- 1School of Postgraduate Education, Shandong Sport University, Jinan, Shandong, China
- 2School of Life Sciences, Ludong University, Yantai, Shandong, China
- 3School of Exercise and Health, Shandong Sport University, Jinan, Shandong, China
Background: Mild Cognitive Impairment (MCI), a transition between normal aging and dementia, is linked to higher dementia risk and potential reversibility. Type 2 Diabetes Mellitus (T2DM), affecting over 537 million adults worldwide, increases susceptibility to MCI, with higher cognitive decline prevalence in diabetic populations. Previous meta-analyses focused on isolated factors, neglecting multidimensional interactions. This study synthesizes T2DM-MCI risk factors across clinical, lifestyle, and biochemical dimensions to support early identification and intervention of cognitive dysfunction in T2DM populations.
Materials and Methods: This systematic review and meta-analysis, following PRISMA guidelines, searched five databases for articles published from January 1, 2014, to December 31, 2024. Studies were screened based on predefined criteria, with data extracted independently by two researchers. Quality was assessed using Newcastle-Ottawa Scale (NOS) and Joanna Briggs Institute (JBI) tools. Data were analyzed using RevMan software, with odds ratio (OR) and 95% CI as effect size measures. Heterogeneity was assessed using I² statistics, and subgroup analyses were conducted for factors with ≥10 studies.
Results: 30 studies with 10,469 participants were included. Prevalence rate of MCI in T2DM was 44.1%. Significant associations were found between T2DM-MCI and age (OR = 1.06, P = 0.01), female sex (OR = 1.23, P = 0.05), diabetes duration (OR = 1.07, P = 0.03), education (OR = 0.82, P = 0.0001), smoking (OR = 1.44, P = 0.003), hypertension (OR = 2.25, P < 0.001), cardiovascular disease (CVD) (OR = 2.61, P < 0.001), glycated hemoglobin (HbA1c) (OR = 1.33, P = 0.001), and homeostasis model assessment of insulin resistance (HOMA-IR) (OR = 1.95, P = 0.02).
Conclusion: This meta-analysis identifies advanced age (≥60 years), female sex, prolonged Diabetes duration (8–9 years), elevated HbA1c (>9%), and low education (≤6 years) as key predictors of MCI in T2DM, with significant dose-response relationships. Vascular comorbidities, insulin resistance, and inflammatory markers further exacerbate risks. Clinical priorities include rigorous glycemic control (HbA1c <7%), targeted cognitive screening for high-risk subgroups, and multidisciplinary care for patients with microvascular complications. However most of the studies included in this study come from Chinese people, so the generalization of the results may be limited.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD420250637336.
1 Introduction
Mild Cognitive Impairment (MCI) represents a transitional phase between normal cognitive aging and dementia, and it is potentially reversible in nature (1). Individuals with MCI exhibit significantly elevated risk of progressing dementia compared to cognitively healthy populations (2). Type 2 Diabetes Mellitus (T2DM), one of the most prevalent metabolic disorders globally, continues to show escalating prevalence rates. According to International Diabetes Federation (IDF), the global adult population with diabetes exceeded 537 million in 2021, over 90% of whom had T2DM, and this figure is projected to surpass 783 million by 2045 (3). Concurrently, cognitive health concerns in T2DM patients, particularly MCI comorbidity, have gained increasing attention. Studies indicated that T2DM not only serves as a risk factor for MCI but also accelerates its progression to dementia (4). Diabetic individuals have a 1.25 to 1.91 times higher likelihood of developing cognitive impairment than non-diabetic individuals (5). Epidemiological data suggest that the prevalence of MCI among T2DM patients ranges from 19.9% to 45.0% (6, 7).
MCI manifests through impairments in core cognitive domains, including memory (8) and executive function (9), and is associated with multiple adverse clinical outcomes. For instance, a meta-analysis revealed that older diabetic patients with comorbid MCI face a higher risk of falling (10). Assessments using the World Health Organization Quality of Life Assessment for Older Adults further demonstrated significantly reduced quality-of-life scores among MCI patients across dimensions such as autonomy, engagement in past/present activities, and social participation (11). These findings collectively highlight that MCI not only serves as an early indicator of cerebral functional decline in T2DM patients but also exacerbates the disease burden through multiple pathways.
Early intervention for MCI in T2DM patients is therefore critical for preserving cognitive function and preventing dementia. The pathophysiological mechanisms underlying T2DM-MCI comorbidity involve complex interactions: chronic hyperglycemia directly impairs cognitive function through the deposition of advanced glycation end-products (12, 13), blood-brain barrier disruption (14, 15), and hippocampal neuronal apoptosis (16, 17). Notably, the MCI stage represents a reversible therapeutic window (18, 19). Meta-analyses indicate that early identification and control of risk factors significantly reduce dementia conversion risks and subsequent healthcare expenditures (20). Comprehensive management strategies could potentially prevent or delay up to 40% of dementia cases (21), underscoring the need for proactive preventive measures.
Despite established evidence on T2DM-MCI determinants, controversies persist regarding the heterogeneity of factors and their relative contributions. Existing meta-analyses predominantly focus on isolated factors, such as glycated hemoglobin (HbA1c) levels (22),or diabetes duration (23), lacking systematic integration of multidimensional elements, including demographic/clinical characteristics, biochemical parameters, lifestyle factors, and disease management. For example, while some studies have reported significant associations between smoking history (24, 25) and T2DM-MCI risk, others have fail to corroborate this relationship (26, 27). Similarly, conflicting evidence exists fasting plasma glucose (FPG) and HbA1c, with some studies finding no significant association (28, 29) and others reporting clear links (30, 31). To address these inconsistencies, the present study conducts a meta-analysis to consolidate current evidence, systematically evaluating risk factors and their weighted contributions across four dimensions: demographic and clinical characteristics (including age, sex, and diabetes duration), lifestyle factors (smoking and alcohol consumption), disease management (hypertension and depression), and biochemical indicators (HbA1c). The aim is to provide evidence-based support for early identification and intervention of cognitive dysfunction in T2DM populations.
2 Methods
This study was conducted as a systematic review and meta-analysis, with the study protocol prospectively registered in the PROSPERO database (Registration ID: CRD420250637336). The methodology strictly adheres to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (32). Institutional ethics committee approval was waived due to the exclusive use of aggregated data from previously published studies.
2.1 Search strategy
As of December 31, 2024, we systematically searched five databases (PubMed, Embase, Web of Science, Google Scholar, and Elsevier) for articles published from January 1, 2014, to December 31, 2024. The search strategy combined the following terms using Boolean operators: (Diabetes Mellitus, Type 2 OR T2DM OR type 2 diabetes) AND (Cognitive Dysfunction OR Mild Cognitive Impairment OR MCI) AND (risk factors OR predictors OR determinants). In addition, manual searches of reference lists from identified articles and relevant reviews were performed to supplement the electronic search.
2.2 Study selection
One researcher (H.L.W.) performed the initial literature search and removed duplicates. Two researchers (Y.Z. and G.H.T.) independently screened titles and abstracts against predefined inclusion and exclusion criteria. Full texts were retrieved if either reviewer deemed an article potentially eligible. The reviewers then independently assessed the full-text articles for final inclusion. Discrepancies were resolved through consultation with the corresponding author (R.L.).
The inclusion criteria were as follows (1): study involving patients diagnosed with T2DM and MCI (2); case-control, cohort, or cross-sectional designs (3); data convertible to odds ratio (OR) with a 95% confidence interval (CI) (4); reporting at least one risk factor (5); use of multivariable logistic regression to identify T2DM-MCI determinants; and (6) clear diagnostic criteria for MCI. The exclusion criteria were (1): duplicate publications (2); reviews, letters, or non-research articles; and (3) non-English publications.
2.3 Data extraction
Data extraction was independently performed by two investigators (Y.Z. and L.L.W.) using standardized forms. The following parameters were recorded: first author’s name, mean age, sex distribution, publication year, study location, sample size, prevalence of T2DM-MCI comorbidity, and reported risk factors. Quantitative measures, including OR with corresponding 95%CI, were extracted for each determinant. The extracted variables were stratified into four etiological domains: 1) Demographic and Clinical Characteristics: age, sex, diabetes duration, body mass index (BMI), and educational attainment. 2) Lifestyle Factors: alcohol consumption, smoking status. 3) Comorbidity Management: depression, hypertension, cardiovascular disease (CVD), and diabetic retinopathy (DR). 4) Biochemical Indicators: HbA1c, homeostasis model assessment of insulin resistance (HOMA-IR), low-density lipoprotein cholesterol (LDL-C), high-sensitivity C-reactive protein (HS-CRP), FPG, and high-density lipoprotein (HDL).
2.4 Quality assessment
Methodological quality was assessed using the Newcastle-Ottawa Scale (NOS) for cohort and case-control studies (33) and the Joanna Briggs Institute (JBI) Critical Appraisal Tool for cross-sectional studies (34). The NOS evaluates three domains: selection, comparability, and exposure/outcome ascertainment, with a maximum score of 9 points. Studies scoring ≤ 5 points were classified as low quality, scoring between 5–7 points were classified as medium quality, and scoring > 8 were classified as high quality. The JBI tool employs a percentage-based scoring system, with a maximum score of 8 points, categorizing studies as high quality (≥ 7), moderate quality (5, 6), or low quality (≤4). Only studies meeting quality thresholds (NOS ≥ 5 or JBI ≥5) were retained. Two investigators (G.H.T. and H.L.W.) independently conducted quality assessments. Discrepancies in scoring were resolved through consultation with the corresponding author (R.L.).
2.5 Data analysis
All statistical analyses were performed using Review Manager (RevMan) software, version 5.4. OR with corresponding 95% CI served as effect size measures. Heterogeneity was quantified using I² statistics and P-values, with thresholds set at P < 0.1 or I² > 50% indicating substantial heterogeneity. The fixed-effects model assumes consistent effect sizes across studies, suitable for low heterogeneity (I² ≤50%) and calculates pooled effect size through weighted averages. The random-effects model assumes variability in effect sizes and is used when significant heterogeneity exists (I² >50%), incorporating study differences through weighted averages. Therefore, a random-effects model is chosen when I² >50%, and a fixed-effects model is used otherwise. Subgroup analyses were conducted for factors with ≥ 10 studies. Sensitivity analyses were performed by switching between fixed-effects and random-effects models for outcomes demonstrating I² > 50%. Publication bias was assessed through funnel plot symmetry evaluation and Egger’s linear regression test, which was restricted to factors with ≥10 studies. A significance level of P < 0.05 was defined for all inferential analyses.
