- 1Shanghai Key Laboratory of Maternal Fetal Medicine, Shanghai Institute of Maternal-Fetal Medicine and Gynecologic Oncology, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai, China
- 2Department of Reproductive Immunology, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai, China
- 3Department of Assisted Reproductive Medicine, Shanghai First Maternity and Infant Hospital, School of Medicine, Tongji University, Shanghai, China
Background: Letrozole is a first-line treatment for anovulatory infertility in women with polycystic ovary syndrome (PCOS). Despite its widespread use, a significant proportion of patients exhibit non-responsive cycles, limiting treatment efficacy. Identifying predictive markers for ovarian response could enhance the efficiency and cost-effectiveness of ovulation induction. Previous studies have suggested an association between follicle-stimulating hormone receptor (FSHR) polymorphisms and ovarian response, though the precise relationship between these polymorphisms and ovarian response to letrozole remains poorly defined.
Methods: This retrospective study analyzed data from 133 women with PCOS (93 responsive to letrozole and 40 resistant) between January 2021 and May 2024. Demographic data were collected, and genotyping of FSHR single nucleotide polymorphisms (c.2039A>G, rs6166 at position 680, and c.919A>G, rs6165 at position 307) was performed using predesigned TaqMan SNP assays. A binary logistic regression model was employed to evaluate the predictive value of FSHR polymorphisms in letrozole resistance.
Results: The Asn/Asn polymorphism at position 680 was significantly more prevalent in the letrozole-resistant group (57.5%) compared to the letrozole-responsive group (34.41%) [OR: 1.543 (95% CI, 1.046-2.278), P = 0.013]. Similarly, the Thr/Thr polymorphism at position 307 was more common in the resistance group (57.5% vs. 30.11%) [OR: 1.645 (95% CI, 1.120-2.415), P = 0.003]. Participants with either the Asn/Asn or Thr/Thr polymorphism were more likely to exhibit resistance to letrozole. Logistic regression analysis indicated that the Asn/Asn polymorphism was a potential risk factor for letrozole resistance [OR: 5.227 (95% CI, 0.994-27.490), P = 0.051], while the Thr/Thr polymorphism significantly influenced letrozole response [OR: 7.04 (95% CI, 1.394-35.559), P = 0.018].
Conclusions: The Asn/Asn polymorphism at position 680 and the Thr/Thr polymorphism at position 307 of the FSHR gene may serve as potential predictors of ovulatory failure during letrozole therapy in PCOS patients.
Introduction
Polycystic ovarian syndrome (PCOS) is a prevalent reproductive endocrine disorder affecting women of childbearing age (1), contributing to approximately 80% of anovulatory infertility cases (2). Ovulation induction is the primary treatment for women with PCOS seeking to conceive (3, 4). However, ovarian responses to induction vary considerably among individuals, complicating the ability to predict successful ovulation. Identifying reliable predictive markers for ovarian response could improve treatment efficiency, reduce costs, and shorten the time to conception.
Follicle-stimulating hormone (FSH) is essential for follicular maturation and the recruitment of the dominant follicle (5). Its action is mediated by the FSH receptor (FSHR), a G protein-coupled receptor expressed exclusively on granulosa cells in the ovary. The FSHR gene, located on chromosome 2p21-p16, consists of 9 introns and 10 exons (6). Several polymorphisms have been identified in the FSHR gene, with two major variants under extensive study. One is a single nucleotide polymorphism (SNP) at position 680 within the intracellular domain, where asparagine (Asn) or serine (Ser) can occupy the position (c.2039A>G, rs6166). The second SNP occurs at position 307 in the extracellular domain, where threonine (Thr) or alanine (Ala) may be present (c.919A>G, rs6165) (7). Studies have shown that the FSHR genotype significantly influences ovarian response during stimulation (8), with the presence of the Ser680 allele in the FSHR gene correlating with ovulation failure following clomiphene citrate (CC) treatment in PCOS patients undergoing ovulation induction (9).
“CC resistance” refers to the failure of ovulation despite CC treatment. For these patients, letrozole is often used, achieving ovulation rates ranging from 54.6% to 84.4% (10, 11). Letrozole functions by inhibiting the conversion of androgens to estrogens, alleviating the negative estrogen feedback on the hypothalamus (12). This results in increased pituitary FSH secretion, thereby promoting follicular development. As a third-generation aromatase inhibitor with a shorter half-life, letrozole has fewer anti-estrogenic effects and side effects compared to CC, making it a preferred option in clinical practice due to its higher ovulation and pregnancy rates (13, 14). Despite its effectiveness, a portion of cycles remain non-responsive to letrozole during ovulation induction (13–15), a phenomenon known as “letrozole resistance” (16–19). To date, the relationship between FSHR polymorphisms and ovarian response to letrozole remains underexplored. This study aims to investigate whether FSHR polymorphisms could serve as a predictive factor for ovarian response to letrozole in women with PCOS undergoing ovulation induction therapy.
Materials and methods
Study design and participants
Women diagnosed with PCOS and undergoing ovulation induction therapy with letrozole at the Department of Assisted Reproductive Medicine, Shanghai First Maternity and Infant Hospital, between January 2021 and May 2024, were included in this study. PCOS diagnosis followed the Rotterdam criteria (20), requiring at least two of the following three features: oligomenorrhea or anovulation, clinical or biochemical hyperandrogenism, and the sonographic appearance of polycystic ovaries. Oligomenorrhea was defined as menstrual cycles occurring every 35 days to 3 months, while amenorrhea was defined as cycles exceeding 3 months. Hyperandrogenemia was diagnosed either clinically (e.g., acne or hirsutism) or biochemically (testosterone ≥ 2.5 nmol/L or free androgen index [FAI] ≥ 5). Polycystic ovaries were characterized by the presence of more than 12 antral follicles (2–9 mm in diameter) on ultrasound, or the presence of at least one ovary with a volume greater than 10 cm3, without accompanying cysts. Exclusion criteria were (1): age ≥ 40 years, (2) use of other pharmacologic agents for ovulation induction, such as metformin, CC, or gonadotropins, during the study. The study received approval from the Research Ethics Committee of Shanghai First Maternity and Infant Hospital.
Interventions
In this study, all patients underwent ovulation induction with letrozole alone. Letrozole (Letrozole Tablets; Yimeishu, Zhejiang Hi Sun Pharmaceutical Co., Ltd., Taizhou, China) was administered at a dose of 5 mg per day from day 2 to day 4 of the menstrual cycle (MC) or following progestin-induced withdrawal bleeding for a total of 5 consecutive days. Follicular development was monitored via transvaginal ultrasound every 2–4 days after the last dose of letrozole to assess the number of growing follicles and endometrial thickness. Serum levels of FSH, luteinizing hormone (LH), estradiol (E2), and progesterone were measured using a chemiluminescent assay (Abbott Biologicals B.V., Weesp, The Netherlands). Ovulation was confirmed by the disappearance of a follicle greater than 14 mm in diameter or a serum progesterone level exceeding 3 ng/mL, followed by either a positive pregnancy test or menstruation (18, 19).
Letrozole resistance was defined as the absence of a dominant follicle (diameter > 10 mm), E2 < 70 pg/mL, and progesterone < 1.0 ng/mL 14 days after completing treatment. In these cases, dydrogesterone (Duphaston; Abbott Biologicals B.V., Weesp, The Netherlands) was administered to induce withdrawal bleeding. Blood samples were collected after the 5-day letrozole treatment and stored at -80°C for subsequent analysis.
