- 1Department of Reproductive Medicine, The Affiliated Taizhou People’s Hospital to Nanjing Medical University, Taizhou, China
- 2Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 3The Affiliated Taizhou People’s Hospital to Nanjing Medical University, Taizhou, China
Background: The debate over the clinical role of atosiban in assisted reproduction continues. The purpose of our study was to explore the efficacy of atosiban on pregnancy outcomes of patients undergoing frozen embryo transfer.
Methods: A total of 1615 frozen embryo transfer cycles between 1 January 2019 and 31 December 2022 were included in this retrospective cohort study. Patients were divided into two groups based on the administration of atosiban before frozen-thawed embryo transfer (FET): the atosiban group (n=339) and the control group (n=1276). The primary outcome was live birth, while the secondary outcomes were biochemical pregnancy, clinical pregnancy, abortion, and ectopic pregnancy.
Results: After propensity score matching (PSM), both univariable and multivariable analyses showed atosiban was not linked to an increased likelihood of biochemical pregnancy or clinical pregnancy, nor a reduced risk of abortion or ectopic pregnancy (p>0.05). When controlling for confounding factors, maternal age (OR, 0.95; 95% CI, 0.91-0.98; p=0.004), history of failed ETs (1: OR, 0.72; 95% CI, 0.53-0.99; p=0.040; ≥2: OR, 0.65; 95% CI, 0.46-0.92; p=0.015), embryo stage (OR, 2.45; 95% CI, 1.85-3.25; p=0.000) and endometrial thickness (OR, 1.12; 95% CI, 1.01-1.24; p=0.025) were found to be associated with the likelihood of live birth. No beneficial effect of atosiban was observed in any of the subgroups based on maternal age, number of previous embryo transfers (ETs), endometrial thickness, or embryo stage in the subgroup analysis of the primary outcome.
Conclusion: These results suggested that adding atosiban in the standard FET cycles might not improve the live birth rate. To confirm this conclusion, more thorough, prospective randomized controlled studies of sizable sample sizes with good design are required.
1 Introduction
Over four decades have elapsed since the birth of the first in vitro fertilization (IVF) baby in the 1970s. Despite advanced progress in assisted reproduction technology (ART) over the past 40 years, embryo implantation remains the bottleneck of assisted reproduction. Embryo transfer (ET) is the final step of IVF-ET, the successful rate was just 23% per embryo transferred (1). There may be many possible factors, such as embryo quality, endometrial receptivity, etc. (2). Embryo quality accounts for around one-third of implantation failure while Suboptimal uterine receptivity accounts for two-thirds (3). A previous study (4) reported that the synchronization of embryo and endometrium is influenced by a variety of factors, such as embryonic and parental inheritance, anatomical factors, maternal immune system, endocrine environment, hematologic factors, and reproductive tract microorganisms. In recent years, a vast number of methods aimed at improving the success rate of IVF-ET through optimizing embryo transfer have been proposed, such as intrauterine infusion with hCG (5–9), hysteroscopy screening (10, 11), and endometrial implantation window detection done before ET (12).
In addition, rhythmic uterine contractions of the non-pregnant uterus have an important role in human reproduction (13). Contractions from the fundus to cervix are observed primarily in the early to mid-follicular phase and decrease as ovulation approaches. A switch in the direction of contractions occurs in the late follicular phase when waves from the cervix to fundus are observed (14–16). These progressive increased cervix-to-fundus contractions during the periovulatory period are thought to assist sperm ascension towards the distal end of the fallopian tubes, where fertilization takes place (14). In this phase, the frequency of uterine contractions is highest (17) and their direction is predominantly ipsilateral to the dominant follicle (18). After ovulation, opposing uterine contractions – defined as simultaneous contractions originating in the cervix and fundal area – appear. The opposing uterine contractions serve to prevent the embryo from being expelled from the cervix or the tubes, providing nutrients, and positioning the embryo before implantation (15, 17).In the luteal phase, the uterus is in a quiescent state, providing an optimal environment for the implantation of the embryo (19, 20). Briefly, the function of uterine contractions seems to be two-fold: providing strong enough contractions to guide spermatozoa to the ovum during ovulation; and creating an optimal, quiescent environment for implantation of an embryo during the luteal phase. Failure in one of these functions can interfere with fertility (21). Some evidence shows that there is a negative association between contractions at the time of ET and the clinical outcome (19), thus, methods or medications that can reduce uterine contractions during ET are attractive options to improve IVF success rates.
Oxytocin receptors (OTRs) are abundantly expressed in the pregnant uterus (particularly during the second and third trimesters), while detectable expression is also present in non-pregnant uterine tissue at lower levels sufficient to mediate uterine contractions (22). Oxytocin receptor antagonists (including atosiban, barusiban, nolasiban, epelsiban, and retosiban) can compete with oxytocin (OT) of oxytocin receptors (OTRs) in uterine smooth muscle cells, decidual cells, and fetal membrane and inhibit OT-induced PGF2a and uterine activity (23). Atosiban is the best-known oxytocin antagonist commonly used to delay premature labor. Studies have revealed a six−fold increase in uterine contractility before ET in IVF cycles compared with that before ovulation in natural cycles (24). Furthermore, there is a considerable reduction in the frequency of uterine contractions from 16/4 minutes to 6–2.6/4 minutes in women undergoing ET following the administration of atosiban (25). However, whether the application of atosiban around ET can improve the clinical outcomes of IVF-ET remains controversial.
While some studies showed that atosiban could improve clinical outcomes after ET (25, 26), other research revealed that atosiban only benefited subjects with a history of implantation failure, especially recurrent implantation failure (RIF) (27–31). Furthermore, some researchers even demonstrated that the application of atosiban could improve neither the implantation nor clinical pregnancy rates of patients undergoing IVF treatment (32, 33).
Considering the inconsistent clinical role of atosiban for infertility people, this retrospective cohort study was conducted to discuss the effects of atosiban in the FET cycle. The results would provide a reliable basis to guide clinicians in the selection of medications during FET cycles.
2 Materials and methods
2.1 Study design and patients
This retrospective cohort study was reviewed and approved by the institutional review board of the Affiliated Taizhou People’s Hospital to Nanjing Medical University (KY 2024-166-01), conducted at the Department of Reproductive Medicine of the hospital from January 2019 to December 2022.
