- 1Department of Medicine, New York University Grossman School of Medicine, New York, NY, United States
- 2Division of Rheumatology, Department of Medicine, New York University Grossman School of Medicine, New York, NY, United States
- 3Department of Population Health, New York University Grossman School of Medicine, New York, NY, United States
- 4Division of Rheumatology, Department of Medicine, Hospital for Special Surgery, Weill Cornell Medical College, New York, NY, United States
- 5Division of Rheumatology, Department of Medicine, Columbia University College of Physicians & Surgeons, New York, NY, United States
- 6Division of Rheumatology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 7Division of Rheumatology, Department of Medicine, SUNY Downstate Health Sciences University, Brooklyn, NY, United States
- 8Azrieli Faculty of Medicine, Zefat, Israel
- 9Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom
- 10Division of Population Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, United States
- 11Division of Disease Control, Bureau of Communicable Disease, New York City Department of Health and Mental Hygiene, Long Island City, NY, United States
Objective: Leveraging the Manhattan Lupus Surveillance Program (MLSP), a population-based registry of cases of systemic lupus erythematosus (SLE) and related diseases, we investigated the proportion of SLE with concomitant rheumatic diseases, including Sjögren’s disease (SjD), antiphospholipid syndrome (APLS), and fibromyalgia (FM), as well as the prevalence of autoantibodies in SLE by sex and race/ethnicity.
Methods: Prevalent SLE cases fulfilled one of three sets of classification criteria. Additional rheumatic diseases were defined using modified criteria based on data available in the MLSP: SjD (anti-SSA/Ro positive and evidence of keratoconjunctivitis sicca and/or xerostomia), APLS (antiphospholipid antibody positive and evidence of a blood clot), and FM (diagnosis in the chart).
Results: 1,342 patients fulfilled SLE classification criteria. Of these, SjD was identified in 147 (11.0%, 95% CI 9.2–12.7%) patients with women and non-Latino Asian patients being the most highly represented. APLS was diagnosed in 119 (8.9%, 95% CI 7.3–10.5%) patients with the highest frequency in Latino patients. FM was present in 120 (8.9%, 95% CI 7.3–10.5) patients with non-Latino White and Latino patients having the highest frequency. Anti-dsDNA antibodies were most prevalent in non-Latino Asian, Black, and Latino patients while anti-Sm antibodies showed the highest proportion in non-Latino Black and Asian patients. Anti-SSA/Ro and anti-SSB/La antibodies were most prevalent in non-Latino Asian patients and least prevalent in non-Latino White patients. Men were more likely to be anti-Sm positive.
Conclusion: Data from the MLSP revealed differences among patients classified as SLE in the prevalence of concomitant rheumatic diseases and autoantibody profiles by sex and race/ethnicity underscoring comorbidities associated with SLE.
Introduction
Systemic lupus erythematosus (SLE) is a potentially fatal systemic autoimmune disease with heterogeneous clinical manifestations (1). SLE can occur as an isolated disease or coexist with other autoimmune diseases (2–4). The epidemiology of concomitant rheumatic diseases in SLE has been reported in several studies, with prevalence estimates varying greatly by study. For example, it is estimated that between 6% and 30% of SLE patients are also diagnosed with Sjögren's disease (SjD) (5–12), up to 30% of SLE patients are diagnosed antiphospholipid syndrome (APLS) (13–16) and between 12% and 27% are diagnosed with fibromyalgia (FM) (17–25). While several publications have reported these estimates, the literature is limited by smaller sample sizes that are not population-based and often do not address prevalence by race and ethnicity.
A hallmark of SLE is the presence of autoantibodies against a myriad of self-antigens, many being complexed with nucleic acids. Autoantibody specificities in SLE can be highly dynamic, such as anti-dsDNA, or relatively constant, such as those reactive with ribonuclear proteins, also referred to as extractable nuclear antigens (ENAs). Autoantibodies often provide insights into disease pathogenesis and may assist with diagnosis and prognosis (26, 27). Several studies investigating ENA positivity in SLE have identified racial and ethnic differences (16, 28). For example, across several studies, anti-Smith seropositivity is more common in Black patients compared with White patients (16, 29–35). In addition to ENAs, patients with SLE can have autoantibodies to phospholipids which increase thrombotic risk (1, 13–16). Several studies suggest the prevalence of anti-phospholipid antibodies among SLE patients to be between 20% and 33% (36, 37); however, much of these data are often limited by single race cohorts, and differences between races in concomitant APLS are not well understood.
SLE has a higher incidence among people from racial and ethnic minority populations, and these patients tend to suffer from worse outcomes (1, 28, 38, 39). These trends persist even after adjusting for socioeconomic status (28). Accordingly, knowledge of clinical phenotypes extending beyond primary SLE and distribution of antibody specificities among people from racial and ethnic minority groups should further contribute to the understanding of health disparities. While existing literature provides some insights into this area, most data are derived from cohort-based studies. The Manhattan Lupus Surveillance Program (MLSP) is a Centers for Disease Control and Prevention (CDC) funded population-based registry that recorded detailed clinical data on the large, multiracial/ethnic population of patients with SLE and related diseases living in Manhattan (1, 40–42). This study leveraged the MLSP to further investigate the proportion of concomitant SjD, APLS, and FM among SLE patients and to report the prevalence of specific autoantibodies in SLE by sex and race/ethnicity.
Methods
A detailed overview of the MLSP has been previously reported (1). Briefly, data were extracted from medical records under the health surveillance exemption to HIPAA privacy rules [45 CFR § 164.512(b)] and as authorized by New York City Charter Sections 556(c)(2) and (d)(2). As a surveillance study, no cases were contacted for this study, and the creation of the MLSP did not require institutional review board (IRB) approval at CDC, New York City Department of Health and Mental Hygiene (DOHMH), or the New York University Grossman School of Medicine. The DOHMH IRB approved secondary analyses, including those reported herein, on a de-identified dataset (NYC DOHMH IRB no. 16–147). The surveillance period was from 1 January 2007 through 31 December 2009 in Manhattan. Manhattan was more diverse than the USA overall based on the 2010 census data with 48% of the population being non-Latino White, 25% Latino, 13% non-Latino Black and 11% non-Latino Asian residents (43).
