- 1Department of Microbiology and Biochemical Pharmacy, National Engineering Research Center of Immunological Products, College of Pharmacy, Third Military Medical University, Chongqing, China
- 2Chongqing Nankai Secondary School, Chongqing, China
- 3Chongqing Technical Center for Drug Evaluation and Certification, Chongqing, China
Over fifty percent of the people around the world is infected with Helicobacter pylori (H. pylori), which is the main cause of gastric diseases such as chronic gastritis and stomach cancer. H. pylori adhesin A (HpaA), which is a surface-located lipoprotein, is essential for bacterial colonization in the gastric mucosa. HpaA had been proposed to be a promising vaccine candidate against H. pylori infection. However, the effect of non-lipidated recombinant HpaA (rHpaA) to stimulate immune response was not very ideal, and the protective effect against H. pylori infection was also limited. Here, we hypothesized that low immunogenicity of rHpaA may attribute to lacking the immunostimulatory properties endowed by the lipid moiety. In this study, two novel lipopeptides, LP1 and LP2, which mimic the terminal structure of the native HpaA (nHpaA), were synthesized and TLR2 activation activity was confirmed in vitro. To investigate whether two novel lipopeptides could improve the protective effect of rHpaA against the infection of H. pylori, groups of mice were immunized either intramuscularly or intranasally with rHpaA together with LP1 or LP2. Compared with rHpaA alone, the bacterial colonization of the mice immunized with rHpaA plus LP2 via intranasal route was significantly decreased and the expression levels of serum IgG2a, IFN-γ, and IL-17 cytokines in spleen lymphocyte culture supernatant increased obviously, indicating that the enhanced protection of LP2 may be associated with elevated specific Th1 and Th17 responses. In conclusion, LP2 has been shown to improve the protective effect of rHpaA against H. pylori infection, which may be closely related to its ability in activating TLR2 by mimicking the terminal structure of nHpaA.
Introduction
Helicobacter pylori (H. pylori) was identified as a category I human carcinogen by the International Agency for Research on Cancer in 1994. Infection with H. pylori was proved to be associated with peptic ulcer, chronic gastritis and gastric carcinoma. It is estimated that over half of the world's population is infected with H. pylori (1–3). Due to the growing antibiotic resistance, the clearance rate of H. pylori has fallen in a great portion of patients (4–6), and the treatment of H. pylori infection has become a challenge in recent years. Thus, vaccination may be one of the most promising means to control H. pylori infection (7).
In the pathogenesis of H. pylori, bacterial attachment to mucosal epithelial cells and gastric epithelial mucosa seems to be a crucial step (8, 9). Because of their important role in host adhesion and survival, membrane proteins are considered as potential targets for vaccine development. HpaA (H. pylori adhesin A), also described as neuraminyllactose-binding haemagglutinin (NLBH), can bind to various glycosylation components on the surface of gastric epithelial cells, which is essential for colonization of the bacteria to gastric mucosa (10, 11). It has previously been found that expression of HpaA is highly conserved in H. pylori isolates. (12). Genomic studies also showed that HpaA has no significant sequence homologies with other known proteins (13). Additionally, several studies had confirmed the immunogenicity and immunostimulatory effect of HpaA, while the expression of serum HpaA-specific antibodies was elevated in those infected with H. pylori (14, 15). HpaA had been considered as a promising H. pylori vaccine candidate antigen.
It was reported that the prophylactic immunization with non-lipidated recombinant HpaA (rHpaA) could induce protection against H. pylori infection in mice (16–19). However, the effect of stimulating human immune response was not satisfactory, and the protection against the infection of H. pylori was also limited (20, 21). However, native HpaA (nHpaA) from H. pylori is a surface-located lipoprotein, which may play an important role in activating innate and adaptive immune responses via TLR2-dependent signaling pathways (22–25). In addition, lipoprotein could activate macrophages and monocytes, which may promote immune response and play a role as an adjuvant (26–28). Based on these studies, we deduce that the lack of lipid modification on the rHpaA in Escherichia coli may be the main reason for its weak immunostimulatory and protective effects.
It had been reported that lipoproteins could be recognized by TLR2 mainly depending on their N-terminal lipid moiety (29, 30). Therefore, the capability of lipoproteins to activate TLR2 could be retained by mimicking the specific N-terminal structure. In this study, the lipid modification sites of nHpaA were predicted by bioinformatics. Accordingly, two novel lipopeptides, including LP1 and LP2, were synthesized. Mice were immunized with the mixture of synthetic lipopeptides and rHpaA through intramuscular or intranasal routes. The protection against the infection of H. pylori was evaluated and the mechanism was explored.
Materials and Methods
Ethics Statement
Animal maintenance and laboratory procedures were carried out according to the National Institutes of Health Guidelines for the Use of Experimental Animals and approved by the Medicine Animal Care Committee of the Third Military Medical University. Well-trained and skilled animal care personnel participated in the current study to minimize the suffering of animals. All procedures were performed under anesthesia with 1% sodium pentobarbital to alleviate pain. The health of the mice we monitored every 8–12 h and CO2 was used for euthanasia.
Animals and Cell Lines
Specific-pathogen-free (SPF) female BALB/c mice aged 6–8 weeks old were purchased from the Experimental Animal Center of the Third Military Medical University, and were kept under pathogen-free conditions. HK-2 (human kidney 2) was purchased from the ATCC (American Type Culture Collection). HEK-Blue™-mTLR2 cells were purchased from InvivoGen (San Diego, CA, USA) and cells were grown in DMEM supplemented with 10% FBS, L-glutamine (2 mM), Normocin (100 μg/mL), penicillin (50 U/mL), streptomycin (50 g/mL) and passaged when reached 70% confluence. Cells were scraped and then resuspended in HEK-Blue™ Detection medium (InvivoGen, San Diego, CA, USA). The induction of SEAP was detected at 620 nm by a spectrophotometer.
