- 1School of Medicine, Xiamen University, Xiamen, Fujian, China
- 2National Institute for Data Science in Health and Medicine, School of Medicine, Xiamen University, Xiamen, Fujian, China
- 3Department of Minimally Invasive and Interventional Oncology, Zhongshan Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, Fujian, China
Purpose: The primary objective of this study is to systematically monitor and comprehensively characterize adverse events (AEs) associated with methimazole, which is utilized in the treatment of hyperthyroidism.
Method: A comprehensive analysis of the US FDA Adverse Event Reporting System (FAERS) database was conducted, covering data from the first quarter of 2004 to the first quarter of 2025. Multiple signal detection algorithms, including the ROR, PRR, BCPNN, and EBGM, were employed for conduct disproportionality analysis to efficiently mine the data and accurately identify signals associated with AEs related to methimazole.
Result: After analyzing 1,908 patient cases with 6,449 reported AEs linked to methimazole, the study confirmed AEs like agranulocytosis, pyrexia, hypothyroidism, and drug-induced liver injury aligning with the drug’s package insert. Interestingly, several previously unreported AEs, such as premature baby, polyarthritis, pleural effusion, septic shock, cholestasis and jaundice cholestatic were identified. The findings indicate potential unrecognized AEs and highlight the importance of continued pharmacovigilance and rigorous drug safety surveillance. The median onset time of methimazole related AEs was 27 days (interquartile range [IQR]: 5–58 days), indicating that the majority of cases occurred early after initiating use methimazole.
Conclusion: This study confirms that agranulocytosis, pyrexia, exposure during pregnancy, and hyperthyroidism are AEs associated with methimazole use. It also identifies new safety signals, including premature infants, polyarthritis, pleural effusion, septic shock, cholestasis, and cholestatic jaundice, that warrant further investigation. These findings support deeper analysis of the relationship between methimazole and adverse events and may help improve patient safety and clinical outcomes.
1 Introduction
Hyperthyroidism, commonly known as “thyroid toxicity”, is a complex endocrine disorder characterized by excessive secretion of thyroid hormones, leading to an abnormally elevated metabolic rate (1). The global prevalence of this condition ranges from 0.2% to 1.3%, with the condition affecting individuals of all age groups but occurring more frequently in women between 20 and 50 years of age (2). The etiology of hyperthyroidism is heterogeneous, and its clinical manifestations vary widely (3). Its main characteristic is that excessive thyroid hormones (mainly T3 and T4) synthesized and secreted by the thyroid gland enter the bloodstream, resulting in a significant increase in the metabolic level of body tissues. This leads to an accelerated metabolism of the body and triggers a series of clinical symptoms (4–8). Among the various causes leading to hyperthyroidism, Graves’ disease accounts for the majority of cases. Global statistics show that the prevalence of Graves’ disease among women is approximately 2%, and among men it is about 0.5%. Patients typically exhibit elevated levels of triiodothyronine (T3) and/or free thyroxine (FT4), which affects approximately 0.2% to 1.4% of the global population. Additionally, there is a condition known as subclinical hyperthyroidism, characterized by a lower concentration of thyroid stimulating hormone (TSH), while the levels of T3 and FT4 remain within the normal range (9, 10). Unlike overt hyperthyroidism, the clinical symptoms of subclinical hyperthyroidism are often atypical or lack obvious manifestations. Patients are usually discovered incidentally during routine physical examinations or when seeking treatment for other diseases. Although its clinical manifestations are concealed, multiple studies have suggested that the continuous inhibition of TSH is closely related to an increased risk of cardiovascular events, atrial fibrillation, osteoporosis, and an increased incidence of fractures, especially in the elderly population (11–13). Hyperthyroidism not only has a multifaceted negative impact on patients’ physical health but can also lead to a range of serious complications. Therefore, accurate diagnosis and the formulation of individualized treatment strategies are essential for improving patient outcomes, with early identification and timely intervention being particularly critical. Therefore, the early identification and intervention of subclinical hyperthyroidism has become one of the focuses of attention in the field of endocrinology. As an anti-thyroid drug widely used in the current market, methimazole has received a lot of research attention. In recent years, studies on the clinical efficacy and safety of methimazole, especially the detection of adverse events, have become an important direction in the evaluation of drug safety. This study, based on real-world data, conducted a systematic analysis of adverse events related to the use of methimazole. It also integrated four algorithms. The research results are helpful for a comprehensive assessment of its safety characteristics and provide a scientific basis for the formulation and management of individualized clinical medication strategies.
The primary mechanism of action of methimazole is to inhibit thyroid peroxidase (TPO), which is a rate-limiting enzyme that plays a crucial role in the synthesis of thyroid hormones and can regulate the synthesis of thyroid hormones production (14). TPO catalyzes the iodination of tyrosine residues on the thyroid globulin and the subsequent coupling reactions between these tyrosine residues. These processes are crucial for the production of thyroid hormones. Methimazole exerts an anti-thyroid hormone synthesis effect by binding to TPO, hindering the iodination and coupling reactions of tyrosine residues, thereby reducing the production of thyroid hormones and exerting an anti-thyroid effect (15).
Methimazole is a common anti-thyroid drug. It blocks the making of thyroid hormones. This can help reduce bone loss caused by thyrotoxicosis. But the drug can cause side effects. In a Danish study at many centers (n=208; average treatment 22 months; range 0.5–49 months), 10% of patients had drug-related side effects. Seventy-five percent happened in the first 6 months. By 24 months, when the dose was 5 mg/day, no new side effects were seen in that group. The most common side effects were skin problems (68%) (16). A clinic study in Brazil found that many adults taking methimazole had a positive ANCA blood test (17).These results show that close follow-up is needed early in treatment, especially in the first 6 months. Watch for common problems such as skin reactions.
These findings show that close monitoring is needed, especially in the first 6 months. Watch for common side effects, such as skin problems. The FDA Adverse Event Reporting System (FAERS) is one of the world’s largest pharmacovigilance databases for adverse drug events, providing a wealth of data for the study of drug safety (18–21). We analyzed the data in the FAERS database using four algorithms, namely ROR (22), PRR (23), BCPNN (24) and MGPS. The PTs that met the criteria of all four algorithms were included in our subsequent study. By leveraging the extensive data resources of the FAERS database and the precise analytical capabilities of signal detection techniques, we are able to conduct a comprehensive and in-depth evaluation of the AEs associated with methimazole. This provides clinical physicians with detailed safety information for reference when using this drug. This helps clinical physicians make more accurate decisions when weighing the efficacy and safety of drugs, reduces the risk of adverse drug events, ensures patient medication safety, and thereby enhances the overall effectiveness and quality of clinical treatment, promoting the continuous improvement of rational drug use in clinical practice.
2 Methods
2.1 Data collection
The FAERS is a publicly accessible database that primarily supports post-marketing surveillance programs for drugs and therapeutic biologics. It covers all adverse event and medication error information directly obtained from patients and healthcare practitioners. In this study, the real-world data related to AEs of methimazole from the first quarter of 2004 to the first quarter of 2025 were downloaded as raw ASCII data packages and imported into SAS 9.4 software for data mining and statistical analysis. After data download, data cleaning was performed, including removing duplicates, deleting missing data, and excluding withdrawn or deleted reports. The adverse event names in the FAERS database were coded using the latest MedDRA dictionary (MedDRA 28.0). According to the hierarchical structure of MedDRA 28.0 terminology, significant AEs were mapped to PT and System Organ Classes (SOC). Based on data from the FAERS database, we conducted differential and subgroup analyses to provide a comprehensive assessment of the distribution and characteristics of methimazole-associated AEs across population groups and clinical strata.
