- 1Center of Reproductive Medicine, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, Sichuan, China
- 3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
- 4Department of Pharmacy Practice, Faculty of Pharmacy, University of Tabuk, Tabuk, Saudi Arabia
Background and Aim: Menopausal hormone therapy (MHT) remains a pivotal approach in managing menopausal symptoms; however, its effects on inflammation and cardiovascular risk markers are still under debate. In particular, the combination of medroxyprogesterone acetate (MPA) and conjugated equine estrogens (CEE) has shown variable impacts on inflammatory biomarkers. This systematic review and meta-analysis aimed to synthesize evidence from randomized controlled trials (RCTs) assessing the effects of oral MPA combined with CEE (MPA/CEE) on systemic inflammation in postmenopausal women.
Methods: Thirteen RCTs (comprising 16 arms) reporting data on inflammatory markers, including C-reactive protein (CRP), fibrinogen, homocysteine, and interleukin-6 (IL-6), were included, with a total sample size of 2,278 participants. A random-effects model was used to calculate pooled weighted mean differences (WMDs) with 95% confidence intervals. Subgroup and sensitivity analyses were performed to explore heterogeneity, and publication bias was assessed using Egger's test and trim-and-fill methods.
Results: MPA/CEE treatment was associated with a significant decrease in CRP levels (WMD = -0.173 mg/dL; 95% CI: -0.25 to -0.10; P < 0.001), particularly among postmenopausal women aged <60 years, trials with MPA doses ≤2.5 mg/day, and those with BMI <25 kg/m². In addition, a significant reduction in fibrinogen levels was observed (WMD = -60.588 mg/dL; 95% CI: -71.436 to -49.741; P < 0.001), especially at MPA doses ≤2.5 mg/day and in women with BMI <25 kg/m². No statistically significant changes were found in homocysteine or IL-6 levels.
Conclusion: While MPA/CEE therapy significantly reduces CRP and fibrinogen, key inflammatory and cardiovascular risk markers, these findings suggest a notable protective effect of oral MPA/CEE on inflammation, highlighting the need for individualized therapeutic strategies based on patient risk profiles.
Introduction
The menopausal transition is characterized by a decline in endogenous estrogen production, which can result in a range of undesirable symptoms, including vasomotor disturbances, vulvovaginal dryness and atrophy, decreased bone mineral density, and adverse changes in lipid metabolism (1). Beyond these clinical manifestations, menopause has also been associated with an increase in systemic inflammation, independent of chronological aging (2). Menopausal hormone therapy (MHT), administered as either estrogen alone or in combination with a progestin, has long been employed to relieve these symptoms and improve quality of life in postmenopausal women (3). Current guidelines from global organizations, including the North American Menopause Society and the International Menopause Society, recommend MHT as the most effective treatment for vasomotor symptoms and vulvovaginal atrophy in appropriately selected postmenopausal women, with an emphasis on individualized risk-benefit assessment (4, 5).
However, estrogen therapy has been shown to elevate circulating levels of C-reactive protein (CRP), a well-established marker of systemic inflammation (6). This finding is of particular clinical relevance, as elevated CRP levels are strongly linked to an increased risk of cardiovascular events (7). Randomized controlled trials (RCTs) evaluating the use of hormone replacement therapy (HRT) for cardiovascular disease prevention have, unexpectedly, revealed a rise in both venous and arterial thrombotic events following initiation of treatment (8). It remains unclear whether the increase in CRP reflects a generalized pro-inflammatory response mediated by upstream cytokines like interleukin-6 (IL-6), or whether alternative pathways are involved. For example, findings from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial indicated that while CRP levels rose during HRT, there were no corresponding increases in fibrinogen, E-selectin, or other acute-phase reactants (9). Although CRP elevation has been proposed as a possible mediator of HRT-associated risks, definitive clinical outcome data supporting this link are lacking. Some evidence suggests that estrogen therapy may provoke or exacerbate inflammation, potentially accelerating the development of atherosclerosis and thrombosis in women with predisposing risk factors (10). However, the inflammatory potential of unopposed estrogen remains a subject of debate (11). The relative contributions of estrogen versus progestins to systemic inflammation remain incompletely understood, with conflicting data on whether estrogen or progestins are the predominant mediators of CRP elevation.
Medroxyprogesterone acetate (MPA) is commonly prescribed as a progestin alongside estrogen in women with an intact uterus, to reduce the risk of endometrial hyperplasia and cancer (12). Notably, androgens possess anti-inflammatory properties (13), and because synthetic progestins such as MPA exhibit androgenic activity, their concurrent use with estrogen may help counterbalance the pro-inflammatory effects of estrogen therapy (14).
Despite the widespread use of MHT and recognition of its benefits, there remains uncertainty regarding the differential effects of various progestin doses on systemic inflammation and cardiovascular risk markers. While estrogen's impact on inflammatory biomarkers like CRP has been extensively studied, the role of progestins, especially MPA, in modulating these effects at different doses remains inadequately characterized. Moreover, conflicting evidence exists about whether lower progestin doses may confer superior anti-inflammatory benefits compared to higher doses (15, 16). Addressing these gaps is critical to optimizing hormone therapy regimens to maximize therapeutic benefits while minimizing cardiovascular risks.
Therefore, this systematic review and meta-analysis aims to evaluate the dose-dependent effects of oral MPA combined with conjugated equine estrogens (MPA/CEE) on inflammatory biomarkers in postmenopausal women.
Materials and methods
Search strategy
Two independent researchers conducted a comprehensive literature search across Scopus, PubMed/MEDLINE, EMBASE, and Web of Science to identify peer-reviewed articles published in English through August 2025. The search aimed to locate studies evaluating the effects of MPA/CEE on inflammatory biomarkers in postmenopausal women. A combination of Medical Subject Headings (MeSH) and free-text keywords was used to maximize the sensitivity and specificity of the search. Full details of the search strategy are provided in Supplementary Table 1.
Inclusion criteria and exclusion criteria
Publications were included in our systematic review and meta-analysis if they met all of the following criteria: Population (P), postmenopausal women; Intervention (I), treatment with MPACEE; Comparison (C), randomized controlled trials featuring a placebo or control group; and Outcomes (O), reported measurable inflammation markers, specifically mean and standard deviation (SD) values for CRP, fibrinogen, homocysteine, and IL-6 at both baseline and at the end of the intervention. In this review, we excluded studies that did not provide adequate outcome data, along with unpublished reports, correspondence, commentaries, narrative reviews, brief communications, meta-analyses, ecological studies, and research conducted on animals.
Data extraction
Two researchers independently screened all relevant RCTs and carefully selected those eligible for the meta-analysis. Data extraction was also performed independently by both researchers using a standardized form within Microsoft Excel (Microsoft Corp, Redmond, WA, USA). Any disagreements were resolved by consensus with the primary author. The following information was systematically collected from each RCT and recorded in the standardized Excel template: number of participants per group, mean age of participants, first author's name, treatment duration, study location, publication year, study design, participants' health status, mean and standard deviation (SD) values for CRP, fibrinogen, homocysteine, and IL-6 before and after intervention, as well as the prescribed MPA/CEE dose.
Quality assessment
Two independent assessors evaluated the quality of evidence in the selected studies using the Cochrane Collaboration's risk of bias tool. This assessment covered key domains including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, handling of incomplete outcome data, and selective outcome reporting. These criteria formed the foundation for judging the overall quality and reliability of the evidence presented in the RCTs (17).