This study included patients who underwent IVF/ICSI cycles and had at least one frozen embryo transfer (FET) cycle. The exclusion criteria were as follows: (a) patients aged 40 years or older; (b) patients with chromosomal abnormalities; (c) pre-implantation genetic testing (PGT) cycles; (d) egg donation cycles; (e) untreated hydrosalpinx, uterine malformation, precancerous lesions, and malignant neoplasm; (f) cycles lost to follow-up; (g) cycles with critical data missing. The information on demographic characteristics, associated laboratory measurements, ET protocol, and pregnancy outcomes for each FET cycle was recorded from electronic medical records, including maternal age, infertility duration, gravidity, parity, history of failed ETs, basic serum hormone levels, body mass index (BMI), anti-mullerian hormone (AMH), FET treatment procedures and reproductive outcomes. Finally, a total of 1,615 FET cycles were categorized into two groups based on the administration of atosiban before FET: the atosiban group (n=339) and the control group (n=1276). The decision to use atosiban was made based on a shared decision-making process involving the patient and the physician. Patients were informed about the potential effects, side effects, and costs of atosiban, and the final decision was influenced by the patient’s preferences, financial situation, and the physician’s clinical judgment and prescribing practices. The patients in the treatment group received intravenous atosiban 30 minutes before FET with a bolus dose of 6.75 mg (Tractocile, Ferring Pharmaceuticals, Kiel, Germany), within 1–2 min infusion time.
2.2 Frozen-thawed embryo transfer
The endometrium was prepared for the subsequent FET cycles, using the natural or artificial protocol, as previously described (34)[1]. Briefly, for ovulatory patients, FET was performed in the natural cycle. Patients underwent transvaginal ultrasound monitoring on days 2–5 of their menstrual cycle. Ovulation was triggered with human chorionic gonadotropin (hCG) (Chorionic Gonadotropin For Injection; Livzon (GROUP) Pharmaceutical Co., Ltd.) when the dominant follicle reached 18 mm. FET was performed 4 to 7 days after the hCG injection, depending on the embryo stage. Luteal phase support was provided using 40 mg of intramuscular progesterone (Progesterone Injection; Tianjin Kingyork Pharmaceuticals Co.) and 40 mg of oral dydrogesterone (Duphaston; Abbott Biologicals B.V.). For anovulatory women, FET was done based on programmed protocol. Whether downregulation was administered prior to hormone replacement (HR) was based on shared decision-making, considering patient preferences, financial status, and the physician’s clinical judgment. HR cycles were conducted with increasing doses of oestradiol valerate (Progynova; Bayer AG), administered at 2 to 8 mg daily for a minimum of 12 days. If endometrial thickness reached 7mm or more and there were no dominant follicles, both estradiol and luteal phase support were given. FET was performed after 3 to 6 days of progesterone supplementation. No more than 2 embryos were transferred per FET cycle. If pregnancy was confirmed, luteal support was continued until the 10th to 12th week of pregnancy.
2.3 Outcome measures
The primary outcome was live birth and the secondary outcomes were biochemical pregnancy, clinical pregnancy, abortion, and ectopic pregnancy. Live birth was defined as the delivery of one or more living infants at 28 weeks’ gestation or later. Biochemical pregnancy was defined as a positive serum β-hCG result 14 days after FET. Clinical pregnancy was defined as the presence of one or more gestational sacs with visible cardiac activity on transvaginal ultrasound. Abortion was defined as the spontaneous loss of a clinically recognized pregnancy within 28 weeks of gestation. Ectopic pregnancy was defined as the implantation of the embryo outside the uterine cavity.
2.4 Statistical analysis
Data were expressed as the numbers (%) for dichotomous variables and median (interquartile range, IQR) for continuous variables as no continuous variables meet the normality criteria of distribution. The missing rates of several demographic characteristics and laboratory measurements were less than 3% and we imputed missing data using multiple imputation.
To address the differences in the baseline characteristics in the two groups, we utilized PSM to mitigate the variations in attributes and reduce the influence of potential confounding factors and selection bias. The variables considered in the PSM model included maternal age, infertility duration, gravidity, parity, number of ET failures, BMI, basal hormone levels, AMH, embryo developmental stage at cryopreservation, number of embryos transferred, and endometrial thickness. Patients in the atosiban group were paired with patients in the control group using the nearest-neighbor method with a caliper width of 0.2 without replacement, in a ratio of 1:2.
Before and after PSM, Mann–Whitney U test, chi-square test or Fisher’s exact test were conducted for comparison between groups. For adjustment purposes, variables in the matched cohort dataset that had a p<0.05 in the univariable analysis were added to the multivariable analysis. Crude and adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are used to display effect estimates.
Subgroup analyses in the matched cohort were based on maternal age (<35 vs ≥35 yr), number of failed ETs (0 vs 1 vs ≥2), endometrial thickness (<8 vs ≥8 mm), and embryo stage (cleavage stage vs blastocyst). A significance level of p<0.05 was used to determine statistical significance. Statistical analyses were conducted using SPSS 27.0 (IBM Corp., USA) and R 4.3.0 (R Foundation, Vienna, Austria).
