- 1Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2Jiangxi Cardiovascular Research Institute, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
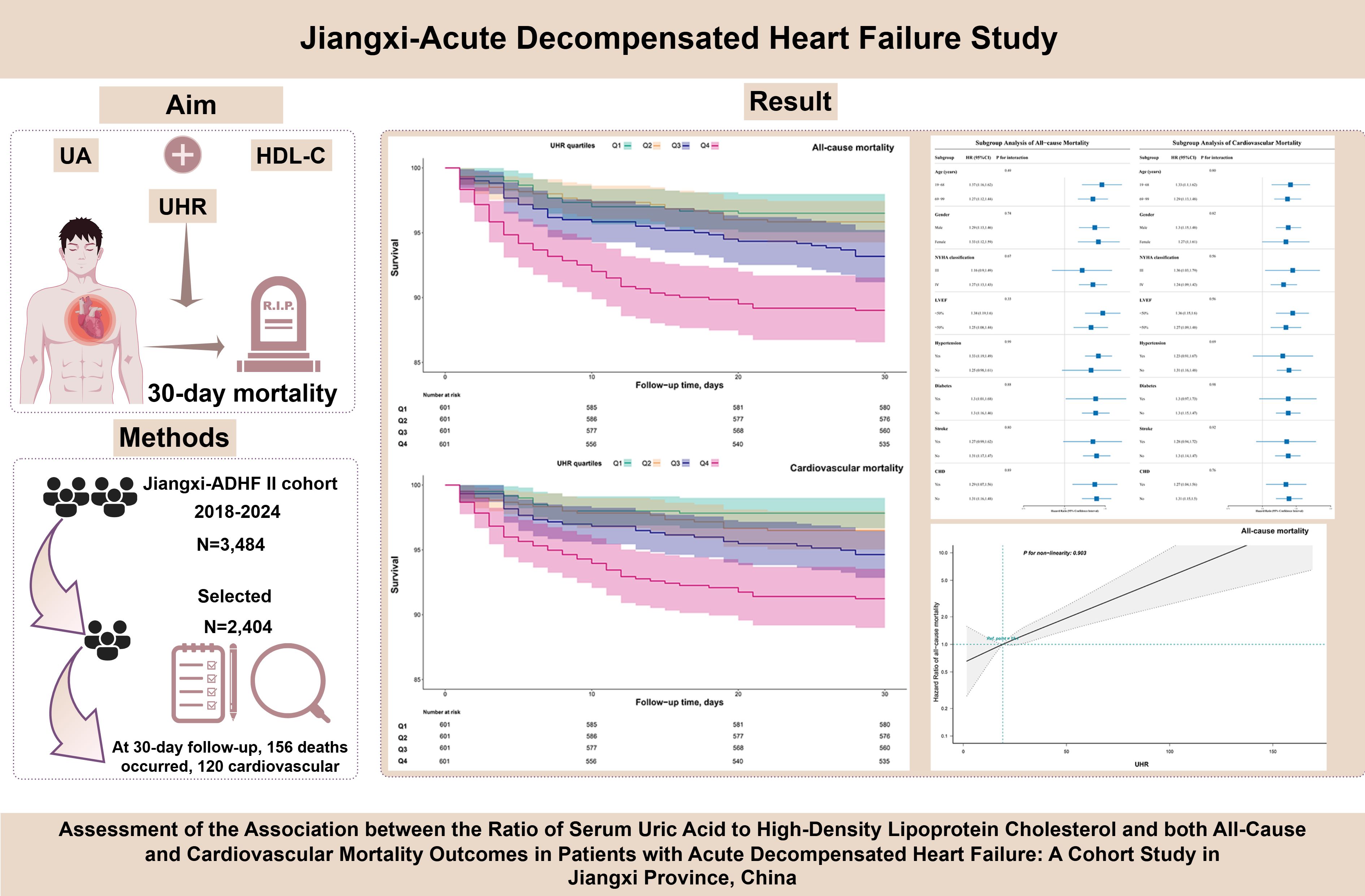
Introduction: Accumulating evidence suggests that both serum uric acid (UA) and high-density lipoprotein cholesterol(HDL-C) play critical roles in the pathogenesis of heart failure. This study aimed to investigate the association between the UA-to-HDL-C ratio(UHR) and short-term all-cause and cardiovascular mortality in patients with acute decompensated heart failure(ADHF).
Methods: A total of 2,404 ADHF patients admitted to Jiangxi Provincial People’s Hospital from 2018 to 2024 were included in this study. The association between the UHR and 30-day all-cause and cardiovascular-specific mortality in patients with ADHF was systematically evaluated using Kaplan-Meier analysis, Cox regression, restricted cubic spline models, and stratified analysis. The robustness of the findings was further validated through multi-faceted sensitivity analyses.
Results: During the 30-day follow-up period, 156 patients(6.49%) died in the entire cohort, with 120 deaths attributed to cardiovascular causes. The all-cause mortality rates across UHR quartiles were as follows: Q1: 3.83%, Q2: 4.16%, Q3: 6.82%, Q4: 11.15%, while cardiovascular mortality rates were Q1: 2.33%, Q2: 3.49%, Q3: 5.32%, Q4: 8.82%. Multivariable Cox regression analysis revealed that each 1-unit increase in UHR was associated with a 30% increased risk of both all-cause and cardiovascular mortality in ADHF patients. Furthermore, compared with patients in the lowest UHR quartile, those in the highest quartile had an 88% increased risk of 30-day all-cause mortality and a 113% increased risk of cardiovascular mortality. Further restricted cubic spline regression analysis demonstrated a linear positive association between UHR and the 30-day risks of all-cause and cardiovascular mortality in ADHF patients. Stratified analysis revealed that the association between UHR and mortality in ADHF patients was not modified by age, gender, New York Heart Association classification, left ventricular ejection fraction, or comorbidities. Finally, multiple sensitivity analyses conducted across four dimensions—population heterogeneity, causal temporality, model adjustment, and data integrity—confirmed the robustness of the primary findings.
Discussion: In this cohort study conducted in Jiangxi, China, we demonstrated for the first time that the UHR could serve as a tool for early prognostic assessment of short-term all-cause and cardiovascular mortality risk in ADHF patients, and elevated UHR levels were independently associated with an increased risk of both outcomes.
Background
Acute decompensated heart failure (ADHF) is a cardiovascular syndrome characterized by sudden worsening of HF symptoms, primarily manifesting as circulatory congestion and organ hypoperfusion due to inadequate cardiac output, which can be life-threatening in severe cases (1–3). According to statistics, ADHF patients have a short-term mortality rate of approximately 12%, a 1-year readmission rate of about 45%, and a 1-year mortality rate of approximately 22% (4, 5). Despite ongoing advancements in treatment strategies, the incidence of short-term adverse outcomes in ADHF patients remains persistently high (6, 7). Therefore, given the significant short-term mortality and adverse prognostic features observed in ADHF patients, establishing an effective early warning system and promptly identifying critical risk factors driving disease progression may hold substantial clinical value for optimizing clinical decision-making and reducing short-term mortality.
UA (Uric Acid) and HDL-C (High-Density Lipoprotein Cholesterol) are two common metabolism-related indicators closely associated with cardiovascular health; research indicates that elevated UA levels significantly increase the risk of all-cause mortality in HF patients, and this association is consistent across both acute and chronic HF patients (8–14). HDL-C is a cardioprotective lipid. On one hand, it exhibits anti-atherosclerotic effects by promoting reverse cholesterol transport to the liver for metabolic clearance, thereby reducing atherosclerotic burden (15, 16). On the other hand, HDL-C possesses anti-inflammatory and antioxidant properties, protecting the cardiovascular system by mitigating oxidative stress and inflammatory responses (17–20). Clinical studies have demonstrated that reduced HDL-C levels are independently associated with an increased risk of HF (21–24). It should be noted that there is an interplay between UA and HDL-C, which is associated with future mortality risk in cardiovascular patients (25, 26). Recently, Kocak et al. proposed a ratio combining UA and HDL-C (UA to HDL-C Ratio, UHR) for the diagnosis of metabolic syndrome (27). Subsequent related studies have further revealed that UHR can also be applied to risk assessment for various cardiovascular diseases, including ischemic heart disease (28), hypertension (29, 30), and acute myocardial infarction (31). Notably, in a recent cross-sectional study, researchers found that elevated UHR is significantly associated with an increased risk of congestive HF (32). These findings collectively support a significant association between UHR and cardiovascular diseases (28–32); however, it remains unclear whether UHR can be applied to assess HF prognosis. Therefore, based on the above research background, this study aims to systematically evaluate the clinical association of UHR with 30-day all-cause mortality and cardiovascular-specific mortality in ADHF patients, using a cohort of ADHF patients from Jiangxi, China.
Methods
Study population and design
Data for this study were derived from the Jiangxi-ADHF II project. Briefly, the Jiangxi-ADHF II project is a retrospective cohort study that consecutively enrolled 3,484 ADHF patients admitted to Jiangxi Provincial People’s Hospital between January 2018 and January 2024. The project aims to investigate key factors influencing short-term adverse outcomes in ADHF patients, with the goal of refining early risk stratification strategies for this population. In this project, the definition of ADHF referred to the latest guidelines from the American College of Cardiology/American Heart Association and the European Society of Cardiology for HF (33–36), taking into account the subjects’ clinical symptoms, signs, and laboratory test results, primarily as follows: (1) At least one symptom worsened from baseline: (a) dyspnea (exertional dyspnea, paroxysmal nocturnal dyspnea, or orthopnea), (b) systemic venous congestion (lower extremity edema, hepatic congestion, or ascites), and (c) tissue hypoperfusion (oliguria or anuria, cold extremities, impaired consciousness, hyperlactatemia, or metabolic acidosis); (2) At least one sign of HF: (a) pulmonary edema detected by physical examination or chest X-ray, (b) elevated levels of B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide (NT-proBNP), and (c) echocardiographic evidence of cardiac structural and/or functional abnormalities.
