- Health Examination Center, Shanghai Health and Medical Center (Huadong Sanatorium), Wuxi, China
Objective: This study aimed to explore the diagnostic value of clinical features in the assessment of malignant thyroid Imaging Reporting and Data System (TIRADS) category 4 thyroid nodules and to provide a more effective reference for clinical diagnostic practices.
Methods: A total of 998 patients with 1,103 TIRADS 4 thyroid nodules underwent conventional ultrasound (US) and clinical information assessment at the Shanghai Health and Medical Center from January 1, 2012, to June 30, 2024. A qualitative assessment of clinical and US features was performed, followed by univariable and multivariable logistic regression analyses using a training cohort, which contributed to the construction of the clinical TIRADS model. A receiver-operating characteristic (ROC) curve, a Hosmer-Lemeshow (HL) test and a decision curve analysis (DCA) were employed to further validate this model in the validation cohort.
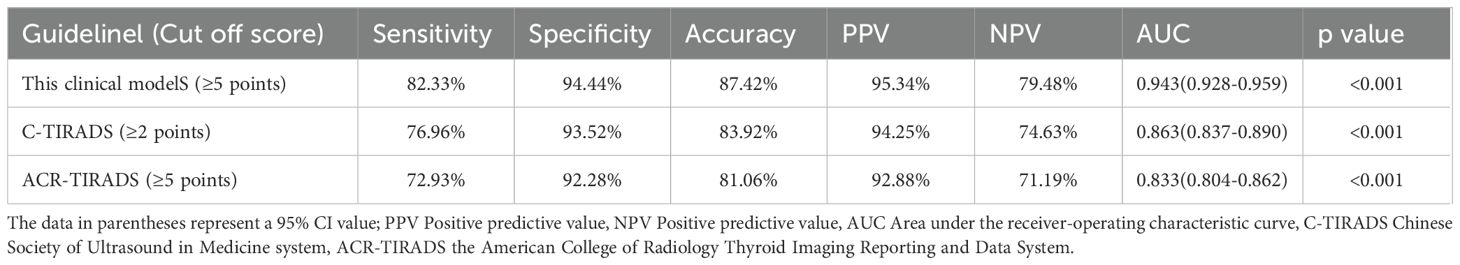
Results: Patient age, body mass index, sex, family history of thyroid carcinoma, and US features—such as vertical orientation, ill-defined or irregular margins or extrathyroidal extensions, microcalcifications, blood flow signals of central or peripheral vessels, and swollen cervical lymph nodes—were identified as independent risk factors in the clinical scoring model for TI-RADS 4 nodules. This diagnostic model achieved an area under the curve (AUC) of 0.943 [0.928, 0.959], with a sensitivity of 82.33%, specificity of 94.44%, diagnostic threshold of 5 points, accuracy of 87.42%, positive predictive value of 95.34%, and negative predictive value of 79.48% in the validation cohort. The HL tests and DCA also demonstrated excellent predictive performances.
Conclusions: The integration of clinical and US features in the construction of the diagnostic model can significantly enhance the diagnosis of TIRADS 4 thyroid nodules and provide a reliable evaluation tool for clinical practice.
Introduction
Due to the popularization of thyroid ultrasound examinations in health examinations, the detection rate of thyroid nodules was obviously increased in recent years (1, 2). Thyroid nodules are found in approximately 60% of the general population, with the malignancy rate of unselected thyroid nodule generally ranges from 1 to 5% (3–5),. The predominant pathological subtype of malignant cases is papillary thyroid carcinoma, which typically exhibits a low mortality rate, mild biological behavior, and is often asymptomatic throughout a lifetime (6, 7). Multiple studies (4, 7, 8) have indicated that a proportion of thyroid nodules are unnecessarily referred for surgery, resulting in an unfavorable risk and cost-benefit ratio. Given the high incidence, low invasiveness, and low mortality rates associated with thyroid nodules, there is a clear need for more cost-effective and risk-adapted management strategies for this prevalent condition.
Characterized by its non-invasiveness, convenience, repeatability, and low cost, ultrasound has become the first choice for screening thyroid nodules. To help diagnostic, ultrasound risk stratification guidelines have been developed in various countries worldwide (9–14). In 2020, the Society of Ultrasound in Medicine of the Chinese Medical Association published the Chinese guidelines for the Thyroid Imaging Reporting and Data System (C-TIRADS) (15). In C-TIRADS, thyroid nodules are categorized into classes 2 to 6 based on various ultrasound features. The nature of the class 2 and 6 nodules are definite, while class 3 nodules (malignancy rate is <2%) and class 5 nodules (malignancy rate is >90%) could also be diagnosed more readily through routine ultrasound. However, the ultrasound features of class 4 nodules exhibit some overlap, rendering the differentiation between benign and malignant nodules challenging.
Clinical factors (3, 16–20) such as patient age, sex, and family history, along with biochemical markers (17, 20) like TSH levels and thyroglobulin antibodies, have been found to be significant associated with malignancy risk of thyroid nodules. Prior research (19–21) has demonstrated that integrating these factors with ultrasound features can help clinicians make more informed decisions regarding diagnosis and management. Our research hopes to establish a model based on clinical and ultrasound risk factors to distinguish between benign and malignant class 4 thyroid nodules.
Materials and methods
Study design
This retrospective cohort study conducted at the Shanghai Health and Medical Center, China. We retrospectively analyzed the questionnaires and electronic medical records of patients with C-TIRADS 4 thyroid nodules who underwent regular health examinations at our hospital from January 1, 2012, to June 30, 2024, A total of 998 patients with 1,103 C-TIRADS 4 nodules were included for retrospective analysis.
The training cohort consisted of 447 thyroid nodule patients with pathologically confirmed malignancies from January 1, 2012, to December 12, 2020, were selected as the case group. In contrast, 324 thyroid nodule patients with pathologically confirmed benign confirmed benign nodules or those whose ultrasound characteristics remained unchanged for more than 9 to 10 years were selected as the control group. The validation cohort included 200 thyroid nodule patients with pathologically confirmed malignancies from January 1, 2021, to June 30, 2024, were selected as the case group. Meanwhile, 132 thyroid nodule patients with pathologically confirmed benign nodules were selected as the control group.
This study was approved by the Institutional Ethics Committee of the Shanghai Health and Medical Center, China. Our study conforms to the guidelines of the Declaration of Helsinki and its later amendments. Informed consent was obtained from all patients for being included in the study. The date of approval was 01 April 2022, reference number 2022(01).
Participants
The research subjects were arranged by their respective companies to undergo annual health examinations at our hospital. All enrolled patients met the following inclusion criteria: (1) nodules classified as C-TIRADS 4, (2) age ≥ 18 years, and (3) availability of complete ultrasonography, clinical, and follow-up data. The exclusion criteria were set as follows: (1) a history of treatment with immunosuppressants, hormones, or iodine-containing medications, (2) a history of thyroid surgery or related radiotherapy, and (3) intolerance to puncture or surgical procedures.
