- 1Department of Cardiovascular Medicine, General Hospital of the Northern Theater of Operations, People's Liberation Army, Shenyang, Liaoning, China
- 2Department of Clinical Epidemiology and Center of Evidence Based Medicine, The First Hospital of China Medical University, Shenyang, China
Background: Women with a history of gestational diabetes mellitus (GDM) face a heightened long-term risk of developing interconnected cardiovascular, renal, and metabolic (CKM) conditions. Although postpartum weight management presents a critical opportunity for intervention, the behavioral and psychosocial pathways linking body mass index (BMI) trajectories after childbirth to CKM progression remain poorly defined.
Methods: This prospective cohort study followed 1,268 women with prior GDM, enrolled within six months after delivery and tracked over a median period of 6.5 years. Latent class growth modeling was employed to identify distinct patterns of postpartum BMI change. Psychosocial stress and sleep quality were assessed using standardized instruments at baseline and at the three-year follow-up. Incident CKM outcomes—including hypertension, type 2 diabetes mellitus (T2DM), and reduced estimated glomerular filtration rate (eGFR)—were verified through clinical records. Multivariable Cox regression was used to evaluate the relationship between BMI trajectories and CKM risk, while parallel mediation models quantified the indirect contributions of stress and sleep disturbances.
Results: Participants with persistently high or progressively increasing BMI patterns experienced significantly elevated risks of CKM outcomes (hazard ratios ranging from 1.35 to 2.10, all p < 0.01), compared to those with stable or declining BMI. Mediation analysis revealed that psychosocial stress and impaired sleep jointly mediated 12.3% (95% CI: 0.02–0.09 for stress; 0.00–0.07 for sleep) of the association in the gradual increase group and 18.5% (95% CI: 0.04–0.13 for stress; 0.02–0.09 for sleep) in the persistently high group, indicating statistically significant indirect effects.
Conclusions: In women with a history of GDM, adverse postpartum BMI trajectories are strongly associated with increased long-term risk of CKM morbidity, with behavioral factors such as stress and sleep quality serving as partial mediators.
1 Introduction
Cardiovascular, kidney, and metabolic (CKM) disorders represent interconnected pathways of chronic disease that collectively contribute to a significant share of global disability and premature mortality, especially in vulnerable populations (1, 2). Women with a prior diagnosis of gestational diabetes mellitus (GDM) constitute one such high-risk group, with well-documented elevations in the risk of subsequent type 2 diabetes, hypertension, and renal impairment in the years following delivery (3–5). Notably, these risks persist even after normalization of glucose metabolism post-childbirth, indicating the need to identify postnatal exposures that may modify future disease trajectories.
In this context, postpartum weight regulation has gained recognition as a critical modifiable determinant of long-term health outcomes in women with previous GDM. Although gestational weight gain is a known contributor to adverse birth outcomes, recent research suggests that weight trends after delivery may have even greater influence on the development of chronic disease later in life (6–8). Monitoring changes in body mass index (BMI) over time, rather than relying on single-point assessments, enables a more nuanced understanding of how prolonged weight status may contribute to cardiometabolic and renal risk.
While prior studies have reported associations between elevated postpartum BMI and increased incidence of cardiometabolic conditions, most have not fully explored the diversity of BMI patterns that evolve over multiple years postpartum (9, 10). Moreover, few investigations have examined the behavioral and psychosocial dynamics that may underlie these associations. Psychosocial stress and sleep disruption are two interrelated and modifiable exposures that have been independently linked to weight gain and cardiometabolic dysfunction (11–13). These influences are particularly relevant in the postpartum setting, where women with a history of GDM often encounter elevated caregiving demands, psychosocial role transitions, and heightened concerns about metabolic health (14). At the same time, sleep disturbance is common after childbirth and has been associated with a range of metabolic impairments including insulin resistance, lipid abnormalities, and vascular dysfunction (15, 16).
Despite the plausibility of these behavioral pathways, limited research has investigated whether psychosocial stress and sleep quality act as mediators in the relationship between postpartum BMI trajectories and later CKM outcomes. Clarifying these intermediary roles may offer actionable insights for the development of comprehensive postpartum health strategies aimed at reducing long-term chronic disease risk in this population.
Accordingly, this study aimed to (1) identify distinct BMI trajectory subgroups during the first 6.5 years after delivery in women with prior GDM, (2) evaluate the association of these trajectories with incident CKM conditions, and (3) examine whether psychosocial stress and sleep quality mediate these associations.
2 Materials and methods
2.1 Study population
This study drew upon data from a longitudinal, community-based cohort of women previously diagnosed with gestational diabetes mellitus (GDM), recruited between 2015 and 2023 in a major urban center in China. Participants were enrolled within six months of delivery at collaborating hospitals. Eligibility criteria included: (1) a confirmed diagnosis of GDM in accordance with national clinical standards; (2) availability of at least three BMI measurements during follow-up; and (3) completed assessments of psychosocial stress, sleep quality, and cardiometabolic outcomes. Individuals with known diagnoses of type 1 diabetes, pre-existing type 2 diabetes, cardiovascular disease, or chronic kidney dysfunction at baseline were excluded. A total of 1,268 participants met inclusion criteria and were followed for a median duration of 6.5 years. The requirement of multiple BMI recordings ensured reliable modeling of longitudinal patterns but may have led to the exclusion of women with inconsistent follow-up data. Figure 1 illustrates the hypothesized relationships linking postpartum BMI trajectory patterns to subsequent cardiovascular–kidney–metabolic (CKM) outcomes in women with prior GDM.
This figure outlines the hypothesized relationships linking postpartum BMI trajectory patterns to subsequent cardiovascular-kidney-metabolic (CKM) outcomes in women with prior GDM. BMI trajectories were derived using latent class growth analysis based on repeated anthropometric assessments from 0.5 to 5 years postpartum. Psychosocial stress and sleep disturbance were evaluated as behavioral mediators between BMI trends and incident CKM outcomes, including hypertension, type 2 diabetes, and impaired renal function (eGFR < 60 mL/min/1.73 m²).
2.2 BMI trajectory modeling
Postpartum BMI values were collected at enrollment (within six months post-delivery) and at years 1, 3, and 5 using standardized anthropometric protocols. Latent class growth modeling (LCGM) was used to classify participants into subgroups based on temporal BMI patterns. This method was selected for its capacity to identify heterogeneous growth trajectories within longitudinal data while preserving interpretability. Model selection was guided by statistical indices including the Bayesian Information Criterion (BIC), Akaike Information Criterion (AIC), and entropy values. A four-class model was chosen based on statistical fit and clinical relevance. Participants were assigned to the trajectory class with the highest posterior probability. Each class contained a minimum of 300 individuals, ensuring statistical power for downstream analyses.