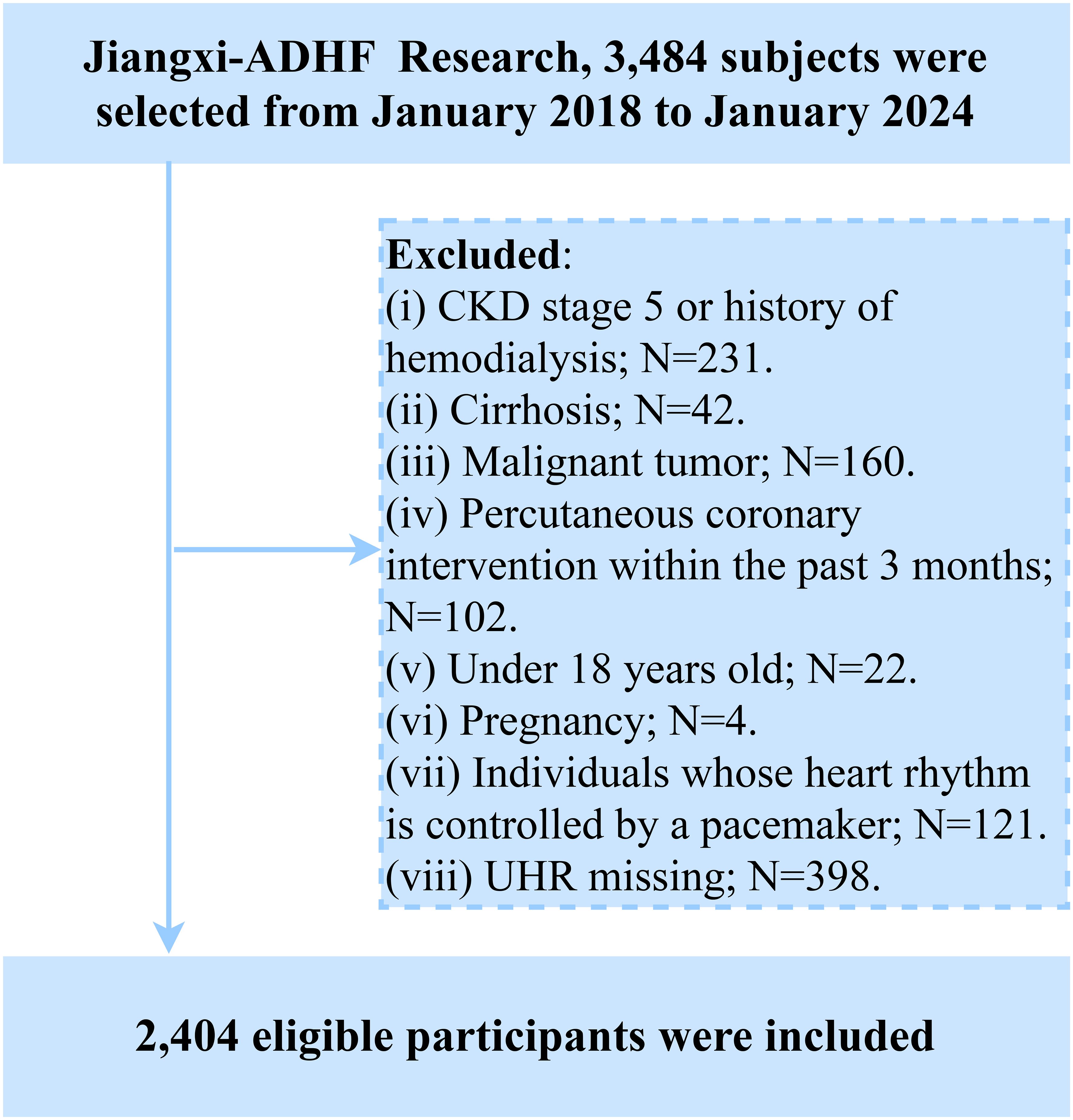
Participants with the following baseline characteristics were excluded: (i) Those with malignant tumors (n=160) were excluded due to a potentially significant compromise in life expectancy; (ii) To mitigate confounding effects of fluid and sodium retention arising from other diseases, we excluded patients undergoing hemodialysis for chronic kidney disease or those with diagnosed uremia (n=231), and individuals with cirrhosis (n=42). (iii) Participants with pacemakers were excluded due to reliance on pacemaker-regulated heart rate (lack of intrinsic autonomic control) (n=121). (iv) To account for the significant impact of reperfusion therapy on short-term prognosis, individuals who underwent percutaneous coronary intervention within 3 months prior to admission were excluded (n=102). (v) Participants aged <18 years were excluded (n=22). (vi) Pregnant participants were excluded (n=4). Additionally, based on the study’s objectives, we further excluded participants with missing UHR data (n=398). Ultimately, 2,404 participants were included in the final analysis. The detailed study flow is illustrated in Figure 1.
Ethics approval
According to Article 39 of the “Ethical Review Measures for Biomedical Research Involving Humans” issued by the National Health Commission of China, the informed consent exemption clause (37) stipulates: Research that utilizes human materials or data containing identifiable personal information may be exempt from the requirement to obtain signed informed consent, provided that the research does not involve personal privacy or commercial interests. In the current study, the design and implementation of the Jiangxi-ADHF II project were reviewed and approved by the Ethics Committee of Jiangxi Provincial People’s Hospital (Approval No. 2024-001), adhering strictly to the ethical principles outlined in the Declaration of Helsinki. Research data were recorded and analyzed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines after obtaining oral informed consent from participants or their legal representatives.
Data collection
Baseline data collection and verification were completed within 24 hours of patient admission by a professionally trained two-member research team. The collected parameters included demographic characteristics (gender, age), New York Heart Association (NYHA) functional classification, comorbidities (hypertension, diabetes, stroke, and coronary heart disease [CHD]), echocardiographic left ventricular ejection fraction (LVEF), lifestyle history (smoking and drinking status), and laboratory test results. Specifically, comorbidities were confirmed through a comprehensive assessment that integrated patient self-reported medical history, current medication regimens, and past diagnostic records.
Laboratory tests were uniformly conducted within 24 hours of admission at the Clinical Laboratory Center of Jiangxi Provincial People’s Hospital. The tested parameters included NT-proBNP, complete blood count (white blood cell count: WBC; red blood cell count: RBC; platelet count), liver function markers (alanine aminotransferase, and aspartate aminotransferase: AST), UA, lipid profiles (total cholesterol, triglycerides, low-density lipoprotein cholesterol, and HDL-C), and fasting plasma glucose (FPG). Fasting blood samples were required for FPG, liver enzyme, and lipid profile tests, and specimens were collected either at admission or early the following morning.
Calculation formula
(27).
Research outcomes
This study used 30-day all-cause mortality as the primary endpoint and cardiovascular mortality as the secondary endpoint. In the current study, cardiovascular deaths primarily included HF-related deaths, arrhythmia/sudden cardiac deaths, and stroke-related deaths; non-cardiovascular deaths primarily included septic shock/sepsis-related deaths, respiratory disease-related deaths, and other deaths attributed to clearly diagnosed non-cardiovascular causes. Follow-up procedures began on the day of patient admission, with survival status verified by uniformly trained researchers through multiple methods (including telephone follow-up, text message confirmation, and outpatient/inpatient follow-up visits).
Handling of missing data
The covariates included in this study exhibited a low rate of missingness (0–4.70%, Supplementary Table 1). A missingness pattern plot (Supplementary Figure 1) confirmed that the data met the criteria for missing at random. Due to these characteristics and the low overall missingness rate, the final analysis was conducted using the original dataset without imputation.
Statistical analysis
Data analysis was performed using R (version 4.2.1) and Empower(R) software (version 2.0). Baseline characteristics were described with appropriate statistical methods based on data types: categorical variables were presented as frequencies (percentages), normally distributed continuous variables as means (standard deviations), and non-normally distributed variables as medians (interquartile ranges). Group comparisons were conducted using one-way ANOVA, Kruskal-Wallis test, or chi-square test according to data characteristics, with a two-sided significance level set at α=0.05.
Kaplan-Meier analysis was used to plot survival curves for subjects stratified by UHR quartiles, and the log-rank test was applied to evaluate statistical differences. Multivariable Cox regression models were constructed to assess the association between UHR and prognosis in ADHF patients, and three levels of models were developed through stepwise adjustment of covariates: Model I (Basic Model): Adjusted for demographic factors (gender, age). Model II (Extended Model): Added lifestyle factors (smoking status, drinking status) and clinical characteristics (hypertension, diabetes, stroke, CHD) along with LVEF, building on Model I. Model III (Final Model): Further incorporated laboratory parameters (WBC, RBC, platelet count, AST, triglycerides, low-density lipoprotein cholesterol, FPG, and NT-proBNP) based on Model II. All models underwent variance inflation factor analysis to verify multicollinearity among covariates (Supplementary Table 2). In addition, the proportional hazards assumption of the model was tested using Schoenfeld residuals, with no violations detected.
Restricted cubic spline (RCS) analysis was employed (with 4 knots at the 5th, 35th, 65th, and 95th percentiles) to examine dose-response relationships between UHR and all-cause/cardiovascular mortality. Stratified analysis was further conducted to explore subgroup-specific associations of UHR with all-cause/cardiovascular mortality among ADHF patients. Likelihood ratio tests were used to evaluate linear/non-linear associations and test for differences across strata. Regarding the selection of knots in the RCS analysis, we referred to the recommendations in Professor Harrell’s book Regression Modeling Strategies (38): With 4 knots, the model achieves a good fit by balancing curve smoothness and avoiding reduced accuracy caused by overfitting. For larger sample sizes, 5 knots are preferable, while 3 knots are recommended for small samples (n < 30).
Sensitivity analysis
Four sensitivity analyses were conducted across four dimensions to test the robustness of results:
1. Considering the potential impact of frailty on adverse outcomes (39), we defined a frailty subgroup as individuals with ≥ 3 comorbidities in this study; we excluded this subgroup and reanalyzed the data.
2. To mitigate potential reverse causality, we excluded participants who experienced death events within 48 hours of admission.
3. A quadratic term for age was incorporated into the final model to account for potential non-linear associations between age and adverse outcomes (40).
4. To reduce potential bias from missing data, we estimated missing values via multiple imputation and repeated the primary analysis.
5. To validate the generalizability of our findings, we conducted external validation using data from the 1998–2018 U.S. (United States) National Health and Nutrition Examination Survey; specifically, we examined the association between UHR and all-cause and cardiovascular mortality in congestive HF patients within this independent sample.
6. To address the potential confounding effects of medications, we further included urate-lowering therapy, statin therapy, and diuretic therapy as covariates and adjusted for these treatment factors in the multivariate model (Sensitivity-6). Additionally, we excluded patients undergoing urate-lowering therapy or statin therapy and adjusted for diuretic therapy in the multivariate analysis (Sensitivity-7).
Results
Baseline characteristics
According to the study population screening process, we excluded 160 patients with malignant tumors, 231 patients diagnosed with uremia or those with chronic kidney disease undergoing hemodialysis treatment, 42 patients with liver cirrhosis, 121 patients with pacemakers, 102 patients who recently underwent percutaneous coronary intervention treatment, 22 minor patients, 4 pregnant patients, and 398 patients with missing UHR data.
A total of 2,404 ADHF patients were included in this study, comprising 58.7% males (n=1,412) and 41.3% females (n=992). As shown in Table 1, comparisons across UHR quartiles revealed that patients in the highest UHR quartile, compared to the lowest quartile, exhibited the following characteristics: (1) Demographic characteristics: A higher proportion of males and younger age. (2) Laboratory parameters: Levels of LVEF, total cholesterol, and HDL-C were lower, while WBC, RBC, alanine aminotransferase, AST, UA, and NT-proBNP levels were significantly elevated. (3) Clinical characteristics: A higher proportion of patients presented with NYHA Class IV. Notably, comorbidity distribution showed no statistical difference across groups (P>0.05).
