- 1Center for Reproductive Medicine, Women and Children’s Hospital of Ningbo University, Ningbo, China
- 2Department of Obstetrics and Gynecology, Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China
- 3Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China
- 4The Central Laboratory of Birth Defects Prevention and Control, Women and Children’s Hospital of Ningbo University, Ningbo, China
- 5Center for Reproductive Medicine, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 6Department of Obstetrics and Gynecology, Shanghai Medical School, Fudan University, Shanghai, China
Objective: To investigate the associations between triglyceride glucose-body mass index (TyG-BMI) and reproductive outcomes in women with polycystic ovary syndrome (PCOS) undergoing frozen embryo transfer (FET).
Methods: This retrospective cohort study included PCOS women undergoing their first in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) cycle followed by FET from January 2018 to January 2024 at a single reproductive medicine center. Patients were categorized into four groups according to the quartiles of TyG-BMI. Multivariable logistic regression, restricted cubic splines (RCS) and stratified analyses were used to evaluate the associations between TyG-BMI and reproductive outcomes. LASSO regression was performed to identify predictors for miscarriage and receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive power.
Results: A total of 744 women were included in the analysis. After adjusting for covariates, TyG-BMI showed a negative correlation with live birth rate and positive correlations with the risks of miscarriage and gestational diabetes mellitus (GDM) (all P trend < 0.05). RCS models demonstrated linear relationships of TyG-BMI with miscarriage rate, GDM risk and large for gestational age risk (P-overall < 0.05, P-nonlinear > 0.05). These associations remained consistent across all subgroups of the population (all P-interaction > 0.05). ROC analysis revealed that TyG-BMI was predictive of miscarriage (area under the curve (AUC) = 0.627, P < 0.001) with a cutoff value of 180.4. Combined with other identified risk factors, including basal luteinizing hormone, basal follicle stimulating hormone, total cholesterol, testosterone, infertility type and controlled ovarian stimulation protocols, the AUC value increased (AUC = 0.667, P < 0.001) and this model showed good miscarriage prediction performance in most subgroups (AUC > 0.650, P < 0.05), especially in patients with normal or low weight (BMI < 24 kg/m2, AUC = 0.743, P < 0.001).
Conclusion: Higher TyG-BMI levels are independently associated with an increased risk of adverse reproductive outcomes in women with PCOS undergoing FET. Additionally, TyG-BMI proves to be a cost-effective tool for the early identification of high-risk groups among PCOS patients, enabling personalized interventions prior to IVF to optimize reproductive outcomes in this population.
1 Introduction
Polycystic Ovary Syndrome (PCOS) is one of the most common endocrine disorders in reproductive-aged women, with a global prevalence of approximately 5-18% (depending on the population studied and the diagnostic criteria used) (1). It is characterized by ovulatory dysfunction, hyperandrogenism, and polycystic ovarian morphology, with a significant impact on both reproductive and metabolic health (2). Notably, PCOS accounts for up to 70% of anovulatory infertility cases requiring assisted reproductive technologies (ART), such as in vitro fertilization (IVF) (3). Beyond its effects on fertility, PCOS predisposes women to gestational complications, including hypertensive disorders and gestational diabetes mellitus (GDM), while also exerting long-term impacts on offspring health, such as low birth weight (LBW), large for gestational age (LGA), neonatal hypoglycemia, and congenital anomalies (4–8).
Though the exact pathophysiology of PCOS remains elusive, emerging evidence indicates that metabolic disorders, particularly insulin resistance (IR), obesity and dyslipidemia, are critical drivers of PCOS pathogenesis and progression (9). IR and compensatory hyperinsulinemia are prevalent in approximately 75% of PCOS patients (10). These conditions exacerbate hyperandrogenemia by stimulating the overproduction of androgen in ovarian theca cells and inhibiting the synthesis of hepatic sex hormone-binding globulin, perpetuating anovulation and hirsutism (11). Obesity, affecting about 50% of PCOS women across populations, exacerbating IR severity and functional ovarian hyperandrogenism, as well as induces ovarian inflammation and reduces oocyte quality (12, 13). Dyslipidemia, prevalent in up to 70% of cases, disrupts follicular steroidogenesis and oocyte maturation through lipotoxicity and oxidative stress, contributing to poor embryo quality and placental dysfunction (14).
Since these metabolic disturbances do not operate in isolation and instead interact synergistically to exacerbate PCOS-related reproductive dysfunction, integrated metabolic indicators are needed to provide a more comprehensive assessment of metabolic status. Individual metabolic parameters, such as homeostatic model assessment for IR (HOMA-IR), body mass index (BMI), and triglycerides, have been studied as potential predictors of IVF outcomes in women with PCOS (15–17). However, findings remain inconsistent and inconclusive, often failing to fully account for the interplay among metabolic factors. Recently, the triglyceride-glucose (TyG) index has gained increasing attention as a cost-effective and robust surrogate marker of IR (18). By combining TyG index with BMI, TyG-BMI not only improves its effectiveness in evaluating IR, but also reflects the obesity-related metabolic dysfunction (19).
Despite TyG-BMI’s acknowledged value in assessing metabolic risk, there have been few studies investigating the relationship between TyG-BMI and reproductive outcomes among women with PCOS undergoing IVF treatments, with most results focusing on fresh embryo transfer (ET) cycles and lack of data on obstetric and neonatal outcomes (20, 21). However, a freeze-all policy is the better choice for PCOS patients in order to reduce the risk of ovarian hyperstimulation syndrome (OHSS) (22). In addition, frozen embryo transfer (FET) provides a more physiological uterine environment than ET, avoiding the adverse effects of controlled ovarian hyperstimulation on embryo-endometrium synchronization (23, 24). To address these concerns, we conducted the present study in PCOS women who underwent their first FET cycles to investigate the effects of TyG-BMI on pregnancy, obstetric and neonatal outcomes.
2 Materials and methods
2.1 Study design and participants
This retrospective cohort study was conducted at the Center for Reproductive Medicine, Women and Children’s Hospital of Ningbo University, from January 2018 to January 2024. The study included women diagnosed with PCOS based on the Rotterdam Criteria (25), who underwent their first IVF or intracytoplasmic sperm injection (ICSI) cycle followed by FET. The diagnosis of PCOS required at least two of the following three criteria: oligomenorrhea or anovulation, clinical or biochemical hyperandrogenism, and polycystic ovaries on ultrasound; and other causes of hyperandrogenism and ovulatory dysfunction are ruled out. Exclusion criteria included: (1) women older than 40 years of age; (2) recurrent spontaneous abortion; (3) chromosome abnormality; (4) abnormal liver, renal or thyroid function; (5) uterine malformations; (6) endometriosis or adenomyosis; (7) severe male factor infertility; (8) missing important baseline or outcome data. The study was approved by the Ethics Committee of The Affiliated Women and Children’s Hospital of Ningbo University (Approval Number: EC2024-197) with a waiver for informed consent.
2.2 ART procedures
The controlled ovarian stimulation (COS) protocols were determined based on physician’s recommendation and patient’s preference. The gonadotropin-releasing hormone (GnRH) agonist protocol involved the administration of a short-acting GnRH agonist (Triptorelin acetate, Lummy, China; 0.1mg daily) starting at the luteal phase or a long-acting GnRH agonist (Beiyi®, Livzon, China; 3.75mg) on days 1–2 of the menstrual cycle. When pituitary down-regulation was achieved, ovarian stimulation was initiated by using daily injections of 100–300 IU urine-derived follicular stimulating hormone (FSH, Lishenbao®, Livzon, China) individualized based on age, BMI, ovarian reserve and prior response. Dose adjustments were guided by serial ultrasonography and serum hormone monitoring. Final oocyte maturation was triggered with human chorionic gonadotropin (hCG, Livzon, China; 4000–10000 IU) when ≥ 2 follicles reached mean diameter of 18 mm. The GnRH antagonist protocol consisted of daily ovarian stimulation with 100-150IU FSH from day 3–4 of the menstrual cycle. GnRH antagonist (Cetrotide®, Merck Serono, Switzerland; 0.25 mg daily) was initiated when leading follicles reached 11–12 mm diameter with serum estradiol (E2) approaching 1000 pg/mL. When 3 follicles reached ≥ 17 mm diameter or at least one follicle reached 18-20mm diameter, 2000 IU of hCG and 0.1mg of GnRH agonist (Decapeptyl®, Ferring, Germany) was administered for triggering. Retrieved oocytes were fertilized either by conventional IVF or ICSI or rescue ICSI based on semen analysis, the couple’s history and fertilization failure of IVF. Fertilization was confirmed by the presence of two pronuclei (2PN) 16–18 hours post-fertilization. Embryos were cultured in a standard medium and evaluated on day 3 (D3) as well as on day 5 (D5) and day 6 (D6), based on the number of cells, degree of fragmentation, and morphological characteristics. On Day 3 after oocyte retrieval, cleavage embryos with at least six cells and < 20% fragmentation were considered as high quality and eligible for transfer or cryopreservation. On day 5 or 6 after oocyte retrieval, blastocysts were graded by Gardner criteria (26). Blastocysts scored ≥ 3BB were considered as high-quality and scored ≥ 4BC were eligible for cryopreserved.
