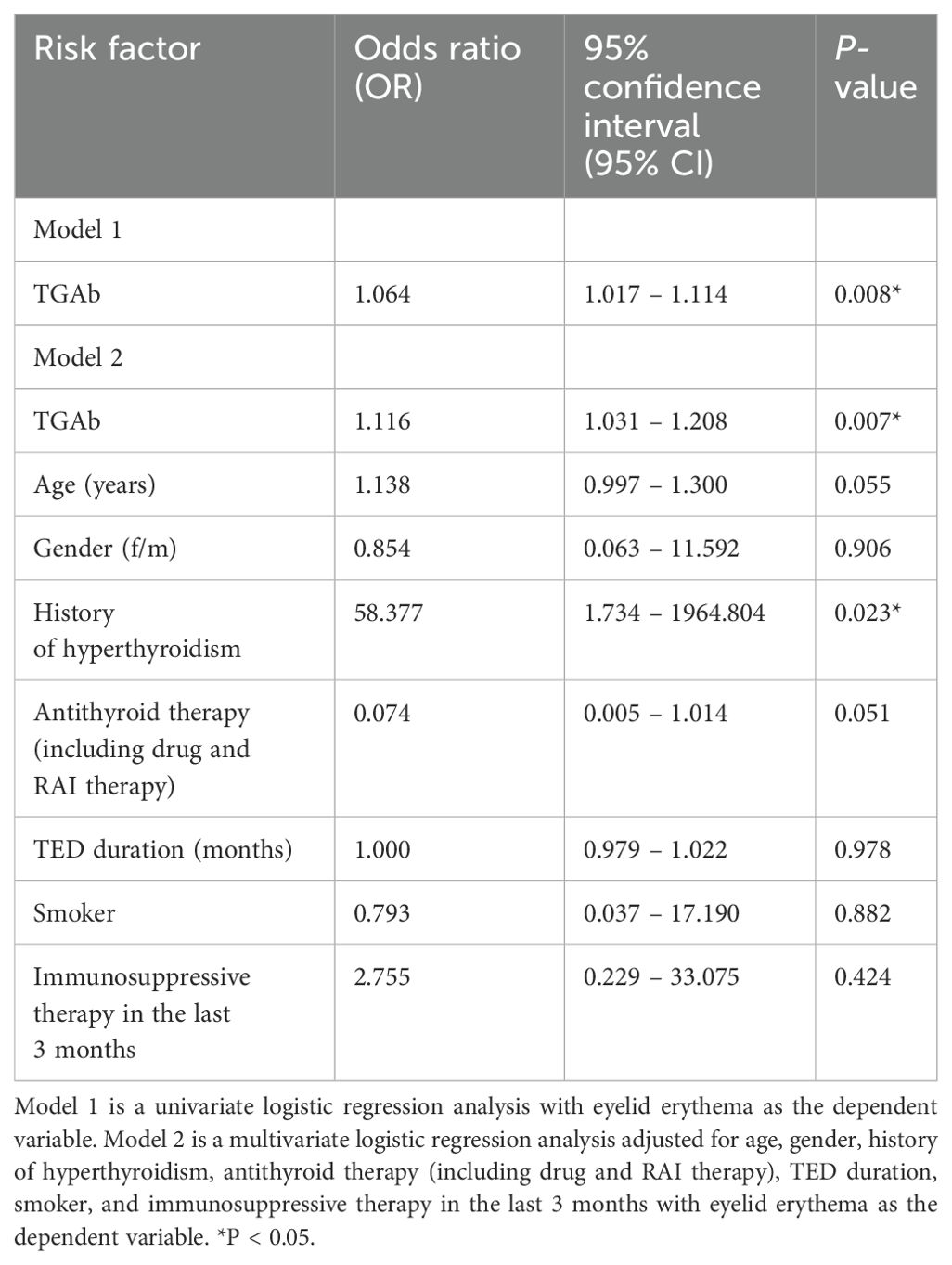
- 1Department of Endocrinology, The First Hospital of Lanzhou University, Lanzhou, China
- 2The First Clinical Medical College, Lanzhou University, Lanzhou, China
- 3Gansu Clinical Medical Research Center for Endocrine Diseases, Lanzhou, China
- 4Senior Department of Ophthalmology, 3rd Medical Center of Chinese People's Liberation Army General Hospital, Beijing, China
Background: Thyrotrophin receptor antibody (TRAb)-negative thyroid eye disease (TED) constitutes a clinically significant subset of TED, yet its features remain inadequately characterized. This study characterizes the clinical features of TRAb-negative TED patients.
Methods: In this cross-sectional study, conducted at a Chinese tertiary hospital, 86 TRAb-negative TED patients underwent comprehensive ocular examinations and thyroid function tests. Clinical characteristics were systematically analyzed.
Results: Of the 86 patients (mean age 45.24 ± 10.78 years; 53.5% female), 86.0% (n=74) exhibited bilateral ocular involvement. The primary manifestations included proptosis, eyelid edema, and eyelid retraction. The TPOAb (-)/TGAb (+) subgroup demonstrated significantly higher frequencies of eyelid erythema and lagophthalmos, along with severe NOSPECS grading, reduced visual acuity, elevated orbital pressure, and marked soft tissue involvement (all p<0.05). TPOAb-positive patients had significantly greater proptosis (p<0.05), while TGAb-positive patients showed an increased incidence of eyelid erythema (p<0.05). TGAb was identified as an independent risk factor for eyelid erythema (p<0.01).ROC analysis for TGAb predicting eyelid erythema yielded an AUC of 0.851 (sensitivity 0.778, specificity 0.831) at 3.980 IU/mL.
Conclusion: TRAb-negative TED displays distinct clinical features. TPOAb and TGAb levels are associated with specific manifestations, highlighting their potential value in the assessment and management of TRAb-negative TED.
1 Introduction
Thyroid eye disease (TED) is the most common orbital autoimmune disorder in adults, affecting up to 40% of patients with Graves’ disease (GD) (1). Its clinical manifestations, including eyelid retraction, proptosis, diplopia, and optic neuropathy, significantly reduce quality of life (2). Thyroid receptor antibody (TRAb) levels are strongly associated with the TED incidence and pathogenesis of TED (3), correlating with clinical features such as clinical activity score (CAS), conjunctival injection, caruncle edema, lagophthalmos, and chemosis (4, 5).
However, TED can also present in TRAb-negative patients, irrespective of TPOAb or TGAb status. These cases often present with milder initial manifestations (6), which are frequently overlooked clinically, thereby delaying intervention. Symptoms may include subtle eyelid edema or diplopia. While progression to more severe complications like headache or vision loss is rare (7–9), prompt recognition of TRAb-negative TED is crucial. Despite documented associations with Hashimoto’s thyroiditis (HT) (8, 9), systematic studies analyzing TRAb-negative TED remain limited.
This study characterizes the clinical features in 86 TRAb-negative TED patients and explores potential underlying mechanisms. Our objectives are to enhance clinical recognition and inform diagnostic and therapeutic innovations.
