Abstract
Objective:
This study aimed to compare the prognosis of unilateral papillary thyroid carcinoma(PTC) patients with ipsilateral cervical lymph node metastasis(IC-LNM) under the treatment of unilateral lobectomy(uLT) vs total thyroidectomy(TT) in order to find out the optimal surgery for these patients without other clinical risk characteristics.
Methods:
PTC patients at Zhejiang Cancer Hospital between 2012 and 2022 were retrospectively reviewed. Additionally, a propensity score matching(PSM) was performed on patients treated with uLT or TT. Recurrence-free survival(RFS), overall survival(OS), hospitalization costs, postoperative complications, and other clinical characteristics were analyzed between the two groups.
Results:
Ultimately, 682 unilateral PTC patients with IC-LNM were available in the study. After PSM with possible prognostic factors(such as gender, age, primary tumor size, multifocality, extrathyroidal invasion, and T-stage), 225 pairs of patients were available. With a median of 81(5-154) months follow-up, 22 patients(9.8%) in the uLT and 12(5.3%) in the TT recurred. There were no significant differences in 5-year RFS and 5-year OS between uLT and TT groups. However, TT group was significantly correlated with higher risk of transient and permanent hypoparathyroidism, higher levothyroxine doses, longer hospital stays, and higher hospitalization costs than uLT group(p<0.05).
Conclusions:
Our study indicated that there were no differences in recurrence and survival between unilateral PTC patients with IC-LNM treated with uLT or TT for the primary tumor. However, uLT group had a lower risk of postoperative complications and a lower hospitalization cost than TT group. Thus, for selected unilateral PTC patients with IC-LNM without other risk features, uLT could be recommended.
Introduction
Papillary thyroid carcinoma (PTC) accounts for more than 90% of all thyroid cancer cases, and its incidence has increased rapidly in recent years (1, 2). Compared with other malignant tumors, most PTCs have a relatively favorable prognosis, so that the treatment is different from that of other tumors (3, 4). PTC patients commonly have cervical lymph node metastasis, especially central lymph node metastasis, which can reach 20%-50% (5–8). However, the relationship between lymph node metastasis and the prognosis of PTC is still controversial (9–11). Lateral cervical lymph node metastasis is relatively rare compared to central lymph node metastasis, but some studies have shown that lateral cervical lymph node metastasis is associated with increased mortality and recurrence (12–15).
The 2015 American Thyroid Association (ATA) Guidelines recommend near-total or total thyroidectomy (TT) for PTC patients with lateral cervical lymph node metastasis (16). However, TT might increase the risks of recurrent laryngeal nerve injury and transient or permanent hypoparathyroidism (17), while unilateral lobectomy (uLT) could reduce the doses and side effects of postoperative levothyroxine (L-T4) intake, and after uLT, remnant thyroid tissue could produce triiodothyronine (T3) to maintain thyroid hormone balance (18). There were a few studies comparing the prognosis of unilateral PTC patients with ipsilateral cervical lymph node metastasis (IC-LNM; level II-V) treated with uLT and TT, and some studies have shown that there was no significant difference in the recurrence between the two managements (19, 20). Therefore, whether the unilateral PTC patients with IC-LNM should be treated with TT deserves further study.
In this study, our aim was to evaluate the risks and benefits of postoperative complications, prognosis, and other clinical features between unilateral PTC patients with IC-LNM treated with uLT and TT.
Patients and methods
Patients
Thyroid cancer patients who received surgical treatment at Zhejiang Cancer Hospital from February 2012 to January 2022 were retrospectively analyzed (Figure 1). We collected clinical data including gender, age, surgery time, postoperative complications (such as superior laryngeal nerve injury, recurrent laryngeal nerve injury, transient or permanent hypoparathyroidism, infection of incisional wound, and postoperative hemorrhage, etc), hospital stays, hospitalization costs, recurrence-free survival (RFS), and overall survival (OS). Tumor stage and initial risk stratification were performed according to the American Joint Committee on Cancer (AJCC) 8th Edition TNM Staging System and the 2015 ATA guidelines. The inclusion criteria of patients were as follows: (1) pathologically confirmed unilateral PTC; (2) imaging suspected and fine-needle aspiration biopsy (FNAB) or intraoperative frozen section confirmed IC-LNM (level II-V); and (3) primary tumor treated with uLT (with or without isthmusectomy) or total (near-total) thyroidectomy and concurrent ipsilateral cervical lymph node dissection. Patients with any of the following characteristics were excluded: bilateral lobe foci; tumor size ≥ 4 cm; extensive extrathyroidal invasion (such as invasion of nerves, trachea, esophagus, prevertebral tissues;excluding micro-invading only the strap muscles); specific histologic subtypes such as tall cell, columnar cell, and poorly differentiated PTCs; with contralateral cervical lymph node metastasis; distant metastasis; non-primary surgery. A propensity score analysis with 1:1 matching based on the individual risk factors of gender, age, primary tumor size, multifocality, extrathyroidal invasion, and T stage was performed on uLT and TT groups. The study was approved by the Ethics Committee of Zhejiang Cancer Hospital.
