- 1Department of Cardiovascular Medicine, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 2Department of Cardiovascular Medicine, Affiliated Hospital of Yan’an University, Yan’an, Shaanxi, China
Objective: Carotid atherosclerosis (CAS) is a significant factor contributing to cardiovascular events and poses a major public health concern. There are still many controversies about the association between serum bilirubin and CAS. This study aims to provide a systematic review and meta-analysis to examine the association between serum bilirubin levels and carotid atherosclerosis.
Methods: An electronic literature search was performed using PubMed, Web of Science and Embase up to December 2023. Articles were screened based on predefined inclusion criteria and assessed for risk of bias and quality of evidence utilizing the Newcastle-Ottawa Scale and GRADE tool. Pooled mean differences were calculated using a random effects model. Subgroup and meta-regression analyses were performed to identify potential sources of heterogeneity.
Results: Nine studies involving 7,023 participants were included in this meta-analysis. The results indicated that patients with carotid atherosclerosis exhibited lower levels of total bilirubin compared to those without (SMD -3.42, 95% CI [-5.18, -1.67]), with a statistically significant difference (z=-3.819, P<0.001). Moreover, a significant inverse association was found between total bilirubin levels and the risk of carotid atherosclerosis (OR 0.79, 95% CI [0.71, 0.88], P<0.001, I²=78.2%). However, substantial heterogeneity was observed (I²=98.0%, P<0.001). Subgroup and meta-regression analyses indicated that sample size and the severity of carotid atherosclerotic lesions might contribute to the heterogeneity observed across studies. The GRADE assessment was low.
Conclusion: Lower serum bilirubin levels are associated with an increased risk of carotid atherosclerosis. This meta-analysis offers new insights into the development of diagnostic biomarkers and therapeutic targets. Further prospective cohort studies are necessary to validate our conclusions.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023447199.
1 Introduction
Cardiovascular disease (CVD) is the leading cause of disability and premature mortality worldwide, imposing a substantial burden on individuals, families, and healthcare systems. According to the Global Burden of Cardiovascular Diseases and Risks Study 1990-2022, the age-standardized mortality rate for CVD has decreased by 34.9%. However, the total number of CVD-related deaths surged from 12.4 million in 1990 to 19.8 million in 2022 (1). This trend underscores the aging global population and the rising prevalence of cardiometabolic risk factors, including high systolic blood pressure, elevated low-density lipoprotein (LDL) cholesterol, increased body mass index (BMI), high fasting glucose, and renal insufficiency (2). Prolonged exposure to these cardiometabolic risk factors predisposes individuals to the formation and development of atherosclerotic plaques, facilitating the transition from subclinical to clinical stages (3).
Atherosclerosis (AS) is a chronic inflammatory disease primarily driven by lipid deposition, typically resulting in the narrowing of the vessel lumen and plaque formation (4). In cases of carotid artery stenosis, carotid intima-media thickness (CIMT) and plaque can be routinely assessed using non-invasive Doppler ultrasonography (5). In the global population aged 30–79 years in 2020, the prevalence of increased carotid intima-media thickness (≥1.0 mm) was estimated at 27.62%, affecting over one billion individuals, while the prevalence of carotid plaque was approximately 21.13%, and carotid stenosis was 1.50% (6).Thus, the considerable disease burden of CAS has prompted efforts toward effective preventive health strategies and early assessment.
Bilirubin, recognized as the terminal product of heme catabolism, has traditionally been viewed as potentially harmful, with elevated levels associated with liver dysfunction and neurological deficits (7, 8). However, emerging evidence indicates that bilirubin possesses significant anti-inflammatory, antioxidant, and neuroprotective properties when acting as a hormone (9, 10). It protects LDL from peroxidation and endothelial cells against oxidative damage, while also enhancing heme oxygenase activity and serum cholesterol solubility (11). Elevated serum bilirubin levels have been positively correlated with total serum antioxidant status and inversely correlated with pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, as well as other atherogenic factors, including ICAM, E-selectin, or VEGF in patient with atherosclerosis (12). Furthermore, bilirubin has demonstrated clinical significance in diagnosising and prognosticating oxidative stress-related diseases, including stroke (13), peripheral arterial disease (14), chronic kidney disease (15) and cancer (16), suggesting that regulating bilirubin levels may offer a promising a therapeutic strategy for these conditions.
The relationship between serum bilirubin levels and carotid atherosclerosis has garnered increasing scholarly attention, yielding conflicting results across studies. Consequently, we conducted a meta-analysis to thoroughly investigate the associations between serum bilirubin levels and carotid atherosclerosis, specifically focusing on carotid intima-media thickness (CIMT), atherosclerotic plaque and stenosis. This study may provide a foundation for understanding the implications of serum bilirubin levels in CAS and offer new insights for early diagnosis and potential therapeutic targets.
2 Methods
2.1 Search strategy
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline (17). A comprehensive electronic literature search was performed to identify eligible articles from PubMed, Web of Science and Embase, covering the period from database inception to December 2023. The search utilized the following terms and keywords: (“Bilirubin”[Mesh] OR “bilirubinaemia” OR “hyperbilirubinemia” AND (“Carotid Artery Diseases”[Mesh]) OR (“Carotid Intima-Media Thickness”[Mesh] OR “Carotid Atherosclerosis” OR “carotid plaque”). The specific search strategy is provided in the Supplementary Table S1. The search was not limited by language. The protocol of this meta-analysis has been registered in PROSPERO (CRD42024498887).
2.2 Study selection
Inclusion criteria for included studies are as follows: (1) observational studies(cohort, case-control, and cross-sectional studies); (2)the relationship between circulating total bilirubin level and carotid atherosclerosis was investigated; (3) studies reporting mean values with standard errors or odds ratio (OR) with the corresponding 95% confidence intervals (CIs) for the risk of carotid atherosclerosis or other adverse clinical outcomes; and (4) examination of carotid atherosclerosis based on ultrasonography. Exclusion criteria are as follows: (1) the study did not report the relationship between total bilirubin level and carotid atherosclerosis; (2) the study did not provide complete effect estimates or data were not available; (3) articles not published in English, and (4) the study did not involve human subjects. Two investigators (EBY/YL) independently assessed the eligibility of all studies for inclusion. If conflicts arose during the process, a third investigator (TH) adjudicated the contradiction through discussion.
2.3 Data extraction and quality assessment
Data extraction was managed in Microsoft Excel. Two investigators (EBY and YL) independently extracted the following information from the included articles, with any disagreements resolved through discussion with a third investigator (TH): (1) Study details, including first author, publication year, country, sample size, and design; (2) Participant characteristics, including number, age, sex, and bilirubin levels; (3) Evaluation/indicator of carotid atherosclerosis.
The methodological quality of each study was assessed independently by two investigators (EBY/YL) using the Newcastle-Ottawa Scale (NOS) (18). Each included study was rated on a scale of 1 to 9 points based on three criteria: (1) study selection criteria; (2) comparability of groups; and (3) evaluation of the outcome/exposure.
2.4 Certainty of evidence assessment
The GRADE tool was employed to evaluate the quality of the pooled outcomes derived from the effect estimates (19). The quality of evidence was classified into four categories: “very low,” “low,” “moderate,” and “high.” A classification of “very low” suggests that the true effect may differ substantially from the estimate, indicating a high degree of uncertainty in the results. In contrast, a classification of “low” reflects limited confidence in the effect, while “moderate” implies that the true effect may be close to the estimate, albeit with a possibility of variation. The scores were reviewed by the senior author (TH).
2.5 Statistical analyses
In our meta-analysis, we utilized the standardized mean difference (SMD) and pooled odds ratios (ORs) to examine the association between serum bilirubin and carotid atherosclerosis. We assessed heterogeneity among the various studies using the I2 test and Cochran’s Q-test (20). A fixed-effects model was applied for low heterogeneity (I²<50%), while a random-effects model was employed for high heterogeneity (I²>50%). Subgroup analyses were conducted based on study variables such as design, average age, sample size, country, and year of publication. To investigate the sources of heterogeneity, we performed univariate meta-regression analysis. Additionally, sensitivity analysis was executed by excluding each study to evaluate the robustness of the results (21). Publication bias was assessed through funnel plot, with Egger’s test and Begg’s test utilized for visualization (22). A two-sided P value of less than 0.05 was considered statistically significant. To maintain unit consistency across all included studies, we converted serum bilirubin levels from mg/dL to μmol/L by multiplying by 17.1. Statistical analyses were performed using Stata (version 18.0).