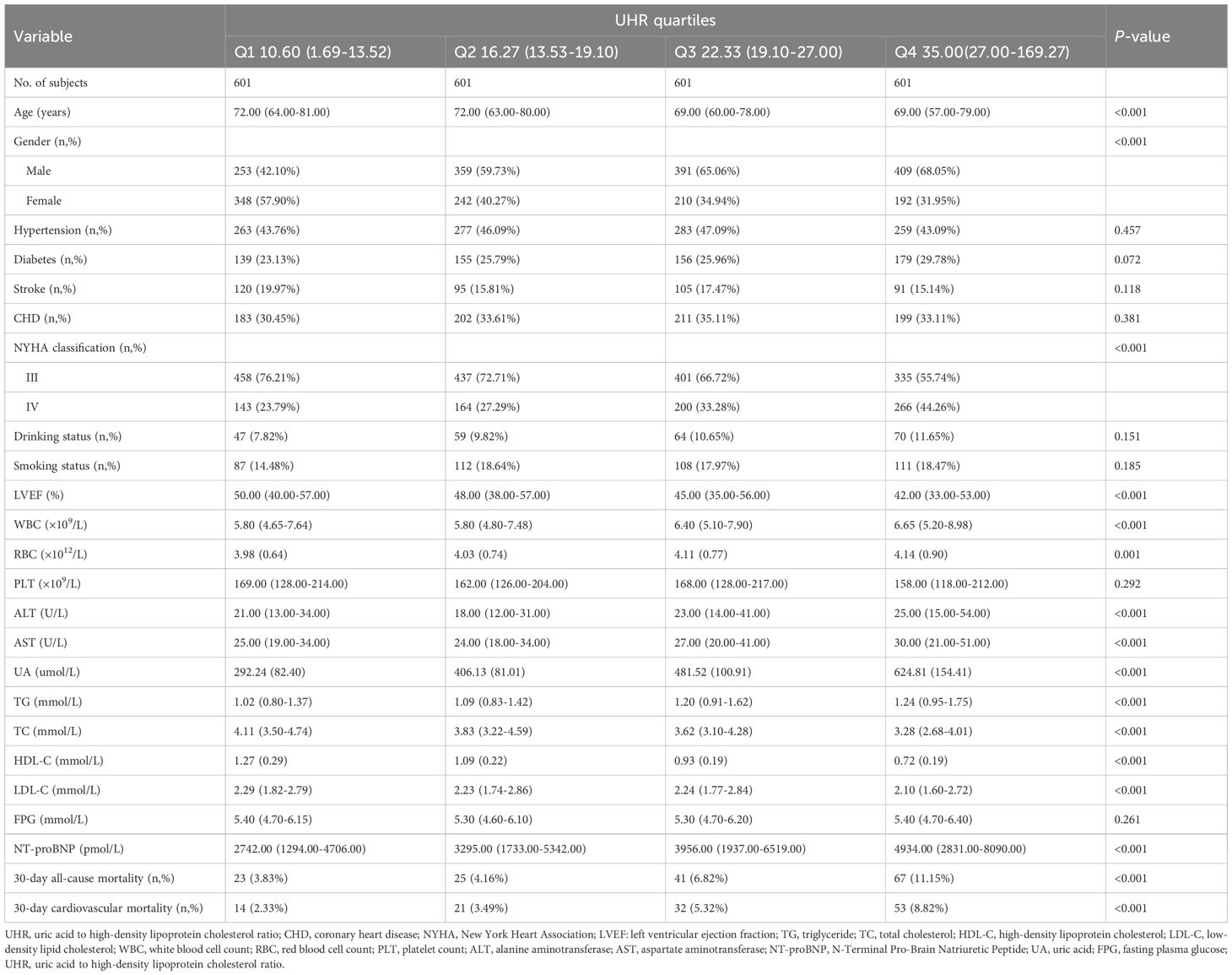
Table 1. Summary of baseline characteristics of the study population according to UHR quartiles group.
Follow-up results
During the 30-day observation period, mortality events occurred in 156 participants (6.49%), with 120 cases (4.99%) attributed to cardiovascular causes. Based on UHR quartile grouping, we generated stacked bar charts for 30-day all-cause and cardiovascular mortality rates (Figure 2), and the results demonstrated a progressive increase in both all-cause and cardiovascular mortality within 30 days among ADHF patients as UHR quartiles rose.
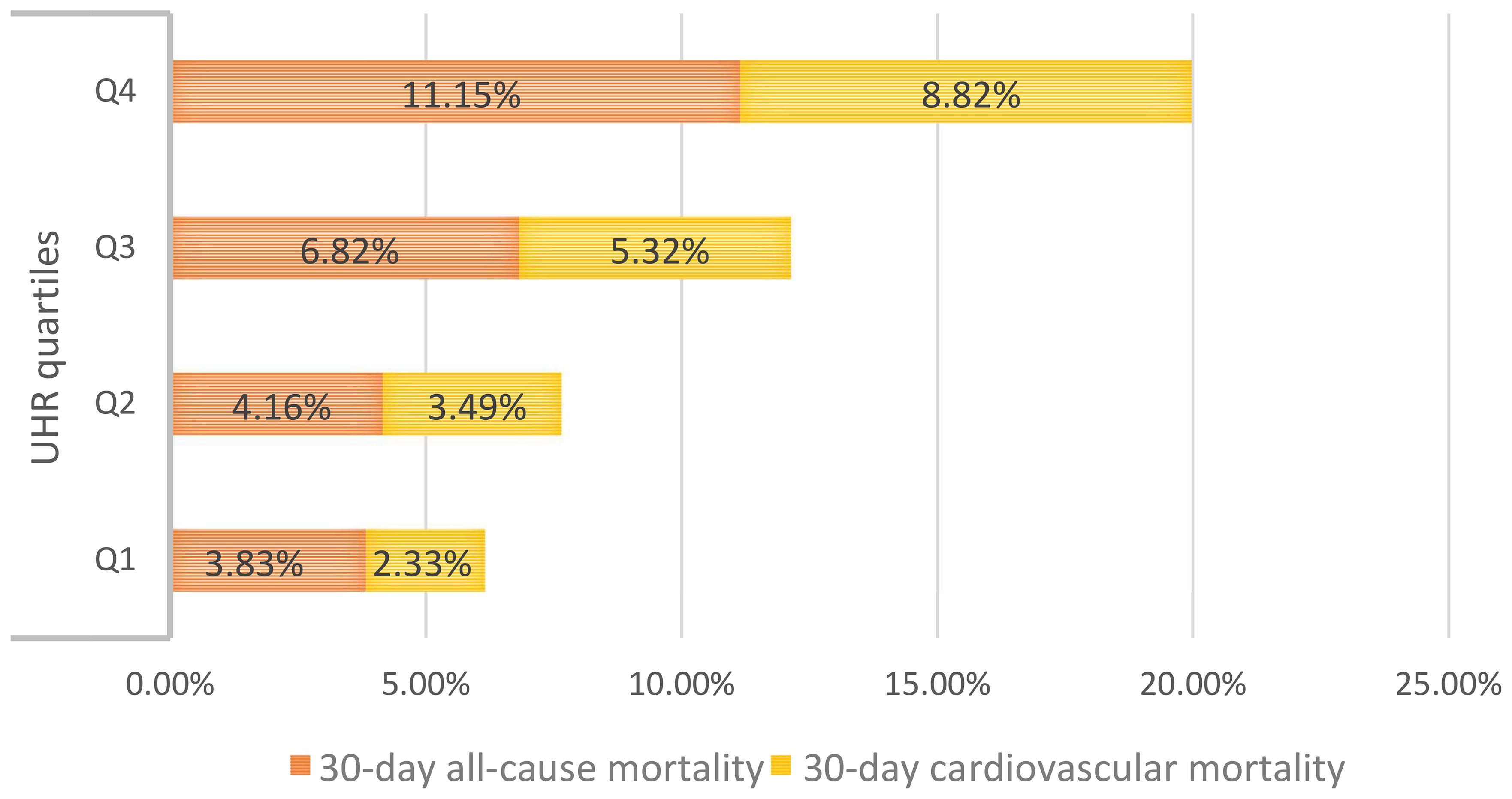
Figure 2. The stacked bar chart shows 30-day all-cause/cardiovascular mortality in ADHF patients according to UHR quartile groups. UHR: uric acid to high-density lipoprotein cholesterol ratio; ADHF: acute decompensated heart failure.
Additionally, 30-day survival curves stratified by UHR groups were plotted using the Kaplan-Meier method (Figure 3), revealing significantly higher mortality in the Q4 group compared to the other three groups (log-rank p < 0.0001).
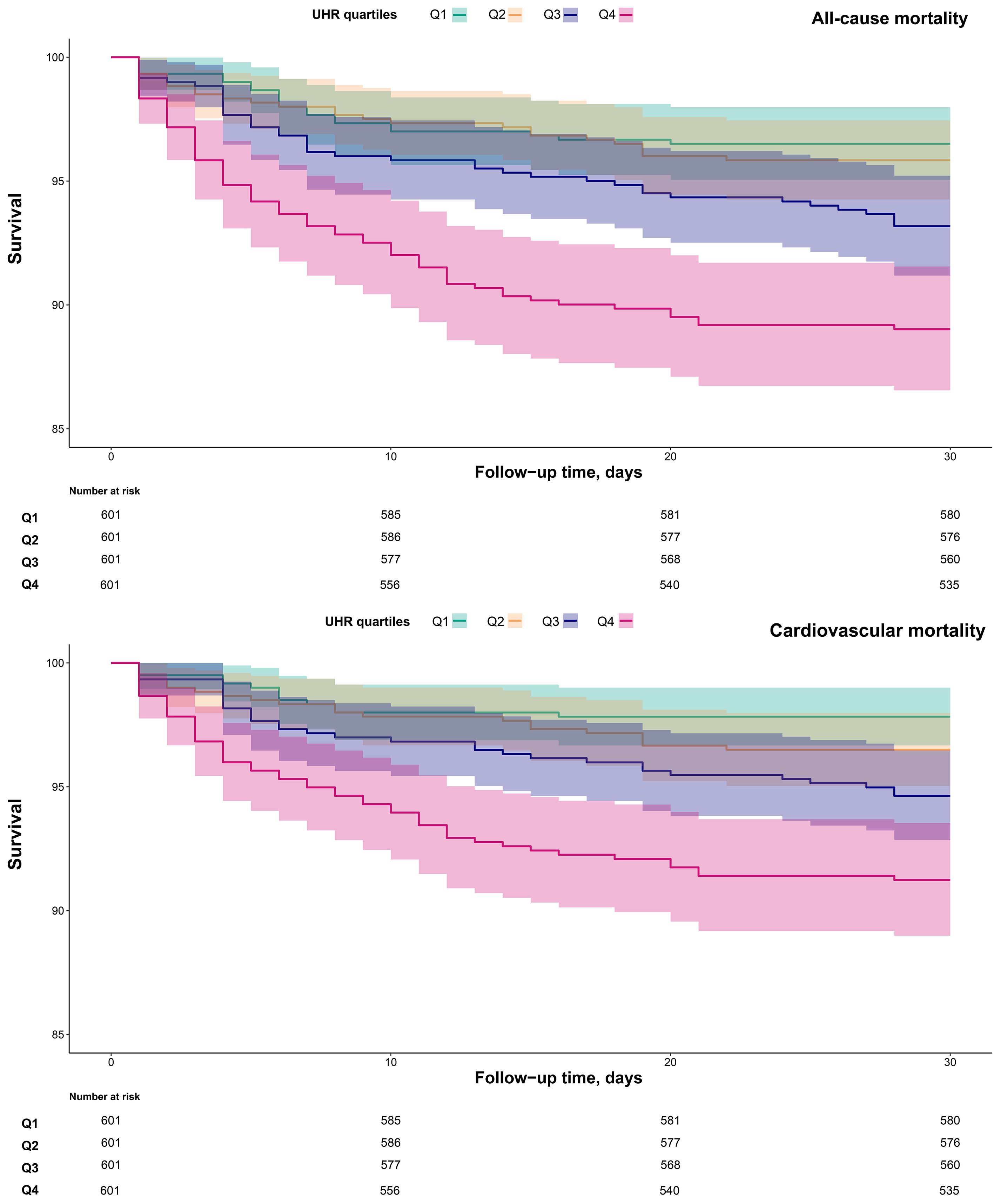
Figure 3. 30-day survival curves of ADHF patients stratified by UHR quartiles. UHR, uric acid to high-density lipoprotein cholesterol ratio; ADHF, acute decompensated heart failure.