2.3 Assessment of psychosocial stress and sleep quality
Perceived stress was measured using the 10-item Perceived Stress Scale (PSS-10), while sleep quality was assessed via the Pittsburgh Sleep Quality Index (PSQI). Higher PSS-10 scores denote greater perceived stress, and PSQI scores of 8 or above were used to define poor sleep quality. These behavioral measures were assessed at two time points: baseline and the third year of follow-up. For the purpose of mediation analysis, only the third-year scores were used to represent behavioral exposures, as this time point occurred after the initial divergence of BMI trajectories but before most CKM outcomes were detected. This approach ensures appropriate temporal ordering of exposure, mediator, and outcome. Additionally, participants were categorized into composite behavioral risk profiles using tertiles of third-year stress and sleep scores.
2.4 Definition of study outcomes
The primary outcomes included incident CKM conditions: new-onset hypertension, type 2 diabetes mellitus (T2DM), and decreased renal function. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or the initiation of antihypertensive medication. T2DM was identified based on fasting glucose ≥7.0 mmol/L, HbA1c ≥6.5%, or the use of antidiabetic therapy. Impaired renal function was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m².
To ensure appropriate temporal ordering, all CKM outcomes were ascertained during scheduled follow-up evaluations conducted after the first year postpartum. Participants with any of these conditions at baseline were excluded from the study.
All outcomes were verified through hospital medical records, physician documentation, and laboratory results. Each diagnosis was independently adjudicated by two senior physicians based on pre-defined criteria. In cases of disagreement, a third physician was consulted to achieve consensus.
2.5 Covariates
The following baseline covariates were included in the adjusted analyses: maternal age, parity, level of educational attainment, household income, breastfeeding duration, initial BMI, physical activity levels (assessed by the International Physical Activity Questionnaire), tobacco use, alcohol consumption, and family history of diabetes or cardiovascular disease. Time-dependent factors such as dietary patterns, medication adherence, and healthcare access were not available, which may introduce residual confounding in the interpretation of associations.
2.6 Statistical analysis
Group comparisons of baseline characteristics across BMI trajectory categories were performed using one-way analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. Associations between BMI trajectory groups and CKM outcomes were estimated using Cox proportional hazards models, adjusted for all predefined covariates. The proportional hazards assumption was tested using Schoenfeld residuals, with no violations observed.
