- 1College of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun, Jilin, China
- 2Department of Endocrinology, The Affiliated Hospital to Changchun University of Chinese Medicine, Changchun, Jilin, China
- 3Institute of Metabolic Diseases, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: Diabetic gastrointestinal diseases not only affect the quality of life of patients, but also bring heavy economic burden to patients. Understanding of the current features of diabetic gastrointestinal diseases-related clinical trials are important for improving designs of clinical trials and identifying neglected areas of study. Despite the high prevalence of gastrointestinal complications among diabetic patients, comprehensive analyses of registered clinical trials are lacking. This study aimed to present a scoping overview of diabetic gastrointestinal diseases-related clinical trials registered in ClinicalTrials.gov, ChiCTR and high-quality diabetic gastrointestinal diseases-related clinical trials published in Pubmed in the past 10 years.
Methods: The trials registered in ClinicalTrials.gov and ChiCTR databases from the establishment of the database to June 14,2024 were searched. Moreover, high-quality trials with impact factors of 5 points or more published in Pubmed from June 2014 to June 2024 were searched. The results were extracted and presented in tabular form.
Results: Most studies focused on diabetic gastroparesis, with drug interventions being the most common. In addition, most studies were small sample sizes (≤100), randomized parallel controlled trials and 69.01% of the studies used different methods of blinding. Most studies did not conduct safety evaluation and follow-up.
Conclusion: The diagnostic criteria of diabetic gastrointestinal diseases were diverse. Furthermore, most studies on diabetic gastrointestinal diseases focused on diabetic gastroparesis. There was considerable heterogeneity in study designs and efficacy evaluations.
1 Introduction
Diabetes is a chronic disease. According to the International Diabetes Federation (IDF) Diabetes Atlas, the global prevalence of adult diabetes was 10.5%, rising to 12.2% in 2045 (1). Gastrointestinal diseases are common in patients with diabetes mellitus (2). Up to 30% -70% of diabetic patients present intestinal-related dysfunction and complications (3). In addition to diabetic gastroparesis, diabetic gastrointestinal diseases also includes diabetic dyspepsia (4). Patients with diabetic gastrointestinal diseases will experience a number of gastrointestinal symptoms. Common gastrointestinal symptoms include nausea, vomiting, early satiety, abdominal bloating, diarrhoea, etc (5). Gastrointestinal symptoms affect quality of life in diabetes negatively (6). In addition, gastrointestinal symptoms also increase diabetes-related health care costs and bring a heavy economic burden to diabetic patients (7). More and more clinical workers had carried out clinical research on diabetic gastrointestinal diseases. Previous trials have mostly focused on gastroparesis and prokinetic therapies, but broader gastrointestinal symptoms manifestations remain underexplored. Moreover, there were great differences in research designs, such as the lack of standardized diagnostic criteria; limited follow-up periods and sparse data on interventions beyond prokinetics and so on.
Clinical trials are the basis of evidence-based medicine and the driving force of medical development (8). Clinical trial registration is an effective measure to promote the transparency of clinical trial design and implementation (9). ClinicalTrials.gov was a web-based registry maintained by the National Library of Medicine and National Institutes of Health (8). Meanwhile, the ClinicalTrials.gov database provided the most comprehensive information about ongoing and completed clinical studies worldwide (10). The Chinese Clinical Trial Register (ChiCTR) was sponsored and established by the Ministry of Health of China in June 2007, which was accepted as one of the main registrars of the World Health Organization International Clinical Trial Registry Platform (WHO ICTRP) (11). PubMed, the most widely used biomedical literature search engine, currently contains over 36 million articles (12). Although there are related reviews on diabetic gastroparesis, enteric neuropathy in diabetes and other diseases, there is lack of comprehensive reviews on clinical trial designs and outcomes for diabetic gastrointestinal diseases (13, 14). Physicians still lack a comprehensive understanding of high-quality clinical trials on diabetic gastrointestinal diseases.
A better understanding of the current research situation of clinical trials related to diabetic gastrointestinal diseases is very important for improving the design of clinical trials and the quality of research. Therefore, the purpose of this scoping overview is to examine how diabetic gastrointestinal disease trials are currently designed, executed, and reported, and to identify recommendations for future research.
2 Methods
2.1 Search strategy
The study used words related to search term “diabetes gastroparesis” or “diabetic gastroparesis” or “diabetic gastropathy” or “diabetes mellitus gastroparesis” or “diabetic gastrointestinal disorders” or “gastrointestinal diseases of diabetes” or “gastrointestinal disorders in diabetes” or “diabetic stomach” or “diabetic enteropathy” to retrieve the trials registered in ClinicalTrials.gov and ChiCTR databases from the establishment of the database to June 14,2024. High-quality trials with impact factors of 5 points or more published in Pubmed from June 2014 to June 2024 were searched with the same search terms. At the same time, clinical trials and randomized controlled trials were selected on the pubmed article type filter to initially include clinical studies related to diabetic gastrointestinal diseases.
2.2 Inclusion and exclusion criteria
The study included relevant clinical trials on diabetic gastrointestinal diseases if these studies 1) were conducted on humans, 2) were retrieved on ClinicalTrials.gov and ChiCTR databases from inception to June 14, 2024, 3) were high-quality clinical studies published in Pubmed from June 2014 to June 2024 (impact factor 5 and above).We did not include studies published on pubmed 10 years ago, because the study is to let clinicians understand the current research situation of diabetic gastrointestinal diseases. Moreover, the older articles are of little significance to understand the recent research status and are inconsistent with the purpose of this study. The study excluded surgical studies and studies unrelated to diabetic gastrointestinal diseases.
