- 1College of Pharmacy, University of Sargodha, Sargodha, Pakistan
- 2School of Economics, University of Wollongong, Wollongong, NSW, Australia
- 3Rai Foundation Pharmacy College, Sargodha, Pakistan
- 4Riphah Institute of Pharmaceutical Sciences, Riphah International University, Lahore, Pakistan
- 5Cadson College of Pharmacy, Kharian, Pakistan
- 6Ahmad Polyclinic and Diabetic Center, Sargodha, Pakistan
- 7Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia
- 8Department of Biochemistry, A.T. Still University of Health Sciences, Kirksville, MO, United States
Introduction: Every one in seven people with Type-I or Type-II diabetes suffers from fear of hypoglycemia (FOH). Its impact on quality of life, glycemic control, and health outcomes is well studied. However, its relationship with sleep quality remains underexplored, particularly in developing societies. We hypothesize that FOH is a key predictor of sleep quality in Type-I and Type-II patients with diabetes and, therefore, needs detailed investigation.
Methods: A multicentric study was conducted across five cities and six centers of Punjab. Data from 310 diabetes patients were analyzed using the Hypoglycemia Fear Survey-II (HFS-II) Scale and the Pittsburgh Sleep Quality Index (PSQI). Statistical analyses explored subgroup variations, correlations, regression models, and receiver operator curve (ROC) estimation.
Results: The study reports 57.70% of patients with poor sleep among whom 47% had elevated FOH. Sleep quality, age, gender, diabetes duration, and insulin route significantly correlated with FOH (p < 0.05), while glycemic control and insulin use did not. Binary logistics regression showed that for every one-unit increase in FOH, the odds of experiencing poor sleep increased by approximately 3.7% (p < 0.001; OR 1.037). Five out of seven sleep components (sleep quality, efficiency, disturbance, medication use, and daytime dysfunction) were significantly related to FOH. We hypothesize that FOH might specifically influence the quality rather than the initiation or termination of the sleep cycle. ROC analysis revealed that HFS-II may be better at diagnosing poor sleep in patients than by chance (p < 0.001) with an AUC of 0.691.
Conclusion: FOH is a key predictor of sleep quality among patients with diabetes. Healthcare providers should prioritize patient education targeting common FOH concerns and assess patients with disturbed sleep for elevated FOH levels, as it may contribute to sleep disturbances.
1 Introduction
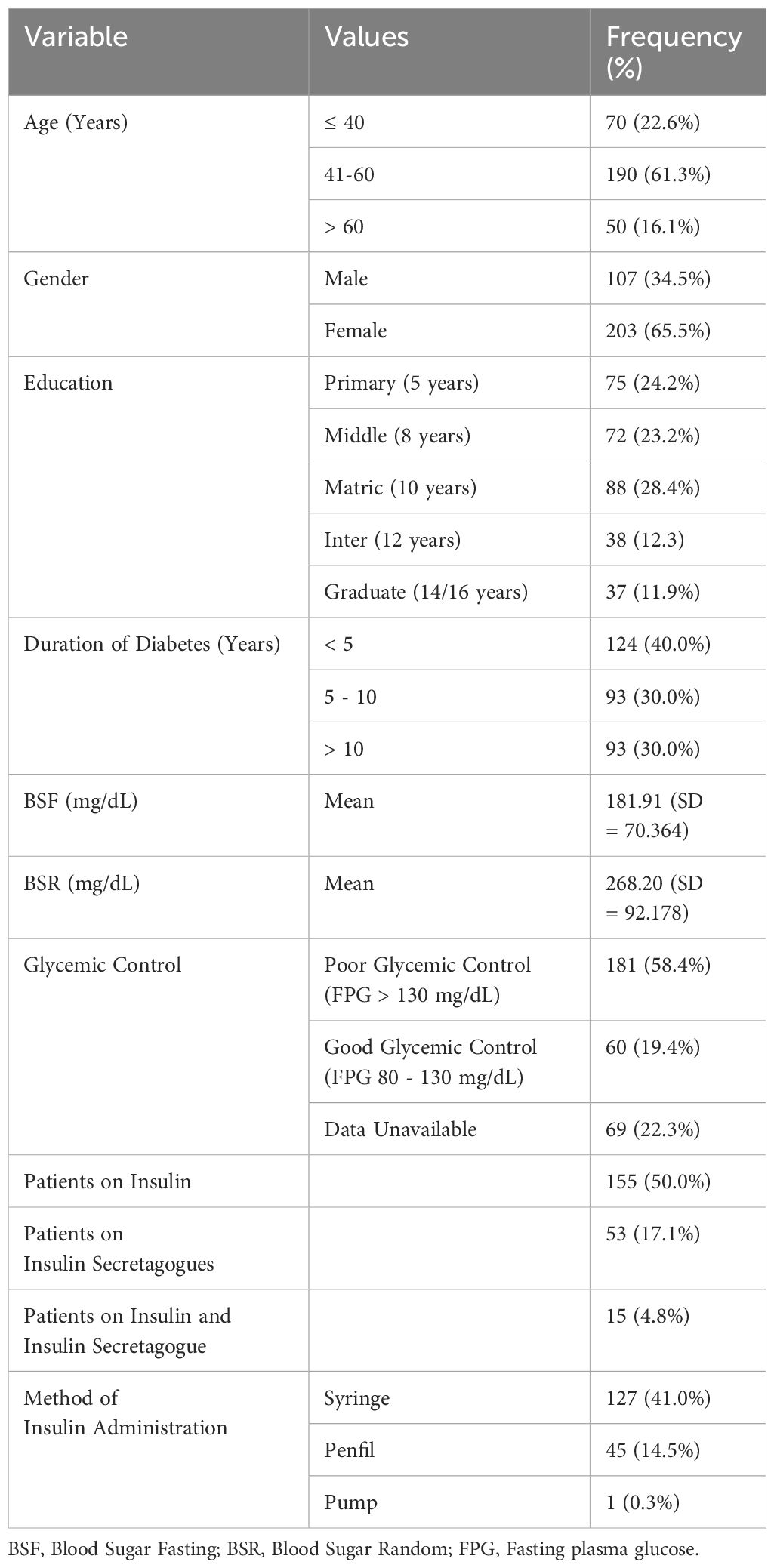
Diabetes is characterized as an increased blood glucose level that is caused by either a defect in insulin action, insulin secretion, or both, causing various metabolic disorders (1, 2). It is one of the most prevalent diseases across the world with an increasing incidence in Pakistan. The most recent data provided by the International Diabetes Foundation (IDF) has shown a 30.8% prevalence of diabetes in the Pakistani adult population, where every 1 in 4 persons is affected by diabetes (3).
Patients with diabetes are required to maintain a balance between their medication and food intake. This balance is essential to avoid poor glycemic control on one side and hypoglycemia on the other side. Hypoglycemia, although acute, yet is one of the most serious acute adverse outcomes of anti-diabetic treatments (4). It can be manifested as either autonomic symptoms (trembling, palpitations, sweating, tingling) or neuroglycopenic symptoms (difficulty concentrating, confusion, drowsiness, vision changes, difficulty speaking) (5). Generally, the symptoms can be alleviated by the administration of fast-acting carbohydrates and do not require any assistance (level 1 and level 2 hypoglycemia), however, if left unrecognized or unaddressed it may lead to a severe form of hypoglycemia (level 3, requiring aid) resulting in loss of consciousness, seizure, coma, or even death (6). These potentially fatal consequences create a psychological state of mind in diabetes patients known as the fear of hypoglycemia (FOH).
