- Department of Obstetrics and Gynecology, Chengdu Shuangliu District Maternal and Child Health Hospital, Chengdu, China
Background: Hypertensive disorders of pregnancy (HDP) are a major public health problem affecting a large number of pregnancies worldwide. Despite extensive research, little is known about the long-term cardiometabolic consequences of HDP exposure in offspring.
Objective: To investigate the long-term cardiometabolic risks in offspring exposed to HDP.
Search strategy: A comprehensive search of relevant studies published in PubMed, EMBASE, the Cochrane Central Register, and Web of Science databases was conducted.
Selection criteria: Inclusion criteria comprised case-control and cohort studies, with outcome measures encompassing blood pressure, body mass index, lipid levels, and glucose metabolism.
Data collection and analysis: Meta-analysis was performed using Review Manager 5.4, and fixed- or random-effects models were selected as appropriate.
Main results: A total of 23 observational studies with 89,982 participants from 10 countries were included. Meta-analysis indicated that offspring exposed to HDP presented with significantly increased systolic blood pressure (MD: 2.44; 95% CI: 2.03–2.85; P < 0.00001), elevated diastolic blood pressure (SMD: 0.19; 95% CI: 0.15–0.23; P < 0.00001), and higher body mass index (MD: 0.34; 95% CI: 0.05–0.64; P < 0.05). Additionally, these offspring demonstrated a decreased likelihood of elevated homeostasis model assessment of insulin resistance (OR: 0.58; 95% CI: 0.34–0.98; P < 0.05). No significant differences were observed in other indicators.
Conclusions: The impact of HDP on offspring cardiometabolism is multifaceted. Elevated blood pressure and body mass index are more likely to be observed in offspring exposed to HDP, while the risk of insulin resistance appears to be reduced.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42025630378, identifier CRD42025630378.
1 Introduction
Hypertensive disorders of pregnancy (HDP) encompass a range of conditions characterized by elevated blood pressure during pregnancy, affecting 5%–10% of pregnancies globally (1, 2). These include pregnancy-induced hypertension, preeclampsia, eclampsia, and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome (3). In the United States, the occurrence of HDP increased from 13% in 2017 to 16% in 2019. During this period, preeclampsia complications were identified in approximately 3% of all pregnancies. There was a notable rise in HDP cases between 2017 and 2019 (4). Globally, these complications occur in 2%–8% of pregnancies (5). HDP poses immediate health risks to pregnant women, such as higher cesarean section rates, placental abruption, increased maternal mortality, and long-term cardiovascular disease, and significantly impacts fetal development, leading to intrauterine growth restriction, preterm birth, and elevated perinatal mortality (6–12).
The impact of HDP on children’s cardiometabolic health remains a subject of debate. There are differing viewpoints regarding the long-term consequences of HDP exposure for offspring. Some studies report significant associations between HDP and elevated blood pressure among offspring. For instance, a comprehensive systematic review conducted by Davis et al. (13) indicated that children exposed to preeclampsia exhibited increased systolic and diastolic blood pressure during childhood and young adulthood. Similarly, a prospective study by Alsnes et al. (14) found that such exposure was linked to higher blood pressure levels in offspring. However, other studies, such as those by Jansen et al. (15) and Tripathi et al. (16), found no significant or only weak associations after adjusting for maternal characteristics. Findings on metabolic indicators are also inconsistent. While Alsnes et al. (14) reported higher body mass index (BMI) in HDP-exposed offspring during adolescence, Tripathi et al. (16) reached the opposite conclusion, and no consensus exists regarding the relationship between HDP and other metabolic indicators, such as blood lipids and glucose (16, 17).
Given the diverse findings and their public health implications, systematic reviews and meta-analyses are essential to synthesize current evidence and accurately assess the effects of HDP on offspring cardiometabolic health. This approach clarifies the strength of the association between HDP and offspring cardiovascular health, provides a foundation for early preventive interventions, and informs the monitoring and management of long-term health outcomes in affected offspring. Understanding these associations is particularly important given the rising incidence of hypertensive disorders in pregnancy and the global burden of cardiovascular and metabolic diseases.
2 Methods
This meta-analysis strictly adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines. The review protocol was registered with PROSPERO on January 17, 2025 (registration number: CRD42025630378).
2.1 Literature retrieval
The literature search included online searches of published databases and archives, with authors contacted when necessary. This review focused on the relationship between hypertensive conditions during pregnancy and cardiometabolic outcomes in offspring, specifically examining population-based links between these hypertensive disorders and their potential impact on offspring cardiometabolic health.
Four comprehensive electronic databases—PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and Web of Science—were searched from their inception through December 2024. The search strategy combined subject headings and free-text terms. Keywords included hypertension, pregnancy-induced hypertension, cardiovascular system, cardiovascular diseases, BMI, blood pressure, triglycerides, cholesterol, and offspring.
The retrieval strategy, using PubMed as an example, was as follows: #1: Hypertension, Pregnancy-Induced[MeSH Terms],#2: (((((((((((Hypertension, Pregnancy-Induced[Title/Abstract]) OR (Hypertension, Pregnancy Induced[Title/Abstract])) OR (Pregnancy-Induced Hypertension[Title/Abstract])) OR (Gestational Hypertension[Title/Abstract])) OR (Hypertension, Gestational[Title/Abstract])) OR (Pregnancy Induced Hypertension[Title/Abstract])) OR (Induced Hypertension, Pregnancy[Title/Abstract])) OR (Transient Hypertension, Pregnancy[Title/Abstract])) OR (Hypertension, Pregnancy Transient[Title/Abstract])) OR (Pregnancy Transient Hypertension[Title/Abstract])) OR (HDP[Title/Abstract])) OR (HDPS[Title/Abstract]),#3: #1 OR #2,#4: ((((Cardiovascular System[MeSH Terms]) OR (Cardiovascular Diseases[MeSH Terms])) OR (Cardiometabolic Risk Factors[MeSH Terms])) OR (Metabolic Syndrome[MeSH Terms])) OR (Endocrine System[MeSH Terms]),#5: ((((((((((((((((((Cardiovascular System[Title/Abstract]) OR (Cardiovascular Diseases[Title/Abstract])) OR (Cardiometabolic Risk Factors[Title/Abstract])) OR (Metabolic Syndrome[Title/Abstract])) OR (Endocrine System[Title/Abstract])) OR (Syndromes, Metabolic[Title/Abstract])) OR (cardiovascular health[Title/Abstract])) OR (Cardiovascular diseases[Title/Abstract])) OR (Disease, Cardiovascular[Title/Abstract])) OR (metabolic health[Title/Abstract])) OR (Glucose[Title/Abstract])) OR (Birthweight[Title/Abstract])) OR (BMI[Title/Abstract])) OR (Insulin[Title/Abstract])) OR (fasting insulin[Title/Abstract])) OR (Insulin Resistance[Title/Abstract])) OR (Cholesterol[Title/Abstract])) OR (Triglycerides[Title/Abstract])) OR (Blood Pressure[Title/Abstract]),#6: #4 OR #5,#7: Child[MeSH Terms],#8: (((Child[Title/Abstract]) OR (Children[Title/Abstract])) OR (Childhood[Title/Abstract])) OR (Offspring[Title/Abstract]),#9: #7 OR #8,#10: #3 AND #6 AND #9.
2.2 Inclusion criteria
(1) Case-control or cohort studies;
(2) Content: Relationship between hypertensive disorders in pregnancy and offspring cardiometabolic outcomes;
(3) Population: Pregnant women with hypertensive disorders;
(4) Outcomes: At least one of the following—systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), waist circumference, fat mass index, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, blood glucose, or homeostasis model assessment of insulin resistance (HOMA-IR).
2.3 Exclusion criteria
(1) Non–case-control and non-cohort studies (e.g., animal studies, protocols, abstracts, case reports, correspondence);
(2) Studies not meeting diagnostic criteria for hypertensive disorders in pregnancy;
(3) Incomplete data with unextractable odds ratios and 95% confidence intervals (CIs);
(4) Duplicate publications;
(5) Unavailable full text.
2.4 Literature screening
Bibliographic management software (EndNote) was used to organize document selection and exclusion. Abstracts and full texts were independently reviewed by two researchers (YL and PL) to determine eligibility, and full texts of potentially eligible studies were retrieved and assessed. Two researchers (YL and PL) independently read the full texts of all potentially eligible articles and identified those meeting the inclusion criteria to ensure accuracy. In case of any discrepancies in inclusion decisions, consensus was reached through discussion or with the assistance of a third independent researcher (HX).
2.5 Data extraction
After reviewing the full texts of studies meeting the inclusion criteria, data were independently extracted by two researchers (YL and PL). Extracted data on the cardiometabolic effects of HDP on offspring included study name (first author and publication year), country or region, study design, study population, follow-up period, exclusion criteria, outcome measures, adjustment factors, and other relevant information.
2.6 Literature quality evaluation
The quality of included studies was independently assessed by two researchers (HX and YL) using the Newcastle–Ottawa Scale (NOS). The scale includes three categories—population selection, comparability, and exposure or outcome evaluation—with a maximum score of 9 points. The scoring system was classified as follows: scores of ≤3 indicated low quality, scores of 4–6 indicated medium quality, and scores of ≥7 reflected high quality. Discrepancies were resolved through discussion or by consulting a third investigator (PL).
2.7 Statistical methods
Meta-analysis was conducted using Review Manager 5.4. For categorical variables, odds ratios (ORs) with 95% confidence intervals (CIs) were used. Continuous variables were evaluated using mean difference (MD) or, as needed, standardized mean difference (SMD), each accompanied by 95% CIs. MD was used for all analyses, while SMD was applied when combining data from studies using different scales. A fixed-effects model was employed when I² < 50%; when I² ≥ 50%, a random-effects model was adopted (18). Publication bias was assessed using funnel plots.
3 Results
3.1 Overview of search results
The initial search yielded 4,500 records. After eliminating 1,329 duplicates and dismissing 3,104 irrelevant records according to titles and abstracts, 67 full-text articles were evaluated. Among these, 44 were excluded: incomplete data (n = 1), reviews (n = 11), conference abstracts (n = 5), case reports (n = 4), animal studies (n = 5), and failure to meet outcome indicators (n = 18). Ultimately, 23 studies satisfied the eligibility requirements. The selection procedure is presented in Figure 1.