2.2 Statistical analysis
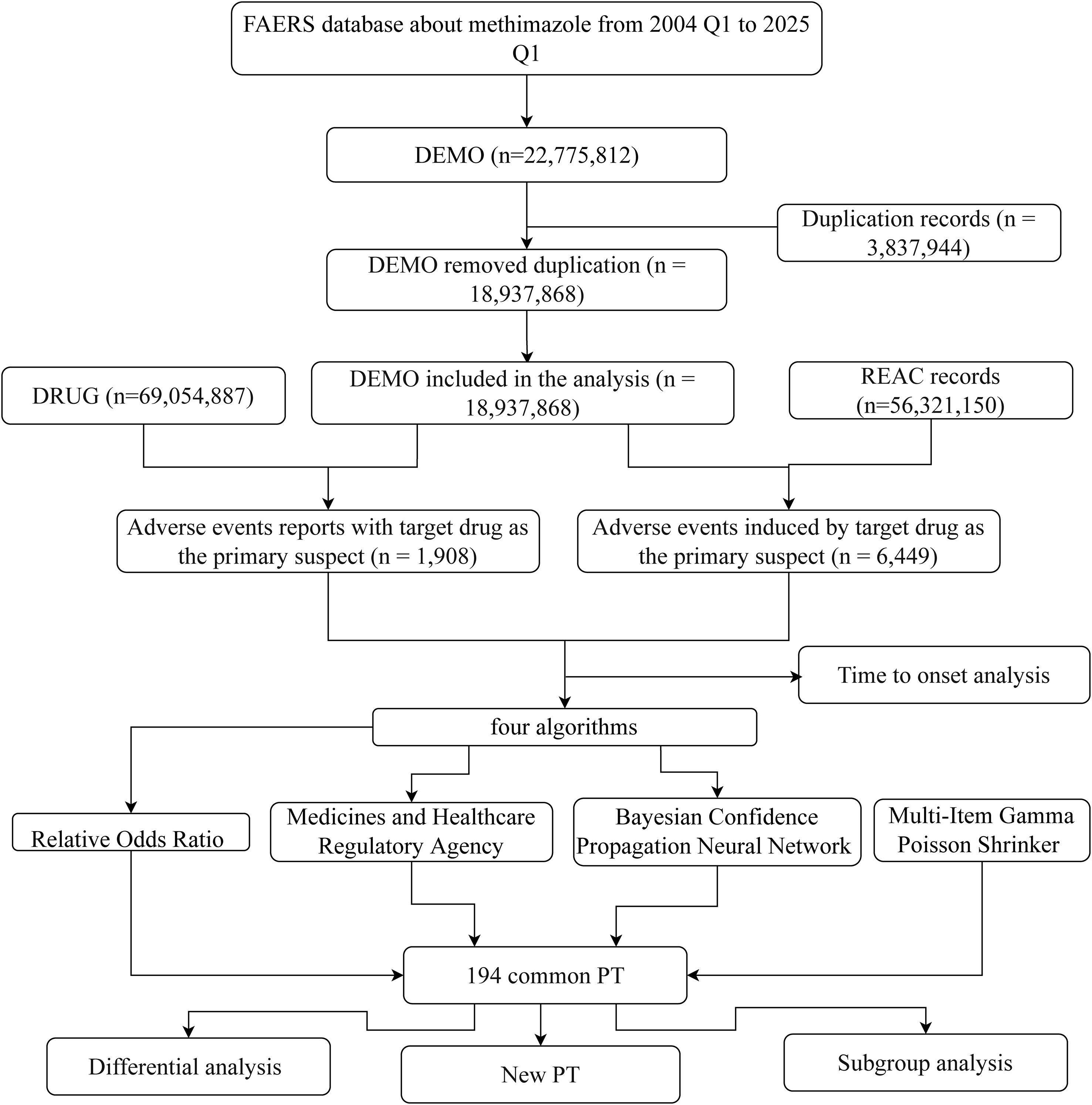
Patients may experience AEs after using methimazole. To look for possible links between methimazole and these AEs, we used four disproportionality methods (25): ROR, PRR, BCPNN, and MGPS. To identify potential safety signals, we applied four disproportionality methods. The Reporting Odds Ratio (ROR) measures how often a specific adverse event (AE) is reported with methimazole compared to other drugs, while the Proportional Reporting Ratio (PRR) assesses the proportion of methimazole-related reports that include a given AE relative to other drugs. In addition, two Bayesian approaches were used: the Bayesian Confidence Propagation Neural Network (BCPNN), which calculates an Information Component (IC) to quantify the strength of drug–AE associations, and the Empirical Bayes Geometric Mean (EBGM), which adjusts for small sample sizes to provide more stable estimates and avoid overestimating rare events. With four carefully selected methods, we systematically compared the adverse event reporting rates of methimazole in the FAERS database. These methods quantified the signal strength through the standardized formulas and thresholds detailed in Supplementary Table 1, providing highly convincing evidence for the safety assessment of methimazole and ensuring the scientific and reliable nature of the assessment results. The flowchart of this study is presented in Figure 1.
3 Result
3.1 Demographic statistics of related to methimazole in the FAERS database
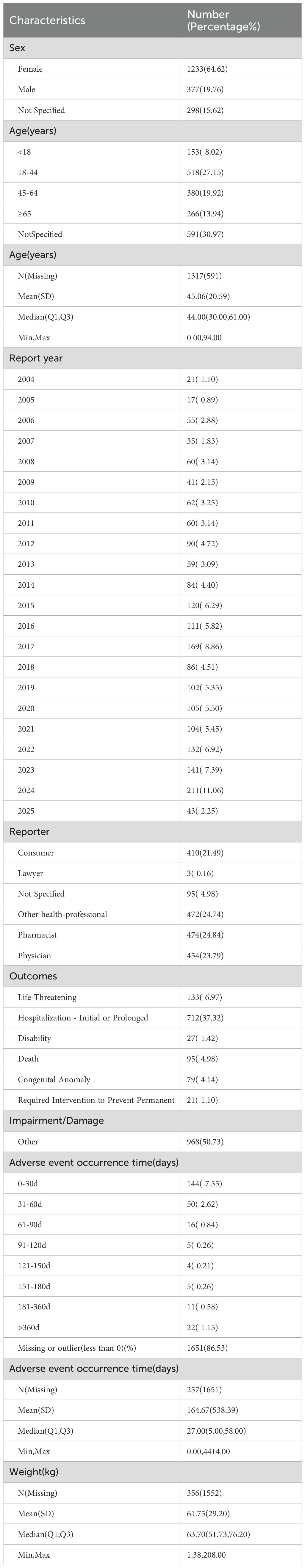
Interrogation of the FAERS database identified 1,908 patients reporting methimazole-associated AEs, accounting for 6,449 individual events overall. Each patient experienced an average of 2.4 AEs. The gender distribution analysis revealed that the proportion of AEs in females (64.62%, n = 1,233), whereas males accounted for 19.76% (n=377) (Figure 2A). The ratio of the female population number to the male population number is 3.27. Geographically, reports originating from North America (n = 1,215; 63.68%) and Asia (n = 299; 15.67%) accounted for -a substantially greater number of AEs compared with other continents (Figure 2B). Age-stratified analysis showed that, excluding patients with unknown age, the largest proportion of AEs occurred in the 18–44-year age group (27.15%, n = 518), followed by the 45–64-year group (19.92%, n = 380), those ≥65 years (13.94%, n = 266), and patients younger than 18 years (8.02%, n = 153). The overall mean age was 45.06 ± 20.59 years (Figure 2C). Temporal trends indicated that the number of reported AEs peaked in 2024 (n = 211), with the second highest in 2017 (n = 169, 8.86%). Overall, the annual trend showed an initial increase, followed by a decline, and then a renewed increase (Figure 2D). In the stratified analysis by reporter type, pharmacists accounted for the largest proportion of AE reports (24.84%, n = 474), whereas lawyers contributed the smallest fraction (Figure 2E). Regarding outcomes, the most frequent category was “other” (50.73%, n = 968), followed by hospitalization (initial or prolonged) (37.32%, n = 712) and life-threatening events (6.97%, n = 133) (Figure 2F) (Table 1).
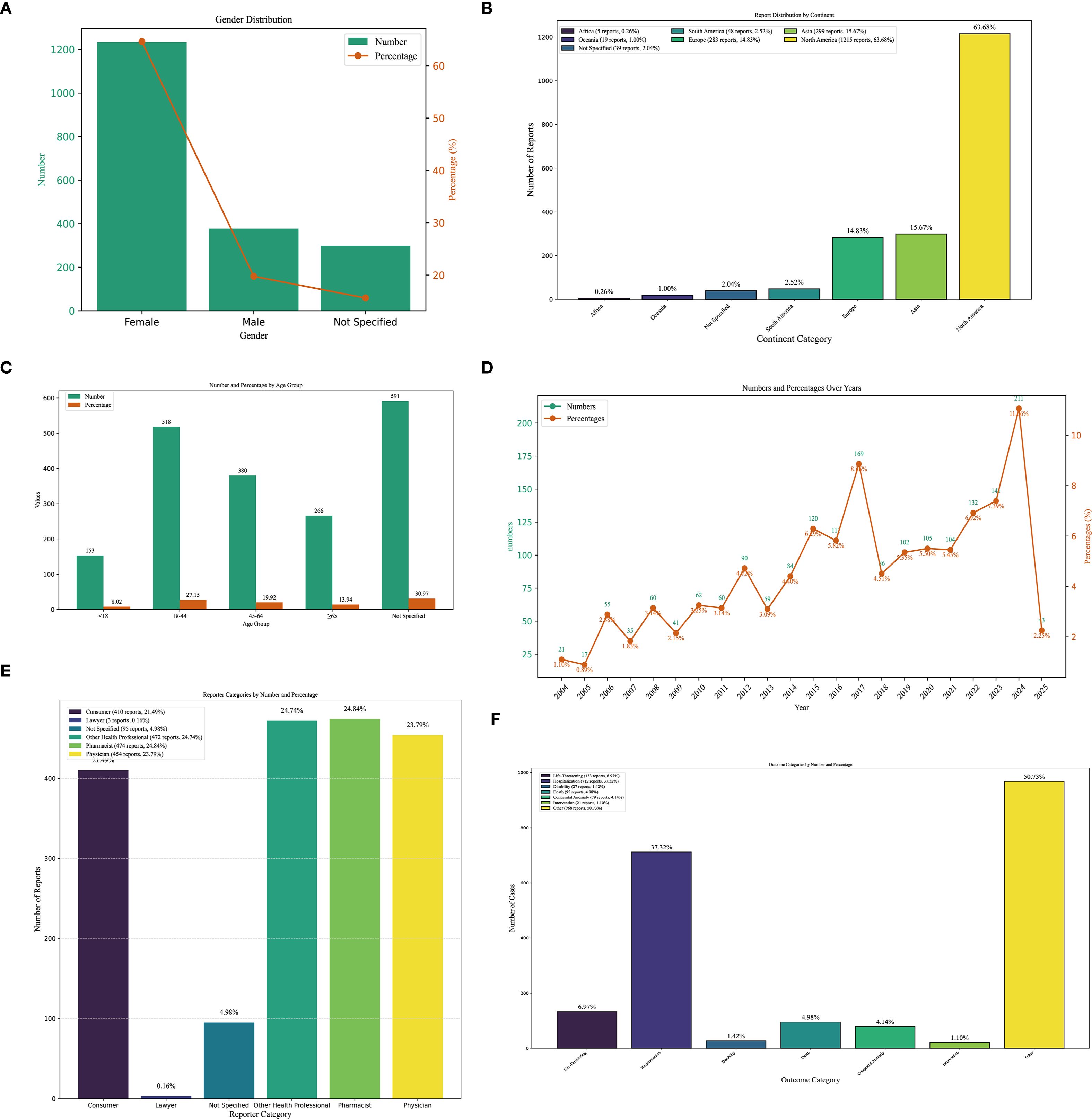
Figure 2. Clinical characteristics of methimazole-associated reports from the FAERS database. (A) Gender. (B) Continent. (C) The occurrence time of adverse events after using methimazole. (D) The frequency of AEs occurring each year from 2004 to 2025. (E) reporter. (F) outcomes.
