-0843-3366
8. Higham CE, Olsson-Brown A, Carroll P, Cooksley T, Larkin J, Lorigan P, et al. SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: Acute management of the endocrine complications of checkpoint inhibitor therapy. Endocr Connect. (2018) 7:G1–7. doi: 10.1530/EC-18-0068
9. Husebye ES, Castinetti F, Criseno S, Curigliano G, Decallonne B, Fleseriu M, et al. Endocrine-related adverse conditions in patients receiving immune checkpoint inhibition: an ESE clinical practice guideline. Eur J Endocrinol. (2022) 187:G1–G21. doi: 10.1530/EJE-22-0689
10. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. (2019) 7:306. doi: 10.1186/S40425-019-0805-8
11. Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. (2020) 6:1952–6. doi: 10.1001/jamaoncol.2020.5012
12. Fan Y, Xie W, Huang H, Wang Y, Li G, Geng Y, et al. Association of immune related adverse events with efficacy of immune checkpoint inhibitors and overall survival in cancers: A systemic review and meta-analysis. Front Oncol. (2021) 11:633032. doi: 10.3389/FONC.2021.633032
13. Cook S, Samuel V, Meyers DE, Stukalin I, Litt I, Sangha R, et al. Immune-related adverse events and survival among patients with metastatic NSCLC treated with immune checkpoint inhibitors. JAMA Netw Open. (2024) 7:e2352302–e2352302. doi: 10.1001/JAMANETWORKOPEN.2023.52302
14. Flatz L, Berner F, Bomze D, Diem S, Ali OH, Fässler M, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. (2019) 5:1043–7. doi: 10.1001/JAMAONCOL.2019.0402
15. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. (2020) 18:87. doi: 10.1186/s12916-020-01549-2
16. Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors – A systematic review and meta-analysis. Cancer Treat Rev. (2021) 92:102134. doi: 10.1016/J.CTRV.2020.102134
17. Pinato DJ, Marron TU, Mishra-Kalyani PS, Gong Y, Wei G, Szafron D, et al. Treatment-related toxicity and improved outcome from immunotherapy in hepatocellular cancer: Evidence from an FDA pooled analysis of landmark clinical trials with validation from routine practice. Eur J Cancer. (2021) 157:140–52. doi: 10.1016/J.EJCA.2021.08.020
18. Available online at: https://nspccro.nihr.ac.uk/working-with-us/research-service-evaluation-or-audit.
19. Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, et al. Thyroid immune-related adverse events following immune checkpoint inhibitor treatment. J Clin Endocrinol Metab. (2021) 106:E3704–13. doi: 10.1210/clinem/dgab263
20. Campredon P, Mouly C, Lusque A, Bigay-Game L, Bousquet E, Mazières J, et al. Incidence of thyroid dysfunctions during treatment with nivolumab for non-small cell lung cancer: Retrospective study of 105 patients. Presse Medicale. (2019) 48:e199–207. doi: 10.1016/j.lpm.2018.10.019
21. Labadzhyan A, Wentzel K, Hamid O, Chow K, Kim S, Piro L, et al. Endocrine autoantibodies determine immune checkpoint inhibitor-induced endocrinopathy: A prospective study. J Clin Endocrinol Metab. (2022) 107:1976–82. doi: 10.1210/clinem/dgac161
22. Lo Preiato V, Salvagni S, Ricci C, Ardizzoni A, Pagotto U, Pelusi C. Diabetes mellitus induced by immune checkpoint inhibitors: type 1 diabetes variant or new clinical entity? Review of the literature. Rev Endocr Metab Disord. (2021) 22:337–49. doi: 10.1007/s11154-020-09618-w
23. Paschou SA, Liontos M, Eleftherakis-Papaiakovou E, Stefanaki K, Markellos C, Koutsoukos K, et al. Oncological patients with endocrine complications after immunotherapy with checkpoint inhibitors present longer progression-free and overall survival. Front Oncol. (2022) 12:847917. doi: 10.3389/fonc.2022.847917
24. Kotwal A, KottsChade L, Ryder M. PD-L1 inhibitor-induced thyroiditis is associated with better overall survival in cancer patients. Thyroid. (2020) 30:177. doi: 10.1089/THY.2019.0250
