- 1Department of Neurosurgery, The First Hospital of Shanxi Medical University, Taiyuan, China
- 2Department of Vascular Surgery, The Second Hospital of Shanxi Medical University, Taiyuan, China
- 3Vascular Institute of Shanxi Medical University, Taiyuan, China
Objective: To identify risk factors for cerebrospinal fluid (CSF) leakage after endoscopic endonasal pituitary adenoma surgery and to develop a clinical prediction model aimed at facilitating early detection and reducing the risk of related complications.
Methods: Clinical data were retrospectively collected from patients who underwent endoscopic endonasal pituitary adenoma resection between January 2021 and September 2024. Postoperative CSF leakage was diagnosed based on clinical manifestations, glucose testing, and expert consensus. Univariable and multivariable logistic regression analyses were performed to determine independent risk factors, which were subsequently incorporated into a nomogram. The predictive performance of the model was evaluated using receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA).
Results: Among 254 patients, 19 (7.48%) developed postoperative CSF leakage. Compared with those without leakage, affected patients exhibited larger tumor diameters, wider sellar floor bony windows, higher intraoperative CSF leak grades, greater suprasellar extension, and higher postoperative pneumocephalus grades. Multivariate logistic regression identified postoperative pneumocephalus grade (OR = 12.90, 95% CI: 3.24-59.80, p < 0.001), sellar floor bony window size (OR = 1.20, 95% CI: 1.07-1.37, p = 0.004), suprasellar extension grade (OR = 5.87, 95% CI: 1.15-46.10, p = 0.049), and intraoperative CSF leak grade (OR = 4.71, 95% CI: 1.14-20.30, p = 0.032) as independent predictors of postoperative CSF leakage. The nomogram incorporating these factors achieved excellent predictive accuracy, with an AUC of 0.948 (95% CI: 0.906–0.991).
Conclusion: This study confirms that a higher grade of pneumocephalus and a larger sellar floor bony window are significant risk factors for postoperative CSF leak following endoscopic endonasal transsphenoidal surgery for pituitary tumors. Building on these findings, we developed a clinical prediction model for postoperative CSF leak by integrating relevant preoperative, intraoperative, and postoperative factors. This model facilitates the early prevention, identification, and management of CSF leaks, which is crucial for reducing the risk of associated complications and improving patient outcomes.
1 Introduction
Pituitary adenomas are benign tumors located in the sellar region and account for approximately 10-15% of all intracranial space-occupying lesions (1). With advances in imaging and surgical techniques, the endoscopic endonasal approach has become the preferred treatment for most pituitary adenomas (2). However, due to the thin skull base bone and complex anatomical structures, complications such as cerebrospinal fluid (CSF) leakage, diabetes insipidus, and hypopituitarism remain relatively common (3, 4). Among these, postoperative CSF leakage is particularly concerning, as it may lead to a range of secondary complications, including intracranial hypotension, meningitis, and tension pneumocephalus, which in severe cases can even be life-threatening (5).
Intracranial pneumocephalus is a common complication following endoscopic endonasal skull base surgery (6). Clinical manifestations can include headache, visual disturbances, and in severe cases, tension pneumocephalus, which may lead to rapid neurological deterioration and even threaten life (7). Recent studies have indicated that both the extent and distribution of intracranial air are closely associated with postoperative CSF leakage (6, 8), yet few reports have incorporated these parameters into clinical prediction models. The sellar floor bony window refers to the area of bone removal during endoscopic endonasal surgery, reflecting the degree of bony destruction and the technical difficulty of skull base reconstruction (9). Although its precise role in postoperative CSF leakage has not been fully elucidated, several studies suggest that the integrity of the sellar floor is crucial for supporting reconstructive materials during skull base repair (9, 10).
Building upon previously reported risk factors, we further evaluated variables such as pneumocephalus grade and the size of the sellar floor bony window to more accurately quantify the potential risks for CSF leak following endoscopic endonasal pituitary adenectomy. We subsequently developed and validated a clinical prediction model for postoperative CSF leak. This model aims to facilitate the early identification, management, and intervention in high-risk patients, thereby reducing the incidence of postoperative complications and improving patient outcomes. Furthermore, we hypothesize that patients with a larger intraoperative sellar floor bony window and a higher postoperative pneumocephalus grade are at a significantly increased risk of developing a CSF leak.
2 Methods
2.1 Study population
This retrospective study was approved by the Ethics Committee of the First Hospital of Shanxi Medical University. Clinical data from 254 patients who underwent endoscopic endonasal surgery for pituitary adenomas in the Department of Neurosurgery between January 2021 and September 2024 were analyzed. Inclusion criteria were: (1) age ≥ 18 years; (2) preoperative cranial MRI diagnosis of pituitary adenoma and planned endoscopic endonasal resection, with postoperative pathological confirmation of pituitary neuroendocrine tumor. Exclusion criteria were: (1) history of craniotomy for pituitary tumor resection; (2) presence of severe cardiovascular, cerebrovascular, or malignant diseases; and (3) incomplete clinical or imaging data (with pre- and postoperative sellar MRI and a 24-hour postoperative CT scan). Data extraction was performed by one medical staff member and verified for statistical analysis by another. All patients or their legal guardians provided informed consent for surgery, and the study was conducted in accordance with the Declaration of Helsinki (Supplementary Figure 1 shows a typical case of postoperative cerebrospinal fluid leakage).
2.2 Study variables
The primary variables included patient demographics, comorbidities, tumor characteristics (tumor size, Knosp grade, suprasellar extension grade, pathological type), intraoperative parameters (extended approach, sellar floor bony window size, intraoperative CSF leak grade, tumor consistency, extent of tumor resection), and postoperative pneumocephalus grade. The maximum diameter of the sellar floor drilled, which was measured jointly by the primary surgeon and the first assistant with all results reaching consensus and being recorded in the operative notes, was documented as the bony window size. The extent of tumor resection was determined based on intraoperative assessment and postoperative sellar MRI. Tumor consistency and use of a nasoseptal flap were obtained from operative records. Suprasellar extension was classified according to Hardy grading. Tumor consistency was assessed using the Rutkowski grading system (11). Intraoperative CSF leakage was graded using the Kelly system (12), and pneumocephalus grade was assessed on routine CT scans performed within 24 hours postoperatively, according to the Banu grading system. (6) (Supplementary Tables 2-4).