Detection of FSHR genotypes
Genomic DNA was extracted from blood samples using the Blood DNA Extraction Kit (EMERTHER Biotech, Shanghai, China). Genotyping of two FSHR SNPs was performed using pre-designed TaqMan SNP assays: c.919A>G (rs6165) with primers (forward: TGCTATACTGGATCTGAGATGTTGA; reverse: CTTAGGGGAGCAGGTCACG) and c.2039A>G (rs6166) with primers (forward: TGCCAACCCCTTCCTCTATG; reverse: GCTCTTTGTGACATACCCTTCAA). Sequencing was conducted using a 3730xl sequencer, and the results were analyzed with DNA Sequencing Analysis software, interpreted using Sequencing Analysis 5.2.0, and aligned using Sequencher 5.1 software.
Statistical analysis
Data are presented as median (IQR) or n (%) values. The Mann-Whitney U test was used for continuous variables due to non-normal distribution. Fisher’s exact test or Pearson’s chi-square test was employed for categorical variables. Spearman’s correlation analysis assessed the association between letrozole resistance and other variables. Binary logistic regression models were used to evaluate the impact of FSHR polymorphisms on letrozole resistance: model 1 (letrozole resistance as the dependent variable and FSHR variant as the independent variable) and model 2 (letrozole resistance as the dependent variable, with FSHR variant, BMI, and menstrual cycle as independent variables). All statistical analyses were performed using SPSS version 26 (IBM Corporation, Armonk, NY, USA). A two-tailed P value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of participants
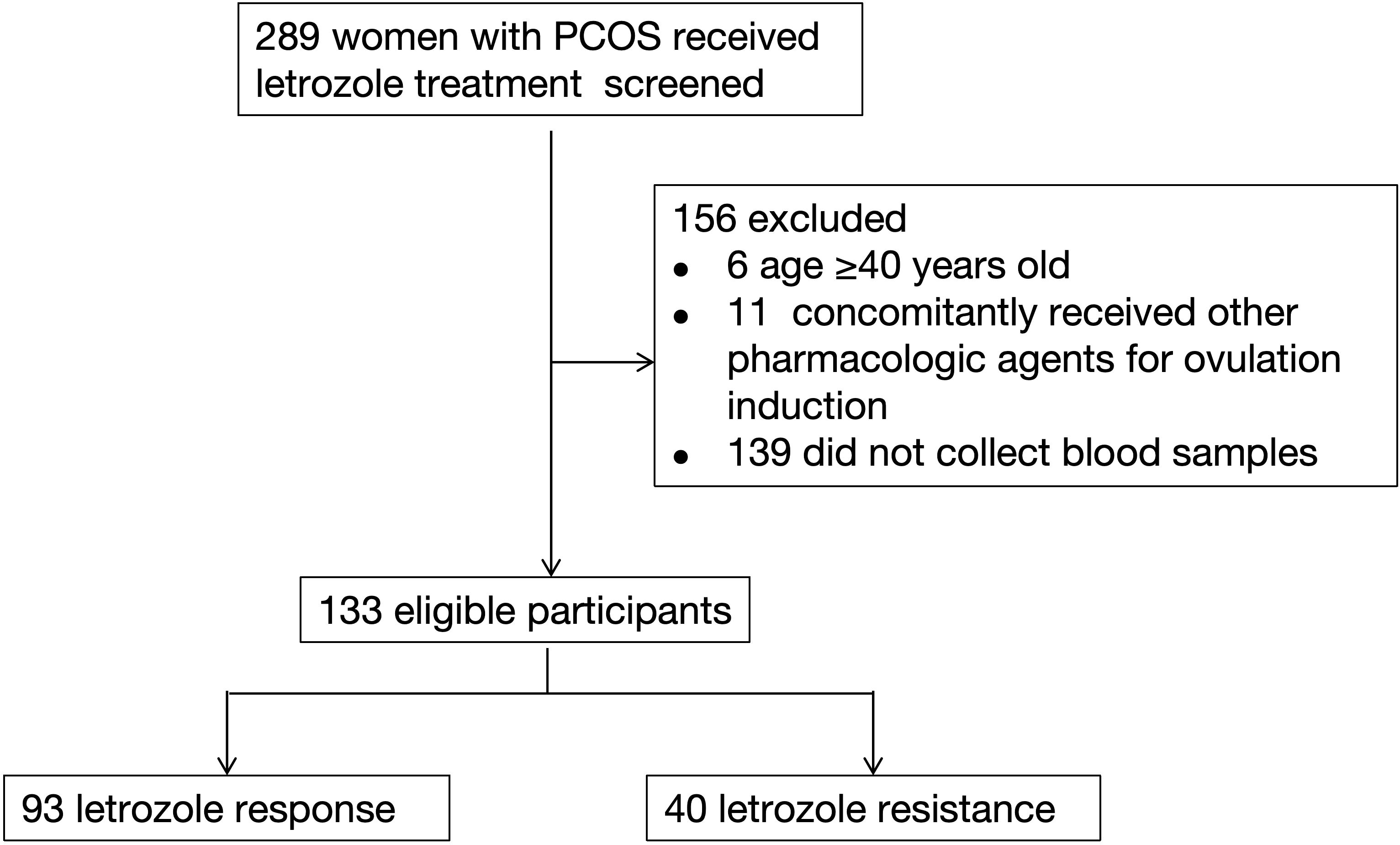
A total of 289 women with PCOS who received letrozole treatment at the Department of Assisted Reproductive Medicine, Shanghai First Maternity and Infant Hospital, between January 2021 and May 2024, were initially screened for inclusion. Following screening, 156 women were excluded based on the following criteria: 6 women aged over 40 years, 11 women receiving concomitant pharmacologic agents for ovulation induction, and 139 women who did not provide blood samples for genetic analysis.
