- 1Department of Pediatrics, Hanyang University College of Medicine, Seoul, Republic of Korea
- 2Department of Pediatrics, Hanyang University Hospital, Seoul, Republic of Korea
Background: Vitamin D deficiency is common among very low birth weight (VLBW) preterm infants due to challenges in achieving adequate enteral nutrition and reduced transplacental transfer. Supplementation with 800 IU/day of vitamin D has been shown to safely and effectively increase serum 25-hydroxyvitamin D [25(OH)D] levels above 30 ng/mL when initiated at two weeks of age and continued until hospital discharge.
Objective: This study aimed to evaluate whether daily supplementation with 800 IU of vitamin D significantly improves bone mineral density, as measured by dual-energy X-ray absorptiometry (DEXA), in VLBW infants at discharge, compared to supplementation with 400 IU/day.
Methods: We retrospectively reviewed the medical records of 215 VLBW infants with birth weights under 1,500 g who were admitted to the neonatal intensive care unit at Hanyang University Seoul Hospital between January 2010 and December 2023. The infants were divided into two groups based on their daily vitamin D intake of either 400 IU or 800 IU, initiated on day 14 of life after trophic feeding was established. Bone mineral apparent density (BMAD) was assessed at discharge using whole-body DEXA (Hologic QDR-4500, infant mode).
Results: Maternal age (32.01 ± 3.43 vs. 33.34 ± 4.39 years, p = 0.017) and birth weight (812.86 ± 141.19 vs. 883.76 ± 260.09 g, p = 0.010) were significantly higher in the 800 IU group, which also had a longer duration of total parenteral nutrition. After adjusting for birth weight and duration of parenteral nutrition, total body BMAD was significantly higher in the 800 IU group (p = 0.008). A general linear model further demonstrated that 800 IU supplementation was positively associated with femoral BMAD at discharge (β = 0.267, p= 0.001).
Conclusion: Daily supplementation of 800 IU of vitamin D was associated with improved bone mineralization, as measured by DEXA, at discharge in VLBW infants.
1 Introduction
Vitamin D deficiency is highly prevalent among very low birth weight (VLBW) preterm infants due to challenges in achieving adequate enteral nutrition, reduced transplacental transfer, limited ultraviolet B exposure, and low fat mass for vitamin D storage (1).
Adequate vitamin D intake is essential for the development of bone mineral density (BMD) and overall growth in these infants (2). Low BMD in preterm infants is associated with an increased risk of skeletal complications, such as rickets and osteopenia of prematurity (OOP). These conditions can be evaluated using clinical, biochemical, and radiological assessments. Common biochemical markers include serum calcium, phosphate, alkaline phosphatase (ALP), and 25-hydroxyvitamin D (25(OH)D). In addition, radiological methods such as wrist X-rays and dual-energy X-ray absorptiometry (DEXA) are critical for evaluating bone health (2). While DEXA is a precise tool for predicting metabolic bone disease, its utility is enhanced when combined with clinical and biochemical markers, given its limitations (3).
Vitamin D plays a fundamental role in bone health by enhancing calcium and phosphate absorption through the regulation of sodium-phosphate co-transporters (NaPi-IIb) and transient receptor potential vanilloid 6 (TRPV6) in enterocytes. Activated vitamin D binds to the vitamin D receptor (VDR) in osteoblasts, stimulating the expression of RANKL (Receptor Activator of Nuclear Factor Kappa-β Ligand), which promotes bone remodeling and osteoclast differentiation. Additionally, vitamin D upregulates ALP and osteocalcin, both of which are essential for osteoblast-mediated bone mineralization (4). This molecular mechanism suggests that higher vitamin D intake in preterm infants may reduce the risk of OOP and improve bone mineralization.
The optimal dosage and timing of vitamin D supplementation in preterm infants remain subjects of ongoing investigation. European guidelines recommend 800–1,000 IU/day, while U.S. guidelines commonly suggest 400 IU/day (5, 6). However, recent studies have questioned the adequacy of the 400 IU dose, suggesting that it may not be sufficient to support optimal bone health in high-risk preterm populations. Our previous research demonstrated that 800 IU of vitamin D safely and effectively raised 25(OH)D levels above 30 ng/mL in VLBW infants from two weeks of age until discharge (7).
This study aims to evaluate whether a daily vitamin D dose of 800 IU significantly improves BMD, as measured by DEXA, in VLBW infants at discharge compared to a 400 IU dose.
2 Methods
2.1 Study design and participants
This retrospective cohort study included 215 very low birth weight infants (VLBWIs; birth weight <1,500 g) admitted to the NICU at Hanyang University Seoul Hospital between January 2011 and December 2022. Following the implementation of a high-dose vitamin D protocol in September 2017, infants were categorized into two groups: 400 IU/day (n = 70; Period 1: January 2011–October 2015) and 800 IU/day (n = 145; Period 2: November 2015–December 2022).
Liquid cholecalciferol (vitamin D3; Sunny D®, GMP Laboratories, USA) was administered via a nasogastric or orogastric tube starting on day 14 of life if enteral feeding was tolerated, and continued until 36 weeks postmenstrual age (PMA) (8). Medical records for Period 1 were retrospectively reviewed with an IRB waiver of consent, while informed consent was obtained for infants in Period 2 (IRB No. 08028019).
2.2 Nutritional protocol and vitamin D supplementation
Once infants achieved 100 mL/kg/day of enteral feeding, they received either fortified human milk (containing 82 mg calcium, 45 mg phosphorus, and 100 IU vitamin D per 100 mL) or mineral-fortified preterm formula (115 mg calcium, 62 mg phosphorus, and 66 IU vitamin D per 100 mL). Total daily vitamin D intake, including supplementation, was limited to 900 IU/day. Supplementation continued until 36 weeks PMA and was discontinued if serum 25(OH)D exceeded 80 ng/mL. In the 800 IU/day group, 25(OH)D levels were measured at birth (cord blood), and at 32 and 36 weeks PMA.
