- 1Shanghai Clinical Research Center of Bone Disease, Department of Osteoporosis and Bone Diseases, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Endocrinology, Punan Hospital of Pudong New District, Shanghai, China
- 3Clinical Research Center, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Purpose: Optimal dosing of denosumab in osteogenesis imperfecta (OI) remains undefined. This prospective cohort study evaluated the 12-month efficacy and safety of denosumab in OI patients, with a historical control study with alendronate.
Materials and methods: Eight pediatric patients (1 mg/kg every 3 months; ≤60 mg/dose) and ten adults (60 mg every 6 months) received denosumab. Outcomes included lumbar spine (LS) and femoral neck (FN) bone mineral density (BMD), bone turnover markers (BTMs), vertebral compression fractures (assessed via AI-assisted Genant grading [AI_OVF_SH system]), fracture incidence, height velocity and adverse events. Historical controls (n=25 alendronate-treated OI patients) were analyzed for comparative efficacy. Sensitivity analyses excluded female pediatric participants (n=4) and peri-/post-menopausal adults (n=4) to assess hormonal confounding.
Results: Pediatric denosumab recipients exhibited significant LS-BMD (+30.3%, P<0.001) and FN-BMD gains (+38.7%, P=0.001) versus baseline, whereas adults showed non-significant increases (LS: +2.6%, P=0.100; FN: +4.4%, P=0.051). Sensitivity analyses revealed attenuated BTMs suppression in adults after excluding peri-/post-menopausal women (only ALP decreased by 27.9%, P=0.028). Rebound hypercalcemia occurred in 62.5% (5/8) of children, peaking at 2.93 mmol/L. Compared to alendronate, denosumab demonstrated comparable BMD improvements and fracture reduction (P>0.050) but superior pediatric height gain (+5.8% vs. +2.5%, P=0.004). Vertebral area loss decreased significantly with denosumab (-14.6%, P=0.029), unlike alendronate (-8.8%, P=0.296). Adverse events were more frequent with denosumab in children (hypercalcemia: 62.5% vs. 0%, P=0.002).
Conclusion: Denosumab demonstrates non-inferior efficacy to alendronate for BMD improvement in OI, with heightened vertebral remodeling and pediatric height gains. However, its overshoot phenomenon in children (rebound hypercalcemia) and hormone-dependent efficacy in adults necessitate risk-stratified use. Age and menopausal status considerations are critical for optimizing denosumab therapy in OI.
Clinical trial registration: https://www.chictr.org.cn/bin/project/edit?pid=184231, identifier ChiCTR2300074207.
1 Introduction
Osteogenesis imperfecta (OI), a monogenic disorder of type I collagen metabolism, is characterized by recurrent fragility fractures, low bone mass, and variable extraskeletal manifestations including dentinogenesis imperfecta, blue sclerae, and joint hyperlaxity (1, 2). Current therapeutic strategies for OI, predominantly adapted from osteoporosis treatments, rely heavily on bisphosphonates (BPs) for their anti-resorptive effects (3). While BPs consistently improve bone mineral density (BMD), their efficacy in fracture risk reduction remains equivocal (4).
Denosumab, a monoclonal antibody targeting RANKL, disrupts osteoclastogenesis through competitive inhibition of the RANKL-RANK pathway (5, 6). In postmenopausal osteoporosis, denosumab demonstrates sustained BMD gains without plateau effects, outperforming BPs in long-term outcomes (7, 8). However, its transient pharmacodynamics—marked by rapid bone turnover markers (BTMs) suppression followed by rebound hyperabsorption upon discontinuation—raise unique safety concerns, particularly in pediatric populations with inherently elevated bone turnover (9, 10).
Emerging evidence supports denosumab’s utility in OI (11–17), but optimal dosing regimens and comparative efficacy against alendronate remain undefined. This 12-month prospective cohort study evaluates denosumab’s safety and efficacy in pediatric and adult OI patients, utilizing a historical alendronate control group to inform clinical decision-making.
2 Materials and methods
2.1 Study population
Inclusion required confirmed OI diagnosis per Sillence criteria (18): 1) With fracture family history: ≥1 fragility fracture + lumbar/hip BMD Z-score ≤-1; 2) Without fracture family history: ≥1 fragility fracture + ≥1 extraskeletal feature (blue sclerae, dentinogenesis imperfecta, hearing loss, ligamentous laxity). Exclusion criteria encompassed other metabolic or hereditary bone diseases (hyper/hypothyroidism, Paget’s disease, hypophosphatemic rickets/osteomalacia et al.), chronic organ dysfunction, malignancy, glucocorticoid use, pregnancy, or lactation.
2.2 Study design
Eligible participants were classified into pediatric (<18 years) or adult groups. A prospective cohort of OI patients was established at the Department of Osteoporosis and Bone Diseases of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, with pediatric and adult subgroups. Historical controls (alendronate-treated patients with ≥12 months of follow-up) were selected from institutional databases using identical inclusion/exclusion criteria as the denosumab cohort.
Pediatric denosumab (Prolia®, Amgen Inc., Thousand Oaks, CA) dosing was 1 mg/kg subcutaneously every 3 months (max 60 mg/dose), while adults received 60 mg every 6 months. Alendronate (Fosamax, Merck Sharp & Dohme, USA) was administered at 70 mg/week. All participants received calcium (300–600 mg/day) and vitamin D (≥400 IU/day).
The primary efficacy endpoints were the changes in BMD and serum levels of BTMs during treatment. Secondary endpoints were the incidence of new fractures, area loss of vertebra and height velocity.
2.3 Measurements
Anthropometric measurements (height, weight) were obtained annually. For non-ambulatory patients, supine length was recorded; limb length discrepancies were addressed by measuring the longer extremity. Pediatric heights were converted to SDS using Chinese reference data (18). The body mass index (BMI) was calculated as weight/height² (kg/m²).
Lumbar spine (LS) and femoral neck (FN) BMD were assessed via dual-energy X-ray absorptiometry (DXA; Lunar/Hologic) at baseline and 12 months (19). Daily phantom calibrations ensured quality control (CV: Lunar LS 1.39%, FN 2.22%; Hologic LS 0.9%, FN 0.08%). Scans with surgical implants or deformities were excluded. All measurements used consistent operators/devices, with Z-scores derived from age-sex references (20–22).
Thoracolumbar radiographs (T4-L4) were analyzed using the AI_OVF_SH system, which demonstrated 96.85% fracture detection accuracy (23). Vertebral compression fractures were graded by area loss: Grade 1 (10-20%), Grade 2 (20-40%), Grade 3 (>40%). Scoliosis was defined as Cobb angle >10° (24).
Fasting morning blood samples collected every 3–6 months were analyzed on Hitachi 7600-020 (calcium(Ca, reference range 2.08-2.60mmol/L), phosphorus (P)) and Roche Cobas 6000 (C-terminal telopeptide of type 1 collagen (CTX), osteocalcin (OC), 25-hydroxyvitamin D (25OHD, reference range ≥20 ng/mL), and intact parathyroid hormone (PTH, reference range 15.00-65.00 pg/mL)) platforms. P, CTX, and OC were matched to age adapted reference data (25, 26). Rebound hypercalcemia was defined as serum calcium >2.60 mmol/L without secondary causes.
