- 1Xiamen Cardiovascular Hospital of Xiamen University, School of Medicine, Fujian Branch of National Clinical Research Center for Cardiovascular Diseases, Xiamen, China
- 2Pharmacy Department, The Affiliated Jiangning Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 3Department of Clinical Nutrition, College of Applied Medical Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
- 4School of Food and Biological Engineering, Jiangsu University, Zhenjiang, China
- 5Department of Preventive Medicine, School of Public Health, Wuhan University, Wuhan, China
- 6Clinical Nutrition Department, The Affiliated Jiangning Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 7Department of Traditional Chinese Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
Background: Gestational diabetes mellitus (GDM) is a metabolic disorder of pregnancy associated with multiple adverse maternal-perinatal outcomes among singleton and twin pregnancies and its incidence is increasing across the globe. We aimed to find the secular trend of GDM and its interaction effect with advanced maternal age (AMA) on adverse maternal-perinatal outcomes among primiparous singleton and twin pregnancies in Hubei, China.
Methods: A retrospective-based cohort study was conducted at the Wuhan University Renmin Hospital, Hubei Province, China, between 2011 and 2019. A chi-square test was used to explore a significant difference in the adverse maternal-perinatal outcomes between younger (18–34 years) and older/AMA (≥35 years) women with singleton and twin gestations. A multiple binary logistic regression model was used to estimate the adverse effect of GDM on maternal-perinatal outcomes among younger and older women with singleton and twin gestations, taking non-GDM as a reference group. We used a joinpoint regression analysis to find the secular trend of GDM prevalence among singleton and twin pregnancies during the study period.
Results: The secular trend of GDM [average annual percentage change (AAPC) 51.3% (95% confidence interval (95%CI): 3.9, 120.5)] significantly increased among singleton pregnant women. Based on age groups, the secular trend of GDM significantly increased in younger women with singleton [AAPC, 53.2% (95%CI: 2.0, 130.0)] and twin gestations [AAPC, 83.7% (95%CI: 36.0, 148.1)] between 2011 and 2019. Among younger women with singleton gestation, GDM showed a higher risk of hypertensive disorders of pregnancy (HDP), C-section, and macrosomia compared with non-GDM. Among younger women with twin gestations, GDM increased the risk of nuchal cord, polyhydramnios, and preterm births. GDM was associated with an increased risk of HDP, nuchal cord, macrosomia, and congenital defects among older women with singleton gestation. The interaction effect between GDM and AMA significantly increased the risk of HDP (adjusted odds ratio (aOR), 2.5; 95% CI: 1.8, 3.6), C-section (aOR, 2.5; 95% CI: 1.9, 3.4), and preterm birth (aOR, 1.5; 95% CI: 1.1, 1.9) among singleton pregnancies.
Conclusion: Among younger women with singleton and twin gestations, the secular trend of GDM significantly increased between 2011 and 2019. Among singleton pregnancies, GDM is associated with an increased risk of several adverse maternal-perinatal outcomes in both younger and older women. The interaction effect between GDM and AMA significantly increased the risk of HDP, C-section, and preterm birth among singleton pregnancies.
Introduction
Gestational diabetes mellitus (GDM) is a metabolic disorder of pregnancy, characterized by hyperglycemia that is first detected in pregnancy. It can affect up to 25% of women during pregnancy (1) and its incidence rate varies across different regions of the world including South-East Asia (25%), Middle East and North Africa (17.5%), Europe (12.6%), and North America and the Caribbean region (10.4%) (2, 3). In China, the pooled GDM prevalence was 14.8% in 2019 and its prevalence varies across different cities and regions (4). The prevalence of GMD was 24.2% in Beijing (5), 19.9% in Xiamen (6), 22.9% in Guangzhou (7), 15.8% in Chengdu (8), and 5.1% in Xinjiang (4). According to the American Diabetes Association, the temporal trend of GMD incidence significantly increased by 85% in China (2001–2016) (7). Moreover, the temporal trend of GMD increased by 28% in Xiamen (2012-2017) (6), by 18% in Beijing (2013-2018) (5), and substantially increased in Hubei from 0.6% to 11.8% during 2011-2019 (9) and in Hebei from 3.0% to 15.0% between 2014 and 2021 (10).
The rising incidence rate of GMD could be attributed to the higher prevalence of obesity and increasing childbearing age in China (11, 12). The mean childbearing age has increased from 27.1 years to 29.7 in Hubei, China (11). In China, the proportion of women with advanced maternal age (AMA, ≥35 years) ranges from 10.0% to 20.2% and the proportion of women with AMA increased by 75% and 223% between 2011 and 2019 and during 2010–2017 respectively, in Hubei, China (11, 13). It is well known that both GDM and AMA are independent risk factors associated with a higher risk of hypertensive disorders of pregnancy (HDP), C-section, placenta previa, congenital defects, perinatal mortality, preterm births, and low birth weight (LBW) (8, 9, 12, 14–17). Several studies observed the independent effect of GDM and AMA on adverse maternal-perinatal outcomes (8–10, 12, 14–17) however, very few studies have determined the combined effect of GDM and AMA on maternal-perinatal outcomes (18–21).
Moreover, these previous studies have focused on pooled primiparous and multiparous pregnant women (18–21), thus data on the GDM and its interaction effect with AMA on adverse maternal-perinatal outcomes among primiparous women is limited (22, 23). The pregnancy outcomes of primiparous women are significantly different than multiparous women and represent a unique and pivotal period for assessing various medical, psychological, and social outcomes without the potential confounding factors introduced by prior births (24, 25). Therefore, to understand the unique pregnancy outcomes of primiparous women, without the bias that may arise from previous births, we aimed to explore the secular prevalence of GDM and the interaction effect of GDM with AMA on adverse maternal-perinatal outcomes among primiparous singleton and twin pregnancies in Hubei, China.
Materials and methods
Study design and population
The present retrospective-based cohort study was conducted in the Department of Obstetrics and Gynecology at Wuhan University Renmin Hospital in Hubei, China according to the strengthening of the reporting of observational studies in epidemiology (STROBE) guidelines (26) between 2011 and 2019. The skilled nurses collected and recorded the data on pregnant women (n=25678) in the obstetrics register and electronic database during their examinations. Based on maternal age, pregnant women were divided into younger (18–24 years) and advanced maternal age (AMA) (≥35 years) groups. Furthermore, according to the results of the 75 g oral glucose tolerance test, both younger and older women were allocated to gestational diabetes mellitus (GDM) and non-GDM groups. The study protocol was approved by the Ethical Review Board of Renmin Hospital (ID: WDRY2019–K034) in accordance with the Declaration of Helsinki.