Association analysis
We incorporated UHR as both continuous and categorical variables into three progressively adjusted Cox regression models to investigate its association with 30-day all-cause and cardiovascular mortality in ADHF patients (Table 2). Whether treated as continuous or categorical variables, a positive association between UHR and both 30-day all-cause and cardiovascular mortality was observed across all adjusted models. In the fully adjusted model (Model III), each unit increase in UHR was associated with a 30% higher risk of all-cause mortality (Hazard ratio [HR]: 1.30, 1.17–1.45) and a 30% higher risk of cardiovascular mortality (HR: 1.30, 1.16–1.46) among ADHF patients. When UHR was analyzed as a categorical variable, the results showed that compared with the lowest quartile (Q1), individuals in the highest quartile (Q4) had significantly elevated risks of both all-cause and cardiovascular mortality. Specifically, in the final model, ADHF patients in Q4 experienced an 88% increased risk of all-cause mortality (HR: 1.88, 1.08–3.26) and a 113% increased risk of cardiovascular mortality (HR: 2.13, 1.06–4.28) within 30 days, compared with those in Q1.
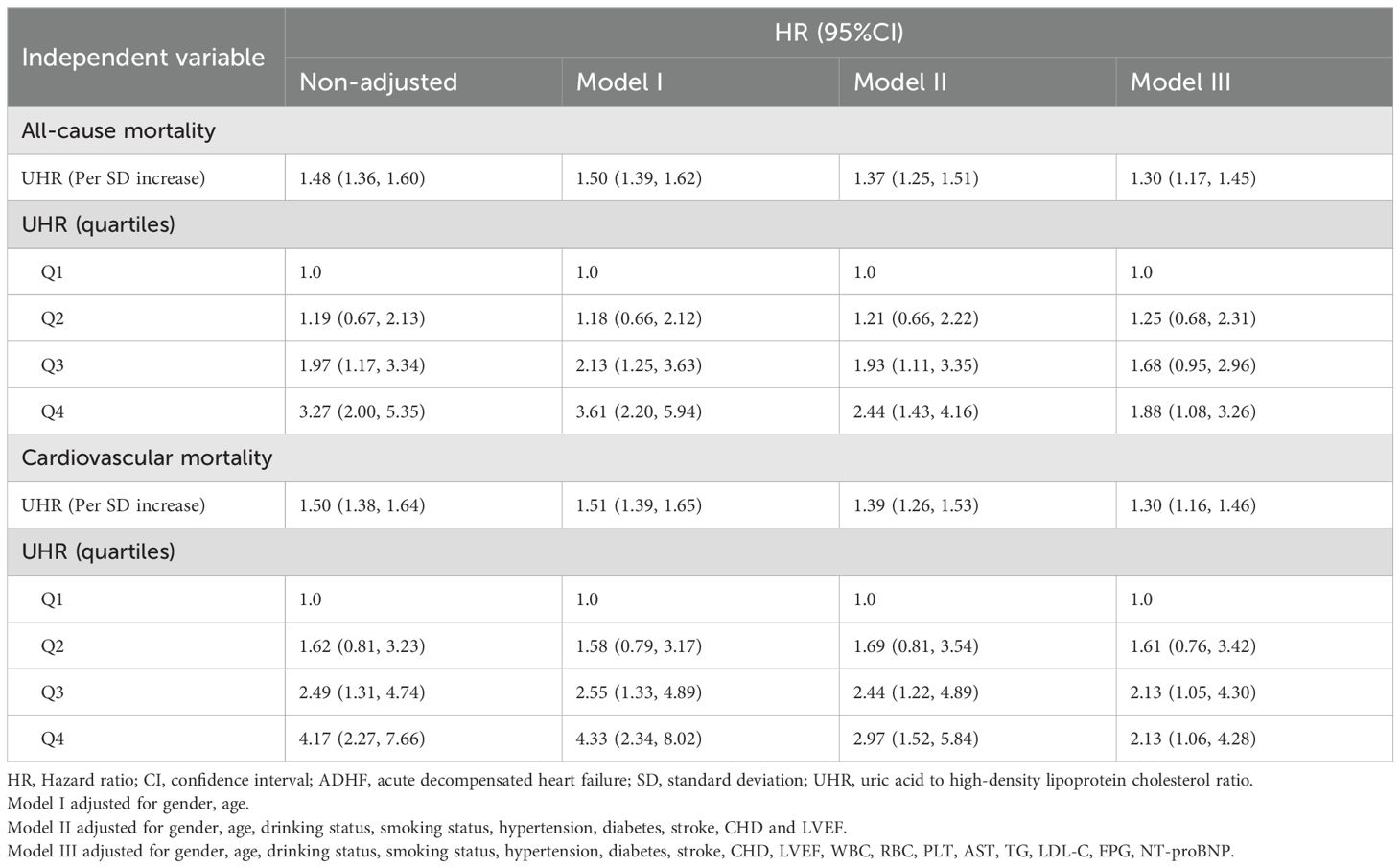
Table 2. Multivariable Cox regression analysis of the association between UHR and 30-day all-cause and cardiovascular mortality in patients with ADHF.
Dose-response relationship
To further investigate the association between UHR and mortality outcomes, adjusted RCS curves were used to visualize the dose-response relationships of UHR with all-cause and cardiovascular mortality (Figure 4). The analysis revealed a linear positive association between UHR and both all-cause mortality (P for non-linearity = 0.903) and cardiovascular mortality (P for non-linearity = 0.590) in ADHF patients.
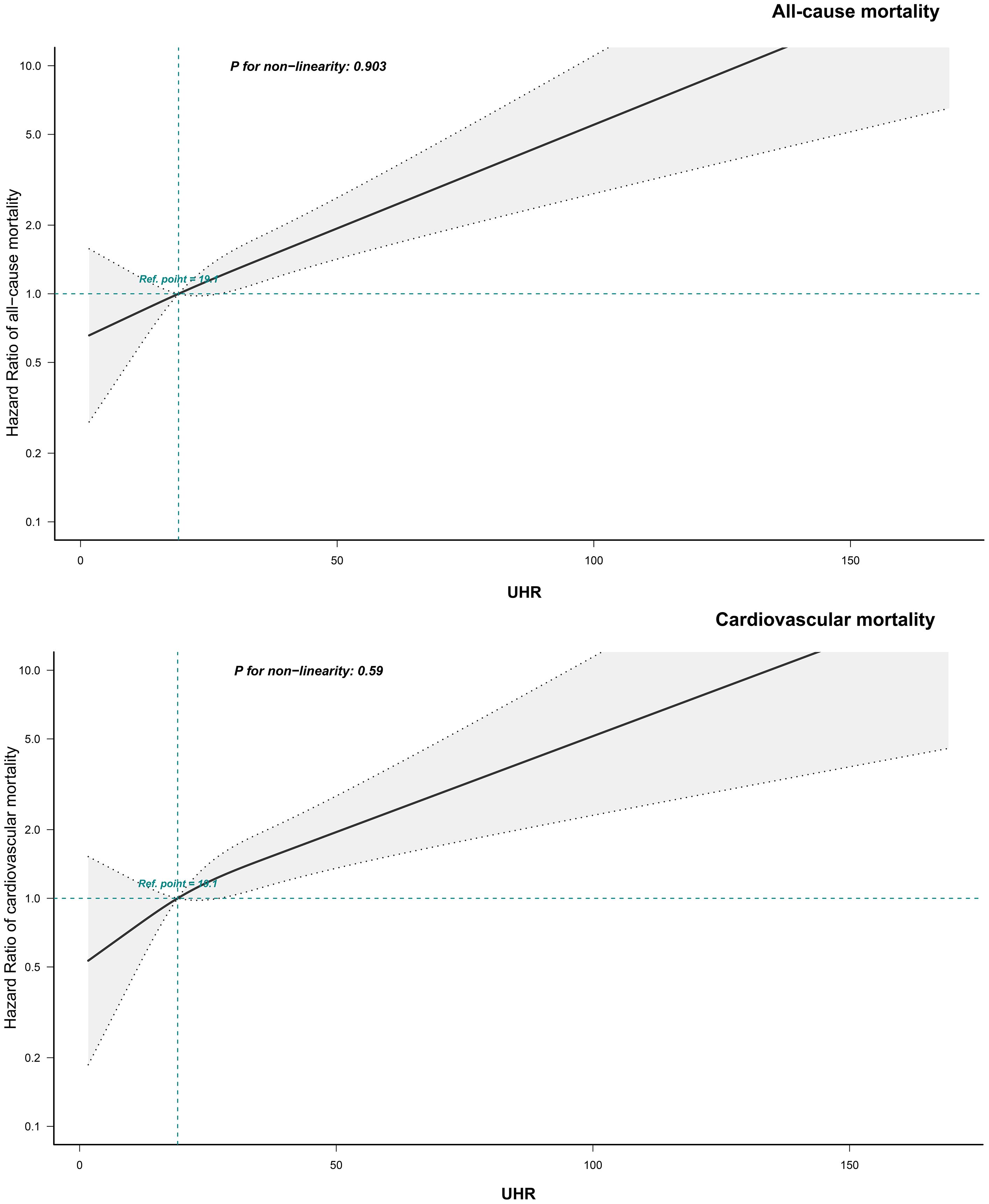
Figure 4. Fitting the dose-response relationship between UHR and 30-day all-cause/cardiovascular mortality in ADHF patients with 4 knots restricted cubic spline. UHR, uric acid to high-density lipoprotein cholesterol ratio; ADHF, acute decompensated heart failure. Adjusted for gender, age, drinking status, smoking status, hypertension, diabetes, stroke, CHD, LVEF, WBC, RBC, PLT, AST, TG, LDL-C, FPG, NT-proBNP.
Subgroup analysis
This study employed a stratified analysis approach to stratify patients into subgroups based on demographic characteristics (age, gender), clinical indicators (NYHA classification, LVEF), and comorbidities (Table 3). The likelihood ratio test was used to evaluate interaction effects between UHR and each stratifying factor, and the results showed no significant interactions across all subgroups (all P for interaction > 0.05; Figure 5 for details). This finding suggests that the association between UHR and adverse outcomes (all-cause and cardiovascular mortality) in ADHF patients is consistent across subgroups and not influenced by the aforementioned clinical characteristics.
