- 1Andrology Unit, Department of Clinical Medicine, Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy
- 2Spinal Unit, San Raffaele Sulmona Institute, Sulmona, Italy
- 3Nephrology and Dialysis Division, Department of Medicine, San Salvatore Hospital, L’Aquila, Italy
- 4Faculty of Bioscience and Technology for Food, Agriculture and Environment, University of Teramo, Teramo, Italy
Objective: The impact of testosterone-based gender affirming hormone therapy (T-GAHT) on kidney function in transgender individuals assigned female at birth (AFAB) remains a matter of clinical uncertainty and debate. This study aimed to quantify through a meta-analytical approach the changes in estimated glomerular filtration rate (eGFR), a widely used clinical parameter that reflects how efficiently the kidneys filter waste products from the blood, and in secondary markers of kidney functions in this population during 24 months of GAHT. The eGFR was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation, which estimates kidney filtration based on serum creatinine, age, and sex.
Methods: A thorough search of MEDLINE, COCHRANE LIBRARY, SCOPUS and WEB OF SCIENCE databases was carried out to identify suitable studies. Quality of the articles was scored using the Effective Public Health Practice Project. Data were combined using random effects models and the between-study heterogeneity was assessed using Cochrane’s Q and I2.
Results: Twenty included studies provided information about an overall sample of 2380 individuals. The pooled estimates documented a significant decrease in eGFR (CKD-EPI equation) at 6 and 12 months with respect to baseline, using the attributed (female) gender. When the CKD-EPI equation was referred to the perceived (male) gender, eGFR significantly decreased after 12 months but not after 6 months of T-GAHT. The trend of eGFR values showed a transient decline during the first year of therapy, followed by stabilization at 18 and 24 months. This pattern is likely attributable to increased creatinine production due to testosterone-induced gains in muscle mass, rather than to a true decline in kidney function. Among the secondary outcomes, pooled estimates revealed significant increases of creatinine and uric acid levels at all follow-up times. On the contrary, blood urea nitrogen (BUN), a waste product filtered by the kidneys and commonly used to assess renal function, did not change significantly after either 6 months or 12 months of T-GAHT.
Conclusions: The influence of T-GAHT on eGFR in the first two years in healthy, young AFAB transgender individuals appears to be statistically significant, but is likely not clinically relevant. This interpretation is supported by the stability of BUN levels and the absence of adverse renal events in the included studies, suggesting preserved kidney function despite changes in creatinine-based estimates. Further research is warranted to identify more accurate tools for evaluating kidney function in this population, particularly during the early months of treatment or in individuals with pre-existing renal conditions.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024596106.
Introduction
Interpretation of laboratory tests in transgender individuals can be challenging in routine care, especially when the analytes have sex-specific reference intervals (1, 2). Health care professionals may be asked whether to use reference ranges based on assigned sex, self-identified gender, or a combined approach (3). Factors such as the type of hormone therapy initiated or its duration may influence this decision (4). Accurate interpretation is crucial for appropriate clinical decision-making.
This issue becomes particularly relevant when assessing the effects of gender-affirming hormone therapy (GAHT) on kidney function, a parameter influenced by several clinical and lifestyle-related factors (e.g., hydration, nutrition, comorbidities, and medications) and monitored through various biochemical markers. Available evidence does not clearly establish whether, and to what extent, testosterone preparations affect renal function in cisgender individuals (5), nor whether GAHT may exacerbate pre-existing renal impairment in transgender people assigned female at birth (AFAB) (6, 7).
Indeed, the assessment of kidney function in this population undergoing testosterone-based GAHT (T-GAHT) presents several challenges. First, testosterone treatment, by increasing muscle mass—and consequently serum creatinine (SCr) levels—could lead to an overestimation of renal dysfunction or may lead to a misclassification of kidney function (8). Second, there is still no specific formula validated for estimating glomerular filtration rate (eGFR) in transgender individuals. Currently, the most widely used formula is the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (9), which incorporates sex as a covariate because creatinine production is lower in cisgender females than in cisgender males due to differences in muscle mass; in addition, substantial sex- and gender-related differences in diet may exist. Consequently, if these factors are not properly accounted for, eGFR may be systematically overestimated in cisgender females (10).
In an attempt to overcome this limitation, some researchers suggest calculating an “intermediate” GFR value by averaging male and female estimates (11), while others recommend using the sex corresponding to the individual’s gender identity if GAHT has been ongoing for more than six months, although this approach is better validated for transgender men (12).
While these methodological challenges are important and deserve careful consideration, the central clinical question remains whether testosterone-based GAHT itself induces measurable changes in renal function in AFAB individuals. To address this question, we conducted a comprehensive systematic review and meta-analysis aimed at evaluating whether T-GAHT leads to clinically meaningful alterations in renal parameters—including eGFR, SCr, uric acid, and blood urea nitrogen (BUN)—over a follow-up period of up to 24 months. By doing so, we aimed to contribute to the expanding field of transgender medicine by offering a clearer understanding of the renal effects of masculinizing hormone therapy, beyond the technical difficulties inherent to laboratory test interpretation.
Methods
The study was conducted according to the statement Preferred Reporting Items for Systematic reviews and Meta-analyses protocols (PRISMA-P) (13); it also complies with the guidelines for Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE) (14). The study protocol was registered in the international prospective registry for systematic reviews (PROSPERO) with registration number CRD42024596106.
Systematic search strategy
A systematic search was carried out in PubMed, Scopus, Web of Science and Cochrane Library to identify the studies published in English on this topic up to November 2024. The databases were queried by means of a purpose-built search string using the biomedical vocabulary Medical Subject Headings (MeSH) of PubMed. For the extraction of publications (records), the following terms were used: “transgender”, “AFAB”, “FtM”, “female to male”, “transmen”, “trans men”, “GAHT”, “gender-affirming hormone therapy”, “testosterone”, “androgen*”, “kidney”, “uric acid”, “BUN”, “urate”, “urea”, “creatinine”, “proteinuria”, “cystatin”, “creatinine clearance”, eGFR, GFR, “Cockcroft-Gault”, “MDRD”, “CKD-EPI”, “SCr/Q”, “CKiDU25”. To combine these key terms we used the Boolean operators AND/OR. If it was not clear from reading the abstract whether the study contained relevant data, the full text was retrieved. Finally, possible additional studies were identified by means of a manual search among the references cited in the articles included.
