- Uniformed Services University, Bethesda, MD, United States
Human immunodeficiency virus (HIV) establishes life-long latency in infected individuals. Although highly active antiretroviral therapy (HAART) has had a significant impact on the course of HIV infection leading to a better long-term outcome, the pool of latent reservoir remains substantial even under HAART. Numerous approaches have been under development with the goal of eradicating the latent HIV reservoir though with limited success. Approaches that combine immune-mediated control of HIV to activate both the innate and the adaptive immune system under suppressive therapy along with “shock and kill” drugs may lead to a better control of the reactivated virus. Interferon-α (IFN-α) is an innate cytokine that has been shown to activate intracellular defenses capable of restricting and controlling HIV. IFN-α, however, harbors numerous functional subtypes that have been reported to display different binding affinities and potency. Recent studies have suggested that certain subtypes such as IFN-α8 and IFN-α14 have potent anti-HIV activity with little or no immune activation, whereas other subtypes such as IFN-α4, IFN-α5, and IFN-α14 activate NK cells. Could these subtypes be used in combination with other strategies to reduce the latent viral reservoir? Here, we review the role of IFN-α subtypes in HIV infection and discuss the possibility that certain subtypes could be potential adjuncts to a “shock and kill” or therapeutic vaccination strategy leading to better control of the latent reservoir and subsequent functional cure.
Introduction
Human immunodeficiency virus (HIV) infections are characterized by severe immunodeficiency and onset of opportunistic infections. Currently, there are over 36 million people worldwide who are living with HIV. Onset of highly active antiretroviral therapy (HAART) has led to better viral control and long-term outcome in HIV-infected patients. As access to therapy becomes more readily available around the world, the number of new infections and transmission are expected to dramatically decrease, raising the hope that the HIV epidemic can be controlled and managed. Encouraging studies (1) showing the efficacy of neutralizing antibodies to control viral rebound and the development of long-lasting drugs are likely to have a major impact on the epidemiology of the disease. As major efforts to control the HIV epidemic gets underway, focus has shifted to finding cure for patients who are already infected with HIV.
Human immunodeficiency virus is a retrovirus that integrates into the host genome. As such, an HIV-infected individual is infected for life. The primary target cell for HIV is the CD4 T cell, with HIV establishing latency in these cells, and this latent reservoir continues to persist during HAART. Except in the case of Timothy Brown who is the only known case of HIV to have been completely cured, complete eradication of HIV reservoir has proven to be challenging not only due to the integration of HIV into the host genome but also due to the large size of the latent persistent reservoir. As such focus has recently shifted to the development of functional cure strategies, where the objective is to obtain complete remission in the absence of antiretroviral drugs.
Evidence for functional cure came rather serendipitously when an infant born to an HIV-infected mother was treated continuously for over 2 years within hours after birth. The child remained free of HIV for about 2 years after withdrawal of therapy raising the prospect that early HAART could potentially achieve full remission in HIV-infected subjects. However, the excitement was short lived as HIV rebounded suggesting the latent reservoir was not eradicated with early therapy and reactivated in the absence of long-term HAART. A number of novel approaches such as “shock and kill” using latency reversing agents (LRA) although somewhat successful in reactivating latent HIV (1), their impact on the viral reservoir has been rather limited, suggesting that LRA would need to be combined with other approaches such as vaccination against HIV that can simultaneously activate the immune system to recognize viral antigens expressed on the surface of latently infected cells following reactivation with LRA. A number of studies are currently underway to explore this strategy.
Other strategies have focused on activating intracellular defense mechanisms using interferon α (IFN-α) in combination with LRA or other immune mediators with some promising data from non-human primate models. Here, we review the progress that has been made to date in understanding the role IFN-α plays in HIV infection and explore the potential for harnessing IFN-α and its subtype as a strategy toward functional cure.
Type I IFN and HIV Infection
Since its initial discovery in 1957 as factors that inhibit viral replication (2), the role of innate IFN in viral infections has been extensively studied. The primary source of IFN-α is the plasmacytoid DC (pDC), whereas IFN-β is produced by most cell types (3, 4). pDC plays a major role in regulating the immune system and are the earliest cells recruited to the sites of virus entry. In response to viral pathogen-associated molecular patterns, pDCs have been shown to produce ~1,000-fold more IFN-α/β than other cell types (5).
Plasmacytoid DC express a variety of pathogen recognition receptors (PRRs) such a Toll-like receptor (TLR) 3, TLR7, TLR8, and TLR9 that can sense viral nucleic acids leading to the secretion of IFN-α (6–8). Recent studies have demonstrated that the cytoplasmic DNA sensor cGAS plays an important role in the secretion of IFN-α during both HIV and SIV infections (9). Lahaye et al. (10) showed that DC’s sense viral cDNA in the cytoplasm that was mediated by cGAS and blocking cGAS or reverse transcription inhibited these responses (11). Likewise, Herzner et al. (12) showed that single-stranded HIV-1 DNA activates cGAS and HIV-1 reverse transcripts was the predominant viral DNA found in the cytoplasm during early infection. George et al. (3) showed that treatment with reverse transcriptase inhibitors immediately after infection completely blocked plasma IFN-α in SIV-infected rhesus macaques. Taken together these studies show that numerous innate sensing PRR contribute to the induction of IFN-α responses during HIV infection.