3.2 Signal detection of methimazole-related adverse events at the SOC level using disproportionality analysis in the FAERS database
The number of AEs reported for thiamazole in different SOCs is presented in Table 2 and Figure 3. We identified a total of 27 AEs related to the system organs. These results are ranked by frequency of case reports, and those with the lower limit of the 95% ROR confidence interval being less than 1 are excluded. Ranked by the number of case reports, the six most frequently reported SOCs were blood and lymphatic system disorders (n=589), investigations (n=459), skin and subcutaneous tissue disorders (n=437), musculoskeletal and connective tissue disorders (n=371), respiratory, thoracic and mediastinal disorders (n=339), and hepatobiliary disorders (n=324).Ranked by the number of ROR, the six most frequently reported SOCs were Endocrine disorders (ROR = 16.97), Congenital, familial and genetic disorders (ROR = 14.19), Blood and lymphatic system disorders (ROR = 5.86), Hepatobiliary disorders (ROR = 5.70), Pregnancy, puerperium and perinatal conditions (ROR = 5.19), and Immune system disorders (ROR = 2.31).
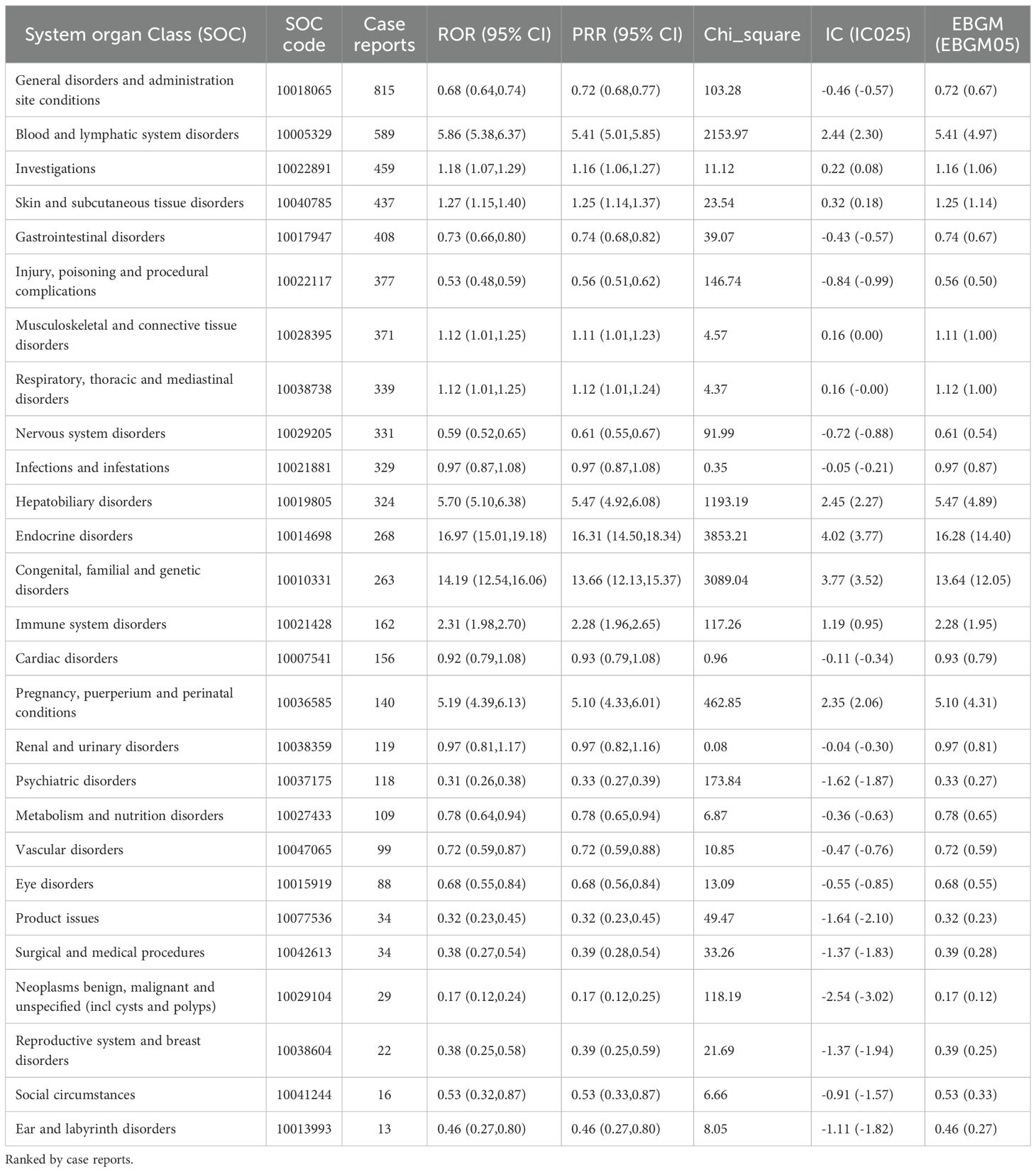
Table 2. The signal intensity of ADEs related to methimazole at the system organ category (SOC) level in the FAERS database.
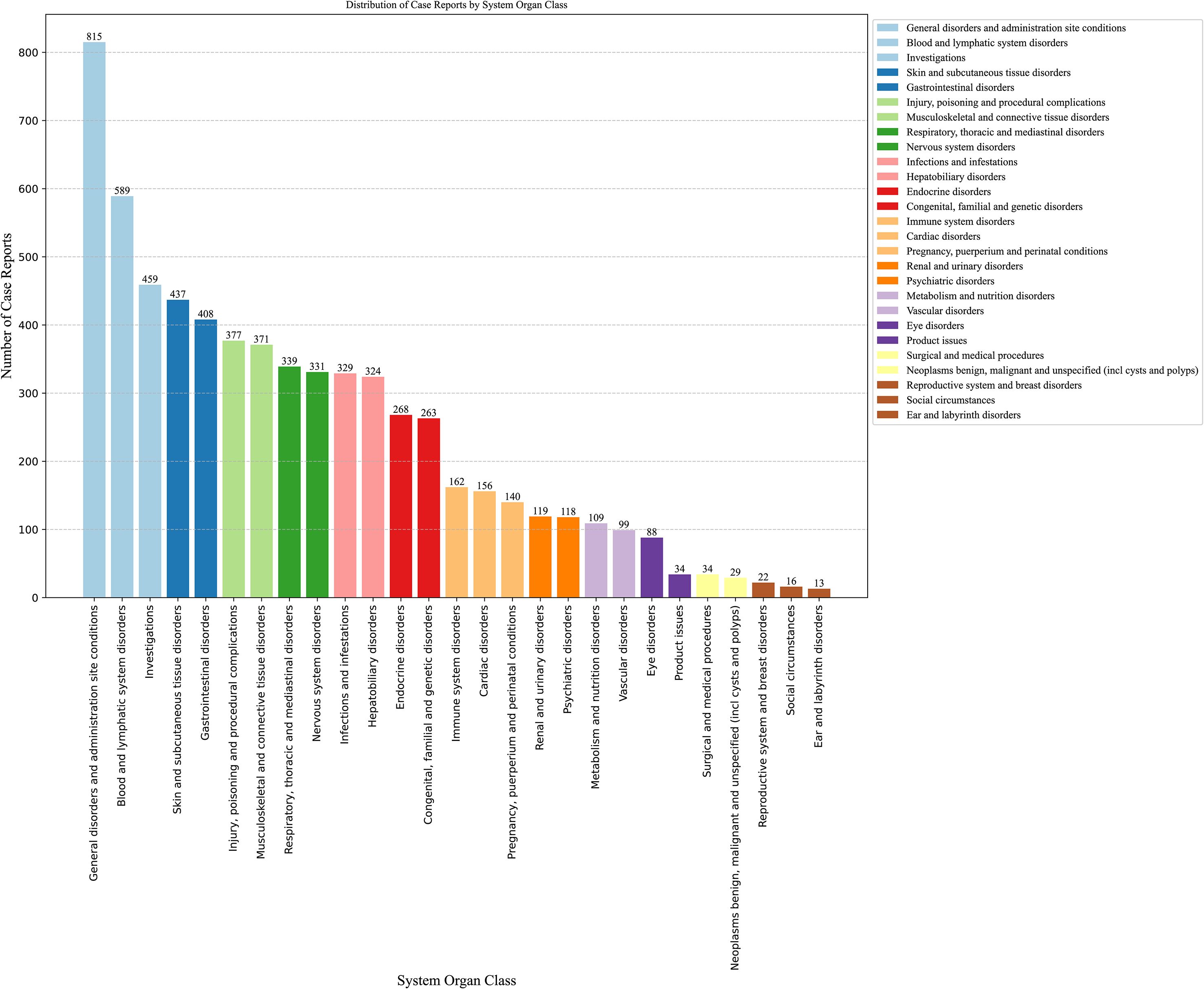
Figure 3. Signal detection related to SOC levels about methimazole (ranking based on number of reports).