Inclusion and exclusion criteria
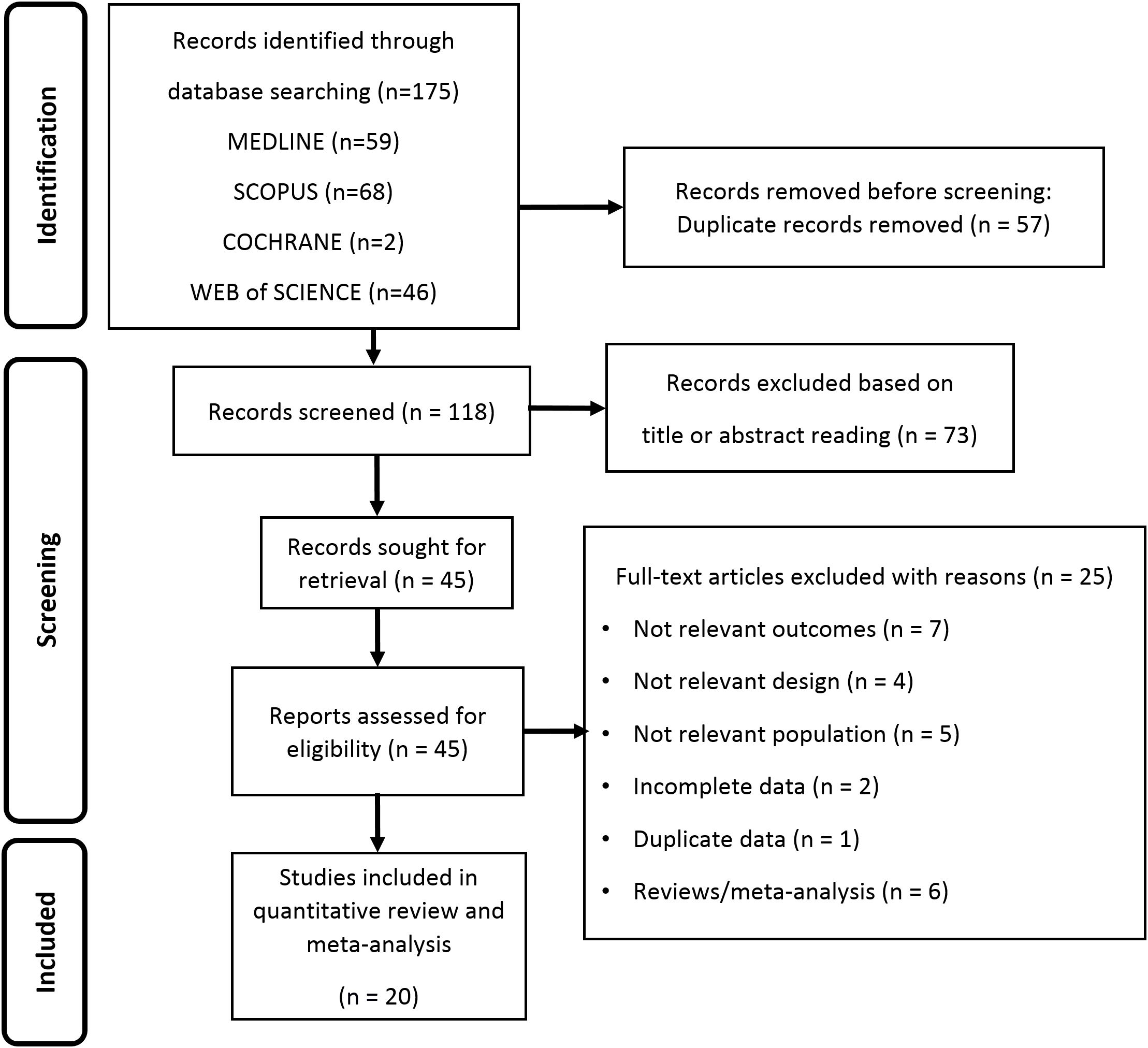
The study selection for inclusion was carried out in several stages. In the identification phase, querying the databases identified potentially eligible studies that could be included in the meta-analysis. Following the removal of repeated articles (same publication found in more than one database), in the second phase, studies of possible interest were screened by reading the title and abstract. In the third phase, the remaining articles were assessed in full text for eligibility. The following criteria were used: (1) studies enrolling AFAB transgender individuals undergoing T-GAHT; (2) availability of pre- and post-intervention values related to the primary outcome and/or one or more of the secondary outcomes, as reported below. Observational studies, as well as longitudinal intervention studies were considered eligible, while we excluded studies that did not focus on the target population, lacked relevant outcomes, used a non-eligible design, or presented incomplete or inconsistent data. Two independent reviewers (D.T., L.S.) assessed the full text of all selected studies to establish eligibility, and any disagreements were resolved through an open discussion involving a third reviewer (A.B.). The flow-chart proposed by Page et al. (15) was used to schematize the steps for the inclusion of studies.
Quality assessment
The methodological quality of the included articles was established using the Quality assessment tool for Quantitative studies developed by the Effective Public Health Practice Project (EPHPP) (16). This quality assessment tool, used for intervention studies as well as randomized controlled and case-control studies, was also validated for systematic reviews (17). It considers the following domains: selection bias, study design, confounding factors, study blindness, data collection method and losses at follow-up. The quality of each domain can be indicated as strong (strong), moderate (moderate) or weak (weak), and in the overall judgment, the quality can be considered strong, if no weak score was assigned, moderate, if only a weak judgment was assigned to one of the domains, and finally, weak, if two or more weak judgments were assigned to several domains. Two independent reviewers (D.T., L.S.) performed the quality assessment.