Although the production of IFN-α during HIV infection has been clearly demonstrated, the exact role these IFN play during infection has been less clear. Blockade of IFN-αR with anti-IFN-αR antibody was associated with higher HIV replication, whereas HIV replicated at lower levels in pDC-depleted cultures treated with IFN-α (13). IFN-α was found to limit HIV-1 replication by decreasing the formation of late reverse transcriptase products in infected cells (14), and treatment of newly infected CD4 T cells with IFN-α for short period time was associated with significant inactivation of HIV during the early stages of replication (15). IFN-α was shown to slow HIV disease progression in randomized, placebo-controlled trials (16), and Asmuth et al. (17) reported that the treatment with pegylated IFN-α2a had a statistically significant anti-HIV effect. Others have shown that IFN-α treatment inhibited HIV and SIV replication in CD4 T-cell lines (18), monocytes (19), and macrophages (20). IFN-α has been reported to affect late stages of HIV-1 replication in chronically infected cells, by inhibiting virus assembly and release and reducing the infectivity of virions (21). Other studies have shown the IFN-α induced IFN-stimulated genes (ISGs) that effectively suppressed HIV replication (22–24).
Interferon α has been shown to induce numerous ISG that are capable of restricting HIV replication namely, apolipoprotein B mRNA-editing (APOBEC3) family of cytidine deaminases, TRIM5α, tetherin (BST-2), SAMHD1, MX2, etc. (25–27). Studies have reported high levels of ISG expression in CD4 T cells very early during infection (28), and increased levels of APOBEC3G was found to correlate with lower levels of infection in macrophages during SIV infection (29). Others have reported that ISG were significantly upregulated during SIV infection (30–33). In addition to the induction of ISG, IFN-α has been shown to prime adaptive immune responses by cross-presenting viral antigens to CD8+ T cells (34–36). Interestingly, Boasso et al. demonstrated that IFN-α-induced indoleamine 2,3-dioxygenase (IDO) from pDC inhibited CD4+ T-cell proliferation during HIV infection (37), and blockade of gp120/CD4 interactions was found to inhibit HIV-mediated induction of IDO and IFN-α (38, 39).
In contrast to the protective effects of IFN-α during HIV infection, increased production of IFN-α was accompanied by an increase in HIV loads (40). Mandl et al. (41) argued that the generalized immune activation and progressive CD4 T cell depletion observed in pathogenic SIV infection was likely due to an aberrant activation of the innate immune system and increased IFN-α production in contrast to natural hosts such as sooty mangabeys. Martinson et al. (42) reported that TLR stimulation and IFN-α secretion by pDC contribute to immune activation during HIV infection. Others have shown that rapid progression of HIV was associated with continuous production of IFN-α, likely through enhanced T cell differentiation and activation (43). Parrish et al (44) demonstrated that transmitted founder viruses replicate and spread more efficiently in CD4 T cells in the presence of IFN-α. Fraietta et al. (45) showed that IFNα/β upregulated the expression of Bak, a pro-apoptotic protein that correlated with increased T cell apoptosis, low CD4+ T cell counts and high viral loads in HIV-infected patients. Patients who progressed to disease were found to have lower levels of pDC but displayed higher levels of IFN-α and MxA compared to healthy individuals (46). Other studies have reported that IFN-α promoted chronic immune activation, apoptosis, and immune dysfunction during HIV-1 infection (47–51). Likewise IFN-α was found to regulate CD4+ T-cell apoptosis induced by noninfectious HIV-1 by upregulating the expression of TNF-related apoptosis-inducing ligand (TRAIL) (38). Cha et al. (52) reported that IFN-α significantly enhanced activation-induced proliferation but not homeostatic proliferation, suggesting that the IFN-α likely promotes the loss of CD4 T cells by accelerating cell turnover and activation-induced cell death. On the other hand, Dondi et al. found that IFN-α displays contrasting proliferation-inducing and proapoptotic properties (53). Chronic IFN-α signaling has been implicated in other persistent viral infections such as LCMV (54, 55).
Type I IFN Subtypes and HIV Infection
Since its initial discovery, numerous isoforms of type I IFN have been identified. These isoforms, encoded by single exon genes include IFN-α (which harbors 13 different subtypes namely, IFN-α1, α2, α4, α5, α6, α7, α8, α10, α13, α14, α16, α17, and α21), IFN-β, IFN-ε, IFN-κ, and IFN-ω (56). All the type I IFN subtypes signal through a common receptor complex consisting of IFN-αR1 and IFN-αR2 subunits. In humans, IFN-α subtypes share ~70–99% amino acid sequence identity with each other and a ~35% identity with IFN-β (57).