2.3 Frozen-thawed embryo transfer
Endometrial preparation was performed in either a hormone replacement cycle, a natural cycle or a stimulated cycle based on patient’s menstrual pattern and physicians’ discretion. When the endometrium reached a sufficient thickness, luteal support was added with vaginal progesterone gel (Crinone®, Merck Serono, Switzerland; 90 mg daily) and oral dydrogesterone (Duphaston®, Abbott, Netherlands; 10 mg, 3 times per day). Embryo transfer was performed under ultrasound guidance after 3 or 5 days of progesterone administration, with a maximum of two embryos transferred per cycle. Luteal support was maintained until 10 weeks of gestation if pregnancy was confirmed and can be extended if vaginal bleeding is present.
2.4 Exposure definitions
Blood samples were collected in the morning after overnight fasting for at least 8 hours. Fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were measured using standard laboratory methods. The basal hormone blood samples were collected during the early follicular phase (cycle days 2–4). Other baseline characteristics, including age, BMI, duration of infertility, blood pressure and ovarian reserve markers (anti-müllerian hormone, AMH; antral follicle account, AFC), were also recorded before commencing IVF treatment. The TyG-BMI was calculated as follows: TyG-BMI = natural logarithm [TG (mg/dL) × FBG (mg/dL)/2] × BMI (kg/m2); BMI = weight (kg)/[height (m)]2 (27).
2.5 Outcome definitions
Biochemical pregnancy was defined as a serum β-hCG level exceeding 25 IU/L measured 14 days after embryo transfer. Clinical pregnancy was confirmed by transvaginal ultrasound visualization of at least one intrauterine gestational sac at 4–5 weeks of gestation. Miscarriage is defined as a pregnancy loss before 24 weeks of gestation. Live birth was recorded as the delivery of a viable infant at ≥ 24 weeks of gestation. Obstetric complications included GDM (10th revision of the International Statistical Classification of Diseases and Related Health Problems [ICD-10] code O24.4), hypertensive disorders of pregnancy (HDP, including pregnancy induced hypertension [ICD-10 code O13] and pre-eclampsia [ICD-10 codes O14-15]) and placental disorders (including placenta previa [ICD-10 code O44], placental abruption [ICD-10 code O45] and placenta accreta [ICD-10 code O43.2]). Neonatal outcomes encompassed gestational age, preterm birth (PTB), birth weight, LBW, macrosomia, small for gestational age (SGA), LGA and birth defects (ICD-10 codes Q00-Q99). PTB was defined as live birth before 37 gestational weeks. Low birth weight was defined as birth weight < 2500g. Macrosomia was defined as birth weight ≥ 4000g. SGA and LGA were respectively defined as birth weight < 10th percentile and > 90th percentile of gender-specific birthweight reference at the same gestational age (28).
2.6 Statistical analysis
Continuous data were expressed as median and interquartile range (25th-75th), whereas categorical data were expressed as frequencies (n) and percentages (%). Differences between groups were analyzed using Kruskal Wallis H test for continuous variables, and chi-square test or Fisher’s exact test for categorical variables. A trend test across TyG-BMI quartiles was performed by modeling quartiles as an ordinal variable (Q1–Q4) in regression analyses. Logistic regression models were employed to investigate the potential association between TyG-BMI and various outcomes. The adjustment models were as follows: (1) no variable was adjusted for in model 1; (2) age and duration of infertility were adjusted for in model 2; and (3) age, duration of infertility, AMH, systolic blood pressure (SBP), COS protocols, total gonadotropin (Gn) dose, duration of Gn, TC, the number of transferred embryos, the developmental stage of transferred embryos and endometrium thickness were adjusted for in model 3. Odds ratios (ORs) were calculated with 95% confidence intervals (CIs). Restricted cubic spline (RCS) models were employed to explore non-linear relationships and dose-response relationships between TyG-BMI and various outcomes. Stratified analysis was performed to assess the impact of female age (≤ 30 and > 30 years), BMI (< 24, 24~28, and ≥ 28 kg/m2) and AMH levels (low and high) on these relationships. Furthermore, the least absolute shrinkage and selection operator (LASSO) regression model was used to identify potential risk variables for miscarriage and receiver operating characteristic (ROC) curve analysis was conducted to evaluate the predictive power. All statistical analyses were performed using R software (version 4.4.1). All P values were two-sided, and P < 0.05 was considered statistically significant.
3 Results
3.1 Demographic and cycle characteristics
A total of 744 PCOS patients were included for analysis. The participants were categorized into four groups according to TyG-BMI quartiles (Quartile 1 (Q1): < 172.6, Quartile 2 (Q2): 172.6-197.5, Quartile 3 (Q3): 197.5-225.0, Quartile 4 (Q4): ≥ 225.0). The baseline characteristics of these groups are presented in Table 1. Compared with women in the lower TyG-BMI quartiles, women in the higher quartiles tend to be older, have higher BMI, SBP, diastolic blood pressure (DBP), TG, TC, LDL and FBG, longer infertility duration, but lower AMH, basal FSH, basal luteinizing hormone (LH) and HDL (all P trend < 0.05). The level of basal E2 was also shown a significant difference across the groups (P < 0.05).
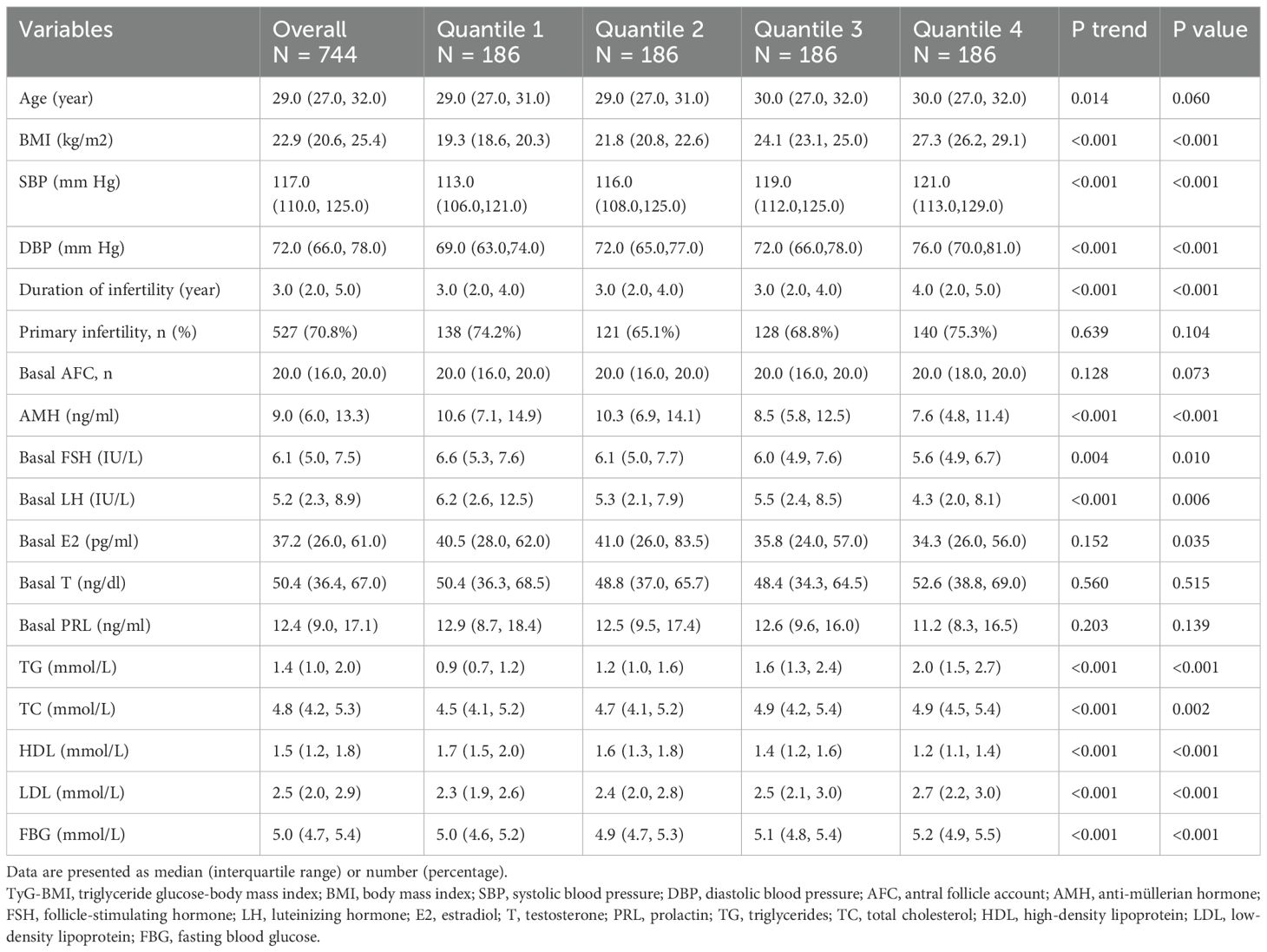
Table 1. Baseline characteristics of the participants according to TyG-BMI quartiles in polycystic ovary syndrome.