Data extraction
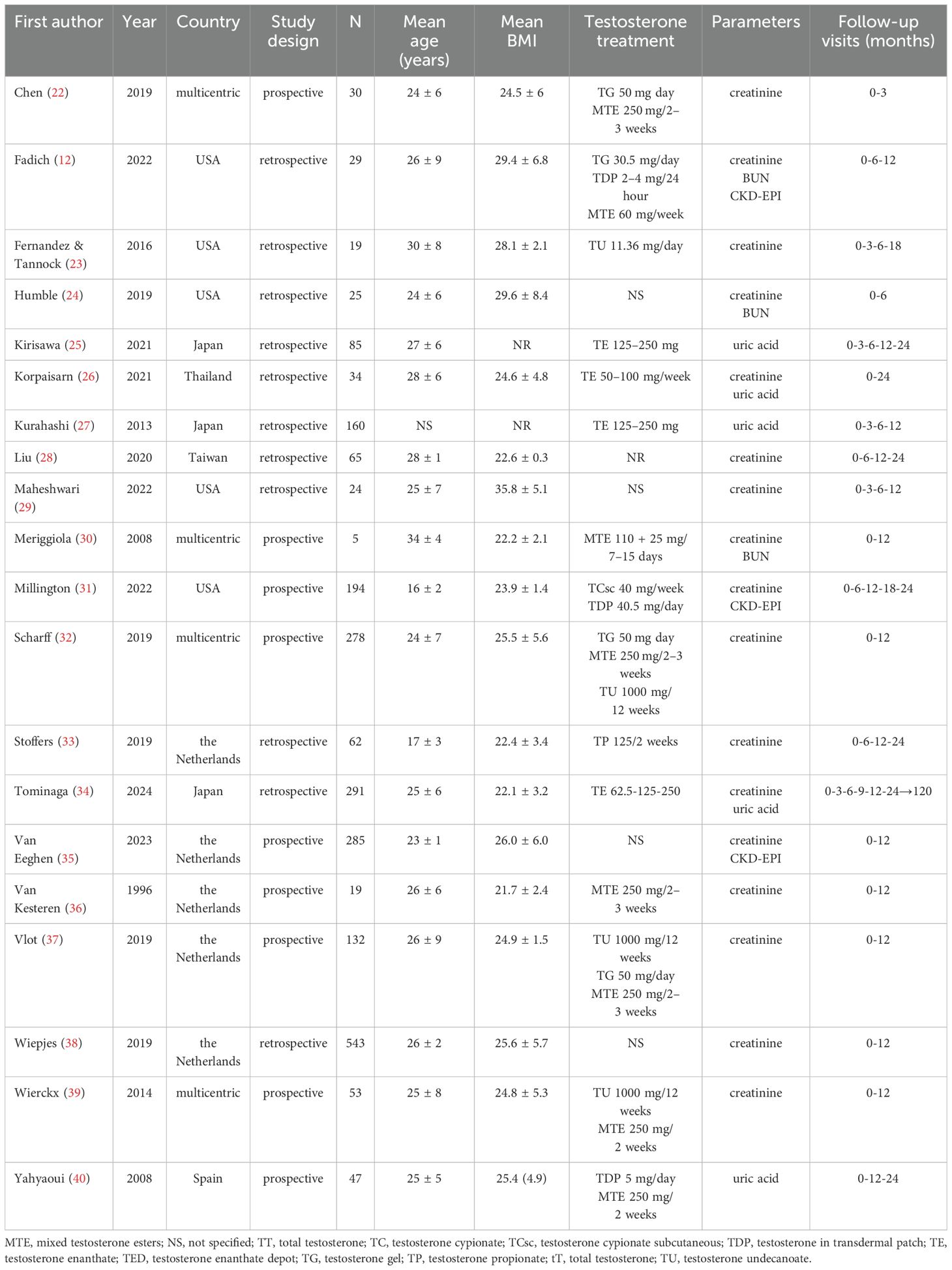
Data were extracted from the studies selected by two independent reviewers (D.T., A.B.). The primary outcome was the mean difference in estimated glomerular filtration rate (eGFR) at 6, 12, 18 and 24 months of GAHT with respect to the baseline, using the CKD-EPI equation for both attributed (female) and self-identified (male) gender. The secondary outcomes were the variations over time in SCr, blood urea nitrogen (BUN), uric acid (UA) levels, systolic (SBP) and diastolic blood pressure (DBP). Additional information extracted, when available, were mean age and body mass index (BMI) of the participants, as well as the type of testosterone preparation administered.
Statistical analysis
The effect of the T-GAHT on kidney parameters was assessed with Mantel-Haenszel estimates using the mean difference (MD) and 95% confidence interval (CI) when different follow-up times were compared with the baseline values. The Cochran’s χ2 (Cochran’s Q) and I2 tests were carried out to analyze statistical heterogeneity between the results of different studies: I2>50% and/or p<0.05 indicated substantial heterogeneity (18, 19). Data were combined using a random effects model. Even when a low heterogeneity was detected, a random-effects model was applied, because the validity of tests for heterogeneity can be limited with a small number of included studies. Publication bias was explored through the funnel plot (20) and Duval and Tweedie trim-and-fill test (21), to help detect presumed missing studies to rebalance the funnel distribution in the presence of a skewed shape. In addition, the test recalculates the combined estimate after the inclusion of these putative missing studies, thus correcting the analysis for publication bias. Data were analyzed using the Review Manager of the Cochrane Library (version5.3; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and the R statistical software (version 3.6.3, 2020; The R Foundation for Statistical Computing, Vienna, Austria) with the “metafor” package.
Results
Study selection
Searching from database yielded a total of 175 studies. Removal of duplicates resulted in a total of 118 publications, of which 73 were judged to be irrelevant simply by reading the title and abstract. Thus, as shown in Figure 1, 45 articles were identified, of which 20 met the inclusion criteria (12, 22–40). Details of the studies included in the quantitative synthesis are summarized in Table 1.
Quality assessment
The quality assessment based on the EPHPP is summarized in Table 2. Overall, most studies (15 of 20) received a methodological quality rating of ‘‘moderate’’ (12, 22, 24, 27–32, 34–37, 39, 40) and 5 studies were scored as ‘‘weak’’ (23, 25, 26, 33, 38). The items ‘‘confounders’’ and “data collection methods” received the highest rating among all the included studies; on the contrary, the item ‘‘blinding’’ was the most lacking, as in none of the studies the participants and the researchers who assessed outcomes were blind to the study conditions. Seven studies received a ‘‘moderate’’ or ‘‘weak’’ methodological quality rating in the item ‘‘withdrawals and dropouts’’ because of the large difference in the number of participants between initial enrollment and the end of follow-up (12, 23, 25, 26, 28, 33, 38).
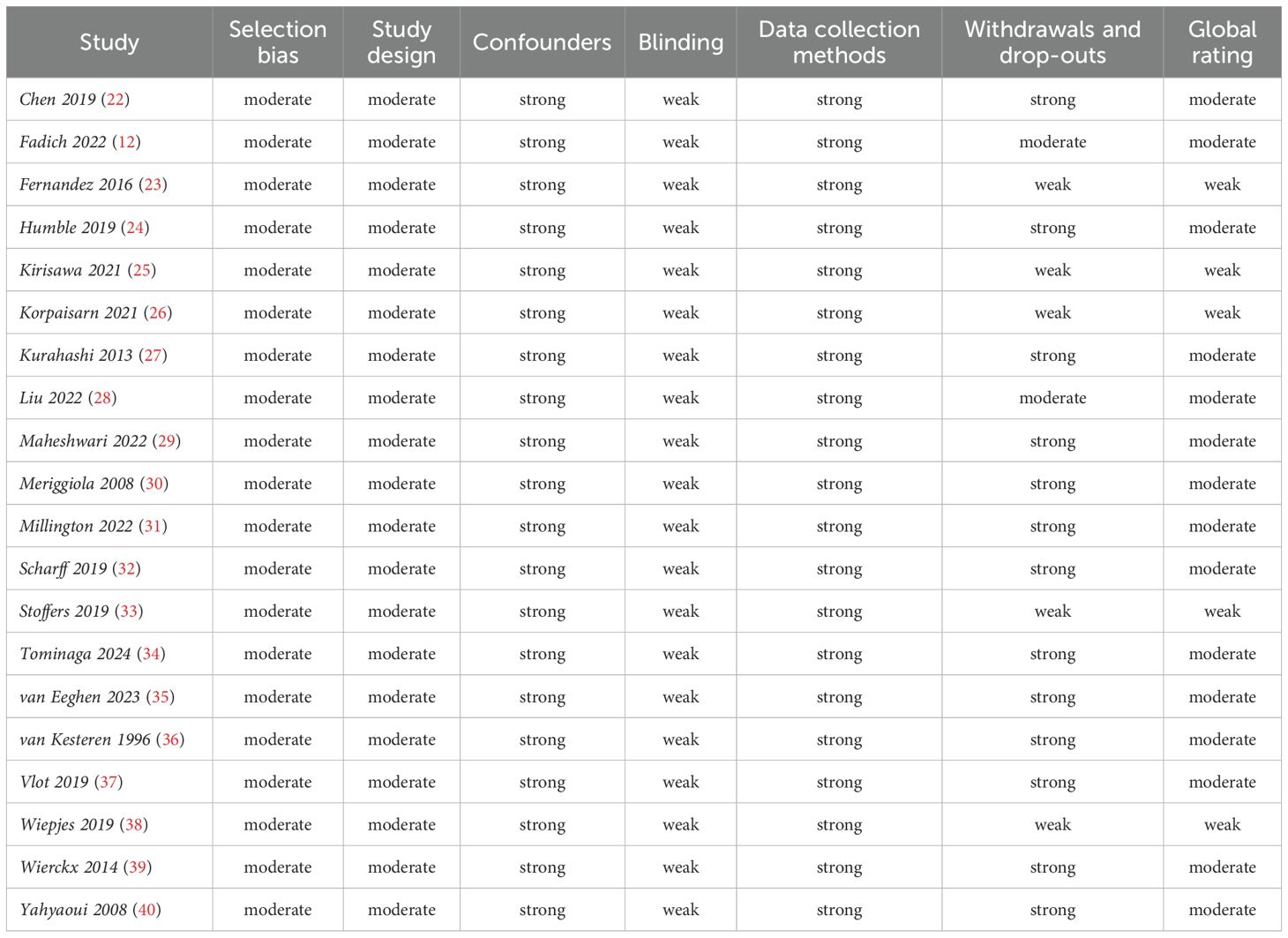
Table 2. Quality assessment of included studies by Effective Public Health Practice Project quality assessment tool.