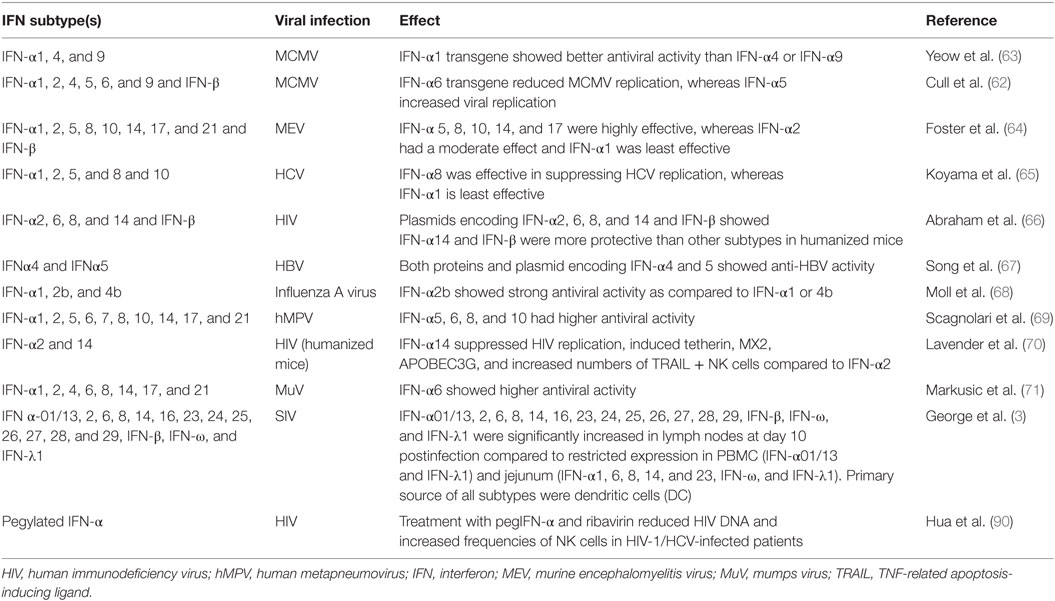
The evolutionary advantage of having multiple isoforms of the same gene that bind to a common receptor complex is not clear. However, there is evidence that the different subtypes display variable binding affinities for the common receptors (58–60), which in turn appears to influence their efficacy and potency (summarized in Table 1). Subtypes such as IFNα-10 binds to the IFN-αR1/2 receptor complex at affinities that is 10- to 100-fold greater than IFNα-1 (61). Interestingly, IFNα-10 was found to be highly effective against Semliki forest virus and Vesicular stomatitis virus, whereas IFN-α1 was the least effective among the nine different subtypes tested (61). Cull et al. (62) examined the expression of IFN-α1, α2, α4, α5, α6, and α9 and IFN-β in murine cytomegalovirus-induced myocarditis and observed that IFN-α6 reduced viral replication and inflammation in contrast to IFN-α2 and α5 that increased replication.
Sperber et al showed that IFNα-2 induced chemotaxis genes and was most effective against HIV-1 (72) whereas IFN-α8 induced ISG’s that were protective against HCV replication (65). Foster et al (64) showed that IFN-α8 has very high antiviral potency compared to some of the other subtypes. On the other hand, Scagnolari et al. (69) reported that IFN-α5, 6, 8, and 10 had high potency against human metapneumovirus, whereas IFN-α2, 17, and 21 were the least potent. Others have shown significant differences in the in vitro antiviral and antiproliferative effects of various subtypes (73–75). Hibbert and Foster (76) examined the effect of various subtypes on human B cells and showed that IFN-α8 induced proliferation at very low concentrations compared to other subtypes with IFN-α1 being largely inactive. Likewise, Hilkens et al. (77) examined the signaling though Janus kinase/STAT and transcriptional responses to selected IFN-α subtypes in human T cells and dendritic cells and reported differences in the potency of various subtypes to induce ISG.
Numerous studies have examined the expression of IFN subtypes during both HIV and SIV infections. Zaritsky et al. (78) evaluated the expression of both total IFNα mRNA and the pattern of IFN-α subtype mRNA expression in macaques during acute SIV infection and found that all 13 subtypes were expressed in the spleen with IFN-α4, 17, and 21 being the least abundant as compared to high levels of IFN-α2, 8, and 13. In contrast, only subtypes IFN-α2, 6, and 13 were expressed in the brain, whereas subtypes IFN-α6 and 13 were upregulated in the lung suggesting to tissue-specific differences in the expression of various subtypes. Lehman et al. (46) reported that IFN-α2 and IFN-α6 were significantly upregulated in HIV-infected patients. On the other hand, Li et al. (79) showed that IFN-α2 and 16 were upregulated during chronic HIV infection. George et al. (3) examined the expression of both type I and III IFN subtypes in peripheral blood, jejunal mucosa, and lymph nodes (LNs) of SIV-infected rhesus macaques and reported that all subtypes (IFN-α01/13, 02, 06, 08, 14, 16, 23, 24, 25, 27, 28, and 29, IFN-β, IFN-ω, and IFN-λ1) were significantly elevated in the LNs at day 10 postinfection compared to a restricted expression in PBMC (IFN-α01/13 and IFN-λ1) and jejunal mucosa (IFN-α1, 6, 8, 14, and 23, IFN-ω, and IFN-λ). Harper et al. (80) evaluated the expression of different IFN-α subtypes and their potency in HIV-1-exposed pDC using the lamina propria aggregate ex vivo culture model and reported that HIV infection induced numerous IFN-α subtypes with IFN-α6, IFN-α8, and IFN-α14 being the most potent at inhibiting HIV infection. Earlier studies (72) have shown that IFN-α2 was effective at suppressing HIV-1 replication although more recent studies (70) have demonstrated that IFN-α14 displayed significantly higher antiviral activity than IFN-α2 against HIV infection in humanized mouse models.
IFN-α Subtypes and Potential for Functional Cure
Given the potential for IFN-α to induce immune activation during HIV infection, there is a potential concern regarding its use in functional cure strategies although there is anecdotal evidence that IFN-α could suppress viral replication during antiretroviral therapy.
