- 1Department of Orthopedic Surgery, Huashan Hospital, Fudan University, Shanghai, China
- 2School of Nursing, Fudan University, Shanghai, China
- 3Department of Orthopedics, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Department of Rehabilitation, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: Both physical activity and nature exposure are associated with several health benefits for both knee osteoarthritis (KOA) and type 2 diabetes mellitus (T2DM). However, the health outcomes when being physically active in nature, called green exercise (GE), are less clear. The purpose of this study is to evaluate the psychosocial and physiological outcomes for green exercise in KOA patients coexisting with T2DM compared to indoor exercise.
Method: A prospective, randomized, open-label, parallel, multi-center clinical trial conducted at two hospitals in Shanghai that included 82 patients T2DM and KOA. Enrollment occurred between January 2020 and March 2022, and follow-up was completed October 2022. Participants were randomized to outdoor green cycling (OGC) group and indoor stationary cycling (ISC) group. Psychosocial and physiological health outcomes were evaluated through questionnaires comprising standard international measures of self-esteem (Rosenberg Self-esteem Scale [RSE]), perceived stress (Perceived Stress Scale [PSS]), mood (Profile of Mood States [POMS]) and nature relatedness (Nature Relatedness Scale – short-form version). Participants’ enjoyment of exercise and intention for a future exercise behavior was measured in a questionnaire using a 100mm visual analogue scale as a continuum from 0 – ‘not at all’ to 100 – ‘very much’.
Results: Among 82 patients, 74 (90.2%) completed the trial. By 24 weeks, the OGC group showed a greater reduction in perceived stress (3.7 vs. 2.8, P = 0.007), and total mood disturbance (POMS) scores decreased more in the OGC group (-7.2 vs. -6.1, P = 0.030). Tension and depression subscales also showed greater reductions in the OGC group (P < 0.001 for both), along with higher improvements in vigor (10.2 vs. 8.1, P < 0.001). Enjoyment of exercise was slightly higher in the OGC group, though this difference did not reach statistical significance (P = 0.061), and their intention to continue exercising was also significantly stronger (P = 0.021). Participants in OGC group achieved significant decrease of HbA1c than participants in ISC group.
Conclusion: Green exercise offers an accessible provision for improving short-term psychological wellbeing than indoor exercises.
Clinical Trial Registration: https://www.chictr.org.cn/, identifier ChiCTR2100042872.
Introduction
Multimorbidity presents a significant public health challenge with crucial implications for health management and policy. A prominent pattern of multimorbidity is the coexistence of cardiometabolic and osteoarticular diseases, notably the common combination of type 2 diabetes mellitus (T2DM) and osteoarthritis (OA) (1). The association between T2DM and knee osteoarthritis (KOA) has received attention due to their concurrent prevalence and mutual risk factors, such as obesity and aging. Studies show a robust link between T2DM and KOA, with one study finding that people with T2DM are more than twice as likely to develop KOA compared to those without diabetes, especially among non-obese individuals (2). This suggests that the impact of diabetes on KOA extends beyond the influence of obesity alone. The body of research underscores a substantial connection between T2DM and KOA, indicating that the underlying mechanisms may transcend common risk factors like obesity (3, 4).
Exercise is recognized as a fundamental component of managing T2DM, alongside diet and effective medications (5, 6). The benefits of exercise, including improved glycemic control and better blood lipid profiles, are well-supported by evidence (7–9). However, the relative benefits of different exercise types remain less certain. Aerobic exercise, which involves activities like brisk walking, cycling, swimming, and jogging that use large muscle groups, is the most extensively studied form of exercise for this purpose (8).
Particularly, the type of exercise environment is believed to play a crucial role in the emotional and stress-reducing effects of short-term exercise sessions. Exercise performed in natural, “green” settings leads to notably better outcomes in mood, focus, and physiological responses compared to exercising indoors or in outdoor “built” environments, where buildings and synthetic materials are predominant (10–15). During physical activity, natural settings are most effective in rejuvenating fatigued cognitive directed attention (the deliberate mental capacity to resist distractions from competing stimuli) (16, 17). This rejuvenation is advantageous for workplace task performance, and it may explain why short episodes of exercise in nature enhance mood and emotional states (18–20).
Psychosocial factors, such as depression, have been linked to the development of T2DM and to poorer results on glucose tolerance tests (21). Additionally, the presence of depression at the start of a study may predict the emergence of impaired glucose regulation and T2DM (22). On the other hand, a systematic review and meta-analysis recently reported that 19.9% of people with OA had depressive symptoms, with a relative risk of depression of 1.17 in those with OA compared to those without (23, 24). However, depression is often under-recognized and under-treated in older adults, particular in patients with OA (25, 26).
To our knowledge, no research has examined the psychosocial and physiological outcomes of outdoor green exercise for patients with multimorbidity of both KOA and T2DM. The purpose of this study was to compare different exercise environments for patients with KOA and T2DM in terms of psychosocial and physiological change, glycemic control, and functional outcomes. The hypotheses were that: 1) The enhancement in mood from pre- to post-exercise will be more apparent in outdoor green exercise settings than in indoor ones; 2) Participants engaging in outdoor green exercise will exhibit superior physiological status during their workout sessions compared to those exercising indoors; 3) Outdoor green exercise environments will lead to a more significant improvement in patients’ blood glucose levels compared to indoor exercise settings; 4) Following outdoor green exercise, participants will demonstrate better functional mobility than those who undertake indoor exercise.
Materials and methods
Study design
This is a prospective, randomized, open-label, parallel, multi-center clinical trial. Eligible participants were recruited from Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine and Huashan Hospital Affiliated to Fudan University. All participants were divided into OGC group and ISC group. The allocation ratio was 1:1. This trial was registered at Chinese Clinical Trial before the first recruitment of the participants (ID: ChiCTR2100042872). This trial was approved by the Institutional Review Board of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (IRB No: 2019-KY-063(K)) and other trial sites acknowledged the IRB approval. All participants provided written informed consents.
Study participants
KOA was confirmed according to the criteria from NICE (27): Osteoarthritis should be diagnosed clinically without the need for imaging in individuals over 45 years who exhibit activity-related joint pain and either no morning stiffness or stiffness lasting less than 30 minutes.