2.3 NICU fluid and feeding protocol
Initial fluid was administered at 60–80 mL/kg/day and advanced to ≥120 mL/kg/day within 7 days. Enteral feeding began on days 1–2 at 10 mL/kg/day and was advanced based on tolerance. Glucose was started at 6–8 g/kg/day and increased up to 17–18 g/kg/day. Amino acids and lipids were initiated at 1.5–2 g/kg/day and progressed to 3–3.5 g/kg/day. The non-protein calorie-to-nitrogen ratio was maintained between 150:1 and 200:1. These TPN and feeding protocols remained consistent throughout the study period, supporting comparability between groups and minimizing potential temporal bias (9).
2.4 Other bone health interventions
Throughout the study period, standard neurodevelopmental physical therapy (NPT) practices were consistently applied as part of routine NICU care. These included daily gentle passive range-of-motion (ROM) exercises, facilitated positioning, and developmental handling techniques to promote musculoskeletal development and prevent disuse-related bone loss. Such mobilization strategies have been shown to positively affect bone mineralization and somatic growth in VLBW infants, as supported by previous randomized trials. Importantly, there were no major changes in the mobilization or rehabilitation protocols between the two study periods, minimizing the risk of confounding due to evolving physical therapy practices (10).
2.5 Data collection and outcome measures
Maternal and neonatal baseline variables (e.g., maternal age, hypertension, gestational diabetes, oligohydramnios, birth weight/height, Apgar scores, mode of delivery) and postnatal morbidities (e.g., respiratory distress syndrome [RDS], patent ductus arteriosus [PDA], intraventricular hemorrhage [IVH], retinopathy of prematurity [ROP], bronchopulmonary dysplasia [BPD], postnatal corticosteroid use) were collected. Biochemical parameters (serum calcium, phosphorus, ALP) were monitored weekly.
2.6 Definitions of prematurity-related conditions
Assessed neonatal morbidities included respiratory distress syndrome (RDS), patent ductus arteriosus (PDA), retinopathy of prematurity (ROP, ≥ stage II), bronchopulmonary dysplasia (BPD, ≥ moderate), postnatal corticosteroid use, intraventricular hemorrhage (IVH, ≥ grade 2), duration of total parenteral nutrition (TPN), and length of hospitalization.
ROP ≥ stage II was defined according to the International Classification of ROP (10); BPD was defined as oxygen dependency ≥21% at 36 weeks PMA (11); and IVH ≥ grade 2 was diagnosed based on Papile’s criteria (11).
2.7 Bone mineralization assessment
At discharge, bone mineralization was assessed using DEXA (Hologic QDR-4500, whole-body infant mode) (2). Bone area (BA), bone mineral content (BMC), and bone mineral density (BMD) were measured for the whole body, spine, and femurs. Bone mineral apparent density (BMAD) was calculated by dividing BMD by the square root of BA.
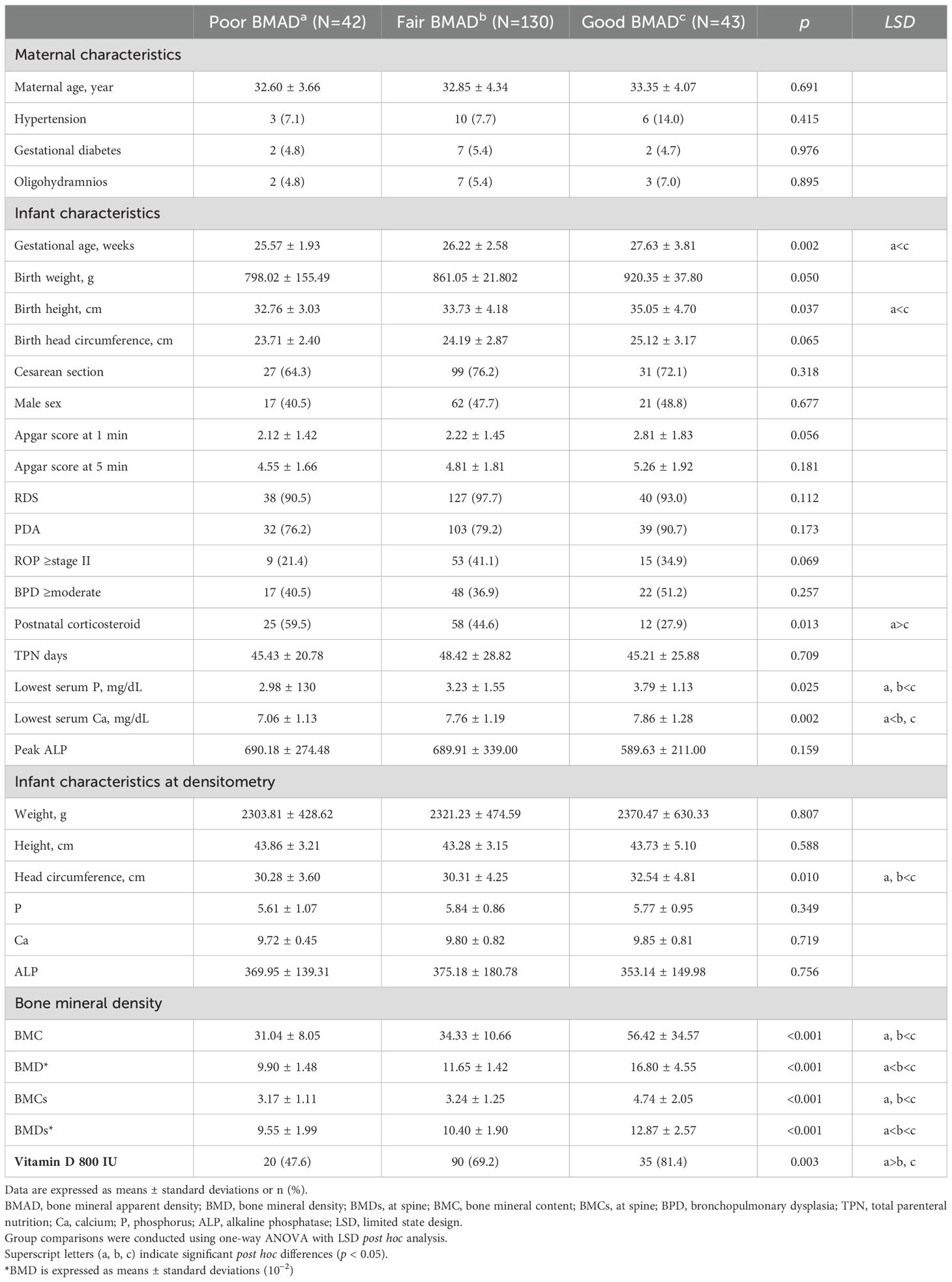
Infants were categorized into BMAD groups as follows: good (>75th percentile, >0.008 g/cm³), fair (25th–75th percentile), and poor (<25th percentile, <0.006 g/cm³). These cutoff values were based on the interquartile distribution of our institutional cohort of VLBW infants who underwent DEXA scans during the study period, given the absence of standardized BMAD reference data for preterm populations. This percentile-based classification approach is consistent with that used by Lee et al. in a previous study of ELBW infants, supporting the validity of using internal cohort distributions for BMAD interpretation in the absence of external normative standards (3) (12–14).