3 Results
3.1 Search results
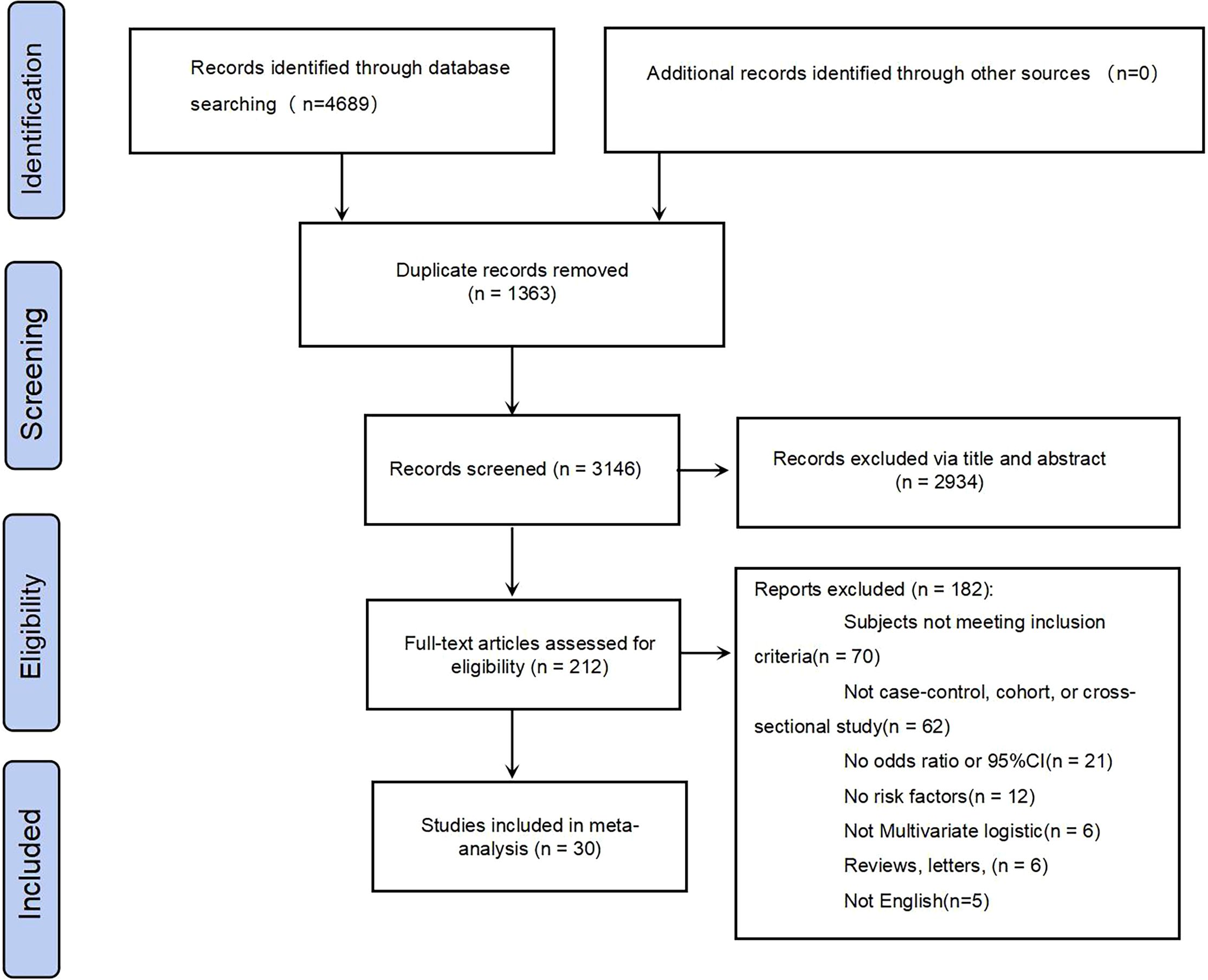
The systematic search initially identified 4,689 citations. Following duplicate removal, 3,326 records were subjected to preliminary screening. Title/abstract screening excluded 2,956 non-eligible studies, leaving 212 articles for full-text assessment. Ultimately, 30 studies met the inclusion criteria and were included in the meta-analysis of T2DM-MCI determinants. The complete screening protocol is presented in the PRISMA flowchart (Figure 1).
3.2 Study characteristics
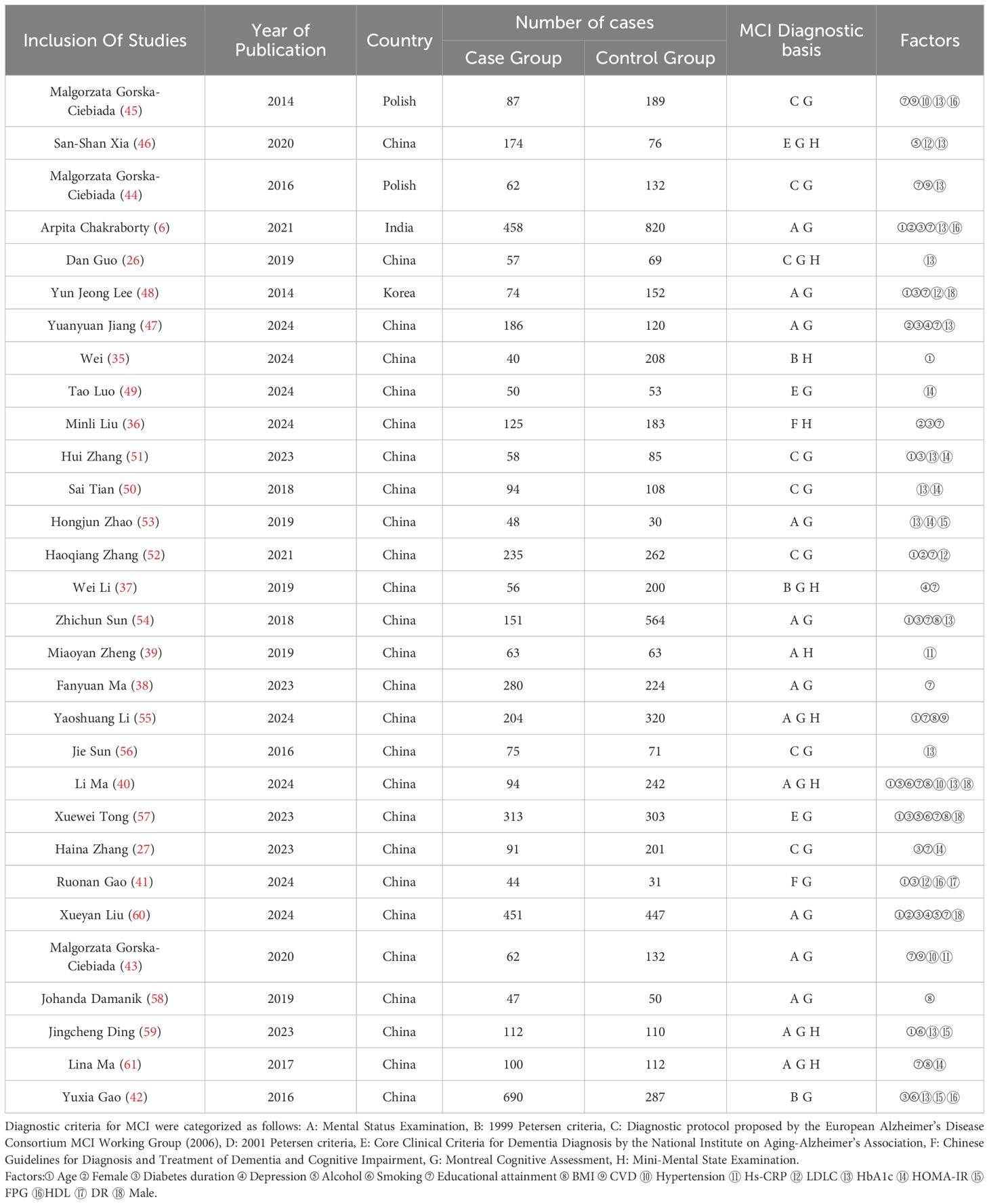
Table 1 summarizes the characteristics of the included studies. The pooled analysis comprised 30 studies conducted across five countries, involving 10,469 participants with T2DM, including 4,516 cases with comorbid MCI (prevalence rate = 44.1%). These investigations, published between 2014 and 2024, had sample sizes ranging from 103 to 1,278 participants. The mean age of the study populations ranged from 50 to 84 years, with female participants representing 41.8% of the total cohort.
3.3 Methodological quality assessment、sensitivity analyses and publication bias assessment
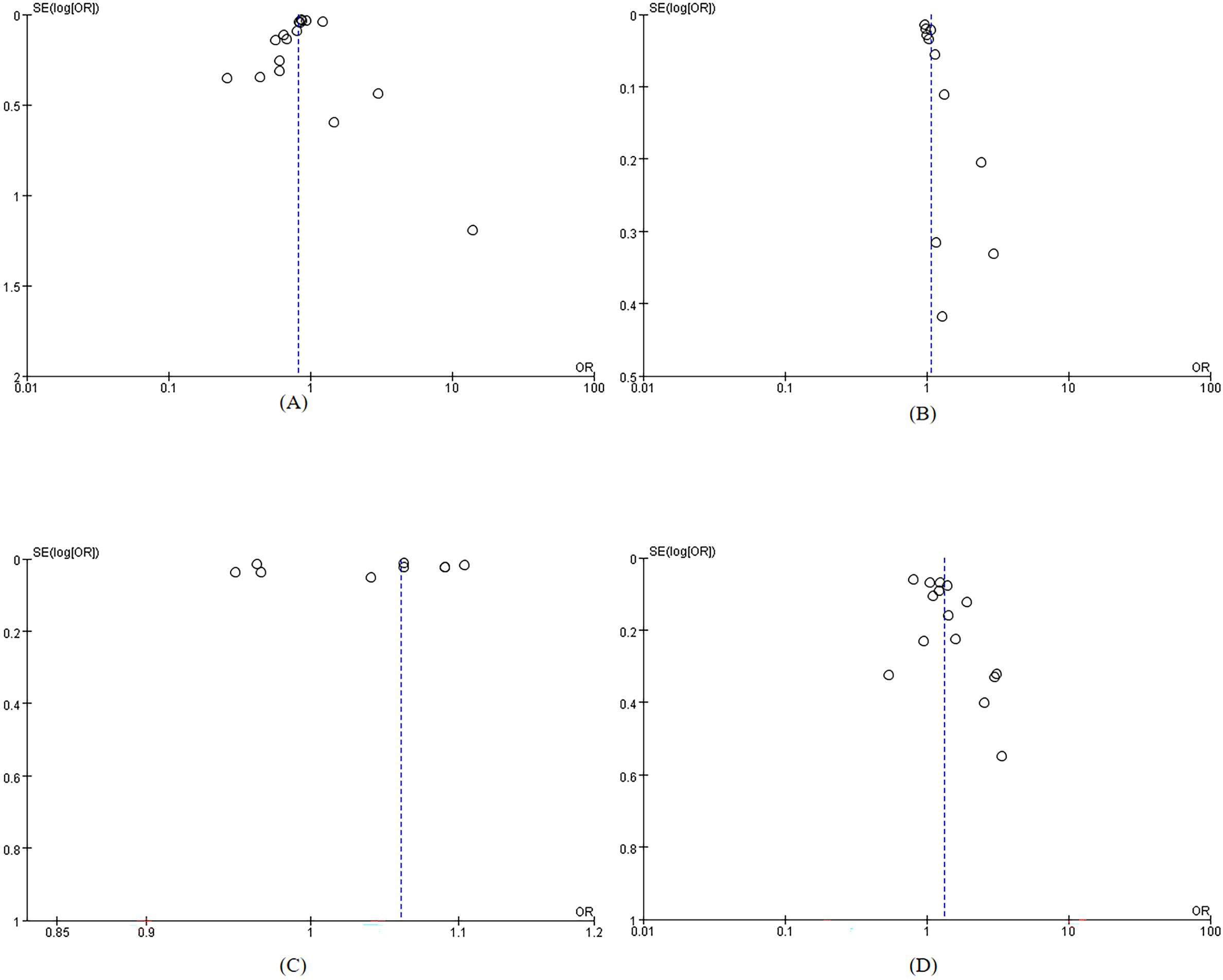
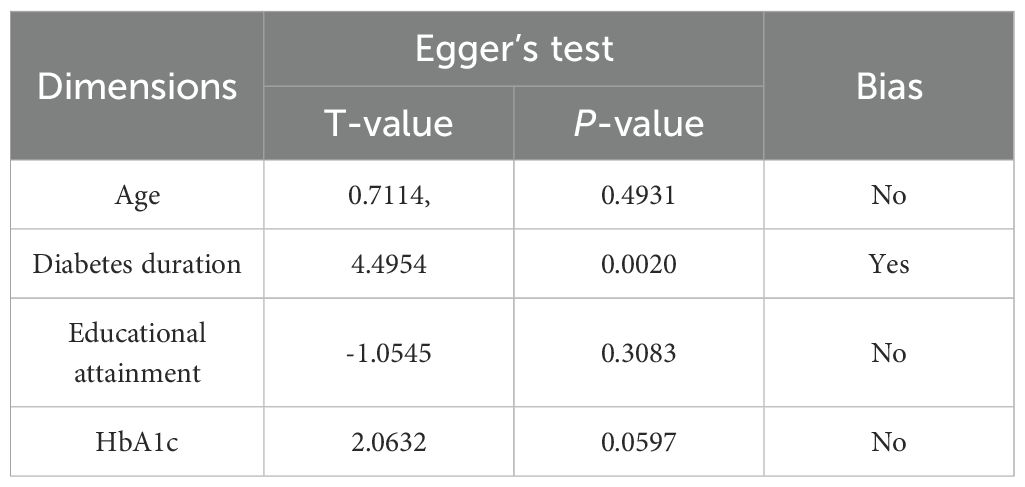
The included studies were of moderate to high quality. Overall quality assessment showed that 8 articles (35–42) were classified as high quality, while 22 articles (6, 26, 27, 43–61) were categorized as medium quality, For specific scores, see Supplementary Table S1 and Supplementary Table S2. The included studies demonstrated stability in sensitivity analyses. Funnel plots indicated no significant publication bias (Figure 2). Table 2 presents the results of Egger’s test, confirming no substantial publication bias. For the sensitivity analysis, see Supplementary Table S3.