3 Results
3.1 Literature search and study characteristics
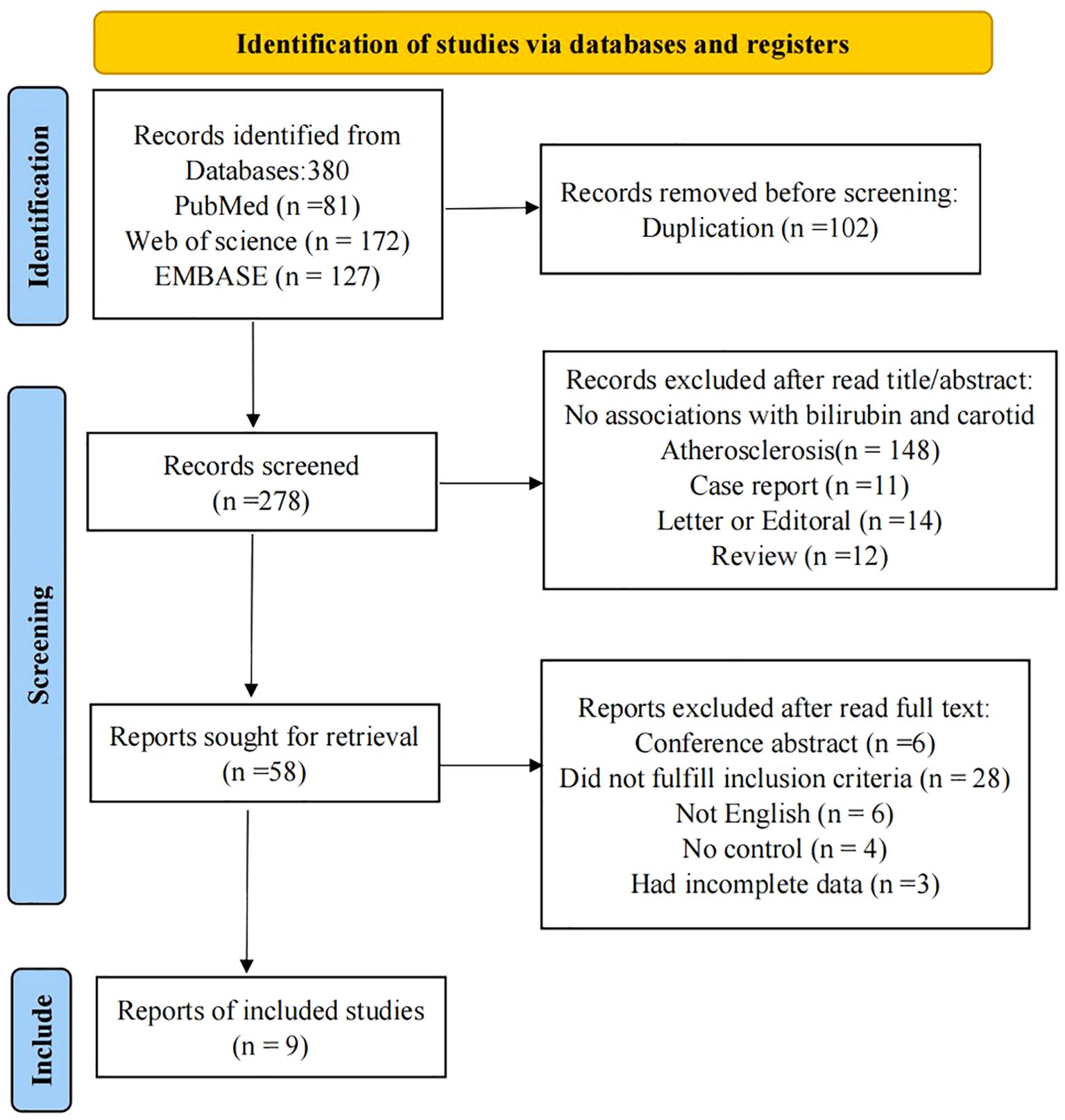
We identified a total of 380 potential studies across six electronic databases: PubMed(81), Web of Science(172), and EMBASE(127), utilizing a comprehensive search strategy. Automation tools facilitated the exclusion of 102 articles. Subsequently, after a thorough review of titles and abstracts, we excluded 220 studies, which comprised 148 studies that did not report the relationship between serum bilirubin and carotid atherosclerosis, 12 reviews, 14 letters, editorials and commentaries, 11 case reports, and 35 studies focused on animal models, chemistry, or cell lines. Following a full-text review, we excluded an additional 47 studies: 6 studies were removed due to being non-English, 4 lacked a control group, and 28 were considered irrelevant to the topic and did not meet our inclusion criteria. During data extraction phase, we found that 3 studies lacked sufficient information. Consequently, a total of 9 studies were ultimately included in our meta-analysis (23–31). The flow chart illustrating the screening process is shown in Figure 1.
3.2 Characteristics of included studies
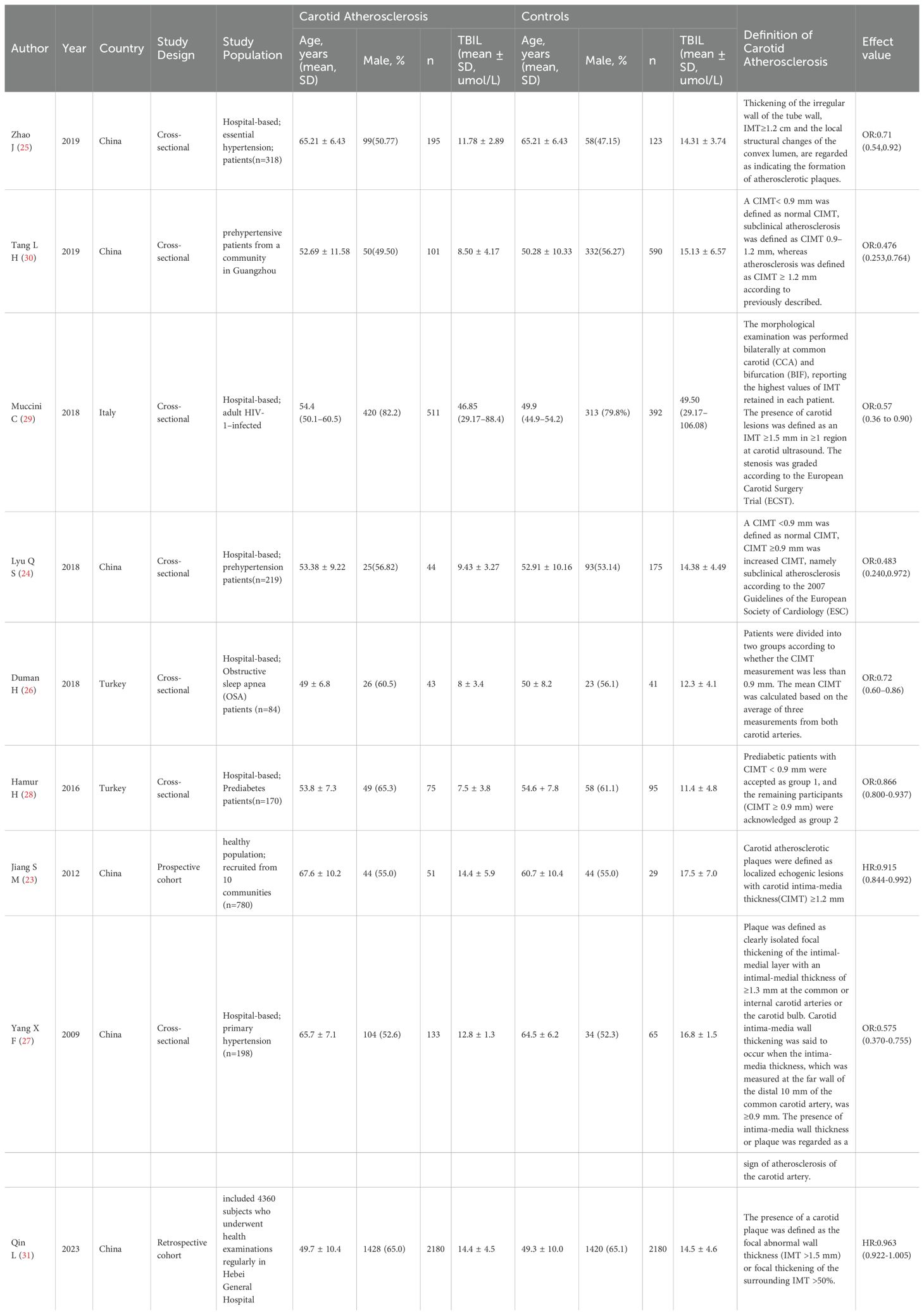
The included studies comprised two longitudinal study and seven cross-sectional studies, yielding a total of 9 articles for this meta-analysis. The combined sample size was 9,398 participants, with 5,708 individuals in the carotid atherosclerosis group and 3,690 in the control group. The study with the largest sample size included 4360 subjects (Lu Qin, 2023, Shijiazhuang, China), while the smallest included 80 participants (JIANG Shi-min, 2012, Beijing, China). Participant ages ranged from 40 to 78 years. Besides healthy middle-aged and elderly individuals, the study population included individuals with prehypertension, essential hypertension, HIV-1 infection, obstructive sleep apnea, prediabetes, and hyperbilirubinemia. Five studies defined subclinical carotid atherosclerosis or carotid atherosclerosis as a carotid intima-media thickness (CIMT) of greater than or equal to 0.9 mm (24, 26–28, 30). Two studies defined a carotid atherosclerotic plaque based on a CIMT of greater than or equal to 1.2 mm or the presence of wall irregularities with thickening or a convex luminal localization (23, 25). The remaining studies established carotid atherosclerotic plaque based on a CIMT of greater than or equal to 1.5 mm or focal thickening exceeding 50% (29, 31). The basic characteristics of the literature are shown in Table 1.