2.3 Data extraction
The retrieved clinical trials were strictly screened according to the inclusion and exclusion criteria. In addition, the following variables that coincidental studies were extracted using the Microsoft Excel table: sample size, diagnostic criteria, intervention type, follow-up duration, study design, and outcome measures. The items that could not be classified in the variables were listed as “other”. Data extraction was performed independently by two reviewers, with discrepancies resolved by consensus.
2.4 Statistical analysis
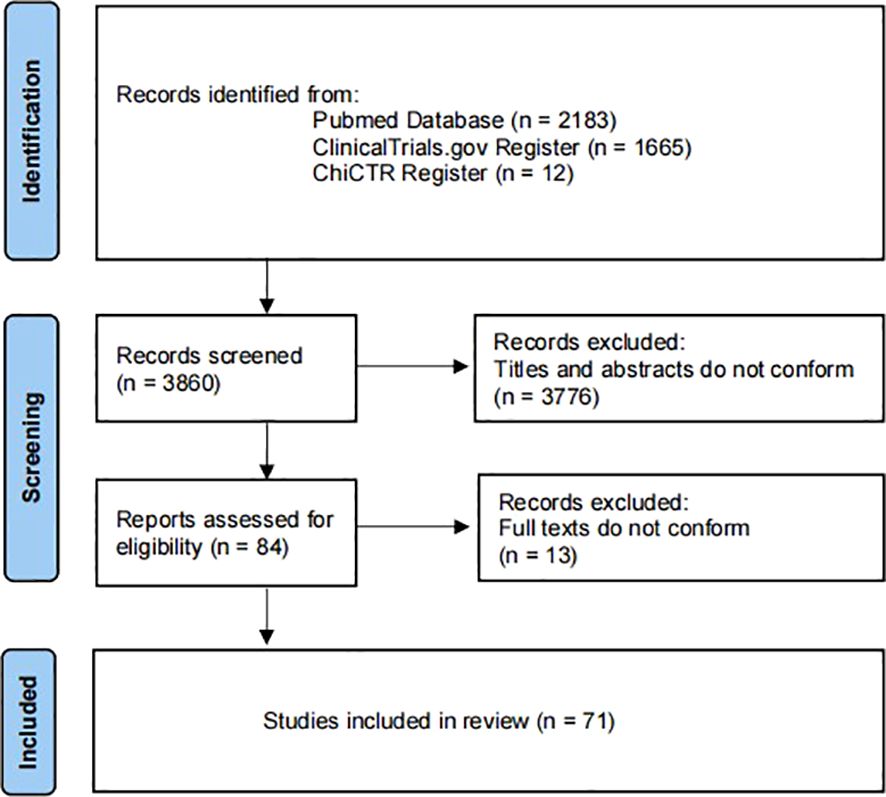
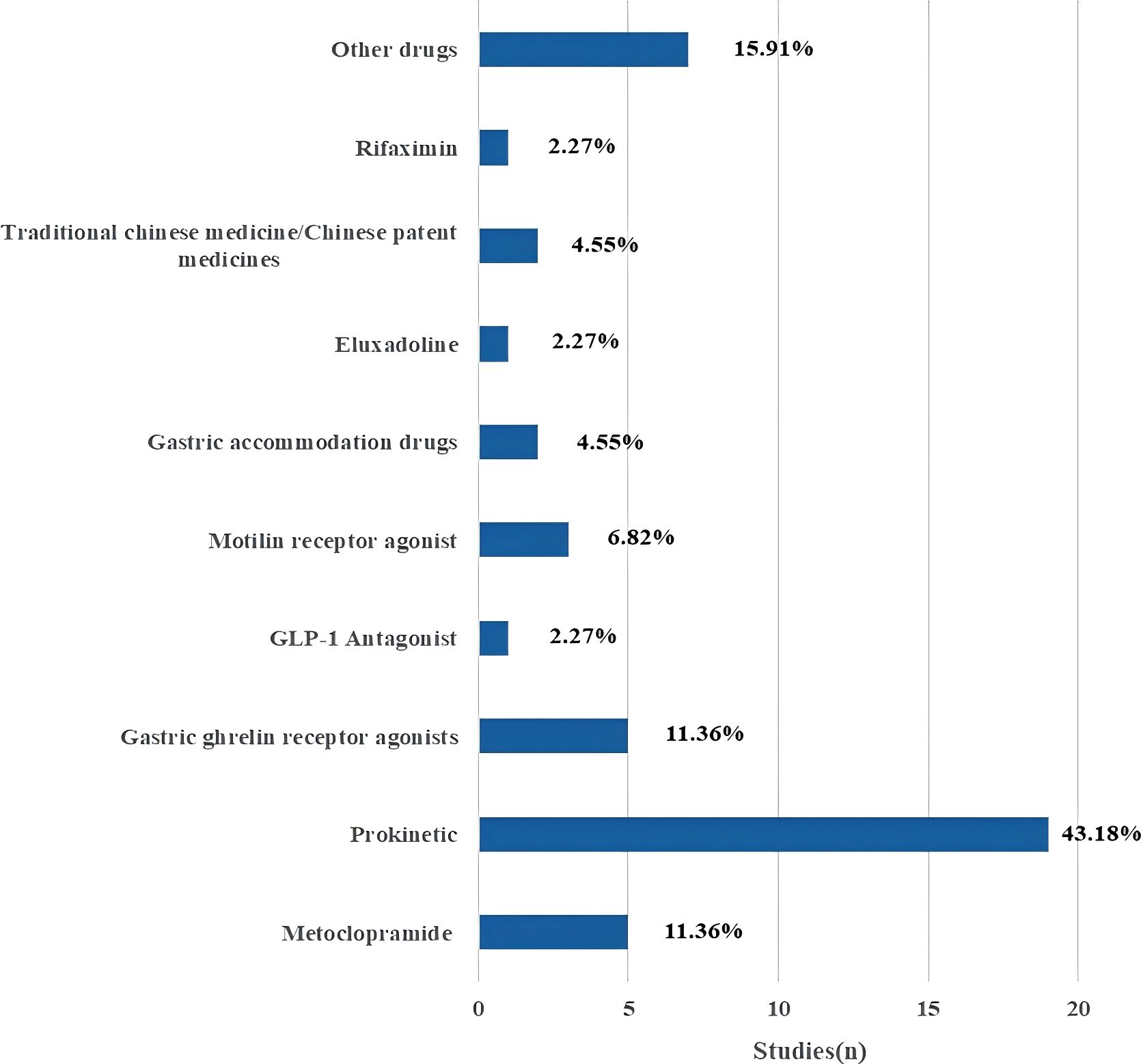
Excel software was used for statistical analysis. This study is a scoping overview of diabetic gastrointestinal diseases-related clinical studies. Therefore, descriptive statistics were performed on the study contents extracted from the included articles in the form of composition ratio and frequency. Furthermore, “Figure 1” for study flow, “Figure 2” for types of drug intervention and “Tables 1-4” for detailed data. Figure 1 flowchart follows the PRISMA guidelines (15).
3 Results
3.1 Study selection
A total of 71 clinical trials were included in the study (Figure 1). The initial search yielded 3860 records from ClinicalTrials.gov, ChiCTR and Pubmed. Of these, 3776 were excluded as they were repetitive and did not meet the defined inclusion criteria. Next, the full texts of the remaining 84 trials were screened. Of these, 13 were excluded as they were not diabetes-related gastrointestinal diseases. Finally, leaving 71 clinical trials for inclusion.