2 Materials and methods
2.1 Participants
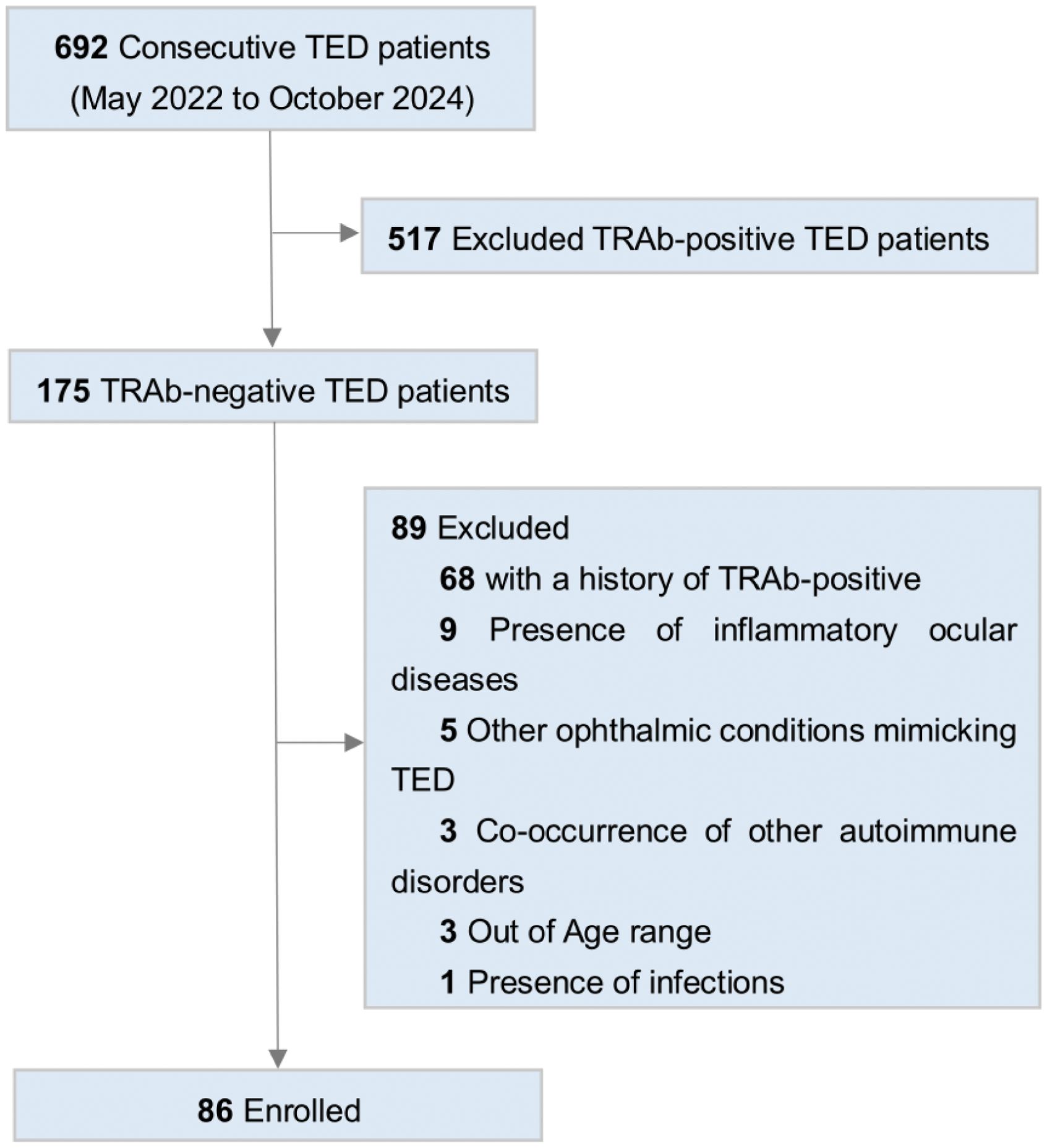
This retrospective study included 86 TRAb-negative TED patients from the Senior Department of Ophthalmology, at the 3rd Medical Center of the Chinese PLA General Hospital. The inclusion criteria comprised: (1) TED diagnosis per Bartley’s criteria (10); (2) age 18–75 years; (3) confirmed TRAb-negative status. The exclusion criteria were: (1) Comorbid psychiatric, hematological, or non-HT autoimmune disorders; (2) Concurrent inflammatory ocular diseases, Immunoglobulin G4-related ophthalmic disease (IgG4-ROD), ocular trauma, infection, high myopia (> -6D), orbital tumors, severe myasthenia gravis, or TED-mimicking conditions; (3) History of TRAb positivity; (4) Pregnancy (Figure 1).
2.2 Ocular examinations
A single ophthalmologist administered a standardized protocol. To assess eyelid retraction, soft tissue involvement, proptosis [Hertel exophthalmometer; >16 mm threshold (11)], eyelid closure, diplopia [Gorman score (12)], visual acuity, intraocular pressure, orbital pressure, and optic nerve function. Disease activity was assessed using the Clinical Activity Score (CAS), with one point assigned foreach of the following signs: spontaneous orbital pain, gaze-evoked orbital pain, eyelid erythema, eyelid edema, conjunctival injection, chemosis, and caruncle edema. CAS ≥ 3 defined active disease; CAS < 3 defined inactive disease. Severity was graded according to EUGOGO guidelines (2) using the NOSPECS scoring system (13).
2.3 Thyroid function assay
Fasting venous blood samples were collected from all patients. TRAb levels were measured using third-generation electrochemiluminescence (Cobas e411, Roche Diagnostics; negative ≤1.75 IU/L). Additional thyroid related parameters were quantified using chemiluminescence assay (Beckman DXI800): free triiodothyronine (FT3, 3.28-6.47 pmol/L), free thyroxine (FT4, 7.64-16.03 pmol/L), thyroid-stimulating hormone (TSH, 0.49-4.91 mIU/L), TPOAb (≤9 IU/mL), and TGAb (≤4 IU/mL). Patients were classified as TPOAb-positive (>9 IU/mL) or TPOAb-negative (≤9 IU/mL), and TGAb-positive (>4 IU/mL) or TGAb-negative (≤4 IU/mL). Thyroid functional status was categorized as follows: euthyroid (all parameters within reference ranges), hyperthyroid (elevated FT3 and/or FT4 or suppressed TSH), or hypothyroid (decreased FT3 and/or FT4 or elevated TSH).
2.4 Statistical analysis
Demographic and clinical characteristics are summarized in Tables 1, 2. Continuous variables are expressed as mean ± standard deviation (SD) or median. Categorical variables were compared using Chi-square or Fisher’s exact tests. For two-group continuous comparisons, independent samples Student’s t-test was applied when normality (Shapiro-Wilk test) and homogeneity of variances (Levene’s test) assumptions were met; otherwise, the Mann-Whiteney U test was used. For three or more groups, one-way ANOVA was used under normality and variance homogeneity; when these assumptions were not met, the Kruskal-Wallis H test was employed, with significant results (P < 0.05) followed by Dunn’s post-hoc test with Bonferroni correction for pairwise comparisons. Logistic regression analyzed eyelid erythema risk factors. Spearman correlation was used to evaluate the relationship between Gorman diplopia scores and TGAb levels. Receiver operating characteristic (ROC) curves were used to evaluate the predictive value of TGAb for eyelid erythema, considering AUC > 0.5 with p < 0.05 as being statistically significant. All analyses were conducted using IBM SPSS Statistics 26 (IBM Corp., Armonk, NY), with statistical significance defined as p<0.05.