3 Results
3.1 Baseline characteristics
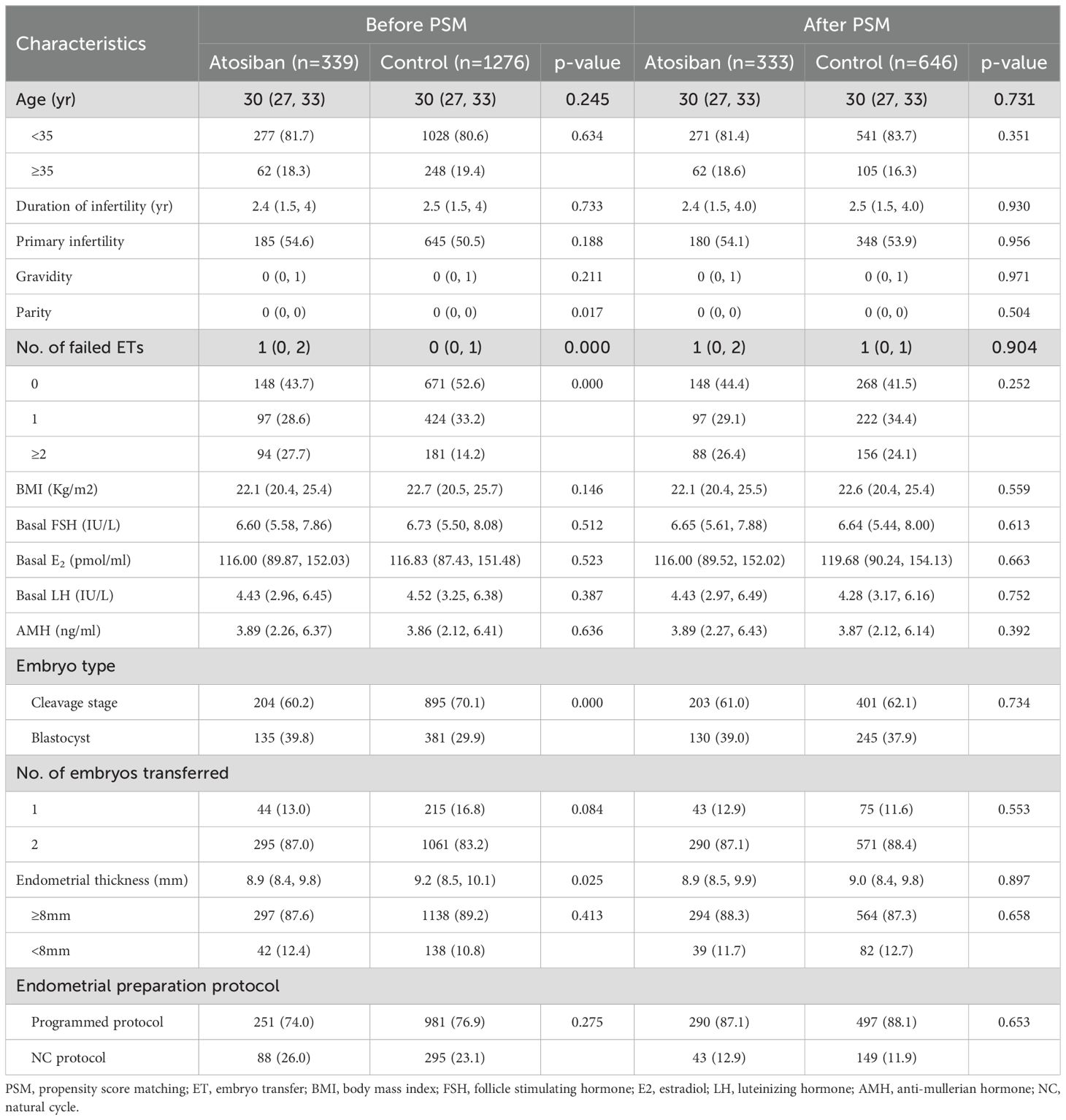
The included FET cycles were divided into the atosiban group and the control group based on whether atosiban was used, with 339 and 1276 cycles in each group, respectively. After PSM, 333 cycles with atosiban administration before FET were matched with 646 cycles without atosiban administration. Standardized mean differences (SMDs) for covariates were below the threshold of 0.1, indicating adequate balance between groups (Supplementary Figure S1). In the original unmatched cohort, there were differences in parity, number of failed ETs, endometrial thickness, and developmental stages at cryopreservation for transferred embryos between the two groups (p<0.05) (Table 1). In the PSM-matched cohort, no significant differences were observed between groups (p>0.05) (Table 1).
3.2 Outcomes
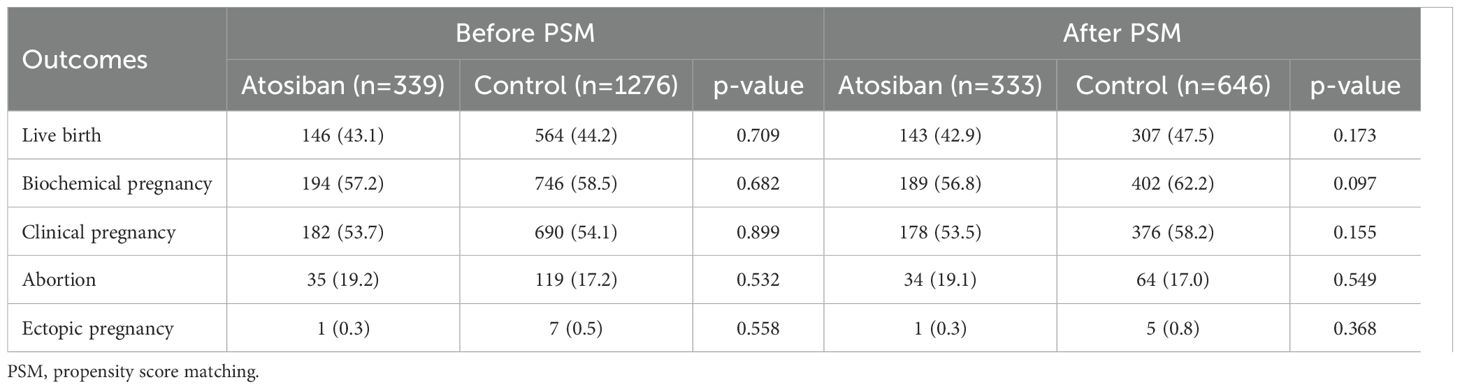
Preliminary comparisons revealed no significant statistical differences in both primary and secondary outcomes between the two groups before and after PSM (p>0.05) (Table 2).
In the PSM-matched cohort, atosiban use was not associated with a higher live birth rate in the univariable analysis (OR, 0.83; 95% CI, 0.64-1.08; p=0.170) as well as in the multivariable analysis (OR, 0.80; 95% CI, 0.61–1.06; p=0.124) (Table 3). After adjusting for confounding factors, maternal age (OR, 0.95; 95% CI, 0.91-0.98; p=0.004), history of failed ETs (1: OR, 0.72; 95% CI, 0.53-0.99; p=0.040; ≥2: OR, 0.65; 95% CI, 0.46-0.92; p=0.015), embryo stage (OR, 2.45; 95% CI, 1.85-3.25; p=0.000) and endometrial thickness (OR, 1.12; 95% CI, 1.01-1.24; p=0.025) were related to the probability of live birth (Table 3). For secondary outcomes, both univariable and multivariable analyses showed atosiban was not linked to an increased likelihood of biochemical pregnancy or clinical pregnancy, nor a reduced risk of abortion or ectopic pregnancy (p>0.05) (Table 4).