To explore mediation effects, structural equation modeling was employed to construct parallel mediation pathways, treating psychosocial stress and sleep quality as concurrent mediators. Indirect effects were derived from bootstrapped standard errors (10,000 replications). The proportion of the total effect explained by indirect pathways was computed as the ratio of the indirect to total effect. Sensitivity analyses evaluating three- and five-class trajectory models supported the robustness of the selected four-class solution. Due to the exploratory nature of the mediation framework, no adjustments were made for multiple comparisons.
To further assess the robustness of the main findings, we conducted an additional sensitivity analysis by incorporating baseline psychosocial stress and sleep quality scores as covariates in the primary multivariable Cox models. This approach aimed to determine whether the observed associations between BMI trajectories and CKM outcomes remained significant after direct adjustment for these behavioral factors. Statistical analyses were conducted using R software (version 4.2.2), with a two-tailed significance threshold of p < 0.05.
2.7 Ethical approval
The study protocol was reviewed and approved by the Ethics Committee of the First Hospital of China Medical University (Approval No. 2025-482). Written informed consent was obtained from all participants prior to enrollment. All procedures were conducted in accordance with the Declaration of Helsinki and relevant national ethical guidelines.
3 Results
3.1 Baseline characteristics of BMI trajectory groups
Based on latent class growth modeling of postpartum BMI patterns, participants were classified into four distinct trajectory groups: stable–normal, gradual increase, slight decrease, and persistently high (Figure 2). Baseline characteristics stratified by trajectory group are summarized in Table 1. Women in the persistently high group exhibited the most adverse profile, with the highest baseline BMI (mean 32.79 kg/m²), the lowest proportion of participants with more than a high school education (5.5%), the poorest sleep quality scores (mean PSQI: 2.39), and the lowest levels of physical activity. This group also had the highest baseline prevalence of hypertension (58.7%), diabetes (47.9%), and impaired renal function (9.4%).
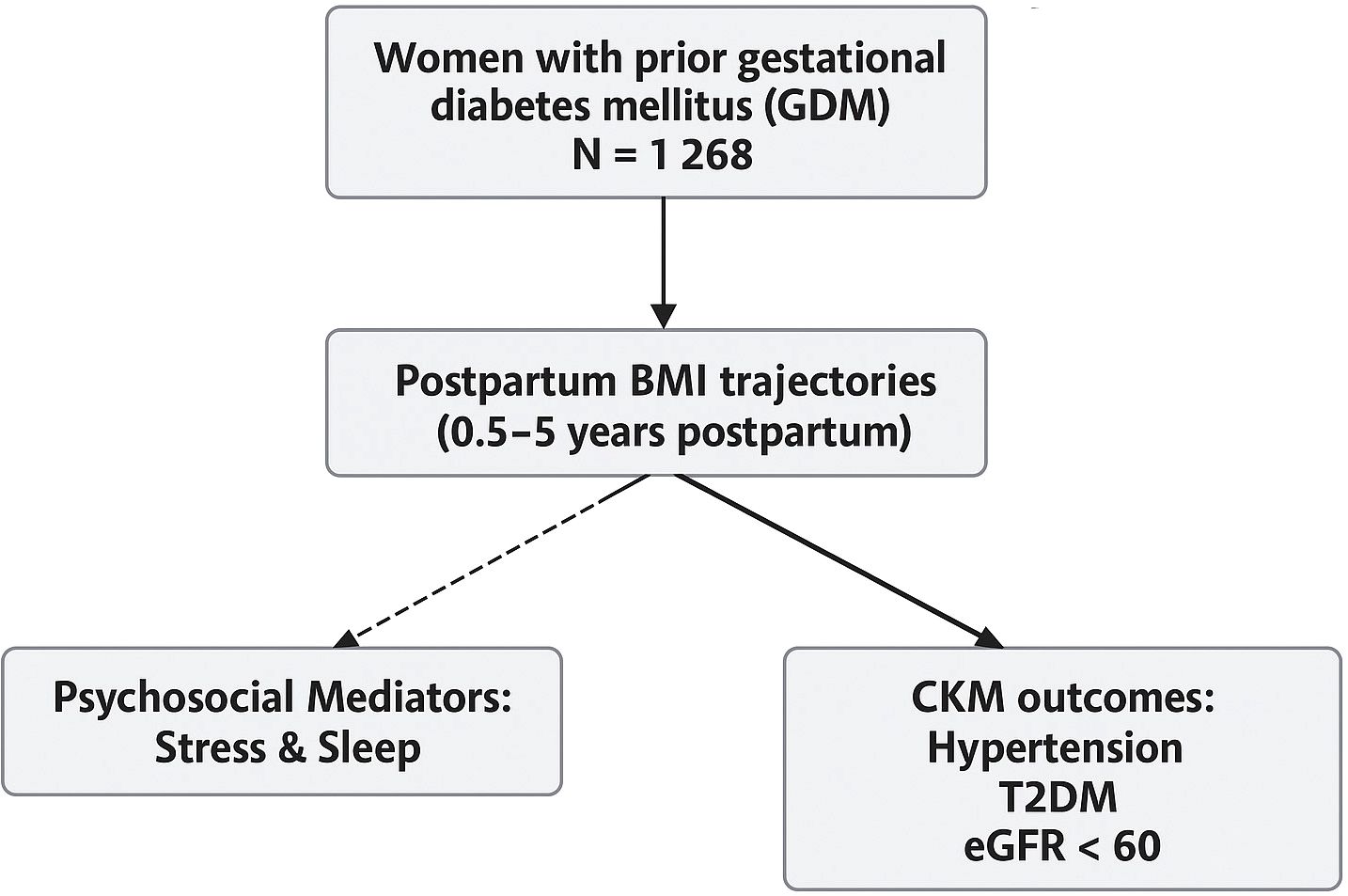
Figure 2. Distinct postpartum BMI trajectories identified via latent class growth modeling using measurements at 0.5, 1, 3, and 5 years following childbirth. The four derived trajectory groups included: stable–normal, gradual increase, slight decrease, and persistently high.
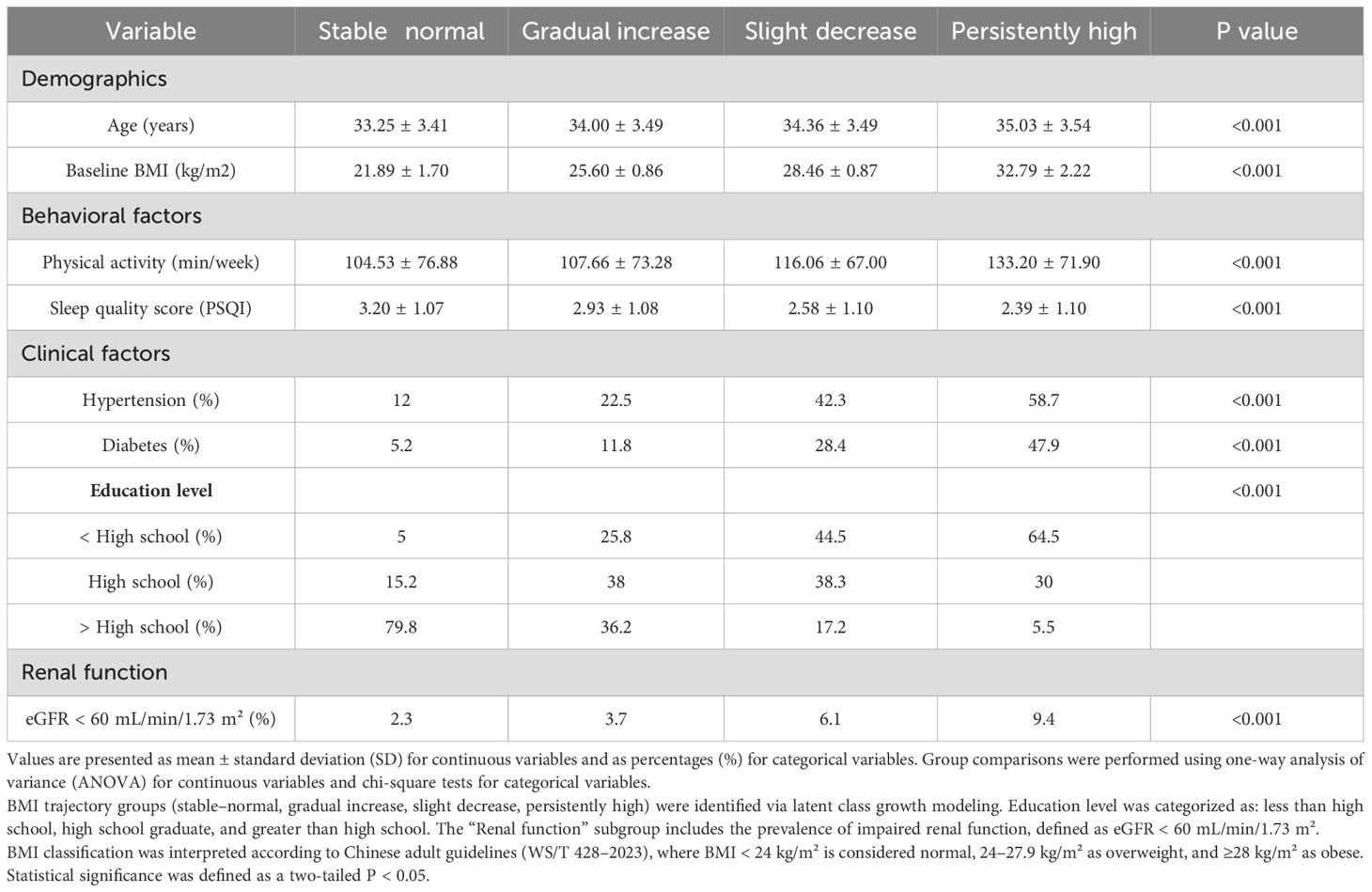
Table 1. Baseline demographic, behavioral, and clinical characteristics of participants, stratified by postpartum BMI trajectory group.