3.4 Comprehensive results analysis
3.4.1 Demographic and clinical characteristics
Significant heterogeneity was observed across studies for age, sex, diabetes duration, educational attainment, and BMI (Figure 3). Pooled effect sizes demonstrated the following outcomes: Age (13 studies; χ² = 80.47, P < 0.001, I² = 85%): OR = 1.06 (95% CI: 1.01–1.11, P = 0.01); Female sex (8 studies; χ² = 21.27, P = 0.03): OR = 1.23 (95% CI: 1.00–1.50, P = 0.05), I² = 67%,; Diabetes duration (11 studies; χ² = 53.57, P < 0.001, I² = 81%): OR = 1.07 (95% CI: 1.01–1.13, P = 0.03); and Educational attainment (17 studies; χ² = 116.98, P < 0.001, I² = 86%): OR = 0.82 (95% CI: 0.73–0.91, P = 0.0001). Forest plots for these factors showed 95% CI that did not overlap with the null line, indicating statistically significant associations with T2DM-MCI comorbidity. In contrast, BMI (6 studies; χ² = 33.81, P < 0.001, I² = 85%) showed a non-significant pooled effect size (OR = 1.18, 95% CI: 0.94–1.49, P = 0.15), with the CI ranges overlapping the null line. For age (I² = 85%), sex (I² = 67%), diabetes duration (I² = 81%), educational attainment (I² = 86%), and BMI (I² = 85%), the heterogeneity was greater than 50%, so a random-effects model was used for all.
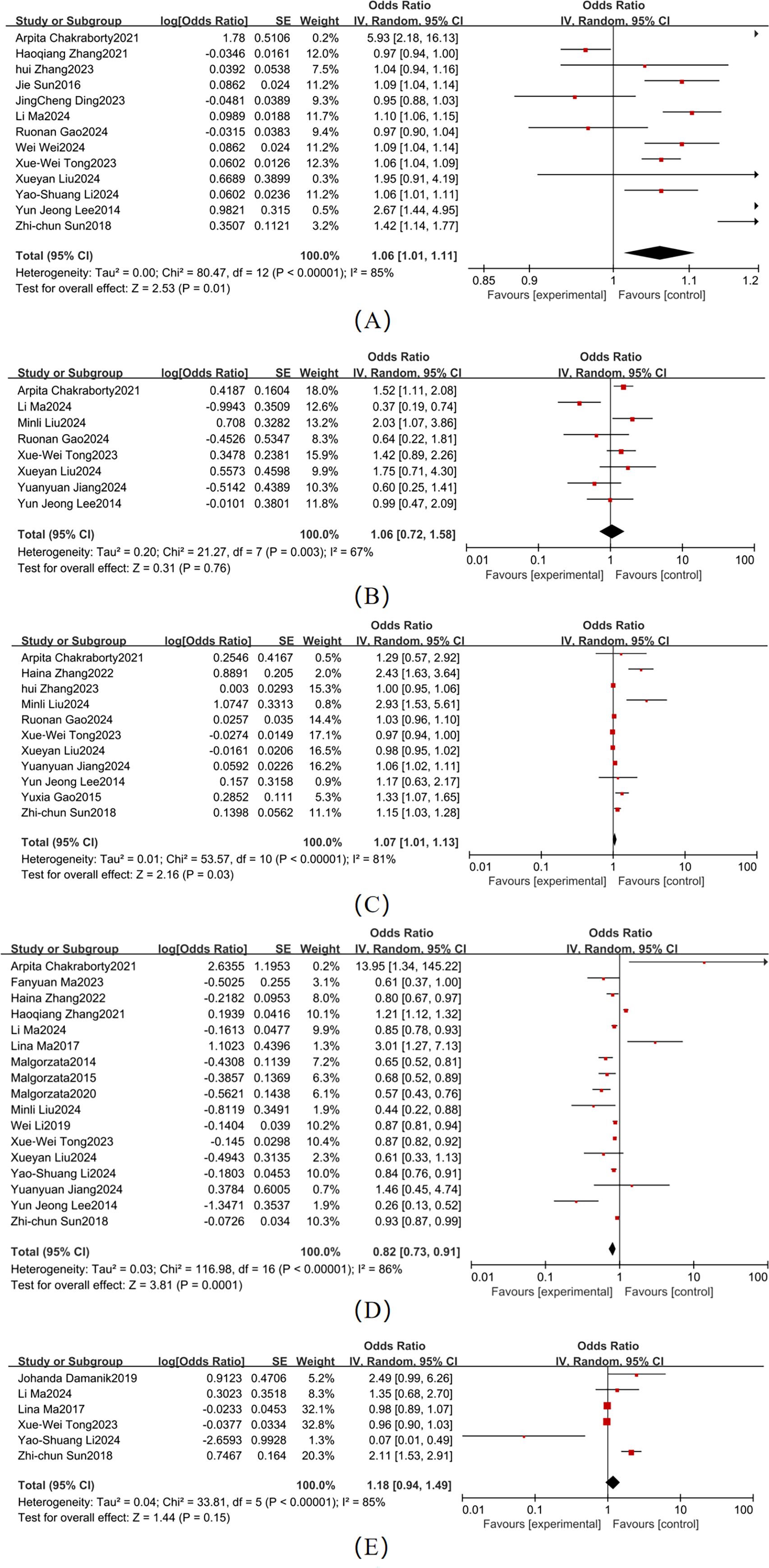
Figure 3. Forest map of demographic and clinical characteristics (A) Age; (B) Sex; (C) Duration; (D) Level of education; (E) BMI.
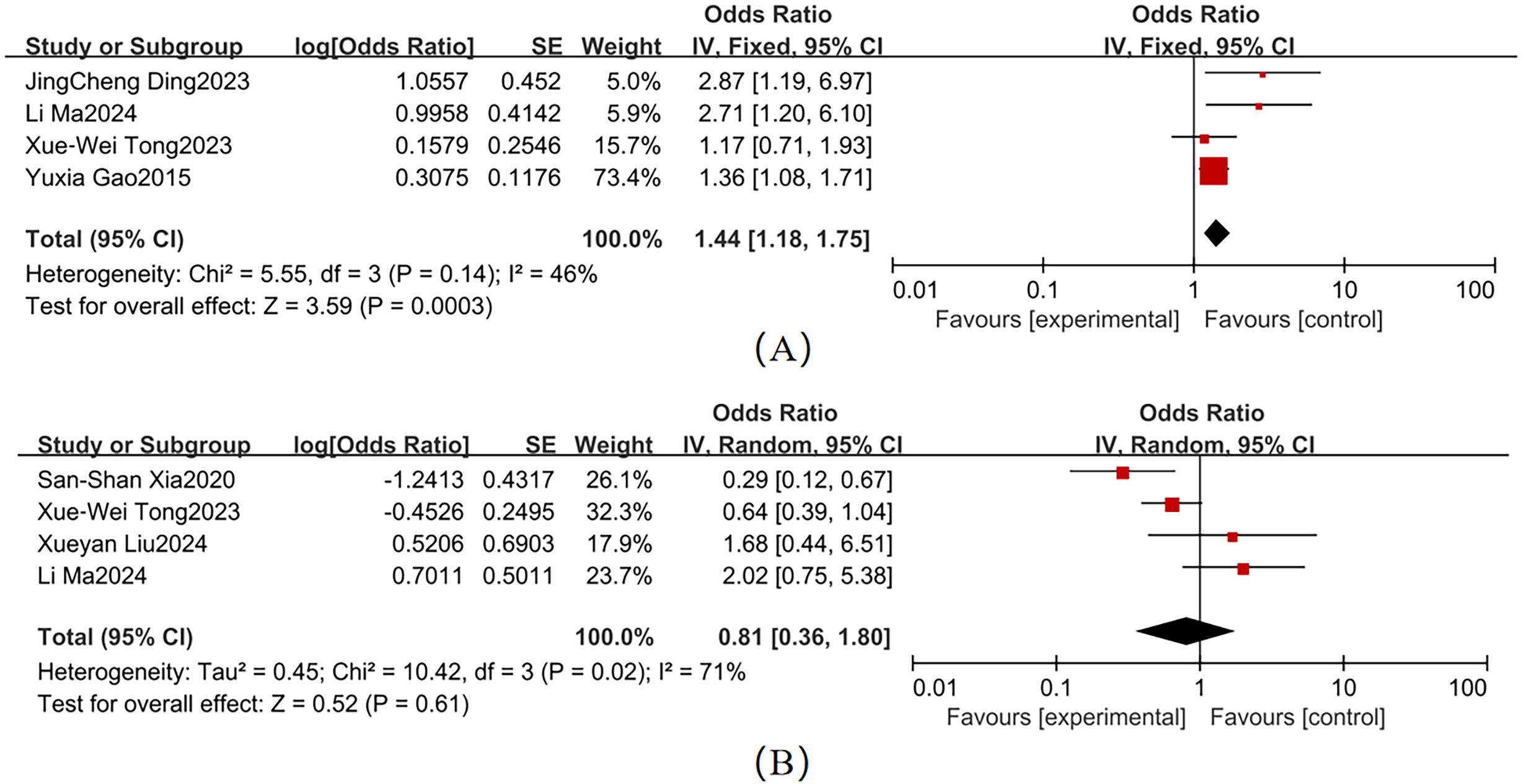
Moderate heterogeneity was observed across studies for smoking and alcohol consumption (Figure 4). Key findings were as follows: Smoking (4 studies; χ² = 5.55, P = 0.14, I² = 46%): Pooled OR = 1.44 (95% CI: 1.18–1.75, P = 0.003), When I² <50%, a fixed-effects model was used. Forest plot analysis showed non-overlapping 95% CI with the null line, indicating a statistically significant association with T2DM-MCI comorbidity. Alcohol consumption (4 studies; χ² = 10.42, P = 0.02, I² = 71%): Pooled OR = 0.81 (95% CI: 0.36–1.80, P = 0.61), When I² >50%, a fixed-effects model was used. The 95% CI ranges overlapped the null line, suggesting no statistically significant association.
3.4.2 Comorbidity management
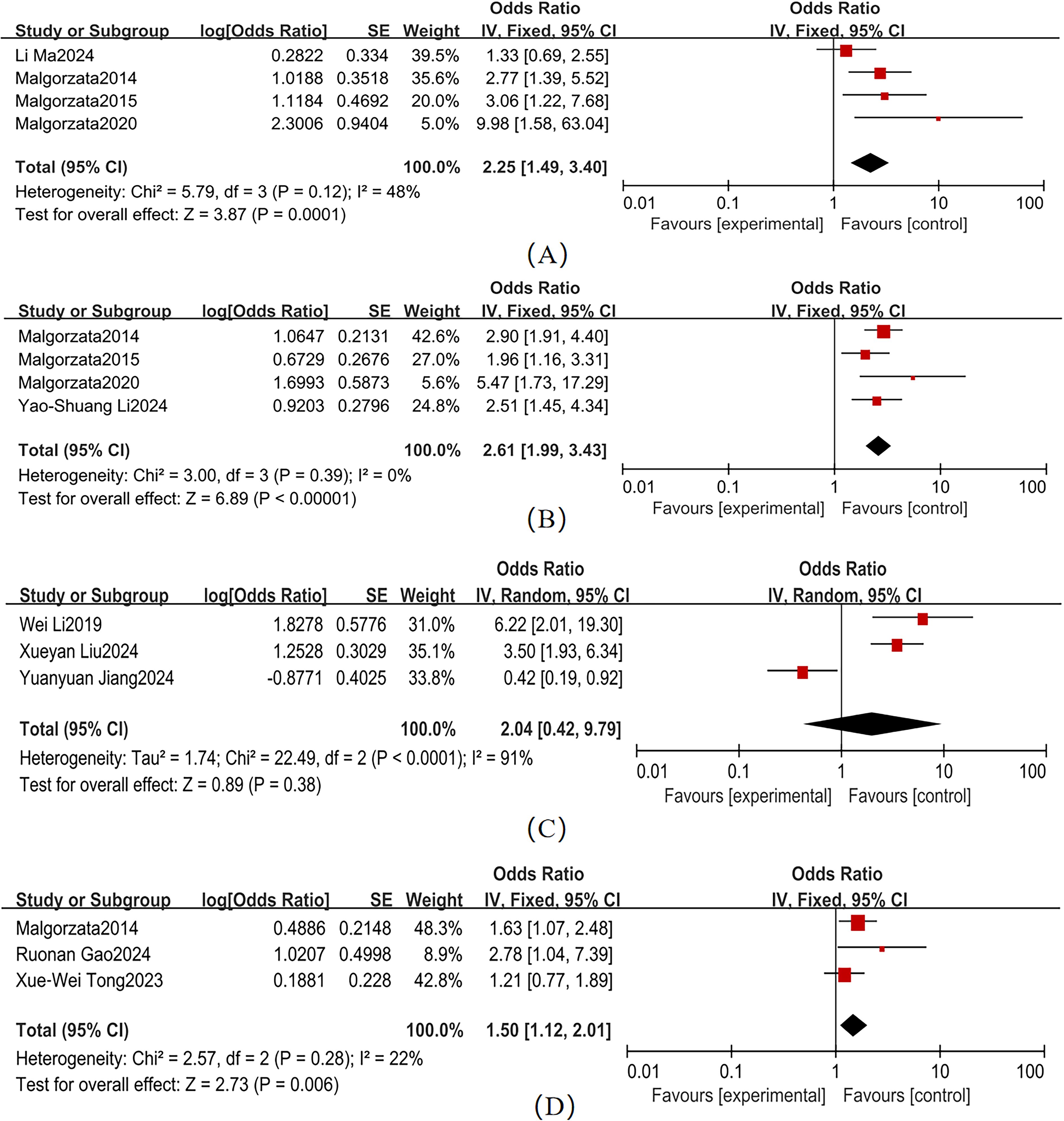
Variable heterogeneity was observed across studies for hypertension, CVD, and DR (Figure 5). The pooled effect sizes were as follows: hypertension (4 studies; χ² = 5.79, P = 0.12, I² = 48%): OR = 2.25 (95% CI: 1.49–3.40, P < 0.001); CVD (4 studies; χ² = 3.00, P = 0.39, I² = 0%): OR = 2.61 (95% CI: 1.99–3.43, P < 0.001); and DR (3 studies; χ² = 2.57, P = 0.28, I² = 22%): OR = 1.50 (95% CI: 1.12–2.01, P = 0.006). Forest plots for these comorbidities showed non-overlapping 95% CI with the null line, indicating statistically significant associations with T2DM-MCI comorbidity. In contrast, depression (3 studies; χ² = 22.49, P < 0.001, I² = 91%) exhibited a non-significant pooled effect size (OR = 2.04, 95% CI: 0.42–9.79, P = 0.38), despite its 95% CI range overlapping the null line. Hypertension (I² = 48%), CVD (I² = 0%), and DR (I² = 22%) had I² < 50%, so a fixed-effects model was used. Depression (I² = 91%) had I² > 50%, so a random-effects model was used.