The definition of T2DM in the present study was formulated according to the SUPREME-DM (28) criteria as follows: a) 1 or more of the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes and Tenth Revision, Clinical Modification (ICD-10-CM) codes for type 2 diabetes associated with in-patient encounters; b) 2 or more ICD codes associated with out-patient encounters on different days within 2 years; c) combination of 2 or more of the following associated with out-patient encounters on different days within 2 years: 1) ICD codes associated with out-patient encounters; 2) fasting glucose level ≥ 126 mg/dl; 3) 2-hour glucose level ≥ 200 mg/dl; 4) random glucose ≥ 200 mg/dl; 5) HbA1c ≥ 6.5%; and 6) prescription for an antidiabetic medication.
Interventions
Before the baseline assessment, all participants received standard care at the Knee Pain Specialized Clinic of Shanghai Sixth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine or Huashan Hospital Affiliated to Fudan University. The usual care for KOA included diagnosis, providing information about the condition, identifying personal risk factors, and developing a tailored treatment plan. This plan typically involved pain medication and advice on knee protection for daily life activities.
To ensure the effectiveness of the cycling exercise and to reduce the risk of injury, all participants were required to set their posture correctly under the guidance of rehabilitation staff before beginning the exercise (see Supplementary Material).
Indoor stationary cycling group
Participants in the ISC group were instructed to use stationary cycling for three times per week for 30–40 minutes. Investigators individualized the program for each participant by estimating target exercise heart rate for the sessions and providing heart rate monitors to help subjects adhere to exercise guidelines (see Supplementary Material).
Outdoor green cycling group
Participants in the OGC group were required to exercise for three times per week for 30–40 minutes step-by-step in groups of maximally 4 people supervised by a physical therapist. Each participant wears a heart rate monitor band around the chest to ensure reaching the target exercise heart rate (see Supplementary Material).
Outcomes
Psychosocial and physiological health outcomes included pre-post changes of Rosenberg Self-Esteem Scale (RSE), Perceived Stress Scale (PSS), and Profile of Mood States (POMS). The RSE is one of the most widely used instruments for measuring self-esteem, which consists of 10 items, rated on a four-point Likert scale ranging from “Strongly Agree” to “Strongly Disagree.” Five of the items are positively worded (e.g., “I feel that I have a number of good qualities”) and five are negatively worded (e.g., “I feel I do not have much to be proud of”). This balance is intended to control for acquiescence bias, where respondents may agree with statements regardless of their content (29).
The PSS is a psychological instrument designed to measure the perception of stress. Developed by Sheldon Cohen and his colleagues in 1983, the PSS assesses the degree to which situations in one’s life are appraised as stressful. It is widely used in psychological research to evaluate the role of stress in the etiology of disease and behavioral disorders. The PSS consists of 10 items (PSS-10), though original versions included 14 items (PSS-14) and a shorter version of 4 items (PSS-4) is also available. Respondents are asked to rate their feelings and thoughts during the last month on a 5-point Likert scale ranging from “Never” to “Very Often” (30).
The POMS is a psychological rating scale used to assess transient, distinct mood states. Developed by Douglas M. McNair, Maurice Lorr, and Leo F. Droppleman in 1971, the POMS is widely utilized in clinical psychology, sports psychology, and research to measure mood fluctuations over a specific period. The POMS originally consists of 65 items, each describing a mood-related adjective (e.g., “angry,” “energetic”). Respondents rate each item on a 5-point Likert scale ranging from “Not at All” to “Extremely,” based on how they have been feeling during the past week, including the day of assessment (31).
Primary functional outcome was the pre-post changes of HbA1c. The secondary functional outcome was changes of Knee Injury and Osteoarthritis Outcome Score (KOOS) from baseline. KOOS is a patient-reported outcome measurement system used to evaluate short-term and long-term symptoms and function in individuals with knee injuries and osteoarthritis. The score consisted of 5 separately scored subscales; Pain, Symptoms, Function in daily living (ADL), Function in Sport and Recreation (Sport/Rec) and knee-related Quality of Life (QoL). The score ranges from 0 to 100 with 0 representing extreme problems and 100 representing no problems. Other secondary outcome included changes in body mass index (BMI).
Sample size
For a two-arm trial with the primary outcome measured by RSE Scale. At the significance level of 0.05, with a noninferiority margin of 5, 36 subjects for each group are needed to achieve 80% power when the mean response difference between treatment and control is 1 and the standard deviation is assumed to be 10 (32). The sample size was a priori increased to at least 40 participants for each group adjusted for a 10% dropout rate (33).
Randomization, blinding and follow-up
The participants were randomly assigned to the intervention and control groups in a 1:1 ratio after screening. The randomization sequence was generated by the institutional staff and was concealed from the physical therapists during follow‐up. Psychosocial and physiological health and functional data were collected from the participants at baseline and at the six weeks and sixth months after initiation of exercise; these data included sex, use of walking aid, BMI, and history of diseases and medications. Height and weight were measured using standardized methods. BMI was calculated as the weight in kilograms divided by the squared height in meters. Other data were collected from questionnaires.
No blinding was performed in this trial. Only the analyst who assessed the outcomes was blind to this trial.
Statistical analysis
The analysis was performed according to the per-protocol principle and the adverse events intention-to-treat (ITT) population. The normality of the data was tested using the Shapiro-Wilk test. Measures that followed a normal distribution were presented as means and standard deviations, and comparisons between groups were conducted using the two independent samples t-test. The effect size was calculated as Cohen’s d for primary outcomes. Variables that did not follow a normal distribution were presented as medians and interquartile ranges, and comparisons between the groups were conducted using the Wilcoxon rank-sum test. Categorical data were presented as frequencies (number of cases) or percentages, and group comparisons were performed using the chi-square test or Fisher’s exact test, depending on the number of categories (columns) and the sample size in the R×C contingency table. The above data were analysed using R (version 4.3.3) and a two-sided test (α=0.05).