3.2 Inclusion criteria for studies on diabetic gastrointestinal diseases
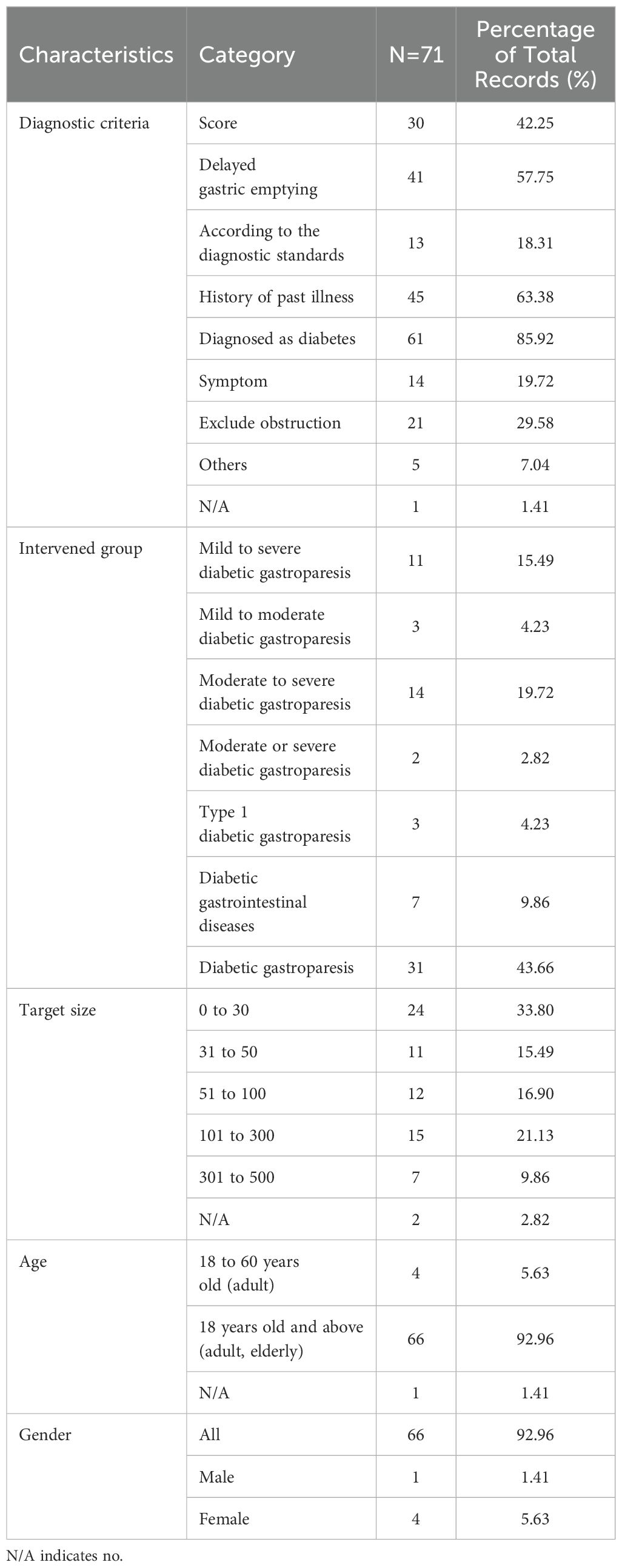
Inclusion criteria for studies on diabetic gastrointestinal diseases were listed in Table 1. In the diagnostic criteria of diabetic gastrointestinal diseases, 61 studies (85.92%) clearly mentioned the diagnosis of diabetes, 45 studies (63.38%) were diagnosed by history of past illness, 41 studies (57.75%) were diagnosed by delayed gastric emptying and 30 studies (42.25%) were diagnosed by scores. Moreover, only 13 studies (18.31%) were diagnosed by meeting the diagnostic criteria of related diseases. In addition, only one study (1.41%) did not specify the diagnostic criteria. Of the 71 studies, only the intervention population of 7 studies (9.86%) were diabetic gastrointestinal diseases, and the rest were diabetic gastroparesis. It could be seen that the studies of diabetic gastroparesis occupied predominance. Among them, 31 studies (43.66%) did not mention the severity of diabetic gastroparesis, 14 studies (19.72%) were mentioned severity as moderate to severe and 11 studies (15.49%) were mild to severe. Of the 71 studies, the participants were mostly concentrated in 30 and less (n=24, 33.8%) and 101 to 300 (n=15, 21.13%). Among them, the target sample size of 47 clinical trials (66%) was less than 100. Only 7 studies (9.86%) had more than 300 participants. In addition, of the 71 studies, the majority of the participants were adults and the elderly (n=66, 92.96%), and only 4 studies (5.63%) were adults. Furthermore, 66 studies (92.96%) were unlimited gender.