3.3 Signal detection related to PT levels about methimazole
We conducted a screening of adverse drug events for methimazole and selected four calculation methods, namely ROR, PRR, BCPNN, and the combined use of MGPS for detecting the detection signals. The threshold was set as follows: a ≥ 3, the lower limit of the 95% ROR confidence interval is greater than 1, PRR ≥ 2, the chi-square value ≥ 4, IC - 2SD > 0, and EBGM05 > 2. Based on the ranking of the frequency of positive signals for the target drug, we selected the top 20 preferred terms. A total of 194 PTs met the criteria across all four calculation methods. Among these reports, the most frequently reported AE was agranulocytosis, with a total of 233 cases. The second most frequently reported adverse event was pyrexia, with a total of 110 cases. The third most frequently reported AE was exposure during pregnancy, with a total of 93 cases. The most common AE was hypothyroidism, with a total of 75 cases. The fourth-ranked AE was hypothyroidism, with a total of 75 cases. The fifth-ranked AE was neutropenia, with a total of 67 cases. Figures 4A–D respectively present the ranking of AEs under the four algorithms: ROR, PRR, IC025, and EBGM05. The above AEs are detailed in the instructions of this drug. It should be noted that, in addition to the AEs explicitly mentioned in the instructions of methimazole, this study also identified several common AEs, such as premature baby, polyarthritis, pleural effusion, septic shock, cholestasis, and jaundice cholestatic (Table 3) (Figure 4E). These need to be particularly noted in clinical settings.
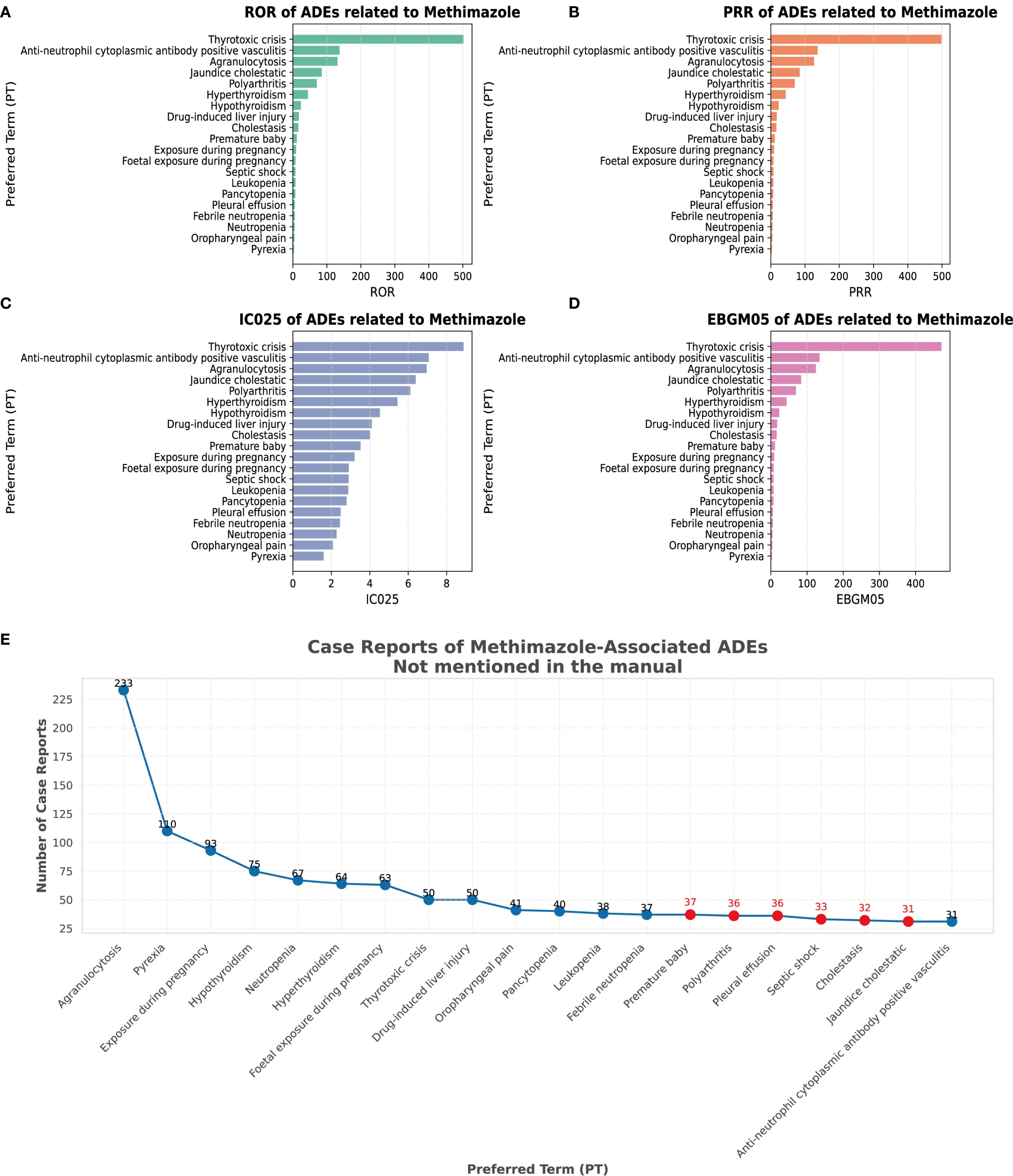
Figure 4. Signal strength at the PT level. (A) Satisfy all four algorithms simultaneously and sort them according to ROR. (B) Satisfy all four algorithms simultaneously and sort them according to PRR. (C) Satisfy all four algorithms simultaneously and sort them according to BCPNN. (D) Satisfy all four algorithms simultaneously and sort them according to MGPS. (E) The top 20 PTs that simultaneously meet the requirements of all 4 algorithms are sorted by case reports.
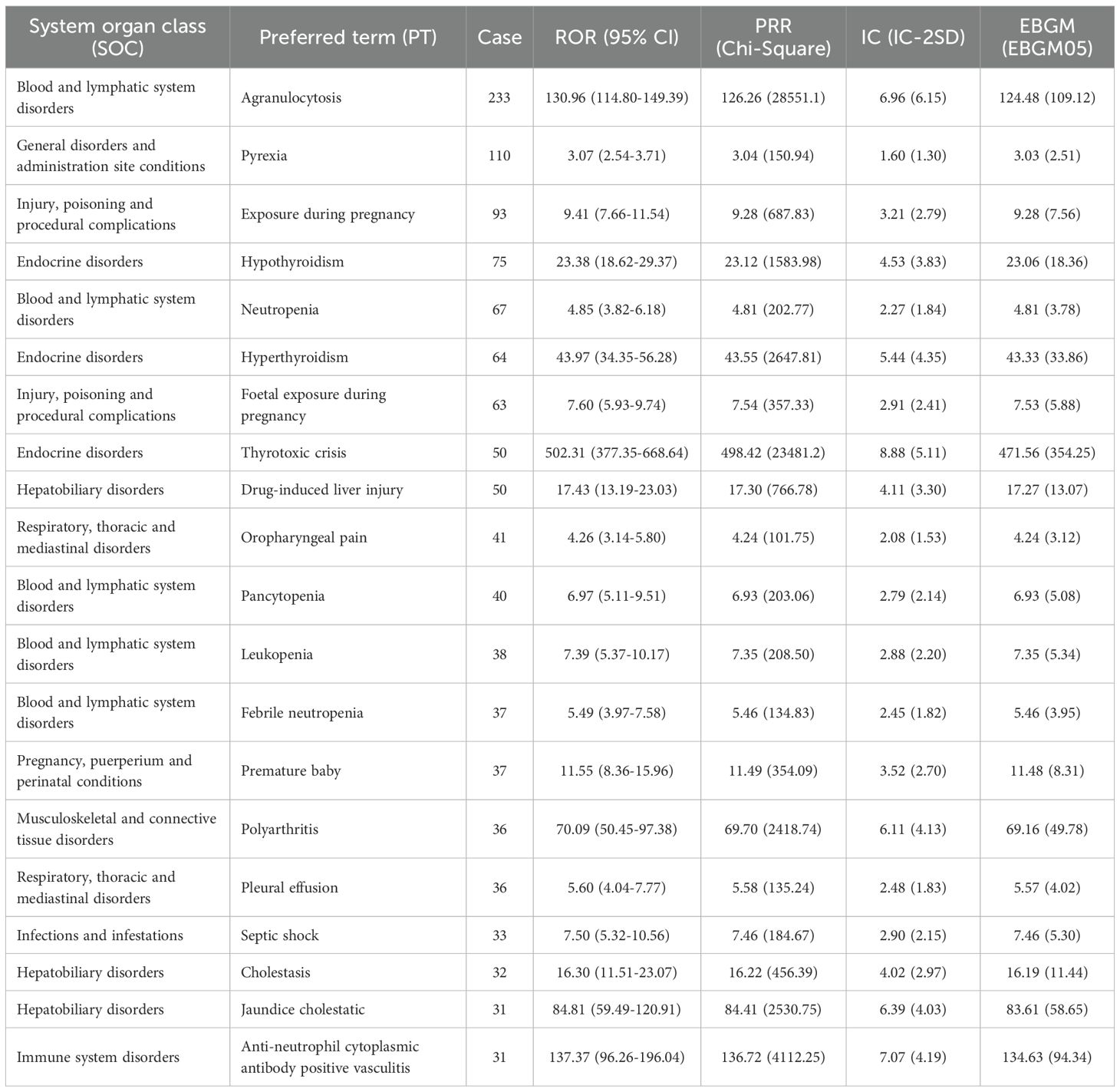
Table 3. Top 20 signal strength of adverse events at the preferred term (PT) level ranked by reports.