Results
Baseline characteristics of the study participants
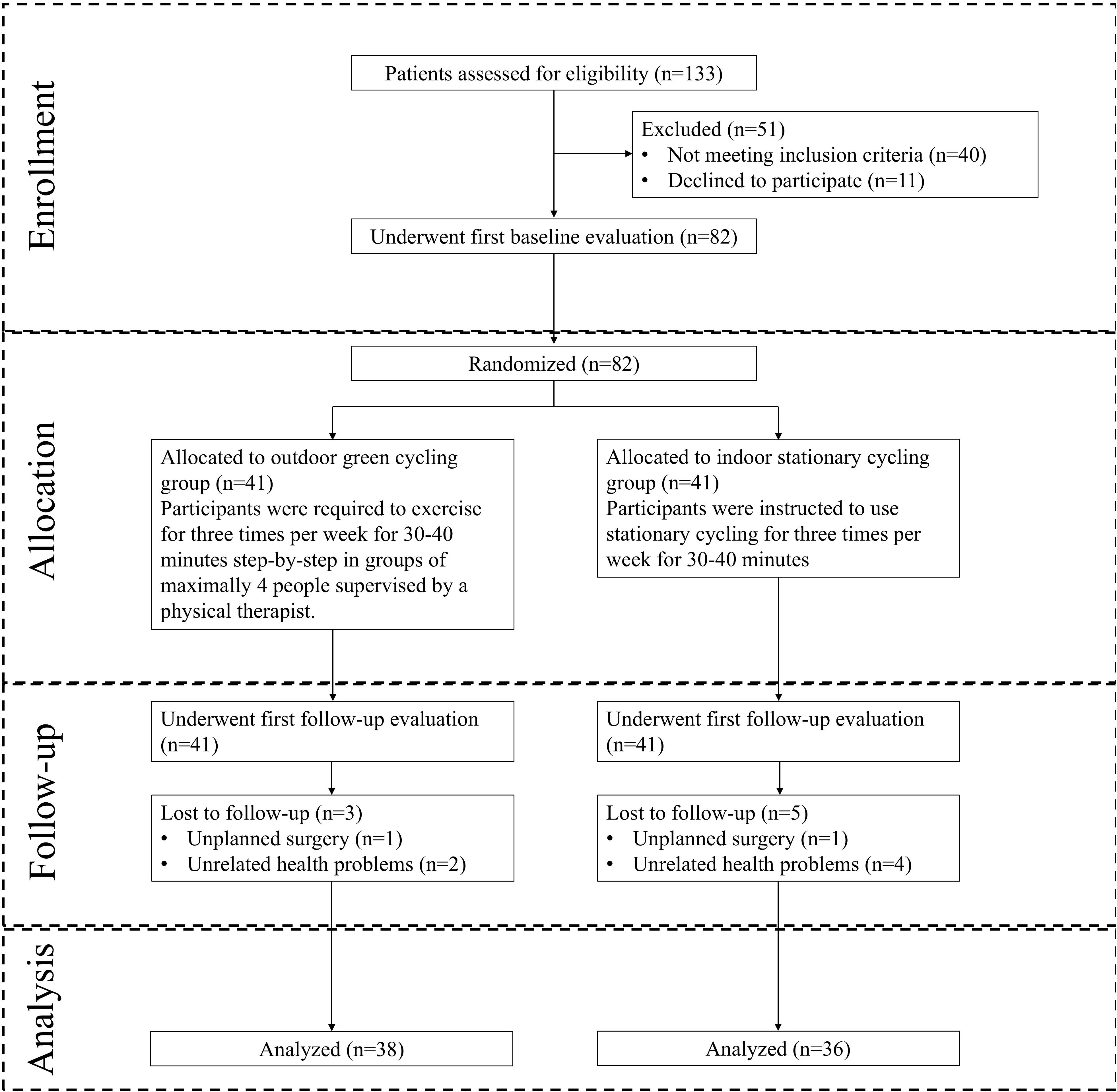
Of the 133 participants who underwent screening in this study, 82 were enrolled into the final analysis and randomized. 41 were in the ISC group, 41 were in the OGC group. Of these, a total of 74 (90.2%) completed the full follow-up visits. 8 participants (5 in ISC group, 3 in OGC group) failed to complete the entire study due to unplanned surgery and injury (N = 2) and reasons unrelated to the study (N = 6) (Figure 1).
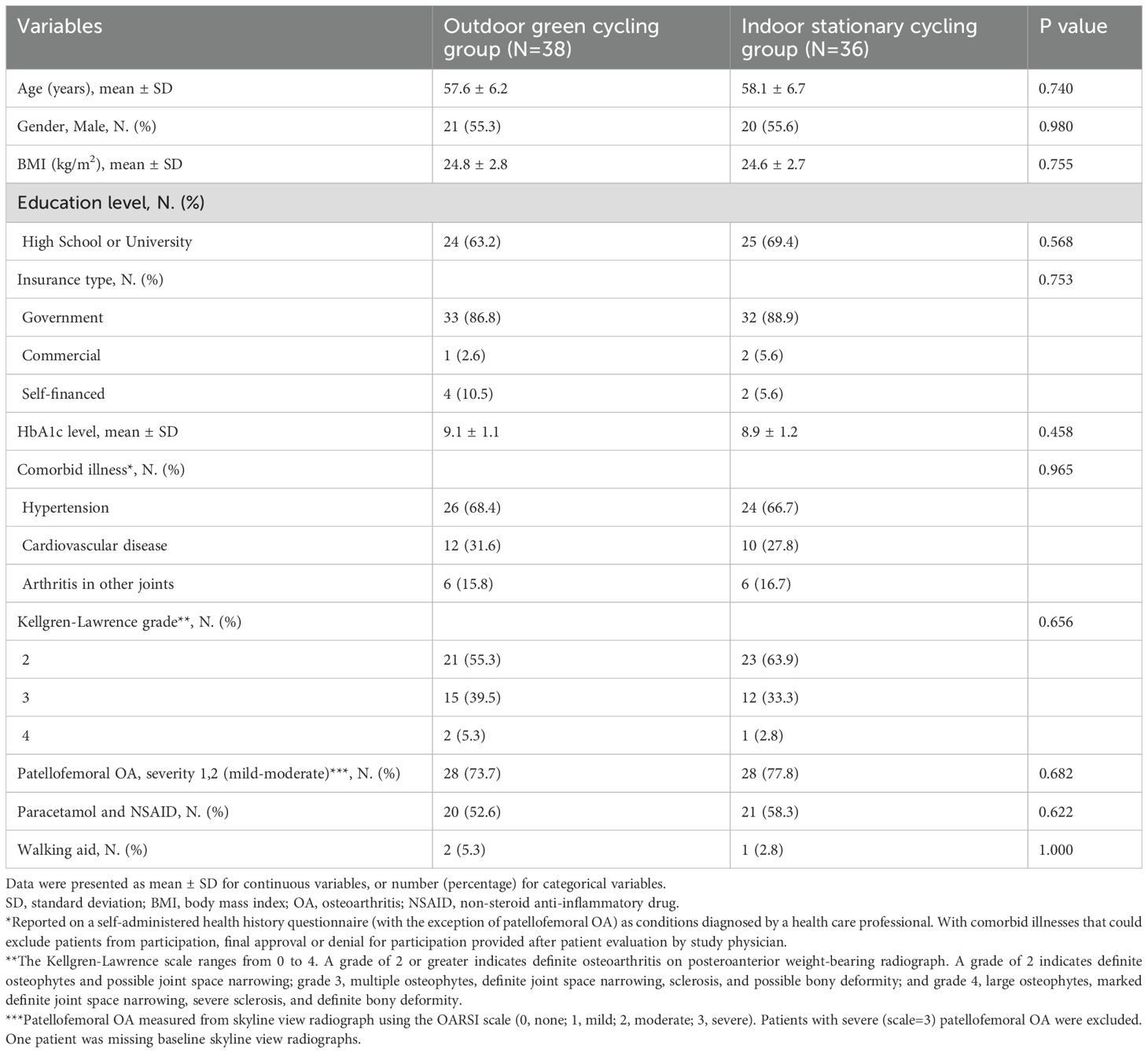
The baseline characteristics are shown in Table 1. The demographic and clinical characteristics of the study participants at baseline show that the OGC group (N = 38) and the ISC group (N = 36) were well-matched. The mean age was 57.6 years in the OGC group and 58.1 years in the ISC group (P = 0.740), and both groups had a similar percentage of males (55.3% vs. 55.6%, P = 0.980). The average BMI was also similar between the groups (24.8 kg/m² in the OGC group and 24.6 kg/m² in