Treatment of HIV-infected subjects under HAART with pegylated-IFN-α2a was associated with the suppression of HIV RNA loads (81). Likewise, Sun et al. (82) demonstrated that the treatment of HIV/HCV co-infected patients with IFN-α/ribavirin during HAART led to a moderate but significant and sustained decline in cell-associated HIV DNA. Recent reports using IFN-α in combination with other factors appear promising. Micci et al. (83) reported that a combination of recombinant IL-21 and pegylated-IFN-α2a limited residual inflammation and viral persistence in SIV-infected rhesus macaques and significantly delayed viral rebound after withdrawal of antiretroviral therapy. Others (84) have shown that pretreatment of CD4 T cells with IFN-α and IFN-β reversed HIV latency in T-cells both in vitro and ex vivo and was associated with a reduction in the number of latently infected cells. Azzoni et al. (81) demonstrated that pegylated-IFN-α2 monotherapy successfully suppressed HIV-1 replication and reduced cell-associated HIV DNA.
Recent studies by Lavender et al. (70) showed that IFN-α14 when delivered at the same clinical dose as IFN-α2 to humanized mice significantly suppressed HIV replication and proviral loads and reduced immune activation that was accompanied by induction of high levels of APOBEC3G, MX2, and tetherin that have been shown to interfere with HIV replication (85–88). Abraham et al. (66) showed that gene therapy with plasmids encoding IFN-β and IFN-α14 significantly suppressed HIV-1 replication in mice for longer periods of time compared to other commonly used subtypes such as IFN-α2. Interestingly, all treated mice rebounded after cessation of IFN-α14 treatment. Additional studies are warranted to determine if the protective efficacy of IFN-α14 activated specific innate defenses during antiretroviral therapy that were different from those induced by other subtypes tested. These studies, however, raise the possibility that IFN subtypes such as IFN-α14 could be a potent adjunct to current approaches exploring functional cure strategies in HIV-infected subjects.
Other studies have shown that specific IFN subtypes were more potent at activating NK cells that could be harnessed to eradiate latently infected cells after reactivation. Gibbert et al. (89) demonstrated that IFN-α11-activated NK cells that enabled cytolytic killing of Friend retrovirus-infected cells compared to other subtypes such as IFN-α2 and IFN-α5. Hua et al. (90) recently reported that the treatment of HIV-1/HCV co-infected subjects on HAART with pegylated-IFN-α induced activation of CD56brightCD16− and CD56brightCD16+ NK cells expressing NKG2D an NKp30 that significantly correlated with a decrease in level of HIV-1 viral reservoir in CD4 T cells. Song et al. (67) examined that the effect of IFN-α subtypes on HBV infection and found that IFN-α4 and IFN-α5 correlated with expansion of effector NK cells in both liver and spleen that was associated with better control of HBV replication. Treatment of HIV-infected humanized mice with IFN-α14 was found to increase the expression of cytotoxic molecule TRAIL in NK cells, whereas Stegmann et al. (91) showed that induction of TRAIL on NK cells by IFN-α was associated with better control of hepatitis C infection. NK cells play an important role in the control of HIV infections (92) and strategies that can enhance NK cell activity could be beneficial in eradicating latently infected cells.
The studies described above suggest that a subset of IFN subtypes may be more effective at controlling infection than the others although there is a significant gap in our knowledge regarding the timing of administering these subtypes in the context of suppressive HAART that could potentially impact their efficacy. Sandler et al. (32) treated SIV-infected rhesus macaques with IFN-α2 during the acute phase of infection and reported that IFN-α2 initially upregulated the expression of antiviral genes, whereas continuous treatment was accompanied by desensitization and an increase in the viral reservoir size. Although the effect of initiating IFN therapy early in infection appears to be apparent, it is not clear if subjects under suppressive HAART regimens when treated would be unresponsive to treatment with various IFN subtypes.
Two exciting new studies (93, 94) using the humanized mouse model have reported that that blocking IFN signaling and reducing IFN-induced activation by treating with an antibody to the IFN receptor could reduce the size the HIV reservoir and delay viral rebound after cessation of HAART. These studies appear to be in contrast to what has been reported earlier using non-human primate models where blockade of IFN-αR was found to have the opposite effect (32). Audige et al. reported that treatment with anti-IFN-αR antibody was associated with increased HIV replication (13). On the other hand, blockade of chronic IFN signaling was shown to decrease immune activation and clear persistent LCMV infection in mice (55). Additional studies are needed to better clarify and confirm these findings in HIV infected subjects.
Conclusion
Functional cure strategies that can eradicate the viral reservoir are urgently needed. A number of approaches are being currently explored to achieve this goal. Although IFN-α therapy has been attempted in the field, there is new evidence suggesting that specific subtypes such as IFN-α8 and 14 may display more potent efficacy against HIV infection than the subtypes such as IFN-α2 that have been used in the past. Whether these subtypes can enhance innate immune defense during suppressive HAART and if these innate defenses would be sufficient to eradicate the reactivated latent reservoir remains to be seen. Studies that use a combination of approaches such as specific IFN-α subtypes along with therapeutic immunization to activate both the innate and adaptive immune responses during suppressive HAART are likely to be more effective at achieving full remission of HIV.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The described project was supported by funds from NIAID (AI125260) and the Uniformed Services University to JJM. The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense, the Uniformed Services University or any other agency of the US Government.