3.4 Subgroup analysis of age and sex
We observed that AE types differed across age groups. Age-stratified subgroup analyses further refined these patterns. Pregnancy exposure and early delivery were reported mainly among individuals aged 18–44 years. Stevens-Johnson Syndrome was observed predominantly among individuals under the age of 18. Guillain-Barré syndrome was observed predominantly among individuals aged 65 years and older. This syndrome was mainly observed in those aged greater than or equal to 65 years old. Agranulocytosis ranked first across all four age groups. Age-stratified analyses revealed distinct age-specific patterns. Among reporters, alopecia, drug hypersensitivity reactions, and urticaria were relatively common; these events were not reported by healthcare professionals (Figures 5A, B).
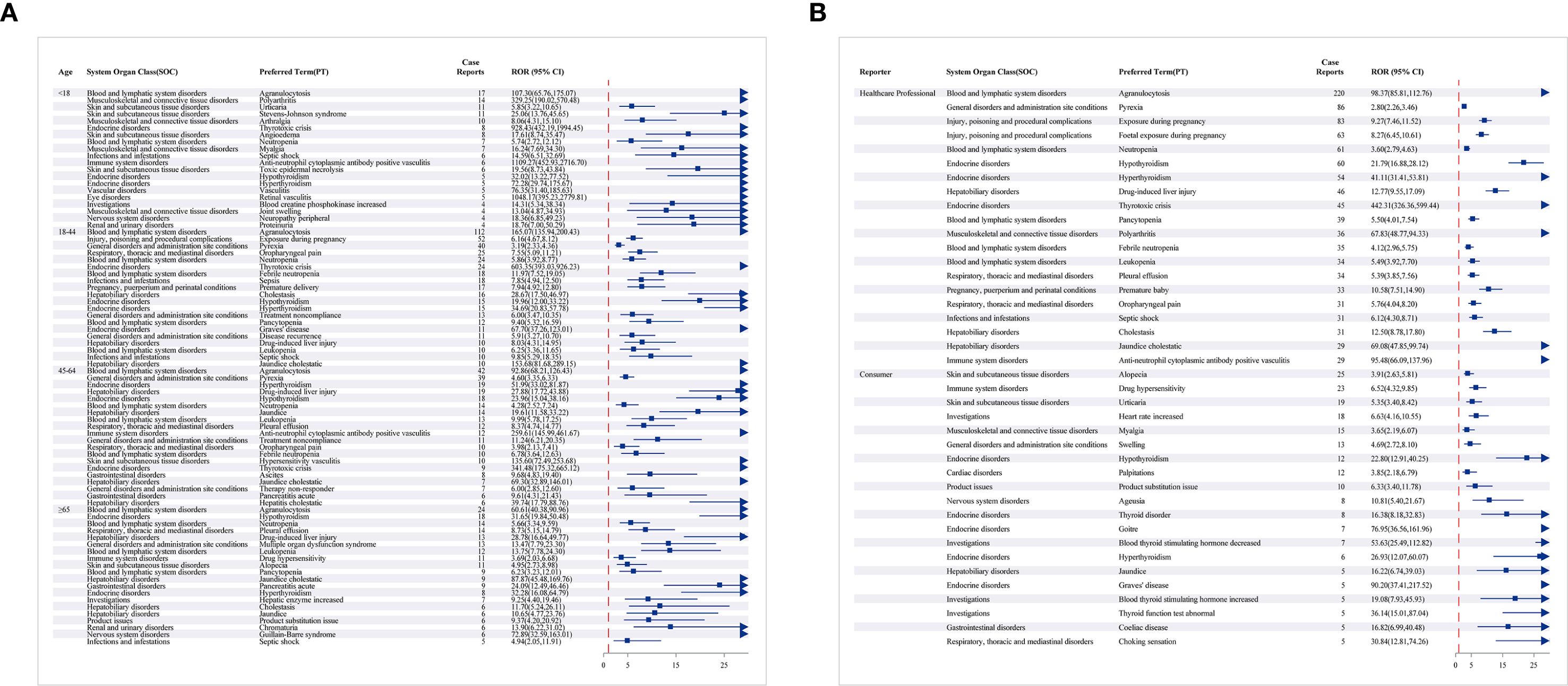
Figure 5. The forest map showing the PT top 20 results for methimazole subgroup analysis. (A) Different age groups; (B) reporter.
3.5 Differential analysis of FAERS data based on age, sex, fatal, and serious
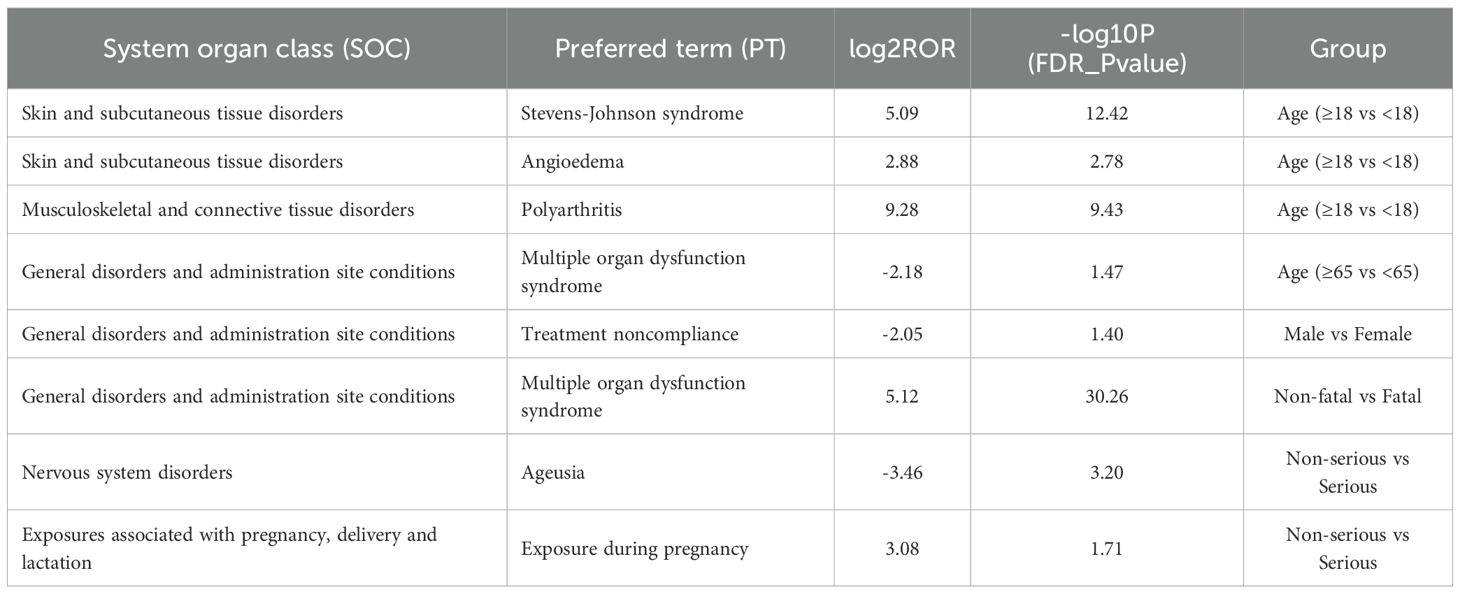
To investigate potential differences in the occurrence of adverse events across age groups, we compared the reporting patterns of adverse reactions between adolescents and non-adolescents, as well as between the elderly and non-elderly. In the comparison between adolescents and non-adolescents, the incidence rates of angioedema, polyarthritis, and Stevens–Johnson syndrome were significantly higher among adolescents, and these differences were statistically significant (Figure 6A). Teenagers may be at a higher risk of certain adverse reactions and therefore require special monitoring and prevention. In the comparison between the non-elderly group and the elderly group, the incidence of multiple organ dysfunction in the elderly group was significantly higher, demonstrating a significant statistical difference (Figure 6B). The analysis of gender differences shows that the probability of male patients not complying with the treatment is higher (Figure 6C). The analysis of the differences between fatal and non-fatal events indicates that fatal events are mainly concentrated in the upper right quadrant. The most significant signals include multiple organ dysfunction syndrome, septic shock, and pancytopenia (Figure 6D). The group with severe illness had a higher incidence of exposure during pregnancy, while in the group with milder illness, the incidence of ageusia was higher, and there was a statistically significant difference (Figure 6E) (Table 4).