3.3 Quality analysis
Quality evaluation indicated a clear diagnosis of carotid atherosclerosis, appropriate methods for detecting total bilirubin levels, satisfactory group comparisons, and complete data (Supplementary Table S2). According to the Newcastle-Ottawa Scale (NOS) guidelines, all included studies achieved a total score of 8 stars or higher, signifying good overall study quality.
3.4 Meta-analysis results
The distribution of studies estimating the association between the serum bilirubin level and carotid atherosclerosis is illustrated in Figure 2. A total of nine studies had calculated the pooled SMD, revealing lower serum bilirubin levels in patients with carotid atherosclerosis compared to those without (SMD -3.42, 95% CI [-5.18, -1.67]), with a statistically significant difference (z=-3.819, P<0.001). Due to significant heterogeneity (I2 = 98.0%, P<0.001), a random-effects model was employed. Subsequently, analyses were conducted for carotid plaques and intima-media thickness, respectively. For intima-media thickness, five studies involving 1,362 individuals were included. Regarding the presence of plaques, four observational studies with 5,661 individuals were analyzed. We found that total bilirubin levels were negatively associated with carotid intima-media thickness (SMD -4.77, 95% CI [-5.87, -3.67], P< 0.001, I2 = 84.0%), but not with carotid plaques (SMD -1.58, 95% CI [-3.21, 0.06], P=0.058, I2 = 92.3%), as shown in Figure 2.
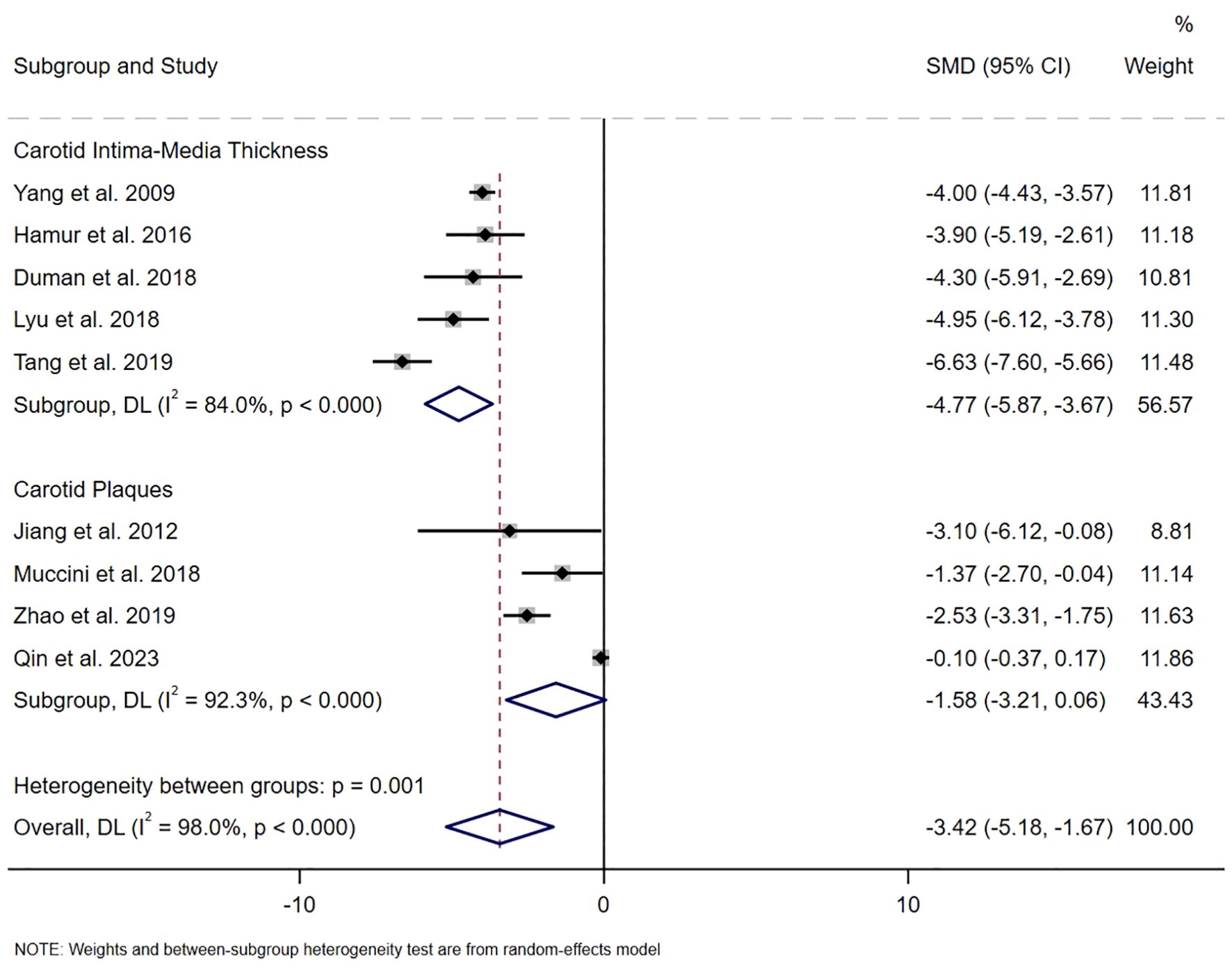
Figure 2. The SMD on the relationship between serum bilirubin level and the risk of carotid atherosclerosis.
Nine studies calculated the pooled ORs, demonstrating a significant negative relationship between serum bilirubin levels and the risk of carotid atherosclerosis (OR 0.79, 95% CI [0.71, 0.88], P<0.001, I2 = 78.2%, Figure 3). Pooled data suggested that the serum bilirubin levels were associated with a 0.68-fold decrease in the odds of having a higher carotid intima-media thickness (OR 0.68, 95% CI [0.55, 0.85], P<0.001, I2 = 70.5%, GRADE: low). The pooled analysis indicated that serum bilirubin levels serve as a protective factor against an increased risk of carotid plaque (OR 0.88, 95% CI [0.79, 0.99], P<0.001, I2 = 71.3%, GRADE: low), indicating a negative correlation between serum bilirubin levels and the occurrence of carotid atherosclerosis. The GRADE evidence ratings are specified in Supplementary Table S3.