3.2 Overview of included studies
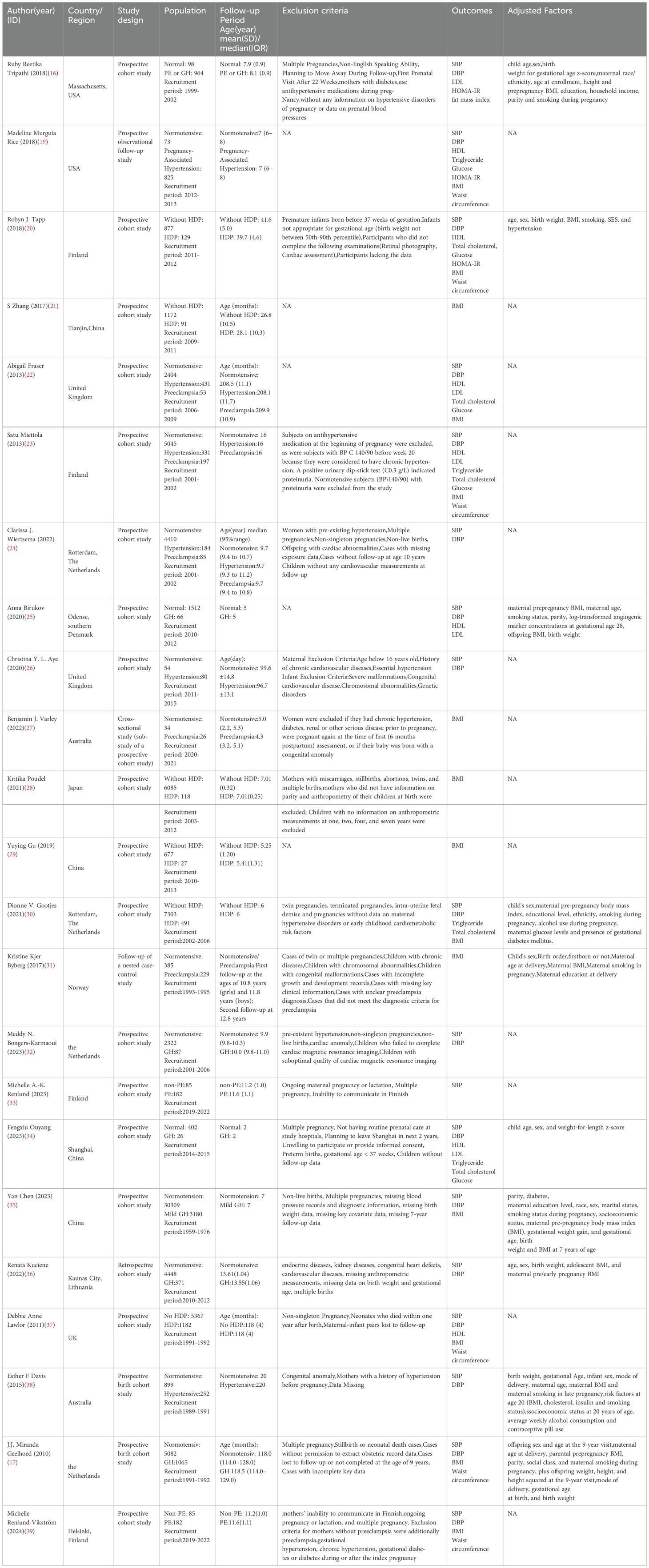
A total of 23 observational studies (16, 17, 19–39) were included, comprising 21 prospective cohort studies, 1 retrospective cohort study, and 1 nested case-control study. These studies involved 89,982 participants (10,854 with gestational hypertension and 79,128 with normal blood pressure) from 10 countries: 4 studies in China, 4 in Finland, 4 in the Netherlands, 3 in the United Kingdom, 2 in the United States, 2 in Australia, and 1 each in Southern Denmark, Japan, Norway, and Lithuania. Of the included studies, 18 reported systolic blood pressure (SBP), 17 reported diastolic blood pressure (DBP), 14 reported body mass index (BMI), 6 reported waist circumference, 5 reported blood glucose, 6 reported high-density lipoprotein (HDL), 2 reported low-density lipoprotein (LDL), 4 reported triglycerides, and 5 reported total cholesterol. Two studies assessed offspring fat mass index, and two studies evaluated offspring HOMA-IR. The detailed characteristics of the 23 included studies are presented in Table 1.
3.3 Methodological quality of included studies
Risk of bias was assessed for the included studies. The 23 articles were evaluated according to the three domains of the Newcastle–Ottawa Scale (NOS) (40). All 23 studies included clearly defined and representative patients with hypertension during pregnancy, a control group of pregnant women with normal blood pressure, and reasonable settings with clear definitions. All 23 studies were comparable, with 10 adjusting key parameters. One study was retrospective with inconclusive exposure assessment, while the remaining 22 studies were prospective. In one study, the follow-up loss rate was slightly higher due to use of questionnaires. Overall, all 23 studies scored above 7 points, indicating high quality (Table 2).
3.4 Statistical results
3.4.1 Results: impact of HDP on offspring blood pressure
A total of 75,980 participants from 18 articles were included to assess the influence of HDP on offspring SBP. A higher offspring SBP is associated with a greater risk. As shown in Figures 2A, B, in 8 studies (19, 22–24, 26, 32, 37, 39), I² = 0% prompted a fixed-effect model, yielding an MD of 2.44 (95% CI: 2.03–2.85; P < 0.00001). In 10 studies (16, 17, 20, 25, 30, 33–36, 38), I² = 91% led to a random-effects model, yielding an OR of 2.24 (95% CI: 1.59–3.15; P < 0.00001). Both results were statistically significant.
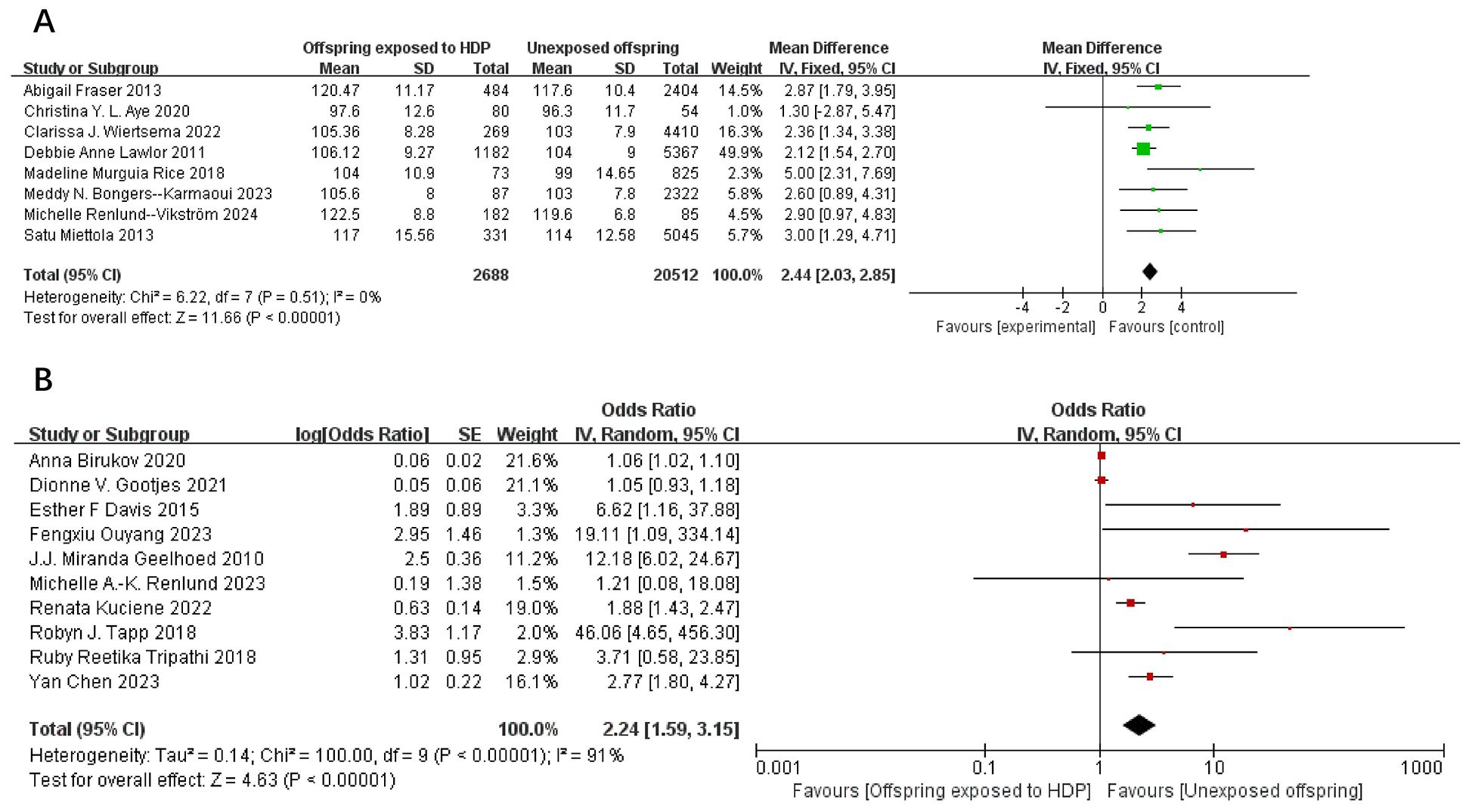
Figure 2. The forest plot shows the impact of HDP on offspring SBP (A) continuous variable (B) categorical variable.
A total of 75,713 participants from 17 articles were included to assess the influence of HDP on offspring DBP. Higher offspring DBP is associated with greater risk. As shown in Figure 3A, B, in 8 studies (19, 22–24, 26, 32, 37, 39), I² = 49% prompted a fixed-effect model, yielding an SMD of 0.19 (95% CI: 0.15–0.23; P < 0.00001). In nine studies (16, 17, 20, 25, 30, 34–36, 38), I² = 87% led to a random-effects model, yielding an OR of 1.79 (95% CI: 1.37–2.34; P < 0.00001). Both results were statistically significant.