Case finding, data collection, and quality control of data entry
Case-finding sources included rheumatology practices (both adult and pediatric), hospitals, and hospitalization discharge and death registry databases (1). Sources were queried retrospectively to identify patients who lived in Manhattan with the following International Classification of Disease Ninth Revision Clinical Modification (ICD-9CM) billing codes: 710.0 (SLE) (1), 695.4 (discoid lupus erythematosus) (41), 710.8 (other specified connective tissue disease which is often used for mixed connective tissue disease) (42), 710.9 (unspecified connective tissue disease), and 710.2 (Sicca syndrome, which is used for SjD) (40). Every patient who lived in Manhattan with one of these ICD-9CM codes had their chart abstracted; the final diagnosis and date of diagnosis were coded by trained abstractors with medical degrees who underwent extensive training and routine quality assurance. Overall, 90.5% of hospitals and 75.8% of rheumatologists’ practices were included in the MLSP (1).
Data elements collected included common autoantibodies that were part of the three sets of classification criteria for lupus, including antinuclear antibody (ANA), anti-double stranded DNA (anti-dsDNA) antibody, anti-Smith antibody, and the anti-phospholipid (aPL) antibodies. In addition, common autoantibodies not part of classification criteria were also collected, including anti- Sjögren's Syndrome A (anti-SSA/Ro) and anti- Sjögren's Syndrome B (anti-SSB/La). These were considered positive if a patient ever had a positive result and negative if a patient had equivocal or borderline results. Since not all patients had evidence of testing, a result was considered negative only if there was information that the test had been performed. Likewise it was clearly noted if the specific laboratory tests were not found in the medical chart. A physician report of a positive test was accepted even if the primary laboratory tests were not found. Lupus anticoagulant was only included if the screen and confirmatory tests were positive. Anti-cardiolipin IgG and IgM were only considered positive if medium or high titer (>40 GPL/MPL units). Anti-beta 2 glycoprotein I IgG and IgM were considered positive by lab results, with equivocal or borderline results considered negative. We combined results and reported if any of the five individual antiphospholipid antibodies (lupus anticoagulant, anti-cardiolipin IgG and IgM antibodies, and anti-β2 glycoprotein I IgG and IgM antibodies) were positive at least once.
Case definitions
SLE was defined as meeting at least one of the following: 1) 1997 American College of Rheumatology (ACR) (44), Systemic Lupus Erythematosus Collaborating Clinics (SLICC) (45), or 2019 European League Against Rheumatism (EULAR)/ACR classification criteria (46) as we previously described (47). Concomitant rheumatic diseases were defined using modified criteria based on available data collected in the MLSP: SjD (anti-SSA/Ro positive and evidence of keratoconjunctivitis sicca and/or xerostomia, which was our restrictive definition in the primary SjD analysis) (40), and APLS (antiphospholipid antibody positive and evidence of an arterial or venous clot). FM was defined by chart diagnosis. A separate analysis was conducted which relied only on a physician's stated diagnosis of SLE with SjD, APLS, and FM that was not restricted to any classification criteria.
Statistical analysis
The proportion of SLE cases associated with each outcome and with autoantibody seropositivity were calculated by sex and race/ethnicity. Univariate differences were evaluated using chi-square tests or Fisher's exact tests when necessary. Bonferoni correction was applied for the ANA subtypes (anti-dsDNA abs, anti-Sm abs, Anti SSA/Ro abs, and anti-SSB/La abs) and only those with p values <0.0125 remained significant. For differences by race/ethnicity, comparisons excluded cases categorized as non-Latino other or unknown as being too small to evaluate. Analyses were also performed to understand whether there were differences in laboratory testing found in the chart. Sensitivity analyses were performed to determine the impact of missing antibody data on our findings on overlapping SjD and APLS.
Results
Concomitant diagnoses
Of the 1,342 SLE patients included in the study 1,226 (91.4%) were women; 400 (29.8%) Latino; 394 (29.4%) non-Latino White; 343 (25.6%) non-Latino Black; 148 (11.0%) non-Latino Asian; and 57 (4.2%) non-Latino Other. The average age of the SLE patients was 44 years. Of the 1342 cases, 1079 met the 1997 ACR classification criteria, 1265 met the SLICC classification critiera and 1029 met the EULAR/ACR criteria. Clinical criteria of the 1342 patients are included in Table 1.
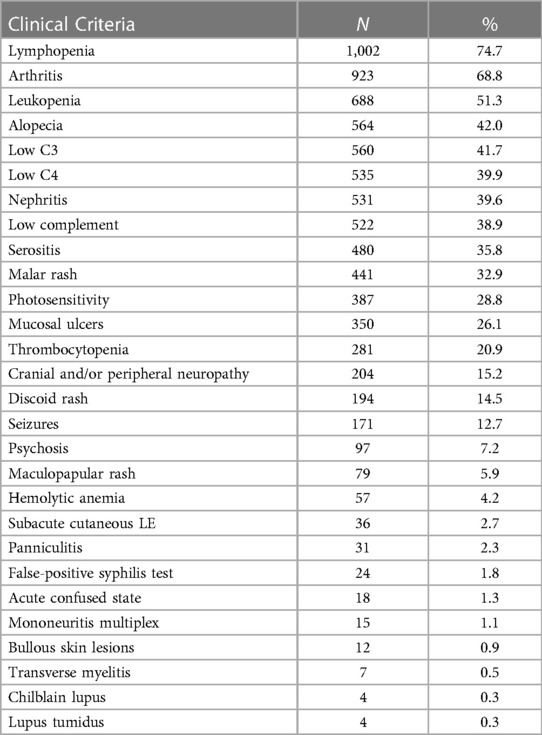
Table 1. Clinical criteria among 1342 prevalent SLE cases meeting the 1997 ACR, SLICC, or EULAR/ACR classification criteria.