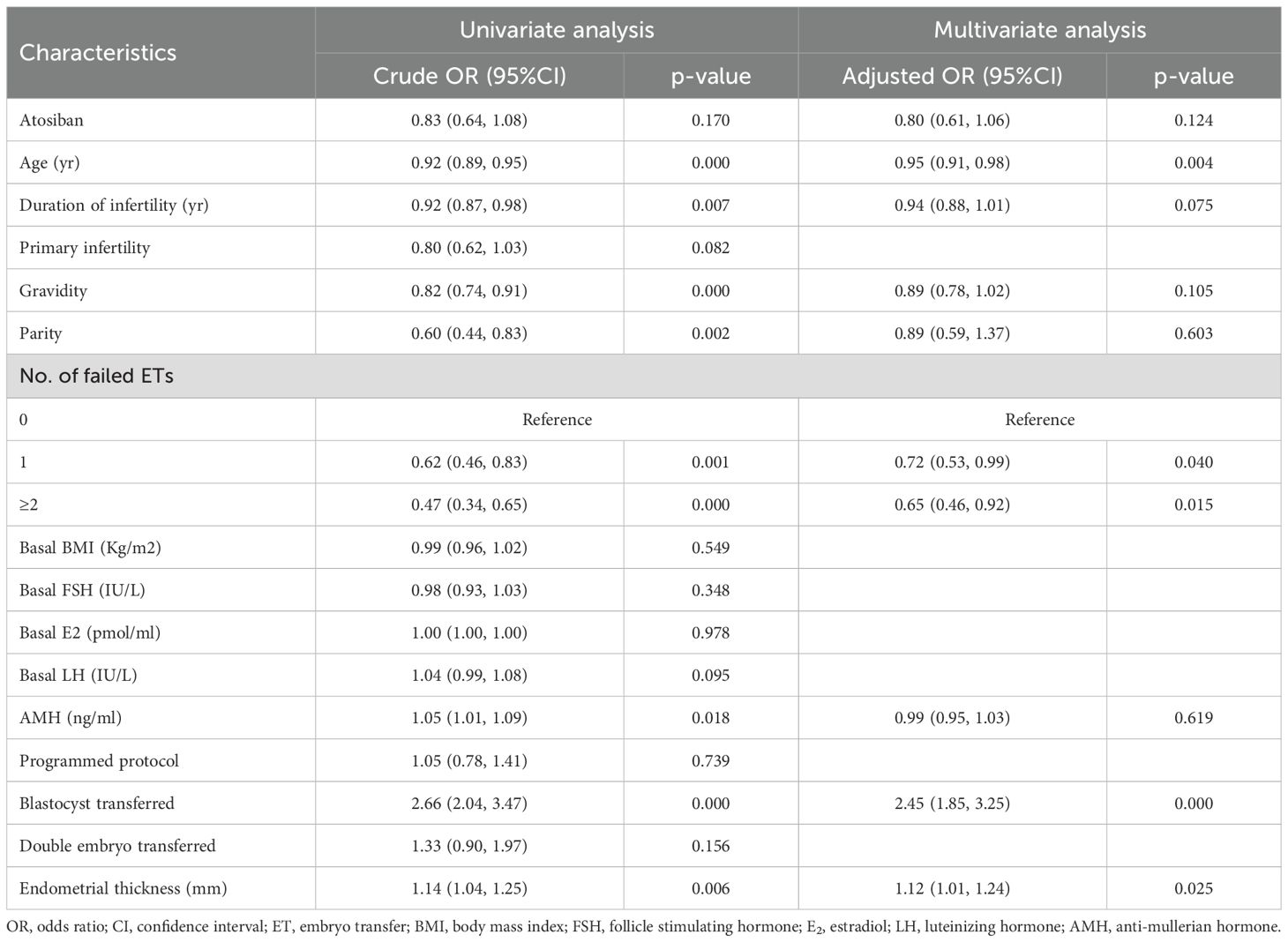
Table 3. Univariate and multivariate analysis of the association between atosiban and primary outcome (live birth) after propensity score matching.
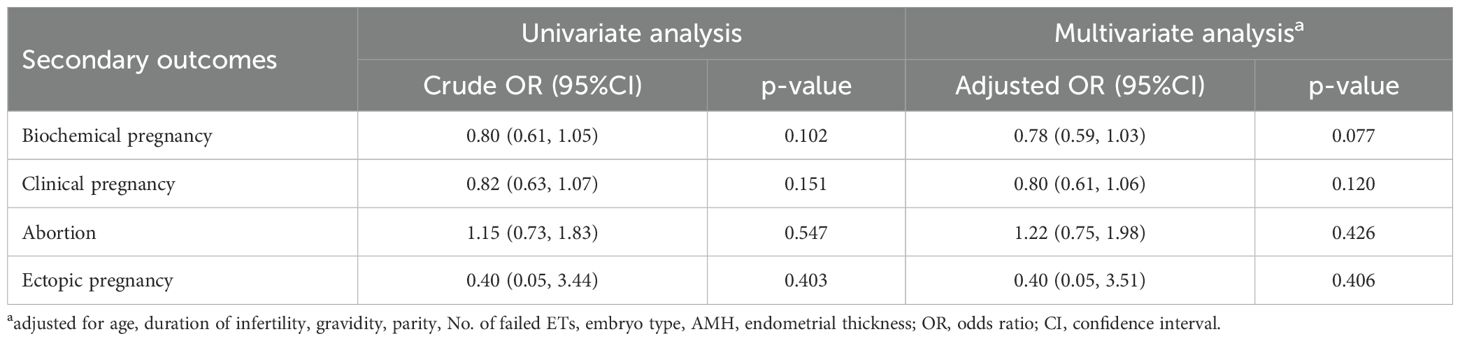
Table 4. Univariate and multivariate analysis of the association between atosiban and secondary outcomes after propensity score matching.
In the subgroup analysis of primary outcome, no beneficial effect of atosiban was observed in the maternal age subgroups (<35 vs ≥35 yr), number of failed ETs subgroups (0 vs 1 vs ≥2), endometrial thickness subgroups (<8 vs ≥8 mm), and embryo stage subgroups (cleavage stage vs blastocyst) (p>0.05) (Table 5).