the ISC group, P = 0.755). Insurance type and comorbid illnesses such as hypertension and cardiovascular disease were comparable across both groups, with no significant differences (Table 1).
The changes in RSS, PSS and POMS
Significant improvements were seen in the OGC group. By week 24, the OGC group experienced a greater reduction in perceived stress compared to the ISC group (3.7 vs. 2.8, P = 0.007). Additionally, the total mood disturbance (POMS) score showed a larger decrease in the OGC group (-7.2 vs. -6.1, P = 0.030). Tension and depression subscales also showed greater reductions in the OGC group (P < 0.001 for both), along with higher improvements in vigor (10.2 vs. 8.1, P < 0.001). Enjoyment of exercise was marginally higher in the OGC group (P = 0.061), and their intention to continue exercising was also significantly stronger (P = 0.021) (Table 2).
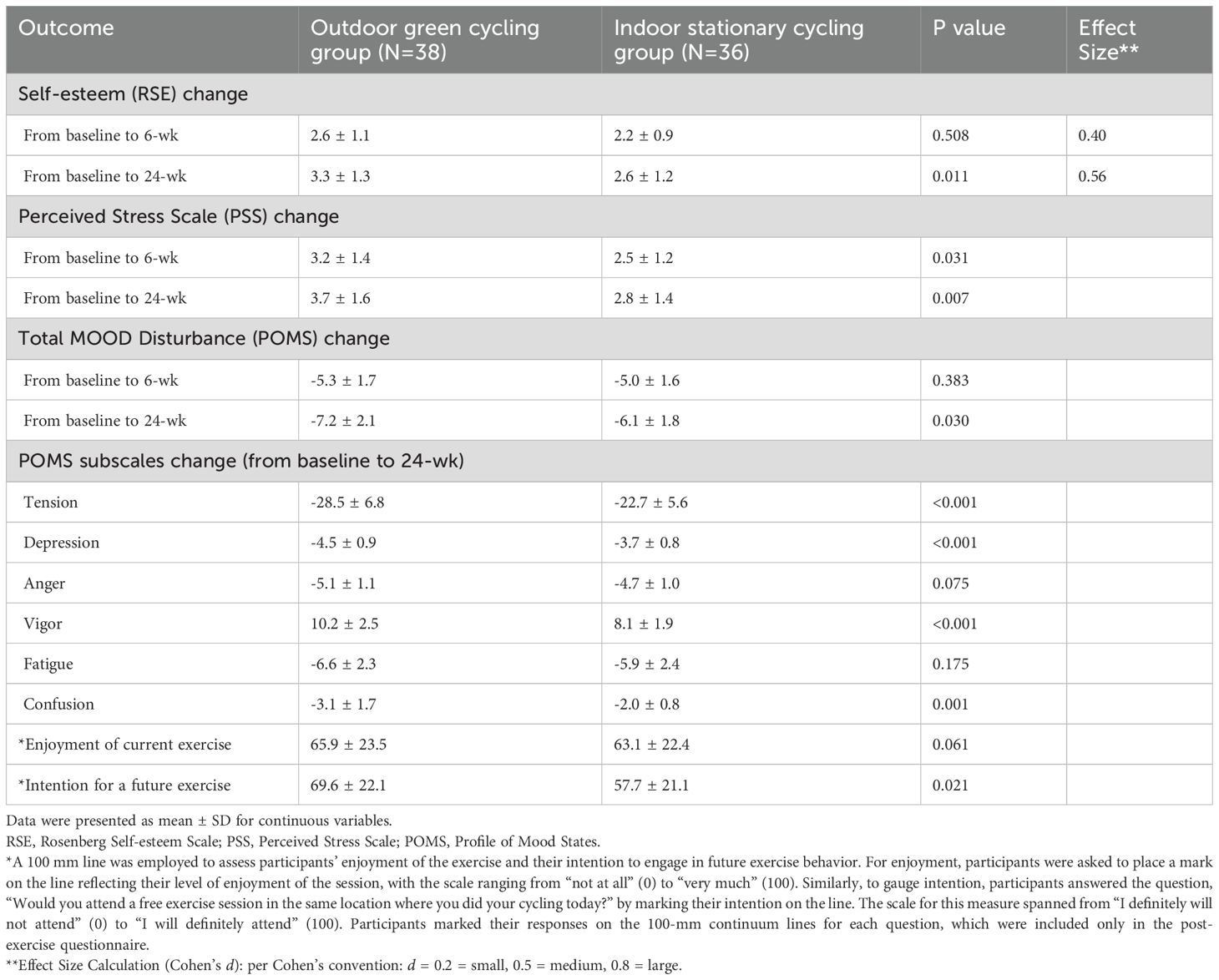
Table 2. Changes in psychosocial and physiological health outcomes at 6 weeks and 24 weeks from baseline in per-protocol population.