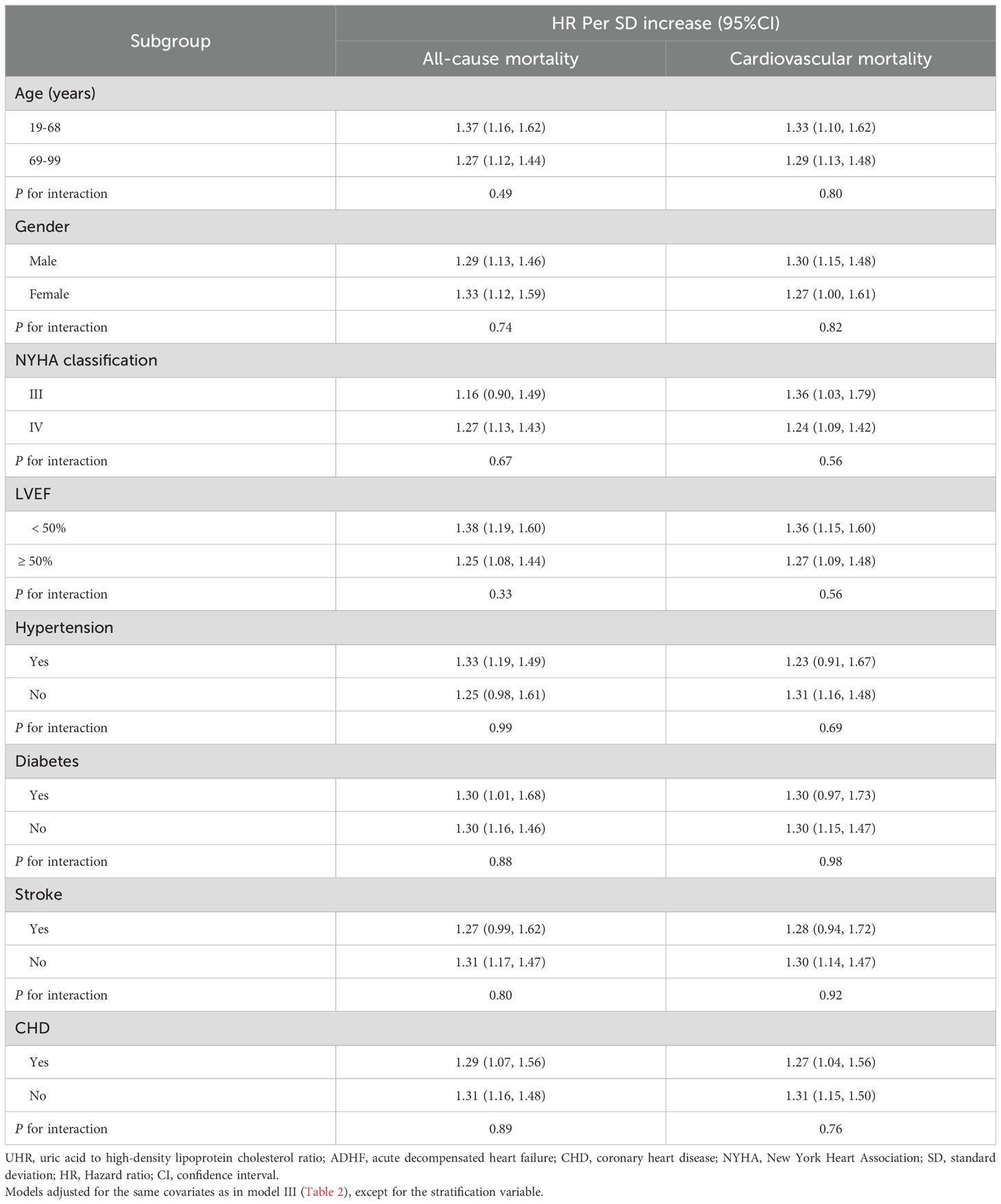
Table 3. Stratified analysis showed the relationship between UHR and 30-day mortality in patients with ADHF in different age, gender, NYHA classification, LVEF and whether combined with hypertension/diabetes/stroke/CHD.
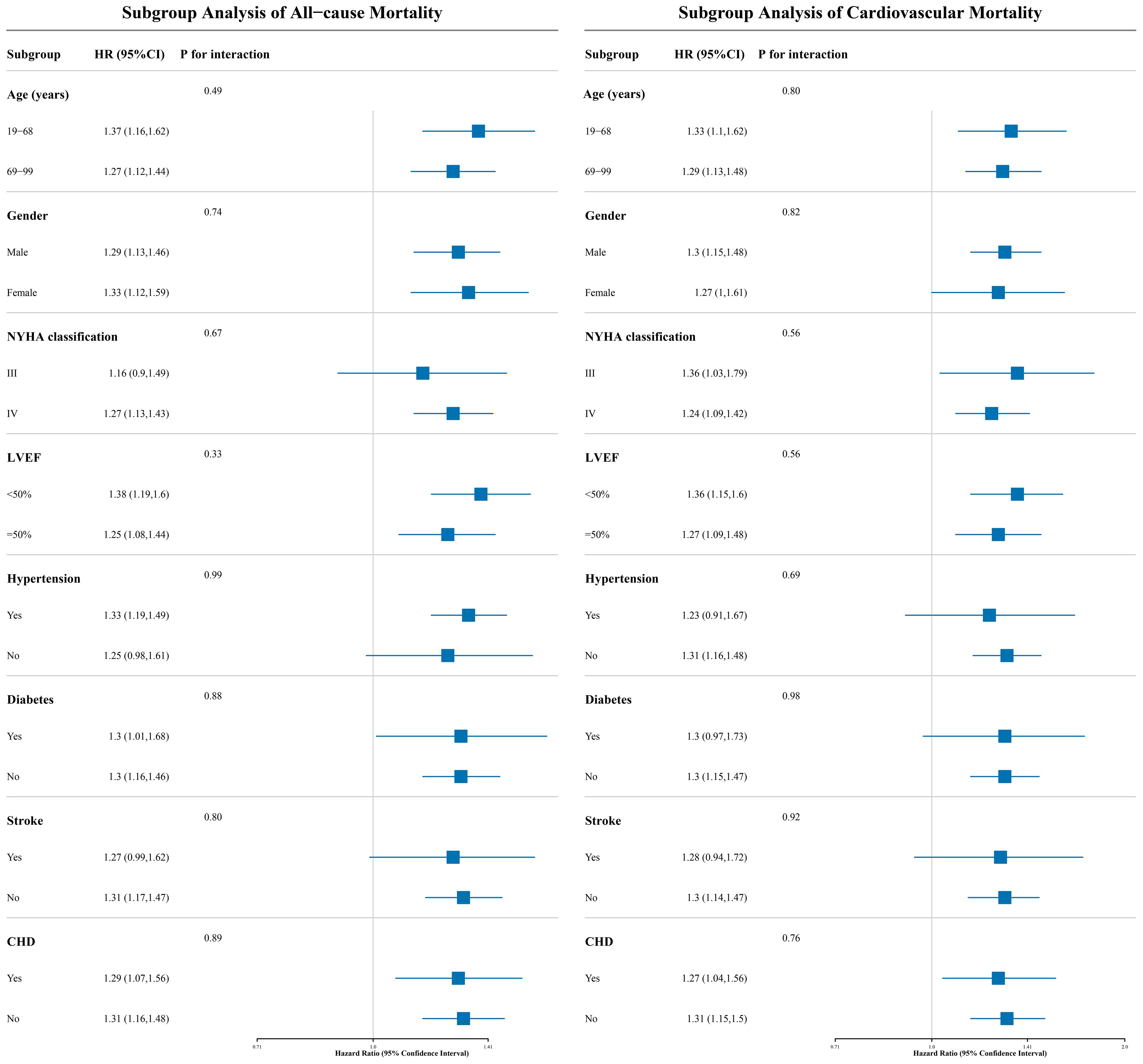
Figure 5. The forest plot shows the association between UHR and 30-day all-cause and cardiovascular mortality in ADHF patients across different subgroups. UHR, uric acid to high-density lipoprotein cholesterol ratio; ADHF, acute decompensated heart failure. Adjusted for gender, age, drinking status, smoking status, hypertension, diabetes, stroke, CHD, LVEF, WBC, RBC, PLT, AST, TG, LDL-C, FPG, NT-proBNP.
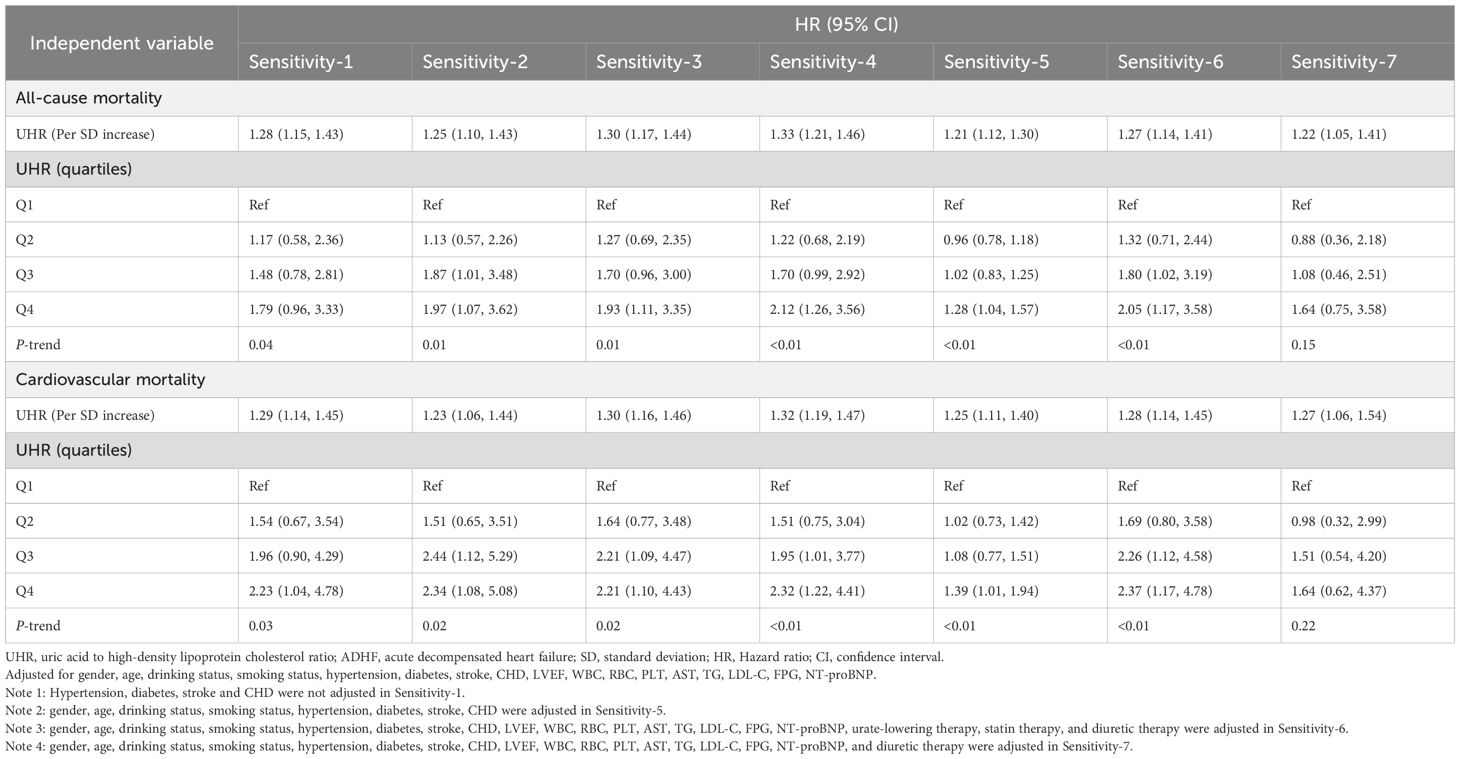
Sensitivity analysis
First, after excluding frail subgroup patients and re-analyzing the data, we observed results consistent with the primary analysis. Second, re-analysis after excluding participants who died within 48 hours of admission yielded findings aligned with the primary analysis. Third, further adjustment for the quadratic term of age did not significantly alter the results. Fourth, validation analysis in the imputed complete dataset confirmed the robustness of the findings (Table 4). Fifth, regarding UHR distribution, our study cohort and the U.S. HF cohort exhibited comparable distribution patterns (Supplementary Figure 2). In terms of association analysis, results from the U.S. HF cohort indicated a positive association between UHR and both all-cause mortality and cardiovascular mortality. These findings were consistent with the results of the present study. Sixth, after accounting for the potential influences of urate-lowering therapy, statin therapy, and diuretic therapy, the new findings remained consistent with the primary results (Sensitivity-6 and Sensitivity-7).