Primary outcome: glomerular filtration rate
Three studies reported information on calculated GFR in a total of 141 and 407 AFAB individuals after 6 and 12 months of T-GAHT, respectively (Figure 2). The overall mean difference (MD) documented a statistically significant decrease in GFR at 6 and 12 months as assessed by CKD-EPI equation, using the attributed (female) gender (6 months: MD = -12.52; 95% CI: -16.65, -8.4, p <0.0001; I2 = 0%, Pfor heterogeneity = 0.35; 12 months: MD = -17.21; 95% CI: -19.44, -14.97, p <0.00001; I2 = 0%, Pfor heterogeneity = 0.82). When the self-identified (male) gender was included in the CKD-EPI equation, a significant decrease in GFR was revealed after 12 months (MD = -0.64; 95% CI: -0.88, -0.40, p <0.00001; I2 = 54%, Pfor heterogeneity = 0.11) but not 6 months of GAHT (MD = -0.27; 95% CI: -0.66, 0.11, p = 0.16; I2 = 50%, Pfor heterogeneity = 0.35). The trend of the weighted averages of the estimated glomerular filtration rate values using the CKD-EPI formula according to attributed or self-identified gender at each follow-up time is presented in Figure 3: it suggests an initial decrease in eGFR during early T-GAHT, likely reflecting changes in creatinine production rather than impaired renal function.
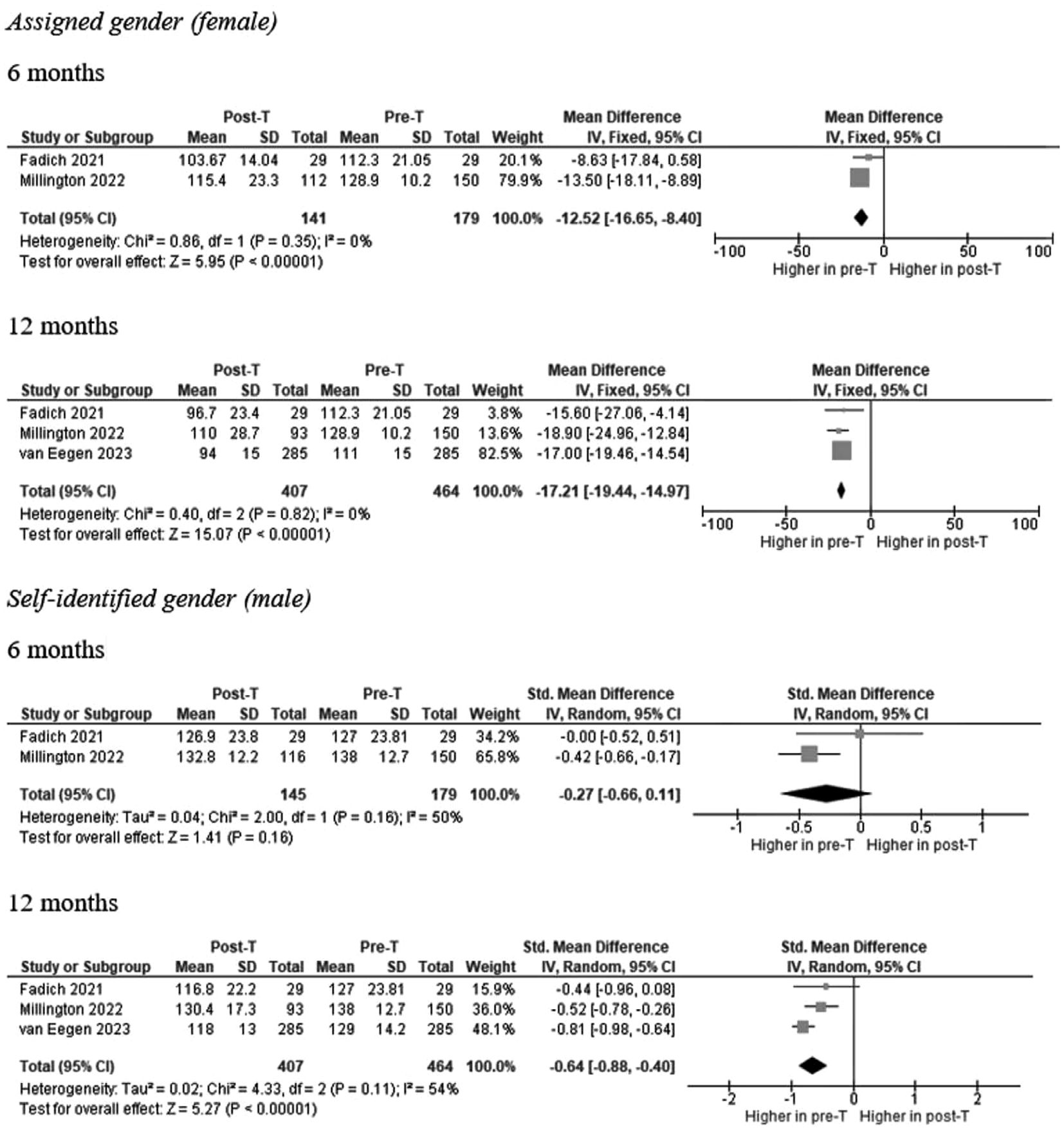
Figure 2. Forests plot of the effects of T-based GAHT on Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) mean values (ml/min/1.73 m²) in TM according to assigned (A) or perceived (B) gender. Diamonds indicate the overall effect estimates (and diamond width the 95% CI); squares indicate the weight of individual studies in the aggregate estimate. CI confidence interval, IV inverse variance, T Testosterone, GAHT gender affirming hormone therapy, TM transmen.
