- 1Henan Human Sperm Bank, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Clinical Laboratory, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
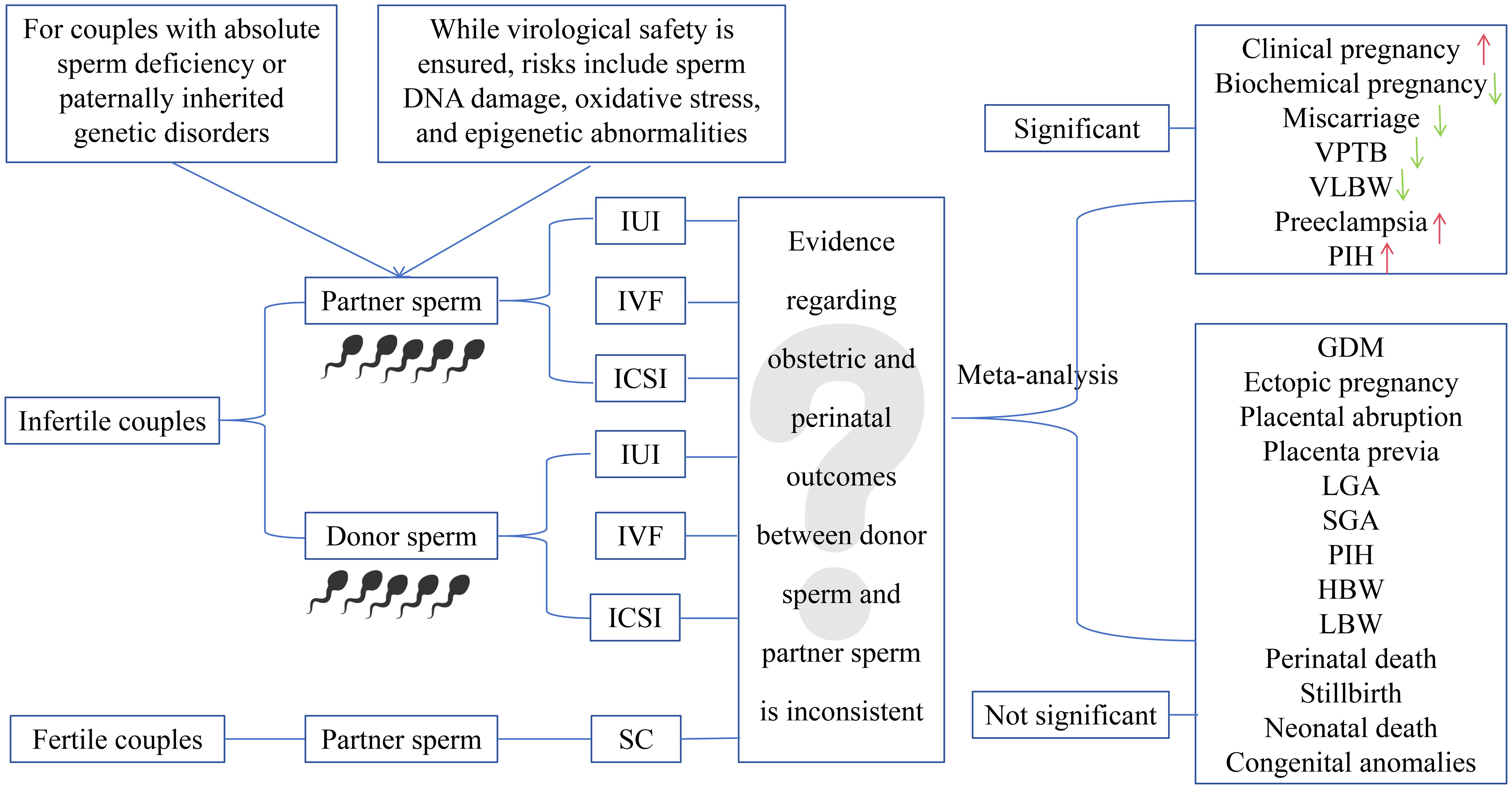
Background: Male-related factors contribute to 30-40% of infertility cases, with donor sperm serving as a critical solution for severe male infertility or paternally inherited genetic disorders. While cryopreservation ensures virological safety, concerns persist regarding sperm DNA damage, oxidative stress, and epigenetic impacts on embryogenesis. Previous studies have shown inconsistent evidence regarding obstetric and perinatal outcomes using donor versus partner sperm. This meta-analysis aimed to compare these outcomes to guide evidence-based clinical decisions.
Methods: To identify studies published up to December 2024, we systematically search Embase, PubMed, Scopus, Wanfang, Web of Science, and China National Knowledge Infrastructure (CNKI). Studies investigating obstetric and perinatal outcomes using donor versus partner sperm were included regardless of the conception method. Adjusted estimates were prioritized, but crude estimates were utilized when necessary. Given the clinical and methodological heterogeneity, random-effects models were utilized to pool relative risks (RRs) and their 95% confidence intervals (CIs).
Results: This analysis included 64 studies. Donor sperm was linked to better clinical pregnancy rates (RR 1.27, 95% CI 1.08–1.48) and decreased incidences of biochemical pregnancy (RR 0.85, 95% CI 0.81–0.88), miscarriage (RR 0.91, 95% CI 0.84–1.00), very preterm birth (RR 0.88, 95% CI 0.80–0.96), and very low birth weight (RR 0.89, 95% CI 0.81–0.98) compared with partner sperm. However, donor sperm conceptions exhibited increased risks of preeclampsia (RR 1.35, 95% CI 1.06–1.74) as well as pregnancy-induced hypertension (RR 1.19, 95% CI 1.05–1.36). For other outcomes, including gestational diabetes mellitus, ectopic pregnancy, placental abruption, placenta previa, large and small for gestational age, preterm birth, high and low birth weight, perinatal death, stillbirth, neonatal death, and congenital anomalies, no significant disparities were observed.
Conclusions: Donor sperm offers improved pregnancy outcomes for severe male infertility or paternally inherited genetic disorders but is linked to elevated risks of preeclampsia and pregnancy-induced hypertension. Additional studies are required to explore potential mechanisms and design specific interventions.
Introduction
Globally, approximately 15% of couples struggle with infertility, with male factors accounting for 30–40% of these instances (1–3). Assisted reproductive technologies (ART) have revolutionized infertility treatment, and donor sperm has become a crucial approach for couples facing absolute sperm deficiency (e.g., non-obstructive azoospermia) or paternally inherited genetic disorders. Clinically, donor sperm is applied through three approaches: artificial insemination (AID), in vitro fertilization (IVF-D), and intracytoplasmic sperm injection (ICSI-D). Among these, ICSI-D is typically reserved for complex cases, such as recurrent IVF failure.
Mandatory six-month cryopreservation of donor sperm effectively eliminates HIV transmission risks but introduces biological challenges. These include ultra-structural sperm damage, mitochondrial dysfunction, and oxidative stress-mediated DNA fragmentation (4–6), which are further exacerbated by laboratory processing techniques such as density gradient centrifugation. These alterations may impair epigenetic reprogramming during fertilization, raising concerns about downstream impacts on blastocyst development and long-term offspring health. These concerns underscore the need for a comprehensive evaluation of donor sperm’s obstetric and perinatal safety profile.
Previous studies comparing donor and partner sperm outcomes remain inconclusive (7–73) due to heterogeneity in study designs, sample sizes, conception methods (e.g., IUI vs. IVF/ICSI), and confounding factors. Although four previous meta-analyses have addressed this topic (74–77), their findings are undermined by several critical limitations (1): reliance on unadjusted estimates (8, 16, 22–26, 28, 29, 31–38, 40–71) (2); the emergence of recent high-quality evidence (7, 12–14, 23, 24, 26–36) (3); the omission of studies that should have been included based on the study period (37–45) (4); incomplete outcome assessment, particularly for clinical pregnancy, biochemical pregnancy, and very high birth weight(VHBW); and (5) language bias due to the exclusion of Chinese studies (29, 31, 33, 35, 36, 40, 41, 43–47).
To address these limitations, we presented the first and largest meta-analysis to date, synthesizing both unadjusted (8, 16, 22–26, 28, 29, 31–38, 40–71) and adjusted data (7–9, 11–14, 17–20, 27, 30, 39, 72, 73) from 64 studies. By systematically evaluating 21 obstetric and perinatal outcomes, we aimed to provide robust evidence to guide clinical decision-making for couples considering the use of donor sperm in ART, while also identifying areas requiring further investigation to optimize maternal and neonatal outcomes.
Materials and methods
Following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 guidelines (Supplementary PRISMA checklist), this systematic review and meta-analysis was prospectively registered on PROSPERO (CRD42024568737).
Literature search
A comprehensive search was conducted across six electronic databases, including PubMed, Embase, Web of Science, Scopus, Wanfang, and China National Knowledge Infrastructure (CNKI), to identify articles published until December 2024. No language or publication date restrictions were applied to minimize selection bias. Detailed search strategies utilized in each database are provided in Supplementary Data.
Outcomes
The outcomes of interest were categorized into three domains: pregnancy outcomes, pregnancy complications, and perinatal outcomes. Pregnancy outcomes included clinical pregnancy (verified by detecting an intrauterine gestational sac), ectopic pregnancy, biochemical pregnancy (defined by serum hCG levels of ≥5 IU/L without ultrasound confirmation), and miscarriage (occurring before 20 weeks of gestation). Pregnancy complications involved pregnancy-induced hypertension (PIH), gestational diabetes mellitus (GDM), preeclampsia (blood pressure ≥140/90 mmHg and proteinuria ≥300 mg/24h), placental abruption, and placenta previa. Perinatal outcomes focused on adverse birth events, including preterm birth (PTB, occurring before 37 weeks’ gestation), very preterm birth (VPTB, occurring before 32 weeks’ gestation), low birth weight (LBW, < 2500 g), high birth weight (HBW, > 4000 g), large for gestational age (LGA, birth weight > 90th percentile), VHBW (> 4500 g), very low birth weight (VLBW, < 1500 g), small for gestational age (SGA, birth weight >10th percentile), stillbirth, neonatal death, perinatal death, and congenital anomalies.
Inclusion and exclusion criteria
To be deemed eligible for inclusion, studies needed to satisfy the following conditions (1): reporting ≥1 predefined outcome comparing donor and partner sperm, regardless of conception method (2); reporting adjusted/crude relative risks (RR)/odds ratio (OR) and their 95% confidence intervals (CIs) (3); availability of peer-reviewed full texts.
The criteria for exclusion included the following (1): studies that were literature reviews, conference abstracts, case reports (2); studies conducted on non-human subjects (3); studies involving mixed donor gametes (e.g., donor oocytes) (4); duplicate datasets (5); studies lacking sufficient data to calculate effect sizes; and (6) studies comparing different ART methods without stratifying by sperm source.
Study selection
Retrieved studies were managed using EndNote X8. After removing duplicate records, two authors independently screened each study for eligibility based on title, abstract, or full text. Any disagreements were addressed by either discussion or by seeking input from a third reviewer. All qualifying articles were incorporated into this study without consideration of quality scores, recognizing that even studies with methodological weaknesses may provide valuable evidence (78, 79).
Data extraction
Two authors independently conducted data extraction from the selected articles, capturing the following information: first author, publication year, study period, study design, country, occurrence of multiple births, model of conception, sample size, controlled confounders, and outcomes. When adjusted estimates were available, they were preferred; otherwise, crude estimates were utilized. Any discrepancies that arose during data extraction were addressed by either discussion or by seeking input from a third reviewer.
Quality assessment
Two researchers independently evaluated the quality of the selected articles utilizing the Joanna Briggs Institute (JBI) critical appraisal checklist (80). Any disagreements were addressed by either discussion or by seeking input from a third reviewer. For each item, a score was assigned as follows: 0 for “no”, 1 for “unclear”, and 2 for “yes”. Studies were classified into three quality categories: high quality (70% or above), medium quality (between 40% and 70%), and low quality (below 40%) based on summary scores (the total score divided by the maximum achievable score) (81).
Statistical methods
The formula RR=OR/[(1-P0)+(P0*OR)] was used to convert the OR to the RR, where P0 represents the outcome incidence rate in the control group (82). To transform the 95% CIs, the following formula was applied: SElog(RR)=SElog(OR)×log(RR)/log(OR) (83). The I2 statistic was utilized to evaluate the extent of heterogeneity among studies, and a value exceeding 50% denotes notable heterogeneity. To explore possible sources of heterogeneity, subgroup analyses were carried out by stratifying the data based on whether confounding factors were adjusted (yes or no), the model of conception (such as donor sperm IUI vs. partner sperm IUI), and location (Asian vs. non-Asian). To assess heterogeneity between subgroups, univariate meta-regression under a random-effects model was performed using R software (version 4.5.1) to obtain the corresponding P values. Sensitivity analyses, including itemized exclusions and effect-size weighting, were utilized to verify the stability of the findings while funnel plots and Egger’s regression test were applied to detect potential publication bias. Owing to clinical and methodological heterogeneity, crude and adjusted RRs were synthesized employing random-effects models. The criteria devised by the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Working Group enabled us to assess the overall evidence quality regarding the relationship between various sperm sources and obstetric as well as perinatal outcomes.
Results
Characteristics of eligible studies
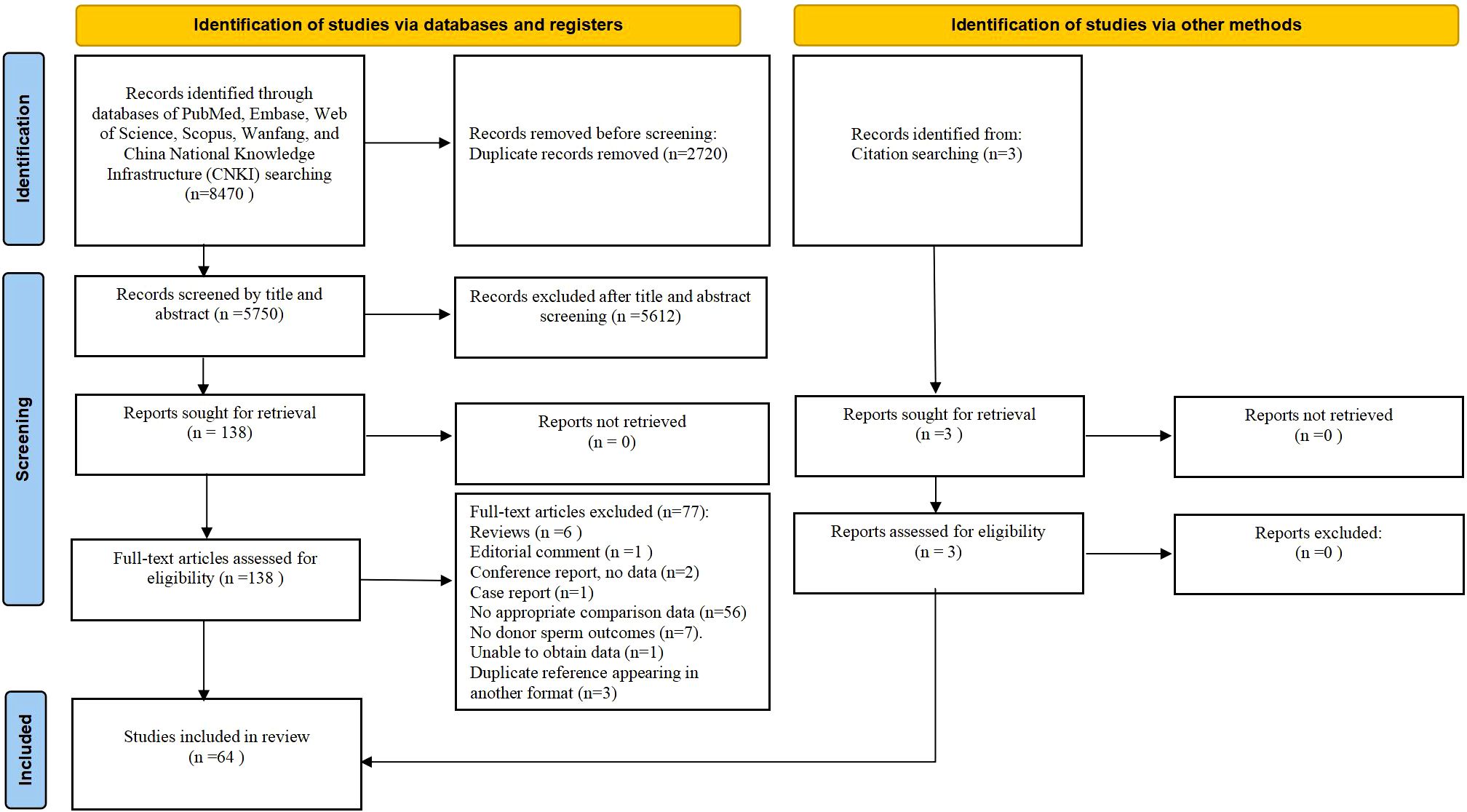
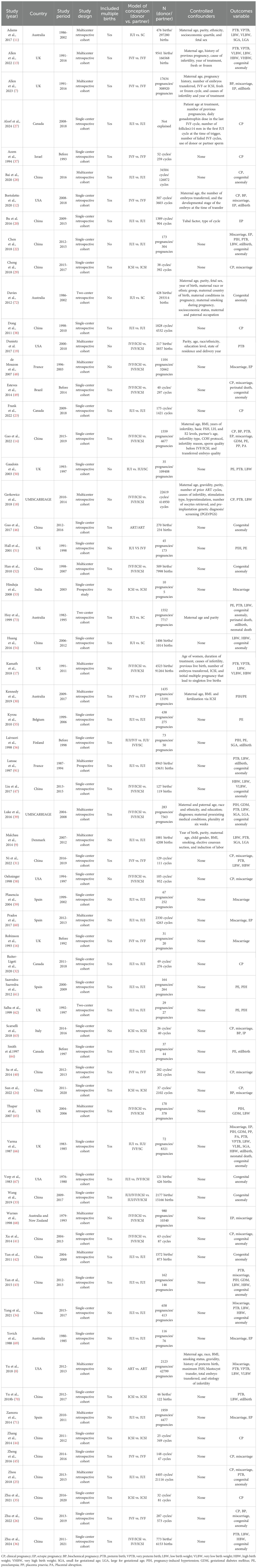
Initial database searches and reference screening identified 8470 articles. After eliminating duplicates and examining titles/abstracts, 141 articles were chosen for full-text assessment. Of these, 77 articles were excluded according to predefined criteria (Supplementary Table S1). The final analysis included 64 studies (Figure 1). Table 1 presents the main characteristics of those eligible articles. Among these, twenty were multicenter (7–11, 13, 17–19, 25, 28, 30, 42, 48, 52, 57, 60, 65, 68, 71), while the remaining were conducted at either single-center (12, 14, 16, 20, 22–24, 26, 27, 29, 31–38, 40, 41, 43–47, 49–51, 53–56, 58, 59, 61, 63, 64, 66, 67, 69, 70) or dual-center settings (62, 72, 73). Adjusted confounders for obstetric/perinatal outcomes were reported in 16 studies (7–9, 11–14, 17–20, 27, 30, 39, 72, 73), whereas others provided unadjusted estimates (8, 16, 22–26, 28, 29, 31–38, 40–71). Quality assessment using the JBI critical appraisal tool revealed 47 studies (73.44%) as medium-quality and 17 studies (26.56%) as high-quality. Most studies had adequate selection of participants and clearly distinguished between donor and partner sperm conception, but control of confounding factors and reporting of follow-up were often insufficient (Supplementary Table S2). Evidence certainty, assessed using the GRADE criteria, was rated as moderate for biochemical pregnancy, preeclampsia, PIH, VPTB, VLB, and VHBW. Evidence for miscarriage, placenta previa, PTB, HBW, stillbirth, neonatal death, perinatal death, and congenital anomalies was classified as low certainty, while the evidence certainty for the remaining outcomes was deemed very low (Supplementary Table S3).
Pregnancy outcomes: donor vs. partner sperm
Donor sperm was linked to a significantly elevated overall clinical pregnancy rate compared to partner sperm (RR 1.27, 95%CI 1.08-1.48; Figure 2A). Consistent findings were observed across all subgroups despite notable heterogeneity (I2 = 97.8%; Supplementary Figure S1). Univariable meta-regression showed that only the model of conception significantly contributed to heterogeneity (QM=16.68, P<0.001, R2 = 51.24%), while location and adjustment for confounding factors had no significant effects (all P>0.05) (Supplementary Table S4).
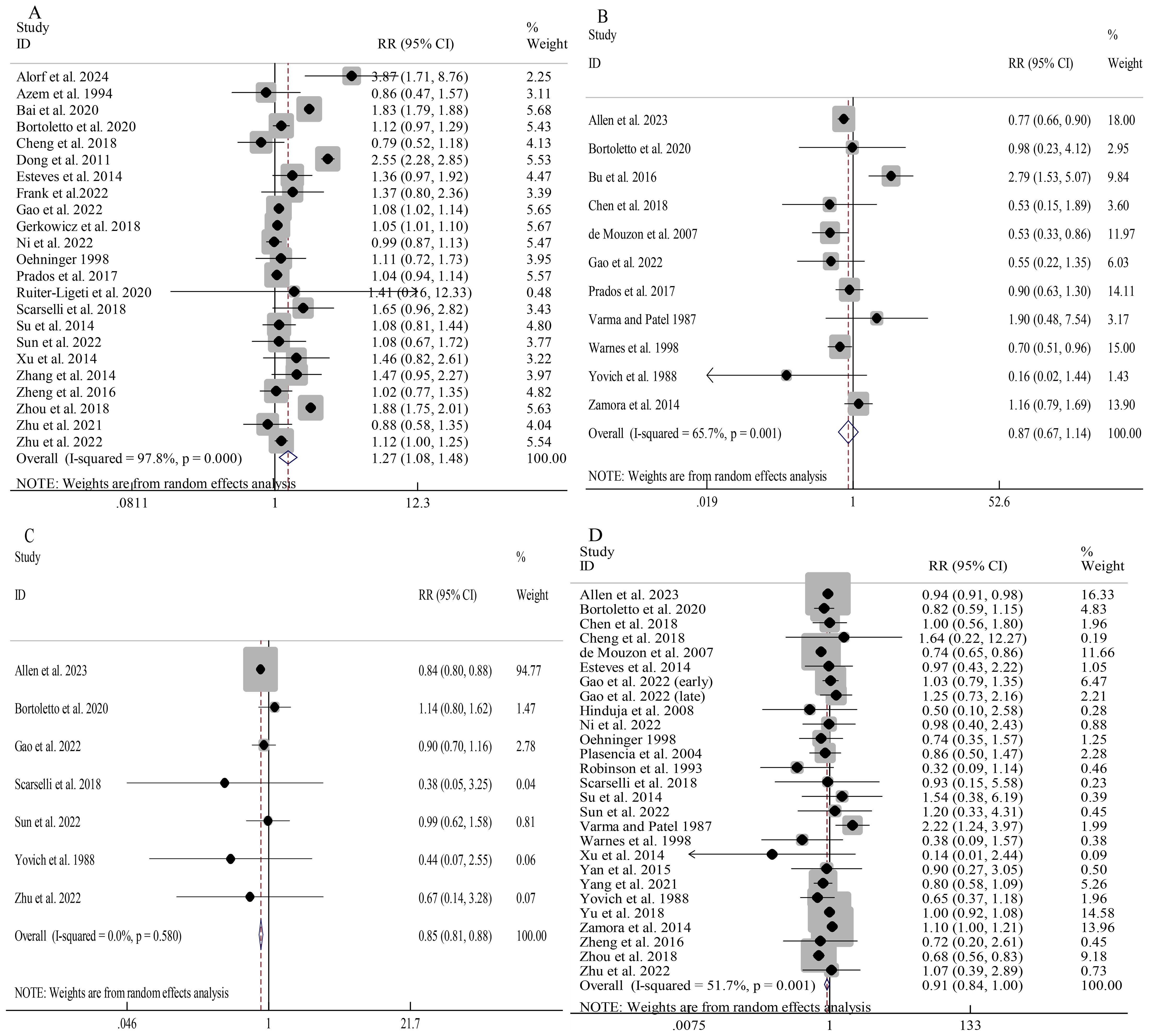
Figure 2. Association between different sperm sources and pregnancy outcomes: clinical pregnancy (A); ectopic pregnancy (B); biochemical pregnancy (C); miscarriage (D).
Donor sperm did not significantly differ from partner sperm in overall risk of ectopic pregnancy (RR 0.87, 95%CI 0.67-1.14; Figure 2B). Subgroup analyses indicated a reduced ectopic pregnancy risk in donor sperm cycles involving IVF/ICSI (RR 0.74, 95%CI 0.65-0.84) as well as non-Asian cohorts (RR 0.80, 95%CI 0.66-0.98) (Supplementary Figure S2). Univariable meta-regression indicated that none of the examined moderators significantly contributed to heterogeneity (all P>0.05), although the model of conception showed a trend toward significance (QM=5.45, P=0.065, R2 = 58.56%) (Supplementary Table S4).
Donor sperm conception was associated with a significantly lower overall risk of biochemical pregnancy than partner sperm conception (RR 0.85, 95%CI 0.81-0.88; I2 = 0.0%; Figure 2C). While the overall miscarriage risk showed a trend toward reduction with donor sperm (RR 0.91, 95%CI 0.84-1.00; Figure 2D), substantial heterogeneity (I2 = 51.7%) prompted prespecified subgroup analyses. No significant associations were observed in unadjusted analyses, IUI vs IUI, and Aisan cohorts, while findings from other subgroups were consistent with the overall results (Supplementary Figure S3). Univariable meta-regression indicated that only the model of conception significantly contributed to heterogeneity (QM=7.80, P=0.020, R2 = 11.31%), whereas location and adjusted confounding factors showed no significant effects (all P>0.05) (Supplementary Table S4).
Pregnancy complications: donor vs. partner sperm
Pooled analyses demonstrated significantly increased risks of preeclampsia (RR 1.35, 95%CI 1.06-1.74; Figure 3B) as well as PIH (RR 1.19, 95%CI 1.05-1.36; Figure 3A) in donor sperm conceptions, with minimal between-study heterogeneity (I2 = 17.6% and I2 = 15.4%, respectively).
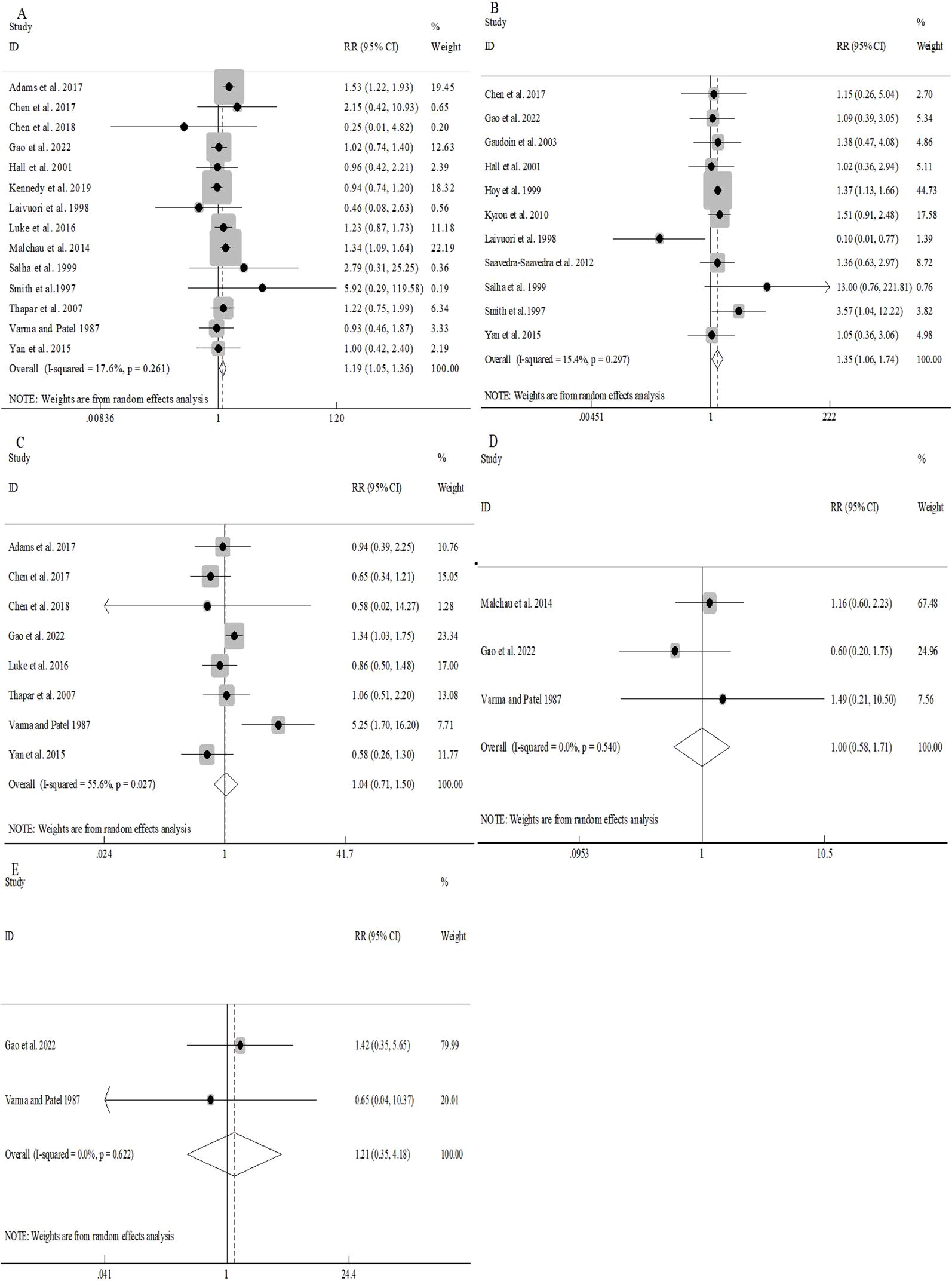
Figure 3. Association between different sperm sources and pregnancy complications: PIH (A); pre-eclampsia (B); GDM (C); placenta praevia (D).
No notable discrepancies were detected in the overall risk of GDM between donor and partner sperm conception (RR 1.04, 95%CI 0.71-1.50; Figure 3C). However, moderate heterogeneity (I2 = 55.6%) prompted stratified analyses, which revealed consistent patterns (Supplementary Figure S4). Univariable meta-regression indicated that location explained a substantial proportion of heterogeneity (R2 = 60.81%, P=0.064), but was not statistically significant. Other moderators accounted for minimal heterogeneity (all R2≈0, P>0.05) (Supplementary Table S4).
Similarly, the overall risks of placenta previa (RR 1.00, 95%CI 0.58-1.71; Figure 3D) as well as abruption (RR 1.21, 95%CI 0.35-4.18; Figure 3E) were comparable between groups with negligible heterogeneity (I2 = 0.0% for both).
Perinatal outcomes: donor vs. partner sperm
Pooled analyses indicated that using donor sperm had comparable risks of PTB, HBW, LGA, neonatal death, stillbirth, congenital anomalies, and perinatal death when compared to partner sperm, with very low statistical heterogeneity across these outcomes (Figures 4A, F, H–L).
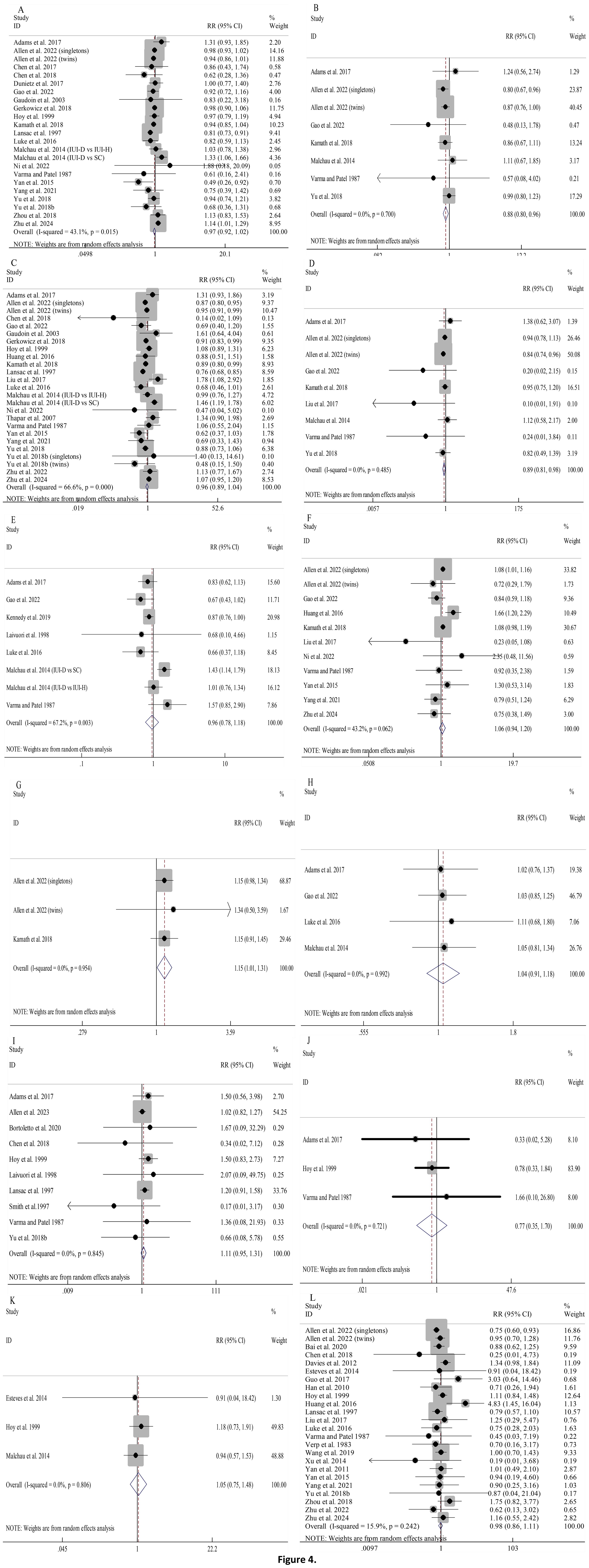
Figure 4. Association between different sperm sources and perinatal outcomes: PTB (A); VPTB (B); LBW (C); VLBW (D); SGA (E); HBW (F); VHBW (G); LGA (H); stillbirth (I); neonatal death (J); perinatal death (K); congenital anomalies (L).
Notably, compared to partner sperm, pregnancies resulting from donor sperm exhibited significantly lower risks of VPTB (RR 0.88, 95%CI 0.80-0.96; Figure 4B) as well as VLBW (RR 0.89, 95%CI 0.81-0.98; Figure 4D). Furthermore, between-study heterogeneity was minimal (I2 = 0.0% for both).
The overall risks of LBW and SGA were comparable between donor and partner sperm conceptions (Figures 4C, E). However, substantial heterogeneity (I2 = 66.6% and I2 = 67.2%, respectively) prompted prespecified subgroup analyses, which were largely consistent with the overall results (Supplementary Figures S5, S6). Univariable meta-regression indicated that, for LBW, only model of conception was a significant moderator (QM=17.623, P=0.002, R2 = 76.56%), while for SGA, none of the examined moderators significantly explained the heterogeneity among studies (Supplementary Table S4).
Sensitivity analysis
Sensitivity analyses using itemized exclusion demonstrated the robustness of pooled estimates for most outcomes. However, exclusion of the study by Allen et al. (7) eliminated the observed association between sperm sources and biochemical pregnancy risk (P=0.658, Supplementary Figures S7-S9). To further assess the robustness of our results, we performed additional sensitivity analyses by increasing the weight of each study in turn. The changes in pooled effect size and CIs were minimal (maximum difference: 0.11), and the overall conclusions remained unchanged, indicating robust findings without dominance by any single study (Supplementary Table S5).
Publication bias
No significant publication bias was observed through funnel plots and Egger’s tests (P>0.05 for all; Supplementary Figures S10-S12).
Discussion
Main findings
This meta-analysis demonstrated that pregnancies achieved using donor sperm had improved clinical pregnancy rates and reduced risks of biochemical pregnancy, miscarriage, VPTB, and VLBW in comparison to those achieved using partner sperm. However, donor sperm conceptions were associated with elevated risks of PIH and preeclampsia. No notable differences were found between pregnancies achieved with donor sperm and those with partner sperm in other obstetric or perinatal outcomes, including GDM, placenta previa, placental abruption, PTB, or congenital anomalies, etc.
Comparison with literature
Four previous meta-analyses had investigated obstetric and perinatal outcomes in donor versus partner sperm conceptions (74–77). A meta-analysis conducted in 2017, which included three papers published before 2012, reported no increased risks of LBW, PTB, or congenital anomalies in donor sperm conceptions compared with natural conceptions (74). In 2018, the same research team updated their analysis with three additional publications, identifying elevated risks of LBW and congenital anomalies in donor conceptions but no notable differences in PTB, SGA, LGA, VPTB, and VLBW risks (75). A meta-analysis conducted in 2021, which included 36 publications up to 2019, found that donor sperm conceptions had elevated risks of preeclampsia and SGA, but a decreased risk of ectopic pregnancy, compared to partner sperm conceptions. There were no notable differences in the risks of miscarriage, GDM, PIH, placental abruption, placenta previa, PTB, VPTB, LBW, VLBW, HBW, LGA, perinatal death, neonatal death, stillbirth, or congenital anomalies between donor sperm and partner sperm conceptions (77). In 2022, another meta-analysis, which included 17 studies published up to 2020, concluded that donor sperm conceptions were associated with increased risks of preeclampsia and PIH but found no increased risks of LBW and PTB (76).
In contrast to these earlier studies, our meta-analysis synthesized data from 64 eligible studies, including those published as recently as 2024, to provide a more updated and comprehensive assessment. Unlike previous meta-analyses that only focused on univariate analyses, our study is the first to incorporate both univariate and multivariate analyses, enabling a more robust and accurate evaluation of the relationship between sperm sources and obstetric as well as perinatal outcomes. Furthermore, this study is the first to evaluate and compare the outcomes of clinical pregnancy, biochemical pregnancy, and VHBW between donor sperm and partner sperm conceptions. By incorporating a broader range of studies and outcomes, our analysis offers a more comprehensive and detailed insight into the possible risks and benefits associated with using donor sperm.
Interpretation of findings
Our meta-analysis reveals both clinically significant benefits and potential risks associated with donor sperm conception. On the one hand, donor sperm use is associated with higher clinical pregnancy rates and reduced risks of biochemical pregnancy, miscarriage, VPTB, and VLBW when compared to partner sperm. These benefits are likely attributable, at least in part, to the rigorous screening procedures implemented for sperm donors. Such screening not only ensures that only healthy individuals with optimal semen parameters can become donors, but also effectively excludes issues such as sexually transmitted infections, genetic diseases, and chromosomal abnormalities through physical examinations and hematological tests, thereby helping to improve early pregnancy outcomes and neonatal health.
On the other hand, an important finding of our study is the significantly elevated risk of PIH and preeclampsia in pregnancies conceived with donor sperm. These hypertensive disorders are associated with significant maternal and neonatal morbidity, underscoring the importance of enhanced surveillance, close monitoring, and early intervention, such as regular blood pressure monitoring and prompt management when necessary. Although the underlying mechanisms are not yet fully understood, potential factors may include immunological incompatibility between mother and fetus (84), as well as possible deleterious effects of sperm cryopreservation on DNA integrity (4, 85, 86) and epigenetic modifications (87–90).
Importantly, our findings did not show an increased risk of several other maternal and perinatal complications, including GDM, placenta previa, placental abruption, LBW, SGA, PTB, HBW, VHBW, neonatal death, stillbirth, congenital anomalies, and perinatal mortality, suggesting that donor sperm conception does not broadly elevate perinatal risk beyond hypertensive disorders. However, sample sizes for rarer outcomes were limited.
Strengths and weaknesses
This meta-analysis has six strengths. First, our meta-analysis included a large sample size, incorporating 17 recently published studies (7, 12–14, 23, 24, 26–36), as well as studies omitted from previous meta-analyses (37–45). This extensive inclusion enhances the generalizability of the study results and improves statistical power. Second, it is the first meta-analysis to integrate both univariate (n=38 studies) and multivariate (n=26 studies) estimates, allowing for a more comprehensive comparison of obstetric and perinatal outcomes between donor and partner sperm. Incorporating multivariate estimates helps to address potential confounding variables, thereby improving the robustness of the findings. Third, unlike earlier meta-analyses, this study expanded the scope of analysis by adding clinical pregnancy, biochemical pregnancy, and VHBW, offering a broader perspective on ART outcomes. Fourth, our meta-analysis minimized selection bias by including eligible studies published in multiple languages, including Chinese (29, 31, 33, 35, 36, 40, 41, 43–47), English, and other languages. Fifth, the statistical heterogeneity for most outcomes was low, indicating consistency across studies and strengthening the reliability of the pooled estimates. Sixth, sensitivity analyses showed no significant change in the pooled estimates after excluding any individual study, indicating that the findings were stable and not overly influenced by individual studies.
Nevertheless, it is important to acknowledge the presence of five limitations in this meta-analysis. First, as the studies included were observational, the findings are inevitably subject to residual or uncontrolled confounding factors, which limits the ability to draw definitive causal inferences. While multivariate analyses were included to address some confounding, the inherent design limitations of observational studies remain. Second, the loss of follow-up may affect the accuracy of the results. Couples receiving ART often experience psychological pressure, influenced by traditional beliefs and societal pressures, which may lead to interrupted contact with medical institutions and loss of follow-up. Third, there was inconsistency in the definitions of key confounders and outcome variables among the included studies. Since most of the original studies only reported aggregated data and some did not provide detailed definitions of these variables, it was not possible to fully harmonize variable definitions in our analysis. This inconsistency may have introduced additional heterogeneity. Such heterogeneity is inevitable in most meta-analyses synthesizing observational studies. Fourth, due to the limited baseline information in the included studies, some key variables (such as maternal age, duration of sperm cryopreservation, and the proportion of multiple pregnancies) were either insufficiently reported or not accompanied by stratified outcome data. This limited our ability to perform subgroup analyses or meta-regression based on these variables, and thus hindered a more comprehensive exploration of potential sources of heterogeneity. Fifth, according to the GRADE assessment, only 6 of the 20 outcomes were rated as moderate quality, while the remaining 14 were of low or very low quality. This indicates that the overall reliability of the study’s conclusions is limited, and the related results should be interpreted with caution.
Implications for clinical practice
For couples facing absolute sperm deficiency or paternally inherited genetic disorders, donor sperm is a viable and effective solution, as it can significantly improve pregnancy success rates and reduce the occurrence of various adverse outcomes. However, clinicians must clearly inform patients of the increased risk of PIH and preeclampsia, strengthen prenatal monitoring, and implement necessary preventive measures. For couples without strict indications for donor sperm use, considering the increased risk of hypertensive disorders, conception with partner sperm remains preferable when feasible. Therefore, for these couples, the potential risks and benefits should be carefully weighed. In addition, this meta-analysis indicates the need for further high-quality studies to clarify the mechanisms underlying the increased risk of PIH and preeclampsia in donor sperm conceptions, and to develop targeted interventions for optimizing ART outcomes.
Conclusions
Donor sperm is a viable and effective solution for male infertility or paternally inherited disorders and should be prioritized for patients with strict medical indications. Due to increased risks of PIH and preeclampsia, careful risk-benefit assessment is necessary for other patients. Enhanced surveillance and counseling can aid in the early detection and management of complications, thereby improving maternal and fetal outcomes. Further research is needed to elucidate the underlying mechanisms and develop targeted interventions.
Author contributions
JL: Conceptualization, Formal Analysis, Software, Writing – original draft, Writing – review & editing. YD: Data curation, Formal Analysis, Methodology, Writing – review & editing. ZS: Data curation, Methodology, Writing – review & editing. XS: Data curation, Methodology, Writing – review & editing. DL: Data curation, Methodology, Writing – review & editing. DZ: Conceptualization, Data curation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Henan Provincial Key Research and Development Program (251111314200) and Henan Province-Ministerial Co-construction Medical Science and Technology Tackling Plan Project (SBGJ202303038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1590261/full#supplementary-material
References
1. Zorzi PM, Kussler APS, Pimentel AM, Capp E, and Corleta HVE. Semen analysis of total motile sperm count based on the 1999 and 2010 WHO criteria. JBRA assisted reproduction. (2022) 26:261–6. doi: 10.5935/1518-0557.20210066
2. Witherspoon L and Flannigan R. Male factor infertility: Initial workup and diagnosis in primary care. Can Family physician Medecin famille canadien. (2021) 67:248–54. doi: 10.46747/cfp.6704248
3. Novák J, Vik V, and Krátká Z. Diagnostics of infertile males in the 21st century - a traditional concept or a modern approach? Casopis lekaru ceskych. (2021) 160:20–6.
4. Zribi N, Feki Chakroun N, El Euch H, Gargouri J, Bahloul A, and Ammar Keskes L. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertility sterility. (2010) 93:159–66. doi: 10.1016/j.fertnstert.2008.09.038
5. Di Santo M, Tarozzi N, Nadalini M, and Borini A. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv urology. (2012) 2012:854837. doi: 10.1155/2012/854837
6. Toro E, Fernández S, Colomar A, Casanovas A, Alvarez JG, López-Teijón M, et al. Processing of semen can result in increased sperm DNA fragmentation. Fertility sterility. (2009) 92:2109–12. doi: 10.1016/j.fertnstert.2009.05.059
7. Allen C, McLernon D, Bhattacharya S, and Maheshwari A. Early pregnancy outcomes of IVF cycles using donor versus partner sperm: analysis of 1 376 454 cycles recorded by the Human Fertilisation and Embryology Authority (1991-2016). Hum Reprod (Oxford England). (2023) 38:1194–201. doi: 10.1093/humrep/dead057
8. Yu B, Fritz R, Xie X, Negassa A, Jindal S, Vega M, et al. The impact of using donor sperm in assisted reproductive technology cycles on perinatal outcomes. Fertility sterility. (2018) 110:1285–9. doi: 10.1016/j.fertnstert.2018.08.012
9. Malchau SS, Loft A, Henningsen AK, Nyboe Andersen A, and Pinborg A. Perinatal outcomes in 6,338 singletons born after intrauterine insemination in Denmark, 2007 to 2012: the influence of ovarian stimulation. Fertility sterility. (2014) 102:1110–6.e2. doi: 10.1016/j.fertnstert.2014.06.034
10. Luke B, Stern JE, Kotelchuck M, Declercq ER, Cohen B, and Diop H. Birth outcomes by infertility diagnosis analyses of the massachusetts outcomes study of assisted reproductive technologies (MOSART). J Reprod Med. (2015) 60:480–90.
11. Adams D, Fernandez R, Moore V, Willson K, Rumbold A, de Lacey S, et al. Sperm donation perinatal outcomes in an Australian population cohort. J obstetrics gynaecology Res. (2017) 43:1830–9. doi: 10.1111/jog.13449
12. Bortoletto P, Willson S, Romanski PA, Davis OK, and Rosenwaks Z. Reproductive outcomes of women aged 40 and older undergoing IVF with donor sperm. Hum Reprod (Oxford England). (2021) 36:229–35. doi: 10.1093/humrep/deaa286
13. Allen CP, McLernon DJ, Bhattahcharya S, and Maheshwari A. Perinatal outcomes of 221,709 singleton and twin pregnancies after the use of donor versus partner sperm. Fertility sterility. (2022) 118:948–58. doi: 10.1016/j.fertnstert.2022.08.015
14. Gao X, Sun S, Xie L, and Lu S. Effects of donor sperm on perinatal and neonatal outcomes resulting from in vitro fertilization-intracytoplasmic sperm injection and embryo transfer cycles: a retrospective cohort study. Ann Trans Med. (2022) 10:819. doi: 10.21037/atm-21-5492
15. Ten J, Peinado P, Guerrero J, Bernabeu A, Llácer J, Orozco-Beltran D, et al. Comparison of the assisted reproductive technology outcomes between conventional IVF and ICSI with donor oocytes in normozoospermic patients. Hum fertility (Cambridge England). (2022) 25:56–62. doi: 10.1080/14647273.2019.1686775
16. Robinson JN, Lockwood GM, Dokras A, Egan DM, Ross C, and Barlow DH. A controlled study to assess the use of in vitro fertilization with donor semen after failed therapeutic donor insemination. Fertility sterility. (1993) 59:353–8. doi: 10.1016/s0015-0282(16)55673-8
17. Kamath MS, Antonisamy B, Selliah HY, La Marca A, and Sunkara SK. Perinatal outcomes following IVF with use of donor versus partner sperm. Reprod biomedicine online. (2018) 36:705–10. doi: 10.1016/j.rbmo.2018.03.016
18. Gerkowicz SA, Crawford SB, Hipp HS, Boulet SL, Kissin DM, and Kawwass JF. Assisted reproductive technology with donor sperm: national trends and perinatal outcomes. Am J obstetrics gynecology. (2018) 218:421.e1–.e10. doi: 10.1016/j.ajog.2017.12.224
19. Dunietz GL, Holzman C, Zhang Y, Li C, Todem D, Boulet SL, et al. Assisted reproduction and risk of preterm birth in singletons by infertility diagnoses and treatment modalities: a population-based study. J assisted Reprod Genet. (2017) 34:1529–35. doi: 10.1007/s10815-017-1003-6
20. Bu Z, Xiong Y, Wang K, and Sun Y. Risk factors for ectopic pregnancy in assisted reproductive technology: a 6-year, single-center study. Fertility sterility. (2016) 106:90–4. doi: 10.1016/j.fertnstert.2016.02.035
21. Castillo CM, Horne G, Fitzgerald CT, Johnstone ED, Brison DR, and Roberts SA. The impact of IVF on birthweight from 1991 to 2015: a cross-sectional study. Hum Reprod (Oxford England). (2019) 34:920–31. doi: 10.1093/humrep/dez025
22. Chen L, Zhu L, Cai C, Yan G, and Sun H. Clinical and neonatal outcomes of intrauterine insemination with frozen donor sperm. Syst Biol Reprod Med. (2018) 64:240–5. doi: 10.1080/19396368.2018.1453563
23. Frank R, Steiner N, Al Shatti M, Ruiter-Ligeti J, and Dahan MH. Outcomes of donor versus partner sperm in intrauterine insemination in women aged 38 years and older. Int J gynaecology obstetrics. (2022) 156:516–20. doi: 10.1002/ijgo.13694
24. Sun X, Yang KL, Zheng QY, Lu QF, Qi ZQ, Liu Y, et al. Effects of different sperm sources on clinical outcomes in intracytoplasmic sperm injection cycles. Andrologia. (2022) 54:e14438. doi: 10.1111/and.14438
25. Zhou Z, Chen L, Wu H, Zheng D, Li R, Mol BW, et al. Assisted reproductive technology in Beijing, 2013-2015. Reprod biomedicine online. (2018) 37:521–32. doi: 10.1016/j.rbmo.2018.08.002
26. Zhu Y, Zhang F, Chen H, Sun X, and Jiang F. The use of frozen embryos and frozen sperm have complementary IVF outcomes: a retrospective analysis in couples experiencing IVF/Donor and IVF/Husband. BMC pregnancy childbirth. (2022) 22:776. doi: 10.1186/s12884-022-05088-x
27. Alorf F, Alani S, Steiner N, and Dahan MH. How successful is intrauterine insemination after failed IVF? A study of 551 women. Reprod biomedicine online. (2024) 48:103684. doi: 10.1016/j.rbmo.2023.103684
28. Bai F, Wang DY, Fan YJ, Qiu J, Wang L, Dai Y, et al. Assisted reproductive technology service availability, efficacy and safety in mainland China: 2016. Hum Reprod (Oxford England). (2020) 35:446–52. doi: 10.1093/humrep/dez245
29. Cheng L, Feng Z, Ma L, and Tan L. Effects of sperm from different sources on the outcomes of intracytoplasmic sperm injection. Chin J Pract Gynecology Obstetrics. (2018) 34:1042–6. doi: 10.19538/j.fk2018090123
30. Kennedy AL, Stern CJ, Tong S, Hastie R, Agresta F, Walker SP, et al. The incidence of hypertensive disorders of pregnancy following sperm donation in IVF: an Australian state-wide retrospective cohort study. Hum Reprod (Oxford England). (2019) 34:2541–8. doi: 10.1093/humrep/dez198
31. Ni X, Chen L, and Zhu L. Impact of donor sperm use on clinical outcomes in assisted technology (ART) IVF-ET. Chin J Reprod Health. (2022) 33:470–3. doi: 10.3969/j.issn.1671-878X.2022.05.013
32. Ruiter-Ligeti J, Dahan MH, Steiner N, Volodarsky-Perel A, and Buckett W. Is intrauterine insemination a viable treatment option for women over 43 years old? An analysis by ovarian stimulation protocol and sperm source. J assisted Reprod Genet. (2020) 37:3103–7. doi: 10.1007/s10815-020-01976-3
33. Wang M. The influence of artificial insemination by donor and in vitro fertilization with donor sperm on the clinical pregnancy outcomes and birth defects of the offspring [dissertation]. Zhengzhou (China): Zhengzhou University (2019).
34. Yang J, Zhang Z, Song J, and Zhou L. Clinical and neonatal outcomes of artificial insemination with sperm from different sources. Natl J Andrology. (2021) 27:991–4. doi: 10.13263/j.cnki.nja.2021.11.006
35. Zhu X, Zhou L, Wang Y, Sun Q, Cao M, Du Y, et al. Correlation analysis of freezing methods with different sperm sources and pregnancy outcomes. J Shandong Univ (Health Sciences). (2021) 59(6):86–93. doi: 10.6040/j.issn.1671-7554.0.2021.0253
36. Zhu Y, Li N, Zhang Y, Zhou L, and Xu C. Aretrospective analysis of neonatal outcomes resulting from IVF/ICSI-ET with donor sperm. J Of Reprod Med. (2024) 33:155–60. doi: 10.3969/j.issn.1004-3845.2024.02.003
37. Azem F, Botchan A, Yaron Y, Lessing JB, Har-toov J, Yavetz H, et al. Outcome of donor versus husband insemination in couples with unexplained infertility treated by in vitro fertilization and embryo transfer. Fertility sterility. (1994) 61:1088–91. doi: 10.1016/s0015-0282(16)56761-2
38. Dong F, Sun Y, Su Y, Guo Y, Hu L, and Wang F. Relationship between processed total motile sperm count of husband or donor semen and pregnancy outcome following intrauterine insemination. Syst Biol Reprod Med. (2011) 57:251–5. doi: 10.3109/19396368.2011.603792
39. Luke B, Stern JE, Kotelchuck M, Declercq ER, Anderka M, and Diop H. Birth outcomes by infertility treatment: analyses of the population-based cohort: massachusetts outcomes study of assisted reproductive technologies (MOSART). J Reprod Med. (2016) 61:114–27.
40. Su D, Bi X, and Wu X. Comparison of clinical outcomes between donor and husband sperm in In vitro fertilization-embryo transfer procedures. J Pract Med Techniques. (2014) 21:656–8.
41. Xu B, Hong Y, Zhao X, Wang Y, Mao L, and Sun Y. Effects of severe oligozoospermia on IVF outcome by comparing with donate sperm. Reprod contraception. (2014) 34:819–23.
42. Yan J, Huang G, Sun Y, Zhao X, Chen S, Zou S, et al. Birth defects after assisted reproductive technologies in China: analysis of 15,405 offspring in seven centers (2004 to 2008). Fertility sterility. (2011) 95:458–60. doi: 10.1016/j.fertnstert.2010.08.024
43. Yan G. Correlative research of the incidence of preeclampsia and artificial insemination by donor [dissertation]. Hangzhou (China): Zhejiang University (2015).
44. Zhang D, Tan L, and Zhao D. Comparison of clinical outcomes of intracytoplasmic sperm injection using three different sources of sperm. Chin Med Innovations. (2014) 11:11–3. doi: 10.3969/j.issn.1674-4985.2014.32.004
45. Zheng W, Tan Y, Zhu X, Chen R, Tan Y, Jiang R, et al. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles of surplus embryo: influence of sperm sources and fertilization method. Reprod Contraception. (2016) 36:845–51. doi: 10.7669/j.issn.0253-357X.2016.10.0845
46. Guo H, Wang S, Liu S, Wang X, Hu J, He W, et al. Analysis on gender ratio of neonates conceived by assisted reproductive technology and the related influencing factors. Maternal Child Health Care China. (2017) 32:5977–80. doi: 10.7620/zgfybj.j
47. Liu S, Wang W, Huang H, Guo W, Liu X, Wang S, et al. Effect of sperm source on birth defects and live birth sex ratio of newborns by assisted reproductive technology. Chin J Family Planning. (2017) 25:51–4. doi: 10.3969/j.issn.1004-8189.2017.01.012
48. de Mouzon J, Levy R, Mourouvin Z, Belaisch-Allart J, Bachelot A, and Royère D. Semen characteristics and quality of the conceptus in fertilization in vitro. Gynecologie obstetrique fertilite. (2007) 35:216–23. doi: 10.1016/j.gyobfe.2007.01.017
49. Esteves SC, Prudencio C, Seol B, Verza S, Knoedler C, and Agarwal A. Comparison of sperm retrieval and reproductive outcome in azoospermic men with testicular failure and obstructive azoospermia treated for infertility. Asian J andrology. (2014) 16:602–6. doi: 10.4103/1008-682x.126015
50. Gaudoin M, Dobbie R, Finlayson A, Chalmers J, Cameron IT, and Fleming R. Ovulation induction/intrauterine insemination in infertile couples is associated with low-birth-weight infants. Am J obstetrics gynecology. (2003) 188:611–6. doi: 10.1067/mob.2003.5
51. Hall G, Noble W, Lindow S, and Masson E. Long-term sexual co-habitation offers no protection from hypertensive disease of pregnancy. Hum Reprod (Oxford England). (2001) 16:349–52. doi: 10.1093/humrep/16.2.349
52. Han J, Chen H, Niu Z, Sun Y, Sun X, Zhao X, et al. A 10-year survey on birth defects after in vitro fertilization-embryo transfer in Shanghai. Chin J Obstetrics Gynecology. (2010) 45:124–7. doi: 10.3788/HPLPB20102209.2186
53. Hinduja I, Zaveri K, and Baliga N. Human sperm centrin levels & outcome of intracytoplasmic sperm injection (ICSI)–a pilot study. Indian J Med Res. (2008) 128:606–10.
54. Huang D, Song S, and Liao A. Short-term safety evaluation of the offspring conceived by 7272 artificial insemination cycles with donor spermatozoon. Andrologia. (2016) 48:817–23. doi: 10.1111/and.12517
55. Kyrou D, Kolibianakis EM, Devroey P, and Fatemi HM. Is the use of donor sperm associated with a higher incidence of preeclampsia in women who achieve pregnancy after intrauterine insemination? Fertility sterility. (2010) 93:1124–7. doi: 10.1016/j.fertnstert.2008.12.021
56. Laivuori HM, Hovatta OL, and Ylikorkala RO. Lack of previous exposure to paternal antigens does not predispose to hypertensive pregnancy complications. Hypertension pregnancy. (1998) 17:291–5. doi: 10.3109/10641959809009602
57. Lansac J, Thepot F, Mayaux MJ, Czyglick F, Wack T, Selva J, et al. Pregnancy outcome after artificial insemination or IVF with frozen semen donor: a collaborative study of the French CECOS Federation on 21,597 pregnancies. Eur J obstetrics gynecology Reprod Biol. (1997) 74:223–8. doi: 10.1016/s0301-2115(97)00102-4
58. Oehninger S, Chaturvedi S, Toner J, Morshedi M, Mayer J, Lanzendorf S, et al. Semen quality: is there a paternal effect on pregnancy outcome in in-vitro fertilization/intracytoplasmic sperm injection? Hum Reprod (Oxford England). (1998) 13:2161–4. doi: 10.1093/humrep/13.8.2161
59. Plasencia W, García R, Torres A, Guillén V, Sánchez V, Domingo J, et al. Analysis of 2304 intrauterine artificial insemination cycles. Rev Iberoamericana Fertilidad y Reproduccion Humana. (2004) 21:217–24.
60. Prados F, Cuevas I, Vidal E, de Andrés M, Hernández J, Zamora S, et al. Registro de inseminación artificial de la Sociedad Espa˜nola de Fertilidad de los a˜nos 2012 y 2013. Medicina Reproductiva y Embriología Clínica. (2017) 4:136–42. doi: 10.1016/j.medre.2017.09.004
61. Saavedra JS, Becerra EP, and Losada PR. Preeclampsia in infertile patients subjected to homologous and heterologous insemination in the centro de Biomedicina Reproductiva del Valle - Fecundar, Cali, Colombia. (2012). doi: 10.18597/rcog.203
62. Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, et al. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod (Oxford England). (1999) 14:2268–73. doi: 10.1093/humrep/14.9.2268
63. Scarselli F, Casciani V, Cursio E, Muzzì S, Colasante A, Gatti S, et al. Influence of human sperm origin, testicular or ejaculated, on embryo morphokinetic development. Andrologia. (2018) 50:e13061. doi: 10.1111/and.13061
64. Smith GN, Walker M, Tessier JL, and Millar KG. Increased incidence of preeclampsia in women conceiving by intrauterine insemination with donor versus partner sperm for treatment of primary infertility. Am J obstetrics gynecology. (1997) 177:455–8. doi: 10.1016/s0002-9378(97)70215-1
65. Thapar A, Harold G, Rice F, Ge X, Boivin J, Hay D, et al. Do intrauterine or genetic influences explain the foetal origins of chronic disease? A novel experimental method for disentangling effects. BMC Med Res methodology. (2007) 7:25. doi: 10.1186/1471-2288-7-25
66. Varma TR and Patel RH. Outcome of pregnancy following investigation and treatment of infertility. Int J gynaecology obstetrics. (1987) 25:113–20. doi: 10.1016/0020-7292(87)90004-x
67. Verp MS, Cohen MR, and Simpson JL. Necessity of formal genetic screening in artificial insemination by donor. Obstetrics gynecology. (1983) 62:474–9. doi: 10.1016/0378-5122(83)90009-9
68. Warnes GM, Petrucco OM, Seamark RF, and Lancaster PA. Is the male involved in the aetiology of ectopic pregnancy? Hum Reprod (Oxford England). (1998) 13:3505–10. doi: 10.1093/humrep/13.12.3505
69. Yovich JL and Matson PL. Early pregnancy wastage after gamete manipulation. Br J obstetrics gynaecology. (1988) 95:1120–7. doi: 10.1111/j.1471-0528.1988.tb06789.x
70. Yu Y, Xi Q, Pan Y, Jiang Y, Zhang H, Li L, et al. Pregnancy and neonatal outcomes in azoospermic men after intracytoplasmic sperm injection using testicular sperm and donor sperm. Med Sci monitor: Int Med J Exp Clin Res. (2018) 24:6968–74. doi: 10.12659/msm.912613
71. Zamora S, de Andrés M, Herrero J, Cabello Y, Prados F, Vidal E, et al. Registro de inseminaciones intrauterinas (conyugales y de donante) de la Sociedad Espaola de Fertilidad. Aos 2010 y 2011. Medicina Reproductiva Y Embriología Clínica. (2014) 1:43–9. doi: 10.1016/j.medre.2014.09.002
72. Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. New Engl J Med. (2012) 366:1803–13. doi: 10.1056/NEJMoa1008095
73. Hoy J, Venn A, Halliday J, Kovacs G, and Waalwyk K. Perinatal and obstetric outcomes of donor insemination using cryopreserved semen in Victoria, Australia. Hum Reprod (Oxford England). (1999) 14:1760–4. doi: 10.1093/humrep/14.7.1760
74. Adams DH, Clark RA, Davies MJ, and de Lacey S. A meta-analysis of sperm donation offspring health outcomes. J Dev origins Health disease. (2017) 8:44–55. doi: 10.1017/s2040174416000489
75. Adams DH, Clark RA, Davies MJ, and de Lacey S. Update on: a meta-analysis of sperm donation offspring health outcomes - 2018 update. J Dev origins Health disease. (2018) 9:561–2. doi: 10.1017/s2040174418000272
76. Pohjonen EM, Söderström-Anttila V, Bergh C, Loft A, Magnusson Å, Pinborg A, et al. Obstetric and perinatal risks after the use of donor sperm: A systematic review and meta-analysis. Eur J obstetrics gynecology Reprod Biol. (2022) 274:210–28. doi: 10.1016/j.ejogrb.2022.05.031
77. Allen CP, Marconi N, McLernon DJ, Bhattacharya S, and Maheshwari A. Outcomes of pregnancies using donor sperm compared with those using partner sperm: systematic review and meta-analysis. Hum Reprod update. (2021) 27:190–211. doi: 10.1093/humupd/dmaa030
78. Popay J, Rogers A, and Williams G. Rationale and standards for the systematic review of qualitative literature in health services research. Qual Health Res. (1998) 8:341–51. doi: 10.1177/104973239800800305
79. Dixon-Woods M, Sutton A, Shaw R, Miller T, Smith J, Young B, et al. Appraising qualitative research for inclusion in systematic reviews: a quantitative and qualitative comparison of three methods. J Health Serv Res policy. (2007) 12:42–7. doi: 10.1258/135581907779497486
80. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E and Munn Z, editors. JBI Manual for Evidence Synthesis Adelaide, Australia: JBI (2020).
81. Mo Y, Zhou Y, Chan H, Evans C, and Maddocks M. The association between sedentary behaviour and sarcopenia in older adults: a systematic review and meta-analysis. BMC geriatrics. (2023) 23:877. doi: 10.1186/s12877-023-04489-7
82. Zhang J and Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Jama. (1998) 280:1690–1. doi: 10.1001/jama.280.19.1690
83. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, and Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ (Clinical Res ed). (2011) 342:d671. doi: 10.1136/bmj.d671
84. Lucio García C, Lucio García CA, Alonso Barreto AS, Camarillo Romero M, del S, Layton Tovar CF, et al. HLA-G in preeclampsia: a pilot study to propose a tolerogenic treatment. Int J Med Students. (2018) 6:56–60. doi: 10.5195/ijms.2018.250
85. Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, and Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod (Oxford England). (2009) 24:2061–70. doi: 10.1093/humrep/dep214
86. Ribas-Maynou J, Fernández-Encinas A, García-Peiró A, Prada E, Abad C, Amengual MJ, et al. Human semen cryopreservation: a sperm DNA fragmentation study with alkaline and neutral Comet assay. Andrology. (2014) 2:83–7. doi: 10.1111/j.2047-2927.2013.00158.x
87. Mani S, Ghosh J, Coutifaris C, Sapienza C, and Mainigi M. Epigenetic changes and assisted reproductive technologies. Epigenetics. (2020) 15:12–25. doi: 10.1080/15592294.2019.1646572
88. Riesco MF and Robles V. Cryopreservation causes genetic and epigenetic changes in zebrafish genital ridges. PloS One. (2013) 8:e67614. doi: 10.1371/journal.pone.0067614
89. Sciorio R, Cantatore C, D’Amato G, and Smith GD. Cryopreservation, cryoprotectants, and potential risk of epigenetic alteration. J assisted Reprod Genet. (2024) 41:2953–67. doi: 10.1007/s10815-024-03287-3
90. Chatterjee A, Saha D, Niemann H, Gryshkov O, Glasmacher B, and Hofmann N. Effects of cryopreservation on the epigenetic profile of cells. Cryobiology. (2017) 74:1–7. doi: 10.1016/j.cryobiol.2016.12.002
91. Lansac J, Thepot F, Mayaux MJ, Czyglick F, Wack T, Selva J, et al. Pregnancy outcome after artificial insemination or IVF with frozen semen donor: a collaborative study of the French CECOS Federation on 21,597 pregnancies. Eur J Obstet Gynecol Reprod Biol. (1997) 74(2):223𠄣8. doi: 10.1016/s0301-2115(97)00102-4
Keywords: meta-analysis, donor conception, semen preservation, reproductive techniques, pregnancy outcome
Citation: Liu J, Dai Y, Song Z, Sun X, Lv D and Zhao D (2025) Obstetric and perinatal outcomes in pregnancies conceived with donor versus partner sperm: a systematic review and meta-analysis. Front. Endocrinol. 16:1590261. doi: 10.3389/fendo.2025.1590261
Received: 09 March 2025; Accepted: 08 July 2025;
Published: 23 July 2025.
Edited by:
Rui Ding, Anhui Medical University, ChinaReviewed by:
Laura Melado, ART Fertility Clinics LLC, United Arab EmiratesXiaohui Hua, Anhui Medical University, China
Copyright © 2025 Liu, Dai, Song, Sun, Lv and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Liu, emRzZnlsampAenp1LmVkdS5jbg==; Dehua Zhao, emhhb2RlaHVhMzY5QDE2My5jb20=
†These authors have contributed equally to this work
 Junjie Liu
Junjie Liu Yanpeng Dai
Yanpeng Dai Zuozhe Song1
Zuozhe Song1 Dehua Zhao
Dehua Zhao