Participant data
Clinical data were obtained from electronic medical records of the physical examination center in our hospital. This dataset includes basic information, physical examinations, disease history, family history, medication history, thyroid ultrasound, and pathology reports.
Covariates: according to World Health Organization standards, age of patient was categorized into 18-44, 45-59, and ≥60 years. Body mass index (BMI) was categorized into 18.5-24.9, 25.0-29.9, and ≥30.0 kg/m2.
Ultrasound examinations were conducted by one thyroid ultrasound examiner and subsequently reviewed by another examiner with over ten years of experience. We implemented a standardized ultrasound reporting protocol based on the TIRADS guidelines to ensure diagnostic consistency (Supplementary File Table S1). Thyroid ultrasound image data, including nodule position, size, aspect ratio, composition, echogenicity, margins, calcifications, blood flow signals, and cervical lymph nodes, were meticulously recorded. Any differences between the two observers were re-evaluated collaboratively, discussed, and resolved through consensus. In cases where agreement could not be reached, a third, more experienced expert ultrasonographer made the final determination.
Statistical analysis
Statistical evaluation was performed by SPSS 22.0. Variables exhibiting non-normal distribution were presented as median (interquartile range [IQR]), while normally distributed variables were shown as mean ± SD. Comparisons were made using independent samples. The t-test or Mann-Whitney U test was used for comparing continuous variables between the case and control groups. Categorical variables were analyzed using the χ2 and Fisher’s exact tests. A p value <0.05 was considered statistically significant.
Additionally, within the training cohort, univariate logistic regression analysis was used to identify risk factors associated with malignant thyroid nodules. Factors with odds ratios>2 or P<0.1 were included in the multivariate regression analysis. Factors with p <0.05 in the multivariate analysis were considered as evaluation indices in the malignant thyroid nodules model. The regression coefficient (β value) for each factor was rounded to the nearest integer to establish the risk score for malignant thyroid nodules.
In the alidation cohort, the area under the curve (AUC) of the receiver operator characteristics (ROC) curve was used to assess the discriminative ability of the model, and the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of the model were calculated. A Hosmer-Lemeshow (H-L) test was used to evaluate the model’s calibration ability by assessing the closeness between the predicted prevalence rate and the observed prevalence rate. A decision curve analysis (DCA) was also used to evaluate the clinical utility of the model.
Results
Baseline demographics of study participants in the training cohort and validation cohorts
Our initial sample size included 1157 TIRADS 4 nodules, Of these, 16 nodules were excluded due to a prior history of treatment with iodine-containing medications (n =1), hormones (n =2) or thyroid surgery (n =13). Besides, 38 nodules were removed because of incomplete ultrasonography, clinical, or follow up data. In total, 447 malignant thyroid nodules and 324 control nodules were included in the training cohort. Additionally, 332 thyroid nodules were selected for the validation cohort, consisting of 200 malignant thyroid nodules and 132 control nodules.
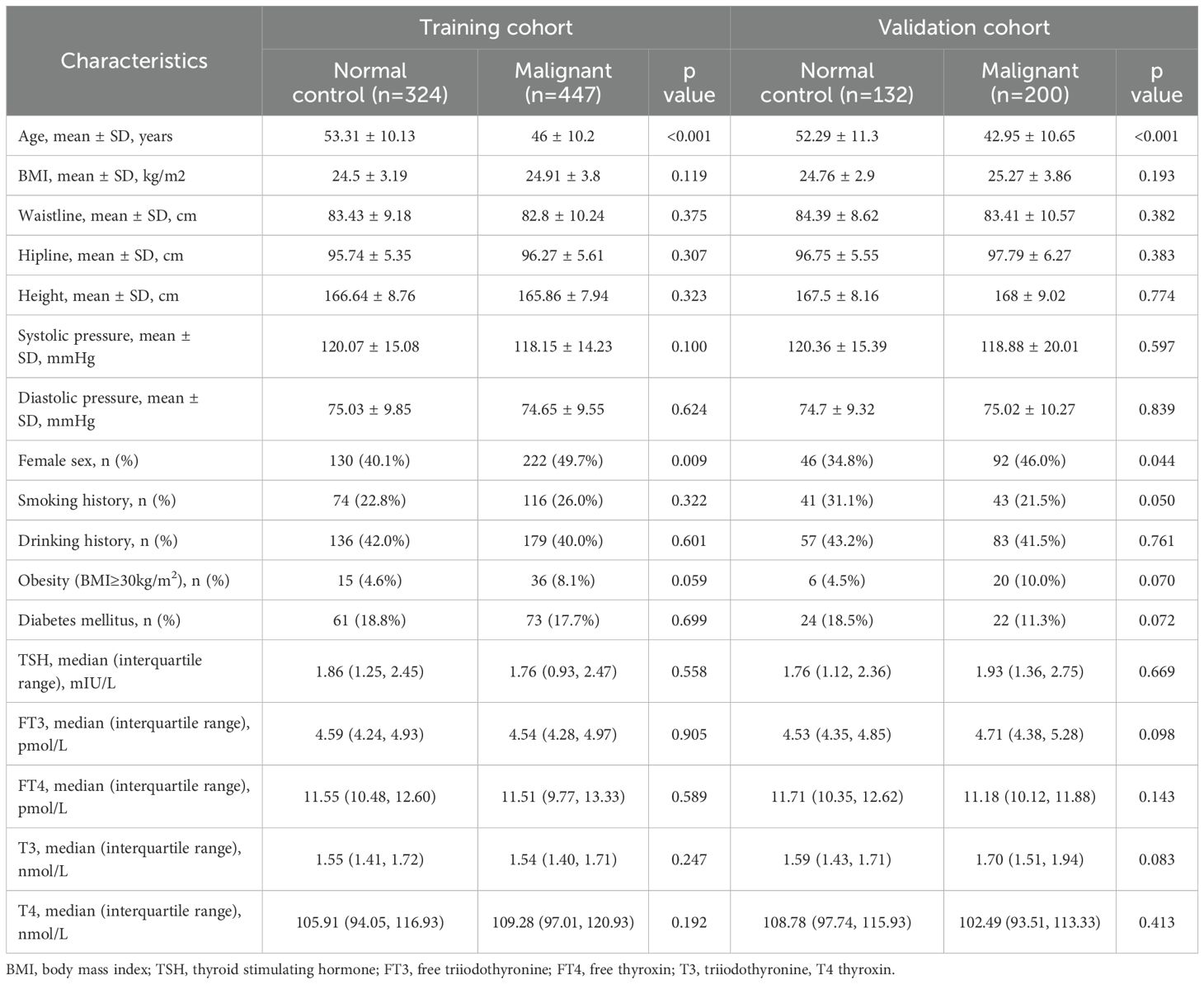
Table 1 presents the descriptive statistics for both the case and control groups. In the training and validation cohorts, no statistically significant differences were observed in BMI, waist circumference, hip circumference, height, smoking status, alcohol consumption, blood pressure, diabetes and thyroid function between the malignant nodule group and the negative control group. However, patients in the malignant nodule group were younger than those in the control group, with an average age of 46 years compared to 53.31 years in the training cohort (P <0.001), and 42.95 years compared to 52.29 years in the validation cohort (P <0.001). Furthermore, the proportion of females in the malignant nodule group was higher than that in the control group, both in the training cohort (49.7% vs. 40.1%, P =0.009) and in the validation cohort (46.0% vs. 34.8%, P =0.044).