References
1. Perreau M, Banga R, Pantaleo G. Targeted immune interventions for an HIV-1 cure. Trends Mol Med (2017) 23(10):945–61. doi:10.1016/j.molmed.2017.08.006
2. Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci (1957) 147(927):258–67. doi:10.1098/rspb.1957.0049
3. George J, Renn L, Verthelyi D, Roederer M, Rabin RL, Mattapallil JJ. Early treatment with reverse transcriptase inhibitors significantly suppresses peak plasma IFNalpha in vivo during acute simian immunodeficiency virus infection. Cell Immunol (2016) 310:156–64. doi:10.1016/j.cellimm.2016.09.003
4. Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol (2014) 14(1):36–49. doi:10.1038/nri3581
5. Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science (1999) 284(5421):1835–7. doi:10.1126/science.284.5421.1835
6. Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med (2005) 202(4):461–5. doi:10.1084/jem.20051395
7. Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol (2005) 26(3):221–9. doi:10.1007/s00281-004-0180-4
8. Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol (2001) 31(7):2154–63. doi:10.1002/1521-4141(200107)31:7<2154::AID-IMMU2154>3.0.CO;2-U
9. Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science (2013) 341(6148):903–6. doi:10.1126/science.1240933
10. Lahaye X, Satoh T, Gentili M, Cerboni S, Conrad C, Hurbain I, et al. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity (2013) 39(6):1132–42. doi:10.1016/j.immuni.2013.11.002
11. Martin-Gayo E, Buzon MJ, Ouyang Z, Hickman T, Cronin J, Pimenova D, et al. Potent cell-intrinsic immune responses in dendritic cells facilitate HIV-1-specific T cell immunity in HIV-1 elite controllers. PLoS Pathog (2015) 11(6):e1004930. doi:10.1371/journal.ppat.1004930
12. Herzner AM, Hagmann CA, Goldeck M, Wolter S, Kubler K, Wittmann S, et al. Sequence-specific activation of the DNA sensor cGAS by Y-form DNA structures as found in primary HIV-1 cDNA. Nat Immunol (2015) 16(10):1025–33. doi:10.1038/ni.3267
13. Audige A, Urosevic M, Schlaepfer E, Walker R, Powell D, Hallenberger S, et al. Anti-HIV state but not apoptosis depends on IFN signature in CD4+ T cells. J Immunol (2006) 177(9):6227–37. doi:10.4049/jimmunol.177.9.6227
14. Baca-Regen L, Heinzinger N, Stevenson M, Gendelman HE. Alpha interferon-induced antiretroviral activities: restriction of viral nucleic acid synthesis and progeny virion production in human immunodeficiency virus type 1-infected monocytes. J Virol (1994) 68(11):7559–65.
15. Chen K, Huang J, Zhang C, Huang S, Nunnari G, Wang FX, et al. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J Virol (2006) 80(15):7645–57. doi:10.1128/JVI.00206-06
16. Lane HC, Davey V, Kovacs JA, Feinberg J, Metcalf JA, Herpin B, et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann Intern Med (1990) 112(11):805–11. doi:10.7326/0003-4819-112-11-805
17. Asmuth DM, Murphy RL, Rosenkranz SL, Lertora JJ, Kottilil S, Cramer Y, et al. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon Alfa-2a in HIV-1-monoinfected participants: a phase II clinical trial. J Infect Dis (2010) 201(11):1686–96. doi:10.1086/652420
18. Agy MB, Acker RL, Sherbert CH, Katze MG. Interferon treatment inhibits virus replication in HIV-1- and SIV-infected CD4+ T-cell lines by distinct mechanisms: evidence for decreased stability and aberrant processing of HIV-1 proteins. Virology (1995) 214(2):379–86. doi:10.1006/viro.1995.0047
19. Bednarik DP, Mosca JD, Raj NB, Pitha PM. Inhibition of human immunodeficiency virus (HIV) replication by HIV-trans-activated alpha 2-interferon. Proc Natl Acad Sci U S A (1989) 86(13):4958–62. doi:10.1073/pnas.86.13.4958
20. Goujon C, Malim MH. Characterization of the alpha interferon-induced postentry block to HIV-1 infection in primary human macrophages and T cells. J Virol (2010) 84(18):9254–66. doi:10.1128/JVI.00854-10
21. Dianzani F, Castilletti C, Gentile M, Gelderblom HR, Frezza F, Capobianchi MR. Effects of IFN alpha on late stages of HIV-1 replication cycle. Biochimie (1998) 80(8–9):745–54. doi:10.1016/S0300-9084(99)80028-5
22. Hansen BD, Nara PL, Maheshwari RK, Sidhu GS, Bernbaum JG, Hoekzema D, et al. Loss of infectivity by progeny virus from alpha interferon-treated human immunodeficiency virus type 1-infected T cells is associated with defective assembly of envelope gp120. J Virol (1992) 66(12):7543–8.
23. Kinzl P, Otani T, Benz R, Minowada J. Interferon-alpha and -gamma differentially reduce rapid immature T-cell death by contact with HIV-1 carrier cell clones in vitro. Microbiol Immunol (1997) 41(9):709–16. doi:10.1111/j.1348-0421.1997.tb01915.x
24. Shirazi Y, Pitha PM. Interferon downregulates CXCR4 (fusin) gene expression in peripheral blood mononuclear cells. J Hum Virol (1998) 1(2):69–76.
