- 1Endocrinology Department, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
- 2Jiangxi Cardiovascular Research Institute, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
- 3Department of Cardiology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
- 4Discipline Construction Office, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
- 5Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 6China-Australia Joint Research Center for Infectious Diseases, School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, Shanxi, China
Introduction: Acute decompensated heart failure (ADHF), a critical cardiovascular emergency, is driven by a metabolic and inflammatory imbalance that serves as the central mechanism of disease progression. This study aims to analyze the heterogeneity of mortality risk in patients with comorbid diabetes mellitus (DM) and HF using the C-reactive protein-triglyceride-glucose index (CTI).
Methods: This study evaluated 1,051 ADHF patients from the Jiangxi-ADHF II cohort. The Boruta algorithm, a fully automated feature selection method, was applied to identify key predictive variables and rank their importance. Cox proportional hazard models were constructed to assess the association between the CTI and 30-day mortality risk in ADHF patients, stratified by DM status. To further elucidate the nonlinear characteristics of risk associations, restricted cubic splines were employed to construct dose-response relationship curves. Additionally, heatmaps were used to assess the joint association of CTI components with mortality risk.
Results: The 30-day follow-up revealed a mortality rate of 8.3%. Through the Boruta algorithm and multivariate Cox regression analysis, we identified CTI as a key prognostic factor for short-term mortality risk in ADHF patients, especially in those with comorbid DM. The restricted cubic splines model further confirmed the linear and non-linear associations between CTI and mortality in ADHF patients with and without DM. Additionally, heatmaps visualized the association between CTI components and mortality: to summarize, the mortality risk is relatively low when the triglyceride-glucose index remains within specific ranges (8.25-9.0 for patients with DM; 7.0-9.0 for non-DM patients) and the C-reactive protein level is maintained below 50 mg/L. Further subgroup analyses highlighted distinct risk modulation patterns: non-DM ADHF patients exhibited mortality risk heterogeneity across gender, hypertension, and stroke subgroups; however, the DM comorbid group demonstrated uniform risk profiles with no statistically significant differences.
Discussion: This study demonstrates the clinical utility of the novel inflammatory-metabolic index CTI in mortality risk assessment for ADHF patients, with superior risk stratification efficacy observed in those with DM comorbidity.
Introduction
Diabetes mellitus (DM) stands as a major global public health challenge in the 21st century, imposing a growing disease burden. According to the 2019 Epidemiology Report by the International Diabetes Federation, the global prevalence of DM among adults reached 9.3% and is projected to rise to 10.9% by 2045 (1). Notably, cardiovascular complications contribute to the majority of (i.e., over 50%) mortality risk among people with DM (2). The Framingham study indicates that the risk of developing heart failure (HF) is significantly higher in DM patients than in non-DM individuals (3). Epidemiological evidence reveals the prevalence of HF among DM patients ranges from 9% to 22%, approximately fourfold higher than in the general population (4); conversely, the prevalence of DM among HF patients is between 10% and 47% (5). In terms of clinical prognosis, this comorbidity profile of metabolic and cardiovascular systems exhibits a significant superimposition of risks for adverse outcomes. Multivariable risk prediction models explicitly categorize DM as an independent risk factor for mortality in HF patients (6). Population-based longitudinal studies demonstrate that concomitant DM significantly elevates future mortality risks in HF populations (7–11). These evidence chains not only uncover the underlying pathological interconnections between DM-HF comorbidity but also underscore the clinical imperative of implementing dual intervention strategies in clinical management.
Acute decompensated HF (ADHF) represents a critical manifestation of cardiovascular disease. In recent years, fundamental research has elucidated that insulin resistance (IR) and chronic inflammation are the core pathological bases driving ADHF pathogenesis and progression. Specifically, IR, as a key driver of metabolic syndrome, not only directly impairs energy metabolism but also exacerbates myocardial fibrosis and diastolic dysfunction through pro-inflammatory cytokine release (12–14). Notably, chronic inflammation aggravates IR states via modulating autocrine effects in inflammatory cells and accelerating ectopic fat deposition, thereby creating a vicious cycle (15). Within this pathological framework, the discovery of novel biomarkers has provided transformative tools for ADHF risk stratification and prognostic assessment. The triglyceride-glucose (TyG) index, as a non-invasive quantitative tool for IR, has been incorporated into HF risk stratification systems due to its efficient IR characterization capability and clinical practicality (16). It has been validated as an independent predictor of mortality risks across various HF subtypes (17–23). Concurrently, C-reactive protein (CRP), recognized as a core mediator of systemic inflammation (24), has expanded its clinical significance beyond a marker for infectious diseases (25–35). Notably, recent studies have demonstrated that a combined assessment of metabolism and inflammation significantly enhances the predictive efficacy of cardiovascular event risk and adverse prognosis (36–39). This multidimensional assessment strategy not only transcends the limitations of traditional risk stratification models but also provides an actionable foundation for individualized interventions in ADHF management within the era of precision medicine.
The combined effect of metabolism and inflammation is a key driver in the progression of multisystem diseases (12–15). Building upon this, Ruan et al. developed the C-reactive protein-triglyceride-glucose index (CTI), which integrates CRP (an inflammatory marker) and the TyG index (a surrogate for IR), and demonstrated its independent predictive utility for mortality risk assessment (40). Subsequent investigations further revealed significant associations between CTI and adverse cardiocerebrovascular outcomes (41–43). However, in high-risk populations for acute cardiovascular events, particularly in DM-ADHF comorbid patients whose pathophysiological characteristics are closely linked to the metabolic-inflammatory axis, the predictive efficacy and threshold effects of CTI for short-term (30-day) mortality risk remain incompletely elucidated. To address this knowledge gap, this study, based on the Jiangxi-ADHF II cohort, aims to investigate the risk stratification capability of CTI for 30-day all-cause mortality in ADHF patients with and without comorbid DM, thereby providing evidence-based guidance for precision risk management of ADHF.
Methods
Study design and population
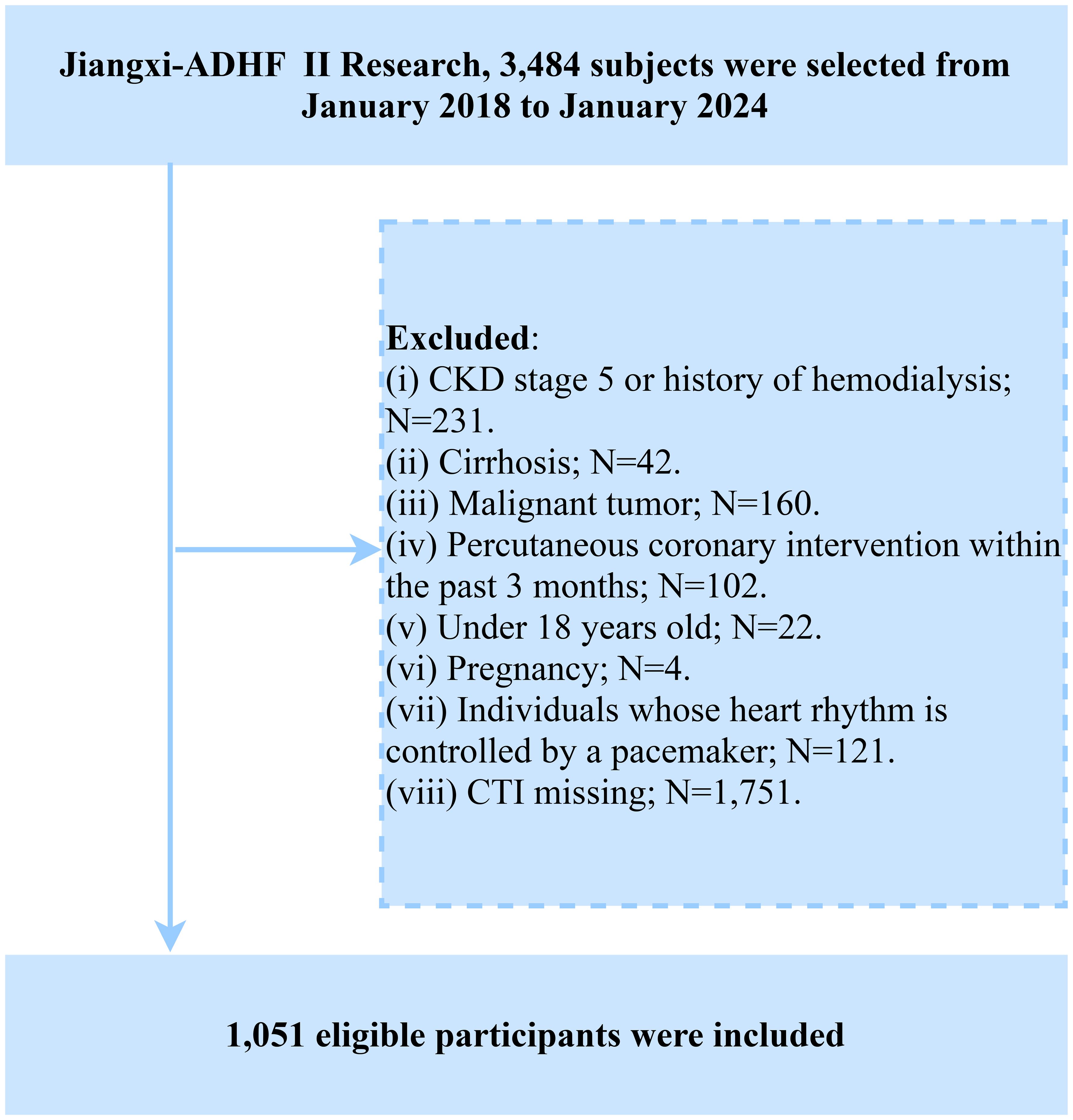
The subjects of this study were recruited from the Jiangxi-ADHF cohort study. Briefly, the Jiangxi-ADHF project is an ongoing cohort study designed to integrate multidimensional clinical data for developing regional, standardized risk stratification models for HF. The Jiangxi-ADHF II cohort was conducted from January 2018 to January 2024, encompassing 3,484 patients with ADHF who met the diagnostic criteria outlined in the then-available guidelines of the European Society of Cardiology and the American College of Cardiology/American Heart Association for HF. We applied the following exclusion criteria to account for pathological heterogeneity and data integrity. Based on considerations of pathological heterogeneity and data integrity, subjects with the following characteristics were excluded: (1) Exclusion of individuals with fluid and sodium retention attributed to non-cardiac causes, including patients with uremia, chronic kidney disease requiring hemodialysis, and liver cirrhosis (n=273). (2) Exclusion of special treatment populations that may interfere with autonomic nervous regulation and short-term prognosis (including patients with pacemaker implantation and those who underwent interventional or reperfusion therapies within 30 days; n=223). (3) Exclusion of individuals with specific physiological states, including malignancies, minors, and pregnancy (n=186). (4) Exclusion of cases with missing CTI data (n=1,751). This ultimately included 1,051 participants in the final analysis, with the detailed screening process illustrated in Figure 1.