3 Results
3.1 Demographic and clinical characteristics of 86 TRAb-negative TED patients
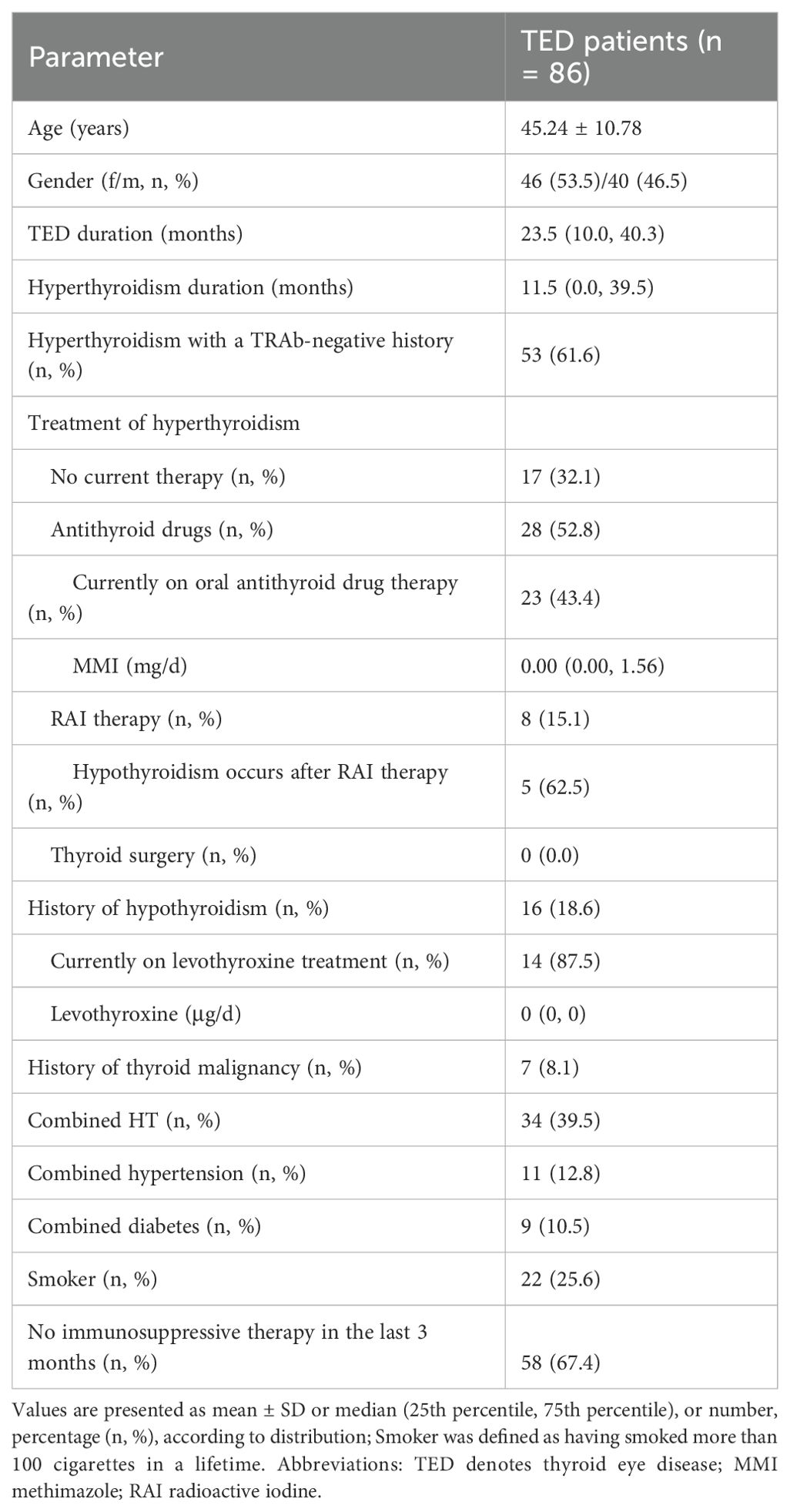
A total of 86 TRAb-negative TED patients met the inclusion criteria, with a mean age of 45.24 ± 10.78 years and a female-to-male ratio of 1.15:1. Twenty-two patients (25.6%) were smokers, with mean TED duration of 23.5 months (IQR 10.0–40.3) months. Fifty-eight patients (67.4%) did not receive immunosuppressive therapy within 3 months. Hyperthyroidism with a TRAb-negative history was present in 53 patients (61.6%), including 8 (15.1%) who received radioiodine (RAI) treatment (5 subsequently developed hypothyroidism). At admission, 57 patients (66.3%) were euthyroid. Seven patients (8.1%) had thyroid malignancy history with surgical treatment (Tables 1, 2).
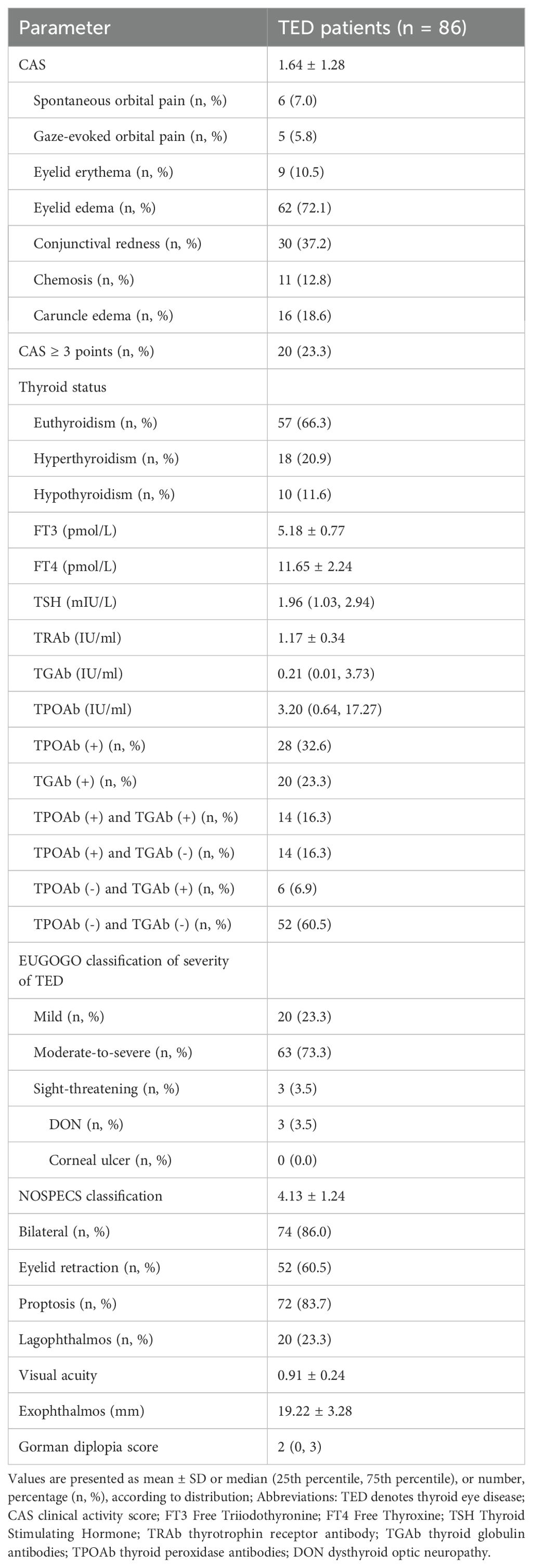
Bilateral ocular involvement was present in 74 patients (86.0%), with the more severely affected eye analyzed. Predominant manifestations included proptosis (83.7%), eyelid edema (72.1%), and eyelid retraction (60.5%). Mean CAS was 1.64 ± 1.28, with active disease in 20 patients (23.3%). EUGOGO classification identified moderate-to-severe disease in 63 patients (73.3%). Mean exophthalmos measured 19.22±3.28mm, visual acuity 0.91 ± 0.24, and Gorman diplopia score 2 (IQR 0–3). Antibody profiles showed mean TRAb 1.17 ± 0.34 IU/mL, TGAb 0.21 (IQR 0.02–3.73) IU/mL, and TPOAb 3.20 (IQR 0.64–17.27) IU/mL. TGAb positivity occurred in 20 patients (23.3%), TPOAb in 28 (32.6%), and both in 14 (16.3%) (Table 2).
