- 1Department of Nutrition, Texas A&M University, College Station, TX, United States
- 2Institute for Advancing Health through Agriculture, Texas A&M AgriLife, College Station, TX, United States
- 3Department of Psychology, Cornell University, Ithaca, NY, United States
- 4Division of Nutritional Sciences, Cornell University, Ithaca, NY, United States
- 5Department of Pediatrics, Section of Adolescent Medicine, Weill Cornell Medicine, New York, NY, United States
- 6Cornell Statistical Consulting Unit, Cornell University, Ithaca, NY, United States
Introduction: PCOS is linked with disproportionately high rates of depression and anxiety that significantly compromise quality of life and pose problems for treatment eligibility and adherence. The overarching objective of the proposed manuscript is to define the presence and severity of psychological symptoms in peri-menarcheal adolescents, and their association with well-described risk-factors for future PCOS.
Methods: Fifty-two pre- and early post-menarcheal participants underwent a non-fasting blood draw to measure reproductive hormones (Anti-Mullerian Hormone (AMH), Luteinizing Hormone (LH), Follicle Stimulating Hormone (FSH), Total Testosterone, Sex Hormone Binding Globulin (SHBG) and HbA1c, anthropometry, menstrual history (if post-menarcheal), and a series of surveys to evaluate depression (CES-DC), anxiety (MASC) and, as a novel approach, rumination, which is a transdiagnostic psychological process and early prodromal risk for psychological disorders. Parents/legal guardians completed a demographics survey. Random Forest analysis was used to predict depression, anxiety, and rumination from a predetermined set of variables in this participant sample.
Results: The overall R2 for depression, anxiety, and rumination from the random forest model were 0.557, 0.555, and 0.597, respectively, suggesting overall good explanatory power for psychological outcomes. Parent education (Portion Sum of Squares (SS) = 11.4%) followed by AMH (Portion SS = 10.9%) and waist-hip-ratio (WHR) (Portion SS = 9.2%) were the most important variables in predicting depression. LH: FSH ratio was the most important variable in the dataset used to differentiate participants along the observed anxiety score continuum (Portion SS = 0.112 (11%) followed by HbA1c (Portion SS = 8.1%) and WHR (Portion SS = 7.9%). SHBG was the most frequently identified variable to differentiate participants reporting rumination (Portion SS = 13.3%) followed by Free Androgen Index (Portion SS = 6.9%) and WHR (Portion SS = 6.9%). Adolescents at high risk for progression to PCOS may already experience psychological vulnerabilities prior to a clinical diagnosis or full manifestation of PCOS. Our study findings highlight PCOS as a lifelong, multifaceted health condition with ramifications earlier than commonly documented.
1 Introduction
Polycystic ovary syndrome (PCOS) is a highly prevalent endocrine condition affecting approximately 1 in 10 females (1–3), and diagnosed by the presence of hyperandrogenism, menstrual irregularity, and in adults enlarged, polycystic ovaries or elevated Anti-Mϋllerian Hormone (AMH) (4). PCOS is a multi-system disease whose pathophysiology is intertwined with significant cardiometabolic abnormalities – including obesity, Type 2 diabetes, hypertension, dyslipidemia, coronary heart disease, and stroke – resulting in disproportionately higher rates of disability and health-related unemployment (4–8). Because PCOS emerges during adolescence, there is a growing scientific and medical consensus that early intervention is crucial for mitigating the lifelong chronic disease burden.
Inexplicably high rates of depression and anxiety have been documented in adolescents and adults with PCOS (9–12), even after consideration of comorbid conditions also closely tied to psychopathology (13). These high rates of psychopathology are poorly understood despite being a crucial consideration for both successful interventions and overall quality of life; mental health comorbidities are known to compromise treatment adherence for both pharmacological and behavioral interventions for PCOS (14, 15) and further increase the risk of cardiometabolic disease among women with PCOS (16). Yet paradoxically, several PCOS interventions (including surgical, pharmacologic, and lifestyle interventions) list psychological comorbidities as exclusionary (17–19), which limits the inference regarding the effectiveness of new and existing interventions and therefore restricts evidence-based options to a subset of those living with PCOS.
Whether psychological symptoms emerge before, after, or concurrent with the diagnostic features of PCOS is neither clear nor well studied. Depression and anxiety are often identified and researched following the diagnosis of PCOS, a disease which has no cure and often is not recognized until after at least a year of actively seeking a diagnosis (20). While the diagnostic experience itself may play an important role in exacerbating pre-existing depression and anxiety, this approach confounds the question of time course and the physiological relationship between PCOS and psychopathology. Thus, whether investigators are measuring psychological vulnerabilities inherent to PCOS – versus the burden of a disorder known to impact quality of life, employment, finances, and childbearing – is impossible to disentangle in the existing literature.
To begin to address this knowledge gap, we conducted a pilot study of pre- and post-menarcheal adolescents to determine whether endocrine or clinical features of PCOS, physical changes of puberty, or age best predicted the severity of depression, anxiety, and rumination. We hypothesized that symptoms of PCOS would emerge as early predictors of PCOS; however we used an unbiased analytical approach without setting priority predictors a priori. While depression and anxiety are well-studied in regard to PCOS, rumination has been considered less frequently. Rumination refers to the tendency to cope with negative emotions by thinking repetitively and passively about distress and the causes and consequences of this distress (21). Ruminative coping has been documented in children as young as 8 years of age (22), rises sharply at puberty (23), and is both a correlate and robust prodromal indicator of depression and anxiety (24). Because rumination often precedes the onset of depression and anxiety, its inclusion in this study offers a chance to understand early connections between psychological states and features of PCOS.
2 Methods
2.1 Participants
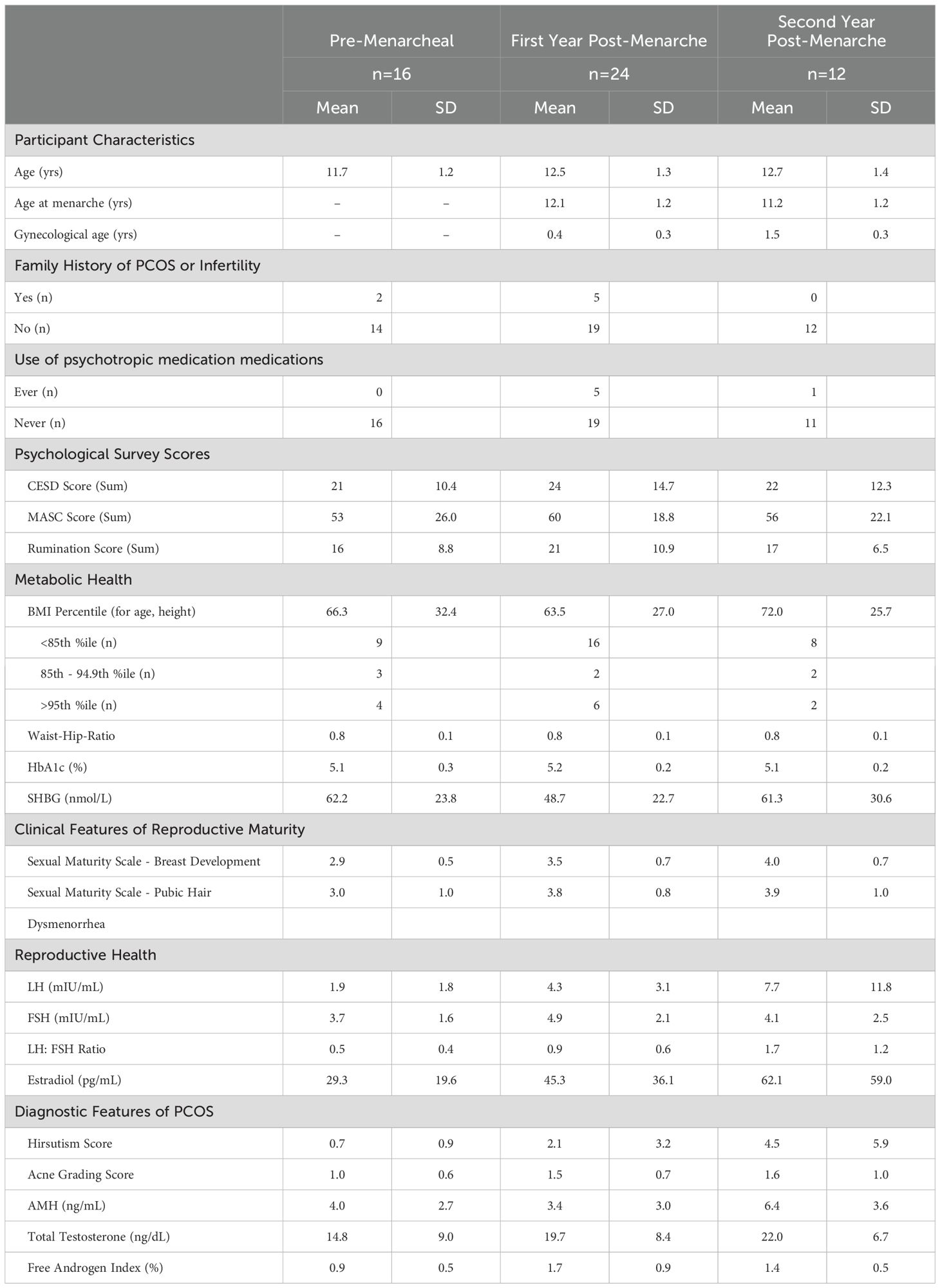
Fifty-two children and adolescents (9–15 years) were enrolled in a cross-sectional study across two sites (Upstate New York; Cornell University, New York City; Weill Cornell). Recruitment was open to all-comers who met eligibility criteria. Recruitment in New York City occurred mainly through an adolescent medicine clinic, although community and employee-based recruitment tools were also employed. Across both sites, study information was distributed using social media, listservs, and flyers posted in clinical and community settings. Participants were recruited based on one of three categories: i) premenarcheal and at least Tanner Stage 3 for either pubic hair or breast development based on self or parent report (Cornell) or physician report (Weill Cornell), ii) within 2 years of menarche with regular menstrual cycles or iii) within 2 years of menarche with irregular menstrual cycles. Regular menstrual cycles were defined as 3 successive cycles 21–45 days apart and irregular cycles were defined as having at least 1 of 3 successive cycles <21 days or >45 days apart (4). All adolescents within the first year of menarche were classified as irregular (4). Exclusion criteria included significant delays in development that influenced social, cognitive, emotional, or behavioral functioning, pregnant or breastfeeding, history of chronic disease (i.e. type 1 or 2 diabetes), precocious puberty, or currently taking medications that affect reproductive functioning (e.g. hormones, metformin, or some anti-seizure medications).