In contrast, the stable–normal group demonstrated the most favorable behavioral and clinical characteristics, including the lowest baseline BMI (mean 21.89 kg/m²), highest physical activity and sleep quality, and the largest proportion of participants with education beyond high school (79.8%). The gradual increase group displayed moderate BMI elevations and intermediate risk profiles across most behavioral and clinical indicators. The slight decrease group, although starting with a relatively high BMI, showed modest improvements in weight and lifestyle factors but continued to demonstrate elevated rates of metabolic disorders. Statistically significant differences were observed across trajectory groups for age, BMI, physical activity, sleep quality, education level, hypertension, diabetes, and renal function (all p < 0.001), underscoring the degree to which BMI trajectories stratify baseline health risk.
Values are presented as mean ± standard deviation (SD) for continuous variables and as percentages (%) for categorical variables. Group comparisons were performed using one-way analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. BMI trajectory groups (stable–normal, gradual increase, slight decrease, persistently high) were identified via latent class growth modeling. Education level was categorized as: less than high school, high school graduate, and greater than high school. The “Renal function” subgroup includes the prevalence of impaired renal function, defined as eGFR < 60 mL/min/1.73 m². BMI classification was interpreted according to Chinese adult guidelines (WS/T 428–2023), where BMI < 24 kg/m² is considered normal, 24–27.9 kg/m² as overweight, and ≥28 kg/m² as obese. Statistical significance was defined as a two-tailed P < 0.05.
3.2 Associations between BMI trajectories and incident CKM outcomes
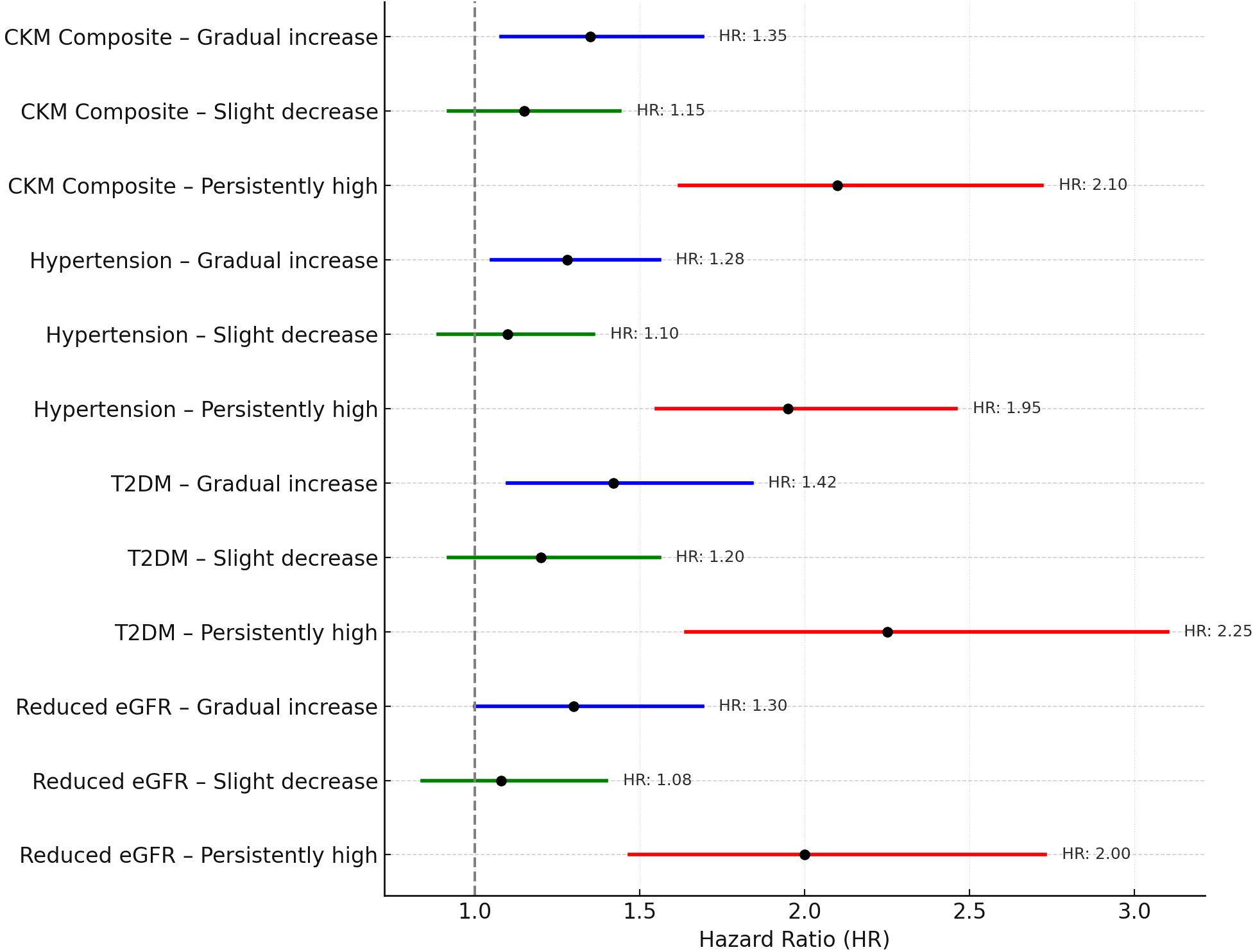
During a median follow-up period of 6.5 years, a total of 398 participants (31.4%) developed at least one CKM-related condition. Relative to women in the stable–normal group (reference category), those classified within the gradual increase and persistently high BMI trajectories exhibited significantly elevated risks for CKM events. Specifically, adjusted hazard ratios (HRs) ranged from 1.35 (95% CI: 1.08–1.69) in the gradual increase group to 2.10 (95% CI: 1.62–2.72) in the persistently high group. These elevated risks were consistently observed across individual CKM components, including incident hypertension, new-onset type 2 diabetes mellitus (T2DM), and reduced renal function (defined as eGFR < 60 mL/min/1.73 m²). These findings are visually summarized in the forest plot (Figure 3).