3.2 Comparison of clinical characteristics of patients grouped based on TPOAb and TGAb
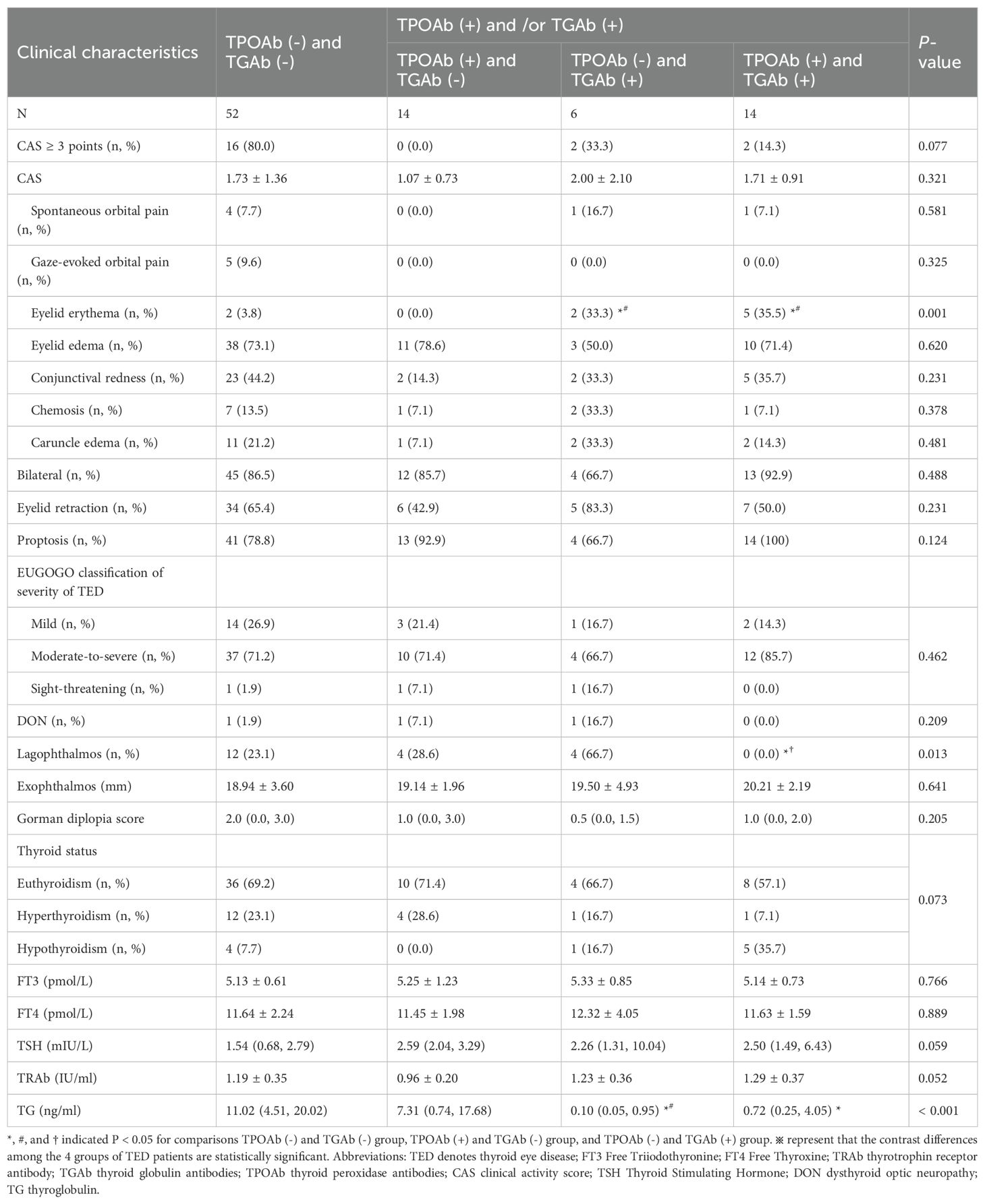
Eighty-six patients were stratified into four groups based on TPOAb/TGAb status: double-negative [TPOAb(-)/TGAb(-)], TPOAb(+)/TGAb(-), TPOAb(-)/TGAb(+), and double-positive [TPOAb(+)/TGAb(+)]. Baseline characteristics showed no significant differences (Supplementary Table S1). However, significant intergroup variations emerged in eyelid erythema and lagophthalmos (both p < 0.05). The TPOAb(-)/TGAb(+) and double-positive groups exhibited higher eyelid erythema incidence than other groups (p < 0.001). The TPOAb(-)/TGAb(+) group demonstrated the highest lagophthalmos frequency among all groups (p < 0.05), along with the lowest thyroglobulin levels (Table 3). This group also featured fewer patients with visual acuity of 1 or higher (Figure 2A), a higher proportion with +++ orbital pressure (Figure 2B), more cases of orbital fixation cases (Figure 2C), and a greater frequency of NOSPECS grade or higher (Figure 2D). Collectively, the TPOAb(-)/TGAb(+) group exhibited the most severe disease profile, characterized by worse NOSPECS grading, reduced visual acuity, orbital pressure, and soft tissue involvement. Analysis of isolated antibody effects revealed distinct clinical associations: TPOAb-positive patients showed increased proptosis (p < 0.05) but less active TED, eyelid retraction, and soft tissue involvement (p < 0.05). Intraocular pressure in this group was predominantly mildly elevated (+) (Supplementary Table S2). TGAb-positive patients presented higher incidences of eyelid erythema and visual impairment incidence but less diplopia (p < 0.05) (Supplementary Table S3).