2.2 Ethics
The study was approved by ethics review boards at Cornell University and Weill Cornell Medicine. All procedures in this study were carried out in accordance with the World Medical Association’s Declaration of Helsinki. Participant assent and parental/legal guardian permission were obtained after confirming that the participant was eligible for participation and before study procedures were initiated.
2.3 Clinical study procedures
Participants attended one non-fasting study visit and underwent the following researcher-guided standardized interviews: participant menstrual history (if post-menarcheal), participant medical history, family medical history, standardized assessment of acne presence and severity (25), and hirsutism scoring with visual aids using the modified Ferriman-Gallwey scoring system (26). Anthropometric measures of weight, height, waist and hip circumference, blood pressure, and heart rate were obtained using standard procedures. A non-fasting blood draw was collected into serum separator and EDTA tubes via antecubital venipuncture. Whole blood, serum, and plasma was processed per protocol and frozen immediately at -80C for long-term storage.
2.4 Psychological measures
2.4.1 Depressive symptoms
The Center for Epidemiologic Studies Depression Scale for Children (CES-DC (27);) is a 20-item, self-report measure of depressive symptoms experienced in the past week. Each item is rated on a scale where 0 =not at all and 3 = a lot, with higher scores indicating a greater frequency and severity of depressive symptoms. A score of 15 is typically used to signify a clinically relevant level of symptoms (27). Scores in this sample ranged from 2 to 46 (M=22.7, SD=12.8). Cronbach’s α = 0.80.
2.4.2 Anxiety symptoms
The Multidimensional Anxiety Scale for Children – 2nd Edition (28) is a 50-item self-report questionnaire assessing physical, cognitive, behavioral and affective symptoms of different forms of anxiety, including worry, panic, social anxiety, separation anxiety, and harm avoidance. Each item is rated on a scale where 0 = never and 3 = often, with higher scores indicated a greater frequency and severity of anxiety symptoms. Scores in this sample ranged from 17-99 (M=56.8, SD=21.7). Cronbach’s α = 0.94.
2.4.3 Rumination
Rumination was assessed using the Ruminative Response Scale of the Children’s Response Styles Questionnaire (22, 29), a 13-item measure of self-focused, cognitive responses to feelings of sadness modeled after the adult version of the Response Styles Questionnaire (30). Each item is scored on a scale where 0 = almost none of the time and 3 = almost all of the time, with higher scores indicating a greater tendency towards rumination. In this sample, scores ranged from 2-39 (M = 18.3, SD = 9.5). Internal consistency was good (Cronbach’s α = 0.91).
2.5 Biochemical assays
Serum androgens were evaluated by Brigham and Women’s Research Assay Core Laboratory using liquid chromatography with tandem mass spectrometry (Boston, MA; interassay CV 6.4%). AMH was measured using the picoAMH enzyme-linked immunosorbent assay (Motive Biosciences Inc., Webster, TX). The remaining analytes were shipped to the Human Nutritional Chemistry Service Laboratory at Cornell University for immunoassay analysis (Immulite 2000, Siemens Medical Solutions Diagnostic, Deerfield, IL). Free Androgen Index (FAI) was calculated as Total Testosterone/Sex Hormone Binding Globulin (SHBG) x 100% (31). FAI is a surrogate for free testosterone and an accepted marker for biochemical hyperandrogenism per the 2023 International Guidelines for PCOS (4).
2.6 Statistical analysis
REDCap® (Research Electronic Data Capture) was used to securely store study data. We conducted Random Forest analysis, which is an ensemble machine learning technique that aggregates a large number of decision trees (32) to optimally predict an outcome (e.g., depression score). A particular strength of this methodology is its ability to handle a large number of covariates per observation and considers complex interactions (32), which we deemed appropriate for our dataset, which included predictors spanning puberty, body habitus, social demographics, and PCOS. A decision tree iteratively searches for predictors on which to divide the dataset so that the residual sum or squares is minimized in each group. It then repeats the process recursively, dividing each subgroup by different predictors until an optimal tree is determined. While analysis using a single decision tree results in models that are biased and prone to overfitting, a random forest analysis generates many different trees that are each trained using a bootstrapped random sample from the original data set (to avoid overfitting) using subsamples of the set of predictor variables (to decorrelate the set of trees). The random forest procedure then averages the predictions of each tree, resulting in a better-fitting model with a lower variance and prediction error than that of any single tree. Another useful angle of a random forest analysis is the ability to calculate the relative variable importance to provide an assessment of each variable’s contribution to the predictive power of the model.
We used JMP Pro (Version 17) to conduct three separate random forest models, one each for the aggregate score for depressive symptoms, anxiety symptoms, and rumination. For each psychological outcome, we trained the model using 1000 trees, requesting 10 minimum splits per tree with a minimum subgroup size of 5. The minimum number of terms (variables) sampled per split was set to 7 for depression, 10 for anxiety, 10 for rumination. These values were arrived at after assessing several options using the tuning design table feature of JMP. The results of the 1000 trees per outcome are aggregated and summarized by listing the rank order of all variables included, from most important to least important, including the number of times each variable was used in a tree (split number). Supplementary Table 1 presents the full list of variables selected for inclusion in the model; these variables were selected based on diagnostic features of PCOS, known risk factors or PCOS, or their identification in previous research as predictive of psychological disorders in adolescence (e.g., Tanner Staging, sociodemographic variables).
3 Results
3.1 Participant characteristics
Participant demographics by reproductive age are depicted in Table 1. Participants were on average between 11–12 years old, most did not have a family history of PCOS or known infertility and most (77%) had never taken psychotropic medications. On average, participants were within a normal BMI and glycemic control (Table 1); however, 23% of participants met criteria for obesity and one participant had an HbA1c of 5.7%, which meets criteria for pre-diabetes (33). Overall, we recruited a diverse sample of youth (65% White, 21% Black/African-American, and 8% Asian, 27% Hispanic; percents do not equal 100 as participants were allowed to select all demographics that applied to them). Differences in depression anxiety, and rumination scores did not differ across pre-menarcheal and first- and second- post-menarcheal age groups (p>0.05).
