Abstract
Craniopharyngiomas are rare intracranial tumors originating from the Rathke’s pouch, affecting the sellar and parasellar regions. Despite their benign nature, they cause significant morbidity and mortality due to their proximity to vital structures such as the optic pathways and the hypothalamic-pituitary axis, resulting in endocrine, visual, neurological impairment, and hypothalamic syndrome. Classified into adamantinomatous (ACP) and papillary (PCP), these tumors differ in epidemiology, histology, and pathophysiology. ACP, the most common type, presents a bimodal peak incidence between 5 and 15 years of age and 45 and 60 years of age, while PCP is more restricted to adults. Traditional treatments such as surgery and radiotherapy face significant challenges, including high recurrence rates. Intracystic chemotherapy is used in monocystic ACP but with limited efficacy and adverse effects related to toxicity. Recent advances in molecular biology have introduced targeted therapies, such as BRAF and MEK inhibitors, which show potential benefits in craniopharyngioma patients, particularly in the PCP. For ACP, however, therapeutic outcomes remain limited despite advances in molecular understanding, including mutations in the CTNNB1 gene and growth factors. Increasing investigation into the inflammatory microenvironment and immune response of these tumors presents new therapeutic possibilities and promising alternatives for tumor control, such as the use of anti-IL-6R, anti-VEGF agents and immune checkpoints inhibitors. This review aims to synthesize advancements in the pathophysiology of craniopharyngiomas and explore emerging therapeutic implications, focusing on precision medicine approaches for the management of this challenging disease.
1 Introduction
Craniopharyngiomas are rare intracranial epithelial tumors that develop from remnants of Rathke’s pouch, predominantly located in the sellar and parasellar regions (1, 2). Two main histological types are recognized: adamantinomatous craniopharyngioma (ACP) and papillary craniopharyngioma (PCP) (1). These tumors account for 1–3% of all primary intracranial tumors in adults and 5–10% of intracranial tumors in children, with an annual incidence ranging from 0.13 to 2 per 100,000 individuals and no gender predilection (2).
ACP is more common in children and young adults, displaying a bimodal age distribution (5–15 years and 45–60 years) and is frequently associated with somatic mutations in the CTNNB1 gene encoding β-catenin. In contrast, PCP predominantly occurs in adults, especially in the fifth and sixth decades of life, and is associated with BRAF V600E mutations (1, 3).
Although histologically classified as WHO low grade I benign tumors, craniopharyngiomas exhibit complex clinical behavior and significant morbidity and mortality (4). Treatment typically involves a multidisciplinary approach combining surgery and radiotherapy to control the tumor while preserving hypothalamic and pituitary functions. Total resection is preferred when feasible, but subtotal resection followed by radiotherapy is often used due to proximity to critical neurovascular structures, especially in cases of hypothalamic invasion (3, 5). While total resection can provide local control, it is associated with a high risk of morbidity, including hypothalamic and pituitary dysfunction (6). Subtotal resection with radiotherapy offers comparable control rates with fewer complications (7, 8). Various radiotherapy modalities are effective, but may cause side effects like visual deterioration and endocrinopathies, depending on tumor characteristics and patient factors (9, 10). The limited efficacy and considerable side effects of traditional therapies underscore the need for innovative strategies to improve therapeutic outcomes and mitigate adverse effects (11).
In light of the limitations of conventional therapeutic approaches, the use of advanced omics technologies – such as next-generation sequencing (NGS) and single-cell transcriptomics (scRNA-seq) – has emerged as a promising strategy to unravel the molecular complexity of craniopharyngiomas. These approaches provide novel insights for developing more effective and personalized therapies. By enabling the identification of specific molecular targets and stratification of patients based on unique molecular profiles, they hold the potential to optimize treatment strategies and improve clinical outcomes (12, 13).
Recent advances in molecular biology have provided new insights into the genetic basis of craniopharyngiomas. Notably, the discovery of the BRAF V600E mutation in PCP has enabled the development of targeted therapeutic interventions. The use of BRAF inhibitors, such as vemurafenib and dabrafenib, either alone or in combination with MEK inhibitors, has shown efficacy in specific cases. This represents a paradigm shift in treatment, as these therapies offer more precise and less invasive alternatives compared to traditional approaches (14, 15).
Targeted therapies offer multiple advantages in the management of craniopharyngiomas. Unlike conventional methods, which often harm surrounding healthy tissues, these therapies specifically inhibit molecular pathways driving tumor growth. Consequently, they promote significant reductions in tumor size and improved clinical outcomes while minimizing complications and preserving essential neurological and endocrine functions. This highlights their potential for achieving better long-term results (14). Understanding the genetic alterations underlying craniopharyngiomas enables a personalized approach, tailoring treatment to the molecular profile of each patient. Furthermore, positive responses in cases refractory to conventional therapies reinforce the potential of these interventions as rescue options, offering new hope to patients with limited therapeutic alternatives (14, 16, 17).
This review aims to provide a comprehensive analysis of the current understanding of craniopharyngioma pathophysiology, with a particular emphasis on targeted therapies. By compiling and synthesizing available evidence, it seeks to highlight targeted therapies evaluated in craniopharyngioma patients with a pathophysiological basis, identify knowledge gaps, address emerging challenges, and delineate areas requiring further research to guide future directions.
2 Papillary craniopharyngioma
PCPs account for approximately 10% of craniopharyngiomas and almost exclusively occur in adults, predominantly in the fifth and sixth decades of life, with a mean age of 44.7 years (18, 19). These tumors typically present as large masses in the suprasellar region, often located above a preserved infundibulum or in the infundibulo-tuberal region of the third ventricle`s floor. Clinically, patients may present with visual deficits, hormonal alterations, memory impairment, and symptoms related to intracranial hypertension (19).
Macroscopically, PCPs consist of solid or mixed round masses, containing yellowish viscous cysts and rare calcifications. Histologically, these tumors are composed of well-defined neoplastic epithelium with cauliflower-shaped papillary projections, without infiltration into adjacent brain tissue. The histological component of PCPs includes pseudopapillae of mature squamous epithelium and an anastomosing fibrovascular stroma with fine capillaries and scattered immune cells, such as macrophages and neutrophils (18, 20) (Figure 1).
Figure 1

(A) Papillary craniopharyngioma. Fibrovascular connective axes forming papillae lined by well-differentiated squamous epithelium with palisading (arrow). (B) Adamantinomatous Craniopharyngioma. Islands and cords of epithelial cells with palisading delineating the stroma composed of stellate reticulum cells (asterisk). Clusters of anucleated squamous cells (arrowhead) and foci of calcification (arrow).
Unlike ACPs, PCPs do not present palisading reticular cells, wet keratin, or collagenous whorls, which are rare and small when present (17). Distinguishing PCPs from other suprasellar masses, such as Rathke’s pouch cysts, can be challenging (1).
In recent years, the genetic characterization of PCPs has advanced significantly. In 2014, the BRAF V600E mutation was identified through exome sequencing in three PCP samples, later confirmed in 36 of 39 additional samples (21, 22). The BRAF V600E mutation constitutively activates the MAPK/ERK pathway, an oncogenic signaling pathway that promotes the proliferation of SOX2+ embryonic cells, transforming them into tumor-initiating cells and stimulating processes such as angiogenesis and apoptosis inhibition (23, 24) (Figure 2).