FOH is defined as “the degree of fear associated with episodes of hypoglycemia and their negative consequences” (7). It is one of the most common psychological manifestations associated with hypoglycemia (8–10).
The state of hypoglycemic fear induces behavioral changes in patients with diabetes to prevent the occurrence of hypoglycemic episodes and thus the consequences. These include having additional meals (11), snacking at night (12), stress-induced eating, decreased physical activity (13), maintaining higher blood glucose levels (14, 15), and mismanagement of insulin doses (11, 16–18). These behaviors can potentially lead to impaired glycemic control (10), reduced quality of life (19, 20), suboptimal diabetes management (21, 22), and development of microvascular complications (7, 22).
Along with these physical behaviors, FOH is also manifested psychologically. Evidence shows that FOH is related to anxiety and depression in patients with diabetes (23). These psychological states are closely linked with sleep quality (24, 25). Similarly, research shows that the improvement in blood glucose monitoring technologies has significantly reduced FOH and improved quality of life, including sleep quality, in these patients (26, 27). Therefore, FOH is a potential factor that may affect the quality of sleep in patients with diabetes.
Only a few studies are available worldwide that have assessed this correlation in patients with diabetes (15, 28), but the data on the Pakistani population is scarce. It has also been reported that there is a high prevalence of sleep disturbances among the diabetes population in Pakistan. The results were confirmed in a recent (and by far the first) study by Farooque et al. where 57% of the patients were found to be poor sleepers (Global PSQI Score > 5) in a sample of 329 patients (29). However, the reasons remain unexplored as in the aforementioned study (29), there was no association found between poor sleep and glycemic control which necessitates further research in this area. In light of this, the present study aims to determine if FOH is a key predictor of sleep quality, describe the relationship between FOH and sleep components, and describe factors associated with FOH for targeted interventions.
2 Methodology
2.1 Study setting and design
This study employed a multicenter cross-sectional research design spanning six months (April 2023 to September 2023). Data was collected from multiple healthcare settings of the Punjab province of Pakistan viz. Ahmad Diabetes and Foot Center, Sargodha; District Headquarter Hospital (DHQ), Sargodha; Non-Communicable Disease Clinic (NCD), DHQ Hospital Jhelum; DHQ Hospital Hafizabad; Tehsil Headquarter Hospital, Lalamusa; and Fatima Memorial Hospital, Sambrial.
2.2 Ethics approval
Ethical approval was obtained on April 19, 2023, from the Ethical Review Committee at the University of Sargodha (Reference Number SU/ORIC/799).
2.3 Study tools
The study employed two pre-validated questionnaires, viz. the Hypoglycemia Fear Survey (HFS-II) (30) and the Pittsburgh Sleep Quality Index (PSQI) (31). The HFS-II consists of 33 items divided among two sub-scales i.e., the behavior scale (HSF-B, 15 items) and the worry scale (HFS-W, 18 items). It is used to measure different aspects of fear related to hypoglycemia. Each item on the scale carries five Likert points from 0-4 marked as ‘Never’, ‘Rarely’, ‘Sometimes’, ‘Often,’ and ‘Almost always’, respectively. Patients were classified into “elevated fear” or “non-elevated fear” groups based on the elevated item (EI) endorsement criterion (32). The criterion describes patients as having elevated fear if they score ‘≥3’ for more than one item on HFS-W.
The PSQI, with its 19 items, is a widely used tool to assess the sleep quality of patients over the past month based on self-reporting (31). Initial scoring of the scale generates seven components i.e., subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, medication use, and daytime dysfunction. Each component is scored from 0-3 (0 being the best and 3 being the worst score), and when all seven are cumulated, a global score is generated from ‘0-21’. The patients were categorized into “good sleep health (PSQI ≤ 5)” or “poor sleep health (PSQI > 5)” groups based on the global score (31). Prior permissions were obtained from the relevant bodies/persons to use these questionnaires in this study.
2.4 Study participants
A total of 310 participants were enrolled from different centers, as mentioned above. Inclusion criteria comprised patients with Type-I or Type-II diabetes, diabetes duration of ≥ 1 year, good cognitive skills, and willingness to participate. Exclusion criteria included pregnancy-related diabetes, mental illness history, and other conditions hindering communication.
Patients were approached during healthcare visits, and provided with study details, and those agreeing to participate gave written informed consent. Selected patients were then interviewed thoroughly for the baseline demographics, HFS-II, and PSQI questionnaires and responses were marked accordingly.
2.5 Statistical analysis
The statistics were applied using the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). Data analysis involved descriptive statistics for means, frequencies, and percentages. The non-normal distribution of dependent variables (HFS-II Total Score and PSQI Global Score) led to the use of non-parametric tests like Kruskal-Wallis and Mann-Whitney tests for significance testing regarding age, gender, education, diabetes duration, and insulin use.
Spearman’s correlation was used to analyze the relationship between fear of hypoglycemia (HFS-II) and sleep quality (PSQI). A binary logistic regression was subsequently performed, treating PSQI as a dichotomous variable for further exploration, with fear of hypoglycemia and insulin use as predictor variables. Finally, ROC curve analysis was conducted to evaluate the diagnostic ability of HFS-II (FOH) in measuring poor sleep quality. The output data on the assumption analysis of regression, normality testing, and ROC curve analysis can be found in the Supplementary File.
3 Results
3.1 Population characteristics
Table 1 summarizes the population’s characteristics. The largest age group was 41-60 years (30.9%), with 65.5% female and 34.5% male participants. Most had 10 years of education (28.4%), while 11.9% were graduates. Around 40.0% were diagnosed with diabetes in the last 5 years, and 30.0% had diabetes for over 10 years. Mean fasting blood sugar (BSF) was 181.91 mg/dL (SD 70.36), and random blood sugar (BSR) was 268.20 mg/dL (SD 92.17). Notably, 58.4% had poor glycemic control (BSF > 130 mg/dL). Type-I diabetes patients constituted 50% of the sample, primarily using syringes for insulin (41.0%). Additionally, 17.1% used insulin secretagogues and 4.8% used both insulin and insulin secretagogues.