3.2 Variable importance for depression scores
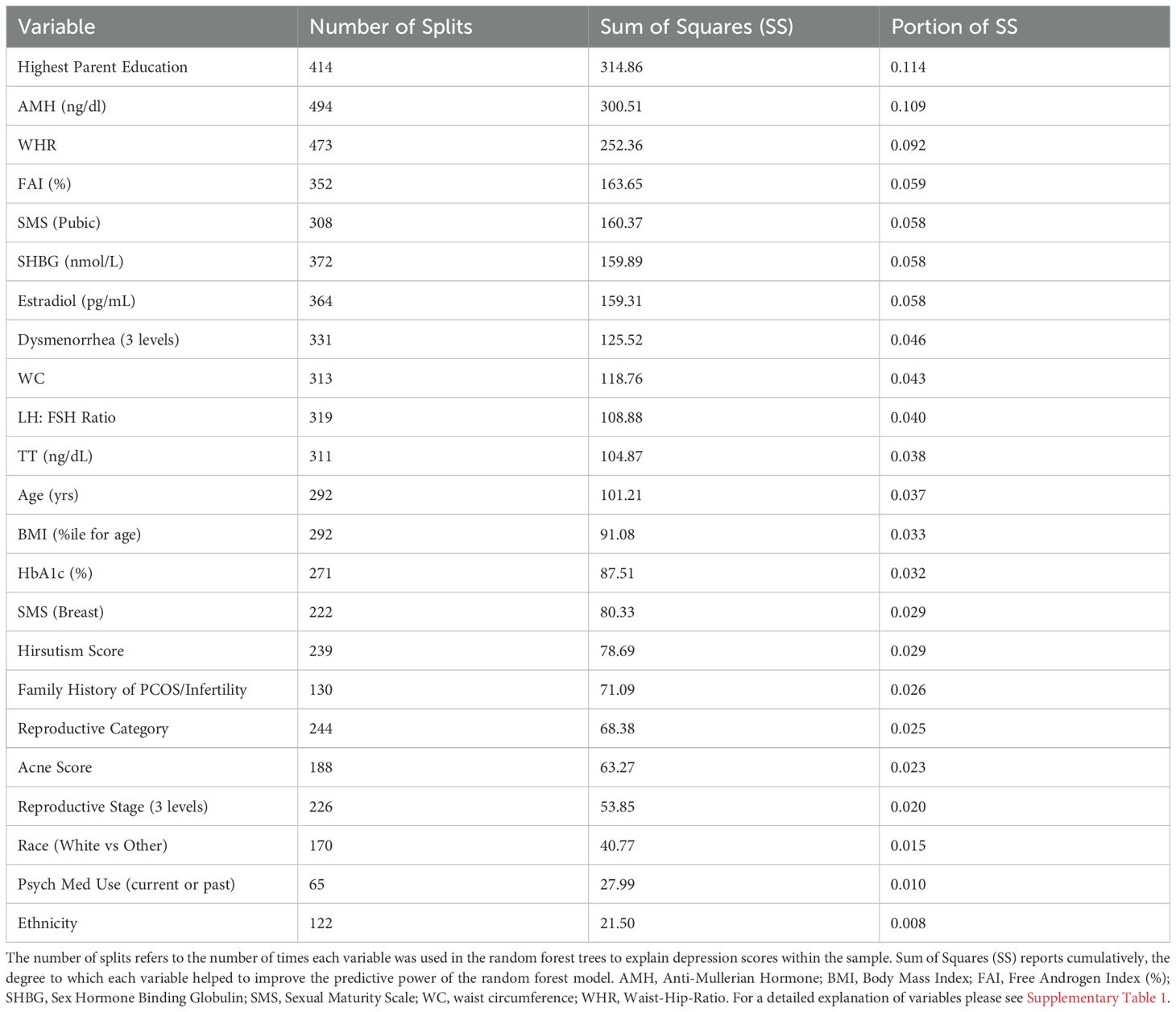
The relative variable importance scores for the observed depression score model are presented in Figure 1. Number of splits per variable and sum of squares presented in Table 2. Of the 52 participants, 51 were included in the analysis (n=1 did not complete the depression score survey). The overall R2 for this random forest model was 0.557 and overall Root Mean Square Error (RMSE) = 10.21, suggesting overall good explanatory power for depression scores in this sample using the variables. Parent education (Portion Sum of Squares = 0.114; Table 2), AMH (Portion Sum of Squares = 0.109; Table 2), and waist-hip-ratio (Portion Sum of Squares = 0.092; Table 2) emerged as top variables (Figure 1). Collectively, these three variables represented 0.32 (32%) of the total Sum of Squares in depression scores.
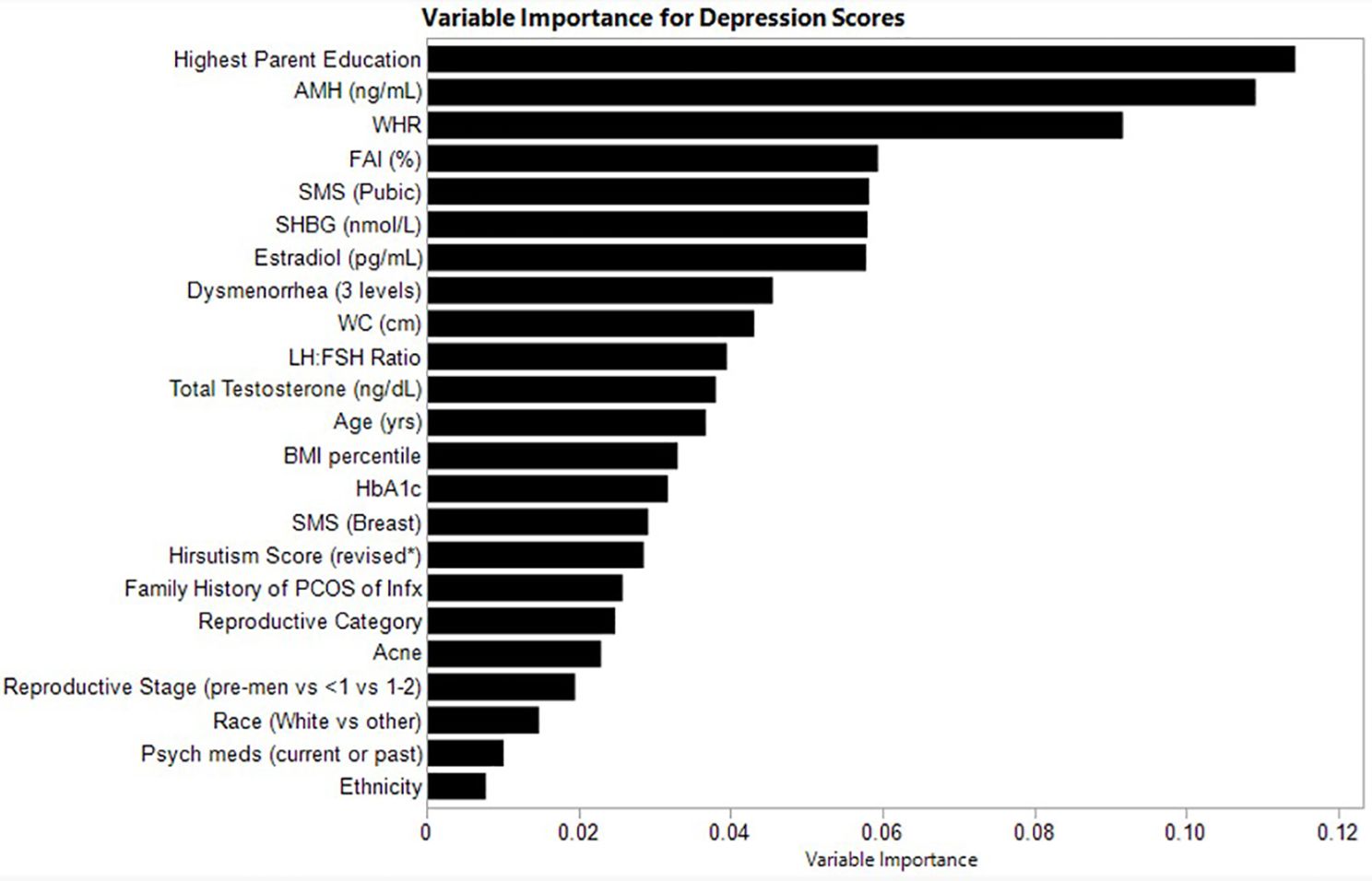
Figure 1. Relative variable importance scores for depression in pre- and post-menarcheal adolescents. Variables are ranked in order of importance, with overall importance (sum of squares) on the x-axis. AMH, Anti-Mullerian Hormone; BMI, Body Mass Index; FAI, Free Androgen Index (%); SHBG, Sex Hormone Binding Globulin; SMS, Sexual Maturity Scale; WC, waist circumference; WHR, Waist-Hip-Ratio. For a detailed explanation of variables please see Supplementary Table 1.