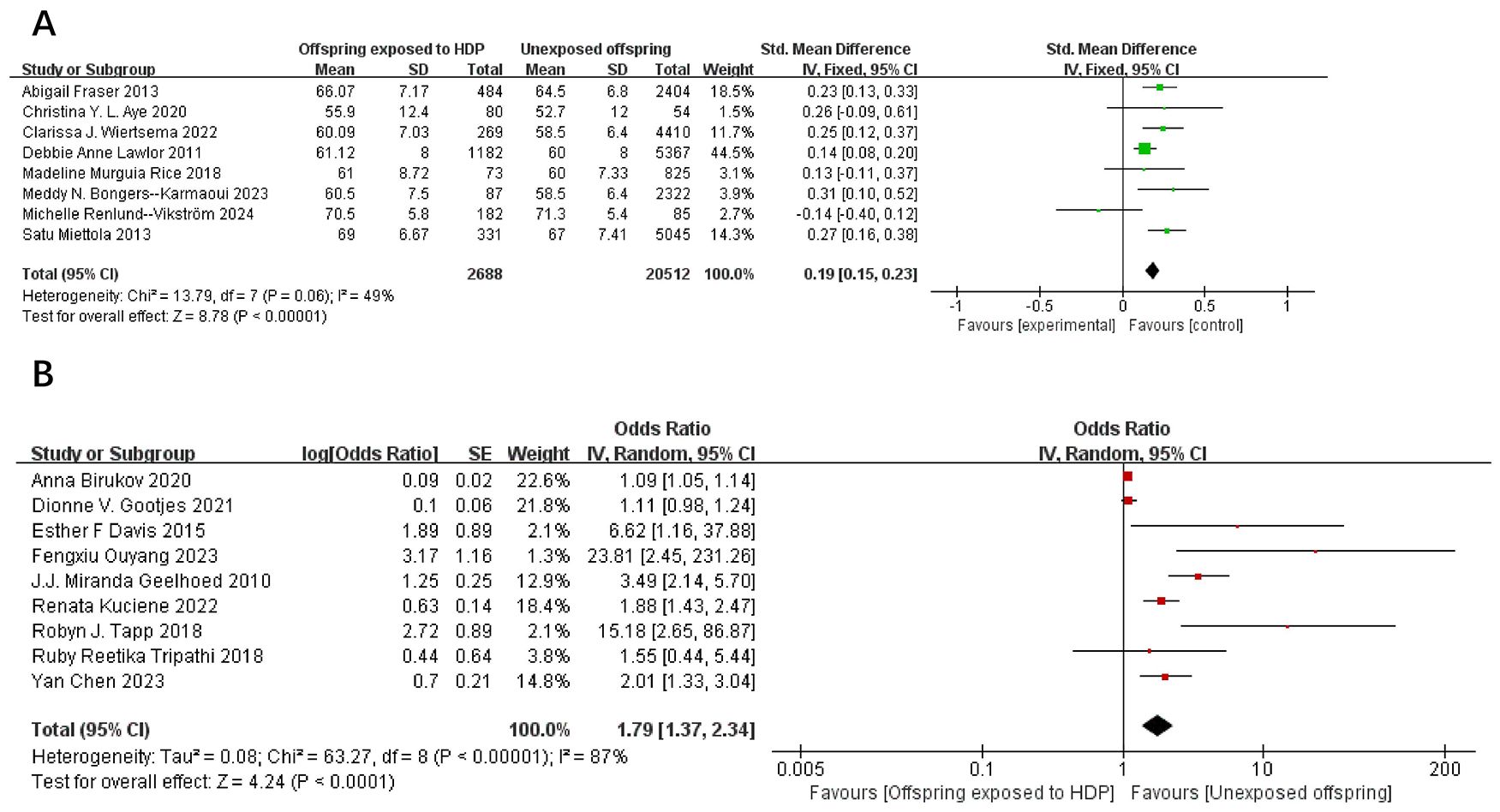
Figure 3. The forest plot shows the impact of HDP on offspring DBP, (A) continuous variable (B) categorical variable.
3.4.2 Results: impact of HDP on offspring obesity
A total of 67,180 participants from 14 articles were included to assess the influence of HDP on offspring BMI. Higher offspring BMI is associated with greater risk. As shown in Figure 4A, B, in 9 studies (19, 21–23, 27–29, 37, 39), I² = 74% prompted a random-effects model, yielding an MD of 0.34 (95% CI: 0.05–0.64; P = 0.02). In five studies (17, 20, 30, 31, 35), I² = 20% prompted a fixed-effect model, yielding an OR of 1.03 (95% CI: 1.02–1.05; P = 0.0004). Both results were statistically significant.
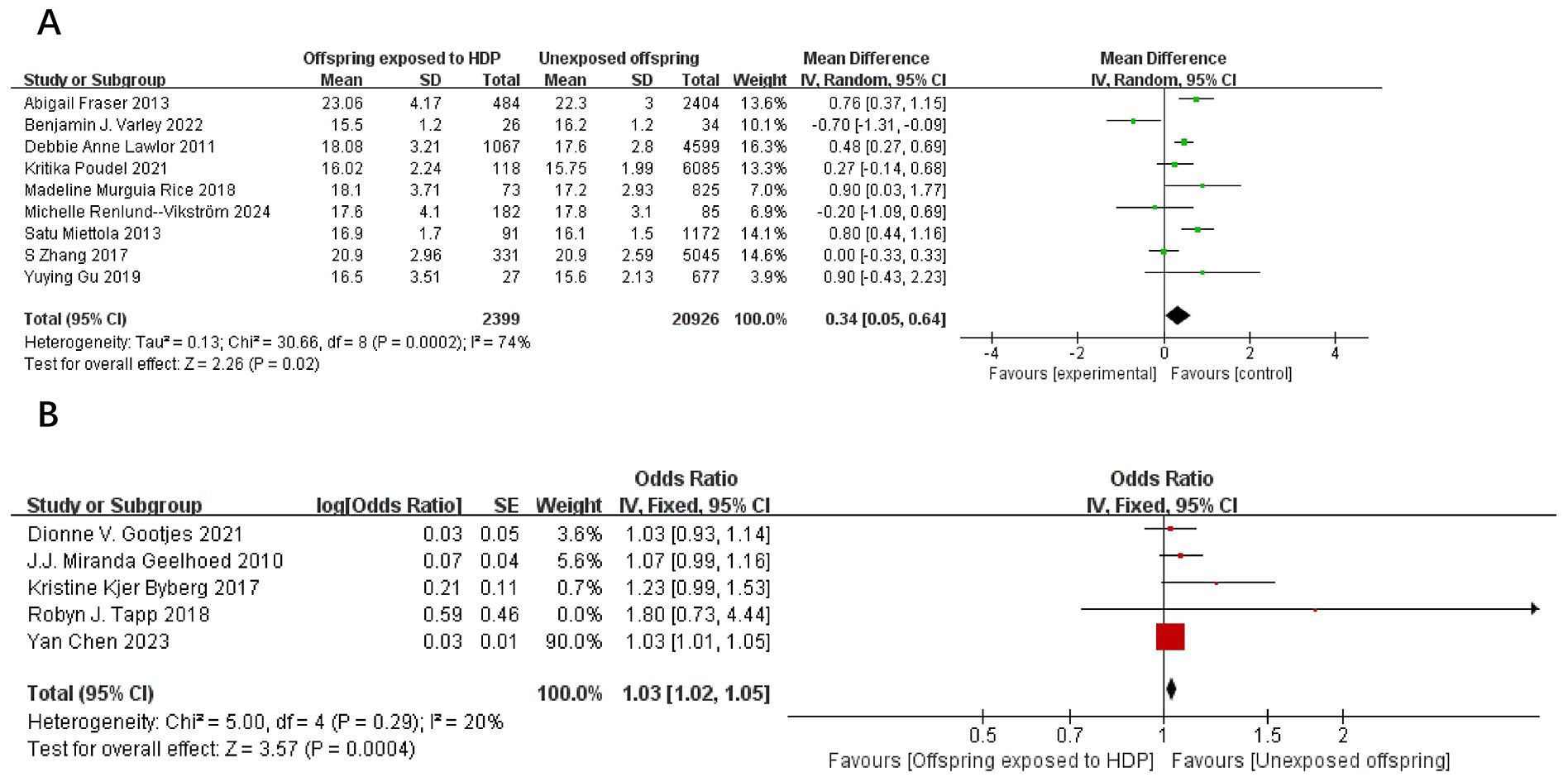
Figure 4. The forest plot shows the impact of HDP on offspring BMI, (A) continuous variable (B) categorical variable.
A total of 14,327 participants from six articles were included to evaluate the effects of HDP on offspring waist circumference. Higher offspring waist circumference is associated with greater risk. As shown in Figures 5A, B, in 4 studies (19, 23, 37, 39), I² = 63% prompted a random-effects model, yielding an SMD of 0.11 (95% CI: −0.00 to 0.23; P = 0.05), which was not statistically significant. In two studies (17, 20), I² = 0% prompted a fixed-effect model, yielding an OR of 1.06 (95% CI: 0.98–1.14; P = 0.15), also not statistically significant.