Figure 1

Flow chart of patient enrollment.
Outcomes and follow-up
The primary endpoints of this study were RFS and OS. RFS was defined as the interval from the date of initial surgery to the detection of locoregional recurrence or distant metastasis during follow-up, confirmed by imaging and either biopsy or postoperative pathological findings. OS was defined as the duration from the date of surgery to the date of death or the end of follow-up. Patients were routinely followed up at 1, 3, and 6 months postoperatively, and subsequently every 6 to 12 months, either through outpatient visits or telephone interviews. Specifically, at 1 month post-surgery, thyroid function and antibody levels—including thyroglobulin (Tg), anti-thyroglobulin antibody (TgAb), triiodothyronine (T3), thyroxine (T4), and thyroid-stimulating hormone (TSH)—were assessed. In subsequent follow-ups, in addition to these serological tests, all patients underwent thyroid and cervical lymph node ultrasound examinations. For patients with persistently elevated Tg or TgAb levels, further evaluation was performed using imaging studies, including neck ultrasound, and suspicious lymph nodes were subjected to FNAB and/or measurement of Tg in the aspirate (FNAB-Tg). If Tg or TgAb levels continued to rise without definite metastatic lesions on ultrasound, contrast-enhanced CT or MRI was recommended to identify potential local recurrence. In high-risk patients, chest CT was also performed to rule out distant metastases. For patients who have undergone TT, a whole-body radioactive iodine scan may be considered when there is a significant elevation in Tg or TgAb levels, despite the absence of evident lesions on ultrasound or CT imaging.
Statistical analysis
Continuous variables were presented as the mean and standard deviation (SD), and tested with Student ‘s t-test; categorical variables were expressed as frequencies and proportions, and analyzed with Pearson’s χ2 test or Fisher’s exact test. RFS and OS were estimated using the Kaplan-Meier method, and intergroup curves were compared using the log rank test. Predictors of RFS were validated using univariate and multivariate Cox regression analyses, and hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. To control for potential confounders, individual patient propensity scores were calculated by logistic regression analysis. Next, propensity scores were used to pair uLT with TT groups in a 1:1 ratio with a tolerance of 0.02. The two-sided p-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software version 25.0 and Graphpad Prism.5.0.730.
Results
Baseline clinicopathological characteristics before and after propensity score matching
Ultimately, 682 unilateral PTC patients with IC-LNM were available in the study. Baseline characteristics of 682 unilateral PTC patients with IC-LNM according to surgical extent are listed in Table 1. A total of 443 patients treated with uLT and 239 patients underwent TT. There were no statistically significant differences in gender and age between the two groups. However, patients in the uLT group were more likely to have smaller tumors (<2 cm, 350 [79.0%]), earlier staging (stage I, 419[94.6%]), fewer numbers of lymph node metastases (median[range], 7[3-11] nodes), and fewer postoperative complication rates compared with the TT group (Tables 1, 2).
Table 1
| Parameters | uLT (n=443) | TT (n=239) | p value |
|---|---|---|---|
| Age (years) | |||
| <55 | 388 (87.6%) | 197 (82.4%) | 0.066 |
| ≥55 | 55 (12.4%) | 42 (17.6%) | |
| Gender | |||
| Male | 174 (39.3%) | 64 (46.0%) | 0.157 |
| Female | 269 (60.7%) | 175 (54.0%) | |
| Size (cm) | |||
| <2 | 350 (79.0%) | 165 (69.0%) | 0.004 |
| ≥2 | 93 (21.0%) | 74 (31.0%) | |
| Stage | |||
| I | 419 (94.6%) | 213 (89.1%) | 0.009 |
| II-III | 24 (5.4%) | 26 (10.9%) | |
| T grade | |||
| T1 | 338 (76.3%) | 152 (63.6%) | <0.001 |
| T2+T3 | 105 (23.7%) | 87 (36.4%) | |
| Overall lymph node metastasis rate | |||
| mean ± SD | 0.24 ± 0.14 | 0.25 ± 0.15 | 0.109 |
| Lateral lymph node metastasis rate | |||
| mean ± SD | 0.17 ± 0.12 | 0.19 ± 0.13 | 0.076 |
| Number of lymph node metastasis | |||
| mean ± SD | 6.97 ± 4.34 | 8.39 ± 5.47 | <0.001 |
Baseline characteristics of 682 unilateral papillary thyroid carcinoma (PTC) patients with ipsilateral cervical lymph node metastasis.
uLT, unilateral lobectomy; TT, total thyroidectomy. Bold values, p < 0.05.
