- Reproductive Medical Center, Hainan Women and Children’s Medical Center, Haikou, China
Melatonin, a neuroendocrine hormone widely present in animals, is a derivative of tryptophan secreted by the pineal gland. This hormone regulates animal circadian rhythms and can affect reproductive performance in many ways; for example, melatonin levels change in response to sunshine duration changes, which can inhibit or promote reproductive performance. In juvenile animals, melatonin inhibits estrus, whereas in mature animals, it promotes estrus. Melatonin regulates animal reproductive activities mainly through the hypothalamus–pituitary–gonad axis and through membrane binding receptor (MT1 and MT2) interactions. It effectively removes cellular free radicals that have strong antioxidant effects and can directly act on the reproductive system and even early embryos by improving tissue and cell anti-inflammatory and antioxidant functions, improving animal reproductive performance. Although modern human fertility is no longer affected by seasonal reproduction, the relationship between melatonin and human reproduction remains unclear. Melatonin is important for improving mitochondrial function, reducing free radical damage, and inducing oocyte maturation, which can improve the fertilization rate, promote embryo development, and positively affect in vitro fertilization and embryo transfer. Here, we describe the biosynthesis and regulation of melatonin and its secretion, the physiological function of melatonin, and its effects on animal reproductive performance and assisted reproduction.
1 Introduction
Melatonin, a hormone produced by the pineal gland, has garnered significant attention owing to its role in reproductive system regulation (1). Melatonin’s influence spans various reproductive stages, including gamete production, embryo implantation, and fetal development (2). Importantly, melatonin exerts diverse regulatory effects on reproduction, mainly through binding with its receptors MT1 and MT2, which are G protein-coupled receptors (GPCRs) that play crucial roles in mediating melatonin signaling (3). These receptors are involved in multiple reproductive processes, such as gametogenesis, gamete quality, reproductive rhythm, endocrine function, and embryonic development (3). Understanding the intricate mechanisms of melatonin biosynthesis and its impact on reproductive processes is crucial for advancing our knowledge of its basic scientific and clinical applications (4). Here, we explore the complexities of melatonin biosynthesis and its regulation in reproduction, shedding light on the latest research findings and their potential implications for reproductive health (5). Neuroendocrine hormones are essential for complex communication between the nervous and endocrine systems and regulate various physiological processes, including reproduction (6). Melatonin, a neuroendocrine hormone, is a potent signaling molecule that coordinates reproductive events by controlling the release of reproductive hormones and interacting with other systems, such as the immune system, to optimize reproductive functions (7). Melatonin has become a research focus in reproductive science in recent years because of its diverse roles in regulating fertility (8).
The suprachiasmatic nucleus in the anterior hypothalamus governs the biological clock, maintains circadian rhythms, and synchronizes physiological processes with the natural light–dark cycle (9). Melatonin, also known as the “hormone of darkness,” plays a central role in this system. In response to darkness, the pineal gland synthesizes and releases melatonin, which reaches peak levels at night and decreases during the day (10). Melatonin also directly affects the reproductive system (11). Melatonin biosynthesis and regulation are important for understanding the complex interplay between melatonin and reproductive processes (12). This interaction has attracted increasing attention because of the diverse and pivotal roles of melatonin in regulating reproductive functions (13). Investigating the intricate processes involved in melatonin biosynthesis and their impact on reproduction can provide valuable insights into the complexities of fertility and potentially result in the development of novel therapeutic approaches addressing reproductive disorders and infertility (14). The multifaceted roles of melatonin in modulating circadian rhythms and reproductive functions have piqued the interest of scientists and clinicians, underscoring the importance of this research area (15). Previous studies have indicated that melatonin plays a role in regulating different reproductive functions (8). For example, it influences the secretion of gonadotropin-releasing hormones, which are crucial for the production of reproductive hormones in the hypothalamus (16). Moreover, melatonin has been linked to the control of follicle development, ovulation, and embryo implantation (17). Thus, the essential role of melatonin in the complex process of reproduction cannot be overlooked.
Melatonin regulates circadian rhythms and significantly influences various aspects of fertility, including the timing of ovulation, sperm production, and embryo implantation (18). Elucidating the mechanisms underlying melatonin biosynthesis and its regulation in reproduction will provide valuable insights into fertility processes and enable the development of innovative treatments for reproductive disorders and infertility (14). Increasing research into the multifaceted roles of melatonin in circadian rhythm and reproductive function regulation has highlighted the importance of scientific and clinical research on this neuroendocrine hormone (19). The diverse roles of melatonin in fertility regulation underscore the importance of further exploration (20), and the complex interplay between melatonin biosynthesis and regulation in reproduction has sparked widespread interest in its potential role in assisted reproductive technology (ART) (21). As the demand for ART increases, there is a growing need to explore the potential impact of melatonin on the success rate of these techniques (22). Investigating the role of melatonin in ART can potentially reveal novel strategies that can increase the efficacy of fertility treatments and improve outcomes for individuals and couples seeking reproductive assistance (23). Melatonin research can potentially revolutionize ART approaches and address evolving challenges in assisted reproduction (24). Furthermore, understanding the influence of melatonin on ART can help advance the personalized and targeted approaches that are increasingly emphasized in reproductive medicine (25).
The potential role of melatonin in ART has recently garnered significant interest. Advancements in ART procedures necessitate the optimization of fertility treatments to increase success rates (25). Melatonin is a promising candidate for enhancing ART outcomes due to its antioxidant nature and ability to modulate reproductive processes. Researchers have investigated the effects of melatonin supplementation in conjunction with ART to assess its potential to improve oocyte quality, enhance embryo development, and optimize the success of in vitro fertilization procedures (26). Furthermore, understanding the specific mechanisms by which melatonin regulates follicle development, ovulation, and embryo implantation in humans can provide valuable insights into reproductive outcomes (27).
Understanding the effects of melatonin on the reproductive system is essential for developing personalized treatment approaches for individuals facing fertility challenges (28). Understanding the influence of melatonin on reproductive function provides insight into potential mechanisms for tailoring fertility treatments according to individuals’ melatonin levels and circadian rhythms (29). In this review, we discuss interactions among melatonin, circadian rhythms, and reproductive processes with a specific focus on the potential of melatonin in the context of ART, providing valuable insights into these processes that could revolutionize reproductive science and contribute to the development of more effective and personalized fertility treatments (30).
In brief, we explore how melatonin biosynthesis and control affect human reproduction, particularly the timing of puberty, the menstrual cycle, follicle development, ovulation, and embryo implantation, seeking to fill the knowledge gap regarding melatonin’s involvement in human reproduction and its potential influence on ART (31). Furthermore, we discuss the regulation and management of melatonin in the aforementioned processes (32), including the impact of melatonin on menstrual cycle regularity and timing and the potential effects of melatonin supplementation on ART, including pregnancy rates and oxidative stress reduction.
We hypothesized that melatonin is crucial for the regulation and enhancement of human reproductive processes; specifically, we hypothesized that melatonin levels and their biosynthesis and regulation contribute to the timing of puberty, the menstrual cycle, follicle development, ovulation, and embryo implantation (33). We further hypothesized that melatonin supplementation could improve the success rate of ART by increasing the quality of oocytes and embryos, increasing pregnancy rates, reducing oxidative stress, and promoting overall reproductive health.
2 Melatonin synthesis and secretion
Various tissues and organs can secrete melatonin; however, only the retinal tissue of the pineal gland and eye exhibits periodic secretory activity. The biosynthesis of melatonin shows a circadian rhythm and seasonal characteristics associated with external light conditions. Functionally, melatonin can link changes in external light signals and multiple physiological activity rhythms (34), has a good protective effect on the nervous system, regulates circadian changes, and has good therapeutic effects on psychiatric diseases (35). In addition, melatonin has broad-spectrum antibacterial (36) and immunoregulatory functions (37).