Reagents
rHpaA was expressed in E. coli and purified as previously described (Figure 1A) (31, 32). Helicobacter Pylori Medium (HB8674) was purchased from Hopebio (Qingdao, Shandong, China). HRP conjugated goat anti-mouse IgG (ab97200), IgG1 (ab97240), IgG2a (ab97245), and IgA (ab97235) were the products of Abcam (Cambridge, MA, USA). Mouse IFN-γ (DKW12-2000-096), IL-17A (DKW12-2170-096), IL-4 (DKW12-2040-096) precoated ELISA kit were purchased from Dakewe (Shenzhen, Guangdong, China). Anti-mouse CD11c-FITC (117305), CD80-APC (104713), CD86-PerCP (105025) were the products of BioLegend (San Diego, MA, USA).
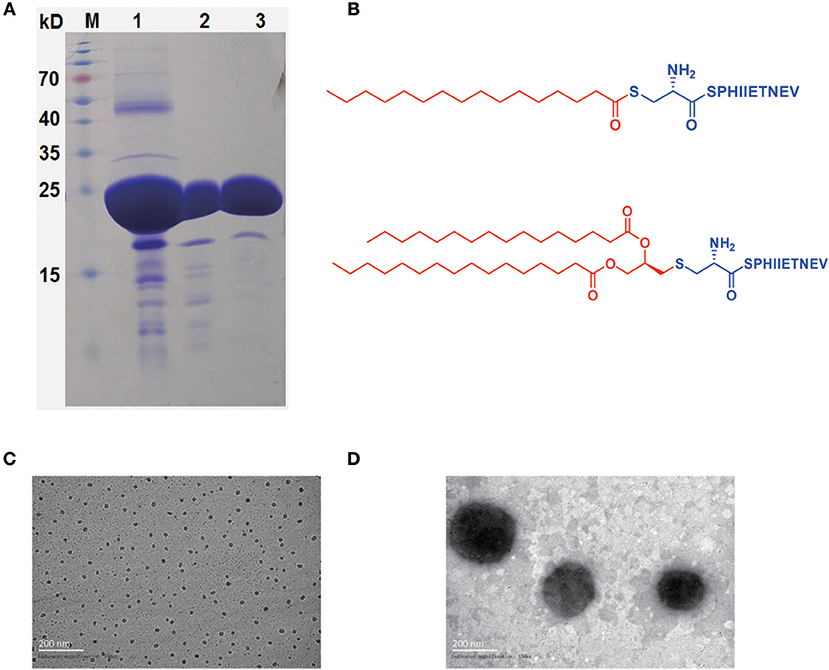
Figure 1. rHpaA expression and lipopeptides characterization. (A) SDS-PAGE. rHpaA was expressed in E. coli. rHpaA was firstly purified by affinity chromatography (lane 1) and then refined by ion exchange chromatography (lane 2). Size exclusion chromatography was used in the final step to polish the purification (lane 3). (B) Chemical structure of LP1 and LP2. Transmission electron microscopy (TEM) image of LP1 (C) and LP2 (D) micelles after dying with 2% phosphotungstic acid.
Synthesis of Lipopeptides
LP1 and LP2 were synthesized with solid-phase methods as previously described (33). LP1 was obtained by S-acylation of the cysteine of the peptide (CSPHIIETNEV) with palmitoyl chloride, and then the lipopeptide is cleaved from the resin and purified. Fmoc-Pam2Cys-OH was introduced to the N terminus of the peptide (SPHIIETNEV) linked to the resin. The Fmoc group was subsequently cleaved. The molecular were detached from the resin and all protecting groups were deprotected to yield the lipopeptide LP2.
Hemolysis and Cell Viability Assay
Hemolysis and cell viability assay was performed using methods described previously (34). Briefly, 0.5 mL of 2% solution of mice red blood cells was mixed with 0.5 mL of diluent containing 100, 200, 400, and 1.25 μg/mL of LP1 or LP2 and then incubated at 37°C for 3 h. The hemolytic activity was determined by measuring the absorbance of the supernatant at 570 nm. For cell viability assay, the trypsin-treated HK-2 cells were cultured with gradient dilutions of LP1 or LP2 at for 48 h. Afterward, 20 μL of a MTT solution (5 mg/mL) was added to each well and incubated for 4 h at 37°C. Subsequently, the mixture was removed from the well and 100 μL DMSO was added. The optical absorption was detected at a wavelength of 570 nm. Percentage of surviving cells = Mean optical absorption of cells exposed to the lipopeptide/Mean optical absorption of control cells × 100.
Transmission Electron Microscopy (TEM)
The morphology of the synthetic lipopeptides was investigated by TEM negative staining technique. LP1 or LP2 at 100 μg/mL was deposited onto a copper grid and negative stained with 2% phosphotungstic acid for 30 s. After removal of excess of the solution and kept dried for at least 6 h, TEM images were taken by a JEOL JEM-1230 (JEOL Ltd, Tokyo, Japan) (35).
TLR2 Signaling Assay
The sample was added to a flat-bottom 96-well plate (20 μL per well) and an equal amount of endotoxin-free water was used as a negative control. Followed by HEK-Blue™ mTLR2 cells were resuspended in the pre-warm HEK-Blue™ Detection, cell suspension (180 μL, 5 ×104 cells) was added to the sample and then incubated at 37°C for 14 h. Samples were analyzed for SEAP activity after which OD was measured in a spectrophotometer at 620 nm (36).
Generation of BMDCs and FACS Assay
Mouse bone marrow cells were isolated from tibia and femurs, and then were cultured in RPMI 1640 medium containing 10% FCS, recombinant murine GM-CSF (6 ng/mL) and IL-4 (20 ng/mL) at 37°C, 5% CO2. The non-adherent cells were removed on day 5 and adherent cells were cultured in a fresh complete medium for another 2 days. BMDCs were harvested and stimulated with LP1, LP2 or PBS for 48 h. After three washes, the cells were incubated with FITC-αCD11c, APC-αCD80, and PerCP-αCD86 in the dark for 30 min at 4°C. Flow cytometry data were acquired with a FACS Canto II (BD Biosciences) (37).
Animal Immunization
For the different delivery routes, mice were divided randomly into two sections. In the first section, mice were intranasally immunized 5 times in 4 weeks with PBS, rHpaA alone, or rHpaA plus one of the two synthetic lipopeptides (2 μg/mouse). The final amount of rHpaA for each intranasal immunization was 10 μg in 20 μL PBS. In the second section, the mice were immunized intramuscularly three times at intervals of 2 weeks with 50 μg of antigen in the same groups to the first section. On day 43, sera and intestinal lavage fluid were collected for further determination.
