- 1Institute of Public Health, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, Abu Dhabi, United Arab Emirates
- 2Zayed Centre for Health Sciences, United Arab Emirates University, Al Ain, Abu Dhabi, United Arab Emirates
- 3College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai Health, Dubai, United Arab Emirates
- 4Endocrinology Division, Tawam Hospital, SEHA, Al Ain, United Arab Emirates
- 5Department of Internal Medicine, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, Abu Dhabi, United Arab Emirates
Background: Gestational diabetes mellitus (GDM) affects 25% of pregnancies in the United Arab Emirates (UAE), and there is a need for evidence-based diagnostic criteria. This study aimed to determine the optimal diagnostic criteria for GDM in the Emirati population based on predicting adverse perinatal outcomes.
Methods: A total of 2,449 eligible pregnancies from “The Mutaba’ah Study” birth cohort were screened using OGTT between 24 and 32 weeks from May 2017 to March 2021. We compared the prediction of adverse perinatal outcomes [Large for Gestational Age (LGA) and Composite Outcome] risk by four GDM diagnostic criteria (IADPSG, NICE2015, WHO1999, and ADIPS1998) using adjusted regression models. We then developed a new GDM diagnostic threshold for this population (using an aOR of 1.75 recommended by the IADPSG consensus panel). The new criteria was validated and compared with other criteria using risk analyses, c-statistic (AUC), integrated discrimination improvement (IDI), and net reclassification improvement (NRI).
Results: Of the four criteria assessed, IADPSG was the best predictor for large for gestational age (LGA) (aOR 1·77, 95%CI 1·36-2·29) and composite outcome (aOR 1·49, 95%CI 1·19-1·86). The newly developed criteria showed even stronger associations than the IADPSG [LGA (aOR 1·93, 95% CI 1·48-2·53); Composite Outcome (aOR 1·62, 95% CI 1·28-2·05)]. The new criteria model had good discrimination properties for LGA prediction (AUC 0·78; 95%CI 0·68-0·88) and composite outcome prediction (AUC 0.73; 95%CI 0.57-0.83). The new criteria model also correctly reclassifies 49.4% of patients based on LGA risk [NRI; 0·494 (p=0·043)], whereas the IADPSG did not significantly reclassify these patients [NRI; 0·202 (p=0·409)]. For composite outcome prediction, the NRIs for both models were not statistically significant. The new criteria model also improved the discrimination slope (IDI) for LGA prediction by 42.2%, whereas IADPSG improved it by only 9.0%. For the composite outcome prediction, the new criteria model improved by 5.0% vs. IADPSG by 1.3%.
Conclusions: Following the development of a new threshold, the GDM diagnostic criteria defined in this study predicted adverse perinatal outcomes better and demonstrated more optimal clinical utility compared with the existing criteria in this population; hence, adopting it could minimize the burden of GDM adverse perinatal outcomes.
1 Introduction
Gestational diabetes mellitus (GDM) is a crucial public health problem with both short- and long-term consequences (1). It affects one in seven live births globally (2) and one in four in the United Arab Emirates (UAE) (3). If undiagnosed and untreated, it is associated with multiple adverse perinatal outcomes (4), future risk of type 2 diabetes (5, 6), childhood adiposity (7), childhood insulin resistance (8), and high economic burden (9, 10).
Although using evidence-based GDM diagnostic criteria is paramount in a population to effectively identify, manage cases, and prevent adverse outcomes (11), the controversy on accurate screening and diagnosing GDM has been ongoing for approximately six decades, and the search for a global consensus guideline has remained elusive (12, 13). There are many international guidelines on GDM diagnostic criteria, and only a few are evidence-based. The International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria (14) is the only GDM diagnostic criteria originally developed based on the risk of developing adverse perinatal outcomes and has been ratified by many international organizations and adopted in many countries, including the UAE (15–17).
The HAPO study, which the IADPSG criteria was based on, was a sizeable multiethnic study; nevertheless, such study is yet to be conducted in the Arab populations (18). A subgroup analysis of the HAPO study data conducted in 2022 showed variations in adverse perinatal outcome predictions by different criteria based on ethnicity (19). The Emirati population of the UAE has one of the highest burdens of GDM worldwide (3, 20).
Several studies have validated using the IADPSG criteria in different populations and compared it with other criteria (21–23). Despite its popularity and use, the IADPSG criteria has still not resolved some issues of utmost concern regarding GDM diagnosis. These include variations in different populations, likely due to resource availability, expertise, burden of GDM adverse perinatal outcomes, and ethnicities (12).
In the UAE, studies have highlighted that different doctors and health facilities are utilizing multiple GDM diagnostic criteria and guidelines (24, 25). Optimal criteria that correctly classify patients based on their risk of developing adverse perinatal outcomes are needed to ensure more optimal management and improved GDM care. This study aimed to compare four GDM diagnostic criteria [IADPSG (14), National Institute for Health and Clinical Excellence (NICE 2015) (26), World Health Organization (WHO 1999) (27), and Australasian Diabetes in Pregnancy Society (ADIPS 1998) (28)] regarding their prediction of adverse perinatal outcomes and to develop the optimal GDM criteria for use among UAE population.
2 Materials and methods
2.1 Study design and setting
This analysis is based on prospective data collected between 2017 and 2021 in the Mutaba’ah Study, the most extensive multicenter mother and child cohort study in the UAE. It recruits and follows up mother–baby pairs from the Emirati population in Al Ain city, which has the largest proportion of Emiratis in the country. The eligibility criteria for recruitment into the Mutaba’ah study include being an 18-year-old and above pregnant woman from the Emirati population. The study was approved by the United Arab Emirates University Human Research Ethics Committee (ERH-2017-5512) and the Abu Dhabi Health Research and Technology Ethics Committee (DOH/CVDC/2022/72). All participants provided written informed consent.
2.2 Study population
For this study, we included the Mutaba’ah study participants with singleton pregnancies screened with a 75-g 2-h oral glucose tolerance test (OGTT) at 24 to 32 weeks between May 2017 and March 2021. Those with at least two OGTT readings were included, and those with preexisting or newly diagnosed diabetes (fasting plasma glucose (FPG) ≥7 mmol/L and/or 2-h OGTT ≥ 11·1 mmol/L) were excluded.
2.3 Procedures
Details of the Mutaba’ah study, including the recruitment process, have been published elsewhere (29). Trained research assistants approached eligible pregnant women during their antenatal care visits to any of the participating hospitals. The assistants administer the questionnaires during the first trimester. After delivery, clinical data, including anthropometric measurements, OGTT results, other laboratory results, and data on the index pregnancy, delivery, and outcomes, were extracted from the medical records. The extraction was automated by the IT department of the hospital.
GDM screening is standardized across the three recruiting hospitals as they all follow the Abu Dhabi Department of Health (DOH) guidelines. Standard quality is achieved through internal and external quality control. All pregnant women during routine antenatal care are offered universal GDM screening with 75-g 2-h OGTT at 24 to 28 weeks. Before 24 weeks of gestation, they undergo screening for preexisting diabetes using fasting plasma glucose (FPG) or HbA1C tests, and those positive are being co-managed with endocrinologists. The OGTT procedures are similar across the hospitals. The pregnant women are requested to fast for 8 to 10 h from the night before testing. A venous blood sample is drawn by an expert phlebotomist using standard practice. The women are then given 75 g oral glucose (Trutol, 10 fluid ounces (296 ml) dextrose beverage, Nerl Diagnostic, Rhode Island, USA). After 1 h, a venous sample is taken, and again after 2 h. The samples taken are immediately processed to avoid preanalytic glycolysis. Glucose analysis is done using the enzymatic reference method with hexokinase (Hexokinase G6PDH/UV Roche Cobas® c500/c303, CA, USA).
This study assessed four GDM diagnostic criteria, namely, the IADPSG, NICE 2015, WHO 1999, and ADIPS 1998. These criteria are among the commonly used by doctors in the UAE as highlighted by different studies (24, 25). Standard definitions of the criteria are given in Supplementary Table S1.