3.4.3 Biochemical indicators
Substantial heterogeneity was observed across studies for HbA1c, HOMA-IR, FPG, and HS-CRP (Figure 6). Pooled effect sizes demonstrated: HbA1c (15 studies; χ² = 97.09, P <0.001, I² = 86%): OR = 1.33 (95% CI: 1.12–1.58, P = 0.001)、HOMA-IR(5 studies; χ² = 23.86, P < 0.001, I² = 83%): OR = 1.95 (95% CI: 1.14–3.35, P = 0.02)、FPG (3 studies; χ² = 0.65, P = 0.06, I² = 0%): OR = 1.15 (95% CI: 1.01–1.32, = 0.04); and HS-CRP (3 studies; χ² = 1.65, P = 0.44, I² = 0%): OR=2.85 (95% CI: 2.09–3.89, P < 0.001). Forest plots showed non-overlapping 95% CI with the null line for these parameters, confirming statistically significant associations with T2DM-MCI comorbidity. HDL (3 studies; χ² = 5.71, P = 0.06, I² = 65%) and LDL-C (3 studies; χ² = 10.17, P = 0.006, I² = 80%) exhibited non-significant pooled effect sizes: HDL: OR = 1.07 (95% CI: 0.79–1.43, P = 0.68); and LDL-C: OR = 0.99 (95% CI: 0.52–1.89, P = 0.99). The overlapping 95% CI with the null line indicated no statistically significant associations for these lipid parameters. HbA1c (I² = 86%), HOMA-IR (I² = 83%), HDL (I² = 65%), and LDL-C (I² = 80%) had I² > 50%, so a random-effects model was used. FPG (I² = 0%) and HS-CRP (I² = 0%) had I² < 50%, so a fixed-effects model was used.
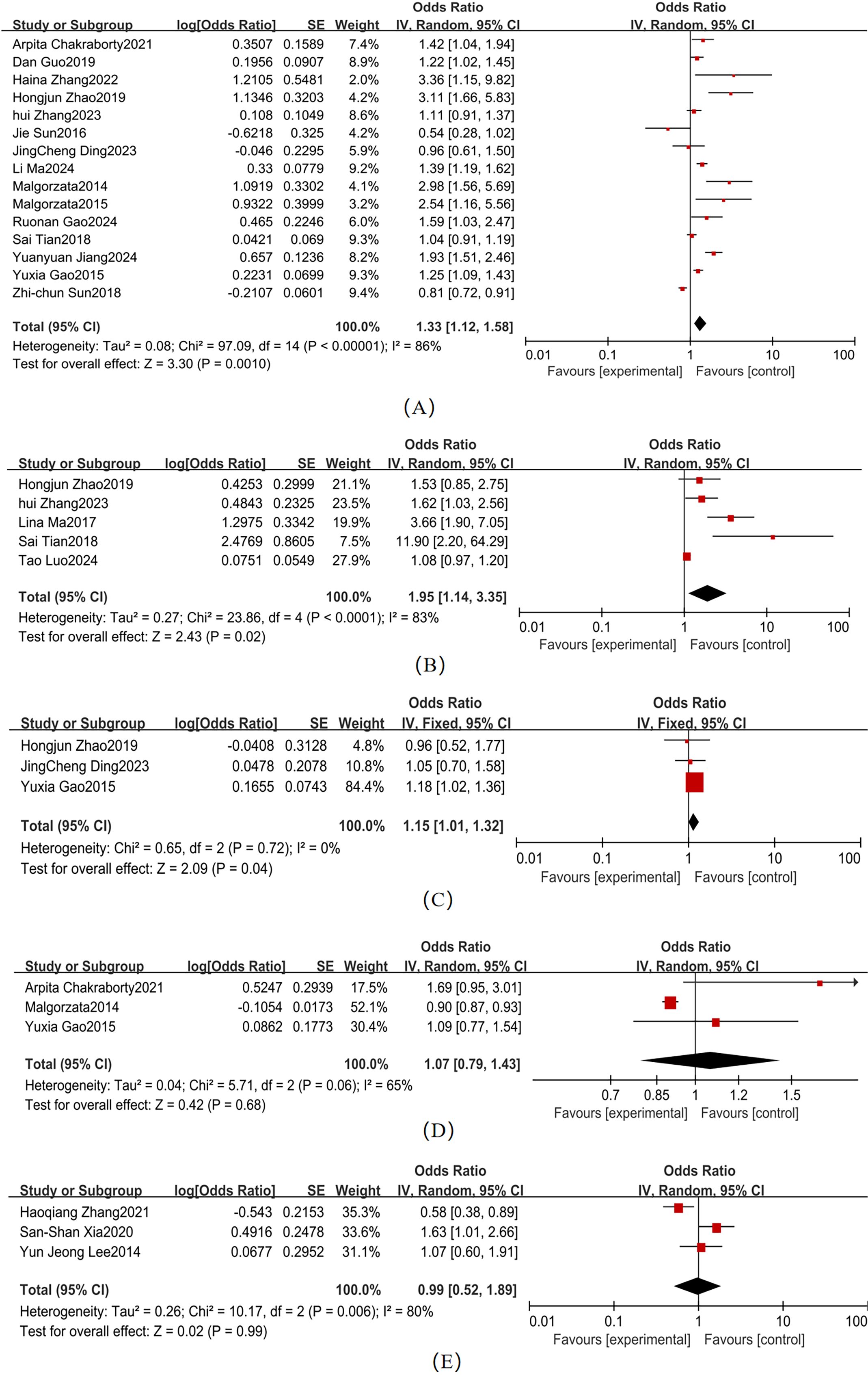
Figure 6. Biochemical Indicators Forest map (A) HbA1c; (B) HOMA-IR; (C) FPG; (D) HDL; (E) LDL-C (2).
3.4.4 Subgroup analysis
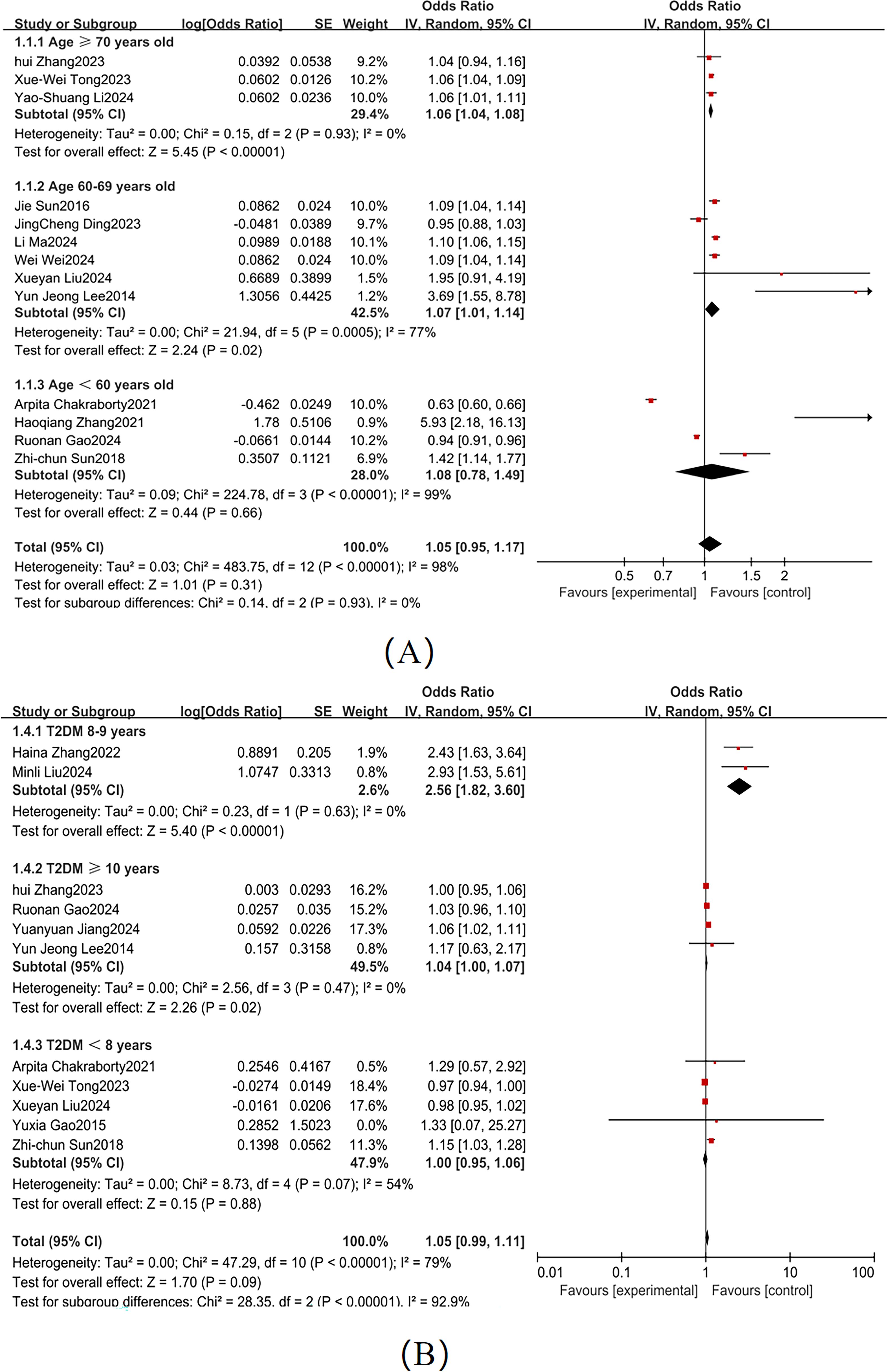
According to the American Diabetes Association guidelines, diabetes patients aged ≥70 are generally at higher risk for complications, particularly in terms of cognitive function and cardiovascular health (62). Based on the dataset, we divided age into three categories: ≥70 years, 60–69 years, and <60 years. Significant heterogeneity was observed across these subgroups (I² = 74%; Figure 7A), so a random-effects model was used. The American Diabetes Association points out that patients with a diabetes duration of ≥10 years have a significantly increased risk of cognitive decline (62). Based on the dataset, we categorized diabetes duration into ≥10 years, 8–9 years, and <8 years, with substantial heterogeneity between subgroups (I² = 81%; Figure 7B). Subgroup analyses identified the following independent risk factors for MCI development in T2DM patients: advanced age (≥ 70 years: OR = 1.06, 95% CI: 1.04-1.08, P < 0.001); prolonged diabetes duration (≥10 years: OR = 1.04, 95% CI: 1.01-1.07, P = 0.02) as independent risk factors for MCI development in T2DM patients.