Development of the clinical risk score model for TIRADS 4 nodules
Clinical and ultrasonographic risk factors for thyroid cancer explored by univariable logistic regression
In terms of clinical risk factors, age was found to be significantly and negatively correlated with the risk of malignant thyroid nodules. Additionally, being female was associated with an increased risk of malignant thyroid nodules, with an odds ratio (OR) of 1.472 (95% CI: 1.102, 1.967). Although obesity may be related to the presence of malignant nodules, it did not reach statistical significance. Concerning ultrasonographic risk factors, the following were identified as risk factors for malignant thyroid nodules: vertical orientation, blood flow in or around the nodule, microcalcifications, solid composition, ill defined or irregular margins or extrathyroidal extensions, and swollen cervical lymph nodes (all P < 0.05) (Table 2).
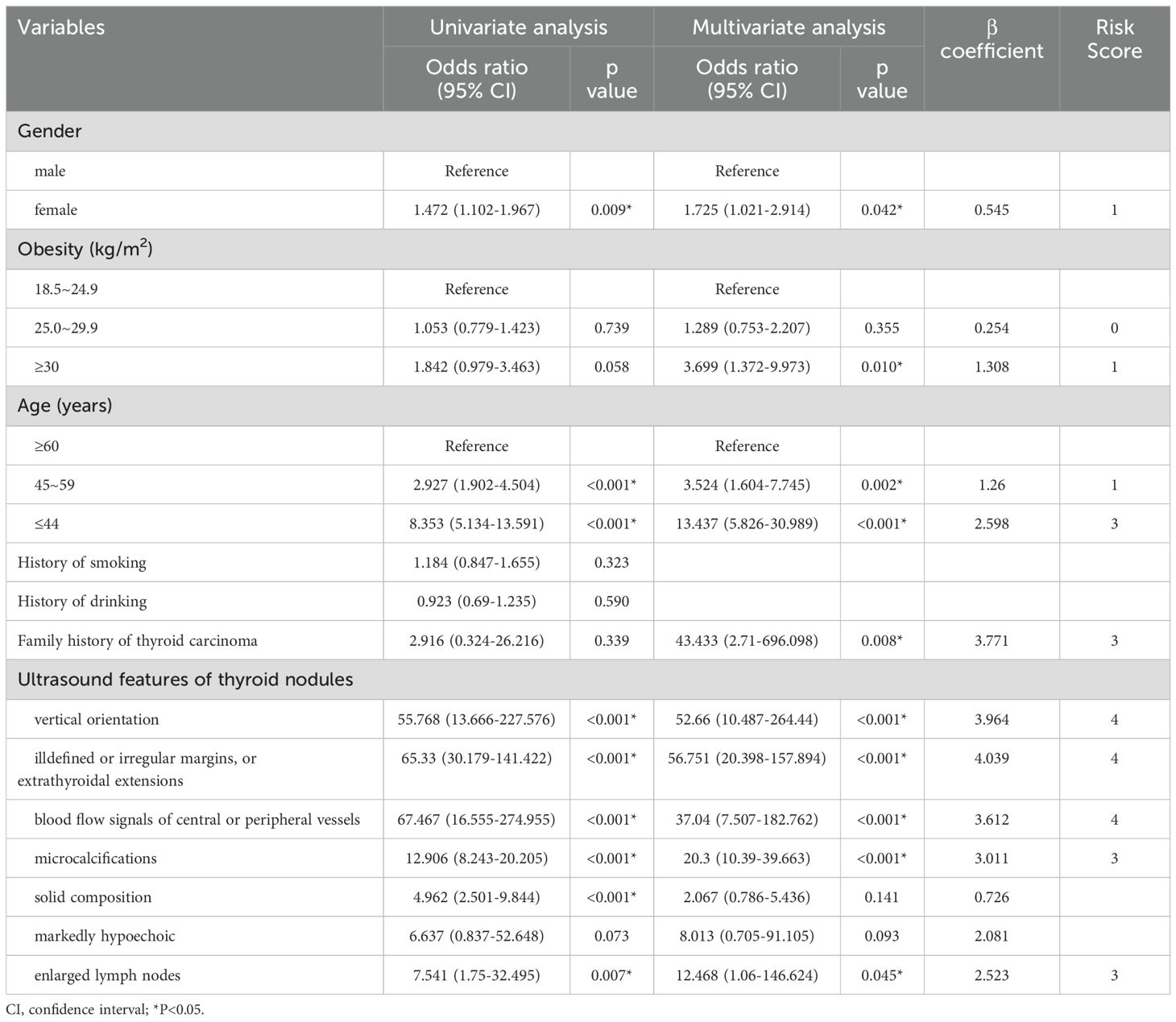
Table 2. Univariate and multivariate analysis to identify factors associated with malignant thyroid nodule and a scoring system developed from β coefficient in training cohort.
Clinical and ultrasonographic risk factors for thyroid cancer explored by multifactorial logistic regression
As shown in Table 2, we established the first clinical risk score model for TIRADS 4 nodules in Chinese individuals. Multivariate logistic regression analysis revealed that obesity, youth (defined as being under 44 years old), a family history of thyroid carcinoma, vertical orientation, blood flow in or around the nodule, microcalcifications, ill-defined or irregular margins or extrathyroidal extensions, and swollen cervical lymph nodes were independent risk factors for malignant nodules (all P < 0.05). We rounded the β values of these independent risk factors to the nearest integer to calculate the risk scores and subsequently created a scoring table.