For SjD, 147 [11.0%, 95% confidence interval (CI) 9.2%–12.7%] fulfilled our criteria (Table 2), with a higher proportion among women (11.7% vs. 3.4%, p = 0.0046). In addition, there were racial/ethnic differences in SjD, with non-Latino Asian patients having the highest proportion (21.0%, 95% CI 14.2–29.7).
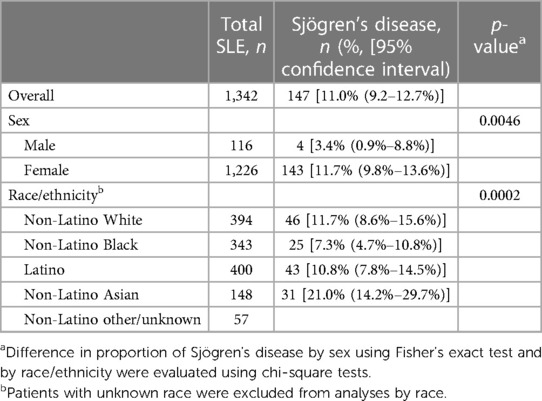
Table 2. Proportion of Sjögren's disease among Manhattan residents with SLE by sex and race/ethnicity.
APLS was seen in 119 (8.9%, 95% CI 7.3%–10.5%) patients with SLE (Table 3), and although no differences were seen between men and women, there were differences by race/ethnicity, with Latino patients having the highest proportion (12.3% 95% CI 9.1%–16.2%).
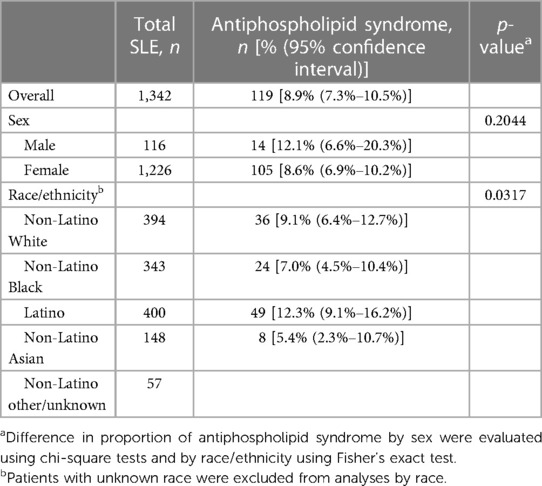
Table 3. Proportion of antiphospholipid syndrome among Manhattan residents with SLE by sex and race/ethnicity.
Finally, FM was diagnosed in 120 (8.9%, 95% CI 7.3%–10.5%) patients who met SLE criteria (Table 4), with women having a higher proportion than men (9.6% vs. 1.7%, p = 0.0018). There were also racial/ethnic differences in patients diagnosed with FM, with non-Latino White patients being the most frequently diagnosed (13.5%, 95% CI 10.1%–17.6%).
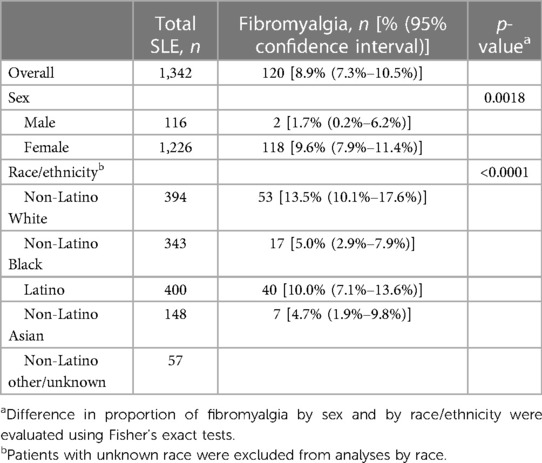
Table 4. Proportion of fibromyalgia of among Manhattan residents with SLE by sex and race/ethnicity.
For each disease, similar patterns were seen when using a physician's clinical diagnosis and not restricting to criteria (data not shown). Sensitivity analyses for SjD and APLS which excluded missing antibody resulted in similar findings, though proportions with each outcome increased given the smaller denominator with complete information available, Supplementary Tables S1A,B.
Proportion of autoantibodies in SLE patients
Overall, 1,285 (95.8%) patients who fulfilled at least one of the three sets of classification criteria for the assignment of SLE had results of an ANA test reported in the chart, 1,222 (95.1%, 95% CI 89.8%–100.4%) of which were positive. ANA positivity did not differ by sex or race/ethnicity. For anti-dsDNA antibodies, results were found for 1,231 (91.7%) patients, 678 (55.1%, 95% CI 50.9%–59.2%) of which were positive. In contrast to the presence of an ANA, anti-dsDNA antibodies significantly differed by race (p = 0.0005) and were most frequent in non-Latino Asian patients (62.3%, 95% CI 49.9%–77.0%) and least frequent in non-Latino White patients (46.5%, 95% CI 39.5%–53.4%), Table 5.