3.3 Intervention characteristics for studies on diabetic gastrointestinal diseases
Intervention characteristics for studies on diabetic gastrointestinal diseases were listed in Table 2. Of the 71 included studies, the intervention time of 24 studies (33.8%) was 4 weeks and less and 18 studies (25.35%) reached 12 weeks and above. Among them, only the intervention time of 2 studies (2.82%) was not necessarily. Through statistical analysis, it was found that most studies the common used of short intervention periods. In terms of the follow-up time of the studies, 62 studies (87.32%) were not followed up, and the follow-up times of only studies were concentrated in 14 days and less (n=4, 5.63%) and 4 weeks and above (n=4, 5.63%). Among them, only one study (1.41%) had an irregular follow-up time. There were eight types for the intervention or treatment of patients with diabetic gastrointestinal diseases: Drug; Iiquid Nutrient; Fecal Microbiota Transplantation; Acupuncture; Inspection/Equipment; Diet; Other and no interventions. Of the 71 studies, 44 studies (61.97%) focused on drugs interventions, followed by 15.49% focusing on Inspection/Equipment (n=11), and 8.45% focusing on acupuncture interventions (n=6). As shown in Figure 2, the 44 drug intervention trials included metoclopramide (5), prokinetic (19), gastric ghrelin receptor agonists (5), GLP-1 Antagonist (1), motilin receptor agonist (3), gastric accommodation drugs (2), eluxadoline (1), traditional chinese medicine/chinese patent medicines (2), rifaximin (1), other drugs (7). Prokinetic drugs were the most common intervention, accounting for 43.18% of drug-related trials.