Statistical analysis
In this meta-analysis, statistical analyses were conducted using Stata version 15 (Stata Corp., College Station, TX, USA). Because the included RCTs varied in participant characteristics, baseline BMI, intervention dosages, and study durations, we anticipated genuine between-study heterogeneity. Therefore, we predefined the use of a random-effects model (DerSimonian and Laird method) to generate pooled estimates, as this approach accounts for both within-study and between-study variance. A fixed-effect model, which assumes a single true effect size, was considered less appropriate in this context. Nevertheless, to ensure the robustness of our pooled results, sensitivity analyses were conducted by systematically excluding each study arm one at a time and recalculating the overall combined effect size.
We utilized a random-effects model based on the DerSimonian and Laird method to calculate pooled estimates of the intervention's impact on inflammation. Weighted mean differences (WMDs) with 95% confidence intervals (CIs) were derived from the mean and standard deviation (SD) values of both the MPA/CEE and control groups. A p-value of less than 0.05 was considered statistically significant. For studies reporting outcomes as percent change from baseline, we converted percent change into post-intervention means using meanpost=meanpre×(1+%Δ/100). When SD of the percent change or post values were not reported, we estimated post-intervention SD by proportional scaling of the baseline SD: SDpost≈ SDpre×(meanpost/meanpre). When SDs for change scores were unavailable, they were estimated using the formula:
SD_change = √[(SD_baseline² + SD_final²) – (2 × R × SD_baseline × SD_final)],
where R represents the correlation coefficient. To ensure consistency, outcome units were converted to mg/dL when reported differently. For data presented as standard errors, medians, interquartile ranges, or ranges, we applied Cochrane Collaboration's recommended formulas to convert them to means and SDs (18, 19).
We utilized Pearson's chi-squared test (χ²) and Higgins' I² statistics to assess statistical heterogeneity among RCT arms. A significance threshold of p < 0.10 was applied, and heterogeneity was classified as low (25–49%), moderate (50–74%), or high (≥75%) based on predefined criteria. To further investigate heterogeneity, subgroup analyses were performed considering intervention duration, baseline characteristics, baseline body mass index (BMI), health status, and daily MPA/CEE dose. Subgroup analyses were performed primarily for outcomes with substantial heterogeneity (I² ≥ 75%) in order to explore potential sources of variability. For parameters with low or moderate heterogeneity, such as homocysteine, subgroup analyses were not conducted because of the limited number of available studies (n = 4), which would have made stratified analyses underpowered and potentially unreliable.
Potential publication bias was evaluated using funnel plots and confirmed with Egger's test, with p-values below 0.1 indicating statistical significance (20). When publication bias was detected, we applied the trim-and-fill method to adjust the effect sizes accordingly (21).
Results
Study selection
We initially identified 16,027 publications across four databases. After removing duplicates, 12,415 unique records remained. A review of titles and abstracts led to the exclusion of 12,382 records, leaving 33 articles for full-text assessment. Of these, 20 were excluded based on eligibility criteria (Figure 1). Ultimately, 13 records with 16 arms were included in the final meta-analysis (22–34). These comprised 7 RCT arms reporting on CRP, 11 on fibrinogen, 4 on homocysteine, and 4 on IL-6.
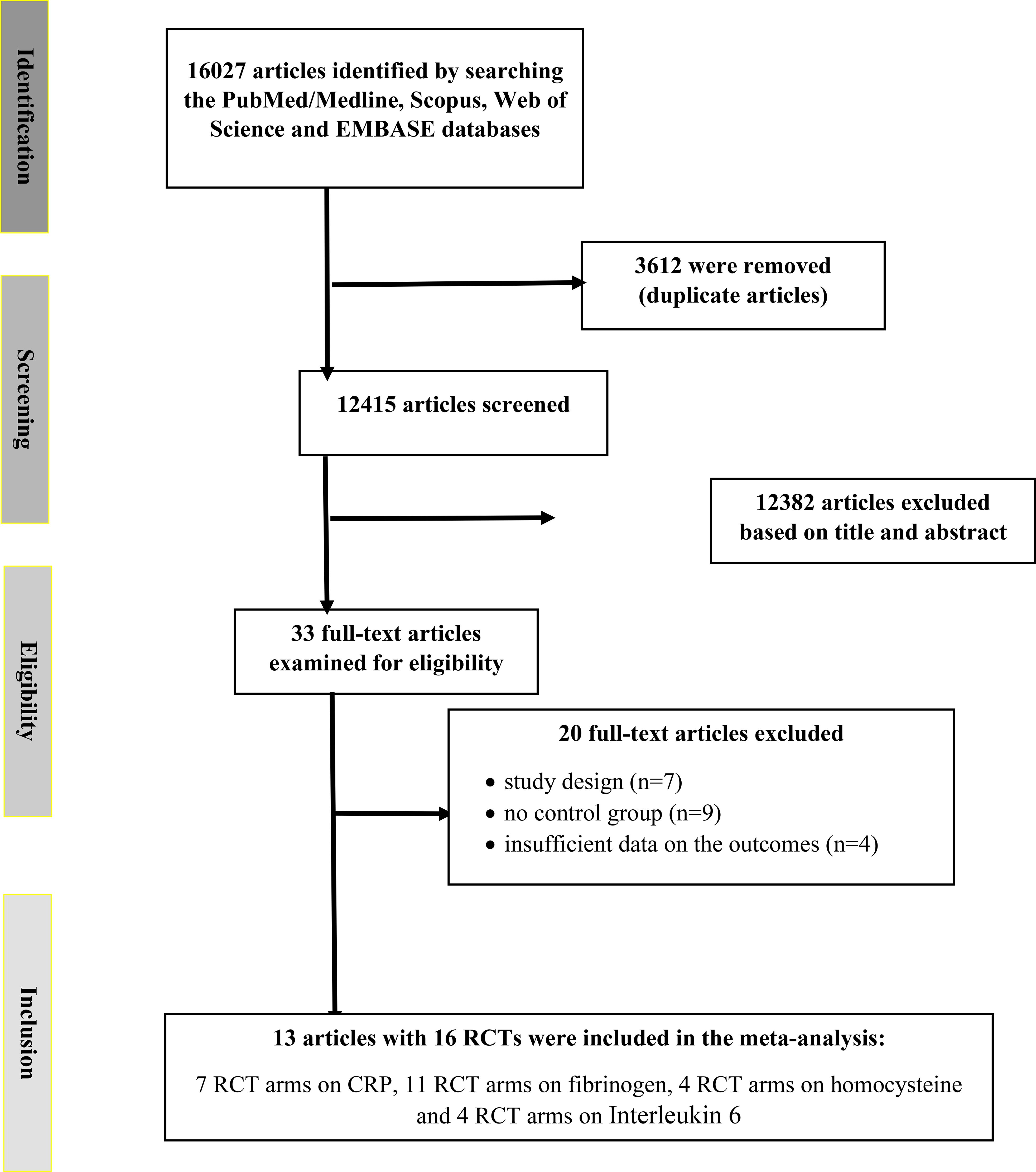
Figure 1. Flowchart depicting the study selection and inclusion process for the present meta analysis. RCT, randomized controlled trial(s).