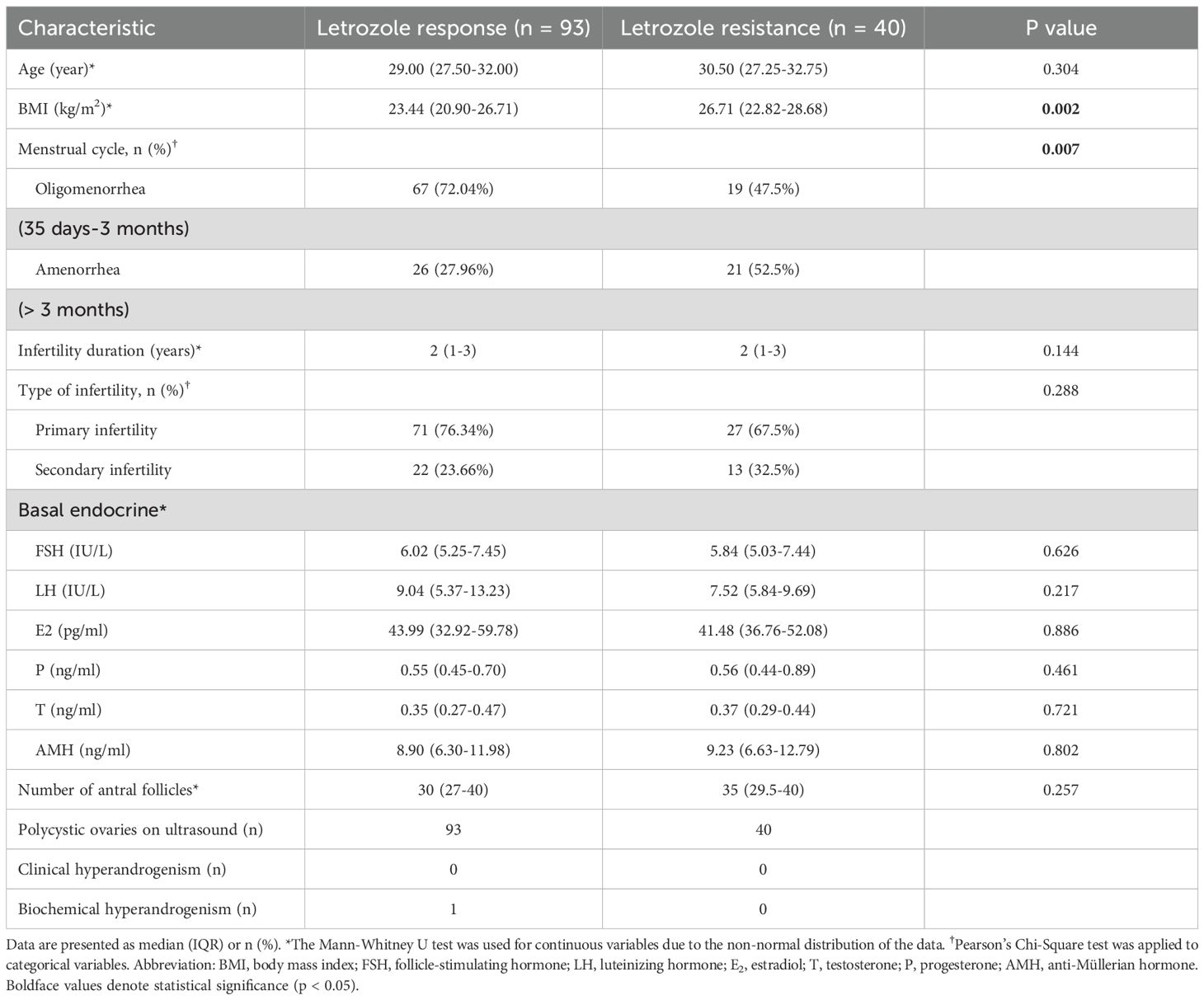
Thus, 133 women with PCOS meeting the inclusion criteria were included in the final study cohort. Of these, 93 were classified into the letrozole response group, and 40 into the letrozole resistance group (Figure 1). No significant differences were observed between the two groups in terms of age, infertility duration, infertility type, or number of antral follicles (Table 1). Basal levels of FSH, LH, E2, progesterone, testosterone, and anti-Müllerian hormone (AMH) were also similar across the groups. However, BMI was significantly higher in the letrozole resistance group [26.71 (22.82-28.68)] compared to the response group [23.44 (20.90-26.71)] (P = 0.002), and the menstrual cycle differed significantly between the groups (P = 0.007). All patients exhibited abnormal menstrual cycles and polycystic ovaries on ultrasound, with only one woman diagnosed with biochemical hyperandrogenism.
Genotype distributions of FSHR polymorphisms
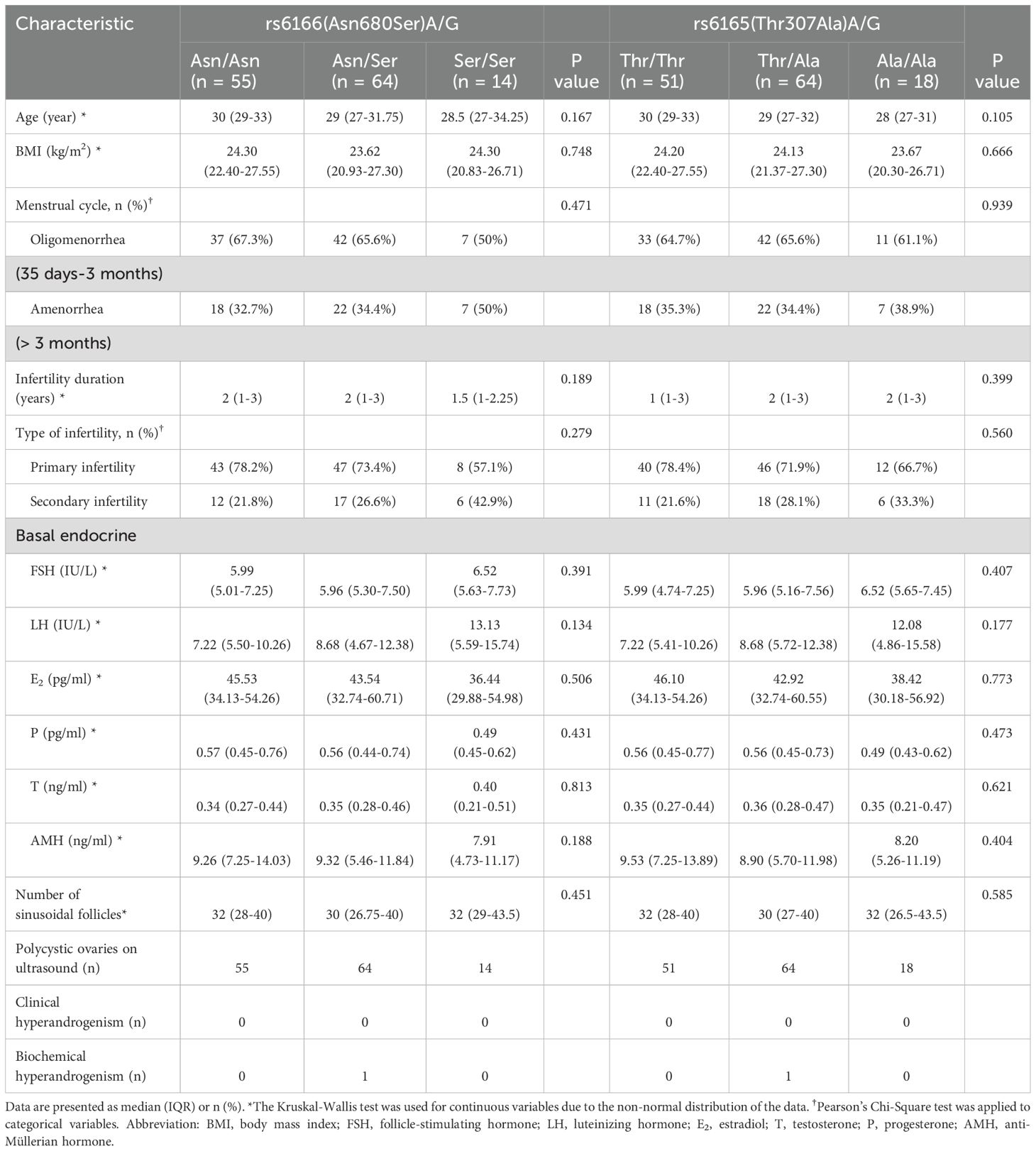
FSHR polymorphisms at rs6166 (Asn680Ser) and rs6165 (Thr307Ala) were analyzed. The baseline characteristics of participants with different FSHR genotypes did not differ significantly (Table 2). For rs6166 (Asn680Ser), the frequency of the Asn/Asn polymorphism was significantly higher in the letrozole resistance group (57.5%) compared to the response group (34.41%) [OR: 1.543 (95% CI, 1.046-2.278), P = 0.013] (Table 3). No significant difference was observed in the distribution of Asn/Ser and Ser/Ser polymorphisms between the groups. For rs6165 (Thr307Ala), the Thr/Thr polymorphism was more frequent in the letrozole resistance group (57.5%) compared to the response group (30.11%) [OR: 1.645 (95% CI, 1.120-2.415), P = 0.003]. No significant differences were found for the other two polymorphisms. Thus, participants with the Asn/Asn or Thr/Thr polymorphisms were more likely to exhibit resistance to letrozole than those with the Asn/Ser or Ser/Ser polymorphisms or the Thr/Ala or Ala/Ala polymorphisms (Figure 2).