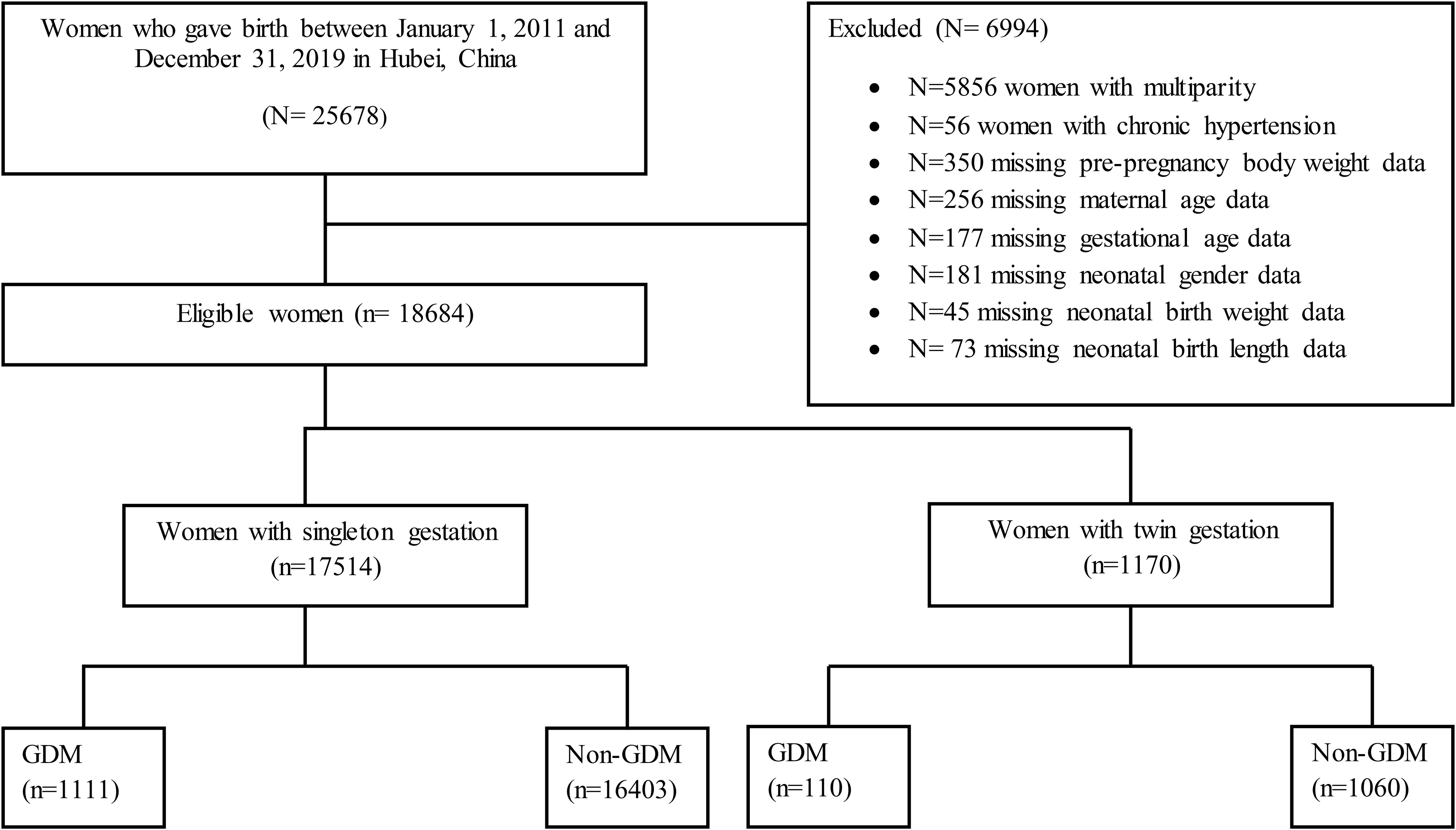
Inclusion and exclusion criteria and sample size calculation
A total of 18,684 primiparous pregnant women were considered for data analysis in the current study, including singleton (n= 17514) and twin pregnancies (n= 1170). The data was excluded on multiparty (n= 5856), chronic hypertension (n=56), and missing data on maternal age (n=256), pre-pregnancy body weight (n=350), gestational age (n=177), and neonatal-related data including neonatal gender (n=181), birth weight (n=45), and birth length (n=73) from the statistical analysis as shown in Figure 1. In our study, the pattern of missing data was missing at random (MAR) and we applied the listwise or case deletion approach for handling missing data which simply means eliminating the subjects with missing data and analyzing the remaining data (27). The power of the study and sample size was determined by using the G*Power software (latest ver. 3.1.9.7; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) (28). We used a priori power method by selecting Z tests (i.e. test family) and logistic regression (i.e. statistical test) to find the power analysis. We assumed that 30% of non-GDM pregnant women had adverse maternal-perinatal outcomes and 50% of pregnant women with GDM had adverse maternal-perinatal outcomes. By considering the aforementioned assumptions and taking the power (1-β =0.80) and significance level (α =0.05), the minimum sample size should be (N=191).
Collection of data, variables, and definitions
Data on maternal characteristics including age, pre-pregnancy body weight, education, occupation gestational age, and parity, and maternal-neonatal outcomes such as GDM, hypertensive disorders of pregnancy (HDP), abnormal placentation, previous history of C-section, C-section, premature rupture of membrane (PROM), nuchal cord, oligohydramnios, polyhydramnios, fetal breech presentation, preterm birth, low birth weight (LBW), perinatal mortality, macrosomia, low Apgar score, fetal distress, intrauterine growth restriction (IUGR), congenital defects, neonatal birth weight, birth length, and gender were retrieved from the obstetrics register and electronic database. Maternal age was divided into two groups (i) 18–34 years, (ii) and ≥35 years at the time of delivery. The last known menstrual cycle date was used to determine gestational age, which was then verified by ultrasound examination in the first and second trimesters. The detailed definition of exposure and outcome variables has been described in our previous article (9) and Supplementary File S1.
Definition of confounding factors
Confounding variables that have been linked to both exposure and perinatal birth outcome in the previous literature were selected such as maternal education, occupation, pre-pregnancy body weight (≤ 45 kg and ≥ 91 kg), and neonatal gender (29).
Statistical analysis
In the first step, we explored a significant difference in the baseline general characteristics and maternal-perinatal outcomes between younger (18–34 years) and older (≥35 years) primiparous singleton and twin pregnancies by using a Chi-square test. In the second step, multiple binary logistic regression models were used (adjusted for maternal education, occupation, pre-pregnancy body weight, and neonatal gender) to estimate the adverse effect of GDM on maternal-perinatal outcomes among younger and older pregnant women in both singleton and twin pregnancies taking non-GDM as a reference group. In our analysis, GDM was a predictor variable and the outcome variables were HDP, abnormal placentation, C-section, PROM, nuchal cord, oligohydramnios, polyhydramnios, fetal breech presentation, preterm birth, LBW, perinatal mortality, macrosomia, low Apgar score, fetal distress, IUGR, and congenital defects. Furthermore, among singleton and twin pregnancies, we determined the interaction effect of GDM and AMA (i.e. GDM×AMA) on maternal-perinatal outcomes. The adjusted odds ratios (aOR) with 95% confidence intervals were used to estimate the association between predictor variables and outcome variables. P-value (two-tailed <0.05) was taken as statistically significant. The data were analyzed by using SPSS (Statistical Package for Social Sciences) for Windows version 22 (IBM Corporation, New York, USA).
In the third step, we used joinpoint regression analysis to find the secular trend of GDM prevalence among primiparous singleton and twin pregnancies between 2011 and 2019. The percentage changes (APC) and average annual percentage changes (AAPC) were estimated for GDM prevalence in the joinpoint regression analysis. The APC shows the trend in the GDM prevalence in each segment and the AAPC indicates trend in the GDM prevalence in the whole study period 2011-2019. The APPC or APC >0 with its 95% confidence interval (CI) shows a positive secular trend, however, the APPC or APC <0 with its 95% CI shows a negative secular trend. Moreover, Monte Carlo methods were used to find each p-value and maintain the overall asymptotic significance level through Bonferroni correction. The joinpoint regression analysis was conducted using the joinpoint regression program version 4.8.0.1 (April 2020) from the Surveillance Research Program of the U.S. National Cancer Institute.
Results
Baseline maternal-perinatal characteristics among primiparous singleton and twin pregnancies
A total of 18,684 primiparous pregnant women including singleton (n= 17514) and twins (n= 1170) were considered in the current study (Figure 1). Singleton and twin pregnancies consisted of 13.2% and 15.1% of women with advanced maternal age (AMA), respectively. Among singleton, women with AMA showed a significantly higher incidence of GDM (11.7% vs. 5.5%), HDP (8.8% vs. 5.8%), abnormal placentation (5.9% vs. 3.3%), C-section (75.8% vs. 56.5%), preterm birth (23.4% vs. 16.5%), LBW (15.5% vs. 12.7%), and perinatal mortality (2.0% vs. 1.2%) compared with younger women. Moreover, women with AMA showed a significantly higher incidence of preterm birth (78.0% vs. 70.4%) compared with younger women among twin pregnancies (Tables 1, 2).
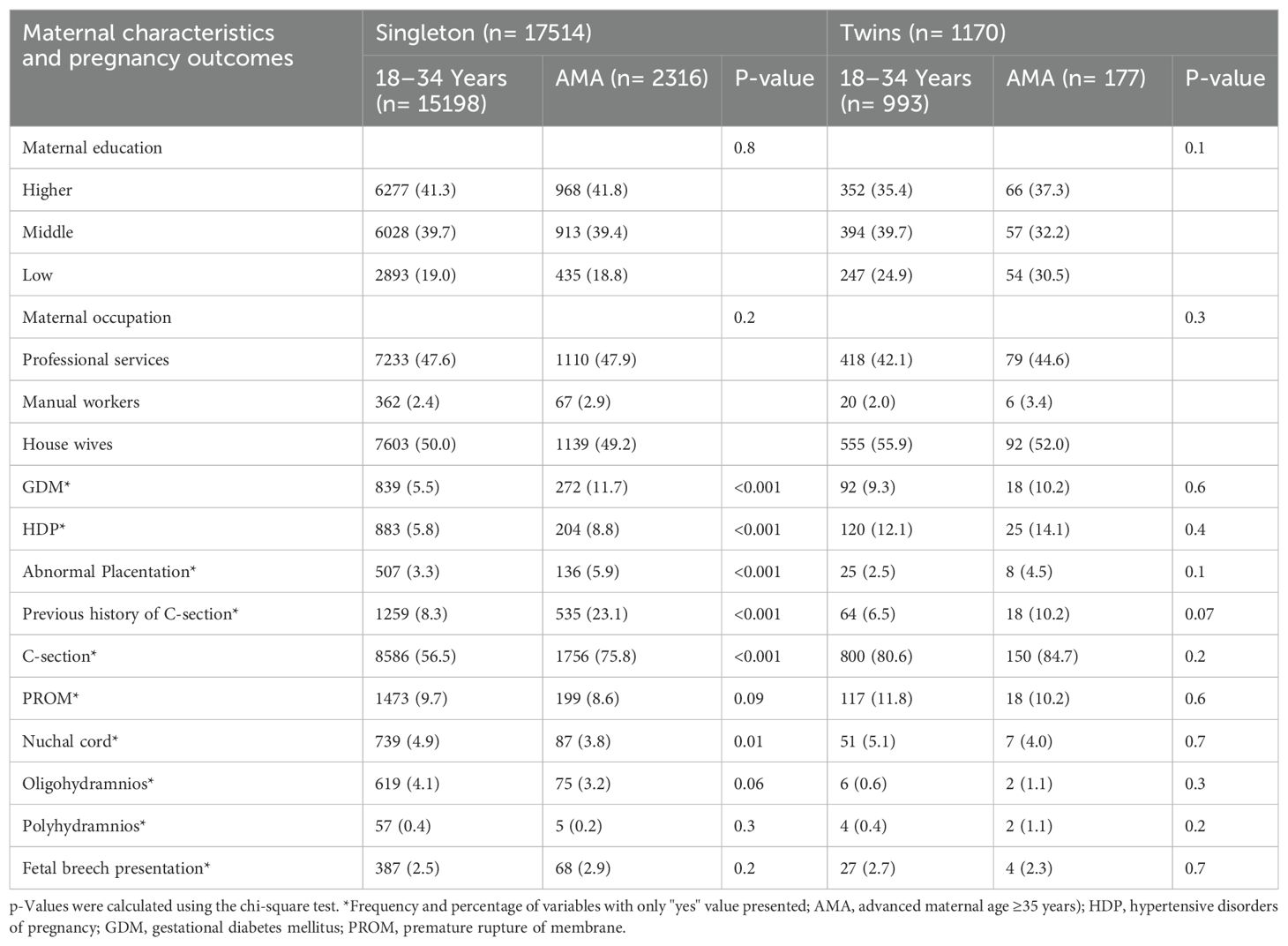
Table 1. Baseline maternal characteristics and pregnancy outcomes among primiparous singleton and twin pregnancies by maternal age groups (N= 18684).