The changes in HbA1c
Pre-post changes of HbA1c were significant in 6- and 24-weeks follow-up in two groups respectively. Participants in OGC group achieved significant decrease of HbA1c than participants in ISC group (Table 3).

Table 3. Changes in functional and other outcome measures at 6 weeks and 24 weeks from baseline in per-protocol population.
The changes in KOOS
BMI reduction was more pronounced in the OGC group at both time points (2.8 kg/m² vs. 2.3 kg/m² at 24 weeks, P = 0.006). Improvements in KOOS scores for quality of life (6.4 vs. 4.8, P < 0.001), and Sport/Rec (5.5 vs. 3.7, P < 0.001) were significantly better in the OGC group by 24 weeks (Table 3).
Adherence and Intention
The result found that enjoyment of current exercise was similar between two groups, but intention for future exercise behavior was significantly positively associated with participants who engaged in outdoor green exercise (Table 2).
Adverse events
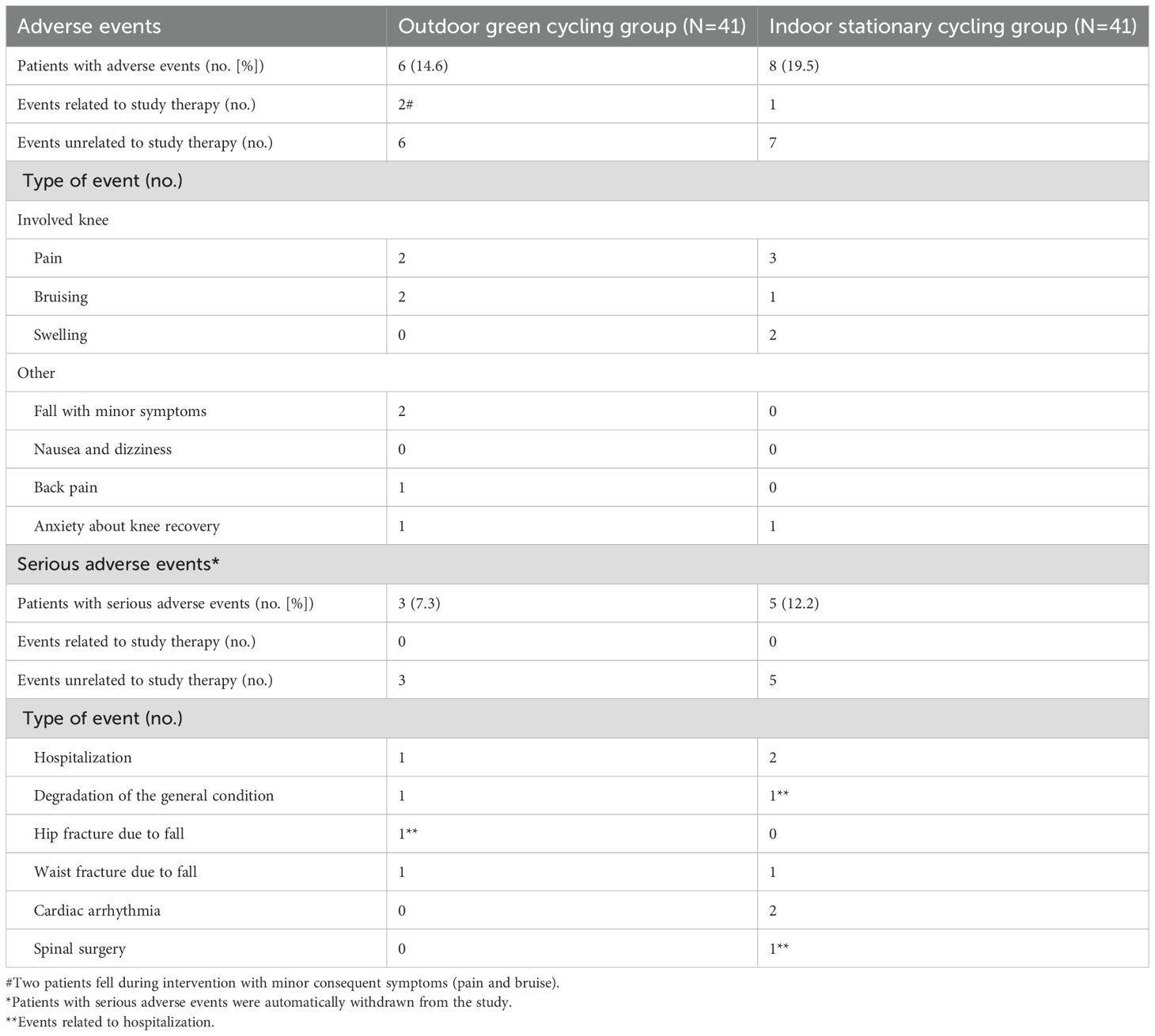
During the follow-up period, a similar proportion of participants in both groups reported adverse events. No serious events were related to intervention, while two minor events were possibly related to the outdoor green cycling intervention (Table 4). The proportions of participants lost to follow-up were equivalent in both groups, with most losses occurring at the final follow-up survey (Figure 1).