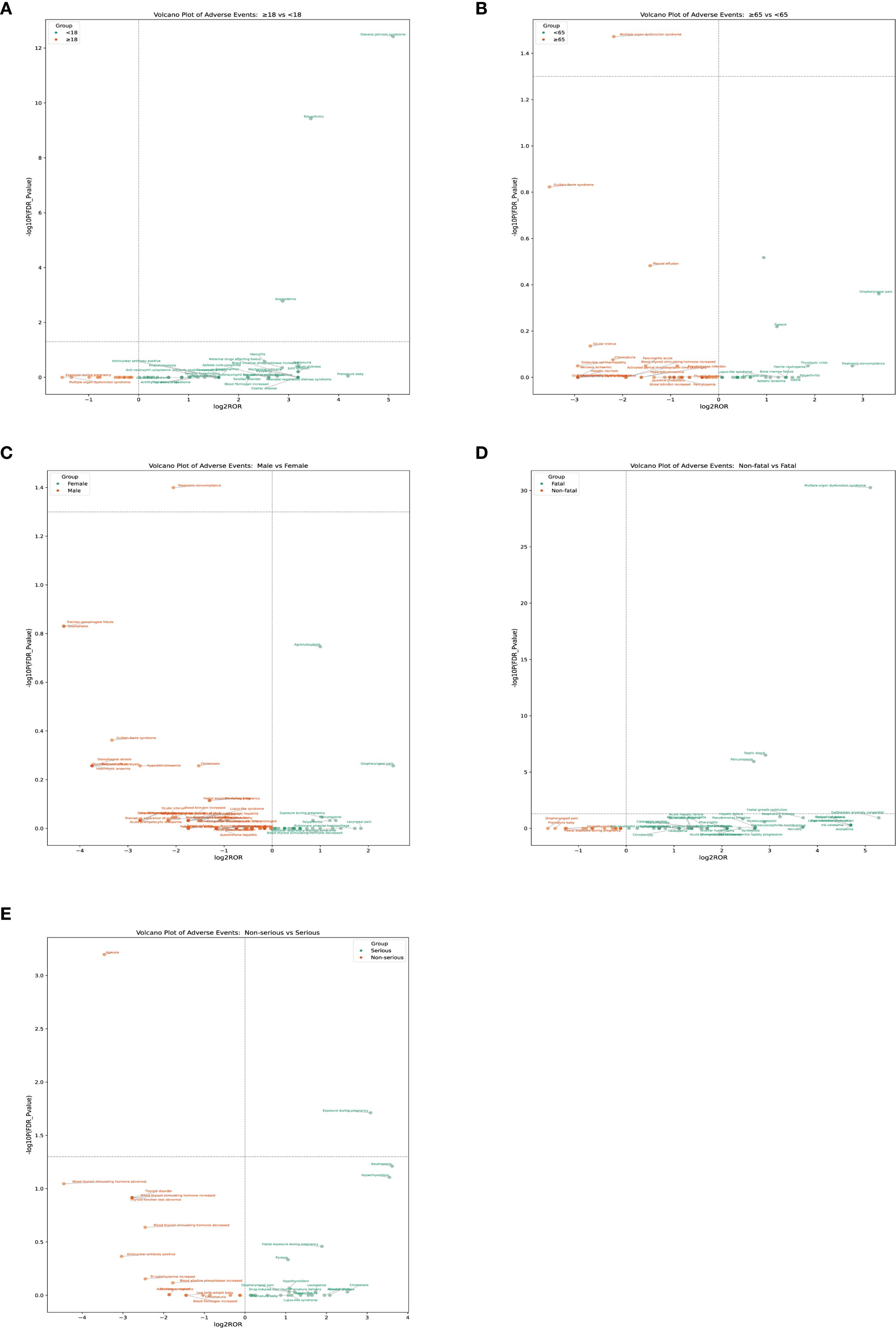
Figure 6. The PT results for methimazole different analysis by age, sex, fatality, and serious. (A) Age: adolescents and non-adolescents (B) Age: elderly and non-elderly; (C) Sex (D) Fatal (E) Serious.
3.6 Time-to-onset and Weibull distribution analysis of AEs based on methimazole
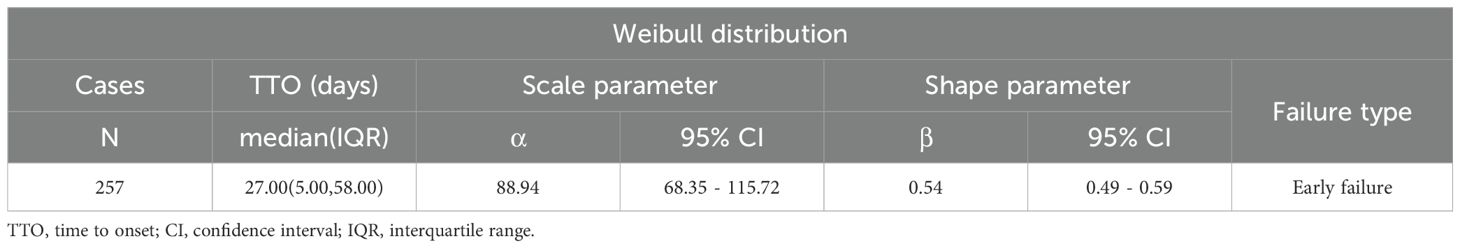
Time of AE occurrence-medication date not included unspecified was mostly clustered in the range of 0–30 days (86.53%), then 31–60 days (n = 50, 19.46%), and >360 days (n = 22, 8.56%) (Figure 7A). The above findings remind us that during the first month of medication, we should pay particular attention to whether AEs occur in the patients. Interestingly, AE can occur at any time within one year after taking methimazole. Subsequent subgroup analysis revealed that the median AE occurrence time-medication date was 27 days (Figure 7B). It is indicated that the majority of patients experienced adverse events 27 days prior to the study. The confidence interval (IQR) for the median time was 5.00 days to 58.00 days. At 0 days, 257 individuals were at risk, and at 360 days, 22 individuals were at risk. In the analysis of outcomes following serious outcome events (Figure 7C), the median onset time for non-severe adverse events was 10 days (IQR: 0–42 days), whereas severe adverse events had a median onset time of 28 days (IQR: 13–62 days). The temporal distribution of adverse events reveals a severity-dependent pattern, whereby non-serious events predominantly occur early, while serious events emerge later. This delay in onset may reflect underlying pathophysiological processes that require longer time to manifest as severe clinical outcomes. In the fatal outcome group (Figure 7D), fatal cases exhibited a median onset time of 68 days (IQR: 35–251 days), substantially later than the 25.5 days (IQR: 4–52.5 days) observed in non-fatal cases. This temporal distinction suggests that fatal events may result from prolonged disease progression or gradual physiological decompensation, requiring extended time to manifest. In contrast, non-fatal events tend to occur earlier and may be amenable to prevention through timely risk stratification and early intervention (Table 5).
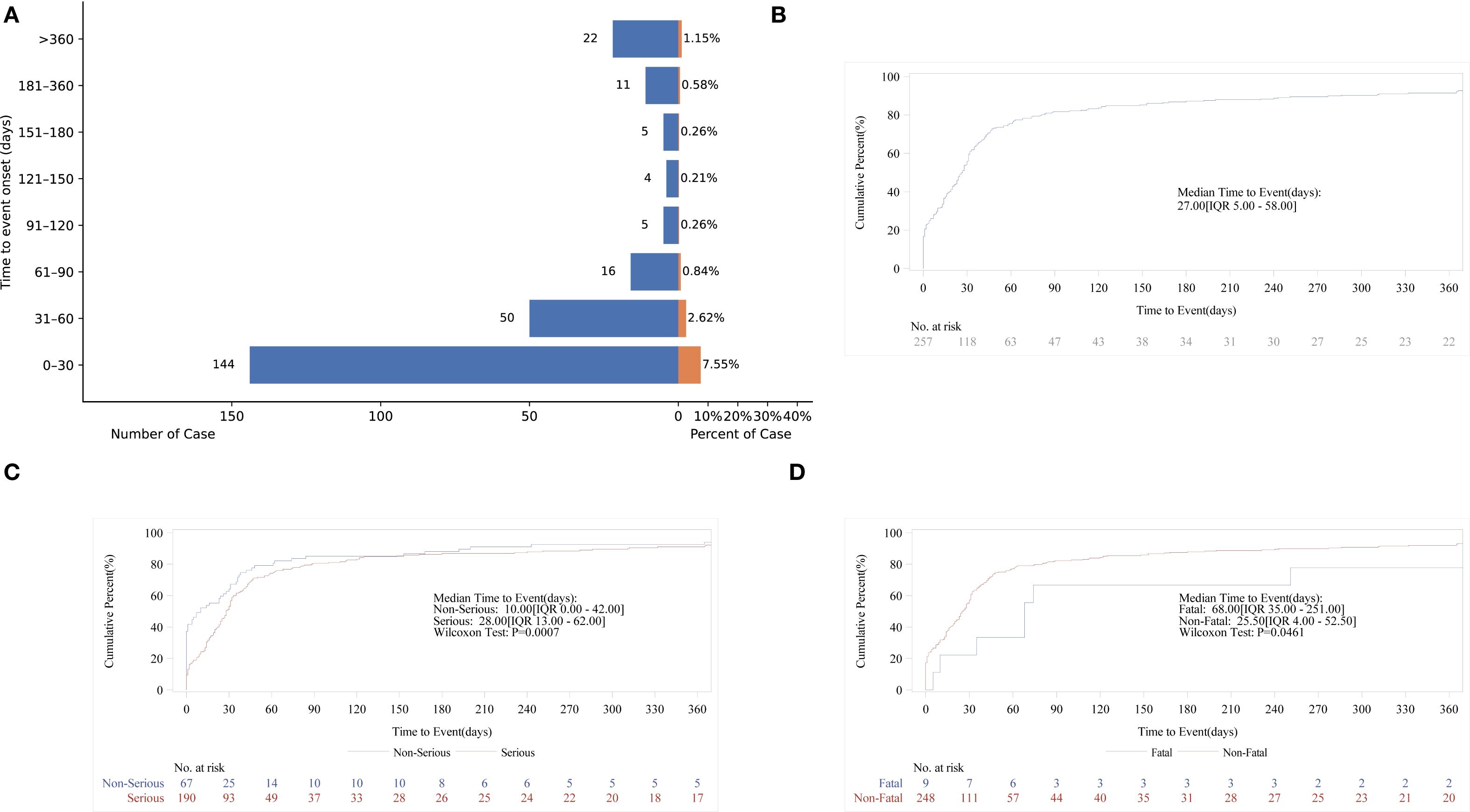
Figure 7. Time-to-onset of methimazole-associated adverse events. (A) Bar chart illustrating the distribution of AE onset times following methimazole administration; (B) Cumulative incidence curve showing the time-dependent accumulation of AE reports; (C) Kaplan–Meier survival curve comparing the onset times of serious versus non-serious AEs; (D) Kaplan–Meier survival curve depicting the onset timing of fatal versus non-fatal events.