2.4 Treatment safety assessment
Adverse events (AEs) were monitored at each visit, including incident fractures. Serious AEs (SAEs) - cellulitis/erysipelas, osteonecrosis of the jaw, atypical femoral fractures, delayed fracture healing, and cardiac disorders - required clinical and radiographic confirmation. Other AEs (hypocalcemia, hypophosphatemia, hypercalcemia, arthralgia, myalgia) were assessed via clinical and biochemical evaluation.
2.5 Statistical analysis
Statistical analyses were performed using SPSS 26.0. Continuous variables were assessed for normality via Kolmogorov-Smirnov test and homogeneity of variance via Levene’s test. Normally distributed continuous variables were expressed as mean ± standard deviation (SD), while non-normally distributed measures were expressed as median (Interquartile range, IQR); categorical variables were expressed as frequencies (%). An independent sample t-test was used for group comparisons of normally distributed data, nonnormally distributed data were compared between groups using nonparametric tests, and rates were compared using chi-square test and Fisher exact test. Within-group comparisons used paired t-tests or Wilcoxon signed-rank tests. Analysis of covariance (ANCOVA) adjusted for age in between-drug analyses. Analyses included all participants receiving ≥1 denosumab dose with baseline assessment (modified intention-to-treat principle). Missing data were addressed via the last observation carried forward. Statistical significance was set at α=0.05 (two-tailed).
2.6 Ethical statement
This study was registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2300074207). All procedures were conducted in accordance with the ethical standards of the institutional and national research committees and the 2013 revision of the Declaration of Helsinki. The Ethics Committee of Shanghai Sixth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine approved the study. Written informed consent was obtained from patients or legal guardians of children younger than 18.
3 Results
3.1 Baseline characteristics
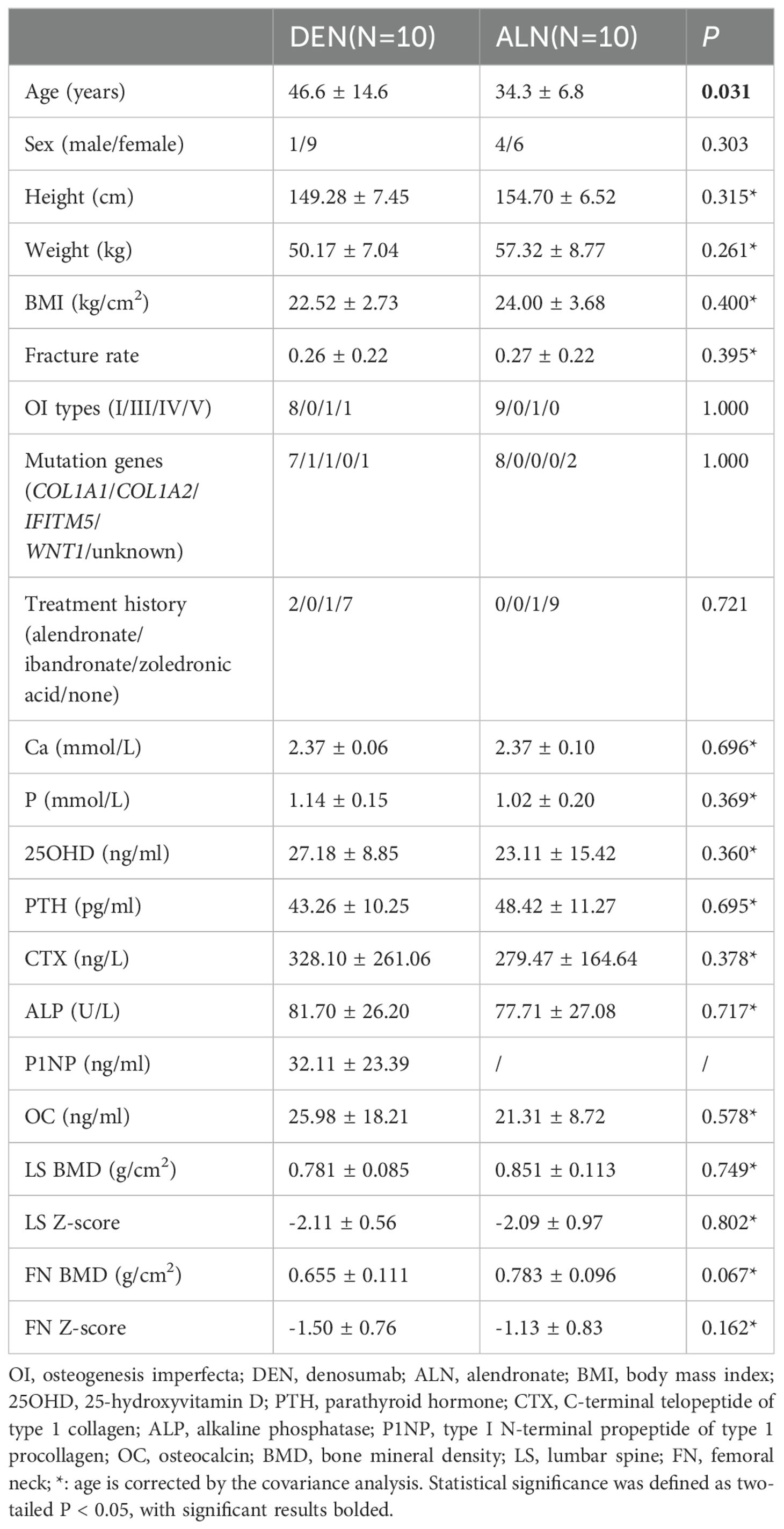
The study included 18 denosumab-treated patients (8 pediatrics, 10 adults) and 25 historical alendronate controls (15 pediatrics, 10 adults) (Figure 1). Baseline demographics and clinical features were comparable between groups (Tables 1, 2), except for prior treatment history in pediatric patients (P=0.002) and age in adults (P=0.031). A total of 8 patients had a medication treatment history to increase BMD in denosumab cohort, including 5 children and 3 adults (Supplementary Figure 1). Treatment switching rationale included suboptimal BMD response (n=5), new fractures (n=2), or alendronate intolerance (n=1). All alendronate patients were treatment-naïve except one adult (36.9 years) with prior zoledronic acid exposure. All patients underwent genetic testing for OI-associated variants (COL1A1, COL1A2, IFITM5, WNT1) and mutations were identified in 40 patients (Supplementary Table 1).
