- 1Department of Obstetrics, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Zhengzhou, China
- 2Institute of Trauma and Metabolism, Zhengzhou Central Hospital Affiliated to Zhengzhou University, Zhengzhou, China
- 3Henan Province Hypertension Precision Prevention and Control Engineering Research Center, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou, Henan, China
Birth weight serves as a critical indicator of neonatal survival. Preeclampsia represents a serious complication during pregnancy and is closely associated with gestational hypothyroidism (GHT), both of which severely affect neonatal birth weight. Preeclampsia and hypothyroidism during pregnancy are usually accompanied by abnormalities of maternal liver function, which frequently leads to adverse pregnancy outcomes including low birth weight (LBW). This retrospective study utilized data from 420 cases of patients with preeclampsia who underwent prenatal examinations and delivery at department of Obstetrics. The association between preeclampsia combined with GHT in pregnancy, maternal liver function and neonatal birth weight was estimated using generalized linear model (GLM), and the potential partial mediating effects of maternal liver function were assessed through mediating models. Among pregnant women with preeclampsia, 11.0% had GHT, and the median (interquartile range) birth weight of all neonates was 2990.0 (2541.3, 3368.8) grams. Neonates born to pregnant women who had preeclampsia combine with GHT showed a higher incidence of LBW (χ²=22.13, P< 0.001), exhibited a significantly lower birth weight compared to those born to women with preeclampsia alone (β=-258.53;95%CI:-398.56, -118.50). Additionally, maternal alanine aminotransferase (ALT) levels were found to partially mediate this association (indirect effect:-50.85, 95%CI:-101.07, -15.07). The findings of this study indicate that compared with pregnant women with preeclampsia alone, neonates born to pregnant women suffering from preeclampsia combined with GHT have significantly lower birth weights, with maternal ALT levels acting as a potential partial mediator in this association. These results provide an important reference for clinicians to monitor thyroid and liver function in patients with preeclampsia.
Introduction
Neonatal birth weight is determined by maternal health, the placenta, and the fetus’ own growth potential, and serving as a critical indicator of neonatal survival (1, 2). Low birth weight (LBW), defined as a birth weight of less than 2500 grams(g), is considered an important factor in neonatal mortality (3, 4). Studies have demonstrated that LBW increases the risk of future cardiovascular morbidity and is associated with an elevated risk of future hypertension in pregnancy (5, 6).
Preeclampsia is a serious complication of pregnancy with hypertension and proteinuria as the main clinical manifestations, and is one of the leading causes of maternal and neonatal mortality (7, 8). Preeclampsia can cause a series of serious obstetric complications, including preterm labor and placental abruption, as well as fetal complications such as fetal respiratory distress, intrauterine growth restriction, oligohydramnios, and stillbirth (9). There is increasing evidence suggests that preeclampsia is closely associated with maternal hypertension, cardiovascular disease, and dementia (10–12). Mechanistically, placental dysfunction induced by preeclampsia profoundly impacts on fetal development, with studies confirming it as an important predictor of neonatal birth weigh (13–15). The thyroid gland is involved in endocrine regulation and plays a crucial role in maternal and fetal development during pregnancy (16). Studies have confirmed the correlation between thyroid dysfunction and preeclampsia, and the prevalence of hypothyroidism in pregnant women with preeclampsia is significantly increased (17–19). Currently, hypothyroidism has become a common complication of preeclampsia, leading to adverse pregnancy outcomes, including LBW, and severely affecting neonatal birth weight and even future growth and development (20).
Preeclampsia and hypothyroidism during pregnancy are closely associated with alterations in liver function. Preeclampsia causes impaired liver function, which has been identified as the third most important predictor after hypertension and proteinuria (21, 22). Simultaneously, hypothyroidism, which is characterized by a feedback increase in thyroid-stimulating hormone (TSH) as a biochemical marker, interacts with hepatic function metabolically (23, 24). Experimental evidence has been presented that hepatic dysfunction in pregnant mice predisposes to placental dysfunction, which results in lower birth weights in newborn mice (25). Population-based studies have also confirmed that pregnant women with abnormal liver function are associated with adverse birth outcomes, such as preterm labor, LBW, intrauterine stillbirth, and fetal respiratory distress (26). Several scholars have investigated the mechanisms underlying the association of preeclampsia and hypothyroidism in pregnancy with LBW, including placental dysfunction in preeclampsia, maternal nutritional deficiencies associated with pregnancy, and maternal thyroid hormone levels (27–29). In conclusion, the mechanism by which preeclampsia combined with hypothyroidism affects neonatal birth weight is multifactorial. But there are currently limited studies exploring the mediating role of liver function as an important factor in the relationship between preeclampsia combined with hypothyroidism and birth weight. As shown in some studies, maternal liver function status is associated with fetal growth and development during pregnancy. Conducting research on the association between liver function indicators and birth weight can provide a basis for early risk monitoring strategies.
Therefore, the objective of this study was to explore the association between preeclampsia in conjunction with gestational hypothyroidism, maternal liver function, and neonatal birth weight. Additionally, the study aimed to explore whether maternal liver function serves as a potential mediating factor in the association between preeclampsia combined with gestational hypothyroidism and neonatal birth weight. This investigation seeks to address existing gaps in the literature regarding the underlying mechanisms and to offer insights for future related studies.
Materials and methods
Study design and population
This study was a retrospective study, and 554 cases who underwent prenatal examinations and deliveries at department of Obstetrics, Zhengzhou Central Hospital Affiliated to Zhengzhou from January 2021 to September 2023 were selected as the study subjects. According to the following inclusion criteria, 482 cases diagnosed with preeclampsia were initially included, and then 62 cases were excluded according to the exclusion criteria, 420 cases of preeclampsia patients were ultimately included in this study, and the median gestational age at diagnosis of preeclampsia (interquartile range) was 35.4(32.5, 37.3) weeks. Among these, 46 cases were complicated by hypothyroidism, and the median gestational age at diagnosis of hypothyroidism (interquartile range) was 32.2(27.0, 36.0) weeks.
The inclusion criteria were as follows: (1) fulfillment of the diagnostic criteria for preeclampsia; (2) maternal age ≥ 18 years; (3) gestational age ≥ 24 weeks; (4) singleton pregnancy; (5) absence of a history of substance abuse, smoking, or alcohol consumption among the pregnant women.