4 Discussion
The demographic pattern of methimazole adverse-event reports appears to reflect underlying disease epidemiology and market exposure rather than intrinsic sex or region specific drug toxicity. Based on the demographic pattern of adverse events with methimazole: first, the higher share of reports from women likely reflects the higher prevalence of hyperthyroidism in women, which matches known patterns. Second, North America and Asia are probably the main markets for this drug, so higher use there may lead to more reported adverse events. The large populations in these regions may also produce more total cases even if the incidence rates are similar. Third, the concentration of reports in the 18eortsrat group is consistent with patterns of hyperthyroidism in that age range. Finally, differences by reporter type may reflect differences in adverse-event awareness, reporting duties, and access to reporting systems across professional groups.
Methimazole shows a strong hematologic toxicity signal in pharmacovigilance data, led by agranulocytosis, pancytopenia, and leukopenia. In our research, we confirmed the aforementioned findings. We believe that these adverse events may also be connected to the mechanism of action of methimazole (26). A case report described a 36-year-old female who was initially diagnosed with Graves’ disease 2 months prior to admission. At the time of diagnosis, her thyroid serology revealed a free T4 level of 3.76 ng/dL (reference range 0.7-1.5 ng/dL), a free T3 level of 15.1 pg/mL (reference range 1.7-3.7 pg/mL), and TSH receptor antibody levels of 31 IU/L (reference range 0.00-1.75 IUnits/L). Subsequently, her endocrinologist initiated treatment with methimazole 20 mg BID and atenolol 25 mg. Two months later, she presented with right axillary and right buttock abscesses, accompanied by symptoms of nausea, dizziness, and fever. She was found to have significant pancytopenia and elevated liver function test results (27). The convergence of disproportionality signals and clinical reports supports a biologically plausible effect of MMI on hematopoiesis. At the same time, spontaneous-report data may over-represent severe early-onset events and lack exposure denominators; intercurrent infection and disease-related hematologic fluctuations in hyperthyroidism can further modulate the apparent risk in FAERS. Our FAERS analysis suggests that MMI may be associated with preterm birth. In pregnant women with hyperthyroidism, propylthiouracil (PTU) is recommended as the first-line treatment during pregnancy, while methimazole (MMI) is rarely chosen due to its association with congenital malformations. Exposure to MMI during early pregnancy has been linked to rare cases of aplasia cutis (a skin and scalp defect), choanal atresia, and esophageal atresia (28, 29). This finding highlights the need for more cautious evaluation when prescribing MMI to pregnant women and consideration of alternative therapies when appropriate. Rare immune-mediated reactions related to antithyroid therapyroida as antithyroid arthritis syndrome (AAS) characterized by myalgia, arthralgia/arthritis, fever, and rashr,lgi been described after MMI initiation. A study from Japan further reported that antithyroid AAS is a rare adverse reaction linked to antithyroid drugs. The clinical manifestations of AAS are complex and often severe. These events typically occur within a certain period after initiation of MMI therapy and include muscle pain, arthralgia, arthritis, fever, and rash caused by the drug. The pathogenesis of AAS remains incompletely understood, but it has been proposed that it may involve immune-mediated hypersensitivity reactions or direct effects of drug metabolites on joint and muscle tissues. All of the AEs related to MMI mentioned above have been disclosed in the drug labeling, where they are described in detail (30). Proposed mechanisms include immune-mediated hypersensitivity and/or reactive metabolite effects on joint and muscle tissues, which provide a coherent explanation for the constellation of musculoskeletal and systemic symptoms.
Pharmacovigilance signals suggest methimazole may be associated with adverse events not captured in current labeling, most notably polyarthritis and pleural effusion, alongside premature birth, septic shock, cholestasis, and cholestatic jaundice. Our study identified several adverse events that have not yet been documented in the official drug labeling. These events included premature birth, polyarthritis, pleural effusion, septic shock, cholestasis, and cholestatic jaundice. Further analysis showed that polyarthritis appeared to be one of the more frequently observed AEs. Clinicians should therefore remain alert to the possibility of polyarthritis when prescribing antithyroid drugs and should promptly consider this diagnosis when relevant clinical symptoms occur. The timing (within weeks of MMI initiation) and systemic features (fever, rash) support an immune-mediated hypersensitivity consistent with AAS. Differences across studies reflect variation in case definitions, ascertainment sources, and whether rates are exposure-adjusted. We also detected a signal for pleural effusion. A published case report also described a 67-year-old woman who had recently been diagnosed with Graves’ disease and was receiving MMI at a daily dose of 20 mg. Upon admission, she presented with dyspnea and new-onset atrial fibrillation with a rapid ventricular rate. Chest radiography revealed a unilateral right-sided pleural effusion, and thoracentesis confirmed transudative characteristics of the pleural fluid. When combined with our analysis of the adverse event reporting database, these findings suggest that pleural effusion may represent a potential adverse reaction associated with MMI. Clinicians should therefore maintain a high index of suspicion for pleural effusion when prescribing MMI and should consider routine chest imaging follow-up to ensure early detection and to reduce the risk of life-threatening complications (31). Immune-mediated serositis remains a plausible pathway, but confounding by comorbid conditions and missing exposure denominators in spontaneous-report systems can inflate apparent disproportionality. Clinicians should maintain a high index of suspicion for pleural effusion when new respiratory symptoms emerge during MMI therapy and pursue targeted imaging when clinically indicated. In our study, adverse events linked to MMI included premature birth, polyarthritis, pleural effusion, septic shock, and cholestasis. The underlying mechanisms responsible for these AEs remain poorly understood, as no definitive explanations have been reported in the current literature. Continued investigation by other researchers will be essential to clarify these mechanisms and guide safer clinical use of MMI in the future.
Subgroup analyses revealed distinct age, sex, and severity specific risk patterns under methimazole (MMI) exposure. In our study, AEs associated with methimazole included premature birth, polyarthritis, pleural effusion, septic shock, and cholestasis. However, the underlying mechanisms of these reactions remain unclear, as no specific reports or studies have yet addressed them. We hope that future research will further explore these areas as scientific knowledge advances. The differential analysis revealed several noteworthy patterns. When adolescents were compared with non-adolescents, the incidence of angioedema, polyarthritis, and Stevens–Johnson syndrome was found to be significantly higher among adolescents. This difference may reflect the unique characteristics of the adolescent immune system as well as variations in drug exposure. Because adolescents appear to be more susceptible to immune-mediated adverse reactions, clinicians should apply closer monitoring when MMI is prescribed in this age group. In the comparison between elderly and non-elderly patients, the elderly group showed a markedly increased risk of multiple organ dysfunction. This finding could be related to the reduced organ reserve, the presence of comorbidities, and diminished drug metabolism that are common in elderly patients. For these individuals, closer and ongoing assessment of organ function is recommended to detect early signs of dysfunction and to reduce the likelihood of serious outcomes. Sex-based differences were also noted. Male patients were more likely to demonstrate poor treatment adherence, suggesting the need for targeted adherence education and closer follow-up for male patients receiving MMI. These measures may help reduce treatment interruptions or incorrect use of medication. Finally, fatal events differed substantially from non-fatal events. Multiple organ dysfunction syndrome, septic shock, and pancytopenia were the most frequent clinical presentations in fatal cases. These conditions often advance rapidly and are associated with poor outcomes, underscoring the need for early recognition and intervention. The analysis also showed that drug exposure during pregnancy was more common in the group with severe disease, whereas loss of taste was observed more frequently in the less severe group, and both differences were statistically significant. These findings suggest that drug exposure during pregnancy may contribute to more serious adverse outcomes and should be further investigated in future studies. Overall, we observed immune-mediated adverse events in different subgroups. Future studies should clearly define the subgroups (such as age stratification, elderly criteria, gender) to distinguish the effects of the drug from the background of the disease and reporting bias.
Methimazole associated AEs concentrate within the first month of therapy, making early detection and intervention critical. This early aggregation phenomenon is consistent with previous studies, especially the occurrence of granulocyte deficiency and other immune-mediated reactions which typically occur several weeks to several months after the medication is administered. When examining the timing of AEs, our findings showed that the majority occurred within the first month of starting methimazole treatment. Detecting these methimazole-related events as early as possible is essential because they have the potential to be life-threatening. This large-sample, real-world observational study has several limitations that must be recognized. First, reports of AEs and medication errors were submitted on a voluntary basis by pharmaceutical companies, healthcare professionals, and patients, which may have led to incomplete data or reporting bias. Second, the submitted reports were not required to demonstrate a definite causal relationship between the drug and the event. Therefore, these findings should be interpreted as safety signals rather than definitive measures of risk. Further prospective clinical trials with larger cohorts and longer follow-up periods are needed to confirm these observations.