Validation of the clinical risk score model for TIRADS 4 nodules
The final risk prediction models were evaluated within the validation cohorts to assess their validity (Table 3, Figure 1). In terms of discrimination, the risk score tested within the validation cohort achieved an AUC of 0.943 (95% CI: 0.928, 0.959). We estimated the optimal cut-off point, sensitivity, and specificity of the risk score model. At a cut-off point of 5, the sensitivity was 82.33% and the specificity was 94.44%. Additionally, the accuracy of the model was 87.42%, with a positive predictive value of 95.34% and a negative predictive value of 79.48%. The C-TIRADS and ACR-TIRADS classifications were also tested within the validation queues. The AUC of the C-TIRADS classification was found to be 0.863 (95% CI: 0.837, 0.890), with an optimal cut-off value of 2 points, a sensitivity of 76.96%, a specificity of 93.52%, an accuracy of 83.92%, a positive predictive value of 94.25%, and a negative predictive value of 74.63%. Moreover, the AUC of the ACR-TIRADS classification was 0.833 (95% CI: 0.804, 0.862), also with an optimal cut-off value of 2 points, a sensitivity of 72.93%, a specificity of 92.28%, an accuracy of 81.06%, a positive predictive value of 92.88%, and a negative predictive value of 71.19% (Table 3).
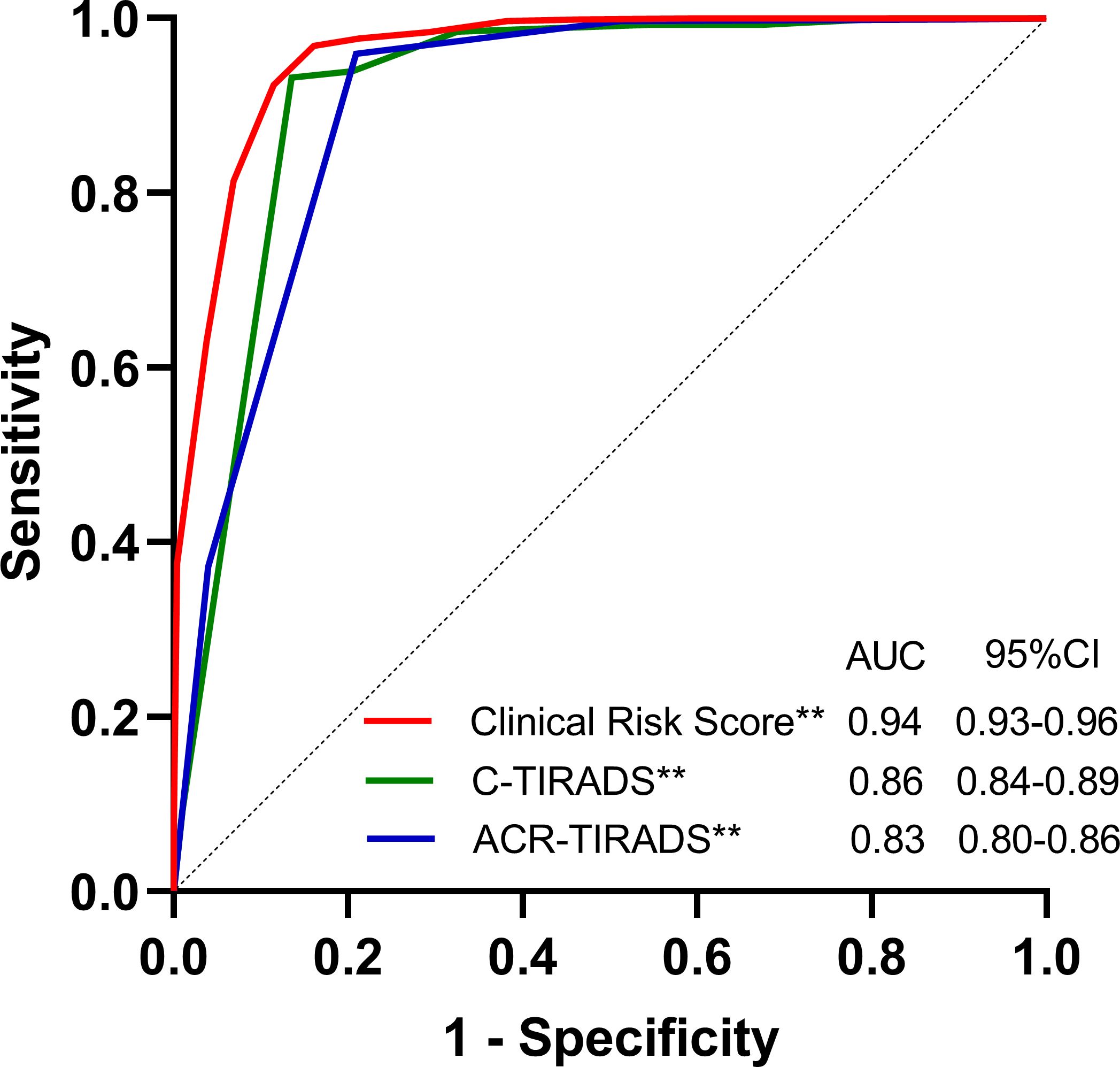
Figure 1. Area under the receiver-operating characteristic curve (AUC) of the Clinical Risk Score Model, C-TIRADS and the ACR-TIRADS in the validation cohorts. AUCs of the Clinical Risk Score Model, C-TIRADS and the ACR-TIRADS were 0.943(95% CI: 0.928, 0.959), 0.863(95%CI: 0.837, 0.890) and 0.833(95% CI: 0.804, 0.862), respectively. **P<0.001.
In the validation cohort, the calibration of the risk score model was assessed using the H-L goodness-of-fit test, which indicated a good fit (χ2 = 13.728; p=0.089). We plotted a calibration graph using data from the validation cohort (Figure 2). In this calibration plot, the horizontal axis represents the actual observed values, while the vertical axis represents the model’s predicted values. The data points were randomly distributed around the reference line (y = x). The trend line drawn from these points closely overlapped with y = x, indicating that the actual values were in close proximity to the predicted values, demonstrating strong model calibration performance and external applicability. Furthermore, to evaluate the clinical applicability of the risk score model, we conducted a DCA in the validation set (Figure 3). We observed that the net clinical benefit of patients was higher than that of the other two extreme curves in the vast majority of the threshold probability range. These results validate that the clinical risk score model had a good clinical predictive ability, effectively balances diagnostic accuracy with reduced overtreatment burdens.