DEXA scans were performed using standardized prone positioning with gentle limb extension and swaddling to minimize motion artifacts. Repeat scans were obtained if any movement was detected during acquisition. Despite these precautions, minor asymmetries in femur alignment or leg rotation may have introduced small degrees of site-specific variability, particularly in femoral measurements, as previously noted in neonatal DEXA guidelines (3, 13).
2.8 Statistical analysis
Clinical characteristics and bone parameters were compared using Student’s t-test or the χ² test. Group differences in DEXA outcomes were analyzed using a general linear model adjusted for birth weight and TPN days. Additionally, estimated marginal means (EMMs) were derived from a weighted linear mixed model (LMM) applied to IPTW-adjusted data to simultaneously account for repeated measurements across body regions and baseline covariate imbalance.
To minimize baseline differences between groups, inverse probability of treatment weighting (IPTW) using propensity scores was performed based on maternal age, birth weight, 5-minute Apgar score, postnatal corticosteroid use, and duration of TPN. For comparisons among poor BMAD, fair BMAD, and good BMAD, one-way ANOVA followed by least significant difference (LSD) post hoc testing was conducted to identify pairwise differences.
3 Results
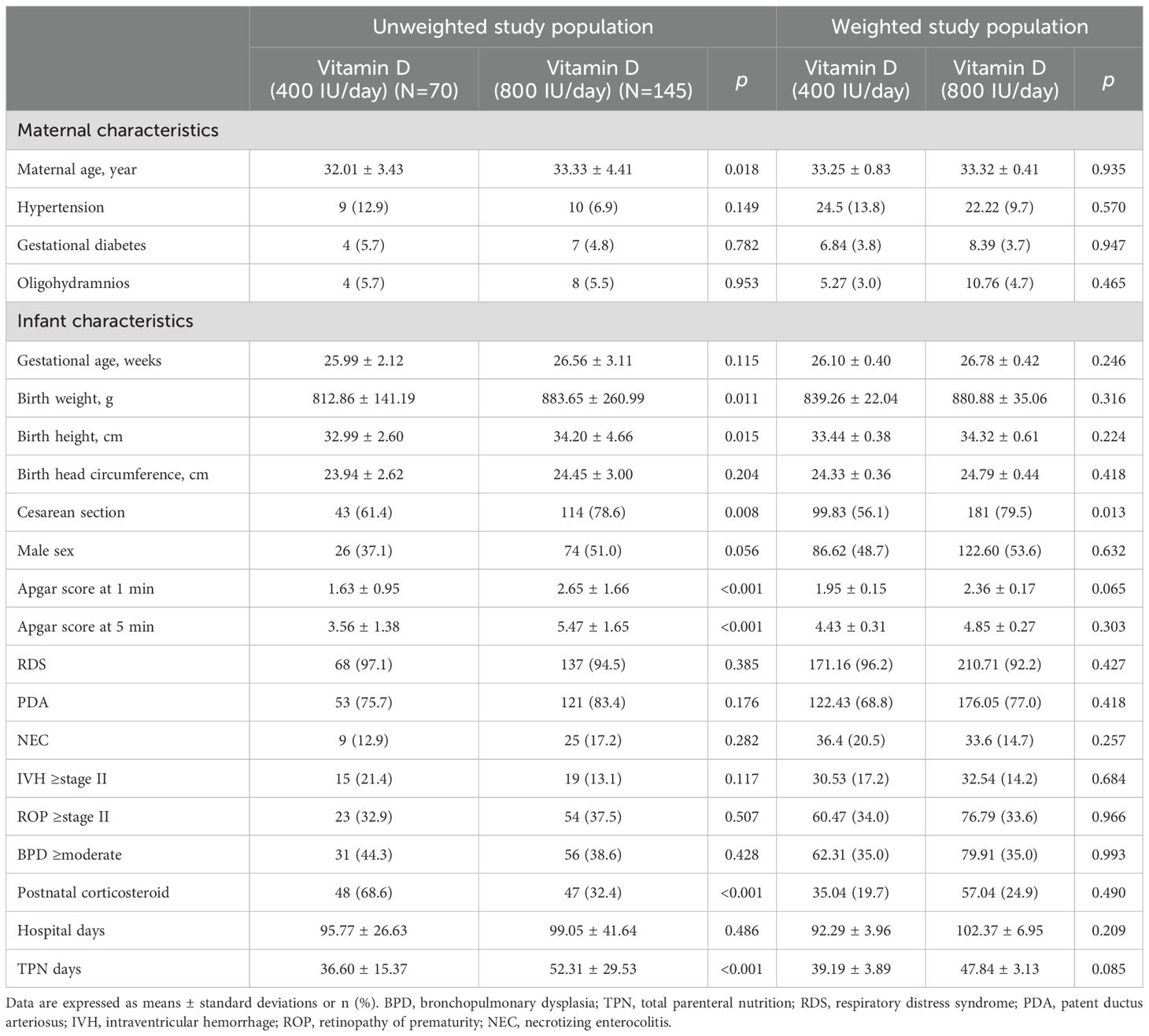
A total of 215 very low birth weight infants (VLBWIs) were included and divided into two groups based on early vitamin D supplementation: 400 IU/day (n=70) and 800 IU/day (n=145). As shown in Table 1, before inverse probability of treatment weighting (IPTW), several baseline characteristics differed significantly between groups. The 800 IU/day group had significantly higher maternal age, birth weight, birth height, cesarean section rate, Apgar scores at 1 and 5 minutes, and total TPN days, while postnatal corticosteroid use and necrotizing enterocolitis (NEC) incidence were more frequent in the 400 IU/day group.