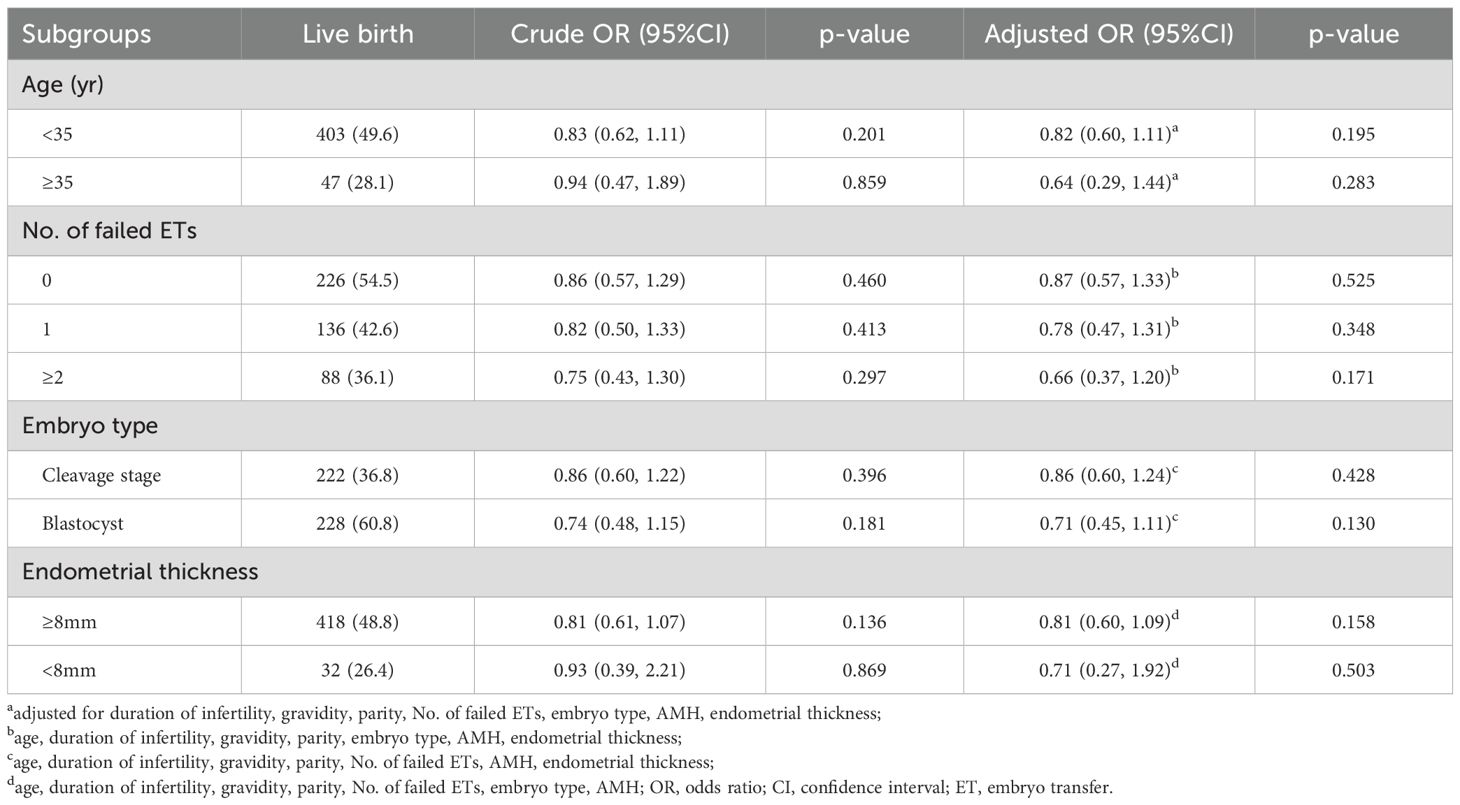
Table 5. Univariate and multivariate analysis of the association between atosiban and primary outcome (live birth) in the subgroups after propensity score matching.
4 Discussion
The current study evaluated the effect of atosiban on pregnancy outcomes in the general population with different numbers of FET cycles. In this retrospective cohort study, our results did not reveal a positive effect of atosiban on pregnancy outcomes. The biochemical pregnancy, clinical pregnancy, and live birth rate were not improved in the atosiban group compared to the control group.
Uterine contraction is one of the basic elements of endometrial receptivity and plays an important role in the embryo implantation process. As the studies showed (35–37), a high level of estradiol (E2), in the ovarian stimulation cycle might stimulate oxytocin and prostaglandin (PG)F2a production from endometrial cells, which may produce strong and frequent uterine contractions. To avoid the effect of high estrogen, our study chose FET cycles rather than fresh cycles. A previous study (33) revealed no difference in the live birth rate between groups stratified by the frequency of endometrial peristalsis. On the other hand, measuring uterine contractions was time-consuming and had intra-variation between different observers, thus the accuracy of the measurement is questionable. So we did not measure endometrial peristalsis.
Patients who experienced constant transvaginal ultrasound supervision or rough manipulation may lead to a hyperactivated autocrine/paracrine OT/OTR system in the endometrial epithelium that can result in a high level of serum OT and PGF2a, thereby leading to a high uterine contraction (30). Thus, gentle manipulation during FET is one of the ways to reduce endometrial contractions. On the other hand, medications that reduce uterine contractions during ET are also attractive options to improve IVF success. Atosiban, the best-known combined oxytocin/vasopressin V1A antagonist, exerts its effect by competing with oxytocin to its receptors located in the myometrium, decidua, and fetal membranes. This competition diminishes the efficacy of oxytocin and reduces the intracellular calcium ion levels in myometrial cells, consequently inhibiting uterine contractions. In addition, it can boost endometrial perfusion (38, 39). In a preclinical study, atosiban had a good embryonic safety profile and did not affect the endocrine profile up to 50-fold therapeutic blood concentrations. It neither affected the survival of the 1‐cell rabbit embryo nor affected the hatched rabbit blastocysts percentage. It had no adverse influence on human spermatozoa during sperm motility bioassays (39). Another study shows that atosiban has no systemic toxicity, mutagenic effects, or carcinogenic effects (25).
Our results agreed with Buddhabunyakan et al. (40), who demonstrated that adding atosiban during FET did not reduce uterine peristalsis and improve pregnancy outcomes in the general population. One RCT compared the live birth rates in women receiving atosiban versus placebo in fresh ET cycles in which day 3 embryos were transferred. The results showed no significant improvement in pregnancy outcomes with atosiban as compared with placebo in the general population undergoing fresh ET cycles (32). In their opinion, atosiban can reduce the frequency and amplitude of uterine contractions, but the proportion of cycles with uterine contractions of >3/min varied widely from 6.2% to 65.0%, and there was no difference between groups stratified by the frequency of endometrial peristalsis. In our multivariate analysis, maternal age, history of failed embryo transfers, embryo stage, and endometrial thickness emerged as significant covariates associated with live birth rates. To evaluate whether these variables might potentially modify the magnitude or direction of the association between the primary exposure (atosiban administration) and clinical outcomes, while simultaneously assessing the robustness of our primary findings across distinct patient populations, we conducted subgroup analyses stratified by these key parameters. In these analyses, atosiban did not show statistically significant treatment benefits. He et al. (30) analyzed several subgroups of infertile women undergoing different numbers of ET cycles and found that the use of atosiban significantly increased clinical and implantation rates among IVF patients undergoing third or subsequent ET cycles, but no statistical significance was observed for the efficacy of atosiban in the first- and second-ET groups. They have found that low levels of serum oxytocin and PGF2α among patients undergoing the first or second ET cycles were associated with low uterine contractions, which implies a stable uterine environment and explains the lack of response to atosiban observed among these patients. Another longitudinal cohort study (29) with larger numbers of ET cycles was consistent with the results of He et al.’ study. However, our subgroup analysis revealed no difference in clinical outcomes between the two groups stratified by the number of FET cycles. Aneuploidy leads to the majority of preclinical pregnancy losses (41). As we know, they did not test the embryos’ aneuploidy status by PGT-A. As a result, patients with possible implantation of aneuploidy embryos were not excluded in those studies and this might have an impact on their results. This may explain why they got positive results. However, the recurrent implantation failure (RIF) subgroup in our study had a limited sample size, and given the retrospective nature of our study design, these findings should be interpreted as exploratory. Definitive confirmation through large-scale randomized controlled trials will be required to validate these observations.