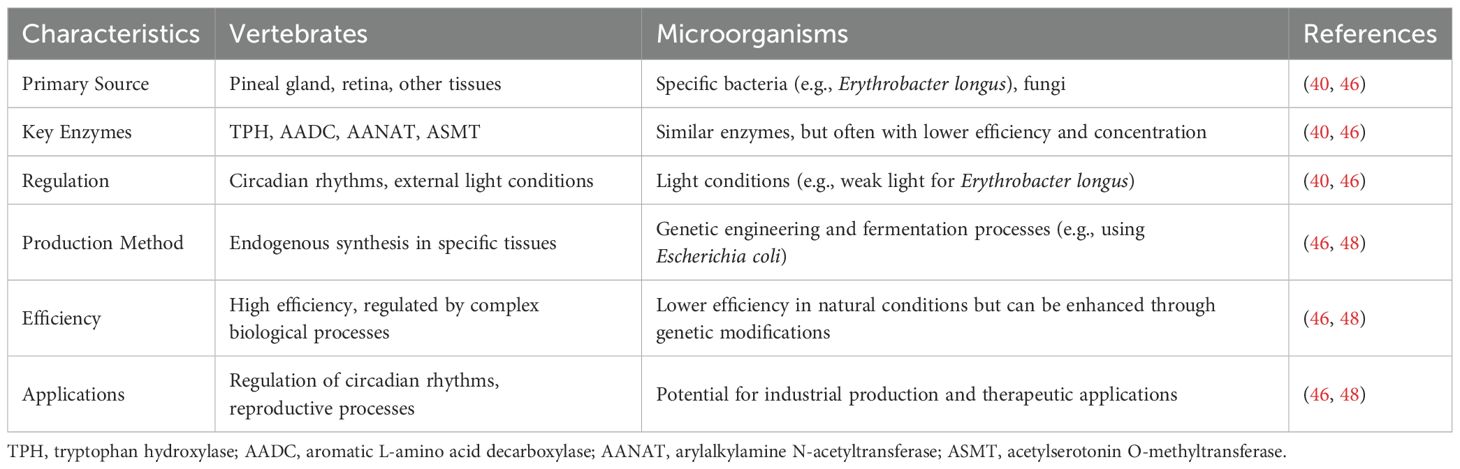
Melatonin was originally isolated and identified from the pineal glands of animals (38). Research has shown that various tissues and organs, including the gut and ovarian follicles, can also synthesize melatonin (39) as autocrine and paracrine signals. Vertebrate gastrointestinal tryptophan is a precursor of melatonin biosynthesis. Tryptophan is excreted from the small intestine into the blood circulation and actively taken up by pineal cells, in which 5-hydroxytryptophan is formed by tryptophan hydroxylase. Aryl alkylamine-N-acetyltransferase (AANAT) converts tryptophan hydroxylase into N-acetylserotonin, and acetylserotonin O-methyltransferase (ASMT) catalyzes the final conversion to melatonin (40). AANAT is the major rate-limiting enzyme in this process, and its biological activity can regulate the melatonin synthesis rate. The binding regulatory/binding sequences in the AANAT gene encode a binding switch for cAMP operation, and cAMP-catalyzed protein kinase promotes the formation of a complex with the 14-3–3 protein. This AANAT/14-3–3 complex shields melatonin from dephosphorylation and AANAT proteolysis and reduces the K (m) of serotonin, enhancing melatonin production (41). Suofu et al. (42) further established that melatonin is synthesized in the mitochondrial matrix. Melatonin is quickly released into the blood and spinal fluid (43), binds to plasma proteins, and is distributed throughout most tissues (44).
Additionally, many studies have demonstrated that some bacteria and fungi can synthesize melatonin (45). Tilden et al. (46) investigated the influence of light conditions on the aerobic photosynthetic bacterium Eryrobacter longusits, demonstrating that it could synthesize and secrete melatonin under weak light conditions. However, the efficiency and concentration of melatonin synthesis in the bacterium’s natural state were low and not conducive to isolation, purification, and application. Advances in research on microbial genomes, genetic engineering technologies, and fermentation engineering have resulted in the use of genetically engineered bacteria to synthesize substances that are of high value or that are difficult to obtain (47). The use of engineered bacteria to ferment and transform substances has the advantages of convenience, speed, safety, efficiency, and cost reduction. Escherichia coli is the most commonly used host bacterium. Some E. coli plasmids can encode T7 lysozyme, express large quantities of target proteins, and inhibit the normal expression of host self-proteins (48). Researchers have used genetic engineering technology to transform Xanthomonas rapeseed carrying the phenylalanine 4-hydroxylase (P4H) gene, rice 3-O-methyltransferase (COMT) gene, and Streptomyces white toxin synthesis gene construction vector into E. coli and used protein engineering and metabolic engineering technology to successfully express melatonin in these bacteria (48). The differences in melatonin synthesis pathways between vertebrates and microorganisms are comprehensively detailed in Table 1.
3 The biological function of melatonin
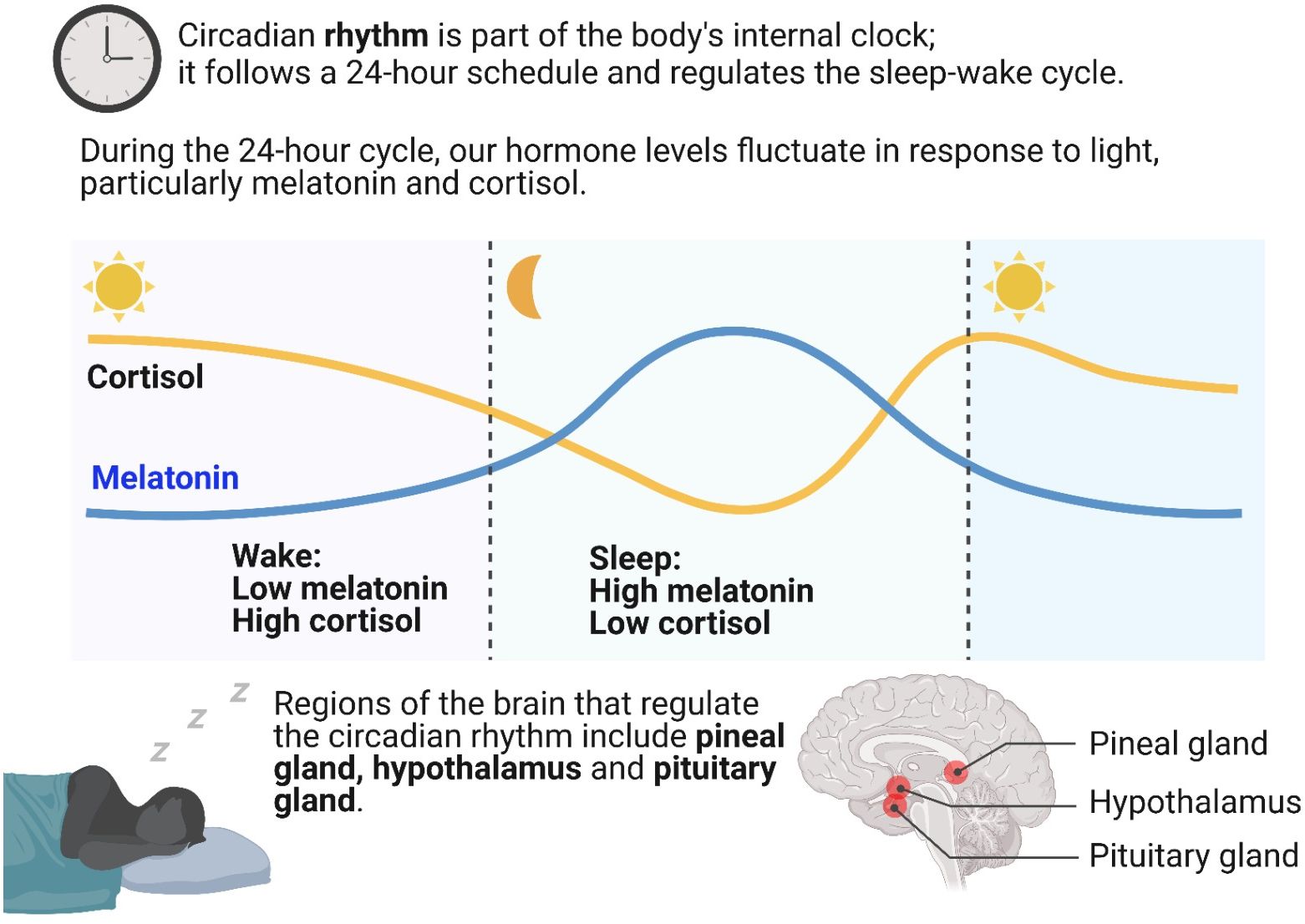
Melatonin regulates circadian rhythms in animals (49). At night, under weak light conditions, endogenous melatonin is synthesized to promote sleep and improve sleep quality. During the day, melatonin secretion by the pineal gland is inhibited by light stimulation (35). Studies have shown that changes in external light conditions can affect the level of melatonin synthesis and secretion by the pineal gland through ocular processes (IOP) and subsequently regulate the body’s sleep state (50). Thus, the body senses changes in external light through the eyes and transmits signals to the pineal gland, which converts these signals into melatonin production responses, regulating the body’s circadian rhythm. A mouse model with downregulated melatonin expression has been constructed via gene editing technology (51), revealing significantly longer sleep durations in these mice compared to those with upregulated melatonin expression. These results demonstrate the regulatory effect of melatonin on the circadian rhythm. Figure 1 shows the biological function of melatonin in circadian rhythm regulation.