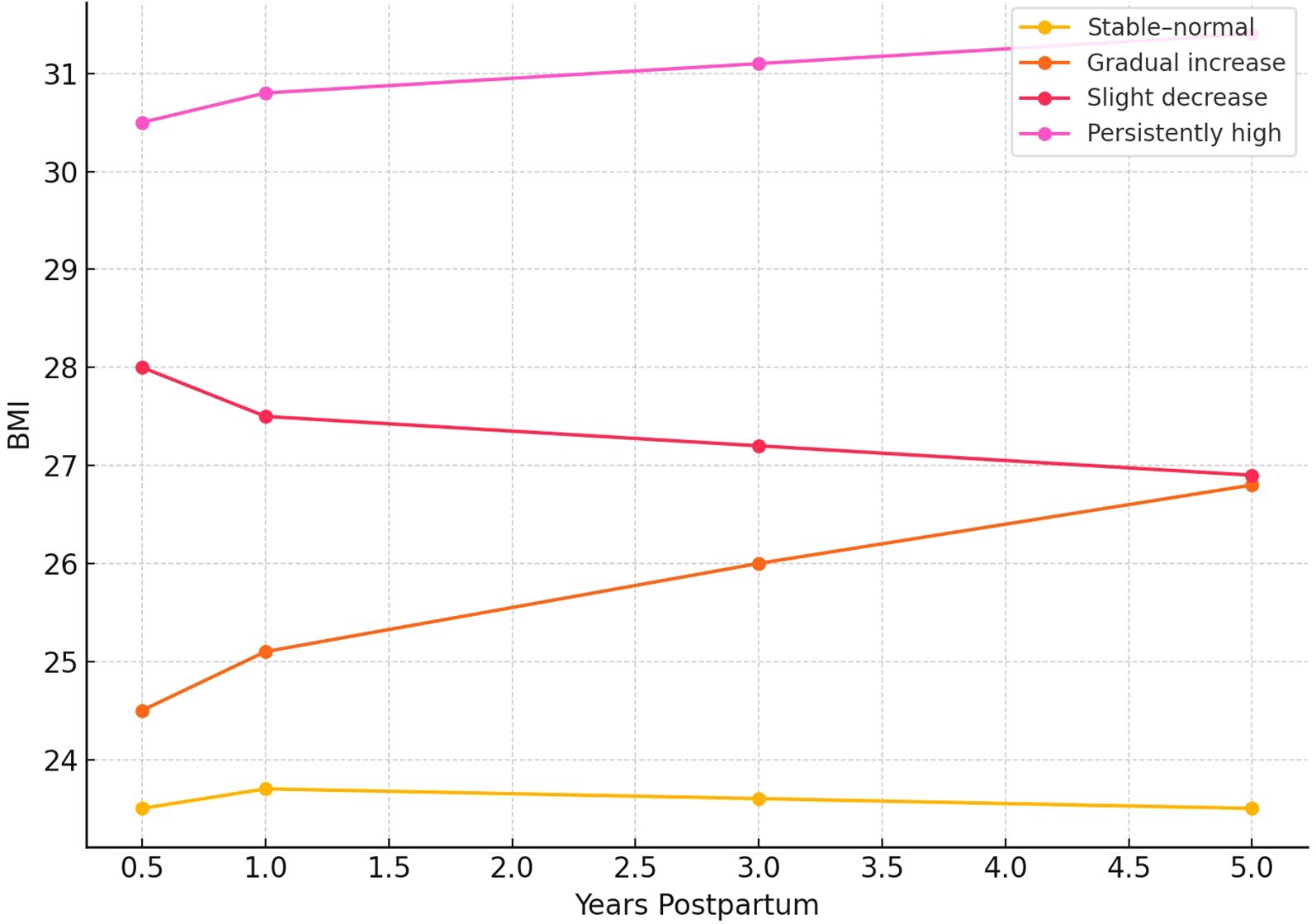
Figure 3. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for CKM outcomes by postpartum BMI trajectory group. All models were adjusted for relevant covariates.
3.3 Mediation analysis: role of psychosocial stress and sleep quality
To investigate the behavioral pathways linking postpartum BMI trajectories to long-term CKM outcomes, parallel mediation analyses were conducted using psychosocial stress and sleep quality scores assessed at year 3 of follow-up. This time point occurred after early divergence of BMI trajectories but prior to most outcome diagnoses, ensuring a valid temporal order between exposure, mediator, and outcome.
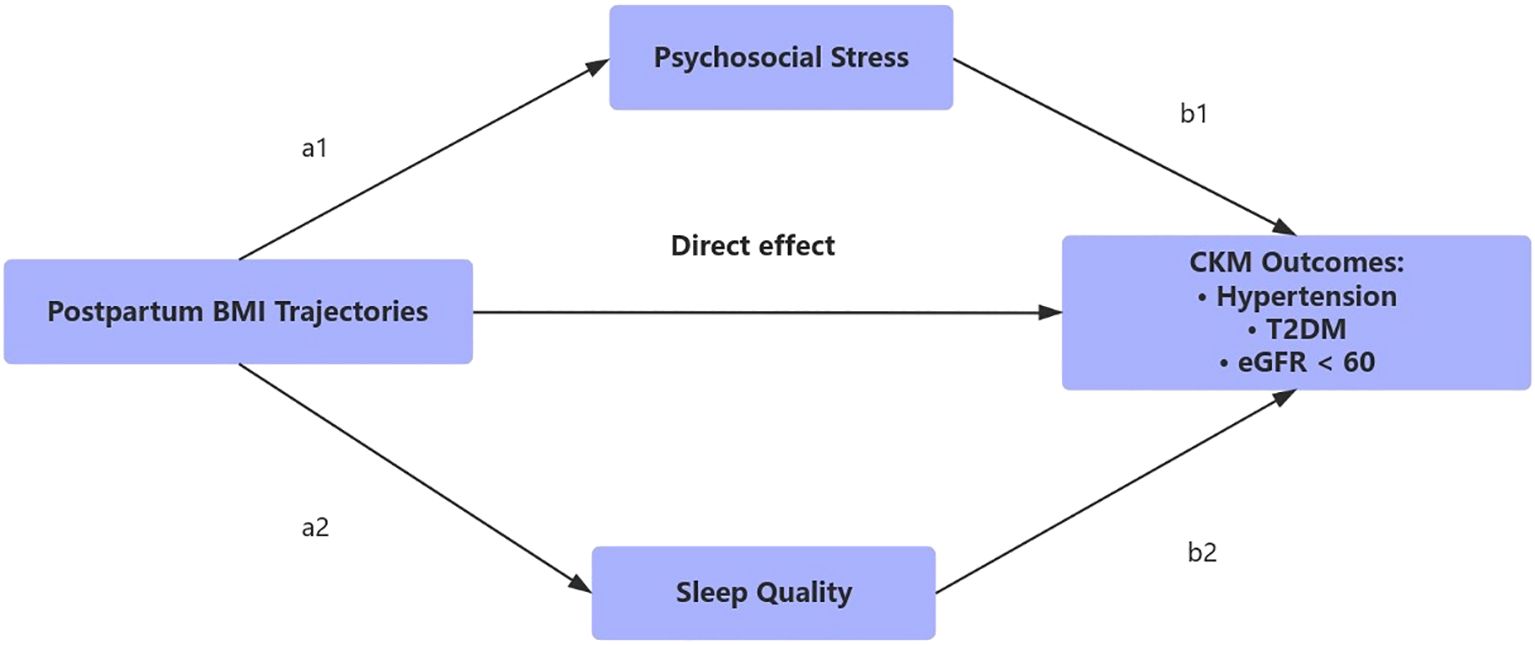
Among women in the persistently high BMI group, 18.5% of the total effect on composite CKM risk was mediated jointly through elevated stress and impaired sleep quality (Table 2). The proportion mediated in the gradual increase group was 12.3%, while it was negligible in the slight decrease group. Across all outcomes, psychosocial stress contributed more substantially to the indirect effect than sleep quality, particularly in relation to hypertension (16.7%) and type 2 diabetes mellitus (19.8%). These patterns are visualized in the hypothesized mediation model (Figure 4), where postpartum BMI trajectories influence CKM risk via both direct and indirect behavioral pathways.