2.3 Skull base dural reconstruction and diagnosis of postoperative CSF leakage
Skull base dural reconstruction was performed according to the intraoperative Kelly grading system. For patients classified as Kelly grade 0 or 1, a unilateral reconstruction strategy was typically employed, such as coverage with a single layer of artificial dura mater. For those with Kelly grade 2 or 3, a multilayer reconstruction technique was adopted, involving the use of artificial dura, fat grafts, fascia lata, or a vascularized nasoseptal flap, with the placement sequence adjusted as appropriate based on intraoperative conditions. Notably, prophylactic lumbar drainage (LD) was not implemented for patients with Kelly grade 2 or 3.
Postoperative CSF leakage was diagnosed when any of the following criteria were met:(1) persistent clear fluid drainage from the nasal cavity with a positive glucose test result; (2) absence of persistent clear nasal discharge but a positive glucose test result confirmed by repeated testing, with the final diagnosis established by two experienced skull base surgeons (HW and RB, each with more than five years of neuroendoscopic surgical experience).Patients without nasal discharge and with negative glucose test results were classified as having no postoperative CSF leakage.
2.4 Feature analysis
Preoperative, intraoperative, and postoperative factors potentially associated with postoperative CSF leakage were analyzed. Continuous variables with a normal distribution were expressed as mean ± standard deviation ( ± s) and compared between groups using the independent-samples t-test. Non-normally distributed continuous variables were presented as median (Q1, Q3) and compared using the Mann–Whitney U test. Categorical variables were summarized as counts and percentages, and differences between groups were assessed using the chi-square test. Factors showing statistical significance in univariate logistic regression were further evaluated using receiver operating characteristic (ROC) curves and included in a multivariable binary logistic regression model to identify independent predictors of postoperative CSF leakage. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using R software (version 4.4.1).
2.5 Predictive model development:
Independent risk factors identified from multivariable logistic regression (p < 0.05) were incorporated into a binary logistic regression model to construct a predictive nomogram using R software. Model performance was assessed by calibration curves and decision curve analysis (DCA), and discriminative ability was evaluated using ROC curves. The ROC and calibration curves quantified the predictive accuracy, while the DCA curve assessed the clinical utility of the model.
3 Results
3.1 Baseline characteristics:
A total of 254 patients who underwent endoscopic endonasal pituitary adenoma resection were included in this study (Table 1). Patients were classified into two groups according to the presence or absence of postoperative CSF leakage. Among them, 19 patients (7.48%) developed postoperative CSF leakage during hospitalization. All affected patients were managed with conservative measures, including strict supine bed rest, therapeutic lumbar drainage, and prophylactic antibiotic therapy. Following these interventions, all patients demonstrated clinical improvement and were discharged in stable condition. The median age (interquartile range) was 58 (37, 63) years in the CSF leakage group and 55 (47, 63) years in the non-leakage group (p = 0.798). The median body mass index (BMI) was 23.31 (22.04, 25.39) in the leakage group and 24.97 (22.49, 27.34) in the non-leakage group (p = 0.063). Tumor maximum diameter was significantly larger in the leakage group (2.8 cm [2.5, 4.0]) compared with the non-leakage group (2.5 cm [2.0, 3.1], p = 0.038). The median sellar floor bony window size was 30 mm (25, 30) in the leakage group versus 20 mm (15, 25) in the non-leakage group (p < 0.001).
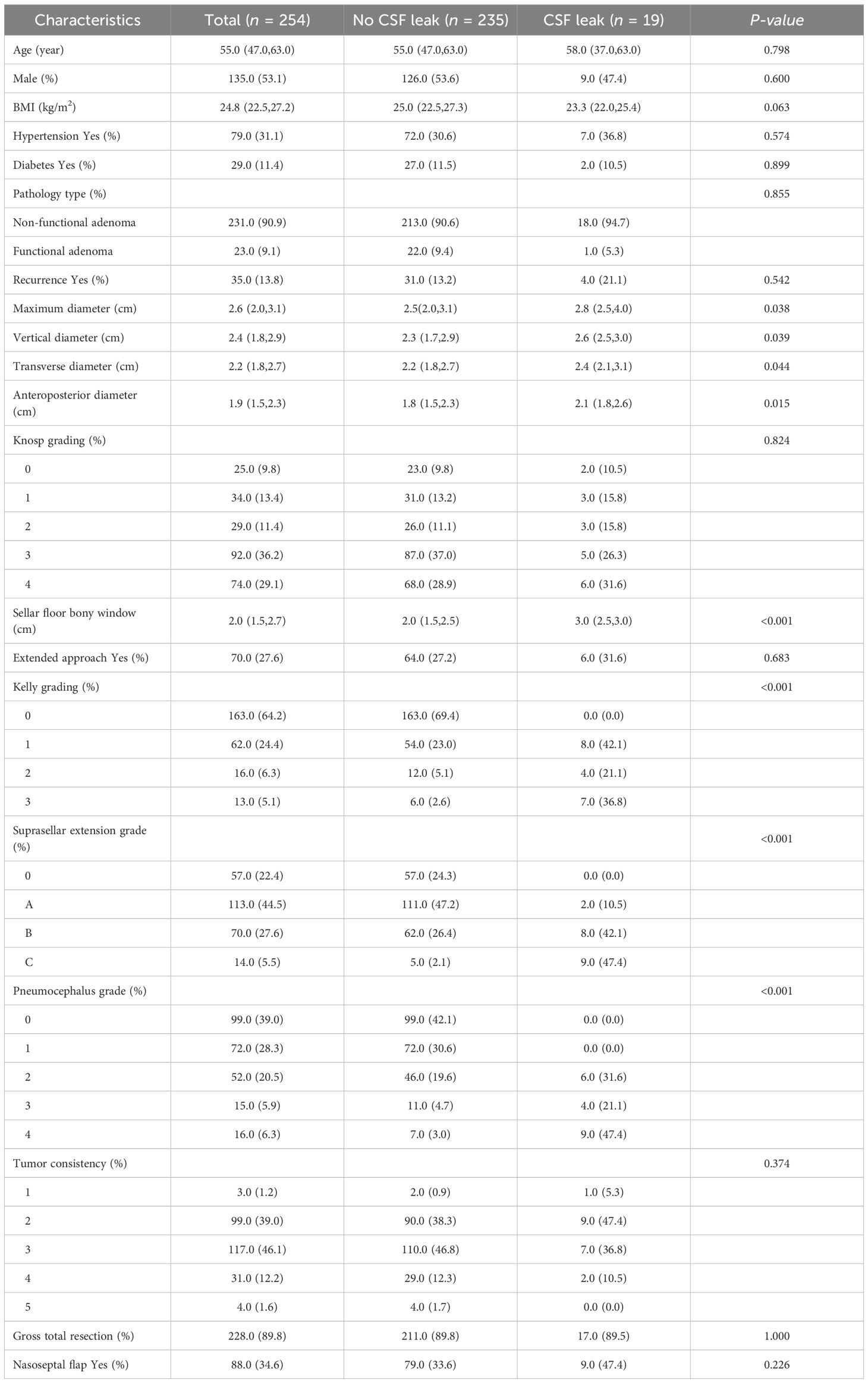
Table 1. Baseline characteristics of pituitary adenoma patients with and without postoperative cerebrospinal fluid leakage.