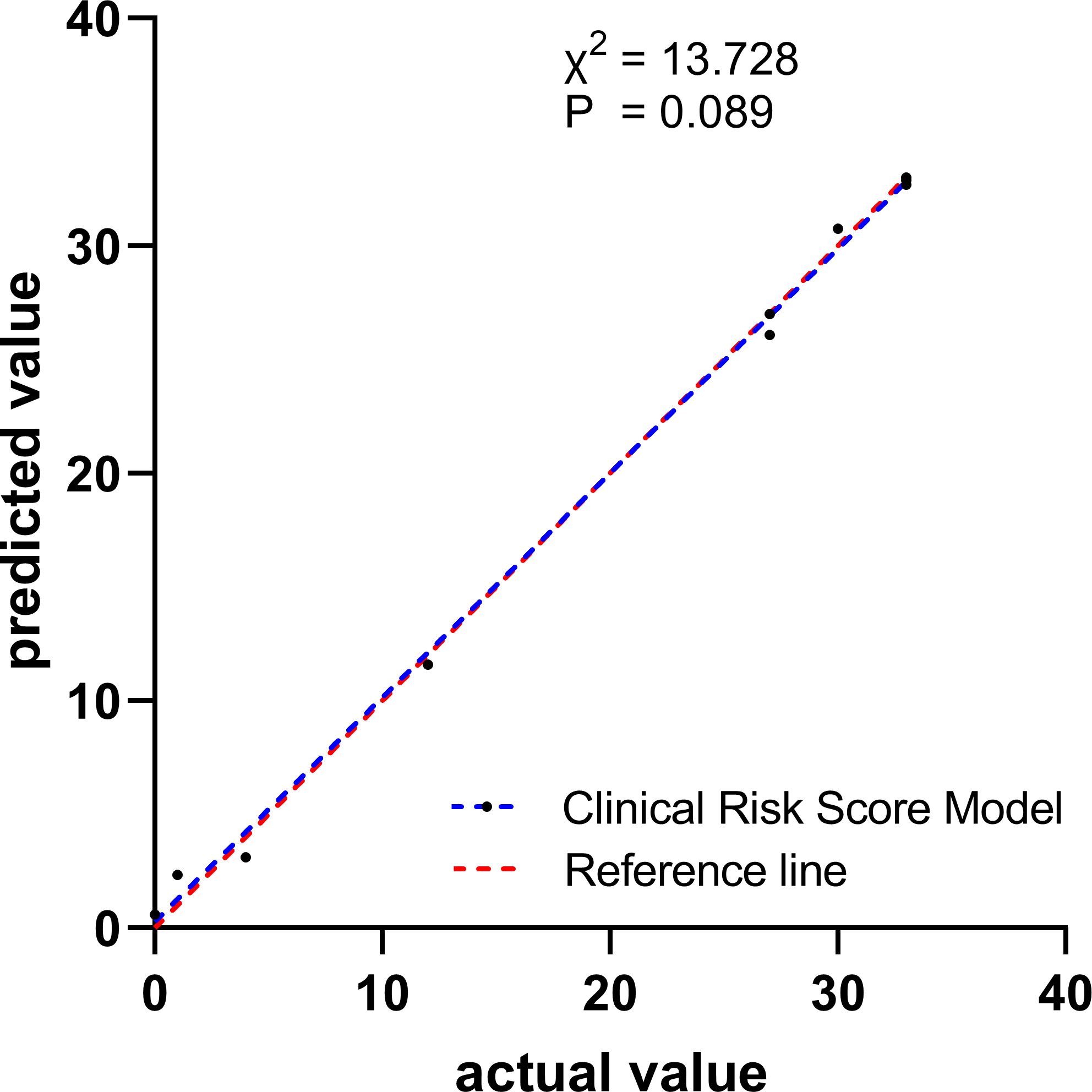
Figure 2. Calibration curve for observed versus predicted risk of developing thyroid cancer for Clinical Risk Score Model in the validation cohort.
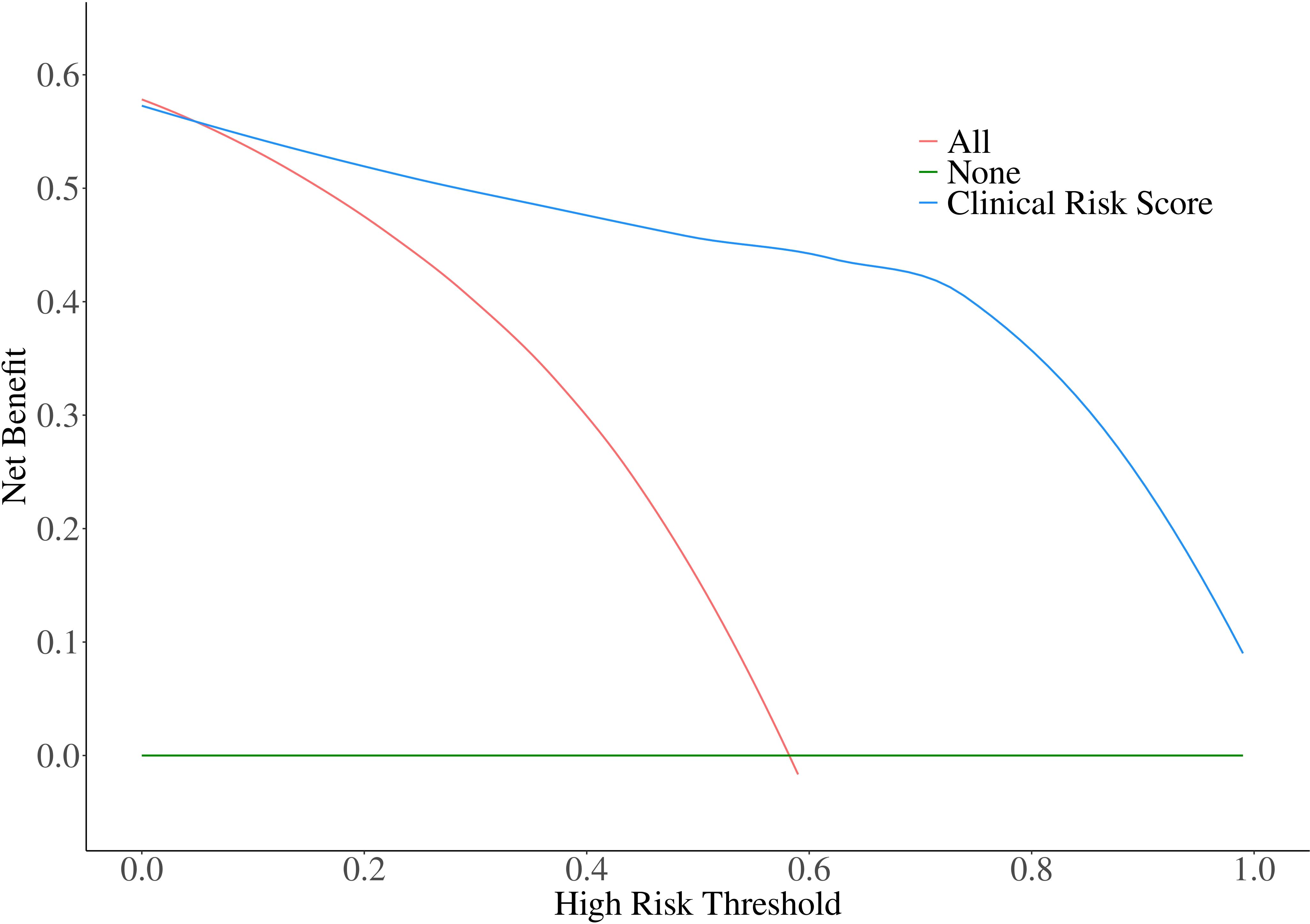
Figure 3. Decision curve analysis for Clinical Risk Score Model in the validation cohort. The net clinical benefit of patients was higher than that of the other two extreme curves in the vast majority of the threshold probability range.