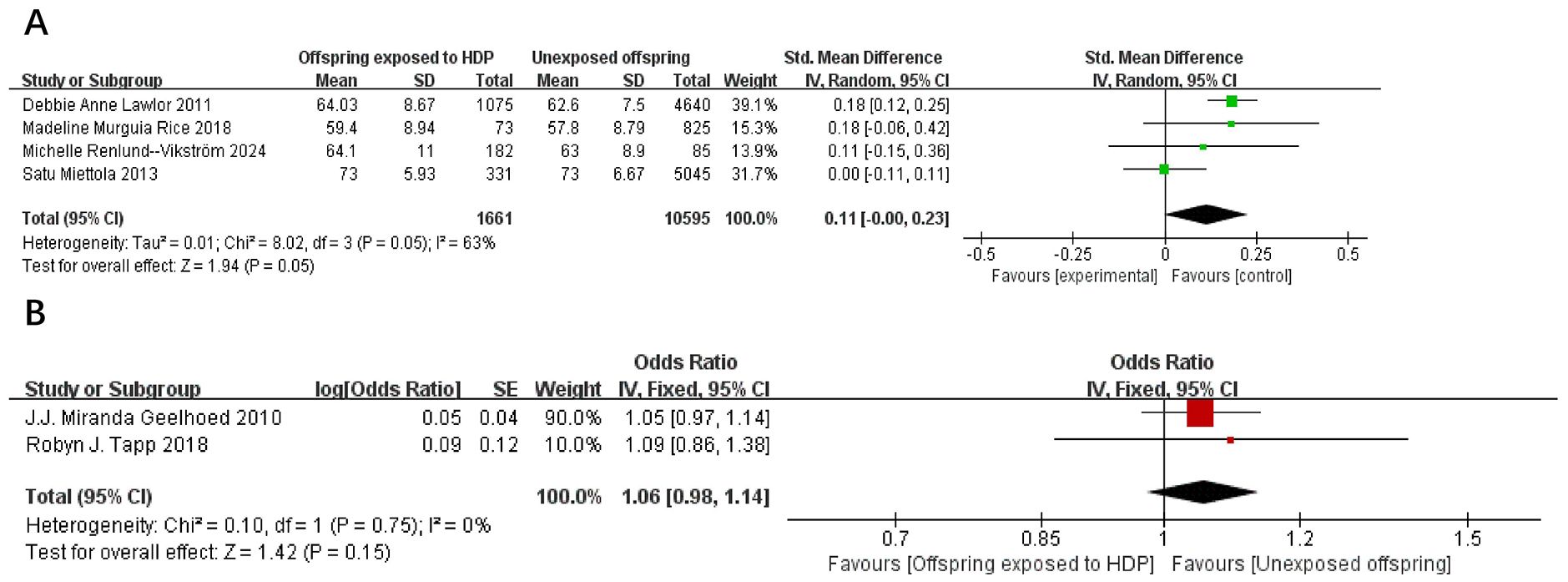
Figure 5. The forest plot shows the impact of HDP on offspring waist circumference, (A) continuous variable (B) categorical variable.
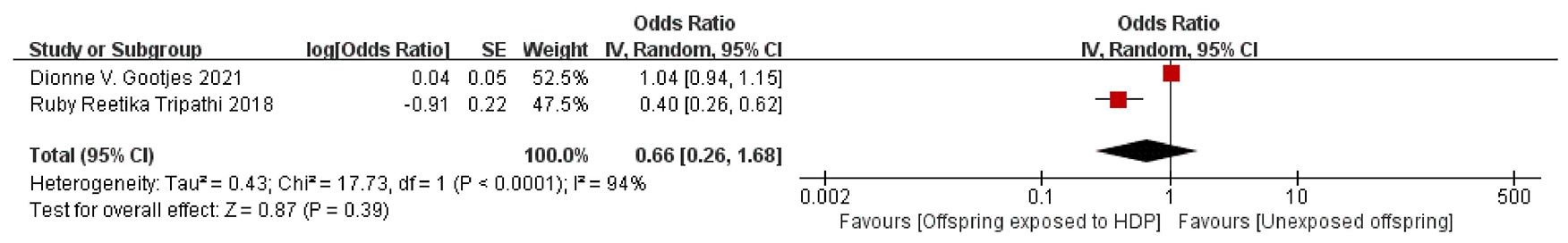
A total of 8,856 participants from 2 articles were included to assess the effects of HDP on offspring fat mass index. Higher offspring fat mass index is associated with greater risk. As shown in Figure 6, in these two studies (16, 30), I² = 94% prompted a random-effects model, yielding an OR of 0.66 (95% CI: 0.26–1.68; P = 0.39), which was not statistically significant.
3.4.3 Results: effect of HDP on offspring lipid metabolism
A total of 14,863 participants from 6 articles were included to assess the effects of HDP on offspring HDL. Higher offspring HDL is associated with lower risk. As shown in Figures 7A, B, in four studies (19, 22, 23, 37), I² = 22% prompted a fixed-effect model, yielding an SMD of −0.03 (95% CI: −0.08 to 0.03; P = 0.34; no statistical significance was found. In two studies (20, 34), I² = 0% prompted a fixed-effect model, yielding an OR of 0.98 (95% CI: 0.93–1.04; P = 0.52), also not significant.
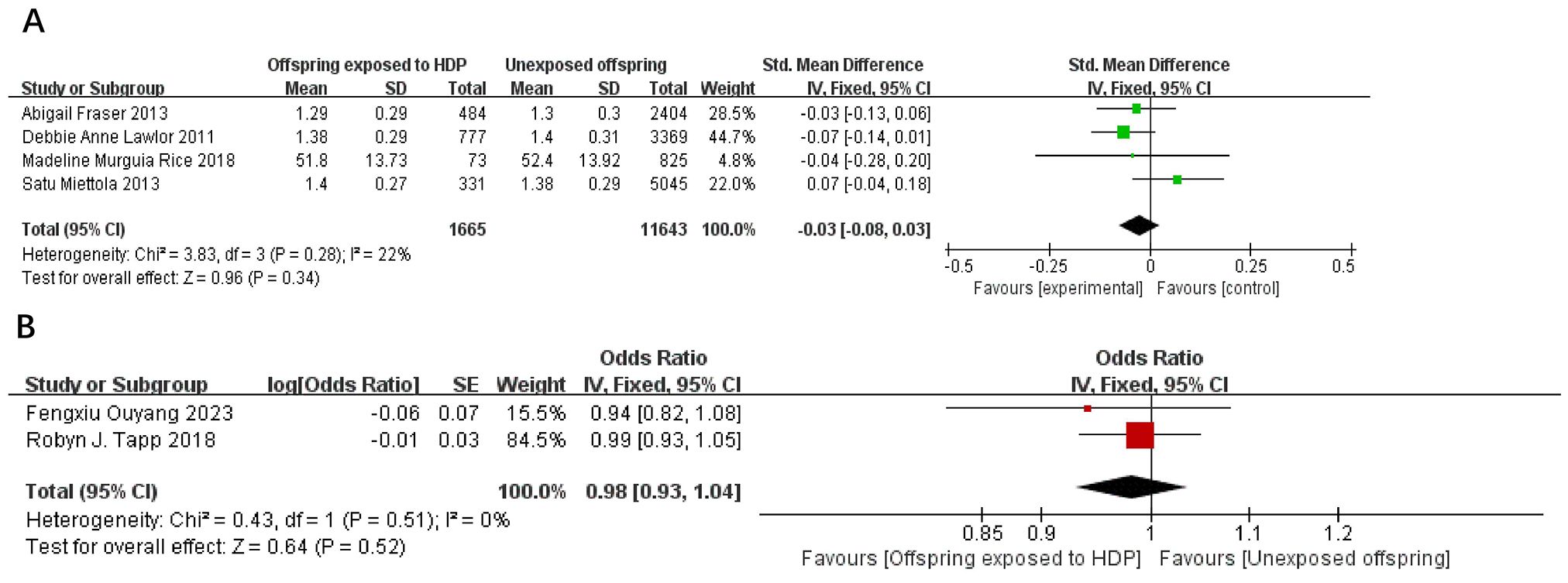
Figure 7. The forest plot shows the impact of HDP on offspring HDL, (A) continuous variable (B) categorical variable.
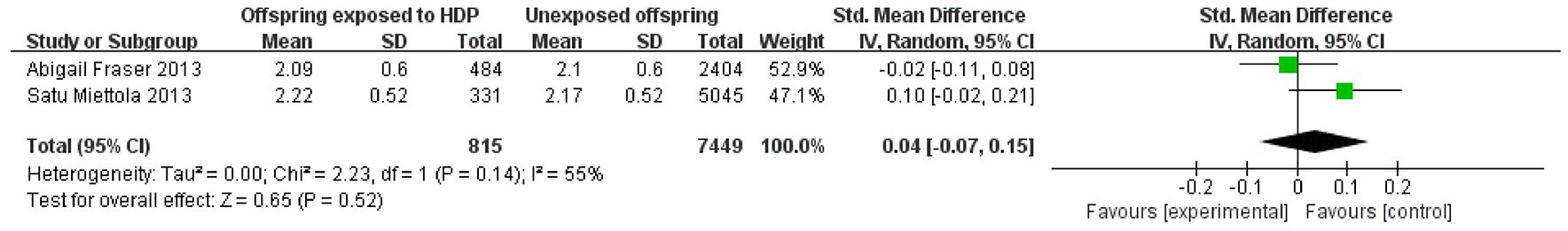
A total of 8,264 participants from 2 articles were included to assess the effects of HDP on offspring LDL. Higher offspring LDL is associated with greater risk. As shown in Figure 8, in two studies (22, 23), I² = 55% prompted a random-effects model, yielding an SMD of 0.04 (95% CI: −0.07 to 0.15; P = 0.52; no statistical significance was found. A total of 14,617 participants from four articles were included to assess the effects of HDP on offspring triglycerides. Higher offspring triglyceride levels are associated with greater risk. As shown in Figure 9A, B, in 2 studies (19, 23), I² = 32% prompted a fixed-effect model, yielding an SMD of −0.07 (95% CI: −0.17 to 0.03; P = 0.17),; no statistical significance was found. In 2 studies (30, 34), I² = 83% prompted a random-effects model, yielding an OR of 1.07 (95% CI: 0.75–1.51; P = 0.72), also not significant.