The COS protocols and laboratory outcomes were shown in Table 2. A significantly higher proportion of GnRH agonist cycles was observed in the Q2-Q4 groups compared to the Q1 group (P trend < 0.05). The starting Gn dose, total Gn dose and duration of Gn increased significantly across TyG-BMI quartiles (all P trend < 0.05). Notably, patients in higher quantiles tended to retrieve fewer oocytes, with the median count decreasing from 16.0 in Q1 to 14.0 in Q4 (P trend = 0.008). Likewise, there was a significant decline in the quantity of metaphase II (MII) oocytes, 2PN zygotes and cleavage embryos from Q1 to Q4 group (all P trend < 0.05). However, no differences were observed in the quantity of high-quality embryos or blastocyst formation rates (all P > 0.05). Fertilization methods, endometrial thickness on the day of embryo transfer, embryo developmental stage at transfer and the number of embryos transferred did not differ significantly among the groups (all P > 0.05).
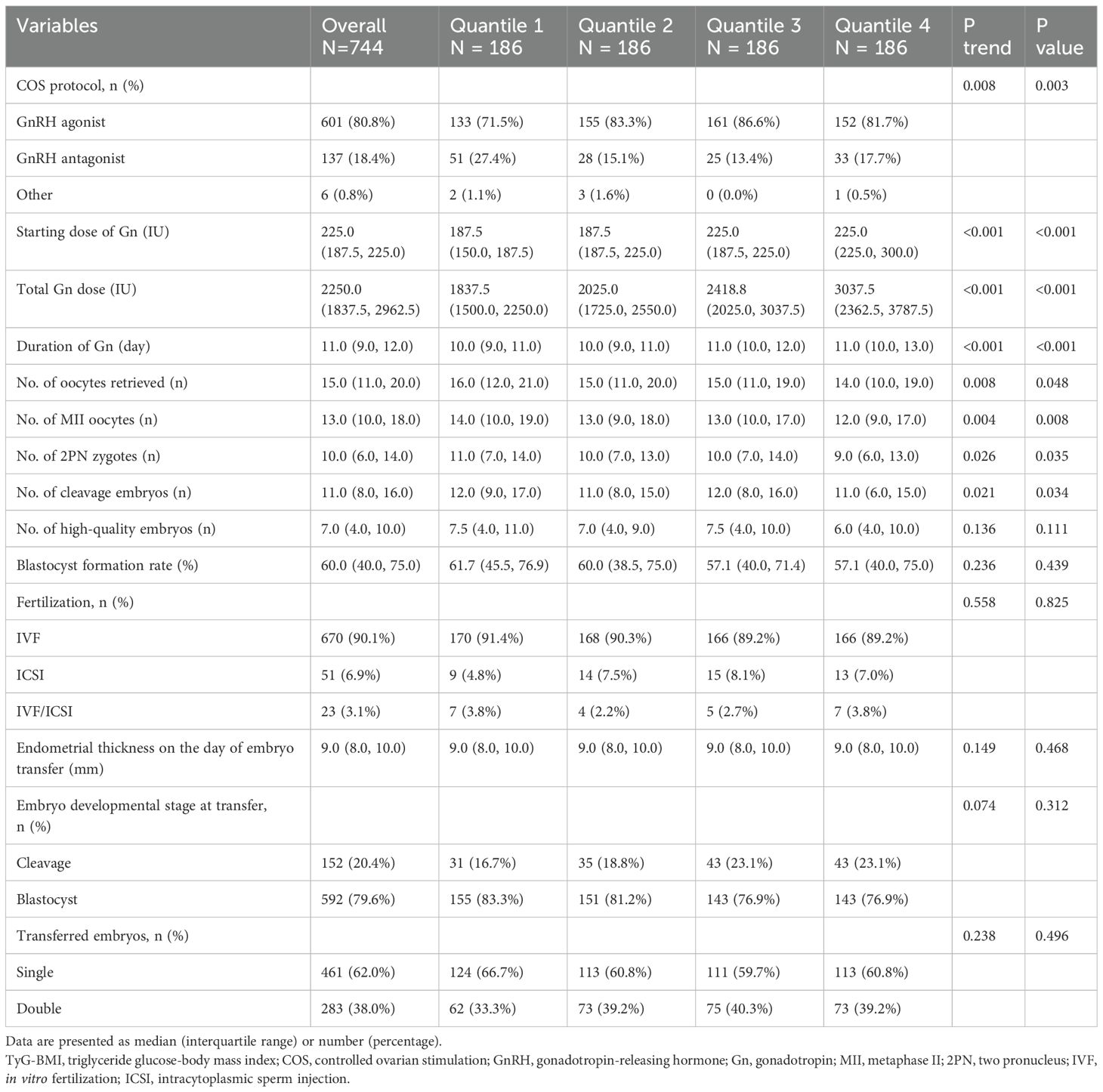
Table 2. Ovarian stimulation, in vitro fertilization, and related outcomes according to TyG-BMI quartiles in polycystic ovary syndrome.
3.2 Pregnancy outcomes
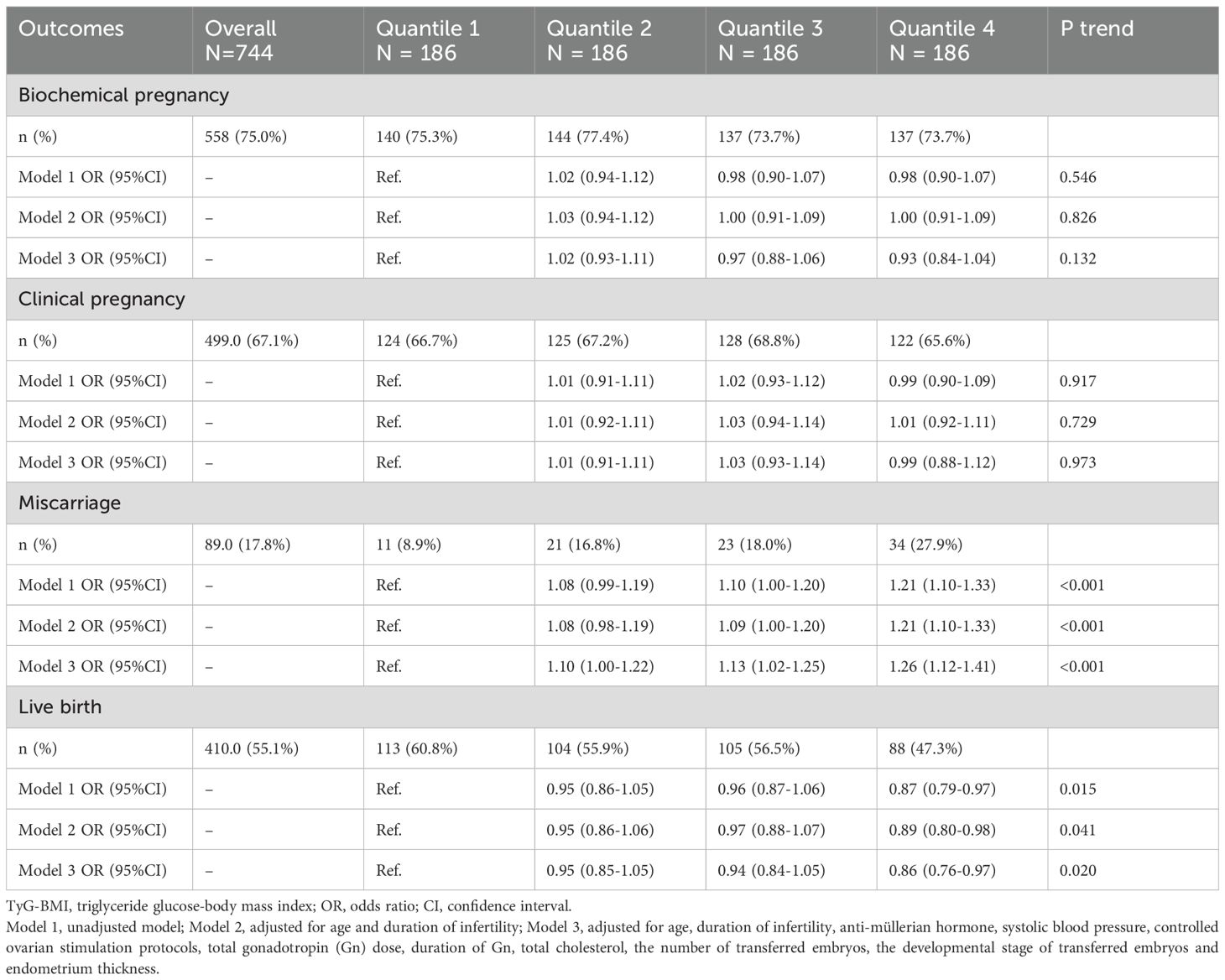
A total of 410 (55.1%) women achieved live births following their FET cycles. The pregnancy outcomes and their associations with TyG-BMI levels assessed by univariate and multivariate analyses were summarized in Table 3. Notably, there was a significant increasing trend in miscarriage rate from Q1 (8.9%) to Q4 (27.9%), and the logistic regression analyses showed stable positive correlations between TyG-BMI and miscarriage rate in all three models (all P trend < 0.001). Compared with Q1, women in Q3 (OR, 1.13; 95% CI, 1.02–1.25) and Q4 (OR, 1.26; 95% CI, 1.12–1.41) showed a significantly higher risk of miscarriage after full adjustment for covariates. The live birth rate decreased significantly from Q1 (60.8%) to Q4 (47.3%) and was negatively correlated with TyG-BMI in all three models (all P trend < 0.05). Compared with Q1, women in Q4 had a significantly lower probability of live birth after fully adjusted (OR, 0.86; 95% CI, 0.76-0.97). However, no statistically significant associations were observed between TyG-BMI and the rates of biochemical pregnancy and clinical pregnancy following the first FET in all three regression models (all P trend > 0.05).