25. Gurney KB, Colantonio AD, Blom B, Spits H, Uittenbogaart CH. Endogenous IFN-alpha production by plasmacytoid dendritic cells exerts an antiviral effect on thymic HIV-1 infection. J Immunol (2004) 173(12):7269–76. doi:10.4049/jimmunol.173.12.7269
26. Sakuma R, Mael AA, Ikeda Y. Alpha interferon enhances TRIM5alpha-mediated antiviral activities in human and rhesus monkey cells. J Virol (2007) 81(18):10201–6. doi:10.1128/JVI.00419-07
27. Sheehy AM, Gaddis NC, Malim MH. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med (2003) 9(11):1404–7. doi:10.1038/nm945
28. Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest (2009) 119(12):3556–72. doi:10.1172/JCI40115
29. Moore AC, Bixler SL, Lewis MG, Verthelyi D, Mattapallil JJ. Mucosal and peripheral Lin- HLA-DR+ CD11c/123- CD13+ CD14- mononuclear cells are preferentially infected during acute simian immunodeficiency virus infection. J Virol (2012) 86(2):1069–78. doi:10.1128/JVI.06372-11
30. Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol (2002) 76(16):8433–45. doi:10.1128/JVI.76.16.8433-8445.2002
31. Kader M, Smith AP, Guiducci C, Wonderlich ER, Normolle D, Watkins SC, et al. Blocking TLR7- and TLR9-mediated IFN-alpha production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection. PLoS Pathog (2013) 9(7):e1003530. doi:10.1371/journal.ppat.1003530
32. Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature (2014) 511(7511):601–5. doi:10.1038/nature13554
33. Schaefer TM, Fuller CL, Basu S, Fallert BA, Poveda SL, Sanghavi SK, et al. Increased expression of interferon-inducible genes in macaque lung tissues during simian immunodeficiency virus infection. Microbes Infect (2006) 8(7):1839–50. doi:10.1016/j.micinf.2006.02.022
34. Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol (2003) 4(10):1009–15. doi:10.1038/ni978
35. Witter F, Barouki F, Griffin D, Nadler P, Woods A, Wood D, et al. Biologic response (antiviral) to recombinant human interferon alpha 2a as a function of dose and route of administration in healthy volunteers. Clin Pharmacol Ther (1987) 42(5):567–75. doi:10.1038/clpt.1987.198
36. Zhu H, Butera M, Nelson DR, Liu C. Novel type I interferon IL-28A suppresses hepatitis C viral RNA replication. Virol J (2005) 2:80. doi:10.1186/1743-422X-2-80
37. Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood (2007) 109(8):3351–9. doi:10.1182/blood-2006-07-034785
38. Herbeuval JP, Hardy AW, Boasso A, Anderson SA, Dolan MJ, Dy M, et al. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A (2005) 102(39):13974–9. doi:10.1073/pnas.0505251102
39. Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest (2008) 118(10):3431–9. doi:10.1172/JCI34823
40. Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol (2009) 83(8):3719–33. doi:10.1128/JVI.01844-08
41. Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, Klucking S, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med (2008) 14(10):1077–87. doi:10.1038/nm.1871
42. Martinson JA, Montoya CJ, Usuga X, Ronquillo R, Landay AL, Desai SN. Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: implication for T-cell activation. Antimicrob Agents Chemother (2010) 54(2):871–81. doi:10.1128/AAC.01246-09
43. Hyrcza MD, Kovacs C, Loutfy M, Halpenny R, Heisler L, Yang S, et al. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J Virol (2007) 81(7):3477–86. doi:10.1128/JVI.01552-06
44. Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A (2013) 110(17):6626–33. doi:10.1073/pnas.1304288110
45. Fraietta JA, Mueller YM, Yang G, Boesteanu AC, Gracias DT, Do DH, et al. Type I interferon upregulates Bak and contributes to T cell loss during human immunodeficiency virus (HIV) infection. PLoS Pathog (2013) 9(10):e1003658. doi:10.1371/journal.ppat.1003658
46. Lehmann C, Taubert D, Jung N, Fatkenheuer G, van Lunzen J, Hartmann P, et al. Preferential upregulation of interferon-alpha subtype 2 expression in HIV-1 patients. AIDS Res Hum Retroviruses (2009) 25(6):577–81. doi:10.1089/aid.2008.0238
47. Herbeuval JP, Nilsson J, Boasso A, Hardy AW, Kruhlak MJ, Anderson SA, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A (2006) 103(18):7000–5. doi:10.1073/pnas.0600363103
48. Sedaghat AR, German J, Teslovich TM, Cofrancesco J Jr, Jie CC, Talbot CC Jr, et al. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol (2008) 82(4):1870–83. doi:10.1128/JVI.02228-07
49. Stylianou E, Aukrust P, Muller F, Nordoy I, Froland SS. Complex effects of interferon-alpha on the cytokine network in HIV infection – possible contribution to immunosuppression. Cytokine (2001) 14(1):56–62. doi:10.1006/cyto.2000.0850
50. Stylianou E, Yndestad A, Sikkeland LI, Bjerkeli V, Damas JK, Haug T, et al. Effects of interferon-alpha on gene expression of chemokines and members of the tumour necrosis factor superfamily in HIV-infected patients. Clin Exp Immunol (2002) 130(2):279–85. doi:10.1046/j.1365-2249.2002.01980.x