H. pylori Challenge and Quantification
Two weeks following the last immunization, mice were orally challenged with 109 BALB/c mouse-adapted H. pylori four times for 4 days. At 2-week post-challenge, the number of H. pylori colonization was quantified by real-time PCR (38). The following primers were used: Forward, 5′-TTTGTTAGAGAAGATAATGACGGTATCTAAC-3′, Reverse, 5′-CATAGGATTTCACACCTGACTGACTATC-3′, Probe, 5′-FAM-CGTGCCAGCAGCCGCGGT-TAMRA-3′. This assay was performed in a Bio-Rad iQ5 multicolor real-time PCR thermocycler (39).
Antibody Production Assay
Antibody levels were measured by standard indirect ELISA as previously described (40). Briefly, 96-well flat bottom microtiter plates were coated with rHpaA antigens in PBS and incubated overnight at 4°C. After blocking with 5% BSA in PBST, the 1:1,000 pre-diluted sera samples (intramuscularly) and the 1:50 pre-diluted sera samples (intranasally) were added to 96-well microtiter plates followed 1:1 gradient dilution and incubated for 1 h at 37°C. HRP-conjugated goat anti-mouse IgG was then used as a secondary antibody to determine antigen-specific IgG level. Each well was added 100 μL TMB substrate solution and then was kept in darkness for 20 min. The reaction was finally quenched by stop solution (2M sulfuric acid). The optical density (OD) was measured at 450 nm in a micro-plate reader (Bio-Rad). As described above, the levels of specific IgG1 and IgG2a in the serum were also measured by ELISA.
Cytokines Assay
Splenocytes were isolated from mice and re-stimulated with the antigen for 48 h. Cytokines IFN-γ, IL-4, and IL-17A in coculture supernatants, which were derived from Th1/Th2/Th17 cells, were detected by ELISA kit. Absorbance at 450 nm was measured in a microplate reader.
In vitro Adhesion Assay With AGS Cells
The in vitro adhesion assay was based on the method as previously described (41, 42). Briefly, H. pylori strain B0 were suspended in DMEM/F12 to an OD600nm of 0.7 (i.e., 108 CFU/ml), and then left untreated or treated with antiserum (1:100 dilution) at 4°C for 1 h. AGS cells were incubated with pretreated bacterial suspension at an MOI of 100 for each species. After 2 h of incubation, the cells were washed with PBS three times in order to remove unbound bacteria. The colonization of H. pylori attached to AGS cells was quantified by real-time PCR as described in section H. pylori Challenge and Quantification.
Statistical Analysis
All statistical analysis were conducted by the SPSS 21.0 statistical package. Data were expressed as means ± standard deviation (SD). One-way ANOVA was used to analyze inter-group differences, and differences with a p-value ≤ 0.05 were considered statistically significant., Bonferroni test were further performed if inter-group differences were statistically significant, with the significant level α' = α/k (k is the number of comparison times).
Results
Synthesis and Characterization of LP1 and LP2
According to bioinformatics analysis using the DOLOP (http://www.mrc-lmb.cam.ac.uk/genomes/dolop) and UniProt (https://www.uniprot.org) database, nHpaA has two lipidative forms, monopalmitoylation (Pam) and dipalmitoylation (Pam2). The peptide containing 10 amino acids (CSPHIIETNEV), derived from the N terminus of the nHpaA, was selected for chemical coupling with Pam and Pam2, respectively, resulting in two novel lipopeptides (Figure 1B). The purity of the synthesized LP1 was 95.58% and of LP2 was 95.14% as determined by HPLC. The structures of LP1 and LP2 were further confirmed by mass spectrometry (Figures S1, S2.)
Given that LP1 and LP2 were amphiphilic molecules, as shown in Figures 1C,D, they were able to self-assemble into micelles in an aqueous environment. The resultant LP1 micelles had a mean diameter of about 20–30 nm (Figure 1C), and LP2 micelles were approximately ten times larger than LP1 micelles, with a diameter about 200 nm (Figure 1D). LP1 and LP2 micelles exhibited sizes in the range of microorganisms, which may be better recognized by antigen-presenting cells, thereby possibly enhanced the immune response against co-administered antigens.
In vitro Safety of LP1 and LP2
To evaluate the safety of LP1 and LP2 in vitro, the cytotoxicity of LP1 and LP2 was evaluated on HK-2 cell lines using the MTT colorimetric assay. As shown in Figure 2A, all doses tested (50–200 μg/ml) showed almost no HK-2 cells toxicity. Exposition of mice red blood cells to LP2 (50–200 μg/ml) did not induce significant hemolysis in vitro. LP1 caused <3% hemolysis at 100 μg/mL (Figure 2B). The above results indicated that synthetic lipopeptides were safe in vitro at concentrations below 100 μg/ml and could be further tested in vivo.
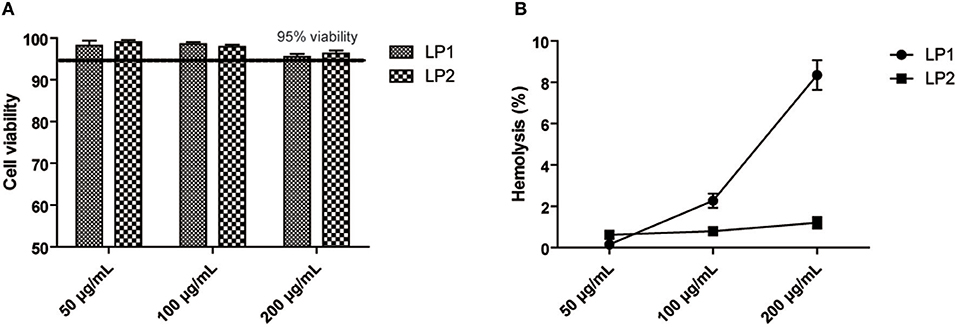
Figure 2. Cell viability and Hemolysis assay. (A) HK-2 cells were incubated with LP1 and LP2 for 48 h, respectively, and then cell viability was quantified with an MTT assay (data are expressed as mean ± S.D., n = 3). (B) Hemolytic effects were investigated by incubating erythrocytes with the diluent containing 200, 100, 50 μg/mL of two synthetic lipopeptides in saline (data are expressed as mean ± S.D., n = 3).