Figure 2

MAPK pathway with presence of BRAF mutation in the pathophysiology of papillary craniopharyngioma. RTK, Receptor tyrosine kinase; RAS, Rat Sarcoma; GDP, Guanosine Diphosphate; GTP, Guanosine Triphosphate; BRAF, B-Rapidly Accelerated Fibrosarcoma; MEK, Mitogen-Activated Protein Kinase; ERK, Extracellular Signal-Regulated Kinase.
The BRAF V600E mutation is identified in 81% to 100% of PCPs, serving as a genetic marker for this type (22, 24). Immunoreactivity for the VE1 antibody using immunohistochemistry confirms the presence of this mutation, while β-catenin remains localized to the cell membranes (21). This mutation is also observed in melanomas, and BRAF and MEK inhibitors such as vemurafenib, dabrafenib, and trametinib have revolutionized the treatment of these neoplasms, with promising results also for PCPs (25) (Figure 3).
Figure 3

Targeted therapies in the MAPK pathway with potential application in papillary craniopharyngiomas. BRAF, B-Rapidly Accelerated Fibrosarcoma; MEK, Mitogen-Activated Protein Kinase; ERK, Extracellular Signal-Regulated Kinase.
Recent transcriptomic studies, including scRNA-seq have revealed distinct gene expression profiles in PCPs, marked by overexpression of genes associated with the MAPK pathway and Rathke’s pouch development (22, 26). Furthermore, characterization of the tumor microenvironment has revealed an immunosuppressive landscape enriched in regulatory T cells (13). These insights support the use of targeted therapy with BRAF and MEK inhibitors, which have yielded superior response rates in recurrent or unresectable PCPs compared to conventional treatment modalities (27, 28).
Transcriptomic data also reinforce the notion that PCPs are a molecularly homogeneous entity, with lower intratumoral heterogeneity than ACPs (13, 21, 22). Moreover, signals originating from fibroblasts and activating immune responses in neutrophils have been reported, highlighting the critical role of the tumor immune microenvironment in disease pathophysiology and the development of targeted therapies (26).
Recent studies report significant responses to treatment with BRAF and MEK inhibitors in cases of PCPs with the BRAF V600E mutation. In one of the first reported cases, there was an 85% reduction in the solid portions and 81% reduction in the cystic portions of the tumor following treatment with MEK/BRAF inhibitors (29). In a series with six patients, treatment with dabrafenib, trametinib, or vemurafenib, either alone or in combination, resulted in a reduction of 80% to 91% of the tumor masses, allowing for subsequent surgical or radiotherapeutic interventions (25).
The efficacy of these therapies with BRAF and MEK inhibitors has been extensively studied. In a phase II study, 94% of patients treated with the combination of vemurafenib and cobimetinib achieved a durable partial response, with a median tumor volume reduction of 91%. Disease progression-free survival was 87% at 12 months and 58% at 24 months, indicating significant tumor control (30). Dabrafenib, in combination with trametinib, has shown promise as a treatment option for papillary craniopharyngiomas with the BRAFV600E mutation. Studies and case reports suggest that this combination may lead to significant reductions in tumor volume and improvement in clinical symptoms, such as visual deficits and neurological dysfunctions (27, 29, 31). In a cohort study, the combination of dabrafenib and trametinib resulted in a partial response or better in 94% of patients, with an average tumor volume reduction of up to 91.8% in some treatment groups (27). A systematic review highlighted that treatment response may range from 24% to 100% volumetric reduction, with near-complete response observed in many cases (28) (Table 1).
Table 1
| Agent | n | Duration | Mechanism | Efficacy | Adverse effects |
|---|---|---|---|---|---|
| Vemurafenib + Cobimetinib Brastianos et al., 2023 (30) | 16 | 8 cycles (28 days each) | BRAF and MEK Inhibition | Partial response in 94%, average tumor volume reduction of 91%, progression-free survival of 87% at 12 months and 58% at 24 months. | Rash, elevated creatine kinase, hyperglycemia |
| Dabrafenib + Trametinib Alcubierre et al., 2024 (27) | 16 | 7.6 ± 5.3 months | BRAF and MEK Inhibition | Tumor volume reduction of 73.3% to 91.8%. Improvement in neurological symptoms. | Low-grade fever, fatigue, cough, peripheral edema, skin rash, anemia, elevated liver enzymes, verrucous keratoses, hyperglycemia |
Targeted therapies for papillary craniopharyngioma (PCP).
These findings strengthen the potential of targeted therapies in the management of PCPs, especially in patients refractory to traditional treatments. The accurate identification of the genetic mutations involved not only facilitates differential diagnosis but also opens new perspectives for personalized and more effective therapeutic approaches (21).
3 Adamantinomatous craniopharyngioma
ACPs represent 90% of craniopharyngiomas and can affect individuals of any age, showing a bimodal distribution with peaks between 5–15 and 45–60 years (32). The mean age at diagnosis in children under 15 years is 8.8 years (1, 18). Studies have shown no significant differences between pediatric and adult ACPs (24, 33).
These tumors are commonly cystic, with calcifications and cholesterol-rich contents resembling “motor oil” (34). Their irregular margins, with palisading basal layers infiltrating into surrounding tissues in finger-like projections, are surrounded by intense gliosis, complicating surgical identification of planes (35). The heterogeneous tumor epithelium is juxtaposed with stellate reticulum and wet keratin nodules, composed of ghost and squamous cells, often associated with calcifications, cholesterol crystals, and hemosiderin deposits from chronic hemorrhage (1) (Figure 1).
In their pathophysiology, the Sonic Hedgehog (SHH) pathway is highly active, particularly in cell clusters and palisaded basal layers marked by Ki67 positivity (36, 37). The SHH pathway, linked to pituitary embryogenesis, appears to sustain tumor stem cells and promote tumor growth, infiltration, and angiogenesis via autocrine and paracrine mechanisms (38–40).
The pathogenesis of ACPs involves somatic mutations in the CTNNB1 gene, which encodes β-catenin (41). Normally, the Wnt pathway regulates crucial processes like growth and metabolism, with β-catenin localized to cell membranes (42). β-catenin also participates in adhesion complexes with E-cadherin, maintaining cellular architecture (43). Activating mutations in CTNNB1 are reported with prevalence rates ranging from 16% to 100%, depending on sequencing techniques (1, 17, 44). The heterogeneity in the prevalence of CTNNB1 mutations may also reflect the complex cellular composition of the tumor, which includes distinct neoplastic and non-neoplastic subpopulations such as senescent cells, tumor germ cells, and cells with different cytokeratin (CK) expression profiles (12).
Mutations in CTNNB1 affect exon 3, encoding β-catenin’s degradation complex, leading to aberrant nucleocytoplasmic accumulation in 96% of ACPs (39). This accumulation hyperactivates the Wnt pathway, critical for cell proliferation and pituitary embryogenesis, as evidenced by targets like AXIN2, LEF1, and BMP4 (37, 45). In ACPs, β-catenin accumulates in small whorled clusters or near infiltrative edges, acting as signaling centers for cell proliferation and differentiation (45, 46) (Figure 4).
Figure 4

The Wnt pathway and its role in tumorigenesis of adamantinomatous craniopharyngiomas. Wnt, Wingless-Integration site; Fz, Frizzled receptor; CK1, Casein Kinase 1; APC, Antigen-presenting Cell; AXIN, Axin protein.
CTNNB1 mutations are specific to ACPs and absent in other sellar region tumors, such as PCPs (17, 46, 47). However, simultaneous mutations in CTNNB1 and BRAF have been identified in tumors with mixed adamantinomatous and papillary features (48). Wnt pathway hyperactivation correlates with aggressive disease and poorer overall survival rates (49).