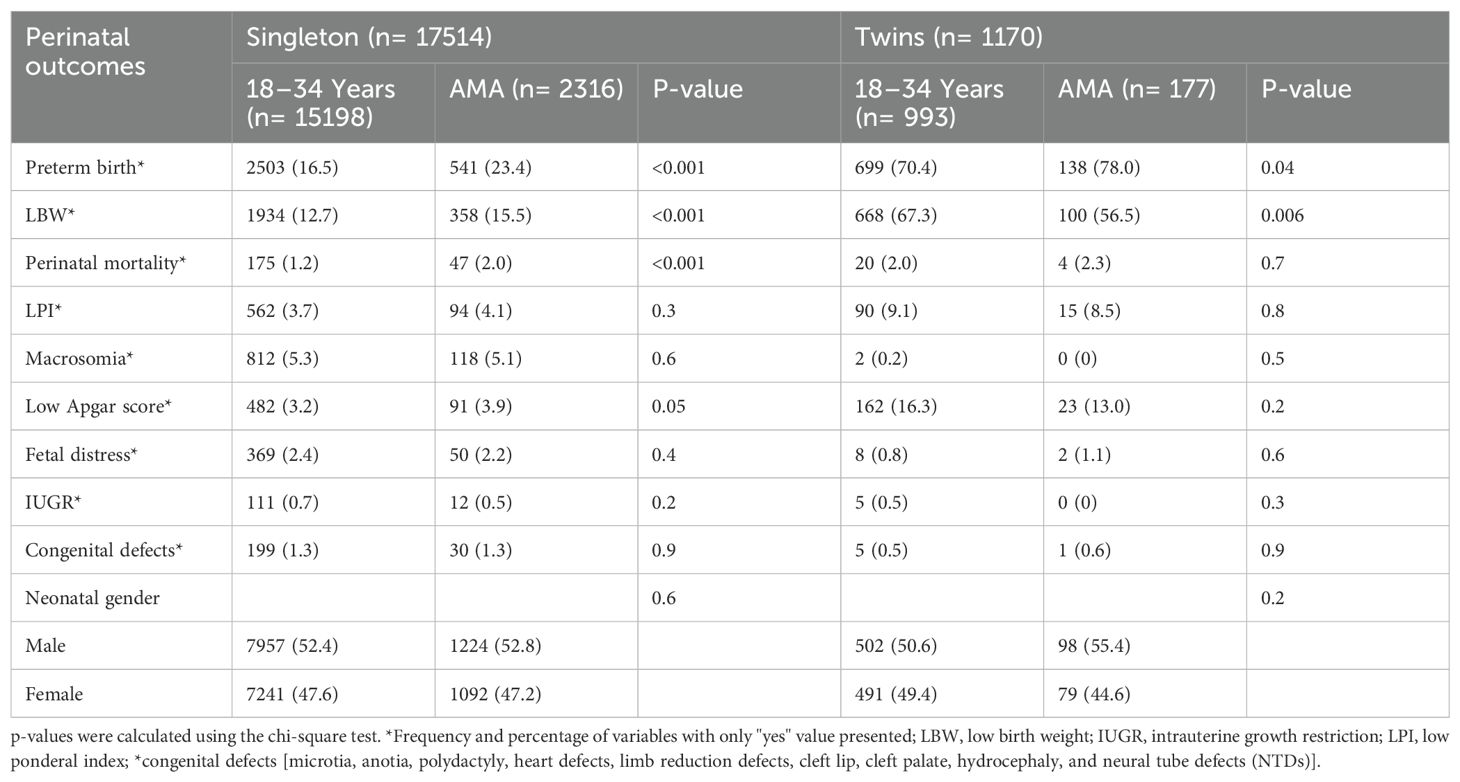
Table 2. Baseline perinatal outcomes among primiparous singleton and twin pregnancies by maternal age groups.
Association of GDM with adverse maternal–perinatal outcomes among younger and older women with singleton gestation
After adjusting for confounding factors, GDM was associated with a higher risk of HDP (aOR, 2.1; 95% CI: 1.6, 2.5), C-section (aOR, 1.6; 95% CI: 1.4, 1.9), and macrosomia (aOR, 1.4; 95% CI: 1.1, 1.8) compared with non-GDM among younger women aged (18–34 years). Among women with AMA, GDM was associated with an increased risk of HDP (aOR, 1.9; 95% CI: 1.3, 2.7), nuchal cord (aOR, 1.8; 95% CI: 1.1, 3.1), macrosomia (aOR, 1.6; 95% CI: 1.1, 2.7), and congenital defects (aOR, 2.4; 95% CI: 1.1, 5.7) compared with non-GDM (Table 3).
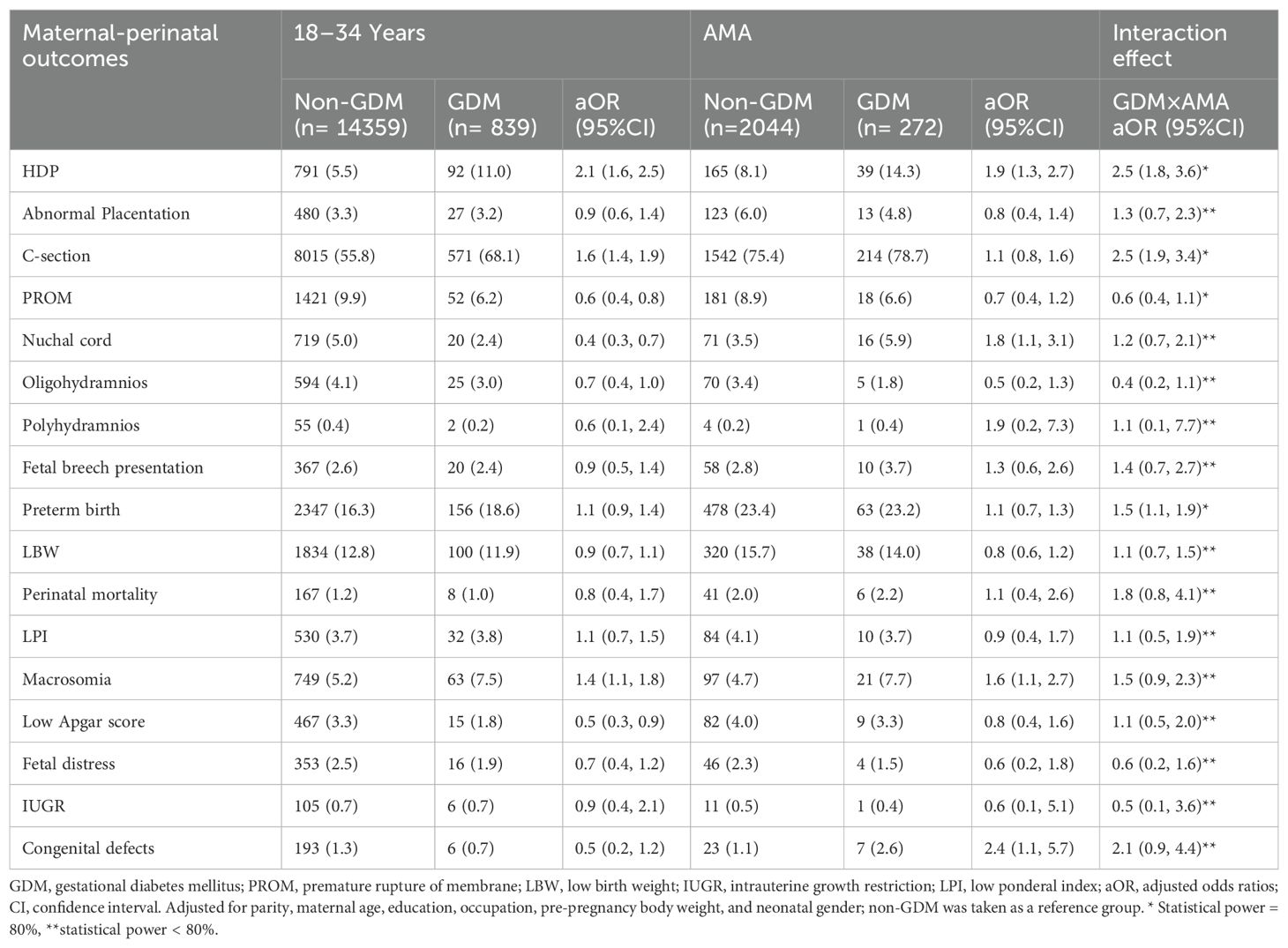
Table 3. Risk of maternal-perinatal outcomes among primiparous singleton women with gestational diabetes mellitus by maternal age groups.