LP1 and LP2 Activate TLR2 and Promote BMDCs Maturation
To confirm whether the two synthesized lipopeptides retained the TLR2-stimulating activity of nHpaA, HEK293-TLR2 cells were used for luciferase reporter assays. As shown in Figure 3A, both LP1 and LP2 significantly enhanced the dose-dependent NF-kB activation in HEK293-TLR2 cells. Compared with LP1, LP2 exhibited significantly stronger TLR2 stimulatory activity.
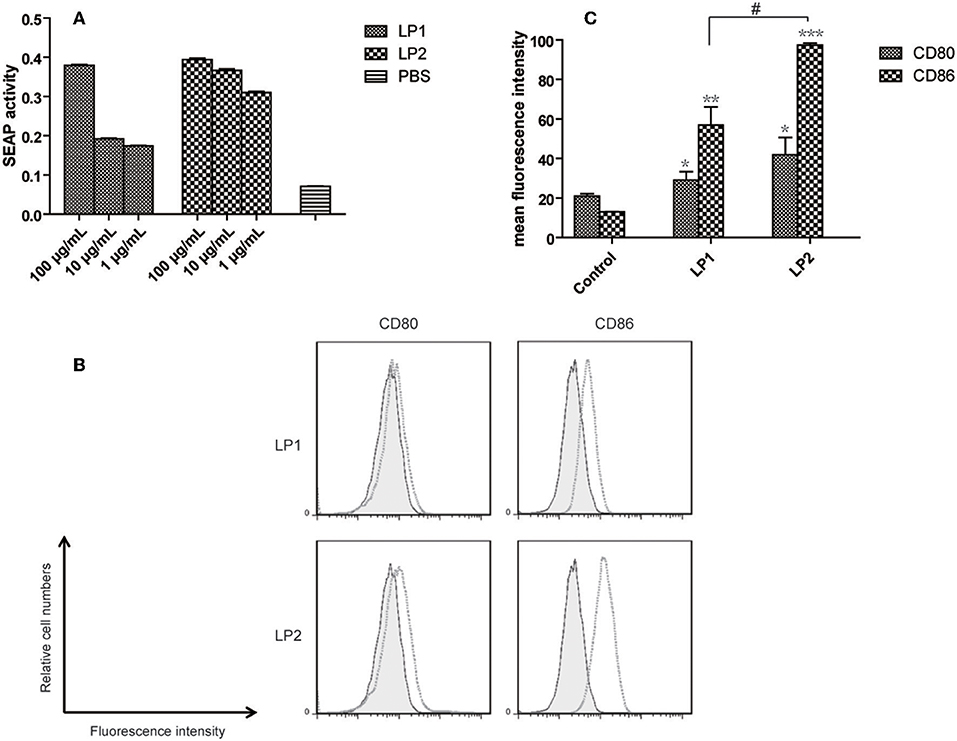
Figure 3. TLR2 signaling assay and expression of maturation surface markers in BMDCs treated with LP1 and LP2. (A) The effect of different lipopeptides treatment on secreted embryonic alkaline phophatase (SEAP) release from HEK-Blue™ mTLR2 cells. Data are expressed as mean ± S.D., n = 3. BMDCs were incubated with PBS, LP1, or LP2 for 48 h and analyzed for surface expression of CD80 and CD86. (B) A representative set of flow cytometry histograms. Black line: cells treated with PBS. (C) Normalized expression level of maturation markers. Data are expressed as mean ± S.D., n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with PBS control; #P < 0.05.
To determine whether the synthetic lipopeptiedes has a direct effect on dendritic cells, BMDCs were stimulated with PBS, LP1 or LP2 for 2 days. FACS results showed that the levels of the costimulatory molecules (CD86 and CD80) expression were significantly elevated in LP1 and LP2 stimulation group compared with PBS group (Figure 3B). LP2 showed more potent stimulating effect (Figure 3C). Together, these data demonstrated that LP1 and LP2 had an ability to induce BMDCs maturation possibly through TLR2 signal pathway.
LP2 Enhanced rHpaA-Induced Protective Effect Against H. pylori Infection via Intranasal Administration
Since LP1 and LP2 could help to induce BMDCs maturation and showed no significant toxicity in vitro, we further investigated whether vaccinated with rHpaA and the synthetic lipopeptides could provide more potent protection against H. pylori. BALB/c mice were intramuscularly or intranasally immunized with rHpaA plus LP1 or LP2, and then orally challenged with H. pylori strain B0. Bacteria colonization in mice stomachs were determined by real-time PCR (Figure 4A). There was no protective effect was observed in the intramuscular immunization groups, whether rHpaA alone or in combination with one of the two lipopeptides (Figure 4B). However, the number of copies of H. pylori in the group intranasally immunized with rHpaA plus LP2 was significantly lower than that in the PBS group. No significant difference in bacterial load was observed in other intranasal immunization groups (Figure 4C). Those results indicated that, of the two lipopeptides, LP2 enhanced rHpaA-mediated protection against H. pylori when combined with rHpaA via intranasal administration.