3.2 HFS-II scores
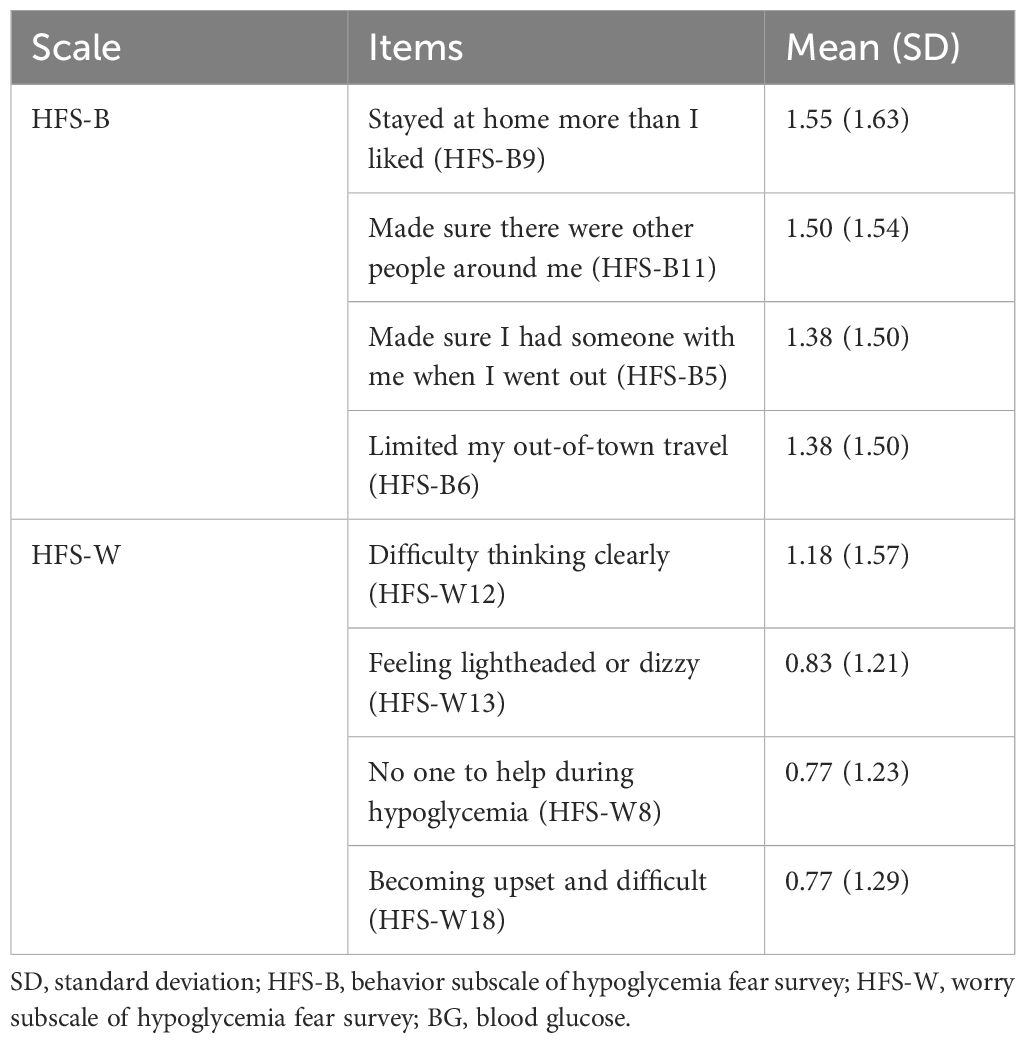
The mean HFS-II total score was 25.18 (SD 23.24) on a scale of 0 – 132. Mean scores on HFS-B and HFS-W scales were 15.40 (SD 13.64) and 9.77 (SD 12.48) respectively. The most rated items on HFS-B and HFS-W have been listed in Table 2. Using the EI criterion (32), we identified 120 patients (38.7%) having an elevated FOH as they scored ‘≥ 3’ for more than one item on HFS-W.
3.3 FOH in different population groups
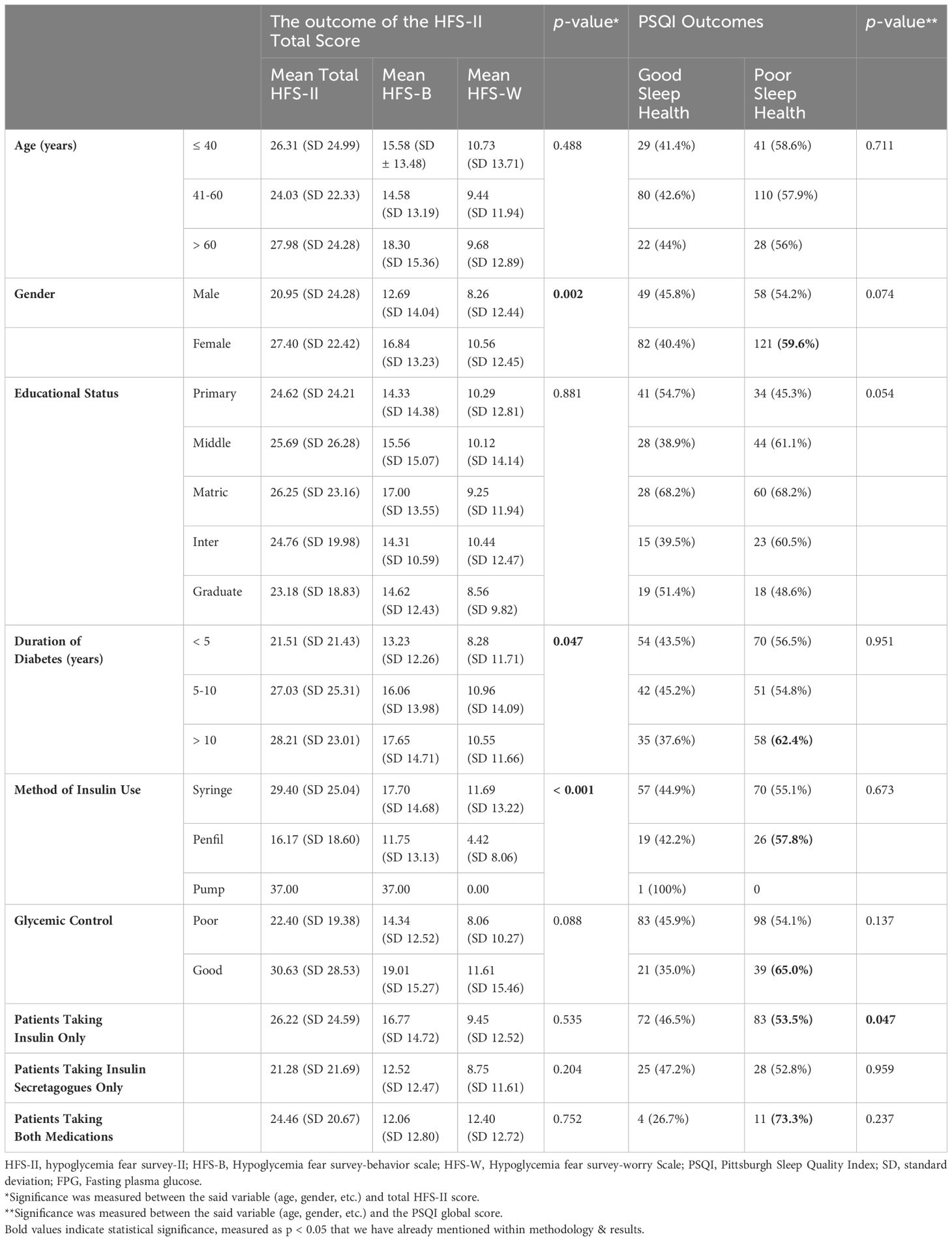
The scores of HFS-II, HFS-B, and HFS-W have been presented in Table 3. Participant’s gender, diabetes duration, and method of insulin administration were significantly related to the FOH scores. The mean HFS-II score was significantly higher in the female gender (M = 27.40 (SD 22.42)) than in males (M = 20.95 (SD 24.28)). Nearly 75% of the females had an elevated fear of hypoglycemia. The most feared items among females related to hypoglycemia were ‘made sure there were other people around,’ ‘stayed at home more than I liked,’ and ‘made sure I had someone with me when I went out.’ Male participants differed only in item (‘ate large snacks’).
Duration of diabetes appears to have a positive correlation with the FOH as the mean HFS-II scores are highest in patients with a duration of > 10 years. Though the overall difference in the groups is marginally significant (p = 0.047), the pairwise comparison highlights a more substantial difference of means between the groups ‘< 5 years’ and ‘> 10 years’ (p = 0.016).
Finally, the way the patients administer insulin seems to have a significant impact on their FOH. The fear is more prevalent among patients using traditional methods such as insulin syringes (M = 29.40; SD 25.04) than those using modern technologies such as penfills (M = 16.17; SD 18.60). No solid inference can be derived from the scores of patients on insulin pumps due to their small proportion.
Glycemic control (good or bad), insulin vs non-insulin users, and the educational status of the participants did not appear to influence the FOH levels significantly. However, the fear was higher in patients with good glycemic control (M = 30.63 (SD 28.53)), use of insulin (M = 26.22 (SD 24.59)), and lower educational level (M = 26.25 (SD 23.16)).