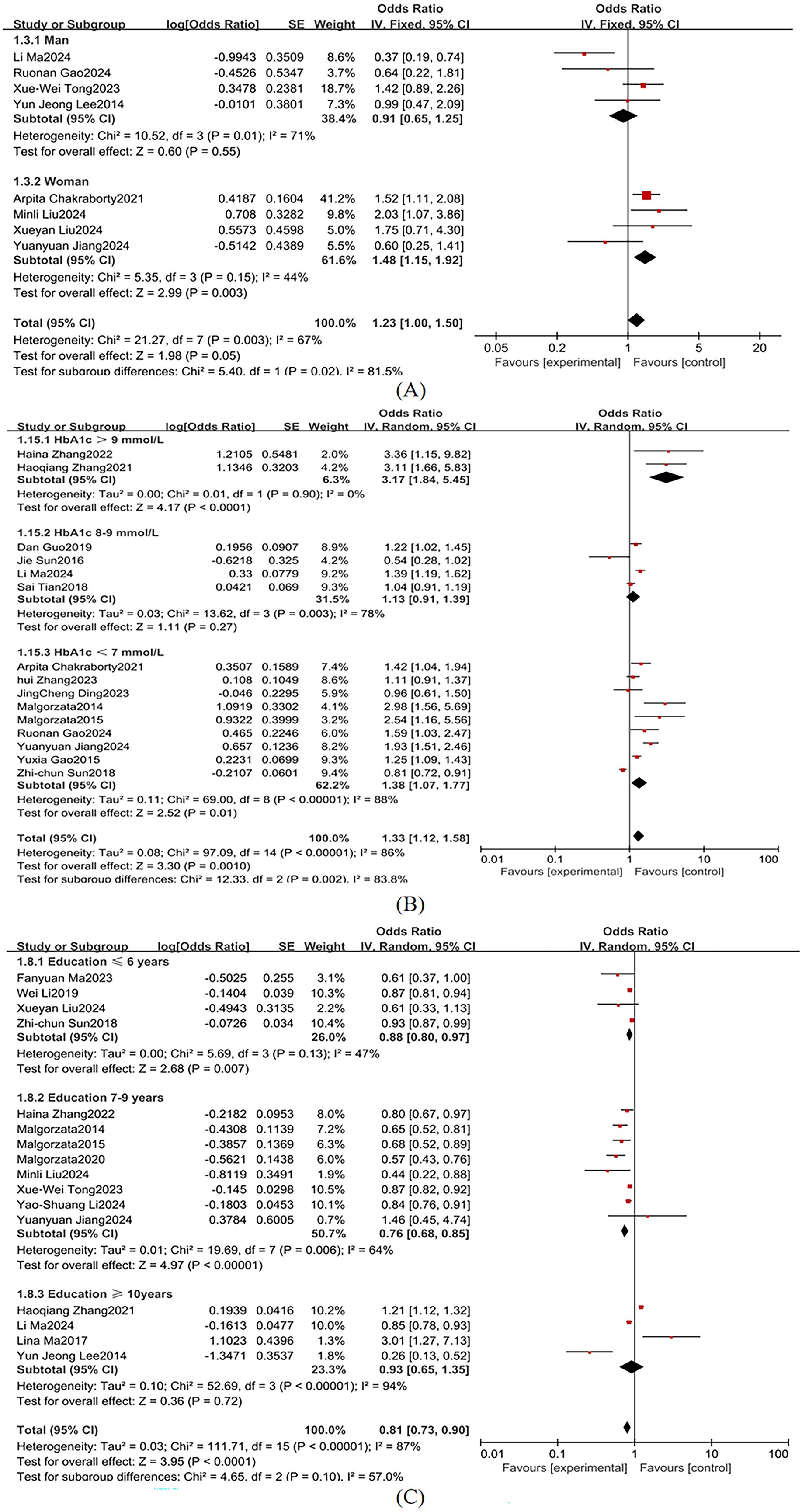
Sex was stratified into male and female subgroups, demonstrating significant between-group heterogeneity in effect sizes (I² = 81.5%; Figure 8A), so a random-effects model was used. According to the American Diabetes Association guidelines, an HbA1c level above 9%, typically indicates poor diabetes control, with a higher risk of complications. An HbA1c level <7% is the treatment goal for most diabetes patients to reduce the risk of diabetes-related complications (62). Therefore, we categorized HbA1c into >9%, 8-9%, and <7%, with substantial heterogeneity across subgroups (I² = 86%; Figure 8B), so a random-effects model was used. Educational attainment was stratified into ≤6 years, 7–9 years, and ≥10 years, demonstrating marked heterogeneity (I² = 87%; Figure 8C), so a random-effects model was used. Key subgroup analyses identified the following independent risk factors for MCI in T2DM populations: female sex: OR = 1.48 (95% CI: 1.15-1.91, P = 0.003); HbA1c >9%: OR = 3.17 (95% CI: 2.35-4.28, P <0.001); educational attainment ≤ 6 years: OR = 1.29 (95% CI: 0.80-0.97, P = 0.007) as independent risk factors for MCI in T2DM populations.
4 Discussion
This study systematically evaluated the influencing factors of MCI comorbidity in patients with T2DM through meta-analysis, revealing multidimensional interactions among demographic/clinical characteristics, lifestyle factors, disease management, and biochemical indicators. The results indicate that age and educational attainment exhibit the highest predictive weights among demographic and clinical characteristics. For lifestyle factors, smoking demonstrates the strongest association with T2DM-MCI comorbidity. In disease management, CVD and hypertension emerge as core risk factors. Among biochemical indicators, HbA1c is identified as the most significant factor, followed by the heavily weighted HOMA-IR.
4.1 Demographic and clinical determinants of T2DM-MCI comorbidity
Subgroup analysis in this study demonstrated that T2DM patients aged ≥ 70 years face an elevated MCI risk (OR = 1.06, 95% CI: 1.04-1.08, P < 0.001), while those with diabetes aged 60–69 years showed a higher risk (OR = 1.07, 95%CI:1.01-1.14, P = 0.005). These findings align with Sun et al. (63), confirming age as a non-modifiable risk factor for MCI. Potential mechanisms include age-related neurodegeneration (e.g., neuronal loss, reduced synaptic density, and impaired cerebral energy metabolism) (64), compounded by elevated advanced glycation end products (AGEs) and oxidative stress in elderly T2DM patients (65), which synergistically accelerate vascular dysfunction and cognitive deterioration (66).
Female sex was identified as a significant risk factor for MCI in T2DM patients (OR = 1.48 vs. 0.91 in men), consistent with You et al. (67). While gender differences remain debated (68, 69), emerging evidence suggests that the postmenopausal decline in estrogen may attenuate neuroprotective pathways (68, 70). Additionally, sex-specific disparities in cardiovascular risks profiles (71), adiposity distribution patterns (72), and chronic inflammatory states (73) likely contribute to this association. Although these mechanisms are not fully understood, existing studies support the significant role of sex in diabetes-related cognitive impairment.
The present study highlights a strong relationship between diabetes duration and MCI risk. Subgroup analyses revealed significantly elevated risks in patients with longer disease duration: those with ≥ 10 years of diabetes exhibited an adjusted OR of 1.04 (95%CI:1.01-1.07, P = 0.02), while those with 8–9 years diabetes showed a higher risk (OR = 2.56, 95%CI:2.13-3.08, P < 0.001). These findings suggest that prolonged hyperglycemia accelerates cognitive dysfunction through cumulative metabolic insults. Longitudinal studies indicate that patients with ≥ 20 years of diabetes have a 3.32-fold increased risk of information processing deficits, a 1.72-fold risk of immediate recall impairment, and a 1.76-fold risk of executive dysfunction compared to those with shorter disease duration (74). Mechanistically, chronic hyperglycemia drives insulin resistance, intermittent hypoglycemia, and microvascular complications (75). Furthermore, extended disease duration may induce structural and functional brain changes (e.g. accelerating cerebral atrophy and reduced synaptic density) and functional neurodegeneration (76), which collectively contribute to cognitive decline.
Low educational attainment (≤ 6 years) was associated with an increased risk of MCI (OR = 1.29, 95% CI: 0.80–0.97, P = 0.007), supporting the cognitive reserve hypothesis (77). Higher educational attainment may enhance neural plasticity and compensatory mechanisms, potentially delaying cognitive decline. Additionally, greater education may optimize neural network efficiency, helping maintain cognitive resilience despite chronic metabolic conditions such as diabetes (36).
No significant association was found between BMI and MCI risk in patients with T2DM (OR = 1.18, 95% CI: 0.94–1.49, P = 0.15). Although elevated BMI is associated with insulin resistance (78), adiposity-related inflammation (79), and cardiovascular risks (80)—factors that may indirectly impair cerebral metabolism and cognition— BMI was not independently associated with MCI in this cohort. This finding may reflect interactions between BMI and confounding variables (e.g., age, glycemic control, sex). Future research should clarify BMI’s role through stratified analyses and longitudinal studies.
4.2 Lifestyle factors and T2DM-MCI comorbidity
It was demonstrated that smoking increases the MCI risk in patients with T2DM (OR = 1.44, 95% CI: 1.18–1.75, P = 0.0003). These findings align with previous studies (81, 82), confirming smoking as a critical risk factor for T2DM-MCI comorbidity. For instance, Hagger-Johnson et al. (83) reported accelerated cognitive decline in middle-aged and elderly smokers, while Xia et al. (84) identified an inverse correlation between smoking intensity and serum brain-derived neurotrophic factor levels—a key mediator of neurogenesis and synaptic plasticity. Mechanistically, nicotine may impair cognition through interactions with nicotinic acetylcholine receptor subunits (e.g., α4, β2, and α7) [ (85). Although precise pathways require further elucidation, the robust association between smoking and cognitive deterioration is well-established.
In contrast, alcohol consumption showed no significant association with T2DM-MCI comorbidity in this study (OR = 0.81, 95% CI: 0.36–1.80, P = 0.61). However, existing evidence (86) suggests a complex dose-response dynamic, including U-shaped or J-shaped relationships. A Finnish cohort study of 1,464 adults aged 65–79 years found that midlife heavy drinking patterns significantly increased MCI risk (OR = 5.08, P = 0.020) (87), while another study reported elevated cognitive decline risks in both heavy drinkers (OR = 1.44, 95% CI: 1.02–2.10) and abstainers (OR = 1.94, 95% CI: 1.10–3.44) compared to moderate drinkers (88). The non-significant association observed here may reflect limitations of the cross-sectional design or population heterogeneity, necessitating longitudinal studies to clarify the role of alcohol in T2DM-related cognitive dysfunction.
4.3 Comorbidity management and T2DM-MCI comorbidity
Hypertension was identified as a significant risk factor for MCI in patients with T2DM (OR = 2.25, 95% CI: 1.49–3.40, P < 0.001), consistent with prior research (89, 90). Chronic hypertension induces structural and functional cerebrovascular damage through ischemic white matter injury and microvascular pathology, reducing cerebral blood flow and accelerating cognitive decline (91). Similarly, CVD significantly elevates MCI risk (OR = 2.61, 95% CI: 1.99–3.43, P < 0.001), corroborating Xie et al. (92). Post-stroke cerebrovascular injuries—particularly those involving extracranial carotid or intracranial vascular lesions (93)—are strongly associated with cognitive impairment in diabetic populations. Notably, diabetic stroke survivors with larger infarct volumes exhibit pronounced cognitive deficits, substantially increasing post-stroke cognitive impairment (PSCI) risk (94). These findings underscore the need for intensified CVD management in T2DM patients to mitigate cognitive deterioration.
Interestingly, depression showed no significant association with MCI risk (OR = 2.04, 95% CI: 0.42–9.79, P = 0.38), contrasting with Carr et al. (95) and Chow et al. (96). While depression-related neurodegeneration in brain regions such as the hippocampus and prefrontal cortex may drive cognitive dysfunction (97), confounding factors [e.g. glycemic control (98), systemic inflammation (99)] and regional population differences likely explain this discrepancy.