Association of GDM with adverse maternal–perinatal outcomes among younger and older women with twin gestations
Among younger women, individuals with GDM had a higher risk of nuchal cord (aOR, 2.3; 95% CI: 1.1, 5.1), polyhydramnios (aOR, 11.6; 95% CI: 1.5, 18.1), and preterm births (aOR, 1.9; 95% CI: 1.2, 3.3) compared with non-GDM. However, GDM was not significantly associated with an increased risk of any maternal-perinatal outcomes among women with AMA (Table 4).
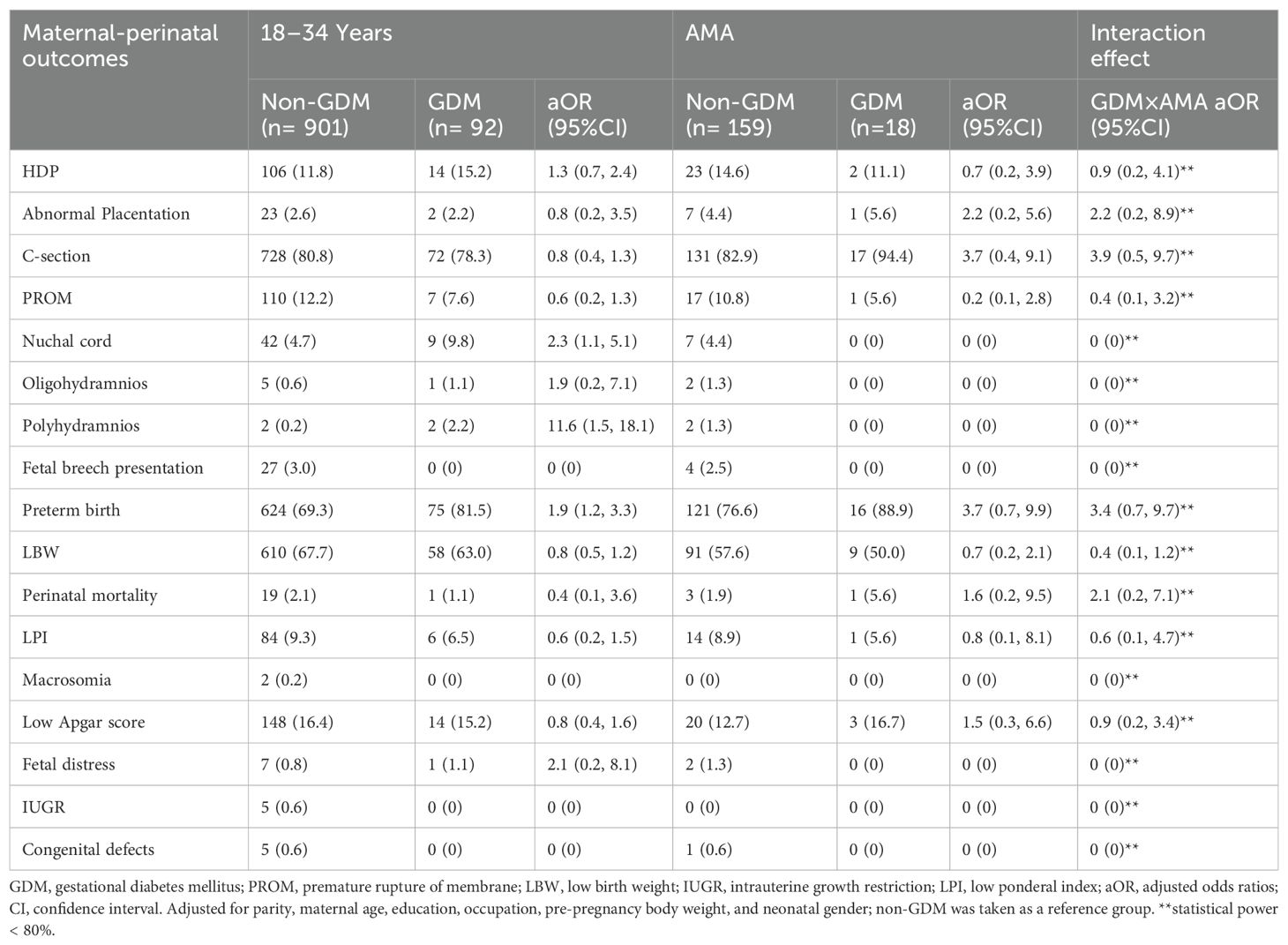
Table 4. Risk of maternal-perinatal outcomes among primiparous twin women with gestational diabetes mellitus by maternal age groups.
Interaction effect between GDM and AMA on adverse maternal-perinatal outcomes among women with singleton and twin gestations
Among singleton, the interaction effect between GDM and AMA (i.e., GDM×AMA) significantly increased the risk of HDP (aOR, 2.5; 95% CI: 1.8, 3.6), C-section (aOR, 2.5; 95% CI: 1.9, 3.4), and preterm birth (aOR, 1.5; 95% CI: 1.1, 1.9) compared with non-GDM. On the other hand, the interaction effect between GDM and AMA is not significantly associated with an increased risk of maternal-perinatal outcomes among twins (Tables 3, 4).
Temporal trend of GDM among primiparous singleton and twin pregnancies (2011-2019)
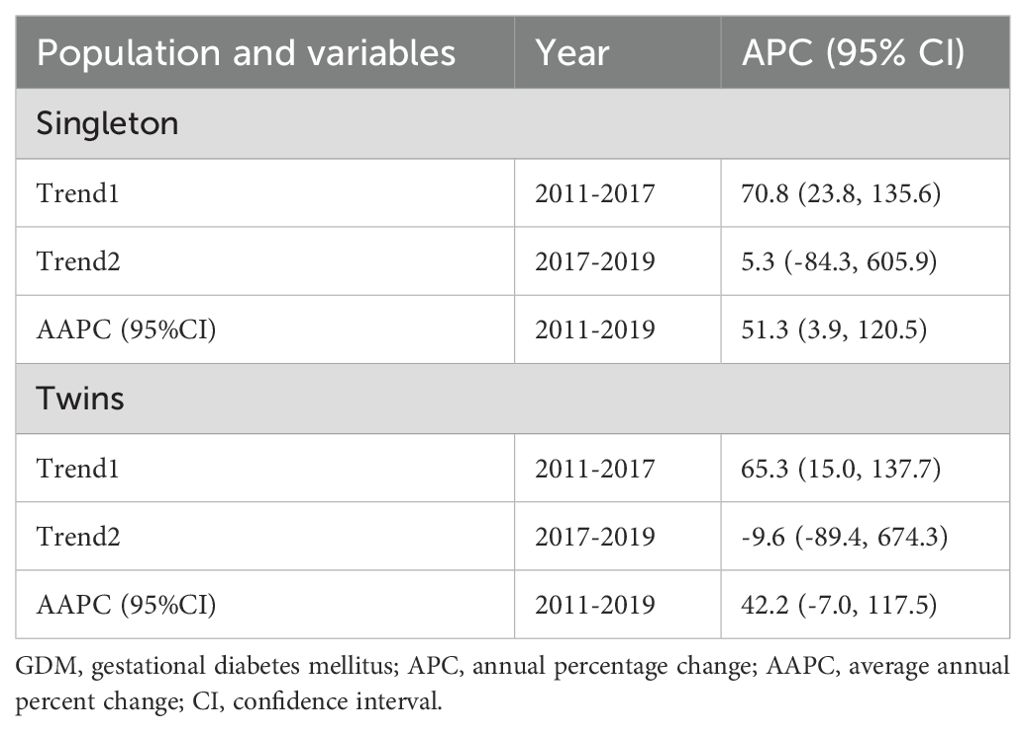
The joinpoint regression analysis revealed that the APC of GDM significantly increased among primiparous singleton (APC, 70.8%; 95%CI: 23.8, 135.6) and twin pregnancies (APC, 65.3%; 95%CI: 15.0, 137.7) between 2011 and 2017. In addition, the AAPC of GDM significantly increased among primiparous singleton (AAPC, 51.3%; 95%CI: 3.9, 120.5) from 2011 to 2019 (Table 5, Figure 2).