Ethical approval
This study adhered strictly to international biomedical research ethical frameworks and received systematic evaluation and approval from the Ethics Review Committee of Jiangxi Provincial People’s Hospital prior to implementation (Ethics Approval No: 2024-01). In accordance with the ethical principles outlined in the Declaration of Helsinki, informed consent was obtained from all participants or their legal guardians for data utilization. The study’s findings are reported in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Data collection
In the clinical data acquisition phase, this study employed a dual independent data entry-blinded verification quality control system: two standardized-trained research assistants independently collected demographic characteristics (gender, age), lifestyle factors (smoking and drinking status), cardiovascular comorbidities [hypertension, DM, stroke, coronary heart disease (CHD)], New York Heart Association (NYHA) functional classification, vital signs (blood pressure), and echocardiographic parameters (left ventricular ejection fraction: LVEF). Following cross-verification between the two datasets, validated information was included in the final analysis. Concurrently, comorbidity diagnoses were substantiated through a comprehensive review of patients' electronic medical records containing specialist consultation records, medication histories, and supporting imaging evidence.
The laboratory analysis incorporated blood sample data collected within 24 hours of admission, with strict fasting criteria implemented for glucose/lipid metabolism-related indicators and liver enzyme tests (minimum interval of ≥8 hours from last meal to blood sampling). Metabolic parameters were measured using the HITACHI LABOSPECT 008 fully automated biochemical analyzer, including liver/kidney function markers [alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), uric acid (UA)], and glucose/lipid profiles [fasting plasma glucose (FPG), total cholesterol, triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C)]. Blood cell (WBC: white blood cell count; RBC: red blood cell count; PLT: platelet count) analysis was performed using the Sysmex XN-3000 5-part differential hematology analyzer. The HF biomarker N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) was quantitatively measured via electrochemiluminescence immunoassay. CRP levels were quantified using an immunoturbidimetric assay. All testing protocols were monitored through standardized quality control protocols to ensure result reliability.
Calculation of TyG index and CTI
TyG index = ln [TG (mg/dL) × FPG (mg/dL)/2] (16).
CTI = CTI = 0.412 × Ln (CRP [mg/L]) + TyG index (40).
Study outcomes
This study used the time of admission for ADHF patients as the observation starting point, with the primary outcome defined as all-cause mortality occurring within 30 days. Outcome ascertainment was conducted through multiple methods, including follow-up via mobile text messages, telephone interviews, and in-person follow-up during outpatient or inpatient visits, all performed by medically trained personnel. In addition to in-hospital deaths, out-of-hospital deaths have also been systematically verified.
Missing data processing
In the current study, there are missing data for LVEF, ALT, AST, Cr, and UA, with missing rates of 4.00%, 0.57%, 0.57%, 0.95%, and 1.05% respectively. The detailed information is provided in Supplementary Table 1. Analysis of the missingness pattern cross-information diagram (Supplementary Figure 1) revealed high correlations in missing patterns between UA and Cr, as well as ALT and AST, suggesting these data are likely missing at random. Conversely, LVEF demonstrated relative independence from other missing variables, indicating its missing data might be missing completely at random. Given the low missing proportion and the observed independence, we used the mice package in R software to perform multiple imputation for missing covariate information: predictive mean matching was used for continuous variables, while logistic regression was applied to categorical variables.
Statistical analysis
This study utilized Free Statistics 1.7, R language 3.4.1, and Empower(R) 2.0 software platforms for data analysis. Participants were stratified into tertile-based groups (low, moderate, high) according to CTI distribution. Baseline characteristics were described following variable-specific conventions: categorical variables as frequencies (percentages), normally distributed continuous variables as mean ± standard deviation, and non-normally distributed continuous variables as median (interquartile range). Between-group comparisons were conducted using chi-square tests, one-way Analysis of Variance, Student's t-test, or non-parametric tests as appropriate. A two-sided significance threshold of p < 0.05 was uniformly applied for all statistical inferences.
In the feature selection phase, the Boruta algorithm was employed to identify potential biomarkers associated with all-cause mortality in ADHF from high-dimensional clinical data (44). In detail, the Boruta method is based on a random forest model and identifies statistically significant features by comparing the importance of "real features" with that of "shadow features". The core steps are as follows: Shadow features with the same distribution as real features are generated (original feature values are randomly shuffled to eliminate their real association with the outcome). Both real and shadow features are input into the random forest model together, and feature importance is evaluated through repeated iterations ("Gini impurity decrease" was used as the importance metric in this study); if the importance of a real feature is significantly higher than that of all shadow features (assessed by Z-test), it is classified as an "important feature"; otherwise, it is labeled as "unimportant" or "tentative".
To optimize model stability, a dual-stage variable screening strategy was implemented: (1) variables exhibiting multicollinearity were first excluded using the variance inflation factor method (Supplementary Table 2), followed by (2) elimination of CTI components (e.g., TG, FPG) considering underlying collinearity concerns. Survival analysis was conducted using the Kaplan-Meier method, generating survival curves and performing log-rank tests, with the proportional hazards assumption visually validated. Three progressively adjusted Cox proportional hazards models were subsequently constructed: Model I was adjusted for demographic parameters (gender, age, smoking, and drinking status); Model II was further adjusted for clinical comorbidities (hypertension, stroke, CHD), NYHA functional class, and LVEF; As the final model, Model III was built upon Model II by additionally adjusting for hematological and functional laboratory parameters, including RBC, PLT, AST, Cr, UA, LDL-C, and NT-proBNP. All analyses were stratified by DM comorbidity status.
To elucidate the nonlinear relationship between CTI and prognosis, this study innovatively integrated multiple visualization techniques. We used restricted cubic spline (RCS) curves with 4 knots at the 5th, 35th, 65th, and 95th percentiles to visualize the dose-response relationship between CTI and mortality. The CTI inflection points where the association changed were identified using a recursive algorithm and piecewise Cox regression. For the knot selection in the RCS analysis, we followed the recommendations of Professor Harrell in Regression Modeling Strategies (45): Four knots provide an optimal balance, ensuring both curve smoothness and protection against overfitting, thereby preventing a loss of precision. Five knots may be more suitable for larger sample sizes, while 3 knots are recommended for small samples (n < 30). We also utilized a heatmap to reveal the joint influence of CTI components on mortality risk. This approach intuitively displays the interplay between inflammatory and metabolic factors in mortality risk evaluation (46).
Further subgroup analyses evaluated potential effect modifiers, including age, gender, cardiac function (LVEF/NYHA classification), and comorbidities (hypertension, stroke, and CHD). First, a stratified analysis was performed to assess the association between CTI and mortality in ADHF patients across different subpopulations. Then, the likelihood ratio test was used to quantify the differences between groups and determine the presence of interaction effects, with particular attention to the differential results in patients with and without DM.
To clarify the clinical application value of CTI, we further compared it with the existing HF risk score, ADHERE (Acute Decompensated Heart Failure National Registry) (47), to evaluate its predictive performance for short-term mortality outcomes. The evaluation metrics included the area under the curve and the net reclassification improvement to determine its incremental predictive value.
Sensitivity analysis
To test the robustness of the association between CTI and mortality, we conducted several sensitivity analyses: (1) For external validation, we used data from the U.S. (United States) National Health and Nutrition Examination Survey between 1998 and 2018 to analyze the association between CTI and all-cause mortality in U.S. patients with congestive HF. (2) To control for potential confounding by relevant medications, we adjusted for the use of statins, sodium-glucose cotransporter-2 inhibitors, and anti-inflammatory drugs in the multivariable model. (3) We further distinguished the types of study outcomes and evaluated the association between CTI and cardiovascular-specific mortality events.
Results
Baseline characteristics of the study population are presented according to CTI groups
This study enrolled 1,051 eligible ADHF patients, including 611 males (58.14%) and 440 females (41.86%), with a mean age of 69 years. Clinical characteristics of the study population were stratified by CTI tertiles (Table 1). Demographic analysis revealed a distinct gender distribution pattern in the high CTI group (9.87-13.34), with a significantly higher proportion of males. Comorbidity analysis showed that patients in the high CTI group not only had significantly elevated rates of hypertension, DM, and CHD but also demonstrated more severe hemodynamic profiles. Baseline characteristics of the high CTI group were further corroborated by multi-parameter laboratory assessments. While inflammatory markers showed significant elevations in WBC count and PLT count, metabolic profiles revealed more pronounced glucolipid metabolic dysregulation, along with hepatorenal dysfunction, characterized by significantly higher levels of FPG, TG, total cholesterol, LDL-C, ALT, Cr, UA, NT-proBNP, and lower HDL-C. Notably, patients with ADHF exhibited a progressive upward trend in TyG index and CRP levels, with significant between-group differences observed.