DR emerged as a significant MCI predictor (OR = 1.50, 95% CI: 1.12–2.01, P = 0.006), corroborating Gorska-Ciebiada et al. (45). As a microvascular complication, DR shares pathophysiological mechanisms with cerebral microangiopathy [e.g. chronic hyperglycemia-induced endothelial dysfunction (100)], suggesting its potential role as a biomarker for concurrent brain microvascular damage (101). Proactive DR screening and management may thus help reduce MCI risk in T2DM patients.
4.4 Biochemical indicators and T2DM-MCI comorbidity
It was found that HbA1c >9% significantly elevates MCI risk in patients with T2DM (OR = 1.33, P = 0.001), with residual risks persisting even within the conventional glycemic target range (HbA1c <7%: OR = 1.38, P = 0.002). Chronic hyperglycemia impairs glial cell function, inducing cerebrovascular pathology and neuronal damage that ultimately compromise cognition (102). Zheng et al. (103) reported that each 1 mmol/mol increase in HbA1c exacerbates declines in cognitive, memory, and executive functions. Hyperglycemia-driven cognitive deterioration involves multiple mechanisms, including disrupted neurotransmitter metabolism (104), aggravated oxidative stress (105), and altered neuronal energy homeostasis (106), solidifying HbA1c’s role as a critical biomarker for T2DM-MCI comorbidity.
Elevated HOMA-IR was independently associated with MCI risk (OR = 1.95, 95% CI: 1.14–3.35, P = 0.02). As a surrogate marker of insulin resistance, HOMA-IR reflects compensatory hyperinsulinemia, which is closely linked to cognitive decline. Kim et al. (107) identified strong associations between hyperinsulinemia and impairments in memory and executive function. Pharmacological interventions improving insulin sensitivity have shown potential to enhance memory performance (108), suggesting therapeutic relevance. Although insulin resistance-MCI relationships are empirically supported (108, 109), underlying mechanisms remain incompletely elucidated, necessitating further investigation of HOMA-IR’s predictive utility.
A potential association between elevated FPG and MCI risk in T2DM patients was also identified (OR = 1.15, 95% CI: 1.01–1.32, P = 0.04). Comparative analysis revealed lower FPG levels in non-MCI groups, suggesting that sustained hyperglycemia may contribute to cognitive dysfunction. Chronic hyperglycemia not only exacerbates microvascular complications but also induces long-term neurological detriment, oxidative stress, and blood-brain barrier disruption (110, 111). Thus, FPG serves dual roles as a glycemic control biomarker and a predictor of cognitive decline in T2DM.
HDL levels showed no significant association with MCI risk in T2DM patients (OR = 1.07, 95% CI: 0.79–1.43, P = 0.68), diverging from studies suggesting HDL’s neuroprotective effects via anti-inflammatory and antioxidant pathways (112, 113). Moderate heterogeneity across studies (I² = 65%, P = 0.06) may stem from methodological variations in HDL measurement or population characteristics.
No significant association was found between LDL-C levels and MCI risk in the current study (OR = 0.99, 95% CI: 0.52–1.89, P = 0.99). Despite proposed U-shaped relationships between LDL-C and cognitive function (114), significant heterogeneity (I² = 80%, P = 0.006) suggests confounding effects from diabetes control status or inflammatory mediators, necessitating further investigation into these complex interactions.
Finally, elevated HS-CRP was strongly associated with MCI risk (OR = 2.85, 95% CI: 2.09–3.89, P <0.001). As a sensitive inflammatory biomarker (115), elevated HS-CRP reflects systemic inflammation that synergizes with oxidative stress to amplify free radical generation, damaging cellular membranes, DNA, and neuronal function (116, 117). These findings highlight HS-CRP’s dual roles as an inflammatory marker and a predictor of early cognitive decline in T2DM.
5 Limitations
This study has several limitations. First, the generalizability of the findings may be constrained by geographical and population homogeneity, as most of the included studies focused on Chinese populations, with limited representation of other ethnic or regional groups. Second, methodological heterogeneity—including variability in study designs (e.g., cross-sectional vs. cohort), sample sizes, and quality—likely contributed to substantial heterogeneity, which introduces a cautious interpretation of pooled estimates. Third, the predominance of cross-sectional designs (which constitute a high proportion of the included studies) precludes causal inference or longitudinal trajectory analysis. To address these limitations, future research should prioritize large-scale, multi-center cohort studies with extended follow-up periods to validate the identified risk factors. Additionally, a systematic investigation of unexplored confounders (e.g., genetic predisposition, lifestyle interactions) and mechanistic pathways is warranted to comprehensively elucidate T2DM-MCI pathophysiology.
6 Conclusions
This meta-analysis identified advanced age (≥ 60 years), female sex, prolonged diabetes duration (8–9 years), elevated HbA1c (> 9%), and low educational attainment (≤ 6 years) as significant independent predictors of MCI in patients with T2DM, demonstrating clear dose-response relationships. Smoking, hypertension, CVD, insulin resistance (as measured by the HOMA-IR), FPG, and HS-CRP were also significantly associated with increased MCI risk.
These findings underscore the need for integrated clinical strategies. Regular cognitive assessments should target high-risk subgroups, including elderly patients with long-standing diabetes (8–9 years), females with poor glycemic control (HbA1c > 9%), and individuals with vascular comorbidities. Glycemic management aiming for HbA1c < 7% may offer cognitive protection, while population with limited education warrant tailored health literacy interventions to mitigate self-management barriers. Multidisciplinary collaboration across endocrinology, neurology, and ophthalmology is critical for patients with microvascular complications, such as diabetic retinopathy.
This study advances the understanding of T2DM-MCI determinants but highlights key research gaps. Future investigations should prioritize longitudinal designs to establish causality, validate biomarkers across diverse populations, and explore mechanistic interactions between metabolic dysregulation and neurodegeneration. Such efforts will be essential for developing precision prevention frameworks against diabetic cognitive decline. Since the results of this study are mainly based on Chinese populations, their applicability to other regions or races may be limited. Follow-up research should include more diverse samples to enhance the external validity of the research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YZ: Writing – review & editing, Writing – original draft. HW: Writing – review & editing, Writing – original draft. GT: Writing – review & editing, Writing – original draft. LW: Writing – review & editing, Writing – original draft. XT: Writing – original draft, Writing – review & editing. RL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Ministry of Education’s Youth Fund Project for Humanities and Social Sciences (24YJC890031).
Acknowledgments
We extend our heartfelt gratitude to all the researchers; their commitment was essential for the completion of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1617248/full#supplementary-material
Abbreviations
MCI, Mild Cognitive Impairment; T2DM, Type 2 Diabetes Mellitus; HbA1c, Glycated Hemoglobin; FPG, Fasting Plasma Glucose; CI, Confidence Intervals; OR, Odds Rations; BMI, Body Mass Index; CVD, Cardiovascular Disease; DR, Diabetic Retinopathy; HOMA-IR, Homeostasis Model Assessment Of Insulin Resistance; LDL-C, Low-Density Lipoprotein Cholesterol; HS-CRP, High-Sensitivity C-Reactive Protein; HDL, High-Density Lipoprotein; NOS, Newcastle-Ottawa Scale; JBI, Joanna Briggs Institute.
References
1. Geda YE. Mild cognitive impairment in older adults. Curr Psychiatry Rep. (2012) 14:320–7. doi: 10.1007/s11920-012-0291-x
2. Pal K, Mukadam N, Petersen I, and Cooper C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. (2018) 53:1149–60. doi: 10.1007/s00127-018-1581-3
3. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin practice. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
4. Yuan XY and Wang XG. Mild cognitive impairment in type 2 diabetes mellitus and related risk factors: a review. Rev neurosciences. (2017) 28:715–23. doi: 10.1515/revneuro-2017-0016
5. Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, et al. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev. (2019) 55:100944. doi: 10.1016/j.arr.2019.100944
6. Chakraborty A, Hegde S, Praharaj SK, Prabhu K, Patole C, Shetty AK, et al. Age related prevalence of mild cognitive impairment in type 2 diabetes mellitus patients in the Indian population and association of serum lipids with cognitive dysfunction. Front endocrinology. (2021) 12:798652. doi: 10.3389/fendo.2021.798652
7. Zheng Y, Ma Q, Qi X, Zhu Z, and Wu B. Prevalence and incidence of mild cognitive impairment in adults with diabetes in the United States. Diabetes Res Clin practice. (2023) 205:110976. doi: 10.1016/j.diabres.2023.110976
8. Jongsiriyanyong S and Limpawattana P. Mild cognitive impairment in clinical practice: A review article. Am J Alzheimer’s Dis other dementias. (2018) 33:500–7. doi: 10.1177/1533317518791401
9. Jung YH, Park S, Jang H, Cho SH, Kim SJ, Kim JP, et al. Frontal-executive dysfunction affects dementia conversion in patients with amnestic mild cognitive impairment. Sci Rep. (2020) 10:772. doi: 10.1038/s41598-020-57525-6
10. Freire LB, Brasil-Neto JP, da Silva ML, Miranda MGC, de Mattos Cruz L, Martins WR, et al. Risk factors for falls in older adults with diabetes mellitus: systematic review and meta-analysis. BMC geriatrics. (2024) 24:201. doi: 10.1186/s12877-024-04668-0
11. Hussenoeder FS, Conrad I, Roehr S, Fuchs A, Pentzek M, Bickel H, et al. Mild cognitive impairment and quality of life in the oldest old: a closer look. Qual Life research: an Int J Qual Life aspects treatment Care rehabilitation. (2020) 29:1675–83. doi: 10.1007/s11136-020-02425-5
12. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21176275
13. Lu X, Xie Q, Pan X, Zhang R, Zhang X, Peng G, et al. Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal transduction targeted Ther. (2024) 9:262. doi: 10.1038/s41392-024-01951-9
14. Wątroba M, Grabowska AD, and Szukiewicz D. Effects of diabetes mellitus-related dysglycemia on the functions of blood-brain barrier and the risk of dementia. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241210069
15. Gao HM, Chen H, Cui GY, and Hu JX. Damage mechanism and therapy progress of the blood-brain barrier after ischemic stroke. Cell bioscience. (2023) 13:196. doi: 10.1186/s13578-023-01126-z
16. Sadeghi A, Hami J, Razavi S, Esfandiary E, and Hejazi Z. The effect of diabetes mellitus on apoptosis in hippocampus: cellular and molecular aspects. Int J Prev Med. (2016) 7:57. doi: 10.4103/2008-7802.178531
17. Zhang L, Chen ZW, Yang SF, Shaer M, Wang Y, Dong JJ, et al. MicroRNA-219 decreases hippocampal long-term potentiation inhibition and hippocampal neuronal cell apoptosis in type 2 diabetes mellitus mice by suppressing the NMDAR signaling pathway. CNS Neurosci Ther. (2019) 25:69–77. doi: 10.1111/cns.2019.25.issue-1
18. Sachdev PS, Lipnicki DM, Crawford J, Reppermund S, Kochan NA, Trollor JN, et al. Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: a population-based study. PloS One. (2013) 8:e59649. doi: 10.1371/journal.pone.0059649