Discussion
Our randomized, controlled trial involving T2DM patients showed that in 24 weeks, the OGC group showed significantly greater reductions in perceived stress, mood disturbance, and depression compared to the ISC group (P < 0.05). Additionally, the Green outdoor group experienced higher vigor and tension reduction, indicating better psychological benefits. Our hypothesis that greater intention for a future exercise behavior and greater enjoyment of the exercise session would be reported following outdoors green exercise compared to indoors exercise were confirmed based on the results of this study. To our knowledge, this is the first study to examine the differential psychosocial and physiological effects of GE compared to indoor training on patients with chronic diseases.
Both OA and T2DM are complex diseases influenced by genetic, demographic, and lifestyle factors, such as older age and obesity (34). Older age, in particular, significantly impacts the prevalence and severity of both OA and T2DM. As individuals age, the cumulative wear and tear on joint cartilage increases the likelihood of OA development, resulting in joint pain and stiffness. Similarly, the prevalence of T2DM rises with age due to progressive beta-cell dysfunction and increasing insulin resistance over time (29, 30, 35, 36). Lifestyle intervention is considered a cornerstone of treatment of both OA and T2DM (4, 8, 31, 37). Effective weight management through dietary modifications and regular physical activity is essential to reduce joint stress and enhance mobility in OA patients. Targeted exercise programs that improve muscle strength and joint flexibility can significantly alleviate pain and enhance functional outcomes. For T2DM, lifestyle interventions focusing on a balanced diet and consistent physical activity are critical for managing blood glucose levels and improving overall metabolic health (38, 39).
On the other hand, physical activity has been shown to release endorphins and other neurochemicals that promote feelings of well-being and reduce symptoms of depression and anxiety (31, 37–39). These benefits are especially pronounced in natural environments (40), which can be especially advantageous for patients managing chronic conditions such as T2DM and OA. In our study, participants in both groups reported reduced levels of depression and anxiety following the initiation of exercise; however, participants in the GE group experienced significantly greater improvements, with statistically significant differences observed. Our results align with previous research. For example, Rogerson et al. demonstrated that a single session of GE improved self-esteem by 7.7%, reduced stress by 18.4%, and enhanced mood by 14.2% (41). Additionally, the observed improvements in tension, depression, vigor, and confusion are consistent with prior findings (42). While these measures may not fully reflect the long-term benefits or clinical significance for participants, they do indicate meaningful improvements in acute psychological well-being. In the short term, such results can provide valuable insights for health promotion initiatives (43). Previous studies have also found that immediate emotional responses to exercise play a critical role in fostering long-term motivation and adherence to regular physical (44, 45).
Here, participation in GE might serve as a tool for maintaining exercise behaviors (10). The increase in POMS fatigue contradicts previous studies. The 40-minute cycling session in our study is likely more intense than the exercises in earlier GE research (11, 12). Higher exercise intensity may lead participants to interpret items on the fatigue subscale (fatigued, worn out, exhausted, sluggish, weary) in a physiological sense rather than the intended psychological sense. Although the exercise in this study might have been more intense than in previous GE studies, which likely remained below ventilatory or lactate thresholds (11, 12). Positive affective responses normally resulted from the situation that exercise intensities below these thresholds, whereas intensities above them often lead to discomfort for most recreational exercisers (46). However, self-pacing can enhance tolerance to higher intensities better than imposed pacing. This self-regulation may have mitigated the negative effects of high-intensity exercise on their overall affect for some individuals (46). The results of both enjoyment of current exercise and intention for future exercise behavior confirmed this theory in both groups.
Previous research suggests that GE offers greater psychological benefits than exercising indoors or in artificial outdoor environments (10, 11, 15, 46). This finding implies that the additional affective benefits may be obtainable through a variety of GE environments. However, our study did not have a non-nature-based exercise environment for comparison. Since acute affective improvement of single exercise bouts are consistently reported, it is hard to distinguish between the contributions of the ‘greenness’ of the exercise environments and the exercise itself in this study. On the other hand, ‘non-green’ exercise may also provide similar affective benefits.
Functional outcomes over 24 weeks show that the Green outdoor group experienced larger improvements in HbA1c, BMI, and KOOS-QoL compared to the ISC group (P < 0.05). Improvements in KOOS-ADL and KOOS-Sport/Rec were also significantly greater in the OGC group. Englund suggested that the modest benefits of exercise interventions for KOA could largely be attributed to the placebo effect, the disease’s natural progression, and statistical regression to the mean (47). Thus, our findings implied that placebo effects of exercise have less connection with environment. Some authors considered the use of exercise treatment in chronic pain conditions should be viewed as a form cognitive therapy, where the goal is to modulate the feeling of pain and thus patients’ thoughts and feelings about it rather than increasing muscle strength and endurance (48, 49).
A key strength of this study was its ecological validity. The results reflect improvements in psychological well-being observed in a real-world setting with individuals acting on their own, rather than through a designed and instructed intervention. This study also has several limitations. First, in the baseline condition, the submaximal test was conducted after participants completed a brief warm-up cycling session. For participants who exercise less frequently, even “extremely light” intensity exercise could influence the heart rate response of a subsequent bout, potentially biasing the estimated 50% HRR intensity levels used for the experimental conditions. To mitigate this potential bias, resting heart rate values were obtained earlier during the baseline session, and participants took a two-minute rest period immediately before the submaximal test. Second, a limitation of this study is the relatively short follow-up period of 24 weeks, which may not fully capture the long-term benefits of green exercise. While this duration is appropriate for assessing immediate and short-term changes in psychosocial and physiological outcomes, it does not provide insights into the sustainability of these effects. Future studies with extended follow-up periods are needed to evaluate whether the positive outcomes observed during the study persist over time and contribute to long-term improvements in both KOA and T2DM. Thirdly, a potential source of bias in our study is observational bias, particularly in relation to the psychosocial outcomes, such as self-esteem, perceived stress, and mood. Participants in the green exercise group may have experienced positive social and environmental factors—such as being outdoors and engaging in group exercise—that could have influenced their responses to these measures. Lastly, while baseline radiographic characteristics (including Kellgren-Lawrence grade and patellofemoral OA status) were documented, longitudinal imaging data were not systematically collected due to ethical and practical constraints. Future studies might prioritize serial radiographic assessments to evaluate structural progression alongside clinical outcomes, providing deeper insights into the relationship between exercise modalities and OA pathophysiology.