Discussion
The risk score was a simple, non-invasive, and practical tool for evaluating thyroid nodules. In this study, we developed the first clinical and ultrasonographic risk score model to evaluate the nature of class 4 thyroid nodules in the Chinese population. The prediction model demonstrated both good discrimination and calibration capabilities for validation cohort.
Clinical risk factors for thyroid cancer
Studies have found that low age (16, 17), female gender (3), radiation exposure (22), genetic predisposition (18), thyroid function and antibodies (17), and disease status (obesity, diabetes (23–28) are associated with an increasing risk of thyroid cancer. Radiation exposure was known as one of the most important risk factors for thyroid cancer, increasing the risk of malignant thyroid from 5% to 50% (22). In addition, thyroid function and antibodies may be associated with the risk of thyroid cancer. A recent study (18) found that thyroid stimulating hormone (TSH) ≥1.79 mIU/L (OR 1.76[1.05, 2.95]) and TgAb positivity (OR 2.59[1.25, 5.37]) were independent risk factors for malignant thyroid nodules. However, our study did not find a correlation between thyroid stimulating hormone and thyroid cancer. Obesity has been associated with the development of many cancers (23, 34), and insulin resistance, chronic systemic inflammatory states, and alterations in the gut microbiota may be the possible inner mechanisms (25). It has been estimated that for every 5% increase in BMI, the risk of thyroid cancer increases by 30% (26). Consistent with our study, a large US study (27) of 457,331 cases indicated that obesity was a significant contributor to the rapid increase in the incidence of papillary thyroid carcinoma between 1995-2015, both overweight and obese were significantly associated with papillary thyroid carcinoma (overweight OR 1.26 [1.05, 1.52]; obese OR 1.30 [1.05-1.62]). In our study, obesity did not present significance in the univariate analysis, but persist with multivariate analysis which indicating its inherent independent predictive value. A meta-analysis (28) of 10725884 cases from 16 studies showed that diabetes mellitus was associated with an increased risk of thyroid cancer (RR 1.20 [1.09, 1.33]), and especially more pronounced in women (RR 1.11 [1.06, 1.17]) than in men (RR 1.14 [1.00, 1.30]). The correlated molecular mechanism may be estrogen receptor alpha (ERα) and beta (ERβ), which have been reported to have an important role in the pathogenesis of thyroid cancer (29). Due to no significant differences were found between diabetes and thyroid cancer in our study, diabetes was not included in the new model.
Ultrasound manifestations for thyroid cancer
A study conducted in 2021 found that ultrasound manifestations, including solidity, microcalcification, very hypoechoicity, blurred margins, irregular margins or extrathyroidal invasion, vertical position and other ultrasound features can indicate a high risk of malignant thyroid nodules (30). What’s more, angioinvasion is a critical indicator of aggressive behavior in differentiated thyroid cancer (DTC), correlating with distant metastasis and recurrence risks (31). Multiple studies have confirmed that blood flow signals (e.g., abnormal elevation of resistance index, the pulsatility index and peak systolic velocity) are associated with the invasiveness of thyroid cancer (32, 33). In our study, ultrasound features variables such as shape, margins, blood flow signals, calcifications, and cervical lymph nodes retained significance in both univariate and multivariate analysis, indicating their robustness as predictors. In contrast, while the composition variable was significant in univariate analysis, it failed to maintain significance in multivariate models, suggesting that its association may be confounded by other factors. In recent years, Chinese experts have proposed the C-TIRADS classification (15) to identify the nature of thyroid nodules recently, with a fluctuating ROC-AUC range of 0.753 to 0.933, a sensitivity of 84.25% to 93.1%, a specificity of 55.3% to 58.76%, an accuracy of 64.82% to 74.6% (34, 35). The C-TIRADS (15) guideline proposes that the probability of malignancy of thyroid nodules of category 4A, 4B and 4C are 2-10%, 10%-50%, and 50-90%, its diagnosis is still clinically difficult.
Comparison and application of models
In recent years, machine learning models have been proved to be effective in thyroid nodule malignancy prediction. Current studies report AUC values ranging from 0.83 to 0.97 across different models (36–39), with accuracy rates between 71.7% to 92.1% in validation cohorts (38–40). However, machine learning models require substantial computational resources, and their performance depends on the image acquisition protocol and annotation quality. The clinical risk score model developed in our study achieves comparable performance, and is more feasible for clinical deployment. Besides, the incorporation of molecular markers, such as BRAF, RAS and TERT detection, enhances the diagnostic value of thyroid nodules exhibiting indeterminate cytology (41, 42), and plays a role in predicting outcomes for patients with papillary thyroid carcinoma (43). Nevertheless, the invasiveness, high cost, insufficient timeliness, and technical barriers associated with these methods limit their application as a first-line screening tool.
The addition of clinical features could enhance diagnostic efficiency for thyroid nodules (19–21). We applied the quantitative analysis parameters of ultrasonography and clinical features to construct a predictive risk score model for category 4 nodules. In the validation cohort, we found that the AUC, accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of the new model were superior to those of the C-TIRADS and ACR-TIRADS stratification. In practice, sonographers perform initial evaluation based on predefined ultrasonographic features, while endocrinologists or surgeons could integrate clinical data to guide strategies on patient management and treatment. When the risk score of the new model reached 5 points, the Youden Index achieved its maximum value. Clinicians could prioritize patients with clinical risk score model scores≥5 points for biopsy or surgery. The diagnostic model could effectively avoid missed diagnoses when the score was 4 points and avoid misdiagnoses when the score was 6 points.
Strengths and weaknesses
Our study has several strengths. The clinical risk score model exhibits good accuracy and calibration. In addition to ultrasound risk factors, the model also included age, gender, BMI and family history of thyroid carcinoma. Without increasing the burden of medical expenses, this study enhances the accuracy of the model in distinguishing between malignant and benign nodules compared to traditional C-TIRADS and ACR-TIRADS stratifications, thereby facilitating the development of more appropriate and beneficial treatment plans in daily clinical practice. Furthermore, the risk score model is highly practical. It serves as a simple and convenient assessment tool that can be easily understood and recognized by both doctors and patients in a short period, which may offer significant translational value for clinical practice.