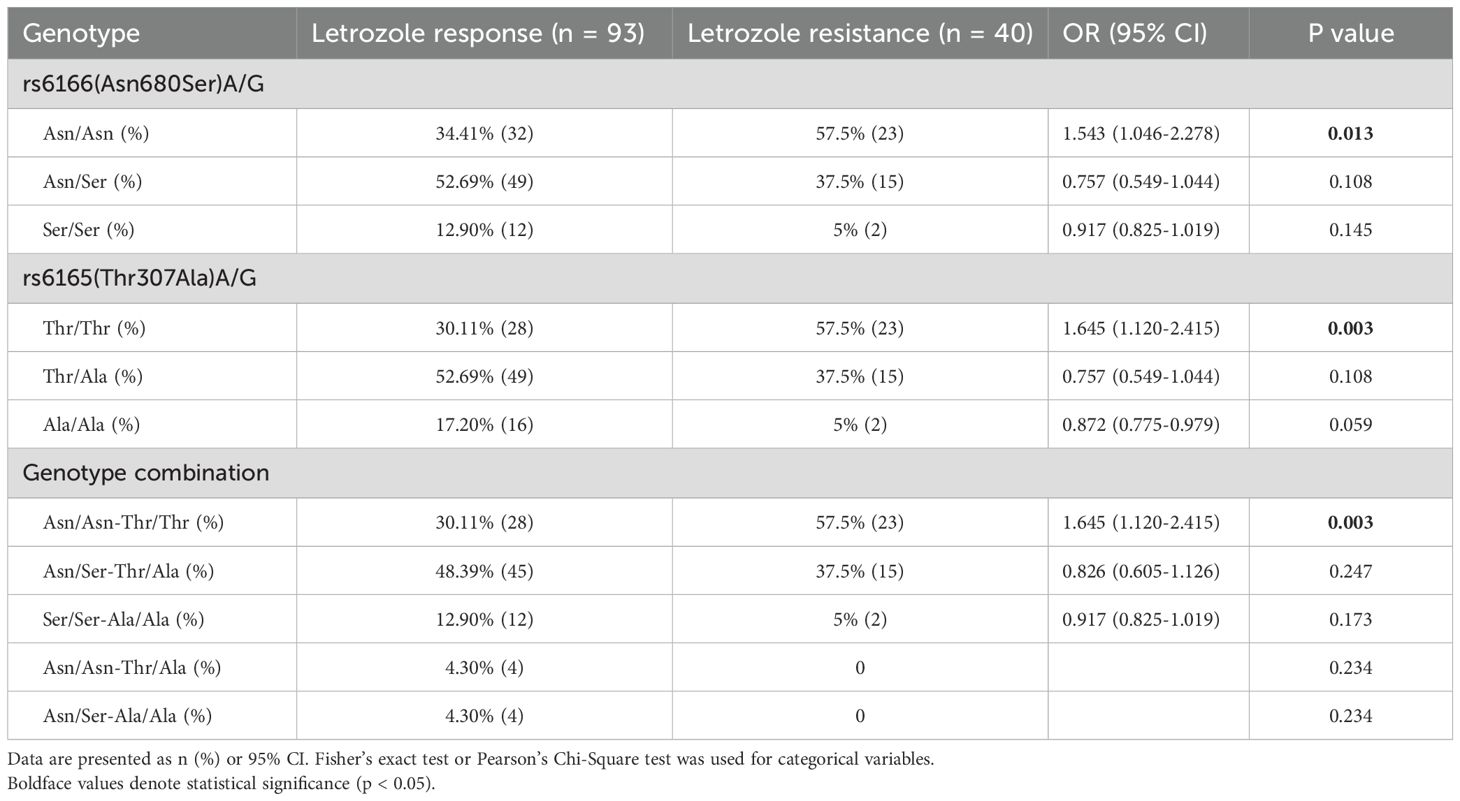
Table 3. Genotype distributions of FSHR polymorphisms in PCOS patients with letrozole response and resistance.
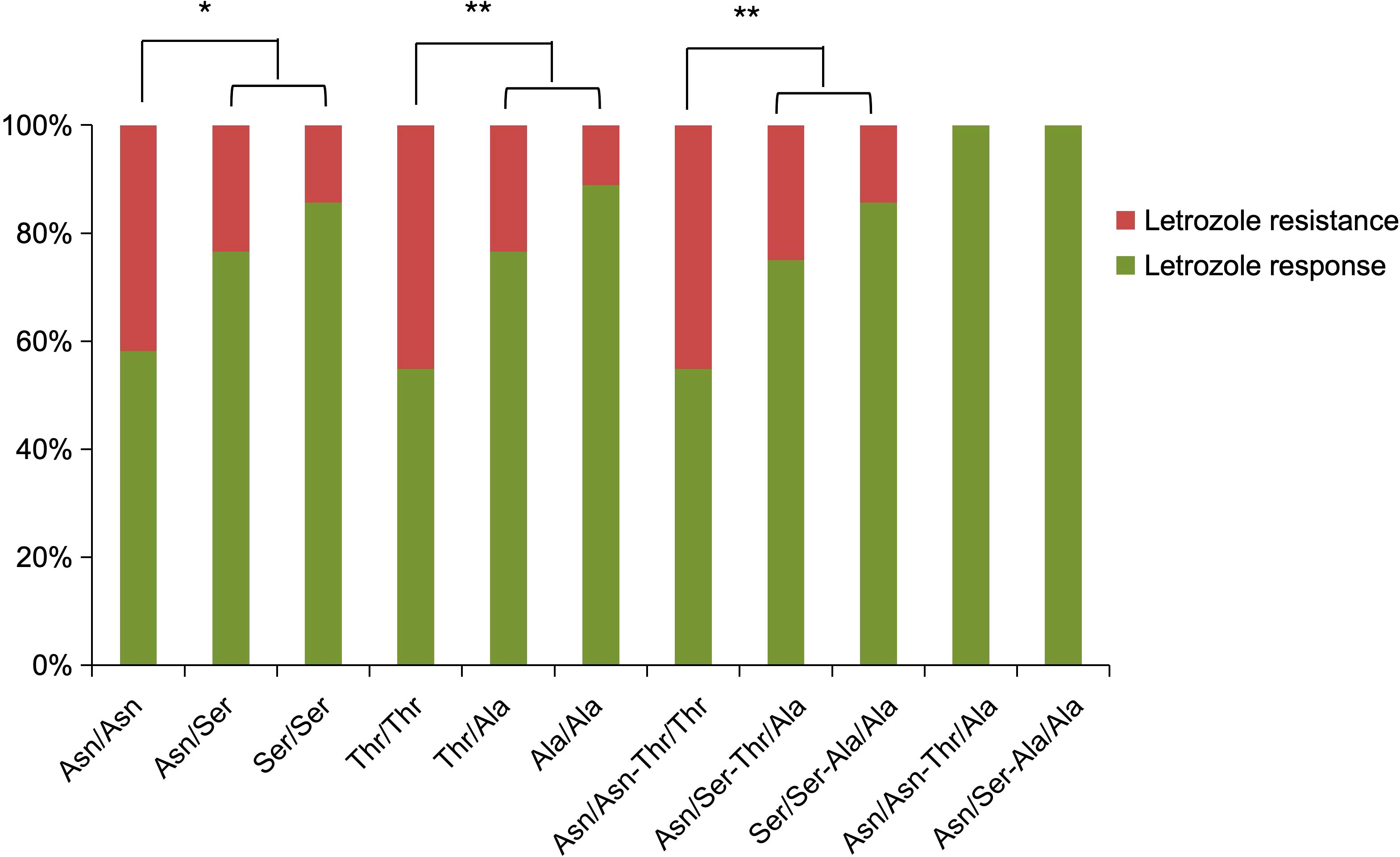
Figure 2. Distribution of FSHR polymorphisms in the letrozole response and resistance groups, expressed as percentages.