The exclusion criteria were as follows: (1) pregnant individuals with other complications, such as gestational diabetes or hypertension, as well as those with underlying medical conditions prior to pregnancy (e.g., thyroid disorders, chronic hypertension, heart disease, liver or biliary diseases, or renal diseases); (2) patients lacking relevant information, such as those with incomplete or missing neonatal birth weight and liver function indicators; (3) individuals who conceived through assisted reproductive technology.
The diagnostic criteria for preeclampsia were based on the “Diagnosis and treatment of hypertension and preeclampsia in pregnancy: a clinical practice guideline in China (2020)” issued by the Obstetrics and Gynecology Branch of the Chinese Medical Association. Specifically, preeclampsia is diagnosed when, after 20 weeks of gestation, a pregnant woman exhibits a systolic blood pressure of ≥140 mmHg and/or a diastolic blood pressure of ≥90 mmHg, accompanied by at least one of the following: (1) a 24-hour urinary protein quantification of ≥0.3 g, or a urinary protein-to-creatinine ratio of ≥0.3, or a random urinary protein result of ≥(+); (2) the absence of proteinuria but the presence of any one of the following organ or system involvements, including abnormalities affecting vital organs such as the heart, lungs, liver, and kidneys, or alterations in the hematological, gastrointestinal, or neurological systems, as well as involvement of the placenta and fetus (30).
The diagnostic criteria for hypothyroidism during pregnancy refer to the revised “Guideline on diagnosis and management of thyroid diseases during pregnancy and postpartum (2nd edition)” by the Chinese Medical Association, which stipulates that serum thyroid-stimulating hormone (TSH) levels exceed the upper limit of the pregnancy-specific reference range, while serum free thyroxine (FT4) levels fall below the lower limit of the specific reference range. Combined with the types of kits and fully automated chemiluminescent immunoassay analyzers used in this study, the guideline recommended reference ranges for TSH and FT4 were as follows: in early pregnancy, TSH 0.05~3.55 mIU/L, FT49.01~15.89 pmol/L; in mid-pregnancy, TSH 0.21~3.31 mIU/L, FT4 6.62~13.51 pmol/L; in late pregnancy, TSH 0.43~3.71 mIU/L, FT4 6.42~10.75 pmol/L (31).
Ethical compliance statement for human participant research
All study participants provided written informed consent, and this study received approved from the Ethics Committee of Zhengzhou Central Hospital Affiliated to Zhengzhou (No. ZXYY202470). All methods were performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki.
Variables and definitions
Measurement of serum liver function indicators
In this study, we collected the levels of liver function indicators from subjects during their hospitalization. Based on previous research, this study selected indicators closely related to liver function, primarily including alanine aminotransferase (ALT 7-40U/L), aspartate aminotransferase (AST 13-35U/L), alkaline phosphatase (ALP 50-135U/L), total bilirubin (TBIL 0-21μmol/L), total protein (TP 60-80g/L), and albumin (Alb 35-55g/L). A volume of 5 mL of fasting antecubital venous blood was collected, and serum was separated by centrifugation at 4000 rpm for 5 minutes. All biochemical analyses were performed using an AU5800 fully automated biochemistry analyzer (Beckman Coulter, USA) with matched reagent kits. All reagents and instruments were subjected to quality control procedures.
Measurement of neonatal birth weight
Neonatal birth weight, measured in grams, was measured and recorded within one hour after birth. The data pertaining to birth weight for this investigation was sourced from medical records.
Measurement of covariates
Participants in this study were requested to complete a baseline questionnaire upon admission, which encompassed demographic characteristics of the pregnant women (age, ethnicity, residence, and educational level), history of cesarean delivery (yes/no), history of adverse pregnancy outcomes (yes/no), primiparity (yes/no), and family history of hypertension (yes/no). Participants self-reported their pre-pregnancy weight (kg) and their height was measured in a barefoot standing position using a medical height and weight measuring device (cm). Subsequently, pre-pregnancy body mass index (PBMI) was calculated using the standard formula BMI = weight (kg)/height (m²). According to World Health Organization (WHO) standards, participants were classified into categories of underweight (BMI< 18.5 kg/m²), normal weight (18.5 ≤ BMI ≤ 24.9 kg/m²), overweight (25 ≤ BMI ≤ 29.9 kg/m²), and obese (BMI ≥ 30 kg/m²). During hospitalization, ultrasound was utilized to assess whether the fetus was experiencing growth restriction, and postpartum data on preterm birth (yes/no) and neonatal sex (male/female) were collected from medical records. The ultrasound diagnostic criteria for fetal growth restriction (FGR) were defined as an ultrasound-estimated fetal weight or abdominal circumference below the 10th percentile for the corresponding gestational age; preterm birth was defined as delivery occurring before 37 weeks of gestation.
Statistical analysis
Data analysis was conducted using SPSS 26.0 statistical software. Initially, descriptive analysis was performed, with categorical data expressed as N (%) and continuous data described using either or M (IQR). For univariate analysis, given that birth weight exhibited a non-normal distribution, the Mann-Whitney U test or Kruskal-Wallis H test were employed to examine differences in birth weight among various characteristic groups. Spearman correlation analysis was utilized to assess the relationship between liver function indicators (ALT/AST/ALP/TP/Alb/TBIL) and birth weight. Subsequently, a generalized linear model (GLM) was applied for multivariate analysis to evaluate the potential association between the presence of GHT, the levels of liver function indicators, and birth weight. Finally, the presence of GHT was treated as the independent variable, liver function indicators as mediating variables, and birth weight as the dependent variable. The mediation effect of liver function indicators was calculated using the SPSS Process macro, employing the bias-corrected Bootstrap method (with 5000 resamples) for validation. A p-value of<0.05 was considered statistically significant.