Our study has several limitations. Firstly, as a single-center, retrospective study, it inevitably suffers from a lack of sample size and external validation, along with inherent biases such as incomplete data collection and recall bias. Secondly, the ultrasonic features were obtained from doctors with varying levels of experience in ultrasound imaging, which may introduce inter-observer variability. Thirdly, our risk score lacked indicators of ionizing radiation, as well as the morphology and blood flow characteristics of cervical lymph nodes. Finally, the pathological types of most malignant nodules were predominantly papillary thyroid carcinomas (92.5%), while other types of malignant thyroid tumors, such as follicular thyroid carcinoma (1.8%) and medullary thyroid carcinoma (1.2%), were not separately classified and analyzed due to the limited sample size. This limitation may restrict the generalizability of our model to other thyroid malignancies. Looking ahead, we hope to design prospective research, expand the sample size, include additional risk factors such as radiation history, the morphology and blood flow characteristics of cervical lymph nodes. In addition, future studies that incorporate surgical pathology and molecular markers may enhance the diagnostic and prognostic utility of the model.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of the Shanghai Health and Medical Center(Huadong Sanatorium). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XD: Data curation, Software, Writing – original draft. JH: Data curation, Formal Analysis, Methodology, Writing – original draft. YJ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. YW: Funding acquisition, Methodology, Writing – review & editing, Data curation, Formal Analysis, Validation. LY: Funding acquisition, Methodology, Writing – review & editing, Data curation, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This publication is supported by the Shanghai Three-Year Action Plan for Public Health System Construction-”Research on health management strategy and application of elderly population”(NO.GWVI-11.1-28), the Special Project for Clinical Research in Health Industry of Shanghai Municipal Health Commission (202440001), “Qi Ming Xing”Young Medical Talents Training Program (grant number 2023QMX08), and the Shanghai “Rising Stars of Medical Talents” Youth Development Program (SHWSRS(2023)_062).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1550034/full#supplementary-material
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
2. Davies L and Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. (2014) 140:317–22. doi: 10.1001/jamaoto.2014.1
3. Hyuna S, Jacques F, Siegel Rebecca L, Mathieu L, Isabelle S, Ahmedin J, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 70:209–49. doi: 10.3322/caac.21660
4. Lim H, Devesa SS, Sosa JA, Check D, and Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
5. Grussendorf M, Ruschenburg I, and Brabant G. Malignancy rates in thyroid nodules: a long-term cohort study of 17,592 patients. Eur Thyroid J. (2022) 11:e220027. doi: 10.1530/ETJ-22-0027
6. Cosimo D, LaszloHegedüs, Agnieszka C, Paschke R, Russ G, Schmitt F, et al. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur Thyroid J. (2023) 12:e230067. doi: 10.1530/ETJ-23-0067
7. La Vecchia C and Negri E. Thyroid cancer: The thyroid cancer epidemic-overdiagnosis or a real increase? Nat Rev Endocrinol. (2017) 13:318–9. doi: 10.1038/nrendo.2017.53
8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
9. Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. (2009) 94:1748–51. doi: 10.1210/jc.2008-1724
10. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. (2011) 260:892–9. doi: 10.1148/radiol.11110206
11. Russ G, Bigorgne C, Royer B, Rouxel A, and BienvenuPerrard M. The Thyroid Imaging Reporting and Data System (TIRADS) for ultrasound of the thyroid. J radiologie. (2011) 92:701–13. doi: 10.1016/j.jradio.2011.03.022
12. Sánchez JF. TI-RADS classifcation of thyroid nodules based on a score modifed according to ultrasound criteria for Malignancy. Rev Argent Radiol. (2014) 78:138–48. doi: 10.1016/j.rard.2014.07.015
13. Zayadeen AR, Abu-Yousef M, and Berbaum K. JOURNAL CLUB: retrospective evaluation of ultrasound features of thyroid nodules to assess Malignancy risk: A step toward TIRADS. AJR. Am J Roentgenol. (2016) 207:460–9. doi: 10.2214/AJR.15.15121
14. Songsaeng D, Soodchuen S, Korpraphong P, and Suwanbundit A. Siriraj thyroid imaging reporting and data system and its efficacy. (2017) 69:262–7. doi: 10.14456/smj.2017.52
15. Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, et al. 2020 Chinese guidelines for ultrasound Malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine. (2020) 70:256–79. doi: 10.1007/s12020-020-02441-y
16. Zhang X, Ze Y, Sang J, Shi X, Bi Y, Shen S, et al. Risk factors and diagnostic prediction models for papillary thyroid carcinoma. Front Endocrinol (Lausanne). (2022) 13:938008. doi: 10.3389/fendo.2022.938008
17. Hu T, Li Z, Peng C, Huang L, Li H, Han X, et al. Nomogram to differentiate benign and Malignant thyroid nodules in the American College of Radiology Thyroid Imaging Reporting and Data System level 5. Clin Endocrinol (Oxf). (2023) 98:249–58. doi: 10.1111/cen.14824
18. Liyanarachchi S, Gudmundsson J, Ferkingstad E, He H, Jonasson JG, Tragante V, et al. Assessing thyroid cancer risk using polygenic risk scores. Proc Natl Acad Sci U S A. (2020) 117:5997–6002. doi: 10.1073/pnas.1919976117
19. Wang Y, Tang Y, Luo Z, Li J, and Li W. Diagnostic nomogram model for ACR TI-RADS 4 nodules based on clinical, biochemical data and sonographic patterns. Clin Endocrinol (Oxf). (2025) 102:79–90. doi: 10.1111/cen.v102.1
20. Trimboli P, Curti M, Colombo A, Scappaticcio L, and Leoncini A. Combining TSH measurement with TIRADS assessment to further improve the detection of thyroid cancers. Minerva Endocrinol (Torino). (2024) 49:125–31. doi: 10.23736/S2724-6507.24.04207-6
21. Feroci F, Perini D, Giordano A, Romoli L, Guagni T, Coppola A, et al. ACR TI-RADS Score combined with cytopathology classification improves the risk stratification of indeterminate thyroid nodules. Minerva Endocrinol (Torino). (2023). doi: 10.23736/S2724-6507.22.03929-X