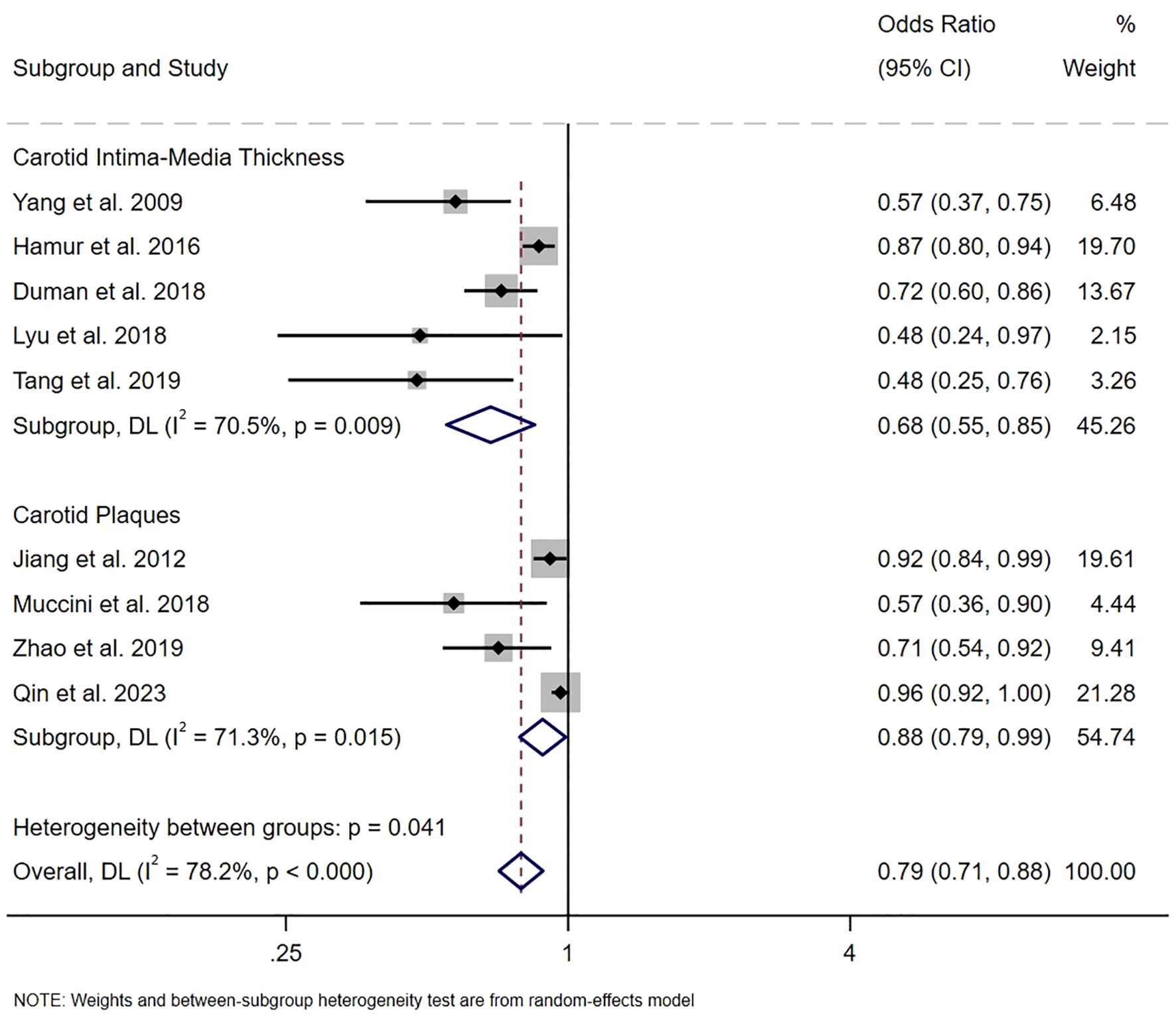
Figure 3. The pooled ORs on the relationship between serum bilirubin level and the risk of carotid atherosclerosis.
3.5 Subgroup analysis
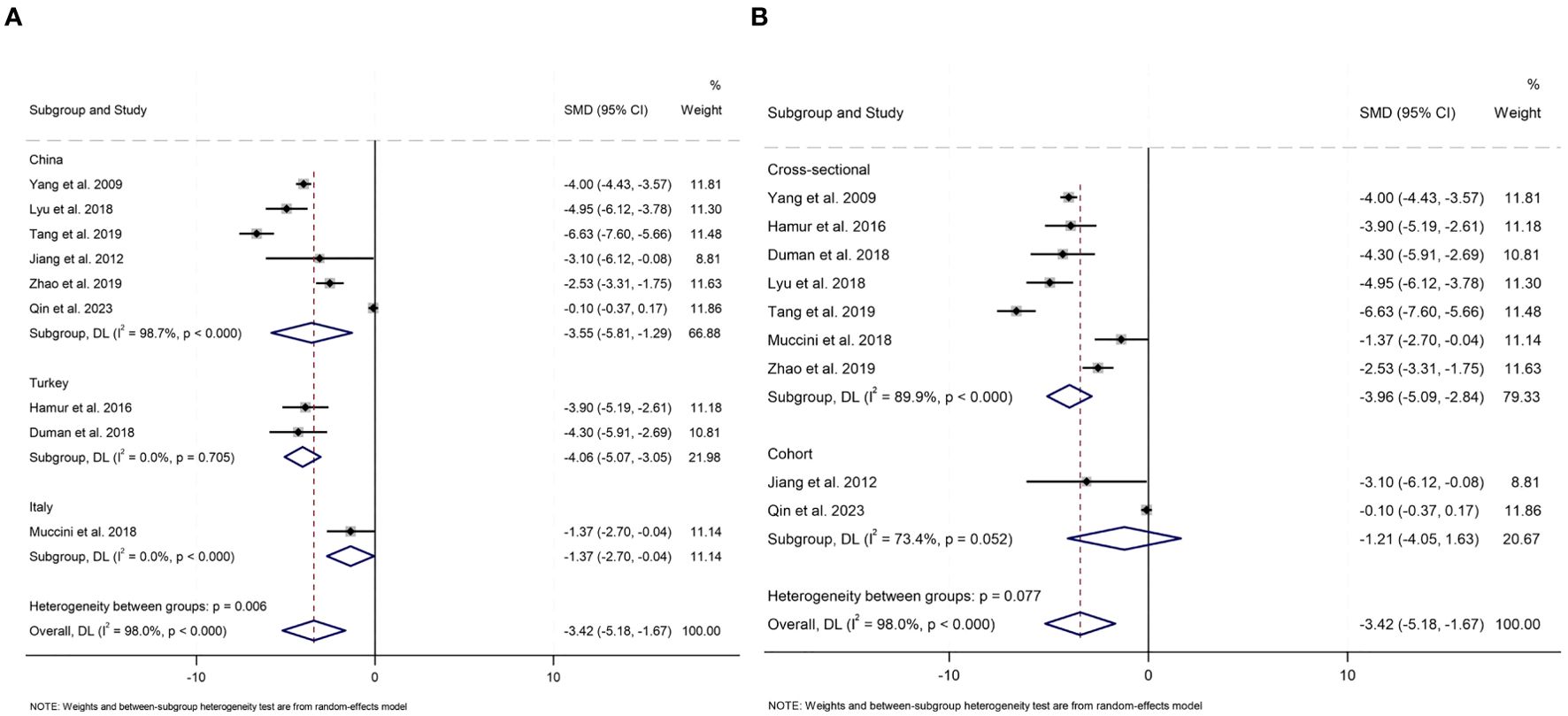
To investigate the impact of study design, geographical ethnicity, publication year, sample size, and average age and diagnostic criteria for carotid atherosclerosis on serum bilirubin levels in carotid atherosclerosis patients and controls, prespecified subgroup analyses were performed (Figure 4, Supplementary Figure S1).
All studies were categorized into three subgroups (China, Turkey and Italy) based on the countries were conducted. Significant differences in serum total bilirubin levels between carotid atherosclerosis patients and controls were observed in subgroups from China (SMD -3.55, 95% CI [-5.81, -1.29], P= 0.002), Turkey (SMD -4.06, 95% CI [-5.07, -3.05], P<0.001), and Italy (SMD -1.37, 95% CI [-2.70, -0.04], P= 0.043). Notably, the heterogeneity in the China group remained high (I2 = 98.7%), whereas the Turkey and Italy groups exhibited a substantial decrease in heterogeneity (I2 = 0.0%). Subgroup analysis based on study design revealed that, among cross-sectional studies, patients with carotid atherosclerosis exhibited significantly lower serum bilirubin levels compared to controls (SMD -3.96, 95% CI [-5.09, -2.84], P<0.001). In contrast, among cohort studies, no significant difference in serum bilirubin levels was observed between patients with carotid atherosclerosis and controls (SMD -1.21, 95% CI [-4.05, 1.63], P=0.404); however, high heterogeneity persisted in both groups (I2 = 89.9%; I2 = 73.4%). Lower levels of serum bilirubin were observed among patients with carotid atherosclerosis across both subgroups stratified by sample size. In the subgroup analysis involving fewer than 200 participants, the SMD was -3.99 (95% CI [-4.38, -3.60], P<0.001) with an I2 of 0.0%. Similarly, in the subgroup analysis of 200 or more participants, the SMD was -3.10 (95% CI [-5.71, -0.50], P = 0.020), with an I2 of 98.3%. As for age, a significant difference in serum bilirubin levels was observed in individuals aged younger than 60 years (SMD -3.53, 95% CI [-6.21, -0.84], P= 0.010, I2 = 98.0%), as well as in those aged 60 years and older (SMD -3.28, 95% CI [-4.53, -2.03], P<0.001, I2 = 81.4%). When analyzing the data by publication year, patients with carotid atherosclerosis consistently exhibited lower serum bilirubin levels compared to controls. Specifically, studies published before 2016 reported an SMD of -3.97 (95% CI [-4.38, -3.57], P<0.001, I2 = 0.0%), while studies published after 2017 showed an SMD of -3.29 (95% CI [-5.66, -0.93], P=0.006, I2 = 98.0%). Subgroup analysis was analyzed according to the diagnostic criteria for carotid atherosclerosis, and when CIMT ≥ 0.9 mm was used as a diagnostic criterion, serum bilirubin levels were significantly lower in carotid atherosclerosis patients than in controls (SMD=-4.77, 95% CI: [-5.87, -3.67], P<0.001, I²=84%). However, in studies where the criteria were CIMT ≥ 1.2 mm or plaque formation, or CIMT ≥ 1.5 mm or focal thickening > 50%, no significant difference in serum bilirubin levels was found between patients and controls (P > 0.05), with reduced heterogeneity (I²=0.0%, I²=70.4%). The subgroup analysis indicated a significant difference in serum bilirubin levels between the carotid atherosclerosis group and the control group.