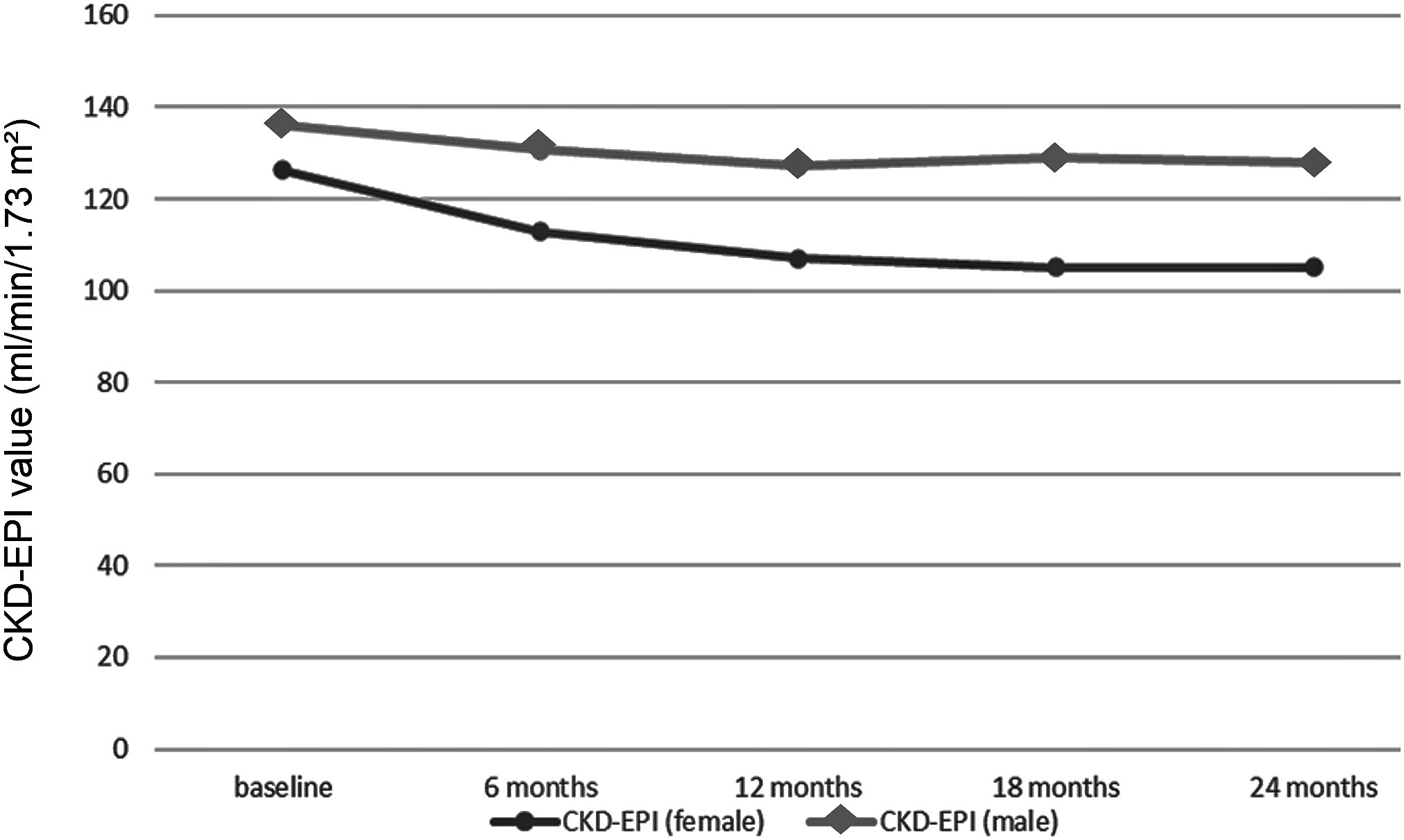
Figure 3. Trends in weighted average glomerular filtration rate values estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula at each follow-up time point. The distinction between eGFR trajectories is represented using different markers: square symbols indicate estimates based on female sex in the CKD-EPI equation, while circles correspond to self-identified male gender.
Secondary outcomes
Secondary endpoints included changes under GAHT in SCr, UA, BUN, SBP and DBP levels. Overall combined estimates documented a significant increase in SCr at all follow-up times (3, 6, 12, 18, and 24 months) compared with baseline, albeit with significant heterogeneity between studies after the third month (Supplementary Figure 1).
Overall, UA levels also increased significantly after 6, 12, and 24 months compared with baseline, with high reproducibility among studies (Supplementary Figure 2).
On the contrary, BUN did not change significantly after either 6 months or 12 months of therapy (Supplementary Figure 3).
Finally, while DBP did not change significantly at either 6 or 12 months (Supplementary Figure 4A), SBP increased significantly after 6 months of therapy, returning to values not significantly different from baseline after 12 months (Supplementary Figure 4B).
Publication bias
Given the unavailability of an adequate number of studies for most of the outcomes analyzed, including the primary outcome, we assessed publication bias only for data on the change in SCrvalues at 12 months of therapy. As shown in Supplementary Figure 5, the asymmetric shape of the funnel plot might suggest the presence of publication bias. However, the trim-and-fill analysis did not identify any putative missing studies.
Discussion
To our knowledge, this is the first systematic review and meta-analysis assessing the impact of testosterone-based gender affirming hormone therapy (T-GAHT) on kidney function in transgender individuals assigned female at birth (AFAB).
The direct effect of testosterone administration on kidney physiology has been explored in several preclinical studies using murine models, yielding inconclusive findings. Sex hormones appear to exert opposing actions on renal tissue: testosterone induces podocyte injury, while estrogens are protective (41–43). Conversely, in male rats, testosterone has been shown to mitigate renal ischemia–reperfusion injury, independently of estradiol (44). Lichtenecker et al. reported that testosterone treatment in female rats increased glomerular area and kidney size after four months, although this was associated with reduced GFR and histological changes (45). Other studies in male rodents have shown that early orchiectomy may prevent proteinuria and delay glomerulosclerosis (46), suggesting a detrimental role of androgens. In humans, androgens have been implicated in increased blood pressure and impaired renal function (47, 48), potentially through enhanced tubular sodium and water reabsorption (49) and activation of vasoconstrictive pathways such as the renin–angiotensin system and endothelin (48, 50–52). In line with these findings, our analysis showed a transient increase in systolic blood pressure after six months of T-GAHT, followed by stabilization.