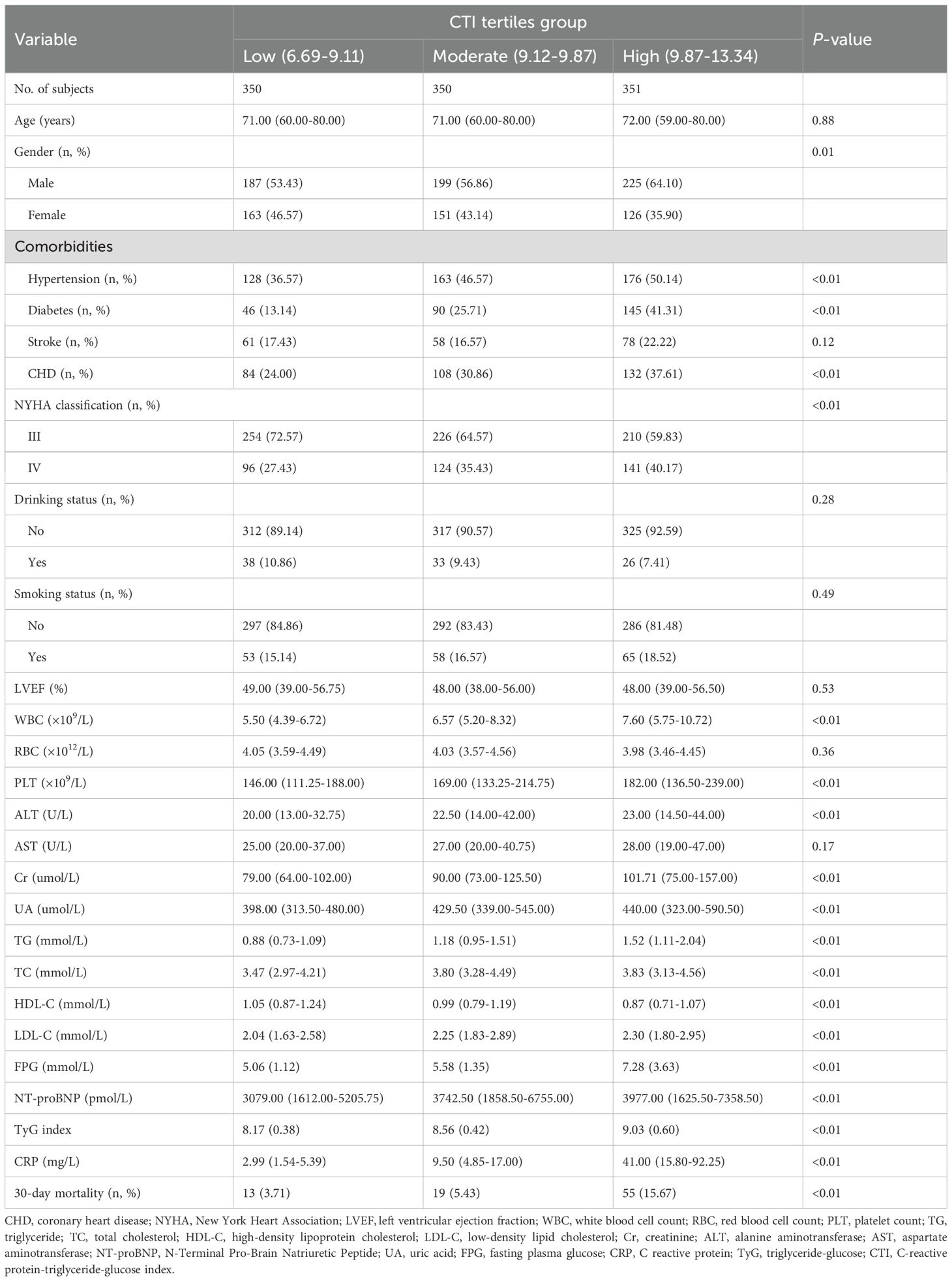
Table 1. Summary of baseline characteristics of the study population according to the CTI tertiles group.
Table 2 further stratifies the clinical baseline characteristics of ADHF patients with or without DM. Compared to non-DM patients, ADHF patients with comorbid DM exhibited a more complex comorbidity network, characterized by higher comorbidity rates of hypertension and CHD, along with more severe cardiac dysfunction deterioration. Laboratory profiles of patients with comorbid DM demonstrated a characteristic metabolic dysregulation pattern: in addition to significant elevations in WBC count, PLT count, FPG, and TG, higher levels of ALT and Cr suggested multi-organ involvement. Critically, both CRP and TyG index were significantly higher in DM patients.

Table 2. Summarize the baseline characteristics of the study population according to whether they are complicated with diabetes or not.
Follow-up results
A 30-day prognostic follow-up was conducted for 1,051 ADHF patients, during which 87 (8.3%) all-cause death events were observed. Survival analysis based on CTI tertile grouping demonstrated that ADHF patients in the high CTI group exhibited significantly higher all-cause mortality compared to those in the low and middle CTI groups (Figure 2, Log-rank p < 0.0001). Notably, this trend persisted in subgroup analyses of patients with and without DM, with DM patients in the high CTI group showing markedly lower survival rates than non-DM patients (Figures 2B, C).

Figure 2. 30-day survival curves of ADHF patients stratified by CTI tertiles (A) Total; (B) Diabetes group; (C) Non-diabetic group. ADHF: acute decompensated heart failure; CTI: C-reactive protein-triglyceride-glucose index.
Feature selection
This study systematically identified predictors significantly associated with 30-day all-cause mortality in ADHF patients using the Boruta ensemble feature selection algorithm (Figure 3). By iteratively comparing the importance scores of original features against randomly generated "shadow features," the Boruta algorithm ultimately confirmed 12 critical predictors of mortality in ADHF patients, including RBC count, NYHA classification, Cr, HDL-C, ALT, TyG index, AST, FPG, WBC count, NT-proBNP, CRP, and the CTI index. Notably, among all significant features, the CTI index demonstrated the highest importance score (Z-score ≈ 15), significantly outperforming other variables and underscoring its dominant role in prognostic assessment for ADHF patients. To assess the stability of the feature selection results, we employed the bootstrap method to generate 100 bootstrap samples. The Boruta algorithm was run independently on each sample, and the frequency of each feature being selected across all runs was recorded. The results showed that the features included in our final report were consistently selected in over 90% of the runs, which fully demonstrates that they are not the result of random fluctuations but rather important features with a robust association with the outcome variable.
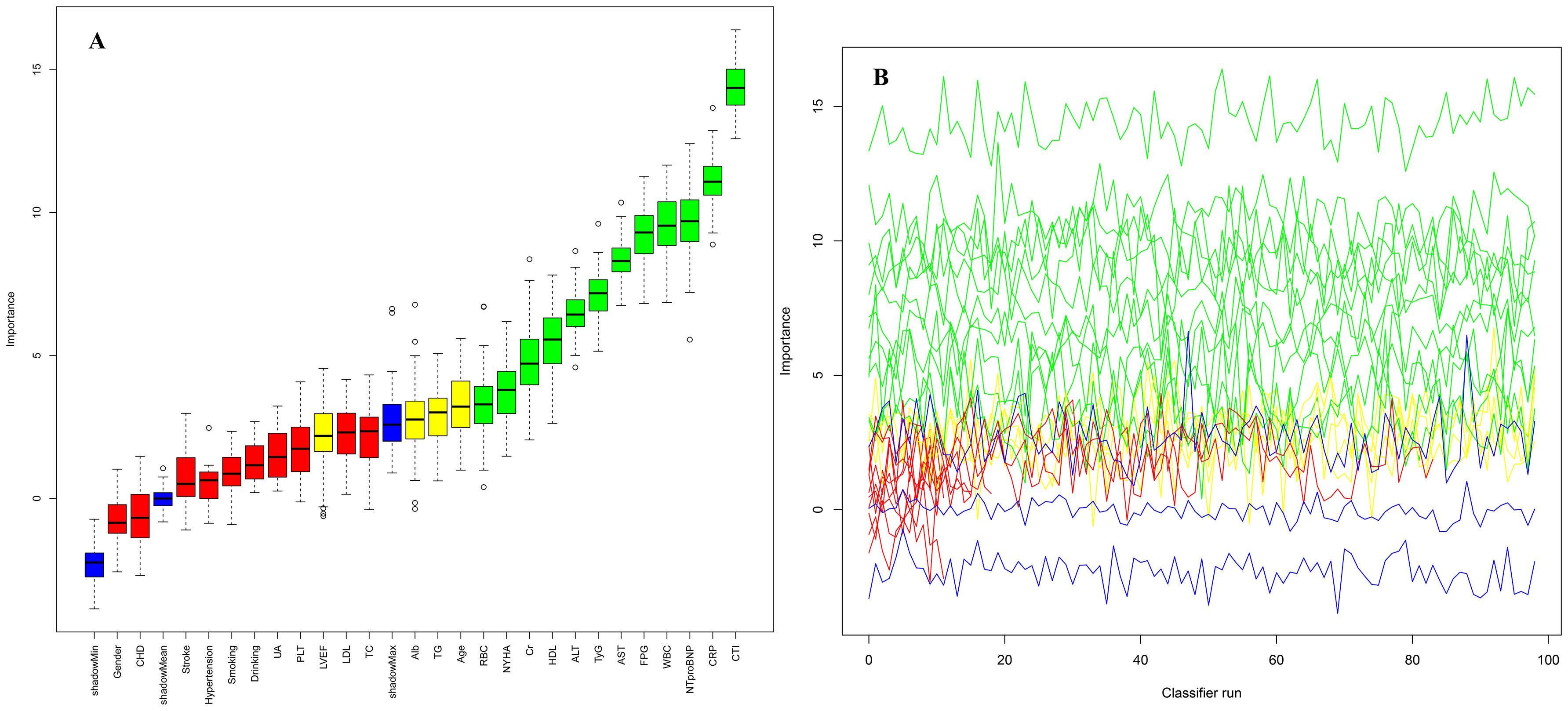
Figure 3. Feature selection for 30-day mortality in ADHF patients using the Boruta algorithm. (A) The process of feature selection; (B) The value evolution of the Z-score in the screening process. The horizontal axis shows the name of each variable and the number of times the classifier is run in (A, B) respectively. The vertical axis represents the Z-value of each variable. The green boxes and lines represent confirmed variables, the yellow ones represent tentative attributes, and the red ones represent rejected variables in the model calculation.
Association between CTI and 30-day mortality in ADHF patients across overall, DM, and non-DM populations
Multivariable Cox proportional hazards model analyses (Table 3) revealed a significant positive association between CTI and 30-day all-cause mortality risk in ADHF patients. The prognostic assessment of CTI as a continuous variable demonstrated robustness across three adjusted models: in the base model (adjusted for demographic characteristics), each 1-unit increase in CTI was associated with a 193% elevated mortality risk [Hazard ratios (HR): 2.93: 2.31, 3.71]. Subsequent adjustment for comorbidities and cardiac function (NYHA classification and LVEF) attenuated the HR to 2.78 (2.19, 3.52). In the fully adjusted model (Model III) incorporating hematological parameters, CTI remained significantly and positively associated with mortality risk (HR: 2.68: 2.09, 3.44). CTI-stratified analysis further highlighted its risk gradient effect: compared to the low CTI group, patients in the high CTI group exhibited a 184% increased mortality risk (HR: 2.84: 1.48, 5.43).