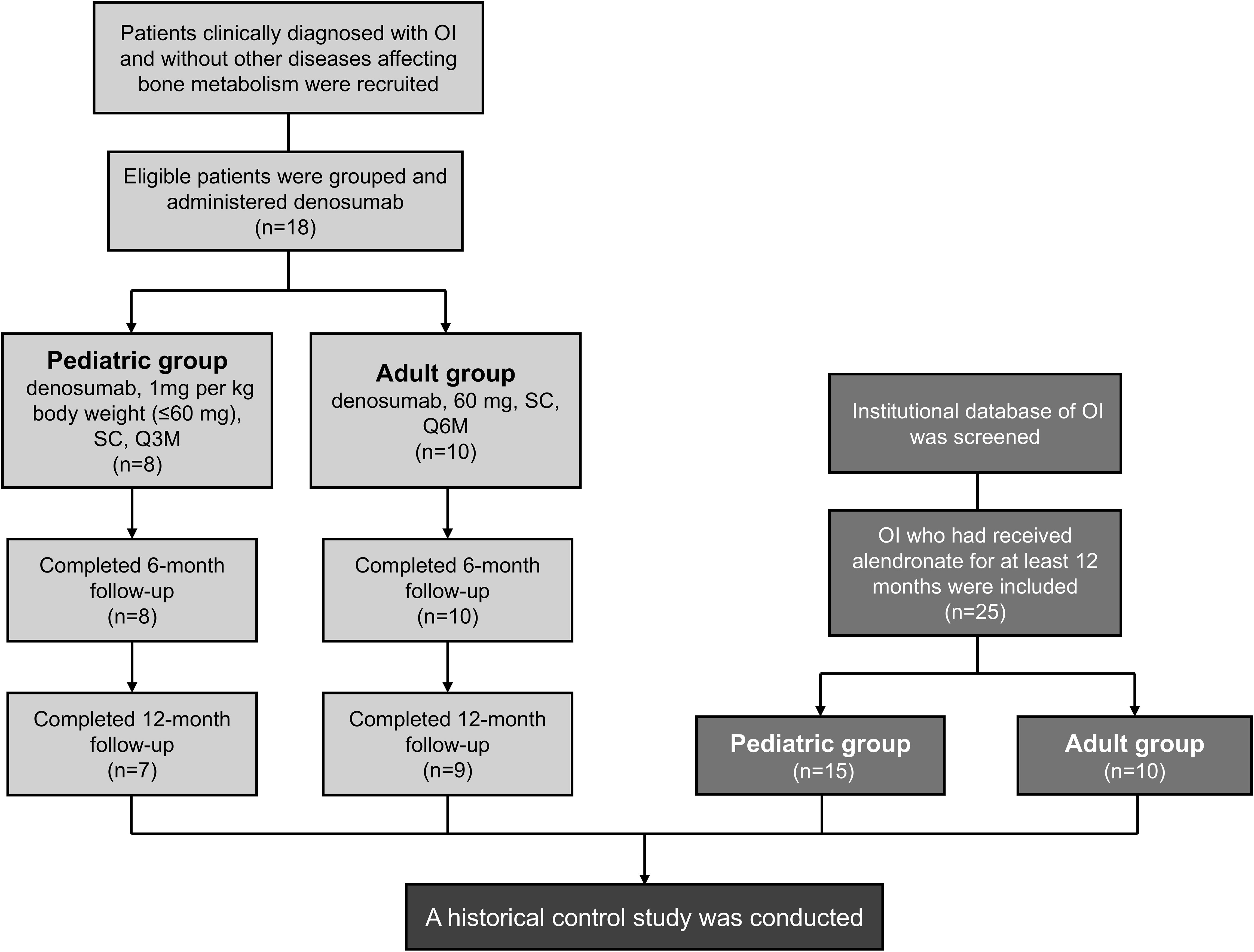
Figure 1. Study flowchart. OI, osteogenesis imperfecta; SC, subcutaneous injection; Q3M, every 3 months; Q6M, every 6 months. Two discontinued at 6 months because of transportation barriers.
3.2 Primary outcomes
3.2.1 Denosumab cohort
7/8 pediatric (87.5%) and 9/10 adult (90%) patients completed 12-month follow-up. Two discontinued at 6 months because of transportation barriers.
After 12 months of denosumab therapy, significant BMD increases at LS (+30.3%, P<0.001) and FN (+38.7%, P=0.001) were observed in pediatric group (Figures 2A, B). CTX levels showed transient suppression (43.5% reduction at 3 months and 32.4% at 6 months) with a rebound to baseline by 9 months (Figure 3A). OC levels exhibited persistent suppression in the whole period (Figure 3B). In the adult group, denosumab treatment led to modest BMD gains (LS: +2.6%, P=0.100; FN: +4.4%, P=0.051; Figures 2C, D) with sustained CTX suppression (48.7% reduction at 6 months and 49.9% at 12 months, P<0.050; Figure 3C).
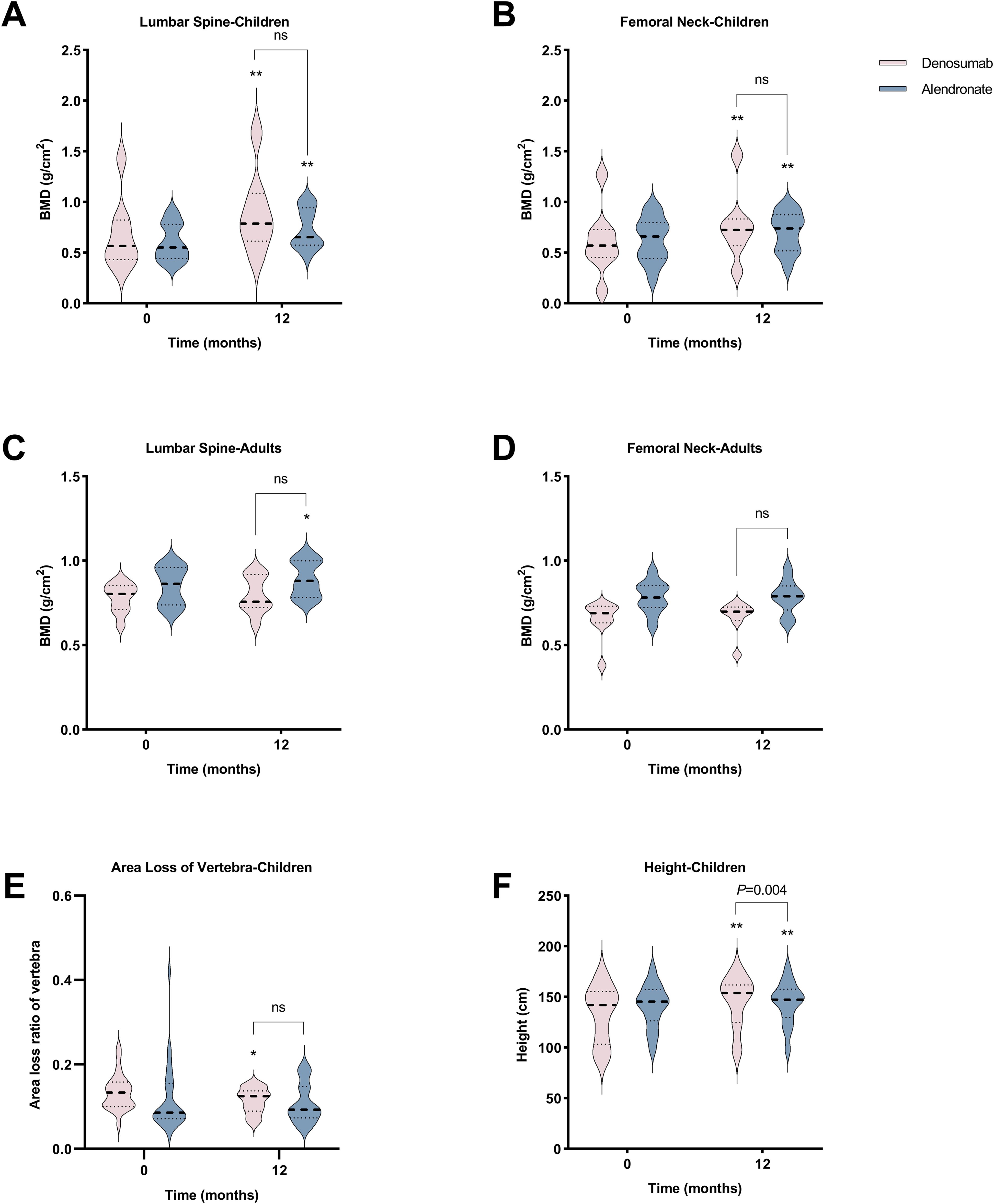
Figure 2. Comparative effects of denosumab and alendronate on bone parameters. (A-D) Longitudinal changes in lumbar spine (LS) and femoral neck (FN) bone mineral density BMD in pediatric and adult cohorts. (E) Vertebral area loss ratio progression in pediatric patients. (F) Height velocity in pediatric cohort. Data are presented as mean ± SD. *P<0.05, **P<0.01 vs baseline; ns, no significant intergroup differences (denosumab vs alendronate).