Further studies identified fibroblast growth factors (FGFs), bone morphogenetic proteins (BMPs), and transforming growth factor-β (TGF-β) as mediators of tumor growth in ACPs (39, 45). Activation of the epidermal growth factor receptor (EGFR) stabilizes β-catenin in tumor cells coexpressing fascin, an actin-binding protein linked to adhesion, migration, invasion, and cytoskeletal reorganization. Fascin expression correlates with invasive growth and poor prognosis in ACP patients (50).
Moreover, reports suggest MAPK/ERK pathway activation in ACPs, with FGF, EGF and ERK1/2 expression colocalized with Ki67 in palisaded epithelium. Ex vivo experiments on ACPs treated with the MEK inhibitor trametinib demonstrated increased apoptosis and reduced cell proliferation, highlighting the relevance of this pathway (51).
Markers like CK, particularly CK8 and CK18, are elevated in β-catenin-positive cells, indicating dedifferentiation and tumor progression. Claudin-1 (CLDN1), a tight junction component, is downregulated in β-catenin-positive clusters and finger-like projections, suggesting a role in invasiveness. Claudins also influence cyst formation through fluid accumulation from endothelial leakage, impairing cellular adhesion (52).
Inflammation plays a central role in ACP pathophysiology. Cholesterol crystals from cystic components can stimulate IL-1β secretion, triggering immune activation (53). Spatial transcriptomic analyses have demonstrated that this inflammatory response involves heterogeneous immune cell infiltration, including tumor-associated macrophages (TAMs) with both M1 and M2 polarization. These processes may be modulated by interactions between tumor cells and the lipid-rich microenvironment (13).
Treating ACPs remains challenging, with no clear consensus on the optimal therapeutic approach. Since β-catenin is critical for normal processes, nonspecific inhibitors could harm healthy tissues (54). Therapeutic approaches vary widely, reflecting the complexity of managing ACPs. Interferon-α (INF-α) shows mixed outcomes, ranging from complete response to disease progression (55, 56). Pegylated Interferon-α-2b (INF-peg-α-2b) appears more effective, with studies showing complete responses or significant cystic reduction (57, 58). Other inflammatory mediators like tocilizumab (anti-IL-6R) and bevacizumab (anti-VEGF) are promising, with reports of significant tumor volume reduction after recurrences (59–61). Understanding the role of the MEK/ERK pathway in ACPs may also enable therapeutic targeting. MEK inhibitor binimetinib reduced tumor volume by over 95% after 21 months of treatment (62) (Table 2).
Table 2
| Agent | n | Duration | Mechanism | Efficacy | Adverse effects |
|---|---|---|---|---|---|
| Interferon-α Kilday et al., 2017 (97) | 56 | 5,1 years | Immunomodulation | Varied response (ranging from complete response to disease progression) | Fever, chills, myalgias, hypotension, lethargy, nausea, vomiting, elevated transaminases, thrombocytopenia, seizures, hyperpigmentation, weight loss |
| Pegylated Interferon-α-2b Goldman et al., 2020 (58) | 19 | 108 weeks | Immunomodulation | Partial response in 28%. Median disease-free survival of 19.5 months. | Nausea, fever, constitutional symptoms, elevated transaminases, thrombocytopenia, fatigue, neutropenia |
| Tocilizumab Grob et al., 2019 (60) | 2 | 6 months | Anti-IL-6R | Reduction of cystic volume and disease stability | Neutropenia |
| Bevacizumab De Rosa et al., 2023 (61) | 1 | 6 weeks | Anti-VEGF | Partial response (66% tumor volume reduction after 3 months) | Not reported |
| Binimetinib Patel et al., 2021 (62) | 1 | 8 months | MEK Inhibition | Disease stability | Skin rash, nail dystrophy, hyponatremia, venous stasis, fatigue, daytime sleepiness, weight gain. |
Targeted therapies for adamantinomatous craniopharyngiomas (ACP).
Despite these advances, therapeutic responses remain highly variable. The molecular and cellular heterogeneity of ACPs – revealed through single-cell and spatial transcriptomic approaches – emphasizes the need for individualized treatments tailored to each tumor’s unique profile (63). Integrated multi-omics analyses encompassing genomic, transcriptomic, proteomic, and spatial data, combined with detailed microenvironment characterization, represent a promising strategy for identifying predictive biomarkers, stratifying patients, and developing more precise and effective therapies (12). A deeper understanding of the cellular and molecular crosstalk within the tumor microenvironment—including the roles of senescent cells, tumor germ cells, and macrophage polarization—may ultimately pave the way for innovative therapeutic approaches in ACP treatment (13, 64–67).
4 Inflammatory mediators and immune response
Inflammation plays a significant role in the pathophysiology of craniopharyngiomas, influencing both the biological behavior of the tumor and the clinical prognosis of patients (11, 51). Several inflammatory mediators, including interleukins (IL-6, IL-8, IL-10), tumor necrosis factor (TNF), and chemokines (CXCL12, CXCR4), are implicated in tumor progression and the pathological inflammatory microenvironment of these tumors (68). The presence of a pronounced inflammatory response correlates with a higher incidence of hypopituitarism and a lower likelihood of recurrence-free survival (68). Moreover, β-catenin, frequently mutated in adamantinomatous craniopharyngiomas (ACPs), not only participates in Wnt signaling but also interacts with inflammatory mediators, amplifying cytokine and chemokine production in the tumor microenvironment. This process establishes a vicious cycle wherein inflammation perpetuates cellular proliferation and fosters tumor recurrence (69).
4.1 Interleukins
Interleukins, such as IL-6 and IL-8, are often found in elevated levels within tumor tissues and are associated with the promotion of a pro-tumorigenic microenvironment (70). IL-6 is known for its ability to enhance tumor invasion and angiogenesis while mediating communication between tumor cells and immune cells, such as tumor-associated macrophages, which can increase tumor aggressiveness. This occurs via epithelial-mesenchymal transition (EMT), a process that boosts the migratory capacity of tumor cells, facilitating local invasion (71). Furthermore, elevated levels of IL-6 and its receptor (IL-6R) suggest that this signaling pathway could be a potential therapeutic target, as demonstrated in studies exploring the use of tocilizumab, a monoclonal antibody that blocks IL-6R, to reduce cystic volume in ACP patients (60).
IL-8 also plays a significant role in the tumor microenvironment of craniopharyngiomas, contributing to local invasion and adhesion to surrounding tissues (72). Studies indicate that IL-8, alongside other pro-inflammatory cytokines such as IL-6, is elevated in the plasma of patients and brain tumor tissues (68, 73). IL-8 is further recognized for its pro-tumorigenic functions across various cancers, including promoting angiogenesis, increasing cellular proliferation and survival, and facilitating cell migration (72). Although specific literature on craniopharyngiomas is limited, IL-8’s role in other tumors suggests that it may contribute to tumor progression through similar mechanisms by modulating the tumor microenvironment (68, 74).