22. Iglesias ML, Schmidt A, Ghuzlan AA, Lacroix L, Vathaire F, Chevillard S, et al. Radiation exposure and thyroid cancer: a review. Arch Endocrinol Metab. (2017) 61:180–7. doi: 10.1590/2359-3997000000257
23. Wang H. Obesity and risk of thyroid cancer: evidence from a meta-analysis of 21 observational studies. Med Sci Monitor Int Med J Exp Clin Res. (2015) 21:283–91. doi: 10.12659/MSM.892035
24. Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, et al. Vital signs: trends in incidence of cancers associated with overweight and obesity-United States, 2005-2014. MMWR Morb Mortal Wkly Rep. (2017) 66:1052–8. doi: 10.15585/mmwr.mm6639e1
25. Franchini F, Palatucci G, Colao A, Ungaro P, Macchia PE, Nettore IC, et al. Obesity and thyroid cancer risk: an update. Int J Environ Res Public Health. (2022) 19:1116–26. doi: 10.3390/ijerph19031116
26. Schmid D, Ricci C, Behrens G, and Leitzmann MF. Adiposity and risk of thyroid cancer: A systematic review and meta-analysis. Obes Rev. (2015) 16:1042–54. doi: 10.1111/obr.v16.12
27. Kitahara CM, Pfeiffer RM, Sosa JA, and Shiels MS. Impact of overweight and obesity on US papillary thyroid cancer incidence trends (1995-2015). Natl Cancer Inst. (2020) 112:810–7. doi: 10.1093/jnci/djz202
28. Li H and Qian J. Association of diabetes mellitus with thyroid cancer risk: A meta-analysis of cohort studies. Med Baltimore. (2017) 96:e8230. doi: 10.1097/MD.0000000000008230
29. Gong Z, Yang S, Wei M, Vlantis AC, Chan JYK, van Hasselt CA, et al. The isoforms of estrogen receptor alpha and beta in thyroid cancer. Front Oncol. (2022) 12:916804. doi: 10.3389/fonc.2022.916804
30. Zhou JQ, Song YY, Zhan WW, Wei X, Zhang S, Zhang RF, et al. Thyroid imaging reporting and data system (TIRADS) for ultrasound features of nodules: multicentric retrospective study in China. Endocrine. (2021) 72:157–70. doi: 10.1007/s12020-020-02442-x
31. Xu B, Roy D, Serrette R, and Ghossein R. Defining angioinvasion and lymphatic invasion in papillary thyroid carcinoma: morphological criteria, utility of D2-40/CD31/ERG immunohistochemistry and correlation with clinicopathological characteristics. Histopathology. (2024) 85:950–8. doi: 10.1111/his.15285
32. Sun X, Wei Z, Luo Y, et al, and Wang M. Exploration of the evaluation value of combined magnetic resonance imaging and ultrasound blood flow parameters in cervical lymph node metastasis of thyroid cancer. Cancer Manag Res. (2025) 17:651–9. doi: 10.2147/CMAR.S505730
33. Huang X, Zhang Y, He D, Lai L, Chen J, and Zhang T. Machine learning-based shear wave elastography elastic index (SWEEI) in predicting cervical lymph node metastasis of papillary thyroid microcarcinoma: A comparative analysis of five practical prediction models. Cancer Manag Res. (2022) 14:2847–58. doi: 10.2147/CMAR.S383152
34. Zhu T, Chen J, Zhou Z, Ma X, and Huang Y. Differentiation of thyroid nodules (C-TIRADS 4) by combining contrast-enhanced ultrasound diagnosis model with chinese thyroid imaging reporting and data system. Front Oncol. (2022) 12:840819. doi: 10.3389/fonc.2022.840819
35. Cai Y, Yang R, Yang S, Lu L, Ma R, Xiao Z, et al. Comparison of the C-TIRADS, ACR-TIRADS, and ATA guidelines in Malignancy risk stratification of thyroid nodules. Quant Imaging Med Surg. (2023) 13:4514–25. doi: 10.21037/qims-22-826
36. Liu X, Yao J, Wang D, Xiao W, Zhou W, Li L, et al. Machine learning model for risk stratification of papillary thyroid carcinoma based on radiopathomics. Acad Radiol. (2025) 32:2545–53. doi: 10.1016/j.acra.2024.12.062
37. Canali L, Gaino F, Costantino A, Guizzardi M, Carnicelli G, Gullàetal F, et al. Development of machine learning models to predict papillary carcinoma in thyroid nodules: The role of immunological, radiologic, cytologic and radiomic features. Auris Nasus Larynx. (2024) 51:922–8. doi: 10.1016/j.anl.2024.09.002
38. Khodabandelu S, Ghaemian N, Khafri S, Ezoji M, and Khaleghi S. Development of a machine learning-based screening method for thyroid nodules classification by solving the imbalance challenge in thyroid nodules data. J Res Health Sci. (2022) 22:e00555. doi: 10.34172/jrhs.2022.90
39. Weng S, Ding C, Hu D, Chen J, Liu Y, Liu W, et al. Utilizing machine learning for early screening of thyroid nodules: a dual-center cross-sectional study in China. Front Endocrinol (Lausanne). (2024) 15:1385167. doi: 10.3389/fendo.2024.1385167
40. Wang X, Niu Y, Liu H, Tian F, Zhang Q, Wang Y, et al. ThyroNet-X4 genesis: an advanced deep learning model for auxiliary diagnosis of thyroid nodules’ Malignancy. Sci reports. (2025) 15:10599. doi: 10.1038/s41598-025-86819-w
41. Decaussin-Petrucci M, Descotes F, Depaepe L, Lapras V, Denier M, Borson-Chazot F, et al. Molecular testing of BRAF, RAS and TERT on thyroid FNAs with indeterminate cytology improves diagnostic accuracy. Cytopathology. (2017) 28:482–7. doi: 10.1111/cyt.2017.28.issue-6
42. Lu SY, Chen YC, Zhu CF, Chen J, Zhou QY, Zhang MM, et al. A five-gene panel refines differential diagnosis of thyroid nodules. J Clin Lab Anal. (2021) 35:e23920. doi: 10.1002/jcla.23920
Keywords: TI-RADS 4 thyroid nodules, thyroid cancer, risk factors, clinical scoring model, diagnostic efficiency
Citation: Hu J, Du X, Jiang Y, Wang Y and Yang L (2025) Incorporation of clinical features into a multivariate logistic regression model for the differential diagnosis of benign and malignant TI-RADS 4 thyroid nodules. Front. Endocrinol. 16:1550034. doi: 10.3389/fendo.2025.1550034
Received: 22 December 2024; Accepted: 06 May 2025;
Published: 29 May 2025.
Edited by:
Chunyu Tan, Sichuan University, ChinaReviewed by:
Giovanni Vitale, University of Milan, ItalyAngelika Buczyńska, Medical University of Bialystok, Poland
Sabine Weidner, Inselspital, Switzerland
Copyright © 2025 Hu, Du, Jiang, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunle Wang, ZWNobzEyMTNAeWVhaC5uZXQ=; Lijuan Yang, eWFuZ2xpanVhbjg1MjdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jun Hu†
Jun Hu† Xian Du
Xian Du Yongbin Jiang
Yongbin Jiang Yunle Wang
Yunle Wang Lijuan Yang
Lijuan Yang