Discussion
In this retrospective cohort study of ADHF patients in Jiangxi, China, we demonstrated for the first time a linear positive association between UHR and short-term (30-day) all-cause and cardiovascular mortality. The robustness of these findings was validated across four dimensions: population heterogeneity, causal temporality, model adjustment, and data integrity.
UA is generated as the final product of purine metabolism and exhibits a dual role in oxidative stress, acting as both an antioxidant and a pro-oxidant (41–43). On one hand, UA functions as a potent antioxidant by donating hydrogen atoms to free radicals, thereby stabilizing them and preventing oxidative damage. Additionally, UA exerts cardiovascular protective effects by inhibiting superoxide radical-mediated degradation of nitric oxide, which promotes vasodilation (44–47). On the other hand, elevated UA levels can induce inflammation and activate the renin-angiotensin-aldosterone system, ultimately contributing to vascular endothelial dysfunction, oxidative stress, and various inflammatory responses (48–51). In recent years, due to significant dietary shifts and rising prevalence of unhealthy lifestyles, the incidence of hyperuricemia has gradually risen. According to statistics, approximately 170 million people in China suffer from hyperuricemia, posing a significant impact on cardiovascular health (52–54). HDL-C is a critical component of lipid metabolism and exhibits a complex relationship with cardiovascular events. Traditionally, the classical theory posited a significant inverse association between HDL-C levels and cardiovascular risk (55, 56). However, with advances in research, recent studies have raised doubts about the protective role of elevated HDL-C in cardiovascular outcomes (57, 58). For instance, in a multicenter prospective cohort study based on the United Kingdom Biobank and the Emory Cardiovascular Biorepository, Liu et al. observed a significant increase in all-cause and cardiovascular mortality among patients when HDL-C levels were ≥ 80 mg/dL (59). This phenomenon may be attributed to conformational and functional alterations of HDL-C at extremely high levels, leading to a loss of its anti-inflammatory and antioxidant properties under inflammatory or oxidative stress conditions (60). Additionally, a meta-analysis of 37 prospective cohort studies revealed a J-shaped association between HDL-C levels and all-cause mortality, with the lowest risk observed at HDL-C concentrations between 54–58 mg/dL (61). Notably, recent research has begun to focus on potential interactions between UA and HDL-C: studies indicate that the combined effect of elevated UA and HDL-C levels may increase mortality risk in cardiovascular populations (25). Therefore, further elucidating the synergistic relationship between UA and HDL-C could be essential for evaluating adverse prognostic outcomes.
As a composite indicator of UA and HDL-C, the UHR was first proposed by Kocak et al. as a metabolic assessment parameter. In their initial study, Kocak et al. demonstrated that UHR exhibits high accuracy in diagnosing metabolic syndrome (27). Subsequently, an increasing number of studies have identified UHR as a significant influencing factor in cardiovascular disease risk assessment, including hypertension, CHD, and HF (28–32). In terms of HF risk assessment, data from the National Health and Nutrition Examination Survey revealed that compared to the low UHR levels (Q1), high UHR levels (Q4) were associated with a 159% increased risk of chronic HF (32). In the current study, we further demonstrated a significant positive correlation between UHR and both all-cause and cardiovascular mortality in Chinese patients with ADHF, thereby expanding the research evidence on UHR’s role in HF prognosis. Collectively, UHR emerges as an important factor in both HF risk stratification and prognostic evaluation, suggesting that incorporating UHR into cardiovascular disease screening protocols may offer significant clinical benefits. It should be noted that UHR, in addition to its utility in assessing mortality risk among HF patients, also plays an important role in evaluating death risk across various chronic disease populations and middle-aged/elderly cohorts (62–66). Specifically, studies have demonstrated the following: Among the United States (US) adult populations, high UHR levels (Q4) were associated with a 16-36% increased risk of all-cause mortality and a 20-37% elevated risk of cardiovascular mortality during long-term follow-up (63, 64). In patients with diabetes or prediabetes, high UHR conferred a 24% higher all-cause mortality risk and a 34-56% increased cardiovascular mortality risk (65, 66). Among peritoneal dialysis patients, high UHR was linked to a 35% greater risk of all-cause death and a 46% higher cardiovascular mortality risk (65, 66). In the current study, we further demonstrated that among ADHF patients, high UHR levels were associated with an 88% increased risk of all-cause mortality and a 113% elevated risk of cardiovascular mortality. Compared to previous studies investigating UHR’s association with mortality across diverse populations (62–66), UHR appears to offer additional prognostic information specifically for ADHF patients, while also expanding the evidence base for its application in acute disease prognosis. Additionally, in the current study, we further explored the impact of multiple subgroup factors on the association between UHR and mortality outcomes. The results showed that no significant interaction effects were detected across all subgroups. This finding further suggests that UHR may have high generalizability for assessing adverse prognosis in ADHF patients in Jiangxi. Certainly, after subgroup stratification, the sample size for each stratum decreases relatively; this may also lead to potential false-negative results in the subgroup analyses in the current study, as detecting interaction effects typically requires a much larger sample size than detecting main effects. Interaction effects are assessed by comparing differences between effect sizes; such differences exhibit greater variability and require more data to stabilize the estimation (67, 68). A widely cited rule of thumb states that detecting interaction effects requires a sample size at least four times larger than that needed to detect main effects of comparable magnitude.
In the current study, we further demonstrated through RCS analysis that UHR exhibits a linear association with both all-cause mortality (P for non-linearity = 0.903) and cardiovascular mortality (P for non-linearity = 0.590) among ADHF patients. Notably, similar linear findings were reported in recent studies (32, 63): In a cross-sectional study conducted by Yi et al., a linear association was observed between UHR and chronic HF (P for non-linearity = 0.564) among U.S. adults. Additionally, among middle-aged and elderly U.S. populations, UHR demonstrated a linear association with cardiovascular mortality (P for non-linearity = 0.408). However, inconsistent findings were also reported in other UHR-related studies: Lai et al. observed a J-shaped association between UHR and both all-cause and cardiovascular mortality among U.S. patients with diabetes or prediabetes (65), with calculated threshold values of 13.73 and 9.39, respectively. In contrast, Huang et al. demonstrated a U-shaped relationship between UHR and mortality outcomes in U.S. diabetic patients (66), where excessively high or low UHR levels were associated with increased mortality risk. The discrepancies between our current findings and UHR-related prognostic outcomes in U.S. populations may be attributed to racial differences and disease heterogeneity, and further studies across diverse populations are warranted to validate these associations.
Adverse outcomes during hospitalization for ADHF patients have consistently been one of the most critical clinical challenges that require resolution. Current research evidence indicates that the UHR serves as an independent predictor of mortality prognosis in ADHF patients. From a computational perspective, UHR is influenced by UA and HDL-C levels; when UHR increases, the balance between these two biomarkers becomes disrupted. This imbalance leads to the accumulation of numerous detrimental factors, which may cause vascular damage and atherosclerosis (28–31), ultimately resulting in HF progression and even death (32). From a mechanistic perspective, elevated UA and low HDL-C share a common pathological basis in promoting the development of HF, primarily including oxidative damage and inflammatory damage to endothelial cells (19, 69–71). High levels of UA activate nicotinamide adenine dinucleotide phosphate oxidase and induce mitochondrial dysfunction, leading to a surge in reactive oxygen species (ROS). These ROS subsequently activate the nuclear factor-kappa B and activator protein-1 pathways, promote renin release and angiotensin-converting enzyme synthesis, thereby forming a ROS-RAS positive feedback loop that exacerbates oxidative stress, inflammation, and endothelial dysfunction (69, 72). HDL is a lipoprotein with multiple protective functions (18, 19); its reduced levels can lead to impaired reverse cholesterol transport, attenuated anti-inflammatory and antioxidant capacities, and endothelial dysfunction, thereby exacerbating cardiac dysfunction (70, 71, 73). Notably, under the stress and inflammatory state of HF exacerbation, the function of HDL particles may shift from protective to pathological; this significantly reduces their antioxidant capacity and exacerbates oxidative stress-induced damage to endothelial cells and cardiomyocytes. In addition, during HF, particularly in the acute decompensated phases, impaired cardiac pumping function reduces effective blood ejection, leading to fluid retention and volume overload. This condition can initiate or worsen renal dysfunction, which in turn causes abnormal metabolism/dysfunction of UA and HDL-C, thereby diminishing their cardiovascular protective effects (73–76). Overall, elevated UA and low HDL-C are not isolated risk factors for ADHF progression; they may act synergistically through multiple mechanisms—such as amplifying inflammation and oxidative stress, exacerbating endothelial dysfunction, and promoting myocardial remodeling and fibrosis—to collectively contribute to the deterioration of cardiac function and adverse outcomes in patients with ADHF.
Notably, researchers in recent reports have evaluated the prognostic benefits of urate-lowering therapy or HDL-C-elevating interventions in HF patients (77, 78). A United Kingdom clinical practice study demonstrated that HF patients receiving urate-lowering treatment (including allopurinol, febuxostat, probenecid, lesinurad, pegloticase, and lesinurad/allopurinol combination) exhibited a significantly reduced risk of adverse clinical outcomes within 5 years (77). Furthermore, results from a multicenter randomized trial in Japan showed that urate-lowering therapy significantly improved 3-year adverse prognosis in HF patients; in contrast, febuxostat demonstrated greater efficacy than allopurinol in treating HF patients with comorbid hyperuricemia (78). Regarding HDL-C-elevating therapy for HF, experimental studies have demonstrated that recombinant HDL infusion improves cardiac diastolic function and attenuates myocardial fibrosis in mice (79); moreover, recombinant HDL infusion also showed significant electrophysiological improvements in HF treatment: electrocardiographic results indicated that recombinant HDL infusion shortened the duration of ventricular electrical systole, manifested as a reduction in heart rate-corrected QT interval (80). Overall, HDL-targeted therapy exerts direct positive inotropic effects on the myocardium, inhibits the development of cardiac hypertrophy, suppresses interstitial and perivascular myocardial fibrosis, increases myocardial capillary density, and thereby inhibits the occurrence and progression of HF (81). These findings collectively demonstrate the significant role of UHR in assessing cardiovascular disease risk and prognosis. Targeting UA reduction or HDL-C elevation may improve clinical outcomes in ADHF patients. However, whether the use of urate-lowering medications or interventions to raise HDL-C provides prognostic benefits in ADHF remains to be quantitatively evaluated in future clinical trials. Such studies should assess the comprehensive benefits of different drug combinations and dosages in HF patients.