IPTW was applied using propensity scores estimated from maternal age, birth weight, Apgar score at 5 minutes, postnatal corticosteroid use, and TPN days. After weighting, all standardized mean differences (SMDs) were below 0.2, except for TPN days (SMD = 0.227), indicating acceptable covariate balance between groups. IPTW-adjusted p-values are presented in the right columns of Tables 1, 2. Linear mixed model estimates using IPTW-weighted data (Table 3) were presented as a complementary approach to support the consistency of the findings.
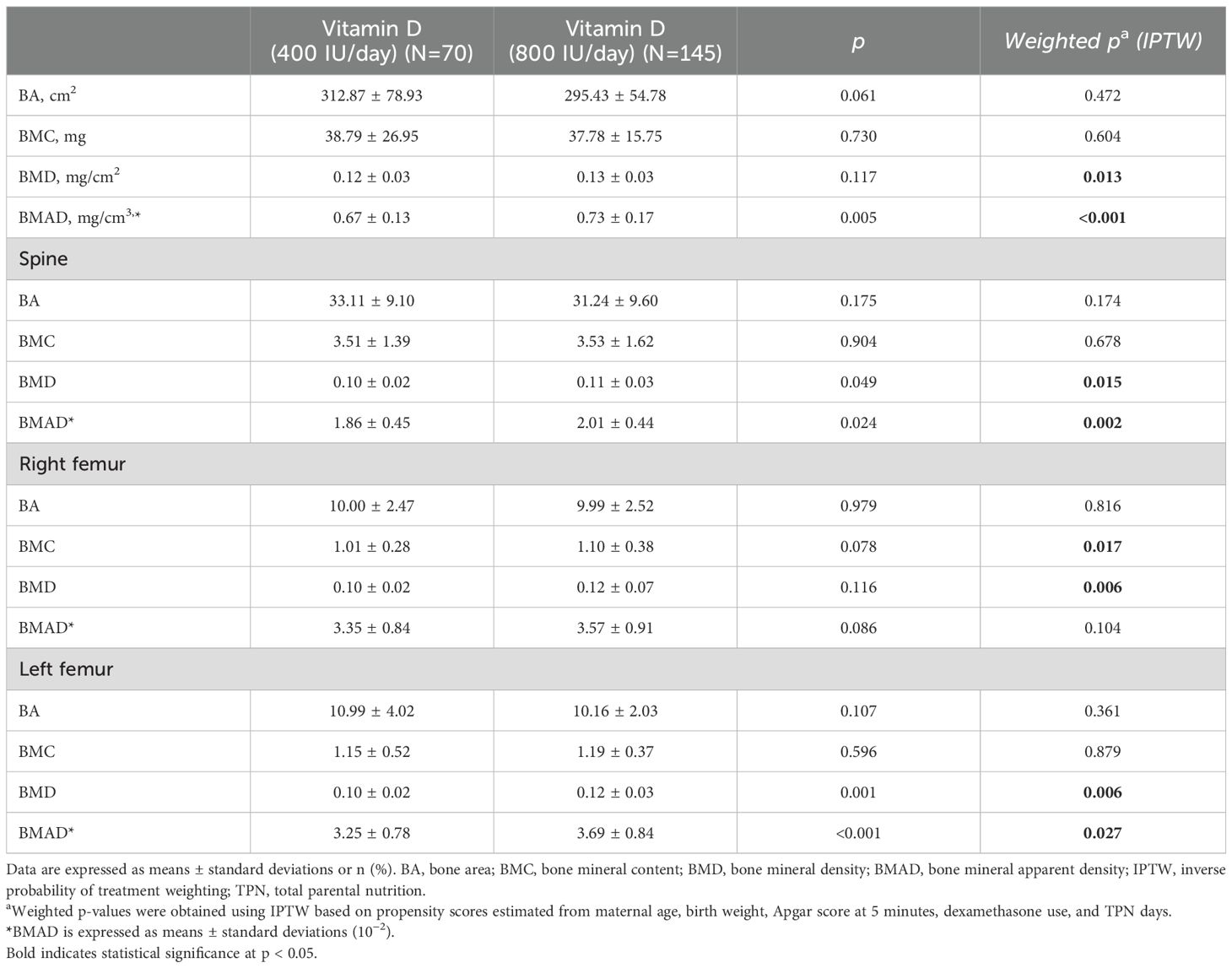
Table 2. Comparison of bone mineral density provided with 400 IU/day and 800 IU/day vitamin D supplementation.