Regarding intraoperative CSF leak (Kelly grading), 0-level was observed in 163 patients (all non-leakage, 69.4%), 1-level in 62 patients (leakage group 8, 42.1%), 2-level in 16 patients (leakage group 4, 21.1%), and 3-level in 13 patients (leakage group 7, 36.8%). Suprasellar extension grades were distributed as follows: 0-level, 57 patients (all non-leakage); A-level, 113 patients (leakage group 2, 10.5%); B-level, 70 patients (leakage group 8, 42.1%); C-level, 14 patients (leakage group 9, 47.4%). Postoperative pneumocephalus grades were: 0-level, 99 patients (all non-leakage); 1-level, 72 patients (all non-leakage); 2-level, 52 patients (leakage group 6, 31.6%); 3-level, 15 patients (leakage group 4, 21.1%); 4-level, 16 patients (leakage group 9, 47.4%). Complete tumor resection was achieved in 211 patients (89.8%) in the non-leakage group and 17 patients (89.5%) in the leakage group.
3.2 Univariate and multivariate logistic regression analysis
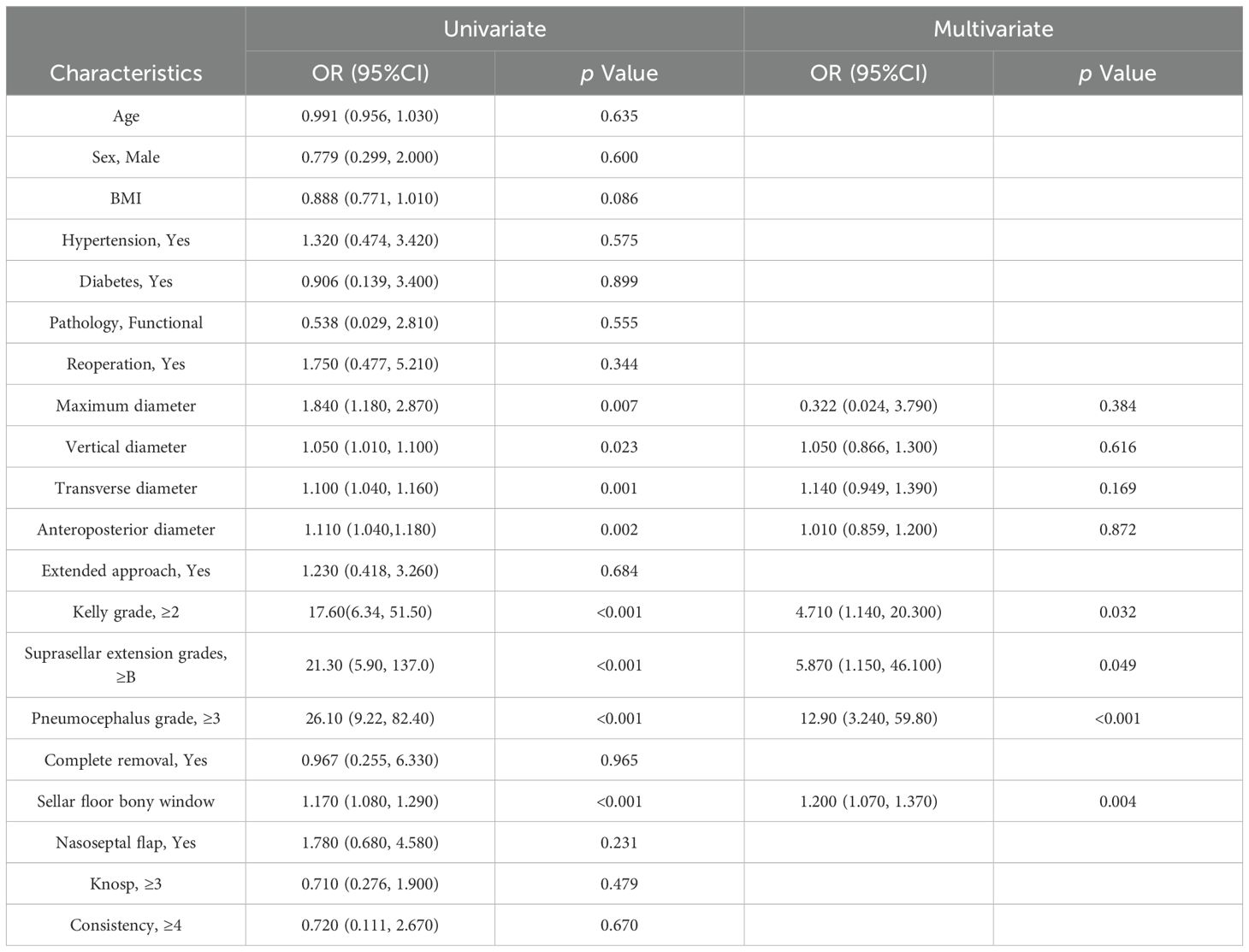
Univariate logistic regression (Table 2) indicated that age, sex, BMI, hypertension, diabetes, pathological type, repeat surgery, extended approach, extent of tumor resection, nasoseptal flap use, Knosp grade, and tumor consistency were not significantly associated with postoperative CSF leakage. Tumor maximum diameter, vertical diameter, anterior-posterior diameter, transverse diameter, Kelly grade, suprasellar extension, pneumocephalus grade, and sellar floor bony window size showed significant differences between groups. Multivariate logistic regression identified the following independent predictors: Kelly grade (OR = 4.71, 95% CI: 1.14-20.30, p = 0.032), suprasellar extension grade (OR = 5.87, 95% CI: 1.15-46.10, p = 0.049), pneumocephalus grade (OR = 12.90, 95% CI: 3.24-59.80, p < 0.001), and sellar floor bony window size (OR = 1.20, 95% CI: 1.07-1.37, p = 0.004).