3.6 Meta-regression
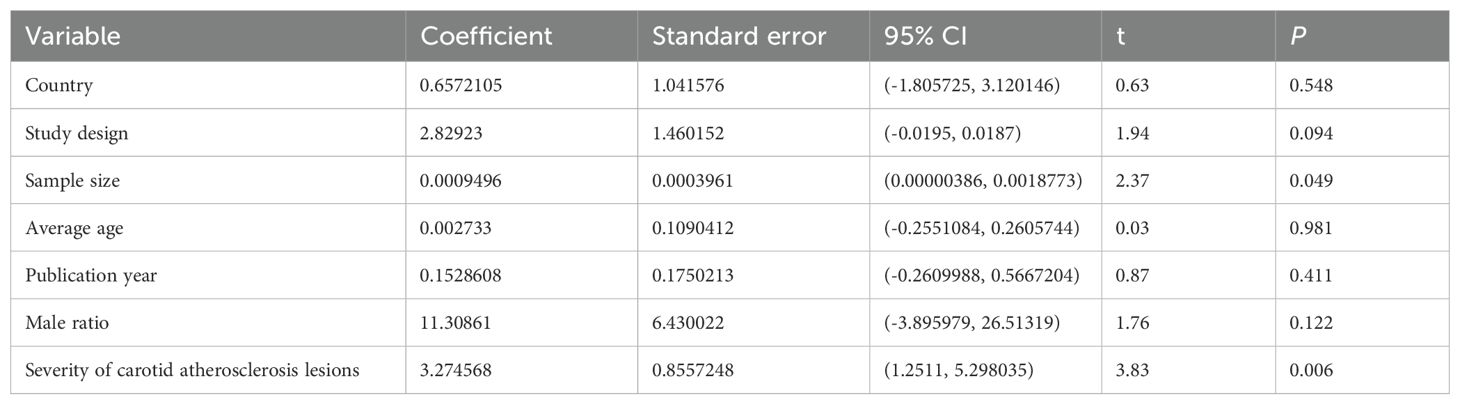
Due to significant heterogeneity (I2 = 98.0%) observed in the meta-analysis, a univariate meta-regression analysis was conducted to identify potential sources of this heterogeneity (Table 2, Supplementary Figure S2). The results of meta-regression analysis, which examined factors such as on country, study design, research sample size, average age, and publication year and male ratio of carotid atherosclerosis groups, indicated that none of them were potential sources of heterogeneity (all P >0.05). However, sample size and the severity of carotid atherosclerosis lesions(CIMT or carotid plaques) demonstrated significant effects on the meta-analysis outcomes (P < 0.05), suggesting they may be sources of heterogeneity.
3.7 Sensitivity analysis and publication bias
Given the pronounced heterogeneity in the association between bilirubin levels and carotid atherosclerosis, an additional sensitivity analysis was performed (Supplementary Figure S3). No significant alternations were detected when excluding one of the included studies and pooling the rest. Overall, this suggests that the primary results of this meta-analysis are relatively robust.
3.8 Publication bias
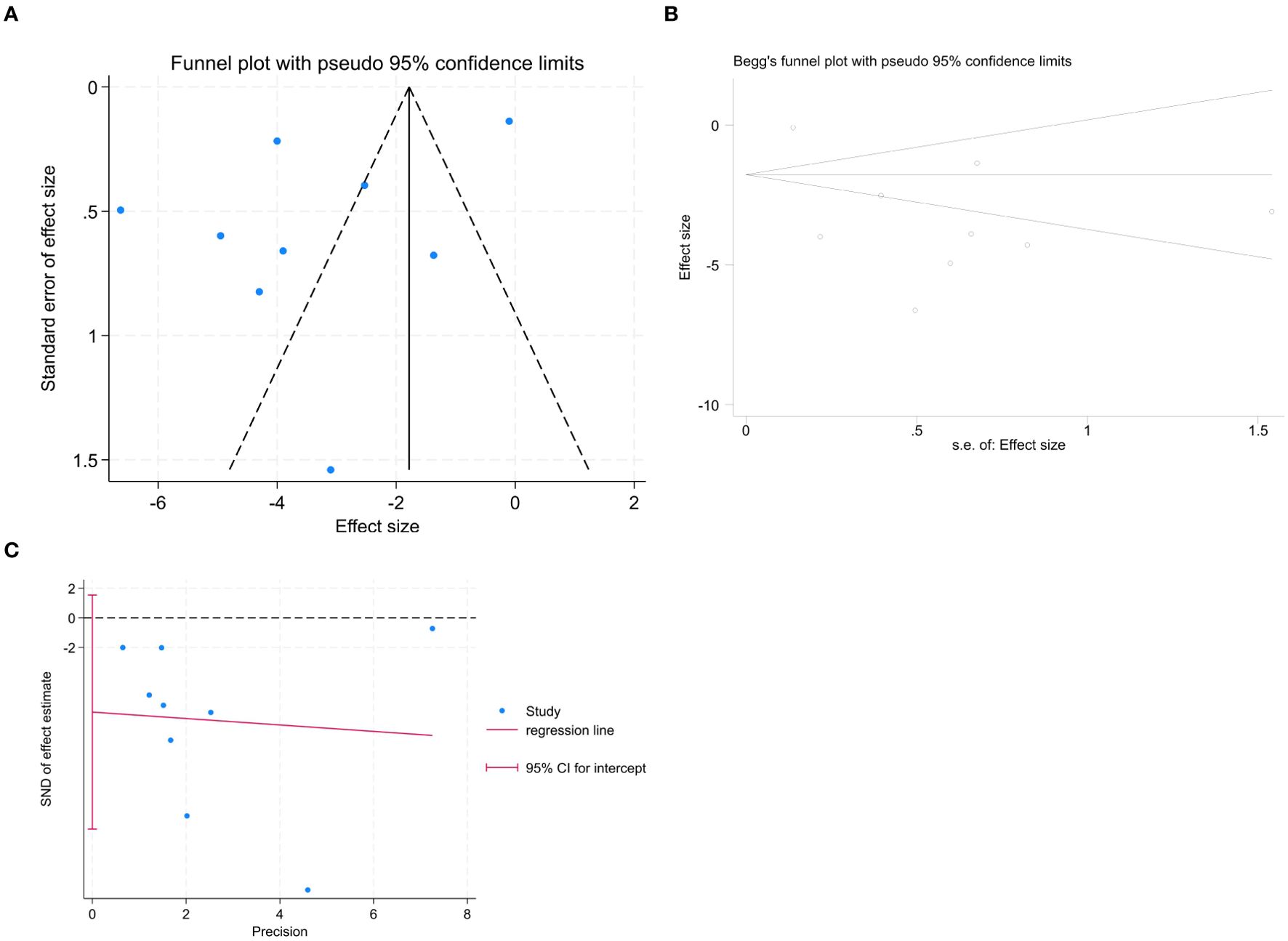
To assess publication bias, a forest plot was drawn. The distribution on both sides of the graph appeared asymmetric (Figure 5A); however, further analyses using Begg’s (Figure 5B) and Egger’s (Figure 5C) tests indicated no significant publication bias among the articles included in this meta-analysis (Begg: z = 0.73, P = 0.466; Egger: P= 0.098, 95% CI [- 14.280, 1.536]).