Results
Descriptive statistics
This study included a total of 420 pregnant women diagnosed with preeclampsia, among whom 46 (11.0%) also had concomitant hypothyroidism. The median age (interquartile range) was 31 (28, 34) years, with the majority of the participants (72.1%) aged between 25 and 35 years. The median neonatal birth weight (interquartile range) was 2990.0 (2541.3, 3368.8) grams. Preterm birth occurred in 111 participants (26.4%), while 24 (5.7%) were diagnosed with fetal growth restriction. Additionally, 15 participants were classified as underweight prior to pregnancy, 123 as overweight, and 52 as obese. Univariate analysis revealed that preterm birth (P<0.001), occurrence of fetal growth restriction during pregnancy (P<0.001), a pre-pregnancy BMI below 18.5 kg/m² (P=0.003), and the presence of GHT (P<0.001) were associated with lower birth weights of the neonates born to women with preeclampsia (Table 1).
Compared with pregnant women with preeclampsia alone, those with preeclampsia combined with GHT had higher rates of preterm delivery (39.1% vs. 24.9%), fetal growth restriction (17.4% vs. 4.3%) and LBW (50.0% vs. 19.3%), were more likely to be primiparous (73.9% vs. 58.6%), and obese (13.0% vs. 12.3%). From the perspective of liver function indicators, the levels of ALT (P<0.001) and AST (P=0.002) showed statistically significant differences between the two subgroups (Table 2).
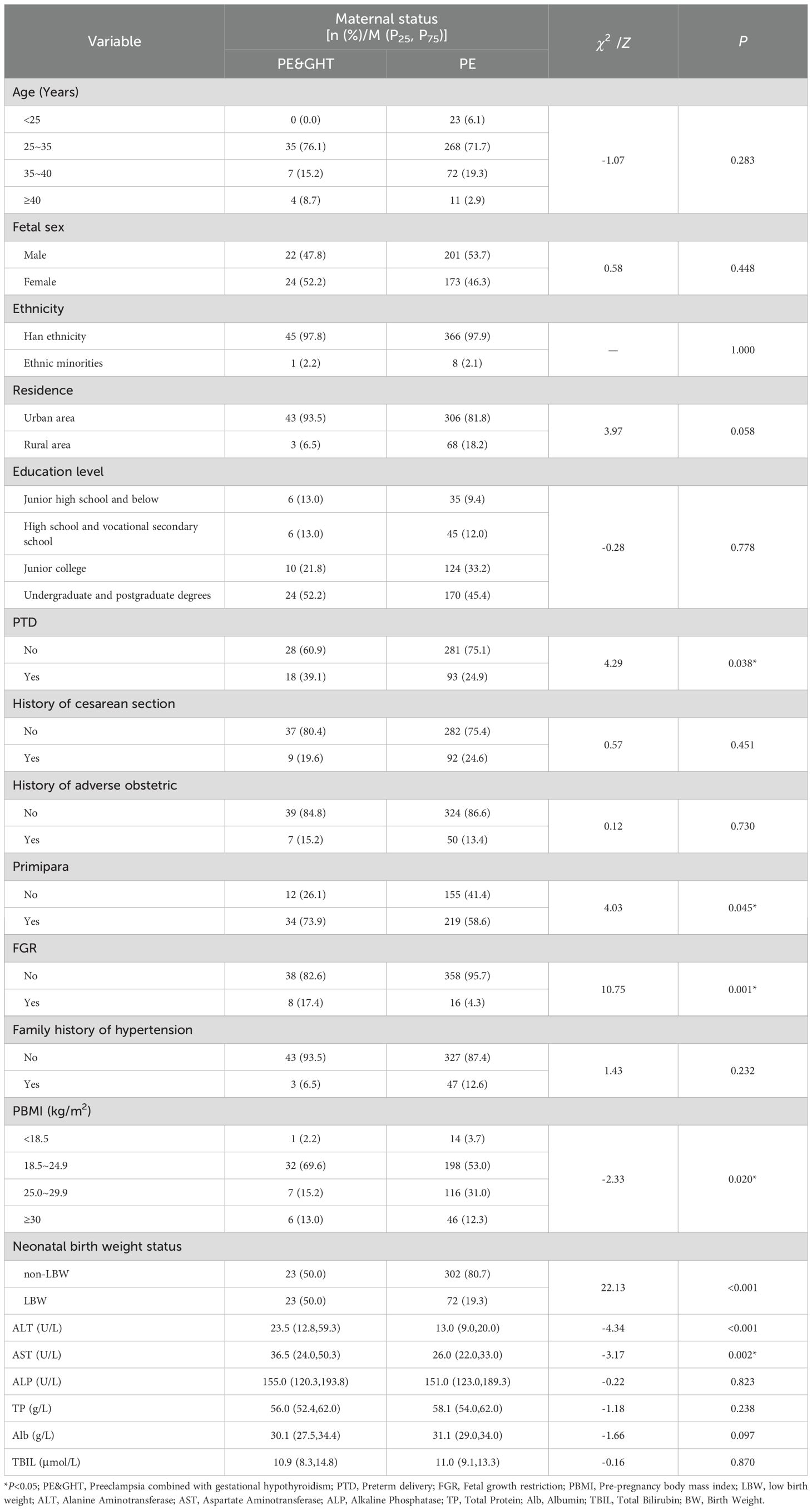
Table 2. Comparison of baseline data between the preeclampsia group and the preeclampsia with hypothyroidism group.
Correlation between maternal liver function indicators and neonatal birth weight
Except for total bilirubin, the levels of other maternal liver function indicators showed significant correlations with neonatal birth weight. Specifically, ALT (r=-0.320) and AST (r=-0.234) levels exhibited negative correlations with neonatal birth weight, while ALP (r=0.193), TP (r=0.165), and ALB (r=0.177) displayed positive correlations with neonatal birth weight (Table 3).

Table 3. Analysis of the correlation of maternal liver function indicators and neonatal birth weight.