51. Zagury D, Lachgar A, Chams V, Fall LS, Bernard J, Zagury JF, et al. Interferon alpha and Tat involvement in the immunosuppression of uninfected T cells and C-C chemokine decline in AIDS. Proc Natl Acad Sci U S A (1998) 95(7):3851–6. doi:10.1073/pnas.95.7.3851
52. Cha L, de Jong E, French MA, Fernandez S. IFN-alpha exerts opposing effects on activation-induced and IL-7-induced proliferation of T cells that may impair homeostatic maintenance of CD4+ T cell numbers in treated HIV infection. J Immunol (2014) 193(5):2178–86. doi:10.4049/jimmunol.1302536
53. Dondi E, Roue G, Yuste VJ, Susin SA, Pellegrini S. A dual role of IFN-alpha in the balance between proliferation and death of human CD4+ T lymphocytes during primary response. J Immunol (2004) 173(6):3740–7. doi:10.4049/jimmunol.173.6.3740
54. Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science (2013) 340(6129):207–11. doi:10.1126/science.1235214
55. Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science (2013) 340(6129):202–7. doi:10.1126/science.1235208
56. Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol (2005) 23:307–36. doi:10.1146/annurev.immunol.23.021704.115843
57. Genin P, Vaccaro A, Civas A. The role of differential expression of human interferon – a genes in antiviral immunity. Cytokine Growth Factor Rev (2009) 20(4):283–95. doi:10.1016/j.cytogfr.2009.07.005
58. Lamken P, Lata S, Gavutis M, Piehler J. Ligand-induced assembling of the type I interferon receptor on supported lipid bilayers. J Mol Biol (2004) 341(1):303–18. doi:10.1016/j.jmb.2004.05.059
59. Piehler J, Roisman LC, Schreiber G. New structural and functional aspects of the type I interferon-receptor interaction revealed by comprehensive mutational analysis of the binding interface. J Biol Chem (2000) 275(51):40425–33. doi:10.1074/jbc.M006854200
60. Roisman LC, Jaitin DA, Baker DP, Schreiber G. Mutational analysis of the IFNAR1 binding site on IFNalpha2 reveals the architecture of a weak ligand-receptor binding-site. J Mol Biol (2005) 353(2):271–81. doi:10.1016/j.jmb.2005.08.042
61. Yamaoka T, Kojima S, Ichi S, Kashiwazaki Y, Koide T, Sokawa Y. Biologic and binding activities of IFN-alpha subtypes in ACHN human renal cell carcinoma cells and Daudi Burkitt’s lymphoma cells. J Interferon Cytokine Res (1999) 19(12):1343–9. doi:10.1089/107999099312803
62. Cull VS, Bartlett EJ, James CM. Type I interferon gene therapy protects against cytomegalovirus-induced myocarditis. Immunology (2002) 106(3):428–37. doi:10.1046/j.1365-2567.2002.01423.x
63. Yeow WS, Lawson CM, Beilharz MW. Antiviral activities of individual murine IFN-alpha subtypes in vivo: intramuscular injection of IFN expression constructs reduces cytomegalovirus replication. J Immunol (1998) 160(6):2932–9.
64. Foster GR, Rodrigues O, Ghouze F, Schulte-Frohlinde E, Testa D, Liao MJ, et al. Different relative activities of human cell-derived interferon-alpha subtypes: IFN-alpha 8 has very high antiviral potency. J Interferon Cytokine Res (1996) 16(12):1027–33.
65. Koyama T, Sakamoto N, Tanabe Y, Nakagawa M, Itsui Y, Takeda Y, et al. Divergent activities of interferon-alpha subtypes against intracellular hepatitis C virus replication. Hepatol Res (2006) 34(1):41–9. doi:10.1016/j.hepres.2005.10.005
66. Abraham S, Choi JG, Ortega NM, Zhang J, Shankar P, Swamy NM. Gene therapy with plasmids encoding IFN-beta or IFN-alpha14 confers long-term resistance to HIV-1 in humanized mice. Oncotarget (2016) 7(48):78412–20. doi:10.18632/oncotarget.12512
67. Song J, Li S, Zhou Y, Liu J, Francois S, Lu M, et al. Different antiviral effects of IFNalpha subtypes in a mouse model of HBV infection. Sci Rep (2017) 7(1):334. doi:10.1038/s41598-017-00469-1
68. Moll HP, Maier T, Zommer A, Lavoie T, Brostjan C. The differential activity of interferon-alpha subtypes is consistent among distinct target genes and cell types. Cytokine (2011) 53(1):52–9. doi:10.1016/j.cyto.2010.09.006
69. Scagnolari C, Trombetti S, Selvaggi C, Carbone T, Monteleone K, Spano L, et al. In vitro sensitivity of human metapneumovirus to type I interferons. Viral Immunol (2011) 24(2):159–64. doi:10.1089/vim.2010.0073
70. Lavender KJ, Gibbert K, Peterson KE, Van Dis E, Francois S, Woods T, et al. Interferon alpha subtype-specific suppression of HIV-1 infection in vivo. J Virol (2016) 90(13):6001–13. doi:10.1128/JVI.00451-16
71. Markusic M, Santak M, Kosutic-Gulija T, Jergovic M, Jug R, Forcic D. Induction of IFN-alpha subtypes and their antiviral activity in mumps virus infection. Viral Immunol (2014) 27(10):497–505. doi:10.1089/vim.2014.0028
72. Sperber SJ, Gocke DJ, Haberzettl C, Kuk R, Schwartz B, Pestka S. Anti-HIV-1 activity of recombinant and hybrid species of interferon-alpha. J Interferon Res (1992) 12(5):363–8. doi:10.1089/jir.1992.12.363
73. Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev (2004) 202:8–32. doi:10.1111/j.0105-2896.2004.00204.x