The immunomodulatory functions of melatonin can have proinflammatory and anti-inflammatory effects under different inflammatory conditions and can improve the body’s resistance and resilience to exogenous or endogenous antigens (52). These effects are partly mediated by the melatonin receptors MT1 and MT2, which can trigger various downstream signaling pathways. These receptors mainly interact with Gi (including Gαi2 and Gαi3) and Gq/11 proteins, leading to the inhibition of adenylate cyclase (AC) activity and cAMP production. Additionally, MT1 receptors can stimulate the Gβγ-dependent PI3K/Akt and PKC/ERK pathways and activate K+ channels (Kir3) and Ca2+ channels (Cav2.2). MT2 receptors can stimulate Gαi-dependent PKC/ERK signaling and decrease intracellular cGMP levels (3). These signaling pathways collectively contribute to the diverse biological functions of melatonin, including its immunomodulatory effects (3). Melatonin stimulates reactive oxygen species (ROS) production in monocytes, which can activate other immune cells (52), promoting immune system defenses. Furthermore, melatonin can act on MT1 and MT2 on the surface of human monocyte U937 cells, stimulating intracellular ROS production; however, the concentration of ROS produced does not cause oxidative stress-related damage (53, 54). Activated monocytes can differentiate into macrophages and synthesize chemokines and related inflammatory factors to perform their immunomodulatory functions. In other studies, melatonin has been found to act on MT1 on the cell membrane surface of immune cells, upregulating the expression of the interleukin 2 (IL-2) receptor and alleviating the inhibitory effects of prostaglandins on IL-2 (55). In contrast, melatonin inhibits severe inflammation; dextran sodium sulfate-induced neuroinflammation and liver injury in mice were relieved by exogenous melatonin administration, which increased short-chain fatty acid production (56). Melatonin has also been found to inhibit the overactivation of intestinal fonnesin X receptor fibroblast growth factor 15 (FXR-FGF 15) and apoptotic signal-regulated kinase 1 (ASK 1) in the liver, alleviating intestinal inflammation and hepatic metabolic disorders.
Melatonin has broad-spectrum antioxidant activity (37) and can limit oxidative damage through multiple mechanisms, including scavenging excess free radicals, stimulating endogenous antioxidant enzymes, and improving the efficiency of other antioxidants (57). Furthermore, melatonin can be transferred by hydrogen atoms (hydrogen atom transfer, HAT), proton-coupled electron transfer (proton-coupled electron transfer, PCET), free radical addition, substances (radical adduct formation, RAF), single-electron transfer (SET), sequential electron-proton transfer (SEPT), and other mechanisms that directly remove peroxy groups (peroxyl radical, ROO), hydroxyl radical (OH), OO groups (nitric oxide, NO), and other free radicals (58). Mekhloufi et al. (59) constructed an in vitro lipid model to assess the scavenging effects of melatonin and hydroperoxides. The results revealed that melatonin could directly react with and remove hydroxyl radicals. Melatonin can also chelate Cu2+, reduce Fe2+, Zn2+, Al3+, Mn2+, and other toxic metal ions (60), reduce metal ion-catalyzed molecular damage, and suppress metal ion interaction with β-amyloid peptides to produce free radicals (61), playing significant antioxidant and protective roles in the body. The effects of melatonin on Cu2+-mediated lipid peroxidation (62) and Cu2+/H2O2-induced metal-catalyzed oxidation and protein damage (63) have been found to be protective. In addition, melatonin can increase the antioxidant effects of glutaglyanin, ascorbic acid, and water-soluble vitamin E through electron transfer (64).
4 Effects of melatonin on reproduction
Melatonin can act on the hypothalamic–pituitary–gonadal axis to regulate animal reproductive activity (65), which has multiple effects on animal reproduction. These effects are largely mediated by the melatonin receptors MT1 and MT2. For example, in the male reproductive system, melatonin can inhibit the expression of key steroidogenic genes (such as p450scc, p450c17, and StAR) in Leydig cells via MT1 receptors, thereby reducing testosterone synthesis (3). In the female reproductive system, MT1 receptors are widely distributed in the ovary and are crucial for melatonin-regulated activities, such as delaying the decline in fertility in female animals (3). Moreover, melatonin can improve oocyte development and fertilization capacity via a receptor-mediated demethylation mechanism, including an increase in Tet1 gene expression and a decrease in Dnmt1 gene expression (3). In immature animals, melatonin can inhibit the secretion of endogenous gonadotropin-releasing hormone (GnRH) (66) to inhibit sexual maturation, spontaneous ovulation, and the estrous response (67). Melatonin also plays a role in mature animals (68). To some extent, this phenomenon shows that melatonin has a protective effect on immature animals, preventing premature reproductive activities and reducing damage to the reproductive system and other tissues and organs. The hypothalamic suprachiasmatic nucleus of mammals receives light stimulation through the retinal hypothalamic bundle, thereby regulating the rhythmicity of pineal melatonin synthesis and secretion. Melatonin is mainly secreted at night, and its secretion is inversely proportional to the duration of sunshine during the day. Thus, the photoperiod signal is transformed into changes in melatonin content in the animal, thereby regulating the reproductive cycle of animals with seasonal estrus (69, 70).
4.1 Effects on the male reproductive system
Melatonin significantly improves the status of the male reproductive system, acting through the hypothalamic–pituitary–gonadal (HPG) axis to regulate the secretion of reproductive hormones, primarily GnRH and luteinizing hormone (LH) (71). Melatonin receptors (MT1 and MT2) mediate not only melatonin signaling but also other hormone signaling, thereby increasing testosterone levels (72). In the embryonic and juvenile stages, melatonin promotes the secretion of male hormones and the development and maturation of the reproductive system by binding to MT1 and MT2 in testicular tissues (73). In adulthood, melatonin continues to promote testosterone secretion and sperm formation, increasing reproductive activity, especially in seasonal breeders (74).
The inherent anti-inflammatory and antioxidant effects of melatonin also contribute to the overall health of the male reproductive system. Studies have shown that melatonin can improve the survival of cryopreserved spermatogonial stem cells (SSCs) by reducing ROS production during freezing and thawing (75). Additionally, melatonin has a protective effect against reproductive diseases in male animals. For example, it can increase the phagocytic capacity of macrophages in the testis, inhibit the p38 MAPK pathway, and promote testosterone secretion by Leydig cells, thereby reducing inflammation (76).
4.2 Effects on the female reproductive system
Melatonin also significantly affects the female reproductive system, influencing oocyte maturation, cumulus cell expansion, and the production of steroid hormones. Recent studies have highlighted the critical role of melatonin in modulating gene expression levels related to these processes.