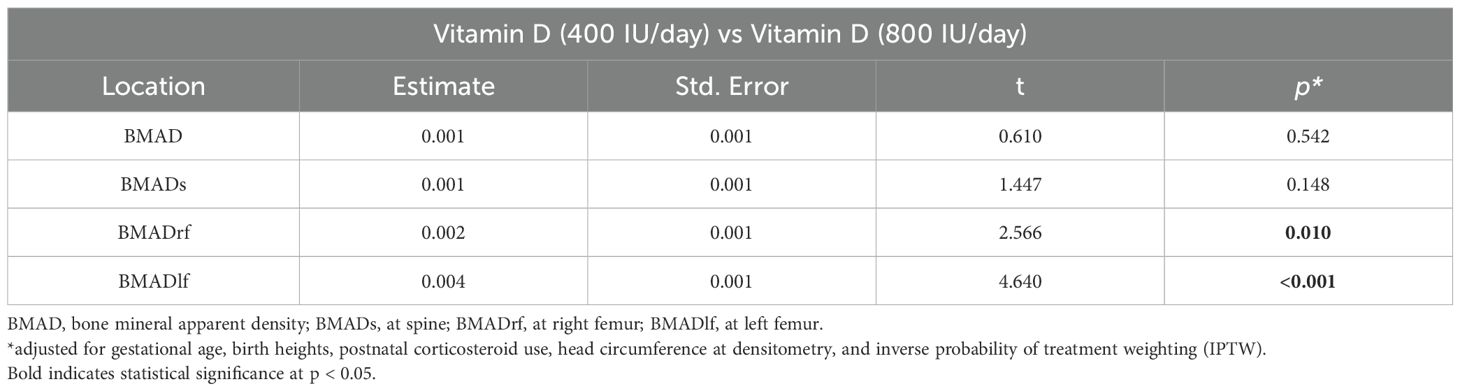
Table 3. Comparison of bone mineral density provided with 400 IU/day and 800 IU/day vitamin D supplementation using estimated marginal means derived from a weighted linear mixed effects model applied to IPTW-adjusted data.
Although a statistically significant improvement was observed in the left femur BMAD, this may partly reflect positioning or measurement variability in very small preterm infants. However, consistent patterns were observed across other skeletal sites, supporting a general beneficial effect of higher-dose vitamin D supplementation. IPTW-adjusted analyses further strengthened the robustness of these findings.
After IPTW, clinical differences in baseline characteristics were no longer statistically significant, except for TPN days (p=0.085), which remained slightly imbalanced. In bone densitometry analyses, the 800 IU/day group showed significantly higher whole-body BMAD compared to the 400 IU/day group even after IPTW adjustment (weighted p < 0.001). Similar but less significant patterns were observed in the spine and femoral regions: spine BMAD (weighted p = 0.002), right femur BMAD (p = 0.104), and left femur BMAD (p = 0.027), indicating a consistent positive effect of higher-dose vitamin D supplementation on skeletal mineralization across multiple sites.
To assess the biochemical safety of the high-dose regimen, serum 25(OH)D levels were monitored in the 800 IU/day group. The mean cord blood level was 29.7 ng/mL, and the mean level at discharge was 43.8 ng/mL. Three infants exceeded 80 ng/mL prior to discharge, with cord blood levels of 24.2, 27.4, and 35.2 ng/mL, and discharge levels of 91.6, 99.6, and 84.0 ng/mL, respectively. In all cases, vitamin D supplementation was promptly discontinued according to protocol, and no clinical signs of toxicity were observed, supporting the safety of the 800 IU/day regimen in this population. Detailed individual data are provided in Supplementary Table X.
Clinical characteristics according to BMAD classification are summarized in Table 4. Infants with good BMAD had a significantly higher gestational age (27.63 ± 3.81 weeks) compared to those with poor (25.57 ± 1.93 weeks) or fair BMAD (26.22 ± 2.58 weeks, p = 0.002). Birth height also showed significant differences among groups (good: 35.05 ± 4.70 cm, poor: 32.76 ± 3.03 cm, fair: 33.73 ± 4.18 cm, p = 0.037). Postnatal corticosteroid use was significantly lower in the good BMAD group (27.9%) than in the poor (59.5%) and fair (44.6%) groups (p = 0.013). The good BMAD group also showed a higher rate of vitamin D 800 IU supplementation (81.4% vs. 47.6% in poor and 69.2% in fair groups, p = 0.003). In laboratory findings, lowest serum calcium differed significantly between groups (good: 7.86 ± 1.28 mg/dL, poor: 7.06 ± 1.13 mg/dL, fair: 7.76 ± 1.19 mg/dL, p = 0.002). In bone densitometry results, good BMAD infants had significantly higher BMC (56.42 ± 34.57 g), BMD (16.80 ± 4.55 × 10−2), BMCs (4.74 ± 2.02 g), and BMDs (12.87 ± 5.27 ×10−2) compared to the other groups (all p < 0.001). These results indicate that higher vitamin D supplementation and greater gestational maturity may contribute to improved bone mineralization in very low birth weight infants.
4 Discussion
Osteopenia of prematurity (OOP) remains a significant concern among very low birth weight (VLBW) infants, who are at high risk for impaired bone mineralization due to multiple factors, including inadequate mineral intake. Extensive research has emphasized the importance of vitamin D supplementation in preventing metabolic bone disease, including OOP, in this vulnerable population.
Initially, our standard regimen involved daily supplementation with 400 IU of vitamin D, a dose widely recommended for preterm infants at the time. However, a substantial proportion of infants remained vitamin D deficient despite this supplementation. Recognizing this gap, our previous research provided compelling evidence supporting the safety and efficacy of 800 IU/day of vitamin D, demonstrating marked improvements in both vitamin D status and bone mineralization (7).