Another reason why we did not observe any benefit of atosiban may be related to the regimen of atosiban infusion used in the present study, which was based on the study of He et al. (30). Atosiban is a very short-acting drug, so it was administered 30 min before the transfer with a bolus dose of 6.75 mg within 1–2 min infusion time in our center. Therefore, the reduction in uterine contractions may not last long enough after stopping the atosiban infusion to produce appreciable effects on the outcome measures. Since embryo implantation takes place 3 days after cleavage embryo transfer and 1 day after blastocyst transfer, a prolonged atosiban infusion over 1–2 days may be associated with a sustained reduction in uterine contractions after ET, leading to a higher live birth rate (33).
As expected, younger age, blastocyst transferring and a thicker endometrial improve the LBR after ET. Advanced maternal age may lead to a compromised competence of the oocytes/embryos because of defective physiological pathways, such as energy production and balance, metabolism, epigenetic regulation, cell cycle checkpoints, and increased meiotic missegregation (42, 43). The consequence of an aging egg is abnormal fertilization and development, such as polyspermy, division arrest, implantation failure, and miscarriage (44–46). Women with advanced maternal age would experience a poor ovarian response, fewer retrieved oocytes, lower fertilization rate, and embryo quality rates, reduced embryo implantation and pregnancy rates, and higher risks of miscarriage, preterm delivery rate, and birth defect rate (47, 48). Studies have shown that early cleavage embryos peristalsis in the uterine cavity and fallopian tube before implantation, leads to a higher rate of ectopic pregnancy. Moreover, they were more likely to induce uterine contractions than blastocysts (49–51). The culture process of blastocyst provides more morphogenetic information to identify and discard embryos with lower implantation potential, thus enabling self-selection of viable embryos. While blastocyst, appropriate age, and endometrial of moderate thickness also promote better embryo-endometrial synchrony (52–56).
However, several limitations of this study need to be acknowledged. Firstly, all participants were from one medical center. Therefore, selection bias could not be eliminated. Secondly, we did not track congenital abnormalities in newborns. Thirdly, this observational study included all patients who attended the study medical center during the study period, so no formal sample size calculations were conducted. Fourth, the sample size of some subgroups (such as older women or patients with recurrent implantation failure) was relatively limited, which may reduce statistical power and potentially underestimate or overestimate the true effect. Nevertheless, with the growing demand for fertility preservation among older patients, we anticipate that future studies will benefit from larger cohort sizes. Lastly, this was a retrospective study, and thus the associated limitations could not be avoided (e.g., selection bias, reporting bias, and incomplete or missing data). The original dataset lacked detailed documentation of specific infertility factors, compounded by the frequent co-existence of multiple infertility factors in our patient population. These limitations prevented meaningful subgroup analysis of individual infertility causes and their potential impact on treatment outcomes.
5 Conclusions
In summary, the use of atosiban given before FET did not improve the live birth rate in general patients. Similar results were found in patients stratified by maternal age, number of previous failed embryo transfers, embryo type, or endometrial thickness on embryo transfer day. The clinical value of using atosiban in conventional embryo transfer strategy needs to be further studied.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YY: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. RL: Formal Analysis, Writing – original draft. SQ: Data curation, Writing – original draft. WZ: Data curation, Writing – original draft. DZ: Writing – review & editing. MY: Writing – review & editing. PZ: Writing – review & editing. JW: Conceptualization, Writing – review & editing. HG: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the National Natural Science Foundation of China to HG (grant number: 81771586), and 2023 Taizhou Science and Technology Support Program (Social Development) Project (grant number: TS202307).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1547694/full#supplementary-material
References
1. Human Fertilisation and Embryology Authority. Fertility treatment 2018: trends and figures (2021). Available online at: https://www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2018trends-and-figures/ (Accessed January 13, 2021).
2. Achache H and Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. (2006) 12:731–46. doi: 10.1093/humupd/dml004
3. Lédée-Bataille N, Laprée-Delage G, Taupin J-L, Dubanchet S, Frydman R, and Chaouat G. Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum Reprod Oxf Engl. (2002) 17:213–8. doi: 10.1093/humrep/17.1.213
4. Franasiak JM, Alecsandru D, Forman EJ, Gemmell LC, Goldberg JM, Llarena N, et al. A review of the pathophysiology of recurrent implantation failure. Fertil Steril. (2021) 116:1436–48. doi: 10.1016/j.fertnstert.2021.09.014
5. Holt-Kentwell A, Ghosh J, Devall A, Coomarasamy A, and Dhillon-Smith RK. Evaluating interventions and adjuncts to optimize pregnancy outcomes in subfertile women: an overview review. Hum Reprod Update. (2022) 28:583–600. doi: 10.1093/humupd/dmac001
6. Conforti A, Longobardi S, Carbone L, Iorio GG, Cariati F, Campitiello MR, et al. Does intrauterine injection of hCG improve IVF outcome? A systematic review and a meta-analysis. Int J Mol Sci. (2022) 23:12193. doi: 10.3390/ijms232012193