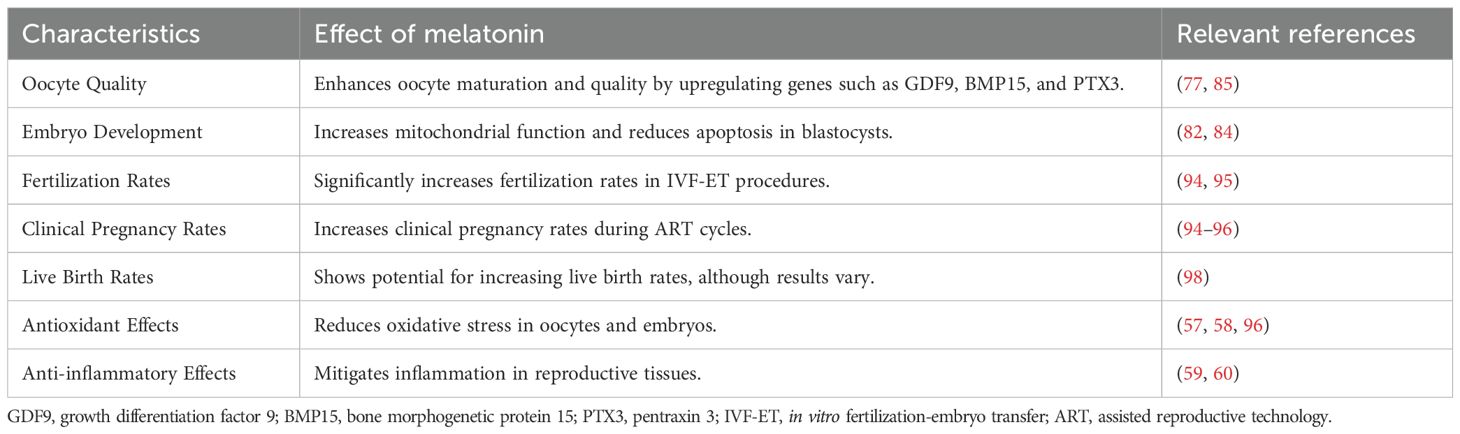
Melatonin has been shown to upregulate the expression of several genes crucial for oocyte maturation and cumulus cell expansion. For example, melatonin supplementation has been found to increase the expression of genes such as growth differentiation factor 9 (GDF9), bone morphogenetic protein 15 (BMP15), and pentraxin 3 (PTX3) (77). These genes are essential for promoting the development and quality of oocytes, thereby enhancing reproductive outcomes. Melatonin also supports the expansion of cumulus cells, which is vital for providing a supportive environment for oocyte maturation.
In addition to its effects on gene expression, melatonin influences the production of steroid hormones by granulosa cells. Growing granulosa cells produce steroid hormones such as 17β-estradiol and androstenedione, which are essential for maintaining the physical connections between granulosa cells and oocytes and facilitating the exchange of necessary substances during oocyte growth (78). Melatonin modulates the synthesis of these hormones, thereby supporting the overall health and development of oocytes.
The antioxidant and anti-inflammatory properties of melatonin contribute to its beneficial effects on the female reproductive system (79). By reducing oxidative stress and inflammation, melatonin helps protect oocytes and surrounding cells from damage, thereby improving the overall reproductive environment (80). This protective effect is particularly important in the context of ART, where oxidative stress can negatively impact oocyte quality and embryo development. The ability of melatonin to mitigate oxidative stress and inflammation ensures a healthier reproductive environment, thereby increasing ART procedure success rates.
The clinical implications of the effects of melatonin on the female reproductive system are significant. In the context of ART, melatonin supplementation has been shown to improve oocyte quality, increase embryo development, and increase pregnancy rates. For example, a systematic review and meta-analysis demonstrated that melatonin improved the fertilization rate and the number of mature oocytes (MII), although it did not significantly affect the clinical pregnancy rate (81). These findings highlight the potential of melatonin as a therapeutic agent for improving reproductive outcomes.
In conclusion, melatonin plays a vital role in modulating reproductive functions in both males and females and is a promising therapeutic agent for improving reproductive outcomes due to its antioxidant properties and effects on gene expression and steroid hormone production. Future research should continue to explore the mechanisms underlying the actions of melatonin and its potential applications in clinical settings.
4.3 Effects of melatonin on early embryonic development and oocyte quality
4.3.1 Effects on early embryonic development
Melatonin affects both gamete formation and early embryo development. Melatonin can increase the mtRNA copy number, mitochondrial membrane potential, and mitochondrial distribution in blastocyst-stage cells; inhibit the expression of apoptotic genes such as p53 and Bax; and promote the expression of antioxidant genes such as SOD1 and GPx 4, thus improving the mitochondrial function of blastocyst-stage embryos, promoting early embryo development, and improving the quality of blastocysts (82). MT1 expression is initiated early in embryonic development (83), and MT1 is distributed mainly on the cell membrane in activated oocytes with no cleavage. With the development of blastomeres, MT1 receptor expression gradually increases, and it is primarily localized on the cell membrane. During embryo development, MT1 is expressed and distributed inside the blastomere, and its expression levels are low in degenerated embryos. Treating IVF-fertilized follicles with 10–9 mol/L melatonin significantly improved the blastocyst formation rate and embryo quality (84). During bovine embryo development, 10–9 mol/L melatonin significantly improved the cleavage rate, blastocyst rate, and number of blastocyst cells. Moreover, melatonin binds to MT2 receptors to promote the establishment of an endometrial receptive state during embryonic colonization (85). The expression of AANAT, the rate-limiting enzyme for melatonin synthesis, increases in the uterus during early pregnancy, and MT2 receptors are specifically expressed in uterine luminal epithelial cells and uterine glands on the second day of pregnancy (86). After the injection of 15 mg/kg melatonin, the endometrial thickness and uterine gland density increased, and the number of implantation sites and litter size increased significantly.
4.3.2 Effects on oocyte quality during in vitro maturation and growth
Recent research has highlighted the significant role of melatonin in improving oocyte quality, particularly in the context of in vitro maturation and growth (87, 88). Melatonin has been shown to enhance the meiotic and developmental competence of oocytes derived from early antral follicles and small antral follicles (89). For example, studies have demonstrated that melatonin supplementation during in vitro maturation (IVM) can upregulate the expression of genes related to oocyte maturation, such as GDF9, BMP15, and PTX3 (77). These genes are essential for promoting oocyte development and improving oocyte quality. Besides, recent studies further demonstrate that melatonin supplementation during in vitro growth (IVG) of oocytes from preantral or early antral follicles significantly improves their developmental competence. For instance, a study has reported that melatonin could enhance the developmental potential of porcine oocyte-granulosa cell complexes derived from preantral follicles (90), while another research has showed that melatonin, combined with cyclic adenosine monophosphate (cAMP) modulators, could promote meiotic and developmental competence in porcine oocytes from early antral follicles during IVG and pre-maturation culture (91). These findings highlight melatonin’s dual role in supporting both oocyte growth and maturation, offering promising avenues for human ART applications.
Moreover, the antioxidant properties of melatonin play crucial roles in protecting oocytes from oxidative stress in vitro (92). Melatonin helps maintain oocyte integrity and functionality by scavenging ROS and enhancing endogenous antioxidant enzyme activity (57, 58). This protection is particularly important in IVM settings, where oocytes are exposed to relatively high levels of oxidative stress due to the absence of the natural protective mechanisms provided by the follicular environment.
Recent studies have also shown that melatonin can improve the survival and developmental competence of oocytes derived from small antral follicles (89). Compared with larger follicles, these oocytes often exhibit lower quality and developmental potential. Melatonin supplementation has been shown to enhance the meiotic maturation and developmental competence of these oocytes, thereby improving their potential for successful fertilization and embryo development (85).
In summary, the effects of melatonin on early embryonic development and oocyte quality are multifaceted. Melatonin enhances mitochondrial function, reduces oxidative stress, and promotes the expression of genes crucial for oocyte maturation and development. These findings underscore the potential of melatonin as a valuable supplement in IVM and in vitro growth (IVG) protocols, thereby improving reproductive outcomes.