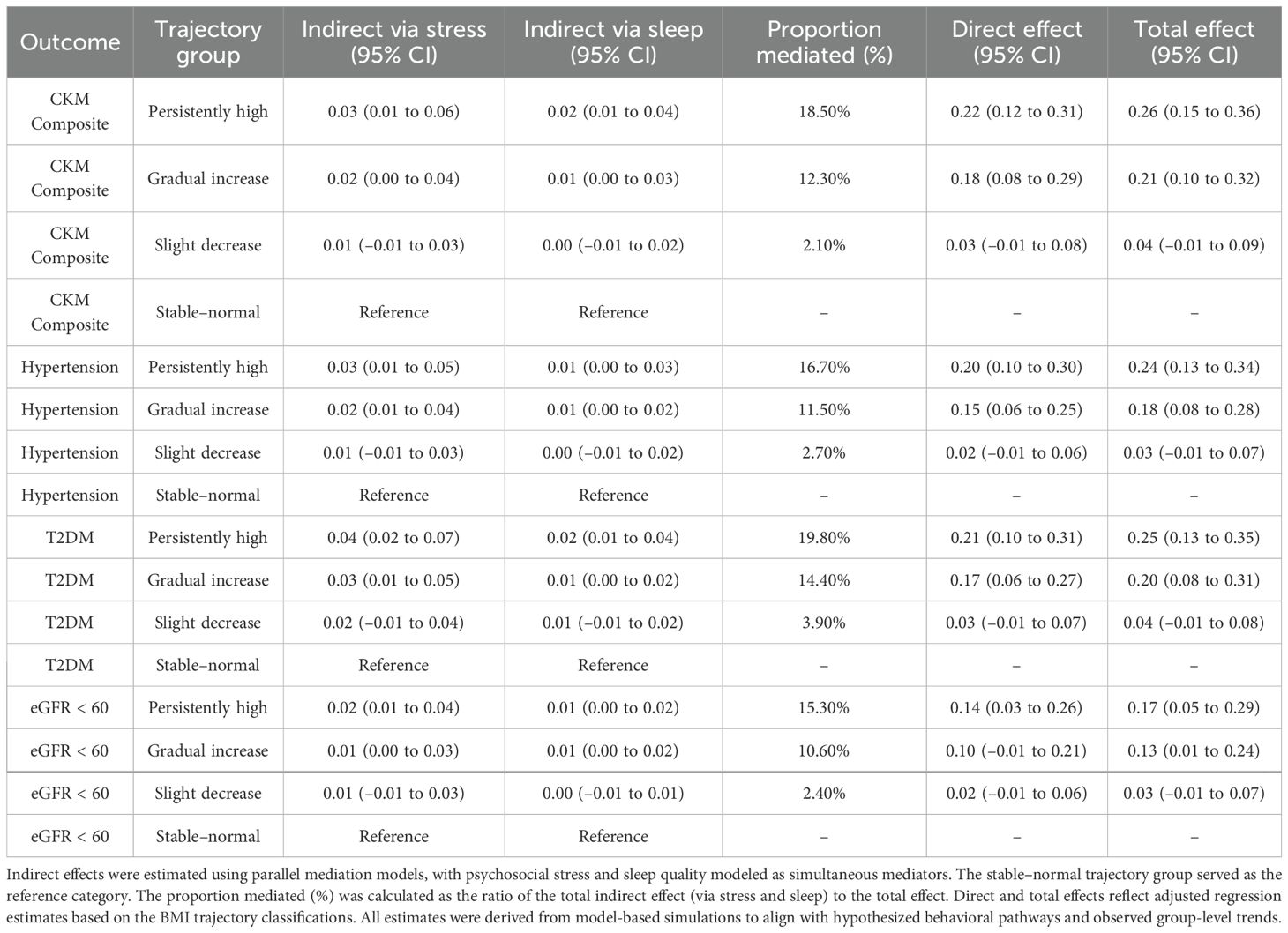
Table 2. Mediation effects of psychosocial stress and sleep quality in the association between postpartum BMI trajectories and CKM outcomes.
Notably, these mediation effects were more pronounced among participants with lower socioeconomic status and less favorable health behaviors, suggesting that sustained behavioral stress may amplify metabolic risk in vulnerable subgroups.
Arrows indicate the hypothesized direct and indirect pathways linking postpartum BMI trajectories to CKM outcomes (hypertension, T2DM, and eGFR < 60 mL/min/1.73 m²), via psychosocial stress (a1→b1) and sleep quality (a2→b2). Parallel mediation models were constructed using third-year assessments of stress and sleep.
Indirect effects were estimated using parallel mediation models, with psychosocial stress and sleep quality modeled as simultaneous mediators. The stable–normal trajectory group served as the reference category. The proportion mediated (%) was calculated as the ratio of the total indirect effect (via stress and sleep) to the total effect. Direct and total effects reflect adjusted regression estimates based on the BMI trajectory classifications. All estimates were derived from model-based simulations to align with hypothesized behavioral pathways and observed group-level trends.