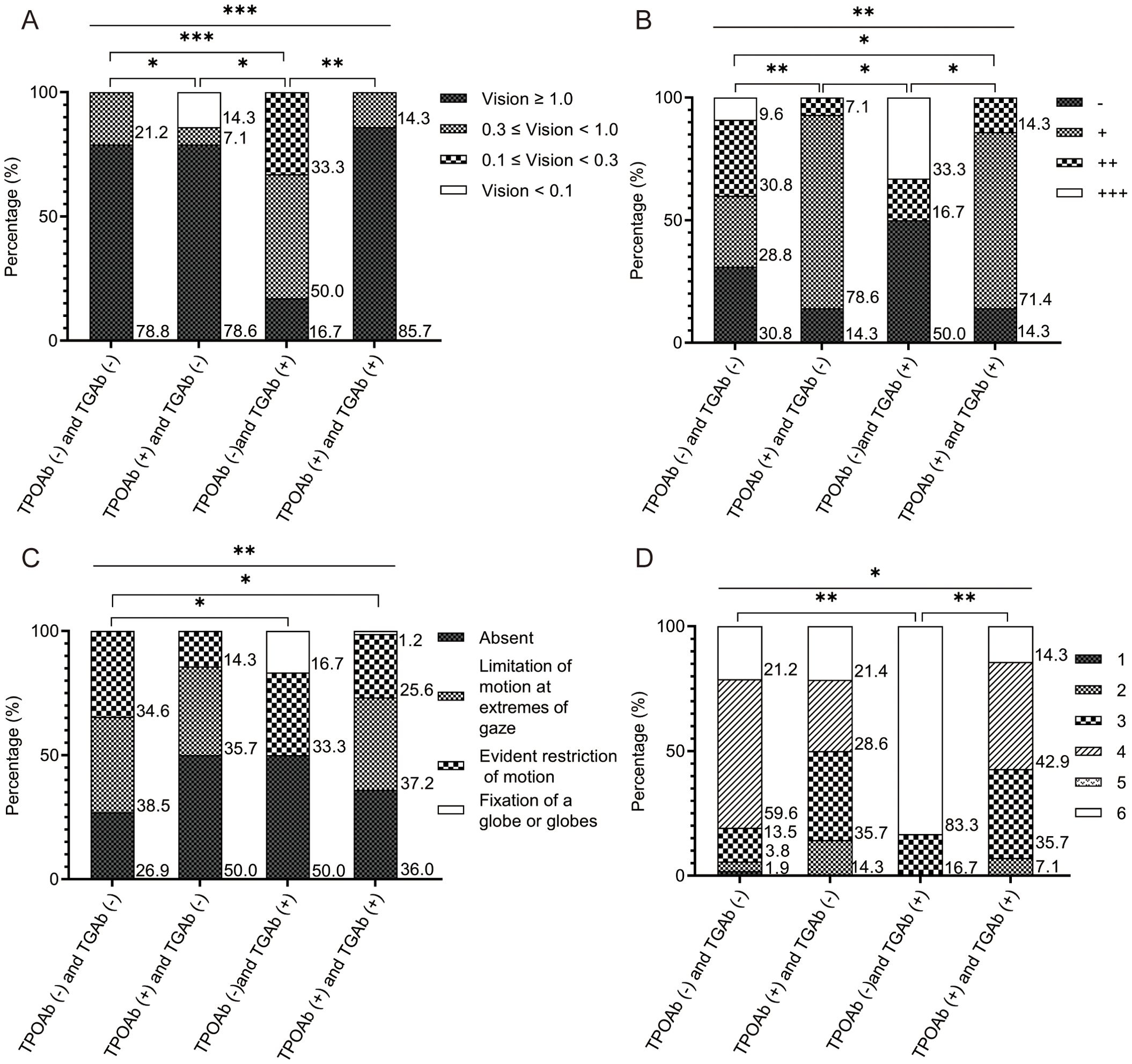
Figure 2. The comparison results of the four groups of patients with TED, based on TPOAb and TGAb. (A) Comparison results of visual acuity among the four groups. The number of patients with visual acuity ≥ 1 was the lowest in the TPOAb (-) and TGAb (+) group; (B) Comparison results of orbital pressure among the four groups. The proportion of patients with orbital pressure of ++ was the highest in the TPOAb (-) and TGAb (+) group; (C) Comparison results of soft tissue involvement among the four groups. The proportion of patients with fixed eyeballs or fixed eyeball clusters was the highest in the TPOAb (-) and TGAb (+) group; (D) Comparison results of NOSPECS classification among the four groups. The proportion of patients with NOSPECS classification of 6 or above was the highest in the TPOAb (-) and TGAb (+) group. The x-axis represents each group. The y-axis represents the percentage of the different groups’ population within each group. P values were showed as: *p < 0.05; **p < 0.01; ***p < 0.001. TED, thyroid eye disease. TPOAb, thyroid peroxidase antibody. TGAb, thyroglobulin antibody.
3.3 Thyroid-related antibodies in TRAb-negative TED patients were significantly correlated with clinical characteristics
Spearman correlation analysis revealed a significant negative association between TGAb levels and Gorman diplopia scores (Rs = -0.231, p < 0.05; Figure 3A). Patients with eyelid erythema demonstrated significantly higher TGAb levels compared to those without this manifestation (p < 0.01; Figure 3B). Multivariate logistic regression adjusted for age, sex, history of hyperthyroidism history, antithyroid therapy (medication/RAI), TED duration, smoking status, and recent immunosuppressive therapy identified TGAb as an independent risk factor for eyelid erythema (Table 4). ROC analysis using TGAb to predict eyelid erythema yielded an AUC of 0.851 (95% CI: 0.721-0.980; p < 0.001). The optimal cutoff of 3.980 IU/mL yielded a sensitivity of 0.778 and a specificity of 0.831 (Figure 3C).