Characteristics of the included studies
The detailed characteristics of the included RCTs are presented in Table 1. The randomized controlled trials (RCTs) included in the analysis enrolled female participants and were conducted between 1997 and 2018. The duration of MPA/CEE treatment ranged from 3 to 36 months. The average age of participants spanned from 51 to 68.4 years, with a median age of 55.65 years. the daily dosage of MPA/CEE varied across studies, ranging from 1.5 mg to 10 mg for MPA and from 0.45 mg/day to 0.625 mg/day for CEE. These trials were conducted in several countries, including the United States, Denmark, Korea, Turkey, Finland, Japan, and Italy. Baseline body mass index (BMI) values ranged from 20.7 kg/m² to 32 kg/m².
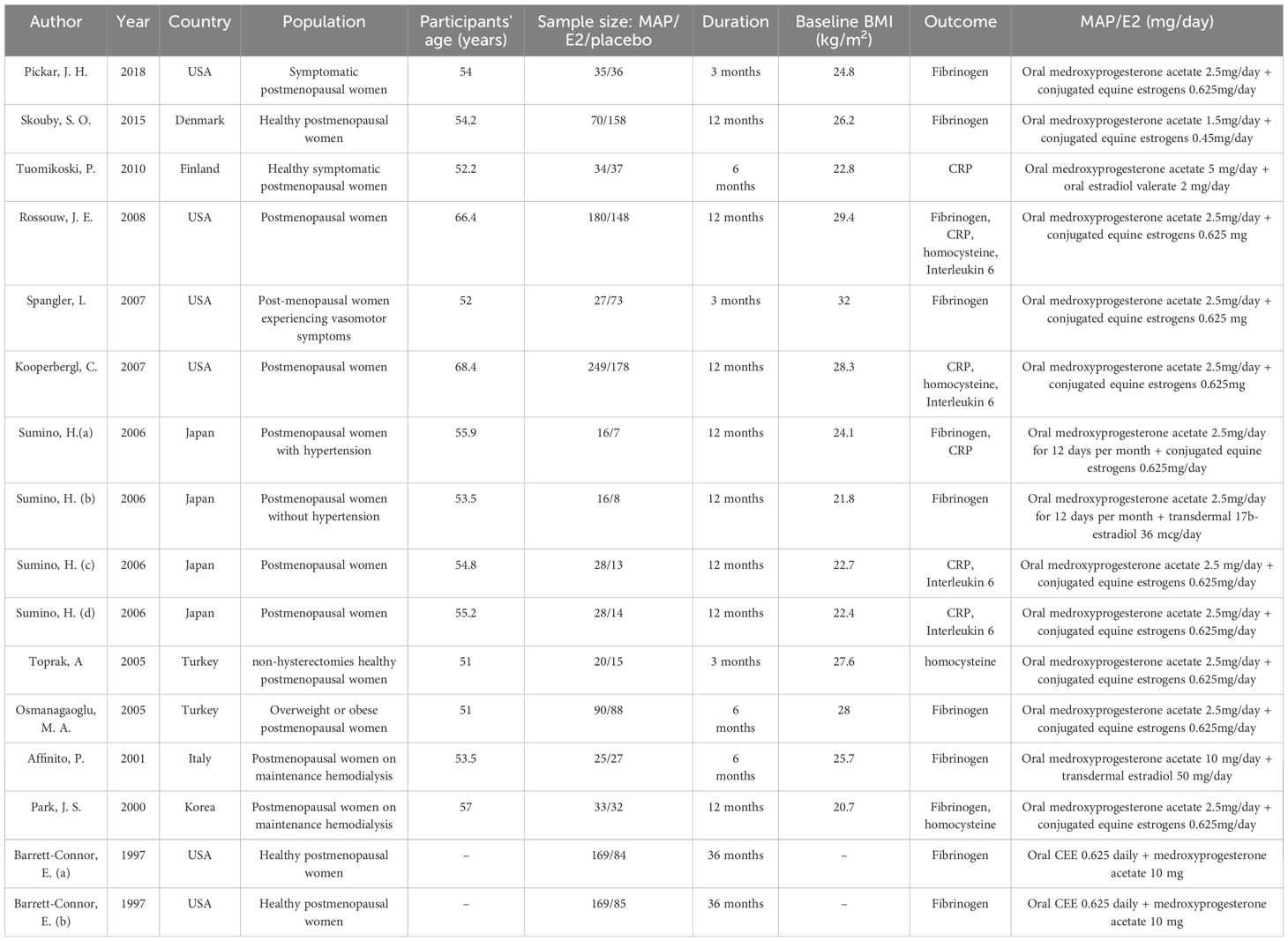
Table 1. Characteristics of the eligible randomized controlled trials (RCTs) included in the meta-analysis.BMI, body mass index.
Participants included a variety of postmenopausal populations: symptomatic and healthy postmenopausal women, those with hypertension, overweight or obese individuals, postmenopausal women with vasomotor symptoms, postmenopausal women without hypertension, non-hysterectomized healthy women, and those undergoing maintenance hemodialysis. The detailed characteristics of the included RCTs are presented in (Table 1). Risk of bias and methodological quality assessments are provided in (Supplementary Table 2).
Findings from the meta-analysis
Effects of MPA/CEE treatment on C-reactive protein levels
After pooling data from 7 RCT arms comprising a total of 998 participants (551 in the intervention group and 447 in the placebo group), we conducted a meta-analysis using a random-effects model. The results showed that MPA/CEE administration was associated with a significant decrease in CRP levels in postmenopausal women (WMD = -0.17 mg/dL; 95% CI: -0.25 to -0.10; P < 0.001) (Figure 2). A heterogeneity analysis indicated substantial variability among the included studies (I² = 98%, P < 0.001). Subgroup analyses revealed a significant decrease in CRP concentrations when MPA/CEE was administered at doses ≤2.5 mg/day (WMD = -0.26 mg/dL; 95% CI: -0.40 to -0.13; P< 0.001) compare to >2.5 mg/day (WMD = 0.02 mg/dL; 95% CI: 0.02 to 0.03; P< 0.001). The decrease was also more pronounced among participants aged <60 years (WMD = -0.29 mg/dL; 95% CI: -0.39 to -0.20; P< 0.001) compared to those aged ≥60 years (WMD = 0.10 mg/dL; 95% CI: 0.07 to 0.14; P< 0.001). Similarly, participants with a BMI <25 kg/m² showed a greater decrease in CRP (WMD = -0.29 mg/dL; 95% CI: -0.392= to -0.20; P< 0.001) compared to those with a BMI ≥25 kg/m² (WMD = 0.10 mg/dL; 95% CI: 0.071to 0.14; P< 0.001) (Supplementary Figure 1).