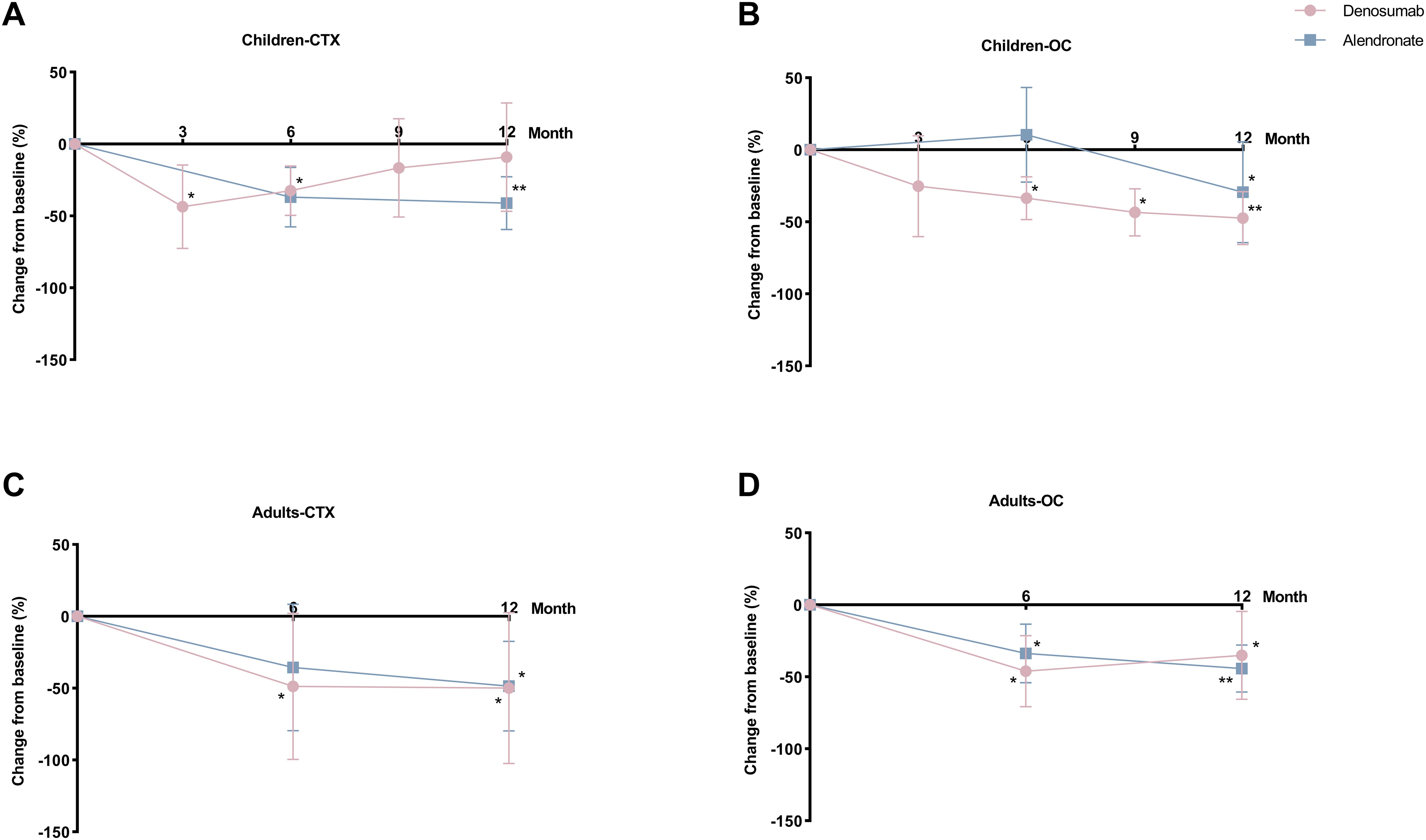
Figure 3. Percent changes in bone turnover markers (BTMs) with denosumab and alendronate. (A, B) Serum C-terminal telopeptide of type 1 collagen (CTX) and osteocalcin (OC) dynamics in pediatric patients. (C, D) Corresponding BTMs changes in adult patients. Data expressed as percentage change from baseline. *P<0.05, **P<0.01 vs baseline.
3.2.2 Alendronate cohort
In the pediatric group, 12-month alendronate treatment significantly increased BMD (LS: +24.0%, P<0.001; FN: +15.7%, P<0.001; Figures 2A, B), with CTX levels declining steadily (P<0.050; Figure 3A). In adult alendronate recipients, a 5.1% LS BMD gain (P=0.010) and 1.6% FN BMD improvement (P=0.051; Figures 2C, D) were accompanied by sustained reductions in serum CTX and OC levels throughout the 12-month treatment period (Figures 3C, D).
3.2.3 Comparative analysis between denosumab and alendronate
No significant differences in BMD changes or BTMs suppression were observed between denosumab and alendronate groups (Figures 2, 3).
3.3 Secondary outcomes
Two pediatric denosumab recipients experienced new fractures (rate: 0.83/year), while no fractures occurred in adults. Alendronate-treated pediatric patients had a similar fracture rate (0.25/year, P>0.050). Denosumab significantly reduced vertebral area loss in pediatric patients (from 13.4% to 11.5%, P=0.029) (Figure 2E), with two cases showing fracture remodeling (Supplementary Figure 2). Alendronate also promoted remodeling but without significant area loss reduction (from 11.8% to 10.8%, P=0.296). Denosumab-treated pediatric patients showed greater height increase compared to alendronate recipients (+5.8% vs +2.5%, P=0.004) (Figure 2F).