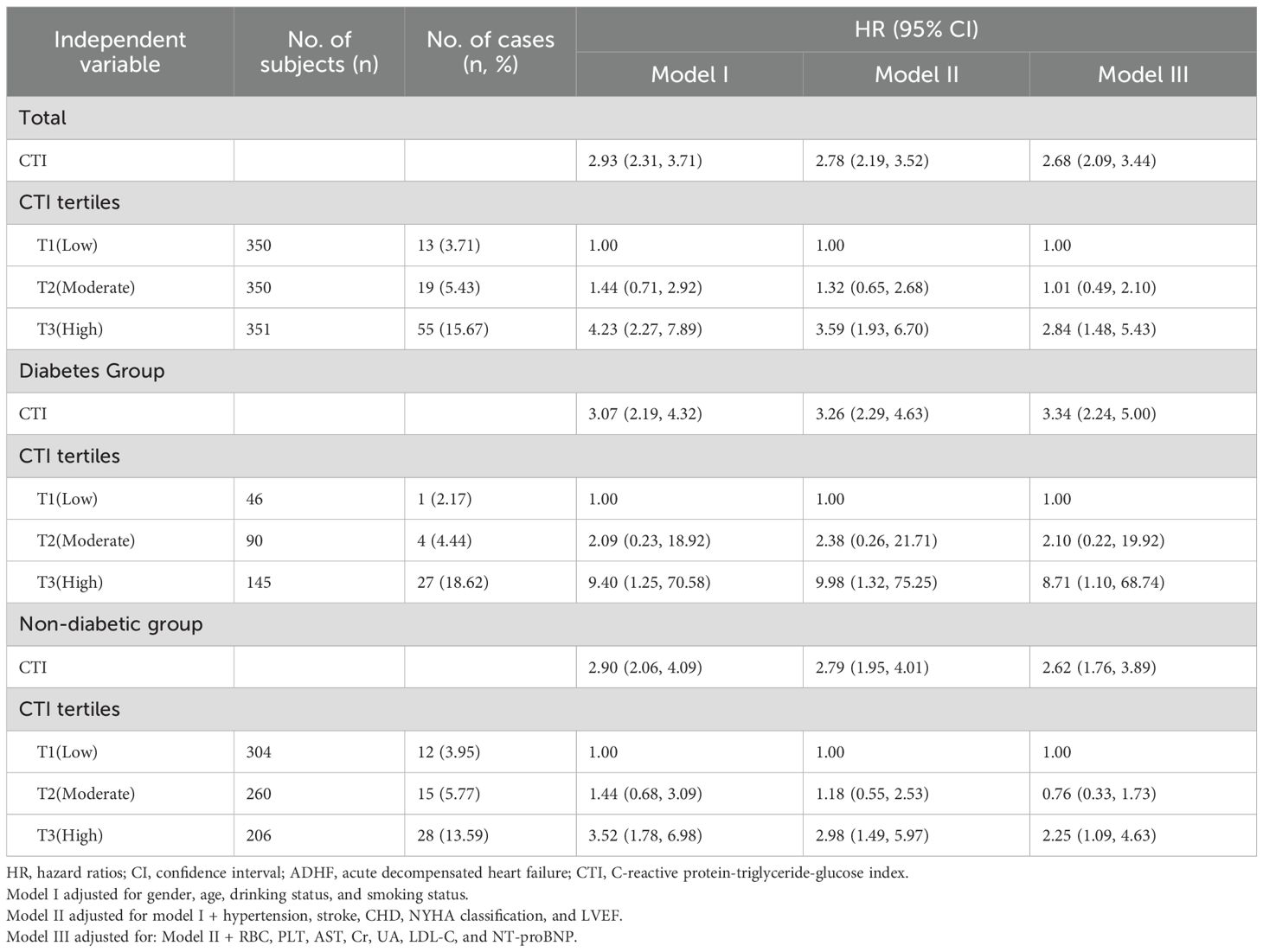
Table 3. Multivariable Cox regression analysis of the associations between CTI and 30-day mortality in patients with ADHF.
Notably, the CTI-associated mortality risk was significantly higher in DM patients. Specifically, each 1-unit increase in CTI corresponded to an elevated mortality risk in patients with comorbid ADHF and DM (HR: 3.34: 2.24, 5.00) compared to non-DM ADHF patients (HR: 2.62: 1.76, 3.89). When stratified by CTI tertiles, this glucose metabolism-dependent risk disparity became more pronounced: ADHF patients with DM in the high CTI group exhibited an 8.71-fold increased mortality risk compared to the low CTI group; significantly exceeding the 2.25-fold risk observed in non-DM patients.
Dose-response association between CTI and 30-day mortality in ADHF patients across overall, DM, and non-DM populations
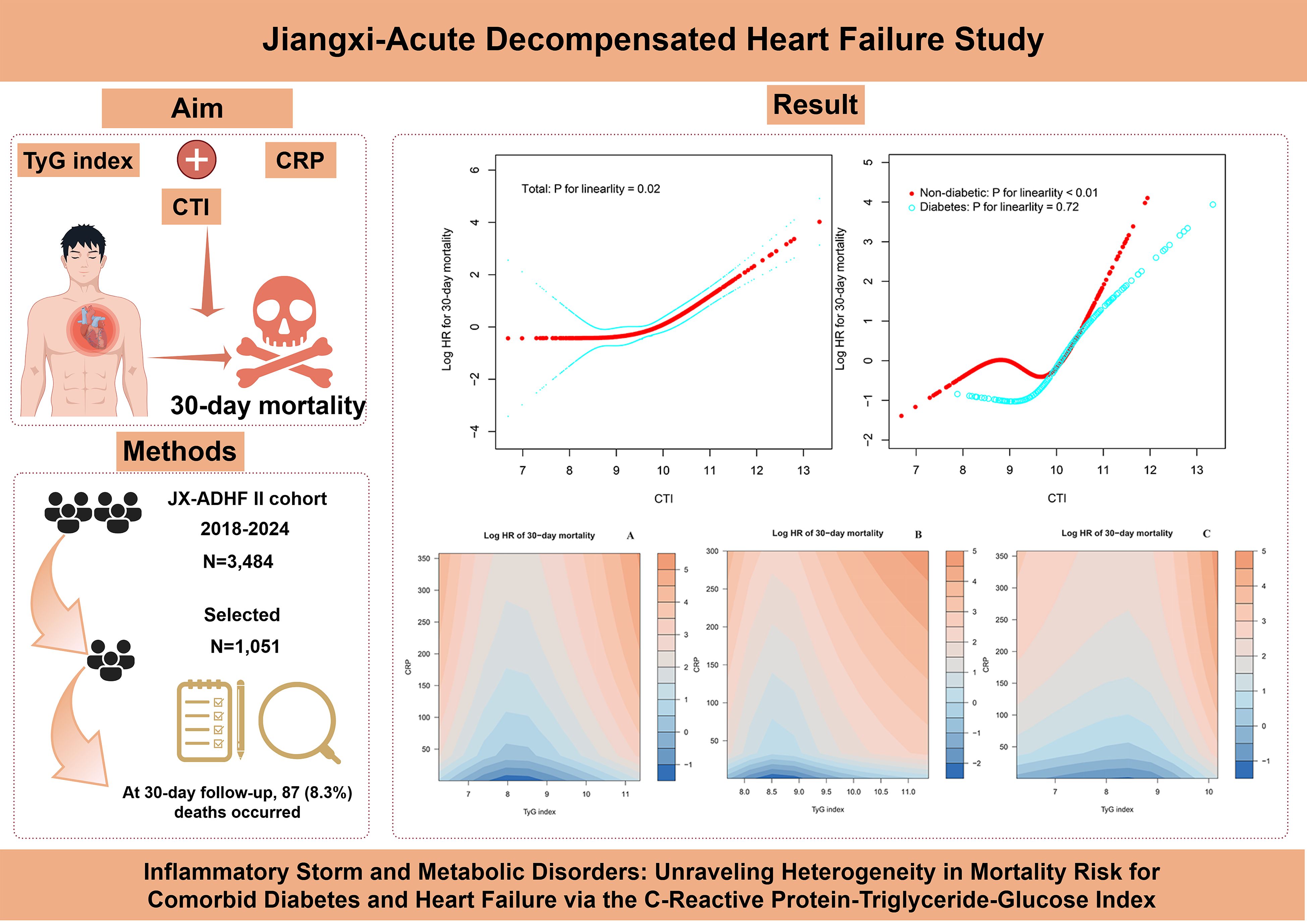
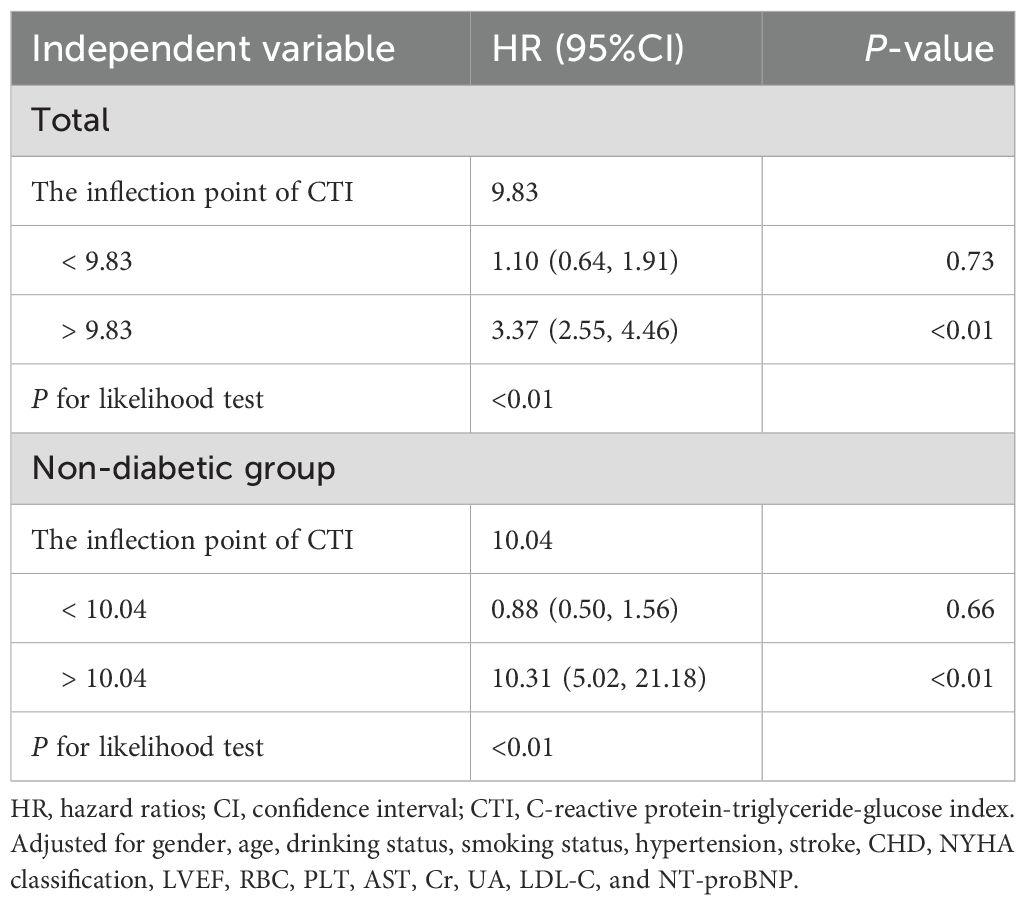
This study employed RCS analysis to further elucidate the dose-response association between CTI and 30-day all-cause mortality in ADHF patients (Figure 4). After adjusting for confounding factors, including demographic characteristics, comorbidities, cardiac function, and hematological parameters, a significant obtuse-angled nonlinear association between CTI and 30-day all-cause mortality was observed in the overall population (P for nonlinearity = 0.02). The inflection point value was calculated as 9.83 using recursive algorithms (Table 4), suggesting a potential threshold effect in the risk model. Specifically, when CTI < 9.83, ADHF patients exhibited a gradual increase in 30-day all-cause mortality risk (HR: 1.10: 0.64, 1.91). However, when CTI > 9.83, the risk curve steepened dramatically (HR: 3.37: 2.55, 4.46), with a 3.06-fold higher risk increment compared to the low-CTI segment (P for likelihood ratio test < 0.01).