3.4 Study designs for studies on diabetic gastrointestinal diseases
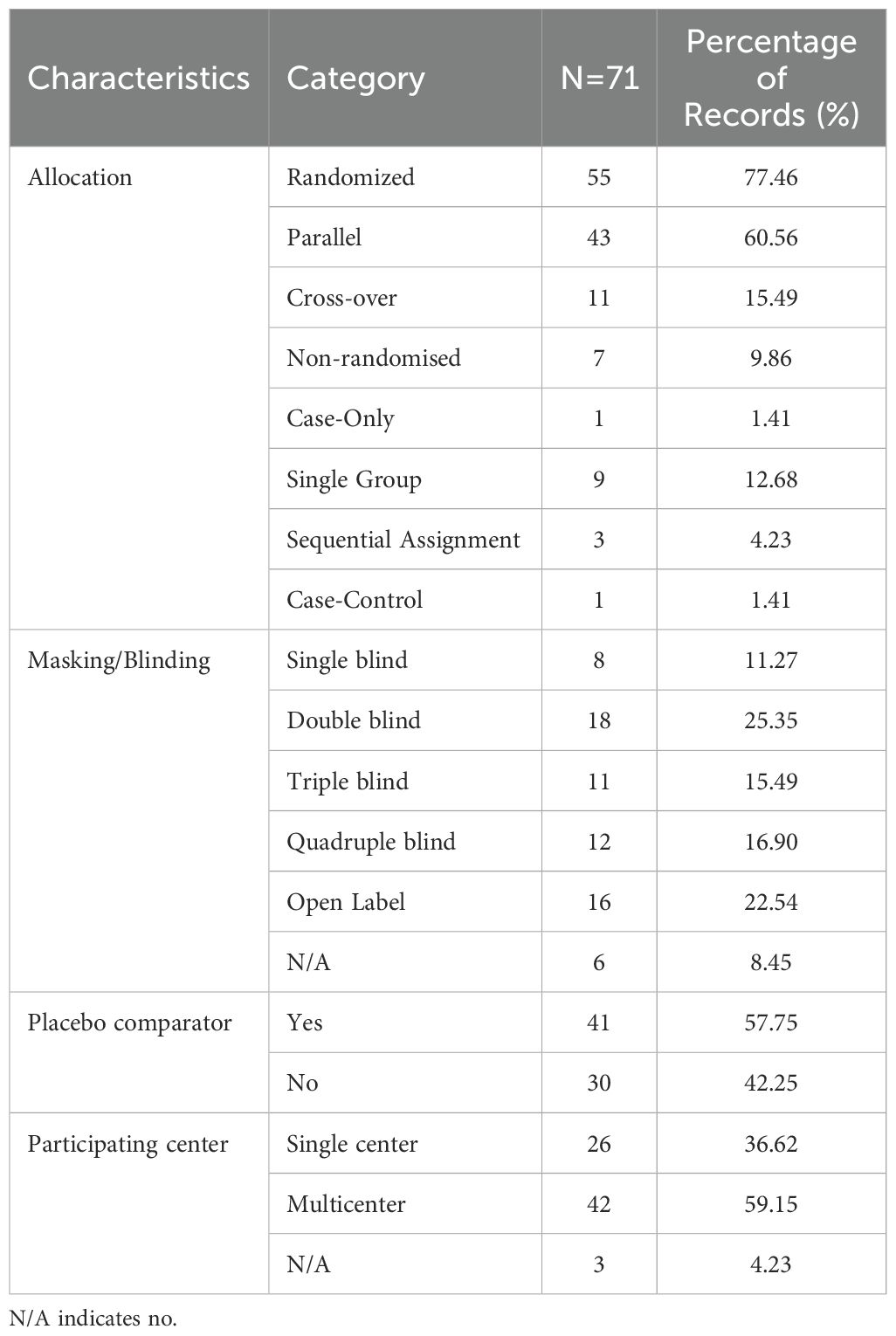
Study designs for studies on diabetic gastrointestinal diseases were listed in Table 3. Of the 71 included studies, most were randomized parallel controlled trials (n = 55, 77.46% VS n = 43, 60.56% VS n = 41, 60.56%). Moreover, 18 studies (25.35%) had a double-blinded research design, while 16 studies (22.54%) did not, and 6 studies failed to provide a description. In addition, 41 studies (57.75%) provided placebo, while 30 studies (42.25%) did not. Of the 71 studies, 42 (59.15%) were multi-center studies, 26 (36.62%) were single-center studies, while 3 studies (4.23%) did not.
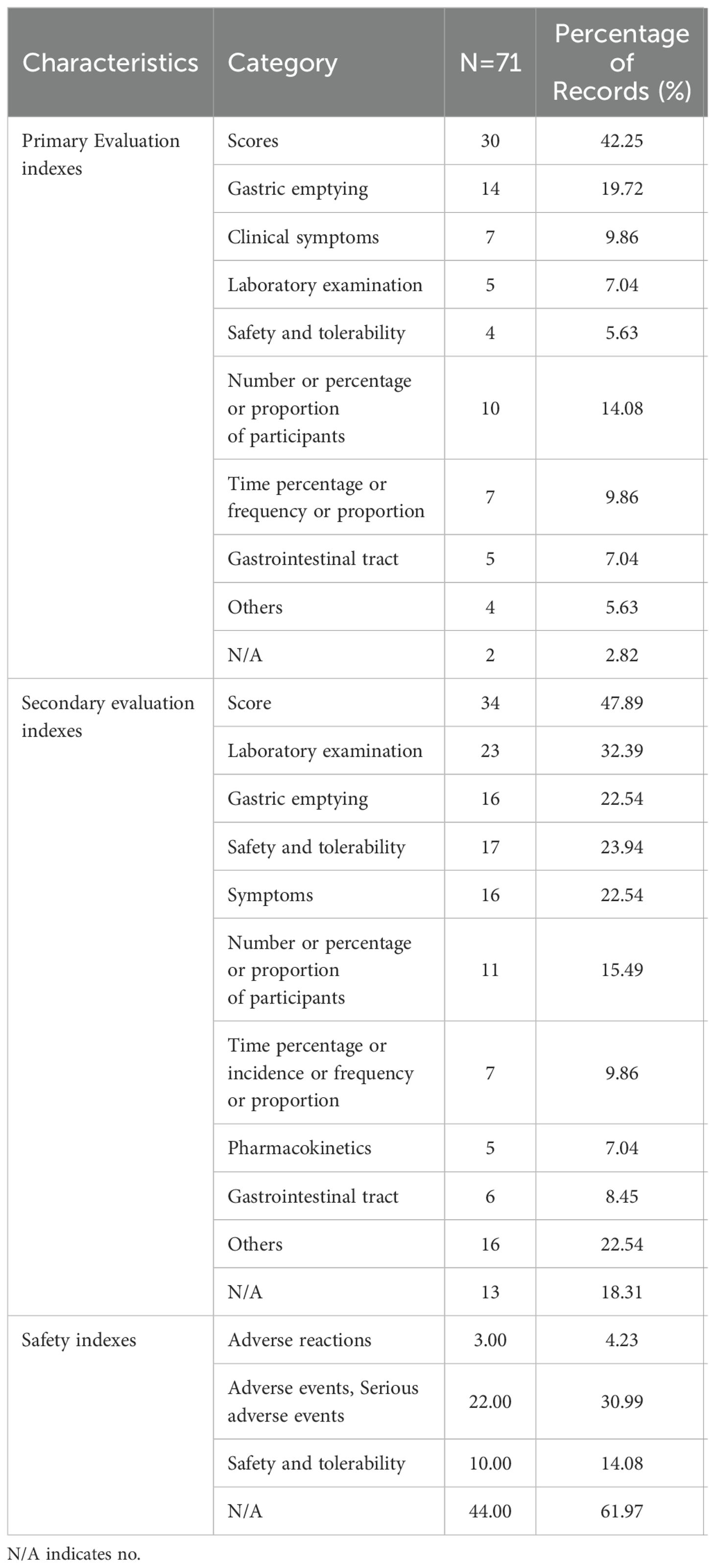
3.5 Outcome measures for studies on diabetic gastrointestinal diseases
Outcome measures for studies on diabetic gastrointestinal diseases were listed in Table 4. Of the 71 included studies, the primary and secondary evaluation indexes were based on the scores (n = 30, 42.25% VS n = 34, 47.89%) as the primary index. In terms of primary evaluation indexes, 14 studies (19.72%) were evaluated by gastric emptying, 10 studies (14.08%) were evaluated by the number or percentage or proportion of participants, while 2 studies (2.82%) did not. In terms of secondary evaluation indexes, 23 studies (32.39%) were evaluated by laboratory tests, 17 studies (23.94%) were evaluated by safety and tolerability. In addition, 13 studies (18.31%) failed to provide explanations.