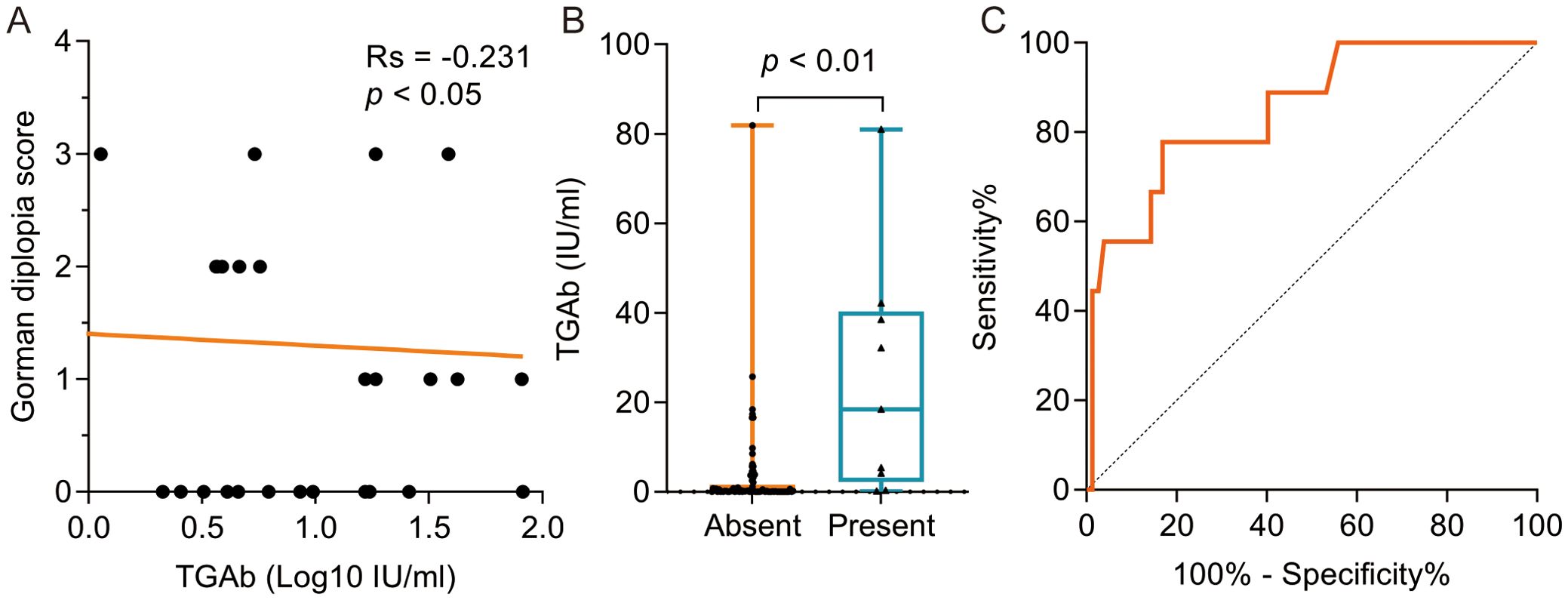
Figure 3. The correlation between TGAb and clinical characteristics in TRAb-negative TED Patient. (A) The negative correlation was evident between TGAb and Gorman diplopia score; (B) The TGAb level was significantly higher in the group with eyelid erythema symptoms compared to the group without symptoms at 18.39 (2.18, 40.32) IU/ml and 0.18 (0.00, 1.63) IU/ml, respectively, p < 0.01; (C) ROC curve analysis was performed using TGAb as the dependent variable and the presence of eyelid erythema as the outcome variable, yielding an AUC of 0.851 (95% CI: 0.721 – 0.980), p < 0.001, sensitivity of 0.778, specificity of 0.831, and a cutoff value of 3.980 IU/ml. TED, thyroid eye disease. TPOAb, thyroid peroxidase antibody. TGAb, thyroglobulin antibody. ROC, receiver operating characteristic. AUC, area under the curve.
Notably, TRAb negativity does not imply absolute absence of TRAb. Within detectable ranges (0.8 - 1.75 IU/ml), TRAb levels showed a positive correlation with CAS (Rs = 0.377, p < 0.01). Patients exhibiting conjunctival injection and caruncle edema had higher TRAb levels than asymptomatic counterparts (p < 0.01; Supplementary Figures S1A, B). TRAb independently predicted these manifestations (p < 0.05; Supplementary Table S4) with significant ROC analysis (Supplementary Figure S1C).
Collectively, TRAb-negative TED presents distinct clinical features. Both TPOAb and TGAb associated with key ocular manifestations including proptosis, lagophthalmos, and elevated orbital pressure. Notably, TGAb serves as an effective predictor for eyelid erythema, highlighting the clinical necessity of monitoring these antibodies in TRAb-negative TED evaluation and management.
4 Discussion
This cross-sectional observational study comprehensively characterized 86 TRAb-negative TED patients, establishing predictive thresholds for specific symptoms such as eyelid erythema, conjunctival injection, and caruncle edema.
Our cohort accounted for 12.4% (86/692) of TED cases, consistent with the reported prevalence (12.0-19.4%) (14, 15). The mean age was 45.24 ± 10.78 years with 53.5% of participants being female (female-to-male ratio 1.15), aligning with demographic patterns (11, 16). Bilateral involvement occurred in 86.0% (74/86) of patients, with the most common clinical signs being proptosis (83.7%), eyelid edema (72.1%), and eyelid retraction (60.5%). While proptosis and eyelid retraction represent common features of TED features (11, 17), our cohort demonstrated higher prevalence of eyelid edema prevalence than HT-associated TED (18). Moderate-to-severe disease affected 73.3% of patients, exceeding rates in HT-TED cohorts (19) but consistent with other TRAb-negative populations (6), potentially reflecting racial/geographic variations (20, 21). Vision-threatening dysthyroid optic neuropathy (DON) occurred in 3.5%, comparable to prior reports (3.2%) (16).
Although thyroid-stimulating immunoglobulins (TSI) demonstrate superior diagnostic performance (22), their clinical implementation remains limited. Therefore, the third-generation electrochemiluminescent-based TRAb assay used in this study, represents the most widely adopted clinical standard (23). While TSH receptor (TSHR)-mediated orbital inflammation remains central to TED pathogenesis (24, 25), our findings suggest that alternative mechanisms may be operational in TRAb-negative patients. The TPOAb(-)/TGAb(+) subgroup exhibited severe manifestations including eyelid erythema, lagophthalmos, visual impairment, elevated orbital pressure, and advanced NOSPECS grading. Contrasting reports of TGAb’s protective role in GD-TED (26), our non-GD cohort demonstrated TGAb positivity associated with multiple severe symptoms. This apparent discrepancy likely stems from fundamental differences in the underlying autoimmune pathogenesis between GD and non-GD (TRAb-negative) populations. In TRAb-negative TED, TGAb positivity may reflect a distinct pathogenic process involving synergistic action with other antibodies, direct effects on orbital tissue effects, or serve as an indicator of heightened inflammation. The particularly severe manifestations in the TPOAb(-)/TGAb(+) subgroup further suggest that the absence of potentially immunomodulatory TPOAb (27, 28) may unmask a more severe disease associated with TGAb in this setting. However, these mechanisms remain speculative, and our study design cannot establish a causal relationship. Unmeasured confounding factors related to the distinct etiologies within TRAb-negative TED could also contribute to the observed association. Future mechanistic studies using cellular models to assess TGAb’s effects on orbital fibroblasts, exploration in relevant animal models, and longitudinal assessments in well-characterized TRAb-negative TED cohorts are crucial to validate these findings and elucidate the precise role of TGAb in this specific TED phenotype. After adjusting for confounders (age, sex, history of hyperthyroidism history, antithyroid therapy [medication/RAI], TED duration, smoking status, and recent immunosuppression) (2, 15), multivariate analysis confirmed TGAb as an independent risk factor for eyelid erythema (optimal cutoff: 3.980 IU/mL). TPOAb positivity correlated with reduced active TED, eyelid retraction, and soft tissue involvement - potentially reflecting orbital TPO-mediated immunomodulation (27, 28).