Heatmaps to highlight the non-linearity of interactions between the top three covariates for depression scores are depicted in Supplementary Figure 1. The heatmaps show the predicted values of depression scores by the top predictor variables, also considering all other covariates included in the random forest model. White squares reflect no observations along the x- and y-axis variables and therefore no data. The relationship between predicted and observed psychological outcomes are depicted in Supplementary Figure 4.
3.3 Variable importance for anxiety scores
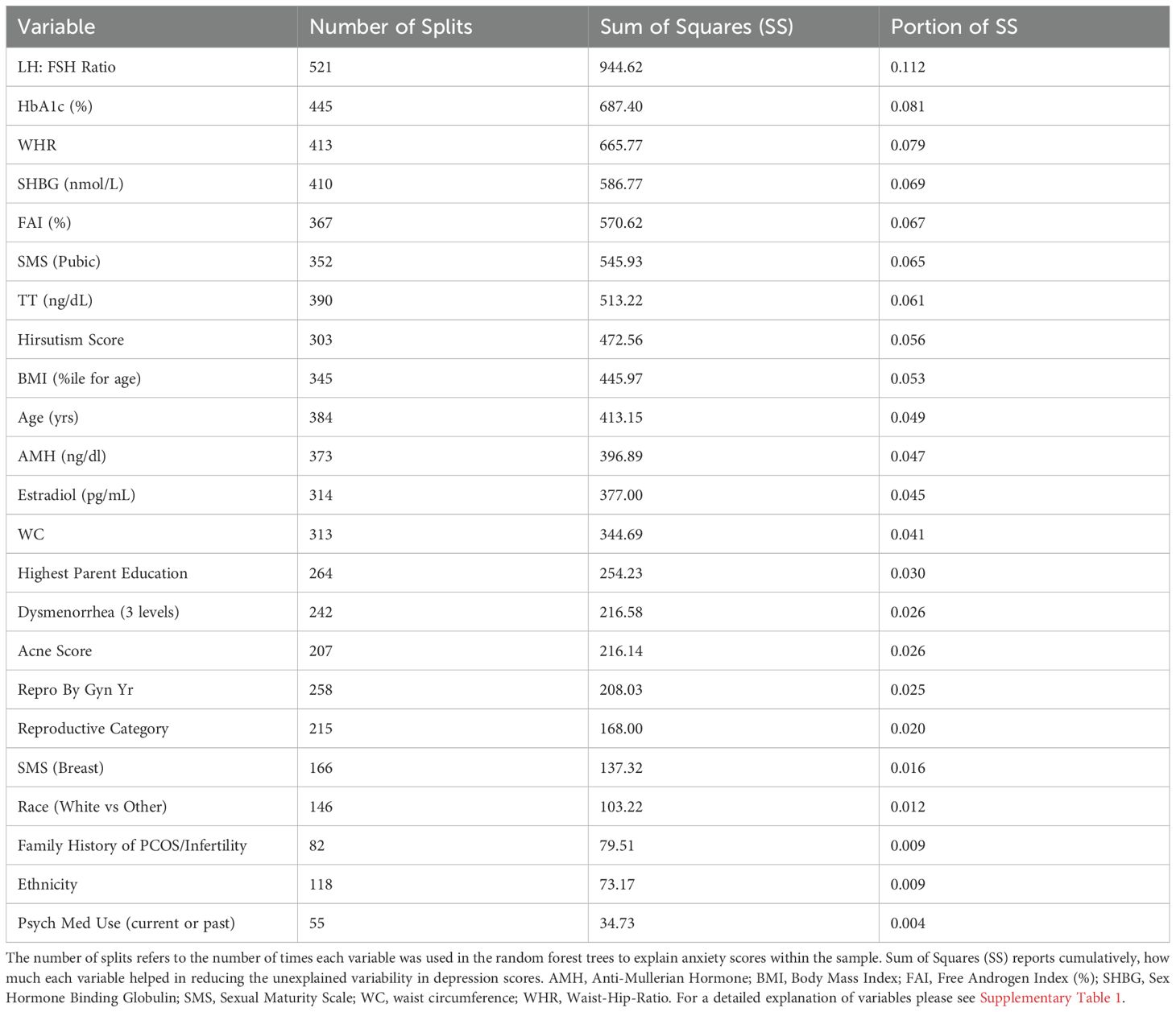
The relative variable importance scores for the observed anxiety score model are presented in Figure 2. Number of splits per variable and sum of squares presented in Table 3. Of the 52 participants, 49 were included in the analysis (n=3 did not complete the anxiety score survey). The overall model fit using random forest trees based on the included variables was R2 = 0.555 and overall RMSE = 14.4, again suggesting good explanatory power of anxiety scores in this sample. The LH: FSH ratio was the most important variable in the dataset used to differentiate participants along the observed anxiety score continuum, explaining 0.112 (11%) of the total Sum of Squares for anxiety scores. HbA1c and waist-hip ratio exhibited similar explanatory power (Portion Sum of Squares = 0.081 and 0.079, respectively; Table 3). Heatmaps highlighting non-linearity of interactions between the top three covariates for anxiety scores are depicted in Supplementary Figure 2; the relationship between predicted and observed psychological outcomes presented in Supplementary Figure 4.
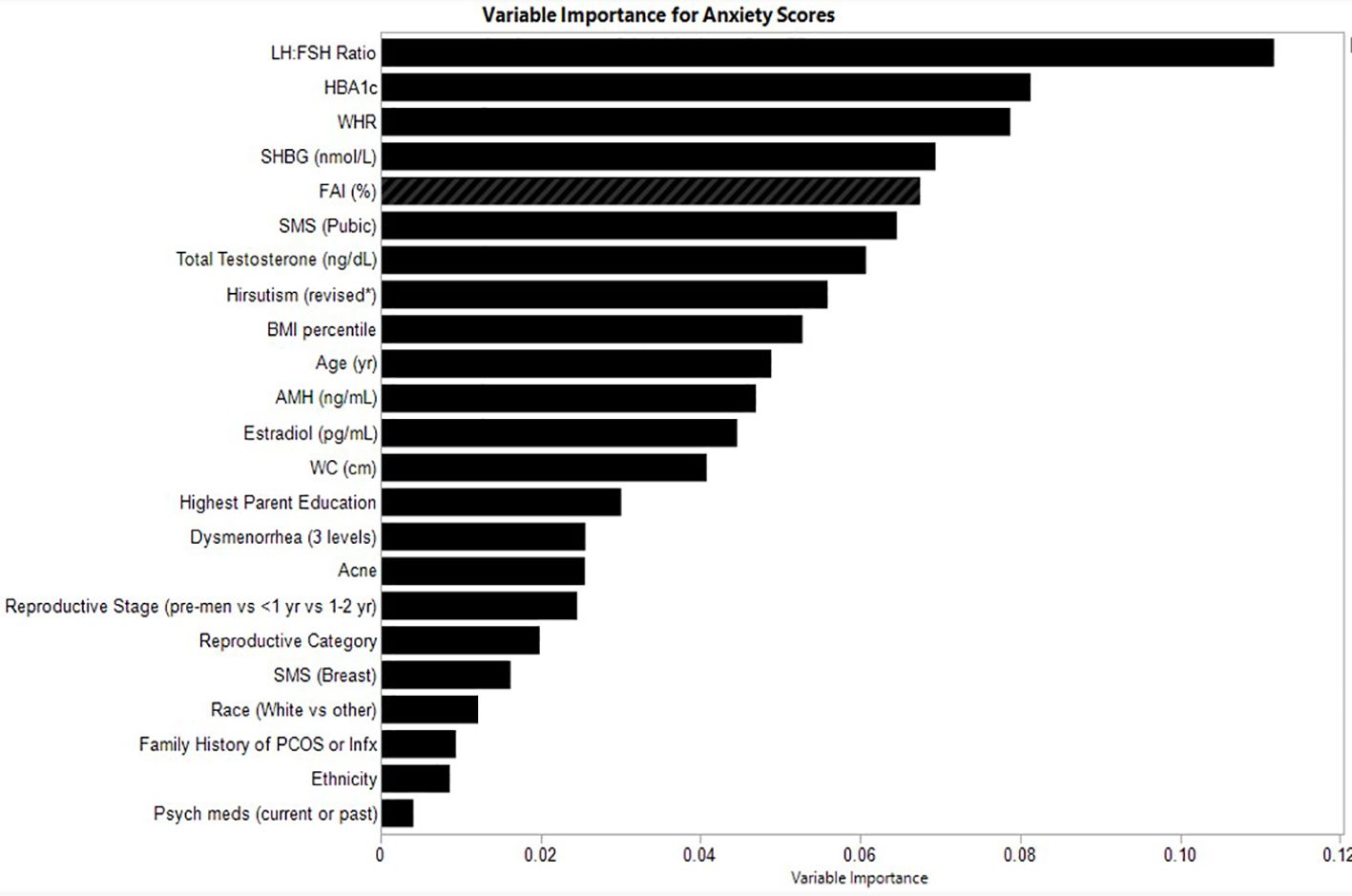
Figure 2. Relative variable importance scores for anxiety in pre- and post-menarcheal adolescents. Variables are ranked in order of importance, with overall importance (sum of squares) on the x-axis. AMH, Anti-Mullerian Hormone; BMI, Body Mass Index; FAI, Free Androgen Index (%); SHBG, Sex Hormone Binding Globulin; SMS, Sexual Maturity Scale; WC, waist circumference; WHR, Waist-Hip-Ratio. For a detailed explanation of variables please see Supplementary Table 1.