Prediction of FSHR polymorphisms for letrozole resistance
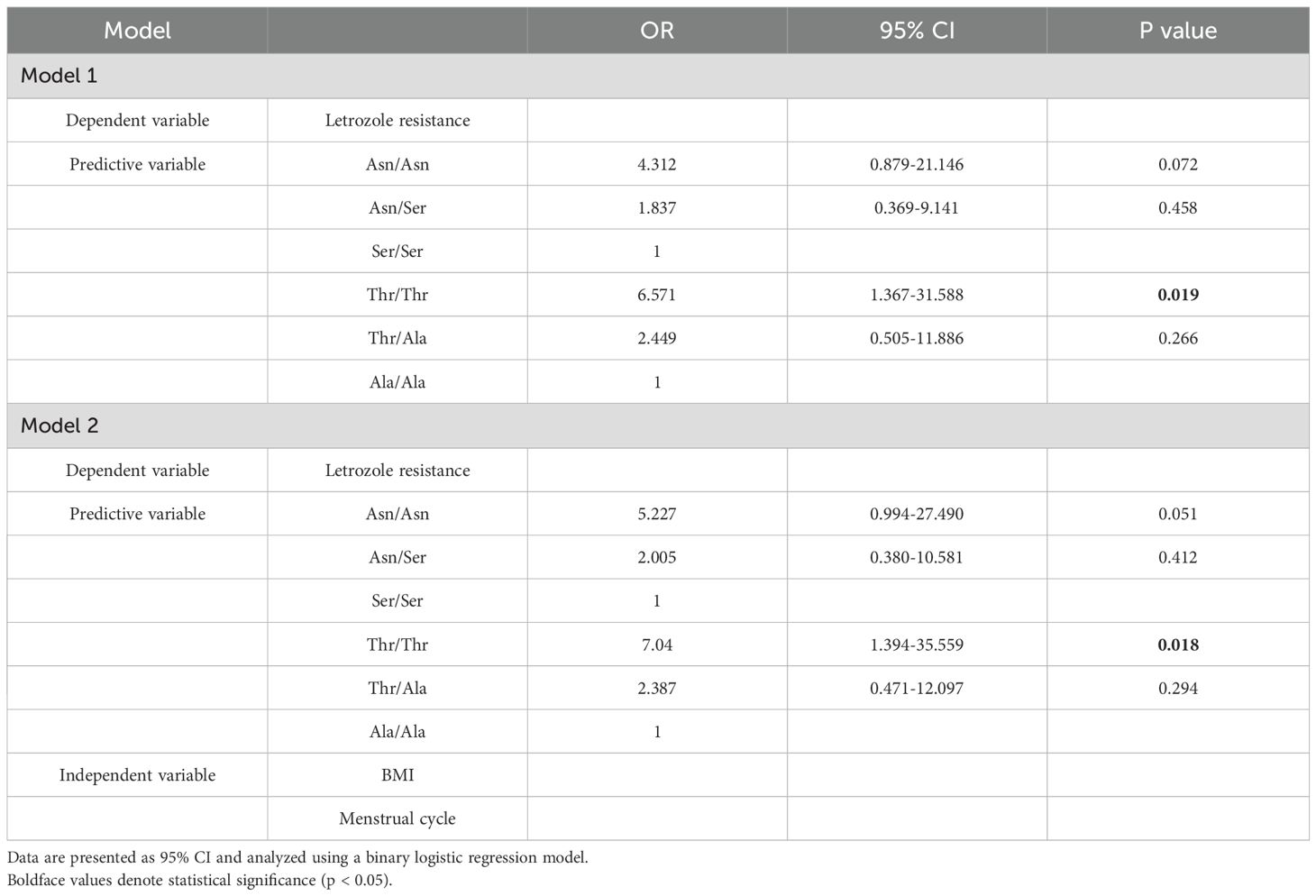
Spearman correlation analysis indicated that letrozole resistance was significantly associated with FSHR polymorphisms and positively correlated with both BMI and menstrual cycle (Figure 3). Logistic regression analysis was conducted with letrozole resistance as the dependent variable and FSHR variant as a predictive factor (Model 1, Table 4). The analysis showed that the Asn/Asn polymorphism tended to affect letrozole response [OR: 4.312 (95% CI, 0.879-21.146), P = 0.072], while the Thr/Thr polymorphism significantly influenced the response [OR: 6.571 (95% CI, 1.367-31.588), P = 0.019]. Given that BMI and menstrual cycle may serve as potential confounders for letrozole resistance, a further analysis using Model 2 was conducted, incorporating these factors as independent variables. The results were consistent with those obtained in Model 1, reinforcing the finding that the Thr/Thr polymorphism is a sensitive predictor for letrozole resistance.