In recent years, several studies have examined how best to estimate GFR in transgender individuals receiving hormone therapy (12, 31, 35), including whether to use the sex assigned at birth or the individual’s self-identified gender. In our analysis, we assessed changes in eGFR using both approaches across all follow-up timepoints. As highlighted by Krasowski, this choice impacts CKD stage classification according to KDIGO 2013 guidelines (53). Most of the included studies relied on the 2009 version of the CKD-EPI equation, which incorporates a race-based correction. Although the updated 2021 race-neutral equation (54) is now recommended, particularly in the United States (55), we used the published eGFR values without recalculating them due to the lack of access to individual-level data. This is acknowledged as a methodological limitation.
Using the female coefficient in CKD-EPI, despite ongoing masculinization and male gender identification, may overestimate renal function during follow-up. This explains the artifactual increase in eGFR observed when switching from sex-assigned-at-birth to affirmed-gender coefficients (Figure 3). However, when a consistent coefficient is applied over time, eGFR values decrease at 6 and 12 months and then stabilize at 18 and 24 months.
Whether this pattern reflects true renal impairment remains uncertain. The observed rise in SCr during the first year of T-GAHT, as reported in several studies (56, 57), likely reflects testosterone-induced increases in muscle mass and altered body composition (58, 59). This interpretation is supported by the concomitant rise in uric acid levels, which plateau after 12 months (Supplementary Figure 2), consistent with increased purine metabolism.
Transient hemodynamic changes, such as the rise in systolic blood pressure at six months (Supplementary Figure 4B), may also contribute to these changes. However, the absence of significant alterations in BUN (Supplementary Figure 3) and the lack of reported cases of new-onset hypertension or antihypertensive treatment suggest that these variations are more likely to represent a physiological adaptation to masculinization rather than early signs of pathological renal involvement (60, 61), consistent with recent insights into testosterone’s cardiovascular effects during gender-affirming therapy (62). Future research should clarify whether T-GAHT poses any clinically significant risk of hypertension.
This meta-analysis has several limitations. The observational nature of all included studies, combined with the absence of control groups, limits the ability to control for confounding variables. Attrition bias is also notable, as several studies reported substantial loss to follow-up. Moreover, most participants were young and had normal kidney function at baseline, which restricts the generalizability of our findings to older individuals or those with pre-existing renal disease. Additionally, none of the included studies assessed alternative biomarkers of renal function, such as cystatin C, which is less influenced by muscle mass and may better reflect glomerular filtration in individuals undergoing masculinizing hormone therapy. Although van Eeghen et al. (35) acknowledged the potential value of cystatin C, no longitudinal data were reported. Finally, while a few studies mentioned the concurrent use of GnRH agonists (30, 33), none stratified renal outcomes by treatment regimen or explored the specific impact of GnRH suppression. This remains an important area for future investigation.
In conclusion, testosterone-based GAHT appears to have a statistically significant impact on eGFR in healthy, young AFAB individuals during the first two years of therapy. However, these changes are unlikely to reflect clinically meaningful renal impairment. eGFR values—regardless of whether calculated using male or female CKD-EPI coefficients—tend to stabilize after 12 months and remain well above thresholds for renal dysfunction. These findings underscore the importance of cautious interpretation of renal function markers during early T-GAHT. Further studies are needed to evaluate these effects in more diverse populations, particularly in older individuals and those with existing kidney disease, and to validate sex-independent tools for accurately monitoring renal function in transgender people receiving gender-affirming hormone therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DT: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. LS: Data curation, Methodology, Writing – original draft, Writing – review & editing. LP: Data curation, Methodology, Writing – original draft, Writing – review & editing. CT: Data curation, Writing – original draft, Writing – review & editing. VD: Data curation, Writing – original draft, Writing – review & editing. GC: Data curation, Writing – original draft, Writing – review & editing. MB: Supervision, Validation, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare(s) that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare(s) that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1537838/full#supplementary-material
Supplementary Figure 1 | Forest plot of the effects of T-based GAHT on creatinine mean values (mg/dl) in TM. Diamonds indicate the overall effect estimates (and diamond width the 95% CI); squares indicate the weight of individual studies in the aggregate estimate. CI confidence interval, IV inverse variance, T Testosterone, GAHT gender affirming hormone therapy, TM transmen.
Supplementary Figure 2 | Forest plot of the effects of T-based GAHT on uric acid values (mg/dl) in TM. Diamonds indicate the overall effect estimates (and diamond width the 95% CI); squares indicate the weight of individual studies in the aggregate estimate. CI confidence interval, IV inverse variance, T Testosterone, GAHT gender affirming hormone therapy, TM transmen.
Supplementary Figure 3 | Forest plot of the effects of T-based GAHT on BUN values in TM. Diamonds indicate the overall effect estimates (and diamond width the 95% CI); squares indicate the weight of individual studies in the aggregate estimate. BUN blood urea nitrogen (mg/dl), CI confidence interval, IV inverse variance, T Testosterone, GAHT gender affirming hormone therapy, TM transmen.
Supplementary Figure 4 | Forest plot of the effects of T-based GAHT in TM on diastolic (A) and systolic (B) blood pressure values (mmHg). Diamonds indicate the overall effect estimates (and diamond width the 95% CI); squares indicate the weight of individual studies in the aggregate estimate. CI confidence interval, IV inverse variance, T Testosterone, GAHT gender affirming hormone therapy, TM transmen.
Supplementary Figure 5 | Funnel plot of results from studies assessing changes in creatinine levels (mg/dl) after 12 months of testosterone-based gender affirming hormone therapy.