In the present study, 52 preterm infants (mean gestational age: 27.1 weeks) received 800 IU/day of vitamin D starting at two weeks of age. Prior to supplementation, 41% of these infants were vitamin D deficient, and an additional 49% had insufficient levels. Following supplementation, no infants remained deficient, and 72% reached sufficient vitamin D levels by 36 weeks postmenstrual age (PMA). Notably, the higher dose was associated with a significantly lower incidence of OOP, underscoring the clinical importance of optimizing vitamin D intake in preterm infants.
Among the skeletal sites evaluated, the left femur showed the most pronounced improvement in BMAD with high-dose vitamin D supplementation. While this may partially reflect technical variability, such as subtle differences in positioning or leg rotation during scanning, similar trends were observed in the spine and right femur, supporting a generalized skeletal effect. Given that DEXA measurements of the femur are more susceptible to alignment artifacts than central sites like the spine, and that preterm infants often have asymmetric posture or low muscle tone, such variability is not unexpected. The ISCD pediatric guidelines acknowledge that small differences in limb position can affect regional DEXA measurements in this population (13). Importantly, bilateral femoral improvements were seen, with greater magnitude on the left, suggesting an exaggerated expression of an overall systemic effect rather than a clinically meaningful unilateral difference. Further research using bilateral imaging and adjunctive modalities such as quantitative ultrasound (QUS) may help validate this interpretation.
In the 400 IU/day supplementation group, serum 25(OH)D levels were not available due to the retrospective nature of the study and institutional practices at the time, which were aligned with ESPEN guidelines (15). These guidelines consider 400 IU/day to be a standard dose that does not require routine vitamin D monitoring in preterm infants. Instead, bone health was routinely assessed using wrist radiographs, discharge DEXA scans, and serial biochemical markers such as serum calcium, phosphorus, and alkaline phosphatase (ALP), which were regularly monitored throughout hospitalization.
4.1 Clinical evidence supporting higher vitamin D supplementation
A randomized controlled trial by Ann Anderson-Berry et al. demonstrated that preterm infants (<32 weeks gestational age) receiving 800 IU/day of vitamin D had significantly greater improvements in serum 25(OH)D levels compared to those receiving 400 IU/day (5). Additionally, the 800 IU/day group showed enhanced bone mineral density (BMD) by DEXA and better linear growth.
Vitamin D supplementation has also been associated with improved physical growth parameters (weight, length, BMI, and head circumference) and neurodevelopmental outcomes in NICU infants. Furthermore, studies have reported no increased risk of serious morbidities such as BPD or NEC with vitamin D supplementation (16). In our study, larger head circumference was significantly associated with higher BMD (p=0.01), supporting the role of vitamin D in skeletal and overall growth. Future long-term follow-up studies on growth and neurodevelopmental outcomes based on vitamin D supplementation are warranted.
Interestingly, the 800 IU/day group had a significantly longer duration of total parenteral nutrition (TPN) (52.31 ± 29.53 days vs. 36.60 ± 15.37 days, p < 0.001), possibly reflecting greater illness severity or delayed enteral feeding. Despite prolonged TPN, this group showed better bone mineralization outcomes, suggesting that high-dose vitamin D may confer protective effects even under suboptimal nutritional conditions. While residual confounding cannot be completely excluded, this finding highlights the robustness of the clinical benefit associated with 800 IU/day supplementation.
4.2 Safety considerations of 800 IU/day vitamin D supplementation
Despite growing evidence, some concerns remain regarding the safety of 800 IU/day supplementation in neonates. However, several studies have confirmed its safety. For example, Natalia Aristizabal et al. reported no evidence of vitamin D or calcium toxicity in extremely preterm infants (<28 weeks) receiving 800 IU/day (17). Similarly, Dominika et al. found no serious neonatal morbidities associated with high-dose vitamin D, although they noted a possible reduction in serum parathyroid hormone levels (18). Hein G et al. raised concerns about hypercalciuria and nephrocalcinosis with excessive vitamin D intake (19). Given these concerns, routine monitoring of serum calcium and 25(OH)D levels is essential in VLBW infants receiving high-dose supplementation to ensure safety and prevent complications.
4.3 Challenges in assessing bone health in preterm infants
Evaluating bone mineral density (BMD) and mineralization in preterm infants remains challenging, as there is no universally accepted gold standard. Current assessment methods include biochemical markers, radiologic imaging, and clinical evaluation. Although serum alkaline phosphatase (ALP) levels are commonly used to assess bone turnover in preterm infants, their diagnostic utility for OOP is limited.
Our previous study examined BMD and BMAD in extremely low birth weight infants at discharge. Among 70 infants included, with an average gestational age of 25.9 weeks and a birth weight of approximately 813 g, the poor BMD group had significantly lower BMD values in the lumbar spine compared to the other groups. Factors associated with low BMD included BPD, prolonged TPN, and elevated ALP levels (3).
4.4 DEXA as a bone health assessment tool in preterm infants
Williams et al. demonstrated the utility of dual-energy X-ray absorptiometry (DEXA) in accurately quantifying bone mineral content (BMC) and BMD in preterm infants (20). Similarly, Rigo et al. showed that preterm infants have significantly lower BMC and BMD values compared to term infants, highlighting their increased risk of osteopenia and fractures (21). These findings reinforce the importance of early detection and timely intervention.
4.5 Future directions: advancing bone health assessment
DEXA is currently the most validated method for assessing bone mineral density in preterm infants, especially in research settings. However, its limitations include the need for infant transport, exposure to radiation, and the lack of standardized reference values for VLBW infants (22). Further research is needed to validate QUS and establish reference standards that could enable safer and more accessible bone health monitoring in this population.
5 Conclusion
Our findings indicate that daily supplementation with 800 IU of vitamin D, initiated at 14 days of life, significantly enhances bone mineralization in very low birth weight (VLBW) preterm infants compared to the standard 400 IU dose. Bone mineral density (BMD), assessed using dual-energy X-ray absorptiometry (DEXA), was significantly higher in the 800 IU group at discharge, supporting the potential benefits of a higher vitamin D intake in this high-risk population.