74. Weck PK, Apperson S, May L, Stebbing N. Comparison of the antiviral activities of various cloned human interferon-alpha subtypes in mammalian cell cultures. J Gen Virol (1981) 57(Pt 1):233–7. doi:10.1099/0022-1317-57-1-233
75. Yanai Y, Sanou O, Kayano T, Ariyasu H, Yamamoto K, Yamauchi H, et al. Analysis of the antiviral activities of natural IFN-alpha preparations and their subtype compositions. J Interferon Cytokine Res (2001) 21(10):835–41. doi:10.1089/107999001753238088
76. Hibbert L, Foster GR. Human type I interferons differ greatly in their effects on the proliferation of primary B cells. J Interferon Cytokine Res (1999) 19(4):309–18. doi:10.1089/107999099314009
77. Hilkens CM, Schlaak JF, Kerr IM. Differential responses to IFN-alpha subtypes in human T cells and dendritic cells. J Immunol (2003) 171(10):5255–63. doi:10.4049/jimmunol.171.10.5255
78. Zaritsky LA, Dery A, Leong WY, Gama L, Clements JE. Tissue-specific interferon alpha subtype response to SIV infection in brain, spleen, and lung. J Interferon Cytokine Res (2013) 33(1):24–33. doi:10.1089/jir.2012.0018
79. Li Y, Sun B, Esser S, Jessen H, Streeck H, Widera M, et al. Expression pattern of individual IFNA subtypes in chronic HIV infection. J Interferon Cytokine Res (2017) 37(12):541–9. doi:10.1089/jir.2017.0076
80. Harper MS, Guo K, Gibbert K, Lee EJ, Dillon SM, Barrett BS, et al. Interferon-alpha subtypes in an ex vivo model of acute HIV-1 infection: expression, potency and effector mechanisms. PLoS Pathog (2015) 11(11):e1005254. doi:10.1371/journal.ppat.1005254
81. Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, et al. Montaner: Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis (2013) 207(2):213–22. doi:10.1093/infdis/jis663
82. Sun H, Buzon MJ, Shaw A, Berg RK, Yu XG, Ferrando-Martinez S, et al. Hepatitis C therapy with interferon-alpha and ribavirin reduces CD4 T-cell-associated HIV-1 DNA in HIV-1/hepatitis C virus-coinfected patients. J Infect Dis (2014) 209(9):1315–20. doi:10.1093/infdis/jit628
83. Micci L, Harper J, Paganini S, King C, Ryan E, Villinger F, et al. Combined IL-21 and IFNα treatment limits residual inflammation and delays viral rebound in SIV-infected macaques (OA4-1). IAS HIV Cure & Cancer Forum. July 2017. Paris, France. J Virus Eradic (2017) 3(Suppl 1):1–5.
84. Van der Sluis RM, Mota T, Kumar NA, Evans AN, Harman S, RTennakoon SG, et al. Plasmacytoid DCs control HIV latency in resting T cells by type I IFNα (P 32). IAS HIV Cure & Cancer Forum. July 2017. Paris, France. J Virus Eradic (2017) 3(Suppl 1):6–19.
85. Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ, et al. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature (2013) 502(7472):563–6. doi:10.1038/nature12653
86. Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature (2003) 424(6944):99–103. doi:10.1038/nature01709
87. Pillai SK, Abdel-Mohsen M, Guatelli J, Skasko M, Monto A, Fujimoto K, et al. Role of retroviral restriction factors in the interferon-alpha-mediated suppression of HIV-1 in vivo. Proc Natl Acad Sci U S A (2012) 109(8):3035–40. doi:10.1073/pnas.1111573109
88. Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature (2002) 418(6898):646–50. doi:10.1038/nature00939
89. Gibbert K, Joedicke JJ, Meryk A, Trilling M, Francois S, Duppach J, et al. Interferon-alpha subtype 11 activates NK cells and enables control of retroviral infection. PLoS Pathog (2012) 8(8):e1002868. doi:10.1371/journal.ppat.1002868
90. Hua S, Vigano S, Tse S, Zhengyu O, Harrington S, Negron J, et al. Pegylated IFN-alpha-induced NK cell activation is associated with HIV-1 DNA decline in ART-treated HIV-1/HCV co-infected patients. Clin Infect Dis (2017). doi:10.1093/cid/cix1111
91. Stegmann KA, Bjorkstrom NK, Veber H, Ciesek S, Riese P, Wiegand J, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology (2010) 138(5):1885–97. doi:10.1053/j.gastro.2010.01.051
92. Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol (2013) 31:163–94. doi:10.1146/annurev-immunol-032712-100001
93. Cheng L, Ma J, Li J, Li D, Li G, Li F, et al. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J Clin Invest (2017) 127(1):269–79. doi:10.1172/JCI90745
Keywords: human immunodeficiency virus, functional cure, interferon-α, interferon-α subtypes, human immunodeficiency virus latency
Citation: George J and Mattapallil JJ (2018) Interferon-α Subtypes As an Adjunct Therapeutic Approach for Human Immunodeficiency Virus Functional Cure. Front. Immunol. 9:299. doi: 10.3389/fimmu.2018.00299
Received: 07 December 2017; Accepted: 02 February 2018;
Published: 22 February 2018
Edited by:
Vijayakumar Velu, Emory University, United StatesReviewed by:
Namal P. M. Liyanage, The Ohio State University, United StatesPaul Urquhart Cameron, University of Melbourne, Australia
Copyright: © 2018 George and Mattapallil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph J. Mattapallil, am9zZXBoLm1hdHRhcGFsbGlsQHVzdWhzLmVkdQ==
 Jeffy George
Jeffy George Joseph J. Mattapallil
Joseph J. Mattapallil