Vascular Endothelial Growth Factor (VEGF), a key mediator of angiogenesis, is an essential component in craniopharyngioma pathophysiology, driving the formation of new blood vessels that supply the tumor with nutrients and oxygen (75). It is present in epithelial cells of both ACP and PCP, and microvascular density related to VEGF expression correlates with tumor recurrence, suggesting the prognostic value of angiogenesis extent. VEGF is also linked to cyst formation in craniopharyngiomas, with its expression appearing to correlate with tumor size (76). Regulated by β-catenin and other molecular pathways, VEGF interacts with matrix metalloproteinases (MMPs), such as MMP-9, to remodel the extracellular matrix and facilitate tumor invasion. This remodeling may be critical for craniopharyngioma progression. MMP-1, for instance, can enhance VEGF-2R expression on endothelial cells, promoting cellular proliferation and angiogenesis via intracellular signaling pathways (77). Evidence also suggests that MMPs may induce the production of the anti-apoptotic protein Bcl-2, which regulates tumor growth and recurrence through autocrine and paracrine mechanisms (78). Dysregulation of apoptosis-related genes, including members of the Bcl-2 family, seems to play a significant role in the pathogenesis of pituitary adenomas (79). Although the specific role of MMPs in craniopharyngiomas remains underexplored, their involvement in angiogenesis and VEGF modulation suggests they contribute to tumor pathogenesis.
The interplay between IL-6 and VEGF establishes a pathological axis that amplifies inflammation and vascularization in the tumor microenvironment. IL-6 can upregulate VEGF expression, creating a feedback loop that intensifies these processes (80). This interaction also contributes to resistance to conventional therapies, highlighting the need for integrated approaches to disrupt this cycle.
While studies on anti-IL-6 and anti-VEGF therapies in craniopharyngiomas remain limited, preliminary evidence suggests therapeutic potential. A study reported the combined use of tocilizumab (anti-IL-6R) and bevacizumab (anti-VEGF) in two patients with recurrent cystic ACPs, resulting in significant responses, including reduced cystic tumor burden and tumor stability, suggesting a viable therapeutic alternative (60).
Inflammatory mediators also play a significant role in PCPs, influencing both tumor invasiveness and the immune microenvironment. The use of preoperative inflammatory markers, such as neutrophil counts and the neutrophil-to-lymphocyte ratio, can aid in the differential diagnosis of PCPs from other sellar region tumors, with higher levels observed in PCPs. These tumors exhibit high immune infiltration but with low activity, attributed to extensive neutrophil infiltration that creates an inactive immune microenvironment (81). Furthermore, elevated IL-6 expression is positively correlated with PCP tumor invasion into the hypothalamus, suggesting that these inflammatory mediators could serve as potential therapeutic targets to prevent tumor invasion (82).
4.2 Chemokines
Chemokines from the CXC and CC families play significant roles in craniopharyngioma pathophysiology, particularly in ACP. The CXCL12/CXCR4 axis is specifically implicated in tumor progression. Overexpression of CXCL12 and CXCR4 in ACP promotes tumor cell proliferation, migration, and invasion, primarily via the activation of the PI3K/AKT signaling pathway (83, 84). This pathway is critical in regulating cell growth and survival, indicating that the CXCL12/CXCR4 axis contributes to tumor aggressiveness. These chemokines are also recognized for their capacity to influence the tumor microenvironment by modulating angiogenesis and immune responses, potentially affecting tumor progression (85). Although the impact of other CC and CXC chemokines on craniopharyngiomas is less studied, their roles in other cancers suggest similar contributions to tumor growth and invasion (86).
4.3 Immune checkpoint
Immune checkpoints are essential molecules and pathways in the immune system that regulate immune responses, preventing them from becoming destructive to the body’s healthy cells. These include inhibitory receptors and ligands that play a crucial role in modulating T-cell activation and function. Under normal conditions, these checkpoints help maintain immune tolerance and prevent collateral damage during antimicrobial immune responses (87). In the context of cancer, tumor cells can exploit these checkpoints to evade immune destruction. This occurs when proteins on the surface of T-cells, known as immune checkpoint proteins, recognize and bind to partner proteins on cancer cells, sending inhibitory signals that suppress the immune response (88). Therapies that block these checkpoints, known as immune checkpoint inhibitors, have shown significant efficacy in restoring T-cell capability to attack tumor cells, leading to robust tumor regressions (89).
Immune checkpoint inhibitors have demonstrated significant potential in the treatment of various cancer types. Blocking programmed cell death protein 1 (PD-1) and its ligand (PD-L1) with agents such as nivolumab and pembrolizumab has improved survival in cases of melanoma and non-small cell lung adenocarcinoma (90, 91). The availability of these agents, combined with their relatively favorable side effect profiles, has prompted numerous studies investigating their efficacy across various tumor types.
Recent studies have demonstrated PD-L1 expression in the epithelial cells lining cysts and intrinsic PD-1 expression in cell clusters in ACPs with β-catenin overexpression. These clusters play a central role in tumor growth in ACPs through various mechanisms, making PD-1 targeting a promising therapy (92, 93). Two more studies also demonstrated elevated PD-L1 expression in ACPs, revealing this as a potential therapeutic target in craniopharyngiomas (94, 95).
5 Ongoing clinical trials
In recent years, the multi-omic characterization of craniopharyngiomas has provided significant insights into their pathogenesis, driving clinical trials aimed at developing more personalized and lower-risk therapeutic approaches. Ongoing studies with binimetinib (NCT05286788), tocilizumab (NCT05233397), and nivolumab (NCT05465174) for ACP as well as vemurafenib and cobimetinib (NCT03224767) for the PCP, offer promising therapeutic alternatives and may help define optimal patient profiles (96) (Figure 5) (Table 3). However, some challenges still remain regarding the optimal timing for introducing these treatments—whether as neoadjuvant therapy to reduce tumor volume before surgery or as adjuvant therapy to minimize recurrence and improve disease control—as well as the appropriate duration of treatment. Furthermore, uncertainties persist regarding their use as monotherapy, in combination with other agents, or in conjunction with surgery and radiotherapy, underscoring the need for further studies to refine the management of these rare tumors.