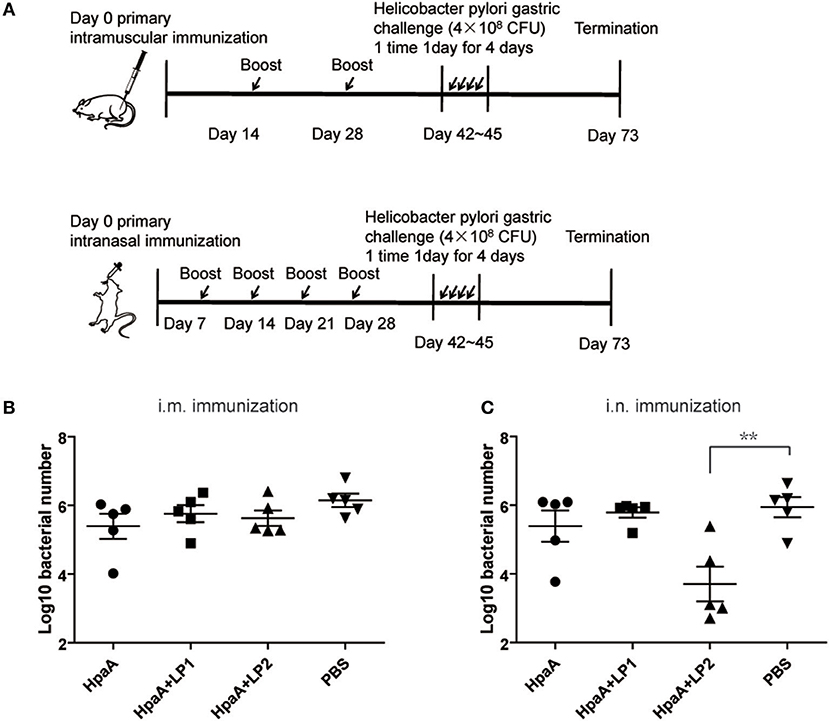
Figure 4. Colonization of H. pylori in stomachs of mice prophylactically immunized with rHpaA plus LP1 or LP2. (A) Timeline representation of immunization schedule and experimental procedures. BALB/c mice were intramuscularly or intranasally immunized with rHpaA or together with one of the two lipopeptides. Controls were treated with PBS. Two weeks after final vaccination, mice were challenged orally four times with H. pylori B0. Four weeks post challenge, levels of gastric H. pylori colonization via intramuscular (B) and intranasal (C) administration were determined by real-time quantitative PCR. Data are expressed as mean ± S.D., n = 5. **P < 0.01 compared with PBS control.
LP2 Elevated rHpaA Specific Serum IgG2a Response
In order to investigate whether the synthetic lipopeptide could enhance rHpaA-specific humoral immune response via TLR2 activation, mice were immunized intranasally or intramuscularly with rHpaA plus LP1 or LP2, and the level of serum specific IgG were measured by ELISA. rHpaA in combination with the lipopeptides elicited significant serum specific IgG antibody response compared with PBS group (Figures 5A,B). Moreover, intramuscular immunization induced more robust IgG level than the same vaccine given intranasally. However, there was no significant difference in titer between rHpaA alone and plus one of the two lipopeptides whether administered intramuscularly or intranasally. In addition, two routes of administration failed to elicit specific IgA response (Figures 5C,D). Those results suggested that LP1 or LP2 could not elevate rHpaA specific systemic or local antibody response. IgG1 and IgG2a as markers for Th2 and Th1 responses respectively, and rHpaA-specific IgG1 and IgG2a levels were also determined. Compared with rHpaA alone group, both intranasal and intramuscular immunization plus LP2 resulted in elevated levels of serum IgG2a (Figures 5E,F). The results showed that enhanced rHpaA specific Th1 response were induced by LP2, which may be associated with improved protective efficacy against H. pylori infection.
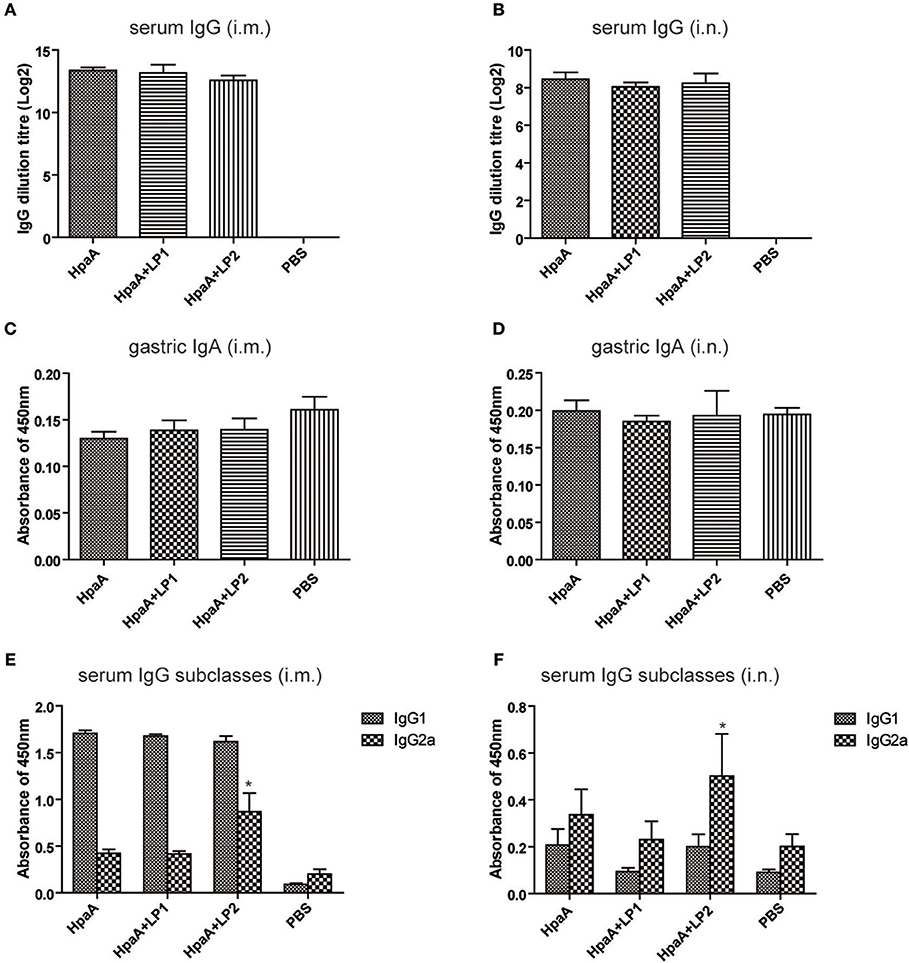
Figure 5. rHpaA-specific antibody profile of mice immunized with rHpaA plus LP1 or LP2. BALB/c mice were immunized intramuscularly or intranasally with rHpaA alone or rHpaA plus one of the two lipopeptides. The serum and intestinal lavage fluid was collected at two weeks after the last immunization. (A,B) rHpaA -specific serum IgG antibody titers were measured by ELISA. (C,D) Secretory IgA level in the intestinal lavage fluid. (E,F) The level of specific IgG1 and IgG2a against rHpaA in serum samples were tested by ELISA. Data are expressed as mean ± S.D., n = 5. *P < 0.05, compared with rHpaA alone group.