3.3 ROC curve analysis:
Factors showing significant differences in univariate logistic regression were further evaluated using ROC curves (Figure 1). The area under the curve (AUC) values were as follows: tumor maximum diameter 0.643, vertical diameter 0.642, transverse diameter 0.639, anterior-posterior diameter 0.668, Kelly grade 0.753, suprasellar extension 0.805, pneumocephalus grade 0.804, and sellar floor bony window size 0.773 (Supplementary Table 1).
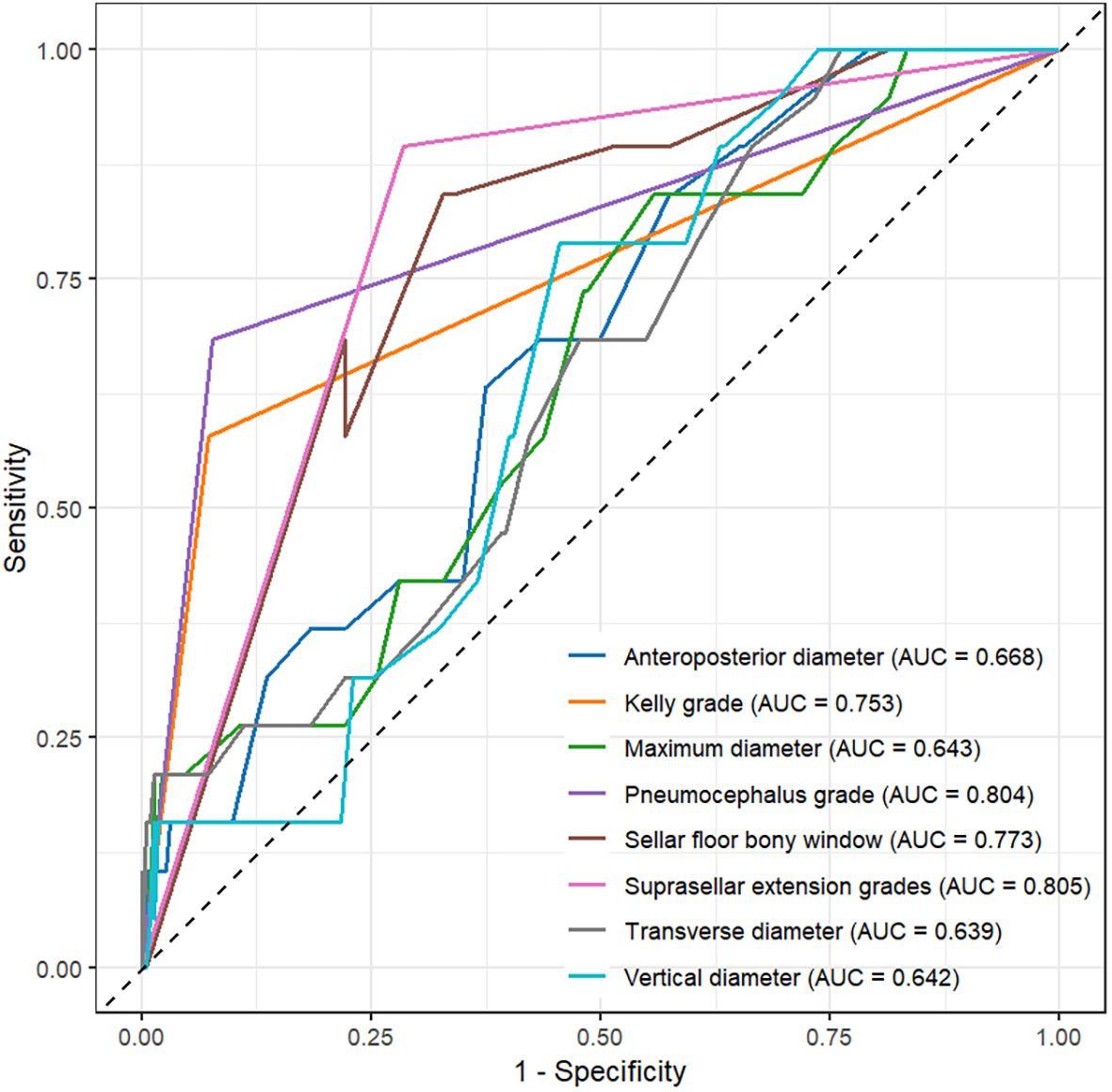
Figure 1. ROC curve analysis of maximum diameter, vertical diameter, transverse diameter, anteroposterior diameter, Kelly grade, suprasellar extension grade, pneumocephalus grade, and sellar floor opening size.
3.4 Predictive model construction and evaluation
A clinical prediction model was developed and presented as a nomogram based on the results of multivariate logistic regression analysis (Figure 2). The scoring system incorporated four parameters. The size of the sellar floor bony window was determined intraoperatively by measuring the maximal diameter of the drilled area. The degree of suprasellar extension was evaluated on preoperative imaging. The intraoperative Kelly grade was assessed during the surgical procedure, and the grade of pneumocephalus was determined using postoperative CT scans. Each parameter was assigned a specific score according to its regression coefficient, and the total score was calculated by summing the individual scores. The total point axis of the nomogram corresponded to the bottom scale, which represented the predicted risk of postoperative CSF leakage. The ROC curve demonstrated excellent discriminative ability with an AUC of 0.948 (95% CI: 0.906-0.991) (Figure 3A). Calibration curves showed that the bias-corrected line closely approximated the ideal line, indicating good predictive accuracy (Figure 3B). Decision curve analysis (DCA) indicated that, for threshold probabilities of 0-80%, using the nomogram provided greater net benefit compared with treating all or no patients (Figure 3C).