Table 2
| Parameters | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| uLT (n=443) | TT (n=239) | p value | uLT (n=225) | TT (n=225) | p value | |
| Transient hypoparathyroidism | ||||||
| Absent | 439 (99.1%) |
147 (61.5%) |
<0.001 | 221 (98.2%) |
140 (62.2%) |
<0.001 |
| Present | 4 (0.9%) |
92 (38.5%) |
4 (1.8%) |
85 (37.8%) |
||
| Permanent hypoparathyroidism | ||||||
| Absent | 443 (100%) |
235 (98.3%) |
0.006 | 225 (100%) |
221 (98.2%) |
0.045 |
| Present | 0(0%) | 4(1.7%) | 0(0%) | 4(1.8%) | ||
| Recurrent laryngeal nerve injury | ||||||
| Absent | 442 (99.8%) |
239 (100%) |
0.462 | 224 (99.6%) |
225 (100%) |
0.317 |
| Present | 1(0.2%) | 0(0%) | 1(0.4%) | 0(0%) | ||
Surgical complications according to surgical extent before and after PSM.
Hypoparathyroidism is characterized by hypocalcemia and low or undetectable levels of parathyroid hormone. Recurrent laryngeal nerve (RLN) injury is defined when the surgical records documented intraoperative RLN injury, or when the patients developed hoarseness after surgery, or when laryngoscopy revealed postoperative restriction of vocal cord movement.
uLT, unilateral lobectomy; TT, total thyroidectomy; PSM, propensity score matching. Bold values, p < 0.05.
After PSM with potential prognostic factors (such as gender, age, primary tumor size, multifocality, extrathyroidal invasion, and T stage), a total of 225 pairs of patients were included in the study. There were no statistically significant differences in the proportion of gender, age, primary tumor size, T stage, number of lymph node metastasis, lymph node metastasis rate, and TNM stage between the two groups (p>0.05; Table 3). However, postoperative complication rates were higher in the TT group than in the uLT group (93[41.3%] vs. 13[5.8%], p<0.001). Of the 225 patients in the TT group, 4 patients (1.8%, p=0.045) developed permanent hypoparathyroidism and 85 patients (37.8%, p<0.001) developed transient hypoparathyroidism (Table 2). All patients with transient hypoparathyroidism recovered within 6 months of healing.
Table 3
| Parameters | uLT (n=225) | TT (n=225) | p value |
|---|---|---|---|
| Age (years) | |||
| <55 | 191 (84.9%) | 186 (82.7%) | 0.523 |
| ≥55 | 34 (15.1%) | 39 (17.3%) | |
| Gender | |||
| Male | 65 (28.9%) | 64 (28.4%) | 0.917 |
| Female | 160 (71.1%) | 161 (71.6%) | |
| Size (cm) | |||
| <2 | 161 (71.6%) | 162 (72.0%) | 0.917 |
| ≥2 | 64 (28.4%) | 63 (28.0%) | |
| Stage | |||
| I | 202 (89.8%) | 202 (89.8%) | 1.000 |
| II-III | 23 (10.2%) | 23 (10.2%) | |
| T grade | |||
| T1 | 144 (64.0%) | 149 (66.2%) | 0.621 |
| T2+T3 | 81 (36.0%) | 76 (33.8%) | |
| Overall lymph node metastasis rate | |||
| mean ± SD | 0.24 ± 0.14 | 0.25 ± 0.15 | 0.156 |
| Lateral lymph node metastasis rate | |||
| mean ± SD | 0.18 ± 0.13 | 0.18 ± 0.13 | 0.500 |
| Number of lymph node metastasis | |||
| mean ± SD | 4.32 ± 3.07 | 4.36 ± 3.08 | 0.481 |
Baseline characteristics of 225 pairs unilateral PTC patients with ipsilateral cervical lymph node metastasis after propensity score matching (PSM).
uLT, unilateral lobectomy; TT, total thyroidectomy. Bold values, p < 0.05.
Comparisons of time and financial factors in both uLT and TT groups
Patients treated with uLT spent less time on surgery (p<0.01) and hospitalization (p<0.01) compared to those treated with TT (Figures 2A, B). Moreover, we also found that this uLT group spent less money (p<0.01) on surgery and hospitalization (p<0.0001; Figures 2C, D). In addition, uLT reduced the L-T4 doses at one month postoperatively (Figure 2E). Thus, uLT is more cost-effective than TT, and these are equally important considerations for clinicians.