3.4 Variable importance for rumination scores
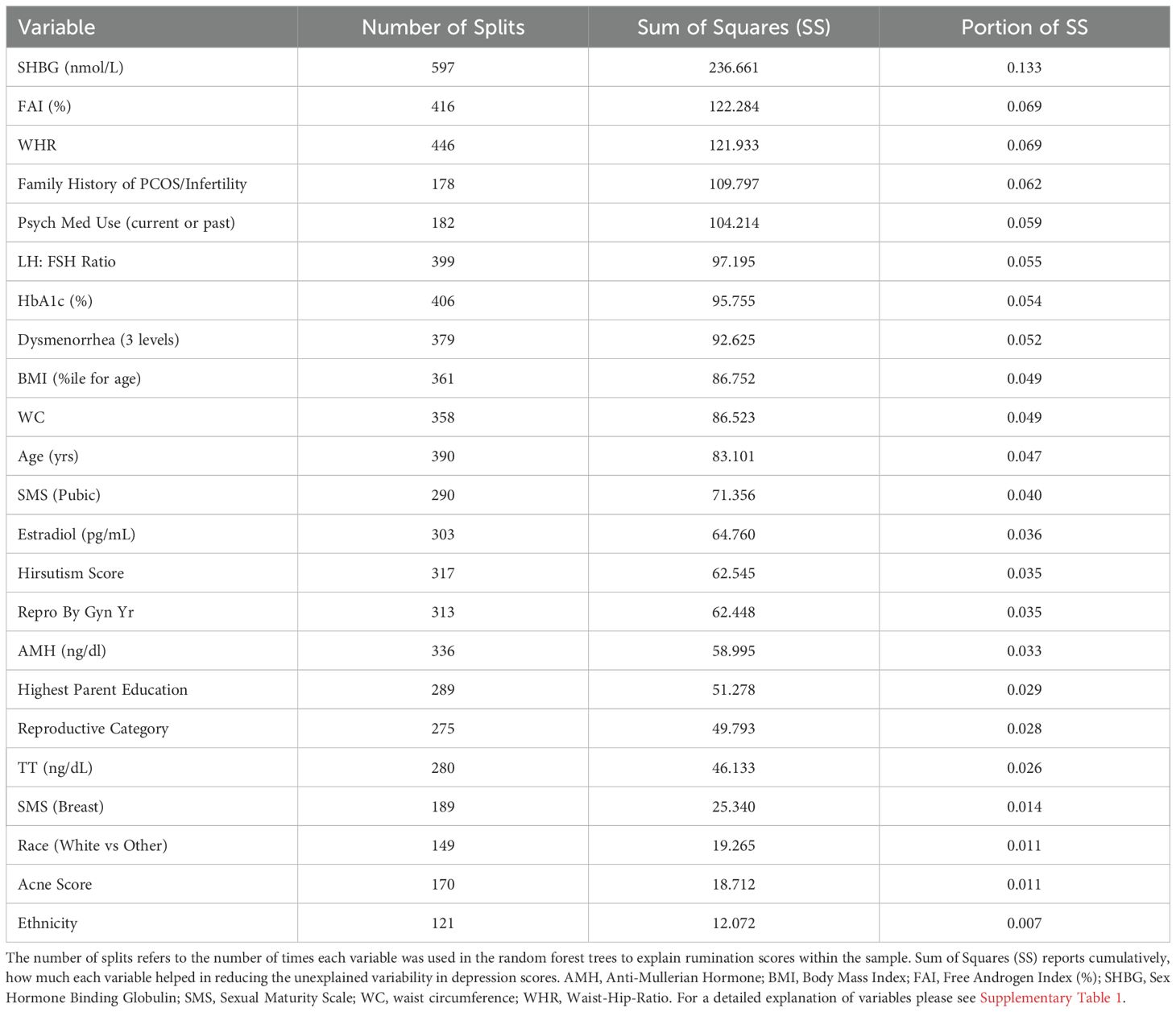
The relative variable importance scores for the observed rumination score model are presented in Figure 3. Number of splits per variable and sum of squares are presented in Table 4. The overall model fit for rumination scores based on the included variables was good at R2 = 0.597, RMSE = 5.99. In predicting rumination, SHBG was the most frequently identified variable in the random forest trees to differentiate participants reporting rumination (Portion Sum of Squares = 0.13; Table 4). The next most important variable was FAI, which uses SHBG in its calculation (Portion Sum of Squares = 0.069), and waist-hip-ratio (Portion Sum of Squares = 0.069). Heatmaps highlighting non-linearity of interactions between the top three covariates for rumination scores are depicted in Supplementary Figure 3.