A sample size of 1,438 pregnancies will allow the detection of a true relative risk of approximately 1·73 (30) of developing large for gestational age (LGA) babies in GDM patients (using IADPSG) with 80% power and at 95% confidence level, accounting for a 20% attrition rate (Fleiss with CC).
2.4 Outcomes
The outcomes assessed were large for gestational age (LGA) and a composite outcome (three maternal and three newborn adverse outcomes). The LGA was defined as a newborn’s birth weight above the 90th percentile for gestational age and sex. The LGA was determined using the reference from the US Centers for Disease Control and Prevention growth chart and the method described by Vidmar S. I. and colleagues in 2013 (31). The composite outcome was defined as having one or more from LGA, neonatal intensive care unit (NICU) admission, abnormal APGAR score, caesarean delivery, premature delivery, and preeclampsia. These were selected based on the evidence from a meta-analysis of adverse perinatal outcomes among more than 7.5 million pregnancies and the most commonly found in the Arab population (32, 33). See Supplementary Material (p 2) for the operational definition of each outcome variable.
2.5 Statistical analysis
We summarized continuous variables using means with standard deviations (SD) and categorical variables using frequencies with percentages (%). Logistic regressions were conducted to assess the associations between the four GDM diagnostic criteria and the adverse perinatal outcomes (LGA and composite outcome). Generalized linear models (GLM) were used to estimate the adjusted relative risks (RR) and risk differences (RD) with 95% confidence intervals for these associations. Logistic regressions were also conducted to assess the associations between OGTT (FPG, 1 h, and 2 h) readings (used as continuous variables) and the outcomes. Regression results were reported using odds ratios with 95% confidence intervals. For the OGTT readings, we calculated odds ratios per unit change (1 mmol/L) in fasting, 1-h, and 2-h plasma glucose levels.
For the multiple regressions, two models (model 1 and model 2) were used. Model 1 was constant for all outcomes and adjusted for baseline maternal characteristics, including age, gravidity, body mass index (BMI) at booking, education, employment, family history of type 2 DM, previous GDM, and study center. Model 2 adjusted for model 1 plus lifestyle factors and other factors. For LGA, model 2 included model 1 plus maternal smoking, passive smoking, physical activity, antepartum hemorrhage, and previous macrosomia. For the composite outcome, a family history of hypertension was added to this list. See Supplementary Material (p 2) for the operational definition of each exposure variable. Missing data in this study were handled using multiple imputations by the chained equation (MICE) method with the number of imputed datasets specified at m = 100.
Following multiple regression with the OGTT results, post-estimation analysis was conducted and expressed using the marginsplot to show the predictive margins for the fitted, adjusted model, which was then used to make predictions. The method adopted by the UCLA Statistical Consulting Group (34), which utilizes multiple imputed data, was used. New cutoff values were identified using the adjusted predictions. New GDM diagnostic criteria was proposed for this population based on these cutoffs.
The new criteria was compared with two others (IADPSG and NICE 2015) in terms of GDM cumulative incidence, adjusted odds ratio (aOR) for outcomes with 95% confidence intervals, and agreements using kappa statistics. These two criteria were selected because they are commonly used in the UAE and were also found to be the most inclusive in GDM diagnosis for this population (3).
Further evaluation of the new criteria and the IADPSG was done using:
● The c-statistic (using area under the curve (AUC) for receiver operating characteristics (ROC)).
● The net reclassification improvement/index (NRI).
● The integrated discrimination improvement (IDI).
The AUC assessed the performance and discrimination of the IADPSG and the new criteria in two separate models containing established risk factors (models 1 and 2) of the outcomes. Results were presented as a graph using the ROC curves and showing the AUC with 95% confidence intervals for both models. AUC values of 0·9–1·0 show that tests have excellent quality; 0·8–0·9 very good quality; 0·7–0·8 good (acceptable) quality; 0·6–0·7 satisfactory; 0·5–0·6 unsatisfactory. The test of equality for the two models was conducted using the chi-square test. P-value was significant at <0·05.
The continuous NRI was used to assess the clinical utility of the new criteria by assessing the incremental value in its risk predictions of the outcomes. Results were presented as the proportions of reclassified cases and non-cases based on risk predictions with their NRI values. The IDI showed the mean difference in discrimination slopes of the two models (extended and traditional) for both criteria. Results were reported as Absolute IDI (standard errors—SE) and Relative IDI (%) (35).
All analyses were conducted using STATA statistical software version 16·1 (StataCorp LLC, College Station, TX, USA).
3 Results
A total of 5,295 participants were recruited in the Mutaba’ah Study from May 2017 to March 2021. Those with multiple pregnancies (323) and those with pending OGTT at the time of extraction (2,386) were excluded. Of the remaining 2,586 participants, 1 known and 39 newly diagnosed patients with diabetes were excluded. There were 97 participants with less than two readings who were also excluded. Hence, 2,449 participants were followed up in this study (Supplementary Figure S1).
3.1 Maternal characteristics
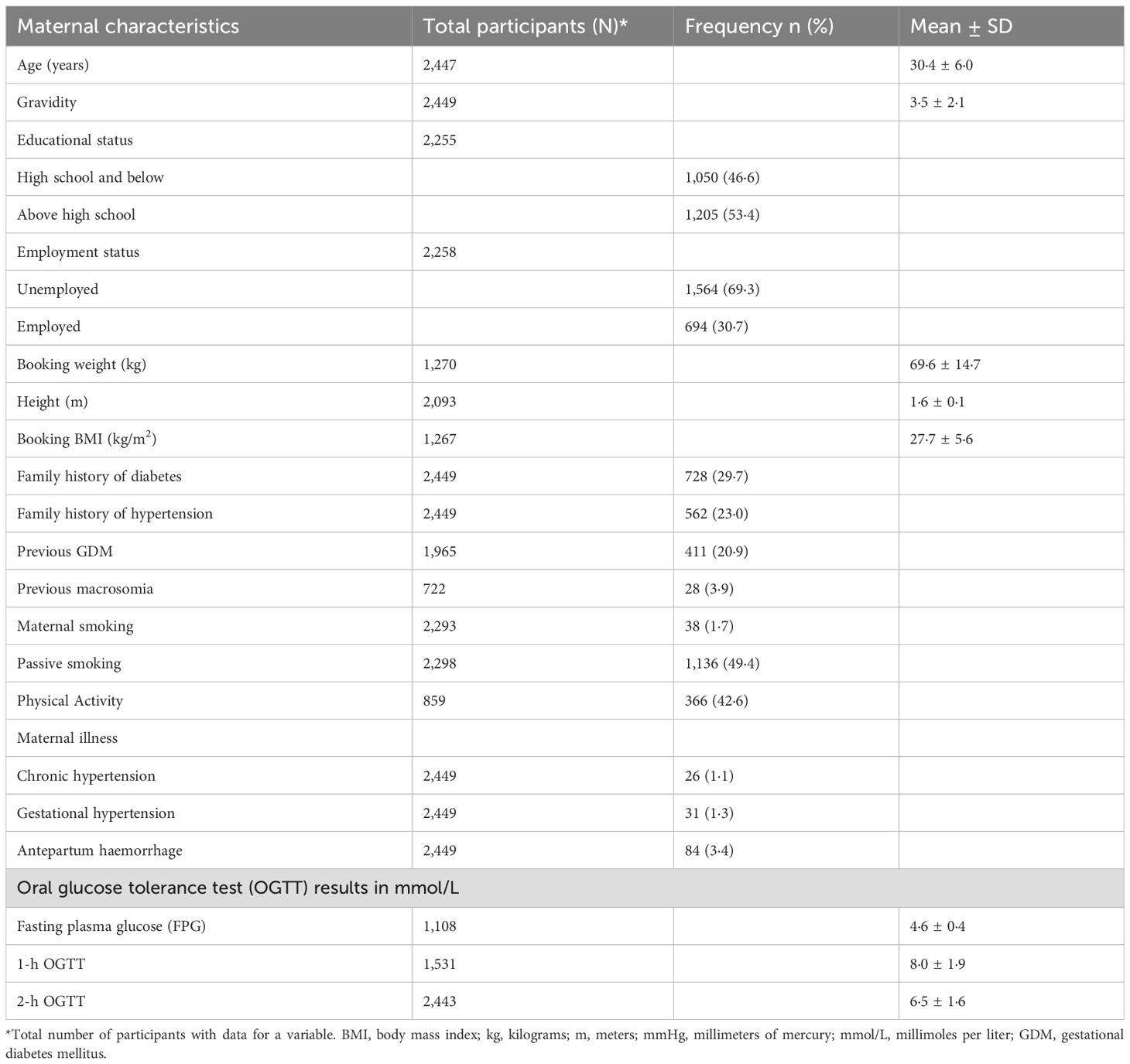
The mean maternal age of participants at booking was 30.4 ± 6.0 years, and the mean booking body mass index (BMI) was 27.7 ± 5.6 kg/m2. 53.4% of the participants had above high school education, and 30.7% were employed. Their mean fasting plasma glucose (FPG) was 4.6 ± 0.4 mmol/L, 1-h OGTT was 8.0 ± 1.9 mmol/L, and 2-h OGTT was 6.5 ± 1.6 mmol/L. Table 1 shows the descriptives for maternal characteristics.