19. Pandya SY, Lacritz LH, Weiner MF, Deschner M, and Woon FL. Predictors of reversion from mild cognitive impairment to normal cognition. Dementia geriatric Cogn Disord. (2017) 43:204–14. doi: 10.1159/000456070
20. Canevelli M, Grande G, Lacorte E, Quarchioni E, Cesari M, Mariani C, et al. Spontaneous reversion of mild cognitive impairment to normal cognition: A systematic review of literature and meta-analysis. J Am Med Directors Assoc. (2016) 17:943–8. doi: 10.1016/j.jamda.2016.06.020
21. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London England). (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
22. Chen JF, Zhang YP, Han JX, Wang YD, and Fu GF. Systematic evaluation of the prevalence of cognitive impairment in elderly patients with diabetes in China. Clin Neurol neurosurgery. (2023) 225:107557. doi: 10.1016/j.clineuro.2022.107557
23. Tabesh M, Sacre JW, Mehta K, Chen L, Sajjadi SF, Magliano DJ, et al. The association of glycaemic risk factors and diabetes duration with risk of heart failure in people with type 2 diabetes: A systematic review and meta-analysis. Diabetes Obes Metab. (2024) 26:5690–700. doi: 10.1111/dom.v26.12
24. Sonoda N, Morimoto A, Ugi S, Morino K, Sekine O, Nemoto KI, et al. Smoking status is associated with mild cognitive impairment assessed with the mini-mental state examination in Japanese diabetic patients. Diabetol Int. (2016) 7:361–7. doi: 10.1007/s13340-016-0256-0
25. Debnath DJ, Ray J, Jah SM, and Marimuthu Y. Smoking and the risk of type 2 diabetes: A cross-sectional analytical study. Indian J Community medicine: Off Publ Indian Assoc Prev Soc Med. (2024) 49:588–92. doi: 10.4103/ijcm.ijcm_1009_22
26. Guo D, Yuan Y, Huang R, Tian S, Wang J, Lin H, et al. Association between plasma adipsin level and mild cognitive impairment in Chinese patients with type 2 diabetes: a cross-sectional study. BMC endocrine Disord. (2019) 19:108. doi: 10.1186/s12902-019-0431-y
27. Zhang H, Yu L, and Yun G. Reduced serum levels of klotho are associated with mild cognitive impairment in patients with type 2 diabetes mellitus. Diabetes Metab syndrome obesity: Targets Ther. (2023) 16:129–37. doi: 10.2147/DMSO.S394099
28. Sartore G, Ragazzi E, Caprino R, and Lapolla A. Long-term HbA1c variability and macro-/micro-vascular complications in type 2 diabetes mellitus: a meta-analysis update. Acta diabetologica. (2023) 60:721–38. doi: 10.1007/s00592-023-02037-8
29. Shao P, Li X, Qin R, Xu H, Sheng X, Huang L, et al. Altered local gyrification and functional connectivity in type 2 diabetes mellitus patients with mild cognitive impairment: A pilot cross-sectional small-scale single center study. Front Aging Neurosci. (2022) 14:934071. doi: 10.3389/fnagi.2022.934071
30. Liu Y, Liu Y, Qiu H, Haghbin N, Li J, Li Y, et al. Association of time in range with cognitive impairment in middle-aged type 2 diabetic patients. BMC endocrine Disord. (2024) 24:241. doi: 10.1186/s12902-024-01772-5
31. Sitasuwan T and Lertwattanarak R. Prediction of type 2 diabetes mellitus using fasting plasma glucose and HbA1c levels among individuals with impaired fasting plasma glucose: a cross-sectional study in Thailand. BMJ Open. (2020) 10:e041269. doi: 10.1136/bmjopen-2020-041269
32. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). (2021) 372:n71. doi: 10.1136/bmj.n71
33. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
34. Vardell E and Malloy M. Joanna briggs institute: an evidence-based practice database. Med Reference Serv Quarterly. (2013) 32:434–42. doi: 10.1080/02763869.2013.837734
35. Wei W, Xu P, Li L, Mao H, Li N, Wang XQ, et al. Association of glycogen synthase kinase-3β with cognitive impairment in type 2 diabetes patients: a six-year follow-up study. Front endocrinology. (2024) 15:1386773. doi: 10.3389/fendo.2024.1386773
36. Zhong T, Li S, Liu P, Wang Y, and Chen L. The impact of education and occupation on cognitive impairment: a cross-sectional study in China. Front Aging Neurosci. (2024) 16:1435626. doi: 10.3389/fnagi.2024.1435626
37. Li W, Sun L, Li G, and Xiao S. Prevalence, influence factors and cognitive characteristics of mild cognitive impairment in type 2 diabetes mellitus. Front Aging Neurosci. (2019) 11:180. doi: 10.3389/fnagi.2019.00180
38. Ma F, Zhang Q, Shi J, Li S, Wu L, and Zhang H. Risk factors for cognitive dysfunction and glycemic management in older adults with type 2 diabetes mellitus: a retrospective study. BMC endocrine Disord. (2023) 23:220. doi: 10.1186/s12902-023-01476-2
39. Zheng M, Chang B, Tian L, Shan C, Chen H, Gao Y, et al. Relationship between inflammatory markers and mild cognitive impairment in Chinese patients with type 2 diabetes: a case-control study. BMC endocrine Disord. (2019) 19:73. doi: 10.1186/s12902-019-0402-3
40. Ma L, Yuan YX, Cheng FJ, Liu Y, Wei Q, Peng YF, et al. The association between blood lipids and cognitive impairment in type 2 diabetes mellitus. Eur J Med Res. (2024) 29:1. doi: 10.1186/s40001-023-01574-w
41. Gao R, Zhan M, Ke S, Wu K, He G, Qi L, et al. Potential risk factors for mild cognitive impairment among patients with type 2 diabetes experiencing hypoglycemia. Diabetes Res Clin practice. (2024) 207:111036. doi: 10.1016/j.diabres.2023.111036
42. Gao Y, Xiao Y, Miao R, Zhao J, Cui M, Huang G, et al. The prevalence of mild cognitive impairment with type 2 diabetes mellitus among elderly people in China: A cross-sectional study. Arch gerontology geriatrics. (2016) 62:138–42. doi: 10.1016/j.archger.2015.09.003
43. Gorska-Ciebiada M and Ciebiada M. Association of hsCRP and vitamin D levels with mild cognitive impairment in elderly type 2 diabetic patients. Exp gerontology. (2020) 135:110926. doi: 10.1016/j.exger.2020.110926
44. Gorska-Ciebiada M, Saryusz-Wolska M, Borkowska A, Ciebiada M, and Loba J. Adiponectin, leptin and IL-1 β in elderly diabetic patients with mild cognitive impairment. Metab Brain disease. (2016) 31:257–66. doi: 10.1007/s11011-015-9739-0
45. Gorska-Ciebiada M, Saryusz-Wolska M, Ciebiada M, and Loba J. Mild cognitive impairment and depressive symptoms in elderly patients with diabetes: prevalence, risk factors, and comorbidity. J Diabetes Res. (2014) 2014:179648. doi: 10.1155/2014/179648
46. Xia SS, Xia WL, Huang JJ, Zou HJ, Tao J, and Yang Y. The factors contributing to cognitive dysfunction in type 2 diabetic patients. Ann Trans Med. (2020) 8:104. doi: 10.21037/atm.2019.12.113
47. Jiang Y, Liu X, Gao H, Yan J, and Cao Y. A new nomogram model for the individualized prediction of mild cognitive impairment in elderly patients with type 2 diabetes mellitus. Front endocrinology. (2024) 15:1307837. doi: 10.3389/fendo.2024.1307837
48. Lee YJ, Kang HM, Kim NK, Yang JY, Noh JH, Ko KS, et al. Factors associated for mild cognitive impairment in older korean adults with type 2 diabetes mellitus. Diabetes Metab J. (2014) 38:150–7. doi: 10.4093/dmj.2014.38.2.150
49. Luo T, Jiang X, Xu N, Zhao X, Xie X, Xia X, et al. Risk factors and metabolomics of mild cognitive impairment in type 2 diabetes mellitus. Front Mol biosciences. (2024) 11:1341290. doi: 10.3389/fmolb.2024.1341290
50. Tian S, Huang R, Han J, Cai R, Guo D, Lin H, et al. Increased plasma Interleukin-1β level is associated with memory deficits in type 2 diabetic patients with mild cognitive impairment. Psychoneuroendocrinology. (2018) 96:148–54. doi: 10.1016/j.psyneuen.2018.06.014
51. Zhang H, Fareeduddin Mohammed Farooqui H, Zhu W, Niu T, Zhang Z, and Zhang H. Impact of insulin resistance on mild cognitive impairment in type 2 diabetes mellitus patients with non-alcoholic fatty liver disease. Diabetol Metab syndrome. (2023) 15:229. doi: 10.1186/s13098-023-01211-w
52. Zhang H, Zhu W, Niu T, Wang Z, An K, Cao W, et al. Inverted U-shaped correlation between serum low-density lipoprotein cholesterol levels and cognitive functions of patients with type 2 diabetes mellitus. Lipids Health Dis. (2021) 20:103. doi: 10.1186/s12944-021-01534-5
53. Zhao H, Wu C, Zhang X, Wang L, Sun J, and Zhuge F. Insulin resistance is a risk factor for mild cognitive impairment in elderly adults with T2DM. Open Life Sci. (2019) 14:255–61. doi: 10.1515/biol-2019-0029
54. Sun ZC, Yu J, Liu YL, Hong ZZ, Ling L, Li GQ, et al. Reduced serum levels of brain-derived neurotrophic factor are related to mild cognitive impairment in chinese patients with type 2 diabetes mellitus. Ann Nutr Metab. (2018) 73:271–81. doi: 10.1159/000493275
55. Li YS, Li JB, Wang JJ, Wang XH, Jiang WR, Qiu HN, et al. Risk factors for cognitive impairment in middle-aged type 2 diabetic patients: a cross-sectional study. BMJ Open. (2024) 14:e074753. doi: 10.1136/bmjopen-2023-074753
56. Sun J, Cai R, Huang R, Wang P, Tian S, Sun H, et al. Cholesteryl ester transfer protein intimately involved in dyslipidemia-related susceptibility to cognitive deficits in type 2 diabetic patients. J Alzheimer’s disease: JAD. (2016) 54:175–84. doi: 10.3233/JAD-160053
57. Tong XW, Zhang YT, Li X, Yu ZW, Pu SD, Xu YX, et al. Uric acid index is a risk for mild cognitive impairment in type 2 diabetes. Hormones (Athens Greece). (2023) 22:425–39. doi: 10.1007/s42000-023-00465-3
58. Damanik J, Mayza A, Rachman A, Sauriasari R, Kristanti M, Agustina PS, et al. Association between serum homocysteine level and cognitive function in middle-aged type 2 diabetes mellitus patients. PloS One. (2019) 14:e0224611. doi: 10.1371/journal.pone.0224611
59. Ding J, Shi Q, Tao Q, Su H, Du Y, Pan T, et al. Correlation between long-term glycemic variability and cognitive function in middle-aged and elderly patients with type 2 diabetes mellitus: a retrospective study. PeerJ. (2023) 11:e16698. doi: 10.21203/rs.3.rs-2504178/v1
60. Liu X, Jiang T, Jiang Y, Li L, and Cao Y. Prevalence of mild cognitive impairment and modifiable risk factors: A cross-sectional study in rural older adults with diabetes. Geriatric Nurs (New York NY). (2024) 59:549–56. doi: 10.1016/j.gerinurse.2024.08.010
61. Ma L and Li Y. Cognitive function and insulin resistance in elderly patients with type 2 diabetes. Neurological Res. (2017) 39:259–63. doi: 10.1080/01616412.2017.1281199
62. Committee ADAPP. 13. Older adults: standards of care in diabetes—2024. Diabetes Care. (2023) 47:S244–S57. doi: 10.2337/dc24-S013
63. Sun L, Diao X, Gang X, Lv Y, Zhao X, Yang S, et al. Risk factors for cognitive impairment in patients with type 2 diabetes. J Diabetes Res. (2020) 2020:4591938. doi: 10.1155/2020/4591938
64. Peters R. Ageing and the brain. Postgraduate Med J. (2006) 82:84–8. doi: 10.1136/pgmj.2005.036665
65. Rhee SY and Kim YS. role of advanced glycation end products in diabetic vascular complications. Diabetes Metab J. (2018). doi: 10.4093/dmj.2017.0105
66. Chen Y, Yao L, Zhao S, Xu M, Ren S, Xie L, et al. The oxidative aging model integrated various risk factors in type 2 diabetes mellitus at system level. Front endocrinology. (2023) 14:1196293. doi: 10.3389/fendo.2023.1196293
67. You Y, Liu Z, Chen Y, Xu Y, Qin J, Guo S, et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta diabetologica. (2021) 58:671–85. doi: 10.1007/s00592-020-01648-9
68. Espeland MA, Carmichael O, Yasar S, Hugenschmidt C, Hazzard W, Hayden KM, et al. Sex-related differences in the prevalence of cognitive impairment among overweight and obese adults with type 2 diabetes. Alzheimer’s dementia: J Alzheimer’s Assoc. Rom J Neurol. (2018) 21(4):342–6. doi: 10.37897/RJN.2022.4.14
69. Putri Laksmidewi AAA and VJRJoN TD. The impact of gender differences on the cognitive function of type 2 Diabetes Mellitus patients. (2022).