25. Yamauchi I, Sakane Y, Fukuda Y, Fujii T, Taura D, Hirata M, et al. Clinical features of nivolumab-induced thyroiditis: A case series study. Thyroid. (2017) 27:894–901. doi: 10.1089/THY.2016.0562
26. Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. (2017) 28:583. doi: 10.1093/ANNONC/MDW640
27. Maillet D, Corbaux P, Stelmes JJ, Dalle S, Locatelli-Sanchez M, Perier-Muzet M, et al. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur J Cancer. (2020) 132:61–70. doi: 10.1016/J.EJCA.2020.03.017
28. Di Dalmazi G, Ippolito S, Lupi I, Caturegli P. Hypophysitis induced by immune checkpoint inhibitors: a 10-year assessment. Expert Rev Endocrinol Metab. (2019) 14:381. doi: 10.1080/17446651.2019.1701434
29. Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. (2018) 124:3706–14. doi: 10.1002/CNCR.31629
30. Faje A, Reynolds K, Zubiri L, Lawrence D, Cohen JV, Sullivan RJ, et al. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol. (2019) 181:211–9. doi: 10.1530/EJE-19-0238
31. Seejore K, Giannoudi M, Osborn D, Lynch JM, Al-Qaissi A, Dunwoodie E, et al. Characterisation of the onset and severity of adrenal and thyroid dysfunction associated with CTLA4-related hypophysitis. Eur J Endocrinol. (2021) 186:83–93. doi: 10.1530/EJE-21-0760
32. Percik R, Criseno S, Adam S, Young K, Morganstein DL. Diagnostic criteria and proposed management of immune-related endocrinopathies following immune checkpoint inhibitor therapy for cancer. Endocr Connect. (2023) 12(5). doi: 10.1530/EC-22-0513
33. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. (2014) 6(230):230ra45. doi: 10.1126/SCITRANSLMED.3008002
34. Grouthier V, Lebrun-Vignes B, Moey M, Johnson DB, Moslehi JJ, Salem J, et al. Immune checkpoint inhibitor-associated primary adrenal insufficiency: WHO VigiBase report analysis. Oncologist. (2020) 25:696. doi: 10.1634/THEONCOLOGIST.2019-0555
35. Bluestone JA, Anderson M, Herold KC, Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. (2018) 67:1471–80. doi: 10.2337/DBI18-0002
36. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune checkpoint inhibitor-induced Type 1 diabetes: a systematic review and meta-analysis. Diabetic Med. (2019) 36:1075–81. doi: 10.1111/DME.14050
37. Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discovery. (2022) 21:495–508. doi: 10.1038/s41573-021-00259-5
38. Cortellini A, Buti S, Santini D, Perrone F, Giusti R, Tiseo M, et al. Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: A real-world transverse study. Oncologist. (2019) 24(6):e327-e337. doi: 10.1634/theoncologist.2018-0618
39. Matsuoka H, Hayashi T, Takigami K, Imaizumi K, Shiroki R, Ohmiya N, et al. Correlation between immune-related adverse events and prognosis in patients with various cancers treated with anti PD-1 antibody. BMC Cancer. (2020) 20(1):656. doi: 10.1186/s12885-020-07142-3
40. Crafa A, Calogero AE, Cannarella R, Mongioi’ LM, Condorelli RA, Greco EA, et al. The burden of hormonal disorders: A worldwide overview with a particular look in Italy. Front Endocrinol (Lausanne). (2021) 12:694325. doi: 10.3389/fendo.2021.694325
41. Lai LT, Gu WG, Hu MB, Wang WJ, Wang SS, Huai YJ, et al. Sex-related differences in the efficacy of immune checkpoint inhibitors in Malignancy: a systematic review and meta-analysis. Aging (Albany NY). (2021) 13:15413. doi: 10.18632/AGING.203100
42. Wang S, Cowley LA, Liu XS. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules. (2019) 24(18):3214 . doi: 10.3390/MOLECULES24183214