LP2 Induced Specific Th1 and Th17 Response When Intranasally Administrated With rHpaA
To further investigate the protective mechanism of LP2 assisted rHpaA against H. pylori, splenic lymphocytes were restimulated with the antigen and the CD4+ T cell response was measured by the expression levels of IFN-γ, IL-4, and IL-17 in supernatants, respectively. As shown in Figures 6A–C, there was no significant difference in the expression of IFN-γ, IL-4, and IL-17 among the intramuscularly immunized groups. When intranasally administrated in combination with rHpaA, LP2 can significantly promote IFN-γ/IL-17 levels Figures 6D–F), which suggested that LP2 could effectively elevate rHpaA specific Th1/Th17 response. Many previous studies have also emphasized the crucial role of vaccine-mediated Th1 and Th17 immune responses in protection against H. pylori infection (43, 44).
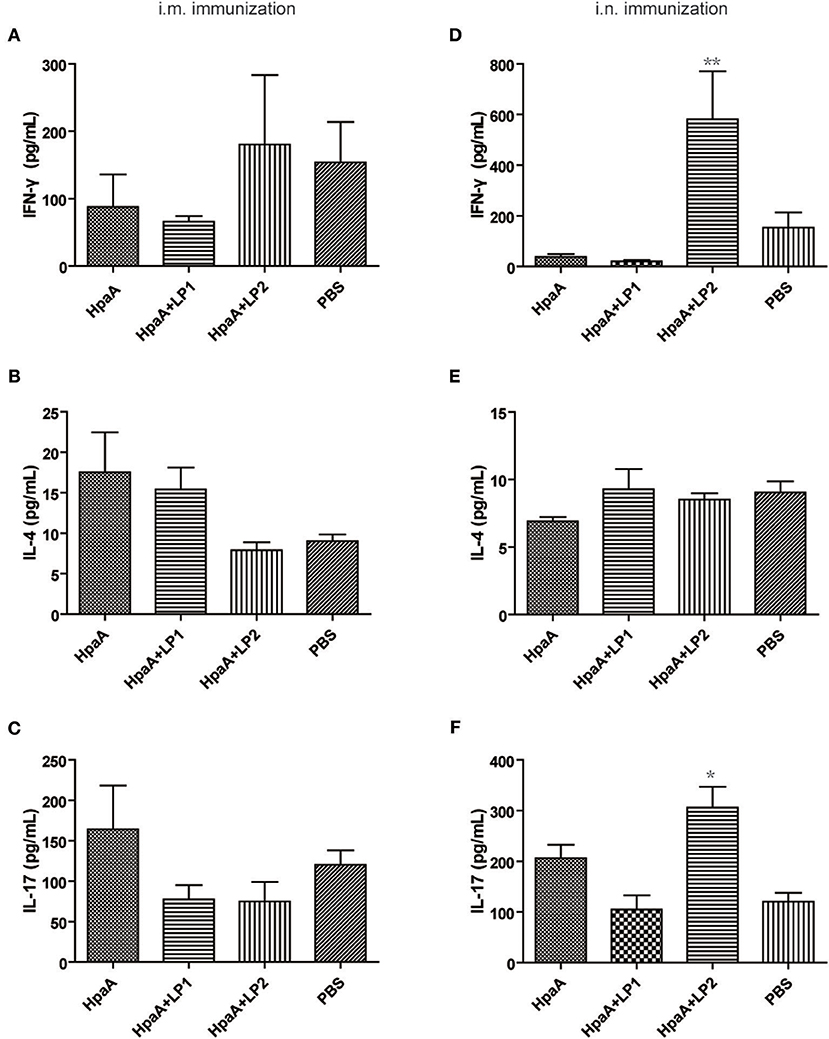
Figure 6. Cytokines production after stimulation of splenic lymphocytes with rHpaA. Mice were immunized intranasally or intramuscularly with rHpaA, rHpaA plus LP1, or rHpaA plus LP2, respectively. Six week after final immunization, splenic lymphocytes from vaccinated group were isolated and stimulated with 10 μg/mL rHpaA for 48 h. ELISA assays were used to measure the accumulation of IFN-γ (A,D), IL-4 (B,E), and IL-17 (C,F) in the supernatants of culture. Data are expressed as mean ± S.D., n = 5. **P < 0.01, *P < 0.05, compared with rHpaA alone group.
LP2- or Peptide-Induced Specific Response Was not Involved in the Protection
Given that intranasal immunization LP2 alone failed to confer protection against H. pylori infection (Figure 7A), we investigated whether the peptide moiety (SPHIIETNEV) of LP2 or LP2 specific response was involved in the protection. LP2 and peptide specific IgG titers in the sera of mice immunized with rHpaA+LP2 were measured. Intranasal immunization with HpaA+LP2 induced low levels of anti-LP2 IgG antibodies (Figure 7B), but anti-peptide antibody response was not observed (Figure 7C). In order to characterize the functional role of the anti-LP2 IgG antibodies, antiserums from mice intranasally immunized with rHpaA alone or plus LP2 were used to determine their effects on H. pylori adhesion. As shown in Figure 7D, both antiserums could significantly reduce binding of the H. pylori to AGS cells. However, there was no difference in the inhibitory effect on adhesion between the two antiserums. The above result suggested that anti-LP2 IgG were not involved in the protection. Further, splenic lymphocytes collected from mice immunized with rHpaA+LP2 were restimulated with LP2 or the peptide, and the levels of IFN-γ, IL-4, and IL-17 production in supernatants were measured, respectively. There was no significant difference in IFN-γ, IL-4, or IL-17 levels between rHpaA+LP2 group and PBS group, suggesting that rHpaA+LP2 could not elicit LP2- or peptide-specific CD4+ T cell response (Figure S3). Together, we concluded that LP2 or peptide moiety specific response was not involved in the protection in the current study.