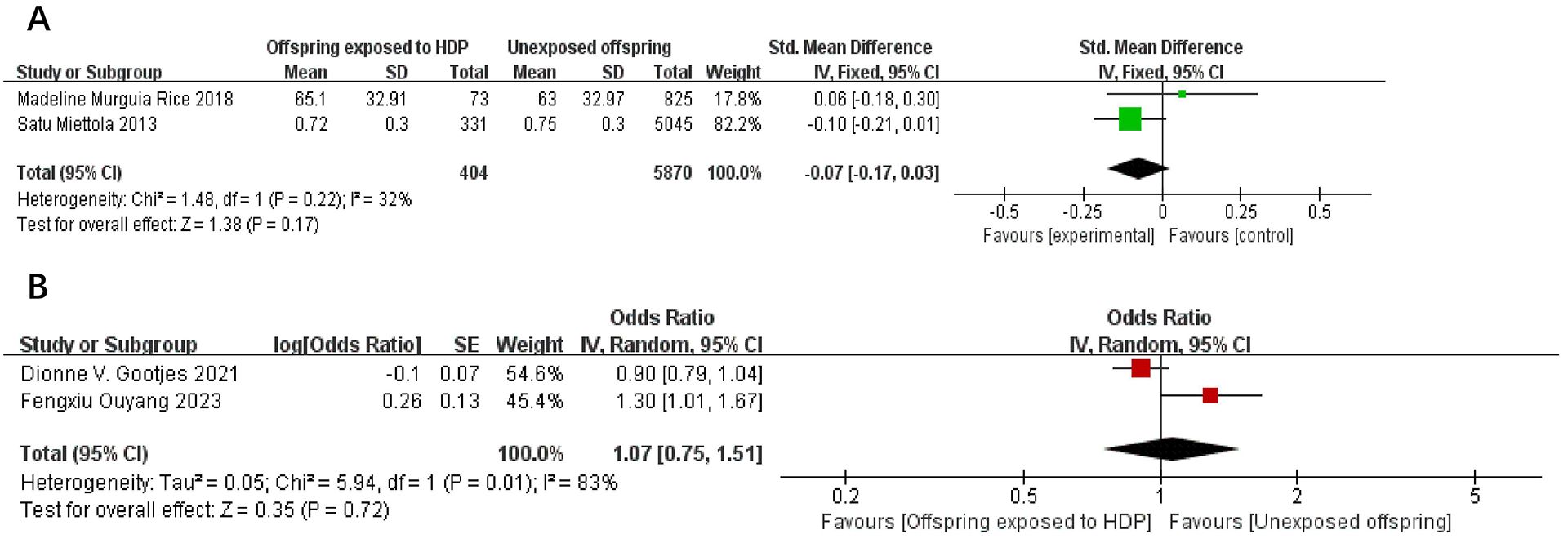
Figure 9. The forest plot shows the impact of HDP on offspring triglyceride, (A) continuous variable (B) categorical variable.
A total of 17,611 participants from five articles were included to assess the effect of HDP on offspring total cholesterol. As shown in Figures 10A, B, in 2 studies (22, 23), I² = 76% prompted a random-effects model, yielding an MD of 0.04 (95% CI: −0.06 to 0.14; P = 0.45; no statistical significance was found. In three studies (20, 30, 34), I² = 0% prompted a fixed-effect model, yielding an OR of 1.01 (95% CI: 0.91–1.12; P = 0.81), also not significant.
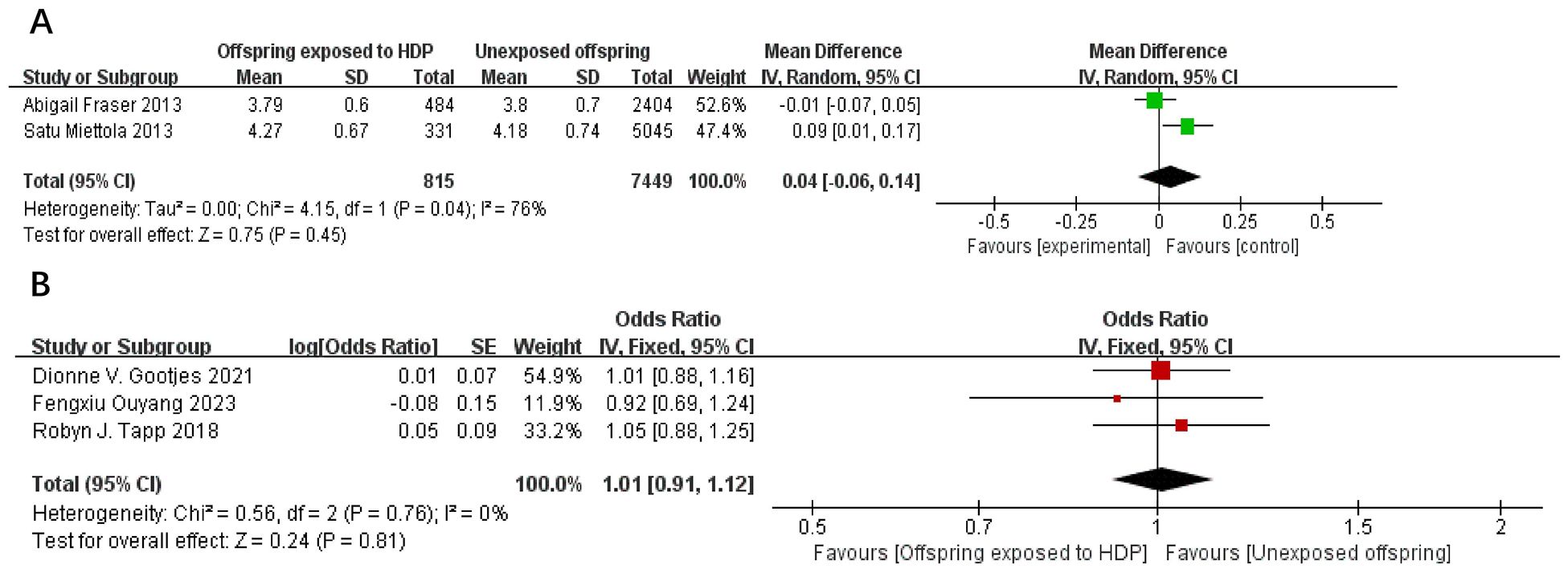
Figure 10. The forest plot shows the impact of HDP on offspring total cholesterol, (A) continuous variable (B) categorical variable.
3.4.4 Results: impact of HDP on offspring glucose metabolism
A total of 10,717 participants from five articles were included to assess the effects of HDP on offspring blood glucose. Higher offspring blood sugar is associated with greater risk. As shown in Figures 11A, B, in 3 studies (19, 22, 23), I² = 70% prompted a random-effects model, yielding an SMD of −0.04 (95% CI: −0.18 to 0.10; P = 0.57), not statistically significant. In two studies (20, 34),I² = 80% prompted a random-effects model, yielding an OR of 1.07 (95% CI: 0.78–1.46; P = 0.67), also not significant.
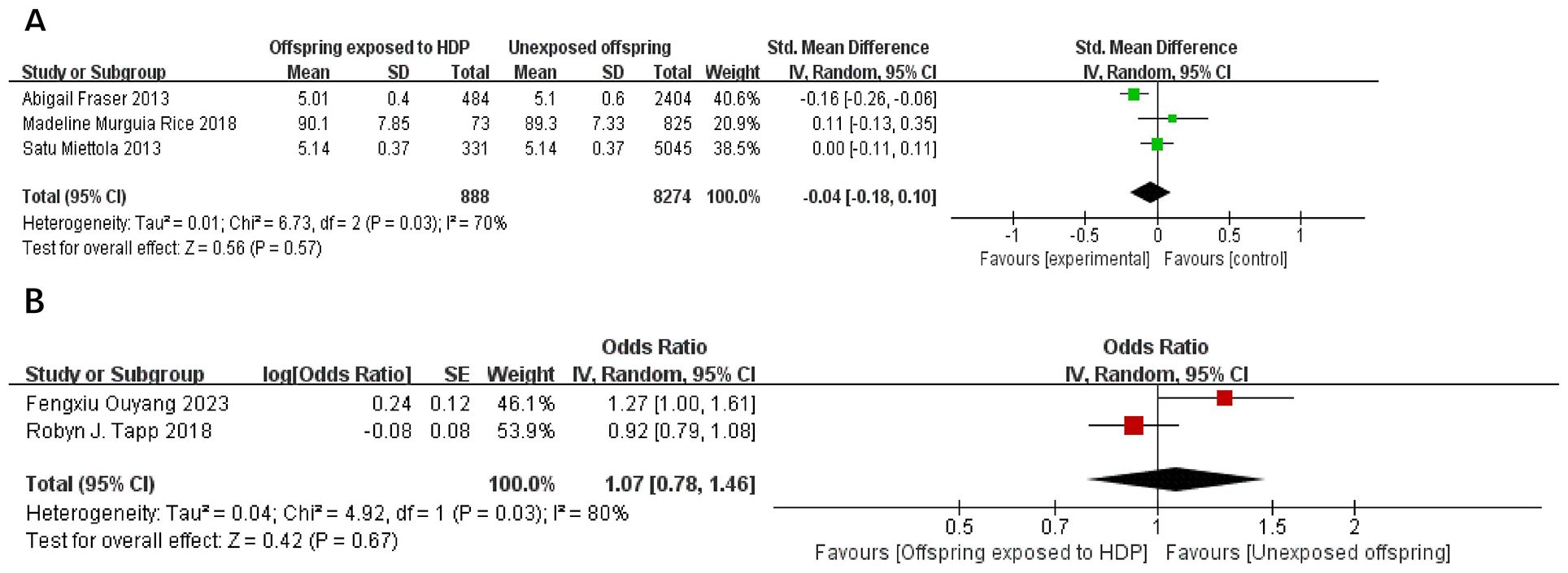
Figure 11. The forest plot shows the impact of HDP on offspring blood glucose, (A) continuous variable (B) categorical variable.
A total of 2,068 participants from two articles were included to assess the effect of HDP on offspring HOMA-IR. As shown in Figure 12, in these two studies (16, 20), I² = 0% prompted a fixed-effect model, yielding an OR of 0.58 (95% CI: 0.34–0.98; P = 0.04), which was statistically significant.
3.5 Meta-regression analysis
To evaluate whether offspring age influences the association between HDP and offspring cardiometabolic indicators, a meta-regression analysis was conducted. HDP was used as the independent variable, cardiometabolic indicators (e.g., SBP, DBP, BMI) as dependent variables, and offspring age as a covariate. The regression coefficient (β) values for offspring age were small, and P > 0.05 (0.53–0.85), indicating no significant influence of offspring age on the association between HDP and cardiometabolic indicators. However, this finding may be affected by the relatively small number of studies. Future research should further investigate the potential impact of offspring age and consider increasing sample size to enhance statistical power. The specific results are presented in Supplementary Table 2 (meta-regression analysis: A, continuous variables; B, categorical variables).
3.6 Publication bias and sensitivity analysis
Results for 11 indicators are shown in Figure 13, including SBP, DBP, BMI, and waist circumference, among others. Funnel plot symmetry was evaluated to detect potential publication bias. The funnel plots were roughly symmetrical, indicating no significant publication bias.