4 Discussion
Carotid atherosclerosis is a main cause of ischemic stroke. Studies have found that nearly one-quarter of Chinese adults have increased CIMT or carotid plaque, which places a substantial burden on socio-economic and health care system (32). The current situation urges us to seek effective strategies for the management of carotid atherosclerosis. Previous studies have found that serum bilirubin and its metabolite HO-1 play a protective role against the progression of carotid atherosclerotic disease (33). However, whether serum bilirubin level is associated with carotid atherosclerosis remains controversial. Our results revealed that lower serum bilirubin levels might be associated with a higher risk of carotid atherosclerosis. What’s more, such an association was likewise significant in further subgroup analyses based on study design, geographic location, publication year, sample size, and average age. Sample size and severity of carotid atherosclerosis lesions(CIMT or carotid plaques) significantly affected the SMD of bilirubin levels in the univariate meta-regression.
To date, the underlying protective mechanism of serum bilirubin against carotid atherosclerosis remains unclear, but there are several possible explanations. Bilirubin, a natural endogenous antioxidant, scavenges reactive oxygen species (ROS) through its conjugated double bonds and active hydrogen atoms, mitigating oxidative stress damage to vascular endothelial cells and inhibiting atherosclerotic plaque formation (34, 35). It also activates the nuclear transcription factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase (HO-1) signaling pathway, enhancing cellular antioxidant capacity and inhibiting apoptosis (36). HO-1 inhibits vascular smooth muscle cell (VSMC) proliferation and migration, reducing intimal accumulation and limiting plaque formation (37). Elevated plasma HO-1 levels are observed in patients with carotid plaques and are positively correlated with the severity of carotid atherosclerosis (38). Bilirubin exhibits anti-inflammatory effects by inhibiting macrophage polarization, complement cascade reactions, and the removal of circulating hemoglobin, protecting the vessel wall from inflammatory damage. It inhibits the NF-κB signaling pathway, reducing the production of pro-inflammatory cytokines such as TNF-α, IL-6, NLRP3, and NLRC4, thereby mitigating inflammatory responses in atherosclerotic lesions (36, 39, 40). Endothelial dysfunction, an early feature of atherosclerosis, is ameliorated by bilirubin through binding to lipids or albumin in atherosclerotic lesions and scavenging hydroxyl radicals and superoxide anions (41). Serum bilirubin levels are negatively correlated with the expression of E-selectin and intercellular adhesion molecule 1 (ICAM-1), which mediate leukocyte extravasation and monocyte recruitment in atherogenesis (12, 41). Animal studies indicate that mild hyperbilirubinemia prevents atherosclerosis progression by improving lipid profiles and modulating the mRNA expression of vascular cell adhesion molecule 1 (VCAM-1), ICAM-1, inducible nitric oxide synthase (iNOS), and lectin-like LDL receptor 1 (LOX-1) (42). Furthermore, bilirubin inhibits VSMC proliferation and migration, suppressing neoplastic intima proliferation and providing arterial wall protection, thus delaying atherosclerotic plaque formation (43). Recent studies show that bilirubin inhibits cholesterol biosynthesis by promoting HMGCR ubiquitination, reducing lipid accumulation in the arterial wall and improving atherosclerosis (44). Bvra deficiency, leading to bilirubin deficiency, enhances the proatherogenic phenotype, increasing atherogenesis risk and causing plaque destabilization (45). Changes in bilirubin levels play pivotal role in the development of carotid atherosclerosis.
Previous studies have proposed that serum bilirubin levels can serve as a predictor for the occurrence and progression of carotid atherosclerosis; however, the relationship between arteriosclerotic cardiovascular disease (ASCVD) and serum bilirubin levels has been inconsistent. Recently, some studies have reported that serum bilirubin is independently and inversely associated with the risks of carotid or femoral atherosclerosis (46, 47), while others have discovered a U-shaped dose-response relationship between bilirubin levels and the risk of coronary heart disease (CHD), particularly in men (48, 49). A Korean study involving patients with type 2 diabetes mellitus (T2DM) over an 8-year follow-up indicated that higher levels of total serum bilirubin were significantly associated with a lower risk of plaque progression in the carotid arteries (50). Furthermore, a meta-analysis encompassing 323,891 patients indicated that higher serum total bilirubin levels are an independent protective factor for ASCVD and are inversely associated with the prognosis of acute myocardial infarction (AMI), stroke, and peripheral arterial disease (PAD), yet positively correlated with in-hospital cardiovascular death and major adverse cardiac events (MACEs) (51).
In our study, serum bilirubin levels were negatively correlated with CIMT thickening ((SMD -4.77, 95% CI [-5.87, -3.67]; OR 0.68, 95% CI [0.55, 0.85])), but only weakly correlated carotid plaque (SMD -1.58, 95% CI [-3.21, 0.06]; OR 0.88, 95% CI [0.79, 0.99]). Subgroup analysis based on diagnostic criteria for carotid atherosclerosis found that serum bilirubin levels were significantly lower in patients with carotid atherosclerosis than in controls when CIMT ≥ 0.9 mm was used as a diagnostic criterion, whereas this was not observed when CIMT ≥ 1.2 mm or plaque formation, or CIMT ≥ 1.5 mm or focal thickening > 50%. A one-way meta-regression analysis revealed a significant relationship between the severity of carotid atherosclerosis lesions and serum total bilirubin levels (P < 0.05), which may account for the observed heterogeneity. CIMT thickening typically signifies the early and subclinical stages of carotid atherosclerosis, while the presence of carotid plaques and stenoses indicates more advanced stages of atherosclerotic progression (52). The formation of plaques involves irreversible and complex pathological processes, such as necrosis of lipid cores, rupture of the fibrous cap, and inflammatory cascades, which may reduce the protective effect of bilirubin. However, a cross-sectional study by Chun-Hua Jin et al. demonstrated that high-normal serum unconjugated bilirubin (UCB) is inversely associated with late carotid atherosclerotic lesions including carotid plaque and stenosis but not with CIMT, an early carotid atherosclerotic lesion (53). The primary distinction lies in the fact that CIMT is routinely measured in the distal 1 cm of the common carotid artery just before the bifurcation, whereas plaque formation and progression predominantly occur downstream in the internal carotid artery (54). In addition, different methods of measuring bilirubin, such as the bilirubin oxidase method and the vanadate oxidation method, may also be a source of heterogeneity.
Regarding study design, subgroup analyses showed that the combined effect in cross-sectional studies was consistent with the overall results, while it was not applicable in cohort studies. A total of 7 cross-sectional studies (24–30) and 2 cohort studies (23, 31) were included in our meta-analysis. Interestingly, we observed that the cross-sectional studies were all in subjects with hypertension, HIV-1 infection, obstructive sleep apnea, prediabetes, and hyperbilirubinemia, whereas the cohort studies were all based on healthy physical examination populations. Therefore, we speculate that the inconsistency may be due to differences in the number of cross-sectional and cohort studies, and the health status of the study populations.
Subgroup analyses of the study sample sizes revealed that serum total bilirubin levels in patients in the CAS group were significantly lower than those in the non-CAS group when the sample sizes were fewer than 200 individuals, exhibiting notably less heterogeneity. Similar conclusions were drawn for sample sizes of 200 or more, yielding heterogeneity did not change significantly. One-way meta-regression analysis showed a significant effect of sample size on serum total bilirubin levels (P<0.05), which may contribute to the observed heterogeneity. For instance, this study involved individuals from three different countries, with the smallest sample size being 80 and the largest reaching 4,360. It is necessary to conduct large-scale, long-term follow-up cohort studies to validate our findings.
To our knowledge, this study is the first systematic review and meta-analysis investigating the correlation between serum bilirubin levels and carotid atherosclerosis. We employed standardized methods for literature identification and assessment, conducted a comprehensive search, and ensured rigorous data processing and quality assessment. Subgroup analyses and meta-regression indicated that sample size severity of carotid atherosclerosis might be the source of heterogeneity. The results suggest that higher serum bilirubin levels could act as a protective factor against carotid atherosclerosis, which may prompt future research scholars to increase attention on this topic. Importantly, our study offers new insights into the clinical application of serum bilirubin levels as a biomarker for the early diagnosis of carotid atherosclerosis or as an adjunct to therapy.