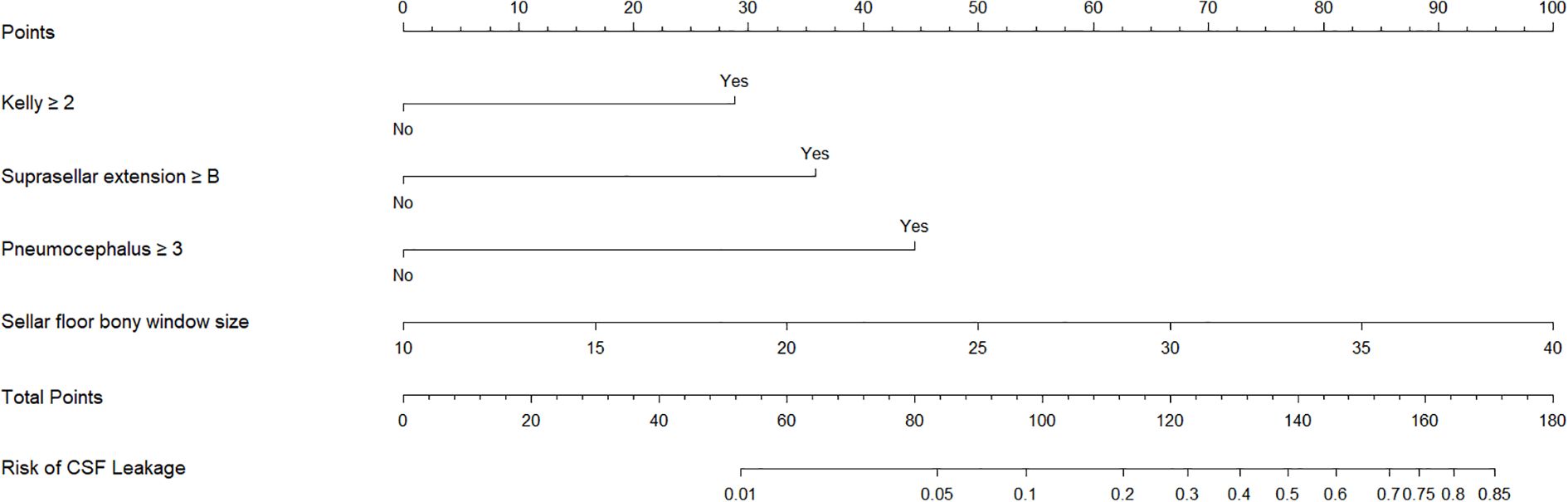
Figure 2. Nomogram construction. Nomogram constructed based on kelly grade, suprasellar extension grade, pneumocephalus grade, and sellar floor bony window.
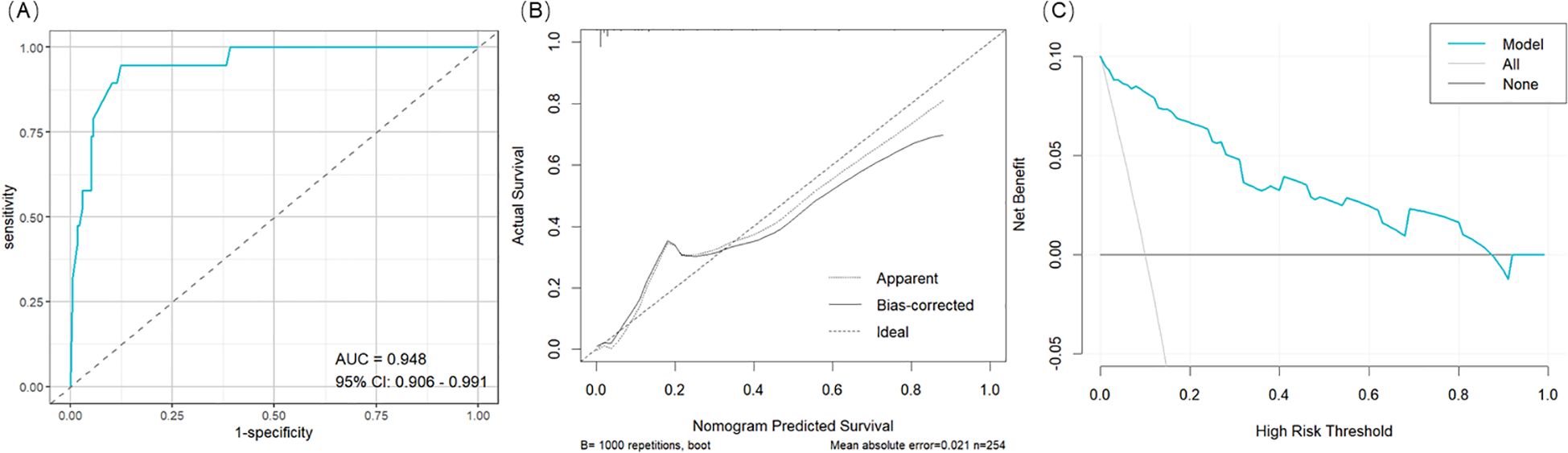
Figure 3. Nomogram Evaluation. (A) The ROC curve for the nomogram. (B) The calibration for the nomogram. (C) The decision curve analysis (DCA) for the nomogram.
4 Discussion
Postoperative cerebrospinal fluid (CSF) leakage is a significant adverse event following transsphenoidal pituitary adenoma surgery (13), with reported incidence ranging from 2% to 15% (14–16). It can lead to serious complications such as postoperative intracranial infection and tension pneumocephalus (5, 17), thereby prolonging hospital stay, adversely affecting patient prognosis, and increasing healthcare costs (18). In the present study, 7.48% of patients experienced postoperative CSF leakage, consistent with findings from a recent systematic review (19).
Our findings identified intraoperative CSF leak grade, suprasellar tumor extension, size of the sellar floor bony window, and postoperative pneumocephalus grade as significant factors influencing postoperative CSF leakage. The intraoperative CSF leak grade has long been recognized as a critical predictor, with studies reporting that patients with intraoperative CSF leaks are 6.33 times more likely to develop postoperative CSF leakage than those without (20, 21). Suprasellar tumor extension has also been reported as a key risk factor for postoperative CSF leakage (22). Anatomically, this association may be explained by the proximity of the pituitary tumor to the diaphragma sellae and arachnoid membrane: the superior portion of the sella is formed by a dural diaphragm, above which lie the arachnoid and pia mater, with CSF residing in the intervening space (23). Greater suprasellar extension increases the contact area between the tumor and arachnoid, raising the risk of arachnoid injury during surgical dissection, consistent with the “sellar barrier” principle described by Villalonga (24). The influence of tumor size on postoperative CSF leakage remains controversial. Some studies suggest that giant adenomas are more prone to CSF leakage than microadenomas or macroadenomas (18), whereas others report no correlation between tumor size and CSF leakage (25). In our study, although maximum tumor diameter and three linear dimensions differed significantly between groups, they were not statistically significant in multivariate analysis. This may reflect that suprasellar extension more directly represents the extent and intensity of tumor-arachnoid contact than tumor diameter alone (24).Intraoperative CSF leakage has been consistently associated with postoperative CSF leakage (26), and multiple studies emphasize controlling intraoperative CSF leak grade as a key preventive strategy (24, 27, 28).The intraoperative CSF leak grade reflects both the extent of arachnoid disruption and the volume of CSF flow (12). Higher-grade leaks often necessitate complex reconstruction using multiple graft materials combined with nasoseptal flaps (29), which not only complicates skull base repair but also increases the risk of postoperative CSF leakage.