5 Clinical application and treatment of melatonin in reproduction
ART has played a pivotal role in helping infertile couples achieve pregnancy. This includes a range of treatments, such as artificial insemination (AI), in vitro fertilization–embryo transfer (IVF–ET), and derivative technologies. The global ART-facilitated birth population has now exceeded 6 million, with ART treatment accounting for 1–3% of the total number of newborns in developed countries (93). One controlled clinical trial revealed that fertilization and pregnancy rates were approximately twice as high in patients treated with melatonin than in those treated without melatonin, indicating the ability of melatonin to improve the success rate of IVF–ET (94). A meta-analysis of randomized trials revealed that melatonin treatment significantly increased clinical pregnancy rates during the ART cycle, as did the number of oocytes collected, mature oocytes, and high-quality embryos (95). Melatonin can also improve the clinical outcomes of IVF–ET by increasing the fertilization rate and the number of mature oocytes and high-quality embryos (96); therefore, melatonin intake during IVF–ET is considered to have some clinical utility (97).
Melatonin is also used as a therapeutic agent for unexplained infertility. A randomized pilot study revealed improvements in intracellular oxidative stress and oocyte quality in patients receiving 3 or 6 mg/day doses and slight increases in clinical pregnancy and live birth rates with IVF–ET (98). The main advantage of melatonin antioxidant therapy is its relatively adequate safety, as confirmed in short-term studies. However, long-term clinical trials are needed to evaluate its application further.
Despite these promising findings, the effectiveness of melatonin supplementation in ART can vary significantly depending on the specific stage of treatment and the individual context. One key limitation is the variability in individual responses to melatonin treatment. Factors such as age, underlying health conditions, and the presence of other antioxidants in the body can significantly impact the efficacy of melatonin. For example, older patients or those with preexisting oxidative stress conditions may not respond as positively to melatonin treatment as younger or healthier individuals do. This variability underscores the need for personalized approaches in ART, where melatonin supplementation is tailored to the specific needs and conditions of each patient.
Another limitation is the lack of standardized protocols for melatonin administration in ART. The optimal dosage, timing, and duration of melatonin supplementation remain the foci of ongoing research. The variability in these parameters across different studies makes it challenging to draw definitive conclusions about the universal effectiveness of melatonin in ART. Standardizing these protocols is crucial for maximizing the benefits of melatonin while minimizing potential side effects.
Furthermore, while melatonin has shown promise in improving oocyte quality and embryo development, its impact on the overall success rates of ART, such as live birth rates, has been less consistent. Some studies have demonstrated significant improvements in live birth rates with melatonin supplementation, whereas others have not reported such effects (99–102). This variability highlights the need for further research to better understand the contexts in which melatonin is most effective. Future studies should focus on elucidating the mechanisms underlying individual variability in response to melatonin treatment, potentially leading to personalized approaches in ART.
Table 2 encapsulates the multifaceted role of melatonin in reproductive medicine, summarizing its beneficial effects on oocyte quality, embryo development, fertilization rates, clinical pregnancy rates, and live birth rates. These findings also underscore the antioxidant and anti-inflammatory properties of melatonin, highlighting the need for further research to elucidate optimal dosing strategies and long-term clinical outcomes.
6 Conclusion
Melatonin is a key signaling molecule that connects changes in external light conditions with changes in physiological activities in the body. After the retina of an animal’s eye receives a light signal, it transmits the signal to the pineal gland. The pineal gland then transforms the light signal into melatonin, thereby participating in various physiological reactions in the body. Melatonin regulation of various antioxidants and immune responses improves animal reproductive performance. ART has enhanced our understanding of the various physiological changes in the reproductive process, and an increasing number of problems restricting reproductive performance have been identified. Previous studies have elucidated the role of melatonin in improving animal reproductive performance, and this factor can potentially improve human-assisted reproduction in the future. However, the mode of administration of melatonin and its isoforms requires further study in different species and breeds.
Author contributions
JZ: Visualization, Conceptualization, Data curation, Writing – original draft, Software, Writing – review & editing, Investigation. ZL: Writing – original draft, Conceptualization, Investigation, Data curation. ZZ: Investigation, Methodology, Writing – original draft. NM: Writing – original draft, Data curation, Investigation. YL: Writing – original draft, Formal Analysis, Software. JH: Writing – original draft, Investigation. BW: Funding acquisition, Conceptualization, Investigation, Writing – original draft. WL: Conceptualization, Funding acquisition, Writing – review & editing, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the Hainan Province Clinical Medical Center (QWYH202175), the Scientific Research Project of Hainan Health Committee (21A200115), the Research and Cultivation Fund of Hainan Medical University (HYPY2020015), the Specific Research Fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202311), the Natural Science Foundation of Hainan Province (820RC771), and the Key R&D Projects of Hainan Province (ZDYF2022SHFZ074).
Acknowledgments
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ART, Assisted reproductive technology; DSS, Dextran sodium sulfate; GC, Granulosa cell; MCO, Metal-catalyzed oxidation; ROS, Reactive oxygen species; SSC, Spermatogonial stem cell; UI, Unexplained infertility.
References
1. Kumar V, Lincoln GA, and Tortonese DJ. Effects of excitatory amino acid receptor agonists and antagonists on the secretion of melatonin, luteinizing hormone and prolactin in the ram. J Neuroendocrinol. (1993) 5:649–54. doi: 10.1111/j.1365-2826.1993.tb00535.x
2. Gurunathan S, Kang M-H, Song H, Kim NH, and Kim JH. The role of extracellular vesicles in animal reproduction and diseases. J Anim Sci Biotechnol. (2022) 13:62. doi: 10.1186/s40104-022-00715-1
3. Gao Y, Zhao S, Zhang Y, and Zhang Q. Melatonin receptors: A key mediator in animal reproduction. Vet Sci. (2022) 9:309. doi: 10.3390/vetsci9070309
4. Lisk K, Agur A, and Woods NN. Exploring cognitive integration of basic science and its effect on diagnostic reasoning in novices. Perspect Med Educ. (2016) 5:147–53. doi: 10.1007/s40037-016-0268-2
5. Hurtado-Gonzalez P and Mitchell R. Analgesic use in pregnancy and male reproductive development. Curr Opin Endocrinol Diabetes Obes. (2017) 24:225–32. doi: 10.1097/med.0000000000000338
6. Babichev VN and Shishkina I. Formation of hypothalamic regulation of the gonadotropic function of the hypophysis under the conditions of hypoinsulinemia in rats. Neurosci Behav Physiol. (1994) 24:297–9. doi: 10.1007/bf02360196
7. Ahmad R and Haldar C. Photoperiod-testicular-immune interaction in a seasonal breeder Indian palm squirrel funambulus pennanti during the reproductively inactive and active phases. J Neuroendocrinol. (2009) 21:2–9. doi: 10.1111/j.1365-2826.2008.01805.x
8. Talpur HS, Chandio IB, Brohi RD, Worku T, Rehman ZU, Bhattarai D, et al. Research progress on the role of melatonin and its receptors in animal reproduction: A comprehensive review. Reprod Domest Anim. (2018) 53:831–49. doi: 10.1111/rda.13188