6 Limitation
This study has several limitations. First, it was a single-center study conducted in a specific geographic region, which may limit the generalizability of the findings. Ethnicity, genetic factors, and maternal conditions such as gestational diabetes mellitus can affect neonatal vitamin D status (23). Given that vitamin D deficiency is more prevalent in Asian and Middle Eastern populations than in Western countries, further multicenter and international studies are needed to validate these results in more diverse populations.
Second, BMAD measurements were conducted only at the time of discharge. We did not assess BMAD before the initiation of vitamin D supplementation because the infants were extremely vulnerable, with birth weights under 1500 g and typically less than 2 weeks of age at the start of supplementation. These preterm infants could not be removed from their incubators or safely transported outside the NICU during this early period. Therefore, performing pre-supplementation BMAD scans was not feasible.
Additionally, serum 25(OH)D levels were not collected in the 400 IU/day group, as retrospective data collection reflected the institutional standard of care at the time. In accordance with ESPEN recommendations, vitamin D status was not routinely monitored at this dosage level (15). Bone health assessments were conducted through standard clinical practice using biochemical markers (calcium, phosphorus, ALP) and radiologic tools, including wrist X-rays and discharge DEXA scans.
Although DEXA is widely used to assess bone mineral density in preterm infants, it has inherent limitations. While DEXA involves minimal radiation exposure and is considered safe even in neonates, especially with modern devices such as the Hologic QDR-4500, reported effective doses are less than 1 μSv, which is lower than natural background radiation levels (24). However, the lack of standardized reference values for VLBW infants remains a critical challenge.
This study highlights the need for larger-scale randomized controlled trials and longitudinal studies to establish standardized protocols and reference values for bone health assessment in VLBW infants. A more comprehensive and clinically practical evaluation strategy is required to facilitate early detection of osteopenia, enabling timely intervention and improved long-term outcomes for this vulnerable population.
This study has certain limitations. The observed differences in Apgar scores likely reflect physiological immaturity rather than perinatal compromise. Recent neonatal resuscitation practices have placed greater emphasis on immediate stabilization instead of Apgar-based assessment, which may have affected these scores (25). The direct application of monitoring devices in the NICU also influenced initial evaluations.
Throughout the study, the TPN protocol and parenteral nutrition composition remained consistent, with no major changes in feeding advancement strategies, mineral supplementation, or other bone health interventions. The only notable change during Period 2 (2015–2022) was the implementation of routine vitamin D level monitoring and the increased supplementation dose. While improvements in overall neonatal care over time may have contributed to better outcomes, the observed enhancements in bone mineralization were temporally aligned with the change in vitamin D practices, supporting a specific effect beyond general advances in NICU care.
Future research should address these limitations through well-designed, prospective randomized controlled trials with adequate sample size calculations. Serial monitoring of vitamin D status, including repeated 25(OH)D measurements, should be incorporated to better evaluate biochemical efficacy. Detailed reporting of gastrointestinal morbidities and corticosteroid exposure is also necessary to reduce potential confounding. Furthermore, the development of standardized reference values for DEXA interpretation in preterm populations, along with the validation of alternative methods such as quantitative ultrasound, is essential. Long-term follow-up beyond hospital discharge will be critical in elucidating the sustained effects of early vitamin D supplementation on bone health and growth in VLBW infants.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Hanyang University Seoul Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
M-JY: Writing – original draft, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Writing – review & editing. JS: Writing – review & editing, Project administration. IL: Writing – review & editing, Project administration. HK: Data curation, Formal analysis, Writing – original draft. JC: Writing – review & editing, Project administration. YC: Writing – review & editing, Software. HL: Conceptualization, Validation, Supervision, Writing – original draft, Software. SY: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1585898/full#supplementary-material
References
1. Cho HN, Lee Y, Oh S, and Heo JS. Risk factors and outcomes of vitamin D deficiency in very preterm infants. Pediatr Neonatology. (2025) 66:31–6. doi: 10.1016/j.pedneo.2024.04.004
2. Koo WW, Gupta JM, Nayanar VV, Wilkinson M, and Posen S. Skeletal changes in preterm infants. Arch Dis Child. (1982) 57:447–52. doi: 10.1136/adc.57.6.447
3. Lee J, Park HK, Kim JH, Choi YY, and Lee HJ. Bone mineral density according to dual energy X-ray absorptiometry is associated with serial serum alkaline phosphatase level in extremely low birth weight infants at discharge. Pediatr Neonatol. (2017) 58:251–7. doi: 10.1016/j.pedneo.2016.05.005