Figure 2
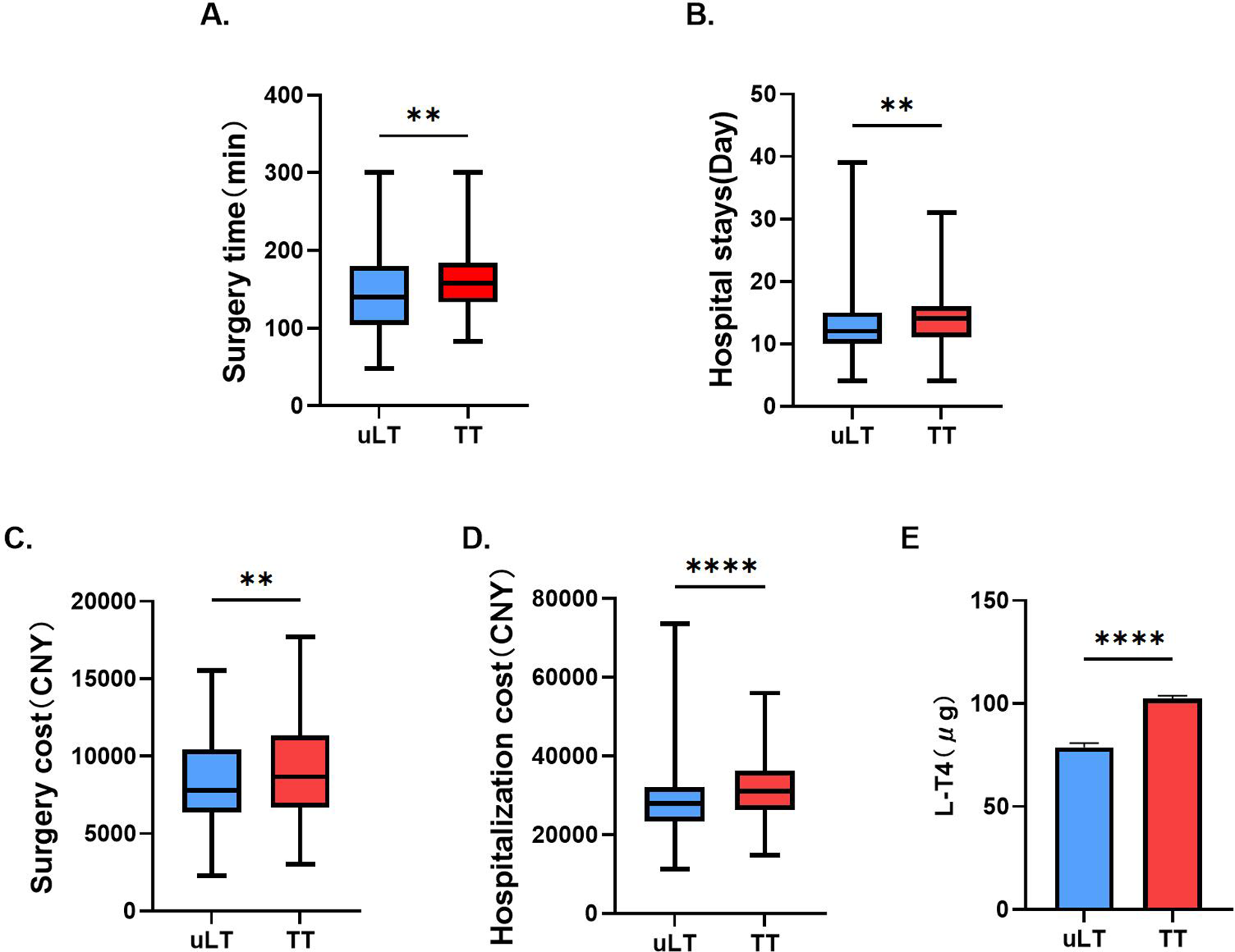
Comparison of surgery time (A), hospital stays (B), surgery cost (C), hospitalization cost (D) and L-T4 doses at one month postoperatively (E) between unilateral lobectomy (uLT) and total thyroidectomy (TT) groups. **p< 0.01, ****p< 0.0001.
Univariate and multivariate analyses of the risk factors of recurrence before and after PSM
Tables 4 , 5 showed the risk factors of recurrence before and after PSM based on the univariate and multivariate analyses, respectively. Based on the univariate analysis, tumor size ≥2 cm, extrathyroidal extension, number of lymph node metastasis, overall lymph node metastasis rate, lateral lymph node metastasis rate and T2-3 stages were significant risk factors of recurrence before PSM, while tumor size ≥2 cm, number of lymph node metastasis, overall lymph node metastasis rate and T2-3 stages were significant risk factors of recurrence after PSM. However, based on the multivariate analysis, there were no significant predictors of recurrence before and after PSM (Tables 4, 5).
Table 4
| Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (HR) | 95% CI | p value | Hazard ratio (HR) | 95% CI | p value | |
| Female gender | 0.723 | 0.407-1.284 | 0.269 | |||
| Age(≥55 years) | 1.419 | 0.687-2.930 | 0.344 | |||
| Total thyroidectomy | 0.710 | 0.380-1.325 | 0.282 | |||
| Tumor size(≥2 cm) | 2.269 | 1.278-4.028 | 0.005 | 1.087 | 0.429-2.757 | 0.861 |
| Multifocality | 1.094 | 0.557-2.148 | 0.794 | |||
| Extrathyroidal extension | 1.920 | 0.999-3.690 | 0.050 | 0.735 | 0.277-1.950 | 0.536 |
| Number of lymph node metastasis | 1.190 | 1.100-1.286 | <0.001 | 1.107 | 0.966-1.268 | 0.143 |
| Overall lymph node metastasis rates | 28.424 | 4.787-168.782 | <0.001 | 2.457 | 0.040-152.084 | 0.669 |
| Lateral lymph node metastasis rates | 38.392 | 5.864-251.341 | <0.001 | 1.513 | 0.007-329.649 | 0.880 |
| T | ||||||
| T1 | Ref | Ref | ||||
| T2-3 | 2.833 | 1.608-4.991 | <0.001 | 2.798 | 0.943-8.299 | 0.064 |
| TNM | ||||||
| I | Ref | |||||
| II-III | 1.957 | 0.876-4.370 | 0.102 | |||
Univariate and multivariate analysis with Cox regression for all patients before PSM (n=682).