Figure 5
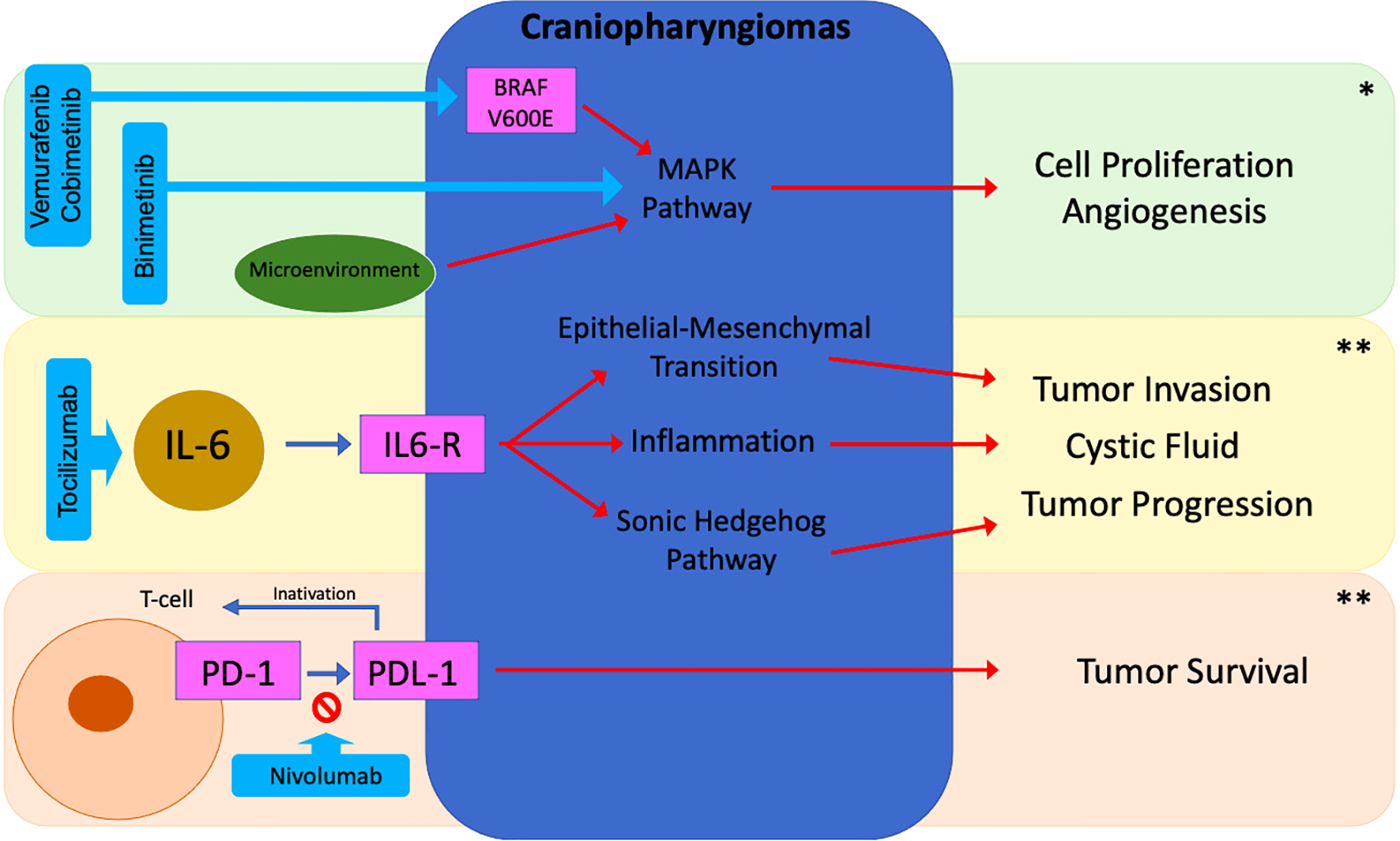
Potential targeted therapies in craniopharyngiomas. *ACP/PCP; **ACP; BRAF, B-Rapidly Accelerated Fibrosarcoma; IL-6, Interleukin 6; PD-1, Programmed Cell Death Protein 1; PD-L1, Programmed Cell Death Protein Ligand 1.
Table 3
| Inflammatory and Immune Target | Mechanism | Therapeutic Potential |
|---|---|---|
| IL-6 (60) | Tumor invasion, angiogenesis, immune cell/tumor communication, and epithelial-to-mesenchymal transition (EMT). | Anti-IL-6R monoclonal antibodies (e.g., tocilizumab) reduce tumor invasion and cystic volume. |
| IL-8 (68) | Angiogenesis, cell proliferation, migration, and cell adhesion. | Blocking IL-8-related pathways to reduce local invasion and angiogenesis. |
| VEGF (61) | Angiogenesis and cyst formation; interacts with MMPs for extracellular matrix remodeling. | VEGF inhibitors (e.g., bevacizumab) to reduce angiogenesis and tumor growth. |
| CXCL12/CXCR4 (83, 84) | Tumor progression, migration, invasion, and angiogenesis through activation of CXCR4 signaling pathways. | Limit tumor progression and invasion. |
| PD-1/PD-L1 (87) | Inhibits the immune response, allowing tumor evasion. | Immune checkpoint inhibitors (e.g., nivolumab, pembrolizumab) to reactivate the immune system against the tumor. |
Targeted therapies for inflammatory mediators and for immune response.
6 Conclusion
Recent advances in understanding the pathophysiology of craniopharyngiomas provide a promising foundation for developing more effective therapeutic strategies. Given the limitations of conventional approaches, targeted therapies, such as BRAF and MEK inhibitors for the PCP, have shown encouraging results. Recently, the growing understanding of the inflammatory behavior and immune response of these tumors has highlighted the therapeutic potential of anti-IL-6R and anti-VEGF agents and immune checkpoints inhibitors, signaling a promising future for the application of precision medicine in this field in both, APC and PCP.
Significant gaps still remain, particularly in managing more aggressive or resistant tumors. Robust clinical trials and interinstitutional collaborations are crucial to validate these therapies on a larger scale and standardize treatment protocols. Additionally, identifying predictive biomarkers and elucidating molecular interactions within the tumor microenvironment may offer new therapeutic opportunities, improve prognostic outcomes, and reduce the morbidity and mortality associated with craniopharyngiomas.
Statements
Author contributions
CP: Conceptualization, Writing – original draft, Writing – review & editing. GN: Writing – original draft, Writing – review & editing. SD: Writing – original draft, Writing – review & editing. MF: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by CNPq (Conselho Nacional de Pesquisa – Finance code 406304/2024-6) and CAPES (Coordenacao de Aperfeiçoamento de Pessoal de Nível Superior - Finance code 001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
MüllerHLMerchantTEWarmuth-MetzMMartinez-BarberaJPPugetS. Craniopharyngioma. Nat Rev Dis Primer. (2019) 5:75. doi: 10.1038/s41572-019-0125-9
2
AlboqamiMNKhalid S AlbaiahyABukhariBHAlkhaibaryAAlharbiAKhairySet al. Craniopharyngioma: A comprehensive review of the clinical presentation, radiological findings, management, and future Perspective. Heliyo. (2024) 10. doi: 10.1016/j.heliyon.2024.e32112
3
PiloniMGagliardiFBailoMLosaMBoariNSpinaAet al. Craniopharyngioma in pediatrics and adults. Adv Exp Med Bio. (2023) 1405:299–329. doi: 10.1007/978-3-031-23705-8_11
4
LouisDNPerryAWesselingPBratDJCreeIAFigarella-BrangerDet al. The 2021 WHO classification of tumors of the Central Nervous System: A summary. Neuro Onco. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
5
HendersonFSchwartzTH. Update on management of craniopharyngiomas. J Neuroonco. (2022) 156:97–108. doi: 10.1007/s11060-021-03906-4
6
MüllerHL. Consequences of craniopharyngioma surgery in children. J Clin Endocrinol Meta. (2011) 96:1981–91. doi: 10.1210/jc.2011-0174
7
PoisetSJSongAIn YoonHHuangJJainSPalmerJDet al. Long-term outcomes of surgery and radiation treatment for adult patients with craniopharyngioma. World Neurosur. (2024) 187:e852–9. doi: 10.1016/j.wneu.2024.04.177
8
KhriguianJTolbaMKhosrow-KhavarFKordlouieSGuiotMCAbdulkarimBet al. Is postoperative radiotherapy needed in the management of adult craniopharyngiomas? Can J Neurol Sc. (2023) 50:428–34. doi: 10.1017/cjn.2022.28
9
HararyPMRajaramSHoriYSParkDJChangSD. The spectrum of radiation therapy options for craniopharyngioma: a systematic review. J Neuroonco. (2025). doi: 10.1007/s11060-025-05001-4
10
RutenbergMSHoltzmanALIndelicatoDJHuhSRaoDFiesterPJet al. Disease control after radiotherapy for adult craniopharyngioma: Clinical outcomes from a large single-institution series. J Neuroonco. (2022) 157:425–33. doi: 10.1007/s11060-022-03983-z
11
WhelanRHengartnerAFolzenlogenZPrinceEHankinsonTC. Adamantinomatous craniopharyngioma in the molecular age and the potential of targeted therapies: a review. Childs Nerv Sys. (2020) 36:1635–42. doi: 10.1007/s00381-020-04677-5
12
AnWLiSAnYLinZ. Molecular subtypes of adamantinomatous craniopharyngiomas. Neuro Onco. (2025). doi: 10.1093/neuonc/noaf030
13
MatsudaTKonoTTakiYSakumaIFujimotoMHashimotoNet al. Deciphering craniopharyngioma subtypes: Single-cell analysis of tumor microenvironment and immune networks. iScienc. (2024) 27:111068. doi: 10.1016/j.isci.2024.111068
14
ReyesMTaghvaeiMYuSSatheACollopySPrashantGNet al. Targeted therapy in the management of modern craniopharyngiomas. Front Biosci (Landmark Ed. (2022) 27:136. doi: 10.31083/j.fbl2704136
15