9. Drunen RV and Eckel-Mahan K. Circadian rhythms of the hypothalamus: from function to physiology. Clocks Sleep. (2021) 3:189–226. doi: 10.3390/clockssleep3010012
10. Millet-Boureima C, Ennis CC, Jamison J, McSweeney S, Park A, and Gamberi C. Empowering melatonin therapeutics with drosophila models. Diseases. (2021) 9:67. doi: 10.3390/diseases9040067
11. Tamura H, Takasaki A, Tanaka T, Tanabe M, Lee L, Tamura I, et al. Melatonin and female reproduction. J Obstet Gynaecol Res. (2014) 40:1–11. doi: 10.1111/jog.12177
12. Raey MAE, Geshi M, Somfai T, Kaneda M, Hirako M, Abdel-Ghaffar AE, et al. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol Reprod Dev. (2011) 78:250–62. doi: 10.1002/mrd.21295
13. Katzman MA and Katzman MP. Neurobiology of the orexin system and its potential role in the regulation of hedonic tone. Brain Sci. (2022) 12:150. doi: 10.3390/brainsci12020150
14. Bandrés-Ciga S, Makarious MB, Ojo OO, Crea PW, Abiodun O, Levine K, et al. Genome-wide association identifies novel etiological insights associated with parkinson’s disease in african and african admixed populations. (2023). doi: 10.1101/2023.05.05.23289529
15. Jan JE, Reíter RJ, Wasdell M, and Bax M. The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J Pineal Res. (2008) 46:1–7. doi: 10.1111/j.1600-079x.2008.00628.x
16. Krsmanović LZ, Stojilković SS, Merelli F, Dufour S, Virmani A, and Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci. (1992) 89:8462–6. doi: 10.1073/pnas.89.18.8462
17. Niringiyumukiza JD, Cai H-C, and Xiang W. Prostaglandin E2 involvement in mammalian female fertility: ovulation, fertilization, embryo development and early implantation. Reprod Biol Endocrinol. (2018) 16:43. doi: 10.1186/s12958-018-0359-5
18. Alvarez J, Hansen AG, Ord T, Bębas P, Chappell PE, Giebułtowicz JM, et al. The circadian clock protein bmal1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. (2008) 23:26–36. doi: 10.1177/0748730407311254
19. Arutjunyan АV, Степанов МГ, Керкешко ГО, and Ailamazyan EК. Impaired hypothalamic regulation of reproductive function when exposed to neurotoxic compounds and melatonin. J Obstet Women S Dis. (2003) 52:77–85. doi: 10.17816/jowd88851
20. Iglesias IS, Santora JA, Fiechter J, and Field JC. Mesopelagic fishes are important prey for a diversity of predators. Front Mar Sci. (2023) 10 - 2023:1220088. doi: 10.3389/fmars.2023.1220088
21. Al-Nasiry S, Ambrosino E, Schlaepfer M, Morré SA, Wieten L, Voncken JW, et al. The interplay between reproductive tract microbiota and immunological system in human reproduction. Front Immunol. (2020) 11:378. doi: 10.3389/fimmu.2020.00378
22. Soubhi H, Bayliss EA, Fortin M, Hudon C, Akker M, Thivierge R, et al. Learning and caring in communities of practice: using relationships and collective learning to improve primary care for patients with multimorbidity. Ann Family Med. (2010) 8:170–7. doi: 10.1370/afm.1056
23. Frullo JM, Elinger J, Pehlivan AU, Fitle K, Nedley K, Francisco GE, et al. Effects of assist-as-needed upper extremity robotic therapy after incomplete spinal cord injury: A parallel-group controlled trial. Front Neurorobot. (2017) 11:26. doi: 10.3389/fnbot.2017.00026
24. Antman EM, Benjamin EJ, Harrington RA, Houser SR, Peterson ED, Bauman MA, et al. Acquisition, analysis, and sharing of data in 2015 and beyond: A survey of the landscape. J Am Heart Assoc. (2015) 4:e002810. doi: 10.1161/jaha.115.002810
25. Woolfenden S, Farrar MA, Eapen V, Masi A, Wakefield CE, Badawi N, et al. Delivering pediatric precision medicine: genomic and environmental considerations along the causal pathway of childhood neurodevelopmental disorders. Dev Med Child Neurol. (2022) 64:1077–84. doi: 10.1111/dmcn.15289
26. Reíter RJ, Sharma R, Romero A, Manucha W, Tan DX, Zuccari D, et al. Aging-related ovarian failure and infertility: melatonin to the rescue. Antioxidants. (2023) 12:695. doi: 10.3390/antiox12030695
27. Hashimoto O, Moore RK, and Shimasaki S. Posttranslational processing of mouse and human bmp-15: potential implication in the determination of ovulation quota. Proc Natl Acad Sci. (2005) 102:5426–31. doi: 10.1073/pnas.0409533102
28. Smith LB, Milne L, Nelson NA, Eddie SL, Brown P, Atanassova N, et al. Katnal1 regulation of sertoli cell microtubule dynamics is essential for spermiogenesis and male fertility. PloS Genet. (2012) 8:e1002697. doi: 10.1371/journal.pgen.1002697
29. Onaolapo OJ and Onaolapo AY. Melatonin in drug addiction and addiction management: exploring an evolving multidimensional relationship. World J Psychiatry. (2018) 8:64–74. doi: 10.5498/wjp.v8.i2.64
30. Millerand M, Sudre L, Nefla M, Pène F, Rousseau C, Ravat A, et al. 14-3-3e, a new alarmin candidate, elicits a catabolic and proinflammatory effect involving innate immunity through tlr signaling in osteoarthritis. Osteoarthritis Cartilage. (2019) 27:S375–S6. doi: 10.1016/j.joca.2019.02.369
31. Valdiani A, Talei D, Lattoo SK, Ortíz R, Rasmussen SK, Batley J, et al. Genoproteomics-assisted improvement of andrographis paniculata: toward a promising molecular and conventional breeding platform for autogamous plants affecting the pharmaceutical industry. Crit Rev Biotechnol. (2017) 37:803–16. doi: 10.1080/07388551.2016.1260525
32. Abecia JA, Meikle A, Vázquez MI, Casao A, Forcada F, and Sosa C. 193 melatonin implants in spring improve embryo production of aged ewes after superovulation regardless of endometrial progesterone receptor expression. Reprod Fertil Dev. (2019) 31:221. doi: 10.1071/rdv31n1ab193
33. Martirosyan Y, Nazarenko T, Birukova A, and Dmitrieva I. O-112 outcomes of random-start ovarian stimulation protocols as a possible evidence of the theory of antral follicles continuous recruitment. Hum Reprod. (2021) 36. doi: 10.1093/humrep/deab126.021
34. Reiter RJ, Tan DX, Korkmaz A, and Rosales-Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update. (2014) 20:293–307. doi: 10.1093/humupd/dmt054
35. Moon E, Kim K, Partonen T, and Linnaranta O. Role of melatonin in the management of sleep and circadian disorders in the context of psychiatric illness. Curr Psychiatry Rep. (2022) 24:623–34. doi: 10.1007/s11920-022-01369-6
36. Ma N, Zhang J, Reiter RJ, and Ma X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med Res Rev. (2020) 40:606–32. doi: 10.1002/med.21628
37. Favero G, Franceschetti L, Bonomini F, Rodella LF, and Rezzani R. Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int J Endocrinol. (2017) 2017. doi: 10.1155/2017/1835195
38. Lerner AB, Case JD, Takahashi Y, Lee TH, and Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes1. J Am Chem Soc. (1958) 80:2587–. doi: 10.1021/ja01543a060
39. Hardeland R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Sci World J. (2012) 2012. doi: 10.1100/2012/640389
40. Axelrod J and Wurtman RJ. Photic and neural control of indoleamine metabolism in the rat pineal gland. Adv Pharmacol. (1968) 6:157–66. doi: 10.1016/S1054-3589(08)61169-2
41. Ganguly S, Gastel JA, Weller JL, Schwartz C, Jaffe H, Namboodiri M, et al. Role of a pineal camp-operated arylalkylamine N-acetyltransferase/14-3-3-binding switch in melatonin synthesis. Proc Natl Acad Sci. (2001) 98:8083–8. doi: 10.1073/pnas.141118798
42. Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, et al. Dual role of mitochondria in producing melatonin and driving gpcr signaling to block cytochrome C release. Proc Natl Acad Sci. (2017) 114:E7997–8006. doi: 10.1073/pnas.1705768114
43. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. (2014) 71:2997–3025. doi: 10.1007/s00018-014-1579-2
44. Cardinali DP and Pévet P. Basic aspects of melatonin action. Sleep Med Rev. (1998) 2:175–90. doi: 10.1016/S1087-0792(98)90020-X
45. Hardeland R and Poeggeler B. Non-vertebrate melatonin. J Pineal Res. (2003) 34:233–41. doi: 10.1034/j.1600-079X.2003.00040.x
46. Tilden AR, Becker MA, Amma LL, Arciniega J, and McGaw AK. Melatonin production in an aerobic photosynthetic bacterium: an evolutionarily early association with darkness. J Pineal Res. (1997) 22:102–6. doi: 10.1111/j.1600-079X.1997.tb00310.x
47. Pan H, Li H, Wu S, Lai C, and Guo D. De novo biosynthesis of N-acetyltyramine in engineered escherichia coli. Enzyme Microbial Technol. (2023) 162:110149. doi: 10.1016/j.enzmictec.2022.110149