The results of this study have important implications for the clinical management of ADHF. The UHR index, due to its testing simplicity (using existing laboratory assays) and cost-effectiveness, is particularly suitable for widespread adoption across healthcare institutions at all levels. Based on empirical evidence, we recommend integrating UHR into the ADHF risk stratification system as a supplementary tool to optimize clinical decision-making. The recommended approach is as follows: By automatically classifying patients’ UHR levels, high-risk ADHF patients at risk of disease progression could be identified early, thereby optimizing intervention decisions. Furthermore, UHR could be integrated into existing risk scoring models and embedded into artificial intelligence-assisted decision-making systems to dynamically predict the probability of adverse mortality outcomes in patients.
Our study has several notable strengths: (1) This is the first report of a linear positive correlation between UHR and both all-cause and cardiovascular mortality in Chinese ADHF patients. These novel findings provide new insights into early risk stratification for ADHF patients. Although we have further obtained similar results based on a U.S. HF cohort, validation in more ethnically diverse cohorts remains necessary. Additionally, exploring improved risk stratification strategies by integrating established risk scores (e.g., ADHFRE, MAGGIC) is also essential. (2) UHR is calculated using UA and HDL-C, both of which are routinely measured in standard health check-ups and easily accessible. The findings of this study therefore hold substantial practical value. Several limitations of this study need to be explained: (1) All study participants were recruited from Jiangxi Province, China, which may limit the generalizability of our findings due to the limited geographic and ethnic diversity of the sample. (2) Dynamic monitoring data of UHR were lacking in the current study, making it difficult to evaluate its temporal variation characteristics and predictive value as a short-term prognostic biomarker. (3) Although the analysis revealed a significant correlation between UHR levels and mortality risk in ADHF patients, the observational design of this study limits the findings to suggesting a potential association rather than establishing a definitive causal relationship. This limitation underscores the need for future mechanistic studies or randomized controlled trials to further validate these results. (4) Due to the retrospective design of this study, some critical disease-related indicators were not fully recorded (e.g., nutritional status, inflammatory markers like C-reactive protein/Interleukin 6). Although we implemented rigorous control of confounding factors through methods such as multivariable adjustment in statistical analyses, residual confounding from unmeasured variables may persist due to limitations of the study design, potentially influencing the study conclusions.
Conclusion
In this retrospective cohort study, we first demonstrated that UHR could be utilized for the early assessment of short-term all-cause and cardiovascular mortality risk in ADHF patients, and that a high UHR was associated with an increased short-term all-cause and cardiovascular mortality risk in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Jiangxi Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZL: Investigation, Methodology, Software, Validation, Writing – original draft. GJ: Investigation, Validation, Writing – original draft. KJ: Investigation, Validation, Writing – original draft. SH: Investigation, Software, Writing – review & editing. ZW: Investigation, Writing – review & editing. JW: Investigation, Writing – review & editing. HL: Investigation, Writing – review & editing. GS: Formal Analysis, Investigation, Supervision, Writing – review & editing. YZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing. SZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82460091), the Natural Science Foundation of Jiangxi Province (20232BAB216004), Jiangxi Province Traditional Chinese Medicine Science and Technology Plan Project (2023B1218); and the Science and technology project of Health Commission of Jiangxi Province (202410011 and 202510148).
Acknowledgments
This study was made possible through the invaluable support from Jiangxi Provincial People’s Hospital. We are deeply grateful to all investigators of the Jiangxi-ADHF research consortium for their dedicated efforts in data acquisition. Special thanks are extended to the Home for Researchers team for their professional graphic abstract preparation services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1667929/full#supplementary-material
References
1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2022) 24:4–131. doi: 10.1002/ejhf.2333
2. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, et al. Acute heart failure. Nat Rev Dis Primers. (2020) 6:16. doi: 10.1038/s41572-020-0151-7
3. Lala A, Hamo CE, Bozkurt B, Fiuzat M, Blumer V, Bukhoff D, et al. Standardized definitions for evaluation of acute decompensated heart failure therapies: HF-ARC expert panel paper. JACC Heart Fail. (2024) 12:1–15. doi: 10.1016/j.jchf.2023.09.030
4. Chinese Society of Cardiology, Chinese Medical Association, Chinese College of Cardiovascular Physician, Chinese Heart Failure Association of Chinese Medical Doctor Association, and Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2024. Zhonghua Xin Xue Guan Bing Za Zhi. (2024) 52:235–75. doi: 10.3760/cma.j.cn112148-20231101-00405
5. Chioncel O, Mebazaa A, Maggioni AP, Harjola VP, Rosano G, Laroche C, et al. Acute heart failure congestion and perfusion status - impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. (2019) 21:1338–52. doi: 10.1002/ejhf.1492
6. Raj L, Maidman SD, and Adhyaru BB. Inpatient management of acute decompensated heart failure. Postgrad Med J. (2020) 96:33–42. doi: 10.1136/postgradmedj-2019-136742
7. Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. (2009) 2:407–13. doi: 10.1161/CIRCOUTCOMES.109.883256
8. Huang G, Qin J, Deng X, Luo G, Yu D, Zhang M, et al. Prognostic value of serum uric acid in patients with acute heart failure: A meta-analysis. Med (Baltimore). (2019) 98:e14525. doi: 10.1097/MD.0000000000014525
9. Okazaki H, Shirakabe A, Kobayashi N, Hata N, Shinada T, Matsushita M, et al. The prognostic impact of uric acid in patients with severely decompensated acute heart failure. J Cardiol. (2016) 68:384–91. doi: 10.1016/j.jjcc.2016.04.013
10. Fujihashi T, Sakata Y, Nochioka K, Miura M, Abe R, Kasahara S, et al. Prognostic impacts of serum uric acid levels in patients with chronic heart failure: insights from the CHART-2 study. ESC Heart Fail. (2021) 8:1027–38. doi: 10.1002/ehf2.12765
11. Hamaguchi S, Furumoto T, Tsuchihashi-Makaya M, Goto K, Goto D, Yokota T, et al. Hyperuricemia predicts adverse outcomes in patients with heart failure. Int J Cardiol. (2011) 151:143–7. doi: 10.1016/j.ijcard.2010.05.002
12. Huang H, Huang B, Li Y, Huang Y, Li J, Yao H, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. (2014) 16:15–24. doi: 10.1093/eurjhf/hft132
13. Coiro S, Carluccio E, Biagioli P, Alunni G, Murrone A, D’Antonio A, et al. Elevated serum uric acid concentration at discharge confers additive prognostic value in elderly patients with acute heart failure. Nutr Metab Cardiovasc Dis. (2018) 28:361–8. doi: 10.1016/j.numecd.2017.12.009
14. Carluccio E, Coiro S, and Ambrosio G. Unraveling the relationship between serum uric acid levels and cardiovascular risk. Int J Cardiol. (2018) 253:174–5. doi: 10.1016/j.ijcard.2017.11.035
15. Rothblat GH and Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. (2010) 21:229–38. doi: 10.1097/mol.0b013e328338472d
16. Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. (2011) 364:127–35. doi: 10.1056/NEJMoa1001689
17. Jia C, Anderson JLC, Gruppen EG, Lei Y, Bakker SJL, Dullaart RPF, et al. High-density lipoprotein anti-inflammatory capacity and incident cardiovascular events. Circulation. (2021) 143:1935–45. doi: 10.1161/CIRCULATIONAHA.120.050808
18. Van Linthout S, Frias M, Singh N, and De Geest B. Therapeutic potential of HDL in cardioprotection and tissue repair. Handb Exp Pharmacol. (2015) 224:527–65. doi: 10.1007/978-3-319-09665-0_17
19. Nagao M, Nakajima H, Toh R, Hirata KI, and Ishida T. Cardioprotective effects of high-density lipoprotein beyond its anti-atherogenic action. J Atheroscler Thromb. (2018) 25:985–93. doi: 10.5551/jat.RV17025
20. deGoma EM, deGoma RL, and Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. (2008) 51:2199–211. doi: 10.1016/j.jacc.2008.03.016
21. Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, and Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation. (2009) 120:2345–51. doi: 10.1161/CIRCULATIONAHA.109.830984
22. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail. (2017) 19:627–34. doi: 10.1002/ejhf.785
23. Mehra MR, Uber PA, Lavie CJ, Milani RV, Park MH, and Ventura HO. High-density lipoprotein cholesterol levels and prognosis in advanced heart failure. J Heart Lung Transplant. (2009) 28:876–80. doi: 10.1016/j.healun.2009.04.026
24. Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective Am Stud Circulation. (1989) 79:8–15. doi: 10.1161/01.cir.79.1.8
25. Palatini P, Virdis A, Masi S, Mengozzi A, Casiglia E, Tikhonoff V, et al. Hyperuricemia increases the risk of cardiovascular mortality associated with very high HdL-cholesterol level. Nutr Metab Cardiovasc Dis. (2023) 33:323–30. doi: 10.1016/j.numecd.2022.11.024
26. Onat A, Can G, Örnek E, Altay S, Yüksel M, and Ademoğlu E. Elevated serum uric acid in nondiabetic people mark pro-inflammatory state and HDL dysfunction and independently predicts coronary disease. Clin Rheumatol. (2013) 32:1767–75. doi: 10.1007/s10067-013-2339-7
27. Kocak MZ, Aktas G, Erkus E, Sincer I, Atak B, and Duman T. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras. (1992) . 2019:65. doi: 10.1590/1806-9282.65.1.9
28. Park B, Jung DH, and Lee YJ. Predictive value of serum uric acid to HDL cholesterol ratio for incident ischemic heart disease in non-diabetic koreans. Biomedicines. (2022) 10:1422. doi: 10.3390/biomedicines10061422
29. Ahari RK, Sahranavard T, Mansoori A, Fallahi Z, Babaeepoor N, Ferns G, et al. Association of atherosclerosis indices, serum uric acid to high-density lipoprotein cholesterol ratio and triglycerides-glucose index with hypertension: A gender-disaggregated analysis. J Clin Hypertens (Greenwich). (2024) 26:645–55. doi: 10.1111/jch.14829
30. Aktas G, Khalid A, Kurtkulagi O, Duman TT, Bilgin S, Kahveci G, et al. Poorly controlled hypertension is associated with elevated serum uric acid to HDL-cholesterol ratio: a cross-sectional cohort study. Postgrad Med. (2022) 134:297–302. doi: 10.1080/00325481.2022.2039007
31. Yang Y, Zhang J, Jia L, Su J, Ma M, and Lin X. The interaction between uric acid and high-density lipoprotein cholesterol on the prognosis of patients with acute myocardial infarction. Front Cardiovasc Med. (2023) 10:1226108. doi: 10.3389/fcvm.2023.1226108
32. Yi Y, Luo Q, Chen J, Chen Z, Aydemir HA, Chen P, et al. Association between the uric acid-to-HDL-cholesterol ratio (UHR) and the risk of cardiovascular disease and dyslipidemia: a population-based study. Lipids Health Dis. (2025) 24:143. doi: 10.1186/s12944-025-02551-4
33. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of america. J Am Coll Cardiol. (2017) 70:776–803. doi: 10.1016/j.jacc.2017.04.025
34. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:e263–421. doi: 10.1016/j.jacc.2021.12.012
35. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
36. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
37. Available online at: https://www.nhc.gov.cn/wjw/c100221/202201/558653bd3d1e4a078904152a30605a03.shtml (Accessed September 10, 2025).