Ref, reference; CI, confidence interval. Bold values, p < 0.05.
Table 5
| Parameters | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (HR) | 95% CI | p value | Hazard ratio (HR) | 95% CI | p value | |
| Female gender | 0.654 | 0.327-1.306 | 0.228 | |||
| Age(≥55 years) | 1.683 | 0.761-3.722 | 0.198 | |||
| Total thyroidectomy | 0.570 | 0.282-1.152 | 0.118 | |||
| Tumor size(≥2 cm) | 2.203 | 1.118-4.338 | 0.022 | 1.983 | 0.728-5.404 | 0.181 |
| Multifocality | 1.169 | 0.528-2.588 | 0.700 | |||
| Extrathyroidal extension | 1.653 | 0.771-3.544 | 0.196 | |||
| Number of lymph node metastasis | 1.132 | 1.029-1.244 | 0.011 | 1.072 | 0.925-1.243 | 0.355 |
| Overall lymph node metastasis rate | 10.009 | 1.024-97.855 | 0.048 | 1.906 | 0.076-47.649 | 0.695 |
| Lateral lymph node metastasis rate | 8.968 | 0.680-118.346 | 0.096 | |||
| T | ||||||
| T1 | Ref | Ref | ||||
| T2-3 | 2.040 | 1.040-4.002 | 0.038 | 1.374 | 0.506-3.729 | 0.533 |
| TNM | ||||||
| I | Ref | |||||
| II-III | 1.814 | 0.751-4.383 | 0.186 | |||
Univariate and multivariate analysis with Cox regression for all patients after PSM(n=225 pairs).
Ref, reference; CI, confidence interval. Bold values, p < 0.05.
Survival analysis before and after PSM
After a median follow-up of 81 (5-154) months, there were 3 deaths in the uLT group, none related to thyroid cancer. In the TT group, there were 5 deaths, of which two patients died of thyroid cancer. Five-year OS rates were 99.4% and 98.3% in the uLT group and TT group, respectively, and the differences were not statistically significant by the log-rank test (p=0.126; Figure 3A). Besides, 34 patients (7.7%) in the uLT group recurred, all of which were locoregional. These patients underwent a second surgery after recurrence, and 9 of them subsequently received postoperative postsurgical radioactive iodine (RAI) therapy, with favorable outcomes. In the TT group, 14 patients (5.9%) experienced recurrence, including one case of distant metastasis. All patients in this group also underwent reoperation, with 3 receiving additional RAI therapy postoperatively, and 2 patients ultimately died, while the remaining patients achieved stable disease control following treatment. Five-year RFS was 93.5% and 94.5% in the uLT group and TT group, respectively, and there were no statistically significant differences, either (p=0.280; Figure 3B). After PSM, the differences between uLT and TT groups in the 5-year OS (99.1% vs 98.2%, p=0.394) and 5-year RFS rates (93.3% vs 95.1%, p=0.113) were not statistically significant, either (Figures 3C, D).
Figure 3

(A, B) Overall survival (OS) and recurrence-free survival (RFS) of uLT and TT groups before Propensity Score Matching (PSM). (C, D) OS and RFS of uLT and TT groups after PSM.
We also verified the prognostic impact of postsurgical RAI therapy on TT. In the TT group, there were 166 patients who received RAI therapy (TT+RAIs) and 59 patients who did not receive RAI therapy (TT-RAIs), and the 5-year OS rates of TT+RAIs group was higher than TT-RAIs group (99.4% vs. 94.8%, p=0.006; Figure 4A). However, there was no significant difference in 5-year RFS (95.2% vs 94.8%, p=0.880; Figure 4B). Due to the differences in 5-year OS, we performed univariate and multivariate analyses of the risk factors of death on these 166 TT+RAIs and 59 TT-RAIs (Supplementary Table 1). The results showed that RAI therapy after TT might reduce the risk of death. Stratified analyses were performed on patients in the uLT group (n=225) and the TT+RAI group (n=166), and there were no statistically significant differences in 5-year OS rates (99.1% vs. 99.4%, p=0.556) and 5-year RFS rates (93.3% vs. 95.2%, p=0.175) between the two groups (Figures 4C, D). After excluding patients who received RAI therapy, the 5-year OS rates in the uLT group (n=225) was higher than that in the TT-RAIs group (n=59) (99.1% vs. 94.8%, p=0.010; Figure 4E). However, the 5-year RFS rates in the uLT group (n=225) and TT-RAIs group (n=59) were 93.3% and 94.8% (p=0.296), respectively, and the differences were not statistically significant (Figure 4F). Univariate and multivariate analyses results indicated no significant predictors of survival (Supplementary Table 2).