3.4 Treatment safety assessment
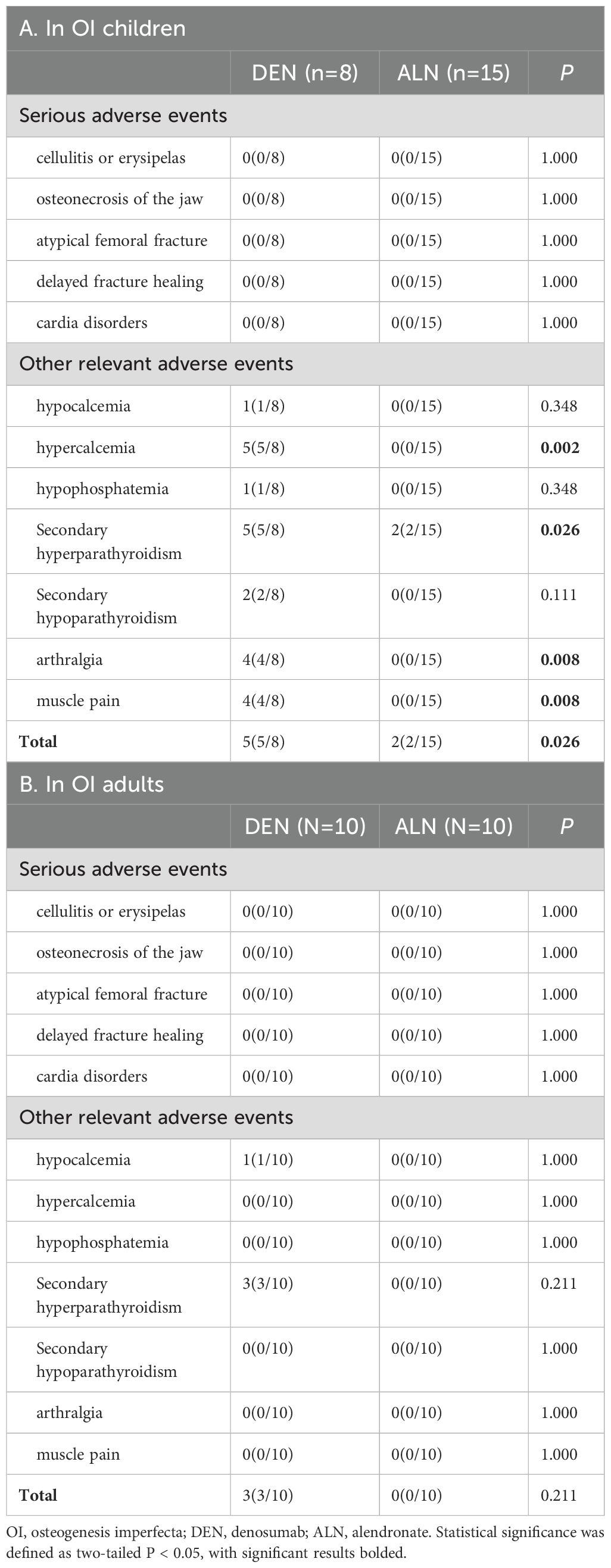
Treatment-emergent AEs are detailed in Table 3. No SAEs occurred in either cohort during the 12-month study period. In denosumab cohort, five children (5/16, 31.3%) developed mild hypercalcemia (peak serum Ca: 2.93 mmol/L), consistently occurring 3 months post-dosing (Figure 4). These episodes coincided with rebound increases in CTX to near-baseline levels. Compared to alendronate, denosumab pediatric recipients demonstrated significantly higher risks of hypercalcemia (62.5% vs. 13.3%; P<0.002), secondary hyperparathyroidism (62.5% vs. 13.3%; P<0.026), and musculoskeletal symptoms (37.5% vs. 0%; P<0.008). Both therapies exhibited favorable safety profiles in adults.
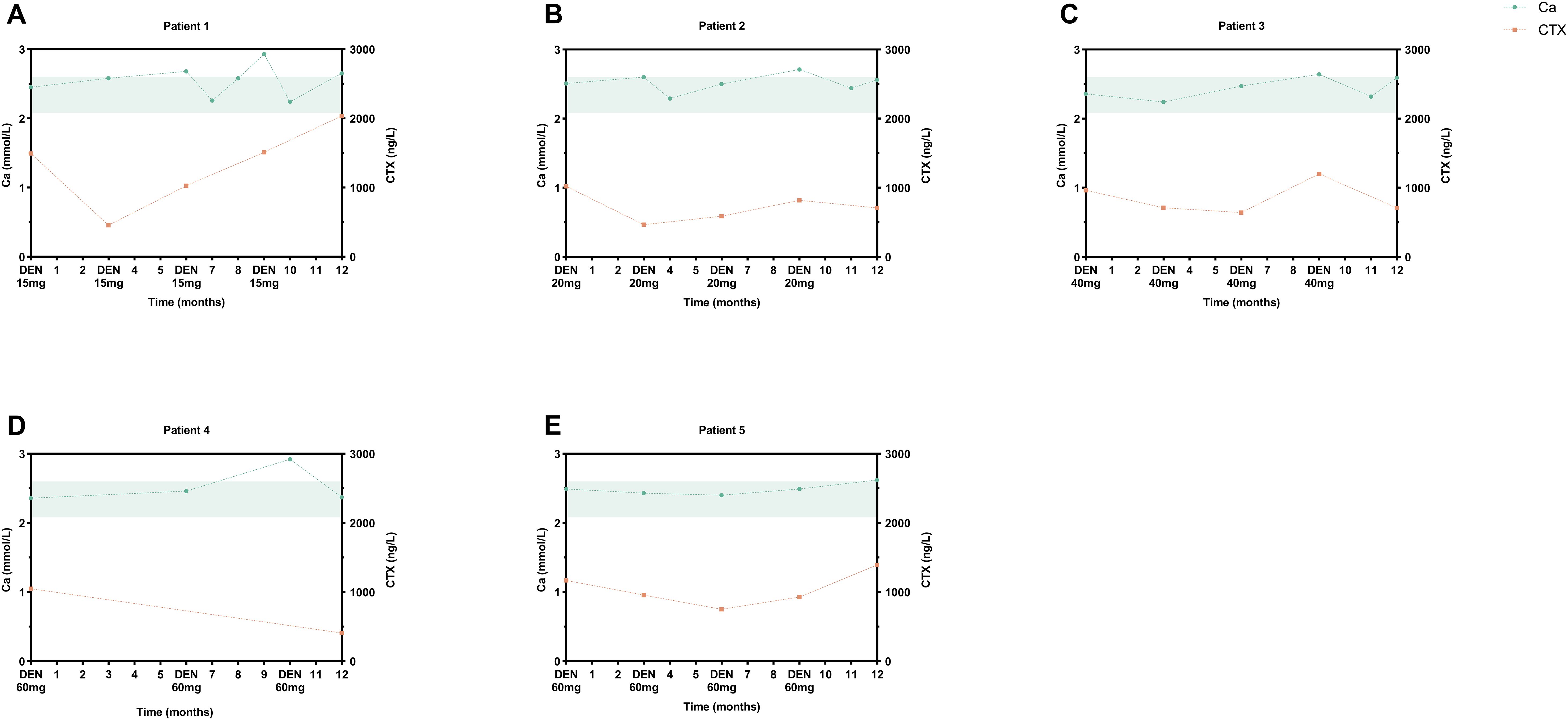
Figure 4. Hypercalcemia events in pediatric denosumab recipients. Serial serum calcium (reference range: 2.08-2.60 mmol/L, shaded green) and CTX profiles of five cases: (A) 2-year-old male (15 mg), transient hypercalcemia at M6/9/12; (B) 5.9-year-old male (20 mg), M9 elevation; (C) 12.3-year-old male (40 mg), M10 event; (D) 13.9-year-old male (60 mg), M10 episode; (E) 11-year-old male (60 mg), M12 occurrence. DEN, denosumab.