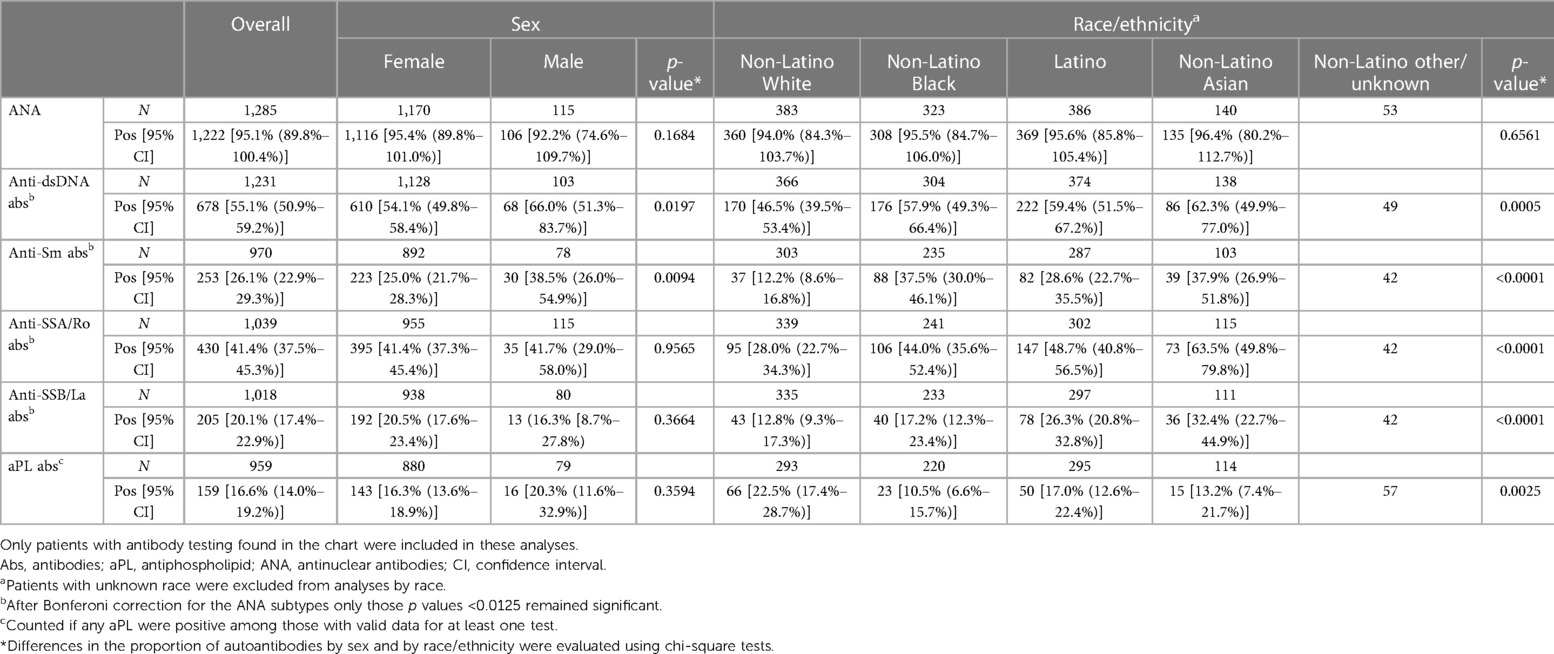
Table 5. Proportion of select autoantibodies among Manhattan residents with SLE by sex and race/ethnicity.
Results for anti-Sm antibodies were found in 970 (72.2%) patients with 26.1% (95% CI 22.9%–29.3%) being positive. Men were more likely to be positive than women (38.5% vs. 25.0%, p = 0.0094), and racial/ethnic differences were similar to that of anti-dsDNA antibodies (p < 0.0001), with non-Latino Asian patients having the highest proportion (37.9%, 95% CI 26.9%–51.8%) and non-Latino White patients having the lowest proportion (12.2%, 95% CI 8.6%–16.8%), Table 5.
Anti-SSA/Ro and anti-SSB/La antibodies were positive in 41.4%, (95% CI 37.5%–45.3%) and 20.1%, (95% CI 17.4%–22.9%) of 1,039 (77.4%) and 1,018 (75.6%) SLE patients with data available, respectively. There were no differences in the proportion positive by sex for having both antibodies, but there were racial/ethnic differences for both (p < 0.0001), with non-Latino Asian patients having the highest rate, and non-Latino White patients having the lowest for anti-SSA/Ro and anti-SSB/La antibodies (Table 5). For combined aPL antibodies, there were no differences by sex, but racial/ethnic differences were present (p = 0.0025), with non-Latino White patients having the highest proportion (22.5.8%, 95% CI 17.4%–28.7%) and non-Latino Black patients having the lowest proportion (10.5%, 95% CI 6.6%–15.7%).
Finding evidence of laboratory testing differed by race/ethnicity for anti-SSA/Ro and anti-SSB/La antibodies. For anti-SSA/Ro and SSB/La antibodies, the proportion with testing found in the chart was highest among non-Latino White patients and lowest among non-Latino Black patients (Supplementary Table S1). Regarding combined aPL antibodies, the proportion with documentation for any individual test was lowest among non-Latino Black patients. However, in cases where clotting was present no differences were found (data not shown).
Discussion
Analysis of the MLSP, a large multi-ethnic CDC-funded population-based registry, provided insight into differences by sex and race/ethnicity in concomitant rheumatic diseases and autoantibody profiles among patients with SLE. In 1,342 patients fulfilling SLE classification criteria, 11.0%, (95% CI 9.2–12.7) met our criteria for SjD, 8.9%, (95% CI 7.3%–10.5%) met our criteria for APLS, and 8.9%, (95% CI 7.3–10.5) carried a diagnosis of FM. The distribution of cases meeting criteria differed by race/ethnicity for SjD, APLS, and FM. Cases meeting criteria for SjD and FM also differed by sex, both being more common in women. These differences were also seen when using a clinical diagnosis irrespective of criteria. The distribution of autoantibody seropositivity differed by race/ethnicity for all autoantibodies evaluated, except ANA. Distribution of autoantibodies only differed by sex for anti-Sm.
The MLSP represents a large and diverse group of patients with SLE, with the prevalence of concomitant rheumatic diseases being largely consistent with prior studies (6–16, 20–25). In this analysis of the MLSP, we found the highest proportion of concomitant SjD with SLE among non-Latino White and non-Latino Asian patients which parallels findings from the MLSP analyzing primary SjD (40). In 2010, Baer et al. found that SLE with overlapping SjD was more prevalent in White women than in other demographics; however, few Latino and Asian patients were in their cohort (10). Other meta-analyses characterizing SLE with SjD offer limited racial and ethnic data (11, 12). While it is not clear why concomitant SjD is more prevalent in these groups, these findings serve to improve recognition of this condition among SLE patients in the future.