Of the 71 studies, 22 studies (30.99%) were evaluated by adverse events and serious adverse events, 10 studies (14.08%) were evaluated by safety and tolerability. Moreover, 3 studies (4.23%) were evaluated by adverse reactions. The other, 44 studies (61.97%) were not mentioned. Through statistics, it was found that the safety evaluations rate of the studies was low.
4 Discussion
The aim of this study was to summarize the clinical research status of diabetic gastrointestinal diseases, in order to provide clinicians with a faster and more convenient understanding of the current research situation. To the best of our knowledge, this is the first comprehensive assessment of the characteristics of diabetic gastrointestinal diseases-related clinical trials. Our results showed that only 13 studies were diagnosed by meeting the diagnostic criteria for related diseases. In the diagnosis of diabetic gastrointestinal diseases, 85.92% of the studies focused on the first diagnosed as diabetes, and then combined with other indications for further diagnosis of diabetic gastrointestinal diseases. After the diagnosis of diabetes, 86 of the studies were based on past medical history, or the participants had delayed gastric emptying diagnosed as diabetic gastrointestinal diseases. In addition, scoring according to the scales or excluding obstruction was also a common method in the diagnosis of diabetic gastrointestinal diseases. Diabetic gastrointestinal diseases are used to describe the gastrointestinal manifestations of diabetes (4). Therefore, diabetic gastrointestinal diseases are a general term, not refer to a single disease. Thus, the diagnostic criteria for diabetic gastrointestinal diseases were slightly different. From the current research, the diagnostic criteria of diabetic gastrointestinal diseases are diverse and more focused on diabetic gastroparesis. The results of this study are similar to the latest clinical research design of diabetic gastroparesis (16), such as diagnostic criteria, safety evaluations, etc. The latest review of diabetic gastroparesis focuses more on pathogenesis and management, while this study focuses more on study designs (13). In terms of diagnostic criteria, this study is similar to the latest review of diabetic gastroparesis (13). However, this study is different from the latest review of enteric neuropathy in diabetes (14). The latest review of diabetic enteric neuropathy focuses more on the specific approaches of diagnosis and pathophysiological mechanisms (14). Furthermore, only 7 studies had studied diabetic gastrointestinal diseases except diabetic gastroparesis. Therefore, diabetic gastrointestinal diseases remain to be further studied, and more studies on diabetic gastrointestinal diseases such as diabetic enteropathy, diabetic dyspepsia and so on can be carried out in the future.
Surprisingly, 66.19% of the studies had a small sample size (≤100). This may be due to many factors can lead to gastrointestinal symptoms (17, 18), which are difficult to distinguish from the gastrointestinal manifestations caused by diabetes. Therefore, the recruitment of participants in the trial was limited. In addition, all participants in the study were satisfied 18 years old. And the age of most participants was spaned two age groups, only 4 studies brought into adults. This may be related to the course of diabetes. Diabetic patients with a long history were prone to diabetic gastroparesis (19). Another potential reason may be that the current studies focused on diabetic gastroparesis. Therefore, the participants were all aged 18 years and above. In addition, the intervention time of 53.52% of the studies focused on 1 month and less, and only 25.35% of the studies lasted for 3 months. This may be related to the compliance of the participants. The cycle of experimental intervention was long, and patient compliance was relatively poor (20, 21), which affected the results and process of the trials, thus limited the intervention time of the trials. Moreover, 62 studies were not followed up, which may be related to the cost of a lot of manpower and time. Meantime, the financial burden on researchers may also limit the follow-up. Therefore, more attention should be paid to the formulation and implementation of diabetic gastrointestinal diseases program.
This study found that 43.18% of the studies focused on prokinetic drugs therapy. Drug-related treatment, especially prokinetic drugs therapy, has been widely used in diabetic gastroparesis (22). Pharmacologic treatment with prokinetics to increase gastric motility formed the mainstay for the treatment of diabetic gastroparesis (23). However, the use of prokinetics was limited by adverse effects and serious adverse effects (23). Therefore, more studies are needed in the future to carry out the effectiveness of other interventions for diabetic gastroparesis or diabetic gastrointestinal diseases in clinical practice.