Relationship between thyroid function, liver function indicators in pregnant women with preeclampsia, and neonatal birth weight
This study explored the relationship between the presence of GHT in preeclamptic pregnant women and their liver function indicators with neonatal birth weight through the construction of a GLM and the adjustment of control variables. The findings revealed that, without adjusting for other variables (Model 1), the neonatal birth weight of infants born to preeclamptic mothers with GHT was significantly lower compared to those born to mothers with uncomplicated preeclampsia [β = -351.36; 95% confidence interval (CI): -532.57, -170.15]. Furthermore, as levels of ALT (β = -2.18; 95% CI: -3.90, -0.47) and AST (β = -4.04; 95% CI: -7.06, 1.02) increased, neonatal birth weight correspondingly decreased, while an elevation in ALP levels (β=1.43; 95% CI: 0.53, 2.32) was associated with an increase in neonatal birth weight. Following adjustments for preterm birth, FGR, and PBMI (Model 2), the presence of GHT (β = -258.53; 95% CI: -398.56, -118.50), ALT (β = -1.88; 95% CI: -3.19, -0.56), and ALP (β=1.02; 95% CI: 0.33, 1.70) levels remained significantly associated with neonatal birth weight, whereas the association between AST levels (β = -1.34; 95% CI: -3.66, 0.98) and neonatal birth weight became statistically insignificant (Table 4).
We also did some extra analysis after sorting out liver function indicators (normal or abnormal) and birth weight (low birth weight or non-low birth weight). Check out the Supplementary Table S1 and Supplementary Table S2 for more details.
Mediating effects of liver function indicators
Since there was no statistically significant difference in ALP levels between the preeclampsia group and the preeclampsia combined with GHT group, this study only included ALT, which was statistically significant in the multifactorial analysis, into a mediation model to investigate whether the factor partially mediate the relationship between preeclampsia in pregnant women with GHT and neonatal birth weight. The path coefficients are detailed in Table 5. The results indicate that pregnant women with preeclampsia combined with GHT exhibited higher ALT levels compared to those with preeclampsia alone (β’=23.19, P=0.002), which was associated with a negative impact on neonatal birth weight (β’=-271.18, P<0.001). Furthermore, ALT levels had a negative effect on neonatal birth weight (β’=-2.40, P<0.001. In light of these path results, the study explored the mediating effect of ALT, as detailed in Table 6. The findings indicate that preeclampsia in conjunction with GHT may indirectly diminish neonatal birth weight by elevating maternal ALT levels. The mediating effect of ALT is quantified at -50.85 with a 95% Bootstrap confidence interval. The interval (95% CI) of [101.07, -15.07] does not encompass zero, indicating a substantial mediating effect that accounts for 15.5% of the total effect. The constructed mediation model is illustrated in Figure 1.
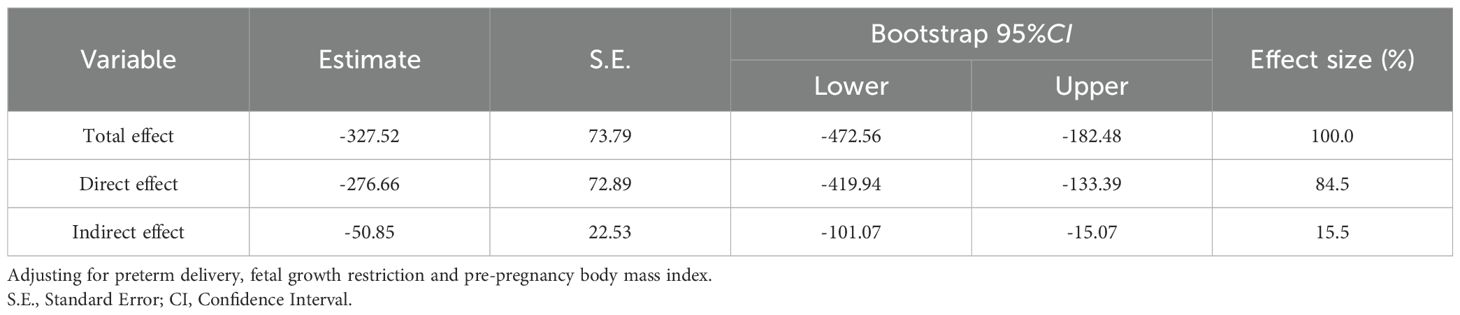
Table 6. Mediating effects of maternal alanine aminotransferase between preeclampsia with gestational hypothyroidism and birth weight.
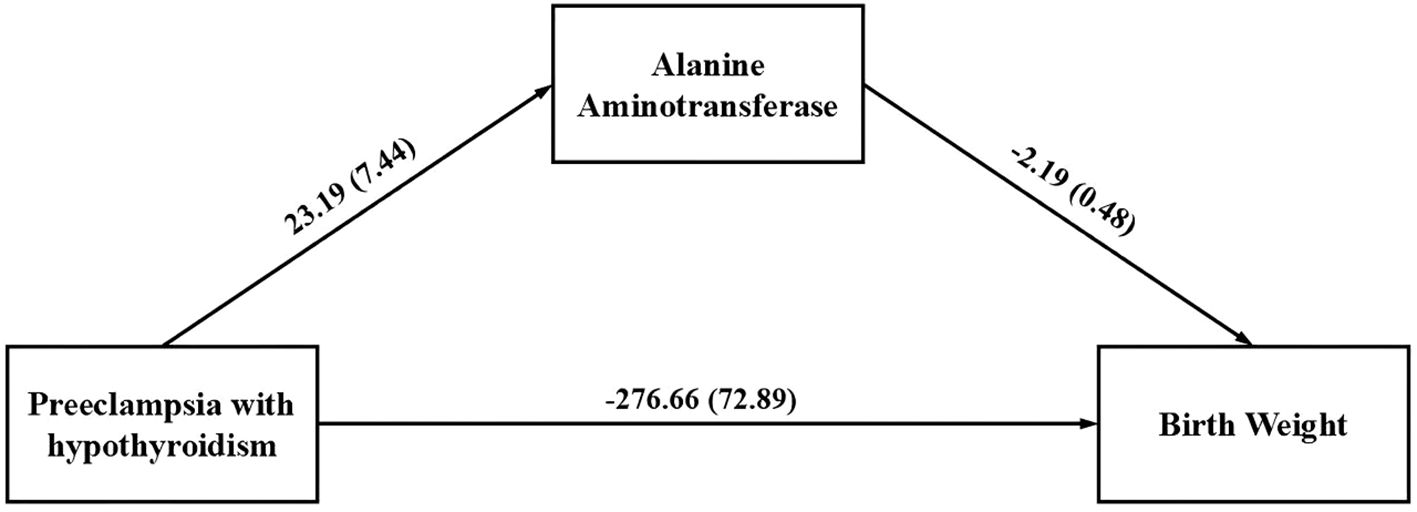
Figure 1. The mediation model examines the indirect correlation between gestational hypothyroidism in pregnant women with preeclampsia and neonatal birth weight through maternal alanine aminotransferase level.