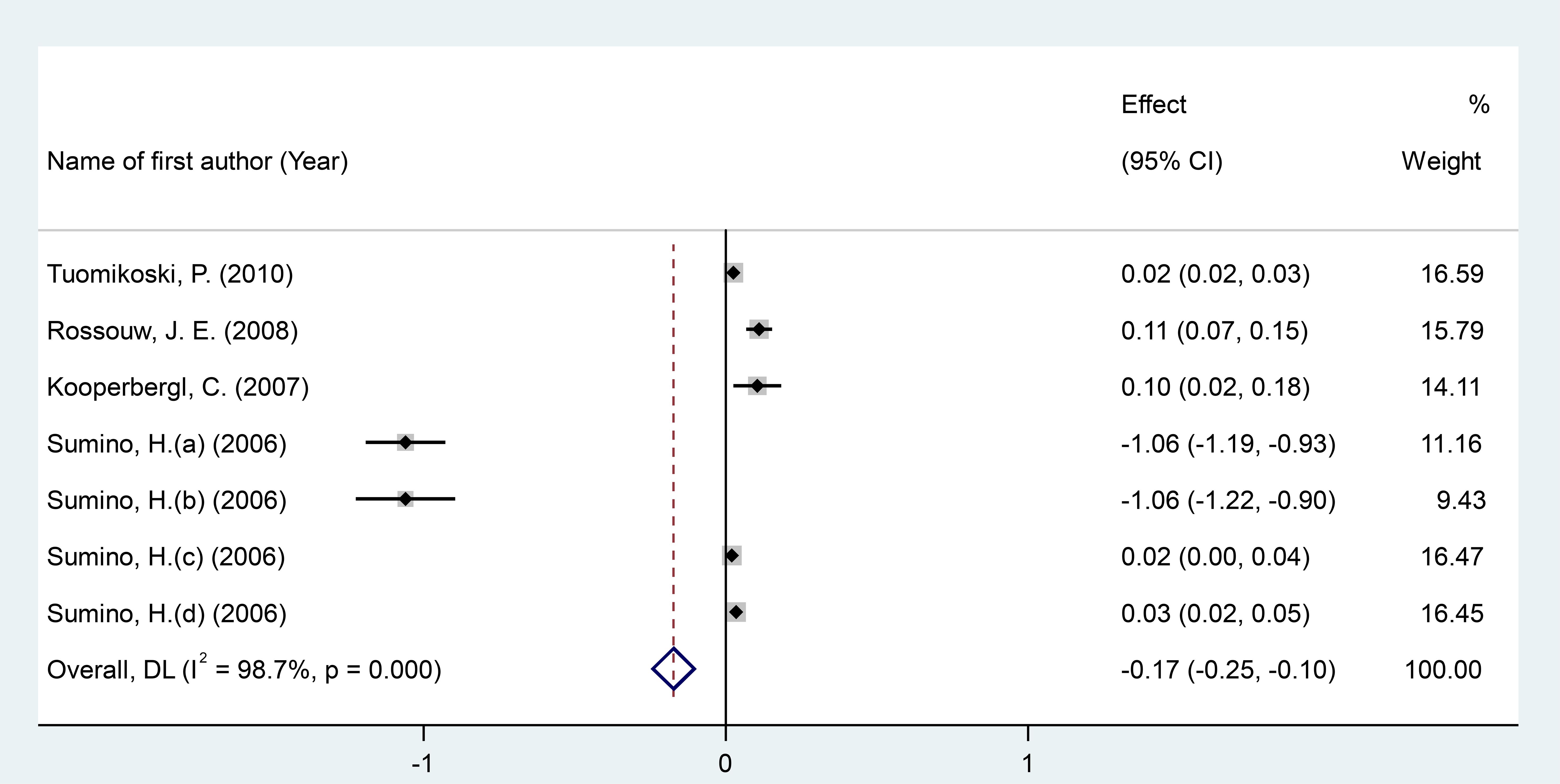
Figure 2. Forest investigating the effects of MAP/E2 on CRP. RCT, randomized controlled trial(s); WMD, Weighted mean difference; CI, confidence interval.
Effects of MPA/CEE treatment on fibrinogen levels
After pooling effect sizes from 11 RCT arms involving a total of 1,760 participants (830 in the intervention group and 930 in the placebo group), a random-effects meta-analysis revealed a significant reduction in fibrinogen levels following MPA/CEE treatment (WMD = -15.40 mg/dL; 95% CI: -20.64 to -10.15; P < 0.001) in postmenopausal women (Figure 3). However, there was considerable heterogeneity across studies (I² = 99.5%, P < 0.001).
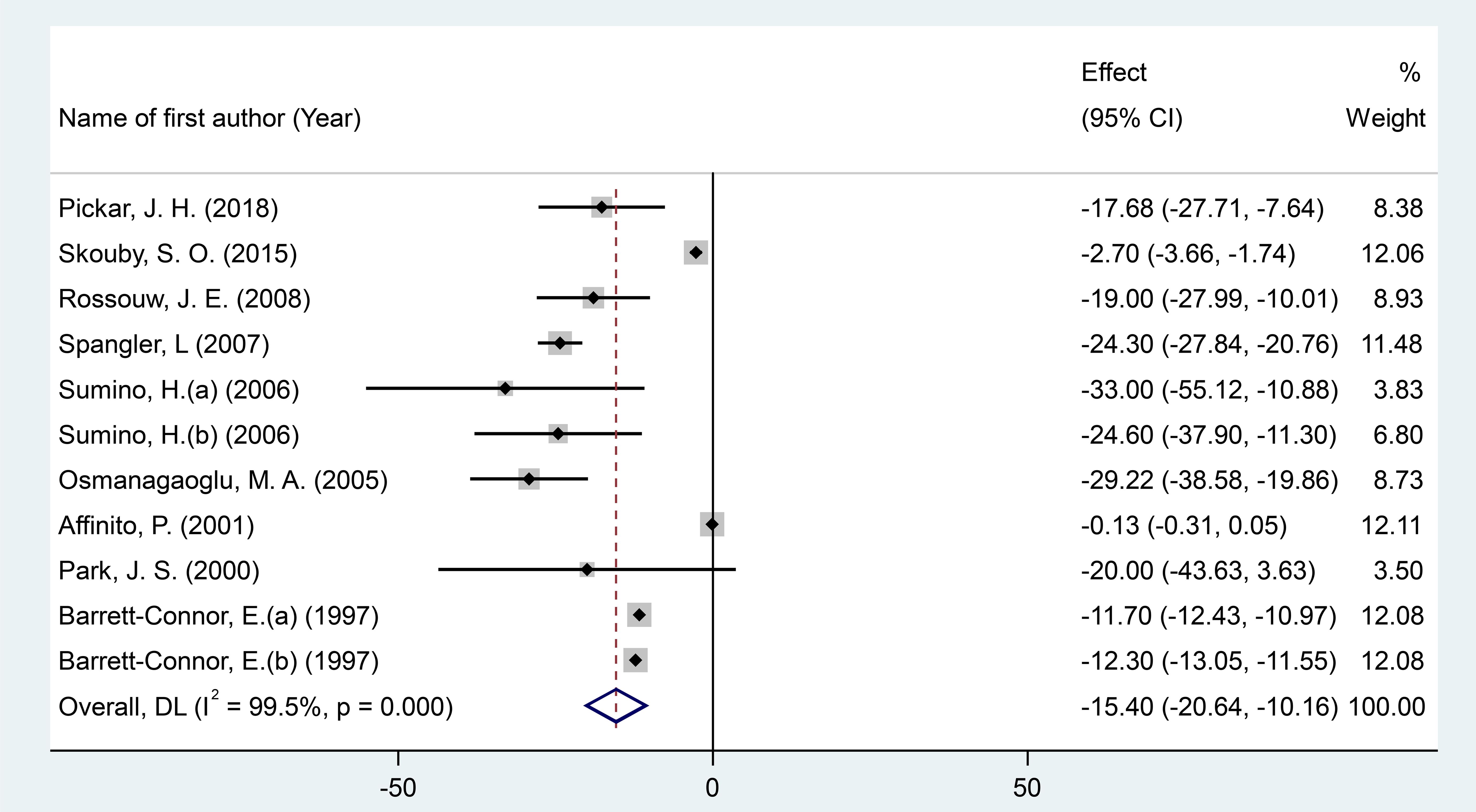
Figure 3. Forest plot of RCTs investigating the effects of MAP/E2 on Homocystteine. RCT, randomized controlled trial(s); WMD, Weighted mean difference; CI, confidence interval.