Regarding APLS, our study shows a higher proportion diagnosed among non-Latino White and Latino SLE patients compared with non-Latino Black and non-Latino Asian patients. Although not a direct comparison to our findings, a previous cohort study found ethnic differences in the incidence of arterial and venous thromboembolism in patients with SLE. Specifically, aPL antibodies and non-Chinese ethnicity were associated with venous thromboembolism (VTE) while Chinese ethnicity was associated with arterial clotting (16). In another study among SLE patients with VTE events, African American patients were less likely to have a clinically significant aPL profile compared with White patients (48). Given the potentially devastating consequences of thrombotic events, understanding which patients face higher risk is important for surveillance.
FM was present in SLE patients in our cohort at a lower rate than has been reported in other studies (17–25, 49). Interestingly, in our cohort, SLE with FM was more prevalent in non-Latino White and Latina women compared with other groups. Friedman et al. similarly found that SLE with concomitant FM was more prevalent in White women compared with Black women; however, their study included few Latina women (23). Because FM is a clinical diagnosis that does not necessarily require pharmacologic treatment, patients may not be universally screened during SLE visits. Without definite screening or required intervention, it is not surprising for chart-documented FM prevalence to be lower than expected. Since FM is a major contributor to decreased health-related quality of life among SLE patients suffering from this comorbidity, our findings highlight a need to increase screening to alleviate disease burden and identify potential target populations most at risk of co-morbid FM (50).
Analysis of autoantibody profiles in the MLSP offers interesting insights compared with similar analyses in prior studies. Anti-dsDNA was most prevalent in non-Latino Asian patients and high in non-Latino Black and Latino patients compared with non-Latino White patients—consistent with findings from other multi-ethnic cohorts (29, 34). Regarding anti-Sm antibodies, our study showed the highest proportion in non-Latino Black and Asian patients compared with Latino and non-Latino White patients. Several studies have demonstrated similar findings (29–34, 48). Additionally, significantly more Latino patients were anti-Sm positive compared with non-Latino White patients, which is also consistent with prior studies (29, 31, 51). Regarding anti-SSA/Ro and anti-SSB/La antibodies, both were most prevalent in non-Latino Asian patients and least prevalent in non-Latino White patients in our cohort. Similar observations were found in a cohort study of newly diagnosed SLE patients with anti-SSA/Ro antibodies which were present in 57% of Chinese patients compared with 28% of African American patients and 18% of Caucasians (48). Recent studies have linked genetic ancestry to the heterogeneous gene expression and immunologic profiles in SLE. Our population level data may help inform future research in this area (52, 53). In addition, by better understanding how autoantibody profiles differ in racial/ethnic groups, we can more appropriately incorporate our patients’ serologic data in their clinical context.
In addition to differences between racial/ethnic groups, our study demonstrated several differences between sexes in both concomitant rheumatic diseases and autoantibody profiles. Men with SLE in our cohort were less likely to have SjD and FM compared with women. These differences have been demonstrated in previous literature for both secondary and primary SjD and FM (10, 20, 40). Regarding autoantibodies, men in our study were more likely to be anti-Sm positive. These differences have also been demonstrated previously (54).
There are several limitations inherent to using the MLSP registry described previously (1). The data used were from 2007 to 2009 but were unique in offering a large registry to study demographic associations in further detail. The data in the MLSP relies on chart documentation, and due to the heterogeneity of the medical records from which the data were abstracted, there is potential for missing data not present in the records accessed, such as antibody testing. In addition, medical care can vary and it is possible data such as keratoconjunctivitis sicca and/or xerostomia symptoms were not solicited by the treating physicians. In 4.2% of patients race/ethnicity was unknown. Differences were detected in the frequencies of laboratory testing with non-Latino Black patients having several antibodies, including anti-SSA/Ro and SSB/La antibodies and individual aPL tests, less likely to be found in the chart. Given the surveillance nature of the MLSP the etiology of these differences cannot be determined. The clinical relevance of the differences in frequency of some of the autoantibodies as a function of race/ethnicity is unknown. The definition of concomitant SjD required anti-SSA/Ro and the presence of keratoconjunctivitis sicca and/or xerostomia leaving the possibility that the differences found in the MLSP between race/ethnicity reflected a greater likelihood that non-Latino White patients were tested and clinically evaluated for keratoconjunctivitis sicca and/or xerostomia symptoms. In addition, our definition of SjD would not capture antibody negative disease. Although there were also differences in the frequencies of testing for individual aPLs, the presence of any thrombotic event prompted testing regardless of race/ethnicity. Finally, we acknowledge that inherent biases could influence our results. For example, physicians may have biases regarding the evaluation of FM in males since the condition is more common in women.
Individual autoantibody data in patients meeting our SLE case definition were incomplete. While we considered equivocal antibodies negative, any positive titer was counted regardless of level of positivity. It is acknowledged that we cannot account for the variation in assays used for antibody testing. This is a limitation, as different assays have different sensitivities and specificities, and accounting for these differences may impact the results. It is also acknowledged that since antibodies, particularly anti-dsDNA and aPL, can fluctuate over time and with disease activity, a positive test at a given point in time may have been missed resulting in a potential underrepresentation of the frequency. In this study, we use modified classification criteria for SjD and APLS, which may not accurately capture these diseases. However, separate analyses looking at diagnoses that are not criteria-based and sensitivity analyses accounting for missing antibody data found similar findings.
The study has several strengths as it used a large population-based registry comprising a multi-racial/ethnic composition to allow analyses by race/ethnicity. The MLSP also included a significant number of male SLE patients to allow for differences to be detected by sex. This study offers interesting insights into the nature of SLE by showing associations of race/ethnicity with coexisting rheumatic disease and autoantibody profiles. The results of this study highlight the importance of delineating co-morbid conditions and measuring autoantibodies in all SLE patients. As SLE is a heterogeneous disease, characterizing a patient’s phenotype is important for understanding their risk profile and their trajectory.These findings open the door for future research directed at answering the questions of why differences exist between demographic groups, and how these differences relate to observed differences in clinical outcomes.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: This study used data from a surveillance initiative which are housed at the NYC DOHMH. Requests to access these datasets should be directed toaHBhcnRvbkBoZWFsdGgubnljLmdvdg==.