Discussions
This study explores the impact of gestational hypothyroidism and liver function indicators on neonatal birth weight in women with preeclampsia by constructing generalized linear models and mediation models, while also evaluates the potential mediating effects of liver function indicators. The results indicate that approximately 11.0% of the preeclamptic participants included in the study concurrently suffered from hypothyroidism. Neonates born to mothers with preeclampsia and hypothyroidism exhibited lower birth weights; specifically, higher levels of ALT in liver function indicators were associated with lower neonatal birth weights, whereas neonatal birth weight increased with rising ALP levels. Notably, after adjusting for covariates such as preterm birth, FGR, and PBMI, the relationship between AST levels and neonatal birth weight became statistically insignificant. Furthermore, the mediation model revealed that hypothyroidism can directly affect the neonatal birth weight of women with preeclampsia and can also indirectly influence neonatal birth weight through elevated ALT levels (mediating effect: -50.85; 95% CI = -101.07, -15.07).
Preeclampsia and gestational hypothyroidism are two common pregnancy complications that can have severe implications for the health of both the mother and the fetus, including miscarriage, preterm birth, fetal growth restriction, and low birth weight (32, 33). Preeclampsia can lead to maternal vascular constriction and reduced blood flow, thereby affecting the blood supply to the placenta and subsequently influencing the nutritional supply to the fetus (34). Particularly, preeclampsia is one of the significant causes of maternal and neonatal mortality, and once diagnosed, there are currently no effective treatment options available aside from the termination of pregnancy (34, 35). Similarly, during pregnancy, thyroid hormones can regulate various metabolic balances in pregnant women and are also involved in the formation and function of the placenta. In cases of hypothyroidism, the resulting deficiency of thyroid hormones may lead to placental dysfunction, causing fetal developmental abnormalities (36). It is worth noting that these two diseases often coexist and influence each other. Previous studies have indicated that hypothyroidism is significantly associated with an increased incidence of preeclampsia (37), and the prevalence of hypothyroidism among patients with preeclampsia is significantly higher than that in the general population (38). Additionally, further research has pointed out that hypothyroidism is correlated with the severity of preeclampsia (39). Therefore, the combination of preeclampsia and gestational hypothyroidism may pose greater risks to both the mother and the fetus. On the other hand, the birth weight of neonates is not only related to their survival rates but also has lasting implications for their physical growth, the development of various systems, and health issues in adulthood (40, 41). That’s why we focused on investigating the effect of hypothyroidism in pregnant women with preeclampsia on neonatal birth weight. Our study findings indicate that neonates born to mothers with preeclampsia combined with hypothyroidism have lower birth weights compared to those born to mothers with preeclampsia alone, which is consistent with previous research results (42). This may indicate that when pregnant women experience preeclampsia in conjunction with hypothyroidism, it may have a more severe impact on the birth weight of the neonate.
Furthermore, our study further investigated the association between maternal liver function indicators and neonatal birth weight. Among pregnant women, the prevalence of liver diseases during pregnancy is approximately 3%, primarily manifested by abnormal changes in transaminases, bilirubin, and other related parameters (43). Pregnancy-related liver diseases are closely associated with fetal growth and development. Some pregnancy-specific liver diseases, such as acute fatty liver of pregnancy (AFLP) and intrahepatic cholestasis of pregnancy (ICP), may result in maternal hepatic dysfunction, which can subsequently affect the placenta’s ability to supply nutrients and oxygen to the fetus. This may lead to complications such as fetal intrauterine distress, preterm birth, and LBW, thereby posing risks to maternal and fetal safety (44). However, during pregnancy, the indicators related to liver function diagnosis do not change independently and may undergo physiological changes, which complicates the diagnosis of liver function in pregnant women. For instance, ALP levels may physiologically increase in the late stages of pregnancy due to placental production and fetal skeletal development. Conversely, albumin levels may decrease due to hemodilution. Nevertheless, when maternal transaminase and bilirubin levels increase, it is generally considered an abnormal phenomenon (45). Consistent with the findings of Sciarrone et, al (46), this study also indicates that maternal ALT levels are negatively correlated with neonatal birth weight. Elevated ALT levels typically indicate liver cell damage or liver dysfunction, which may lead to a decrease in the liver’s synthetic capacity and subsequently affect fetal nutrition supply (47). Additionally, pro-inflammatory factors released due to liver damage can cross the placental barrier and inhibit fetal growth (48), ultimately resulting in reduced the neonatal birth weight (49). This study also found a positive correlation between maternal serum ALP levels and neonatal birth weight, which is consistent with previous research findings (50, 51). The variation in ALP levels is associated with gestational age; although elevated ALP levels are related to ICP (52), which may impair placental function, the increase in ALP during pregnancy is generally considered physiological. From another perspective, ALP is involved in the transport and metabolic processes within the placenta. Elevated ALP levels may reflect robust placental function, which is beneficial for fetal growth and development, thereby contributing to increased birth weight (53). Interestingly, after adjusting for covariates, the statistical significance between AST levels and neonatal birth weight dissipated. This may be attributed to the substantial influence of these covariates on neonatal birth weight, thereby obscuring the effect of AST levels. Additionally, there may be a high correlation between AST levels and the covariates, as indicated by the research conducted by Zhuang et al., which suggests that AST is an independent risk factor for preterm birth (54).