CalvaneseFJacquessonTManetRVasiljevicALasolleHDucrayFet al. Neoadjuvant B-RAF and MEK inhibitor targeted therapy for adult Papillary Craniopharyngiomas: A new treatment paradigm. Front Endocrinol (Lausanne. (2022) 13:882381. doi: 10.3389/fendo.2022.882381
16
HengartnerACPrinceEVijmasiTHankinsonTC. Adamantinomatous craniopharyngioma: moving toward targeted therapies. Neurosurg Focu. (2020) 48:E7. doi: 10.3171/2019.10.FOCUS19705
17
LarkinSKaravitakiN. Recent advances in molecular pathology of craniopharyngioma. F1000Re. (2017) 6:1202. doi: 10.12688/f1000research.11549.1
18
KaravitakiNWassJAH. Craniopharyngiomas. Endocrinol Metab Clin North A. (2008) 37:173–93,ix–x. doi: 10.1016/j.ecl.2007.10.012
19
JannelliGCalvaneseFPaunLRaverotGJouanneauE. Current advances in papillary craniopharyngioma: State-of-the-art therapies and overview of the literature. Brain Sc. (2023) 13. doi: 10.3390/brainsci13030515
20
LarkinSJAnsorgeO. Pathology and pathogenesis of craniopharyngiomas. Pituitar. (2013) 16:9–17. doi: 10.1007/s11102-012-0418-4
21
BrastianosPKSantagataS. ENDOCRINE TUMORS: BRAF V600E mutations in papillary craniopharyngioma. Eur J Endocrino. (2016) 174:R139–44. doi: 10.1530/EJE-15-0957
22
BrastianosPKTaylor-WeinerAManleyPEJonesRTDias-SantagataDThornerARet al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Gene. (2014) 46:161–5. doi: 10.1038/ng.2868
23
HastonSPozziSCarrenoGManshaeiSPanousopoulosLGonzalez-MeljemJMet al. MAPK pathway control of stem cell proliferation and differentiation in the embryonic pituitary provides insights into the pathogenesis of papillary craniopharyngioma. Developmen. (2017) 144:2141–52. doi: 10.1242/dev.150490
24
HölskenASillMMerkleJSchweizerLBuchfelderMFlitschJet al. Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol Commu. (2016) 4:20. doi: 10.1186/s40478-016-0287-6
25
JuratliTAJonesPSWangNSubramanianMAylwinSJBOdiaYet al. Targeted treatment of papillary craniopharyngiomas harboring BRAF V600E mutations. Cance. (2019) 125:2910–4. doi: 10.1002/cncr.32197
26
ZhuXJiaYZhaoZZhangXZhaoYGuiSet al. Cell signaling communication within papillary craniopharyngioma revealed by an integrated analysis of single-cell RNA sequencing and bulk RNA sequencing. J Transl Me. (2025) 23:124. doi: 10.1186/s12967-025-06149-3
27
De AlcubierreDGkasdarisGMordrelMJoncourABrietCAlmairacFet al. BRAF and MEK inhibitor targeted therapy in papillary craniopharyngiomas: a cohort study. Eur J Endocrino. (2024) 191:251–61. doi: 10.1093/ejendo/lvae091
28
CossuGRamsayDSCDanielRTEl CadhiAKerherveLMorlaixEet al. Update on neoadjuvant and adjuvant BRAF inhibitors in papillary craniopharyngioma: A systematic review. Cancers (Basel. (2024) 16. doi: 10.3390/cancers16203479
29
BrastianosPKShankarGMGillCMTaylor-WeinerANayyarNPankaDJet al. Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Ins. (2016) 108:djv310. doi: 10.1093/jnci/djv310
30
BrastianosPKTwohyEGeyerSGerstnerERKaufmannTJTabriziSet al. BRAF–MEK inhibition in newly diagnosed papillary Craniopharyngiomas. New Engl J Medicin. (2023) 389:118–26. doi: 10.1056/NEJMoa2213329
31
FasanoMDella CorteCMCaterinoMPirozziMRausoRTroianiTet al. Dramatic therapeutic response to dabrafenib plus trametinib in BRAF V600E mutated papillary craniopharyngiomas: A case report and literature review. Front Med (Lausann. (2021) 8:652005. doi: 10.3389/fmed.2021.652005
32
MominAARecinosMACioffiGPatilNSoniPAlmeidaJPet al. Descriptive epidemiology of craniopharyngiomas in the United States. Pituitar. (2021) 24:517–22. doi: 10.1007/s11102-021-01127-6
33
PrinceEWhelanRDonsonAStaulcupSHengartnerAVijmasiTet al. Transcriptional analyses of adult and pediatric adamantinomatous craniopharyngioma reveals similar expression signatures regarding potential therapeutic targets. Acta Neuropathol Commu. (2020) 8:68. doi: 10.1186/s40478-020-00939-0
34
PetitoCKDeGirolamiUEarleKM. Craniopharyngiomas: a clinical and pathological review. Cance. (1976) 37:1944–52. doi: 10.1002/1097-0142(197604)37:4<1944::aid-cncr2820370446>3.0.co
35
PrabhuVCBrownHG. The pathogenesis of craniopharyngiomas. Childs Nerv Sys. (2005) 21:622–7. doi: 10.1007/s00381-005-1190-9
36
GomesDCJamraSALealLFColliLMCampaniniMLOliveiraRSet al. Sonic Hedgehog pathway is upregulated in adamantinomatous craniopharyngiomas. Eur J Endocrino. (2015) 172:603–8. Available at: https://www.embase.com/search/results?subaction=viewrecord&id=L604241794&from=export (Accessed January 5, 2025).
37
Gaston-MassuetCAndoniadouCLSignoreMJayakodySACharolidiNKyeyuneRet al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A. (2011) 108:11482–7. doi: 10.1073/pnas.1101553108
38
CarrenoGBoultJKRAppsJGonzalez-MeljemJMHastonSGuihoRet al. SHH pathway inhibition is protumourigenic in adamantinomatous craniopharyngioma. Endocr Relat Cancer. (2019) 26:355–66. doi: 10.1530/ERC-18-0538
39
MüllerHLMartinez-BarberaJP. Adamantinomatous craniopharyngioma: genomics, radiologic findings, clinical, and prognosis. Contemp Endocrinology. (2019), 41–70. Available at: https://www.embase.com/search/results?subaction=viewrecord&id=L627371563&from=export (Accessed January 5, 2025).
40
Garcia-LavandeiraMSaezCDiaz-RodriguezEPerez-RomeroSSenraADieguezCet al. Craniopharyngiomas express embryonic stem cell markers (SOX2, OCT4, KLF4, and SOX9) as pituitary stem cells but do not coexpress RET/GFRA3 receptors. J Clin Endocrinol Meta. (2012) 97. doi: 10.1210/jc.2011-2187
41
CampaniniMLColliLMPaixaoBMCCabralTPFAmaralFCMaChadoHRet al. CTNNB1 gene mutations, pituitary transcription factors, and microRNA expression involvement in the pathogenesis of adamantinomatous craniopharyngiomas. Horm Cancer. (2010) 1:187–96. Available at: https://www.embase.com/search/results?subaction=viewrecord&id=L51134991&from=export (Accessed January 5, 2025).