3.5 Sensitivity analysis
Notably, the study cohort exhibited marked sex disparities: all pediatric denosumab recipients were male (8), while 90% (9/10) of adult denosumab users were female, including three peri-/post-menopausal individuals. To address potential confounding from hormonal status and sex-specific effects, sensitivity analyses were performed: 1) Exclusion of female pediatric participants: Removal of 4 girls from the alendronate cohort (Supplementary Table 2). 2) Exclusion of peri-/post-menopausal adults: Omission of 3 denosumab-treated and 1 alendronate-treated women (Supplementary Table 3). After excluding peri-/post-menopausal females, denosumab’s effect on BTMs was attenuated, with only alkaline phosphatase (ALP) demonstrating a significant 27.9% reduction post-treatment (P=0.028 vs. baseline). Results remained stable in the denosumab pediatric subgroup. Comparative efficacy between denosumab and alendronate remained robust across all sensitivity analyses (P>0.050).
4 Discussion
This study provides the first head-to-head comparison of denosumab and alendronate in OI. Pediatric denosumab recipients exhibited marked BMD gains and vertebral remodeling, consistent with prior reports (11–17). However, the high incidence of rebound hypercalcemia (62.5%) underscores metabolic instability in children.
Since Semler et al. (2012) first reported safe BTMs suppression in four pediatric OI-VI patients with quarterly 1 mg/kg denosumab (27), subsequent studies (11–15, 28, 29) have confirmed its BMD-enhancing effects but identified pediatric-specific rebound risks. Our protocol adopted weight-based pediatric dosing (1 mg/kg/Q3M, ≤60 mg) versus fixed adult regimens (60 mg/Q6M). At 12 months, pediatric LS/FN BMD increased significantly versus non-significant adult changes, potentially attributable to ongoing skeletal growth (30). Pharmacodynamic analyses reveal accelerated drug metabolism in children: BTMs rebound to baseline within 6–8 weeks (27, 31), correlating with 30-50% higher baseline BTMs versus adults (32). Notably, 31% (13/42) pediatric patients in a 6-month interval trial developed rebound hypercalcemia (17), while our 3-month regimen still showed pre-dose CTX recovery. These findings suggest current intervals may inadequately suppress resorption, warranting exploration of compressed schedules (e.g., ≤10 weeks) as proposed by Semler (29).
Fracture patterns in denosumab-treated OI patients demonstrate age-related disparities. While adult fracture rates decreased versus baseline, two pediatric cases developed new fractures - consistent with established epidemiology showing fracture burden predominantly in pediatric populations (33). This inherent limitation (small sample, non-randomized design) precludes definitive fracture risk assessment in our cohort.
Overshoot phenomena remain a critical safety concern. Pediatric patients exhibited CTX rebound by month 3 post-dose, with 5 cases developing mild hypercalcemia (2 with hypoparathyroidism, 3 with musculoskeletal symptoms). A review of denosumab in pediatric bone diseases summarized that 45 children treated with denosumab for various conditions developed “severe hypercalcemia” in 5 cases after discontinuation, and mild or moderate asymptomatic hypercalcemia was reported in 6 of the 18 children with OI treated with denosumab (34). Younger age (<5 years) and elevated resorption markers correlate with risk (17). Mechanistically, rapid CTX rebound drives osteoclast-mediated calcium release (35), occurring earlier in children (3 vs. 6–10 months in adults) due to accelerated bone turnover (36). These findings align with MHRA’s 2022 warning against pediatric denosumab use (37).
Comparative analyses show denosumab’s BMD superiority over BPs in osteoporosis (38–40), though fracture reduction equivalence persists. Our historical controls revealed comparable BMD and BTMs responses between denosumab and alendronate, but denosumab demonstrated superior vertebral remodeling and greater height velocity despite preclinical growth concerns (34). Notably, denosumab carried higher hypercalcemia risk, attributable to its potent resorption inhibition (36). While both agents enable vertebral reshaping in OI (17, 41–43), denosumab’s unique pharmacodynamics warrant careful risk-benefit evaluation in pediatric use.
Our sensitivity analyses revealed that the BTMs-lowering effect of denosumab in adults was predominantly driven by peri-/post-menopausal women. This aligns with the known acceleration of bone turnover during estrogen withdrawal, which may amplify denosumab’s anti-resorptive potency (44). The attenuation of BTMs suppression after excluding these individuals underscores the importance of hormonal milieu in modulating therapeutic responses (45). Our findings highlight the need for stratified therapeutic approaches: in postmenopausal OI women, denosumab may offer enhanced anti-resorptive efficacy, whereas pediatric patients may require closer monitoring of BTMs to detect early rebound effects.
This pioneering real-world analysis provides the first comparative assessment of denosumab versus alendronate in OI patients across pediatric and adult populations. Key limitations merit consideration: 1) 12-month duration precluded evaluation of long-term risks (e.g., ONJ, atypical fractures); 2) Underpowered sample limits fracture risk assessment validity, particularly in pediatric subgroups; 3) Prior BP exposure in 35% of denosumab recipients may confound therapeutic comparisons; 4) Absence of serial renal ultrasounds prevented hypercalcemia-related calcification monitoring; 5) Historical control design introduces residual confounding (e.g., age/sex disparities)., however, such limitations are unavoidable in rare disease contexts.
5 Conclusions
Denosumab demonstrates potential for improving BMD and vertebral structure in OI, particularly in children. However, its association with rebound hypercalcemia and metabolic instability necessitates caution. Alendronate remains a safer alternative for pediatric OI until further evidence establishes denosumab’s risk-benefit balance. Clinicians should prioritize individualized treatment plans considering age and menopausal status and vigilant monitoring for patients on denosumab.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Shanghai Sixth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YM: Data curation, Formal analysis, Investigation, Writing – original draft. SC: Data curation, Investigation, Writing – review & editing. YJ: Data curation, Investigation, Writing – review & editing. YT: Data curation, Investigation, Writing – review & editing. LS: Formal analysis, Methodology, Writing – review & editing. JG: Conceptualization, Funding acquisition, Writing – review & editing. CW: Conceptualization, Writing – review & editing. ZZ: Conceptualization, Project administration, Writing – review & editing. HZ: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was founded by the National Natural Science Foundation of China (No. 81870618, No. 82270933).