Subgroup analyses showed significant decreases in fibrinogen concentrations with MPA/CEE doses ≤2.5 mg/day (WMD = -18.39 mg/dL; 95% CI: -24.35 to -12.44; P< 0.001), and in participants aged ≥60 years (WMD = -19 mg/dL; 95% CI: -27.99 to -10; P< 0.001) compared to those under 60 years (WMD = -14.77 mg/dL; 95% CI: -19.70 to -9.84; P< 0.001). Notable reductions were also observed in studies with treatment durations of ≤12 months (WMD = -17.59 mg/dL; 95% CI: -34.62 to -0.57; P = 0.043) compared to >12 months (WMD = -12.79 mg/dL; 95% CI: -17.49 to -8.08; P< 0.001), and in participants with a BMI <25 kg/m² (WMD = -21.52 mg/dL; 95% CI: -28.69 to -14.34; P< 0.001), compared to those with BMI ≥25 kg/m² (WMD = -12.87 mg/dL; 95% CI: -18.09 to -7.61; P< 0.001) (Supplementary Figure 1).
Effects of MPA/CEE treatment on homocysteine levels
After pooling effect sizes from 4 RCT arms, involving a total of 855 participants (482 in the intervention group and 373 in the placebo group), a random-effects meta-analysis found no significant reduction in homocysteine levels following MPA/CEE treatment (WMD = -0.03 mg/dL; 95% CI: -0.07 to 0.01; P = 0.186) in postmenopausal women (Figure 4). The analysis showed moderate heterogeneity among the studies, which was not statistically significant (I² = 57.1%, P = 0.072). Although moderate heterogeneity was observed for homocysteine (I² = 57.1%), subgroup analyses were not performed due to the small number of included RCTs, which would have limited the reliability of stratified results.
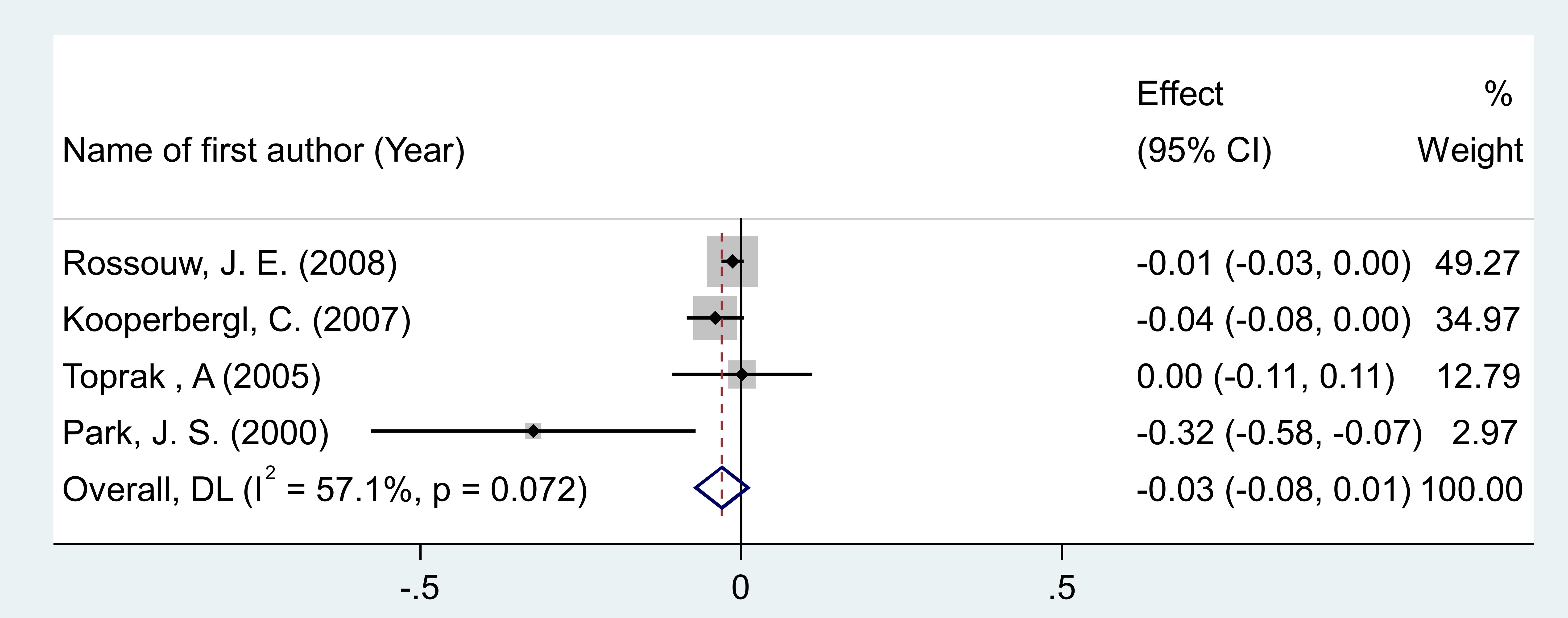
Figure 4. Forest plot of RCTs investigating the effects of MAP/E2 on Interleukin-6. RCT, randomized controlled trial(s); WMD, Weighted mean difference; CI, confidence interval.
Effects of MPA/CEE treatment on interleukin-6 levels
After pooling effect sizes from 4 RCT arms, involving a total of 865 participants (485 in the intervention group and 380 in the placebo group), a random-effects meta-analysis found no significant reduction in IL-6 concentrations following MPA/CEE treatment (WMD = -0.018 pg/mL; 95% CI: -0.09 to 0.05; P = 0.635) in postmenopausal women (Figure 5). The analysis revealed low and non-significant heterogeneity among the included studies (I² = 9.9%, P = 0.344).
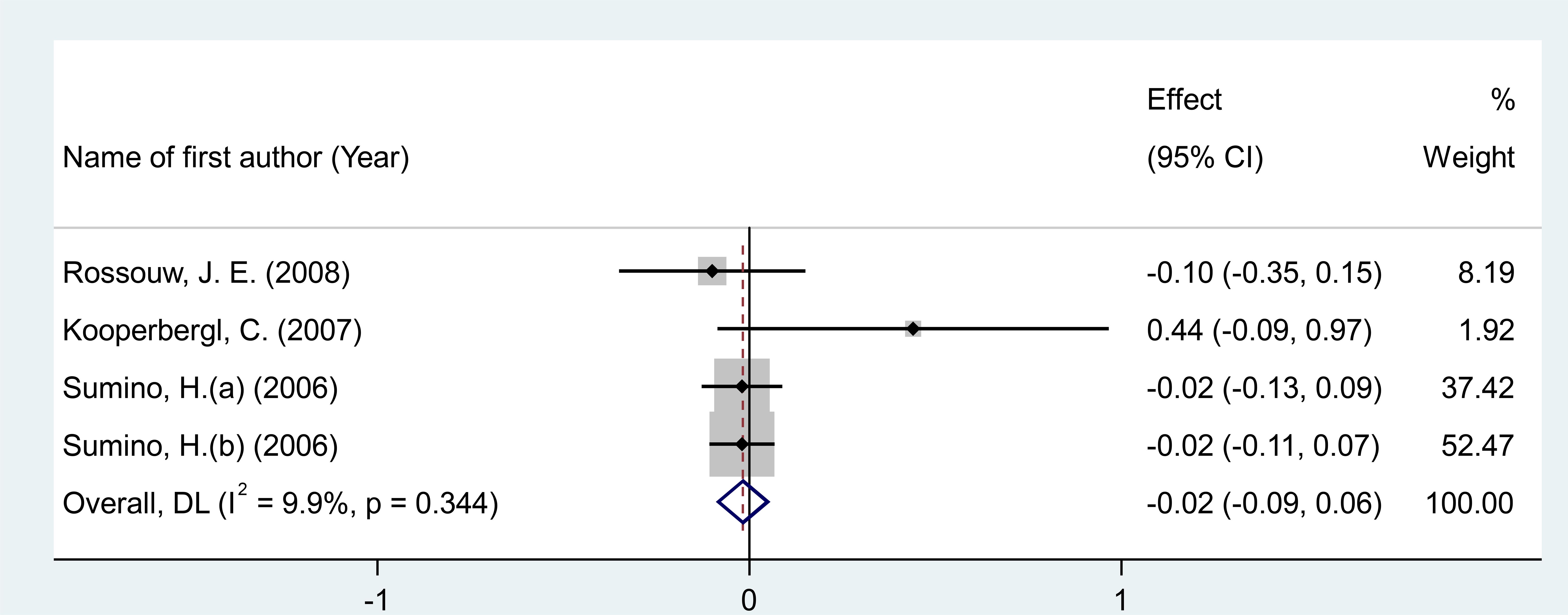
Figure 5. Forest plot of RCTs investigating the effects of MAP/E2 on fibrinogen. RCT, randomized controlled trial(s); WMD, Weighted mean difference; CI, confidence interval.