Figure 4

Kaplan-Meier plots for RFS and OS stratified by RAI therapy after PSM. (A, B) OS and RFS in TT treated with RAI therapy (TT+RAI) and TT untreated with RAI therapy (TT-RAI) groups. (C, D) OS and RFS in uLT and TT+RAI groups. (E, F) OS and RFS in uLT and TT-RAI groups.
Discussion
Most PTC patients have a favorable prognosis. Precise stratification of treatment according to the different levels of risk for PTC can help to better reduce the risk of complications associated with surgery and other treatments while improving the long-term survival of the patients, thereby improving the quality of life of the patients and reducing the socio-economic burden (21, 22). A growing number of studies have shown that patients undergoing TT have no significant survival advantages over patients undergoing lobectomy, especially for 1-4 cm tumors, with a low- to intermediate-risk of recurrence (10, 23). Adam et al. evaluated the National Cancer Database data of 61775 PTC patients, and the results showed that TT had no survival advantage compared with lobectomy (9). The 2015 ATA guidelines have made some changes to the surgical treatment of PTC, with the option of lobectomy or TT for 1-4 cm PTC without high-risk features (16). However, it remains to be explored which surgery is optimal for PTC patients with IC-LNM.
In this retrospective cohort study, we compared the differences in risk and benefit between unilateral PTC patients with IC-LNM in treatment with uLT and TT in terms of postoperative complications, prognosis, total hospitalization cost, etc. To minimize the selection bias, we performed PSM analysis to correct for confounding factors such as tumor stage and size. In the pre-matched and post-matched outcomes, TT and uLT group had similar recurrence and survival, while TT group had a higher risk of complications, including hypoparathyroidism, which is consistent with previous studies (23–25). In addition, we found that uLT had a lower surgical cost and shorter hospital stays than TT. For uLT patients, the production of thyroid hormones by the contralateral thyroid gland could be preserved, and the doses and side effects of postoperative L-T4 intake might be reduced. Therefore, considering that differentiated thyroid carcinoma patients under treatment wish to not only improve survival and reduce recurrence/persistence, but also improve postoperative quality of life, as well as reduce the socioeconomic burden, uLT might be a more economical, efficient, and patient-friendly surgery.
For patients undergoing TT, RAI therapy can be performed after surgery, thus facilitating the use of Tg as a sensitive tumor marker for residual thyroid tissue or recurrence during follow-up (26–28). In our study, after PSM for major risk factors to minimize selection bias, 59 patients undergoing TT (26.2%) were not treated with RAI postoperatively, and 4 of these patients died. The cause of 2 of these patients’ deaths were related to thyroid cancer, due to the occurrence of tumor metastasis. There were no significant differences in prognosis between TT+RAI and uLT groups; after excluding patients with TT+RAI, the OS was worse in the TT group than in the uLT group (p=0.010). Our studies demonstrated that patients not received RAI therapy after TT might affect their prognosis, while the prognosis of patients who received RAI were not statistically significantly different from that of patients with uLT. These results showed the survival impact of RAI therapy on TT. In other words, TT treatment did not prevail compared with uLT, and the impact of RAI therapy should be taken into account, especially for those older age people (≥55 years). However, the effect of RAI therapy in improving prognosis remains controversial. Referring to the ATA guidelines for recurrence risk stratification, postoperative RAI therapy would be recommended in both the intermediate- and high- risk groups. RAI therapy in the low-risk group is controversial for patient prognosis and is not routinely recommended (16). Seo et al. showed that postoperative RAI therapy did not significantly reduce the risk of recurrence in intermediate-risk PTC patients, PTC patients should be considered on the basis of specific risk re-stratification (29). Youngmin et al. speculated that RAI therapy might be ineffective in reducing the recurrence of multifocal PTC patients (30). RAI refractoriness occurs in some PTC patients and dramatically decreased RAI uptake, which affects prognosis (31, 32). In addition, preparation for RAI therapy requires discontinuation of thyroid hormone or recombinant TSH and a low iodine diet, which reduces quality of life, and the risk of complications associated with repeated iodine-131 treatments with high iodine uptake, such as xerostomia, recurrent sialoadenitis and pulmonary fibrosis, also need to be taken into account (33, 34). All in all, available studies have not been able to assess the impact of RAI therapy on clinical outcomes in patients treated with TT.
On the one hand, previous studies were limited to small numbers of patients, excluding adequate multivariate adjustment. In our study, the larger number of patients enabled us to perform PSM for possible prognostic factors in PTC, which could minimize the selection bias. Prospective studies are challenging to implement since most PTCs have a favorable prognosis. On the other hand, the current study also had limitations. Our study was a single-center retrospective study, might limit the conclusions. So that further large-sample and multicenter studies were needed for validation. Besides, while serum Tg levels can be effectively used for early detection of recurrence in patients who have undergone TT, this method is not applicable to those who underwent uLT, as residual thyroid tissue compromises the reliability of Tg-based monitoring.