7. Wang M, Deng H, and Ye H. Intrauterine injection of human chorionic gonadotropin improves pregnancy outcome in patients with repeated implantation failure in frozen-thawed embryo transfer. Zhong Nan Xue Xue Bao Yi Xue Ban. (2019) 44:1247–51. doi: 10.11817/j.issn.1672-7347.2019.180469
8. Abdallah KS, Makhlouf A, Badran E, El-Nashar IM, Al-Hussaini TK, Farghaly T, et al. Intrauterine injection of HCG before embryo transfer: a parallel, double-blind randomized trial. Reprod BioMed Online. (2021) 43:663–9. doi: 10.1016/j.rbmo.2021.06.011
9. Osman A, Pundir J, Elsherbini M, Dave S, El-Toukhy T, and Khalaf Y. The effect of intrauterine HCG injection on IVF outcome: a systematic review and meta-analysis. Reprod BioMed Online. (2016) 33:350–9. doi: 10.1016/j.rbmo.2016.05.010
10. Di Spiezio Sardo A, Di Carlo C, Minozzi S, Spinelli M, Pistotti V, Alviggi C, et al. Efficacy of hysteroscopy in improving reproductive outcomes of infertile couples: a systematic review and meta-analysis. Hum Reprod Update. (2016) 22:479–96. doi: 10.1093/humupd/dmw008
11. Kamath MS, Bosteels J, D’Hooghe TM, Seshadri S, Weyers S, Mol BWJ, et al. Screening hysteroscopy in subfertile women and women undergoing assisted reproduction. Cochrane Database Syst Rev. (2019) 4:CD012856. doi: 10.1002/14651858.CD012856.pub2
12. Enciso M, Aizpurua J, Rodríguez-Estrada B, Jurado I, Ferrández-Rives M, Rodríguez E, et al. The precise determination of the window of implantation significantly improves ART outcomes. Sci Rep. (2021) 11:13420. doi: 10.1038/s41598-021-92955-w
13. IJland MM, Evers JLH, and Hoogland HJ. Velocity of endometrial wavelike activity in spontaneous cycles. Fertil Steril. (1997) 68:72–5. doi: 10.1016/S0015-0282(97)81478-1
14. Ijland MM, Evers JLH, Dunselman GAJ, Van Katwijk C, Lo CR, and Hoogland HJ. Endometrial wavelike movements during the menstrual cycle. Fertil Steril. (1996) 65:746–9. doi: 10.1016/S0015-0282(16)58207-7
15. Ijland MM, Evers JLH, Dunselman GAJ, and Hoogland HJ. Subendometrial contractions in the nonpregnant uterus: an ultrasound study. Eur J Obstet Gynecol Reprod Biol. (1996) 70:23–4. doi: 10.1016/S0301-2115(96)02571-7
16. IJland MM, Hoogland HJ, Dunselman GAJ, Lo CR, and Evers JLH. Endometrial wave direction switch and the outcome of in vitro fertilization. Fertil Steril. (1999) 71:476–81. doi: 10.1016/S0015-0282(98)00501-9
17. Bulletti C, De Ziegler D, Polli V, Diotallevi L, Ferro ED, and Flamigni C. Uterine contractility during the menstrual cycle. Hum Reprod. (2000) 15:81–9. doi: 10.1093/humrep/15.suppl_1.81
18. Kunz G, Beil D, Deininger H, Wildt L, and Leyendecker G. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Hum Reprod. (1996) 11:627–32. doi: 10.1093/HUMREP/11.3.627
19. Fanchin R, Righini C, Olivennes F, Taylor S, de Ziegler D, and Frydman R. Uterine contractions at the time of embryo transfer alter pregnancy rates after in-vitro fertilization. Hum Reprod Oxf Engl. (1998) 13:1968–74. doi: 10.1093/humrep/13.7.1968
20. Bulletti C, De Ziegler D, Rossi S, Polli V, Massoneau M, Rossi E, et al. Abnormal uterine contractility in nonpregnant women. Ann N Y Acad Sci. (1997) 828:223–9. doi: 10.1111/j.1749-6632.1997.tb48543.x
21. Kuijsters NPM, Methorst WG, Kortenhorst MSQ, Rabotti C, Mischi M, and Schoot BC. Uterine peristalsis and fertility: current knowledge and future perspectives: a review and meta-analysis. Reprod BioMed Online. (2017) 35:50–71. doi: 10.1016/j.rbmo.2017.03.019
22. Gimpl G and Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. (2001) 81:629–83. doi: 10.1152/physrev.2001.81.2.629
23. He Y, Wu H, He X, Xing Q, Zhou P, Cao Y, et al. Administration of atosiban in patients with endometriosis undergoing frozen-thawed embryo transfer. A prospect random study. Fertil Steril. (2016) 106:416–22. doi: 10.1016/j.fertnstert.2016.04.019
24. Ayoubi JM, Epiney M, Brioschi PA, Fanchin R, Chardonnens D, and de Ziegler D. Comparison of changes in uterine contraction frequency after ovulation in the menstrual cycle and in in vitro fertilization cycles. Fertil Steril. (2003) 79:1101–5. doi: 10.1016/s0015-0282(03)00179-1
25. Mishra V, Agarwal H, Goel S, Roy P, Choudhary S, and Lamba S. A Prospective Case-control Trial to Evaluate and Compare the Efficacy and Safety of Atosiban versus Placebo in In vitro Fertilization-embryo Transfer Program. J Hum Reprod Sci. (2018) 11:155–60. doi: 10.4103/jhrs.JHRS_7_17
26. Schwarze JE, Crosby J, and Mackenna A. Atosiban improves the outcome of embryo transfer. A systematic review and meta-analysis of randomized and non-randomized trials. JBRA Assist Reprod. (2020) 24:421–7. doi: 10.5935/1518-0557.20200016
27. Huang Q-Y, Rong M-H, Lan A-H, Lin X-M, Lin X-G, He R-Q, et al. The impact of atosiban on pregnancy outcomes in women undergoing in vitro fertilization-embryo transfer: A meta-analysis. PloS One. (2017) 12:e0175501. doi: 10.1371/journal.pone.0175501
28. Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod BioMed Online. (2014) 28:14–38. doi: 10.1016/j.rbmo.2013.08.011
29. Wu M-H, Lin C-W, Su P-F, Lai EC-C, Sie F-C, Mau Y-L, et al. Atosiban and pregnancy outcomes following in vitro fertilization treatment for infertile women requiring one, two, or more embryo transfer cycles: A longitudinal cohort study. Reprod Sci. (2020) 27:853–9. doi: 10.1007/s43032-019-00088-3
30. He Y, Wu H, He X, Xing Q, Zhou P, Cao Y, et al. Application of atosiban in frozen-thawed cycle patients with different times of embryo transfers. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. (2016) 32:811–5. doi: 10.1080/09513590.2016.1180680
31. Lan VTN, Khang VN, Nhu GH, and Tuong HM. Atosiban improves implantation and pregnancy rates in patients with repeated implantation failure. Reprod BioMed Online. (2012) 25:254–60. doi: 10.1016/j.rbmo.2012.05.014
32. Ng EHY, Li RHW, Chen L, Lan VTN, Tuong HM, and Quan S. A randomized double blind comparison of atosiban in patients undergoing IVF treatment. Hum Reprod. (2014) 29:2687–94. doi: 10.1093/humrep/deu263