3.3 Obstetric and neonatal outcomes
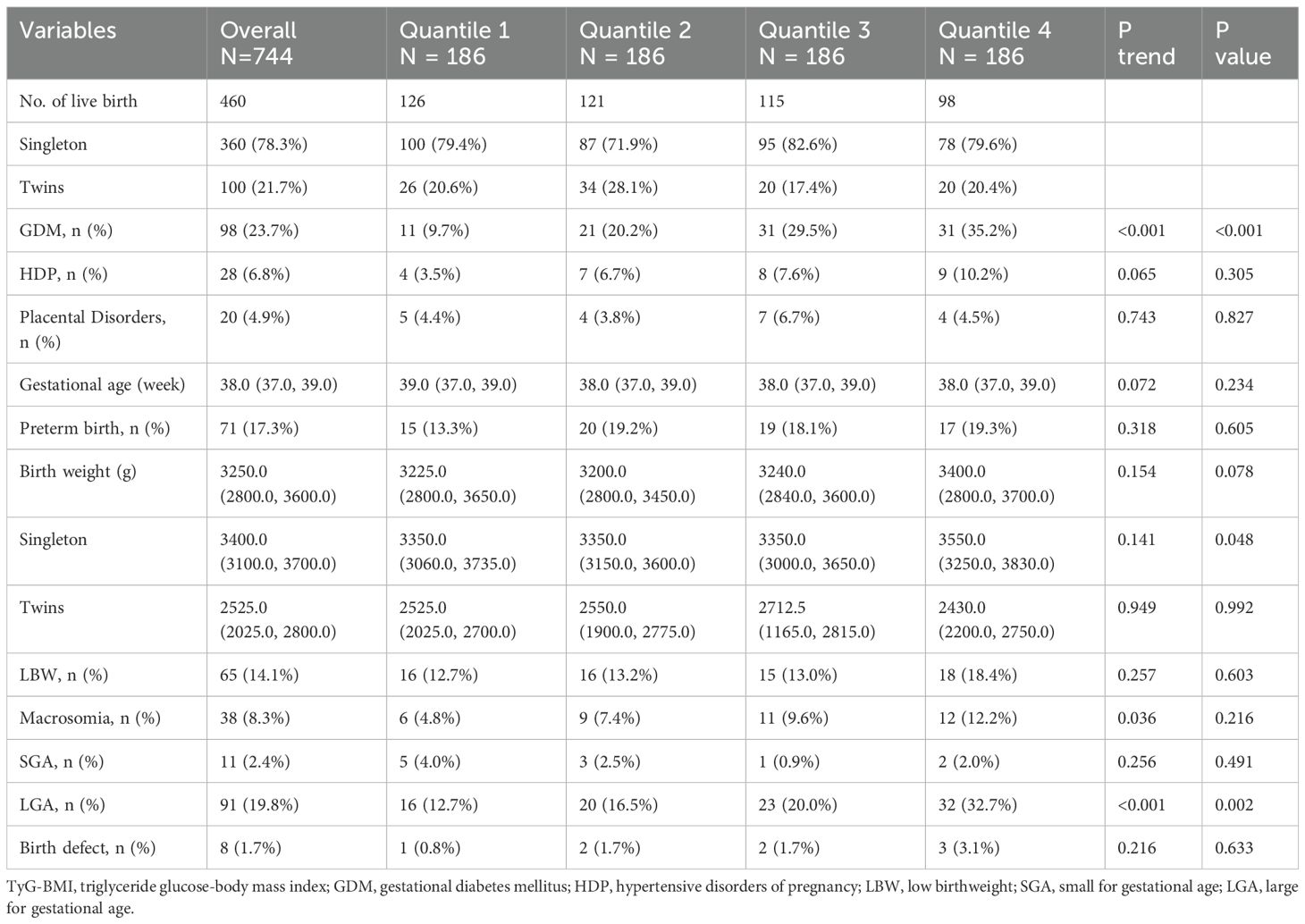
There were 360 singletons (78.3%) and 100 twins (21.7%) born during the study period. The obstetric complications and neonatal outcomes stratified by TyG-BMI quartiles are presented in Table 4. The incidence of GDM increased from 9.7% in Q1 to 35.2% in Q4 (P trend < 0.001), with parallel increases observed in macrosomia rates (Q1: 4.8% vs Q4: 12.2%, P trend = 0.036) and LGA rates (Q1: 12.7% vs Q4: 32.7%, P trend < 0.001). Singleton birth weights in Q4 exceeded those in Q1–Q3 (P = 0.048), whereas twin birth weights remained comparable across quartiles (P > 0.05). Low birth weight incidence demonstrated a nonsignificant upward trend from Q1 (12.7%) to Q4 (18.4%) (P trend = 0.257). No statistical differences were noticed amongst TyG-BMI quartiles in HDP, placental disorders, gestational age, PTB, SGA and birth defect (all P trend > 0.05).
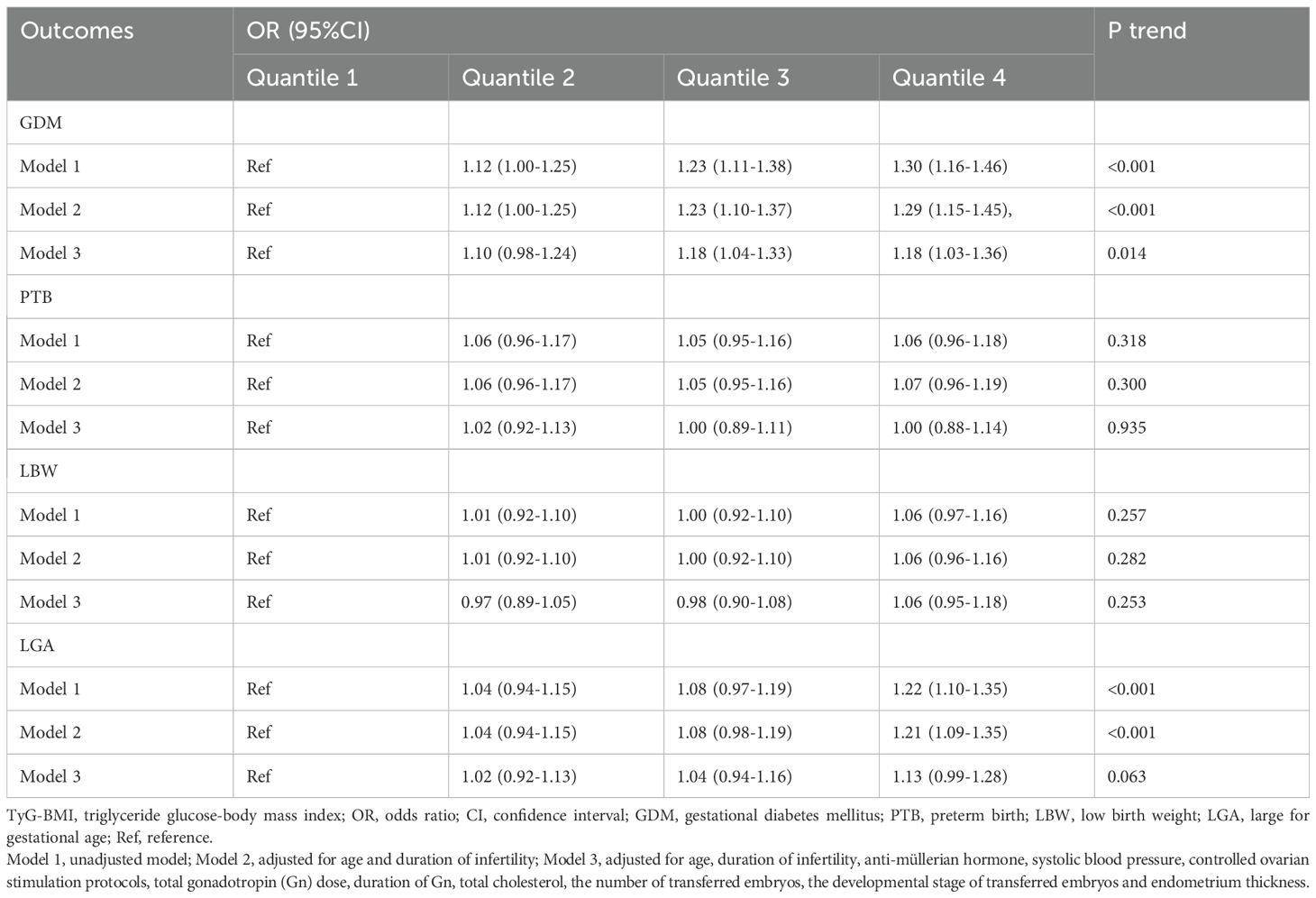
Table 5 presents the associations between TyG-BMI quartiles and perinatal outcomes using regression analyses with or without adjustments. The incidence of GDM was positively associated with TyG-BMI quartiles in all three models (all P trend < 0.05). In the fully adjusted model, women in Q3 (OR 1.18, 95%CI 1.04-1.33) and Q4 (OR 1.18, 95%CI 1.03-1.36) showed a 18% elevated risk of GDM compared with those in Q1. Notably, the risk of LGA showed a positive correlation with TyG-BMI after adjusting for maternal age and duration of infertility (P trend < 0.001), with a 21% increased risk of LGA in Q4 compared with that in Q1 (OR 1.21, 95%CI 1.09-1.35). However, with further adjustment for SBP, AMH, TC, COS protocols, total Gn dose, duration of Gn, the number of embryos transferred, the developmental stage of transferred embryos and endometrial thickness in model 3, no significant correlation was found between the incidence of LGA and TyG-BMI (P trend > 0.05). In addition, the incidences of PTB and LBW remained consistently nonsignificant across all models (all P trend > 0.05).