48. Pontrelli S, Chiu T-Y, Lan EI, Chen FY-H, Chang P, and Liao JC. Escherichia coli as a host for metabolic engineering. Metab Eng. (2018) 50:16–46. doi: 10.1016/j.ymben.2018.04.008
49. Auld F, Maschauer EL, Morrison I, Skene DJ, and Riha RL. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev. (2017) 34:10–22. doi: 10.1016/j.smrv.2016.06.005
50. Gubin D, Neroev V, Malishevskaya T, Cornelissen G, Astakhov SY, Kolomeichuk S, et al. Melatonin mitigates disrupted circadian rhythms, lowers intraocular pressure, and improves retinal ganglion cells function in glaucoma. J Pineal Res. (2021) 70:e12730. doi: 10.1111/jpi.12730
51. Zhang C, Clough SJ, Adamah-Biassi EB, Sveinsson MH, Hutchinson AJ, Miura I, et al. Impact of endogenous melatonin on rhythmic behaviors, reproduction, and survival revealed in melatonin-proficient C57bl/6j congenic mice. J Pineal Res. (2021) 71:e12748. doi: 10.1111/jpi.12748
52. Moradkhani F, Moloudizargari M, Fallah M, Asghari N, Heidari Khoei H, and Asghari MH. Immunoregulatory role of melatonin in cancer. J Cell Physiol. (2020) 235:745–57. doi: 10.1002/jcp.29036
53. Radogna F, Paternoster L, De Nicola M, Cerella C, Ammendola S, Bedini A, et al. Rapid and transient stimulation of intracellular reactive oxygen species by melatonin in normal and tumor leukocytes. Toxicol Appl Pharmacol. (2009) 239:37–45. doi: 10.1016/j.taap.2009.05.012
54. Kwak H-J, Liu P, Bajrami B, Xu Y, Park S-Y, Nombela-Arrieta C, et al. Myeloid cell-derived reactive oxygen species externally regulate the proliferation of myeloid progenitors in emergency granulopoiesis. Immunity. (2015) 42:159–71. doi: 10.1016/j.immuni.2014.12.017
55. Carrillo-Vico A, Lardone PJ, Fernández-Santos JM, Martín-Lacave Is, Calvo JR, Karasek M, et al. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J Clin Endocrinol Metab. (2005) 90:992–1000. doi: 10.1210/jc.2004-1429
56. Lv W-j, Liu C, Yu L-z, Zhou J-h, Li Y, Xiong Y, et al. Melatonin alleviates neuroinflammation and metabolic disorder in dss-induced depression rats. Oxid Med Cell Longev. (2020) 2020. doi: 10.1155/2020/1241894
57. Galano A, Tan DX, and Reiter RJ. Melatonin: A versatile protector against oxidative DNA damage. Molecules. (2018) 23:530. doi: 10.3390/molecules23030530
58. Galano A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys Chem Chem Phys. (2011) 13:7178–88. doi: 10.1039/c0cp02801k
59. Mekhloufi J, Bonnefont-Rousselot D, Yous S, Lesieur D, Couturier M, Thérond P, et al. Antioxidant activity of melatonin and a pinoline derivative on linoleate model system. J Pineal Res. (2005) 39:27–33. doi: 10.1111/j.1600-079X.2005.00208.x
60. Limson J, Nyokong T, and Daya S. The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study. J Pineal Res. (1998) 24:15–21. doi: 10.1111/j.1600-079X.1998.tb00361.x
61. Zatta P, Tognon G, and Carampin P. Melatonin prevents free radical formation due to the interaction between B-amyloid peptides and metal ions [Al (Iii), Zn (Ii), Cu (Ii), Mn (Ii), Fe (Ii). J Pineal Res. (2003) 35:98–103. doi: 10.1034/j.1600-079X.2003.00058.x
62. Parmar P, Limson J, Nyokong T, and Daya S. Melatonin protects against copper-mediated free radical damage. J Pineal Res. (2002) 32:237–42. doi: 10.1034/j.1600-079X.2002.01859.x
63. Mayo J, Tan D, Sainz R, Natarajan M, Lopez-Burillo S, and Reiter R. Protection against oxidative protein damage induced by metal-catalyzed reaction or alkylperoxyl radicals: comparative effects of melatonin and other antioxidants. Biochim Biophys Acta (BBA)-General Subj. (2003) 1620:139–50. doi: 10.1016/S0304-4165(02)00527-5
64. Gitto E, Tan DX, Reiter RJ, Karbownik M, Manchester LC, Cuzzocrea S, et al. Individual and synergistic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferrrioxamine (Desferoxamine) in rat liver homogenates. J Pharm Pharmacol. (2001) 53:1393–401. doi: 10.1211/0022357011777747
65. Zhao D, Yu Y, Zhao Z, Sharma R, and Reiter RJ. Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol (Lausanne). (2019) 10:441357. doi: 10.3389/fendo.2019.00249
66. Sorrentino J,S. Ovulation in pms-treated rats with gonadotropin releasing hormone after pentobarbital and melatonin block. Neuroendocrinology. (1975) 19:170–6. doi: 10.1159/000122437
67. Tamura H, Jozaki M, Tanabe M, Shirafuta Y, Mihara Y, Shinagawa M, et al. Importance of melatonin in assisted reproductive technology and ovarian aging. Int J Mol Sci. (2020) 21:1135. doi: 10.3390/ijms21031135
68. Shi JM, Tian XZ, Zhou GB, Wang L, Gao C, Zhu SE, et al. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J Pineal Res. (2009) 47:318–23. doi: 10.1111/j.1600-079X.2009.00717.x
69. Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. (2001) 16:283–301. doi: 10.1177/074873001129001980
70. Zhang L, Zhang Z, Wang J, Lv D, Zhu T, Wang F, et al. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via mt1/ampk pathway. J Pineal Res. (2019) 66:e12550. doi: 10.1111/jpi.12550
71. Ishizuka B, Fusama S, Hirai K, Hosaka T, Hamada N, Amemiya A, et al. Melatonin secretion from organ-cultured pineal glands of rats: modulation by gonadectomy and gonadotropin-releasing hormone agonist administration. Eur J Endocrinol. (2000) 142:387–92. doi: 10.1530/eje.0.1420387
72. Gao Y, Wu X, Zhao S, Zhang Y, Ma H, Yang Z, et al. Melatonin receptor depletion suppressed hcg-induced testosterone expression in mouse leydig cells. Cell Mol Biol Lett. (2019) 24:21. doi: 10.1186/s11658-019-0147-z
73. Johnston JD, Klosen P, Barrett P, and Hazlerigg DG. Regulation of mt melatonin receptor expression in the fetal rat pituitary. J Neuroendocrinol. (2006) 18:50–6. doi: 10.1111/j.1365-2826.2005.01389.x
74. Tabecka-Lonczynska A, Mytych J, Solek P, Kulpa M, and Koziorowski M. New insight on the role of melatonin receptors in reproductive processes of seasonal breeders on the example of mature male european bison (Bison bonasus, linnaeus 1758). J Photochem Photobiol B. (2017) 173:84–91. doi: 10.1016/j.jphotobiol.2017.05.026
75. Kazemzadeh S, Mohammadpour S, Madadi S, Babakhani A, Shabani M, and Khanehzad M. Melatonin in cryopreservation media improves transplantation efficiency of frozen-thawed spermatogonial stem cells into testes of azoospermic mice. Stem Cell Res Ther. (2022) 13:346. doi: 10.1186/s13287-022-03029-1
76. Deng SL, Zhang BL, Reiter RJ, and Liu YX. Melatonin ameliorates inflammation and oxidative stress by suppressing the P38mapk signaling pathway in lps-induced sheep orchitis. Antioxid (Basel). (2020) 9. doi: 10.3390/antiox9121277
77. Tian X, Wang F, He C, Zhang L, Tan D, Reiter RJ, et al. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J Pineal Res. (2014) 57:239–47. doi: 10.1111/jpi.12163
78. Tao JL, Zhang X, Zhou JQ, Li CY, Yang MH, Liu ZJ, et al. Melatonin alleviates hypoxia-induced apoptosis of granulosa cells by reducing ros and activating mtnr1b-pka-caspase8/9 pathway. Antioxid (Basel). (2021) 10. doi: 10.3390/antiox10020184
79. Showell MG, Mackenzie-Proctor R, Jordan V, and Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev. (2020) 8:Cd007807. doi: 10.1002/14651858.CD007807.pub4
80. Sagrillo-Fagundes L, Assunção Salustiano EM, Ruano R, Markus RP, and Vaillancourt C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J Pineal Res. (2018) 65:e12520. doi: 10.1111/jpi.12520
81. Veiga ECA, Samama M, Ikeda F, Cavalcanti GS, Sartor A, Parames SF, et al. Melatonin improves fertilization rate in assisted reproduction: systematic review and meta-analysis. Clinics (Sao Paulo). (2024) 79:100397. doi: 10.1016/j.clinsp.2024.100397
82. He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int J Mol Sci. (2016) 17:939. doi: 10.3390/ijms17060939
83. Izzo G, Francesco A, Ferrara D, Campitiello MR, Serino I, Minucci S, et al. Expression of melatonin (Mt1, mt2) and melatonin-related receptors in the adult rat testes and during development. Zygote. (2010) 18:257–64. doi: 10.1017/S0967199409990293
84. Jahromi BN, Sadeghi S, Alipour S, Parsanezhad ME, and Alamdarloo SM. Effect of melatonin on the outcome of assisted reproductive technique cycles in women with diminished ovarian reserve: A double-blinded randomized clinical trial. Iranian J Med Sci. (2017) 42:73.