42
PredaVLarkinSJKaravitakiNAnsorgeOGrossmanAB. The wnt signalling cascade and the adherens junction complex in craniopharyngioma tumorigenesis. Endocr Patho. (2015) 26:1–8. doi: 10.1007/s12022-014-9341-8
43
HuberAHWeisWI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. (2001) 105:391–402. doi: 10.1016/s0092-8674(01)00330-0
44
AppsJRStacheCGonzalez-MeljemJMGutteridgeAChalkerJJacquesTSet al. CTNNB1 mutations are clonal in adamantinomatous craniopharyngioma. Neuropathol Appl Neurobio. (2020) 46:510–4. doi: 10.1111/nan.12613
45
AndoniadouCLGaston-MassuetCReddyRSchneiderRPBlascoMALe TissierPet al. Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol. (2012) 124:259–71. doi: 10.1007/s00401-012-0957-9
46
Martinez-BarberaJP. Molecular and cellular pathogenesis of adamantinomatous craniopharyngioma. Neuropathol Appl Neurobio. (2015) 41:721–32. doi: 10.1111/nan.12226
47
BusleiRNoldeMHofmannBMeissnerSEyupogluIYSiebzehnrüblFet al. Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol. (2005) 109:589–97. doi: 10.1007/s00401-005-1004-x
48
PrietoRPascualJM. Can tissue biomarkers reliably predict the biological behavior of craniopharyngiomas? A comprehensive overview. Pituitary. (2018) 21:431–42. doi: 10.1007/s11102-018-0890-6
49
LiZXuJHuangSYouC. Aberrant membranous expression of β-catenin predicts poor prognosis in patients with craniopharyngioma. Ann Diagn Patho. (2015) 19:403–8. doi: 10.1016/j.anndiagpath.2015.10.002
50
HölskenAGebhardtMBuchfelderMFahlbuschRBlümckeIBusleiR. EGFR signaling regulates tumor cell migration in craniopharyngiomas. Clin Cancer Re. (2011) 17:4367–77. doi: 10.1158/1078-0432.CCR-10-2811
51
AppsJRCarrenoGGonzalez-MeljemJMHastonSGuihoRCooperJEet al. Tumour compartment transcriptomics demonstrates the activation of inflammatory and odontogenic programmes in human adamantinomatous craniopharyngioma and identifies the MAPK/ERK pathway as a novel therapeutic target. Acta Neuropathol. (2018) 135:757–77. doi: 10.1007/s00401-018-1830-2
52
StacheCHölskenAFahlbuschRFlitschJSchlafferSMBuchfelderMet al. Tight junction protein claudin-1 is differentially expressed in craniopharyngioma subtypes and indicates invasive tumor growth. Neuro Onco. (2014) 16:256–64. doi: 10.1093/neuonc/not195
53
BurghausSHölskenABuchfelderMFahlbuschRRiedererBMHansVet al. A tumor-specific cellular environment at the brain invasion border of adamantinomatous craniopharyngiomas. Virchows Archiv. (2010) 456:287–300. doi: 10.1007/s00428-009-0873-0
54
de Pellegars-MalhortieAPicque LasorsaLMazardTGranierFPrévostelC. Why is Wnt/β-catenin not yet targeted in routine cancer care? Pharmaceuticals (Basel). (2024) 17:949. doi: 10.3390/ph17070949
55
AgostiEZeppieriMAntoniettiSPiazzaAIusTFontanellaMMet al. Advancing craniopharyngioma management: A systematic review of current targeted therapies and future perspectives. Int J Mol Sc. (2024) 25. doi: 10.3390/ijms25020723
56
JakackiRICohenBHJamisonCMathewsVPArensonELongeeDCet al. Phase II evaluation of interferon-alpha-2a for progressive or recurrent craniopharyngiomas. J Neurosurg. (2000) 92:255–60. Available at: https://thejns.org/view/journals/j-neurosurg/92/2/article-p255.xml (Accessed January 5, 2025).
57
YeungJTPollackIFPanigrahyAJakackiRI. Pegylated interferon-α-2b for children with recurrent craniopharyngioma. J Neurosurg Pediat. (2012) 10:498–503. Available at: https://thejns.org/pediatrics/view/journals/j-neurosurg-pediatr/10/6/article-p498.xml (Accessed January 5, 2025).
58
GoldmanSPollackIFJakackiRIBillupsCAPoussaintTYAdesinaAMet al. Phase II study of peginterferon alpha-2b for patients with unresectable or recurrent craniopharyngiomas: a Pediatric Brain Tumor Consortium report. Neuro Onco. (2020) 22:1696–704. doi: 10.1093/neuonc/noaa119
59
de Vos-KerkhofEBuisDRLequinMHBennebroekCAAronicaEHullemanEet al. Tocilizumab for the fifth progression of cystic childhood craniopharyngioma-a case report. Front Endocrinol (Lausanne). (2023) 14:1225734. doi: 10.3389/fendo.2023.1225734
60
GrobSMirskyDMDonsonAMDahlNForemanNKHoffmanLMet al. Targeting IL-6 is a potential treatment for primary cystic craniopharyngioma. Front Onco. (2019) 9:791. doi: 10.3389/fonc.2019.00791
61
De RosaACalvaneseFDucrayFVasiljevicAManetRRaverotGet al. First evidence of anti-VEGF efficacy in an adult case of adamantinomatous craniopharyngioma: Case report and illustrative review. Ann Endocrinol (Paris). (2023) 84:727–33. doi: 10.1016/j.ando.2023.10.003
62
PatelKAllenJZagzagDWisoffJRadmaneshAGindinTet al. Radiologic response to MEK inhibition in a patient with a WNT-activated craniopharyngioma. Pediatr Blood Cancer. (2021) 68:e28753. doi: 10.1002/pbc.28753
63
JiangYYangJLiangRZanXFanRShanBet al. Single-cell RNA sequencing highlights intratumor heterogeneity and intercellular network featured in adamantinomatous craniopharyngioma. Sci Ad. (2023) 9:eadc8933. doi: 10.1126/sciadv.adc8933
64
ChenYLiuXAiniwanYLiMPanJChenYet al. Axl as a potential therapeutic target for adamantinomatous craniopharyngiomas: Based on single nucleus RNA-seq and spatial transcriptome profiling. Cancer Let. (2024) 592:216905. doi: 10.1016/j.canlet.2024.216905
65
PrinceEWAppsJRJeangJCheeKMedlinSJacksonEMet al. Unraveling the complexity of the senescence-associated secretory phenotype in adamantinomatous craniopharyngioma using multimodal machine learning analysis. Neuro Onco. (2024) 26:1109–23. doi: 10.1093/neuonc/noae015
66
XuCWuJYeJSiYZhangJWuBet al. Multiomics integration-based immunological characterizations of adamantinomatous craniopharyngioma in relation to keratinization. Cell Death Di. (2024) 15:439. doi: 10.1038/s41419-024-06840-1
67
WangXLinJLiuHZhaoCTuZXuDet al. Single-cell and spatial sequencing identifies senescent and germinal tumor cells in adamantinomatous craniopharyngiomas. Cell Biosc. (2024) 14:112. doi: 10.1186/s13578-024-01299-1
68
PengJYangLPanJWangCNieJLiuYet al. Clinical features and prognosis of pediatric infradiaphragmatic craniopharyngioma relative to the tumor inflammatory response. Pediatr Re. (2021) 89:1119–25. doi: 10.1038/s41390-020-1013-4
69
DonsonAMAppsJGriesingerAMAmaniVWittDAAndersonRCEet al. Molecular analyses reveal inflammatory mediators in the solid component and cyst fluid of human adamantinomatous craniopharyngioma. J Neuropathol Exp Neuro. (2017) 76:779–88. doi: 10.1093/jnen/nlx061