It is noteworthy that both preeclampsia and hypothyroidism can impair maternal liver function (35, 55), and that preeclampsia, hypothyroidism, and liver function all have an impact on neonatal birth weight. Therefore, we constructed a mediation model to explore whether preeclampsia combined with hypothyroidism could indirectly influence neonatal birth weight by altering liver function indicators. This investigation serves as an extension of our understanding of the impact of hypothyroidism on fetal development. The results of this study indicate that ALT levels partially mediate the relationship between preeclampsia combined with hypothyroidism and neonatal birth weight, that is, compared to pregnant women with preeclampsia alone, those with preeclampsia combined with hypothyroidism not only directly contribute to a reduction in neonatal birth weight but may also indirectly lower birth weight through increased ALT levels. Hypothyroidism is characterized by elevated serum TSH levels and decreased FT4 levels, leading to thyroid hormone deficiency, which plays a crucial role in hepatic cellular activity and liver metabolism. Thus, hypothyroidism may lead to hepatic dysfunction, commonly manifested as impaired lipid metabolism (56) and hepatic steatosis (57). Previous studies have primarily focused on the lipid metabolism of pregnant women with hypothyroidism and its impact on pregnancy outcomes (58). However, there is a paucity of research examining the effects of changes in liver function indicators caused by hypothyroidism on pregnancy outcomes. In fact, hypothyroidism can lead to abnormalities in serum liver enzymes. A study analyzing serum data from 10292 outpatient adults indicated a negative correlation between serum GGT and ALT concentrations and FT4 levels (59), suggesting that hypothyroidism may result in elevated concentrations of ALT and gamma-glutamyl transferase (GGT).
Certainly, this study has several limitations. Firstly, the scope of our investigation is not comprehensive enough, as it lacks details on factors such as the nutritional status of pregnant women and the treatment received during hospitalization, which may confound the relationship with neonatal birth weight. Secondly, given that this study employs an observational design, it cannot establish causal relationships, necessitating cautious interpretation of the findings. Thirdly, the study only included women with singleton live births, which may introduce selection bias; additionally, there is a lack of relevant data from normal pregnant women to serve as a control group. Fourthly, since the focus of research designs was on examining the effects of preeclampsia combined with or without hypothyroidism on maternal liver function and neonatal birth weight, the impact of the severity of preeclampsia on liver function and birth weight was overlooked. Consequently, some classification criteria lacked comprehensive data monitoring, making it impossible to conduct more in-depth exploratory research. Finally, there may exist a bidirectional relationship between thyroid function and liver function; this study only explored whether hypothyroidism could induce changes in liver function indicators that indirectly affect neonatal birth weight. Therefore, future research should consider conducting larger-scale prospective studies to gain a more comprehensive understanding of the intricate interplay between preeclampsia with hypothyroidism, liver function, and adverse pregnancy outcomes.
Conclusions
This study provides new insights by exploring the impact of hypothyroidism and liver function indicators in pregnant women with preeclampsia on neonatal birth weight. The findings support the notion that hypothyroidism adversely affects fetal development and suggest that maternal serum ALT levels may serve as a potential partial mediator linking preeclampsia combined with hypothyroidism and neonatal birth weight. Clinicians should closely monitor thyroid and liver function in pregnant women with preeclampsia and implement appropriate interventions to improve neonatal birth weight and health outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All study participants provided written informed consent, and this study received approved from the Ethics Committee of Zhengzhou Central Hospital Affiliated to Zhengzhou (No. ZXYY202470). All methods were performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FZ: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. QH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. XY: Conceptualization, Resources, Writing – original draft. FS: Methodology, Software, Writing – original draft. YZ: Project administration, Validation, Writing – original draft. XX: Validation, Visualization, Writing – original draft. XT: Supervision, Writing – review & editing. GT: Data curation, Formal Analysis, Writing – original draft. LL: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Henan Province Key Scientific Research Project Plan for Higher Education Institutions (24A320037).
Acknowledgments
We would like to express our gratitude to the pregnant women who took part in this project for providing us with comprehensive study data and Dr Tian Gang of Henan Provincial People’s Hospital for his invaluable guidance in statistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1555277/full#supplementary-material
References
1. Svensson AC, Pawitan Y, Cnattingius S, Reilly M, and Lichtenstein P. Familial aggregation of small-for-gestational-age births: the importance of fetal genetic effects. Am J Obstet Gynecol. (2006) 194:475–9. doi: 10.1016/j.ajog.2005.08.019
2. Basso O, Wilcox AJ, and Weinberg CR. Birth weight and mortality: causality or confounding? Am J Epidemiol. (2006) 164:303–11. doi: 10.1093/aje/kwj237
3. McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. (1985) 312:82–90. doi: 10.1056/NEJM198501103120204
4. McIntire DD, Bloom SL, Casey BM, and Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. (1999) 340:1234–8. doi: 10.1056/NEJM199904223401603
5. Osmond C and Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. (2000) 108:545–53. doi: 10.1289/ehp.00108s3545
6. Kurabayashi T, Mizunuma H, Kubota T, Nagai K, and Hayashi K. Low birth weight and prematurity are associated with hypertensive disorder of pregnancy in later life: A cross-sectional study in Japan. Am J Perinatol. (2021) 38:1096–102. doi: 10.1055/s-0040-1705134
7. Shennan AH and Hurrell A. The evolving definition of preeclampsia. BJOG. (2021) 128:1383. doi: 10.1111/1471-0528.16632
8. Sibai B, Dekker G, and Kupferminc M. Preeclampsia. Lancet. (2005) 365:785–99. doi: 10.1016/S0140-6736(05)17987-2
9. Bokslag A, van Weissenbruch M, Mol BW, and de Groot CJ. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. (2016) 102:47–50. doi: 10.1016/j.earlhumdev.2016.09.007
10. Phipps EA, Thadhani R, Benzing T, and Karumanchi SA. Preeclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. (2019) 15:275–89. doi: 10.1038/s41581-019-0119-6
11. Benschop L, Duvekot JJ, and Roeters van Lennep JE. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. (2019) 105:1273–8. doi: 10.1136/heartjnl-2018-313453
12. Andolf EG, Sydsjö GC, Bladh MK, Berg G, and Sharma S. Hypertensive disorders in pregnancy and later dementia: a Swedish National Register Study. Acta Obstet Gynecol Scand. (2017) 96:464–71. doi: 10.1111/aogs.13096
13. Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, and Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. (2000) 96:950–5.