3.2 Adverse perinatal outcomes
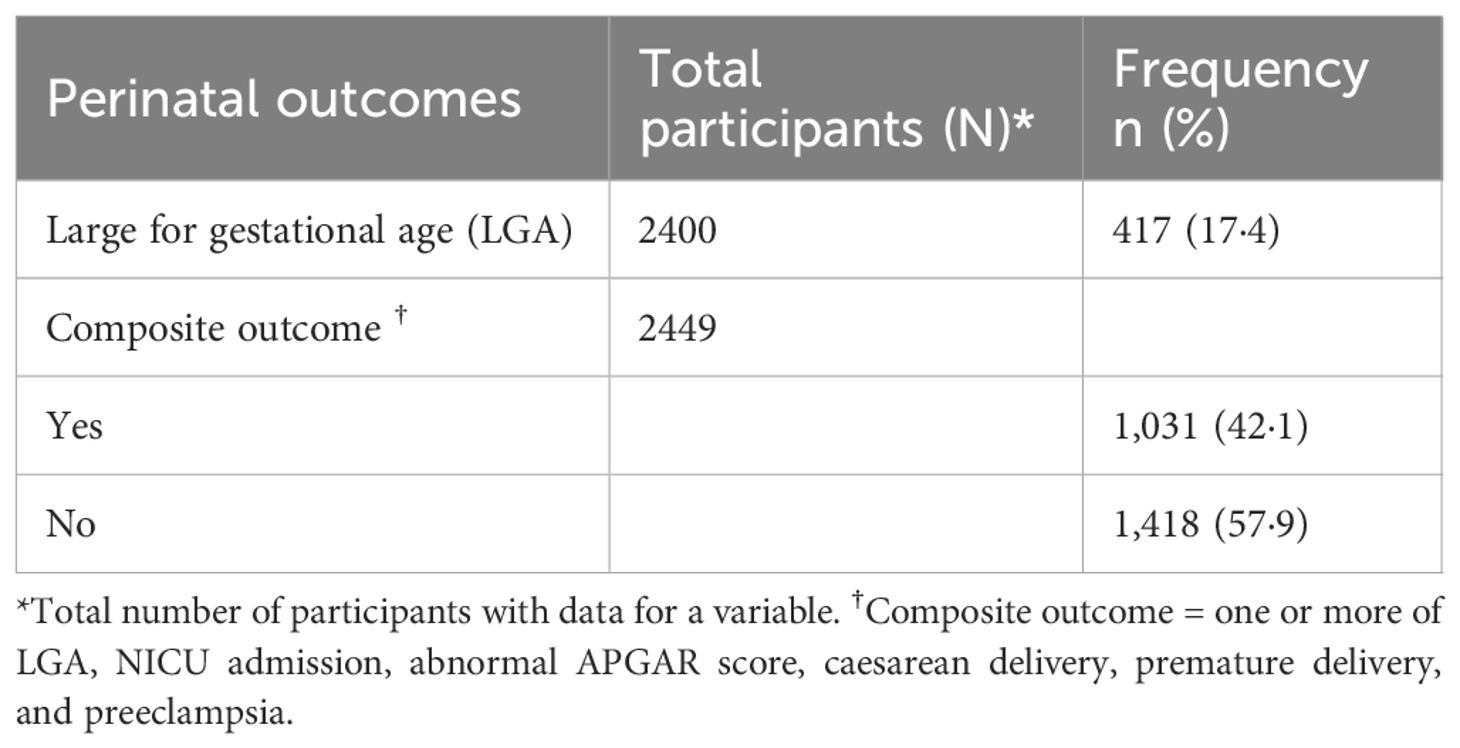
Table 2 summarizes the adverse perinatal outcomes among the participants. 17.4% of the participants had large for gestational age (LGA) babies, and 42.1% had the composite outcome (at least one of the six specified outcomes). The descriptives of the six specified outcomes (LGA, NICU admission, abnormal APGAR score, caesarean delivery, premature delivery, and preeclampsia) are highlighted in Supplementary Table S2.
3.3 Predictions of adverse perinatal outcomes by the four GDM diagnostic criteria
Table 3 shows the associations between the GDM diagnostic criteria (IADPSG, NICE 2015, WHO 1999, and ADIPS 1998) and the outcomes. The IADPSG criteria had the highest odds ratio for the LGA (aOR 1.77, 95% CI 1.36–2.29) compared with the remaining three criteria after adjusting for models 1 and 2. The IADPSG criteria also had the highest adjusted odds ratio for the composite outcome (aOR 1·49, 95% CI 1.19–1.86).