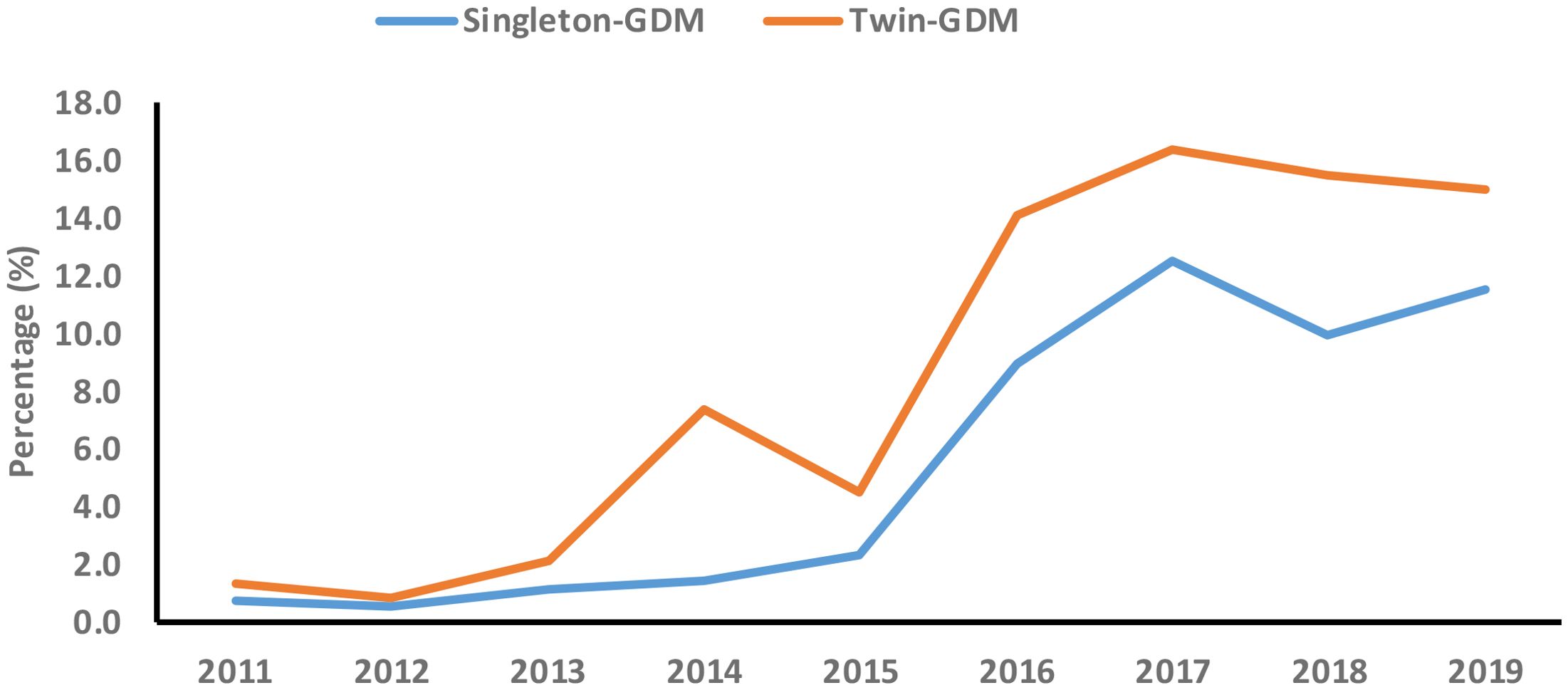
Figure 2. Secular trend of gestational diabetes mellitus (GDM) among primiparous singleton and twin pregnancies (2011-2019).
Temporal trend of GDM among primiparous singleton and twin pregnancies by maternal age groups (2011-2019)
The secular trend of GDM significantly increased in both singleton (AAPC, 53.2%; 95%CI: 2.0, 130.0) and twins (AAPC, 83.7%; 95%CI: 36.0, 148.1) younger (18-34 years) women during the study period (2011-2019). Moreover, the secular trend of GDM significantly increased in both singleton (APC, 60.1%; 95%CI: 20.4, 112.9) and twins (APC, 184.8%; 95%CI: 7.0, 658.4) older (≥35years) women between 2011 and 2017 (Table 6, Figure 3).
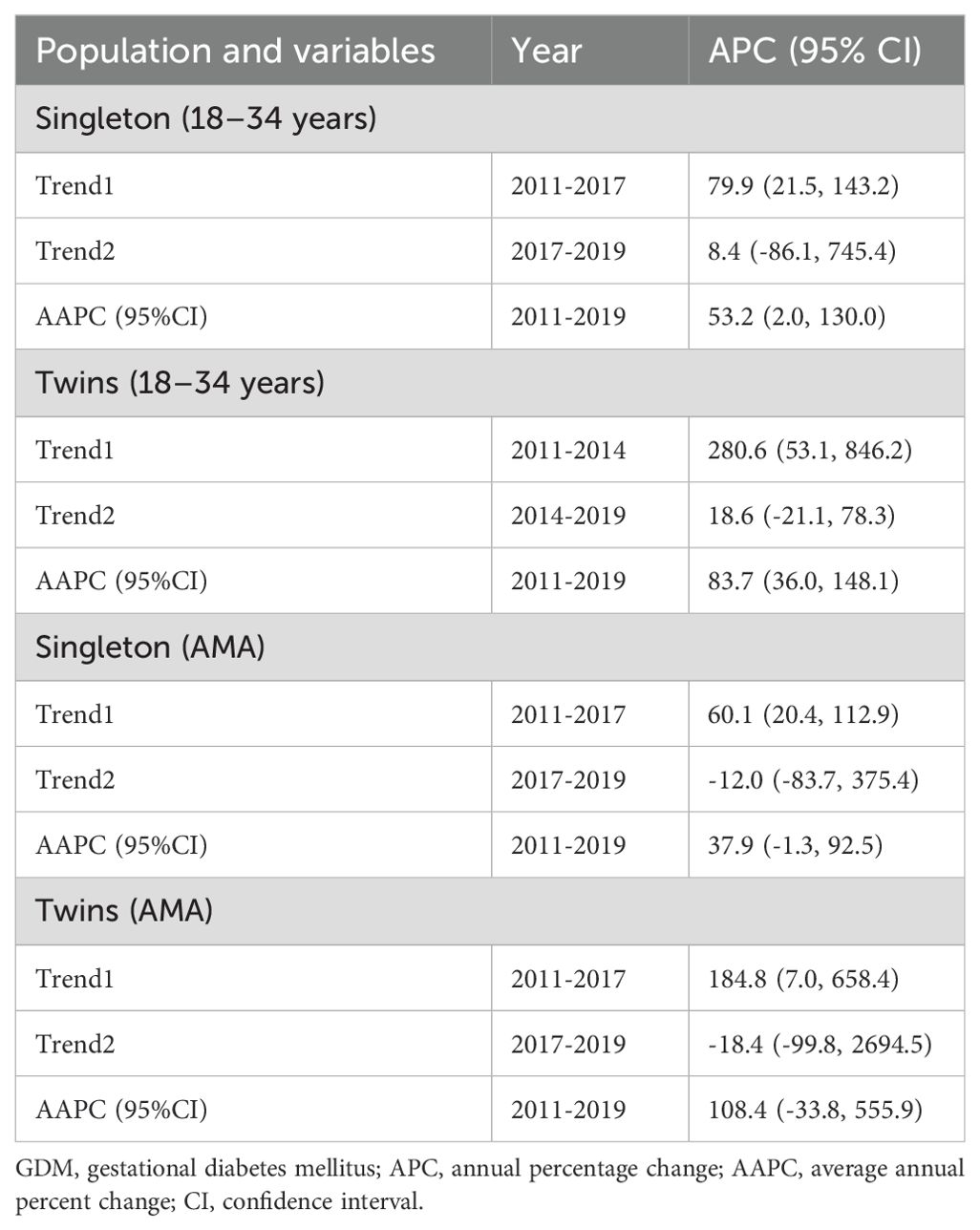
Table 6. Secular trend of GDM among primiparous singleton and twin pregnancies by maternal age groups from 2011 to 2019.