In recent years, no studies have explicitly reported intraoperative sellar floor window size as a risk factor for postoperative CSF leakage. However, Li et al. (22) reported that the degree of tumor invasion into the sellar floor significantly influences postoperative CSF leakage. Similarly, Conger et al.in a large retrospective study (9), emphasized that excessive exposure of the lesion during surgery should be avoided to prevent extensive bony defects, which can complicate skull base reconstruction by hindering graft support within the sphenoid sinus and thereby increasing the risk of postoperative CSF leakage. These findings highlight the importance of sellar floor window size for effective skull base reconstruction. In this study, we quantified the sellar floor window and included it in the analysis of factors affecting postoperative CSF leakage. Our results demonstrated that larger window sizes were associated with higher postoperative CSF leak risk and exhibited good predictive performance in ROC analysis. Although the precise mechanism by which a larger sellar floor window increases CSF leak risk remains unclear, it is likely related to the increased difficulty of skull base reconstruction. Specifically, a larger bony window reduces the available bony support, increasing the risk of graft displacement (10) and complicating the adhesion of reconstructive materials and mucosal flaps.
Intracranial pneumocephalus is observed in 30-40% of patients following endoscopic transnasal surgery, with a markedly higher prevalence in those with pituitary adenomas (57.14%) (17, 30). Both the grade and distribution of pneumocephalus are closely associated with the risk of postoperative CSF leakage (6, 8). To our knowledge, this study is the first to incorporate pneumocephalus grading into a clinical prediction model for postoperative CSF leakage, and the model maintained good predictive performance. Using the Banu classification, we evaluated pneumocephalus (6) and found that postoperative pneumocephalus grade demonstrated the strongest predictive effect for CSF leakage (AUC = 0.804), playing a critical role in the overall accuracy of the model. The association between pneumocephalus and postoperative CSF leakage may be explained by two mechanisms: the ball-valve mechanism and the inverted bottle mechanism (31–33). Gas can enter the sellar, parasellar, and convexity regions through gaps between the sellar floor and reconstruction materials, but it is difficult to evacuate. In addition, CSF leakage from a disrupted arachnoid generates negative pressure in the subarachnoid space, further facilitating gas entry. These mechanisms indicate that pneumocephalus grade reflects both the integrity of sellar floor reconstruction and the extent of suprasellar arachnoid disruption, which likely accounts for its strong predictive value for postoperative CSF leakage.
Based on univariate and multivariate logistic regression analyses, we constructed a nomogram integrating independent risk factors to predict the probability of postoperative CSF leakage. The scoring system incorporates four variables: preoperative assessment of suprasellar tumor extension (≥grade B), intraoperative measurement of sellar floor window size, intraoperative CSF leak grade (≥grade 2), and postoperative 24-hour pneumocephalus grade (≥grade 3). Each variable contributes a corresponding score, and the sum of these scores corresponds to the predicted probability of postoperative CSF leakage, ranging from 0.01 to 0.85. This nomogram enables probability-based risk prediction across preoperative, intraoperative, and postoperative dimensions. The predictive performance of the model was evaluated using ROC, calibration, and decision curve analyses (DCA), all indicating good clinical applicability. The nomogram provides a practical tool for early identification of patients at high risk of postoperative CSF leakage and can inform clinical decision-making.
This study confirms that a larger sellar floor bony window and a higher grade of pneumocephalus are significantly associated with an increased risk of postoperative CSF leakage, which is consistent with our initial hypothesis. Removal of the sellar bone is an essential step in transsphenoidal surgery (29). A larger bony window often indicates a thinner sellar floor structure and consequently increases the difficulty of skull base reconstruction (9, 10). Therefore, based on preoperative imaging findings regarding tumor size, location, and invasiveness, the bony window should be carefully controlled to minimize the extent of bone removal while ensuring adequate tumor exposure. Additionally, a multi-layer skull base reconstruction strategy is recommended. For instance, Esposito et al. proposed a tailored multi-layer repair protocol based on the intraoperative Kelly grading system (12), and the “3F” reconstruction strategy introduced by Solari et al. is also noteworthy (34).Furthermore, the pneumocephalus grade may reflect the integrity of skull base reconstruction and the extent of diaphragmatic sellae disruption, serving as an early indicator for postoperative CSF leakage. Recent studies have shown that prophylactic lumbar drainage (LD) is widely adopted by skull base surgeons to reduce the risk of CSF leakage (35–37). A randomized controlled trial further demonstrated the beneficial role of LD in decreasing the incidence of postoperative CSF leakage (38). However, another randomized study reported no significant difference in CSF leak rates between patients who received prophylactic LD and those who did not, while the LD group experienced a higher rate of complications. These findings suggest that the clinical efficacy of prophylactic LD remains uncertain and warrants further high-quality prospective investigation. In our center’s experience, for high-risk patients—such as those requiring extensive removal of the sellar floor bone or presenting with severe postoperative pneumocephalus—multilayer skull base reconstruction is the key strategy for preventing postoperative CSF leakage, whereas the added benefit of prophylactic LD still requires additional clinical validation. In contrast, when LD is used as a therapeutic intervention in patients who have already developed postoperative CSF leaks, it can effectively lower intracranial pressure, reduce tension at the repair site, and thereby promote sellar floor healing while minimizing associated complications. Therefore, the use of LD should be individualized and selectively applied, taking into account patient-specific risk factors and intraoperative findings.
However, this study has several limitations. First, as a retrospective single-center study with a limited sample size, the results may be subject to inherent bias, and individualized risk assessment is recommended for different populations. Second, the limited sample size precluded the creation of internal and external validation sets to assess the model’s predictive accuracy. Future studies will expand the sample size and incorporate multicenter data to enhance the reliability and generalizability of the model.