3.4 Sleep quality in different population groups
The mean PSQI score was 7.11 (SD 4.31) where 57.7% (179) of the participants were poor sleepers (PSQI > 5) and 42.3% (131) had better sleep (PSQI ≤ 5). Age, gender, diabetes duration, use of oral hypoglycemic agents, glycemic control, and insulin regimen were not related to sleep quality significantly (p > 0.05). The use of insulin only, however, had a significant impact on the quality of sleep (p = 0.047). The proportion of poor sleep health was higher in all patients with diabetes without regard to their therapeutic regimens i.e., insulin, insulin secretagogues, or both medications. However, sleep health was relatively more compromised in patients using insulins in combination with insulin secretagogues (73.3% poor sleep health). Among other groups, sleep health was more compromised in patients aged between 21-30 years (78.6%), females (59.6%), diabetes duration > 10 years (62.4%), penfil users (57.8%), and those having a good glycemic control (65.0%) (Table 3). Interestingly, the FOH also seems to be higher within the same population groups particularly ‘21-30 years old’, ‘females’, ‘diabetes duration >10 years’, and ‘good glycemic control’ (Table 3), setting up a base for a more in-depth relation between these two variables explained as follows.
3.5 Comparison of FOH and sleep quality
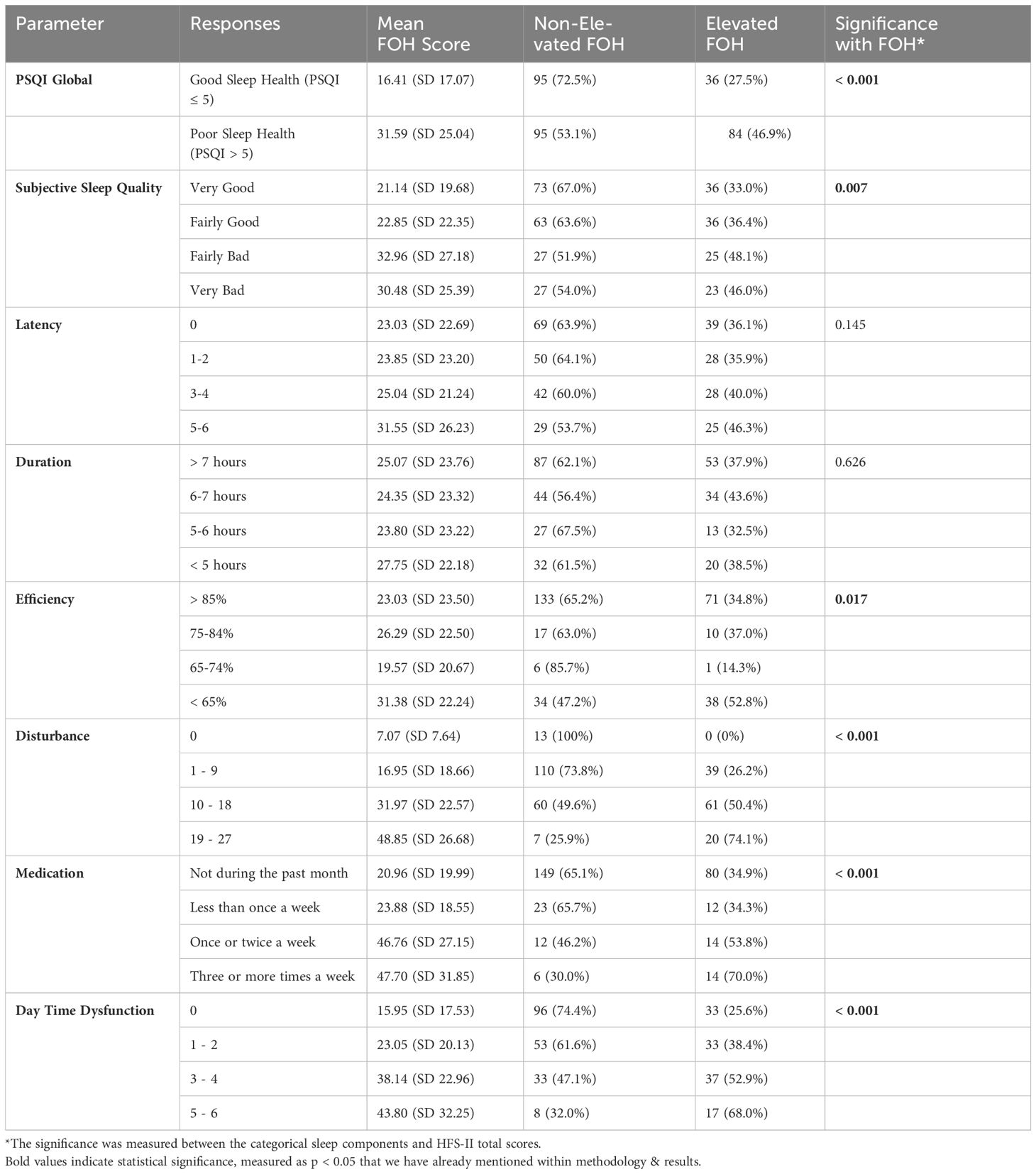
The sleep outcomes were measured as Global PSQI Score (sleep health) and seven sleep components (Table 4). A Spearman’s correlation between FOH and sleep health showed a highly significant positive monotonic relation between the two variables (p < 0.001, rs = 0.397, n = 310). The relationship with behavior and worry dimensions of HFS-II scale was equally significant (p < 0.001, rs = 0.379; p < 0.001, rs = 0.304 respectively). Notably, the correlation was positive, meaning the PSQI score increases with the increments in HFS-II scores. Moreover, the mean HFS-II scores were higher among patients with poor sleep health compared to those with good sleep health (Table 4). In addition, among the 179 patients with poor sleep health, 46.9% had an elevated FOH according to the EI criterion.
Going ahead, we attempted to understand the relationship between FOH and the sleep components to highlight grey areas in overall sleep health. The relationship of five out of seven components was significant (p < 0.05). These were subjective sleep quality, sleep efficiency, sleep disturbance, sleep medications, and daytime dysfunction. Sleep latency and duration showed an insignificant relationship with FOH. With few exceptions, the mean FOH scores as well as the proportion of elevated fear tend to rise with the worsening outcome of each sleep component. For example, a ‘fairly bad’ subjective sleep quality in patients is associated with 48.1% elevated FOH compared to 36.4% for ‘fairly good’ sleep quality. A similar trend is followed in other components except for the sleep duration where a relatively large deviation can be seen.