3.4 The analyses of non-linear relationship
Considering the continuous nature of TyG-BMI, we employed RCS models to analyze its non-linear relationships with various outcomes in our study (Figure 1). After full adjustment for covariates in the master analytical model 3 above, significant linear correlations were observed between TyG-BMI and miscarriage rate, GDM incidence and LGA incidence (P for nonlinear > 0.05, P for overall < 0.05). However, no significant association was found between TyG-BMI and live birth rate (P for nonlinear > 0.05, P for overall > 0.05).
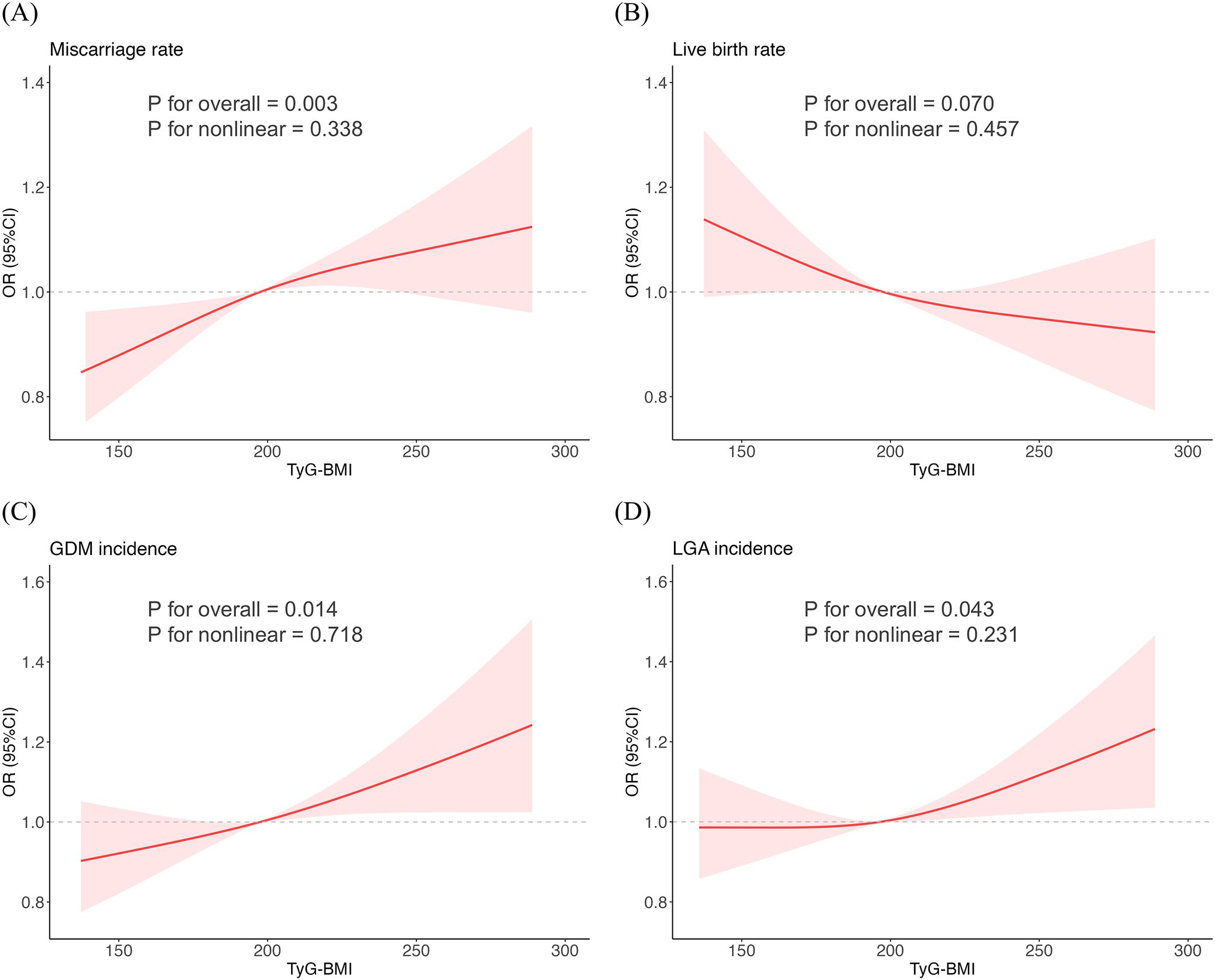
Figure 1. Restricted cubic spline fitting for the association between TyG-BMI and reproductive outcomes. (A) Miscarriage rate. (B) Live birth rate. (C) GDM incidence. (D) LGA incidence.
3.5 Stratified analyses
Stratified analyses were conducted to evaluate the impact of potential confounding factors on the relationship between TyG-BMI and reproductive outcomes. As shown in Supplementary Figure S1, no interaction was found between TyG-BMI and stratification factors on the associations with miscarriage rate, live birth rate, the risk of GDM and the risk of LGA (all P for interaction >0.05).
3.6 ROC analyses for predicting miscarriage
Using LASSO regression, seven variables were identified as independent predictors of miscarriage: TyG-BMI, basal LH, basal FSH, TC, T, infertility type (primary vs. secondary) and COS protocols. The coefficient track diagram and the ten-fold cross-validation error curve were provided in Supplementary Figure S2. ROC curve analyses demonstrated that TyG-BMI alone was predictive of miscarriage (area under the curve (AUC) = 0.627, P < 0.001) with a cutoff value of 180.4. The full model integrating TyG-BMI with baseline characteristics (LH, FSH, TC, T, infertility type and COS protocols) achieved higher discriminative power than other two models (AUC = 0.667, P < 0.001) (Figure 2A, Supplementary Table S1). And the full model showed good miscarriage prediction performance in most subgroups (AUC > 0.650), particularly in patients with normal/low weight (BMI < 24 kg/m2, AUC = 0.743, P < 0.001) or young age (Age ≤ 30, AUC = 0.702, P < 0.001) (Figures 2B–D, Supplementary Table S2).