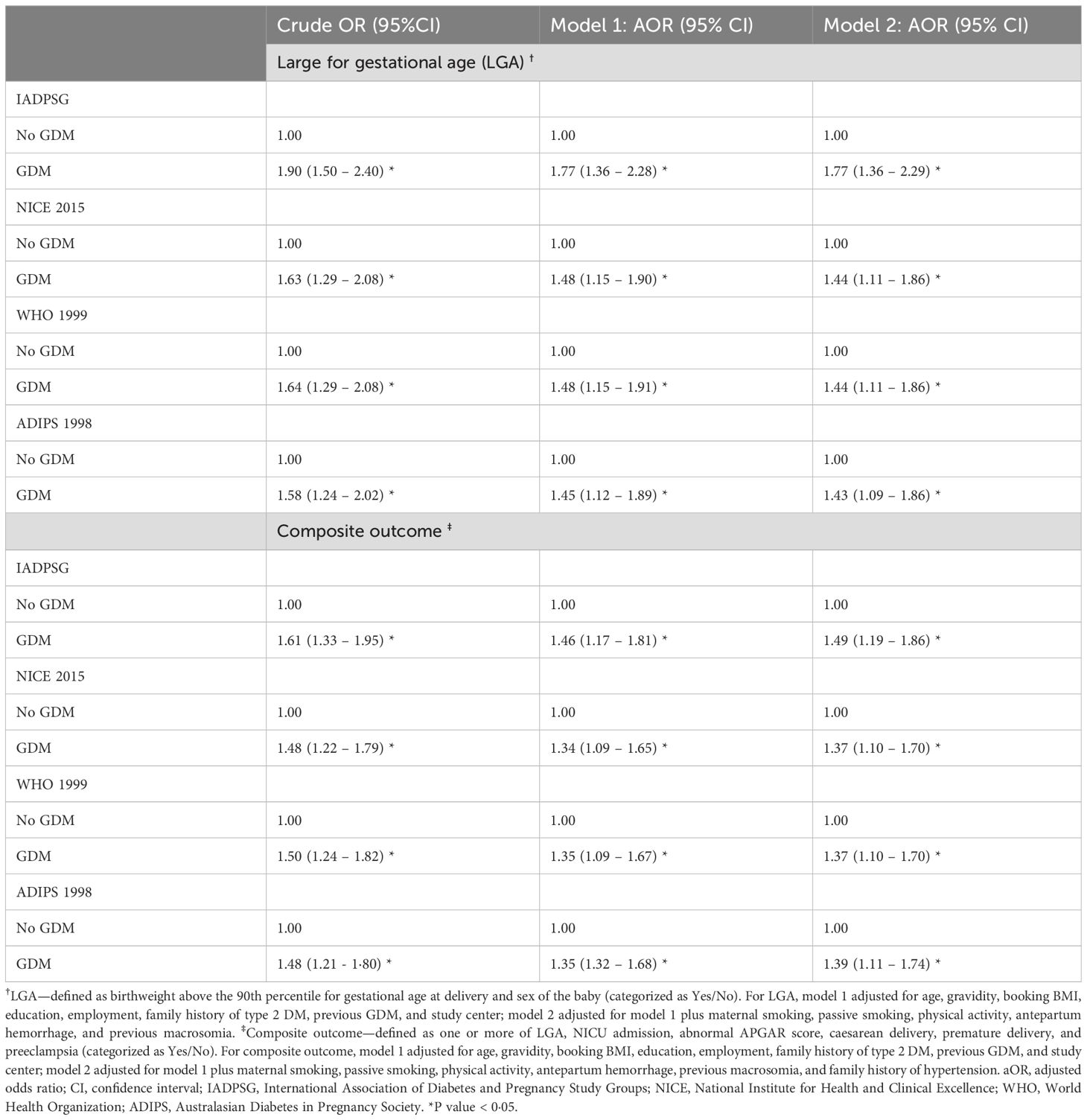
Table 3. Comparing four GDM diagnostic criteria regarding their associations with large for gestational age (N = 2,400) and the composite outcome (N = 2,449) in the Emirati population of the UAE.
Risk analysis results are reported in Supplementary Table S3, and the outcomes’ adjusted relative risks (RR) and risk differences (RD) were compared for the four criteria. It confirmed the IADPSG criteria as the strongest predictor for LGA (aRR 1·55, 95% CI 1.27–1.88) and composite outcome (aRR 1.22, 95% CI 1.09–1.35). The analysis showed that the excess risk of LGA identified by the IADPSG criteria was 9%, whereas it was 6% for the other three criteria. Similarly, for the composite outcome, the RD was highest with the IADPSG criteria.
3.4 Associations of OGTT results (as continuous variables) with adverse perinatal outcomes
Table 4 shows the adjusted odds ratio denoting the strength of association between the FPG, 1-h, and 2-h OGTT results and the outcomes (LGA and composite outcome). FPG had the strongest positive association with both outcomes compared with 1-h and 2-h OGTT. Following adjustments for models 1 and 2, only the association between FPG and the composite outcome remained significant (aOR 1.67, 95% CI 1.21–2.28).
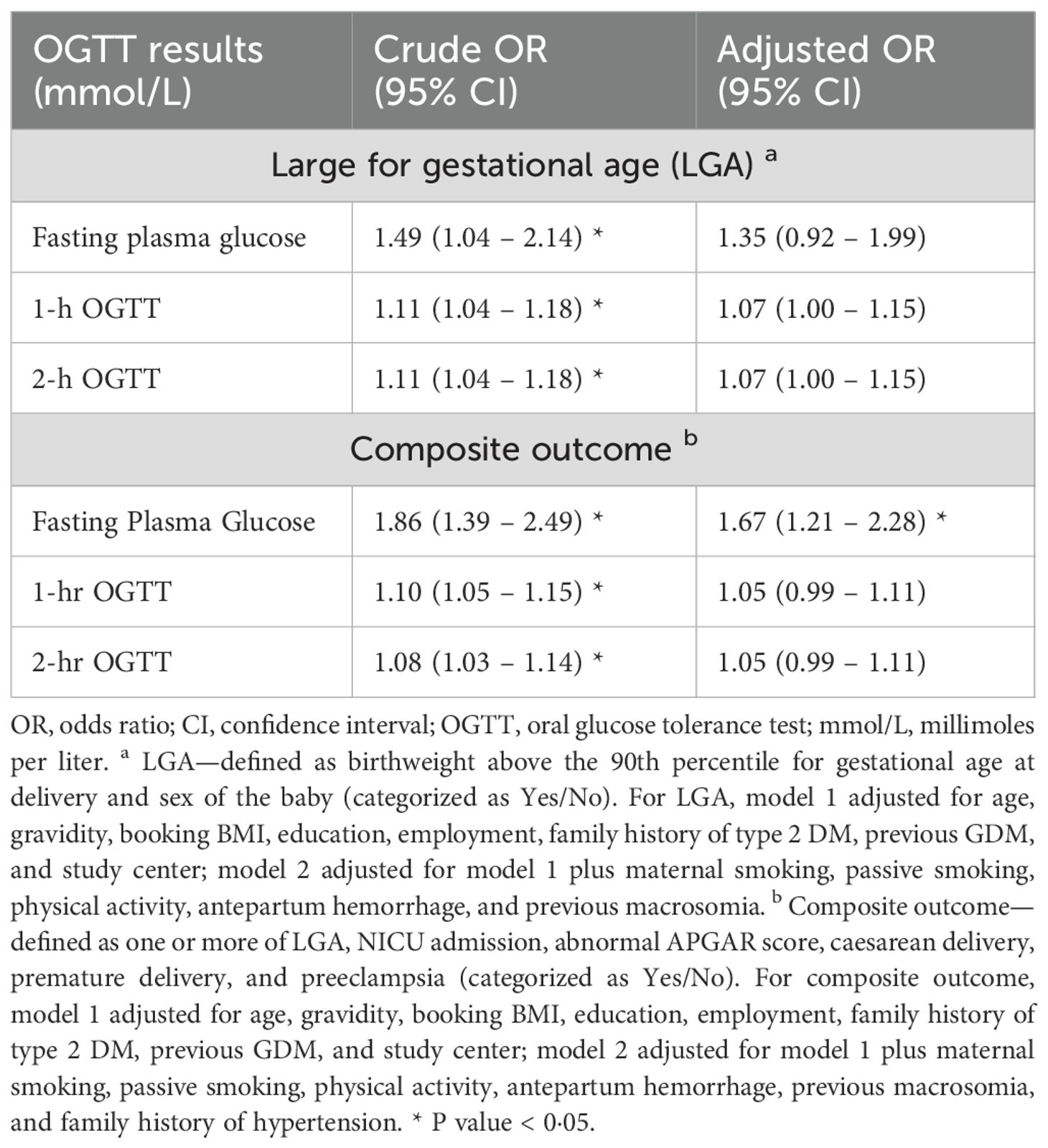
Table 4. Comparing the associations of fasting plasma glucose, 1-hr & 2-hr OGTT with large for gestational age (N=2400) and the composite outcome (N=2449) in the Emirati population of the UAE.
3.5 Prediction plot for the adjusted association between fasting plasma glucose and the composite outcome
Only FPG showed a significant association with the composite outcome after adjusting for models 1 and 2 (Table 4); hence, post-estimation was limited to its analysis. Figure 1 shows the post-estimation prediction graphed by marginsplot showing the fitted, adjusted model for FPG and composite outcome. The graph shows that the association with the composite outcome starts to become positive at a maternal fasting glucose level of 5.00 mmol/L (aOR—1·01 approximately). At the recommended aOR of 1.75 (as specified by the IADPSG consensus panel), this population’s mean maternal FPG level was approximately 6.00 mmol/L.