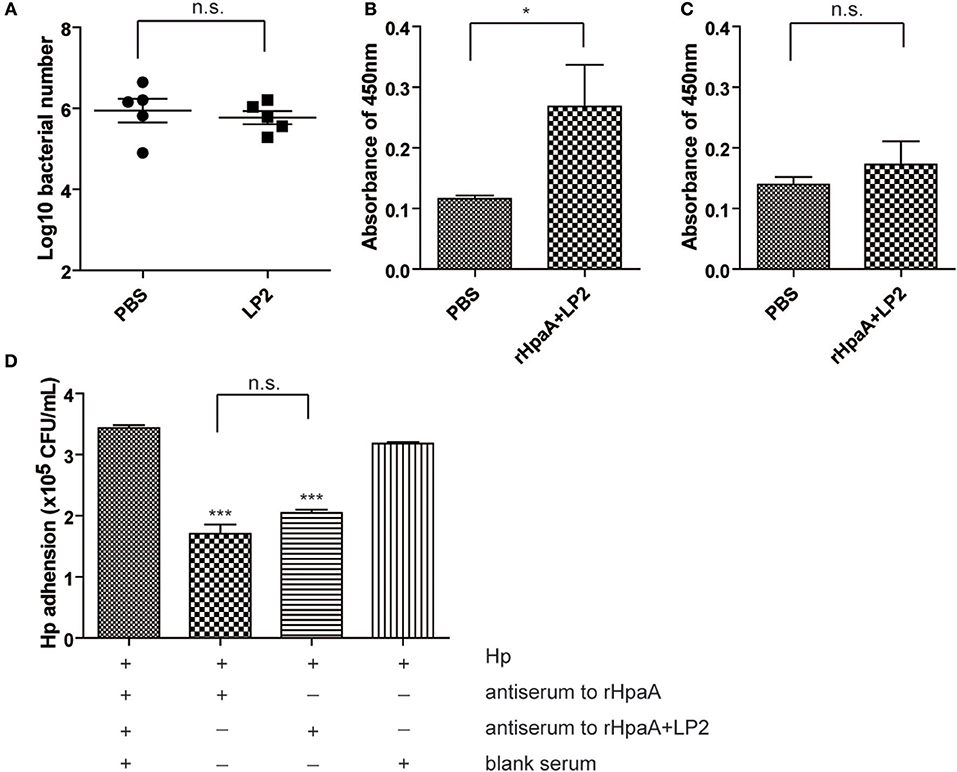
Figure 7. BALB/c mice were intranasally immunized with LP2 alone or PBS. Two weeks after final vaccination, mice were challenged orally four times with H. pylori B0. Four weeks post challenge, levels of gastric H. pylori colonization were determined by real-time quantitative PCR (A). LP2-specific (B) or peptide-specific (C) antibody profile of mice intranasally immunized with rHpaA plus LP2. Data are expressed as mean ± S.D., n = 5. *P < 0.05. Adhesion of the H. pylori strain B0 to AGS cells alone or in combination with antiserum to rHpaA or antiserum to rHpaA plus LP2 (D). The numbers of CFU/ml were determined by real-time PCR using the TaqMan method. The data shown are representative of three independent experiments with triplicate samples. Data are expressed as mean ± S.D., n = 3. ***P < 0.001, compared with blank serum group.
Discussion
H. pylori infection causes gastritis, gastric adenocarcinoma, gastroduodenal ulcers and mucosa-associated tissue lymphoma. With the increasing resistance to antibiotics, the success rate of standard therapy for H. pylori infection has recently declined to low levels, which highlights the need for a vaccine to control the pathogen. Some subunit vaccines have been studied as candidates for H. pylori vaccine, including single (UreB, VacA, CagA, NAP,) and multi-component vaccines (45). Also, HpaA has been found to be a promising vaccine candidate, which is essential for colonization and establishment of infection. In the previous studies, the antigenicity and immunogenicity of rHpaA were confirmed and intragastric immunization with rHpaA could confer protection against H. pylori in mice (14, 15, 17).
However, rHpaA-induced protective efficacy was usually limited or partially effective. Though intragastricly or intranasally immunized with rHpaA plus cholera toxins induced specific antibodies, bacterial colonization was slightly reduced compared with the control group. Our previous study also showed that intranasal immunization with rHpaA adjuvanted with CpG failed to confer protection against H. pylori (46). Compared with nHpaA, the absence of lipid modification on rHpaA may relate to the weak immunostimulatory and protective effects. In the current study, to simulate lipidative N terminus structure of nHpaA, two novel lipopeptides, LP1 and LP2, were synthesized with solid-phase methods. When the concentrations were lower than 100 μg/mL, LP1 and LP2 showed satisfactory hemolytic tolerance and no significant toxicity to HK-2 cells. TEM results indicated that LP1 and LP2 were able to self-assemble into micelles with diameters in the range of microorganisms, and thus they could be easily recognized by DC. In addition, LP1 and LP2 can activate TLR2 in vitro in a dose-dependent manner, indicating that the synthetic lipopeptides that mimic the lipid moiety of nHpaA retained TLR2-stimulating activity. Many studies indicated that co-administration of TLR2 agonists with antigen was able to induce DC maturation, which led to the up-regulation of costimulatory molecules (e.g., CD80, CD86) (28), and promote lymphocytes maturation and activation. In our study, LP1 and LP2 were also able to significantly promote DC maturation, which may be associated with their ability to activate TLR2.
One essential question remains: what type of immunity is key to eliminate H. pylori infection? Recent studies suggested that high levels of IgG antibodies may not be necessary for vaccine protection (47). In this study, no significant difference in serum IgG antibody levels was observed, regardless of the combination with LP1 or LP2. Given earlier studies indicating elevated levels of IgA levels were related to lower bacterial density, which suggested that sIgA might has protective role against H. pylori infection (48, 49), we also evaluated the level of intestinal IgA. However, levels of specific IgA did not significantly increase after immunization with rHpaA alone or plus the lipopeptides. Studies had shown that vaccines against H. pylori could be successfully inoculated in mice with deficiencies of the antibody or Th2 response, confirming that specific cellular immunity, instead of humoral immunity, was essential to eradicate H pylori (50), especially Th1 and Th17 cellular immune responses (51–54). In our study, as indicated by the increase of IgG2a level and IFN-γ/IL-17 cytokine concentrations, Th1 and Th17 response were elicited in mice intranasally immunized with rHpaA plus LP2. We hypothesized that LP2 acquired the ability to activate TLR2 by mimicking the terminal structure of nHpaA, and elevated rHpaA specific Th1 and Th17 response via intranasal administration, which resulted in the enhanced protection of rHpaA against H. pylori infection.