38. Harrell FE. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2nd Vol. 582. . Cham, Switzerland: Springer (2015).
39. Fried LP, Ferrucci L, Darer J, Williamson JD, and Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. (2004) 59:255–63. doi: 10.1093/gerona/59.3.m255
40. Zhang YB, Chen C, Pan XF, Guo J, Li Y, Franco OH, et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ. (2021) 373:n604. doi: 10.1136/bmj.n604
41. Ames BN, Cathcart R, Schwiers E, and Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. (1981) 78:6858–62. doi: 10.1073/pnas.78.11.6858
42. Allegrini S, Garcia-Gil M, Pesi R, Camici M, and Tozzi MG. The good, the bad and the new about uric acid in cancer. Cancers (Basel). (2022) 14:4959. doi: 10.3390/cancers14194959
43. Sautin YY and Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. (2008) 27:608–19. doi: 10.1080/15257770802138558
44. Gherghina ME, Peride I, Tiglis M, Neagu TP, Niculae A, and Checherita IA. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int J Mol Sci. (2022) 23:3188. doi: 10.3390/ijms23063188
45. Agnoletti D, Cicero AFG, and Borghi C. The impact of uric acid and hyperuricemia on cardiovascular and renal systems. Cardiol Clin. (2021) 39:365–76. doi: 10.1016/j.ccl.2021.04.009
46. Glantzounis GK, Tsimoyiannis EC, Kappas AM, and Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. (2005) 11:4145–51. doi: 10.2174/138161205774913255
47. Du L, Zong Y, Li H, Wang Q, Xie L, Yang B, et al. Hyperuricemia and its related diseases: mechanisms and advances in therapy. Signal Transduct Target Ther. (2024) 9:212. doi: 10.1038/s41392-024-01916-y
48. Yu MA, Sánchez-Lozada LG, Johnson RJ, and Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. (2010) 28:1234–42. doi: 10.1097/HJH.0b013e328337da1d
49. Kato M, Hisatome I, Tomikura Y, Kotani K, Kinugawa T, Ogino K, et al. Status of endothelial dependent vasodilation in patients with hyperuricemia. Am J Cardiol. (2005) 96:1576–8. doi: 10.1016/j.amjcard.2005.07.068
50. Ma P, Zhao M, Li Y, Zhang G, Ma Y, Shi Y, et al. The protective effects of uric acid against myocardial ischemia via the Nrf2 pathway. Eur J Pharmacol. (2023) 959:176062. doi: 10.1016/j.ejphar.2023.176062
51. Zha X, Yang B, Xia G, and Wang S. Combination of uric acid and pro-inflammatory cytokines in discriminating patients with gout from healthy controls. J Inflammation Res. (2022) 15:1413–20. doi: 10.2147/JIR.S357159
52. Song Z, Deng D, and Wu H. Association of serum uric acid to all-cause and cardiovascular mortality in patients with cardiovascular disease. Sci Rep. (2024) 14:21808. doi: 10.1038/s41598-024-72527-4
53. Sharaf El Din UAA, Salem MM, and Abdulazim DO. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J Adv Res. (2017) 8:537–48. doi: 10.1016/j.jare.2016.11.004
54. Yu W and Cheng JD. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharmacol. (2020) 11:582680. doi: 10.3389/fphar.2020.582680
55. Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. (1990) 322:1700–7. doi: 10.1056/NEJM199006143222403
56. Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, KK R, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. (2009) 302:1993–2000. doi: 10.1001/jama.2009.1619
57. Trimarco V, Izzo R, Morisco C, Mone P, Virginia Manzi M, Falco A, et al. (High-density lipoprotein) cholesterol increases cardiovascular risk in hypertensive patients. Hypertension. (2022) 79:2355–63. doi: 10.1161/HYPERTENSIONAHA.122.19912
58. Madsen CM, Varbo A, and Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. (2017) 38:2478–86. doi: 10.1093/eurheartj/ehx163
59. Liu C, Dhindsa D, Almuwaqqat Z, Ko YA, Mehta A, Alkhoder AA, et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol. (2022) 7:672–80. doi: 10.1001/jamacardio.2022.0912
60. Barter PJ and Rye KA. HDL cholesterol concentration or HDL function: which matters? Eur Heart J. (2017) 38:2487–9. doi: 10.1093/eurheartj/ehx274
61. Zhong GC, Huang SQ, Peng Y, Wan L, Wu YQ, Hu TY, et al. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: a pooled analysis of 37 prospective cohort studies. Eur J Prev Cardiol. (2020) 27:1187–203. doi: 10.1177/2047487320914756
62. Liu R, Peng Y, Wu H, Diao X, Ye H, Huang X, et al. Uric acid to high-density lipoprotein cholesterol ratio predicts cardiovascular mortality in patients on peritoneal dialysis. Nutr Metab Cardiovasc Dis. (2021) 31:561–9. doi: 10.1016/j.numecd.2020.10.005
63. Cui Y and Zhang W. Long-term cardiovascular risk and mortality associated with uric acid to HDL-C ratio: a 20-year cohort study in adults over 40. Sci Rep. (2025) 15:14242. doi: 10.1038/s41598-025-99205-3
64. Li Z, Liu Q, and Yao Z. The serum uric acid-to-high-density lipoprotein cholesterol ratio is a predictor for all-cause and cardiovascular disease mortality: a cross-sectional study. Front Endocrinol (Lausanne). (2024) 15:1417485. doi: 10.3389/fendo.2024.1417485
65. Lai X and Chen T. Association of serum uric acid to high-density lipoprotein cholesterol ratio with all-cause and cardiovascular mortality in patients with diabetes or prediabetes: a prospective cohort study. Front Endocrinol (Lausanne). (2024) 15:1476336. doi: 10.3389/fendo.2024.1476336
66. Huang X, Hu L, Li J, and Wang X. U-shaped association of uric acid to HDL cholesterol ratio (UHR) with ALL-cause and cardiovascular mortality in diabetic patients: NHANES 1999-2018. BMC Cardiovasc Disord. (2024) 24:744. doi: 10.1186/s12872-024-04436-3
67. Smith PG and Day NE. The design of case-control studies: the influence of confounding and interaction effects. Int J Epidemiol. (1984) 13:356–65. doi: 10.1093/ije/13.3.356
68. Kerin M and Marchini J. Inferring gene-by-environment interactions with a bayesian whole-genome regression model. Am J Hum Genet. (2020) 107:698–713. doi: 10.1016/j.ajhg.2020.08.009
69. Feig DI, Kang DH, and Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. (2008) 359:1811–21. doi: 10.1056/NEJMra0800885
70. Rosenson RS, Brewer HB Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. (2016) 13:48–60. doi: 10.1038/nrcardio.2015.124
71. Tabet F and Rye KA. High-density lipoproteins, inflammation and oxidative stress. Clin Sci (Lond). (2009) 116:87–98. doi: 10.1042/CS20080106
72. Zhou H, Yang J, Yuan X, Song X, Zhang X, Cao T, et al. Hyperuricemia research progress in model construction and traditional Chinese medicine interventions. Front Pharmacol. (2024) 15:1294755. doi: 10.3389/fphar.2024.1294755
73. Xing L, Liu Y, Wang J, Tian P, and Liu P. High-density lipoprotein and heart failure. Rev Cardiovasc Med. (2023) 24:321. doi: 10.31083/j.rcm2411321
74. Tang HY, Huang JE, Tsau MT, Chang CJ, Tung YC, Lin G, et al. Metabolomics assessment of volume overload-induced heart failure and oxidative stress in the kidney. Metabolites. (2023) 13:1165. doi: 10.3390/metabo13111165
75. Martinelli AEM, Maranhão RC, Carvalho PO, Freitas FR, Silva BMO, Curiati MNC, et al. Cholesteryl ester transfer protein (CETP), HDL capacity of receiving cholesterol and status of inflammatory cytokines in patients with severe heart failure. Lipids Health Dis. (2018) 17:242. doi: 10.1186/s12944-018-0888-0
76. Emmens JE, Jia C, Ng LL, van Veldhuisen DJ, Dickstein K, Anker SD, et al. Impaired high-density lipoprotein function in patients with heart failure. J Am Heart Assoc. (2021) 10:e019123. doi: 10.1161/JAHA.120.019123
77. Kiddle SJ, Sundell KA, Perl S, Nolan S, and Bjursell M. Urate-lowering therapy in patients with hyperuricemia and heart failure: A retrospective cohort study using the UK Clinical Practice Research Datalink. Clin Cardiol. (2024) 47:e24297. doi: 10.1002/clc.24297
78. Suzuki S, Yoshihisa A, Yokokawa T, Kobayashi A, Yamaki T, Kunii H, et al. Comparison between febuxostat and allopurinol uric acid-lowering therapy in patients with chronic heart failure and hyperuricemia: a multicenter randomized controlled trial. J Int Med Res. (2021) 49:3000605211062770. doi: 10.1177/03000605211062770
79. Aboumsallem JP, Mishra M, Amin R, Muthuramu I, Kempen H, and De Geest B. Successful treatment of established heart failure in mice with recombinant HDL (Milano). Br J Pharmacol. (2018) 175:4167–82. doi: 10.1111/bph.14463
80. Aboumsallem JP, Muthuramu I, Mishra M, Kempen H, and De Geest B. Effective treatment of diabetic cardiomyopathy and heart failure with reconstituted HDL (Milano) in mice. Int J Mol Sci. (2019) 20:1273. doi: 10.3390/ijms20061273
Keywords: uric acid to high-density lipoprotein cholesterol ratio, heart failure, acute decompensated heart failure, uric acid, high-density lipoprotein cholesterol
Citation: Lu Z, Jian G, Jiang K, He S, Wang Z, Wang J, Lan H, Sheng G, Zou Y and Zhang S (2025) Impact of serum uric acid to high-density lipoprotein cholesterol ratio on short-term outcomes in acute decompensated heart failure: a cohort study in Jiangxi Province, China. Front. Endocrinol. 16:1667929. doi: 10.3389/fendo.2025.1667929
Received: 17 July 2025; Accepted: 22 September 2025;
Published: 07 October 2025.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Stefano Coiro, Hospital of Santa Maria della Misericordia in Perugia, ItalyBerwin Singh Swami Vetha, East Carolina University, United States
Rashu Barua, New York University, United States
Copyright © 2025 Lu, Jian, Jiang, He, Wang, Wang, Lan, Sheng, Zou and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhua Zhang, enNoMTIyOEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Zihao Lu1,2†
Zihao Lu1,2† Guoan Jian
Guoan Jian Kun Jiang
Kun Jiang Shiming He
Shiming He Guotai Sheng
Guotai Sheng Yang Zou
Yang Zou Shuhua Zhang
Shuhua Zhang