Acknowledgments
We thank all OI patients and their families for their participation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1445093/full#supplementary-material
Supplementary Figure 1 | Prior treatment history of denosumab-treated OI patients. Pediatric cohort: P1 (14M) - 4-year alendronate (10-14y); P2 (11M) - single ibandronate dose (9y); P3 (11M) - 3-month alendronate (10y) with 6-month gap; P5 (12M) - ongoing alendronate (10y-). Adult cohort: P6 (68F) - 4-year alendronate (64-68y); P7 (33F) - 4-month alendronate; P8 (40F) - sequential alendronate (2y) and zoledronic acid (2y) (36-40y).
Supplementary Figure 2 | Radiographic evidence of vertebral remodeling. (A) Thoracolumbar series (13M): Pre-treatment vs 12-month DEN. (B) Lumbar progression (13.9M): Baseline to 4-year ALN to 1-year DEN. (C) 6-month ALN effects (12.7M). (D) 12-month ALN outcomes (10.9M). (E) Lower extremity cortical changes (15M): 24-month ALN comparison. Orange arrows denote vertebral remodeling sites/cortical thickening.
References
1. Jovanovic M, Guterman-Ram G, and Marini JC. Osteogenesis imperfecta: mechanisms and signaling pathways connecting classical and rare OI types. Endocr Rev. (2022) 43:61–90. doi: 10.1210/endrev/bnab017
2. Forlino A and Marini JC. Osteogenesis imperfecta. Lancet. (2016) 387:1657–71. doi: 10.1016/S0140-6736(15)00728-X
3. Liu W, Lee B, Nagamani SCS, Nicol L, Rauch F, Rush ET, et al. Approach to the Patient: Pharmacological therapies for fracture risk reduction in adults with osteogenesis imperfecta. J Clin Endocrinol Metab. (2023) 108(7):1787–96. doi: 10.1210/clinem/dgad035
4. Dwan K, Phillipi CA, Steiner RD, and Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev. (2016) 10:CD005088. doi: 10.1002/14651858.CD005088.pub4
5. McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. (2006) 354:821–31. doi: 10.1056/NEJMoa044459
6. Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. (2012) 11:401–19. doi: 10.1038/nrd3705
7. Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, de Gregorio LH, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. (2009) 24:153–61. doi: 10.1359/jbmr.0809010
8. Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. (2017) 5:513–23. doi: 10.1016/S2213-8587(17)30138-9
9. Li G, Jin Y, Levine MAH, Hoyer-Kuhn H, Ward L, and Adachi JD. Systematic review of the effect of denosumab on children with osteogenesis imperfecta showed inconsistent findings. Acta Paediatr. (2018) 107:534–7. doi: 10.1111/apa.2018.107.issue-3
10. Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, and Palermo A. Denosumab discontinuation and the rebound phenomenon: A narrative review. J Clin Med. (2021) 10(1):152. doi: 10.3390/jcm10010152
11. Hoyer-Kuhn H, Semler O, and Schoenau E. Effect of denosumab on the growing skeleton in osteogenesis imperfecta. J Clin Endocrinol Metab. (2014) 99:3954–5. doi: 10.1210/jc.2014-3072
12. Hoyer-Kuhn H, Franklin J, Allo G, Kron M, Netzer C, Eysel P, et al. Safety and efficacy of denosumab in children with osteogenesis imperfect–a first prospective trial. J Musculoskelet Neuronal Interact. (2016) 16:24–32.
13. Kobayashi T, Nakamura Y, Suzuki T, Yamaguchi T, Takeda R, Takagi M, et al. Efficacy and safety of denosumab therapy for osteogenesis imperfecta patients with osteoporosis-case series. J Clin Med. (2018) 109(7):1873–82. doi: 10.3390/jcm7120479
14. Hoyer-Kuhn H, Rehberg M, Netzer C, Schoenau E, and Semler O. Individualized treatment with denosumab in children with osteogenesis imperfecta - follow up of a trial cohort. Orphanet J Rare Dis. (2019) 14:219. doi: 10.1186/s13023-019-1197-z
15. Trejo P, Rauch F, and Ward L. Hypercalcemia and hypercalciuria during denosumab treatment in children with osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact. (2018) 18:76–80.
16. Lin X, Hu J, Zhou B, Wang X, Zhang Q, Jiang Y, et al. Efficacy and safety of denosumab versus zoledronic acid in OI adults: A prospective, open-label, randomized study. J Clin Endocrinol Metab. (2024) 109(7):1873–82. doi: 10.1210/clinem/dgae012
17. Liu J, Lin X, Sun L, Zhang Q, Jiang Y, Wang O, et al. Safety and efficacy of denosumab in children with osteogenesis imperfecta-the first prospective comparative study. J Clin Endocrinol Metab. (2024) 109(7):1827–36. doi: 10.1210/clinem/dgad732
18. Zong X-N and Li H. Construction of a new growth references for China based on urban Chinese children: comparison with the WHO growth standards. PloS One. (2013) 8:e59569. doi: 10.1371/journal.pone.0059569
19. Zhang H, He J-w, Gao G, Yue H, Yu J-b, Hu W-w, et al. Polymorphisms in the HOXD4 gene are not associated with peak bone mineral density in Chinese nuclear families. Acta Pharmacol Sin. (2010) 31:977–83. doi: 10.1038/aps.2010.91
20. Xu H, Zhao Z, Wang H, Ding M, Zhou A, Wang X, et al. Bone mineral density of the spine in 11,898 Chinese infants and young children: a cross-sectional study. PloS One. (2013) 8:e82098. doi: 10.1371/journal.pone.0082098
21. Khadilkar AV, Sanwalka NJ, Chiplonkar SA, Khadilkar VV, and Mughal MZ. Normative data and percentile curves for Dual Energy X-ray Absorptiometry in healthy Indian girls and boys aged 5–17 years. Bone. (2011) 48:810–9. doi: 10.1016/j.bone.2010.12.013
22. Zhang ZQ, Ho SC, Chen ZQ, Zhang CX, and Chen YM. Reference values of bone mineral density and prevalence of osteoporosis in Chinese adults. Osteoporos Int. (2014) 25:497–507. doi: 10.1007/s00198-013-2418-2
23. Shen L, Gao C, Hu S, Kang D, Zhang Z, Xia D, et al. Using artificial intelligence to diagnose osteoporotic vertebral fractures on plain radiographs. J Bone Miner Res. (2023) 38:1278–87. doi: 10.1002/jbmr.v38.9
24. Sato A, Ouellet J, Muneta T, Glorieux FH, and Rauch F. Scoliosis in osteogenesis imperfecta caused by COL1A1/COL1A2 mutations - genotype-phenotype correlations and effect of bisphosphonate treatment. Bone. (2016) 86:53–7. doi: 10.1016/j.bone.2016.02.018
25. Diemar SS, Lylloff L, Rønne MS, Møllehave LT, Heidemann M, Thuesen BH, et al. Reference intervals in Danish children and adolescents for bone turnover markers carboxy-terminal cross-linked telopeptide of type I collagen (β-CTX), pro-collagen type I N-terminal propeptide (PINP), osteocalcin (OC) and bone-specific alkaline phosphatase (bone ALP). Bone. (2021) 146:115879. doi: 10.1016/j.bone.2021.115879
26. Zhang C, Zhao Z, Sun Y, Xu L, JiaJue R, Cui L, et al. Clinical and genetic analysis in a large Chinese cohort of patients with X-linked hypophosphatemia. Bone. (2019) 121:212–20. doi: 10.1016/j.bone.2019.01.021
27. Semler O, Netzer C, Hoyer-Kuhn H, Becker J, Eysel P, and Schoenau E. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact. (2012) 12:183–8.