Though both LP1 and LP2 were synthetic lipopeptides based on the structure of nHpaA, there was significant difference in assisting rHpaA to provide protection against H. pylori infection. LP2, other than LP1, induced rHpaA specific cellular immunity and reduced bacterial colonization in mice. We speculated that the possible reason was that LP2 was more capable of activating TLR2 and promoting DC maturation. In addition, the vaccination route is also important for protective immunity (55). Previous studies have also reported that intranasal administration could provide more pronounced protective effect against H. pylori compared with intramuscular immunization, which was consistent with our results. The possible reason is that the upper respiratory tract (nasal-associated lymphoid tissue, NALT) is rich in APCs, especially mucosal DCs which express TLR on the surfaces (56, 57). Synthetic lipopeptides binded to TLR2 may promote DCs to uptake antigens and then present them to Th cells to induce a stronger immune response.
In this study, the synthetic lipopeptide LP2 was proved to enhance the protective effect of rHpaA against H. pylori infection via intranasal immunization, which might be mediated by specific Th1 and Th17 responses. In future studies, the mechanisms which were involved in the immune-stimulating activity of LP2 will be further investigated. Other antigens will be administered in combination with LP2 to investigate whether LP2 also could enhance their ability to confer protection against H. pylori. For the improvement of antigen specific immune response, co-delivery system that delivers both the antigen and LP2 will be designed in the following studies.
Data Availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.mrc-lmb.cam.ac.uk/genomes/dolop.
Ethics Statement
This study was carried out in accordance with the recommendations of the Animal Ethical and Experimental Committee of the Third Military Medical University. The protocol was approved by the Animal Ethical and Experimental Committee of the Third Military Medical University.
Author Contributions
H-BL and Q-MZ designed experiments. R-YX, M-FG, LG, CL, SL, LJ, LN, C-JM, and D-QC carried out experiments. H-BL, JL, ZJ, and SS analyzed experimental results. H-BL, Q-MZ, R-YX, and MG wrote the manuscript.
Funding
This work was supported by Chinese National Natural Science Foundation Project (No. 31670936/31400788).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01372/full#supplementary-material
References
1. Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. (1999) 284:1328–33. doi: 10.1126/science.284.5418.1328
2. Momtaz H, Souod N, Dabiri H. Comparison of the virulence factors of Helicobacter pylori isolated in stomach and saliva in Iran. Am J Med Sci. (2010) 340:345–9. doi: 10.1097/MAJ.0b013e3181d94fbc
3. Niv Y, Hazazi R. Helicobacter pylori recurrence in developed and developing countries: meta-analysis of 13C-urea breath test follow-up after eradication. Helicobacter. (2008) 13:56–61. doi: 10.1111/j.1523-5378.2008.00571.x
4. Urgesi R, Cianci R, Riccioni ME. Update on triple therapy for eradication of Helicobacter pylori: current status of the art. Clin Exp Gastroenterol. (2012) 5:151–7. doi: 10.2147/CEG.S25416
5. Zullo A, Hassan C, Ridola L, De Francesco V, Vaira D. Standard triple and sequential therapies for Helicobacter pylori eradication: an update. Eur J Intern Med. (2013) 24:16–9. doi: 10.1016/j.ejim.2012.07.006
6. Kim JS, Park SM, Kim BW. Sequential or concomitant therapy for eradication of Helicobacter pylori infection: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2015) 30:1338–45. doi: 10.1111/jgh.12984
7. Opekun AR, El-Zaimaity HM, Osato MS, Gilger MA, Malaty HM, Terry M, et al. Novel therapies for Helicobacter pylori infection. Aliment Pharmacol Ther. (1999) 13:35–42. doi: 10.1046/j.1365-2036.1999.00435.x
8. Lindén SK, Wickström C, Lindell G, Gilshenan K, Carlstedt I. Four modes of adhesion are used during Helicobacter pylori binding to human mucins in the oral and gastric niches. Helicobacter. (2008) 13:81–93. doi: 10.1111/j.1523-5378.2008.00587.x
9. McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. (2011) 9:265–78. doi: 10.1038/nrmicro2538
10. Kavermann H, Burns BP, Angermuller K, Odenbreit S, Fischer W, Melchers K, et al. Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J Exp Med. (2003) 197:813–22. doi: 10.1084/jem.20021531
11. Carlsohn E, Nyström J, Bölin I, Nilsson CL, Svennerholm AM. HpaA is essential for Helicobacter pylori colonization in mice. Infect Immun. (2006) 74:920–6. doi: 10.1128/IAI.74.2.920-926.2006
12. Yan J, Mao YF, Shao ZX. Frequencies of the expression of main protein antigens from Helicobacter pylori isolates and production of specific serum antibodies in infected patients. World J Gastroenterol. (2005) 11:421–5. doi: 10.3748/wjg.v11.i3.421
13. Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. (1999) 397:176–80. doi: 10.1038/16495
14. Voland P, Hafsi N, Zeitner M, Laforsch S, Wagner H, Prinz C. Antigenic properties of HpaA and Omp18, two outer membrane proteins of Helicobacter pylori. Infect Immun. (2003) 71:3837–43. doi: 10.1128/IAI.71.7.3837-3843.2003
15. Mattsson A, Lönroth H, Quiding-Järbrink M, Svennerholm AM. Induction of B cell responses in the stomach of Helicobacter pylori- infected subjects after oral cholera vaccination. J Clin Invest. (1998) 102:51–6. doi: 10.1172/JCI22
16. Mao YF, Yan J, Xu Y. [Immunoprotective effects of Helicobacter pylori UreB and HpaA bivalence recombinant vaccine with inner adjuvant on experimental infection in mice]. Zhejiang Da Xue Xue Bao Yi Xue Ban. (2005) 34:405–11. doi: 10.3785/j.issn.1008-9292.2005.05.006
17. Sutton P, Doidge C, Pinczower G, Wilson J, Harbour S, Swierczak A, et al. Effectiveness of vaccination with recombinant HpaA from Helicobacter pylori is influenced by host genetic background. FEMS Immunol Med Microbiol. (2007) 50:213–9. doi: 10.1111/j.1574-695X.2006.00206.x
18. Huang X, Xu B, