- 1Department of Ultrasound, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, China
- 2Department of Ultrasound, Traditional Chinese Medicine Hospital of Nanjing Lishui District, Nanjing, Jiangsu, China
- 3Department of Thyroid Surgery, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, China
- 4Department of Geriatrics, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, China
- 5Laboratory Center, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, China
- 6Department of Ophthalmology, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, China
- 7Department of Endocrinology, Yancheng city No.6 People’s Hospital, Yancheng, Jiangsu, China
- 8Department of Pathology, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, China
- 9Department of Cardiology, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu, China
- 10Medical Marketing Department, Nanjing D.A. Medical Laboratory, Nanjing, Jiangsu, China
- 11Department of Ultrasound, Jiangsu Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing, Jiangsu, China
- 12Department of Vascular Thyroid Hernia Surgery, Xuzhou Central Hospital, Xuzhou Clinical School of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 13Department of Ultrasound Medicine, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Yangzhou, Jiangsu, China
Introduction: Papillary thyroid carcinoma is the most common pathological subtype of thyroid cancer in both children/adolescents (TCCA) and adults (TCA). TCCA manifests more aggressive and invasive behaviors than TCA, which may be attributed to specific genomic alterations.
Methods: To better understand the specific molecular, pathological and clinical manifestations of TCCA, we retrospectively analyzed a cohort of 60 patients with sporadic papillary thyroid carcinoma, including 20 TCCAs and 40 TCAs. Fine-needle aspiration tissue samples from these cases were analyzed using next-generation sequencing. Demographics, ultrasound features, postoperative pathology and radiation exposure history were compared between TCCAs and TCAs. To validate our findings, we integrated data from 28 prior studies, resulting in a larger cohort of 1,483 sporadic TCCAs.
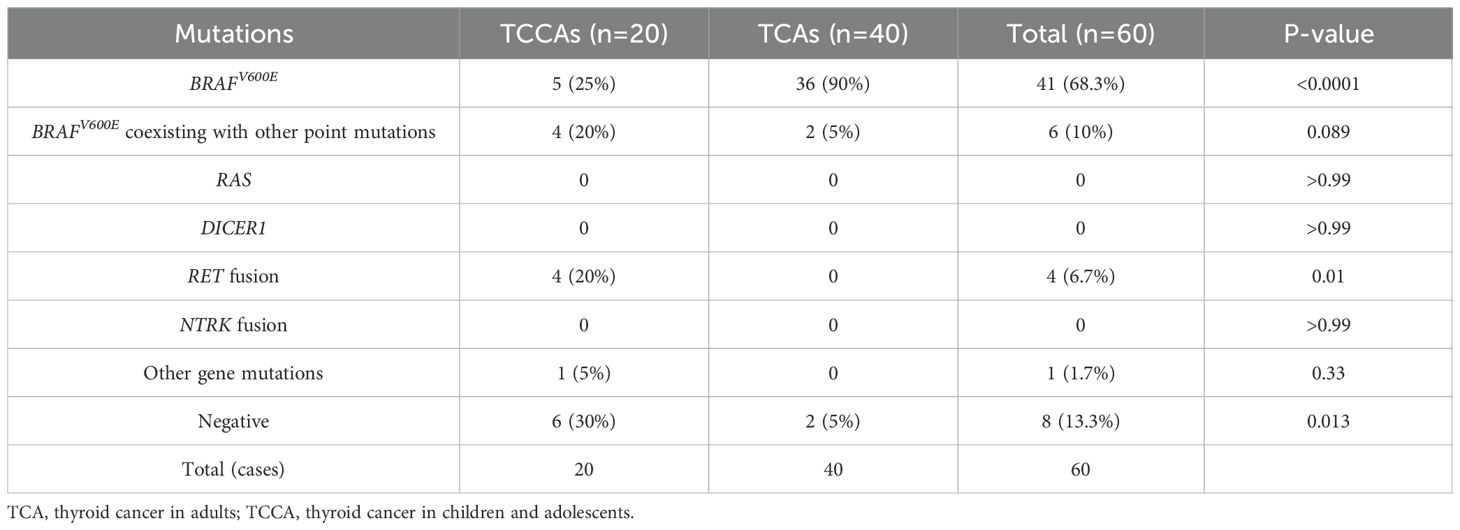
Results: Multiple gene mutations were more prevalent in TCCAs than TCAs (p=0.013), such as BRAFV600E coexisting with KMT2 family genes or PTEN. Although BRAFV600E was the most common single nucleotide variant in TCCAs (25%, 5/20), its prevalence was significantly lower than in TCAs (95%, 38/40, p<0.0001). RET oncogenic fusions were detected exclusively in TCCAs, with an incidence of 20% (4/20). Compared with TCAs, TCCAs were associated with larger tumor diameters (p<0.001), more advanced tumor staging (T3–T4, p<0.001; N2, p=0.002), higher incidence of extrathyroidal extension (TCCA: 25%, TCA: 5%, p=0.036) and more frequent lymph node metastasis (TCCA: 70%, TCA: 27.5%, p=0.0024). Importantly, TCCAs harboring BRAFV600E alongside other mutations (e.g., ATM, PTEN or KMT2 family genes) exhibited more severe clinical manifestations, including larger tumors and higher rates of lymph node metastasis, compared with those harboring BRAFV600E alone.
Discussion: TCCAs exhibit more aggressive and invasive clinical manifestations than TCAs, particularly in cases with RET fusions or BRAFV600E coexisting with other point mutations. Targeted comprehensive molecular profiling may aid in the diagnosis and treatment of TCCA.
1 Introduction
Thyroid cancer is the most common endocrine malignancy, with rising incidence in both pediatric and adult populations (1). It is the second most common malignant neoplasm in adolescents worldwide, with an annual incidence of 0.44/100,000 and a mortality rate of 0.02/100,000 in children and adolescents in China (2–5). To account for differences in clinicopathological characteristics and management strategies, the American Thyroid Association classifies thyroid cancer into thyroid cancer in children and adolescents (TCCA, age ≤18) and in adults (TCA, age >18) (6).
Although TCCA management is largely based on TCA guidelines due to shared pathological features (7–9), TCCAs exhibit distinct clinical behaviors, pathophysiology, and long-term outcomes (10). TCCAs are generally more aggressive, often presenting with multifocality, extrathyroidal invasion, and direct involvement of the recurrent laryngeal nerve, trachea, blood vessels and esophagus (11). Moreover, while thyroid nodules are more common in TCAs (19–68%), only 5–10% are malignant. In contrast, nodules are less common in TCCAs (1–3%) but carry a significantly higher malignancy rate (22–26%), highlighting the need for pediatric-specific diagnostic and therapeutic strategies.
With the rapid advancement of next-generation sequencing (NGS) (12), many studies have characterized the genomic landscape of TCA (13–15). Common genomic alterations, such as BRAFV600E, have been identified and widely used to diagnose TCA (16, 17). However, current guidelines are primarily based on adult data and may not capture the unique genomic features of TCCA. The genetic underpinnings of TCCA remain underexplored, despite their relevance to clinical behavior and treatment. Therefore, investigating these differences is essential to improve diagnostic accuracy and inform tailored therapeutic approaches.
In this study, we compared the genomic alterations between TCCAs and TCAs in an in-house cohort and analyzed the differences in their clinicopathological and ultrasound characteristics. The findings were validated in a large cohort of 1,483 sporadic TCCAs from 28 published studies. Our findings underscored the importance of genomic detection in predicting outcomes and informing therapeutic guidelines for TCCA.
2 Materials and methods
2.1 Ethics declarations
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Affiliated People’s Hospital of Jiangsu University, Traditional Chinese Medicine Hospital of Nanjing Lishui District (IRB approval number: 2022LW037), and Xuzhou Central Hospital (XZXY-LK-20230314-027), Xuzhou Clinical School of Xuzhou Medical University (K-20230090-W). All samples were collected with written informed consent and relevant institutional approval.
2.2 Patient selection
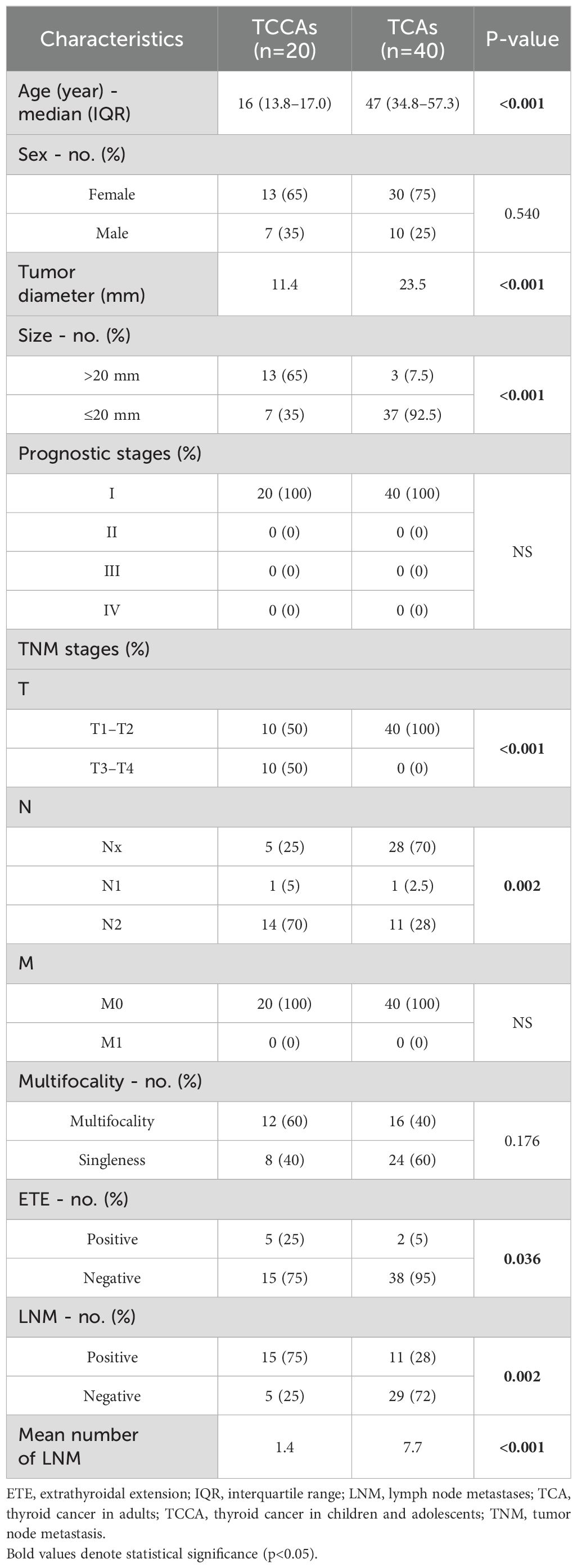
Twenty TCCAs and forty TCAs with a histopathological diagnosis of papillary thyroid cancer (PTC) were recruited from the Traditional Chinese Medicine Hospital of Nanjing Lishui District, Xuzhou Central Hospital and Affiliated People’s Hospital of Jiangsu University between 2021 and 2023. Retrospectively collected data included demographics (age, sex, and radiation exposure history), NGS-detected genomic alterations, histopathologic subtypes, maximum tumor diameters, extrathyroidal extension (ETE), multifocality, lymph node metastases (LNM), distant metastases (DM), and treatment history. A schematic illustration of the study workflow is presented in Figure 1.
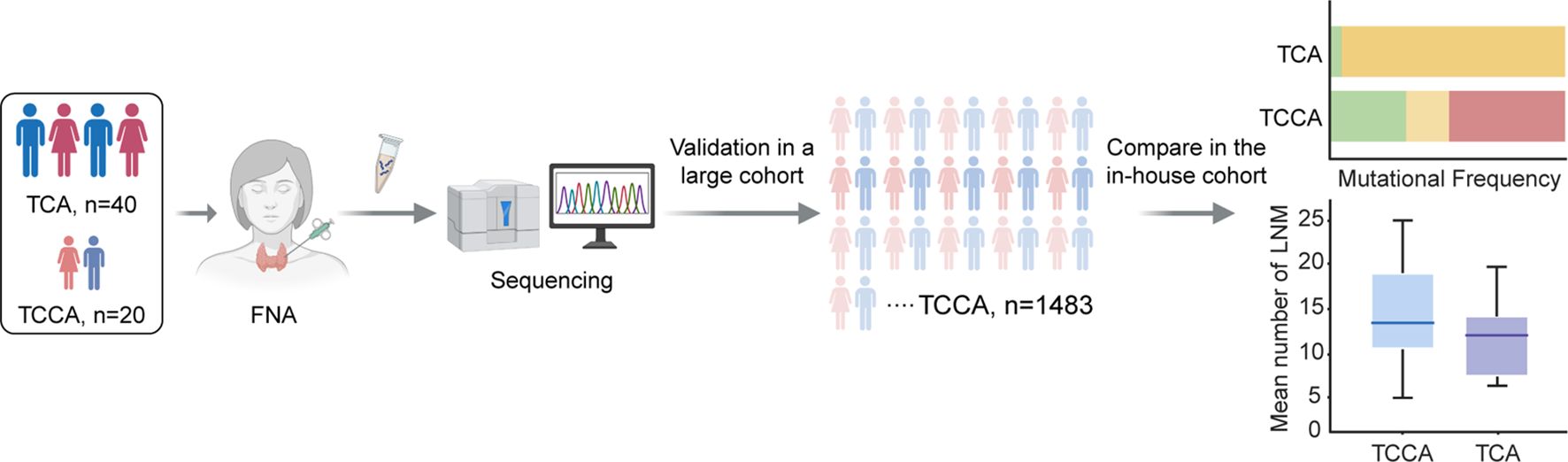
Figure 1. Schematic illustration of workflow in this study. FNA, fine-needle aspiration; TCA, thyroid cancer in adults; TCCA, thyroid cancer in children and adolescents.
Minimal extrathyroidal extension (ETE, T3) refers to microscopic invasion into the perithyroidal soft tissues or sternothyroid muscle, whereas extensive ETE (T4a) involves macroscopic invasion into structures such as the subcutaneous tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve. Extensive ETE is typically identified via preoperative ultrasound, CT, or MRI, and confirmed by pathological examination after surgery. LNM is suspected when ultrasound reveals features such as microcalcifications, cystic changes, peripheral vascularity, hyperechogenicity, or a round shape. Definitive diagnosis requires ultrasound-guided fine-needle aspiration cytology of the washout fluid, with biopsy recommended for central nodes ≥8 mm and lateral nodes ≥10 mm. Chest CT, whole-body radioactive iodine scans, and F-fluorodeoxyglucose positron emission tomography/CT are used to detect distant metastases, particularly in the lungs, bones, and liver. Pathological confirmation through surgical resection or biopsy remains essential, as ETE in lymph nodes and vascular invasion in the primary tumor influence risk stratification. To enhance diagnostic precision and inform staging, patient-specific factors such as tumor size, multifocality, and molecular markers (e.g., BRAF mutations) were also considered.
2.3 Selection criteria for the large TCCA cohort from prior studies
We identified 108 relevant studies, with 104 from PubMed and 4 from Cochrane Library database. After removing duplicates and excluding 57 studies due to non-TCCA genomic alterations, 28 studies were included (Supplementary Figure 1; Supplementary Table 1). To ensure that all TCCAs were sporadic cases, 31 cases with radiation exposure history were excluded. Ultimately, a large cohort of 1,483 TCCAs was included in the analysis.
2.4 Ultrasound imaging
Experienced ultrasound radiologists performed preoperative standard ultrasound scans on the patients using a GE LOGIQ s8, LOGIQ E9, LOGIQ E20 (American General’s GE Medical Systems), Mindray Resona 7, Philips Q5 (Healthcare, Eindhoven, the Netherlands), Philips iU22 ultrasound machine equipped with a 5–12 MHz linear array transducer. These machines were operated using a program specifically designed for thyroid imaging.
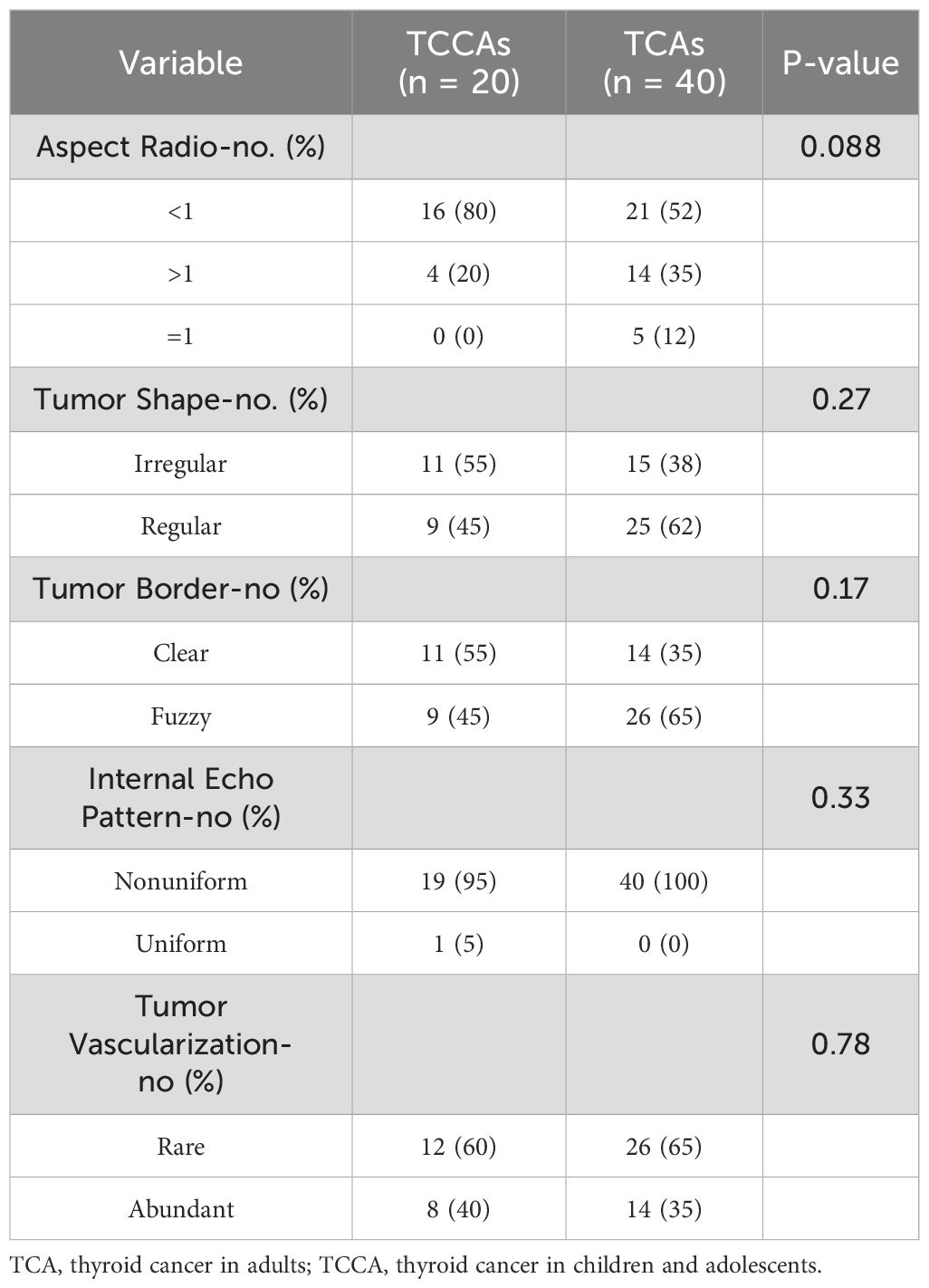
During the procedure, patients were positioned horizontally without a pillow, with their heads slightly inclined to maximize the exposure of the cervical region. Ultrasound assessments of the thyroid and surrounding neck structures were conducted using both cross-sectional and longitudinal scanning. Key ultrasound characteristics assessed included tumor diameter (maximum long axis of the lesion), multifocality (singleness or multifocality), aspect ratio (height divided by width on transverse views, A/T), tumor shape (irregular or regular), tumor border (clear or fuzzy), internal echo pattern (uniform, nonuniform), tumor vascularization (none, rare or abundant), and ultrasonic diagnosis of ETE (positive or negative) and LNM (positive or negative).
2.5 Molecular genetic analysis
2.5.1 Total nucleic acid extraction
Total DNA and RNA were extracted from fine-needle aspiration biopsy (FNAB) samples using the QIAamp DNA Mini Kit (QIAGEN, Germany) following the manufacturer’s protocol, and quantified using Nanodrop 1000 (Thermos Fisher, USA).
2.5.2 Library construction
For quality control, at least 10 ng of total DNA of each sample was extracted and fragmented. Q30 rate of DNA base was above 80%, and DNA sequence alignment rate was above 90%. Mean sequencing depth was over 500×. Q30 rate of RNA base was above 80%, and total reads in the RNA target region was 300,000 reads.
Genomic DNA sequencing libraries were created using the TruSeq DNA Library Preparation Kit following the manufacturer’s protocol. DNA sequencing was performed on the Illumina NextSeq sequencing system (San Diego, CA), using 2100 bp peer read. BWA (Burrows-Wheeler aligner) 10 was used to compare reads with the human genome construction GRCh37. MuTect2 (3.4-46-gbc02625) 11 was used to identify single nucleotide variants (SNVs) and GATK was used to identify small insertions and deletions. Final candidate variants were confirmed with the Integrated Genomics Viewer browser.
2.5.3 NGS
Genetic variations of FNAB samples were detected by NGS (DIAN Diagnostics), including SNVs, fusion alterations (FAs) and copy number alterations. Sixty samples per NGS Illumina NextSeq run were pooled in an equimolar ratio 6 pM library pool. Targeted deep sequencing including a 6% PhiX control library was prepared for sequencing according to the Illumina NextSeq System user guide (Illumina, San Diego, CA). Subsequently, sequencing was carried out on a NextSeq instrument (Illumina) using the v2 chemistry as recommended by the manufacturer.
2.5.4 Statistical analysis
All statistical analyses were carried out using IBM SPSS statistical software (statistic 26.0 version; IBM SPSS, Armonk, NY, USA) or R-4.2.0. The continuous variables between two groups were compared using the Wilcoxon rank-sum test, and Fisher’s exact test was used to compare the categorical variables. All statistical tests were two-sided, and p<0.05 was considered statistically significant.
3 Results
3.1 TCCAs exhibit more aggressive and invasive clinical features than TCAs
Given the significance of ultrasonography in thyroid carcinoma detection, we first investigated differences in ultrasound imaging characteristics between TCCAs and TCAs. TCCAs had larger tumor diameters than TCAs (p<0.001), with no significant differences in other ultrasound characteristics (Tables 1, 2). Also, lesions in TCCAs appeared relatively hyperechoic, with diffusely distributed microcalcifications, irregular morphology, and fuzzy borders on the grayscale ultrasound image (Figures 2A–C). In contrast, TCAs were hypoechoic with irregular morphology (Figures 2A, D). Color Doppler imaging showed more abundant vascularization inside the nodules in TCCAs than in TCAs (Figures 2B, E). Elastography images confirmed the nodules were harder than TCAs, with elastic scores of ≥3 (Figures 2C, F).
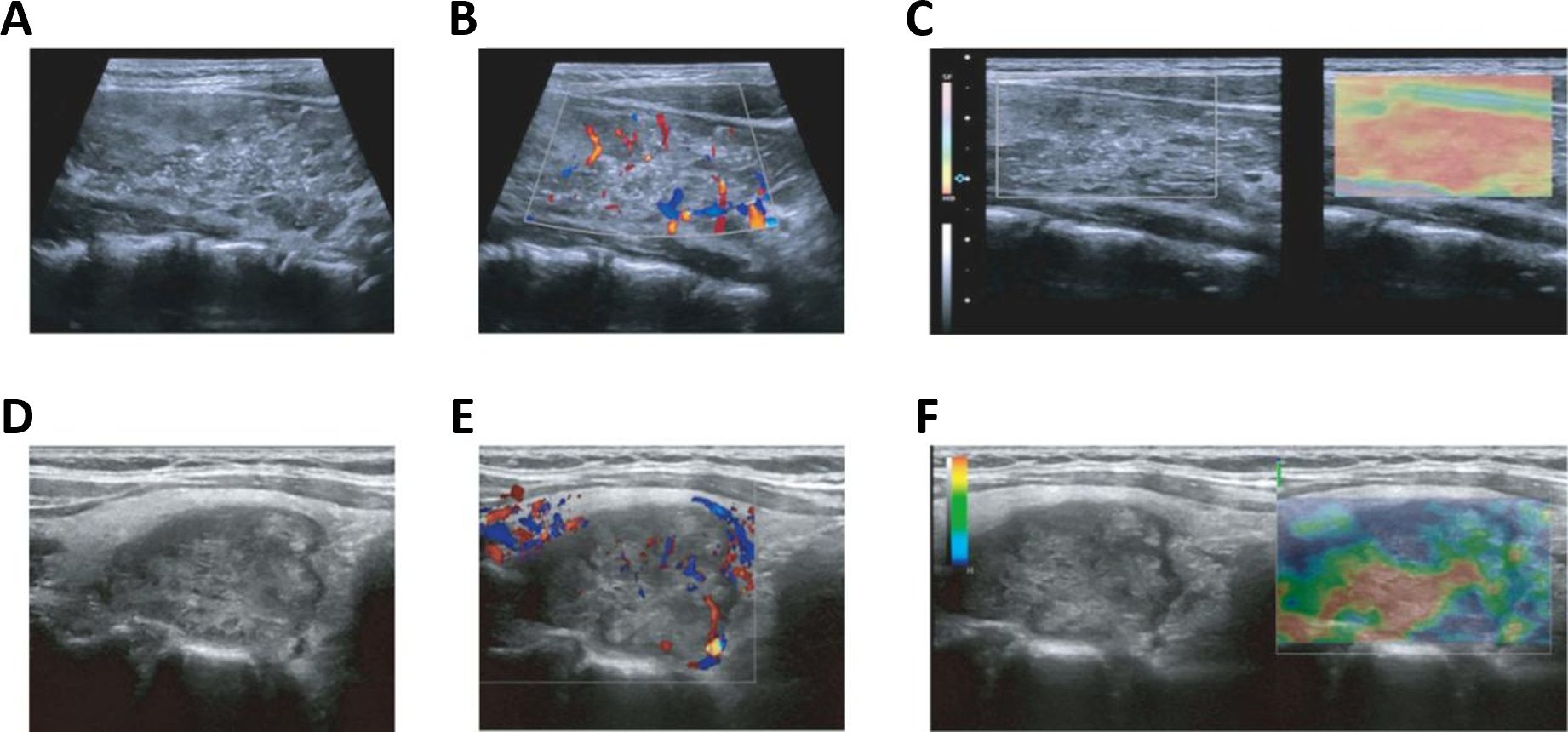
Figure 2. Ultrasound images of TCCAs and TCAs. (A–F) Multimodal ultrasound images of a 15-year-old female (A–C) and a 44-year-old male (D–F) with PTC. Postoperative pathology confirmed TCCA in the female patient and TCA in the male patient. PTC, papillary thyroid cancer; TCA, thyroid cancer in adults; TCCA, thyroid cancer in children and adolescents. Statistical significance: *p<0.05, **p<0.01, ***p<0.001.
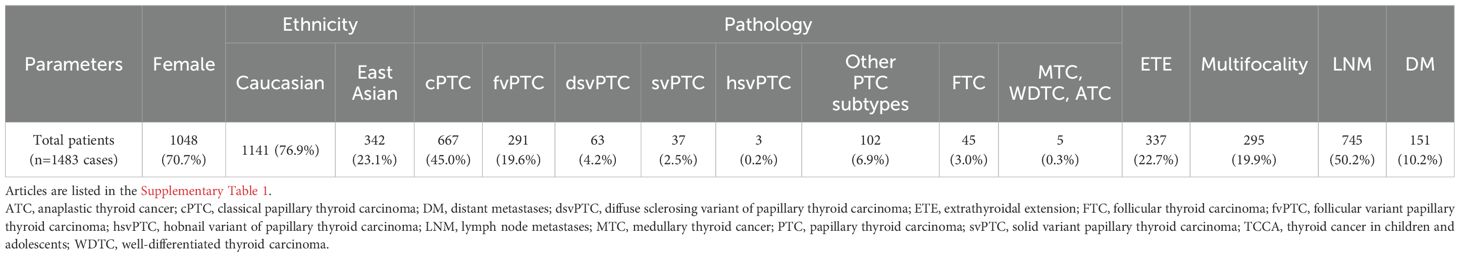
Analysis of baseline characteristics and clinical manifestations showed no significant differences between TCCAs and TCAs in sex (p=0.54) and multifocality (p=0.176) (Table 2). However, TCCAs exhibited more aggressive and invasive clinical features, including larger tumor sizes (p<0.001), more advanced tumor staging (T3–T4, p<0.001; N2, p=0.002), higher incidence of ETE (TCCA: 25%, TCA: 5%, p=0.036), LNM (TCCA: 75%, TCA: 28%, p=0.002) and more positive lymph nodes (p<0.001) (Figure 3). To validate these findings, we analyzed clinical manifestations in the large cohort. As expected, consistent with TCAs, classical PTC was the dominant histopathologic subtype of TCCAs (667/1483, 45%), followed by the follicular variant of PTC (291/1483, 19.6%) (Table 3). Nearly half of TCCAs exhibited LNM (745/1483, 50.2%) or DM (151/1483, 10.2%), 22.7% harbored ETE, and 19.9% had multifocality, indicating the aggressiveness and invasiveness of TCCAs (Table 3).
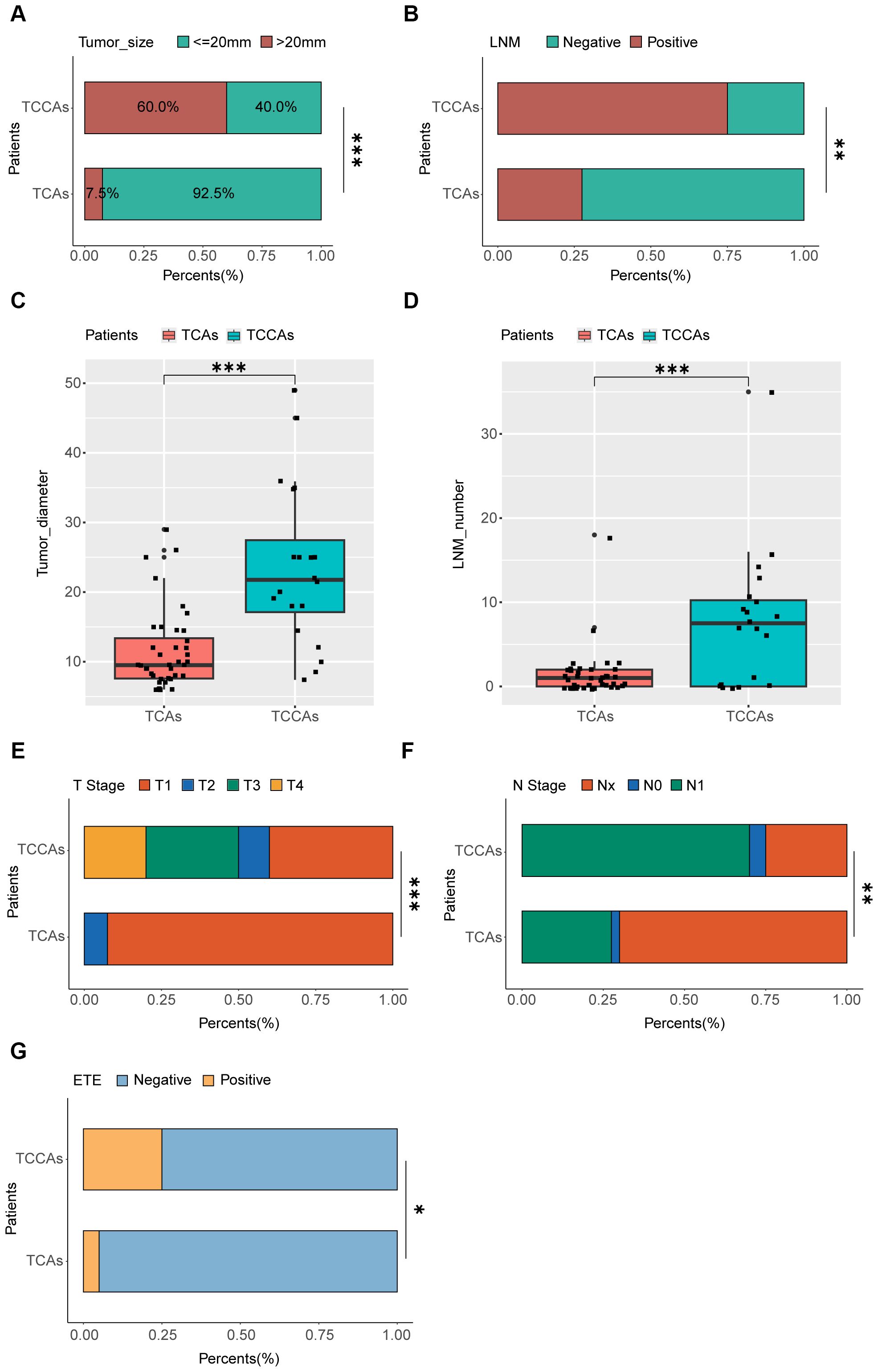
Figure 3. Comparison of clinic-pathological manifestations between TCCAs and TCAs. (A) Proportions of patients with different tumor sizes among the TCCAs and TCAs. (B) Proportions of patients with LNM among the TCCAs and TCAs. (C) Boxplot showing the tumor diameters among TCCAs and TCAs. Wilcoxon rank-sum test was used to measure the differences between groups. (D) Boxplot showing the number of positive lymph nodes among TCCAs and TCAs. Wilcoxon rank-sum test was used to measure the differences between groups. (E) Proportions of patients with T stage among the TCCAs and TCAs. (F) Proportions of patients with N stage among the TCCAs and TCAs. (G) Proportions of patients with ETE among the TCCAs and TCAs. ETE, extrathyroidal extension; LNM, lymph node metastases; TCAs, thyroid cancer in adults; TCCAs, thyroid cancer in children and adolescents.
3.2 TCCAs harbor distinct genomic alterations compared to TCAs
We compared mutation types and frequencies between TCCAs and TCAs in our in-house cohort and validated these findings in the large cohort (Supplementary Figure 1, Supplementary Table 1). The genomic alterations and clinical characteristics of 40 TCAs (30 females and 10 males) and 20 TCCAs (13 girls and 7 boys) are presented in Figure 4; Supplementary Table 2. The distribution of genomic alterations in the large cohort is presented in Table 4.
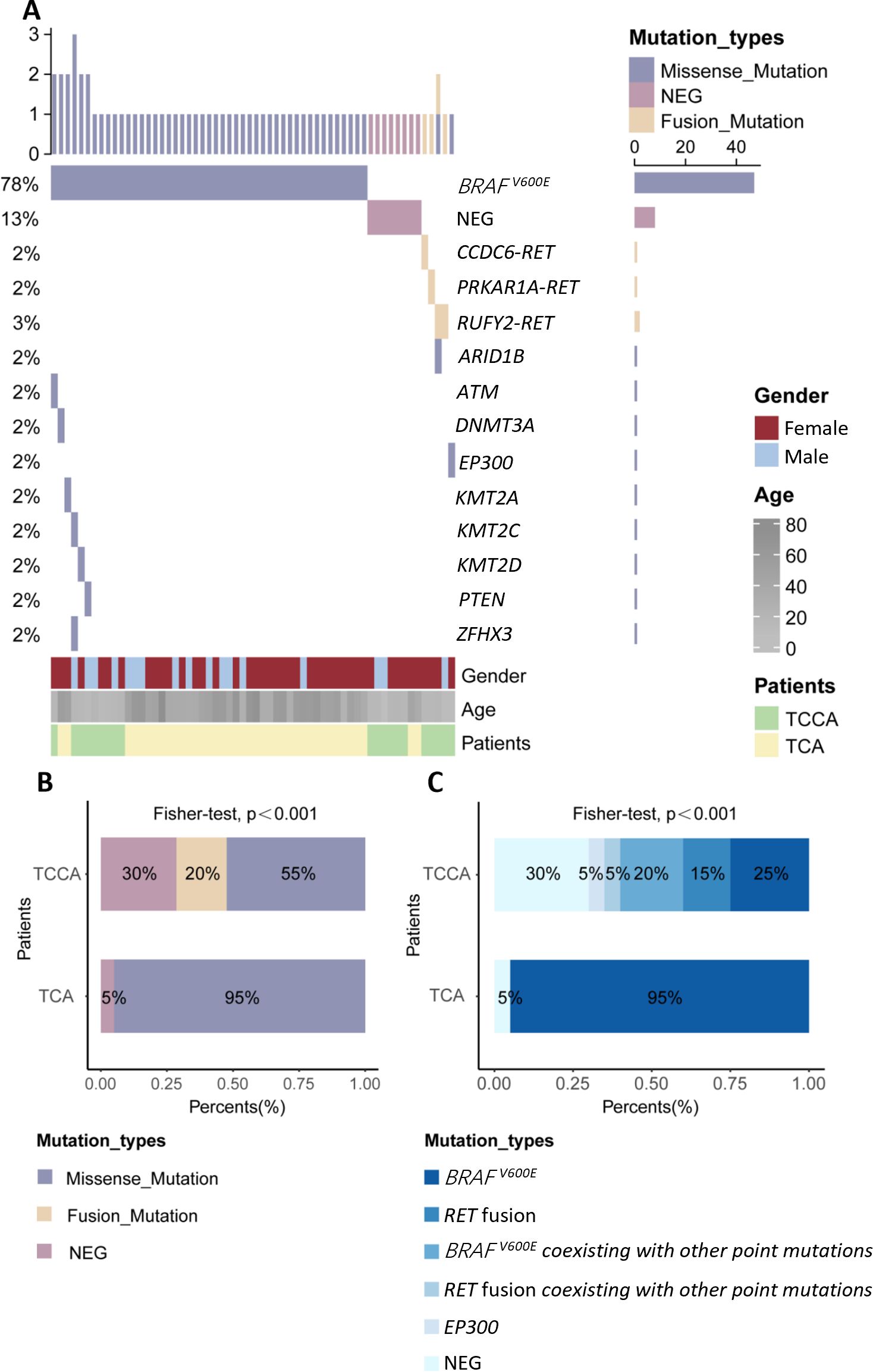
Figure 4. Genomic landscape of TCCAs and TCAs in this in-house cohort. (A) Waterfall Plots showing gene mutations of TCCAs and TCAs in this in-house cohort; (B) Bar plot showing the frequencies of detected mutation types in TCCAs and TCAs; (C) Proportions of detected gene mutations in TCCAs and TCAs in this in-house cohort. NEG, negative; TCAs, thyroid cancer in adults; TCCAs, thyroid cancer in children and adolescents.
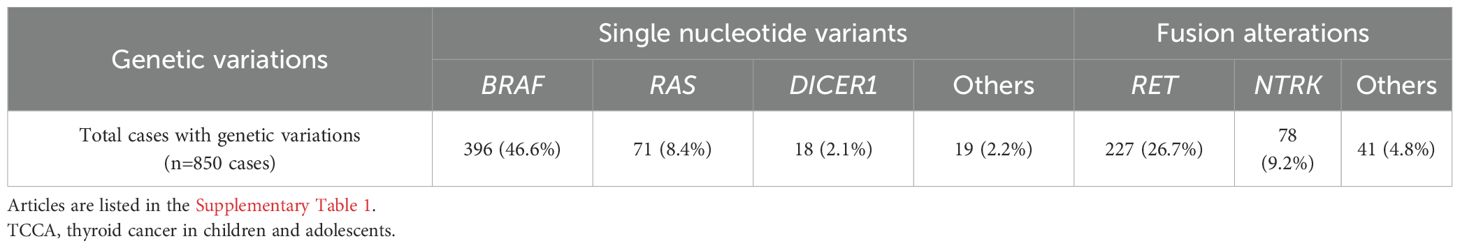
Approximately 70% (14/20) of TCCAs in our in-house cohort harbored genetic alterations, higher than the 57.3% (850/1,483) in the large cohort. BRAFV600E was the most common SNV in TCCAs, found in 46.6% (396/850) of the cases in the large cohort, followed by RET oncogenic fusions (26.7%, 227/850) (Table 4). Consistently, in the in-house cohort, 45% (9/20) of TCCAs had BRAFV600E (five with BRAFV600E alone and four with BRAFV600E and other mutations), and 20% (4/20) had RET fusion. RAS (8.4%, 71/850), DICER1 (2.1%, 18/850) and NTRK (9.2%, 78/850) alterations, reported in the large TCCA cohort, were absent in our in-house cohort (Tables 4; Table 5). Notably, N-methyltransferase 2 (KMT2) gene family mutations (e.g., KMT2C and KMT2D) coexisting with BRAFV600E were detected only in TCCAs in our in-house cohort. One case each of PTEN and EP300 mutation was found in our in-house cohort (Figure 4A).
Furthermore, we compared genetic variants between TCCAs and TCAs (Table 5, Figures 4B, C). BRAFV600E was significantly more prevalent in TCAs (36/40, 90.0%) than in TCCAs (9/20, 45%, p<0.0001). Notably, RET oncogenic fusions were detected in 20% (4/20) of TCCAs but absent in TCAs (p=0.01), highlighting RET fusions as TCCA-specific events. Additionally, uncommon gene mutations included one case of KMT2A in TCA and one case each of KMT2C and KMT2D in TCCA.
3.3 TCCAs with BRAFV600E and other point mutations exhibited more severe clinical manifestations than those with BRAFV600E alone
Although the mutational frequency of BRAFV600E alone was comparable to that of BRAFV600E coexisting with other point mutations, TCCAs harboring BRAFV600E and other point mutations manifested larger tumor diameters and size (p=0.048) than those with BRAFV600E alone. Moreover, TCCAs with multiple gene mutations had more LNMs, as reflected by more positive LNMs (p=0.048) and a greater mean number of LNMs (p=0.017). No significant differences were observed in multifocality or ETE (Table 6).
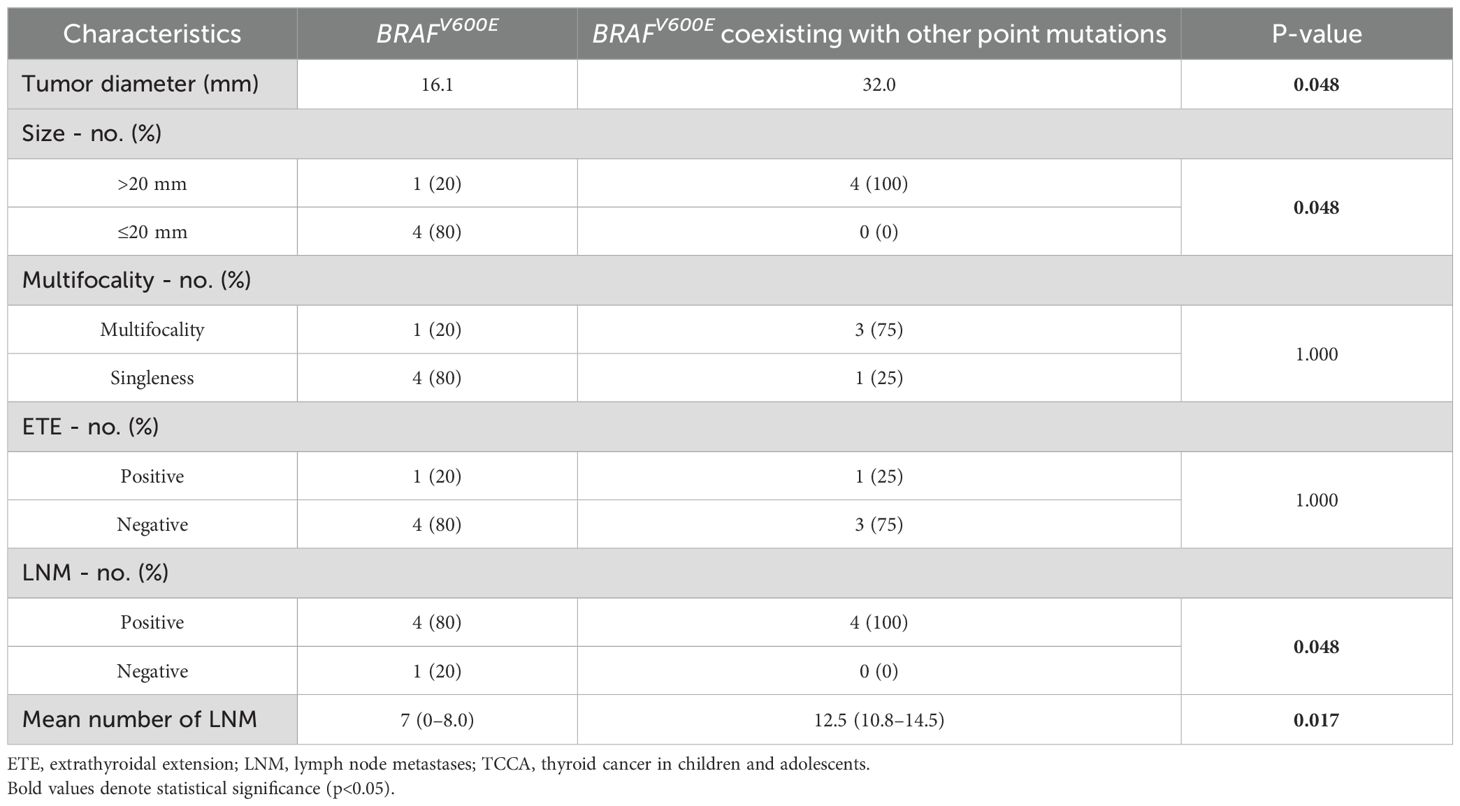
Table 6. Comparison of clinical manifestations between TCCAs with BRAFV600E and BRAFV600E coexisting with other gene mutations.
4 Discussion
4.1 TCCAs manifested more aggressive and invasive clinical features than TCAs, particularly in cases with RET fusions or BRAFV600Eco-mutations
A 2021 population-based study reported incidence rates of TCCA ranged from 0.4–13.4 cases per 1 million person-years (2), primarily due to the diverse PTC subtypes. Incidence increases with age and is higher in girls than in boys. Similarly, in our in-house cohort, female cases outnumbered male cases in both TCCA and TCA.
TCCAs exhibit distinct ultrasound features compared to TCAs. While both groups commonly showed microcalcifications and irregular margins, TCCAs were more likely to present with lymph node involvement, whereas an aspect ratio >1 was more typical of TCAs (18). Hypoechoic features are not specific in the diagnosis of TCA and TCCA. In our study, TCCAs had significantly larger tumors than TCAs (p<0.001), although other ultrasound features did not differ significantly. TCCAs also demonstrated more aggressive histopathological features, including higher rates of ETE, LNM, and multifocality. These nodules often appeared hyperechoic with irregular morphology, diffuse microcalcifications, and increased vascularity. PTC is typically multifocal, aggressive, with a strong tendency to invade the thyroid capsule and directly involve surrounding structures such as the laryngeal nerve, trachea, blood vessels and esophagus (4, 19). Microcalcifications and suspicious cervical lymph nodes are highly indicative of PTC, whereas benign lesions are more likely to be cystic, hyperechoic, and have regular margins with marginal blood flow. Suspicious lymph nodes are typically evaluated based on size, shape, disappearance of lymphatic portal, strong echo, cystic changes, microcalcification and increased blood flow. While microcalcification and increased blood flow have the highest specificity, their sensitivity remains low (11). Notably, no single ultrasound feature is sufficient to assess the malignancy of a thyroid nodule or determine LNM. To improve diagnostic accuracy, we performed ultrasound-guided FNAB, the current preferred preoperative diagnostic tool, especially in children with nodules >1 cm, normal or low thyroid function, and suspicious ultrasonographic features (10, 18). While nodule size may be influenced by age-related changes, our findings suggest that TCCAs exhibit more aggressive clinical features than TCAs.
The malignancy rates of thyroid nodules are significantly higher in TCCAs (22–26%) than in TCA (5–10%) (20, 21). In our study, nearly half of the TCCAs in the large cohort had LNM, 9.7% had DM, 21.7% had ETE, and 19.0% had multifocality, consistent with the in-house cohort. These rates are consistent with previous studies reporting higher recurrence risk (40–80%) and metastatic potential in TCCAs compared to TCAs (18).
TCCAs exhibit more aggressive and invasive clinical features than TCAs, especially when BRAFV600E coexists with other mutations, primarily due to synergistic activation of oncogenic pathways. In children, the developing thyroid microenvironment is more susceptible to epigenetic reprogramming by co-mutations, enhancing proliferation, apoptosis, evasion, and angiogenesis. For instance, BRAFV600E activates the MAPK pathway, while TERT promoter mutations increase telomerase activity, accelerating tumor growth and invasion. Additionally, higher metabolic and growth factor signaling in children may further amplify these effects, promoting rapid tumor expansion and lymph node metastasis. These interactions are less frequent in adults, contributing to clinical differences by age.
Multi-gene panel testing helps identify high-risk cases, as co-mutations often correlate with classic PTC histology and extrathyroidal extension, guiding aggressive staging. Treatment may require total thyroidectomy, higher-dose radioactive iodine, or adjuvant targeted therapies (e.g., BRAF/MEK inhibitors plus TERT pathway modulators) in resistant or advanced cases. Co-mutated tumors have higher recurrence and metastasis risk, necessitating close long-term surveillance with imaging and molecular monitoring. Understanding these mutational profiles allows for personalized risk stratification, ensuring pediatric patients receive targeted treatments that improve survival and reduce treatment-related complications.
Overall, these findings suggest that TCCAs exhibit more aggressive and invasive clinicopathological features than TCAs, highlighting the importance of early identification.
4.2 The genomic landscape of TCCAs requires comprehensive consideration beyond RET and NTRK fusions
Genetic alterations play a critical role in the clinicopathological features of TCCAs, highlighting the need for a comprehensive understanding of its genomic landscape to inform specific management guidelines. However, previous studies on TCCAs often focus solely on FAs or copy number alterations due to childhood radiation exposure from events like Chernobyl and Fukushima accidents (22–24). Additionally, earlier studies primarily focused on the common SNVs (e.g., BRAFV600E) or FAs (e.g., RET) (25, 26), overlooking other critical tumor-suppressor gene mutations. Recent large-cohort studies in thyroid cancer have highlighted additional genetic aberrations relevant to immunotherapy (27, 28). In our analysis of 1,483 TCCAs from 28 prior studies, BRAFV600E, previously considered less frequent in TCCAs (29), was present in 46.6% (396/850) of cases. RAS mutations appeared mainly in the follicular variant PTC and follicular thyroid carcinoma, while RET and NTRK fusions remained common FAs, consistent with previous literature.
In our in-house cohort, the detection rate of genomic alterations was 57.3% (70.0% for TCCAs and 95.0% for TCAs). Although BRAFV600E is the dominant single point mutation in TCA with PTC cases, its prevalence and link to aggressive features in sporadic TCCAs remain understudied (25, 29–32). A study of 19 classical PTC TCCAs reported a 63.6% BRAFV600E prevalence, higher than the 45% and 50.9% reported in two meta-analyses of TCAs (19). Consistently, our study reported a higher incidence of BRAFV600E in TCAs (90.0%, 36/40) than that in TCCAs (19). Also, BRAFV600E was the most prevalent genomic alteration in TCCAs (45%, 9/20), lower than the large cohort (46.6%) and TCAs (90%). TCCAs with BRAFV600E are usually at a more advanced stage. Only two U.S. studies reported the prevalence of BRAFV600E in TCCAs, without reviewing aggressive clinical characteristics. Geographic variation may influence oncogene mutation rates (33, 34), and our study contributes valuable data on the genetic mutation landscape of TCCAs in China.
RAS, BRAF, RET/PTC and PAX8/PPARg mutations are strongly associated with TCCA malignancy, especially in cases with uncertain FNAB cytology (35). The presence of these mutations can increase the positive predictive value of FNAB, with overall sensitivity and specificity reaching 80% and 100%. Notably, RET oncogenic fusions were detected exclusively in TCCAs (4/20, 20%), compared to a much lower prevalence (1.69%) in a large TCA cohort, suggesting these fusions are TCCA-specific (36). RET fusions are primarily associated with sporadic and radiation-induced PTCs (37–40). In younger patients, NCOA4-RET fusion is more prevalent in early radiation-related cases, while CCDC6-RET is more prevalent in sporadic cases (41). In this study, all TCCAs harboring RET fusions (one CCDC6-RET, two RUFY2-RET and one PRKAR1A-RET) were sporadic, consistent with previous studies (36).
The carcinogenic mechanism of RET fusion involves ligand-independent dimerization and constitutive activation of RET kinase, which drives tumorigenesis through key signaling pathways, including MAPK, PI3K, JAX-STAT, PKA, and PKC signaling pathways, resulting in excessive cell proliferation (42, 43). In addition, kinase activation caused by RET fusion promotes cytokine production, contributing to specific biological phenotypes and facilitating tumor initiation and progression (37, 44). Therefore, TCCAs with RET fusions, particularly CCDC6-RET, NCOA4-RET, and KIF5B-RET, exhibit more aggressive and invasive clinicopathological features due to this enhanced carcinogenic activity (45–47).
Notably, although less frequent, KMT2 and PTEN coexisting with BRAFV600E were detected in TCCAs in our in-house cohort and should not be overlooked. The KMT2 family mainly includes KMT2A, KMT2B, KMT2C and KMT2D. KMT2A and KMT2B regulate gene expression by depositing H3K4me2 and H3K4me3 marks at gene promoters, upregulating target genes and potentially promoting tumorigenesis through various signaling pathways. Overexpression of KMT2A has been implicated in thyroid cancer, with its knockdown suppressing thyroid cancer cell proliferation. The National Cancer Institute Genomic Data Commons analyzed more than 33,000 cases and identified KMT2D and KMT2C as the third and seventh most frequently mutated cancer genes. Most mutations are nonsense or frameshift, causing early translation termination and reduced protein expression. While further research is needed to clarify the role of KMT2 mutations in TCCA, these mutations warrant attention.
4.3 Tumor mutational burden from multiple gene mutations may drive aggressive clinical features
In this in-house cohort, TCCAs with BRAFV600E combined with additional mutations such as ATM, PTEN or KMT2 family genes exhibited more aggressive clinical features, including larger tumor size and higher rates of LNM. BRAFV600E coexisting with other point mutations such as PTEN, EP300 or TP53 has been linked to poorer prognosis in PTC (48). Our prior study also demonstrated that BRAFV600E coexisting with TERT promoter mutations contributes to adverse clinical outcomes in radioiodine-refractory differentiated thyroid cancer (49). BRAFV600E with additional mutations is associated with increased tumor mutational burden, which may promote recurrence by altering the immune microenvironment, reducing CD8+ T cells and M1 macrophages (50). These findings underscore that focusing solely on common gene variants such as RET or NTRK in the early stage is insufficient for predicting clinical outcomes in TCCAs. Comprehensive molecular profiling is essential for accurate prognosis and treatment planning.
In summary, TCCAs exhibit distinct genomic alterations, particularly RET fusions, compared with TCAs, consistent with previous reports. In both our in-house and large cohort, BRAFV600E was more frequently observed in TCCAs than in previous reports (29), although it was lower than TCAs in our cohort. Importantly, tumor mutational burden from multiple gene mutations, such as BRAFV600E coexisting with KMT2 or PTEN, should be considered in TCCAs. Overall, in contrast to previous reports (32, 37), our findings underscore the need for a comprehensive analysis of genomic alterations in TCCAs, beyond RET and NTRK.
This study has several limitations. First, the small number of pediatric tissue samples may impact the robustness of the findings, necessitating validation in a larger cohort. Second, despite the broad coverage of our NGS panel, undetected driver alterations in the six NGS-negative samples may have led to an underestimation of the overall detection rate. Third, the NGS method used was unable to detect structural variants, leaving the potential role of these alterations unexplored. Future studies should consider using complementary approaches to capture structural variants and provide a more complete picture of the genetic landscape in thyroid cancer. Nonetheless, to address this limitation, we validated our findings in a large external cohort of 1,483 TCCAs and compared them with TCAs. Our study thus offers valuable insights into the specific genomic alterations and aggressive features of TCCAs.
5 Conclusion
In this study, we delineated the genomic landscape of TCCA and identified TCCA-specific genomic alterations. TCCAs display more aggressive and invasive clinical features than TCAs, particularly in cases with RET fusions, and BRAFV600E coexisting with other point mutations. Integrating molecular and cytological testing improves the predictive value of FNAB and reduces the need for secondary surgery. Overall, targeted molecular testing can support subtype diagnosis and inform treatment strategies for TCCA.
Data availability statement
The datasets presented in this study can be found in online repositories. Raw data have been deposited to National Center for Biotechnology Information under the BioProject number PRJNA1279436.
Ethics statement
The studies involving humans were approved by Ethics Committee of Affiliated People’s Hospital of Jiangsu University, Traditional Chinese Medicine Hospital of Nanjing Lishui District, and Xuzhou Central Hospital, Xuzhou Clinical School of Xuzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
GT: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft. YW: Resources, Writing – review & editing. GZ: Writing – review & editing, Resources. XWa: Writing – review & editing, Investigation, Visualization. YR: Resources, Writing – review & editing. QG: Investigation, Visualization, Writing – review & editing. FX: Resources, Supervision, Writing – review & editing. ZM: Investigation, Visualization, Writing – review & editing. CS: Investigation, Visualization, Writing – review & editing. HW: Writing – review & editing. TW: Investigation, Visualization, Writing – review & editing. XWe: Investigation, Visualization, Writing – review & editing. TZ: Writing – original draft, Data curation. XL: Investigation, Visualization, Writing – review & editing. YX: Investigation, Visualization, Writing – review & editing. SO: Resources, Supervision, Writing – review & editing. XWu: Resources, Writing – review & editing. GJ: Resources, Supervision, Writing – review & editing. XQ: Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study is supported by the General Program of National Natural Science Foundation of China (Grant No. 82471987), the Special Project for Cross Cooperation at the Hospital Level of Northern Jiangsu People’s Hospital (Project No. SBJC23001), Jiangsu Province Elderly Health Research (Project No. LKM2023051), 2024 Zhenjiang Fundamental Research Fund (Project No. JC2024034), 2023 Medical Research Project of Jiangsu Provincial Health Commission (Project No. X0534), Development Fund of Affiliated Hospital of Xuzhou Medical University (Project No. XYFM202204), Research Project of Jiangsu Provincial Health Commission (Project No. Z2021071), Zhenjiang Key R&D Plan - Social Development (Project No. SH2023049), Jiangsu University Medical Education Collaborative Innovation Fund (Project No. JDYY2023015), 22nd College Students Research Project of Jiangsu University (Project No. 22A485), 2023 Clinical Research Project of Affiliated People’s Hospital of Jiangsu University (Project No. YL2023001).
Acknowledgments
Nanjing D.A. Medical Laboratory is acknowledged to provide the genomic detection panels.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1603571/full#supplementary-material
Supplementary Figure 1 | Process for screening previous literature from public database.
References
1. Juhlin CC, Mete O, and Baloch ZW. The 2022 who classification of thyroid tumors: novel concepts in nomenclature and grading. Endocr Relat Cancer. (2023) 30:e220293. doi: 10.1530/erc-22-0293
2. Vaccarella S, Lortet-Tieulent J, Colombet M, Davies L, Stiller CA, Schüz J, et al. Global patterns and trends in incidence and mortality of thyroid cancer in children and adolescents: A population-based study. Lancet Diabetes Endocrinol. (2021) 9:144–52. doi: 10.1016/S2213-8587(20)30401-0
3. Ren P-Y, Liu J, Xue S, and Chen G. Pediatric differentiated thyroid carcinoma: the clinicopathological features and the coexistence of Hashimoto’s thyroiditis. Asian J Surg. (2019) 42:112–9. doi: 10.1016/j.asjsur.2017.10.006
4. Collini P, Massimino M, Leite SF, Mattavelli F, Seregni E, Zucchini N, et al. Papillary thyroid carcinoma of childhood and adolescence: A 30-year experience at the istituto nazionale tumori in Milan. Pediatr Blood Cancer. (2005) 46:300–6. doi: 10.1002/pbc.20474
5. Jung CK, Bychkov A, and Kakudo K. Update from the 2022 world health organization classification of thyroid tumors: A standardized diagnostic approach. Endocrinol Metab (Seoul). (2022) 37:703–18. doi: 10.3803/EnM.2022.1553
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
7. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. (2015) 25:716–59. doi: 10.1089/thy.2014.0460
8. Dermody S, Walls A, and Harley EH Jr. Pediatric thyroid cancer: an update from the seer database 2007-2012. Int J Pediatr Otorhinolaryngol. (2016) 89:121–6. doi: 10.1016/j.ijporl.2016.08.005
9. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 who classification of thyroid neoplasms. Endocrine Pathol. (2022) 33:27–63. doi: 10.1007/s12022-022-09707-3
10. Matalka L, Rahman A, Sparks S, Lindeman B, and Iyer P. Evaluation and Management of Pediatric Thyroid Nodules and Thyroid Cancer at a Single Institution after Adoption of the American Thyroid Association 2015 Guidelines. J Pediatr Endocrinol Metab. (2023) 36:659–66. doi: 10.1515/jpem-2022-0334
11. Guo K, Qian K, Shi Y, Sun T, Chen L, Mei D, et al. Clinical and molecular characterizations of papillary thyroid cancer in children and young adults: A multicenter retrospective study. Thyroid. (2021) 31:1693–706. doi: 10.1089/thy.2021.0003
12. Morganti S, Tarantino P, Ferraro E, D’Amico P, Duso BA, and Curigliano G. Next generation sequencing (NGS): A revolutionary technology in pharmacogenomics and personalized medicine in cancer. Adv Exp Med Biol. (2019) 1168:9–30. doi: 10.1007/978-3-030-24100-1_2
13. Xing M, Haugen BR, and Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. (2013) 381:1058–69. doi: 10.1016/s0140-6736(13)60109-9
14. Morton LM, Karyadi DM, Stewart C, Bogdanova TI, Dawson ET, Steinberg MK, et al. Radiation-related genomic profile of papillary thyroid carcinoma after the chernobyl accident. Science. (2021) 372:725–9. doi: 10.1126/science.abg2538
15. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. (2016) 126:1052–66. doi: 10.1172/jci85271
16. Henderson YC, Shellenberger TD, Williams MD, El-Naggar AK, Fredrick MJ, Cieply KM, et al. High rate of BRAF and RET/PTC dual mutations associated with recurrent papillary thyroid carcinoma. Clin Cancer Res. (2009) 15:485–91. doi: 10.1158/1078-0432.Ccr-08-0933
17. Guerra A, Zeppa P, Bifulco M, and Vitale M. Concomitant BRAF(V600e) mutation and RET/PTC rearrangement is a frequent occurrence in papillary thyroid carcinoma. Thyroid. (2014) 24:254–9. doi: 10.1089/thy.2013.0235
18. Creo A, Alahdab F, Al Nofal A, Thomas K, Kolbe A, and Pittock ST. Ultrasonography and the American thyroid association ultrasound-based risk stratification tool: utility in pediatric and adolescent thyroid nodules. Horm Res Paediatr. (2018) 90:93–101. doi: 10.1159/000490468
19. Network CGAR. Integrated genomic characterization of papillary thyroid carcinoma. Cell. (2014) 159:676–90. doi: 10.1016/j.cell.2014.09.050
20. Paulson VA, Rudzinski ER, and Hawkins DS. Thyroid cancer in the pediatric population. Genes. (2019) 10:723. doi: 10.3390/genes10090723
21. Karapanou O, Tzanela M, Vlassopoulou B, and Kanaka-Gantenbein C. Differentiated thyroid cancer in childhood: A literature update. Hormones (Athens). (2017) 16:381–7. doi: 10.14310/horm.2002.1758
22. Zitzelsberger H and Unger K. DNA copy number alterations in radiation-induced thyroid cancer. Clin Oncol. (2011) 23:289–96. doi: 10.1016/j.clon.2011.01.154
23. Efanov AA, Brenner AV, Bogdanova TI, Kelly LM, Liu P, Little MP, et al. Investigation of the relationship between radiation dose and gene mutations and fusions in post-chernobyl thyroid cancer. J Natl Cancer Institute. (2018) 110:371–8. doi: 10.1093/jnci/djx209
24. Suchy B, Waldmann V, Klugbauer S, and Rabes HM. Absence of RAS and P53 mutations in thyroid carcinomas of children after chernobyl in contrast to TCA thyroid tumours. Br J Cancer. (1998) 77:952–5. doi: 10.1038/bjc.1998.157
25. Givens DJ, Buchmann LO, Agarwal AM, Grimmer JF, and Hunt JP. BRAF V600e does not predict aggressive features of pediatric papillary thyroid carcinoma. Laryngoscope. (2014) 124:E389–E93. doi: 10.1002/lary.24668
26. Romei C, Ciampi R, Faviana P, Agate L, Molinaro E, Bottici V, et al. BRAFv600e mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocrine Related Cancer. (2008) 15:511–20. doi: 10.1677/erc-07-0130
27. Singh A, Ham J, Po JW, Niles N, Roberts T, and Lee CS. The genomic landscape of thyroid cancer tumourigenesis and implications for immunotherapy. Cells. (2021) 10:1082. doi: 10.3390/cells10051082
28. Acuña-Ruiz A, Carrasco-López C, and Santisteban P. Genomic and epigenomic profile of thyroid cancer. Best Pract Res Clin Endocrinol Metab. (2023) 37:101656. doi: 10.1016/j.beem.2022.101656
29. Penko K, Livezey J, Fenton C, Patel A, Nicholson D, Flora M, et al. Braf mutations are uncommon in papillary thyroid cancer of young patients. Thyroid. (2005) 15:320–5. doi: 10.1089/thy.2005.15.320
30. Kumagai A, Namba H, Saenko VA, Ashizawa K, Ohtsuru A, Ito M, et al. Low frequency of BRAFT1796a mutations in childhood thyroid carcinomas. J Clin Endocrinol Metab. (2004) 89:4280–4. doi: 10.1210/jc.2004-0172
31. Henke LE, Perkins SM, Pfeifer JD, Ma C, Chen Y, DeWees T, et al. BRAF V600e mutational status in pediatric thyroid cancer. Pediatr Blood Cancer. (2014) 61:1168–72. doi: 10.1002/pbc.24935
32. Rosenbaum E, Hosler G, Zahurak M, Cohen Y, Sidransky D, and Westra WH. Mutational activation of BRAF is not a major event in sporadic childhood papillary thyroid carcinoma. Modern Pathol. (2005) 18:898–902. doi: 10.1038/modpathol.3800252
33. Agrawal N, Akbani R, Aksoy BA, Ally A, Arachchi H, Asa Sylvia L, et al. Integrated genomic characterization of papillary thyroid carcinoma. Cell. (2014) 159:676–90. doi: 10.1016/j.cell.2014.09.050
34. Al-Qurayshi Z, Hauch A, Srivastav S, Aslam R, Friedlander P, and Kandil E. A national perspective of the risk, presentation, and outcomes of pediatric thyroid cancer. JAMA Otolaryngology–Head Neck Surg. (2016) 142:472–8. doi: 10.1001/jamaoto.2016.0104
35. Macerola E, Proietti A, Poma AM, Ugolini C, Torregrossa L, Vignali P, et al. Molecular alterations in relation to histopathological characteristics in a large series of pediatric papillary thyroid carcinoma from a single institution. Cancers. (2021) 13:3123. doi: 10.3390/cancers13133123
36. Tali G, Payne AE, Hudson TJ, da Silva SD, Pusztaszeri M, Tamilia M, et al. The difference in clinical behavior of gene fusions involving RET/PTC fusions and THADA/IGF2BP3 fusions in thyroid nodules. Cancers. (2023) 15:3394. doi: 10.3390/cancers15133394
37. Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, and Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. (2000) 85:1170–5. doi: 10.1210/jcem.85.3.6472
38. Franco AT, Ricarte-Filho JC, Laetsch TW, and Bauer AJ. Oncogene-specific inhibition in the treatment of advanced pediatric thyroid cancer. J Clin Invest. (2021) 131:e152696. doi: 10.1172/jci152696
39. Alzahrani AS, Alswailem M, Alswailem AA, Al-Hindi H, Goljan E, Alsudairy N, et al. Genetic alterations in pediatric thyroid cancer using a comprehensive childhood cancer gene panel. J Clin Endocrinol Metab. (2020) 105:3324–34. doi: 10.1210/clinem/dgaa389
40. Cordioli MICV, Moraes L, Cury AN, and Cerutti JM. Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Endocrine-Related Cancer. (2015) 22:R311–R24. doi: 10.1530/erc-15-0381
41. Lee YA, Lee H, Im SW, Song YS, Oh DY, Kang HJ, et al. NTRK and RET fusion-directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake. J Clin Invest. (2021) 131:e144847. doi: 10.1172/JCI144847
42. Mulligan LM. Ret revisited: expanding the oncogenic portfolio. Nat Rev Cancer. (2014) 14:173–86. doi: 10.1038/nrc3680
43. Plaza-Menacho I, Mologni L, and McDonald NQ. Mechanisms of ret signaling in cancer: current and future implications for targeted therapy. Cell Signal. (2014) 26:1743–52. doi: 10.1016/j.cellsig.2014.03.032
44. Yakushina VD, Lerner LV, and Lavrov AV. Gene fusions in thyroid cancer. Thyroid. (2018) 28:158–67. doi: 10.1089/thy.2017.0318
45. Cordioli MICV, Moraes L, Bastos AU, Besson P, Alves M, Delcelo R, et al. Fusion oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid. (2017) 27:182–8. doi: 10.1089/thy.2016.0387
46. Fagin JA and Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. (2016) 375:1054–67. doi: 10.1056/NEJMra1501993
47. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in ret-altered thyroid cancers. New Engl J Med. (2020) 383:825–35. doi: 10.1056/NEJMoa2005651
48. Bandoh N, Goto T, Kato Y, Kubota A, Sakaue S, Takeda R, et al. Braf V600e mutation co-existing with oncogenic mutations is associated with aggressive clinicopathologic features and poor prognosis in papillary thyroid carcinoma. Asian J Surg. (2024) 47:413–9. doi: 10.1016/j.asjsur.2023.09.049
49. Tan G, Jin B, Qian X, Wang Y, Zhang G, Agyekum EA, et al. TERT promoter mutations contribute to adverse clinical outcomes and poor prognosis in radioiodine refractory differentiated thyroid cancer. Sci Rep. (2024) 14:23719. doi: 10.1038/s41598-024-75087-9
Keywords: thyroid cancer, children and adolescents, adults, genomic alterations, clinical ultrasound manifestations
Citation: Tan G, Wang Y, Zhang G, Wang X, Ren Y, Gu Q, Xu F, Mao Z, Shi C, Wang H, Wu T, Wei X, Zhang T, Li X, Xu Y, Ou S, Wu X, Jia G and Qian X (2025) Specific genomic alterations and aggressive clinical features of sporadic thyroid carcinomas in children and adolescents: findings from an in-house cohort study. Front. Endocrinol. 16:1603571. doi: 10.3389/fendo.2025.1603571
Received: 31 March 2025; Accepted: 03 July 2025;
Published: 15 August 2025.
Edited by:
Luca Giacomelli, Polistudium srl, ItalyReviewed by:
Tomohiro Chiba, Cancer Institute Hospital of Japanese Foundation for Cancer Research, JapanAlper Küçüker, Izmir Katip Celebi University, Türkiye
Copyright © 2025 Tan, Wang, Zhang, Wang, Ren, Gu, Xu, Mao, Shi, Wang, Wu, Wei, Zhang, Li, Xu, Ou, Wu, Jia and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinping Wu, eGl5dWx1b3lvdW1AMTI2LmNvbQ==; Gaolei Jia, amlhZ2FvbGVpQDE2My5jb20=; Xiaoqin Qian, eXpfdHl6MTAzMEAxMjYuY29t
 Gongxun Tan
Gongxun Tan Yuguo Wang2
Yuguo Wang2 Xian Wang
Xian Wang Zhenwei Mao
Zhenwei Mao Ting Wu
Ting Wu Tengxu Zhang
Tengxu Zhang Xiuying Li
Xiuying Li Xiaoqin Qian
Xiaoqin Qian