Conversely, participants in the indoor cycling group may have been less exposed to such external influences. This could result in differences in reported psychosocial outcomes that are not solely attributable to the exercise modality but may also reflect the impact of the environment in which the exercise took place. We acknowledge that these biases may have influenced the observed differences in psychosocial outcomes and recommend that future studies incorporate strategies to control for such factors, such as blinding or more objective measures of psychosocial well-being. Furthermore, another limitation of our study is the lack of control for environmental factors, such as air quality, temperature, and weather conditions, which may have influenced the outcomes observed in the OGC group. Future studies should consider controlling for environmental factors, either by monitoring these variables or by conducting exercises in more controlled outdoor settings, to better isolate the effects of green exercise from other external influences.
Conclusion
Green exercise enhances self-esteem and mood while reducing stress, regardless of the type of green setting, and this is particularly beneficial for patients coping with chronic conditions like T2DM and OA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of Shanghai Sixth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YJ: Formal Analysis, Methodology, Software, Writing – original draft. XG: Data curation, Methodology, Writing – original draft. QW: Investigation, Methodology, Writing – original draft. FX: Investigation, Writing – original draft. SL: Supervision, Validation, Writing – review & editing. LH: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1560536/full#supplementary-material
References
1. Williams MF, London DA, Husni EM, Navaneethan S, Kashyap SR. Type 2 diabetes and osteoarthritis: A systematic review and meta-analysis. J Diabetes Complications. (2016) 30:944–50. doi: 10.1016/j.jdiacomp.2016.02.016
2. Dubey NK, Ningrum DNA, Dubey R, Deng YH, Li YC, Wang PD, et al. Correlation between diabetes mellitus and knee osteoarthritis: A dry-to-wet lab approach. Int J Mol Sci. (2018) 19(10):3021. doi: 10.3390/ijms19103021
3. Louati K, Vidal C, Berenbaum F, Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open. (2015) 1:e000077. doi: 10.1136/rmdopen-2015-000077
4. Alenazi AM, Alhowimel AS, Alshehri MM, Alqahtani BA, Alhwoaimel NA, Segal NA, et al. Osteoarthritis and diabetes: where are we and where should we go? Diagnost (Basel). (2023) 13(8):1386. doi: 10.3390/diagnostics13081386
5. Sigal RJ, Armstrong MJ, Bacon SL, Boulé NG, Dasgupta K, Kenny GP, et al. Physical activity and diabetes. Can J Diabetes. (2018) 42:S54–63. doi: 10.1016/j.jcjd.2017.10.008
6. Myers J, Atwood JE, Froelicher V. Active lifestyle and diabetes. Circulation. (2003) 107:2392–4. doi: 10.1161/01.cir.0000067882.00596.fc
7. Chudyk A, Petrella RJ. Effects of exercise on cardiovascular risk factors in type 2 diabetes: A meta-analysis. Diabetes Care. (2011) 34:1228–37. doi: 10.2337/dc10-1881
8. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. Jama. (2001) 286:1218–27. doi: 10.1001/jama.286.10.1218
9. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. (2006) 2006:Cd002968. doi: 10.1002/14651858.CD002968.pub2
10. Thompson Coon J, Boddy K, Stein K, Whear R, Barton J, Depledge MH. Does participating in physical activity in outdoor natural environments have a greater effect on physical and mental wellbeing than physical activity indoors? A systematic review. Environ Sci Technol. (2011) 45:1761–72. doi: 10.1021/es102947t
11. Focht BC. Brief walks in outdoor and laboratory environments: effects on affective responses, enjoyment, and intentions to walk for exercise. Res Q Exercise Sport. (2009) 80:611–20. doi: 10.1080/02701367.2009.10599600
12. Pretty J, Peacock J, Sellens M, Griffin M. The mental and physical health outcomes of green exercise. Int J Environ Health Res. (2005) 15:319–37. doi: 10.1080/09603120500155963
13. Rogerson M, Barton J. Effects of the visual exercise environments on cognitive directed attention, energy expenditure and perceived exertion. Int J Environ Res Public Health. (2015) 12:7321–36. doi: 10.3390/ijerph120707321
14. Bodin M, Hartig T. Does the outdoor environment matter for psychological restoration gained through running? Psychol Sport Exercise. (2003) 2:141–53. doi: 10.1016/S1469-0292(01)00038-3
15. Bowler DE, Buyung-Ali LM, Knight TM, Pullin AS. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health. (2010) 10:456. doi: 10.1186/1471-2458-10-456
16. Kaplan S, Berman MG. Directed attention as a common resource for executive functioning and self-regulation. Perspect psychol Sci: A J Assoc psychol Sci. (2010) 5:43–57. doi: 10.1177/1745691609356784
18. Berman MG, Jonides J, Kaplan S. The cognitive benefits of interacting with nature. psychol Sci. (2008) 19:1207–12. doi: 10.1111/j.1467-9280.2008.02225.x
19. Kaplan R. The role of nature in the context of the workplace. Landscape Urban Plann. (1993) 26:193–201. doi: 10.1016/0169-2046(93)90016-7
20. Kaplan S. The restorative benefits of nature: toward an integrative framework. J Environ Psychol. (1995) 15:169–82. doi: 10.1016/0272-4944(95)90001-2
21. Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall Ii study. Arch Internal Med. (2004) 164:1873–80. doi: 10.1001/archinte.164.17.1873
22. Everson-Rose SA, Meyer PM, Powell LH, Pandey D, Torréns JI, Kravitz HM, et al. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. (2004) 27:2856–62. doi: 10.2337/diacare.27.12.2856
23. Stubbs B, Aluko Y, Myint PK, Smith TO. Prevalence of depressive symptoms and anxiety in osteoarthritis: A systematic review and meta-analysis. Age Ageing. (2016) 45:228–35. doi: 10.1093/ageing/afw001
24. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (Ncs-R). Jama. (2003) 289:3095–105. doi: 10.1001/jama.289.23.3095
25. Gleicher Y, Croxford R, Hochman J, Hawker G. A prospective study of mental health care for comorbid depressed mood in older adults with painful osteoarthritis. BMC Psychiatry. (2011) 11:147. doi: 10.1186/1471-244x-11-147
26. Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. (2009) 5:363–89. doi: 10.1146/annurev.clinpsy.032408.153621
27. Excellence N. Osteoarthritis in over 16s: Diagnosis and Management (Nice Guideline Cg177). National Institute for Health and Care Excellence (2014). Available at: https://www.nice.org.uk/guidance/cg177 (Accessed August 16, 2024).