70. Chen HF, Jiang JY, Chen MH, Lin R, Jerence SWO, Chang CH, et al. Gender differences in cognitive function and its associated factors among older adults with type 2 diabetes. Geriatric Nurs (New York NY). (2023) 52:165–71. doi: 10.1016/j.gerinurse.2023.05.017
71. Volgman AS, Bairey Merz CN, Aggarwal NT, Bittner V, Bunch TJ, Gorelick PB, et al. Sex differences in cardiovascular disease and cognitive impairment: another health disparity for women? J Am Heart Assoc. (2019) 8:e013154. doi: 10.1161/JAHA.119.013154
72. Karnes JH, Arora A, Feng J, Steiner HE, Sulieman L, Boerwinkle E, et al. Racial, ethnic, and gender differences in obesity and body fat distribution: An All of Us Research Program demonstration project. PloS One. (2021) 16:e0255583. doi: 10.1371/journal.pone.0255583
73. Dong C, Zhou C, Fu C, Hao W, Ozaki A, Shrestha N, et al. Sex differences in the association between cardiovascular diseases and dementia subtypes: a prospective analysis of 464,616 UK Biobank participants. Biol sex differences. (2022) 13:21. doi: 10.1186/s13293-022-00431-5
74. Tang X, Wang Y, Simó R, Stehouwer CDA, and Zhou JB. The association between diabetes duration and domain-specific cognitive impairment: A population-based study. J Alzheimer’s disease: JAD. (2023) 91:1435–46. doi: 10.3233/JAD-220972
75. Ab-Hamid N, Omar N, Ismail CAN, and Long I. Diabetes and cognitive decline: Challenges and future direction. World J diabetes. (2023) 14:795–807. doi: 10.4239/wjd.v14.i6.795
76. Kim J, Han KD, Lee JY, Yang YS, Cheon DY, Lee JJ, et al. Diabetes status, duration, and risk of dementia among ischemic stroke patients. Alzheimer’s Res Ther. (2025) 17:58. doi: 10.1186/s13195-025-01708-8
77. Pettigrew C and Soldan A. Defining cognitive reserve and implications for cognitive aging. Curr Neurol Neurosci Rep. (2019) 19:1. doi: 10.1007/s11910-019-0917-z
78. Westphal SA. Obesity, abdominal obesity, and insulin resistance. Clin cornerstone. (2008) 9:23–9. doi: 10.1016/s1098-3597
79. Sweatt K, Garvey WT, and Martins C. Strengths and limitations of BMI in the diagnosis of obesity: what is the path forward? Curr Obes Rep. (2024) 13:584–95. doi: 10.1007/s13679-024-00580-1
80. Iyen B, Weng S, Vinogradova Y, Akyea RK, Qureshi N, and Kai J. Long-term body mass index changes in overweight and obese adults and the risk of heart failure, cardiovascular disease and mortality: a cohort study of over 260,000 adults in the UK. BMC Public Health. (2021) 21:576. doi: 10.1186/s12889-021-10606-1
81. Chang SA. Smoking and type 2 diabetes mellitus. Diabetes Metab J. (2012) 36:399–403. doi: 10.4093/dmj.2012.36.6.399
82. Abdel-Latif GA, Hassan AM, Gabal MS, Hemeda SA, El-Chami NH, and IIJOAMJoMS S. Mild cognitive impairment among type II diabetes mellitus patients attending university teaching hospital. Open Access Maced J Med Sci. (2020) 8:105–11. doi: 10.3889/oamjms.2020.4245
83. Hagger-Johnson G, Sabia S, Brunner E, Shipley M, Bobak M, Marmot M, et al. Combined impact of smoking and heavy alcohol use on cognitive decline in early old age: Whitehall II prospective cohort study. Br J psychiatry: J Ment Sci. (2013) 203:120–5. doi: 10.1192/bjp.bp.112.122960
84. Xia H, Du X, Yin G, Zhang Y, Li X, Cai J, et al. Effects of smoking on cognition and BDNF levels in a male Chinese population: relationship with BDNF Val66Met polymorphism. Sci Rep. (2019) 9:217. doi: 10.1038/s41598-018-36419-8
85. Valentine G and Sofuoglu M. Cognitive effects of nicotine: recent progress. Curr neuropharmacology. (2018) 16:403–14. doi: 10.2174/1570159X15666171103152136
86. Sachdeva A, Chandra M, Choudhary M, Dayal P, and Anand KS. Alcohol-related dementia and neurocognitive impairment: A review study. Int J High Risk Behav addiction. (2016) 5:e27976. doi: 10.5812/ijhrba.27976
87. Kilian C, Klinger S, Rehm J, and Manthey J. Alcohol use, dementia risk, and sex: a systematic review and assessment of alcohol-attributab le dementia cases in Europe. BMC geriatrics. (2023) 23:246. doi: 10.1186/s12877-023-03972-5
88. Anttila T, Helkala EL, Viitanen M, Kåreholt I, Fratiglioni L, Winblad B, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ (Clinical Res ed). (2004) 329:539. doi: 10.1136/bmj.38181.418958.BE
89. Nagar SD, Pemu P, Qian J, Boerwinkle E, Cicek M, Clark CR, et al. Investigation of hypertension and type 2 diabetes as risk factors for dementia in the All of Us cohort. Sci Rep. (2022) 12:19797. doi: 10.1038/s41598-022-23353-z
90. Petrova M, Prokopenko S, Pronina E, and Mozheyko E. Diabetes type 2, hypertension and cognitive dysfunction in middle age women. J Neurological Sci. (2010) 299:39–41. doi: 10.1016/j.jns.2010.08.057
91. Santisteban MM, Iadecola C, and Carnevale D. Hypertension, neurovascular dysfunction, and cognitive impairment. Hypertension (Dallas Tex: 1979). (2023) 80:22–34. doi: 10.1161/HYPERTENSIONAHA.122.18085
92. Xie Q, Nie M, Zhang F, Shao X, Wang J, Song J, et al. An unexpected interaction between diabetes and cardiovascular diseases on cognitive function: A cross-sectional study. J Affect Disord. (2024) 354:688–93. doi: 10.1016/j.jad.2024.03.040
93. Ward R, Valenzuela JP, Li W, Dong G, Fagan SC, and Ergul A. Poststroke cognitive impairment and hippocampal neurovascular remodeling: the impact of diabetes and sex. Am J Physiol Heart Circulatory Physiol. (2018) 315:H1402–h13. doi: 10.1152/ajpheart.00390.2018
94. Xu L, Shan D, and Wu D. Infarct volume as a predictor and therapeutic target in post-stroke cognitive impairment. Front Med. (2025) 12:1519538. doi: 10.3389/fmed.2025.1519538
95. Carr AL, Sluiman AJ, Grecian SM, Forster R, McLachlan S, Strachan MWJ, et al. Depression as a risk factor for dementia in older people with type 2 diabetes and the mediating effect of inflammation. Diabetologia. (2021) 64:448–57. doi: 10.1007/s00125-020-05301-6
96. Chow YY, Verdonschot M, McEvoy CT, and Peeters G. Associations between depression and cognition, mild cognitive impairment and dementia in persons with diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin practice. (2022) 185:109227. doi: 10.1016/j.diabres.2022.109227
97. Miskowiak KW, Ott CV, Petersen JZ, and Kessing LV. Systematic review of randomized controlled trials of candidate treatments for cognitive impairment in depression and methodological challenges in the field. Eur neuropsychopharmacology: J Eur Coll Neuropsychopharmacol. (2016) 26:1845–67. doi: 10.1016/j.euroneuro.2016.09.641
98. Medina TA, del Águila Montemayor D, Guajardo Álvarez G, Gonzalez García BI, Hawing Zarate JA, Gamez Treviño DG, et al. Relation between glycemic control with depression, mci, and dementia in elderly with type 2 diabetes. Innovation Aging. (2017) 1:1104. doi: 10.1093/geroni/igx004.4045
99. Pan H, Huang X, Li F, Ren M, Zhang J, Xu M, et al. Association among plasma lactate, systemic inflammation, and mild cognitive impairment: a community-based study. Neurological Sci. (2019) 40:1667–73. doi: 10.1007/s10072-019-03900-9
100. Chen B, Shen C, and Sun B. Current landscape and comprehensive management of glycemic variability in diabetic retinopathy. J Trans Med. (2024) 22:700. doi: 10.1186/s12967-024-05516-w
101. Feuer DS, Handberg EM, Mehrad B, Wei J, Bairey Merz CN, Pepine CJ, et al. Microvascular dysfunction as a systemic disease: A review of the evidence. Am J Med. (2022) 135:1059–68. doi: 10.1016/j.amjmed.2022.04.006
102. Chen Y and Swanson R. Astrocytes and brain injury. J Cereb Blood Flow metabolism: Off J Int Soc Cereb Blood Flow Metab. (2003) 23:137–49. doi: 10.1097/01.WCB.0000044631.80210.3C
103. Zheng F, Yan L, Yang Z, Zhong B, and Xie W. HbA1c, diabetes and cognitive decline: the English Longitudinal Study of Ageing. Diabetologia. (2018) 61:839–48. doi: 10.1007/s00125-017-4541-7
104. Xu P, Ning J, Jiang Q, Li C, Yan J, Zhao L, et al. Region-specific metabolic characterization of the type 1 diabetic brain in mice with and without cognitive impairment. Neurochemistry Int. (2021) 143:104941. doi: 10.1016/j.neuint.2020.104941
105. Sun Y, Zhu S, Chen J, Zhao Y, and Xiao Q. Sirt1 mediated high-glucose-induced Aβ Deposition and cognitive impairment through activation of the TLR9/p53 pathway. (2020). doi: 10.21203/rs.3.rs-34315/v1
106. Van Dyken P and Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front Neurosci. (2018) 12:930. doi: 10.3389/fnins.2018.00930
107. Kim A and Arvanitakis Z. Insulin resistance, cognition, and Alzheimer disease. Obesity. (2023) 31:1486–98. doi: 10.1002/oby.23761
108. Gruber JR, Ruf A, Süß ED, Tariverdian S, Ahrens KF, Schiweck C, et al. Impact of blood glucose on cognitive function in insulin resistance: novel insights from ambulatory assessment. Nutr Diabetes. (2024) 14:74. doi: 10.1038/s41387-024-00331-0
109. Ahlawat A, Walia V, and Garg M. Brain insulin resistance mediated cognitive impairment and neurodegeneration: Type-3 diabetes or Alzheimer’s Disease. Acta Neurologica Belgica. (2025). doi: 10.1007/s13760-024-02706-7
110. Kanoski SE, Zhang Y, Zheng W, and Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimer’s disease: JAD. (2010) 21:207–19. doi: 10.3233/JAD-2010-091414
111. Schwartz SS, Herman ME, Tun MTH, Barone E, and Butterfield DA. The double life of glucose metabolism: brain health, glycemic homeostasis, and your patients with type 2 diabetes. BMC Med. (2024) 22:582. doi: 10.1186/s12916-024-03763-8
112. Huang H, Yang B, Yu R, Ouyang W, Tong J, and Le Y. Very high high-density lipoprotein cholesterol may be associated with higher risk of cognitive impairment in older adults. Nutr J. (2024) 23:79. doi: 10.1186/s12937-024-00983-9
113. Townsend RF, Logan D, O’Neill RF, Prinelli F, Woodside JV, and McEvoy CT. Whole dietary patterns, cognitive decline and cognitive disorders: A systematic review of prospective and intervention studies. Nutrients. (2023) 15. doi: 10.3390/nu15020333
114. Zhang H, Zhu W, Niu T, Wang Z, An K, Cao W, et al. Inverted U-shaped correlation between serum low-density lipoprotein cholesterol levels and cognitive functions of patients with type 2 diabetes mellitus. Lipids Health Disease. (2021) 20:103. doi: 10.1186/s12944-021-01534-5
115. Lutfi AM and Sciences MSNJAJoM. Evaluating the role of high-sensitivity C-reactive protein in asthmatic Iraqi patients and its correlation with parameters of patients¡¯ clinical characteristics and pulmonary function tests. Asian J Med Sci. (2016) 7:47–53. doi: 10.3126/ajms.v7i3.13880
116. Dutta S, Sengupta P, Slama P, and Roychoudhury S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms221810043
Keywords: mild cognitive impairment, type 2 diabetes, risk factors, diabetes duration, HbA1c, meta-analysis
Citation: Zhao Y, Wang H, Tang G, Wang L, Tian X and Li R (2025) Risk factors for mild cognitive impairment in type 2 diabetes: a systematic review and meta-analysis. Front. Endocrinol. 16:1617248. doi: 10.3389/fendo.2025.1617248
Received: 24 April 2025; Accepted: 26 May 2025;
Published: 16 June 2025.
Edited by:
Thorsten Siegmund, Isar Clinic, GermanyCopyright © 2025 Zhao, Wang, Tang, Wang, Tian and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuewen Tian, WHVld2VudGlhbjE5NzhAMTYzLmNvbQ==; Ran Li, bGlyYW5Ac2RwZWkuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yi Zhao
Yi Zhao Hanlin Wang2†
Hanlin Wang2† Ran Li
Ran Li