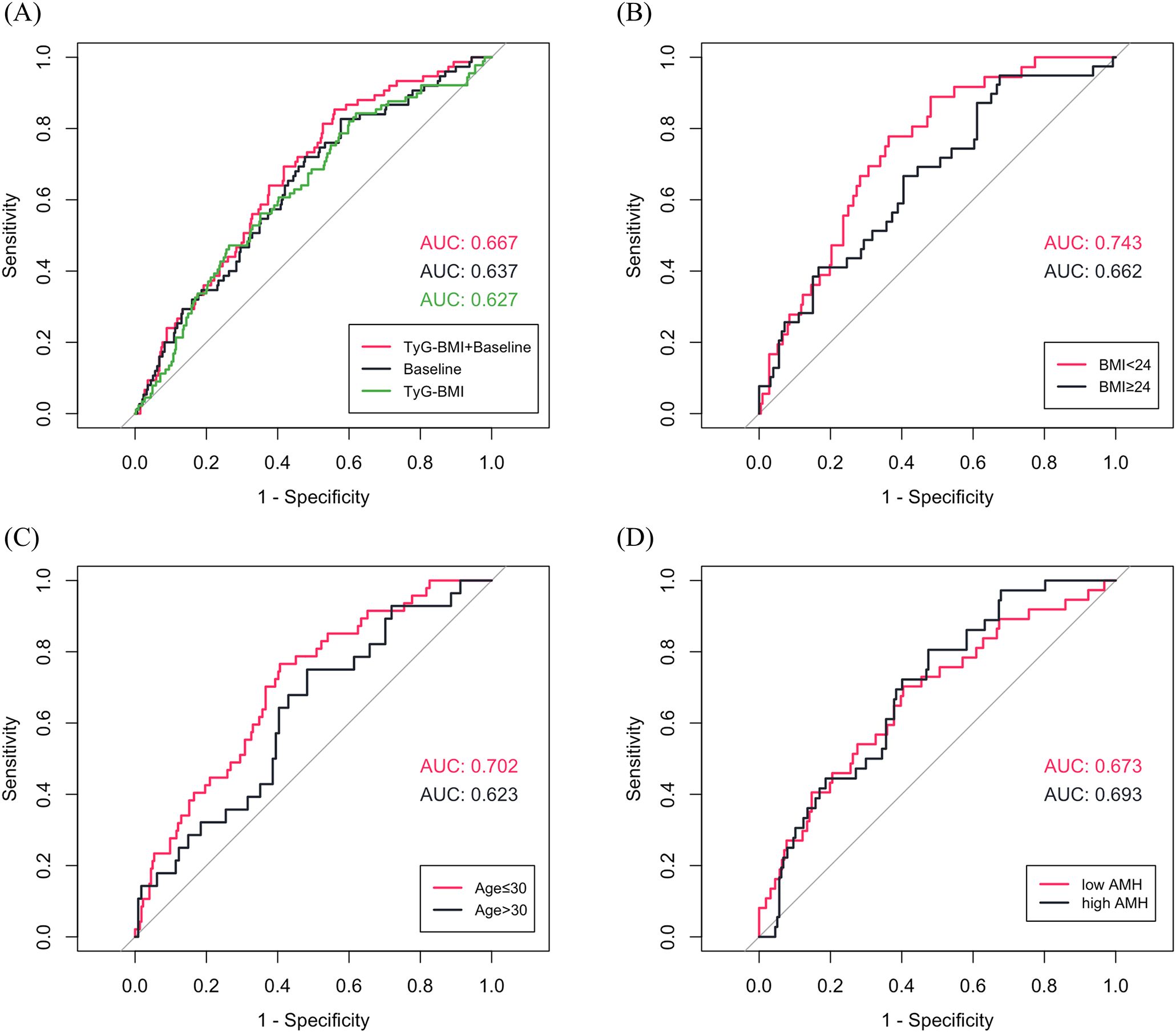
Figure 2. (A) ROC curves for predicting miscarriage in total patients (Baseline characteristics: LH, FSH, total cholesterol, testosterone, infertility type and controlled ovarian stimulation protocols). (B-D) ROC curves of TyG-BMI combined with baseline characteristics for predicting miscarriage in subgroups.
4 Discussion
This study is the first to systematically explore the impact of TyG-BMI on pregnancy outcomes, obstetric complications and neonatal outcomes following the first FET in patients with PCOS. The results showed that an elevated TyG-BMI was independently associated with an increased risk of miscarriage, a decreased probability of live birth, and an increased risk of GDM and LGA. These findings suggest that TyG-BMI is not only a predictor of pregnancy outcomes in patients with PCOS, but also a potential early warning marker for long-term maternal and infant health.
IR is prevalent in women with PCOS, which is strongly associated with metabolic and reproductive complications (11). In contrast to the time-consuming and complex hyperinsulin-normoglycemic clamp technique, which is considered the gold standard for diagnosing IR, HOMA-IR serves as an alternative biomarker of IR (29). However, fasting insulin levels are not routinely measured in clinical practice, which may miss the optimal time to intervene in various metabolic risk factors. In contrast, the TyG-BMI, with its multiple advantages of low cost, easy accessibility, independence from insulin therapy, and more comprehensive assessment of metabolic health, not only correlates strongly with HOMA-IR, but also better predicts metabolic diseases such as diabetes and cardiovascular disease (27, 30, 31). It has been shown that the TyG-BMI is a clinically convenient and practical marker for the early identification of IR and metabolic syndrome in patients with PCOS (32, 33). However, its correlation with the IVF outcomes in women with PCOS has not been fully investigated. Li et al. (20) reported that higher TyG-BMI was associated with fewer available embryos and reduced high-quality embryos, but not significantly correlated with the pregnancy outcomes of fresh ET. However, due to the higher risk of OHSS in patients with PCOS, the strategy of whole embryo freezing and subsequent FET would be more commonly used in clinical practice, resulting in a limited number of fresh ET cycles in this study (only 49 fresh ET cycles out of 966 IVF cycles), which limits the reliability of the results. Subsequently, a study by Wu et al. (21) comparing the correlation between different surrogate indicators of IR and IVF outcomes in patients with PCOS, which included a total of 358 fresh ET cycles and 185 FET cycles, showed that TyG-BMI, TyG, and HOMA-IR were negatively correlated with the live birth rate in the fresh ET cycles, and that the predictive efficacy of TyG-BMI was superior than TyG and HOMA-IR in terms of the live birth rate. However, this relationship did not exist in FET cycles. In contrast, our study, which included PCOS patients with freeze-all strategy, further controlled for confounders and expanded the sample size (a total of 744 FET cycles), found that an elevated TyG-BMI was independently associated with an increased miscarriage rate and a decreased live birth rate after the first FET in PCOS patients. Compared with the lowest TyG-BMI quartile (TyG-BMI < 172.6), the highest quartile (TyG-BMI ≥ 225.0) showed an 19% higher rate of miscarriage and a 13.5% lower rate of live birth. This trend persisted after adjustment for confounding factors.
Miscarriage is the most common adverse pregnancy outcome, affecting 15% of recognized pregnancies (34). It not only leads to both physical (e.g., hemorrhage, infection) and psychological (e.g., anxiety, depression and elevated suicide risk) detriments, but may also serve as a sentinel risk marker for obstetric complications (e.g., preterm birth, placental abruption) (35–37). PCOS has long been suggested to have an independent association with miscarriage, with some research reporting rates as high as 20%–45% for spontaneous miscarriage (38). Therefore, it is critical to identify potential risk factors and develop a low-cost and efficient prediction model based on routine clinical parameters to optimize pregnancy management for patients with PCOS. In our study, we identified for the first time that TyG-BMI was a potential predictor of miscarriage in FET among patients with PCOS, with a cutoff value of 180.4 (AUC = 0.627, P < 0.001). In addition, the potential risk factors included the serum levels of basal LH, basal FSH, TC and T, infertility type and COS protocols, which were consistent with previous related studies (39–44). The baseline model incorporating risk factors other than TyG-BMI achieved an AUC of 0.637. After adding TyG-BMI to the model, the AUC increased to 0.667 and this full model had a good predictive performance in most subgroups (AUC > 0.650, P < 0.05). Notably, the full model had the highest AUC value for predicting miscarriage in the normal or low weight population (BMI < 24 kg/m2, AUC = 0.743, P < 0.001), where metabolic dysregulation is often overlooked. It also indicates heterogeneous etiologies of miscarriage across BMI strata (AUC = 0.662 in the population with a BMI ≥ 24 kg/m2). For obese PCOS patients, miscarriage risk may be more multifactorial, involving mechanisms that are not fully captured by the current model variables, such as chronic low-grade inflammation (45). Future studies should explore additional biomarkers to refine the predictive model.
Maternal metabolic disorders not only impair oocyte quality by affecting the ovarian microenvironment, but may also affect endometrial and placental function, thereby increasing the risk of gestational complications and adverse birth outcomes (46–48). Emerging evidence indicates that early-pregnancy TyG-BMI is associated with the risk of gestational complications and adverse birth outcomes. Meng et al. (49) studied 1136 singleton pregnancies and found that the first-trimester TyG-BMI was strongly associated with the risk of HDP, GDM, and LGA. The study also analyzed TyG-BMI trajectories throughout pregnancy and found that all three observed TyG-BMI trajectories increased over time, suggesting the need for early assessment of TyG-BMI. However, the impact of pre-pregnancy TyG-BMI on maternal and neonatal health has not been studied. Moreover, ART-related techniques (including ovarian stimulation, in vitro embryo culture, cryopreservation, endometrial preparation protocols, and embryo transfer) and PCOS are risk factors for adverse perinatal outcomes, highlighting the need for early screening and intervention in women with PCOS undergoing IVF to improve maternal and neonatal health (50–53). Therefore, our study represents the first analysis for the association of pre-pregnancy TyG-BMI with obstetric and neonatal outcomes after FET in patients with PCOS, and the results showed that the highest TyG-BMI quartile group had a 25.5% higher incidence of GDM, a 6.7% higher incidence of HDP, and a 20% higher incidence of LGA compared to the lowest quartile group. After adjusting for confounders, TyG-BMI was positively associated with the risk of GDM and LGA, but not significantly associated with HDP, which may be related to its low incidence in our study.