70
QuXTangYHuaS. Immunological approaches towards cancer and inflammation: A cross talk. Front Immunol. (2018) 9:563. doi: 10.3389/fimmu.2018.00563
71
ZhouJZhangCPanJChenLQiST. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human adamantinomatous craniopharyngioma cells and promotes tumor cell migration. Mol Med Re. (2017) 15:4123–31. doi: 10.3892/mmr.2017.6538
72
FousekKHornLAPalenaC. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol The. (2021) 219:107692. doi: 10.1016/j.pharmthera.2020.107692
73
AlbulescuRCodriciEPopescuIDMihaiSNeculaLGPetrescuDet al. Cytokine patterns in brain tumour progression. Mediators Inflam. (2013) 2013:979748. doi: 10.1155/2013/979748
74
ZhaoHWuLYanGChenYZhouMWuYet al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target The. (2021) 6:263. doi: 10.1038/s41392-021-00658-5
75
VidalSKovacsKLloydRVMeyerFBScheithauerBW. Angiogenesis in patients with craniopharyngiomas: correlation with treatment and outcome. Cancer. (2002) 94:738–45. doi: 10.1002/cncr.10281
76
VaqueroJZuritaMde OyaSCocaSMoralesCSalasC. Expression of vascular permeability factor in craniopharyngioma. J Neurosurg. (1999) 91:831–4. doi: 10.3171/jns.1999.91.5.0831
77
MazorRAlsaighTShakedHAltshulerAEPocockESKistlerEBet al. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J Biol Che. (2013) 288:598–607. doi: 10.1074/jbc.M112.417451
78
YuCFChenFHLuMHHongJHChiangCS. Dual roles of tumour cells-derived matrix metalloproteinase 2 on brain tumour growth and invasion. Br J Cancer. (2017) 117:1828–36. doi: 10.1038/bjc.2017.362
79
FacundoANMagalhãesMNascimentoGCAzulayRSSantosRMFreitasLAet al. The expression of VDACs and Bcl2 family genes in pituitary adenomas: clinical correlations and postsurgical outcomes. Front Endocrinol (Lausanne). (2024) 15:1481050. doi: 10.3389/fendo.2024.1481050
80
LoefflerSFayardBWeisJWeissenbergerJ. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer J Int du cancer. (2005) 115:202–13. doi: 10.1002/ijc.20871
81
ChenMZhengSHYangMChenZHLiST. The diagnostic value of preoperative inflammatory markers in craniopharyngioma: a multicenter cohort study. J Neurooncol. (2018) 138:113–22. doi: 10.1007/s11060-018-2776-x
82
JiaYMaLCaiKZhangBWuWXiaoYet al. Immune infiltration in aggressive papillary craniopharyngioma: High infiltration but low action. Front Immuno. (2022) 13:995655. doi: 10.3389/fimmu.2022.995655
83
YinXLiuZZhuPWangYRenQChenHet al. CXCL12/CXCR4 promotes proliferation, migration, and invasion of adamantinomatous craniopharyngiomas via PI3K/AKT signal pathway. J Cell Bioche. (2019) 120:9724–36. doi: 10.1002/jcb.28253
84
GongJZhangHLXingSSLiCDMaZYJiaGet al. High expression levels of CXCL12 and CXCR4 predict recurrence of adamantinomatous craniopharyngiomas in children. Cancer Biomarkers. (2014) 14:241–51. doi: 10.3233/CBM-140397
85
KeeleyECMehradBStrieterRM. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp Cell Re. (2011) 317:685–90. doi: 10.1016/j.yexcr.2010.10.020
86
BikfalviABillottetC. The CC and CXC chemokines: major regulators of tumor progression and the tumor microenvironment. Am J Physiol Cell Physio. (2020) 318:C542–54. doi: 10.1152/ajpcell.00378.2019
87
BandayAHAbdallaM. Immune checkpoint inhibitors: Recent clinical advances and future prospects. Curr Med Che. (2023) 30:3215–37. doi: 10.2174/0929867329666220819115849
88
QiuJChengZJiangZGanLZhangZXieZ. Immunomodulatory precision: A narrative review exploring the critical role of immune checkpoint inhibitors in cancer treatment. Int J Mol Sc. (2024) 25:5490. doi: 10.3390/ijms25105490
89
RibasAWolchokJD. Cancer immunotherapy using checkpoint blockade. Science. (2018) 359:1350–5. doi: 10.1126/science.aar4060
90
GaronEBRizviNAHuiRLeighlNBalmanoukianASEderJPet al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Me. (2015) 372:2018–28. doi: 10.1056/nejmoa1501824
91
RobertCLongGVBradyBDutriauxCMaioMMortierLet al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Me. (2015) 372:320–30. doi: 10.1056/NEJMoa1412082
92
GoschzikTGessiMDreschmannVGebhardtUWangLYamaguchiSet al. Genomic alterations of adamantinomatous and papillary craniopharyngioma. J Neuropathol Exp Neuro. (2017) 76:126–34. doi: 10.1093/jnen/nlw116
93
Martinez-BarberaJPAndoniadouCL. Concise review: Paracrine role of stem cells in pituitary tumors: A focus on adamantinomatous craniopharyngioma. Stem Cell. (2016) 34:268–76. doi: 10.1002/stem.2267
94
CoySRashidRLinJRDuZDonsonAMHankinsonTCet al. Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway vulnerabilities in craniopharyngioma. Neuro Onco. (2018) 20:1101–12. doi: 10.1093/neuonc/noy035
95
TopalianSLHodiFSBrahmerJRGettingerSNSmithDCMcDermottDFet al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Me. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
96
JoshiNMuellerSKlineC. Current clinical trials for craniopharyngiomas: what’s on the horizon? J Neuroonco. (2025) 172:281–8. doi: 10.1007/s11060-024-04899-6
97
KildayJPCaldarelliMMassimiLChenRHHLeeYYLiangMLet al. Intracystic interferon-alpha in pediatric craniopharyngioma patients: an international multicenter assessment on behalf of SIOPE and ISPN. Neuro Onco. (2017) 19:1398–407. doi: 10.1093/neuonc/nox056
Summary
Keywords
craniopharyngioma, adamantinomatous craniopharyngioma, papillary craniopharyngioma, target therapies, precision medicine
Citation
Pires de Oliveira Neto C, Nascimento GC, Damianse SdSP and Faria MdS (2025) Recent advances in craniopharyngioma pathophysiology and emerging therapeutic approaches. Front. Endocrinol. 16:1562942. doi: 10.3389/fendo.2025.1562942
Received
18 January 2025
Accepted
28 April 2025
Published
13 May 2025
Volume
16 - 2025
Edited by
Andrea Glezer, University of São Paulo, Brazil
Reviewed by
Simona Galoiu, Carol Davila University of Medicine and Pharmacy, Romania
Clarissa Silva Martins, Federal University of Mato Grosso do Sul, Brazil
Updates
Copyright
© 2025 Pires de Oliveira Neto, Nascimento, Damianse and Faria.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manuel dos Santos Faria, mfaria1949@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.