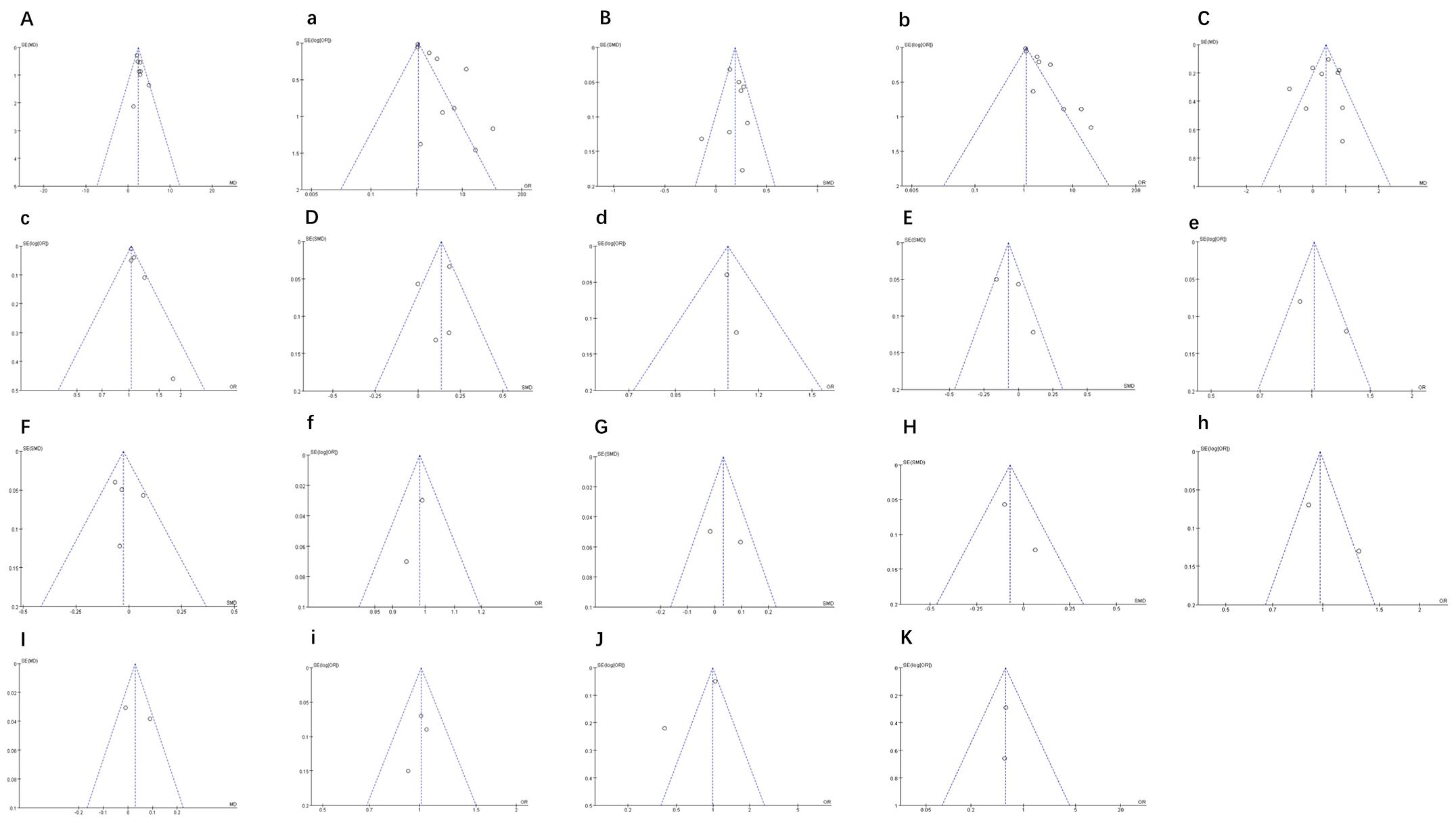
Figure 13. (A, a) Funnel plot of SBP. (B, b) Funnel plot of DBP. (C, c) Funnel plot of BMI. (D, d) Funnel plot of waist circumference. (E, e) Funnel plot of blood glucose. (F, f) Funnel plot of HDL. (G) Funnel plot of LDL. (H, h) Funnel plot of triglyceride. (I, i) Funnel plot of total cholesterol. (J) Funnel plot of total Fat Mass Index. (K) Funnel plot of HOMA-IR.
4 Discussion
4.1 Main results
This review analyzed the cardiometabolic effects of gestational hypertension on offspring based on 23 studies, comparing them with offspring of mothers without hypertension. It included 11 outcome measures and involved 89,982 participants, representing a relatively large sample. Although follow-up periods varied, all studies included measures of HDP and cardiometabolic outcomes. The results show that offspring cardiometabolic health is affected in multiple ways by HDP: SBP, DBP, and BMI were adversely affected, while the risk of insulin resistance was lower. No significant associations were observed for blood glucose or blood lipids.
4.2 Interpretation (in light of other evidence)
4.2.1 Effects of HDP on offspring blood pressure
This study reveals a significant link between HDP and elevated offspring blood pressure. Meta-analysis showed that offspring of hypertensive pregnant women had higher SBP (MD: 2.44; 95% CI: 2.03-2.85, P < 0.00001) and DBP (SMD: 0.19; 95% CI: 0.15-0.23, P < 0.00001). Davis et al. (13) reported a 2.39 mmHg increase in SBP in children born to preeclamptic mothers, consistent with our findings. Thoulass et al. (41) also found higher SBP in adolescence among HDP-exposed offspring. Alsnes et al. (14) showed that HDP exposure independently increased SBP by 2.2 mmHg (95% CI: 1.2–3.1) in offspring, even after adjusting for confounders. In this study, the elevated blood pressure observed in offspring exposed to HDP may be attributed to vascular endothelial dysfunction during the fetal period (42, 43). This dysfunction can alter the responsiveness of blood vessels to pressure changes, thereby increasing the risk of hypertension in these offspring. Additionally, epigenetic modifications within the renin-angiotensin system (44) may contribute to blood pressure dysregulation in HDP-exposed offspring. Glucocorticoid imbalances (23), which can affect fetal adrenal cortex function, may also play a role in blood pressure regulation. Furthermore, HDP may have lasting effects on cardiovascular development through placental dysfunction and fetal nutrition (45). These mechanisms align with the elevated blood pressure findings in our study, underscoring the importance of early intervention and long-term monitoring for offspring exposed to HDP.
4.2.2 Influence of HDP on offspring obesity
Our study disclosed a significant link between HDP and offspring BMI (MD: 0.34; 95% CI: 0.05–0.64; P < 0.05). However, there were no significant differences noted in waist circumference or fat mass index. In a follow-up of 15,778 adolescents, Alsnes et al. (14) reported higher BMI in HDP-exposed individuals (0.66; 95% CI: 0.31–1.01). Chen et al. (35) also observed elevated BMI at age 7 in offspring of mothers with mild HDP (adjusted β: 0.03; 95% CI: 0.01–0.05). These findings suggest that mild HDP may increase the risk of offspring overweight or obesity (46). The link between HDP and offspring BMI may be mediated by epigenetic modifications that influence adipose tissue development and function (19, 47, 48) The pre-pregnancy BMI of the mother is also a significant factor, as a higher pre-pregnancy BMI is associated with an increased risk of HDP and may jointly impact offspring weight through both genetic and environmental pathways. Additionally, the intrauterine environment’s effect on adipose tissue development should not be overlooked. Yan et al. (49) highlighted a gender difference, with female offspring experiencing a significant increase in BMI (MD: 1.04; 95% CI: 0.67–1.42; P < 0.05), suggesting potential sex hormone regulation. This gender difference aligns with the observed increase in BMI in our study, indicating that sex hormones may play a crucial role in the impact of HDP on offspring weight.
4.2.3 Effects of HDP on offspring lipid metabolism
Abnormal lipid metabolism is a significant risk factor for cardiovascular diseases in offspring. However, the relationship between HDP and offspring lipid metabolism remains controversial. Our study found no significant correlation between HDP and offspring lipid indices (HDL, LDL, triglycerides, and total cholesterol). This aligns with several large cohort studies showing no significant association between HDP and early lipid levels in offspring (20, 23, 37). However, some studies report increased dyslipidemia risk in HDP offspring in early childhood, possibly due to fetal nutritional changes, inflammation, oxidative stress, and genetic factors (22, 34). While this study did not identify a significant association between HDP and offspring lipid metabolism, several potential mechanisms have been proposed in prior research. For example, alterations in fetal nutrition, particularly during critical developmental periods, may impact lipid metabolism (22, 34). Moreover, inflammation and oxidative stress may also influence lipid metabolism in offspring exposed to HDP. Notably, Gootjes et al. found a negative association between maternal hypertension and triglyceride levels in 6-year-old girls, potentially related to sex hormone regulation of lipid metabolism (30, 50–52). In mice, preeclampsia exposure reduced lipid transporters and binding proteins in female placentas, affecting lipid metabolism (53). The “no association” conclusion must be interpreted cautiously, as dyslipidemia may take longer to manifest. Factors such as HDP severity, maternal lifestyle, and genetics may influence this relationship (30). Most studies lack long-term dynamic lipid assessments, limiting current understanding. Thus, while no direct causal link is evident, long-term follow-up studies are needed to elucidate the correlation between HDP and offspring lipid metabolism, as metabolic abnormalities may develop later in life.
4.2.4 Effects of HDP on offspring glucose metabolism
Current research on how HDP impacts offspring blood glucose metabolism has yielded inconsistent results. Studies have yet to reach a consensus on this matter. In our study, offspring exposed to HDP showed a significant decrease in HOMA-IR (OR: 0.58; 95% CI: 0.34–0.98; P = 0.04). However, no significant correlation was observed with blood glucose levels.
Some studies report associations between maternal hypertension and abnormal offspring metabolism. For example, a Chinese study showed elevated blood glucose in 2-year-olds exposed to maternal hypertension (β = 0.24; 95% CI: 0.01–0.47) (34). Conversely, Tripathi et al. found lower insulin resistance in children (mean age 8 years) from hypertensive pregnancies (β = −0.54; 95% CI: −1.10 to 0.01) (16), while other studies found no significant associations (19, 20, 22). The impact of HDP on offspring glucose metabolism may be associated with insulin secretion and insulin sensitivity during the fetal period. In this study, the observed decrease in HOMA-IR may suggest that offspring exposed to HDP have certain advantages in terms of insulin sensitivity. This could be related to metabolic adaptation during the fetal period, such as adjustments in insulin secretion to cope with changes in the intrauterine environment. However, this advantage may not persist into adulthood, as other studies have shown inconsistent results for glucose metabolism in offspring at different ages. These inconsistencies may arise from differences in subjects’ ages, metabolic indicators, methodological approaches, and the heterogeneity of gestational hypertension. Future large-scale prospective trials with long-term follow-up are needed to clarify the long-term impact on offspring glucose metabolism and to adjust for confounding factors using a unified standard.