5 Conclusion
Methimazole remains a key therapeutic option in the management of hyperthyroidism. While its ability to effectively lower serum thyroid hormone concentrations is well established, it is essential to implement thorough monitoring strategies to detect and address potential adverse events that may occur during treatment. Through a systematic evaluation, this study identified that, beyond the adverse events commonly recognized, particular vigilance should be directed toward premature birth, polyarthritis, pleural effusion, septic shock, and cholestasis. In addition, the spectrum and frequency of adverse events differed among specific patient subgroups, suggesting that certain populations may carry distinct risk profiles. These observations highlight the critical need for individualized monitoring and risk assessment when methimazole is prescribed, thereby enhancing patient safety and informing clinical decisions. Collectively, the present findings provide meaningful insights for healthcare professionals, patients, and other stakeholders, contributing to a more comprehensive understanding of the safety profile of methimazole in real-world clinical practice and supporting future refinements in risk evaluation and management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. SW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. XZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Natural Science Foundation of Fujian Province (2021J011336). Project (Exploration of the therapeutic effect and potential mechanism of the triterpenoid plant metabolite nomicine on head and neck cancer based on single-cell sequencing and network pharmacology) supported by Traditional Chinese Medicine Foundation of Xiamen (XWZY-2025-0501).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1680281/full#supplementary-material
Supplementary Table 1 | Calculation table.
References
1. Wu J, Niu JX, Jia CY, Yang QK, Li DW, Dong XQ, et al. A higher composite dietary antioxidant index is associated with a decreased risk of subclinical hyperthyroidism in adults: evidence from epidemiological studies. Front Nutr. (2025) 12:1613223. doi: 10.3389/fnut.2025.1613223
2. Wiersinga WM, Poppe KG, and Effraimidis G. Hyperthyroidism: aetiology, pathogenesis, diagnosis, management, complications, and prognosis. Lancet Diabetes Endocrinol. (2023) 11:282–98. doi: 10.1016/S2213-8587(23)00005-0
3. Hetzel BS. aetiology and pathogenesis of hyperthyroidism. Postgrad Med J. (1968) 44:363–76. doi: 10.1136/pgmj.44.511.363
4. Atis G, Dalkilinc A, Altuntas Y, Atis A, Gurbuz C, Ofluoglu Y, et al. Hyperthyroidism: a risk factor for female sexual dysfunction. J Sex Med. (2011) 8:2327–33. doi: 10.1111/j.1743-6109.2011.02354.x
5. Lisco G, Accardo G, Pupilli C, Malandrino P, Geronimo VD, Triggiani V, et al. Perchlorates in the treatment of hyperthyroidism and thyrotoxicosis: a comprehensive review. Endocrine. (2024) 85:1–10. doi: 10.1007/s12020-023-03679-y
6. Zimmerman D. Fetal and neonatal hyperthyroidism. Thyroid. (1999) 9:727–33. doi: 10.1089/thy.1999.9.727
7. Chiera M, Draghetti S, Ronchi DD, Scaramelli AR, Fabbri C, Fanelli G, et al. Hyperthyroidism and depression: a clinical case of atypical thyrotoxicosis manifestation. Int Clin Psychopharmacol. (2023) 38:269–72. doi: 10.1097/YIC.0000000000000438
8. Dierickx I, Decallonne B, Billen J, Vanhole C, Lewi L, Catte LD, et al. Severe fetal and neonatal hyperthyroidism years after surgical treatment of maternal Graves’ disease. J Obstet Gynaecol. (2014) 34:117–22. doi: 10.3109/01443615.2013.831044
9. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. (2016) 26:1343–421. doi: 10.1089/thy.2016.0229
10. Tonacchera M, Chiovato L, Bartalena L, Cavaliere AF, and Vitti P. Treatment of Graves’ hyperthyroidism with thionamides: a position paper on indications and safety in pregnancy. J Endocrinol Invest. (2020) 43:257–65. doi: 10.1007/s40618-019-01148-w
11. Biondi B and Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. (2008) 29:76–131. doi: 10.1210/er.2006-0043
12. Boogaard E, Vissenberg R, Land JA, Wely MV, Post JAM, Goddijn M, et al. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. (2011) 17:605–19. doi: 10.1093/humupd/dmr024
13. Urabe K, Hotokebuchi T, Oles KJ, Bronk JT, Jingushi S, Iwamoto Y, et al. Inhibition of endochondral ossification during fracture repair in experimental hypothyroid rats. J Orthop Res. (1999) 17:920–5. doi: 10.1002/jor.1100170617
14. Azizi F, Amouzegar A, Tohidi M, Hedayati M, Khalili D, Cheraghi L, et al. Increased remission rates after long-term methimazole therapy in patients with graves’ Disease: results of a randomized clinical trial. Thyroid. (2019) 29:1192–200. doi: 10.1089/thy.2019.0180
15. Zhang R, Mao YW, Li JQ, Ni LJ, Lin L, Wang AJ, et al. Fe single atoms encapsulated in N, P-codoped carbon nanosheets with enhanced peroxidase-like activity for colorimetric detection of methimazole. Spectrochim Acta A Mol Biomol Spectrosc. (2024) 310:123934. doi: 10.1016/j.saa.2024.123934
16. Karmisholt J, Andersen SL, Bulow-Pedersen I, Krejbjerg A, Nygaard B, Carlé A, et al. Long-term methimazole therapy in Graves’ hyperthyroidism and adverse reactions: a Danish multicenter study. Eur Thyroid J. (2022) 11:e220031. doi: 10.1530/ETJ-22-0031
17. Andrade GC, Maia FCP, Mourão GF, Rosario PW, and Calsolari MR. Antineutrophil cytoplasmic antibodies in patients treated with methimazole: a prospective Brazilian study. Braz J Otorhinolaryngol. (2019) 85:636–41. doi: 10.1016/j.bjorl.2018.05.014
18. Zhao B, Fu YM, Cui SC, Chen XN, Liu S, Luo L, et al. A real-world disproportionality analysis of Everolimus: data mining of the public version of FDA adverse event reporting system. Front Pharmacol. (2024) 15:1333662. doi: 10.3389/fphar.2024.1333662
19. Yu RJ, Krantz MS, Phillips EJ, and Jr CAS. Emerging causes of drug-induced anaphylaxis: A review of anaphylaxis-associated reports in the FDA adverse event reporting system (FAERS). J Allergy Clin Immunol Pract. (2021) 9:819–829.e2. doi: 10.1016/j.jaip.2020.09.021
20. Yu Q, Yao JY, Li E, Xia MK, Hu Z, Xiao Y, et al. Neurological adverse events associated with antidepressants: a comprehensive 22-year analysis of the FDA adverse event reporting system. Front Pharmacol. (2025) 16:1644241. doi: 10.3389/fphar.2025.1644241
21. Wang M, Chen XH, Liu ZQ, Li ZY, Zhu ZH, Liu S, et al. Exploring potential associations between GLP-1RAs and depressive disorders: a pharmacovigilance study based on FAERS and VigiBase data. EClinicalMedicine. (2025) 86:103385. doi: 10.1016/j.eclinm.2025.103385
22. Rothman KJ, Lanes S, and Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. (2004) 13:519–23. doi: 10.1002/pds.1001
23. Evans SJ, Waller PC, and Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. (2001) 10:483–6. doi: 10.1002/pds.677
24. Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. (1998) 54:315–21. doi: 10.1007/s002280050466
25. Hauben M, Zou C, Bright S, and Hung E. More extreme duplication in the U.S. FDA FAERS database and a suggested check point for disproportionality analysis. Pharmacoepidemiol Drug Saf. (2021) 30:1140–1. doi: 10.1002/pds.5265
26. Taylor PN and Vaidya B. Side effects of anti-thyroid drugs and their impact on the choice of treatment for thyrotoxicosis in pregnancy. Eur Thyroid J. (2012) 1:176–85. doi: 10.1159/000342920
27. Valasareddy S, Tariq F, and Zaheer M. Methimazole-induced pancytopenia with ANCA positivity: diagnostic and management challenges. Am J Case Rep. (2025) 26:e947323. doi: 10.12659/AJCR.947323
28. Liu Y, Li QQ, Xu Y, Chen YX, and Men YY. Comparison of the safety between propylthiouracil and methimazole with hyperthyroidism in pregnancy: A systematic review and meta-analysis. PloS One. (2023) 18:e0286097. doi: 10.1371/journal.pone.0286097
29. Abalovich M, Amino N, Barbour LA, Cobin RH, Groot LJD, Glinoer D, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. (2007) 92:S1–47. doi: 10.1210/jc.2007-0141
30. Kawasumi M, Kubota M, Yoshii Y, and Tokunaga T. Antithyroid arthritis syndrome caused by methimazole in a patient with Graves’ disease. Endocrinol Diabetes Metab Case Rep. (2023) 2023:23–0031. doi: 10.1530/EDM-23-0031
Keywords: methimazole, pharmacovigilance, FAERS, ROR, BCPNN, EBGM
Citation: Sun Y, Wang S and Zhou X (2025) Adverse events of the thyroid peroxidase inhibitor methimazole in the treatment of hyperthyroidism: a comprehensive analysis from the first quarter of 2004 to the first quarter of 2025. Front. Endocrinol. 16:1680281. doi: 10.3389/fendo.2025.1680281
Received: 05 August 2025; Accepted: 08 September 2025;
Published: 01 October 2025.
Edited by:
Chukwuka Elendu, American College of Cardiology, United StatesReviewed by:
Kyriakos Vamvakidis, Henry Dunant Hospital, GreeceOnyinye Okolo, University of Southern Mississippi, United States
Copyright © 2025 Sun, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Zhou, emhvdXh1MjdAeG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Yixin Sun
Yixin Sun Shuai Wang1,2†
Shuai Wang1,2†