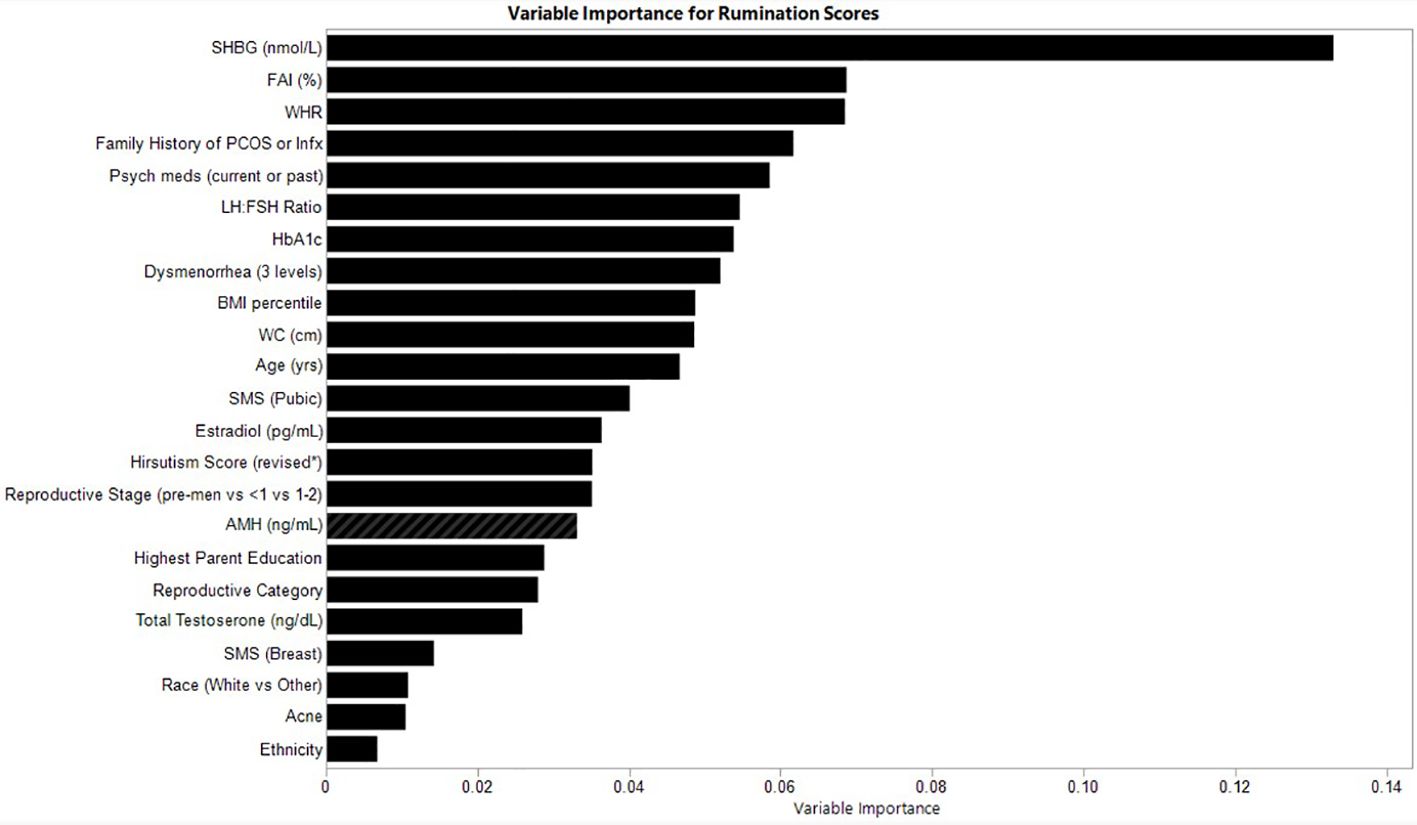
Figure 3. Relative variable importance scores for rumination in pre- and post-menarcheal adolescents. Variables are ranked in order of importance, with overall importance (sum of squares) on the x-axis. AMH, Anti-Mullerian Hormone; BMI, Body Mass Index; FAI, Free Androgen Index (%); SHBG, Sex Hormone Binding Globulin; SMS, Sexual Maturity Scale; WC, waist circumference; WHR, Waist-Hip-Ratio. For a detailed explanation of variables please see Supplementary Table 1.
4 Discussion
The International Guideline for PCOS advises routine screening for depression and anxiety, recognizing the established burden of psychological well-being in women and adolescents with PCOS (4). In the present study, we sought to determine whether known predictors or early indicators of future PCOS among pre- and early post-menarcheal adolescents were associated with the degree of depression, anxiety, and rumination symptoms. Early endocrine predictors of PCOS and metabolic dysfunction – notably insulin resistance, hyperandrogenism, and central adiposity – consistently emerged as top variables in predicted depression, anxiety, and rumination scores. These results suggest that psychopathology may emerge early in PCOS, even before a clinical diagnosis.
The mechanistic basis for psychopathology in PCOS is poorly understood. Numerous connections have been proposed, including abnormalities in the hypothalamic-pituitary-adrenal axis, the emotional toll of infertility, chronic inflammation, and chronic cardiovascular problems secondary to PCOS (34). Yet none of these explanations have been consistently supported within the literature – and most would be unlikely to explain the established elevations of psychopathology in adolescents with PCOS. Our results align with several lines of human and pre-clinical evidence supporting a shared pathophysiology between PCOS and psychopathology. For example, the relevance of insulin receptor signaling in the brain was demonstrated in a brain-specific insulin receptor knockout mouse model establishing the importance of insulin signaling for dopamine turnover, reduction of which was associated with depression and anxiety (35). Likewise, rodent and sheep models to study genetic and epigenetic transmission of PCOS demonstrate that offspring of maternal PCOS animals exhibit frequent and persistent anxiety in offspring (36, 37).
The majority of literature on depression and anxiety in PCOS has been conducted following a prolonged diagnostic experience (20) and accompanied by increased awareness of PCOS-related health concerns (such as infertility, unwanted hair growth, weight management, risk of Type 2 Diabetes) and other long-term comorbidities (38, 39). The present study represents a novel, exploratory analysis in children and adolescents, most of whom were too young to be evaluated for PCOS (4) but may already have emerging symptoms of PCOS or evidence of reproductive dysfunction consistent with a trajectory towards PCOS. The alignment of PCOS correlates with psychological outcomes in our data suggest that the psychological challenges associated with PCOS begin even earlier than previously thought and may be part of the pathogenesis of the condition.
Notably, PCOS-related variables were more closely aligned with psychological outcomes than pubertal development, including menarche and Tanner Staging. This is surprising, given the robust research literature documenting rises in psychopathology in youth at puberty. This suggests there may be an early divergence in mental health symptoms for youth at risk for PCOS, over and above the well-established and expected rise in psychological distress during this window of development (40). Ultimately, this underscores the importance of continued research into the interdependence between reproductive maturation, PCOS pathogenesis, and psychological well-being.
In addition to endocrine correlates of PCOS and insulin resistance, parent education emerged as a top variable for depression, with higher parental education associated with more severe symptoms of depression. In general, the reverse pattern has been documented in the research literature to date (41, 42). Although interesting, we suspect this may be an artifact of our recruitment timeline: we launched this study during the height of the COVID-19 global pandemic. The COVID-19 pandemic associated shut-downs and disruptions were marked by increased depression among youth generally and females from socioeconomic backgrounds similar to our Upstate NY participants specifically (43). Our assumption is that it is unlikely associations of higher parental education and depressive symptoms would persist were data collection to be repeated during a less volatile time period.
Unique to the present study was our inclusion of rumination. Rumination is a familiar risk within psychological science, associated with both the onset and exacerbation of internalizing disorders. It is considered transdiagnostic, because it is linked with multiple psychological disorders versus just one, and it is malleable in evidence-based paradigms in both youth and adults (44, 45). Our findings confirm that, like depression and anxiety, rumination is also strongly connected to endocrine indicators and known risks for PCOS. Because rumination often precedes the onset of these disorders, clinicians and health practitioners may find assessments of rumination beneficial when treating or evaluating PCOS risk.
Our study holds several strengths. First, our choice to target early adolescence is unusual, as participants have not been diagnosed with PCOS and some have not even reached menarche. These results confirm that the interdependence of psychopathology with endocrine indicators of PCOS is present far earlier than generally discussed and reaffirms the value of a lifespan approach to PCOS. Second, in an effort to take an unbiased approach to identifying variables of interest for psychological outcomes we did not use linear models or test mean differences, which are more common and relevant for case-control designs (10, 12, 34). Instead, we employed a sophisticated, machine learning approach. As a result, analyses reveal a collection of traits – potentially representing an early pre-PCOS phenotype – that may be connected to greater symptoms of depression, anxiety, and rumination. While these analyses do not provide insight into causal pathways, our analytical approach enables the development of testable hypotheses to better delineate the intersection of reproductive maturation and psychological health. Second, biochemical analytes were measured across three core laboratories. Our choice of laboratories was deliberate in that total testosterone was measured using liquid chromatography mass spectrometry by a laboratory approved by the Center for Disease Control Hormone Standardization Program and consistent with recommendations put forth by the latest International Guidelines for PCOS (4). Of note, all analytes were measured consistent with other adolescent research from our research group (46) to improve comparability of findings across studies. This study also had limitations which should guide future research. These include a small sample size and cross-sectional study design; without following the participants, we were unable to ascertain which youth went on to develop PCOS versus those who did not. In addition, this study, much like current research on psychopathology in PCOS, measured psychological symptoms and endocrine markers at a single, non-fasting and random time point and therefore cannot offer insight whether there might be cyclical variations in mood, emotion, and behavior consistent with hormonal fluctuations. Future studies should include serial assessments to measure daily changes in psychological symptoms with fluctuations in reproductive endocrinology. Finally, participant self-report of Tanner Staging, hirsutism, and acne may reflect youth perceptions and experiences but not perfectly agree with physician assessment (47–49). For these reasons, we view our study as a preliminary one, requiring replication in larger, prospective samples.