References
1. Horn PS and Pesce AJ. Reference intervals: an update. Clin Chim Acta. (2003) 334:5–23. doi: 10.1016/s0009-8981(03)00133-5
2. Roberts TK, Kraft CS, French D, Ji W, Wu AH, Tangpricha V, et al. Interpreting laboratory results in transgender patients on hormone therapy. Am J Med. (2014) 127:159–62. doi: 10.1016/j.amjmed.2013.10.009
3. Irwig MS. Which reference range should we use for transgender and gender diverse patients? J Clin Endocrinol Metab. (2021) 106:e1479–80. doi: 10.1210/clinem/dgaa671
4. Cheung AS, Lim HY, Cook T, Zwickl S, Ginger A, Chiang C, et al. Approach to interpreting common laboratory pathology tests in transgender individuals. J Clin Endocrinol Metab. (2021) 106:893–901. doi: 10.1210/clinem/dgaa546
5. Filler G, Ramsaroop A, Stein R, Grant C, Marants R, So A, et al. Is testosterone detrimental to renal function? Kidney Int Rep. (2016) 1:306–10. doi: 10.1016/j.ekir.2016.07.004
6. Eckenrode HE, Carwie JC, and Curtis LM. Does gender affirming hormone therapy increase the risk of kidney disease? Semin Nephrol. (2022) 42:151284. doi: 10.1016/j.semnephrol.2022.10.010
7. Eckenrode HE, Gutierrez OM, Osis G, Agarwal A, and Curtis LM. Kidney disease prevalence in transgender individuals. Clin J Am Soc Nephrol. (2022) 17:280–2. doi: 10.2215/CJN.04660421
8. Krupka E, Curtis S, Ferguson T, Whitlock R, Askin N, Millar AC, et al. The effect of gender-affirming hormone therapy on measures of kidney function: A systematic review and meta-analysis. Clin J Am Soc Nephrol. (2022) 17:1305–15. doi: 10.2215/CJN.01890222
9. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
10. Collister D, Saad N, Christie E, and Ahmed S. Providing care for transgender persons with kidney disease: A narrative review. Can J Kidney Health Dis. (2021) 8:2054358120985379. doi: 10.1177/2054358120985379
11. Collister D, Saad N, Christie E, and Ahmed S. Response to “Assessment of renal function in transgender patients with kidney disease. Can J Kidney Health Dis. (2021) 8:20543581211020178. doi: 10.1177/20543581211020178
12. Fadich SK, Kalayjian A, Greene DN, and Cirrincione LR. A retrospective analysis of creatinine-based kidney function with and without sex assigned at birth among transgender adults. Ann Pharmacother. (2022) 56:791–9. doi: 10.1177/10600280211050120
13. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation [published correction appears in BMJ. 2016;354:i4086. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
14. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
16. Thomas J, Harden A, Oakley A, Oliver S, Sutcliffe K, Rees R, et al. Integrating qualitative research with trials in systematic reviews. BMJ. (2004) 328:1010–2. doi: 10.1136/bmj.328.7446.1010
17. Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, and Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. (2012) 18(1):12–8. doi: 10.1111/j.1365-2753.2010.01516.x
18. DerSimonian R and Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
19. Higgins JP and Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
20. Sterne JA and Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. (2001) 54:1046–55. doi: 10.1016/s0895-4356(01)00377-8
21. Boekhout-Berends ET, Wiepjes CM, Nota NM, Schotman HH, Heijboer AC, and den Heijer M. Changes in laboratory results in transgender individuals on hormone therapy - a retrospective study and practical approach. Eur J Endocrinol. (2023) 24:lvad052. doi: 10.1093/ejendo/lvad052
22. Chen H, Wiepjes CM, van Schoor NM, Heijboer AC, de Jongh RT, den Heijer M, et al. Changes of vitamin D-binding protein, and total, bioavailable, and free 25-hydroxyvitamin D in transgender people. J Clin Endocrinol Metab. (2019) 104:2728–34. doi: 10.1210/jc.2018-02602
23. Fernandez JD and Tannock LR. Metabolic effects of hormone therapy in transgender patients. Endocr Pract. (2016) 22:383–8. doi: 10.4158/EP15950.OR
24. Humble RM, Imborek KL, Nisly N, Greene DN, and Krasowski MD. Common hormone therapies used to care for transgender patients influence laboratory results. J Appl Lab Med. (2019) 3:799–814. doi: 10.1373/jalm.2018.027078
25. Kirisawa T, Ichihara K, Sakai Y, Morooka D, Iyoki T, and Masumori N. Physical and psychological effects of gender-affirming hormonal treatment using intramuscular testosterone enanthate in Japanese transgender men. Sex Med. (2021) 9:100306. doi: 10.1016/j.esxm.2020.100306
26. Korpaisarn S, Chiewchalermsri D, Arunakul J, Chinthakanan O, Poomthavorn P, and Sriphrapradang C. Effects of testosterone treatment on transgender males: A single-institution study. SAGE Open Med. (2021) 9:20503121211051546. doi: 10.1177/20503121211051546
27. Kurahashi H, Watanabe M, Sugimoto M, Ariyoshi Y, Mahmood S, Araki M, et al. Testosterone replacement elevates the serum uric acid levels in patients with female to male gender identity disorder. Endocr J. (2013) 60:1321–7. doi: 10.1507/endocrj.ej13-0203
28. Liu YH, Wu TH, Chu CH, Lin YC, and Lin LY. Metabolic effects of cross-sex hormone therapy in transgender individuals in Taiwan. J Chin Med Assoc. (2021) 84:267–72. doi: 10.1097/JCMA.0000000000000475
29. Maheshwari A, Dines V, Saul D, Nippoldt T, Kattah A, and Davidge-Pitts C. The effect of gender-affirming hormone therapy on serum creatinine in transgender individuals. Endocr Pract. (2022) 28:52–7. doi: 10.1016/j.eprac.2021.08.009
30. Meriggiola MC, Armillotta F, Costantino A, Altieri P, Saad F, Kalhorn T, et al. Effects of testosterone undecanoate administered alone or in combination with letrozole or dutasteride in female to male transsexuals. J Sex Med. (2008) 5:2442–53. doi: 10.1111/j.1743-6109.2008.00909.x
31. Millington K, Barrera E, Daga A, Mann N, Olson-Kennedy J, Garofalo R, et al. The effect of gender-affirming hormone treatment on serum creatinine in transgender and gender-diverse youth: implications for estimating GFR. Pediatr Nephrol. (2022) 37:2141–50. doi: 10.1007/s00467-022-05445-0
32. Scharff M, Wiepjes CM, Klaver M, Schreiner T, T’Sjoen G, and den Heijer M. Change in grip strength in trans people and its association with lean body mass and bone density. Endocr Connect. (2019) 8:1020–8. doi: 10.1530/EC-19-0196
33. Stoffers IE, de Vries MC, and Hannema SE. Physical changes, laboratory parameters, and bone mineral density during testosterone treatment in adolescents with gender dysphoria. J Sex Med. (2019) 16:1459–68. doi: 10.1016/j.jsxm.2019.06.014
34. Tominaga Y, Kobayashi T, Matsumoto Y, Moriwake T, Oshima Y, Okumura M, et al. Trans men can achieve adequate muscular development through low-dose testosterone therapy: A long-term study on body composition changes. Andrology. (2024) 13(2):275–85. doi: 10.1111/andr.13640