85. Nishihara T, Hashimoto S, Ito K, Nakaoka Y, Matsumoto K, Hosoi Y, et al. Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol Endocrinol. (2014) 30:359–62. doi: 10.3109/09513590.2013.879856
86. He C, Wang J, Li Y, Zhu K, Xu Z, Song Y, et al. Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J Pineal Res. (2015) 58:300–9. doi: 10.1111/jpi.12216
87. Xi H, Huang L, Qiu L, Li S, Yan Y, Ding Y, et al. Enhancing oocyte in vitro maturation and quality by melatonin/bilirubin cationic nanoparticles: A promising strategy for assisted reproduction techniques. Int J Pharm X. (2024) 8:100268. doi: 10.1016/j.ijpx.2024.100268
88. Zhao Z, Yang L, Zhang D, Zheng Z, Li N, Li Q, et al. Elevation of mpf and mapk gene expression, gsh content and mitochondrial distribution quality induced by melatonin promotes porcine oocyte maturation and development in vitro. PeerJ. (2020) 8:e9913. doi: 10.7717/peerj.9913
89. Jitjumnong J and Tang PC. Improving the meiotic competence of small antral follicle-derived porcine oocytes by using dibutyryl-camp and melatonin. Anim Biosci. (2024) 37:1007–20. doi: 10.5713/ab.23.0371
90. Cao Z, Gao D, Tong X, Xu T, Zhang D, Wang Y, et al. Melatonin improves developmental competence of oocyte-granulosa cell complexes from porcine preantral follicles. Theriogenology. (2019) 133:149–58. doi: 10.1016/j.theriogenology.2019.05.003
91. Phuong LDT, Thien LC, Su Pham CD, Minh NU, Huy Bao NT, Thien Truc LN, et al. Melatonin and cyclic adenosine monophosphate enhance the meiotic and developmental competence of porcine oocytes from early antral follicles during in vitro growth and pre-maturation culture. Theriogenology. (2025) 237:129–42. doi: 10.1016/j.theriogenology.2025.02.026
92. Zhu T, Yan L, Deng S, Ma W, Xia F, Wang L, et al. Mitochondria of porcine oocytes synthesize melatonin, which improves their in vitro maturation and embryonic development. Antioxid (Basel). (2024) 13. doi: 10.3390/antiox13070814
93. Yong W, Ma H, Na M, Gao T, Zhang Y, Hao L, et al. Roles of melatonin in the field of reproductive medicine. Biomed Pharmacother. (2021) 144:112001. doi: 10.1016/j.biopha.2021.112001
94. Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. (2008) 44:280–7. doi: 10.1111/j.1600-079X.2007.00524.x
95. Hu K-L, Ye X, Wang S, and Zhang D. Melatonin application in assisted reproductive technology: A systematic review and meta-analysis of randomized trials. Front Endocrinol (Lausanne). (2020) 11:526732. doi: 10.3389/fendo.2020.00160
96. Van Dalum J, Melum VJ, Wood SH, and Hazlerigg DG. Maternal photoperiodic programming: melatonin and seasonal synchronization before birth. Front Endocrinol (Lausanne). (2020) 10:501607. doi: 10.3389/fendo.2019.00901
97. Cosme P, Rodríguez AB, Garrido M, and Espino J. Coping with oxidative stress in reproductive pathophysiology and assisted reproduction: melatonin as an emerging therapeutical tool. Antioxidants. (2022) 12:86. doi: 10.3390/antiox12010086
98. Espino J, Macedo M, Lozano G, Ortiz Á, Rodríguez C, Rodríguez AB, et al. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants. (2019) 8:338. doi: 10.3390/antiox8090338
99. Tsui KH, Li CJ, and Lin LT. Melatonin supplementation attenuates cuproptosis and ferroptosis in aging cumulus and granulosa cells: potential for improving ivf outcomes in advanced maternal age. Reprod Biol Endocrinol. (2024) 22:138. doi: 10.1186/s12958-024-01311-w
100. Mokhtari F, Akbari Asbagh F, Azmoodeh O, Bakhtiyari M, and Almasi-Hashiani A. Effects of melatonin administration on chemical pregnancy rates of polycystic ovary syndrome patients undergoing intrauterine insemination: A randomized clinical trial. Int J Fertil Steril. (2019) 13:225–9. doi: 10.22074/ijfs.2019.5717
101. Fernando S, Wallace EM, Vollenhoven B, Lolatgis N, Hope N, Wong M, et al. Melatonin in assisted reproductive technology: A pilot double-blind randomized placebo-controlled clinical trial. Front Endocrinol (Lausanne). (2018) 9:545. doi: 10.3389/fendo.2018.00545
Keywords: melatonin, biosynthesis, antioxidant, inflammatory response and assisted reproduction, MT1, MT2
Citation: Zhong J, Lu Z, Zhou Z, Ma N, Li Y, Hu JJ, Wan B and Lu W (2025) Melatonin biosynthesis and regulation in reproduction. Front. Endocrinol. 16:1630164. doi: 10.3389/fendo.2025.1630164
Received: 17 May 2025; Accepted: 09 July 2025;
Published: 28 July 2025.
Edited by:
Nazli Akin, Vrije University Brussels, Brussels, BelgiumReviewed by:
Yuan Gao, Gansu Agricultural University, Lanzhou, ChinaHong-Thuy Bui, International University, Vietnam
Copyright © 2025 Zhong, Lu, Zhou, Ma, Li, Hu, Wan and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bangbei Wan, OTM5MzEzNjEyQHFxLmNvbQ==; Weiying Lu, aG4xMjEwMTgwMjFAMTYzLmNvbQ==
 Jingjing Zhong
Jingjing Zhong Zhiyong Lu
Zhiyong Lu JiaJia Hu
JiaJia Hu Bangbei Wan
Bangbei Wan Weiying Lu
Weiying Lu