3.6 Relationship between FOH and sleep quality
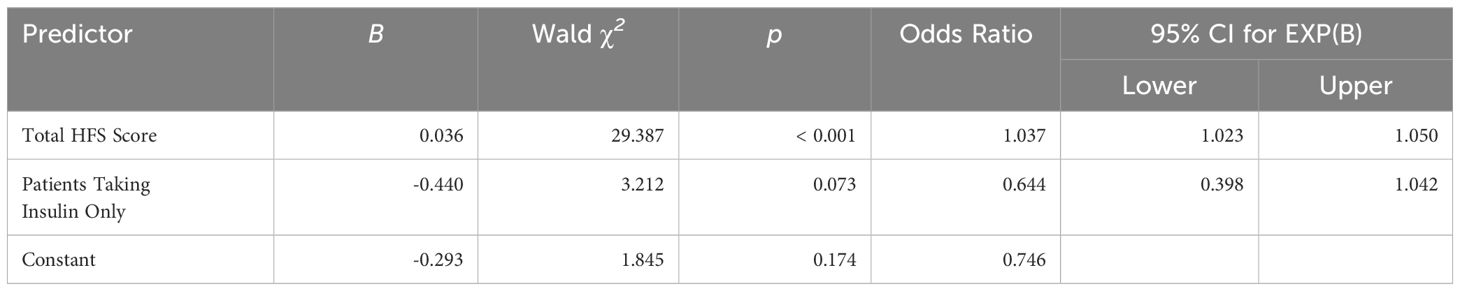
To further understand the nature of the relationship between FOH and sleep quality, logistic regression analysis was performed between the two variables (Table 5). The global PSQI score (continuous scale, 0-21) was converted into a binary variable ‘sleep health’ with two outputs, good sleep health (PSQI ≤ 5) and poor sleep health (PSQI > 5), to perform a binary logistic regression. The ‘HFS-II score’ (continuous variable) was added as the independent variable with ‘insulin use (dichotomous)’ as a covariate since it was found to be significantly related to sleep quality (Table 3). The model demonstrated an overall satisfactory fit, as evidenced by the -2 Log Likelihood value of 382.370 and the Cox & Snell R Square (12.1%) and Nagelkerke R Square (16.2%). For the predictor variable “Fear of hypoglycemia,” the coefficient (B) was 0.036, and the Wald chi-square test yielded a statistically significant result (χ² (1) = 29.387, p < 0.001). The odds ratio (Exp(B)) was 1.037, suggesting that for every one-unit increase in fear of hypoglycemia, the odds of experiencing poor sleep increased by approximately 3.7%. As for “Insulin use,” the coefficient (B) was -0.440, and the Wald chi-square test produced a p-value of 0.073, indicating marginal significance. The odds ratio (Exp(B)) for insulin use was 0.644, suggesting that individuals using insulin had approximately 60% lower odds of experiencing poor sleep compared to those not using insulin.
3.7 Diagnostic ability of FOH to predict poor sleep quality – receiver operating characteristic (ROC) curve analysis
ROC curve analysis is a graphical representation used to evaluate the diagnostic ability of a binary classifier system. In the present case, the ROC curve analysis was conducted to evaluate the diagnostic accuracy of three scales—HFS-B (Behavior score), HFS-W (Worry score), and HFS-T (Total HFS score)—in identifying poor sleep quality (Table 6, Figure 1). The dataset comprised 179 cases with severe sleep difficulty and 131 cases without. The Area Under the Curve (AUC) values were 0.691 for the Total HFS Score, 0.681 for the HFS-B Scores, and 0.647 for the HFS-W Scores, with all p-values being < 0.001. This indicates that all three scales are significantly better than chance at distinguishing between individuals with and without severe sleep difficulty. Following this, we analyzed sensitivity and 1 – specificity values of the three scales at a cutoff value of 3.5. The total HFS-II score showed the highest sensitivity, followed by HFS-B and HFS-W. However, as a trade-off between sensitivity and specificity, HFS-B appears to be more optimal with a specificity of 83.8% and a false-positive rate of 61.1%.
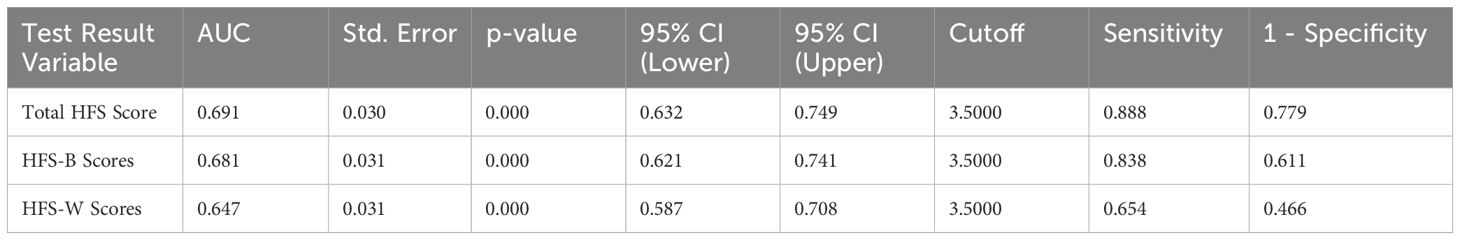
Table 6. Diagnostic performance of Total HFS, HFS-B, and HFS-W scores for identifying poor sleep quality using ROC.
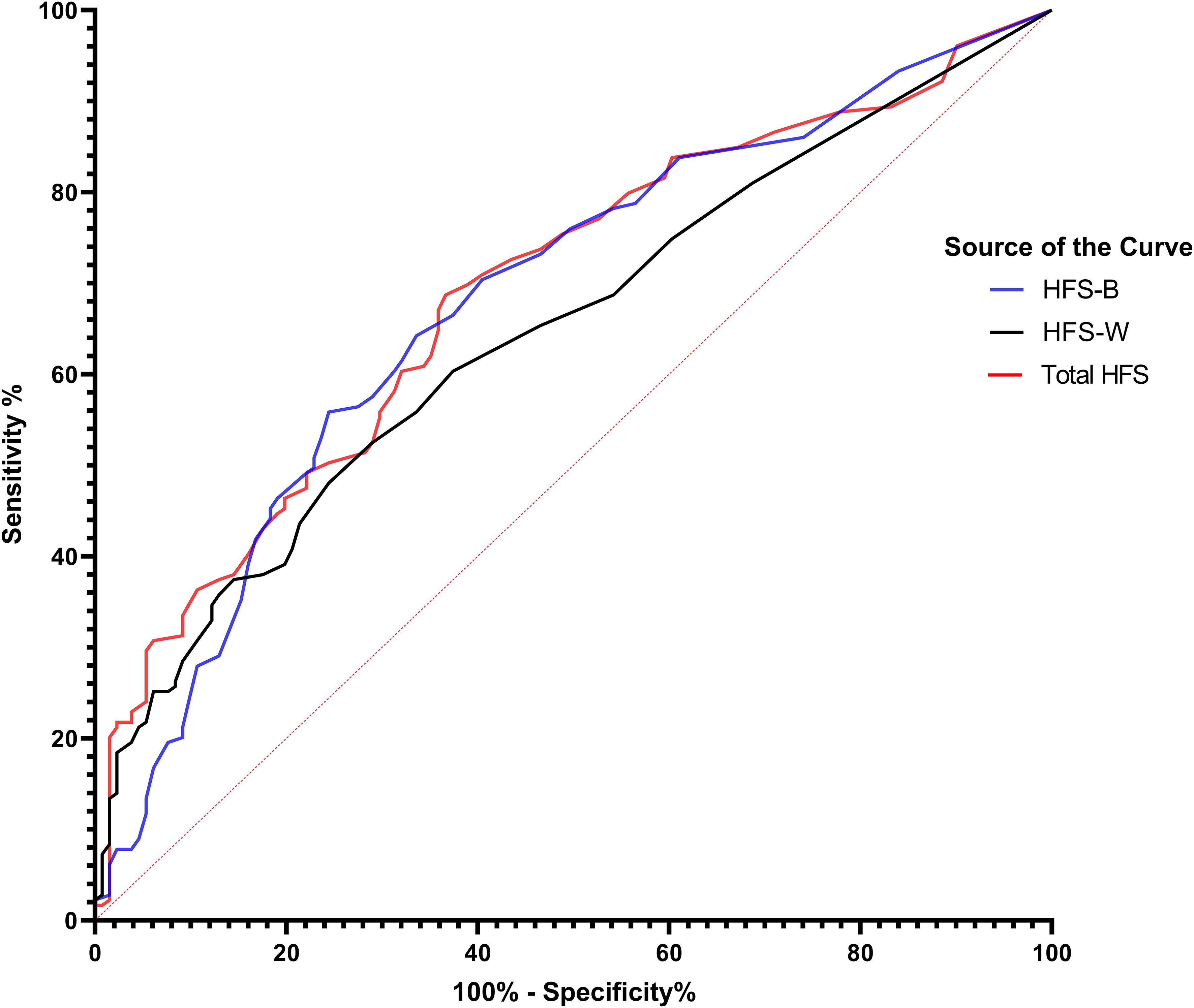
Figure 1. ROC Curves of fear of hypoglycemia scores predicting sleep quality in patients with diabetes. This figure shows ROC curves for components of the Fear of Hypoglycemia Survey (HFS) in predicting sleep quality among patients with diabetes. The blue curve (HFS-B) represents the behavioral component, the black curve (HFS-W) represents the worry component, and the red curve (Total HFS) represents the combined score. The diagonal red dashed line indicates no discrimination (AUC = 0.5). Higher AUC values denote better predictive accuracy.