4.3 Strengths and limitations
The strengths of this study include its large sample size (n = 89,982) and its comprehensive evaluation of 11 cardiometabolic indicators across diverse populations. The findings provide a scientific basis for early intervention in HDP-exposed offspring and emphasize the importance of perinatal care in preventing cardiovascular diseases. However, limitations include the natural occurrence of HDP, long follow-up durations, potential data loss due to population mobility, and the complexity of gene–environment interactions. Additionally, diagnostic criteria for HDP are not standardized, and the prevalence of HDP varies geographically, which may affect the study’s generalizability.
5 Conclusion
This study systematically analyzed the impact of HDP on offspring cardiometabolic outcomes. The comprehensive nature of the review and meta-analysis provided a robust foundation for the findings. The results demonstrated that HDP is significantly associated with offspring cardiovascular and metabolic health. These effects are mainly reflected in increased systolic and diastolic blood pressure and higher body mass index, as well as a reduced risk of insulin resistance. However, no significant associations were found with other metabolic markers, including blood glucose and lipids. Further research is needed to explore the underlying mechanisms and long-term effects to better provide targeted health guidance and intervention measures for affected populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HX: Writing – original draft. YL: Writing – original draft. PL: Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1641563/full#supplementary-material
Supplementary Table 1 | The complete search strategy for all databases
Supplementary Table 2 | Meta-regression analysis (A) Continuous Variables (B) Categorical Variables.
References
1. Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. (2014) 4:97–104. doi: 10.1016/j.preghy.2014.02.001
2. Butalia S, Audibert F, Côté AM, Firoz T, Logan AG, Magee LA, et al. Hypertension Canada’s 2018 guidelines for the management of hypertension in pregnancy. Can J Cardiol. (2018) 34:526–31. doi: 10.1016/j.cjca.2018.02.021
3. Hypertension in pregnancy. Report of the american college of obstetricians and gynecologists’ Task force on hypertension in pregnancy. Obstet Gynecol. (2013) 122:1122–31. doi: 10.1097/01.Aog.0000437382.03963.88
4. Traub A, Sharma A, and Gongora MC. Hypertensive disorders of pregnancy: A literature review - pathophysiology, current management, future perspectives, and healthcare disparities. US Cardiol. (2024) 18:e03. doi: 10.15420/usc.2023.01
5. Practice Bulletin No ACOG. 202: gestational hypertension and preeclampsia. Obstet Gynecol. (2019) 133:1. doi: 10.1097/aog.0000000000003018
6. Shen M, Smith GN, Rodger M, White RR, Walker MC, and Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PloS One. (2017) 12:e0175914. doi: 10.1371/journal.pone.0175914
7. Hutcheon JA, Lisonkova S, and Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. (2011) 25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006
8. Khan KS, Wojdyla D, Say L, Gülmezoglu AM, and Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. (2006) 367:1066–74. doi: 10.1016/s0140-6736(06)68397-9
9. Makino Y, Shibata K, Makino I, Kangawa K, and Kawarabayashi T. Alteration of the adrenomedullin receptor components gene expression associated with the blood pressure in pregnancy-induced hypertension. J Clin Endocrinol Metab. (2001) 86:5079–82. doi: 10.1210/jcem.86.10.8099
10. Rosenberg EA and Seely EW. Long-term cardiovascular disease after adverse pregnancy outcomes. J Clin Endocrinol Metab. (2024) 109:e883–e91. doi: 10.1210/clinem/dgad600
11. Garovic VD, Dechend R, Easterling T, Karumanchi SA, McMurtry Baird S, Magee LA, et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: A scientific statement from the american heart association. Hypertension. (2022) 79:e21–41. doi: 10.1161/hyp.0000000000000208
12. Gagliardi L, Rusconi F, Bellù R, and Zanini R. Association of maternal hypertension and chorioamnionitis with preterm outcomes. Pediatrics. (2014) 134:e154–61. doi: 10.1542/peds.2013-3898
13. Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. (2012) 129:e1552–61. doi: 10.1542/peds.2011-3093
14. Alsnes IV, Vatten LJ, Fraser A, Bjørngaard JH, Rich-Edwards J, Romundstad PR, et al. Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: prospective and sibling studies in the HUNT study (Nord-trøndelag health study) in Norway. Hypertension. (2017) 69:591–8. doi: 10.1161/hypertensionaha.116.08414
15. Jansen MA, Pluymen LP, Dalmeijer GW, Groenhof TKJ, Uiterwaal CS, Smit HA, et al. Hypertensive disorders of pregnancy and cardiometabolic outcomes in childhood: A systematic review. Eur J Prev Cardiol. (2019) 26:1718–47. doi: 10.1177/2047487319852716
16. Tripathi RR, Rifas-Shiman SL, Hawley N, Hivert MF, and Oken E. Hypertensive disorders of pregnancy and offspring cardiometabolic health at midchildhood: project viva findings. J Am Heart Assoc. (2018) 7. doi: 10.1161/jaha.117.007426
17. Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. (2010) 122:1192–9. doi: 10.1161/circulationaha.110.936674
18. Wang Y, Guo X, Obore N, Ding H, Wu C, and Yu H. Aspirin for the prevention of preeclampsia: A systematic review and meta-analysis of randomized controlled studies. Front Cardiovasc Med. (2022) 9:936560. doi: 10.3389/fcvm.2022.936560
19. Rice MM, Landon MB, Varner MW, Casey BM, Reddy UM, Wapner RJ, et al. Pregnancy-associated hypertension and offspring cardiometabolic health. Obstet Gynecol. (2018) 131:313–21. doi: 10.1097/aog.0000000000002433
20. Tapp RJ, Hughes AD, Kähönen M, Wong TY, Witt N, Lehtimäki T, et al. Cardiometabolic health among adult offspring of hypertensive pregnancies: the cardiovascular risk in young finns study. J Am Heart Assoc. (2018) 7. doi: 10.1161/jaha.117.006284
21. Zhang S, Wang L, Leng J, Liu H, Li W, Zhang T, et al. Hypertensive disorders of pregnancy in women with gestational diabetes mellitus on overweight status of their children. J Hum Hypertens. (2017) 31:731–6. doi: 10.1038/jhh.2017.17
22. Fraser A, Nelson SM, Macdonald-Wallis C, Sattar N, and Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. (2013) 62:614–20. doi: 10.1161/hypertensionaha.113.01513
23. Miettola S, Hartikainen AL, Vääräsmäki M, Bloigu A, Ruokonen A, Järvelin MR, et al. Offspring’s blood pressure and metabolic phenotype after exposure to gestational hypertension in utero. Eur J Epidemiol. (2013) 28:87–98. doi: 10.1007/s10654-013-9763-5
24. Wiertsema CJ, Jaddoe VWV, Mulders A, and Gaillard R. Childhood blood pressure, carotid intima media thickness, and distensibility after in utero exposure to gestational hypertensive disorders. J Am Heart Assoc. (2022) 11:e023163. doi: 10.1161/jaha.121.023163
25. Birukov A, Herse F, Nielsen JH, Kyhl HB, Golic M, Kräker K, et al. Blood pressure and angiogenic markers in pregnancy: contributors to pregnancy-induced hypertension and offspring cardiovascular risk. Hypertension. (2020) 76:901–9. doi: 10.1161/hypertensionaha.119.13966
26. Aye CYL, Lewandowski AJ, Lamata P, Upton R, Davis E, Ohuma EO, et al. Prenatal and postnatal cardiac development in offspring of hypertensive pregnancies. J Am Heart Assoc. (2020) 9:e014586. doi: 10.1161/jaha.119.014586