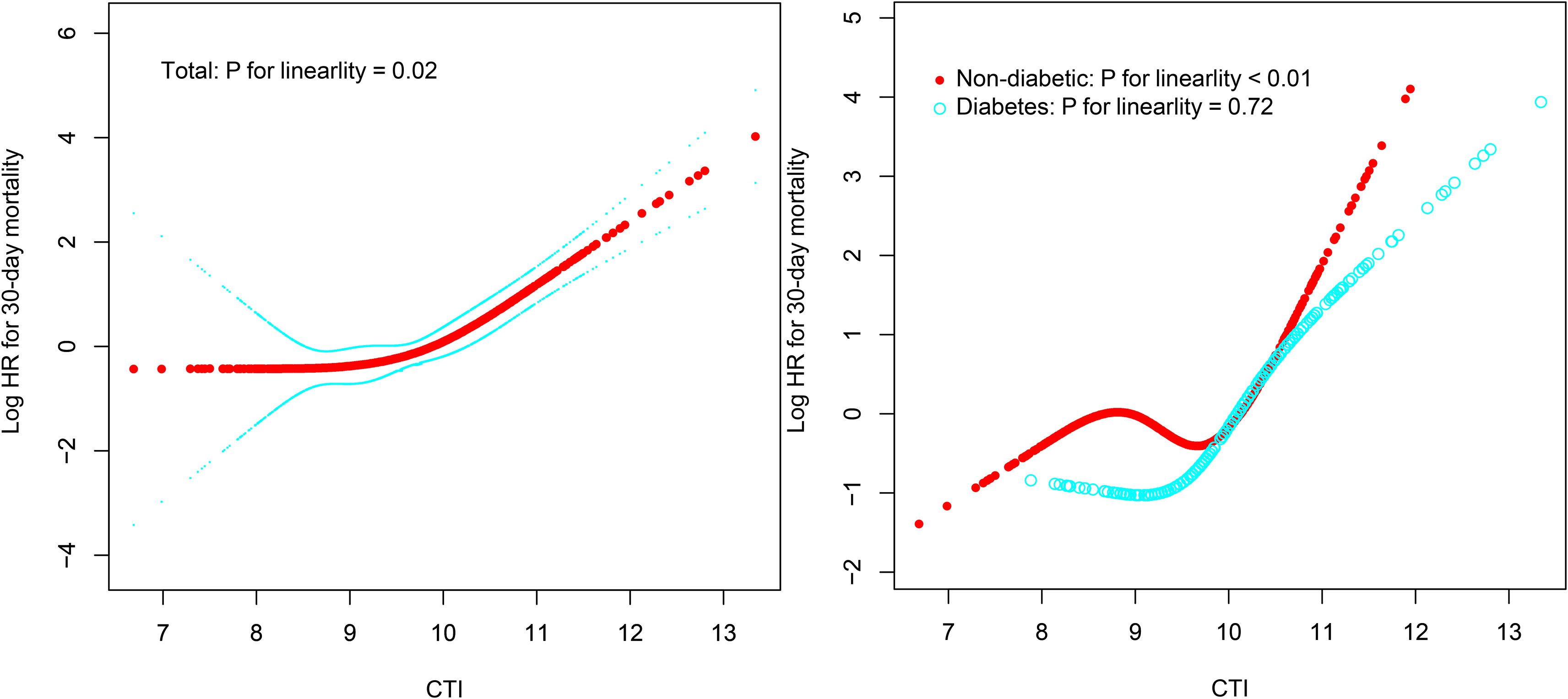
Figure 4. Fitting the dose-response relationship between CTI and 30-day all-cause mortality in ADHF patients with 4 knots restricted cubic spline. ADHF: acute decompensated heart failure; CTI: C-reactive protein-triglyceride-glucose index.
The study further evaluated the dose-response association between CTI and 30-day all-cause mortality risk in ADHF patients with and without DM. Results demonstrated a nonlinear association between CTI and all-cause mortality risk in non-DM ADHF patients (P for nonlinearity < 0.01), with an inflection point at 10.04 (Table 4). Notably, the high-CTI group exhibited a steeper risk escalation (HR: 10.31 vs. 0.88, P for likelihood ratio test < 0.01). In stark contrast, among ADHF patients with DM, CTI demonstrated a positive linear association with all-cause mortality risk (P for nonlinearity = 0.72).
Exploratory analysis of the combined association between TyG and CRP (components of CTI) and 30-day mortality in ADHF patients across overall, DM, and non-DM populations
Based on the adjustment strategy of Model III, we identified the potential interactive association between CTI components (TyG index and CRP) and 30-day mortality risk in ADHF patients using heatmaps. As shown in Figure 5, for the total population, the mortality risk is lowest (indicated by the darker blue band) when the TyG index remains between 7.5 and 9 and the CRP level is maintained below 50 mg/L. Notably, this association trend exhibits distinct patterns between patients with and without DM. For DM patients, the mortality risk is in a relatively low range when the TyG index is approximately between 8.25 and 9.0; for non-DM patients, the mortality risk is in a relatively low range when the TyG index is approximately between 7.0 and 9.0. Overall, the TyG index exerted a dominant effect on mortality risk in ADHF patients, while CRP further amplified the risk by modulating the effect intensity of TyG index.
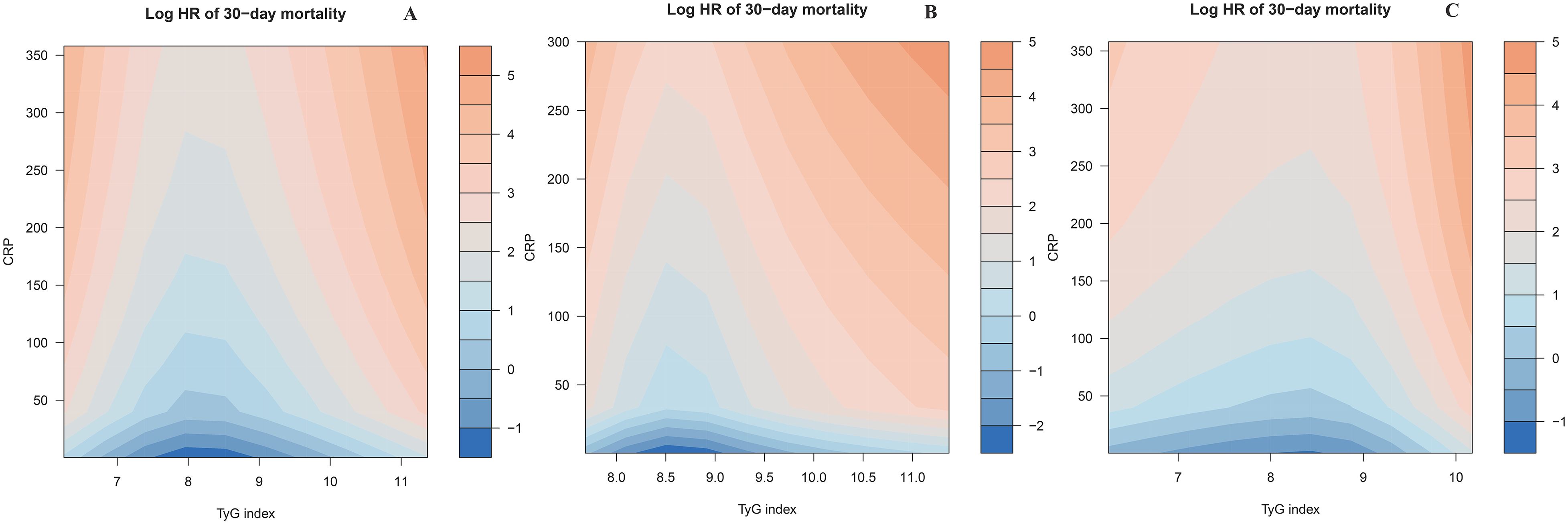
Figure 5. Heatmap of potential interaction effect between TyG index and CRP on 30-day all-cause mortality in ADHF patients (A) Total; (B) Diabetes group; (C) Non-diabetic group). The color gradient represents the combined effect of TyG index and CRP levels on mortality risk: the deeper the blue, the stronger the negative association with mortality, while the deeper the orange, the stronger the positive association with mortality. CRP: C-reactive protein; TyG index: triglyceride-glucose index; ADHF: acute decompensated heart failure.
Subgroup analysis stratified by DM and non-DM populations
In ADHF patients with and without DM, we further conducted stratified interaction analyses based on age, gender, LVEF, NYHA classification, and comorbidities (Table 5). The results revealed that in non-DM patients, the association between CTI and prognosis exhibited significant interaction effects in gender subgroups, hypertension subgroups, and stroke subgroups. Specifically, the association between CTI levels and all-cause mortality risk was stronger in male patients than in females (P for interaction = 0.03). Moreover, the mortality risk was further elevated in subgroups with comorbid hypertension or stroke (All P for interaction < 0.05). Notably, among patients with comorbid DM, the prognostic value of CTI demonstrated no subgroup differences, with risk associations remaining consistent across all subgroups (All P for interaction > 0.05).