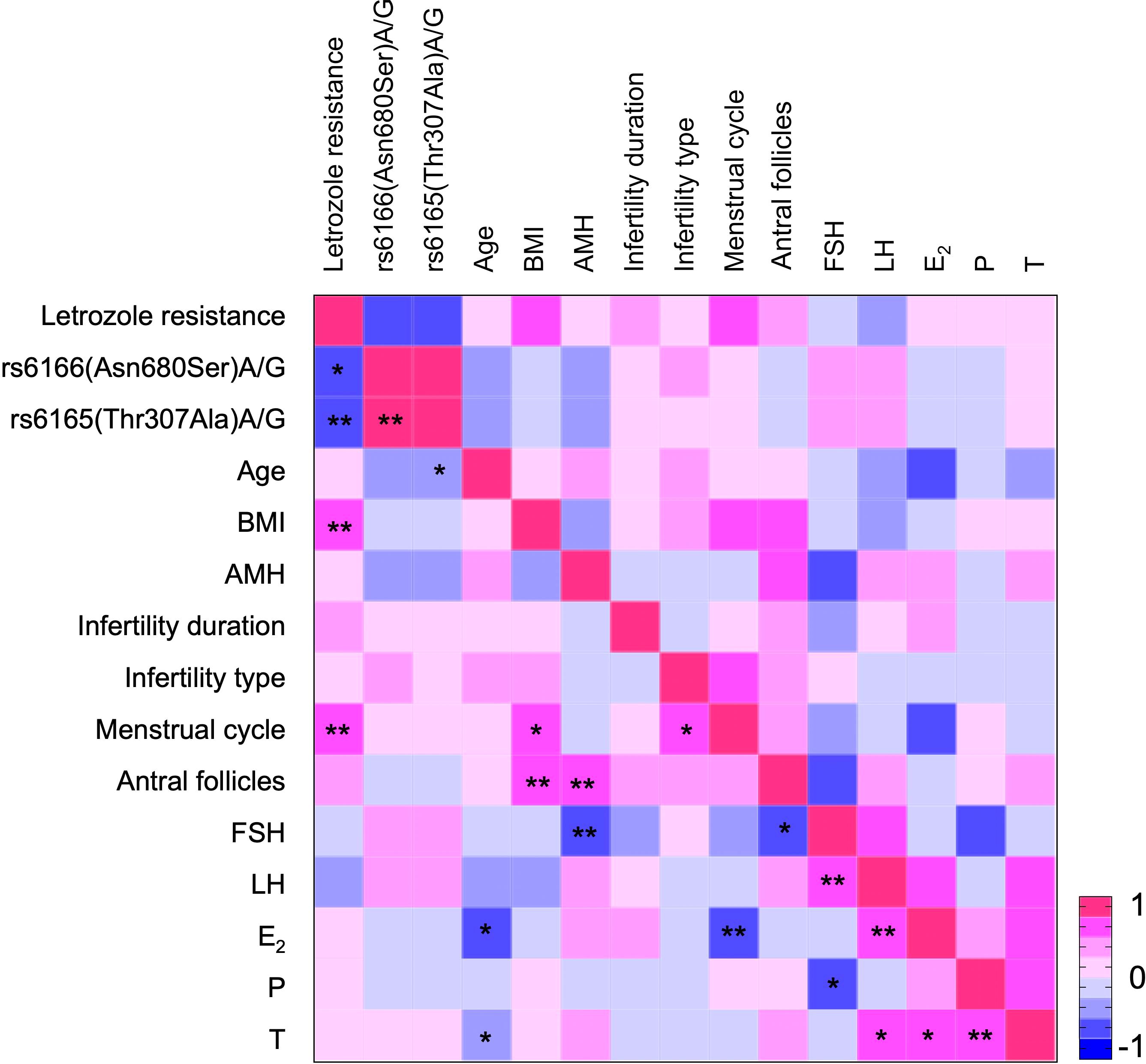
Figure 3. Heatmap showing the association between letrozole resistance and various variables. Red indicates a positive correlation, while blue denotes a negative correlation. The intensity of the color reflects the magnitude of the correlation coefficient. *P < 0.05, **P < 0.01, indicating statistical significance of the correlation coefficient.
Discussion
This study represents the first investigation into whether FSHR polymorphisms can predict ovarian response to letrozole in women with PCOS during ovulation induction. The data reveal that participants with the Asn/Asn polymorphism at position 680 or the Thr/Thr polymorphism at position 307 of FSHR were significantly more likely to experience ovulatory failure following letrozole treatment. Among these, the Thr/Thr polymorphism emerged as the most sensitive predictor of such failure.
FSHR polymorphisms have been extensively studied in both PCOS and non-PCOS populations, with genotype distribution varying across different ethnic groups (21, 22). In this study, the distribution of FSHR genotypes in women with PCOS was 41.35% Asn/Asn, 48.12% Asn/Ser, and 10.53% Ser/Ser at position 680, and 38.35% Thr/Thr, 48.12% Ala/Thr, and 13.53% Ala/Ala at position 307. These results align with those reported in Japanese (23) and South Korean (24) populations but diverge from findings in Caucasian women (22). Kim et al. observed a significant difference in FSHR genotype distribution between women with PCOS and age-matched controls, suggesting an association between FSHR polymorphisms and PCOS susceptibility. Specifically, the genotype distribution in the PCOS group was 39.5%, 47.2%, and 13.3% for Asn/Asn, Asn/Ser, and Ser/Ser, respectively, compared to 46.4%, 45.4%, and 8.2% in controls; and 38.5%, 46.7%, and 14.9% for Thr/Thr, Thr/Ala, and Ala/Ala in the PCOS group, compared to 46.6%, 45.4%, and 8.0% in controls (24). However, this increased susceptibility was not confirmed in Chinese and Thai populations (25). Valkenburg et al. reported that the Ser680 allele correlated with higher levels of FSH, LH, and testosterone, as well as a higher frequency of hyperandrogenism, but was not associated with PCOS risk itself (26). This suggests that while FSHR polymorphisms may affect hormonal levels, their role in PCOS pathogenesis remains uncertain. The impact of FSHR variants on basal FSH levels has been observed in several studies (8, 27). In contrast, our study observed no significant association between FSHR genotypes and basal sex hormone levels, consistent with the findings of Overbeek et al. (9).
The relationship between FSHR polymorphisms and ovarian response to ovulation induction remains unclear. Although numerous studies have examined the impact of FSHR variants on ovarian response during IVF/ICSI cycles, demonstrating that Ser/Ser and Ala/Ala polymorphisms necessitate higher doses of exogenous FSH for ovarian stimulation (28–34), few have focused on their effect during ovulation induction with drugs like letrozole and CC (9, 26, 35). For instance, Overbeek et al. reported that PCOS patients with the Ser/Ser genotype were more likely to exhibit CC resistance (28%) compared to those with Asn/Ser (14%) or Asn/Asn (15%) genotypes, suggesting that FSHR polymorphisms may influence CC resistance (9). Similarly, Valkenburg et al. found that patients with the Ser/Ser genotype at position 680 had an 89% higher likelihood of CC resistance (OR = 1.9, 95% CI, 1.1–3.3) (26). In contrast, Laven et al. observed no significant differences in ovarian response or required FSH doses among anovulatory women with varying FSHR genotypes at positions 307 and 680 (35), implying that the role of FSHR polymorphisms in ovarian responsiveness may not be straightforward.
In the present study, the Asn/Asn 680 and Thr/Thr 307 polymorphisms were found to be more likely associated with resistance to letrozole treatment, contrasting with previous studies suggesting that Ser/Ser 680 and Ala/Ala 307 polymorphisms are linked to a better response (9, 26, 32). Logistic regression analysis, both unadjusted and adjusted for other factors, confirmed this association between FSHR polymorphisms and letrozole resistance. These results highlight that FSHR genotype plays a critical role in determining ovarian sensitivity to ovulation induction medications. Additionally, even individuals with the same genotype may exhibit varying responses to different treatments.
The underlying cause of this variation remains unclear, but it may be attributed to the distinct mechanisms through which letrozole and CC exert their effects. Unlike CC, which primarily influences the hypothalamic-pituitary axis, letrozole increases ovarian sensitivity to FSH by elevating local androgen levels within the ovaries. This mechanism may explain why patients resistant to CC might respond favorably to letrozole (10, 11). These findings support the potential benefit of tailoring ovulation induction treatments based on FSHR genotype. Specifically, patients with the Ser/Ser or Ala/Ala polymorphism may exhibit a better response to letrozole, while those with the Asn/Asn or Thr/Thr polymorphism might derive greater benefit from CC as a first-line treatment. Such personalized approaches could enhance treatment efficiency, minimize cycle cancellations, and ultimately increase the likelihood of success in a shorter time frame.