Ethics statement
The data used in the manuscript were extracted from medical records under the health surveillance exemption to HIPAA privacy rules [45 CFR § 164.512 (b)] and as authorized by New York City Charter Sections 556(c) (2) and (d) (2). As a surveillance study, the creation of the MLSP did not require institutional review board (IRB) approval at CDC, New York City Department of Health and Mental Hygiene (DOHMH), or the New York University Grossman School of Medicine. The DOHMH IRB approved secondary analyses, including those reported herein, on a de-identified dataset (NYC DOHMH IRB no. 16-147). Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
BD: Conceptualization, Writing – original draft, Writing – review & editing. PC: Conceptualization, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. KC: Conceptualization, Writing – review & editing. JBu: Conceptualization, Writing – review & editing. HB: Conceptualization, Writing – review & editing. HG: Conceptualization, Writing – review & editing. JS: Conceptualization, Data curation, Writing – review & editing. AA: Data curation, Writing – review & editing. JB: Data curation, Writing – review & editing. LG-P: Data curation, Writing – review & editing. YA: Writing – review & editing, Data curation. EG: Data curation, Writing – review & editing. CP: Data curation, Writing – review & editing. CG: Supervision, Writing – review & editing. KB: Project administration, Supervision, Writing – review & editing. CH: Project administration, Supervision, Writing – review & editing. HP: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. PI: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Writing – review & editing, Investigation, Resources, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by co-operative agreements between the Centers for Disease Control and Prevention and The New York City Department of Health and Mental Hygiene (grant no. U58/DP002827), a co-operative agreement between the New York City Department of Health and Mental Hygiene and New York Grossman University School of Medicine, and a co-operative agreement between the Centers for Disease Control and Prevention and New York University Grossman School of Medicine (grant no. U01DP006700).
Acknowledgments
The authors would like to acknowledge Benjamin Wainwright for assistance in preparing the manuscript.
Conflict of interest
PI- consulting fees from Momenta/Janssen; JB- consulting fees from Bristol-Myers Squibb, GlaxoSmithKline, Related Sciences LLC, Ventus Therapeutics; and CG-consulting fees from Alumis, Amgen, Astra-Zeneca, Sanofi, and UCB.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AA and CP declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fepid.2024.1334859/full#supplementary-material
References
1. Izmirly PM, Wan I, Sahl S, Buyon JP, Belmont HM, Salmon JE, et al. The incidence and prevalence of systemic lupus erythematosus in New York county (Manhattan), New York: the Manhattan Lupus Surveillance Program. Arthritis Rheumatol. (2017) 69:2006–17. doi: 10.1002/art.40192
2. McDonagh JE, Isenberg DA. Development of additional autoimmune diseases in a population of patients with systemic lupus erythematosus. Ann Rheum Dis. (2000) 59:230. doi: 10.1136/ard.59.3.230
3. Ordoñez-Cañizares MC, Mena-Vázquez N, Redondo-Rodriguez R, Manrique-Arija S, Jimenez-Núñez FG, Ureña-Garnica I, et al. Frequency of polyautoimmunity in patients with rheumatoid arthritis and systemic lupus erythematosus. J Clin Rheumatol. (2022) 28:e38–43. doi: 10.1097/RHU.0000000000001574
4. Mena-Vázquez N, Fernández-Nebro A, Pego-Reigosa JM, Galindo M, Melissa-Anzola A, Uriarte-Isacelay E, et al. Hydroxychloroquine is associated with a lower risk of polyautoimmunity: data from the RELESSER registry. Rheumatol (Oxford, England). (2020) 59:2043–51. doi: 10.1093/rheumatology/kez562
5. Grennan DM, Ferguson M, Williamson J, Mavrikakis M, Dick WC, Buchanan WW. Sjogren’s syndrome in SLE: part I. The frequency of the clinical and subclinical features of sjogren’s syndrome in patients with SLE. N Z Med J. (1977) 86:374–6.272546
6. Nossent JC, Swaak AJ. Systemic lupus erythematosus VII: frequency and impact of secondary sjøgren’s syndrome. Lupus. (1998) 7:231–4. doi: 10.1191/096120398678920046
7. Andonopoulos AP, Skopouli FN, Dimou GS, Drosos AA, Moutsopoulos HM. Sjögren’s syndrome in systemic lupus erythematosus. J Rheumatol. (1990) 17:201–4.2319519
8. Manoussakis MN, Georgopoulou C, Zintzaras E, Spyropoulou M, Stavropoulou A, Skopouli FN, et al. Sjögren’s syndrome associated with systemic lupus erythematosus: clinical and laboratory profiles and comparison with primary sjögren’s syndrome. Arthritis Rheum. (2004) 50:882–91. doi: 10.1002/art.20093
9. Pan HF, Ye DQ, Wang Q, Li WX, Zhang N, Li XP, et al. Clinical and laboratory profiles of systemic lupus erythematosus associated with Sjögren syndrome in China: a study of 542 patients. Clin Rheumatol. (2008) 27:339–43. doi: 10.1007/s10067-007-0720-0
10. Baer AN, Maynard JW, Shaikh F, Magder LS, Petri M. Secondary Sjogren’s syndrome in systemic lupus erythematosus defines a distinct disease subset. J Rheumatol. (2010) 37:1143–9. doi: 10.3899/jrheum.090804
11. Yao Q, Altman RD, Wang X. Systemic lupus erythematosus with Sjögren syndrome compared to systemic lupus erythematosus alone: a meta-analysis. J Clin Rheumatol. (2012) 18:28–32. doi: 10.1097/RHU.0b013e31823ecbdf