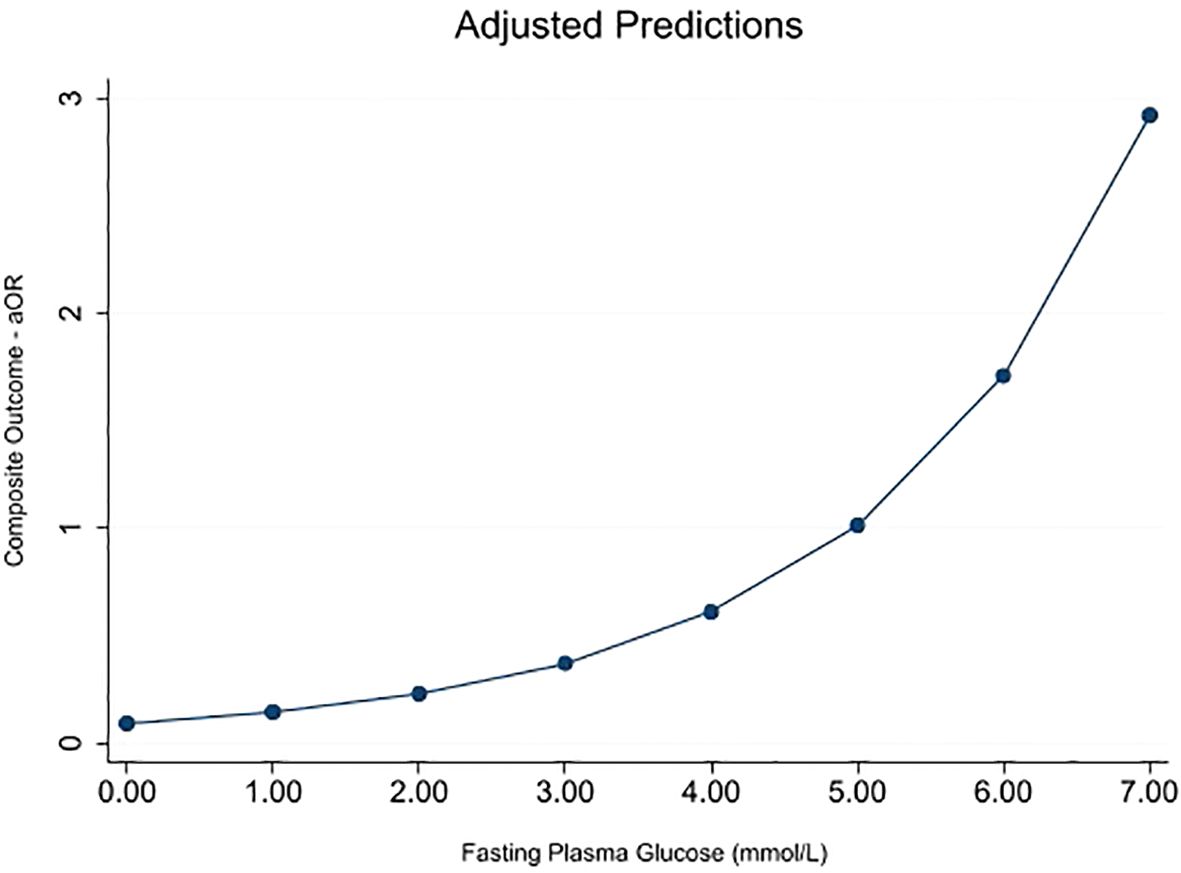
Figure 1. Marginsplot graphing statistics from the fitted, adjusted model (FPG vs. composite outcome) (N = 2,449). The composite outcome is defined as one or more of LGA, NICU admission, abnormal APGAR score, caesarean delivery, premature delivery, and preeclampsia. Model adjusted for age, gravidity, booking BMI, education, employment, family history of type 2 DM, previous GDM, study center, maternal smoking, passive smoking, physical activity, antepartum hemorrhage, previous macrosomia, and family history of hypertension.
3.6 Proposed new GDM criteria for the Emirati population
Based on the above prediction, we developed new GDM diagnostic criteria for the Emirati population using the FPG level of 6.0 mmol/L. For the 1-h and 2-h OGTT, thresholds of the IADPSG criteria were maintained, as it was found to be the best predictor of adverse perinatal outcomes in this population compared with other existing criteria. The new criteria was defined as maternal fasting plasma glucose (FPG) of ≥6.0 mmol/L and/or 1-hr OGTT of ≥10.0 mmol/L and/or 2-hr OGTT of ≥8·5 mmol/L following the 75-g 2-h oral glucose tolerance test.
3.7 Assessment of the newly proposed GDM diagnostic criteria using regression models, risk analysis, kappa statistics, and measures of performance/discrimination
3.7.1 Criteria assessment (incidence, regression, risk analysis, Kappa statistics results)
Supplementary Table S4 shows comparisons between the newly proposed criteria and two existing criteria (IADPSG and NICE) regarding their GDM incidences and adjusted odds ratios of the outcomes. The new criteria gave lower cumulative incidence (GDM Incidence—18.1%, 95% CI 16.6—19.7) than the IADPSG (21.3, 95% CI 19.8–23.0) and NICE criteria (21.5, 95% CI 19.9–23.1). However, it was a better predictor of LGA (aOR 1·93, 95% CI 1.48–2.53; aRR 1·65, 95% CI 1.35–2.01) and composite outcome (aOR 1·62, 95% CI 1.28–2.05; aRR 1.26, 95% CI 1.12–1.40) compared with the two criteria. The risk difference for both outcomes by the new criteria was 11% (Supplementary Table S3).
There was a strong agreement between the new criteria and the IADPSG criteria (k = 0·89; p<0·001) and a moderate agreement with the NICE 2015 criteria (k = 0·71; p<0·001) (Supplementary Table S5).
3.7.2 Area under the ROC curve
The new criteria and the IADPSG predictive models were found to have good predictive power for both outcomes. For LGA (Supplementary Figure S2), the IADPSG model showed an AUC of 0.759 (95% CI; 0.661–0.858) and the new model showed an AUC of 0.776 (95% CI; 0.675–0.875), (p=0·534). For the composite outcome (Supplementary Figure S3), the IADPSG (AUC 0.729, 95% CI; 0.567–0.833) and the new criteria (AUC 0.730, 95% CI; 0.572–0.837) models had very similar predictive power (p=0·879).
3.7.3 Net reclassification improvement
The net reclassification improvement (from the traditional model) for LGA prediction was better and more significant with the new criteria [NRI; 0·494 (p=0·043)] than with the IADPSG criteria [NRI; 0.202 (p=0·409)]. The new criteria reclassified more cases (47.4%) upward and correctly reclassified up to 76.3% of non-cases downward (Supplementary Table S6). The NRI for both the IADPSG and new criteria in the composite outcome prediction were not statistically significant [NRI; 0.312 (p=0·088) vs. NRI; 0.162 (p=0·376), respectively] (Supplementary Table S7).
3.7.4 Integrated discrimination improvement
The IDI assessment showed that the new criteria model increased the discrimination slope (from the traditional model) by 42.2% for LGA and 5.0% for the composite outcome. This was much higher than the IADPSG criteria in both instances (9.0% for LGA and 1.3% for composite outcome) (Supplementary Table S8).
4 Discussion
From this representative sample of the Emirati population of the United Arab Emirates (UAE), findings show that of the four commonly used GDM diagnostic criteria in this population (25); the IADPSG criterion was the best predictor of adverse perinatal outcomes. This finding was statistically and clinically significant and corroborates the findings of other studies in the Arabian Gulf region (21–23). Further assessment showed that after adjusting for potential confounders, the maternal fasting plasma glucose in this population better predicted adverse perinatal outcomes than the 1-h and 2-h OGTT results. From post-estimation analysis, a new GDM diagnostic criteria was developed using the prediction of adverse perinatal outcomes, giving rise to a new cutoff value for the Emirati population. Since the HAPO study (14), research proposing new GDM criteria have been conducted but not based on predicting adverse perinatal outcomes (36, 37). The authors of this study propose to term this newly proposed criteria as “UAE-modified-IADPSG”. Although it is stricter in diagnosing GDM than the currently recommended IADPSG criteria, the new criterion was found to be a stronger predictor of adverse perinatal outcomes among Emirati GDM patients and was more clinically relevant with better performance and discrimination properties.