5 Conclusion
This study identified suprasellar tumor extension, sellar floor window size, intraoperative CSF leak grade, and postoperative pneumocephalus grade as independent risk factors for postoperative CSF leakage following endoscopic transnasal pituitary adenoma resection. Based on these factors, we developed a nomogram prediction model, which demonstrated good usability and reliability. The model effectively predicts postoperative CSF leakage risk and facilitates early identification of high-risk patients. It provides clinicians with a valuable tool to implement timely preventive and interventional strategies, potentially reducing the incidence of CSF leakage and related complications, and improving patient outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Hospital of Shanxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Conceptualization, Data curation, Formal Analysis, Project administration, Software, Visualization, Writing – original draft. YH: Data curation, Software, Visualization, Writing – original draft. YN: Formal Analysis, Visualization, Writing – review & editing. RB: Project administration, Supervision, Writing – review & editing. HW: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Clinical Key Speciality Construction Fund (Grant Number: 24090519).
Acknowledgments
The authors thank colleagues and patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2025.1695573/full#supplementary-material
References
1. Lake MG, Krook LS, and Cruz SV. Pituitary adenomas: an overview. Am Fam Physician. (2013) 88:319–27.
2. Joshi SM and Cudlip S. Transsphenoidal surgery. Pituitary. (2008) 11:353–60. doi: 10.1007/s11102-008-0094-6
3. Stefanidis P, Kyriakopoulos G, Athanasouli F, Mytareli C, Tauzanis G, Korfias S, et al. Postoperative complications after endoscope-assisted transsphenoidal surgery for pituitary adenomas: a case series, systematic review, and meta-analysis of the literature. Hormones (Athens). (2022) 21:487–99. doi: 10.1007/s42000-022-00362-1
4. Li XJ, Peng Z, Wang YF, Wang J, Yan HY, Jin W, et al. Analysis of factors influencing the occurrence of diabetes insipidus following neuroendoscopic transsphenoidal resection of pituitary adenomas and risk assessment. Heliyon. (2024) 10:e38694. doi: 10.1016/j.heliyon.2024.e38694
5. Han ZL, He DS, Mao ZG, and Wang HJ. Cerebrospinal fluid rhinorrhea following trans-sphenoidal pituitary macroadenoma surgery: experience from 592 patients. Clin Neurol Neurosurg. (2008) 110:570–9. doi: 10.1016/j.clineuro.2008.02.017
6. Banu MA, Szentirmai O, Mascarenhas L, Salek AA, Anand VK, and Schwartz TH. Pneumocephalus patterns following endonasal endoscopic skull base surgery as predictors of postoperative CSF leaks. J Neurosurg. (2014) 121:961–75. doi: 10.3171/2014.5.Jns132028
7. Li W, Liu Q, Lu H, Wang H, Zhang H, Hu L, et al. Tension pneumocephalus from endoscopic endonasal surgery: A case series and literature review. Ther Clin Risk Manag. (2020) 16:531–8. doi: 10.2147/tcrm.S258890
8. Gao W, Wang X, Fang Y, Hong Y, Yan W, Zhang S, et al. Diagnostic value of non-contrast CT in cerebrospinal fluid leakage after endoscopic transnasal surgery for sellar and suprasellar tumors. Front Oncol. (2021) 11:735778. doi: 10.3389/fonc.2021.735778
9. Conger A, Zhao F, Wang X, Eisenberg A, Griffiths C, Esposito F, et al. Evolution of the graded repair of CSF leaks and skull base defects in endonasal endoscopic tumor surgery: trends in repair failure and meningitis rates in 509 patients. J Neurosurg. (2019) 130:861–75. doi: 10.3171/2017.11.Jns172141
10. Lee IH, Kim DH, Park JS, Jeun SS, Hong YK, and Kim SW. Cerebrospinal fluid leakage repair of various grades developing during endoscopic transnasal transsphenoidal surgery. PloS One. (2021) 16:e0248229. doi: 10.1371/journal.pone.0248229
11. Rutkowski MJ, Chang KE, Cardinal T, Du R, Tafreshi AR, Donoho DA, et al. Development and clinical validation of a grading system for pituitary adenoma consistency. J Neurosurg. (2021) 134:1800–7. doi: 10.3171/2020.4.Jns193288
12. Esposito F, Dusick JR, Fatemi N, and Kelly DF. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Oper Neurosurg. (2007) 60:295–303. doi: 10.1227/01.Neu.0000255354.64077.66
13. Inoshita N and Nishioka H. The 2017 WHO classification of pituitary adenoma: overview and comments. Brain Tumor Pathol. (2018) 35:51–6. doi: 10.1007/s10014-018-0314-3
14. Cappabianca P, Cavallo LM, Colao A, and de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. (2002) 97:293–8. doi: 10.3171/jns.2002.97.2.0293
15. Shiley SG, Limonadi F, Delashaw JB, Barnwell SL, Andersen PE, Hwang PH, et al. Incidence, etiology, and management of cerebrospinal fluid leaks following trans-sphenoidal surgery. Laryngoscope. (2003) 113:1283–8. doi: 10.1097/00005537-200308000-00003
16. Messerer M, De Battista JC, Raverot G, Kassis S, Dubourg J, Lapras V, et al. Evidence of improved surgical outcome following endoscopy for nonfunctioning pituitary adenoma removal. Neurosurg Focus. (2011) 30:E11. doi: 10.3171/2011.1.Focus10308
17. Sreenivasan S, Arora C, Agarwal N, Rallo M, Jaikumar V, Miglani R, et al. Tension pneumocranium following transsphenoidal surgeries-A case report and systematic review of literature with analysis of predisposing factors and treatment regimens: is early skull base repair better than conservative treatment? World Neurosurg. (2023) 176:115–26. doi: 10.1016/j.wneu.2023.04.109
18. Zhang C, Ding X, Lu Y, Hu L, and Hu G. Cerebrospinal fluid rhinorrhoea following transsphenoidal surgery for pituitary adenoma: experience in a Chinese centre. Acta Otorhinolaryngol Ital. (2017) 37:303–7. doi: 10.14639/0392-100x-1086