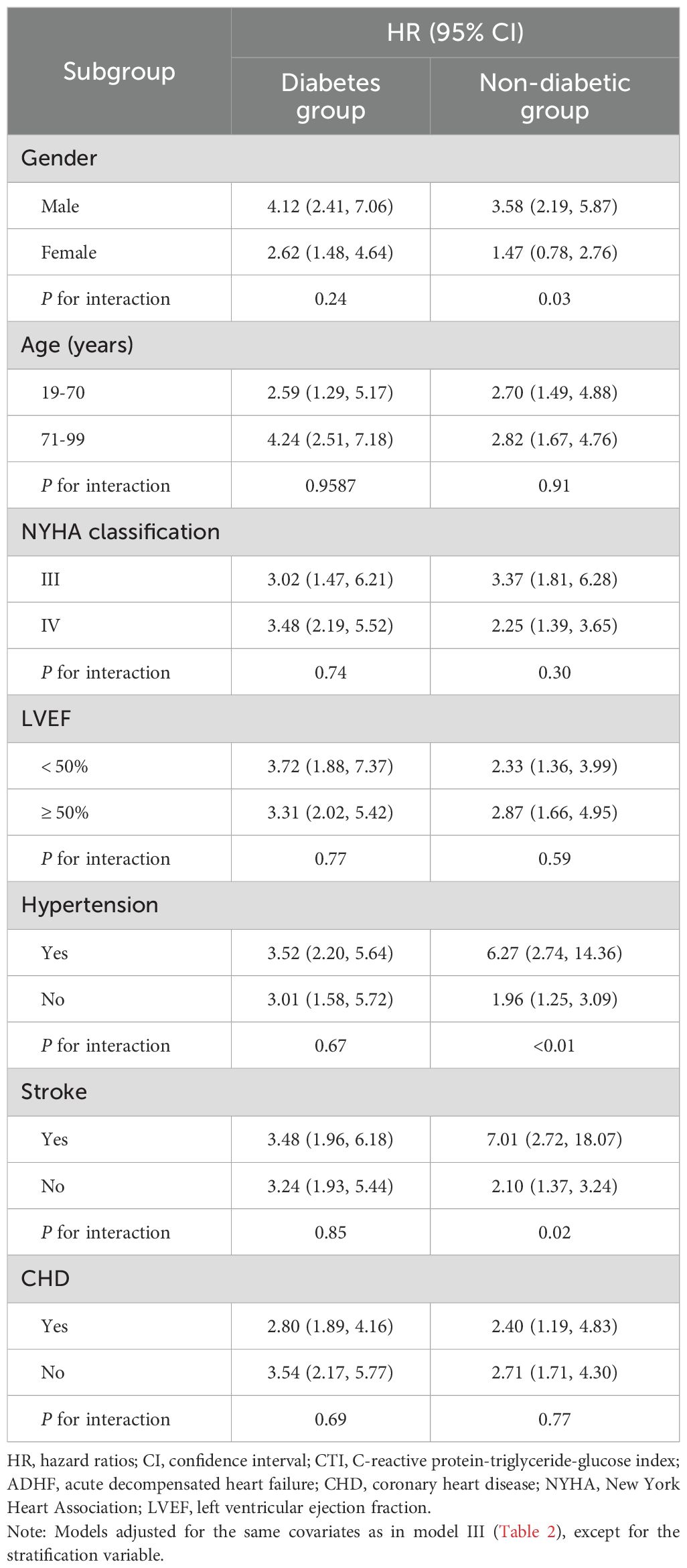
Table 5. Stratified analysis showed the relationship between CTI and 30-day mortality in patients with ADHF in different age, gender, NYHA classification, LVEF and whether combined with hypertension/stroke/CHD.
Comparative performance of the CTI and ADHERE models in mortality prediction
As shown in Supplementary Table 3, we compared the predictive performance of the CTI model and the ADHERE model. The study results indicated that the area under the curve of the CTI model was 0.73, which was significantly higher than the 0.64 of the ADHERE model (Delong P < 0.05). Continuous net reclassification analysis showed that the CTI model achieved a significant net improvement compared with the ADHERE model, with a net reclassification improvement of 0.18 (P < 0.05).
Sensitivity analysis
The validation results in the cohort of U.S. patients with congestive HF further revealed a positive association between CTI and all-cause mortality, reproducing the core findings of this study (Supplementary Table 4). In addition, even after further adjusting for statins, sodium-glucose cotransporter-2 inhibitors, and anti-inflammatory drugs, the main conclusions of this study remained robust (Supplementary Table 5). Finally, the analysis based on cardiovascular-specific mortality yielded results consistent with those of all-cause mortality, further supporting the robustness of the study findings (Supplementary Table 6).
Discussion
Based on the Jiangxi-ADHF II cohort study, CTI was confirmed as an independent risk factor for 30-day all-cause mortality in ADHF patients, with its risk assessment value being more pronounced among those with comorbid DM. Notably, heatmaps further demonstrated that the prognostic impact of CTI components exhibited distinct risk patterns between ADHF patients with and without DM.
This study focuses on the complex role of multimorbidity and its clinical translational value, with particular emphasis on the joint lethal effects of HF-DM comorbidity (6–11, 48). The findings demonstrate a significant positive correlation between CTI and short-term mortality risk in ADHF patients, with the risk gradient further amplified in those with comorbid DM. This suggests that metabolic-inflammatory interactions may exacerbate clinical deterioration in comorbid states. This finding aligns closely with the cross-disease risk prediction value of CTI as a systemic biological marker: Xu et al. demonstrated a linear positive correlation between CTI and CHD risk in cardiovascular research (43), while Huo's team and Tang's team independently revealed its positive association with stroke risk across different cohorts (41, 42). In oncology cohorts, Shi et al. reported that elevated CTI conferred a 225% increased 90-day mortality risk in gastrointestinal cancer patients (49), and the INSCOC cancer cachexia cohort further showed that each 1-standard deviation increase in CTI elevated short-term mortality risk by 22% (50). Additional studies have identified significant associations between CTI and multisystem endocrine-metabolic/immune regulatory disorders (51–55), collectively suggesting CTI's strong generalizability as a trans-disease risk stratification tool.
This study employed Cox regression models, combined with RCS, and heatmaps, to systematically elucidate the joint effects of the CTI and its components (TyG index and CRP) on all-cause mortality risk in ADHF patients with DM comorbidity. Among ADHF patients with comorbid DM, CTI demonstrated a significant nonlinear positive association with 30-day all-cause mortality risk (P for non-linearity = 0.02), manifesting as a threshold effect; whereas in non-DM ADHF patients, a linearly increasing risk pattern was observed (P for non-linearity = 0.72). The heatmap results further elucidated: We found that the lowest mortality risk occurred when the TyG index was within a certain range concurrently with a CRP level under 50 mg/L, a pattern consistent in both DM and non-DM patients. This finding is closely aligned with the theoretical threshold of metabolic homeostasis in chronic diseases (56–64). Notably, the longitudinal CRP gradient effect demonstrated a linear association, with all-cause mortality risk in ADHF patients progressively increasing alongside rising CRP levels. Furthermore, when comparing ADHF patients with DM to those without DM, the former exhibited relatively higher CRP levels under equivalent mortality risk. This discrepancy may be attributed to the DM microenvironment, which potentially amplifies inflammatory signaling transduction efficiency through increased accumulation of advanced glycation end-products (65). At the level of interaction between the TyG index and CRP, joint effect analysis revealed that the TyG index exerted a dominant effect on mortality risk, while CRP further amplified this risk by modulating the magnitude of the TyG index's effect. Specifically, when the TyG index exceeded 9 and CRP levels progressively increased, patients exhibited a significantly elevated 30-day mortality risk compared to baseline levels. This joint effect may stem from the vicious cycle formed between TyG index-induced IR and CRP-mediated chronic inflammation. This cycle escalates mortality risk through multiple pathways, including disrupting myocardial energy metabolism and exacerbating mitochondrial dysfunction (12–15).
This study further revealed population heterogeneity in the association between CTI and 30-day all-cause mortality risk in ADHF patients through subgroup analysis. Specifically, among ADHF patients without DM, CTI-related mortality risk demonstrated gender- and comorbidity-specific stratification characteristics: male patients exhibited significantly higher mortality risk compared to females, and this risk was further elevated in patients with comorbid hypertension or stroke. Previous studies have consistently validated the survival advantage of female HF patients, with this gender-based benefit persisting across different ejection fraction subtypes (66, 67). The underlying mechanisms can be elucidated from multiple dimensions: Biologically, analyses of sample data revealed that male patients exhibit significantly higher median CTI values compared to females, which may be attributed to male-specific visceral fat accumulation patterns and the cardioprotective effects of estrogen in females (68, 69). Behaviorally, adverse lifestyle factors such as smoking and drinking consumption habits are more prevalent in males compared to females, which further exacerbates the risk of future adverse events (70–72). In terms of comorbidity modification effects, patients with comorbid hypertension or stroke exhibit higher CTI levels. This phenomenon might be driven by enhanced IR and systemic inflammation associated with comorbid conditions (73–76). Additionally, the comorbid states of hypertension or stroke inherently contribute to further elevated mortality risk (77–79).
The value of this study lies in providing a novel and actionable strategy for the clinical management of ADHF. Owing to its simplicity and cost-effectiveness, the CTI possesses significant advantages for clinical translation (40–43). We advocate for its systematic integration at the frontline of patient management to establish an evidence-based risk stratification system. The core applications include: embedding an automated CTI assessment module in the information system to achieve early warning and precise intervention for high-risk ADHF patients; meanwhile, integrating it with mature risk models or Artificial Intelligence-assisted systems to enable dynamic, intelligent mortality risk prediction, thereby paving the way for smarter clinical pathways.
Strengths and limitations
This study represents a positive association between CTI and all-cause mortality risk in ADHF patients, and further reveals that DM comorbidity significantly amplifies this correlation. Additionally, through the heatmap visualization analysis, we have further investigated the relationship between CTI components (TyG index and CRP) and mortality risk. These findings on cross-domain biomarkers offer a new framework for personalizing risk management in ADHF, informing clinical decision-making.
The study has several limitations: (1) As a regional observational cohort study, the generalizability of findings may be limited by regional and ethnic constraints, necessitating validation in large-scale multicenter cohorts before broader extrapolation; (2) Although multiple confounders have been corrected for, unmeasured residual confounders may still affect the effect estimates; (3) The dynamic impacts of metabolic-modulating medications on TyG index and CRP levels were not quantitatively assessed, which may result in systematic underestimation of the true exposure-outcome associations. (4) Since CRP is not a routine test for HF patients, a large number of cases with missing CRP data were consequently excluded from our analysis. This may have introduced selection bias and could affect the generalizability of the results. This limitation requires further validation in future studies. (5) While 30-day follow-up effectively captures acute-phase events, it may underestimate the time-dependent associations between metabolic dysfunction, inflammation, and long-term prognosis. Future studies should employ extended follow-up duration with dynamic predictive modeling to elucidate longitudinal effect trajectories, and incorporate additional study outcomes (such as readmission rates) to enhance the applicability of the findings.