43. Scott SC, Shao XM, Niknafs N, Balan A, Pereira G, Marrone KA, et al. Sex-specific differences in immunogenomic features of response to immune checkpoint blockade. Front Oncol. (2022) 12:945798/BIBTEX. doi: 10.3389/fonc.2022.945798
44. Mammen JSR, Cappola AR. Autoimmune thyroid disease in women. JAMA. (2021) 325:2392–3. doi: 10.1001/JAMA.2020.22196
45. Unger JM, Vaidya R, Albain KS, Leblanc M, Minasian LM, Gotay CC, et al. Sex differences in risk of severe adverse events in patients receiving immunotherapy, targeted therapy, or chemotherapy in cancer clinical trials. J Clin Oncol. (2022) 40:1474–86. doi: 10.1200/JCO.21.02377/SUPPL_FILE/DS_JCO.21.02377.PDF
46. Izawa N, Shiokawa H, Onuki R, Hamaji K, Morikawa K, Saji H, et al. The clinical utility of comprehensive measurement of autoimmune disease-related antibodies in patients with advanced solid tumors receiving immune checkpoint inhibitors: a retrospective study. ESMO Open. (2022) 7(2):100415. doi: 10.1016/J.ESMOOP.2022.100415
47. Luongo C, Morra R, Gambale C, Porcelli T, Sessa F, Matano E, et al. Higher baseline TSH levels predict early hypothyroidism during cancer immunotherapy. J Endocrinol Invest. (2021) 44:1927–33. doi: 10.1007/s40618-021-01508-5
48. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling preexisting antibodies in patients treated with anti–PD-1 therapy for advanced non–small cell lung cancer. JAMA Oncol. (2019) 5:376. doi: 10.1001/JAMAONCOL.2018.5860
49. Atkinson M, Lansdown AJ. Endocrine immune-related adverse events: Adrenal, parathyroid, diabetes insipidus, and lipoatrophy. Best Pract Res Clin Endocrinol Metab. (2022) 36(3):101635. doi: 10.1016/j.beem.2022.101635
50. Al Ashi SI, Thapa B, Flores M, Ahmed R, Rahim SEG, Amir M, et al. Endocrine toxicity and outcomes in patients with metastatic Malignancies treated with immune checkpoint inhibitors. J Endocr Soc. (2021) 5(8):1-15. doi: 10.1210/JENDSO/BVAB100
51. Cheung YMM, Wang W, McGregor B, Hamnvik OPR. Associations between immune-related thyroid dysfunction and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Immunol Immunother. (2022) 71:1795–812. doi: 10.1007/S00262-021-03128-7
52. Snyders T, Chakos D, Swami U, Latour E, Chen Y, Fleseriu M, et al. Ipilimumab-induced hypophysitis, a single academic center experience. Pituitary. (2019) 22:488–96. doi: 10.1007/S11102-019-00978-4
53. Johnson J, Goldner W, Abdallah D, Qiu F, Ganti AK, Kotwal A. Hypophysitis and secondary adrenal insufficiency from immune checkpoint inhibitors: diagnostic challenges and link with survival. J Natl Compr Canc Netw. (2023) 21:281–7. doi: 10.6004/JNCCN.2022.7098
Keywords: immune checkpoint inhibitor, endocrinopathy, cancer, survival, immune related adverse effects (irAEs)
Citation: Yang L, Murthy S, Cortellini A, Lim EA, Gonzalez M, Pinato DJ, Abdel-Malek M, Mahmoud S and Martin NM (2024) Effects of immune checkpoint inhibitor associated endocrinopathies on cancer survival. Front. Endocrinol. 15:1369268. doi: 10.3389/fendo.2024.1369268
Received: 11 January 2024; Accepted: 12 March 2024;
Published: 12 April 2024.
Edited by:
Dario Giuffrida, Mediterranean Institute of Oncology (IOM), ItalyReviewed by:
Ana Perdigoto, Yale University, United StatesRoberta Modica, University of Naples Federico II, Italy
Anupam Kotwal, University of Nebraska Medical Center, United States
Copyright © 2024 Yang, Murthy, Cortellini, Lim, Gonzalez, Pinato, Abdel-Malek, Mahmoud and Martin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niamh M. Martin, bi5tYXJ0aW5AaW1wZXJpYWwuYWMudWs=
†ORCID: David J. Pinato, orcid.org/0000-0002-3529-0103
Niamh M. Martin, orcid.org/0000-0003-1185-2762
 Lisa Yang
Lisa Yang Sruthi Murthy1
Sruthi Murthy1 David J. Pinato
David J. Pinato Niamh M. Martin
Niamh M. Martin