19. Zhao J, Wang S, Zhao X, Cui H, and Zou C. Risk factors of cerebrospinal fluid leakage after neuroendoscopic transsphenoidal pituitary adenoma resection: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2023) 14:1263308. doi: 10.3389/fendo.2023.1263308
20. Schuss P, Hadjiathanasiou A, Klingmüller D, Güresir Á, Vatter H, and Güresir E. Transsphenoidal pituitary surgery: comparison of two sellar reconstruction techniques and their effect on postoperative cerebrospinal fluid leakage. Neurosurg Rev. (2018) 41:1053–8. doi: 10.1007/s10143-018-0949-x
21. Zhou Z, Zuo F, Chen X, Zhao Q, Luo M, Jiang X, et al. Risk factors for postoperative cerebrospinal fluid leakage after transsphenoidal surgery for pituitary adenoma: a meta-analysis and systematic review. BMC Neurol. (2021) 21:417. doi: 10.1186/s12883-021-02440-0
22. Li B, Zhao S, Fang Q, Nie D, Cheng J, Zhu H, et al. Risk factors and management associated with postoperative cerebrospinal fluid leak after endoscopic endonasal surgery for pituitary adenoma. Front Surg. (2022) 9:973834. doi: 10.3389/fsurg.2022.973834
23. Shaw O and Uppoor R. Anatomy of the pituitary region. Bmj. (2012) 345:e6963. doi: 10.1136/bmj.e6963
24. Villalonga JF, Solari D, Cavallo LM, Cappabianca P, Prevedello DM, Carrau R, et al. The sellar barrier on preoperative imaging predicts intraoperative cerebrospinal fluid leak: a prospective multicenter cohort study. Pituitary. (2021) 24:27–37. doi: 10.1007/s11102-020-01082-8
25. Nishioka H, Haraoka J, and Ikeda Y. Risk factors of cerebrospinal fluid rhinorrhea following transsphenoidal surgery. Acta Neurochir (Wien). (2005) 147:1163–6. doi: 10.1007/s00701-005-0586-3
26. Guerra GA, Kashif Z, Cote DJ, Feng JJ, Renn A, Yang M, et al. Association between pituitary adenoma consistency, resection techniques, and patient outcomes: a single-institution experience. J Neurosurg. (2025) 142:1674–81. doi: 10.3171/2024.8.Jns232715
27. Lu B, Zhang Y, Liu C, Ma X, Liu G, Bie Z, et al. Intraoperative cerebrospinal fluid leakage and residual tumors in endoscopic transsphenoidal surgery for pituitary adenoma: risk analysis and nomogram development. Acta Neurochir (Wien). (2023) 165:4131–42. doi: 10.1007/s00701-023-05830-0
28. Wang J, Li Z, Wang Y, Peng Z, Li X, Chen C, et al. Analysis of risk factors and development of a prediction model for intraoperative cerebrospinal fluid leakage during transsphenoidal pituitary adenoma surgery. J Evid Based Med. (2025) 18:e70013. doi: 10.1111/jebm.70013
29. Chaskes M.B., Barton B., Karsy M., Chitguppi C., McKnight T., McCambridge J., et al. An algorithm for sellar reconstruction following endoscopic transsphenoidal surgery for pituitary adenoma: A review of 582 cases. Int Forum Allergy Rhinol. (2022) 12(9), 1120–1130. doi: 10.1002/alr.22966
30. Castle-Kirszbaum M, Wang YY, King J, Uren B, Kim M, Danks RA, et al. Tension pneumocephalus from positive pressure ventilation following endoscopic skull base surgery: case series and an institutional protocol for the management of postoperative respiratory distress. World Neurosurg. (2020) 141:357–62. doi: 10.1016/j.wneu.2020.06.079
31. Lunsford LD, Maroon JC, Sheptak PE, and Albin MS. Subdural tension pneumocephalus. Report of two cases. J Neurosurg. (1979) 50:525–7. doi: 10.3171/jns.1979.50.4.0525
32. Ozturk E, Kantarci M, Karaman K, Basekim CC, and Kizilkaya E. Diffuse pneumocephalus associated with infratentorial and supratentorial hemorrhages as a complication of spinal surgery. Acta Radiol. (2006) 47:497–500. doi: 10.1080/02841850600644766
33. Biju RD, Wu J, and Hussain Z. Tension pneumocephalus after skull base surgery. A case report and review of literature. J Clin Neurosci. (2020) 75:218–20. doi: 10.1016/j.jocn.2020.03.041
34. Cavallo LM, Solari D, Somma T, and Cappabianca P. The 3F (Fat, flap, and flash) technique for skull base reconstruction after endoscopic endonasal suprasellar approach. World Neurosurg. (2019) 126:439–46. doi: 10.1002/alr.22966
35. Garcia-Navarro V, Anand VK, and Schwartz TH. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg. (2013) 80:563–8. doi: 10.1016/j.wneu.2011.08.034
36. Roxbury CR, Lobo BC, Kshettry VR, D’Anza B, Woodard TD, Recinos PF, et al. Perioperative management in endoscopic endonasal skull-base surgery: a survey of the North American Skull Base Society. Int Forum Allergy Rhinol. (2018) 8:631–40. doi: 10.1002/alr.22066
37. Hannan CJ, Kelleher E, and Javadpour M. Methods of skull base repair following endoscopic endonasal tumor resection: A review. Front Oncol. (2020) 10:1614. doi: 10.3389/fonc.2020.01614
38. Zwagerman NT, Wang EW, Shin SS, Chang YF, Fernandez-Miranda JC, Snyderman CH, et al. Does lumbar drainage reduce postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery? A prospective, randomized controlled trial. J Neurosurg. (2019) 131:1172–8. doi: 10.3171/2018.4.Jns172447
Keywords: pneumocephalus, sellar floor bony window, cerebrospinal fluid leakage (CSF leakage), pituitary adenoma, predictive model
Citation: Zhang J, He Y, Ning Y, Bai R and Wang H (2025) Risk factors and predictive model for postoperative cerebrospinal fluid leakage following endoscopic endonasal pituitary adenoma surgery: a retrospective study focusing on pneumocephalus and sellar floor bony window. Front. Endocrinol. 16:1695573. doi: 10.3389/fendo.2025.1695573
Received: 01 September 2025; Accepted: 17 October 2025;
Published: 31 October 2025.
Edited by:
Hermann Lothar Mueller, Klinikum Oldenburg, GermanyReviewed by:
Vicki Marie Butenschoen, Technical University of Munich, GermanyShankar Ayyappan Kutty, NMC Healthcare, United Arab Emirates
Copyright © 2025 Zhang, He, Ning, Bai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqin Wang, d2hxMTk2OGhxQDE2My5jb20=
†These authors have contributed equally to this work and share last authorship
 Jiawei Zhang
Jiawei Zhang Yurui He1
Yurui He1 Yijie Ning
Yijie Ning Hongqin Wang
Hongqin Wang