Conclusion
This study supports the clinical value of CTI as a novel biomarker for short-term mortality in ADHF patients and elucidates the enhancing effect of DM comorbidity on this positive association. Further heatmap analyses suggest that controlling TyG and CRP levels in ADHF patients, particularly those with DM comorbidity, may significantly reduce short-term all-cause mortality. These findings underscore the pivotal role of the metabolic-inflammatory pathway in ADHF progression, offering valuable references for early risk stratification and targeted therapeutic interventions in ADHF management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Review Committee of Jiangxi Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
NZ: Formal Analysis, Funding acquisition, Investigation, Methodology, Software, Writing – original draft. LX: Investigation, Writing – review & editing. SZ: Investigation, Writing – review & editing. QW: Investigation, Writing – review & editing. HL: Investigation, Writing – review & editing. ZX: Investigation, Writing – review & editing. GX: Investigation, Supervision, Validation, Writing – review & editing. GS: Investigation, Supervision, Writing – review & editing. HY: Investigation, Writing – review & editing. SH: Formal Analysis, Investigation, Validation, Writing – original draft. TL: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. WW: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing. YZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82460078, 82460091, 81670370, and 82360073); the Natural Science Foundation of Jiangxi Province (20232BAB216004, 20224ACB206004, 20151BAB215046 and 20224BAB216015); the Jiangxi Province Traditional Chinese Medicine Science and Technology Plan Project (2023B1218).
Acknowledgments
We would like to thank Jiangxi Provincial People’s Hospital for its strong support to the research project and the members of the JX-ADHF research team for their great efforts in the data collection process. We thank Home for Researchers editorial team (www.home-for-researchers.com) for Graphic Abstract editing service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1689238/full#supplementary-material
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Morrish NJ, Wang SL, Stevens LK, Fuller JH, and Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. (2001) 44 Suppl 2:S14–21. doi: 10.1007/pl00002934
3. Kannel WB, Hjortland M, and Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. (1974) 34:29–34. doi: 10.1016/0002-9149(74)90089-7
4. Nichols GA, Gullion CM, Koro CE, Ephross SA, and Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. (2004) 27:1879–84. doi: 10.2337/diacare.27.8.1879
5. Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. (2019) 140:e294–324. doi: 10.1161/CIR.0000000000000691
6. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, et al. Predicting survival in heart failure: a risk score based on 39–372 patients from 30 studies. Eur Heart J. (2013) 34:1404–13. doi: 10.1093/eurheartj/ehs337
7. Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail. (2016) 9:e002560. doi: 10.1161/CIRCHEARTFAILURE.115.002560
8. From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. (2006) 119:591–9. doi: 10.1016/j.amjmed.2006.05.024
9. Dauriz M, Targher G, Laroche C, Temporelli PL, Ferrari R, Anker S, et al. Association between diabetes and 1-year adverse clinical outcomes in a multinational cohort of ambulatory patients with chronic heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Diabetes Care. (2017) 40:671–8. doi: 10.2337/dc16-2016
10. Johansson I, Dahlström U, Edner M, Näsman P, Rydén L, and Norhammar A. Prognostic implications of type 2 diabetes mellitus in ischemic and nonischemic heart failure. J Am Coll Cardiol. (2016) 68:1404–16. doi: 10.1016/j.jacc.2016.06.061
11. Targher G, Dauriz M, Laroche C, Temporelli PL, Hassanein M, Seferovic PM, et al. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. (2017) 19:54–65. doi: 10.1002/ejhf.679
12. Lopaschuk GD, Karwi QG, Tian R, Wende AR, and Abel ED. Cardiac energy metabolism in heart failure. Circ Res. (2021) 128:1487–513. doi: 10.1161/CIRCRESAHA.121.318241
13. Riehle C and Abel ED. Insulin signaling and heart failure. Circ Res. (2016) 118:1151–69. doi: 10.1161/CIRCRESAHA.116.306206
14. Sebastian SA, Padda I, and Johal G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr Probl Cardiol. (2024) 49:102344. doi: 10.1016/j.cpcardiol.2023.102344
15. Wu H and Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. (2020) 126:1549–64. doi: 10.1161/CIRCRESAHA.119.315896
16. Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: A systematic review. Int J Endocrinol. (2020) 2020:4678526. doi: 10.1155/2020/4678526
17. Cheng H, Huang W, Huang X, Miao W, Huang Y, and Hu Y. The triglyceride glucose index17 predicts short-term mortality in non-diabetic patients with acute heart failure. Adv Clin Exp Med. (2024) 33:103–10. doi: 10.17219/acem/166043
18. Zhou Q, Yang J, Tang H, Guo Z, Dong W, Wang Y, et al. High triglyceride-glucose (TyG) index is associated with poor prognosis of heart failure with preserved ejection fraction. Cardiovasc Diabetol. (2023) 22:263. doi: 10.1186/s12933-023-02001-4
19. Zhou Y, Wang C, Che H, Cheng L, Zhu D, Rao C, et al. Association between the triglyceride-glucose index and the risk of mortality among patients with chronic heart failure: results from a retrospective cohort study in China. Cardiovasc Diabetol. (2023) 22:171. doi: 10.1186/s12933-023-01895-4
20. Han S, Wang C, Tong F, Li Y, Li Z, Sun Z, et al. Triglyceride glucose index and its combination with the Get with the Guidelines-Heart Failure score in predicting the prognosis in patients with heart failure. Front Nutr. (2022) 9:950338. doi: 10.3389/fnut.2022.950338
21. Özcan KS, Hayıroğlu MI, and Çınar T. Admission triglyceride-glucose index is predictor of long-term mortality and appropriate implantable cardiac defibrillator therapy in patients with heart failure. biomark Med. (2023) 17:487–96. doi: 10.2217/bmm-2023-0113
22. Sun T, Huang X, Zhang B, Ma M, Chen Z, Zhao Z, et al. Prognostic significance of the triglyceride-glucose index for patients with ischemic heart failure after percutaneous coronary intervention. Front Endocrinol (Lausanne). (2023) 14:1100399. doi: 10.3389/fendo.2023.1100399
23. Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. (2022) 21:88. doi: 10.1186/s12933-022-01507-7
24. Yao Z, Zhang Y, and Wu H. Regulation of C-reactive protein conformation in inflammation. Inflammation Res. (2019) 68:815–23. doi: 10.1007/s00011-019-01269-1
25. Huang J, Baum Y, Alemozaffar M, Ogan K, Harris W, Kucuk O, et al. C-reactive protein in urologic cancers. Mol Aspects Med. (2015) 45:28–36. doi: 10.1016/j.mam.2015.04.001
26. Bittoni MA, Focht BC, Clinton SK, Buckworth J, and Harris RE. Prospective evaluation of C-reactive protein, smoking and lung cancer death in the Third National Health and Nutrition Examination Survey. Int J Oncol. (2015) 47:1537–44. doi: 10.3892/ijo.2015.3141
27. Mc Causland FR, Claggett B, Burdmann EA, Eckardt KU, Kewalramani R, Levey AS, et al. C-reactive protein and risk of ESRD: results from the trial to reduce cardiovascular events with aranesp therapy (TREAT). Am J Kidney Dis. (2016) 68:873–81. doi: 10.1053/j.ajkd.2016.07.022
28. Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G, MB P, SG T, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. (2010) 375:132–40. doi: 10.1016/S0140-6736(09)61717-7
29. Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Pennells L, AM W, IR W, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. (2012) 367:1310–20. doi: 10.1056/NEJMoa1107477
30. Badimon L, Peña E, Arderiu G, Padró T, Slevin M, Vilahur G, et al. C-reactive protein in atherothrombosis and angiogenesis. Front Immunol. (2018) 9:430. doi: 10.3389/fimmu.2018.00430
31. Avan A, Tavakoly Sany SB, Ghayour-Mobarhan M, Rahimi HR, Tajfard M, and Ferns G. Serum C-reactive protein in the prediction of cardiovascular diseases: Overview of the latest clinical studies and public health practice. J Cell Physiol. (2018) 233:8508–25. doi: 10.1002/jcp.26791
32. Lakhani I, Wong MV, Hung JKF, Gong M, Waleed KB, Xia Y, et al. Diagnostic and prognostic value of serum C-reactive protein in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart Fail Rev. (2021) 26:1141–50. doi: 10.1007/s10741-020-09927-x
33. Matsumoto H, Kasai T, Sato A, Ishiwata S, Yatsu S, Shitara J, et al. Association between C-reactive protein levels at hospital admission and long-term mortality in patients with acute decompensated heart failure. Heart Vessels. (2019) 34:1961–8. doi: 10.1007/s00380-019-01435-9
34. Minami Y, Kajimoto K, Sato N, Hagiwara N, Takano T, and ATTEND Study Investigators. C-reactive protein level on admission and time to and cause of death in patients hospitalized for acute heart failure. Eur Heart J Qual Care Clin Outcomes. (2017) 3:148–56. doi: 10.1093/ehjqcco/qcw054
35. Burger PM, Koudstaal S, Mosterd A, Fiolet ATL, Teraa M, van der Meer MG, et al. C-reactive protein and risk of incident heart failure in patients with cardiovascular disease. J Am Coll Cardiol. (2023) 82:414–26. doi: 10.1016/j.jacc.2023.05.035
36. Cui C, Liu L, Qi Y, Han N, Xu H, Wang Z, et al. Joint association of TyG index and high sensitivity C-reactive protein with cardiovascular disease: a national cohort study. Cardiovasc Diabetol. (2024) 23:156. doi: 10.1186/s12933-024-02244-9
37. Huang Y, Zhou Y, Xu Y, Wang X, Zhou Z, Wu K, et al. Inflammatory markers link triglyceride-glucose index and obesity indicators with adverse cardiovascular events in patients with hypertension: insights from three cohorts. Cardiovasc Diabetol. (2025) 24:11. doi: 10.1186/s12933-024-02571-x
38. Wan B, Wang S, Hu S, Han W, Qiu S, Zhu L, et al. The comprehensive effects of high-sensitivity C-reactive protein and triglyceride glucose index on cardiometabolic multimorbidity. Front Endocrinol (Lausanne). (2025) 16:1511319. doi: 10.3389/fendo.2025.1511319
39. Feng G, Yang M, Xu L, Liu Y, Yu J, Zang Y, et al. Combined effects of high sensitivity C-reactive protein and triglyceride-glucose index on risk of cardiovascular disease among middle-aged and older Chinese: Evidence from the China Health and Retirement Longitudinal Study. Nutr Metab Cardiovasc Dis. (2023) 33:1245–53. doi: 10.1016/j.numecd.2023.04.001
40. Ruan GT, Xie HL, Zhang HY, Liu CA, Ge YZ, Zhang Q, et al. A novel inflammation and insulin resistance related indicator to predict the survival of patients with cancer. Front Endocrinol (Lausanne). (2022) 13:905266. doi: 10.3389/fendo.2022.905266
41. Huo G, Tang Y, Liu Z, Cao J, Yao Z, and Zhou D. Association between C-reactive protein-triglyceride glucose index and stroke risk in different glycemic status: insights from the China Health and Retirement Longitudinal Study (CHARLS). Cardiovasc Diabetol. (2025) 24:142. doi: 10.1186/s12933-025-02686-9
42. Tang S, Wang H, Li K, Chen Y, Zheng Q, Meng J, et al. C-reactive protein-triglyceride glucose index predicts stroke incidence in a hypertensive population: a national cohort study. Diabetol Metab Syndr. (2024) 16:277. doi: 10.1186/s13098-024-01529-z
43. Xu M, Zhang L, Xu D, Shi W, and Zhang W. Usefulness of C-reactive protein-triglyceride glucose index in detecting prevalent coronary heart disease: findings from the National Health and Nutrition Examination Survey 1999-2018. Front Cardiovasc Med. (2024) 11:1485538. doi: 10.3389/fcvm.2024.1485538
44. Kursa M and Rudnicki W. Feature selection with the Boruta package. J Stat Software. (2010) 36:1–13. doi: 10.18637/jss.v036.i11
45. Harrell FE. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2nd ed Vol. 582. . Cham, Switzerland: Springer (2015).