Pathogenesis extends beyond TSHR autoimmunity, involving potential roles for: eye muscle antigens (calsequestrin, collagen XIII) (29–31); Vitamin D deficiency (32); Müller’s muscle autoimmunity (33); and shared thyroid-orbital epitopes (G2s protein) (34). Novel findings include a negative correlation between the TGAb-Gorman diplopia score and Rs (-0.231, p < 0.05), necessitating a mechanistic investigation. Detectable subthreshold TRAb levels positively correlated with CAS (Rs=0.377, p<0.01) and predicted conjunctival injection/caruncle edema, suggesting that clinical management may require achieving lower TRAb thresholds beyond mere negativity. The limitations of this study include: a mean disease duration of >18 months (32.6% received recent immunosuppression); potential recall bias regarding historical TRAb status; and a modest sample size despite representing the largest TRAb-negative TED cohort reported. Additionally, while TGAb and TPOAb levels correlated significantly with specific clinical features, they do not imply causation, as unmeasured confounders may influence these associations. Future longitudinal studies and mechanistic investigations using cellular/animal models are warranted to validate these findings and elucidate pathogenesis.
5 Conclusions
The main clinical manifestations in TRAb-negative TED patients included proptosis, eyelid edema, and eyelid retraction. TGAb and TPOAb levels were significantly associated with specific clinical features, necessitating routine monitoring of these antibodies in TRAb-negative TED management. The TSHR-mediated pathogenesis cannot fully explain all ocular manifestations, warranting investigation of additional pathogenic mechanisms. Systematic assessment of ocular characteristics—particularly in TRAb-negative patients with Hashimoto’s thyroiditis—enables the earlier recognition of TED recognition and the implementation of preventive interventions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The regional research ethics committee approved the study in the Senior Department of Ophthalmology, 3rd Medical Center of Chinese PLA General Hospital (project number KY2024-002). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SF: Formal analysis, Writing – original draft. XY: Writing – review & editing. YL: Writing – review & editing. RM: Writing – review & editing. WW: Data curation, Software, Supervision, Writing – review & editing. XT: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Military Young Sci-tech Talents Project and the Gansu Province Natural Science Foundation (25JRRA1004).
Acknowledgments
We thank the patients who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1655598/full#supplementary-material
Supplementary Figure 1 | The correlation between TRAb and Clinical Characteristics in TRAb-Negative TED Patients. (A) The TRAb level was significantly higher in the group with conjunctival redness symptoms compared to the group without symptoms at 1.33 ± 0.36 IU/ml and 1.08 ± 0.29 IU/ml, IU/ml, respectively, p < 0.01; (B) The TRAb level was significantly higher in the group with caruncular oedema symptoms compared to the group without symptoms, at 1.39 ± 0.28 IU/ml and 1.12 ± 0.34 IU/ml, respectively, p < 0. 01; (C) ROC curve analysis was performed using TRAb as the dependent variable and conjunctival redness and caruncle edema, yielding AUCs of 0.708 (95% CI: 0.589 – 0.827, p < 0. 01) for conjunctival congestion, sensitivity of 0.406, specificity of 0.944 and a cutoff value of 1.580 IU/ml; and 0.711 (95% CI: 0.587 – 0.836, p < 0.01) for lacrimal duct swelling, sensitivity of 0.875, specificity of 0.586 and a cutoff value of 1.155 IU/ml.
Abbreviations
AUC, area under the curve; CAS, clinical activity score; DON, dysthyroid optic neuropathy; EUGOGO, European Group on Graves’ Orbitopathy; FT3, free triiodothyronine; FT4, free thyroxine; GD, Graves’ disease; HT, Hashimoto’s thyroiditis; OF, orbital fibroblast; RAI, radioactive iodine; ROC, receiver operating characteristic; TED, thyroid eye disease; TGAb, thyroid globulin antibodies; TPOAb, thyroid peroxidase antibodies; TRAb, thyrotrophin receptor antibody; TSH, thyroid-stimulating hormone; TSHR, thyroid-stimulating hormone receptor.
References
1. Chin YH, Ng CH, Lee MH, Koh JWH, Kiew J, Yang SP, et al. Prevalence of thyroid eye disease in Graves' disease: A meta-analysis and systematic review. Clin Endocrinol (Oxf). (2020) 93:363–74. doi: 10.1111/cen.14296
2. Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur J Endocrinol. (2021) 185:G43–g67. doi: 10.1530/EJE-21-0479
3. Diana T, Ponto KA, and Kahaly GJ. Thyrotropin receptor antibodies and Graves' orbitopathy. J Endocrinol Invest. (2021) 44:703–12. doi: 10.1007/s40618-020-01380-9
4. Nicolì F, Lanzolla G, Mantuano M, Ionni I, Mazzi B, Leo M, et al. Correlation between serum anti-TSH receptor autoantibodies (TRAbs) and the clinical feature of Graves' orbitopathy. J Endocrinol Invest. (2021) 44:581–5. doi: 10.1007/s40618-020-01353-y
5. Sarić Matutinović M, Kahaly GJ, Žarković M, Ćirić J, Ignjatović S, Nedeljković Beleslin B, et al. The phenotype of Graves' orbitopathy is associated with thyrotropin receptor antibody levels. J Endocrinol Invest. (2023) 46:2309–17. doi: 10.1007/s40618-023-02085-5
6. Ponto KA, Binder H, Diana T, Matheis N, Otto AF, Pitz S, et al. Prevalence, phenotype, and psychosocial well-being in euthyroid/hypothyroid thyroid-associated orbitopathy. Thyroid. (2015) 25:942–8. doi: 10.1089/thy.2015.0031
7. Yoshihara A, Yoshimura Noh J, Nakachi A, Ohye H, Sato S, Sekiya K, et al. Severe thyroid-associated orbitopathy in Hashimoto's thyroiditis. Report of 2 cases. Endocr J. (2011) 58:343–8. doi: 10.1507/endocrj.K11E-019
8. El Othman R, Ephrem C, Touma E, Hallit S, and El Othman R. A case report of thyroid-associated Orbitopathy with elevated TPO antibodies. BMC Endocr Disord. (2020) 20:176. doi: 10.1186/s12902-020-00658-6
9. Cyranska-Chyrek E, Olejarz M, Szczepanek-Parulska E, Stajgis P, Pioch A, Ruchala M, et al. Severe unilateral orbitopathy in a patient with Hashimoto's thyroiditis -a case report. BMC Ophthalmol. (2019) 19:9. doi: 10.1186/s12886-018-1018-5
10. Bartley GB and Gorman CA. Diagnostic criteria for Graves' ophthalmopathy. Am J Ophthalmol. (1995) 119:792–5. doi: 10.1016/S0002-9394(14)72787-4