4 Discussion
This is the first study examining the FOH among patients with diabetes and its influence on the quality of sleep in Pakistan. While the clinical practice is intensely oriented to reducing glycemic levels, the resultant hypoglycemic events and associated psychological implications are often ignored. FOH is one of the most common psychological manifestations of hypoglycemia. It is known to impact behavioral and worry patterns in individuals with diabetes. However, its impact on psychological states, such as sleep, is still in its infancy. Few studies that examined this particular relationship were related to Type-I patients (28) or adolescents (33).
In our study, with the inclusion of both Type-I and Type-II diabetics and no age restriction, the mean HFS-II score is 25.18 (SD 23.24) which is relatively lower compared to similar studies measuring FOH (10, 30, 34, 35). However, like our study, some other studies have also reported lower mean values for HFS scores (36). The EI criterion classified 120 individuals (38.7%) as having an elevated FOH which is relatively higher than other studies by Hajos et al. (32) (26%) and Majanovic et al. (37) (11.1%). The mean differences as well as fear characteristics could be attributed to the population differences in these studies.
In our study, FOH was higher in younger age (<10 – 30 years) as supported by other studies (35, 38, 39). Also, higher FOH was seen in patients above 60 years which is also evident from the literature (10, 11). We have also noted that the most vulnerable age groups with respect to FOH are those below 40 years and above 50 years. However, we agree with the interpretation of Martyn-Nemet et al. (40) that there is no consistent pattern of FOH with regard to age and that the relationship with age is complex. For gender comparisons, FOH was higher in females which are in uniform agreement with the previous literature (38, 41, 42).
Education seems to have a positive impact on the FOH. The fear is higher among participants with lower educational levels and vice versa. This appears to be a negative correlation as reported by Gonder-Fredrick et al. (30), however, we could not perform a direct correlation statistic due to the categorical nature of our variable. The relationship, nonetheless, remains insignificant and consistent with other studies (41).
Duration of diabetes was significantly associated with FOH in our study. The scores of HFS-II increased with the increase in the duration of diabetes. The findings are consistent with previous studies by Hongmei et al. (43) and Erol and Enc (41) where a positive correlation existed between the course of disease (diabetes) and FOH. Opposite findings also surfaced in some populations where there was no correlation between duration of diabetes and FOH or the FOH decreased with an increase in the duration of diabetes (30, 44).
The impact of glycemic control on FOH is debated. Some studies suggest improved glycemic control is associated with increased FOH (35, 40, 45), while others show higher FOH in patients with poor control (36). In our study, glycemic control showed a marginally insignificant relationship with FOH. However, patients with good control (FPG 80-130 mg/dL) were more likely to have FOH than those with poorer control (FPG > 130 mg/dL). Tight control lowers glucose levels intensively, raising hypoglycemia risk and concerns. Regular glucose monitoring and insulin dose management are key, especially in T2DM.
The FOH did not differ significantly among insulin users and those on oral anti-diabetic agents. However, the proportion of FOH was greater among insulin users compared to other groups. Studies have found a similar pattern of FOH among Type-I and Type-II diabetes patients, that is, individuals with T1DM have more FOH than individuals with T2DM, but the difference is not statistically significant (35, 36, 41).
As for the technology used for insulin administration, our data suggested that FOH was more prevalent in those administering insulin via syringes than those using pen fills. This means that technology infuses confidence and security among patients and is a reliable tool for reducing FOH. These results are consistent with a previous study showing that FOH was higher in patients using multiple-dose injection treatment compared to those receiving insulin via pump (38). However, in our study patients on insulin pumps showed a higher proportion of FOH but given the small number of users, the results cannot be generalized.
The relationship between FOH and sleep appears to be fairly significant. The univariate analysis as well as the multivariate logistic regression found the relationship significant. Moreover, five out of seven PSQI sleep components showed a statistically significant link with FOH. Sleep latency and sleep duration were not significantly linked with FOH. This generates the hypothesis that the FOH may not affect the initiation or termination of the sleep cycle rather it is more specifically linked with the quality of sleep. This could be disturbed sleep (p < 0.001), less efficient sleep (p = 0.017), medication-dependent sleep (p < 0.001), or sleep leading to daytime dysfunction (p < 0.001). These findings represent a novel contribution to the existing literature, signaling a need for further exploration and in-depth investigation into the specific mechanisms through which FOH manifests its impact on the qualitative aspects of sleep.
The ROC curve estimation also provided some novel insights into utilization of HFS-II scores in determining the quality of sleep. The results revealed that the HFS scores were better at predicting sleep quality than by chance. However, we couldn’t determine a specific cut-off score for a reliable diagnosis as the false positive rates were high. For the sake of comparison, a cut-off value of 3.5 was selected, which was closest to the maximum possible score on one item. This approach showed that the HFS-B scale was better at predicting poor sleep quality than the other forms of the scale in terms of sensitivity and specificity. This could be because of the underlying condition that the means of the HFS-B scores were higher in our study participants (15.40 (SD 13.64)) compared to the HFS-W scores (9.77 (SD 12.48)) indicating that fear derived from behavioral aspects may be more limiting towards sleep quality. However, the findings need clinical support to reflect more authenticity.
The identification of these nuanced relationships provides a foundation for targeted interventions aimed at improving sleep quality in individuals grappling with the psychological challenges associated with FOH. As the field advances, this newfound understanding paves the way for tailored strategies that encompass both glycemic control and psychological well-being in the holistic care of patients with diabetes. Further research endeavors should delve into the intricate dynamics of FOH and its implications on sleep, contributing to the evolving landscape of diabetes management and patient-centric care.
The strength of this study is that, for the first time, it provides comprehensive data on FOH in the diabetes population of Pakistan. While most of the studies utilize only the worry scale of HFS-II, we used the complete 33-item HFS-II scale in our sample population. Another strength of the study is that patients with both T1DM and T2DM were included in this study, unlike previous studies where the choice was selective. Finally, the study outlines an in-depth association between FOH and sleep quality and evaluates the diagnostic ability of FOH in terms of sleep quality. Moreover, it proposes a new hypothesis that the FOH may influence not the initiation and termination of the sleep cycle but its quality in an individual.
5 Conclusion
In conclusion, this study sheds light on the underexplored issue of FOH in the Pakistani population with diabetes, revealing its significant impact on both psychological well-being and sleep quality. The findings underscore the prevalence of FOH, with particular vulnerability among younger and older age groups, females, syringe users, and those with longer diabetes durations. Notably, FOH is closely linked with poor sleep quality, emphasizing the need for holistic diabetes management that addresses both glycemic control and psychological aspects. Healthcare practitioners should prioritize patient education and counseling, targeting the most common FOH concerns identified in this study. Furthermore, patients with disturbed sleep should be assessed for an elevated level of FOH as it could be a contributing factor to poor sleep quality. Future research should develop validated methods to better understand the intricate relationship between FOH and sleep disturbances.