28. Pathak RD, Schroeder EB, Seaquist ER, Zeng C, Lafata JE, Thomas A, et al. Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in U.S. Integrated health care delivery systems: 2005-2011. Diabetes Care. (2016) 39:363–70. doi: 10.2337/dc15-0858
29. Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. (2006) 20:3–25. doi: 10.1016/j.berh.2005.09.007
30. Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults: A consensus report. J Am Geriatr Soc. (2012) 60:2342–56. doi: 10.1111/jgs.12035
31. Raposo F, Ramos M, Lúcia Cruz A. Effects of exercise on knee osteoarthritis: A systematic review. Musculoskelet Care. (2021) 19:399–435. doi: 10.1002/msc.1538
32. Huang C, Dong N. Factor structures of the Rosenberg self-esteem scale. Eur J psychol Assess. (2012) 28:132–8. doi: 10.1027/1015-5759/a000101
33. Yanhong Zhou YY, Jack Lee J, Haitao P. Sample Size Calculation - Continuous Endpoint V.2.0.2 ed. (2019). Available online at: www.trialdesign.org.
34. Veronese N, Cooper C, Reginster JY, Hochberg M, Branco J, Bruyère O, et al. Type 2 diabetes mellitus and osteoarthritis. Semin Arthritis Rheum. (2019) 49:9–19. doi: 10.1016/j.semarthrit.2019.01.005
35. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet (London England). (2019) 393:1745–59. doi: 10.1016/s0140-6736(19)30417-9
36. Association AD. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. (2020) 44:S15–33. doi: 10.2337/dc21-S002
37. Mangione KK, McCully K, Gloviak A, Lefebvre I, Hofmann M, Craik R. The effects of high-intensity and low-intensity cycle ergometry in older adults with knee osteoarthritis. J Gerontol Ser A Biol Sci Med Sci. (1999) 54:M184–90. doi: 10.1093/gerona/54.4.m184
38. Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Cochrane Database Syst Rev. (2014) 2014:Cd007912. doi: 10.1002/14651858.CD007912.pub2
39. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Engl J Med. (2013) 369:145–54. doi: 10.1056/NEJMoa1212914
40. Pretty J, Griffin M, Sellens M, Pretty C. Green exercise: complementary roles of nature, exercise and diet in physical and emotional well-being and implications for public health policy. (University Essex CES Occasional Paper). (2003).
41. Rogerson M, Brown DK, Sandercock G, Wooller JJ, Barton J. A comparison of four typical green exercise environments and prediction of psychological health outcomes. Perspect Public Health. (2016) 136:171–80. doi: 10.1177/1757913915589845
42. Barton J, Bragg R, Pretty J. The health benefits of walking in greenspaces of high natural and heritage value. J Integr Environ Sci. (2009) 6:261–78. doi: 10.1080/19438150903378425
43. Barton J, Griffin M, Pretty J. Exercise-, nature- and socially interactive-based initiatives improve mood and self-esteem in the clinical population. Perspect Public Health. (2012) 132:89–96. doi: 10.1177/1757913910393862
44. Williams DM, Dunsiger S, Ciccolo JT, Lewis BA, Albrecht AE, Marcus BH. Acute affective response to a moderate-intensity exercise stimulus predicts physical activity participation 6 and 12 months later. Psychol Sport Exerc. (2008) 9:231–45. doi: 10.1016/j.psychsport.2007.04.002
45. Kwan BM, Bryan AD. Affective response to exercise as a component of exercise motivation: attitudes, norms, self-efficacy, and temporal stability of intentions. Psychol Sport Exerc. (2010) 11:71–9. doi: 10.1016/j.psychsport.2009.05.010
46. Ekkekakis P, Parfitt G, Petruzzello SJ. The pleasure and displeasure people feel when they exercise at different intensities: decennial update and progress towards a tripartite rationale for exercise intensity prescription. Sports Med (Auckland NZ). (2011) 41:641–71. doi: 10.2165/11590680-000000000-00000
47. Englund M. Bout of the corner men and not the boxers? Contextual effects flex their muscles. Ann Rheumatic Dis. (2018) 77:159–61. doi: 10.1136/annrheumdis-2017-211664
48. Tuttle AH, Tohyama S, Ramsay T, Kimmelman J, Schweinhardt P, Bennett GJ, et al. Increasing placebo responses over time in U.S. Clinical trials of neuropathic pain. Pain. (2015) 156:2616–26. doi: 10.1097/j.pain.0000000000000333
Keywords: green exercise, knee osteoarthritis, type 2 diabetes mellitus, psychosocial and physiological health, physical activity
Citation: Jin Y, Geng X, Wang Q, Xue F, Lu S and Huang L (2025) Psychosocial and physiological health outcomes of outdoor green exercise versus indoor exercise in knee osteoarthritis patients coexisting with type 2 diabetes mellitus: a randomized controlled trial. Front. Endocrinol. 16:1560536. doi: 10.3389/fendo.2025.1560536
Received: 14 January 2025; Accepted: 22 April 2025;
Published: 14 May 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Jiawei Meng, Trine University, United StatesLiang Wang, Fidelity Investments, United States
Copyright © 2025 Jin, Geng, Wang, Xue, Lu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengdi Lu, bHVzaGVuZ2RpQHNoc211LmVkdS5jbg==; Lihua Huang, aHVhbmdsaWh1YTkwNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yilin Jin
Yilin Jin Xiang Geng1†
Xiang Geng1† Qiaojie Wang
Qiaojie Wang Shengdi Lu
Shengdi Lu Lihua Huang
Lihua Huang