35. van Eeghen SA, Wiepjes CM, T’Sjoen G, Nokoff NJ, den Heijer M, Bjornstad P, et al. Cystatin C-Based eGFR Changes during Gender-Affirming Hormone Therapy in Transgender Individuals. Clin J Am Soc Nephrol. (2023) 18:1545–54. doi: 10.2215/CJN.0000000000000289
36. van Kesteren P, Lips P, Gooren LJ, Asscheman H, and Megens J. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol (Oxf). (1998) 48:347–54. doi: 10.1046/j.1365-2265.1998.00396.x
37. Vlot MC, Wiepjes CM, de Jongh RT, T’Sjoen G, Heijboer AC, and den Heijer M. Gender-affirming hormone treatment decreases bone turnover in transwomen and older transmen. J Bone Miner Res. (2019) 34:1862–72. doi: 10.1002/jbmr.3762
38. Wiepjes CM, de Jongh RT, de Blok CJ, Vlot MC, Lips P, Twisk JW, et al. Bone safety during the first ten years of gender-affirming hormonal treatment in transwomen and transmen. J Bone Miner Res. (2019) 34:447–54. doi: 10.1002/jbmr.3612
39. Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Toye K, et al. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. (2014) 11:1999–2011. doi: 10.1111/jsm.12571
40. Yahyaoui R, Esteva I, Haro-Mora JJ, Almaraz MC, Morcillo S, Rojo-Martínez G, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. (2008) 93:2230–3. doi: 10.1210/jc.2007-2467
41. Lima-Posada I and Bobadilla NA. Understanding the opposite effects of sex hormones in mediating renal injury. Nephrol (Carlton). (2021) 26:217–26. doi: 10.1111/nep.13806
42. Conte C, Antonelli G, Melica ME, Tarocchi M, Romagnani P, and Peired AJ. Role of sex hormones in prevalent kidney diseases. Int J Mol Sci. (2023) 24:8244. doi: 10.3390/ijms24098244
43. Doublier S, Lupia E, Catanuto P, Periera-Simon S, Xia X, Korach K, et al. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. (2011) 79:404–13. doi: 10.1038/ki.2010.398
44. Soljancic A, Ruiz AL, Chandrashekar K, Maranon R, Liu R, Reckelhoff JF, et al. Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. Am J Physiol Regul Integr Comp Physiol. (2013) 304:R951–8. doi: 10.1152/ajpregu.00360.2012
45. Lichtenecker DCK, Argeri R, Castro CHM, Dias-da-Silva MR, and Gomes GN. Cross-sex testosterone therapy modifies the renal morphology and function in female rats and might underlie increased systolic pressure. Clin Exp Pharmacol Physiol. (2021) 48:978–86. doi: 10.1111/1440-1681.13495
46. Gilboa N, Magro AM, Han Y, and Rudofsky UH. Contrasting effects of early and late orchiectomy on hypertension and renal disease in fawn-hooded rats. Life Sci. (1987) 41:1629–34. doi: 10.1016/0024-3205(87)90731-4
47. Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, and Granger JP. Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol. (2005) 289:F941–8. doi: 10.1152/ajprenal.00034.2005
48. van Kesteren PJ, Kooistra T, Lansink M, van Kamp GJ, Asscheman H, Gooren LJ, et al. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb Haemost. (1998) 79:1029–33.
49. Quan A, Chakravarty S, Chen JK, Chen JC, Loleh S, Saini N, et al. Androgens augment proximal tubule transport. Am J Physiol Renal Physiol. (2004) 287:F452–9. doi: 10.1152/ajprenal.00188.2003
50. Chen YF, Naftilan AJ, and Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension. (1992) 19:456–63. doi: 10.1161/01.hyp.19.5.456
51. Guldan M, Unlu S, Abdel-Rahman SM, Ozbek L, Gaipov A, Covic A, et al. Understanding the role of sex hormones in cardiovascular kidney metabolic syndrome: toward personalized therapeutic approaches. J Clin Med. (2024) 13:4354. doi: 10.3390/jcm13154354
52. Diamanti-Kandarakis E, Spina G, Kouli C, and Migdalis I. Increased endothelin-1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy. J Clin Endocrinol Metab. (2001) 86:4666–73. doi: 10.1210/jcem.86.10.7904
53. Krasowski MD, Hines NG, Imborek KL, and Greene DN. Impact of sex used for assignment of reference intervals in a population of patients taking gender-affirming hormones. J Clin Transl Endocrinol. (2024) 36:100350. doi: 10.1016/j.jcte.2024.100350
54. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. (2021) 385:1737–49. doi: 10.1056/NEJMoa2102953
55. Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. (2022) 79:268–88. doi: 10.1053/j.ajkd.2021.08.003
56. Allen AN, Jiao R, Day P, Pagels P, Gimpel N, and SoRelle JA. Dynamic impact of hormone therapy on laboratory values in transgender patients over time. J Appl Lab Med. (2021) 6:27–40. doi: 10.1093/jalm/jfaa192
57. SoRelle JA, Jiao R, Gao E, Veazey J, Frame I, Quinn AM, et al. Impact of hormone therapy on laboratory values in transgender patients. Clin Chem. (2019) 65:170–9. doi: 10.1373/clinchem.2018.292730
58. Tienforti D, Castellini C, Di Giulio F, Spagnolo L, Muselli M, Fisher AD, et al. Metabolic features of assigned female at birth transgender people on gender-affirming hormone therapy: A meta-analysis. Transgend Health. (2024) 9:466–83. doi: 10.1089/trgh.2023.0040
59. Cheung AS, Zwickl S, Miller K, Nolan BJ, Wong AFQ, Jones P, et al. The impact of gender-affirming hormone therapy on physical performance. J Clin Endocrinol Metab. (2024) 109:e455–65. doi: 10.1210/clinem/dgad414
60. Tienforti D, Savignano G, Spagnolo L, Di Giulio F, Baroni MG, and Barbonetti A. Biochemical liver damage during gender affirming therapy in trans adults assigned female at birth: a meta-analysis. J Endocrinol Invest. (2024) 48(1):161–71. doi: 10.1007/s40618-024-02418-y
61. Tienforti D, Pastori D, and Barbonetti A. Effects of gender affirming hormone therapy with testosterone on coagulation and hematological parameters in transgender people assigned female at birth: A systematic review and meta-analysis. Thromb Res. (2024) 236:170–8. doi: 10.1016/j.thromres.2024.02.029
Keywords: AFAB, testosterone, creatinine, gender dysphoria, gender incongruence, kidney
Citation: Tienforti D, Spagnolo L, Piscitani L, Tonni C, Donatelli V, Cordeschi G, Baroni MG and Barbonetti A (2025) Effects of gender affirming hormone therapy with testosterone on renal function of assigned female at birth transgender people: a meta-analysis. Front. Endocrinol. 16:1537838. doi: 10.3389/fendo.2025.1537838
Received: 23 December 2024; Accepted: 29 May 2025;
Published: 12 June 2025.
Edited by:
Sarah Burke, University of Groningen, NetherlandsReviewed by:
Matthew C. Babcock, University of Colorado Anschutz Medical Campus, United StatesAdriana De Sousa Lages, Braga Hospital, Portugal
Copyright © 2025 Tienforti, Spagnolo, Piscitani, Tonni, Donatelli, Cordeschi, Baroni and Barbonetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Tienforti, ZGFuaWVsZXRpZW5mb3J0aUBnbWFpbC5jb20=
†ORCID: Daniele Tienforti, orcid.org/0000-0002-9359-7955
 Daniele Tienforti
Daniele Tienforti Luca Spagnolo
Luca Spagnolo Luca Piscitani3
Luca Piscitani3 Giuliana Cordeschi
Giuliana Cordeschi Marco Giorgio Baroni
Marco Giorgio Baroni Arcangelo Barbonetti
Arcangelo Barbonetti