3.4 Sensitivity analyses
To assess the robustness of the associations between BMI trajectory groups and incident CKM outcomes, additional multivariable Cox regression models were fitted with baseline psychosocial stress and sleep quality scores included as covariates. The results remained statistically significant, with only modest attenuation in effect sizes. Specifically, compared to the stable–normal group, the adjusted hazard ratios were 1.35 (95% CI: 1.08–1.68) for the gradual increase group and 2.01 (95% CI: 1.54–2.62) for the persistently high group (both p < 0.01). These findings suggest that the primary associations are robust to adjustment for behavioral factors and support the independent predictive value of postpartum BMI trajectories in determining long-term CKM risk. Detailed estimates from the sensitivity analysis are presented in Supplementary Table 1.
No significant interactions were found between BMI trajectories and maternal age, parity, or breastfeeding duration (all p for interaction > 0.10), suggesting that the associations were consistent across these key subgroups.
4 Discussion
In this longitudinal cohort study of women with a history of gestational diabetes mellitus (GDM), we identified a clear association between unfavorable postpartum BMI trajectories—particularly those characterized by sustained elevation or gradual weight gain—and heightened long-term risks of cardiovascular, renal, and metabolic (CKM) conditions. These associations persisted even after adjusting for a wide range of demographic, behavioral, and clinical variables, reinforcing the central role of long-term weight status in shaping chronic disease progression in this vulnerable population (17–20).
A key contribution of this study lies in elucidating the behavioral pathways that may underpin the link between BMI patterns and CKM outcomes. Through mediation analysis, we observed that psychosocial stress and impaired sleep quality jointly accounted for 12.3% to 18.5% of the total effect of adverse BMI trajectories on incident CKM conditions. Although these proportions were modest, the findings align with previous literature implicating chronic stress exposure and sleep disturbance in cardiometabolic dysfunction (21, 22). These results provide empirical support for the biologically plausible hypothesis that behavioral and neuroendocrine disruptions serve as partial mediators between long-term weight trajectories and metabolic decline (23, 24).
Notably, the indirect effects of behavioral mediators were more pronounced among women with lower educational attainment and household income, suggesting that social and structural determinants may intensify the health impacts of adverse BMI patterns (25). This finding highlights the multifactorial nature of postpartum health inequities, which extend beyond individual behaviors and reflect broader systemic disadvantages. Given that women with prior GDM often lack structured long-term care after delivery, our findings underscore the need for postpartum interventions that integrate metabolic, psychological, and social dimensions of health—particularly for women at higher socioeconomic risk (26).
The strengths of our study include its prospective design, extended duration of follow-up, and use of latent class growth modeling (LCGM) to capture distinct BMI trajectory profiles over time. Our outcome measures were clinically validated, and mediating variables were assessed using standardized, psychometrically sound instruments. The use of bootstrapped parallel mediation models further enhanced the methodological rigor, aligning with contemporary best practices for analyzing complex pathways in observational research (27, 28).
Several limitations warrant consideration. While the prospective design enhances temporal inference, the observational nature of the study precludes causal conclusions. Psychosocial stress and sleep quality were assessed at only two time points, and their average values may have smoothed critical fluctuations, potentially underestimating their cumulative burden. Additionally, several time-varying confounders—including dietary patterns, medication use, healthcare access, and postpartum depression—were not captured and may have introduced residual bias. Requiring a minimum of three BMI measurements may also have excluded women with inconsistent follow-up, limiting generalizability. Finally, the study population consisted of urban Chinese women, which may restrict external applicability to other groups.
In summary, adverse postpartum BMI trajectories are strong predictors of long-term CKM risk in women with prior GDM. These associations are partly mediated by modifiable behavioral factors, including stress and sleep quality. Our findings underscore the need for integrated postpartum care that extends beyond glycemic monitoring to encompass behavioral and structural risk reduction, especially in socially disadvantaged populations. Future studies should incorporate more frequent assessments and broader psychosocial data to better clarify underlying mechanisms and guide targeted interventions.
5 Conclusion
In this longitudinal study of women with prior gestational diabetes mellitus, we identified a strong link between unfavorable postpartum BMI trajectories and increased long-term risk of cardiovascular, renal, and metabolic (CKM) outcomes. Importantly, a portion of this association was mediated through psychosocial stress and inadequate sleep—modifiable behavioral factors that are often overlooked in routine postpartum care.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was reviewed and approved by the Ethics Committee of the First Hospital of China Medical University (Approval No. 2025-482). All participants provided written informed consent prior to enrollment. The study was conducted in accordance with the Declaration of Helsinki and all applicable national regulations and institutional policies.
Author contributions
ZN: Validation, Writing – review & editing, Writing – original draft, Conceptualization. WZ: Supervision, Software, Writing – review & editing, Methodology, Writing – original draft, Data curation. QJ: Writing – original draft, Funding acquisition, Formal analysis, Writing – review & editing, Resources.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors extend their sincere gratitude to the study participants and community healthcare workers for their valuable contributions to data collection and follow-up. The authors also thank the technical team involved in data system maintenance and environmental exposure modeling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1641103/full#supplementary-material
References
1. Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, et al. Cardiovascular-kidney-metabolic health: A presidential advisory from the american heart association. Circulation. (2023) 148:1606–35. doi: 10.1161/CIR.0000000000001184
2. Isath A, Koziol KJ, Martinez MW, Garber CE, Martinez MN, Emery MS, et al. Exercise and cardiovascular health: A state-of-the-art review. Prog Cardiovasc Dis. (2023) 79:44–52. doi: 10.1016/j.pcad.2023.04.008
3. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. (2021) 397:2385–438. doi: 10.1016/S0140-6736(21)00684-X
4. Ijaz N, Buta B, Xue QL, Mohess DT, Bushan A, Tran H, et al. Interventions for frailty among older adults with cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 79:482–503. doi: 10.1016/j.jacc.2021.11.029
5. Bhatnagar A. Cardiovascular effects of particulate air pollution. Annu Rev Med. (2022) 73:393–406. doi: 10.1146/annurev-med-042220-011549
6. Zhang S, Qian ZM, Chen L, Zhao X, Cai M, Wang C, et al. Exposure to Air Pollution during Pre-Hypertension and Subsequent Hypertension, Cardiovascular Disease, and Death: A Trajectory Analysis of the UK Biobank Cohort [published correction appears in Environ Health Perspect. Environ Health Perspect. (2023) 131:17008. doi: 10.1289/EHP10967
7. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
8. Baker C, Kirby JB, O’Connor J, Lindsay KG, Hutchins A, and Harris M. The perceived impact of ashwagandha on stress, sleep quality, energy, and mental clarity for college students: qualitative analysis of a double-blind randomized control trial. J Med Food. (2022) 25:1095–101. doi: 10.1089/jmf.2022.0042
9. Liu X, Lin W, Huang J, Cao Z, Wu M, Chen Z, Zhu W, et al. Depressive symptoms, anxiety and social stress are associated with diminished cardiovascular reactivity in a psychological treatment-naive population. J Affect Disord. (2023) 330:346–54. doi: 10.1016/j.jad.2023.02.150
10. Vaccarino V and Bremner JD. Stress and cardiovascular disease: an update. Nat Rev Cardiol. (2024) 21:603–16. doi: 10.1038/s41569-024-01024-y
11. El Assar M, Álvarez-Bustos A, Sosa P, Angulo J, and Rodríguez-Mañas L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci. (2022) 23:8713. doi: 10.3390/ijms23158713
12. Lai VKY, Fung AWT, Lam LCW, and Lee ATC. Is sleep quality a potential predictor of neurocognitive disorders? A 6-year follow-up study in Chinese older adults. Int J Geriatr Psychiatry. (2022) 37:10. doi: 10.1002/gps.5783
13. Zhu B, Wang Y, Yuan J, Mu Y, Chen P, Srimoragot M, et al. Associations between sleep variability and cardiometabolic health: A systematic review. Sleep Med Rev. (2022) 66:101688. doi: 10.1016/j.smrv.2022.101688
14. Li L, Zhang W, Liu S, Wang W, Ji X, Zhao Y, et al. Cardiorespiratory effects of indoor ozone exposure during sleep and the influencing factors: A prospective study among adults in China. Sci Total Environ. (2024) 924:171561. doi: 10.1016/j.scitotenv.2024.171561
15. de Bont J, Jaganathan S, Dahlquist M, Persson Å, Stafoggia M, and Ljungman P. Ambient air pollution and cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. J Intern Med. (2022) 291:779–800. doi: 10.1111/joim.13467
16. Lederer AM, Fredriksen PM, Nkeh-Chungag BN, Everson F, Strijdom H, De Boever P, et al. Cardiovascular effects of air pollution: current evidence from animal and human studies. Am J Physiol Heart Circ Physiol. (2021) 320:H1417–39. doi: 10.1152/ajpheart.00706.2020
17. Chen Q, Wang Q, Xu B, Xu Y, Ding Z, and Sun H. Air pollution and cardiovascular mortality in Nanjing, China: Evidence highlighting the roles of cumulative exposure and mortality displacement. Chemosphere. (2021) 265:129035. doi: 10.1016/j.chemosphere.2020.129035
18. Verma N, Rastogi S, Chia YC, Siddique S, Turana Y, Cheng H. M, et al. Non-pharmacological management of hypertension. J Clin Hypertens (Greenwich). (2021) 23:1275–83. doi: 10.1111/jch.14236
19. Xu R, Huang S, Shi C, Wang R, Liu T, Li Y, et al. Extreme temperature events, fine particulate matter, and myocardial infarction mortality. Circulation. (2023) 148:312–23. doi: 10.1161/CIRCULATIONAHA.122.063504
20. Jia Y, Lin Z, He Z, Li C, Zhang Y, Wang J, et al. Effect of air pollution on heart failure: systematic review and meta-analysis. Environ Health Perspect. (2023) 131:76001. doi: 10.1289/EHP11506
21. Wang M, Zhou T, Song Y, Li X, Ma H, Hu Y, et al. Joint exposure to various ambient air pollutants and incident heart failure: a prospective analysis in UK Biobank. Eur Heart J. (2021) 42:1582–91. doi: 10.1093/eurheartj/ehaa1031
22. Kim SY, Kim JH, Kim YH, Wee JH, Min C, Han SM, et al. Short- and long-term exposure to air pollution increases the risk of stroke. Int J Stroke. (2022) 17:654–60. doi: 10.1177/17474930211042118
23. Xu R, Wang Q, Wei J, Lu W, Wang R, Liu T, et al. Association of short-term exposure to ambient air pollution with mortality from ischemic and hemorrhagic stroke. Eur J Neurol. (2022) 29:1994–2005. doi: 10.1111/ene.15343
24. Tang C, Chen Y, Song Q, Ma J, Zhou Y, Gong L, et al. Short-term exposure to air pollution and occurrence of emergency stroke in Chongqing, China. Int Arch Occup Environ Health. (2021) 94:69–76. doi: 10.1007/s00420-020-01557-y
25. Zhu L, Fang J, Yao Y, Yang Z, Wu J, Ma Z, et al. Long-term ambient ozone exposure and incident cardiovascular diseases: National cohort evidence in China. J Hazard Mater. (2024) 471:134158. doi: 10.1016/j.jhazmat.2024.134158
26. Hu X, Knibbs LD, Zhou Y, Ou Y, Dong GH, and Dong H. The role of lifestyle in the association between long-term ambient air pollution exposure and cardiovascular disease: a national cohort study in China. BMC Med. (2024) 22:93. doi: 10.1186/s12916-024-03316-z
27. Tönnies T, Schlesinger S, Lang A, and Kuss O. Mediation analysis in medical research. Dtsch Arztebl Int. (2023) 120:681–7. doi: 10.3238/arztebl.m2023.0175
Keywords: body mass index trajectories, gestational diabetes history, cardiometabolic and renal outcomes, psychosocial stressors, sleep quality disruption, behavioral mediation modeling
Citation: Nie Z, Zhao W and Jing Q (2025) Longitudinal patterns of postpartum body mass index and their impact on cardiometabolic and renal risk among women with prior gestational diabetes: a prospective cohort analysis. Front. Endocrinol. 16:1641103. doi: 10.3389/fendo.2025.1641103
Received: 04 June 2025; Accepted: 30 June 2025;
Published: 21 July 2025.
Edited by:
Yun Shen, Pennington Biomedical Research Center, United StatesReviewed by:
Daniela Lopes Gomes, Federal University of Pará, BrazilChih-Fu Wei, National Taiwan University Hospital Yunlin Branch, Taiwan
Laura Chaves Cerdas, University of Arkansas for Medical Sciences, United States
Copyright © 2025 Nie, Zhao and Jing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanmin Jing, anFtODgwNkAxMjYuY29t
†These authors have contributed equally to this work
 Zicheng Nie
Zicheng Nie Wenbo Zhao
Wenbo Zhao Quanmin Jing
Quanmin Jing