6 Limitations
The cross-sectional design, missing confounding variables (e.g., history of hypoglycemic episodes, comorbidities e.g., obesity), and a higher proportion of female participants are some of the limitations of this study. Moreover, the data on glycemic control was generated from a single value of either BSR or BSF, which may not reflect the chronic condition of glycemic control. HbA1c could have been a better measure; however, we could not find enough data for this. Additionally, the PSQI tool assesses sleep quality over a relatively short period (past month) and may not capture long-term sleep patterns accurately. Furthermore, using HFS-II scores alone to classify patients into “elevated fear” or “non-elevated fear” groups may oversimplify FOH, which varies in intensity and impact among individuals. Similarly, future studies are also recommended to explore the relationship between fear of hypoglycemia and sleep quality in patients on non-hypoglycemic therapies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB), University of Sargodha. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HH: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing, Writing – original draft. NA: Formal analysis, Investigation, Writing – original draft. MWA: Formal analysis, Software, Validation, Writing – review & editing, Writing – original draft. FG: Resources, Supervision, Writing – review & editing, Writing – original draft. JK: Writing – original draft, Formal analysis, Writing – review & editing. MA: Resources, Visualization, Writing – review & editing, Writing – original draft. ST: Resources, Visualization, Writing – original draft. TA: Project administration, Supervision, Writing – review & editing, Writing – original draft. AM: Resources, Supervision, Validation, Writing – review & editing, Writing – original draft. SA: Resources, Supervision, Validation, Writing – review & editing, Writing – original draft. AS: Formal analysis, Validation, Writing – original draft. MN: Data curation, Writing – original draft. MP: Data curation, Writing – original draft. MU: Data curation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors extend their appreciation to King Saud University for funding this work through research supporting project (RSP2025R376), Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1456641/full#supplementary-material
References
1. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2009) 32:S62–7. doi: 10.2337/DC09-S062
2. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2014) 37:S81–90. doi: 10.2337/dc14-S081
3. International Diabetes Federation. Pakistan - International diabetes federation(2021). Available online at: https://idf.org/our-network/regions-and-members/middle-east-and-north-africa/members/Pakistan/ (Accessed August 21, 2023).
4. Almomani HY, Pascual CR, Grassby P, Ahmadi K. Effectiveness of the SUGAR intervention on hypoglycaemia in elderly patients with type 2 diabetes: A pragmatic randomised controlled trial. Res Soc Adm Pharm. (2023) 19:322–31. doi: 10.1016/j.sapharm.2022.09.017
5. Clayton D, Woo V, Yale JF. Hypoglycemia. Can J Diabetes. (2013) 37:S69–71. doi: 10.1016/j.jcjd.2013.01.022
6. Elsayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care. (2023) 46:S19–40. doi: 10.2337/DC23-S002
7. Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: Implications for diabetes management and patient education. Patient Educ Couns. (2007) 68:10–5. doi: 10.1016/j.pec.2007.05.003
8. Anarte MT, Carreira M, MaChado A, Domínguez M, Tapia MJ, Valdés S, et al. Identification of risk factors for suffering fear of hypoglycemia in type 1 diabetes mellitus patients. Scand J Psychol. (2014) 55:554–7. doi: 10.1111/sjop.12158
9. Johnson SR, Cooper MN, Davis EA, Jones TW. Hypoglycaemia, fear of hypoglycaemia and quality of life in children with Type 1 diabetes and their parents. Diabetes Med. (2013) 30:1126–31. doi: 10.1111/dme.12247
10. Wang Y, Zeng Z, Ding J, Yuan R, Wang R, Zhang Y, et al. Fear of hypoglycaemia among patients with type 2 diabetes mellitus: a cross-sectional study. Sci Rep. (2021) 11:7971. doi: 10.1038/S41598-021-86954-0
11. Böhme P, Bertin E, Cosson E, Chevalier N. Fear of hypoglycaemia in patients with type 1 diabetes: Do patients and diabetologists feel the same way? Diabetes Metab. (2013) 39:63–70. doi: 10.1016/j.diabet.2012.10.006
12. Desjardins K, Brazeau AS, Strychar I, Rabasa-Lhoret R. Are bedtime nutritional strategies effective in preventing nocturnal hypoglycaemia in patients with type 1 diabetes? Diabetes Obes Metab. (2014) 16:577–87. doi: 10.1111/dom.12232
13. Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. (2008) 31:2108–9. doi: 10.2337/dc08-0720
14. Martyn-Nemeth P, Quinn L, Penckofer S, Park C, Hofer V, Burke L. Fear of hypoglycemia: Influence on glycemic variability and self-management behavior in young adults with type 1 diabetes. J Diabetes Complications. (2017) 31:735–41. doi: 10.1016/j.jdiacomp.2016.12.015
15. Martyn-Nemeth P, Phillips SA, Mihailescu D, Farabi SS, Park C, Lipton R, et al. Poor sleep quality is associated with nocturnal glycemic variability and fear of hypoglycemia in adults with type 1 diabetes. J Adv Nurs. (2018) 74:2373. doi: 10.1111/JAN.13765
16. Di Battista AM, Hart TA, Greco L, Gloizer J. Type 1 diabetes among adolescents: reduced diabetes self-care caused by social fear and fear of hypoglycemia. Diabetes Educ. (2009) 35:465–75. doi: 10.1177/0145721709333492
17. Fidler C, Elmelund Christensen T, Gillard S, Nordisk NA, Samantha Gillard D. Hypoglycemia: An overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ. (2011) 14:646–55. doi: 10.3111/13696998.2011.610852
18. Martyn-Nemeth P, Quinn L, Hacker E, Park H, Kujath AS. Diabetes distress may adversely affect the eating styles of women with type 1 diabetes. Acta Diabetol. (2014) 51:683–6. doi: 10.1007/s00592-014-0575-1
19. Davis RE, Morrissey M, Peters JR, Wittrup-Jensen K, Kennedy-Martin T, Currie CJ. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin. (2005) 21:1477–83. doi: 10.1185/030079905X61929
20. Rossi MC, Nicolucci A, Ozzello A, Gentile S, Aglialoro A, Chiambretti A, et al. Impact of severe and symptomatic hypoglycemia on quality of life and fear of hypoglycemia in type 1 and type 2 diabetes. Results of the Hypos-1 observational study. Nutr Metab Cardiovasc Dis. (2019) 29:736–43. doi: 10.1016/J.NUMECD.2019.04.009
21. Gonder-Frederick LA, Vajda KA, Schmidt KM, Cox DJ, Devries JH, Erol O, et al. Examining the behaviour subscale of the hypoglycemia fear survey: An international study. Diabetes Med. (2013) 30:603–9. doi: 10.1111/dme.12129