28. Uehara M, Nakamura Y, Takahashi J, Kamimura M, Ikegami S, Suzuki T, et al. Efficacy of denosumab for osteoporosis in three female patients with osteogenesis imperfecta. Tohoku J Exp Med. (2017) 242:115–20. doi: 10.1620/tjem.242.115
29. Hoyer-Kuhn H, Netzer C, Koerber F, Schoenau E, and Semler O. Two years’ experience with denosumab for children with osteogenesis imperfecta type VI. Orphanet J Rare Dis. (2014) 9:145. doi: 10.1186/s13023-014-0145-1
30. Stagi S, Cavalli L, Iurato C, Seminara S, Brandi ML, and de Martino M. Bone metabolism in children and adolescents: main characteristics of the determinants of peak bone mass. Clin cases Miner Bone Metab. (2013) 10:172–9.
31. Ward L, Bardai G, Moffatt P, Al-Jallad H, Trejo P, Glorieux FH, et al. Osteogenesis imperfecta type VI in individuals from northern Canada. Calcif Tissue Int. (2016) 98:566–72. doi: 10.1007/s00223-016-0110-1
32. Sato J, Hasegawa K, Tanaka H, and Morishima T. Urinary N-telopeptides of type I collagen in healthy children. Pediatr Int. (2010) 52:398–401. doi: 10.1111/j.1442-200X.2010.03086.x
33. Folkestad L, Hald JD, Ersbøll AK, Gram J, Hermann AP, Langdahl B, et al. Fracture rates and fracture sites in patients with osteogenesis imperfecta: A nationwide register-based cohort study. J Bone Miner Res. (2017) 32:125–34. doi: 10.1002/jbmr.2920
34. Boyce AM. Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep. (2017) 15:283–92. doi: 10.1007/s11914-017-0380-1
35. Anastasilakis AD, Makras P, Doulgeraki A, Polyzos SA, Guarnieri V, and Papapoulos SE. Denosumab for the treatment of primary pediatric osteoporosis. Osteoporos Int. (2021) 32(11):2377–81. doi: 10.1007/s00198-021-06002-5
36. Camponovo C, Aubry-Rozier B, Lamy O, and Gonzalez Rodriguez E. Hypercalcemia upon denosumab withdrawal in primary hyperparathyroidism: a case report and literature review. Osteoporos Int. (2020) 31:2485–91. doi: 10.1007/s00198-020-05676-7
37. Denosumab 60mg (Prolia): should not be used in patients under 18 years due to the risk of serious hypercalcaemia 2022 . Available online at: https://www.gov.uk/drug-safety-update/denosumab-60mg-prolia-should-not-be-used-in-patients-under-18-years-due-to-the-risk-of-serious-hypercalcaemia. (Accessed May 20, 2025).
38. Kang T, Park SY, Lee SH, Park JH, and Suh SW. Comparison of denosumab and zoledronic acid in postmenopausal women with osteoporosis: bone mineral density (BMD) and trabecular bone score (TBS). J Korean Med Sci. (2022) 37:e68. doi: 10.3346/jkms.2022.37.e68
39. Wu J, Zhang Q, Yan G, and Jin X. Denosumab compared to bisphosphonates to treat postmenopausal osteoporosis: a meta-analysis. J Orthop Surg Res. (2018) 13:194. doi: 10.1186/s13018-018-0865-3
40. Beaudoin C, Jean S, Bessette L, Ste-Marie LG, Moore L, and Brown JP. Denosumab compared to other treatments to prevent or treat osteoporosis in individuals at risk of fracture: a systematic review and meta-analysis. Osteoporos Int. (2016) 27:2835–44. doi: 10.1007/s00198-016-3607-6
41. Wang HD, Boyce AM, Tsai JY, Gafni RI, Farley FA, Kasa-Vubu JZ, et al. Effects of denosumab treatment and discontinuation on human growth plates. J Clin Endocrinol Metab. (2014) 99:891–7. doi: 10.1210/jc.2013-3081
42. Land C, Rauch F, Munns CF, Sahebjam S, and Glorieux FH. Vertebral morphometry in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate treatment. Bone. (2006) 39:901–6. doi: 10.1016/j.bone.2006.04.004
43. Semler O, Beccard R, Palmisano D, Demant A, Fricke O, Schoenau E, et al. Reshaping of vertebrae during treatment with neridronate or pamidronate in children with osteogenesis imperfecta. Horm Res Paediatr. (2011) 76:321–7. doi: 10.1159/000331128
44. Riggs BL, Khosla S, and Melton Iii LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. (2002) 23:279–302. doi: 10.1210/edrv.23.3.0465
Keywords: osteogenesis imperfecta, denosumab, alendronate, hypercalcemia, bone mineral density
Citation: Mei Y, Cai S, Jiang Y, Tian Y, Shen L, Gu J, Wang C, Zhang Z and Zhang H (2025) Denosumab in patients with osteogenesis imperfecta and a historical control study with alendronate. Front. Endocrinol. 16:1445093. doi: 10.3389/fendo.2025.1445093
Received: 06 June 2024; Accepted: 09 May 2025;
Published: 27 May 2025.
Edited by:
Alberto Falchetti, Grande Ospedale Metropolitano Niguarda, ItalyReviewed by:
Kamyar Asadipooya, University of Kentucky, United StatesEmanuela Galliera, University of Milan, Italy
Copyright © 2025 Mei, Cai, Jiang, Tian, Shen, Gu, Wang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Zhang, emhhbmdoYW96Y2xAMTYzLmNvbQ==; Zhenlin Zhang, emhhbmd6bEBzanR1LmVkdS5jbg==; Chun Wang, d2FuZ2NodW42NkBzanR1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Yazhao Mei
Yazhao Mei Shiya Cai1,2†
Shiya Cai1,2† Li Shen
Li Shen Jiemei Gu
Jiemei Gu Chun Wang
Chun Wang Zhenlin Zhang
Zhenlin Zhang Hao Zhang
Hao Zhang