27. Varley BJ, Henry A, Roberts L, Davis G, Skilton MR, Craig ME, et al. Intrauterine exposure to preeclampsia does not impair vascular health in children. Front Public Health. (2022) 10:1071304. doi: 10.3389/fpubh.2022.1071304
28. Poudel K, Kobayashi S, Miyashita C, Yamaguchi T, Tamura N, Ikeda-Araki A, et al. Hypertensive Disorders during Pregnancy and Anthropometric Measurement of Children up to 7 Years of Age: The Hokkaido Birth Cohort Study in Japan. Int J Environ Res Public Health. (2021) 18. doi: 10.3390/ijerph182010951
29. Gu Y, Lu J, Li W, Liu H, Wang L, Leng J, et al. Joint associations of maternal gestational diabetes and hypertensive disorders of pregnancy with overweight in offspring. Front Endocrinol (Lausanne). (2019) 10:645. doi: 10.3389/fendo.2019.00645
30. Gootjes DV, Posthumus AG, Jaddoe VWV, van Rijn BB, and Steegers EAP. Maternal hypertensive disorders in pregnancy and early childhood cardiometabolic risk factors: The Generation R Study. PloS One. (2021) 16:e0261351. doi: 10.1371/journal.pone.0261351
31. Byberg KK, Øymar K, Eide GE, Forman MR, and Júlíusson PB. Exposure to preeclampsia in utero affects growth from birth to late childhood dependent on child’s sex and severity of exposure: Follow-up of a nested case-control study. PloS One. (2017) 12:e0176627. doi: 10.1371/journal.pone.0176627
32. Bongers-Karmaoui MN, Wiertsema CJ, Mulders A, Helbing WA, Hirsch A, Roest AAW, et al. Gestational hypertensive disorders and blood pressure and childhood cardiac outcomes: A prospective cohort study. Bjog. (2023) 130:1226–37. doi: 10.1111/1471-0528.17468
33. Renlund MA, Jääskeläinen TJ, Kivelä ASE, Heinonen ST, Laivuori HM, and Sarkola TA. Blood pressure, arterial stiffness, and cardiovascular risk profiles in 8-12-year-old children following preeclampsia (FINNCARE-study). J Hypertens. (2023) 41:1429–37. doi: 10.1097/hjh.0000000000003485
34. Ouyang F, Wells JC, Zhang GH, Du K, Wang X, Shen L, et al. Maternal prenatal factors and child adiposity in associations with cardiometabolic risk factors in term-born chinese children at the age of 2 years. Nutrients. (2023) 15. doi: 10.3390/nu15153342
35. Chen Y, Wang Y, Li Y, Ding G, and Zhang Y. Association of the severity of hypertensive disorders in pregnancy with birthweight, childhood obesity, and blood pressure at age 7. Nutrients. (2023) 15. doi: 10.3390/nu15143104
36. Kuciene R and Dulskiene V. Associations of maternal gestational hypertension with high blood pressure and overweight/obesity in their adolescent offspring: a retrospective cohort study. Sci Rep. (2022) 12:3800. doi: 10.1038/s41598-022-07903-z
37. Lawlor DA, Macdonald-Wallis C, Fraser A, Nelson SM, Hingorani A, Davey Smith G, et al. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J. (2012) 33:335–45. doi: 10.1093/eurheartj/ehr300
38. Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, et al. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. (2015) 5:e008136. doi: 10.1136/bmjopen-2015-008136
39. Renlund-Vikström M, Jääskeläinen TJ, Kivelä A, Heinonen S, Laivuori H, and Sarkola T. Cardiac structure and function in 8- to 12-year-old children following in-utero exposure to preeclampsia (FINNCARE study). J Am Heart Assoc. (2024) 13:e034494. doi: 10.1161/jaha.124.034494
40. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. (2015) 8:2–10. doi: 10.1111/jebm.12141
41. Thoulass JC, Robertson L, Denadai L, Black C, Crilly M, Iversen L, et al. Hypertensive disorders of pregnancy and adult offspring cardiometabolic outcomes: a systematic review of the literature and meta-analysis. J Epidemiol Community Health. (2016) 70:414–22. doi: 10.1136/jech-2015-205483
42. Jayet PY, Rimoldi SF, Stuber T, Salmòn CS, Hutter D, Rexhaj E, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. (2010) 122:488–94. doi: 10.1161/circulationaha.110.941203
43. Yu GZ, Aye CY, Lewandowski AJ, Davis EF, Khoo CP, Newton L, et al. Association of maternal antiangiogenic profile at birth with early postnatal loss of microvascular density in offspring of hypertensive pregnancies. Hypertension. (2016) 68:749–59. doi: 10.1161/hypertensionaha.116.07586
44. Washburn LK, Brosnihan KB, Chappell MC, Diz DI, Gwathmey TM, Nixon PA, et al. The renin-angiotensin-aldosterone system in adolescent offspring born prematurely to mothers with preeclampsia. J Renin Angiotensin Aldosterone Syst. (2015) 16:529–38. doi: 10.1177/1470320314526940
45. Barker DJ, Larsen G, Osmond C, Thornburg KL, Kajantie E, and Eriksson JG. The placental origins of sudden cardiac death. Int J Epidemiol. (2012) 41:1394–9. doi: 10.1093/ije/dys116
46. Zheng JS, Liu H, Ong KK, Huang T, Guan Y, Huang Y, et al. Maternal blood pressure rise during pregnancy and offspring obesity risk at 4 to 7 years old: the jiaxing birth cohort. J Clin Endocrinol Metab. (2017) 102:4315–22. doi: 10.1210/jc.2017-01500
47. Stojanovska V, Scherjon SA, and Plösch T. Preeclampsia as modulator of offspring health. Biol Reprod. (2016) 94:53. doi: 10.1095/biolreprod.115.135780
48. Rice MM, Landon MB, Varner MW, Casey BM, Reddy UM, Wapner RJ, et al. Pregnancy-associated hypertension in glucose-intolerant pregnancy and subsequent metabolic syndrome. Obstet Gynecol. (2016) 127:771–9. doi: 10.1097/aog.0000000000001353
49. Yan S, Lyu J, Liu Z, Zhou S, Ji Y, and Wang H. Association of gestational hypertension and preeclampsia with offspring adiposity: A systematic review and meta-analysis. Front Endocrinol (Lausanne). (2022) 13:906781. doi: 10.3389/fendo.2022.906781
50. Seidell JC, Cigolini M, Charzewska J, Ellsinger BM, Björntorp P, Hautvast JG, et al. Fat distribution and gender differences in serum lipids in men and women from four European communities. Atherosclerosis. (1991) 87:203–10. doi: 10.1016/0021-9150(91)90022-u
51. Vaidya D, Dobs A, Gapstur SM, Golden SH, Hankinson A, Liu K, et al. The association of endogenous sex hormones with lipoprotein subfraction profile in the Multi-Ethnic Study of Atherosclerosis. Metabolism. (2008) 57:782–90. doi: 10.1016/j.metabol.2008.01.019
52. Johnson JL, Slentz CA, Duscha BD, Samsa GP, McCartney JS, Houmard JA, et al. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. (2004) 176:371–7. doi: 10.1016/j.atherosclerosis.2004.05.018
Keywords: hypertensive disorders of pregnancy, HDP, cardiometabolic, offspring, meta-analysis
Citation: Xu H, Liang Y and Li P (2025) Association of hypertensive disorders of pregnancy with offspring cardiometabolic indicators: a systematic review and meta-analysis. Front. Endocrinol. 16:1641563. doi: 10.3389/fendo.2025.1641563
Received: 05 June 2025; Accepted: 09 October 2025;
Published: 03 November 2025.
Edited by:
Gopal Chandra Ghosh, Rabindranath Thakur Diagnostic and Medical Care Center, IndiaReviewed by:
Yixiao Wang, Southeast University, ChinaTrini Suryowati, Universitas Kristen Indonesia, Indonesia
Copyright © 2025 Xu, Liang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peishan Li, NTM0NDA1MTVAcXEuY29t
 Hong Xu
Hong Xu Yujie Liang
Yujie Liang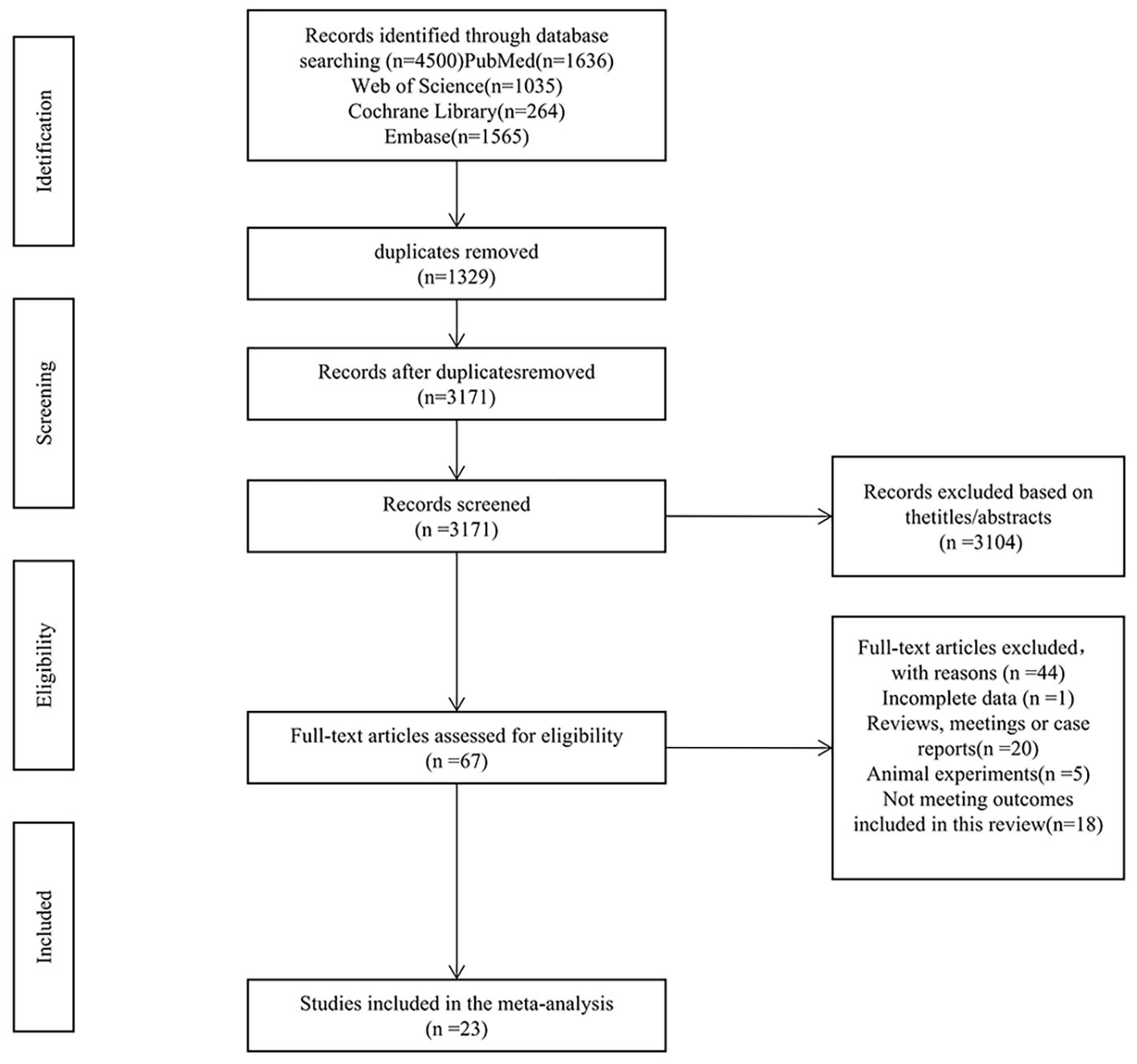
![Forest plot showing odds ratios for two studies: Robyn J. Tapp (2018) and Ruby Reetika Tripathi (2018). Combined odds ratio is 0.58 with a 95% confidence interval of [0.34, 0.98]. The plot favors offspring exposed to HDP over unexposed offspring. Heterogeneity is zero, with a chi-squared value of 0.00 and a P-value of 0.97 for heterogeneity. Test for overall effect shows Z = 2.05, P = 0.04.](https://www.frontiersin.org/files/Articles/1641563/fendo-16-1641563-HTML/image_m/fendo-16-1641563-g012.jpg)