22. Krawczyk J, Duda-Sobczak A, Zozulińska-Ziółkiewicz D. Fear of hypoglycaemia — from normality to pathology. Diagnostic criteria and therapeutic directions. Clin Diabetol. (2020) 9:487–92. doi: 10.5603/DK.2020.0046
23. Polonsky WH, Davis CL, Jacobson AM, Anderson BJ. Correlates of hypoglycemic fear in type I and type II diabetes mellitus. Heal Psychol. (1992) 11:199–202. doi: 10.1037//0278-6133.11.3.199
24. Shan W, Peng X, Tan W, Zhou Z, Xie H, Wang S. Prevalence of insomnia and associations with depression, anxiety among adults in guangdong, China: A large-scale cross-sectional study. Sleep Med. (2024) 115:39–47. doi: 10.1016/j.sleep.2024.01.023
25. Lee S-A, Im K, Seo JY, Jung M. Association between sleep apnea severity and symptoms of depression and anxiety among individuals with obstructive sleep apnea. Sleep Med. (2023) 101:11–8. doi: 10.1016/j.sleep.2022.09.023
26. Cobry EC, Bisio A, Paul Wadwa R, Breton MD. Improvements in parental leep, fear of hypoglycemia, and diabetes distress with use of an advanced hybrid closed-loop system. Diabetes Care. (2022) 45:1292–5. doi: 10.2337/dc21-1778
27. Speight J, Choudhary P, Wilmot EG, Hendrieckx C, Forde H, Cheung WY, et al. Impact of glycaemic technologies on quality of life and related outcomes in adults with type 1 diabetes: A narrative review. Diabetes Med. (2023) 40:e14944. doi: 10.1111/dme.14944
28. Suteau V, Saulnier PJ, Wargny M, Gonder-Frederick L, Gand E, Chaillous L, et al. Association between sleep disturbances, fear of hypoglycemia and psychological well-being in adults with type 1 diabetes mellitus, data from cross-sectional VARDIA study. Diabetes Res Clin Pract. (2020) 160:107988. doi: 10.1016/J.DIABRES.2019.107988
29. Farooque R, Herekar F, Iftikhar S, Patel MJ. The frequency of poor sleep quality in patients with diabetes mellitus and its association with glycemic control. Cureus. (2020) 12:e11608. doi: 10.7759/cureus.11608
30. Gonder-Frederick LA, Schmidt KM, Vajda KA, Greear ML, Singh H, Shepard JA, et al. Psychometric properties of the hypoglycemia fear survey-II for adults with type 1 diabetes. Diabetes Care. (2011) 34:801–6. doi: 10.2337/DC10-1343
31. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
32. Hajós TRS, Polonsky WH, Pouwer F, Gonder-Frederick L, Snoek FJ. Toward defining a cutoff score for elevated fear of hypoglycemia on the hypoglycemia fear survey worry subscale in patients with type 2 diabetes. Diabetes Care. (2014) 37:102–8. doi: 10.2337/DC13-0971
33. Hitt TA, Hershey JA, Olivos-Stewart D, Forth E, Stuart F, Garren P, et al. The impact of fear of hypoglycaemia on sleep in adolescents with type 1 diabetes. Diabetes Med. (2023) 40:e15066. doi: 10.1111/dme.15066
34. Glocker V, Bachmann S, Hess M, Szinnai G, Burckhardt MA. Fear of hypoglycemia and quality of life in young people with type 1 diabetes and their parents in the era of sensor glucose monitoring. Front Endocrinol (Lausanne). (2022) 13:958671/BIBTEX. doi: 10.3389/FENDO.2022.958671/BIBTEX
35. Hapunda G, Abubakar A, Pouwer F, van de Vijver F. Correlates of fear of hypoglycemia among patients with type 1 and 2 diabetes mellitus in outpatient hospitals in Zambia. Int J Diabetes Dev Ctries. (2020) 40:619–26. doi: 10.1007/S13410-020-00835-2
36. Lam AYR, Xin X, Tan WB, Gardner DSL, Goh SY. Psychometric validation of the hypoglycemia fear survey-II (HFS-II) in Singapore. BMJ Open Diabetes Res Care. (2017) 5:e000329. doi: 10.1136/bmjdrc-2016-000329
37. Al AA, Asirvatham H, Robert A, Braham Besher RB, Issa A, Al Sabaan FS. Predictive risk factors for fear of hypoglycemia and anxiety-related emotional disorders among adolescents with type 1 diabetes. Med Princ Pract. (2015) 24:222–30. doi: 10.1159/000375306
38. Majanovic SK, Janez A, Lefterov I, Tasic S, Cikac T. The real-life effectiveness and care patterns of diabetes management study for Balkan Region (Slovenia, Croatia, Serbia, Bulgaria): a multicenter, observational, cross-sectional study. Diabetes Ther. (2017) 8:929–40. doi: 10.1007/s13300-017-0288-x
39. Graue M, Iversen MM, Wentzel-Larsen T, Rokne B, Haugstvedt A. Assessing fear of hypoglycemia among adults with type 1 diabetes–psychometric properties of the Norwegian version of the Hypoglycemia Fear Survey II. Nor Epidemiol. (2013) 23:75–81. doi: 10.5324/nje.v23i1.1605
40. Martyn-Nemeth P, Schwarz Farabi S, Mihailescu D, Nemeth J, Quinn L. Fear of hypoglycemia in adults with type 1 diabetes: impact of therapeutic advances and strategies for prevention - a review. J Diabetes Complications. (2016) 30:167–77. doi: 10.1016/j.jdiacomp.2015.09.003
41. Erol O, Enc N. Hypoglycemia fear and self-efficacy of Turkish patients receiving insulin therapy. Asian Nurs Res (Korean Soc Nurs Sci). (2011) 5:222–8. doi: 10.1016/J.ANR.2011.12.001
42. Gjerløw E, Bjørgaas MR, Nielsen EW, Olsen SE, Åsvold BO. Fear of hypoglycemia in women and men with type 1 diabetes. Nurs Res. (2014) 63:143–9. doi: 10.1097/NNR.0000000000000020
43. Hongmei X, Chun M, Di B. Assessing fear of hypoglycemia and related factors in patients with type 2 diabetes mellitus. Chin J Diabetes Mellit. (2018) 10:735–9.
44. Beléndez M, Hernández-Mijares A. Beliefs about insulin as a predictor of fear of hypoglycaemia. Chronic Illn. (2009) 5:250–6. doi: 10.1177/1742395309346464
Keywords: fear of hypoglycemia, quality of sleep, diabetes mellitus, HFS-II scale, PSQI
Citation: Hussain HR, Ahmed N, Akram MW, Gulzar F, Khan JA, Asad M, Tahseen S, Ahmed T, Malik A, Akhtar S, Shahid A, Noor M, Pervaiz M and Rahman MU (2025) Fear of hypoglycemia: a key predictor of sleep quality among the diabetic population. Front. Endocrinol. 16:1456641. doi: 10.3389/fendo.2025.1456641
Received: 28 June 2024; Accepted: 27 March 2025;
Published: 28 April 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Cosmin Mihai Vesa, University of Oradea, RomaniaAlberto Casertano, University of Naples Federico II, Italy
Sethu Reddy, Central Michigan University, United States
Copyright © 2025 Hussain, Ahmed, Akram, Gulzar, Khan, Asad, Tahseen, Ahmed, Malik, Akhtar, Shahid, Noor, Pervaiz and Rahman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hafiz Rashid Hussain, cmFzaGlkLmh1c3NhaW5AdW9zLmVkdS5waw==; Abdul Malik, YW1vaW51ZGRpbkBrc3UuZWR1LnNh
†Present address: Nabeel Ahmed, School of Pharmacy, Kunming Medical University, Kunming, Yunnan, China
 Hafiz Rashid Hussain1*
Hafiz Rashid Hussain1* Nabeel Ahmed
Nabeel Ahmed Muhammad Waseem Akram
Muhammad Waseem Akram Faisal Gulzar
Faisal Gulzar Sana Tahseen
Sana Tahseen Abdul Malik
Abdul Malik Suhail Akhtar
Suhail Akhtar Mah Noor
Mah Noor Maryam Pervaiz
Maryam Pervaiz