This study also identified a significant association between higher BMI and longer menstrual cycles with ovulatory failure following letrozole treatment, aligning with previous research. Palomba et al. found that PCOS patients who failed to ovulate after CC treatment had a significantly higher BMI compared to those who ovulated (32.3 vs. 25.9) (36). Similarly, Overbeek et al. observed significant BMI differences between CC non-responders and responders (26.3 vs. 23.6) (9). Furthermore, a prospective study by Küniger et al. revealed a significant correlation between BMI and the required FSH dose for achieving monofollicular development in CC-resistant PCOS patients (37). These findings suggest that BMI may serve as an independent predictor of ovarian sensitivity in PCOS. After adjusting for BMI and menstrual cycle length, the predictive value of FSHR polymorphisms for resistance to letrozole remained statistically significant, further reinforcing their role in predicting ovarian response to ovulation induction.
This study has several strengths. It is the first to examine the predictive effect of FSHR genotypes on ovarian response to letrozole in women with PCOS, utilizing both Spearman correlation analysis and logistic regression models to validate the results. However, several limitations must be acknowledged. One major limitation is the relatively low prevalence of clinical and biochemical hyperandrogenism in our cohort, which may reflect ethnic differences. In a previous randomized controlled trial (RCT) conducted by our group, a similarly low prevalence of hyperandrogenism was observed among PCOS patients, with only 4.05% (3/74) in the study group and 1.35% (1/74) in the control group presenting with clinical hyperandrogenism (19). This suggests that ethnic factors may influence the manifestation of hyperandrogenism in PCOS, potentially limiting the generalizability of our findings to populations with higher prevalence rates of hyperandrogenism. Further studies involving more diverse populations are necessary to validate these conclusions. Another limitation is the retrospective design of our study and the relatively small sample size, highlighting the need for larger, prospective trials to confirm these findings. Additionally, the underlying mechanisms by which FSHR genotypes affect ovarian response to letrozole remain unclear, warranting further exploration.
Conclusion
In conclusion, our study provides novel evidence that FSHR polymorphisms at rs6165 (position 307) and rs6166 (position 680) are associated with ovarian response in women with PCOS undergoing ovulation induction with letrozole. Specifically, the Asn/Asn 680 and Thr/Thr 307 polymorphisms may serve as valuable predictors of ovulatory failure. These findings contribute to a deeper understanding of the genetic factors influencing ovulation induction outcomes and highlight the potential for personalized treatment strategies. By considering FSHR genotypes, clinicians can better tailor ovulation induction agents and their dosages, enhancing treatment efficacy, minimizing costs, and ultimately improving patient care for women with PCOS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Research Ethics Committee of Shanghai First Maternity and Infant Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XZ: Supervision, Writing – review & editing, Methodology, Validation, Writing – original draft. QW: Methodology, Validation, Writing – original draft, Writing – review & editing, Data curation, Project administration, Resources. JL: Methodology, Project administration, Resources, Validation, Writing – original draft, Writing – review & editing. YZ: Methodology, Validation, Writing – review & editing, Software. YF: Writing – review & editing, Conceptualization, Funding acquisition, Investigation, Resources, Supervision. SB: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Natural Science Foundation of Shanghai (grant number: 23ZR1450100 to SB), the National Natural Science Foundation of China (grant number: 82200935 to QW), the Science and Technology Commission of Shanghai Municipality (grant number: 18411963800 to YF), and the Shanghai First Maternity and Infant Hospital, affiliated with Tongji University School of Medicine (grant numbers: 2023B03 to YF, 2023B18 to XZ, 2023B13 to JL, and 2020RC02 to YF).
Acknowledgments
The authors would like to thank the staff of the Department of Assisted Reproduction at Shanghai First Maternity and Infant Hospital for their cooperation and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1585655/full#supplementary-material
References
1. Shrivastava S and Conigliaro RL. Polycystic ovarian syndrome. Med Clin North Am. (2023) 107:227–34. doi: 10.1016/j.mcna.2022.10.004
2. Carson SA and Kallen AN. Diagnosis and management of infertility: A review. JAMA. (2021) 326:65–76. doi: 10.1001/jama.2021.4788
3. Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. (2016) 22:687–708. doi: 10.1093/humupd/dmw025
4. Teede HJ, Tay CT, Laven J, Dokras A, Moran LJ, Piltonen TT, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome†. Hum Reprod. (2023) 38:1655–79. doi: 10.1093/humrep/dead156
5. Scheele F and Schoemaker J. The role of follicle-stimulating hormone in the selection of follicles in human ovaries: a survey of the literature and a proposed model. Gynecol Endocrinol. (1996) 10:55–66. doi: 10.3109/09513599609041271
6. Gromoll J, Ried T, Holtgreve-Grez H, Nieschlag E, and Gudermann T. Localization of the human FSH receptor to chromosome 2 p21 using a genomic probe comprising exon 10. J Mol Endocrinol. (1994) 12:265–71. doi: 10.1677/jme.0.0120265
7. Simoni M, Gromoll J, and Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. (1997) 18:739–73. doi: 10.1210/edrv.18.6.0320
8. Perez Mayorga M, Gromoll J, Behre HM, Gassner C, Nieschlag E, and Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab. (2000) 85:3365–9. doi: 10.1210/jcem.85.9.6789
9. Overbeek A, Kuijper EA, Hendriks ML, Blankenstein MA, Ketel IJ, Twisk JW, et al. Clomiphene citrate resistance in relation to follicle-stimulating hormone receptor Ser680Ser-polymorphism in polycystic ovary syndrome. Hum Reprod. (2009) 24:2007–13. doi: 10.1093/humrep/dep114
10. Al-Omari WR, Sulaiman WR, and Al-Hadithi N. Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. (2004) 85:289–91. doi: 10.1016/j.ijgo.2003.11.010
11. Elnashar A, Fouad H, Eldosoky M, and Saeid N. Letrozole induction of ovulation in women with clomiphene citrate-resistant polycystic ovary syndrome may not depend on the period of infertility, the body mass index, or the luteinizing hormone/follicle-stimulating hormone ratio. Fertil Steril. (2006) 85:511–3. doi: 10.1016/j.fertnstert.2005.08.016