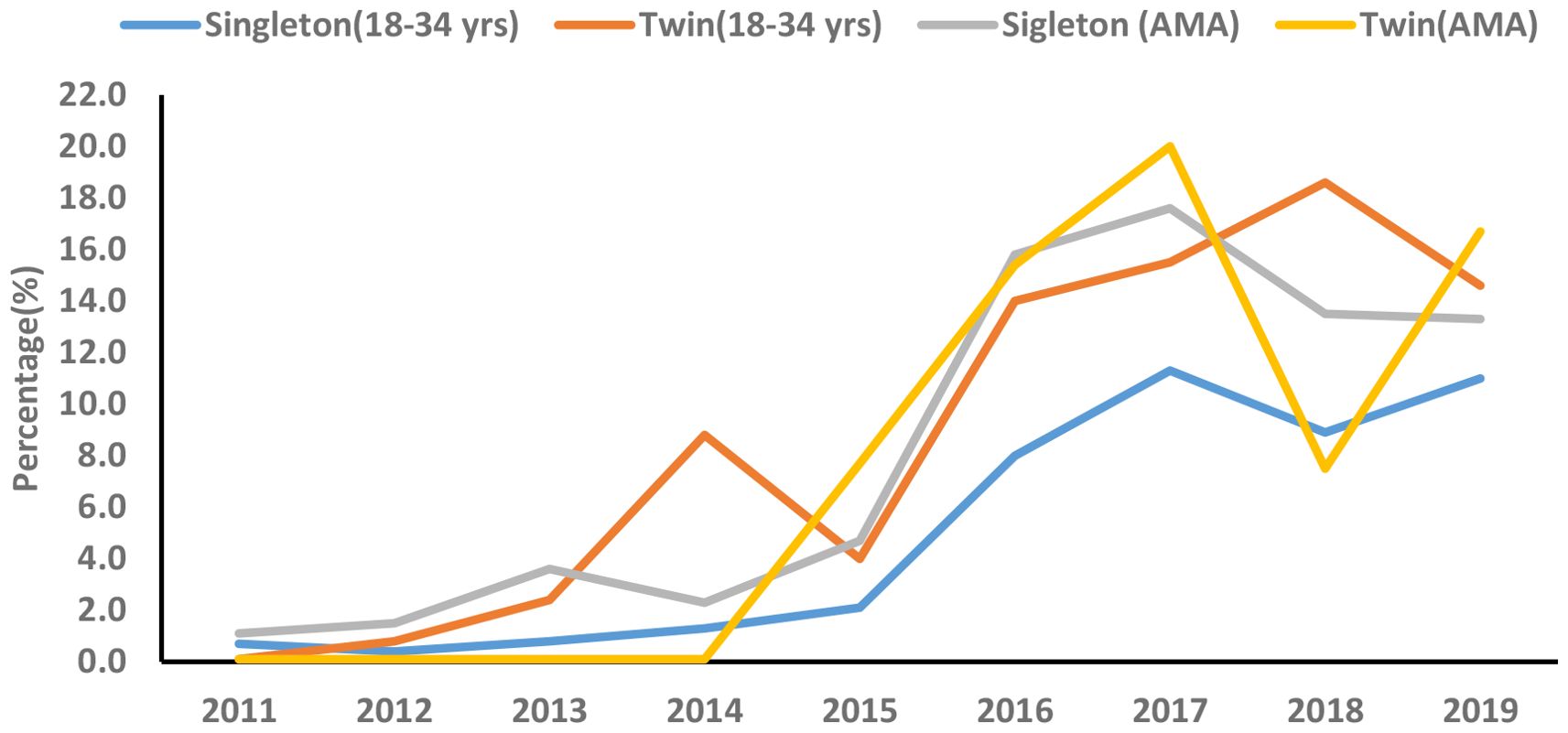
Figure 3. Secular trend of gestational diabetes mellitus (GDM) among primiparous singleton and twin pregnancies by maternal age groups (2011-2019).
Discussion
In the current retrospective cohort study, we observed that older women had a higher incidence of several adverse maternal-perinatal outcomes compared with younger women in singleton pregnancies. Moreover, older women showed a significantly higher incidence of preterm birth compared with younger women among twin pregnancies. Among singleton pregnancies, GDM is associated with several adverse maternal-perinatal outcomes in both younger and older women. Among twin pregnancies, GDM showed a higher risk of nuchal cord, polyhydramnios, and preterm births in younger women but was not significantly associated with adverse maternal-perinatal outcomes in older women. The additive interaction effect between GDM and AMA significantly increased the risk of HDP, C-section, and preterm birth among singleton pregnancies. The secular trend of GDM significantly increased among singleton pregnancies. Based on age groups, the secular trend of GDM significantly increased in younger women with both singleton and twin gestations during the study period.
Adverse maternal-perinatal outcomes among singleton and twin pregnancies by maternal age groups
We found that among singleton pregnancies, older women had a significantly higher incidence of GDM, HDP, abnormal placentation, C-section, preterm birth, LBW, and perinatal mortality than younger women. In addition, older women showed a significantly higher incidence of preterm birth compared with younger women among twin pregnancies. Our findings that older women had a higher incidence of adverse maternal-perinatal outcomes than younger women replicate the results of previous studies (13, 22, 23, 30–32). The higher incidence of GDM among older women could be attributed to progressive vascular endothelial damage in older ages (33), impaired glucose tolerance, reduction in insulin sensitivity (34), and pancreatic β-cell dysfunction as maternal age increases (35). Moreover, older women had high oxidative stress and low nitric oxide levels that could be attributed to atherosclerotic changes in the uterine blood vessels and linked with placental vascular pathology (36, 37). Besides these changes, progesterone deficiency in older women may partially explain the higher incidence of maternal-perinatal outcomes in women with AMA (38).
Association of GDM with adverse maternal–perinatal outcomes among singleton and twin pregnancies by maternal age group
We observed that GDM is associated with an increased risk of several adverse maternal-perinatal outcomes among younger and older women regardless of the number of gestations (i.e. singleton and twins). Among younger women with singleton gestation, GDM was associated with a higher risk of HDP, C-section, and macrosomia compared with non-GDM. Among older women with singleton gestation, GDM increased the risk of HDP, nuchal cord, macrosomia, and congenital defects. Our findings show that GDM increased the risk of HDP and macrosomia among singleton pregnancies regardless of maternal age groups which is in accordance with the previous studies (18, 20). It has been observed that GDM increases the occurrence of gestational hypertension and preeclampsia and insulin resistance plays a significant role in the pathogenies of hypertension during pregnancy (18, 39, 40). We found that among singleton pregnancies, GDM increased the risk of macrosomia by 1.4-fold and 1.6-fold in both younger and older women respectively, compared with non-GDM. The robust association between GMD and neonatal macrosomia was also observed in the previous studies in China (18), the United States (41), Canada (42), and Germany (43). It reflects that fetal exposure to maternal hyperglycemia over a prolonged gestational period resulted in neonatal macrosomia (44).
We showed that among younger women with twin gestations, GDM is associated with a higher risk of nuchal cord, polyhydramnios, and preterm births. However, GDM was not significantly associated with an increased risk of any maternal-perinatal outcomes among older women with twin gestations. It could be partially explained by the small sample size of twin older women with GDM in our study. Several studies observed the adverse maternal-perinatal outcomes in twin pregnancies complicated by GDM (9, 45, 46) or adverse maternal-perinatal outcomes in twin pregnancies by maternal age (32, 47, 48); however, the impact of GDM on adverse maternal-perinatal outcomes among twin pregnancies by maternal age groups has not yet been reported. The present study suggests that a large population-based study should be conducted to investigate the independent effect of GDM on adverse maternal-perinatal outcomes among younger and older twin pregnancies.
Interaction effect between GDM and AMA on adverse maternal-perinatal outcomes
Our findings show that the interaction effect between GDM and AMA significantly increased the risk of HDP, C-section, and preterm birth among singleton pregnancies. On the other hand, the interaction effect between GDM and AMA is not significantly associated with an increased risk of any adverse maternal-perinatal outcome among twin pregnancies. In accordance with our findings, Li et al. (18) also observed that the interaction effect between GDM and AMA significantly increased the risk of aforementioned adverse maternal-neonatal outcomes among singleton pregnancies. This study (18) investigated the individual effect as well as the interaction effect of GDM and AMA on adverse maternal-neonatal outcomes. However, our study only explored the interaction effect of GDM and AMA on adverse maternal-neonatal outcomes.
Zhang et al. (30) showed that compared with women aged 25–39 years, older women with GDM had a higher risk of several adverse pregnancy outcomes. However, this study failed to determine the interaction effect of AMA and GDM on adverse pregnancy outcomes (30). In another study, Wang et al. (21) investigated the independent effect of GDM and the interaction effect of GDM and maternal age on adverse pregnancy outcomes. The study showed that the interaction effect between GDM and young age (i.e. women aged less than 35 years) significantly increased the risk of C-section among Chinese urban women. However, we haven't explored the interaction effect of GDM and young age on adverse pregnancy outcomes. Overall, these findings suggest that besides the independent effect of GMD and AMA on adverse pregnancy outcomes, future studies should consider the interaction effect between GDM and both young and old age on adverse pregnancy outcomes. Moreover, our findings underscore the significant synergistic effect of GDM and AMA on adverse pregnancy outcomes in singleton women and urge a lifestyle intervention to control blood sugar and minimize the risk of HDP and preterm births among older pregnant women with GDM.