12. Alani H, Henty JR, Thompson NL, Jury E, Ciurtin C. Systematic review and meta-analysis of the epidemiology of polyautoimmunity in Sjögren’s syndrome (secondary Sjögren’s syndrome) focusing on autoimmune rheumatic diseases. Scand J Rheumatol. (2018) 47:141–54. doi: 10.1080/03009742.2017.1324909
13. Pons-Estel GJ, Andreoli L, Scanzi F, Cervera R, Tincani A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J Autoimmun. (2017) 76:10–20. doi: 10.1016/j.jaut.2016.10.004
14. Borchers AT, Naguwa SM, Shoenfeld Y, Gershwin ME. The geoepidemiology of systemic lupus erythematosus. Autoimmun Rev. (2010) 9:A277–87. doi: 10.1016/j.autrev.2009.12.008
15. Ruiz-Irastorza G, Egurbide M-V, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med. (2004) 164:77–82. doi: 10.1001/archinte.164.1.77
16. Mok CC, Tang SS, To CH, Petri M. Incidence and risk factors of thromboembolism in systemic lupus erythematosus: a comparison of three ethnic groups. Arthritis Rheum. (2005) 52:2774–82. doi: 10.1002/art.21224
17. Moon SJ, Kang KY, Kwok SK, Ju JH, Hong YS, Park SH, et al. Differences in quality of life determinants according to the presence of fibromyalgia in middle-aged female patients with systemic lupus erythematosus: a multicenter, cross-sectional, single-ethnicity cohort. Int J Rheum Dis. (2018) 21:1173–84. doi: 10.1111/1756-185X.13320
18. Raghunath S, Guymer EK, Glikmann-Johnston Y, Golder V, Kandane Rathnayake R, Morand EF, et al. Fibromyalgia, mood disorders, cognitive test results, cognitive symptoms and quality of life in systemic lupus erythematosus. Rheumatol (Oxford). (2022) 62:190–9. doi: 10.1093/rheumatology/keac207
19. de Araujo AL, Paliares IC, de Araujo MI, Novo NF, Cadaval RA, Martinez JE. The association of fibromyalgia and systemic lupus erythematosus change the presentation and severity of both diseases? Rev Bras Reumatol. (2015) 55:37–42. doi: 10.1016/j.rbr.2014.08.004
20. Torrente-Segarra V, Salman-Monte TC, Rúa-Figueroa Í, Pérez-Vicente S, López-Longo FJ, Galindo-Izquierdo M, et al. Fibromyalgia prevalence and related factors in a large registry of patients with systemic lupus erythematosus. Clin Exp Rheumatol. (2016) 34:S40–7.26575317
21. Middleton GD, McFarlin JE, Lipsky PE. The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum. (1994) 37:1181–8. doi: 10.1002/art.1780370812
22. Valencia-Flores M, Cardiel MH, Santiago V, Resendiz M, Castaño VA, Negrete O, et al. Prevalence and factors associated with fibromyalgia in Mexican patients with systemic lupus erythematosus. Lupus. (2004) 13:4–10. doi: 10.1191/0961203304lu480oa
23. Friedman AW, Tewi MB, Ahn C, McGwin G Jr, Fessler BJ, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups: XV. Prevalence and correlates of fibromyalgia. Lupus. (2003) 12:274–9. doi: 10.1191/0961203303lu330oa
24. Haliloglu S, Carlioglu A, Akdeniz D, Karaaslan Y, Kosar A. Fibromyalgia in patients with other rheumatic diseases: prevalence and relationship with disease activity. Rheumatol Int. (2014) 34:1275–80. doi: 10.1007/s00296-014-2972-8
25. Wolfe F, Petri M, Alarcón GS, Goldman J, Chakravarty EF, Katz RS, et al. Fibromyalgia, systemic lupus erythematosus (SLE), and evaluation of SLE activity. J Rheumatol. (2009) 36:82–8. doi: 10.3899/jrheum.080212
26. Hoffman IE, Peene I, Meheus L, Huizinga TW, Cebecauer L, Isenberg D, et al. Specific antinuclear antibodies are associated with clinical features in systemic lupus erythematosus. Ann Rheum Dis. (2004) 63:1155–8. doi: 10.1136/ard.2003.013417
27. To CH, Petri M. Is antibody clustering predictive of clinical subsets and damage in systemic lupus erythematosus? Arthritis Rheum. (2005) 52:4003–10. doi: 10.1002/art.21414
28. Lewis MJ, Jawad AS. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology. (2017) 56:i67–77. doi: 10.1093/rheumatology/kex200
29. González LA, Toloza SMA, McGwin G, Alarcón GS. Ethnicity in systemic lupus erythematosus (SLE): its influence on susceptibility and outcomes. Lupus. (2013) 22:1214–24. doi: 10.1177/0961203313502571
30. Arnett FC, Hamilton RG, Roebber MG, Harley JB, Reichlin M. Increased frequencies of sm and nRNP autoantibodies in American blacks compared to whites with systemic lupus erythematosus. J Rheumatol. (1988) 15:1773–6.3230562
31. Bruner BF, Guthridge JM, Lu R, Vidal G, Kelly JA, Robertson JM, et al. Comparison of autoantibody specificities between traditional and bead-based assays in a large, diverse collection of patients with systemic lupus erythematosus and family members. Arthritis Rheum. (2012) 64:3677–86. doi: 10.1002/art.34651
32. Arroyo-ávila M, Santiago-Casas Y, McGwin G Jr, Cantor RS, Petri M, Ramsey-Goldman R, et al. Clinical associations of anti-smith antibodies in PROFILE: a multi-ethnic lupus cohort. Clin Rheumatol. (2015) 34:1217–23. doi: 10.1007/s10067-015-2941-y
33. Morais SA, Isenberg DA. A study of the influence of ethnicity on serology and clinical features in lupus. Lupus. (2016) 26:17–26. doi: 10.1177/0961203316645204
34. Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Cohen PL, et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the Southeastern United States. Lupus. (2002) 11:161–7. doi: 10.1191/0961203302lu161oa
35. Santos MJ, Capela S, Figueira R, Nero P, Matos AA, Silva C, et al. Characterization of a Portuguese population with systemic lupus erytematosus. Acta Reumatol Port. (2007) 32:153–61.17576395