To the best of our knowledge, the present study has several strengths. First, based on a large sample size, this is the first study that revealed the independent predictive value of TyG-BMI in IVF-FET outcomes in women with PCOS, suggesting that PCOS patients with elevated TyG-BMI may have a higher risk of miscarriage and a lower probability of live birth, and that metabolic optimization (e.g., metformin treatment, lifestyle interventions) should be recommended prior to IVF to improve the pregnancy outcomes. Second, we included obstetric and neonatal outcomes in the analysis and revealed for the first time the correlation between pre-conception TyG-BMI and the risk of GDM and LGA after FET in PCOS patients, suggesting that metabolic health tracking during pregnancy and maternal and infant health follow-up should be strengthened in PCOS patients with higher TyG-BMI before IVF, which provided a more comprehensive clinical perspective for the study. In addition, we applied multivariate logistic regression models to fully adjust for confounders, profiled the nonlinear relationships, and performed stratified interaction analysis to enhance the reliability of the results. Inevitably, this study has some limitations. First, due to the single-center retrospective design, selection bias should not be ignored, although we have reduced the effects of confounding factors by strict inclusion and exclusion criteria. Second, residual confounding from unmeasured factors (e.g., diet, physical activity) may have affected the results to some extent. In addition, although the FET strategy excludes the potential effects of ovarian stimulation on the endometrium, the TyG-BMI was measured before COS and fluctuations before FET were not tracked. It might have led to overlook of potential variations in TyG-BMI values before FET due to metabolic interventions such as medication and weight loss. Therefore, our findings should be interpreted with caution, and prospective cohort studies—particularly those evaluating metabolic interventions guided by TyG-BMI—are needed to confirm our results and optimize clinical outcomes in the future.
5 Conclusion
In conclusion, this study establishes the TyG-BMI as a robust predictor of adverse pregnancy outcomes in PCOS women undergoing their first FET. Elevated TyG-BMI demonstrated independent associations with increased miscarriage risk, reduced live birth rate, higher GDM incidence and LGA incidence. These findings highlight the critical role of pre-conception metabolic management in achieving reproductive success and safeguarding long-term maternal and offspring health. By incorporating TyG-BMI into routine clinical screening, physicians can identify high-risk PCOS patients who may benefit from targeted metabolic optimization prior to IVF, such as lifestyle interventions or pharmacologic therapies, to reduce the risk of adverse outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of The Affiliated Women and Children’s Hospital of Ningbo University (Approval Number: EC2024-197). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZD: Data curation, Methodology, Conceptualization, Investigation, Writing – review & editing, Software, Visualization, Writing – original draft, Formal Analysis. KaL: Writing – review & editing, Investigation, Data curation. KuL: Data curation, Writing – review & editing, Investigation. SZ: Methodology, Writing – review & editing. ZP: Methodology, Writing – review & editing. FZ: Supervision, Validation, Writing – review & editing, Funding acquisition. LZ: Resources, Funding acquisition, Writing – review & editing, Validation, Supervision. CX: Supervision, Project administration, Writing – review & editing, Funding acquisition, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Ningbo Clinical Medical Research Center (2024L002), Ningbo Top Medical and Health Research Program (No.2024021020), Shanghai Clinical Research Center for Gynecological Diseases (22MC1940200), the National Key Research & Developmental Program of China (2022YFC2703800), and grants from the Shanghai Natural Science Foundation Project (22ZR1409100).
Acknowledgments
We gratefully acknowledge all the staff of the center for reproductive medicine in Women and Children’s Hospital of Ningbo university for their support and cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1629837/full#supplementary-material
Glossary
PCOS: polycystic ovary syndrome
ART: assisted reproductive technologies
IVF: in vitro fertilization
GDM: gestational diabetes mellitus
LBW: low birthweight
LGA: large for gestational age
IR: insulin resistance
HOMA-IR: homeostatic model assessment for IR
BMI: body mass index
TyG: triglyceride glucose
ET: embryo transfer
OHSS: ovarian hyperstimulation syndrome
FET: frozen embryo transfer
ICSI: intracytoplasmic sperm injection
COS: controlled ovarian stimulation
GnRH: gonadotropin-releasing hormone
2PN: two pronuclei
FBG: fasting blood glucose
TC: total cholesterol
TG: triglycerides
LDL: low-density lipoprotein
HDL: high-density lipoprotein
AMH: anti-müllerian hormone
AFC: antral follicle account
HDP: hypertensive disorders of pregnancy
PTB: preterm birth
SGA: small for gestational age
SBP: systolic blood pressure
Gn: gonadotropin
OR: odds ratio
CI: confidence interval
RCS: restricted cubic spline
LASSO: least absolute shrinkage and selection operator
ROC: receiver operating characteristic
DBP: diastolic blood pressure
FSH: follicle-stimulating hormone
LH: luteinizing hormone
E2: estradiol
T: testosterone
PRL: prolactin
GnRH: gonadotropin-releasing hormone
AUC: area under the curve.
References
1. Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. (2022) 10:668–80. doi: 10.1016/S2213-8587(22)00163-2
2. McCartney CR and Marshall JC. Polycystic ovary syndrome. N Engl J Med. (2016) 375:54–64. doi: 10.1056/NEJMcp1514916
3. Carson SA and Kallen AN. Diagnosis and management of infertility: a review. JAMA. (2021) 326:65. doi: 10.1001/jama.2021.4788
4. Bahri Khomami M, Shorakae S, Hashemi S, Harrison CL, Piltonen TT, Romualdi D, et al. Systematic review and meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Nat Commun. (2024) 15:5591. doi: 10.1038/s41467-024-49749-1
5. Ban M, Sun Y, Chen X, Zhou X, Zhang Y, and Cui L. Association between maternal polycystic ovarian syndrome undergoing assisted reproductive technology and pregnancy complications and neonatal outcomes: a systematic review and meta-analysis. J Ovarian Res. (2024) 17:6. doi: 10.1186/s13048-023-01331-x
6. Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Arora C, Silagy M, et al. The role of maternal obesity in infant outcomes in polycystic ovary syndrome-A systematic review, meta-analysis, and meta-regression. Obes Rev. (2019) 20:842–58. doi: 10.1111/obr.12832
7. Foroozanfard F, Moosavi SGA, Mansouri F, and Bazarganipour F. Obstetric and neonatal outcome in PCOS with gestational diabetes mellitus. J Family Reprod Health. (2014) 8:7–12.
8. Talmo MSA, Fløysand IS, Nilsen GØ, Løvvik TS, Ødegård R, Juliusson PB, et al. Growth restriction in the offspring of mothers with polycystic ovary syndrome. JAMA Netw Open. (2024) 7:e2430543. doi: 10.1001/jamanetworkopen.2024.30543
9. Liao B, Qiao J, and Pang Y. Central regulation of PCOS: abnormal neuronal-reproductive-metabolic circuits in PCOS pathophysiology. Front Endocrinol (Lausanne). (2021) 12:667422. doi: 10.3389/fendo.2021.667422
10. Tong C, Wu Y, Zhang L, and Yu Y. Insulin resistance, autophagy and apoptosis in patients with polycystic ovary syndrome: Association with PI3K signaling pathway. Front Endocrinol. (2022) 13:1091147. doi: 10.3389/fendo.2022.1091147
11. Zhao H, Zhang J, Cheng X, Nie X, and He B. Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment. J Ovarian Res. (2023) 16:9. doi: 10.1186/s13048-022-01091-0
12. Glueck CJ and Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metab Clin Exp. (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002
13. Snider AP and Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. (2019) 158:R79–90. doi: 10.1530/REP-18-0583
14. Liu Q, Xie Y, Qu L, Zhang M, and Mo Z. Dyslipidemia involvement in the development of polycystic ovary syndrome. Taiwanese J Obstetr Gynecol. (2019) 58:447–53. doi: 10.1016/j.tjog.2019.05.003
15. Song H, Yu Z, Li P, Wang Y, and Shi Y. HOMA-IR for predicting clinical pregnancy rate during IVF. Gynecol Endocrinol. (2022) 38:33–8. doi: 10.1080/09513590.2021.1952976
16. Alenezi SA, Khan R, and Amer S. The impact of high BMI on pregnancy outcomes and complications in women with PCOS undergoing IVF-A systematic review and meta-analysis. J Clin Med. (2024) 13:1578. doi: 10.3390/jcm13061578