Large for gestational age (LGA) was chosen as the primary outcome because it is a direct effect of hyperglycemia in pregnancy (38). In fully adjusted regression models, out of the four GDM diagnostic criteria assessed in this study, the IADPSG criterion was found to be the strongest predictor of LGA. The NICE 2015, WHO 1999, and ADIPS 1998 predict LGA almost equally. A study in Canada showed similar results when comparing the IADPSG criteria to their national criteria (39). On the other hand, a meta-analysis of studies conducted in the US, Australia, Asia, and Europe showed that the predictions of LGA using different GDM criteria, including IADPSG, were not significant (40). Our predictions could not be compared with other studies in the Gulf region as the odds/risk ratios of perinatal outcomes were not assessed in these studies (21–23).
This study showed that of the three OGTT results, the fasting plasma glucose (FPG) was the strongest predictor for both LGA and composite outcome. However, the association between FPG and LGA was no longer significant after adjusting for the risk models. This result is consistent with the findings of a study in China (41). Using FPG alone instead of the widely accepted first-line OGTT for GDM diagnosis has been explored and recommended in some settings (42).
In 1997, at the ADA-sponsored 4th GDM International Workshop conference (43), a consensus was made to base the development of GDM diagnostic criteria on the risk of adverse perinatal outcomes, hence the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study in 2008 (14). One of the strengths of the HAPO study included being a multicenter, multiethnic study with more than 25,000 participants. 48% of the participants were white, 12% were black, 8% were Hispanic, 29% were either Asian or Oriental, and 3% were unknown (44). Arab populations were not adequately represented in the sample. With the growing burden of GDM among the Arab population (2), the importance of accurately diagnosing the condition cannot be overemphasized.
In this study, we developed an ideal GDM diagnostic criteria for the Emirati population using the recommendations of the IADPSG consensus panel (44). Only the FPG threshold was redefined because it was the only parameter that remained significantly associated with the outcomes following adjustments in multiple regressions. This result is consistent with the findings of studies in Asia (41, 45). The pathophysiology of raised FPG and the other two OGTT results differ. Increased FPG levels have been linked to higher baseline insulin resistance compared with the 1-h and 2-h OGTT levels (46). This could explain our results among the Emirati population, which is known to have a high burden of insulin resistance and its complications (47).
Currently, the IADPSG is the locally recommended GDM diagnostic criteria in the UAE, although there is evidence that different doctors in the country use different criteria (25). Our study reiterates the relevance of the IADPSG criteria over other existing criteria in the UAE. It also revealed new evidence of a more optimal criteria than the IADPSG. Following risk analyses, the new criteria identified approximately 50 more women from our sample at risk of GDM adverse perinatal outcomes than the IADPSG did. Moreover, at least 100 more than the other criteria. Considering the fertility rate in our population (48), the new tool could identify approximately 1,000 more Emirati women at risk annually than the IADPSG. The new criteria and the IADPSG both had good and acceptable predictive power, with the AUCs of the new criteria models slightly larger than those of the IADPSG. Our findings are similar to the study on multiethnic Australian population, where the AUC of the IADPSG model was also found to be satisfactory (AUC—0.68) (11). One of the limitations of AUC is its inability to capture the clinical utility of a diagnostic tool; hence, we employed NRI and IDI for this assessment (35).
The Net Reclassification Improvement (NRI) is an index that quantifies how well a new model reclassifies cases and non-cases, correctly or incorrectly, based on the risk of outcomes compared with a baseline (traditional) risk model. It gives the proportion of cases or non-cases reclassified upward, i.e., to increased risk and vice versa for downward reclassification (35). The traditional model used in this study was a predictive model consisting of established risk factors (models 1 and 2). Our study highlighted that none of the IADPSG models (for LGA and composite outcome) significantly improved over the traditional model. The new criteria, however, significantly improved risk reclassification from the traditional model for LGA by 49.4%. This means that approximately half of the patients whose risk status changed were reclassified correctly by the new criteria. This was not significant for the composite outcome. The Integrated Discrimination Improvement (IDI) takes this one step further because it shows us the magnitude of the discrimination slopes, compared between the traditional risk model and both criteria (35). The IDI reinforced the clinical relevance of the new criteria by showing that the new criteria can predict patients with a high risk of adverse perinatal outcomes (LGA) better than the traditional model by 42.2%, whereas it is only by 9.0% for the IADPSG (compared with the same traditional model). This relevance is also reflected in the IDI for the composite outcome (new criteria by 5.0% vs. IADPSG by 1.3%).
The implications of these results are 3-fold. Firstly, our study confirms the superiority of the IADPSG criteria over existing criteria in the UAE regarding adverse perinatal outcomes risk prediction. This gives the evidence-based backing to unify doctors’ practice in the country. Secondly, this study has proved the newly proposed criteria to be a valid tool for diagnosing GDM in the Emirati population. This is the first study to develop evidence-based GDM diagnostic criteria based on adverse perinatal outcomes risk in the Arabian Gulf region. The new criteria was found to be a more optimal GDM diagnostic tool for this population than even the locally recommended IADPSG regarding risk prediction of adverse perinatal outcomes. Adopting the new criteria could lead to more targeted and effective management of GDM and its complications than the current practice by avoiding under- and overdiagnosis. Further validation of this tool is needed in reducing future type 2 diabetes risk. External validation and comparison with international data are also needed. Finally, the fact that the newly developed criteria was clinically more ideal than the IADPSG criteria in this population suggests that the worldwide unification of GDM diagnostic criteria might be challenging due to differences in the risks of GDM adverse outcomes in different populations. Hence, we recommend that experts focus on developing the optimal guidelines for unique populations, preferably at regional or national levels, to reduce the disease burden effectively (19). Strategies for translation into practice should include clinical practice evaluation, GDM guidelines and policies, education, research and development, and advocacy (Supplementary Figure S4).
4.1 Strengths and limitations
Our study’s strengths include being multicentered and conducted in a large Emirati population of the UAE, thereby increasing its generalizability and power. Rigorous methodological approaches were employed to ensure good internal validity. Regression models addressed potential predictors while keeping the bias/variance issue in mind. Finally, risk and prediction analyses provided a more relevant result for translation into clinical practice.
Limitations in this study include using non-probability (consecutive) sampling, which might affect the representativeness of our sample. Nevertheless, this issue was mitigated by the multicentered nature of our study. We did not assess all adverse perinatal outcomes due to the unavailability of the data. However, we included the primary GDM adverse perinatal outcomes expected in this population (49). The use of medical records for some variables provided incomplete data; however, we conducted a missing data analysis as described in the methods section.
4.2 Conclusion
Firstly, our study highlighted that the IADPSG was the best predictor of adverse perinatal outcomes out of the four commonly used GDM diagnostic criterion (IADPSG, NICE 2015, WHO 1999, and ADIPS 1998) in the UAE. Secondly, from the study data, new evidence-based GDM diagnostic criteria was developed based on the risk of adverse perinatal outcomes, which we found to be a more optimal diagnostic tool in the Emirati population than the other criteria. The new criteria could improve GDM care and reduce the burden of its perinatal complications better than the current clinical practice in this population. Following clinical trials and cost-effectiveness studies in multiethnic settings, the new criteria could be adopted widely. A multi-sectoral approach is needed to ensure the translation of this research into practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available on request from The Mutaba’ah Study. Approval from the research ethics committee may be required.