Whether or not psychopathology and PCOS are entangled early in the disease trajectory matters. Earlier onset of psychopathology tends to predict recurrent and more severe mental health challenges and a greater likelihood of developing major depressive episodes in the face of comparatively small amounts of life stress (50, 51). As the chronicity of depression, and anxiety are linked with treatment adherence and eligibility for interventions for PCOS, adolescent onset of psychopathology holds repercussions both for the severity of PCOS symptoms and comorbid disease management (52–54). Psychological disorders tend to be more malleable in adolescence than adulthood (55), presenting a window of opportunity for effective intervention historically overlooked in PCOS practice. Our results suggest that endocrine indicators of PCOS and correlates of dysfunction are connected to depression and anxiety early in adolescence, raising important questions about disease pathogenesis and new directions for research and clinical management.
Data availability statement
The de-identified data supporting the conclusions of this article will be made available by the authors, upon reasonable request and per institutional guidelines.
Ethics statement
The studies involving humans were approved by by the ethics review boards at Cornell University and Weill Cornell Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written permission and assent for participation in this study were obtained from the participant and the participants' parent and/or legal guardian.
Author contributions
HVB: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. KM: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. ML: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JC: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. LI: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing. EM: Formal analysis, Visualization, Writing – review & editing. AA: Data curation, Project administration, Writing – review & editing. HL: Data curation, Writing – review & editing. JK: Data curation, Writing – review & editing. JM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Office of Academic Integration Cornell-Weill Collaborative Seed Grant (PIs: Mendle, Vanden Brink, Lujan, Ipp, Chang), Texas A&M AgriLife (PI: Vanden Brink), Cornell University (PI: Mendle). Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002384. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Cornell University, or Texas A&M AgriLife.
Acknowledgments
We are grateful to the participants and their parents and/or guardians who participated in this research, without whom this research would not be possible. We are also grateful to Bailey Drewes, and Rene Black-Hellwitz from the Lujan Women's Imaging Lab who supported data collection in Ithaca, and to the nurses and scientific staff who supported data collection locally at Weill Cornell.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1551958/full#supplementary-material.
References
1. Neven ACH, Forslund M, Ranashinha S, Mousa A, Tay CT, Peña A, et al. Prevalence and accurate diagnosis of polycystic ovary syndrome (PCOS) in adolescents across world regions: a systematic review and meta-analysis. Eur J Endocrinol. (2024) 191(4):S15-27. doi: 10.1093/ejendo/lvae125
2. Carmina E and Azziz R. Diagnosis, phenotype, and prevalence of polycystic ovary syndrome. Fertil Steril. (2006) 86 Suppl 1:S7–8. doi: 10.1016/j.fertnstert.2006.03.012
3. Wolf WM, Wattick RA, Kinkade ON, and Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health. (2018) 15:2589. doi: 10.3390/ijerph15112589
4. Teede HJ, Tay CT, Laven JJE, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur J Endocrinol. (2023) 189:G43–64. doi: 10.1093/ejendo/lvad096
5. Kujanpää L, Arffman RK, Vaaramo E, Rossi HR, Laitinen J, Morin-Papunen L, et al. Women with polycystic ovary syndrome have poorer work ability and higher disability retirement rate at midlife: a Northern Finland Birth Cohort 1966 study. Eur J Endocrinol. (2022) 187:479–88. doi: 10.1530/EJE-22-0027
6. Hudnut-Beumler J, Kaar JL, Taylor A, Kelsey MM, Nadeau KJ, Zeitler P, et al. Development of type 2 diabetes in adolescent girls with polycystic ovary syndrome and obesity. Pediatr Diabetes. (2021) 22:699–706. doi: 10.1111/pedi.13206
7. Wekker V, van Dammen L, Koning A, Heida KY, Painter RC, Limpens J, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. (2020) 26:942–60. doi: 10.1093/humupd/dmaa029
8. Wang Z, Jukic AMZ, Baird DD, Wilcox AJ, Li H, Curry CL, et al. Irregular cycles, ovulatory disorders, and cardiometabolic conditions in a US-based digital cohort. JAMA Netw Open. (2024) 7:e249657. doi: 10.1001/jamanetworkopen.2024.9657
9. Alur-Gupta S, Lee I, Chemerinski A, Liu C, Lipson J, Allison K, et al. Racial differences in anxiety, depression, and quality of life in women with polycystic ovary syndrome. F S Rep. (2021) 2:230–7. doi: 10.1016/j.xfre.2021.03.003
10. Barry JA, Kuczmierczyk AR, and Hardiman PJ. Anxiety and depression in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod. (2011) 26:2442–51. doi: 10.1093/humrep/der197
11. Dokras A, Stener-Victorin E, Yildiz BO, Li R, Ottey S, Shah D, et al. Androgen Excess- Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. (2018) 109:888–99. doi: 10.1016/j.fertnstert.2018.01.038
12. Li Y, Zhang J, Zheng X, Lu W, Guo J, Chen F, et al. Depression, anxiety and self-esteem in adolescent girls with polycystic ovary syndrome: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2024) 15. doi: 10.3389/fendo.2024.1399580
13. Anderson RJ, Freedland KE, Clouse RE, and Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. (2001) 24:1069–78. doi: 10.2337/diacare.24.6.1069
14. Parker M, Warren A, Nair S, and Barnard M. Adherence to treatment for polycystic ovarian syndrome: A systematic review. PloS One. (2020) 15:e0228586. doi: 10.1371/journal.pone.0228586
15. Alur-Gupta S, Dokras A, and Cooney LG. Management of polycystic ovary syndrome must include assessment and treatment of mental health symptoms. Fertil Steril. (2024) 121:384–99. doi: 10.1016/j.fertnstert.2024.01.018
16. Lee IT, Rees J, King S, Kim A, Cherlin T, Hinkle S, et al. Depression, anxiety, and risk of metabolic syndrome in women with polycystic ovary syndrome: A longitudinal study. J Clin Endocrinol Metab. (2025) 110(3):e750-6. doi: 10.1210/clinem/dgae256
17. Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity. JAMA. (2021) 325:1403. doi: 10.1001/jama.2021.1831
18. Armstrong SC, Bolling CF, Michalsky MP, Reichard KW, Haemer MA, Muth ND, et al. Pediatric metabolic and bariatric surgery: evidence, barriers, and best practices. Pediatrics. (2019) 144(6):e20193223. doi: 10.1542/peds.2019-3223
19. Oldfield AL, Carter FE, Reeves RE, Jarrett BY, Vanden Brink H, and Lujan ME. Impact of a hypocaloric dietary intervention on antral follicle dynamics in eumenorrheic women with obesity. Hum Reprod. (2024) 39:801–11. doi: 10.1093/humrep/deae017
20. Gibson-Helm M, Teede H, Dunaif A, and Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2017) 102:604–12. doi: 10.1210/jc.2016-2963
21. Nolen-Hoeksema S, Wisco BE, and Lyubomirsky S. Rethinking rumination. Perspect psychol Science. (2008) 3:400–24. doi: 10.1111/j.1745-6924.2008.00088.x
22. Abela JRZ, Brozina K, and Haigh EP. An examination of the response styles theory of depression in third- and seventh-grade children: a short-term longitudinal study. J Abnorm Child Psychol. (2002) 30:515–27. doi: 10.1023/A:1019873015594
23. Mendle J, Beam CR, McKone KMP, and Koch MK. Puberty and transdiagnostic risks for mental health. J Res Adolesc. (2020) 30:687–705. doi: 10.1111/jora.12552
24. Alloy LB, Hamilton JL, Hamlat EJ, and Abramson LY. Pubertal development, emotion regulatory styles, and the emergence of sex differences in internalizing disorders and symptoms in adolescence. Clin psychol Science. (2016) 4:867–81. doi: 10.1177/2167702616643008
25. Adityan B, Kumari R, and Thappa D. Scoring systems in acne vulgaris. Indian J Dermatol Venereol Leprol. (2009) 75:323–6. doi: 10.4103/0378-6323.51258
26. Yildiz BO, Bolour S, Woods K, Moore A, and Azziz R. Visually scoring hirsutism. Hum Reprod Update. (2010) 16:51–64. doi: 10.1093/humupd/dmp024
27. Faulstich M, Carey M, Ruggiero L, Enyart P, and Gresham F. Assessment of depression in childhood and adolescence: an evaluation of the Center for Epidemiological Studies Depression Scale for Children (CES-DC). Am J Psychiatry. (1986) 143:1024–7. doi: 10.1176/ajp.143.8.1024
28. March J. Multidimensional anxiety scale for children 2nd edition–self-report. 2nd ed. North Tonawanda, NY: Multi-Health Systems (2012).