46. He S, Xie L, Xie G, Jian G, Jiang K, Lu Z, et al. Independent and combined associations of high-density lipoprotein cholesterol-modified triglyceride-glucose index with all-cause and cardiovascular mortality in patients with acute decompensated heart failure. Front Endocrinol (Lausanne). (2025) 16:1629066. doi: 10.3389/fendo.2025.1629066
47. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. (2005) 149:209–16. doi: 10.1016/j.ahj.2004.08.005
48. Skou ST, Mair FS, Fortin M, Guthrie B, Nunes BP, Miranda JJ, et al. Multimorbidity. Nat Rev Dis Primers. (2022) 8:48. doi: 10.1038/s41572-022-00376-4
49. Ruan GT, Shi JY, Xie HL, Zhang HY, Zhao H, Liu XY, et al. Prognostic importance of an indicator related to systemic inflammation and insulin resistance in patients with gastrointestinal cancer: a prospective study. Front Oncol. (2024) 14:1394892. doi: 10.3389/fonc.2024.1394892
50. Ruan GT, Deng L, Xie HL, Shi JY, Liu XY, Zheng X, et al. Systemic inflammation and insulin resistance-related indicator predicts poor outcome in patients with cancer cachexia. Cancer Metab. (2024) 12:3. doi: 10.1186/s40170-024-00332-8
51. Huang C, You H, Zhang Y, Li Z, Li M, Feng X, et al. Association between C-reactive protein-triglyceride glucose index and depressive symptoms in American adults: results from the NHANES 2005 to 2010. BMC Psychiatry. (2024) 24:890. doi: 10.1186/s12888-024-06336-4
52. Mei Y, Li Y, Zhang B, Xu R, and Feng X. Association between the C-reactive protein-triglyceride glucose index and erectile dysfunction in US males: results from NHANES 2001-2004. Int J Impot Res. (2025) 37:612–22. doi: 10.1038/s41443-024-00945-z
53. Zhang B, Gu Y, Chen Y, Xia W, Shao N, Zhuang Q, et al. Association between C-reactive protein-triglyceride glucose index and testosterone levels among adult men: analyses of NHANES 2015–2016 data. Sex Med. (2025) 13:qfaf012. doi: 10.1093/sexmed/qfaf012
54. Zhou Y, Lin H, Weng X, Dai H, and Xu J. Correlation between hs-CRP-triglyceride glucose index and NAFLD and liver fibrosis. BMC Gastroenterol. (2025) 25:252. doi: 10.1186/s12876-025-03870-7
55. Ren Y, Xu R, Zhang J, Jin Y, Zhang D, Wang Y, et al. Association between the C-reactive protein-triglyceride-glucose index and endometriosis: a cross-sectional study using data from the national health and nutrition examination survey 1996-2006. BMC Womens Health. (2025) 25:13. doi: 10.1186/s12905-024-03541-x
56. Zhu H, Chen Y, Ding D, and Chen H. Association between different insulin resistance indices and all-cause mortality in patients with diabetic kidney disease: a prospective cohort study. Front Endocrinol (Lausanne). (2025) 15:1427727. doi: 10.3389/fendo.2024.1427727
57. Xu H, Xie J, Xia Y, Niu H, Wang H, and Zhan F. Association of TyG index with mortality at 28 days in sepsis patients in intensive care from MIMIC IV database. Sci Rep. (2025) 15:2344. doi: 10.1038/s41598-025-86746-w
58. Zhang HJ, Han LL, Luo W, Hu M, Zhang HZ, and Liao YL. The triglyceride-glucose index: a predictor of mortality risk among myocardial infarction survivors. Sci Rep. (2024) 14:27512. doi: 10.1038/s41598-024-78056-4
59. Liu C, Liang D, Xiao K, and Xie L. Association between the triglyceride-glucose index and all-cause and CVD mortality in the young population with diabetes. Cardiovasc Diabetol. (2024) 23:171. doi: 10.1186/s12933-024-02269-0
60. Hao B, Lyu L, Xu J, Zhu X, Xu C, Gao W, et al. The relationship between triglyceride-glucose index and prospective key clinical outcomes in patients hospitalised for coronary artery disease. Cardiovasc Diabetol. (2024) 23:40. doi: 10.1186/s12933-024-02132-2
61. Zhang Q, Xiao S, Jiao X, and Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001-2018. Cardiovasc Diabetol. (2023) 22:279. doi: 10.1186/s12933-023-02030-z
62. Zhang H, Wang L, Zhang Q, Song Y, Cai M, Bao J, et al. Non-linear association of triglyceride-glucose index with cardiovascular and all-cause mortality in T2DM patients with diabetic kidney disease: NHANES 2001–2018 retrospective cohort study. Lipids Health Dis. (2024) 23:253. doi: 10.1186/s12944-024-02249-z
63. Zhao M, Xiao M, Tan Q, and Lu F. Triglyceride glucose index as a predictor of mortality in middle-aged and elderly patients with type 2 diabetes in the US. Sci Rep. (2023) 13:16478. doi: 10.1038/s41598-023-43512-0
64. Yu Y, Wang J, Ding L, Huang H, Cheng S, Deng Y, et al. Sex differences in the nonlinear association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Diabetol Metab Syndr. (2023) 15:136. doi: 10.1186/s13098-023-01117-7
65. Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. (2019) 20:247–60. doi: 10.1016/j.redox.2018.09.025
66. Martínez-Sellés M, Doughty RN, Poppe K, Whalley GA, Earle N, Tribouilloy C, et al. Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta-analysis. Eur J Heart Fail. (2012) 14:473–9. doi: 10.1093/eurjhf/hfs026
67. Qiu W, Wang W, Wu S, Zhu Y, Zheng H, and Feng Y. Sex differences in long-term heart failure prognosis: a comprehensive meta-analysis. Eur J Prev Cardiol. (2024) 31:2013–23. doi: 10.1093/eurjpc/zwae256
68. Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obes (Silver Spring). (2007) 15:2984–93. doi: 10.1038/oby.2007.356
69. Ciarambino T, Crispino P, Guarisco G, and Giordano M. Gender differences in insulin resistance: new knowledge and perspectives. Curr Issues Mol Biol. (2023) 45:7845–61. doi: 10.3390/cimb45100496
70. Wang S, Ungvari GS, Forester BP, Chiu HFK, Wu Y, Kou C, et al. Gender differences in general mental health, smoking, drinking and chronic diseases in older adults in Jilin province, China. Psychiatry Res. (2017) 251:58–62. doi: 10.1016/j.psychres.2017.02.007
71. Jha P, Peto R, Zatonski W, Boreham J, Jarvis MJ, and Lopez AD. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet. (2006) 368:367–70. doi: 10.1016/S0140-6736(06)68975-7
72. GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2018) 392:1015–35. doi: 10.1016/S0140-6736(18)31310-2
73. Sinha S and Haque M. Insulin resistance is cheerfully hitched with hypertension. Life (Basel). (2022) 12:564. doi: 10.3390/life12040564
74. Ding PF, Zhang HS, Wang J, Gao YY, Mao JN, Hang CH, et al. Insulin resistance in ischemic stroke: Mechanisms and therapeutic approaches. Front Endocrinol (Lausanne). (2022) 13:1092431. doi: 10.3389/fendo.2022.1092431
75. Kelly PJ, Lemmens R, and Tsivgoulis G. Inflammation and stroke risk: A new target for prevention. Stroke. (2021) 52:2697–706. doi: 10.1161/STROKEAHA.121.034388
76. Guzik TJ and Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. (2017) 70:660–7. doi: 10.1161/HYPERTENSIONAHA.117.07802
77. Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: A systematic review and network meta-analysis. JAMA Cardiol. (2017) 2:775–81. doi: 10.1001/jamacardio.2017.1421
78. Oh JY, Allison MA, and Barrett-Connor E. Different impacts of hypertension and diabetes mellitus on all-cause and cardiovascular mortality in community-dwelling older adults: the Rancho Bernardo Study. J Hypertens. (2017) 35:55–62. doi: 10.1097/HJH.0000000000001145
Keywords: C-reactive protein-triglyceride-glucose index, diabetes mellitus, acute decompensated heart failure, TyG index, C-reactive protein
Citation: Zhang N, Xie L, Zhang S, Wang Q, Lu H, Xiong Z, Xie G, Sheng G, Yang H, He S, Liao T, Wang W and Zou Y (2025) Inflammatory storm and metabolic disorders: unraveling heterogeneity in mortality risk for comorbid diabetes mellitus and heart failure via the C-reactive protein-triglyceride-glucose index. Front. Endocrinol. 16:1689238. doi: 10.3389/fendo.2025.1689238
Received: 20 August 2025; Accepted: 30 October 2025;
Published: 19 November 2025.
Edited by:
Margalida Monserrat-Mesquida, Fundació Institut d’Investigació Sanitaria Illes Balear, SpainReviewed by:
Azadeh Anna Nikouee, Loyola University Chicago, United StatesSixtus Aguree, Indiana University, United States
Copyright © 2025 Zhang, Xie, Zhang, Wang, Lu, Xiong, Xie, Sheng, Yang, He, Liao, Wang and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiming He, aHNtOTcxMjI2QDE2My5jb20=; Tanghong Liao, bGlhb3Rhbmdob25nQDEyNi5jb20=; Wei Wang, d3dhbmdjdnJpQDE2My5jb20=; Yang Zou, anh5eHl6eUAxNjMuY29t
 Na Zhang
Na Zhang Lin Xie2
Lin Xie2 Shuhua Zhang
Shuhua Zhang Guobo Xie
Guobo Xie Guotai Sheng
Guotai Sheng Shiming He
Shiming He Yang Zou
Yang Zou