Ethics statement
The studies involving humans were approved by United Arab Emirates University Human Research Ethics Committee (ERH-2017-5512), UAE University, Al Ain, United Arab Emirates and the Abu Dhabi Health Research and Technology Ethics Committee (DOH/CVDC/2022/72), Department of Health Abu Dhabi, Abu Dhabi, United Arab Emirates. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MB: Methodology, Validation, Investigation, Data curation, Writing – review & editing, Visualization, Resources, Conceptualization, Writing – original draft, Formal Analysis. LA: Methodology, Project administration, Resources, Data curation, Supervision, Conceptualization, Investigation, Validation, Writing – review & editing, Funding acquisition. RA-R: Writing – review & editing, Supervision, Validation, Conceptualization, Resources, Investigation, Project administration. IE: Resources, Methodology, Project administration, Validation, Investigation, Writing – review & editing. TL: Resources, Project administration, Funding acquisition, Investigation, Validation, Writing – review & editing. BA: Visualization, Validation, Resources, Methodology, Investigation, Writing – review & editing. JA: Investigation, Resources, Visualization, Validation, Writing – review & editing, Methodology. FA-M: Methodology, Writing – review & editing, Investigation, Writing – original draft, Supervision, Visualization, Data curation, Validation, Resources, Formal Analysis, Conceptualization, Project administration.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The study was funded by the Zayed Center for Health Sciences at UAE University, Grant #12R080. The funding body had no role in the study design, data collection, data analysis, interpretation, report writing, or the decision to submit for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1641326/full#supplementary-material
References
1. Simmons D, Gupta Y, Hernandez TL, Levitt N, van Poppel M, Yang X, et al. Call to action for a life course approach. Lancet. (2024) 404:193–214. doi: 10.1016/S0140-6736(24)00826-2
2. Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
3. Bashir MM, Ahmed LA, Elbarazi I, Loney T, Al-Rifai RH, Alkaabi JM, et al. Incidence of gestational diabetes mellitus in the United Arab Emirates; comparison of six diagnostic criteria: The Mutaba’ah Study. Front Endocrinol. (2022) 13:1069477. doi: 10.3389/fendo.2022.1069477
4. Greco E, Calanducci M, Nicolaides KH, Barry EVH, Huda MSB, and Iliodromiti S. Gestational diabetes mellitus and adverse maternal and perinatal outcomes in twin and singleton pregnancies: a systematic review and meta-analysis. Am J Obstetrics Gynecol. (2024) 230:213–25. doi: 10.1016/j.ajog.2023.08.011
5. Wood AJ, Boyle JA, Barr ELM, Barzi F, Hare MJL, Titmuss A, et al. Type 2 diabetes after a pregnancy with gestational diabetes among first nations women in Australia: The PANDORA study. Diabetes Res Clin Pract. (2021) 181. doi: 10.1016/j.diabres.2021.109092
6. Andersson-Hall U, Kristiansson E, Zander M, Wallenius K, Sengpiel V, and Holmäng A. Glucose tolerance two years after gestational diabetes classified by old Swedish or new WHO diagnostic criteria. Diabetes Res Clin Pract. (2024) 216. doi: 10.1016/j.diabres.2024.111831
7. Leth-Møller M, Hulman A, Kampmann U, Hede S, Ovesen PG, and Knorr S. Effect of gestational diabetes on fetal growth rate and later overweight in the offspring. J Clin Endocrinol Metab. (2024) 110:1350–1357. doi: 10.1210/clinem/dgae428
8. Lowe WL Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. (2019) 42:372–80. doi: 10.2337/dc18-1646
9. Moon JH and Jang HC. Gestational diabetes mellitus: diagnostic approaches and maternal-offspring complications. Diabetes Metab J. (2022) 46:3–14. doi: 10.4093/dmj.2021.0335
10. Sweeting A, Hannah W, Backman H, Catalano P, Feghali M, Herman WH, et al. Epidemiology and management of gestational diabetes. Lancet. (2024) 404:175–92. doi: 10.1016/S0140-6736(24)00825-0
11. Cooray SD, Boyle JA, Soldatos G, Allotey J, Wang H, Fernandez-Felix BM, et al. Development, validation and clinical utility of a risk prediction model for adverse pregnancy outcomes in women with gestational diabetes: The PeRSonal GDM model. eClinicalMedicine. (2022) 52. doi: 10.1016/j.eclinm.2022.101637
12. Agarwal MM. Consensus in gestational diabetes MELLITUS: looking for the holy grail. J Clin Med. (2018) 7:123. doi: 10.3390/jcm7060123
13. McIntyre HD, Kampmann U, Dalsgaard Clausen T, Laurie J, and Ma RCW. Gestational diabetes: an update 60 years after O’Sullivan and mahan. J Clin Endocrinol Metab. (2024) 110:e19–e31. doi: 10.1210/clinem/dgae709
14. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy,” (in eng). Diabetes Care. (2010) 33:676–82. doi: 10.2337/dc09-1848
15. Agarwal MM, Dhatt GS, and Othman Y. Gestational diabetes: differences between the current international diagnostic criteria and implications of switching to IADPSG. J Diabetes Complications. (2015) 29:544–9. doi: 10.1016/j.jdiacomp.2015.03.006
16. Aubry EM, Raio L, and Oelhafen S. Effect of the IADPSG screening strategy for gestational diabetes on perinatal outcomes in Switzerland. Diabetes Res Clin Pract. (2021) 175:108830. doi: 10.1016/j.diabres.2021.108830
17. Agarwal MM. Gestational diabetes in the arab gulf countries: sitting on a land-mine. Int J Environ Res Public Health. (2020) 17. doi: 10.3390/ijerph17249270
18. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. (2008) 358:1991–2002. doi: 10.1056/NEJMoa0707943
19. He Y, Ma RCW, McIntyre HD, Sacks DA, Lowe J, Catalano PM, et al. Comparing IADPSG and NICE diagnostic criteria for GDM in predicting adverse pregnancy outcomes,” (in eng). Diabetes Care. (2022) 45:2046–54. doi: 10.2337/dc22-0579
20. Zhu Y and Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diabetes Rep. (2016) 16:7. doi: 10.1007/s11892-015-0699-x
21. Alfadhli E. Gestational diabetes in Saudi women identified by the International Association of Diabetes and Pregnancy Study Group versus the former American Diabetes Association criteria: a prospective cohort study. Ann Saudi Med. (2015) 35:428–34. doi: 10.5144/0256-4947.2015.428
22. Al Subhi SK, Al Kindi RM, Al Rawahi A, Al Seyabi IS, and Al Mukhaini A. Prevalence of gestational diabetes mellitus using the latest world health organization diagnostic criteria among Omani women in muscat, Oman. Oman Med J. (2021) 36:e215–5. doi: 10.5001/omj.2021.08
23. Bashir M, Ibrahim I, Eltaher F, Beer S, Baagar K, Aboulfotouh M, et al. Screening pregnant women in a high-risk population with WHO-2013 or NICE diagnostic criteria does not affect the prevalence of gestational diabetes. Sci Rep. (2021) 11:5604. doi: 10.1038/s41598-021-84918-y
24. Al-Maskari F, Bashir MM, Ahmed LA, Al-Rifai RH, Elbarazi I, Aldhanhani S, et al. Gaps in doctors’ practice: gestational diabetes mellitus diagnosis in the United Arab Emirates. Popul Med. (2023) 5. doi: 10.18332/popmed/163779
25. Agarwal MM, Shah SM, Al Kaabi J, Saquib S, and Othman Y. Gestational diabetes mellitus: Confusion among medical doctors caused by multiple international criteria,” (in eng). J Obstet Gynaecol Res. (2015) 41:861–9. doi: 10.1111/jog.12661
26. National Institute for Health and Care Excellence (NICE)Overview | Diabetes in pregnancy: management from preconception to the postnatal period | Guidance. London: NICE (2015). Available online at: https://www.nice.org.uk/guidance/ng3 (Accessed March 6, 2022).
27. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation. Part 1: Diagnosis and Class ification of Diabetes Mellitus (1999). Available online at: https://apps.who.int/iris/bitstream/handle/10665/66040/WHO_NCD_NCS_99.2.pdf?sequence=1 (Accessed March 6, 2022).
28. Hoffman L, Nolan C, Wilson JD, Oats JJN, and Simmons D. Gestational diabetes mellitus - management guidelines: The Australasian Diabetes in Pregnancy Society. Med J Aust. (1998) 169:93–7. doi: 10.5694/j.1326-5377.1998.tb140192.x
29. Amal Al H, Nasloon A, Iffat E, Haba E, Fatima A-M, Hassib N, et al. Mutaba’ah—Mother and Child Health Study: protocol for a prospective cohort study investigating the maternal and early life determinants of infant, child, adolescent and maternal health in the United Arab Emirates. BMJ Open. (2019) 9:e030937. doi: 10.1136/bmjopen-2019-030937
30. Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes–a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. (2012) 12. doi: 10.1186/1471-2393-12-23
31. Vidmar SI, Cole TJ, and Pan H. Standardizing anthropometric measures in children and adolescents with functions for egen: Update. Stata J. (2013) 13:366–78. Available online at: echo(www).stata-journal.com/article.html?article=dm0004_1 (Accessed October 8, 2022).
32. Ye W, Luo C, Huang J, Li C, Liu Z, and Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. (2022) 377:e067946. doi: 10.1136/bmj-2021-067946
33. Wahabi H, Fayed A, Esmaeil S, Mamdouh H, and Kotb R. Prevalence and complications of pregestational and gestational diabetes in saudi women: analysis from riyadh mother and baby cohort study (RAHMA). BioMed Res Int. (2017) 2017:6878263. doi: 10.1155/2017/6878263
34. Group USC. Available online at: https://stats.oarc.ucla.edu/stata/faq/how-can-i-get-margins-and-marginsplot-with-multiply-imputed-data/ (Accessed 2022).
35. Barkhordari M, Padyab M, Hadaegh F, Azizi F, and Bozorgmanesh M. Stata modules for calculating novel predictive performance indices for logistic models. Int J Endocrinol Metab. (2016) 14:e26707. doi: 10.5812/ijem.26707
36. Sfameni SF, Wein P, and Sfameni AM. Establishing novel diagnostic criteria for the glucose tolerance test for the diagnosis of gestational diabetes and gestational hyperglycemia. Int J Gynaecol Obstet. (2024) 164:758–62:no. doi: 10.1002/ijgo.15074
37. Behboudi Gandevani S, Garshasbi A, Shahpari Niri S, and Naghizade MM. New criteria for gestational diabetes in Tehran, Iran. Iran J Reprod Med. (2012) 10:237–42. https://ijrm.ir/article-1-281-en.html (Accessed July 7, 2024).
38. NICE. National institute for health and clinical excellence: guidance. In: Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. RCOG Press Copyright © 2008, National Collaborating Centre for Women’s and Children’s Health., London (2008).
39. Shah BR and Sharifi F. Perinatal outcomes for untreated women with gestational diabetes by IADPSG criteria: a population-based study. Bjog. (2020) 127:116–22. doi: 10.1111/1471-0528.15964
40. Tehrani FR, Naz MSG, Bidhendi-Yarandi R, and Behboudi-Gandevani S. Effect of different types of diagnostic criteria for gestational diabetes mellitus on adverse neonatal outcomes: A systematic review, meta-analysis, and meta-regression,” (in eng). Diabetes Metab J. (2022) 46:605–19:no. doi: 10.4093/dmj.2021.0178
41. Feng H, Zhu WW, Yang HX, Wei YM, Wang C, Su RN, et al. Relationship between Oral Glucose Tolerance Test Characteristics and Adverse Pregnancy Outcomes among Women with Gestational Diabetes Mellitus,” (in eng). Chin Med J (Engl). (2017) 130:1012–8:no. doi: 10.4103/0366-6999.204928
42. Zhang X, Wei Y, Fan L, Zhao Y, Li Y, Liu Y, et al. A multicenter all-inclusive prospective study on the relationship between glycemic control markers and maternal and neonatal outcomes in pregnant women. J Maternal-Fetal Neonatal Med. (2021) 34:3154–61. doi: 10.1080/14767058.2019.1678139
43. Metzger BE, Coustan DR, and The Organizing C. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus,” (in English). Diabetes Care. (1998) 21:B161. Available online at: https://www.proquest.com/scholarly-journals/summary-recommendations-fourth-international/docview/223025729/se-2?accountid=62373 (Accessed October 19, 2022).
44. Coustan DR, Lowe LP, Metzger BE, Dyer AR, International Association of D, and Pregnancy Study G. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus,” (in eng). Am J Obstet Gynecol. (2010) 202:no. doi: 10.1016/j.ajog.2010.04.006
45. Song Q, Song X, Li L, and Ding H. Fasting or 2-hour postprandial plasma glycemic criteria for gestational diabetes mellitus are aassociated with distinct adverse outcomes,” (in eng). BMC Pregnancy Childbirth. (2024) 24:no570. doi: 10.1186/s12884-024-06770-y
46. Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, and Temelkova-Kurktschiev T. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in impaired glucose tolerance for atherosclerosis and diabetes study. Diabetes Care. (2003) 26:868–74. doi: 10.2337/diacare.26.3.868
47. Mahmoud I and Sulaiman N. Prevalence of metabolic syndrome and associated risk factors in the United Arab Emirates: A cross-sectional population-based study. Front Public Health. (2021) 9:811006. doi: 10.3389/fpubh.2021.811006
48. Centre FCAS. Total fertility rate for emiratis . Available online at: https://uaestat.fcsc.gov.ae/?lc=en&fs[0]=FCSC%20-%20Statistical%20Hierarchy%2C0%7CBirths%23VTS_BIR%23&pg=0&fc=FCSC%20-%20Statistical%20Hierarchy&snb=5 (Accessed December 19, 2024).
Keywords: gestational diabetes mellitus (GDM), diabetes, diagnostic criteria, IADPSG, perinatal outcomes, oral glucose tolerance test (OGTT), risk stratification, United Arab Emirates
Citation: Bashir MM, Ahmed LA, Al-Rifai RH, Elbarazi I, Loney T, Afandi B, Alkaabi JM and Al-Maskari F (2025) Optimizing gestational diabetes diagnostic criteria to predict adverse perinatal outcomes in the United Arab Emirates: The Mutaba’ah Study. Front. Endocrinol. 16:1641326. doi: 10.3389/fendo.2025.1641326
Received: 04 June 2025; Accepted: 20 August 2025;
Published: 10 October 2025.
Edited by:
Xilin Yang, Tianjin Medical University, ChinaCopyright © 2025 Bashir, Ahmed, Al-Rifai, Elbarazi, Loney, Afandi, Alkaabi and Al-Maskari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatma Al-Maskari, ZmF0bWEuYW1AdWFldS5hYy5hZQ==
†ORCID: Maryam M. Bashir, orcid.org/0000-0003-1738-0276
Luai A. Ahmed, orcid.org/0000-0001-5292-8212
Iffat Elbarazi, orcid.org/0000-0001-7151-2175
Tom Loney, orcid.org/0000-0003-1687-6587
Rami H. Al-Rifai, orcid.org/0000-0001-6102-0353
Bachar Afandi, orcid.org/0000-0002-5842-9016
Juma Alkaabi, orcid.org/0000-0003-2477-0408
Fatma Al-Maskari, orcid.org/0000-0001-6598-4942
 Maryam M. Bashir
Maryam M. Bashir Luai A. Ahmed
Luai A. Ahmed Rami H. Al-Rifai
Rami H. Al-Rifai Iffat Elbarazi
Iffat Elbarazi Tom Loney
Tom Loney Bachar Afandi4,5†
Bachar Afandi4,5† Juma M. Alkaabi
Juma M. Alkaabi Fatma Al-Maskari
Fatma Al-Maskari