Many studies included strong design elements such as randomization, parallel, double blinding, and placebo control groups. In addition, 15.49% of the studies implemented cross-over design. This study design can better measure the effect of the trials and improve patients compliance. Furthermore, 69.01% of the studies used different methods of blinding. The experimental design of the blind method could reduce the difference assessment of outcomes and avoid analytic bias (24). Surprisingly, the number of double-blind and open study designs was almost the same, accounting for 47.89% of the entire studies. The primary and secondary evaluation indicators of the studies contained similar content, but the focus was slightly different. This may be related to the different purposes of the studies. In addition, there were no secondary evaluation indicators in 13 studies. It is recommended to increase the evaluation of secondary relevant indicators to expand the richness and depth of studies. Moreover, the secondary evaluation indicators of 22.54% of the studies could not be classified because there were no similarities. Meantime, 90.15% of the studies were diabetic gastroparesis. It can be seen that there is no consensus on the evaluation indexes of diabetic gastroparesis at present. It is recommended to further explore its related evaluation indicators in the future to form a relatively standardized and comprehensive unified efficacy evaluation. Surprisingly, 61.97% of the studies did not conduct safety evaluation. This may be related to factors such as funding limitations or study design constraints. The safety of the intervention remained to be further investigated, and it was also important to continue to report the safety of the trials. To observe the occurrence of adverse events in the study participants, whether slight or serious, safety evaluation is an important part of the efficacy evaluation indexes. Therefore, it is suggested that future studies should take safety evaluation as a part of efficacy evaluations. However, small sample sizes, short intervention durations and inconsistent safety evaluations may influence the generalizability of findings, thereby hindering their translation into clinical applications. Moreover, there are also considerable heterogeneity in diagnostic criteria and intervention methods, which will have a certain influence on the treatment, management and development of guidelines for patients with diabetic gastrointestinal diseases in the future.
This summary for diabetic gastrointestinal diseases clinical trials had several limitations. Firstly, there may be potential publication bias, such as registry data incompleteness, and the exclusion of unpublished or non-English studies. Secondly, although as widely as possible to retrieve the relevant studies, there may still be missed trials, which may lead to selection bias and reporting bias. These factors might lead to deviations and non-universality in the results.
5 Conclusion
This study presented the first comprehensive overview of the clinical research status of diabetic gastrointestinal diseases. In addition, this study played a key role in pinpointing methodological shortcomings in diabetic gastrointestinal diseases trials. It also paved the way for more robust future research. Our results indicated that the diagnostic criteria of diabetic gastrointestinal diseases are diverse and more focused on diabetic gastroparesis. In the future, researchers should carry out more studies on diabetic gastrointestinal diseases other than diabetic gastroparesis. In addition, most studies had small sample size, short intervention time, large differences in secondary evaluation indicators, and less follow-up. It is recommended to the development of standardized diagnostic protocols and perform longer follow-up periods. Meantime, it is also recommended that comprehensive safety endpoints be included in future trial designs to provide high-quality clinical evidence. Furthermore, the longer the duration of diabetes, the more easy to develop related complications. Therefore, it is not only necessary to pay more attention to patients with diabetic gastrointestinal diseases, but also to pay more attention to diabetic patients in daily care. By conducting large-sample, long-term, multi-angle clinical studies, patient outcomes and the development of evidence-based guidelines can be further improved. Meanwhile, enhanced standardization of study methodology and comprehensiveness of result evaluation are clearly warranted for continued improvements to studies in this field. In addition, the efficacy of different methods in the treatment of diabetic gastrointestinal diseases can be further explored through systematic evaluation and or meta-analysis in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XF: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. YW: Conceptualization, Writing – original draft, Writing – review & editing. JC: Conceptualization, Data curation, Methodology, Writing – original draft. JY: Conceptualization, Data curation, Methodology, Writing – original draft. JT: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing. JM: Conceptualization, Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by The National Natural Science Foundation of China (grant number 82205039), Jilin Province Natural Science Foundation Project (grant number YDZJ202401120ZYTS), High Level Chinese Medical Hospital Promotion Project (grant number HLCMHPP20230CZ40907), CACMS Outstanding Young Scientific and Technological Talents Program (grant number ZZ13-YQ-026), GAMIMD Special Fund (grant number 2022LYJSZX12), and Research Project of Dose-Effect Relationship of Prescription Dose-Effect Branch of China Association of Chinese Medicine (grant number 202428-002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1568552/full#supplementary-material