14. Li F, Wang T, Chen L, Zhang S, Chen L, Qin J, et al. Adverse pregnancy outcomes among mothers with hypertensive disorders in pregnancy: A meta-analysis of cohort studies. Pregnancy Hypertens. (2021) 24:107–17. doi: 10.1016/j.preghy.2021.03.001
15. Nakimuli A, Starling JE, Nakubulwa S, Namagembe I, Sekibubo M, Nakabembe E, et al. Relative impact of preeclampsia on birth weight in a low resource setting: A prospective cohort study. Pregnancy Hypertens. (2020) 21:1–6. doi: 10.1016/j.preghy.2020.04.002
16. Krassas GE, Poppe K, and Glinoer D. Thyroid function and human reproductive health. Endocr Rev. (2010) 31:702–55. doi: 10.1210/er.2009-0041
17. Wang J, Gong XH, Peng T, and Wu JN. Association of thyroid function during pregnancy with the risk of preeclampsia and gestational diabetes mellitus. Endocr Pract. (2021) 27:819–25. doi: 10.1016/j.eprac.2021.03.014
18. Khadir F, Rahimi Z, Vaisi-Raygani A, Shakiba E, Naseri R, et al. Gestational diabetes mellitus (GDM), hypothyroidism, and gene variants (Keap1 rs11085735) in patients with preeclampsia. Rep Biochem Mol Biol. (2022) 11:493–501. doi: 10.52547/rbmb.11.3.493
19. Lintula A, Keski-Nisula L, and Sahlman H. Hypothyroidism and the increased risk of preeclampsia-interpretative factors? Hypertens Pregnancy. (2020) 39:411–7. doi: 10.1080/10641955.2020.1800030
20. Maraka S, Ospina NM, O’Keeffe DT, Espinosa De Ycaza AR, Gionfriddo MR, Erwin PJ, et al. Subclinical hypothyroidism in pregnancy: A systematic review and meta-analysis. Thyroid. (2016) 26:580–90. doi: 10.1089/thy.2015.0418
21. Abraham C and Kusheleva N. Management of preeclampsia and eclampsia: A simulation. MedEdPORTAL. (2019) 23:10832. doi: 10.15766/mep_2374-8265.10832
22. Thangaratinam S, Ismail K, Sharp S, Coomarasamy A, O’Mahony F, Khan KS, et al. TIPPS (Tests in Prediction of Preeclamsia’s Severity) Review Group. Prioritisation of tests for the prediction of preeclampsia complications: a Delphi survey. Hypertens Pregnancy. (2007) 26:131–8. doi: 10.1080/10641950601148000
23. Vidal-Cevallos P, Murúa-Beltrán Gall S, Uribe M, Chávez-Tapia NC, et al. Understanding the relationship between nonalcoholic fatty liver disease and thyroid disease. Int J Mol Sci. (2022) 24:14605. doi: 10.3390/ijms241914605
24. Di Sessa A, Sambiase Sanseverino NC, De Simone RF, Marrapodi MM, Cirillo G, Umano GR, et al. Association between non-alcoholic fatty liver disease and subclinical hypothyroidism in children with obesity. J Endocrinol Invest. (2023) 46:1835–42. doi: 10.1007/s40618-023-02041-3
25. Kawakami T, Yoshimi M, Kadota Y, Inoue M, Sato M, and Suzuki S. Prolonged endoplasmic reticulum stress alters placental morphology and causes low birth weight. Toxicol Appl Pharmacol. (2014) 275:134–44. doi: 10.1016/j.taap.2013.12.008
26. Jhirwal M, et al. Maternal and perinatal outcome in pregnancy complicated by intrahepatic cholestasis. Cureus. (2022) 14:e28512. doi: 10.7759/cureus.28512
27. Yu X, Yao Y, Wang D, Tang J, and Lu J. Prediction of fetal growth restriction for fetal umbilical arterial/venous blood flow index evaluated by ultrasonic doppler under intelligent algorithm. Comput Math Methods Med. (2022) 2022:7451185. doi: 10.1155/2022/7451185
28. Devarshi PP, Grant RW, Ikonte CJ, and Hazels Mitmesser S. Maternal omega-3 nutrition, placental transfer and fetal brain development in gestational diabetes and preeclampsia. Nutrients. (2019) 11:1107. doi: 10.3390/nu11051107
29. He X, Wang Y, Wu M, Wei J, Sun X, Wang A, et al. Secoisolariciresinol diglucoside improves ovarian reserve in aging mouse by inhibiting oxidative stress. Front Mol Biosci. (2022) 8:806412. doi: 10.3389/fmolb.2021.806412
30. Hypertensive Disorders in Pregnancy Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. Diagnosis and treatment of hypertension and preeclampsia in pregnancy: a clinical practice guideline in China (2020). Chin J Obstet Gynecol. (2020) 55:227–38. doi: 10.3760/cma.j.cn112141-20200114-00039
31. Ad Hoc Writing Committee for Guidelines on diagnosis and management of thyroid diseases during pregnancy and postpartum, Chinese Society of Endocrinology, Chinese Medical Association, and Chinese Society of Perinatology, Chinese Medical Association. Guideline on diagnosis and management of thyroid diseases during pregnancy and postpartum (2nd edition). Chin J Endocrinol Metab. (2019) 35:636–65.
32. Atamamen TF, Naing NN, Oyetunji JA, and Wan-Arfah N. Systematic literature review on the neonatal outcome of preeclampsia. Pan Afr Med J. (2022) 41:82. doi: 10.11604/pamj.2022.41.82.31413
33. Korevaar TIM, Medici M, Visser TJ, and Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol. (2017) 13:610–22. doi: 10.1038/nrendo.2017.93
34. Rana S, Lemoine E, Granger JP, and Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. (2019) 124:1094–112. doi: 10.1161/CIRCRESAHA.118.313276
35. Dimitriadis E, et al. Preeclampsia. Nat Rev Dis Primers. (2023) 9:8. doi: 10.1038/s41572-023-00417-6
36. Adu-Gyamfi EA, Wang YX, and Ding YB. The interplay between thyroid hormones and the placenta: a comprehensive review. Biol Reprod. (2020) 102:8–17. doi: 10.1093/biolre/ioz182
37. Lee SY, Cabral HJ, Aschengrau A, and Pearce EN. Associations between maternal thyroid function in pregnancy and obstetric and perinatal outcomes. J Clin Endocrinol Metab. (2020) 105:e2015–23. doi: 10.1210/clinem/dgz275
38. Medjedovic E, Stanojevic M, Kurjak A, Begic E, Iglica A, and Jonuzovic-Prosic S. Association between maternal thyroid function and risk of gestational hypertension and preeclampsia. J Perinat Med. (2022) 50:904–9. doi: 10.1515/jpm-2022-0121
39. Su X, Liu Y, Li G, Liu X, Huang S, Duan T, et al. Associations of hypothyroxinemia with risk of preeclampsia-eclampsia and gestational hypertension. Front Endocrinol. (2021) 12:777152. doi: 10.3389/fendo.2021.777152
40. Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, and Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. (2002) 346:149–57. doi: 10.1056/NEJMoa010856
41. Grillo MA, Mariani G, and Ferraris JR. Prematurity and low birth weight in neonates as a risk factor for obesity, hypertension, and chronic kidney disease in pediatric and adult age. Front Med. (2022) 8:769734. doi: 10.3389/fmed.2021.769734
42. Liu CJ and Zheng YJ. Comparison of thyroid function and pregnancy outcomes in patients with severe preeclampsia complicated with hypothyroidism. Med Sci J Cent South China. (2017) 45:397–400.