33. Tang CL, Li QY, Chen FL, Cai CT, Dong YY, Wu YY, et al. A randomized double blind comparison of atosiban in patients with recurrent implantation failure undergoing IVF treatment. Reprod Biol Endocrinol. (2022) 20:124. doi: 10.1186/s12958-022-00999-y
34. Luo R, Wang J, Yang Y, Xu C, Yang M, Zhu D, et al. The role of serum vitamin D in patients with normal ovarian reserve undergoing the first IVF/ICSI cycle. Front Endocrinol. (2023) 14:1249445. doi: 10.3389/fendo.2023.1249445
35. Liedman R, Hansson SR, Howe D, Igidbashian S, McLeod A, Russell RJ, et al. Reproductive hormones in plasma over the menstrual cycle in primary dysmenorrhea compared with healthy subjects. Gynecol Endocrinol. (2008) 24:508–13. doi: 10.1080/09513590802306218
36. Li J, Mo S, Lin Z, and Shi Q. Atosiban application in fresh ET cycle is effective for women undergoing repeated embryo implantation failures, especially for advanced-age obese patients. Sci Rep. (2023) 13:23044. doi: 10.1038/s41598-023-49773-z
37. Richter ON. Oxytocin receptor gene expression of estrogen-stimulated human myometrium in extracorporeally perfused non-pregnant uteri. Mol Hum Reprod. (2004) 10:339–46. doi: 10.1093/molehr/gah039
38. Tsatsaris V, Carbonne B, and Cabrol D. Atosiban for preterm labour. Drugs. (2004) 64:375–82. doi: 10.2165/00003495-200464040-00003
39. Pierzynski P. Oxytocin and vasopressin V(1A) receptors as new therapeutic targets in assisted reproduction. Reprod BioMed Online. (2011) 22:9–16. doi: 10.1016/j.rbmo.2010.09.015
40. Buddhabunyakan N, Sothornwit J, Seejorn K, Buppasiri P, and Salang L. Effects of atosiban on uterine peristalsis following frozen embryo transfer: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. (2021) 265:96–101. doi: 10.1016/j.ejogrb.2021.08.017
41. Bashiri A, Halper KI, and Orvieto R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. (2018) 16:121. doi: 10.1186/s12958-018-0414-2
42. Santonocito M, Guglielmino MR, Vento M, Ragusa M, Barbagallo D, Borzì P, et al. The apoptotic transcriptome of the human MII oocyte: characterization and age-related changes. Apoptosis. (2013) 18:201–11. doi: 10.1007/s10495-012-0783-5
43. Capalbo A, Hoffmann ER, Cimadomo D, Maria Ubaldi F, and Rienzi L. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update. (2017) 23:706–22. doi: 10.1093/humupd/dmx026
44. Attali E and Yogev Y. The impact of advanced maternal age on pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. (2021) 70:2–9. doi: 10.1016/j.bpobgyn.2020.06.006
45. Balasch J. Ageing and infertility: an overview. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. (2010) 26:855–60. doi: 10.3109/09513590.2010.501889
46. Tatone C. Oocyte senescence: a firm link to age-related female subfertility. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. (2008) 24:59–63. doi: 10.1080/09513590701733504
47. The ESHRE Capri Workshop Group. Genetic aspects of female reproduction. Hum Reprod Update. (2008) 14:293–307. doi: 10.1093/humupd/dmn009
48. Schmidt L, Sobotka T, Bentzen JG, and Nyboe Andersen A. on behalf of the ESHRE Reproduction and Society Task Force. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update. (2012) 18:29–43. doi: 10.1093/humupd/dmr040
49. Jwa SC, Takamura M, Kuwahara A, Kajihara T, and Ishihara O. Effect of endometrial preparation protocols on the risk of ectopic pregnancy for frozen embryo transfer. Sci Rep. (2021) 11:17453. doi: 10.1038/s41598-021-97044-6
50. Trindade VD, Hentschke MR, Dornelles VC, Ferri-Guerra J, Kira ATF, Colombo T, et al. Tubal factor, cleavage stage and more than one embryo transferred were risk factors associated with ectopic pregnancy after assisted reproductive treatment. JBRA Assist Reprod. (2021) 26(2):321–8. doi: 10.5935/1518-0557.20210074
51. Krishnamoorthy K, Greenberg P, Perlman BE, Morelli SS, Jindal SK, and McGovern PG. The incidence of ectopic/heterotopic pregnancies after blastocyst-stage frozen-thawed embryo transfers compared with that after cleavage-stage: A society for assisted reproductive technologies clinical outcomes reporting system study. FS Rep. (2021) 2:421–7. doi: 10.1016/j.xfre.2021.06.010
52. Mahutte N, Hartman M, Meng L, Lanes A, Luo Z-C, and Liu KE. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril. (2022) 117:792–800. doi: 10.1016/j.fertnstert.2021.12.025
53. Glujovsky D, Quinteiro Retamar AM, Alvarez Sedo CR, Ciapponi A, Cornelisse S, et al. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. (2022) (5):CD002118. doi: 10.1002/14651858.CD002118.pub6
54. Shapiro BS, Daneshmand ST, Desai J, Garner FC, Aguirre M, and Hudson C. The risk of embryo-endometrium asynchrony increases with maternal age after ovarian stimulation and IVF. Reprod BioMed Online. (2016) 33:50–5. doi: 10.1016/j.rbmo.2016.04.008
55. Clua E, Rodríguez I, Arroyo G, Racca A, Martínez F, and Polyzos NP. Blastocyst versus cleavage embryo transfer improves cumulative live birth rates, time and cost in oocyte recipients: a randomized controlled trial. Reprod BioMed Online. (2022) 44:995–1004. doi: 10.1016/j.rbmo.2022.01.001
Keywords: atosiban, live birth rate, frozen-thawed embryo transfer, assisted reproduction, endometrial peristalsis
Citation: Yang Y, Luo R, Qin S, Zhu W, Yang M, Zhu D, Zhang P, Wang J and Ge H (2025) The effect of atosiban on pregnancy outcomes in different FET cycles: a single-center matched retrospective cohort study. Front. Endocrinol. 16:1547694. doi: 10.3389/fendo.2025.1547694
Received: 18 December 2024; Accepted: 09 June 2025;
Published: 24 June 2025.
Edited by:
Richard Ivell, University of Nottingham, United KingdomReviewed by:
Jing Wang, Nanjing Medical University, ChinaMaw-Sheng Lee, Lee Women’s Hospital, Taiwan
Masashi Yoshida, Miyake Women’s Clinic, Japan
Copyright © 2025 Yang, Luo, Qin, Zhu, Yang, Zhu, Zhang, Wang and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Wang, MzI3MzU2NDRAcXEuY29t; Hongshan Ge, aG9uZ3NoYW5nZUBuam11LmVkdQ==
 Yu Yang
Yu Yang Rong Luo
Rong Luo Shilei Qin3
Shilei Qin3