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Chandran M, Chu NV, Edelman SV. Gastrointestinal disturbances in diabetes. Curr Diabetes Rep. (2003) 3:43–8. doi: 10.1007/s11892-003-0052-7
3. Drewes AM, Søfteland E, Dimcevski G, Farmer AD, Brock C, Frøkjær JB, et al. Brain changes in diabetes mellitus patients with gastrointestinal symptoms. World J Diabetes. (2016) 7:14–26. doi: 10.4239/wjd.v7.i2.14
4. Bharucha AE, Kudva YC, Prichard DO. Diabetic gastroparesis. Endocr Rev. (2019) 40:1318–52. doi: 10.1210/er.2018-00161
5. Caley LR, Zagoya C, Duckstein F, White H, Shimmin D, Jones AM, et al. Diabetes is associated with increased burden of gastrointestinal symptoms in adults with cystic fibrosis. J Cystic Fibrosis: Off J Eur Cystic Fibrosis Soc. (2023) 22:275–81. doi: 10.1016/j.jcf.2023.01.010
6. Talley NJ, Young L, Bytzer P, Hammer J, Leemon M, Jones M, et al. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. (2001) 96:71–6. doi: 10.1111/j.1572-0241.2001.03350.x
7. Du YT, Rayner CK, Jones KL, Talley NJ, Horowitz M. Gastrointestinal symptoms in diabetes: prevalence, assessment, pathogenesis, and management. Diabetes Care. (2018) 41:627–37. doi: 10.2337/dc17-1536
8. Long J, Liang R, Zheng Q, Yuan G, Xin Z, Chen X, et al. Overview of clinical trials on type 2 diabetes mellitus: A comprehensive analysis of the ClinicalTrials.gov database. Diabetes Metab Syndrome Obesity: Targets Ther. (2021) 14:367–77. doi: 10.2147/DMSO.S288065
9. Zhang T, Li W, Chen L. Analysis of hypertension-related clinical trial registration in China based on ClinicalTrials.gov and Chinese Clinical Trial Registry database. West China Med J. (2019) 34:419–24. doi: 10.7507/1002-0179.201901179
10. Zwierzyna M, Davies M, Hingorani AD, Hunter J. Clinical trial design and dissemination: comprehensive analysis of clinicaltrials.gov and PubMed data since 2005. BMJ (Clinical Res ed.). (2018) 361:k2130. doi: 10.1136/bmj.k2130
11. Xu P, Xing X, Yu K, Lv Z, Cui H, Shi Y, et al. Profiles of COVID-19 clinical trials in the Chinese Clinical Trial Registry. Emerg Microbes Infect. (2020) 9:1695–701. doi: 10.1080/22221751.2020.1791736
12. Jin Q, Leaman R, Lu Z. PubMed and beyond: biomedical literature search in the age of artificial intelligence. EBioMedicine. (2024) 100:104988. doi: 10.1016/j.ebiom.2024.104988
13. Uppaluri S, Jain MA, Ali H, Shingala J, Amin D, Ajwani T, et al. Pathogenesis and management of diabetic gastroparesis: An updated clinically oriented review. Diabetes Metab Syndrome. (2024) 18:102994. doi: 10.1016/j.dsx.2024.102994
14. Abdalla MMI. Enteric neuropathy in diabetes: Implications for gastrointestinal function. World J Gastroenterol. (2024) 30:2852–65. doi: 10.3748/wjg.v30.i22.2852
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed.). (2021) 372:n71. doi: 10.1136/bmj.n71
16. McCallum RW, Parkman HP, Fass R, Bhandari BR, Carlson MR, Buck RD. Metoclopramide nasal spray in women with symptomatic diabetic gastroparesis: A randomized, double-blind, placebo-controlled phase 3 study. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterological Assoc. (2024) 22:2497–2505.e5. doi: 10.1016/j.cgh.2023.10.022
17. Camilleri M. Gastrointestinal motility disorders in neurologic disease. J Clin Invest. (2021) 131:e143771. doi: 10.1172/JCI143771
18. Sayuk GS, Gyawali CP. Functional dyspepsia: diagnostic and therapeutic approaches. Drugs. (2020) 80:1319–36. doi: 10.1007/s40265-020-01362-4
19. Feng R, Ma J. Research progresses in pathogenesis, diagnosis, and treatment of diabetic gastroparesis. J Shanghai Jiaotong Univ (Medical Sci). (2016) 36:761–6. doi: 10.3969/j.issn.1674-8115.2016.05.029
20. Feehan M, Morrison MA, Tak C, Morisky DE, DeAngelis MM, Munger MA. Factors predicting self-reported medication low adherence in a large sample of adults in the US general population: a cross-sectional study. BMJ Open. (2017) 7:e014435. doi: 10.1136/bmjopen-2016-014435
21. Audrain-Pontevia AF, Menvielle L, Ertz M. Effects of three antecedents of patient compliance for users of peer-to-peer online health communities: cross-sectional study. J Med Internet Res. (2019) 21:e14006. doi: 10.2196/14006
22. Jalleh R, Marathe CS, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis and glycaemic control. Curr Diabetes Rep. (2019) 19:153. doi: 10.1007/s11892-019-1281-8
23. Kumar M, Chapman A, Javed S, Alam U, Malik RA, Azmi S. The investigation and treatment of diabetic gastroparesis. Clin Ther. (2018) 40:850–61. doi: 10.1016/j.clinthera.2018.04.012
Keywords: diabetic gastrointestinal diseases, diabetic gastroparesis, clinical study, study status, clinical design
Citation: Fan X, Wang Y, Cao J, Yu J, Tian J and Mi J (2025) Clinical study status of diabetic gastrointestinal diseases. Front. Endocrinol. 16:1568552. doi: 10.3389/fendo.2025.1568552
Received: 30 January 2025; Accepted: 26 March 2025;
Published: 09 April 2025.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Rohit Kumar Tiwari, Sharda University, IndiaÇağdaş Erdoğan, Ankara Etlik City Hospital, Türkiye
Eclésio Batista De Oliveira Neto, Centro Universitário de Maceió (Unima) - Maceió University Center, Brazil
Copyright © 2025 Fan, Wang, Cao, Yu, Tian and Mi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaxing Tian, dGluYV95YWlAMTI2LmNvbQ==; Jia Mi, bWlqaWEwMTAxQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Xuechun Fan
Xuechun Fan Yanyan Wang
Yanyan Wang Jingsi Cao1
Jingsi Cao1 Jiaxing Tian
Jiaxing Tian Jia Mi
Jia Mi