However, our meta-analysis has several limitations. First, the included studies, primarily observational, indicate a potential association between serum bilirubin and carotid atherosclerosis, but could not be used to diagnose carotid atherosclerosis or establish causality. Second, our meta-analysis included only studies published in English, predominantly from Europe and Asia, specifically involving populations from China, Turkey, and Italy. Some of the studies had small sample sizes, which may limit the representativeness of the results. Caution is therefore warranted in interpreting these findings. Additionally, the variables adjusted for varied across studies, potentially introducing bias in the estimation of the pooled SMD and OR. Fourth, the criteria for defining carotid atherosclerosis differed among the original studies, with varying thresholds affecting the frequency of exposure. Finally, results based on observational studies cannot demonstrate a causal relationship between serum total bilirubin levels and carotid atherosclerosis. Consequently, there is a pressing need for higher-quality, large-scale, prospective studies with long-term follow-ups, as well as foundational research, to yield more reliable evidence.
5 Conclusion
The results of the meta-analysis revealed that serum bilirubin levels were significantly lower in patients with carotid atherosclerosis compared to controls, revealing a significant relationship between serum bilirubin levels and the risk of carotid atherosclerosis. However, the magnitude of this association may depend on the stage of carotid atherosclerosis development. By examining the relationship between bilirubin and carotid atherosclerosis, this meta-analysis establishes a basis for the development of more convenient diagnostic biomarkers and effective therapeutic targets. Further prospective cohort studies and randomized controlled trials are necessary to validate our findings.
Author contributions
B-EY: Conceptualization, Formal Analysis, Writing – original draft, Investigation. YL: Formal Analysis, Methodology, Writing – original draft. M-JZ: Data curation, Investigation, Writing – review & editing. Q-JW: Data curation, Writing – review & editing, Investigation. JX: Data curation, Writing – review & editing, Investigation. YZ: Funding acquisition, Visualization, Writing – review & editing. C-YZ: Visualization, Writing – review & editing. JZ: Funding acquisition, Supervision, Writing – review & editing, Validation. TH: Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (No. 82100359), the Natural Science Foundation of Shaanxi (No. 2020JQ-552) and the Science and Technology Programme Projects of Yan’an (2022SLSFGG-025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1451465/full#supplementary-material
References
1. Mensah GA, Fuster V, Murray CJL, and Roth GA. Global burden of cardiovascular diseases and risks, 1990-2022. J Am Coll Cardiol. (2023) 82:2350–473. doi: 10.1016/j.jacc.2023.11.007
2. Stefan N and Schulze MB. Metabolic health and cardiometabolic risk clusters: implications for prediction, prevention, and treatment. Lancet Diabetes Endocrinol. (2023) 11:426–40. doi: 10.1016/S2213-8587(23)00086-4
3. Sarraju A and Nissen SE. Atherosclerotic plaque stabilization and regression: a review of clinical evidence. Nat Rev Cardiol. (2024) 21:487–97. doi: 10.1038/s41569-023-00979-8
4. Gusev E and Sarapultsev A. Atherosclerosis and inflammation: insights from the theory of general pathological processes. Int J Mol Sci. (2023) 24:7910. doi: 10.3390/ijms24097910
5. Spence JD. Assessment of atherosclerosis: should coronary calcium score and intima-media thickness be replaced by ultrasound measurement of carotid plaque burden and vessel wall volume? Curr Opin Lipidol. (2023) 34:126–32. doi: 10.1097/MOL.0000000000000880
6. Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, et al. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. (2020) 8:e721–9. doi: 10.1016/S2214-109X(20)30117-0
7. Li L, Li S, Pan Z, Zhang Y, and Hua Z. Bilirubin impacts microglial autophagy via the Akt-mTOR signaling pathway. J Neurochem. (2023) 167:582–99. doi: 10.1111/jnc.v167.4
8. Liu HW, Gong LN, Lai K, Yu XF, Liu ZQ, Li MX, et al. Bilirubin gates the TRPM2 channel as a direct agonist to exacerbate ischemic brain damage. Neuron. (2023) 111:1609–1625.e6. doi: 10.1016/j.neuron.2023.02.022
9. Vitek L, Hinds TD Jr., Stec DE, and Tiribelli C. The physiology of bilirubin: health and disease equilibrium. Trends Mol Med. (2023) 29:315–28. doi: 10.1016/j.molmed.2023.01.007
10. Paul BD and Pieper AA. Neuroprotective roles of the biliverdin reductase-A/bilirubin axis in the brain. Biomolecules. (2024) 14:155. doi: 10.3390/biom14020155
11. Chen Z, Vong CT, Gao C, Chen S, Wu X, Wang S, et al. Bilirubin nanomedicines for the treatment of reactive oxygen species (ROS)-mediated diseases. Mol Pharm. (2020) 17:2260–74. doi: 10.1021/acs.molpharmaceut.0c00337
12. Vítek L, Jirásková A, Malíková I, Dostálová G, Eremiášová L, Danzig V, et al. Serum bilirubin and markers of oxidative stress and inflammation in a healthy population and in patients with various forms of atherosclerosis. Antioxid (Basel). (2022) 11:2118. doi: 10.3390/antiox11112118
13. Wang G, Qiao L, Tang Z, Zhou S, Min J, and Li M. Association between bilirubin levels and risk of stroke: a systematic review and meta-analysis. BMJ Open. (2023) 13:e064433. doi: 10.1136/bmjopen-2022-064433
14. Zhong K, Wang X, Ma X, Ji X, Sang S, Shao S, et al. Association between serum bilirubin and asymptomatic intracranial atherosclerosis: results from a population-based study. Neurol Sci. (2020) 41:1531–8. doi: 10.1007/s10072-020-04268-x
15. Zhao P, Xu H, Shi Y, Song X, Qiu G, Ding C, et al. Association between bilirubin and chronic kidney disease in hypertensive patients: The China hypertension registry study. J Clin Hypertens (Greenwich). (2023) 25:1185–92. doi: 10.1111/jch.v25.12
16. Yuan X, Ma C, Li J, Li J, Yu R, Cai F, et al. Indirect bilirubin impairs invasion of osteosarcoma cells via inhibiting the PI3K/AKT/MMP-2 signaling pathway by suppressing intracellular ROS. J Bone Oncol. (2023) 39:100472. doi: 10.1016/j.jbo.2023.100472
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
19. Jayedi A, Zargar MS, Emadi A, and Aune D. Walking speed and the risk of type 2 diabetes: a systematic review and meta-analysis. Br J Sports Med. (2024) 58:334–42. doi: 10.1136/bjsports-2023-107336
20. Melsen WG, Bootsma MC, Rovers MM, and Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. (2014) 20:123–9. doi: 10.1111/1469-0691.12494
21. Zuo X, Li X, Tang K, Zhao R, Wu M, Wang Y, et al. Sarcopenia and cardiovascular diseases: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2023) 14:1183–98. doi: 10.1002/jcsm.13221
22. Afonso J, Ramirez-Campillo R, Clemente FM, Büttner FC, and Andrade R. The perils of misinterpreting and misusing “Publication bias” in meta-analyses: an education review on funnel plot-based methods. Sports Med. (2024) 54:257–69. doi: 10.1007/s40279-023-01927-9
23. Jiang SM, Sun XF, Gu HX, Chen YS, Xi CS, Qiao X, et al. Effects of decline in renal function with age on the outcome of asymptomatic carotid plaque in healthy adults: A 5-year follow-up study. Chin Med J. (2012) 125:2649–57. doi: 10.3760/cma.j.issn.0366-6999.2012.15.002
24. Lyu QS and Huang YQ. The relationship between serum total bilirubin and carotid intima-media thickness in patients with prehypertension. Ann Clin Lab Sci. (2018) 48:757–63.
25. Zhao J, Quan X, Liu H, Xue C, and Liu J. Correlation analysis of carotid atherosclerotic plaques and serum markers in elderly patients with essential hypertension. Acta Med Mediterr. (2019) 35:943–8.