Sensitivity analysis and publication bias
We performed sensitivity analyses to assess the robustness of the overall findings by systematically excluding each RCT arm one at a time and recalculating the pooled effect sizes. None of these exclusions significantly impacted the overall results (Supplementary Figure 2). Additionally, no evidence of publication bias was detected for the pooled effect sizes of CRP, homocysteine, and IL-6 levels, as indicated by visual inspection of the funnel plots. These findings were further supported by Egger's test results (Supplementary Figure 3). However, we found significant publication bias for fibrinogen (P = 0.053); yet, the trim-and-fill test did not identify any potentially missing (unpublished) studies.
Discussions
This comprehensive meta-analysis of RCTs aimed to consolidate all available evidence on the effects of MPA/CEE on inflammation-related biomarkers in postmenopausal women. The analysis included data from 16 studies, covering 7 trial arms on CRP, 11 on fibrinogen, 4 on homocysteine, and 4 on IL-6, based on clearly defined inclusion and exclusion criteria. The primary focus was on postmenopausal women, encompassing both healthy individuals and those with existing health conditions.
A key finding of this review was the significant reduction in fibrinogen levels following MPA/CEE administration. Fibrinogen is a critical glycoprotein involved in coagulation and inflammatory processes, including macrophage adhesion and cytokine production (35). Beyond its role in acute inflammation, elevated fibrinogen contributes to chronic low-grade inflammation (36), serving as a reliable biomarker for systemic inflammation and an established predictor of cardiovascular disease risk (37). Subgroup analyses revealed that the reduction in fibrinogen was particularly significant in women aged ≥60 years and among those receiving MPA/CEE doses of ≤2.5 mg/day. These findings align with previous RCTs showing favorable changes in fibrinogen with lower MPA/CEE doses (38). Since fibrinogen levels tend to rise with age, the pronounced effect in older women may be attributed to higher baseline concentrations (39). Indeed, elevated plasma fibrinogen has been associated with a 15% increased risk of cardiovascular disease, underscoring its clinical relevance as a risk stratification tool (40). While our results suggest that MPA/CEE may mitigate inflammation via reductions in fibrinogen, it is important to recognize that biomarkers offer only indirect insights into actual cardiovascular outcomes. Therefore, large-scale prospective studies are needed to further explore this association.
MPA/CEE use was associated with a modest but statistically significant decrease in CRP levels (WMD = -0.16 mg/dL). However, no significant effects were observed for homocysteine (WMD = -0.03 mg/dL) or IL-6 (WMD = -0.018 pg/mL). These findings corroborate earlier reports that estrogen therapy, either alone or combined with a progestin, can decrease CRP levels (41). However, the mechanism behind the stimulation of CRP is debated; various studies report differing outcomes regarding the impact of estrogen, with or without progestins, on IL-6 (41). Moreover, subgroup analyses revealed a significant decrease in CRP concentrations when MPA/CEE was administered at doses ≤2.5 mg/day (WMD = -0.26 mg/dL). One possible explanation for the greater reductions in CRP and fibrinogen at lower progestin doses relates to the nuanced immunomodulatory effects of progestins. Lower doses may more effectively balance the suppression of pro-inflammatory cytokines and inhibition of nuclear factor-kappa B activity, thereby reducing inflammation without inducing receptor desensitization or counter-regulatory mechanisms that could blunt anti-inflammatory responses at higher doses (42, 43). This dose-dependent effect has been observed in studies of MPA and other progestins, suggesting that minimal effective dosing may optimize anti-inflammatory benefits.
The underlying mechanisms driving CRP declined remain unclear, especially given inconsistent data on the role of IL-6 in this process. Notably, findings from the PEPI study revealed that changes in IL-6 and CRP were positively correlated in progestin-treated groups, but negatively correlated in those receiving estrogen alone. This suggests that progestins may amplify IL-6-mediated CRP production, while alternative pathways may be responsible in estrogen-only regimens (44). These results support the hypothesis that progestins, rather than oral estrogens, are the primary contributors to CRP elevation through inflammatory signaling in the context of combined hormone therapy. Interestingly, no meaningful differences were observed based on the type of progestin or regimen (cyclic vs. continuous). While these findings provide important insights into the inflammatory signaling of combined hormone therapy, they do not originate from the present analysis.
Our results build upon this framework by demonstrating dose-dependent effects of MPA/CEE on inflammatory biomarkers, suggesting that progestin dosing may be a critical factor in modulating systemic inflammation. This distinction may partly explain the conflicting evidence regarding estrogen's role in CRP elevation described in the introduction. Whereas estrogen alone may elevate CRP through hepatic metabolic pathways unrelated to classical inflammatory cytokines, the progestin component in combined therapy appears to be a more direct contributor to inflammatory signaling and CRP production (45).
Therefore, rather than contradicting earlier observations, our findings refine the understanding of hormone therapy's inflammatory effects by highlighting the complex and dose-dependent interplay between estrogen and progestins. Further mechanistic studies are needed to clarify these pathways and optimize therapeutic strategies.
Importantly, our subgroup analyses revealed that MPA/CEE therapy at higher progestin doses, in older women, and in those with higher BMI was associated with significant increases in CRP levels, indicating a possible pro-inflammatory response under these conditions. This contrasts with the overall CRP reductions observed at lower doses and in younger or leaner subgroups, underscoring a complex interplay between hormone dose, patient characteristics, and inflammatory outcomes. The molecular mechanisms underlying this dose- and context-dependent pro-inflammatory effect remain to be fully elucidated. Potential pathways may involve enhanced activation of pro-inflammatory cytokines and nuclear factor-kappa B signaling at higher MPA doses, as well as synergistic effects with adipose tissue–derived inflammatory mediators in individuals with elevated BMI (46). Moreover, age-related changes in immune function and hormone metabolism could amplify these responses, increasing systemic inflammation and cardiovascular risk. Further research is needed to dissect these mechanisms, including studies exploring gene expression profiles, cytokine networks, and receptor-mediated effects of MPA and estrogen in diverse patient populations.