29. Abela JRZ, Aydin CM, and Auerbach RP. Responses to depression in children: reconceptualizing the relation among response styles. J Abnorm Child Psychol. (2007) 35:913–27. doi: 10.1007/s10802-007-9143-2
30. Nolen-Hoeksema S and Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. J Pers Soc Psychol. (1991) 61:115–21. doi: 10.1037/0022-3514.61.1.115
31. Vermeulen A, Verdonck L, and Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. (1999) 84:3666–72. doi: 10.1210/jcem.84.10.6079
32. Boulesteix A, Janitza S, Kruppa J, and König IR. Overview of random forest methodology and practical guidance with emphasis on computational biology and bioinformatics. WIREs Data Min Knowledge Discovery. (2012) 2:493–507. doi: 10.1002/widm.1072
33. Arslanian S, Bacha F, Grey M, Marcus MD, White NH, and Zeitler P. Evaluation and management of youth-onset type 2 diabetes: A position statement by the American diabetes association. Diabetes Care. (2018) 41:2648–68. doi: 10.2337/dci18-0052
34. Cooney LG, Lee I, Sammel MD, and Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome : a systematic review and meta-analysis. Hum Reprod. (2017) 32:1075–91. doi: 10.1093/humrep/dex044
35. Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, Vienberg SG, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci U S A. (2015) 112:3463–8. doi: 10.1073/pnas.1500877112
36. Risal S, Manti M, Lu H, Fornes R, Larsson H, Benrick A, et al. Prenatal androgen exposure causes a sexually dimorphic transgenerational increase in offspring susceptibility to anxiety disorders. Transl Psychiatry. (2021) 11:45. doi: 10.1038/s41398-020-01183-9
37. Chasles M, Fleurot R, Giacobini P, and Tillet Y. Prenatal androgen exposure induces anxiety-like behavior in ewes. Neuroendocrinology. (2024) 114:721–32. doi: 10.1159/000539111
38. Cinar N, Kizilarslanoglu MC, Harmanci A, Aksoy DY, Bozdag G, Demir B, et al. Depression, anxiety and cardiometabolic risk in polycystic ovary syndrome. Hum Reproduction. (2011) 26:3339–45. doi: 10.1093/humrep/der338
39. Tan S, Hahn S, Benson S, Janssen OE, Dietz T, Kimmig R, et al. Psychological implications of infertility in women with polycystic ovary syndrome. Hum Reproduction. (2008) 23:2064–71. doi: 10.1093/humrep/den227
40. Mendle J. Why puberty matters for psychopathology. Child Dev Perspect. (2014) 8:218–22. doi: 10.1111/cdep.2014.8.issue-4
41. Devenish B, Hooley M, and Mellor D. The pathways between socioeconomic status and adolescent outcomes: A systematic review. Am J Community Psychol. (2017) 59:219–38. doi: 10.1002/ajcp.2017.59.issue-1pt2
42. Mendle J, Moore SR, Briley DA, and Harden KP. Puberty, socioeconomic status, and depression in girls. Clin Psychol Sci. (2016) 4:3–16. doi: 10.1177/2167702614563598
43. Madigan S, Racine N, Vaillancourt T, Korczak DJ, Hewitt JMA, Pador P, et al. Changes in depression and anxiety among children and adolescents from before to during the COVID-19 pandemic. JAMA Pediatr. (2023) 177:567. doi: 10.1001/jamapediatrics.2023.0846
44. Barlow DH and Long LJ. The Unified Protocol: Current status, future directions. Clin Psychology: Sci Practice. (2023) 30:222–5. doi: 10.1037/cps0000152
45. Ehrenreich-May J, Queen AH, Bilek EL, Remmes CS, and Marciel KK. The unified protocols for the treatment of emotional disorders in children and adolescents. In: Ehrenreich J and Chu BC (Eds.). Transdiagnostic treatments for children and adolescents: Principles and practice. New York, NY: The Guilford Press (2014). p. 267–92.
46. Vanden Brink H, Burgert TS, Barral R, Malik A, Gadiraju M, and Lujan ME. Ovarian morphology in girls longitudinal cohort study: pilot evaluation of ovarian morphology as a biomarker of reproductive and metabolic features during the first gynecological year. J Pediatr Adolesc Gynecol. (2024) 37:315–22. doi: 10.1016/j.jpag.2024.02.004
47. Campisi SC, Marchand JD, Siddiqui FJ, Islam M, Bhutta ZA, and Palmert MR. Can we rely on adolescents to self-assess puberty stage? A systematic review and meta-analysis. J Clin Endocrinol Metab. (2020) 105:2846–56. doi: 10.1210/clinem/dgaa135
48. Pasch L, He SY, Huddleston H, Cedars MI, Beshay A, Zane LT, et al. Clinician vs self-ratings of hirsutism in patients with polycystic ovarian syndrome. JAMA Dermatol. (2016) 152:783. doi: 10.1001/jamadermatol.2016.0358
49. Morss-Walton P, McGee JS, Santillan MR, Cukras A, and Porter ML. 27469 Accuracy of self-reported acne severity in a small study of 16 adolescents. J Am Acad Dermatol. (2021) 85:AB146. doi: 10.1016/j.jaad.2021.06.599
50. Monroe SM, Anderson SF, and Harkness KL. Life stress and major depression: The mysteries of recurrences. Psychol Rev. (2019) 126:791–816. doi: 10.1037/rev0000157
51. Kovacs M, Obrosky S, and George C. The course of major depressive disorder from childhood to young adulthood: Recovery and recurrence in a longitudinal observational study. J Affect Disord. (2016) 203:374–81. doi: 10.1016/j.jad.2016.05.042
52. Greenwood EA, Pasch LA, Shinkai K, Cedars MI, and Huddleston HG. Clinical course of depression symptoms and predictors of enduring depression risk in women with polycystic ovary syndrome: Results of a longitudinal study. Fertil Steril. (2019) 111:147–56. doi: 10.1016/j.fertnstert.2018.10.004
53. Livadas S, Chaskou S, Kandaraki AA, Skourletos G, Economou F, Christou M, et al. Anxiety is associated with hormonal and metabolic profile in women with polycystic ovarian syndrome. Clin Endocrinol (Oxf). (2011) 75:698–703. doi: 10.1111/j.1365-2265.2011.04122.x
54. Lalonde-Bester S, Malik M, Masoumi R, Ng K, Sidhu S, Ghosh M, et al. Prevalence and etiology of eating disorders in polycystic ovary syndrome: A scoping review. Adv Nutrition. (2024) 15:100193. doi: 10.1016/j.advnut.2024.100193
Keywords: PCOS, puberty, depression, anxiety, mental health, menarche
Citation: Vanden Brink H, McCormick KC, Lujan ME, Chang J, Ipp L, Mudrak EL, Alladeen A, Lamar H, Kim JY and Mendle J (2025) Psychological symptoms in perimenarcheal adolescents: association with PCOS risk factors. Front. Endocrinol. 16:1551958. doi: 10.3389/fendo.2025.1551958
Received: 26 December 2024; Accepted: 12 May 2025;
Published: 12 June 2025.
Edited by:
Bassel H. Al Wattar, Anglia Ruskin University, United KingdomReviewed by:
Diana Speelman, LECOM Health, United StatesSubia Jamil, Jinnah University for Women, Pakistan
Copyright © 2025 Vanden Brink, McCormick, Lujan, Chang, Ipp, Mudrak, Alladeen, Lamar, Kim and Mendle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heidi Vanden Brink, aGVpZGkudmFuZGVuYnJpbmtAYWcudGFtdS5lZHU=; Jane Mendle, amVtNDgyQGNvcm5lbGwuZWR1
 Heidi Vanden Brink
Heidi Vanden Brink Kathleen C. McCormick
Kathleen C. McCormick Marla E. Lujan4
Marla E. Lujan4 Erika L. Mudrak
Erika L. Mudrak Joy Y. Kim
Joy Y. Kim Jane Mendle
Jane Mendle