4. Xue Y, Karaplis AC, Hendy GN, Goltzman D, and Miao D. Exogenous 1,25-dihydroxyvitamin D3 exerts a skeletal anabolic effect and improves mineral ion homeostasis in mice that are homozygous for both the 1alpha-hydroxylase and parathyroid hormone null alleles. Endocrinology. (2006) 147:4801–10. doi: 10.1210/en.2006-0403
5. Anderson-Berry A, Thoene M, Wagner J, Lyden E, Jones G, Kaufmann M, et al. Randomized trial of two doses of vitamin D3 in preterm infants <32 weeks: Dose impact on achieving desired serum 25(OH)D3 in a NICU population. PloS One. (2017) 12:e0185950. doi: 10.1371/journal.pone.0185950
6. Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. (2010) 50:85–91. doi: 10.1097/mpg.0b013e3181adaee0
7. Cho SY, Park HK, and Lee HJ. Efficacy and safety of early supplementation with 800 IU of vitamin D in very preterm infants followed by underlying levels of vitamin D at birth. Ital J Pediatr. (2017) 43:45. doi: 10.1186/s13052-017-0361-0
8. Embleton ND, Jennifer Moltu S, Lapillonne A, Akker CHPvd, Carnielli V, Fusch C, et al. A position paper from the ESPGHAN committee on nutrition and invited experts. J Pediatr Gastroenterol Nutr. (2022) 76:248–68. doi: 10.1097/01.mpg.0000181841.07090.f4
9. Sung SI, Chang YS, Choi JH, Ho Y, Kim J, Ahn SY, et al. Increased risk of refeeding syndrome-like hypophosphatemia with high initial amino acid intake in small-for-gestational-age, extremely-low-birthweight infants. PloS One. (2019) 14:e0221042. doi: 10.1371/journal.pone.0221042
10. El-Farrash RA, Abo-Seif IS, El-Zohiery AK, Hamed GM, and Abulfadl RM. Passive range-of-motion exercise and bone mineralization in preterm infants: A randomized controlled trial. Am J Perinatol. (2020) 37:313–21. doi: 10.1055/s-0039-1678559
11. Papile LA, Burstein J, Burstein R, and Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/s0022-3476(78)80282-0
12. Jeddi M, Roosta MJ, Dabbaghmanesh MH, Omrani GR, Ayatollahi SM, Bagheri Z, et al. Normative data and percentile curves of bone mineral density in healthy Iranian children aged 9–18 years. Arch Osteoporos. (2013) 8:114. doi: 10.1007/s11657-012-0114-z
13. Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. (2014) 17:225–42. doi: 10.1016/j.jocd.2014.01.003
14. Lim JS, Hwang JS, Lee JA, Kim DH, Park KD, Cheon GJ, et al. Bone mineral density according to age, bone age, and pubertal stages in korean children and adolescents. J Clin densitometry. (2010) 13(1):68–76. doi: 10.1016/j.jocd.2009.09.006
15. Bronsky J, Campoy C, Braegger C, Braegger C, Bronsky J, Cai W, et al. Vitamins, clinical nutrition. Clin Nutr. (2018) 9:1–31. doi: 10.1016/j.clnu.2018.06.951
16. Kumar M, Shaikh S, Sinha B, Upadhyay RP, Choudhary TS, Chandola TR, et al. Enteral vitamin D supplementation in preterm or low birth weight infants: A systematic review and meta-analysis. Pediatrics. (2022) 150:S1–S7. doi: 10.1542/peds.2022-057092k
17. Aristizabal N, Holder MP, Durham L, Ashraf AP, Taylor S, and Salas AA. Safety and efficacy of early vitamin D supplementation in critically ill extremely preterm infants: an ancillary study of a randomized trial. J Acad Nutr Diet. (2023) 123:87–94. doi: 10.1016/j.jand.2022.06.012
18. Paw D, Bokiniec R, and Kołodziejczyk-Nowotarska A. High initial dose of monitored vitamin D supplementation in preterm infants (HIDVID trial): study protocol for a randomized controlled study. Nutrients (2024) 16:700. doi: 10.3390/nu16050700
19. Hein G, Richter D, Manz F, Weitzel D, and Kalhoff H. Development of nephrocalcinosis in very low birth weight infants. Pediatr Nephrol. (2004) 19:616–20. doi: 10.1007/s00467-004-1428-x
20. Williams JE, Wilson CM, Biassoni L, Suri R, and Fewtrell MS. Dual energy x-ray absorptiometry and quantitative ultrasound are not interchangeable in diagnosing abnormal bones. Arch Dis Child. (2012) 97:822–4. doi: 10.1136/archdischild-2011-301326
21. Rigo J, Nyamugabo K, Picaud JC, Gerard P, Pieltain C, and De Curtis M. Reference values of body composition obtained by dual energy X-ray absorptiometry in preterm and term neonates. J Pediatr Gastroenterol Nutr. (1998) 27:184–90. doi: 10.1097/00005176-199808000-00011
22. Rack B, Lochmüller EM, Janni W, Lipowsky G, Engelsberger I, Friese K, et al. Ultrasound for the assessment of bone quality in preterm and term infants. J Perinatol. (2012) 32:218–26. doi: 10.1038/jp.2011.82
23. Levin GP, Robinson-Cohen C, de Boer IH, Houston DK, Lohman K, Liu Y, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. Jama. (2012) 308:1898–905. doi: 10.1001/jama.2012.17304
24. Damilakis J, Solomou G, Manios GE, and Karantanas A. Pediatric radiation dose and risk from bone density measurements using a GE Lunar Prodigy scanner. Osteoporos Int. (2013) 24:2025–31. doi: 10.1007/s00198-012-2261-x
Keywords: vitamin D, bone mineral density, VLBW (very low birth weight), DEXA, preterm
Citation: Yoo M-J, Seo JH, Lee I, Kim H, Choi J, Choe Y, Lee HJ and Yang S (2025) Effects of high-dose vitamin D supplementation on bone mineral density in very low birth weight preterm infants. Front. Endocrinol. 16:1585898. doi: 10.3389/fendo.2025.1585898
Received: 01 March 2025; Accepted: 14 July 2025;
Published: 01 August 2025.
Edited by:
Gendie Lash, Guangzhou Medical University, ChinaReviewed by:
Han Zo Choi, Kyung Hee university hospital at Gangdong, Republic of KoreaMar Romero-Lopez, University of Texas Health Science Center at Houston, United States
Copyright © 2025 Yoo, Seo, Lee, Kim, Choi, Choe, Lee and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung Yang, anhpc2ZyaWVuZEBoYW55YW5nLmFjLmty
 Myoung-Jin Yoo
Myoung-Jin Yoo Jeong Ho Seo1,2
Jeong Ho Seo1,2 Hyuna Kim
Hyuna Kim Jinjoo Choi
Jinjoo Choi Yunsoo Choe
Yunsoo Choe Hyun Ju Lee
Hyun Ju Lee Seung Yang
Seung Yang