26. Duman H and Ozyurt S. Low serum bilirubin levels associated with subclinical atherosclerosis in patients with obstructive sleep apnea. Intervent Med And Appl Sci. (2018) 10:179–85. doi: 10.1556/1646.10.2018.39
27. Yang XF, Chen YZ, Su JL, Wang FY, and Wang LX. Relationship between serum bilirubin and carotid atherosclerosis in hypertensive patients. Internal Med. (2009) 48:1595–9. doi: 10.2169/internalmedicine.48.2286
28. Hamur H, Duman H, Demirtas L, Bakirci EM, Durakoglugil ME, Degirmenci H, et al. Total bilirubin levels predict subclinical atherosclerosis in patients with prediabetes. Angiology. (2016) 67:909–15. doi: 10.1177/0003319716632394
29. Muccini C, Galli L, Poli A, Carbone A, Maillard M, Giusti MC, et al. Hyperbilirubinemia is associated with a decreased risk of carotid atherosclerosis in HIV-infected patients on virological suppression. Jaids-J Of Acquired Immune Deficiency Syndromes. (2018) 79:617–23. doi: 10.1097/QAI.0000000000001854
30. Tang LH, Huang C, and Feng YQ. Serum total bilirubin concentration is associated with carotid atherosclerosis in patients with prehypertension. Clin Exp Hypertens. (2019) 41:682–6. doi: 10.1080/10641963.2018.1539094
31. Qin L, Li L, Fan H, Gu Y, He W, Zhang K, et al. Longitudinal associations between serum bilirubin level and carotid atherosclerosis plaque in a health screening population. Angiology. (2023) 74:452–60. doi: 10.1177/00033197221110966
32. Fu J, Deng Y, Ma Y, Man S, Yang X, Yu C, et al. National and provincial-level prevalence and risk factors of carotid atherosclerosis in chinese adults. JAMA Netw Open. (2024) 7:e2351225. doi: 10.1001/jamanetworkopen.2023.51225
33. Kishimoto Y, Kondo K, and Momiyama Y. The protective role of heme oxygenase-1 in atherosclerotic diseases. Int J Mol Sci. (2019) 20:3628. doi: 10.3390/ijms20153628
34. Dorresteijn MJ, Dekker D, Zwaag J, Heemskerk S, Roelofs HMJ, Smits P, et al. Atazanavir-induced unconjugated hyperbilirubinemia prevents vascular hyporeactivity during experimental human endotoxemia. Front Immunol. (2023) 14:1176775. doi: 10.3389/fimmu.2023.1176775
35. Jiang X, Huang S, Cai W, Wang P, Jiang Z, Wang M, et al. Glutamine-based metabolism normalization and oxidative stress alleviation by self-assembled bilirubin/V9302 nanoparticles for psoriasis treatment. Adv Healthc Mater. (2023) 12:e2203397. doi: 10.1002/adhm.202203397
36. Zhao X, Duan B, Wu J, Huang L, Dai S, Ding J, et al. Bilirubin ameliorates osteoarthritis via activating Nrf2/HO-1 pathway and suppressing NF-κB signalling. J Cell Mol Med. (2024) 28:e18173. doi: 10.1111/jcmm.18173
37. Alonso-Piñeiro JA, Gonzalez-Rovira A, Sánchez-Gomar I, Moreno JA, and Durán-Ruiz MC. Nrf2 and heme oxygenase-1 involvement in atherosclerosis related oxidative stress. Antioxid (Basel). (2021) 10:1463. doi: 10.3390/antiox10091463
38. Kishimoto Y, Sasaki K, Saita E, Niki H, Ohmori R, Kondo K, et al. Plasma heme oxygenase-1 levels and carotid atherosclerosis. Stroke. (2018) 49:2230–2. doi: 10.1161/STROKEAHA.118.022256
39. Casper E. The crosstalk between Nrf2 and NF-κB pathways in coronary artery disease: Can it be regulated by SIRT6? Life Sci. (2023) 330:122007. doi: 10.1016/j.lfs.2023.122007
40. Li Y, Huang B, Ye T, Wang Y, Xia D, and Qian J. Physiological concentrations of bilirubin control inflammatory response by inhibiting NF-κB and inflammasome activation. Int Immunopharmacol. (2020) 84:106520. doi: 10.1016/j.intimp.2020.106520
41. Punzo A, Silla A, Fogacci F, Perillo M, Cicero AFG, and Caliceti C. Bile acids and bilirubin role in oxidative stress and inflammation in cardiovascular diseases. Diseases. (2024) 12:103. doi: 10.3390/diseases12050103
42. Maleki MH, Vakili O, Tavakoli R, Nadimi E, Noori Z, Taghizadeh M, et al. Protective and curative effects of unconjugated bilirubin on gene expression of LOX-1 and iNOS in the heart of rats receiving high-fat diet and low dose streptozotocin: a histomorphometric approach. J Inflammation (Lond). (2024) 21:26. doi: 10.1186/s12950-024-00397-8
43. Durante W. Targeting heme oxygenase-1 in the arterial response to injury and disease. Antioxid (Basel). (2020) 9:829. doi: 10.3390/antiox9090829
44. Wen G, Yao L, Hao Y, Wang J, and Liu J. Bilirubin ameliorates murine atherosclerosis through inhibiting cholesterol synthesis and reshaping the immune system. J Transl Med. (2022) 20:1. doi: 10.1186/s12967-021-03207-4
45. Chen W, Tumanov S, Stanley CP, Kong SMY, Nadel J, Vigder N, et al. Destabilization of atherosclerotic plaque by bilirubin deficiency. Circ Res. (2023) 132:812–27. doi: 10.1161/CIRCRESAHA.122.322418
46. Zhao CC, Wang JW, Chen MY, Ke JF, Li MF, and Li LX. High-normal serum bilirubin decreased the risk of lower limb atherosclerosis in type 2 diabetes: a real-world study. Diabetol Metab Syndr. (2023) 15:105. doi: 10.1186/s13098-023-01088-9
47. Su Q, Chen H, Du S, Dai Y, Chen C, He T, et al. Association between serum bilirubin, lipid levels, and prevalence of femoral and carotid atherosclerosis: A population-based cross-sectional study. Arterioscler Thromb Vasc Biol. (2023) 43:136–45. doi: 10.1161/ATVBAHA.122.318086
48. Li C, Wu W, Song Y, Xu S, and Wu X. The nonlinear relationship between total bilirubin and coronary heart disease: A dose-response meta-analysis. Front Cardiovasc Med. (2021) 8:761520. doi: 10.3389/fcvm.2021.761520
49. Zuo L, Huang J, Zhang H, Huang B, Wu X, Chen L, et al. Dose-response association between bilirubin and cardiovascular disease: A systematic review and meta-analysis. Angiology. (2022) 73:911–9. doi: 10.1177/00033197211059693
50. Lee I, Lee HH, Cho Y, Choi YJ, Huh BW, Lee BW, et al. Association between serum bilirubin and the progression of carotid atherosclerosis in type 2 diabetes. J Lipid Atheroscler. (2020) 9:195–204. doi: 10.12997/jla.2020.9.1.195
51. Lan Y, Liu H, Liu J, Zhao H, and Wang H. Is serum total bilirubin a predictor of prognosis in arteriosclerotic cardiovascular disease? A Meta-analysis Med (Baltimore). (2019) 98:e17544. doi: 10.1097/MD.0000000000017544
52. Zhang B, Ke J, Zhang N, Zhao W, and Zhong L. Mildly elevated serum prolactin level may be a protective factor for preventing thickening of the carotid intima-media in patients with type 2 diabetes mellitus. Endocr Connect. (2025) 14:e240285. doi: 10.1530/EC-24-0285
53. Jin CH, Wang JW, Ke JF, Li JB, Li MF, and Li LX. Low-normal serum unconjugated bilirubin levels are associated with late but not early carotid atherosclerotic lesions in T2DM subjects. Front Endocrinol (Lausanne). (2022) 13:948338. doi: 10.3389/fendo.2022.948338
Keywords: carotid atherosclerosis, carotid intima-media thickness, carotid plaque, bilirubin, meta-analysis
Citation: Yan B-E, Li Y, Zhu M-J, Wang Q-J, Xiao J, Zhang Y, Zhang C-Y, Zhou J and Han T (2025) Association between serum bilirubin levels and carotid atherosclerosis: a systematic review and meta-analysis. Front. Endocrinol. 16:1451465. doi: 10.3389/fendo.2025.1451465
Received: 19 June 2024; Accepted: 02 June 2025;
Published: 19 June 2025.
Edited by:
Yashendra Sethi, PearResearch, IndiaReviewed by:
Hector A. Cabrera-Fuentes, Imam Abdulrahman bin Faisal University, Saudi ArabiaArrigo Francesco Giuseppe Cicero, University of Bologna, Italy
Jun Wen, First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2025 Yan, Li, Zhu, Wang, Xiao, Zhang, Zhang, Zhou and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tuo Han, aGFudHVvMDIyOEAxNjMuY29t; Jing Zhou, MjU4OTEwODJAcXEuY29t
†These authors have contributed equally to this work
 Bao-E Yan
Bao-E Yan Ying Li
Ying Li Min-Jie Zhu2
Min-Jie Zhu2 Tuo Han
Tuo Han