36. Taraborelli M, Leuenberger L, Lazzaroni MG, Martinazzi N, Zhang W, Franceschini F, et al. The contribution of antiphospholipid antibodies to organ damage in systemic lupus erythematosus. Lupus. (2016) 25:1365–8. doi: 10.1177/0961203316637431
37. Sebastiani GD, Galeazzi M, Tincani A, Piette JC, Font J, Allegri F, et al. Anticardiolipin and anti-beta2GPI antibodies in a large series of European patients with systemic lupus erythematosus. Prevalence and clinical associations. European concerted action on the immunogenetics of SLE. Scand J Rheumatol. (1999) 28:344–51.10665739
38. Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcón GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with medicaid coverage, 2000–2004. Arthritis Rheum. (2013) 65:753–63. doi: 10.1002/art.37795
39. Izmirly PM, Ferucci ED, Somers EC, Wang L, Lim SS, Drenkard C, et al. Incidence rates of systemic lupus erythematosus in the USA: estimates from a meta-analysis of the Centers for Disease Control and Prevention national lupus registries. Lupus Sci Med. (2021) 8:e000614. doi: 10.1136/lupus-2021-000614
40. Izmirly PM, Buyon JP, Wan I, Belmont HM, Sahl S, Salmon JE, et al. The incidence and prevalence of adult primary Sjogren’s syndrome in New York county. Arthritis Care Res (Hoboken). (2019) 71:949–60. doi: 10.1002/acr.23707
41. Izmirly P, Buyon J, Belmont HM, Sahl S, Wan I, Salmon J, et al. Population-based prevalence and incidence estimates of primary discoid lupus erythematosus from the Manhattan Lupus Surveillance Program. Lupus Sci Med. (2019) 6:e000344-e. doi: 10.1136/lupus-2019-000344
42. Hasan G, Ferucci ED, Buyon JP, Belmont HM, Salmon JE, Askanase A, et al. Population-based prevalence and incidence estimates of mixed connective tissue disease from the Manhattan Lupus Surveillance Program. Rheumatol (Oxford). (2023) 62:2845–9. doi: 10.1093/rheumatology/keac703
43. U.S. Census Bureau. 2010 Census, Summary File 1, Table P2. Available online at: https://data.census.gov/cedsci/ (accessed May 9, 2013).
44. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. (1997) 40:1725. doi: 10.1002/art.1780400928
45. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. (2012) 64:2677–86. doi: 10.1002/art.34473
46. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71:1400–12. doi: 10.1002/art.40930
47. Guttmann A, Denvir B, Aringer M, Buyon JP, Belmont HM, Sahl S, et al. Evaluation of the EULAR/American College of Rheumatology classification criteria for systemic lupus erythematosus in a population-based registry. Arthritis Care Res (Hoboken). (2023) 75(5):1007–16. doi: 10.1002/acr.24960
48. Gkrouzman E, Peng M, Davis-Porada J, Kirou KA. Venous thromboembolic events in African American lupus patients in association with antiphospholipid antibodies compared to white patients. Arthritis Care Res (Hoboken). (2022) 74:656–64. doi: 10.1002/acr.24508
49. Corbitt K, Carlucci PM, Cohen B, Masson M, Saxena A, Belmont HM, et al. Clinical and serologic phenotyping and damage indices in patients with systemic lupus erythematosus with and without fibromyalgia. ACR Open Rheumatol. (2024). doi: 10.1002/acr2.11641
50. Gladman DD, Urowitz MB, Gough J, MacKinnon A. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol. (1997) 24:2145–8.9375874
51. Weckerle CE, Franek BS, Kelly JA, Kumabe M, Mikolaitis RA, Green SL, et al. Network analysis of associations between serum interferon-alpha activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum. (2011) 63:1044–53. doi: 10.1002/art.30187
52. Carter LM, Alase A, Wigston Z, Psarras A, Burska A, Sutton E, et al. Gene expression and autoantibody analysis revealing distinct ancestry-specific profiles associated with response to rituximab in refractory systemic lupus erythematosus. Arthritis Rheumatol. (2023) 75:697–710. doi: 10.1002/art.42404
53. Catalina MD, Bachali P, Yeo AE, Geraci NS, Petri MA, Grammer AC, et al. Patient ancestry significantly contributes to molecular heterogeneity of systemic lupus erythematosus. JCI Insight. (2020) 5:e140380. doi: 10.1172/jci.insight.140380
Keywords: systemic lupus erythematosus, Sjögren's disease, antiphospholipid syndrome, fibromyalgia, autoantibodies
Citation: Denvir B, Carlucci PM, Corbitt K, Buyon JP, Belmont HM, Gold HT, Salmon JE, Askanase A, Bathon JM, Geraldino-Pardilla L, Ali Y, Ginzler EM, Putterman C, Gordon C, Barbour KE, Helmick CG, Parton H and Izmirly PM (2024) Prevalence of concomitant rheumatologic diseases and autoantibody specificities among racial and ethnic groups in SLE patients. Front. Epidemiol. 4:1334859. doi: 10.3389/fepid.2024.1334859
Received: 7 November 2023; Accepted: 15 February 2024;
Published: 6 March 2024.
Edited by:
Glinda Cooper, Retired, Washington DC, United StatesReviewed by:
Marisa Gallant Stahl, University of Colorado, United StatesMaxime Desmarets, University of Franche-Comté, France
© 2024 Denvir, Carlucci, Corbitt, Buyon, Belmont, Gold, Salmon, Askanase, Bathon, Geraldino-Pardilla, Ali, Ginzler, Putterman, Gordon, Barbour, Helmick, Parton and Izmirly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter M. Izmirly cGV0ZXIuaXptaXJseUBueXVsYW5nb25lLm9yZw==
†These authors have contributed equally to this work
‡ORCID Peter M. Izmirly orcid.org/0000-0001-5445-2182
 Brendan Denvir1,†
Brendan Denvir1,† Philip M. Carlucci
Philip M. Carlucci Jane E. Salmon
Jane E. Salmon Anca Askanase
Anca Askanase Laura Geraldino-Pardilla
Laura Geraldino-Pardilla Ellen M. Ginzler
Ellen M. Ginzler Chaim Putterman
Chaim Putterman Kamil E. Barbour
Kamil E. Barbour Peter M. Izmirly
Peter M. Izmirly