In conclusion, this study showed that the uLT group and TT group presented similar prognostic outcomes, while the uLT group had a lower risk of postoperative complications and lower hospitalization costs than the TT group. Therefore, for reasonably selected unilateral PTC patients without other high-risk features (such as bilateral lobe foci; tumor size ≥ 4 cm; extensive extrathyroidal invasion; with contralateral cervical lymph node metastasis; distant metastasis), uLT could be recommended with IC-LNM.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Zhejiang Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SZ: Data curation, Formal Analysis, Writing – original draft. KZ: Formal Analysis, Writing – original draft. JW: Formal Analysis, Writing – original draft. MZ: Data curation, Writing – original draft. XM: Funding acquisition, Writing – original draft. JS: Data curation, Writing – original draft. XL: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2023RC138, 2022PY005, 2020KY482), and Zhejiang Provincial Natural Science Foundation of China under Grant No. LY24H160019.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1564752/full#supplementary-material
References
1
Chen W Zheng R Baade PD Zhang S Zeng H Bray F et al . Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
2
Xu S Han Y . The overdiagnosis of thyroid micropapillary carcinoma: the rising incidence, inert biological behavior, and countermeasures. J Oncol. (2021) 2021:5544232. doi: 10.1155/2021/5544232
3
Wang W Shen C Zhao Y Sun B Qiu X Yin S et al . The Role of m6A RNA Methylation-Related lncRNAs in the Prognosis and Tumor Immune Microenvironment of Papillary Thyroid Carcinoma. Front Cell Dev Biol. (2021) 9:719820. doi: 10.3389/fcell.2021.719820
4
Miller KD Nogueira L Devasia T Mariotto AB Yabroff KR Jemal A et al . Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. (2022) 72:409–36. doi: 10.3322/caac.21731
5
Kim YS Park WC . Clinical predictors of right upper paraesophageal lymph node metastasis from papillary thyroid carcinoma. World J Surg Oncol. (2012) 10:164. doi: 10.1186/1477-7819-10-164
6
Lundgren CI Hall P Dickman PW Zedenius J . Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. (2006) 106:524–31. doi: 10.1002/cncr.21653
7
Fagin JA Wells SA Jr . Biologic and clinical perspectives on thyroid cancer. N Engl J Med. (2016) 375:1054–67. doi: 10.1056/NEJMra1501993
8
Ho AS Luu M Barrios L Chen I Melany M Ali N et al . Incidence and mortality risk spectrum across aggressive variants of papillary thyroid carcinoma. JAMA Oncol. (2020) 6:706–13. doi: 10.1001/jamaoncol.2019.6851
9
Adam MA Pura J Gu L Dinan MA Tyler DS Reed SD et al . Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg. (2014) 260:601–605; discussion 605-607. doi: 10.1097/SLA.0000000000000925
10
Nixon IJ Ganly I Patel SG Palmer FL Whitcher MM Tuttle RM et al . Thyroid lobectomy for treatment of well differentiated intrathyroid Malignancy. Surgery. (2012) 151:571–9. doi: 10.1016/j.surg.2011.08.016
11
Wang W Ding Y Zhao Y Li X . m6A reader IGF2BP2 promotes lymphatic metastasis by stabilizing DPP4 in papillary thyroid carcinoma. Cancer Gene Ther. (2024) 31:285–99. doi: 10.1038/s41417-023-00702-2
12
Siddiqui S White MG Antic T Grogan RH Angelos P Kaplan EL et al . Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. (2016) 26:807–15. doi: 10.1089/thy.2015.0429
13
So YK Kim MJ Kim S Son YI . Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg. (2018) 50:94–103. doi: 10.1016/j.ijsu.2017.12.029
14
Ito Y Kudo T Takamura Y Kobayashi K Miya A Miyauchi A . Lymph node recurrence in patients with N1b papillary thyroid carcinoma who underwent unilateral therapeutic modified radical neck dissection. World J Surg. (2012) 36:593–7. doi: 10.1007/s00268-011-1391-1
15
Baek SK Jung KY Kang SM Kwon SY Woo JS Cho SH et al . Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. (2010) 20:147–52. doi: 10.1089/thy.2008.0243
16
Haugen BR Alexander EK Bible KC Doherty GM Mandel SJ Nikiforov YE et al . 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
17
Yuan Y Pan B Tang E Mo H Zhu J Yi Z et al . Surgical methods of total thyroidectomy for differentiated thyroid cancer: a systematic review and Bayesian network meta-analysis. Int J Surg. (2024) 110:529–40. doi: 10.1097/JS9.0000000000000819
18
Ito M Miyauchi A Kang S Hisakado M Yoshioka W Ide A et al . Effect of the presence of remnant thyroid tissue on the serum thyroid hormone balance in thyroidectomized patients. Eur J Endocrinol. (2015) 173:333–40. doi: 10.1530/EJE-15-0138
19
Wang Z Xiang J Gui Z Qin Y Sun W Huang J et al . Unilateral Tnm T1 and T2 papillary thyroid carcinoma with lateral cervical lymph node metastasis: total thyroidectomy or lobectomy? Endocr Pract. (2020) 26:1085–92. doi: 10.4158/EP-2020-0125
20
Song J Qiu W Yan T Wu B Guo M Fan Y et al . Comparison of lobectomy and total thyroidectomy in unilateral papillary thyroid microcarcinoma patients with ipsilateral lateral lymph node metastasis without gross extrathyroidal extension. World J Surg. (2020) 44:2677–84. doi: 10.1007/s00268-020-05514-1
21
Pelizzo MR Boschin IM Toniato A Pagetta C Piotto A Bernante P et al . Natural history, diagnosis, treatment and outcome of papillary thyroid microcarcinoma (PTMC): a mono-institutional 12-year experience. Nucl Med Commun. (2004) 25:547–52. doi: 10.1097/01.mnm.0000126625.17166.36
22
Sugitani I Ito Y Takeuchi D Nakayama H Masaki C Shindo H et al . Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid. (2021) 31:183–92. doi: 10.1089/thy.2020.0330
23
Kwon H Jeon MJ Kim WG Park S Kim M Song DE et al . A comparison of lobectomy and total thyroidectomy in patients with papillary thyroid microcarcinoma: a retrospective individual risk factor-matched cohort study. Eur J Endocrinol. (2017) 176:371–8. doi: 10.1530/EJE-16-0845
24
Kandil E Krishnan B Noureldine SI Yao L Tufano RP . Hemithyroidectomy: a meta-analysis of postoperative need for hormone replacement and complications. ORL J Otorhinolaryngol Relat Spec. (2013) 75:6–17. doi: 10.1159/000345498
25
Hauch A Al-Qurayshi Z Randolph G Kandil E . Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann Surg Oncol. (2014) 21:3844–52. doi: 10.1245/s10434-014-3846-8
26
Cabanillas ME McFadden DG Durante C . Thyroid cancer. Lancet. (2016) 388:2783–95. doi: 10.1016/S0140-6736(16)30172-6
27
Lee SH Roh JL Gong G Cho KJ Choi SH Nam SY et al . Risk factors for recurrence after treatment of N1b papillary thyroid carcinoma. . Ann Surg. (2019) 269:966–71. doi: 10.1097/SLA.0000000000002710
28
Padovani RP Robenshtok E Brokhin M Tuttle RM . Even without additional therapy, serum thyroglobulin concentrations often decline for years after total thyroidectomy and radioactive remnant ablation in patients with differentiated thyroid cancer. Thyroid. (2012) 22:778–83. doi: 10.1089/thy.2011.0522
29
Kim SK Woo JW Lee JH Park I Choe JH Kim JH et al . Radioactive iodine ablation may not decrease the risk of recurrence in intermediate-risk papillary thyroid carcinoma. Endocr Relat Cancer. (2016) 23:367–76. doi: 10.1530/ERC-15-0572
30
Kim Y An S Park J Bae JS Kim JS Kim K . Clinical implication of bilateral and unilateral multifocality in papillary thyroid carcinoma: A propensity score-matched study. Cancers (Basel). (2023) 15:3596. doi: 10.3390/cancers15143596
31
Wang W Bai N Li X . Comprehensive analysis of the prognosis and drug sensitivity of differentiation-related lncRNAs in papillary thyroid cancer. Cancers (Basel). (2022) 14:1353. doi: 10.3390/cancers14051353
32
Oh JM Ahn BC . Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics. (2021) 11:6251–77. doi: 10.7150/thno.57689
33
Davies L Welch HG . Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch Otolaryngol Head Neck Surg. (2010) 136:440–4. doi: 10.1001/archoto.2010.55
34
Lee SL . Complications of radioactive iodine treatment of thyroid carcinoma. J Natl Compr Canc Netw. (2010) 8:1277–1286; quiz 1287. doi: 10.6004/jnccn.2010.0094
Summary
Keywords
unilateral lobectomy, total thyroidectomy, papillary thyroid carcinoma, recurrence-free survival, propensity score matching
Citation
Zhang S, Zhou K, Wang J, Zhao M, Mao X, Shang J and Lan X (2025) Lobectomy vs total thyroidectomy for unilateral papillary thyroid carcinoma with ipsilateral cervical lymph node metastasis. Front. Endocrinol. 16:1564752. doi: 10.3389/fendo.2025.1564752
Received
22 January 2025
Accepted
09 July 2025
Published
24 July 2025
Volume
16 - 2025
Edited by
Takahisa Hiramitsu, Japanese Red Cross Nagoya Daini Hospital, Japan
Reviewed by
Kyriakos Vamvakidis, Henry Dunant Hospital, Greece
Sasi Mouli V V H P K, Sri Shankara Cancer Research Foundation, India
Updates
Copyright
© 2025 Zhang, Zhou, Wang, Zhao, Mao, Shang and Lan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiabin Lan, lanxb@zjcc.org.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.