It is worth noting that while changes in homocysteine and IL-6 were not statistically significant, the decreases in fibrinogen and CRP were of a magnitude considered potentially meaningful in reducing inflammatory burden.
Clinical implications
From a clinical perspective, MPA/CEE may be a favorable option for postmenopausal women with a lower risk of inflammation. However, individualized treatment remains essential. Clinicians should consider the total daily dose of MPA/CEE, along with patient-specific risk factors, when prescribing this therapy, particularly for those susceptible to inflammation-related complications.
Strengths and limitations
A major strength of this systematic review and meta-analysis is that it represents the first comprehensive synthesis of RCT data evaluating the impact of MPA/CEE on inflammatory biomarkers in women.
Nonetheless, this study is not without limitations. Considerable heterogeneity was observed among the included trials, which may be attributed to variations in treatment duration, study populations, and demographic characteristics. Additionally, the methodological quality of some trials raised concerns, necessitating cautious interpretation of the results. The limited number of studies evaluating homocysteine and IL-6 (only four arms each) restricts the generalizability of findings for these specific markers. Furthermore, because fewer than 10 studies were available for some biomarkers, the assessment of publication bias using funnel plots, Egger's test, or trim-and-fill was unreliable, and these results should therefore be interpreted with caution.
Conclusion
This systematic review and meta-analysis demonstrates that MPA/CEE therapy significantly reduces fibrinogen and CRP levels, indicating a potential anti-inflammatory effect. As such, MPA/CEE could be considered for postmenopausal women at reduced risk of inflammation. However, due to the modest effects on other biomarkers and the inherent limitations of using surrogate markers like fibrinogen, clinical decision-making should be personalized. Future large-scale epidemiological studies are needed to confirm the long-term cardiovascular benefits of MPA/CEE and to further elucidate its role in modulating inflammation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
QS: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. YH: Methodology, Investigation, Writing – original draft. JL: Formal analysis, Writing – review & editing, Methodology. MS: Methodology, Writing – original draft. KP: Methodology, Writing – original draft. JQ: Writing – original draft, Conceptualization, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the following sources: Natural Science Foundation of Sichuan Province 2025ZNSFSC1668.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1643413/full#supplementary-material
References
1. Whiteley J, DiBonaventura M, Wagner J-S, Alvir J, and Shah S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J women's Health. (2013) 22:983–90. doi: 10.1089/jwh.2012.3719
2. El Khoudary SR, Chen X, Qi M, Matthews KA, Karlamangla AS, Gold EB, et al. The relation between systemic inflammation and the menopause transition: the study of women's health across the nation. J Clin Endocrinol Metab. (2025), dgaf175. doi: 10.1210/clinem/dgaf175
3. Zhou F, Prabahar K, and Shu J. The effects of transdermal estrogens combined with Medroxyprogesterone Acetate on cardiovascular disease risk factors in postmenopausal women: a meta-analysis of randomized controlled trials. Diabetol Metab syndrome. (2025) 17:111. doi: 10.1186/s13098-025-01664-1
4. Faubion SS, Crandall CJ, Davis L, El Khoudary SR, Hodis HN, Lobo RA, et al. The 2022 hormone therapy position statement of the North American Menopause Society. Menopause (New York NY). (2022) 29:767–94. doi: 10.1097/GME.0000000000002028
5. Baber R, Panay N, and Fenton A. 2016 IMS Recommendations on women's midlife health and menopause hormone therapy. Climacteric. (2016) 19:109–50. doi: 10.3109/13697137.2015.1129166
6. Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, et al. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women's Health Initiative observational study. Jama. (2002) 288:980–7. doi: 10.1001/jama.288.8.980
7. Attiq A, Afzal S, Ahmad W, and Kandeel M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur J Pharmacol. (2024) 966:176338. doi: 10.1016/j.ejphar.2024.176338
8. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. (2002) 288:321–33. doi: 10.1001/jama.288.3.321
9. Hu P, Greendale GA, Palla SL, Reboussin BA, Herrington DM, Barrett-Connor E, et al. The effects of hormone therapy on the markers of inflammation and endothelial function and plasma matrix metalloproteinase-9 level in postmenopausal women: the postmenopausal estrogen progestin intervention (PEPI) trial. Atherosclerosis. (2006) 185:347–52. doi: 10.1016/j.atherosclerosis.2005.06.011
10. Kurtz EG, Ridker PM, Rose LM, Cook NR, Everett BM, Buring JE, et al. Oral postmenopausal hormone therapy, C-reactive protein, and cardiovascular outcomes. Menopause. (2011) 18:23–9.
11. Hodis HN, John JAS, Xiang M, Cushman M, Lobo RA, and Mack WJ. Inflammatory markers and progression of subclinical atherosclerosis in healthy postmenopausal women (from the Estrogen in the Prevention of Atherosclerosis Trial). Am J Cardiol. (2008) 101:1131–3. doi: 10.1016/j.amjcard.2007.09.120
12. Feng L, Wang L, Hernández-Wolters B, Prabahar K, and Kord-Varkaneh H. The impact of medroxyprogesterone acetate on lipid profiles in Women: A time and dose-response meta-analysis of randomized controlled trials. Diabetes Res Clin Practice. (2024) 209:111073. doi: 10.1016/j.diabres.2023.111073
13. Pace S and Werz O. Impact of androgens on inflammation-related lipid mediator biosynthesis in innate immune cells. Front Immunol. (2020) 11:1356. doi: 10.3389/fimmu.2020.01356
14. Cheng Q, Yan X, Prabahar K, and Ye Z. The effect of medroxyprogesterone acetate on apolipoproteins and lipoprotein (a) concentrations in postmenopausal women: a meta-analysis of randomized controlled trials. Prostaglandins Other Lipid Mediators. (2024) 106919. doi: 10.1016/j.prostaglandins.2024.106919
15. Piróg M, Jach R, and Kacalska-Janssen O. Differential effect of the ultra-low dose and standard estrogen plus dydrogesterone therapy on thrombin generation and fibrinolysis in postmenopausal women. Acta obstetricia gynecologica Scandinavica. (2017) 96:1438–45. doi: 10.1111/aogs.13239
16. Gocer S, Guven S, Sal H, and Guven ESG. The effect of low-dose combined hormone therapy (oestradiol and norethindrone acetate) on serum C-reactive protein levels and life quality in natural menopause women. Menopause Review/Przegląd Menopauzalny. (2021) 20:177–83. doi: 10.5114/pm.2021.110935
17. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Higgins JPT and Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). The Cochrane Collaboration, Chichester, UK. (2011).
19. Hozo SP, Djulbegovic B, and Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res methodology. (2005) 5:13. doi: 10.1186/1471-2288-5-13
20. Egger M, Smith GD, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
21. Duval S. The trim and fill method. In: Publication bias in meta-analysis: Prevention, assessment and adjustments. Wiley. Chichester, UK. (2005). p. 127–44.