12. Mitwally MF and Casper RF. Aromatase inhibitors in ovulation induction. Semin Reprod Med. (2004) 22:61–78. doi: 10.1055/s-2004-823028
13. Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. (2014) 371:119–29. doi: 10.1056/NEJMoa1313517
14. Liu Z, Geng Y, Huang Y, Hu R, Li F, Song Y, et al. Letrozole compared with clomiphene citrate for polycystic ovarian syndrome: A systematic review and meta-analysis. Obstet Gynecol. (2023) 141:523–34. doi: 10.1097/AOG.0000000000005070
15. Dai X, Li J, Fu T, Long X, Li X, Weng R, et al. Ovulation induction using sequential letrozole/gonadotrophin in infertile women with PCOS: a randomized controlled trial. Reprod BioMed Online. (2023) 46:352–61. doi: 10.1016/j.rbmo.2022.08.002
16. Guo Z, Chen S, Chen Z, Hu P, Hao Y, and Yu Q. Predictors of response to ovulation induction using letrozole in women with polycystic ovary syndrome. BMC Endocr Disord. (2023) 23:90. doi: 10.1186/s12902-023-01336-z
17. Neblett MF 2nd, Baumgarten SC, Babayev SN, and Shenoy CC. Ovulation induction with letrozole and dexamethasone in infertile patients with letrozole-resistant polycystic ovary syndrome. J Assist Reprod Genet. (2023) 40:1461–6. doi: 10.1007/s10815-023-02817-9
18. Zhu X and Fu Y. Extending letrozole treatment duration is effective in inducing ovulation in women with polycystic ovary syndrome and letrozole resistance. Fertil Steril. (2023) 119:107–13. doi: 10.1016/j.fertnstert.2022.09.018
19. Zhu X, Lang J, Wang Q, and Fu Y. Extended versus conventional letrozole regimen in patients with polycystic ovary syndrome undergoing their first ovulation induction cycle: a prospective randomized controlled trial. Hum Reprod Open. (2024) 2024:hoae046. doi: 10.1093/hropen/hoae046
20. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
21. Kaur M, Singh S, and Kaur A. Polymorphisms in FSHR modulating susceptibility to polycystic ovary syndrome: an updated meta-analysis. J Ovarian Res. (2023) 16:183. doi: 10.1186/s13048-023-01238-7
22. Polyzos NP, Neves AR, Drakopoulos P, Spits C, Alvaro Mercadal B, Garcia S, et al. The effect of polymorphisms in FSHR and FSHB genes on ovarian response: a prospective multicenter multinational study in Europe and Asia. Hum Reprod. (2021) 36:1711–21. doi: 10.1093/humrep/deab068
23. Sudo S, Kudo M, Wada S, Sato O, Hsueh AJ, and Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. (2002) 8:893–9. doi: 10.1093/molehr/8.10.893
24. Kim JJ, Choi YM, Hong MA, Chae SJ, Hwang K, Yoon SH, et al. FSH receptor gene p. Thr307Ala and p. Asn680Ser polymorphisms are associated with the risk of polycystic ovary syndrome. J Assist Reprod Genet. (2017) 34:1087–93. doi: 10.1007/s10815-017-0953-z
25. Laven JSE. Follicle stimulating hormone receptor (FSHR) polymorphisms and polycystic ovary syndrome (PCOS). Front Endocrinol (Lausanne). (2019) 10:23. doi: 10.3389/fendo.2019.00023
26. Valkenburg O, Uitterlinden AG, Piersma D, Hofman A, Themmen AP, de Jong FH, et al. Genetic polymorphisms of GnRH and gonadotrophic hormone receptors affect the phenotype of polycystic ovary syndrome. Hum Reprod. (2009) 24:2014–22. doi: 10.1093/humrep/dep113
27. Conforti A, Tuttelmann F, Alviggi C, Behre HM, Fischer R, Hu L, et al. Effect of genetic variants of gonadotropins and their receptors on ovarian stimulation outcomes: A delphi consensus. Front Endocrinol (Lausanne). (2022) 12:797365. doi: 10.3389/fendo.2021.797365
28. Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. (2005) 15:451–6. doi: 10.1097/01.fpc.0000167330.92786.5e
29. Daelemans C, Smits G, de Maertelaer V, Costagliola S, Englert Y, Vassart G, et al. Prediction of severity of symptoms in iatrogenic ovarian hyperstimulation syndrome by follicle-stimulating hormone receptor Ser680Asn polymorphism. J Clin Endocrinol Metab. (2004) 89:6310–5. doi: 10.1210/jc.2004-1044
30. de Castro F, Moron FJ, Montoro L, Real LM, and Ruiz A. Pharmacogenetics of controlled ovarian hyperstimulation. Pharmacogenomics. (2005) 6:629–37. doi: 10.2217/14622416.6.6.629
31. de Castro F, Ruiz R, Montoro L, Perez-Hernandez D, Sanchez-Casas Padilla E, Real LM, et al. Role of follicle-stimulating hormone receptor Ser680Asn polymorphism in the efficacy of follicle-stimulating hormone. Fertil Steril. (2003) 80:571–6. doi: 10.1016/s0015-0282(03)00795-7
32. Dieamant F, Petersen CG, Vagnini LD, Petersen B, Ricci J, Nicoletti A, et al. The Ala307Thr polymorphism of the follicle-stimulating hormone receptor (FSHR) gene is associated with the dose of recombinant FSH received during IVF/ICSI treatment. JBRA Assist Reprod. (2023) 27:78–84. doi: 10.5935/1518-0557.20230009
33. Jun JK, Yoon JS, Ku SY, Choi YM, Hwang KR, Park SY, et al. Follicle-stimulating hormone receptor gene polymorphism and ovarian responses to controlled ovarian hyperstimulation for IVF-ET. J Hum Genet. (2006) 51:665–70. doi: 10.1007/s10038-006-0005-5
34. Loutradis D, Patsoula E, Minas V, Koussidis GA, Antsaklis A, Michalas S, et al. FSH receptor gene polymorphisms have a role for different ovarian response to stimulation in patients entering IVF/ICSI-ET programs. J Assist Reprod Genet. (2006) 23:177–84. doi: 10.1007/s10815-005-9015-z
35. Laven JS, Mulders AG, Suryandari DA, Gromoll J, Nieschlag E, Fauser BC, et al. Follicle-stimulating hormone receptor polymorphisms in women with normogonadotropic anovulatory infertility. Fertil Steril. (2003) 80:986–92. doi: 10.1016/s0015-0282(03)01115-4
36. Palomba S, Falbo A, Orio F Jr., Tolino A, and Zullo F. Efficacy predictors for metformin and clomiphene citrate treatment in anovulatory infertile patients with polycystic ovary syndrome. Fertil Steril. (2009) 91:2557–67. doi: 10.1016/j.fertnstert.2008.03.011
Keywords: letrozole, ovulation induction, polycystic ovary syndrome, follicle-stimulating hormone polymorphism, ovarian response
Citation: Zhu X, Wang Q, Lang J, Zhi Y, Fu Y and Bao S (2025) Role of follicle-stimulating hormone receptor polymorphism in predicting ovarian response to letrozole in women with polycystic ovary syndrome during ovulation induction. Front. Endocrinol. 16:1585655. doi: 10.3389/fendo.2025.1585655
Received: 01 March 2025; Accepted: 03 June 2025;
Published: 19 June 2025.
Edited by:
Fred Sinowatz, Ludwig Maximilian University of Munich, GermanyReviewed by:
Gagandeep Gahlay, Guru Nanak Dev University, IndiaYali Liu, Shanghai Jiao Tong University, China
Ozlem Karabay Akgul, Bagcilar Education and Research Hospital, Türkiye
Copyright © 2025 Zhu, Wang, Lang, Zhi, Fu and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihua Bao, YmFvc2hpaHVhQHRvbmdqaS5lZHUuY24=; Yonglun Fu, ZnV5b25nbHVuaXZmQDE2My5jb20=
†These authors have contributed equally to this work
‡ORCID: Yonglun Fu, orcid.org/0000-0003-0047-3396
Shihua Bao, orcid.org/0000-0003-1341-1653
 Xiuxian Zhu
Xiuxian Zhu Qiaoling Wang1,3†
Qiaoling Wang1,3† Yonglun Fu
Yonglun Fu Shihua Bao
Shihua Bao