Secular trend of GDM among primiparous singleton and twin pregnancies (2011–2019)
The secular trend of GDM significantly increased among singleton pregnancies. Based on age groups, the secular trend of GDM significantly increased in younger women with both singleton and twin gestations during the study period (2011–2019). Moreover, the secular trend of GDM significantly increased in older women with singleton and twin gestations between 2011 and 2017. The increasing secular trend of GDM has been observed across different cities and regions in China. Between 2001 and 2016, the secular trend of GDM incidence significantly increased by 85% in China (7). Moreover, the secular trend of GMD increased by 28% in Xiamen (2012–2017) (6), by 18% in Beijing (2013–2018) (5), and substantially increased in Hubei from 0.6% to 11.8% during 2011-2019 (9) and in Hebei from 3.0% to 15.0% between 2014 and 2021 (10). These studies estimated the secular trend of GDM regardless of parity and maternal age groups (5–7, 9, 10). However, our findings show the increasing secular trend of GDM among primiparous younger and older women with singleton and twin gestations. In our study, the increasing trend of GDM among younger women with singleton and twin gestations is alarming and is associated with an increased risk of several adverse maternal-perinatal outcomes. In China, the increasing secular trend of GDM could be attributed to smoking, urbanization, lifestyle changes, pre-pregnancy higher body mass index (BMI), and higher gestational weight gain (6, 49). However, we failed to determine the risk factors associated with the increasing secular trend of GDM in our study due to the lack of certain data including smoking, pre-pregnancy BMI, gestational weight gain, dietary habits, and sedentary lifestyle. Future studies should be encouraged to estimate the secular trend of GDM and its associated risk factors among younger and older women with singleton and twin gestation at the population level in China.
We acknowledge that our study had several limitations. First, our study was a retrospective cohort study conducted in a single-center tertiary hospital, which could be a potential selection bias in our study. Second, our study had missing data and lacked data on maternal smoking, drinking alcohol, dietary habits, sedentary lifestyle, assisted reproductive technology, pre-pregnancy BMI, and gestational weight gain, which may bias our findings. Third, overall we had a small sample size particularly twin older women with GDM, thus the results cannot be generalized to the overall population.
Conclusion
Among women with singleton gestation, GDM increased the risk of several adverse maternal-perinatal outcomes in both younger and older women. Among women with twin gestations, GDM showed a higher risk of nuchal cord, polyhydramnios, and preterm births in younger women but was not significantly associated with any adverse maternal-perinatal outcomes in older women. The additive interaction effect between GDM and AMA significantly increased the risk of HDP, C-section, and preterm birth among singleton pregnancies. The secular trend of GDM significantly increased in younger singleton and twin pregnancies between 2011 and 2019. Our findings suggest that gynecologists and clinicians should prioritize singleton pregnancies with both GDM and AMA. Additionally, policymakers should implement lifestyle interventions (e.g., physical activity, diet) and ensure timely, free GDM screenings and treatment, particularly for younger women, to address the growing trend of GDM and its impact on maternal and perinatal outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethical Review Board of Renmin Hospital (ID: WDRY2019–K034) in accordance with the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because it was a retrospective study.
Author contributions
N: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. H-TS: Conceptualization, Methodology, Writing – review & editing. IH: Formal analysis, Methodology, Visualization, Writing – review & editing. GN: Methodology, Validation, Writing – review & editing. SN: Data curation, Methodology, Validation, Writing – review & editing. J-LX: Conceptualization, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing. XN: Conceptualization, Investigation, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work is supported by the Xiamen’s Science and Technology Program (Grant No: 3502Z20209007, 3502Z20224032, 3502Z20241002) and by Deanship for Scientific Research, King Faisal University (Grant No: KFU252621).
Acknowledgments
We are thankful to the staff of Obstetrics and Gynecology department of Renmin Hospital, Wuhan for helping in data collection. We also extend our appreciation to the Deanship For Scientific Research, King Faisal University (Grant # KFU252621) for support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1439592/full#supplementary-material
References
1. Choudhury AA and Devi Rajeswari V. Gestational diabetes mellitus - A metabolic and reproductive disorder. Biomedicine Pharmacotherapy. (2021) 143:112183. doi: 10.1016/j.biopha.2021.112183
2. Zhu H, Zhao Z, Xu J, Chen Y, Zhu Q, Zhou L, et al. The prevalence of gestational diabetes mellitus before and after the implementation of the universal two-child policy in China. Front Endocrinol. (2022) 13:960877. doi: 10.3389/fendo.2022.960877
3. Guariguata L, Linnenkamp U, Beagley J, Whiting DR, and Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. (2014) 103:176–85. doi: 10.1016/j.diabres.2013.11.003
4. Gao C, Sun X, Lu L, Liu F, and Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and metaewaticl. J Diabetes Invest. (2019) 10:154–62. doi: 10.1111/jdi.12854
5. Wang C, Jin L, Tong M, Zhang J, Yu J, Meng W, et al. Prevalence of gestational diabetes mellitus and its determinants among pregnant women in Beijing. J Maternal-Fetal Neonatal Med. (2022) 35:1337–43. doi: 10.1080/14767058.2020.1754395
6. Yan B, Yu Y, Lin M, Li Z, Wang L, Huang P, et al. High, but sta ble, trend in the prevalence of gestational diabetes mellitus: A populationlbased study in Xiamen, China. J Diabetes Invest. (2019) 10:1358–64. doi: 10.1111/jdi.13039
7. He Z, Xie H, Liang S, Tang Y, Ding W, Wu Y, et al. Influence of different diagnostic criteria on gestational diabetes mellitus incidence and medical expenditures in China. J. Diabetes Investig. (2019) 10:1347–57. doi: 10.1111/jdi.13008
8. Mak JK, Lee AH, Pham NM, Pan XF, Tang L, Binns CW, et al. Gestational diabetes incidence and delivery outcomes in Western China: A prospective cohort study. Birth. (2019) 46:166–72. doi: 10.1111/birt.12397
9. Nawsherwan, Liu Z, Le Z, Mubarik S, Sun Y, Naeem S, et al. The adverse effect of gestational diabetes mellitus and hypertensive disorders of pregnancy on maternal–perinatal outcomes among singleton and twin pregnancies: a retrospective cohort study (2011–2019). Front Endocrinol. (2023) 14:1267338. doi: 10.3389/fendo.2023.1267338
10. Tian M-L, Du L-Y, Ma G-J, Zhang T, Ma X-Y, Zhang Y-K, et al. Secular increase in the prevalence of gestational diabetes and its associated adverse pregnancy outcomes from 2014 to 2021 in Hebei province, China. Front Endocrinol. (2022) 13:1039051. doi: 10.3389/fendo.2022.1039051
11. Cao J, Xu W, Liu Y, Zhang B, Zhang Y, Yu T, et al. Trends in maternal age and the relationship between advanced age and adverse pregnancy outcomes: a population-based register study in Wuhan, China, 2010–2017. Public Health. (2022) 206:8–14. doi: 10.1016/j.puhe.2022.02.015
12. Song Z, Cheng Y, Li T, Fan Y, Zhang Q, and Cheng H. Effects of obesity indices/GDM on the pregnancy outcomes in Chinese women: a retrospective cohort study. Front Endocrinol. (2022) 13:1029978. doi: 10.3389/fendo.2022.1029978
13. Li H, Nawsherwan, Fan C, Mubarik S, Nabi G, and Ping YX. The trend in delayed childbearing and its potential consequences on pregnancy outcomes: a single center 9-years retrospective cohort study in Hubei, China. BMC Pregnancy Childbirth. (2022) 22:514. doi: 10.1186/s12884-022-04807-8
14. Shan D, Qiu P-Y, Wu Y-X, Chen Q, Li A-L, Ramadoss S, et al. Pregnancy outcomes in women of advanced maternal age: a retrospective cohort study from China. Sci Rep. (2018) 8:1–9. doi: 10.1038/s41598-018-29889-3