22. Pickar JH, Lavenberg J, Pan K, and Komm BS. Initial investigation into the optimal dose ratio of conjugated estrogens and bazedoxifene: A double-blind, randomized, placebo-controlled phase 2 dose-finding study. Menopause. (2018) 25:273–85. doi: 10.1097/GME.0000000000000992
23. Skouby SO, Pan K, Thompson JR, Komm BS, and Mirkin S. Effects of conjugated estrogens/bazedoxifene on lipid and coagulation variables: A randomized placebo-and active-controlled trial. Menopause. (2015) 22:640–9. doi: 10.1097/GME.0000000000000362
24. Tuomikoski P, Mikkola TS, Tikkanen MJ, and Ylikorkala O. Hot flushes and biochemical markers for cardiovascular disease: a randomized trial on hormone therapy. Climacteric. (2010) 13:457–66. doi: 10.3109/13697131003624656
25. Rossouw JE, Cushman M, Greenland P, Lloyd-Jones DM, Bray P, Kooperberg C, et al. Inflammatory, lipid, thrombotic, and genetic markers of coronary heart disease risk in the women's health initiative trials of hormone therapy. Arch Intern Med. (2008) 168:2245–53. doi: 10.1001/archinte.168.20.2245
26. Spangler L, Newton KM, Grothaus LC, Reed SD, Ehrlich K, and LaCroix AZ. The effects of black cohosh therapies on lipids, fibrinogen, glucose and insulin. Maturitas. (2007) 57:195–204. doi: 10.1016/j.maturitas.2007.01.002
27. Kooperbergl C, Cushman M, Hsia J, Robinson JG, Aragaki AK, Lynch JK, et al. Can biomarkers identify women at increased stroke risk? The Women's Health Initiative hormone trials. P. LoS Clin Trials. (2007) 2. doi: 10.1371/journal.pctr.0020028
28. Sumino H, Ichikawa S, Takahashi T, Sakamoto H, Goto-Onozato K, Koya S, et al. Conjugated estrogen plus medroxyprogesterone does not impair blood rheological properties in hypertensive postmenopausal women. Maturitas. (2006) 53:306–14. doi: 10.1016/j.maturitas.2005.05.018
29. Sumino H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, et al. Different effects of oral conjugated estrogen and transdermal estradiol on arterial stiffness and vascular inflammatory markers in postmenopausal women. Atherosclerosis. (2006) 189:436–42. doi: 10.1016/j.atherosclerosis.2005.12.030
30. Toprak A, Erenus M, Ilhan AH, Haklar G, Fak AS, and Oktay A. The effect of postmenopausal hormone therapy with or without folic acid supplementation on serum homocysteine level. Climacteric. (2005) 8:279–86. doi: 10.1080/13697130500191040
31. Osmanağaoğlu MA, Osmanağaoğlu S, Osmanağaoğlu T, Okumuş B, and Bozkaya H. Effect of different preparations of hormone therapy on lipid and glucose metabolism, coagulation factors, and bone mineral density in overweight and obese postmenopausal women. Fertil Steril. (2005) 84:384–93. doi: 10.1016/j.fertnstert.2005.01.131
32. Affinito P, Palomba S, Bonifacio M, Fontana D, Izzo R, Trimarco B, et al. Effects of hormonal replacement therapy in postmenopausal hypertensive patients. Maturitas. (2001) 40:75–83. doi: 10.1016/s0378-5122(01)00196-7
33. Park JS, Jung HH, Yang WS, Kim SB, Min WK, and Chi HS. Effects of hormonal replacement therapy on lipid and haemostatic factors in post-menopausal ESRD patients. Nephrology dialysis transplantation: Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc. (2000) 15:1835–40. doi: 10.1093/ndt/15.11.1835
34. Barrett-Connor E, Slone S, Greendale G, Kritz-Silverstein D, Espeland M, Johnson SR, et al. The postmenopausal estrogen/progestin interventions study: Primary outcomes in adherent women. Maturitas. (1997) 27:261–74. doi: 10.1016/S0378-5122(97)00041-8
35. Wolberg AS. Fibrinogen and fibrin: synthesis, structure, and function in health and disease. J Thromb Haemostasis. (2023) 21:3005–15. doi: 10.1016/j.jtha.2023.08.014
36. Luyendyk JP, Schoenecker JG, and Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood J Am Soc Hematology. (2019) 133:511–20. doi: 10.1182/blood-2018-07-818211
37. Nencini F, Giurranna E, Borghi S, Taddei N, Fiorillo C, and Becatti M. Fibrinogen oxidation and thrombosis: shaping structure and function. Antioxidants. (2025) 14:390. doi: 10.3390/antiox14040390
38. Lobo RA, Bush T, Carr BR, and Pickar JH. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on plasma lipids and lipoproteins, coagulation factors, and carbohydrate metabolism. Fertility sterility. (2001) 76:13–24. doi: 10.1016/S0015-0282(01)01829-5
39. Gligorijević N, Križáková MZ, Penezić A, Katrlík J, and Nedić O. Structural and functional changes of fibrinogen due to aging. Int J Biol macromolecules. (2018) 108:1028–34. doi: 10.1016/j.ijbiomac.2017.11.016
40. Collaboration ERF. C-reactive protein, fibrinogen, and cardiovascular disease prediction. New Engl J Med. (2012) 367:1310–20. doi: 10.1056/NEJMoa1107477
41. Langer RD, Pradhan AD, Lewis CE, Manson JE, Rossouw JE, Hendrix SL, et al. Baseline associations between postmenopausal hormone therapy and inflammatory, haemostatic, and lipid biomarkers of coronary heart disease. Thromb haemostasis. (2005) 93:1108–16. doi: 10.1160/TH04-09-0608
42. Wakatsuki A, Okatani Y, Ikenoue N, and Fukaya T. Effect of medroxyprogesterone acetate on vascular inflammatory markers in postmenopausal women receiving estrogen. Circulation. (2002) 105:1436–9. doi: 10.1161/hc1202.105945
43. Fedotcheva TA, Fedotcheva NI, and Shimanovsky NL. Progesterone as an anti-inflammatory drug and immunomodulator: new aspects in hormonal regulation of the inflammation. Biomolecules. (2022) 12:1299. doi: 10.3390/biom12091299
44. Reuben DB, Palla SL, Hu P, Reboussin BA, Crandall C, Herrington DM, et al. Progestins affect mechanism of estrogen-induced C-reactive protein stimulation. Am J Med. (2006) 119:167. e1–. e8. doi: 10.1016/j.amjmed.2005.08.012
45. Rossi R, Bursi F, Veronesi B, Cagnacci A, and Modena MG. Effects of progestins on estrogen-induced increase in C-reactive protein in postmenopausal women. Maturitas. (2004) 49:315–20. doi: 10.1016/j.maturitas.2004.02.016
Keywords: medroxyprogesterone acetate, conjugated equine estrogens, inflammation, postmenopausal women, meta-analysis, hormone therapy, CRP, fibrinogen
Citation: Qiu J, He Y, Li J, Safargar M, Prabahar K and Shi Q (2025) The effects of oral medroxyprogesterone acetate combined with conjugated equine estrogens on inflammation in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. 16:1643413. doi: 10.3389/fendo.2025.1643413
Received: 08 June 2025; Accepted: 29 September 2025;
Published: 15 October 2025.
Edited by:
Andrew Libby, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Xuezhi (Daniel) Jiang, Reading Hospital, United StatesVishal Dubey, Sundyota Numandis Probioceuticals Pvt. Ltd., India
Copyright © 2025 Qiu, He, Li, Safargar, Prabahar and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingquan Shi, c3FxcXVhbjE5OTBAMTYzLmNvbQ==
 Jiahui Qiu
Jiahui Qiu Yunan He
Yunan He Jinhong Li1,2
Jinhong Li1,2 Kousalya Prabahar
Kousalya Prabahar Qingquan Shi
Qingquan Shi