17. Jamro EL, Bloom MS, Browne RW, Kim K, Greenwood EA, and Fujimoto VY. Preconception serum lipids and lipophilic micronutrient levels are associated with live birth rates after IVF. Reprod BioMed Online. (2019) 39:665–73. doi: 10.1016/j.rbmo.2019.06.004
18. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
19. Lim J, Kim J, Koo SH, and Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PloS One. (2019) 14:e0212963. doi: 10.1371/journal.pone.0212963
20. Li X, Luan T, Wei Y, Zhang J, Zhao C, and Ling X. The association between triglyceride glucose-body mass index and in vitro fertilization outcomes in women with polycystic ovary syndrome: A cohort study. J Ovarian Res. (2024) 17:90. doi: 10.1186/s13048-024-01416-1
21. Wu S, Wu Y, Fang L, and Lu X. Association of insulin resistance surrogates with live birth outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. BMC Pregnancy Childbirth. (2025) 25:25. doi: 10.1186/s12884-024-07131-5
22. Chen Z, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. (2016) 375:523–33. doi: 10.1056/NEJMoa1513873
23. Devroey P, Bourgain C, Macklon NS, and Fauser BCJM. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. (2004) 15:84–90. doi: 10.1016/j.tem.2004.01.009
24. Järvelä IY, Pelkonen S, Uimari O, Mäkikallio K, Puukka K, Ruokonen A, et al. Controlled ovarian hyperstimulation leads to high progesterone and estradiol levels during early pregnancy. Hum Reprod. (2014) 29:2393–401. doi: 10.1093/humrep/deu223
25. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
26. Gardner DK, Lane M, Stevens J, Schlenker T, and Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. (2000) 73:1155–8. doi: 10.1016/S0015-0282(00)00518-5
27. Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals. PloS One. (2016) 11:e0149731. doi: 10.1371/journal.pone.0149731
28. Villar J, Ismail LC, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. (2014) 384:857–68. doi: 10.1016/S0140-6736(14)60932-6
29. Gastaldelli A. Measuring and estimating insulin resistance in clinical and research settings. Obes (Silver Spring). (2022) 30:1549–63. doi: 10.1002/oby.23503
30. Wang H, He S, Wang J, Qian X, Zhang B, Yang Z, et al. Assessing and predicting type 2 diabetes risk with triglyceride glucose-body mass index in the Chinese nondiabetic population—Data from long-term follow-up of Da Qing IGT and Diabetes Study. J Diabetes. (2024) 16:e70001. doi: 10.1111/1753-0407.70001
31. Cheng W, Kong F, and Chen S. Comparison of the predictive value of four insulin resistance surrogates for the prevalence of hypertension: a population-based study. Diabetol Metab Syndr. (2022) 14:137. doi: 10.1186/s13098-022-00907-9
32. Zheng Y, Yin G, Chen F, Lin L, and Chen Y. Evaluation of triglyceride glucose index and homeostasis model of insulin resistance in patients with polycystic ovary syndrome. Int J Womens Health. (2022) 14:1821–9. doi: 10.2147/IJWH.S387942
33. Zhang L, Wang H, Ma Q, Liu Y, Chen A, Lu J, et al. Value of the triglyceride-glucose index and non-traditional blood lipid parameters in predicting metabolic syndrome in women with polycystic ovary syndrome. Hormones (Athens). (2023) 22:263–71. doi: 10.1007/s42000-023-00438-6
34. Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, et al. Miscarriage matters: The epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. (2021) 397:1658–67. doi: 10.1016/S0140-6736(21)00682-6
35. Farren J, Jalmbrant M, Falconieri N, Mitchell-Jones N, Bobdiwala S, Al-Memar M, et al. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: A multicenter, prospective, cohort study. Am J Obstetr Gynecol. (2020) 222:367.e1–367.e22. doi: 10.1016/j.ajog.2019.10.102
36. Gunnarsdottir J, Stephansson O, Cnattingius S, Åkerud H, and Wikström A-K. Risk of placental dysfunction disorders after prior miscarriages: A population-based study. Am J Obstetr Gynecol. (2014) 211:34.e1–8. doi: 10.1016/j.ajog.2014.01.041
37. Sugiura-Ogasawara M, Ebara T, Yamada Y, Shoji N, Matsuki T, Kano H, et al. Adverse pregnancy and perinatal outcome in patients with recurrent pregnancy loss: Multiple imputation analyses with propensity score adjustment applied to a large-scale birth cohort of the Japan environment and children’s study. Am J Reprod Immunol. (2019) 81:e13072. doi: 10.1111/aji.13072
38. Bui LM, Aghajanova L, Lathi RB, and Sokalska A. Polycystic ovary syndrome and miscarriage: a narrative review. F&S Rev. (2024) 5:100078. doi: 10.1016/j.xfnr.2024.100078
39. Wartena R and Matjila M. Polycystic ovary syndrome and recurrent pregnancy loss, a review of literature. Front Endocrinol. (2023) 14:1183060. doi: 10.3389/fendo.2023.1183060
40. Cai WY, Luo X, Ma HL, Shao XG, and Wu XK. Association between preconception serum lipid concentrations and treatment outcomes in women with PCOS who underwent ovulation induction. Reprod BioMed Online. (2022) 45:805–14. doi: 10.1016/j.rbmo.2022.04.013
41. Wang B and Li Z. Hypersecretion of basal luteinizing hormone and an increased risk of pregnancy loss among women with polycystic ovary syndrome undergoing controlled ovarian stimulation and intrauterine insemination. Heliyon. (2023) 9:e16233. doi: 10.1016/j.heliyon.2023.e16233
42. Yusuf ANM, Amri MF, Ugusman A, Hamid AA, Wahab NA, and Mokhtar MH. Hyperandrogenism and its possible effects on endometrial receptivity: A review. Int J Mol Sci. (2023) 24:12026. doi: 10.3390/ijms241512026
43. Jie HY, Zhou X, Zhao MP, Hu M, Mai QY, and Zhou CQ. Pregnancy outcomes in patients with polycystic ovary syndrome who conceived after single thawed blastocyst transfer: A propensity score-matched study. BMC Pregnancy Childbirth. (2022) 22:718. doi: 10.1186/s12884-022-05011-4
44. Kdous M, Chaker A, Bouyahia M, Zhioua F, and Zhioua A. Increased risk of early pregnancy loss and lower live birth rate with GNRH antagonist vs. long GNRH agonist protocol in PCOS women undergoing controlled ovarian hyperstimulation. Tunis Med. (2009) 87:834–42.
45. Rudnicka E, Suchta K, Grymowicz M, Calik-Ksepka A, Smolarczyk K, Duszewska AM, et al. Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci. (2021) 22:3789. doi: 10.3390/ijms22073789
46. Ou XH, Li S, Wang ZB, Li M, Quan S, Xing F, et al. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum Reprod. (2012) 27:2130–45. doi: 10.1093/humrep/des137
47. De Bem THC, Tinning H, Vasconcelos EJR, Wang D, and Forde N. Endometrium on-a-chip reveals insulin- and glucose-induced alterations in the transcriptome and proteomic secretome. Endocrinology. (2021) 162:bqab054. doi: 10.1210/endocr/bqab054
48. Zhang Y, Hu M, Jia W, Liu G, Zhang J, Wang B, et al. Hyperandrogenism and insulin resistance modulate gravid uterine and placental ferroptosis in PCOS-like rats. J Endocrinol. (2020) 246:247–63. doi: 10.1530/JOE-20-0155
49. Meng Z, Lin M, Song L, Chen Y, Deng S, Xia S, et al. The first-trimester triglyceride glucose-body mass index is a valuable predictor for adverse pregnancy outcomes. BMC Pregnancy Childbirth. (2025) 25:142. doi: 10.1186/s12884-025-07258-z
50. Luke B, Gopal D, Cabral H, Stern JE, and Diop H. Pregnancy, birth, and infant outcomes by maternal fertility status: the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol. (2017) 217:327.e1–327.e14. doi: 10.1016/j.ajog.2017.04.006
51. Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, and Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update. (2018) 24:35–58. doi: 10.1093/humupd/dmx031
52. Luke B, Brown MB, Eisenberg ML, Callan C, Botting BJ, Pacey A, et al. In vitro fertilization and risk for hypertensive disorders of pregnancy: associations with treatment parameters. Am J Obstet Gynecol. (2020) 222:350.e1–350.e13. doi: 10.1016/j.ajog.2019.10.003
Keywords: triglyceride glucose-body mass index, polycystic ovary syndrome, adverse reproductive outcomes, miscarriage, frozen embryo transfer
Citation: Ding Z, Liao K, Liang K, Zhang S, Pei Z, Zhang F, Zhou L and Xu C (2025) The triglyceride glucose-body mass index predicts adverse reproductive outcomes in women with polycystic ovary syndrome undergoing frozen embryo transfer. Front. Endocrinol. 16:1629837. doi: 10.3389/fendo.2025.1629837
Received: 16 May 2025; Accepted: 14 July 2025;
Published: 30 July 2025.
Edited by:
Milan Perovic, Gynecology and Obstetrics Clinic Narodni Front, SerbiaReviewed by:
Bojana Salovic, University of Oxford, United KingdomMichael Schenk, Medical University of Graz, Austria
Copyright © 2025 Ding, Liao, Liang, Zhang, Pei, Zhang, Zhou and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feifei Zhang, ZmVpZmVpemhhbmdAZnVkYW4uZWR1LmNu; Liming Zhou, emhvdS5saS5taW5nQDE2My5jb20=; Congjian Xu, eHVjb25namlhbkBmdWRhbi5lZHUuY24=
†These authors have contributed equally to this work
 Ziyin Ding
Ziyin Ding Kai Liao
Kai Liao Kun Liang
Kun Liang Shuo Zhang
Shuo Zhang Zhenle Pei
Zhenle Pei Feifei Zhang
Feifei Zhang Liming Zhou
Liming Zhou Congjian Xu
Congjian Xu