43. Joshi D, James A, Quaglia A, Westbrook RH, and Heneghan MA. Liver disease in pregnancy. Lancet. (2010) 375:594–605. doi: 10.1016/S0140-6736(09)61495-1
44. Terrault NA and Williamson C. Pregnancy-associated liver diseases. Gastroenterology. (2022) 163:97–117. doi: 10.1053/j.gastro.2022.01.060
45. Westbrook RH, Dusheiko G, and Williamson C. Pregnancy and liver disease. J Hepatol. (2016) 64:933–45. doi: 10.1016/j.jhep.2015.11.030
46. Sciarrone SS, Ferrarese A, Bizzaro D, Volpato S, Maria Donato F, Invernizzi F, et al. Safe pregnancy after liver transplantation: Evidence from a multicenter Italian collaborative study. Dig Liver Dis. (2022) 54:669–75. doi: 10.1016/j.dld.2021.08.013
47. Vaughan OR, Rosario FJ, Powell TL, and Jansson T. Regulation of placental amino acid transport and fetal growth. Prog Mol Biol Transl Sci. (2017) 145:217–51. doi: 10.1016/bs.pmbts.2016.12.008
48. Gomes J, Au F, Basak A, Cakmak S, Vincent R, Kumarathasan P, et al. Maternal blood biomarkers and adverse pregnancy outcomes: a systematic review and meta-analysis. Crit Rev Toxicol. (2019) 49:461–78. doi: 10.1080/10408444.2019.1629873
49. Li L, Chen YH, Yang YY, and Cong L. Effect of intrahepatic cholestasis of pregnancy on neonatal birth weight: A meta-analysis. J Clin Res Pediatr Endocrinol. (2018) 10:38–43. doi: 10.4274/jcrpe.4930
50. Liu Y, Hou W, Meng X, Zhao W, Pan J, Tang J, et al. Early elevated alkaline phosphatase increases the risk of large-for-gestational-age birth weight in pregnant women with normal glucose tolerance. Diabetes Res Clin Pract. (2018) 141:209–16. doi: 10.1016/j.diabres.2018.04.024
51. Yarrington CD, Cantonwine DE, Seely EW, Mcelrath TF, and Zera CA. The association of early unexplained elevated alanine aminotransferase with large-for-gestational-age birthweight. Am J Obstet Gynecol. (2016) 215:474.e1–e5. doi: 10.1016/j.ajog.2016.04.051
52. Monrose E, Bui A, Rosenbluth E, Dickstein D, Acheampong D, Sigel K, et al. Burden of future liver abnormalities in patients with intrahepatic cholestasis of pregnancy. Am J Gastroenterol. (2021) 116:568–75. doi: 10.14309/ajg.0000000000001132
53. Makris K, Mousa C, and Cavalier E. Alkaline phosphatases: biochemistry, functions, and measurement. Calcif Tissue Int. (2023) 112:233–42. doi: 10.1007/s00223-022-01048-x
54. Zhuang X, Cui AM, Wang Q, Cheng XY, Shen Y, Cai WH, et al. Liver dysfunction during pregnancy and its association of with preterm birth in China: A prospective cohort study. EBioMedicine. (2017) 26:152–6. doi: 10.1016/j.ebiom.2017.11.014
55. Piantanida E, Ippolito S, Gallo D, Masiello E, Premoli P, Cusini C, et al. The interplay between thyroid and liver: implications for clinical practice. J Endocrinol Invest. (2020) 43:885–99. doi: 10.1007/s40618-020-01208-6
56. Sinha RA, Singh BK, and Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. (2018) 14:259–69. doi: 10.1038/nrendo.2018.10
57. Hatziagelaki E, Paschou SA, Schön M, Psaltopoulou T, and Roden M. NAFLD and thyroid function: pathophysiological and therapeutic considerations. Trends Endocrinol Metab. (2022) 33:755–68. doi: 10.1016/j.tem.2022.08.001
58. Qin Y, Wu Y, Zang H, Cong X, Shen Q, Chen L, et al. Lipid metabolism in pregnancy women with hypothyroidism and potential influence on pregnancy outcome. J Lipids. (2024) 2024:5589492. doi: 10.1155/2024/5589492
Keywords: birth weight, preeclampsia, gestational hypothyroidism, liver function, mediating model
Citation: Zhang F, Hua Q, You X, Shi F, Zhou Y, Xu X, Tian X, Tian G and Li L (2025) The association between gestational hypothyroidism in pregnant women with preeclampsia, maternal liver function indicators, and neonatal birth weight: a study in Chinese pregnant women. Front. Endocrinol. 16:1555277. doi: 10.3389/fendo.2025.1555277
Received: 04 January 2025; Accepted: 28 August 2025;
Published: 22 September 2025.
Edited by:
Sijung Yun, Predictiv Care, Inc., United StatesReviewed by:
Geng-dong Chen, Foshan Women and Children Hospital, ChinaSatyajit Kulkarni, Gujarat Ayurved University, India
Copyright © 2025 Zhang, Hua, You, Shi, Zhou, Xu, Tian, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Li, WlpTWUxpTGlAMTYzLmNvbQ==; Gang Tian, R2FuZ190aWFuQHllYWgubmV0; Xiaona Tian, MjMxMjg5MDkxOUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Fang Zhang
Fang Zhang Qing Hua1†
Qing Hua1† Gang Tian
Gang Tian