11. Li Q, Ye H, Ding Y, Chen G, Liu Z, Xu J, et al. Clinical characteristics of moderate-to-severe thyroid associated ophthalmopathy in 354 Chinese cases. PloS One. (2017) 12:e0176064. doi: 10.1371/journal.pone.0176064
12. Bahn RS and Gorman CA. Choice of therapy and criteria for assessing treatment outcome in thyroid-associated ophthalmopathy. Endocrinol Metab Clin North Am. (1987) 16:391–407. doi: 10.1016/S0889-8529(18)30485-7
13. Werner SC. Modification of the classification of the eye changes of Graves' disease. Am J Ophthalmol. (1977) 83:725–7. doi: 10.1016/0002-9394(77)90140-4
14. Barbesino G and Tomer Y. Clinical review: Clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab. (2013) 98:2247–55. doi: 10.1210/jc.2012-4309
15. Lai KKH, Aljufairi FMAA, Sebastian JU, Wei Y, Jia R, Chan KKW, et al. Epidemiology of thyroid-stimulating immunoglobulin in recent-onset symptomatic thyroid eye disease. Eur Thyroid J. (2024) 13. doi: 10.1530/ETJ-23-0129
16. Du B, Wang Y, Yang M, and He W. Clinical features and clinical course of thyroid-associated ophthalmopathy: a case series of 3620 Chinese cases. Eye (Lond). (2021) 35:2294–301. doi: 10.1038/s41433-020-01246-7
17. Lim SL, Lim AK, Mumtaz M, Hussein E, Wan Bebakar WM, Khir AS, et al. Prevalence, risk factors, and clinical features of thyroid-associated ophthalmopathy in multiethnic Malaysian patients with Graves' disease. Thyroid. (2008) 18:1297–301. doi: 10.1089/thy.2008.0044
18. Tjiang H, Lahooti H, McCorquodale T, Parmar KR, and Wall JR. Eye and eyelid abnormalities are common in patients with Hashimoto's thyroiditis. Thyroid. (2010) 20:287–90. doi: 10.1089/thy.2009.0199
19. Kan E, Kan EK, Ecemis G, and Colak R. Presence of thyroid-associated ophthalmopathy in Hashimoto's thyroiditis. Int J Ophthalmol. (2014) 7:644–7. doi: 10.3980/j.issn.2222-3959.2014.04.10
20. Marinò M, Rotondo Dottore G, Menconi F, Comi S, Cosentino G, Rocchi R, et al. Role of genetics and epigenetics in Graves' orbitopathy. Eur Thyroid J. (2024) 13. doi: 10.1530/ETJ-24-0179
21. Ramesh S, Zhang QE, Sharpe J, Penne R, Haller J, Lum F, et al. Thyroid eye disease and its vision-threatening manifestations in the academy IRIS registry: 2014-2018. Am J Ophthalmol. (2023) 253:74–85. doi: 10.1016/j.ajo.2023.04.013
22. van Balkum M, Schreurs MWJ, Visser WE, Peeters RP, and Dik WA. Comparison of two different TSH-receptor antibody assays: A clinical practice study. Heliyon. (2023) 9:e22468. doi: 10.1016/j.heliyon.2023.e22468
23. Higgins V, Patel K, Kulasingam V, Beriault DR, Rutledge AC, Selvaratnam R, et al. Analytical performance evaluation of thyroid-stimulating hormone receptor antibody (TRAb) immunoassays. Clin Biochem. (2020) 86:56–60. doi: 10.1016/j.clinbiochem.2020.08.007
24. Burch HB and Wartofsky L. Graves' ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. (1993) 14:747–93. doi: 10.1210/edrv-14-6-747
25. Lee ACH and Kahaly GJ. Pathophysiology of thyroid-associated orbitopathy. Best Pract Res Clin Endocrinol Metab. (2023) 37:101620. doi: 10.1016/j.beem.2022.101620
26. Constantinescu SM, Hospel J, Daumerie C, Alexopoulou O, Maiter D, Burlacu MC, et al. Significance of thyroperoxidase and thyroglobulin antibodies in medically treated Graves' disease. Eur Thyroid J. (2023) 12. doi: 10.1530/ETJ-23-0193
27. Khoo DH, Ho SC, Seah LL, Fong KS, Tai ES, Chee SP, et al. The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in Graves' disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid. (1999) 9:1175–80. doi: 10.1089/thy.1999.9.1175
28. Lai OF, Zaiden N, Goh SS, Mohamed NE, Seah LL, Fong KS, et al. Detection of thyroid peroxidase mRNA and protein in orbital tissue. Eur J Endocrinol. (2006) 155:213–8. doi: 10.1530/eje.1.02205
29. Lahooti H, Parmar KR, and Wall JR. Pathogenesis of thyroid-associated ophthalmopathy: does autoimmunity against calsequestrin and collagen XIII play a role? Clin Ophthalmol. (2010) 4:417–25. doi: 10.2147/opth.s6534
30. Wall JR and Lahooti H. Pathogenesis of thyroid eye disease–does autoimmunity against the TSH receptor explain all cases? Endokrynol Pol. (2010) 61:222–7. doi: not applicable
31. Wall JR, Lahooti H, El Kochairi I, Lytton SD, and Champion B. Thyroid-stimulating immunoglobulins as measured in a reporter bioassay are not detected in patients with Hashimoto's thyroiditis and ophthalmopathy or isolated upper eyelid retraction. Clin Ophthalmol. (2014) 8:2071–6. doi: 10.2147/OPTH.S67098
32. Heisel CJ, Riddering AL, Andrews CA, and Kahana A. Serum vitamin D deficiency is an independent risk factor for thyroid eye disease. Ophthalmic Plast Reconstr Surg. (2020) 36:17–20. doi: 10.1097/IOP.0000000000001437
33. Barsouk A, Peele KA, Kiljanski J, Stolarski C, Nebes V, Kennerdell JS, et al. Antibody-dependent cell-mediated cytotoxicity against orbital target cells in thyroid-associated ophthalmopathy and related disorders; close relationship between serum cytotoxic antibodies and parameters of eye muscle dysfunction. J Endocrinol Invest. (1996) 19:334–41. doi: 10.1007/BF03344966
34. Gunji K, De Bellis A, Li AW, Yamada M, Kubota S, Ackrell B, et al. Cloning and characterization of the novel thyroid and eye muscle shared protein G2s: autoantibodies against G2s are closely associated with ophthalmopathy in patients with Graves' hyperthyroidism. J Clin Endocrinol Metab. (2000) 85:1641–7. doi: 10.1210/jcem.85.4.6553
Keywords: thyrotrophin receptor antibody-negative, thyroid eye disease, clinical characteristics, anti-thyroglobulin antibody, clinical management
Citation: Jing G, Fu S, Yang X, Li Y, Ma R, Wu W and Tang X (2025) Clinical profiling of TPOAb and TGAb in patients with thyrotrophin receptor antibody-negative thyroid eye disease: A single-center observational study in China. Front. Endocrinol. 16:1655598. doi: 10.3389/fendo.2025.1655598
Received: 28 June 2025; Accepted: 22 August 2025;
Published: 22 September 2025.
Edited by:
Sijie Fang, Shanghai Jiao Tong University, ChinaReviewed by:
Yongze Li, The First Affiliated Hospital of China Medical University, ChinaJixiong Xu, The First Affiliated Hospital of Nanchang University, China
Copyright © 2025 Jing, Fu, Yang, Li, Ma, Wu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xulei Tang, eGx0YW5nQGx6dS5lZHUuY24=; Wei Wu, d3V3ZWkxQDMwMWhvc3BpdGFsLmNvbS5jbg==
†These authors have contributed equally to this work
 Gaojing Jing
Gaojing Jing Songbo Fu1,2,3†
Songbo Fu1,2,3†