15. Londero AP, Rossetti E, Pittini C, Cagnacci A, and Driul L. Maternal age and the risk of adverse pregnancy outcomes: a retrospective cohort study. BMC pregnancy childbirth. (2019) 19:261. doi: 10.1186/s12884-019-2400-x
16. Timofeev J, Reddy UM, Huang C-C, Driggers RW, Landy HJ, and Laughon SK. Obstetric complications, neonatal morbidity, and indications for cesarean delivery by maternal age. Obstetrics gynecology. (2013) 122:1184. doi: 10.1097/aog.0000000000000017
17. Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, et al. Impact of maternal age on obstetric outcome. Obstetrics Gynecology. (2005) 105:983–90. doi: 10.1097/01.AOG.0000158118.75532.51
18. Li J, Yan J, Ma L, Huang Y, Zhu M, and Jiang W. Effect of gestational diabetes mellitus on pregnancy outcomes among younger and older women and its additive interaction with advanced maternal age. Front Endocrinol. (2023) 14:1158969. doi: 10.3389/fendo.2023.1158969
19. Yong HY, Mohd Shariff Z, Mohd Yusof BN, Rejali Z, Tee YYS, Bindels J, et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. (2020) 10:8486. doi: 10.1038/s41598-020-65251-2
20. Lee CS, Zhu S, Wu Q, Hu Y, Chen Y, Chen D, et al. Independent and joint associations of age, pre-pregnancy BMI, and gestational weight gain with adverse pregnancy outcomes in gestational diabetes mellitus. Diabetes Ther. (2023) 14:363–75. doi: 10.1007/s13300-022-01352-7
21. Wang X, Zhang X, Zhou M, Juan J, and Wang X. Association of gestational diabetes mellitus with adverse pregnancy outcomes and its interaction with maternal age in chinese urban women. J Diabetes Res. (2021) 2021:5516937. doi: 10.1155/2021/5516937
22. Laine MK, Kautiainen H, Gissler M, Raina M, Aahos I, Järvinen K, et al. Gestational diabetes in primiparous women–impact of age and adiposity: a registery:ct/5 cohort study. Acta obstetricia gynecologica Scandinavica. (2018) 97:187–94. doi: 10.1111/aogs.13271
23. Kim EH, Lee J, Lee SA, and Jung YW. Impact of maternal age on singleton pregnancy outcomes in primiparous women in South Korea. J Clin Med. (2022) 11(4):969. doi: 10.3390/jcm11040969
24. Schwartz N, Nachum Z, and Green MS. The prevalence of gestational diabetes mellitus recurrence—effect of ethnicity and parity: a metaanalysis. Am J Obstetrics Gynecology. (2015) 213:310–7. doi: 10.1016/j.ajog.2015.03.011
25. Almahmeed B, Shah B, Mukerji G, Ling V, Booth G, and Feig D. Effect of multiparity and ethnicity on the risk of development of diabetes: a large populationtedrg/cohort study. Diabetic Med. (2017) 34:1637–45. doi: 10.1111/dme.13441
26. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. (2019) 13:S31–s4. doi: 10.4103/sja.SJA_543_18
27. Kang H. The prevention and handling of the missing data. Korean J anesthesiology. (2013) 64:402. doi: 10.4097/kjae.2013.64.5.402
28. Kang H and Huh S. Sample size determination and power analysis using the G*Power software. jeehp. (2021) 18:17–0. doi: 10.3352/jeehp.2021.18.17
29. Xiong X, Demianczuk NN, Saunders LD, Wang F-L, and Fraser WD. Impact of preeclampsia and gestational hypertension on birth weight by gestational age. Am J Epidemiol. (2002) 155:203–9. doi: 10.1093/aje/155.3.203
30. Zhang T, Tian M, Zhang P, Du L, Ma X, Zhang Y, et al. Risk of adverse pregnancy outcomes in pregnant women with gestational diabetes mellitus by age: a multicentric cohort study in Hebei, China. Sci Rep. (2024) 14:807. doi: 10.1038/s41598-023-49916-2
31. Lisonkova S, Sheps SB, Janssen PA, Lee SK, and Dahlgren L. Effect of older maternal age on birth outcomes in twin pregnancies: a population-based study. J Perinatology. (2011) 31:85–91. doi: 10.1038/jp.2010.114
32. Yang M, Xiao L-L, and Wang J-M. Association between maternal age and adverse pregnancy outcome in twin pregnancy. Zhongguo dang dai er ke za zhi = Chin J Contemp Pediatr. (2020) 22:238–44. doi: 10.7499/j.issn.1008-8830.2020.03.011
33. He Y and Wu NJD. Research progress on gestational diabetes mellitus and endothelial dysfunction markers. Diabet Metab Synd Ob. (2021) 14:983. doi: 10.2147/DMSO.S295737
34. Fulop T, Larbi A, and Douziech N. Insulin receptor and ageing. Pathologie Biologie. (2003) 51:574–80. doi: 10.1016/j.patbio.2003.09.007
35. Szoke E, Shrayyef MZ, Messing S, Woerle HJ, Van Haeften TW, Meyer C, et al. Effect of aging on glucose homeostasis: accelerated deterioration of β-cell function in individuals with impaired glucose tolerance. Diabetes Care. (2008) 31:539–43. doi: 10.2337/dc07-1443
36. Williams MA and Mittendorf R. Increasing maternal age as a determinant of placenta previa. More important than increasing parity? J Reprod Med. (1993) 38:425–8.
37. Taddei S, Virdis A, Ghiadoni L, Versari D, and Salvetti A. Endothelium, aging, and hypertension. Curr Hypertens Rep. (2006) 8:84–9. doi: 10.1007/s11906-006-0045-4
38. Norwitz ER and Caughey AB. Progesterone supplementation and the prevention of preterm birth. Rev Obstetrics Gynecology. (2011) 4(2):60–72. doi: 10.3909/riog0163
39. Reinders P, Zoellner Y, and Schneider U. Real-world evaluation of adverse pregnancy outcomes in women with gestational diabetes mellitus in the German health care system. Primary Care Diabetes. (2020) 14:633–8. doi: 10.1016/j.pcd.2020.04.009
40. Negrato CA, Jovanovic L, Tambascia MA, Geloneze B, Dias A, IdMP C, et al. Association between insulin resistance, glucose intolerance, and hypertension in pregnancy. Metab Syndrome Related Disord. (2008) 7:53–9. doi: 10.1089/met.2008.0043
41. Deng L, Ning B, and Yang H. Association between gestational diabetes mellitus and adverse obstetric outcomes among women with advanced maternal age: A retrospective cohort study. Medicine. (2022) 101(40):e30588. doi: 10.1097/MD.0000000000030588
42. Lai FY, Johnson JA, Dover D, and Kaul P. Outcomes of singleton and twin pregnancies complicated by preplicatedv diabetes and gestational diabetes: A populationlve008 study in A lberta, C anada, 2005–11. J Diabetes. (2016) 8:45–55. doi: 10.1111/1753-0407.12255
43. Domanski G, Lange AE, Ittermann T, Allenberg H, Spoo RA, Zygmunt M, et al. Evaluation of neonatal and maternal morbidity in mothers with gestational diabetes: a population-based study. BMC Pregnancy Childbirth. (2018) 18:367. doi: 10.1186/s12884-018-2005-9
44. Neiger RAN. Fetal macrosomia in the diabetic patient. Clin Obstetrics Gynecology. (1992) 35(1):138-50. doi: 10.1097/00003081-199203000-00019
45. Hiersch L, Berger H, Okby R, Ray JG, Geary M, McDonald SD, et al. Gestational diabetes mellitus is associated with adverse outcomes in twin pregnancies. Am J Obstetrics Gynecology. (2019) 220:102.e1–.e8. doi: 10.1016/j.ajog.2018.10.027
46. Zhang Z, Mei L, Li L, Xiao J, Wu X, and Yuan Y. Maternal and neonatal outcomes of twin pregnancies complicated by gestational diabetes mellitus. Endocrine. (2024) 84:388–98. doi: 10.1007/s12020-023-03588-0
47. Fox NS, Rebarber A, Dunham SM, and Saltzman DH. Outcomes of multiple gestations with advanced maternal age. J Maternal-Fetal Neonatal Med. (2009) 22:593–6. doi: 10.1080/14767050902801819
48. McLennan AS, Gyamfi-Bannerman C, Ananth CV, Wright JD, Siddiq Z, D'Alton ME, et al. The role of maternal age in twin pregnancy outcomes. Am J Obstetrics Gynecology. (2017) 217:80.e1–.e8. doi: 10.1016/j.ajog.2017.03.002
Keywords: singleton, twins, gestational diabetes mellitus, advanced maternal age, adverse outcomes
Citation: Nawsherwan, Shen H-t, Haq IU, Nabi G, Naeem S, Xu J-l and Ni X (2025) Secular trend of gestational diabetes mellitus and its interaction effect with advanced maternal age on adverse maternal-perinatal outcomes among primiparous singleton and twin pregnancies in Hubei, China (2011-2019). Front. Endocrinol. 16:1439592. doi: 10.3389/fendo.2025.1439592
Received: 28 May 2024; Accepted: 18 July 2025;
Published: 02 September 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Sumaira Mubarik, University of Groningen, NetherlandsPatricia Canto, National Autonomous University of Mexico, Mexico
Copyright © 2025 Nawsherwan, Shen, Haq, Nabi, Naeem, Xu and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie-lian Xu, ODU0NDU1NDE4QHFxLmNvbQ==; Xiaoqiu Ni, eHFpdW5pd211QDE2My5jb20=
†These authors have contributed equally to this work
 Nawsherwan
Nawsherwan Hong-tao Shen2†
Hong-tao Shen2† Ijaz Ul Haq
Ijaz Ul Haq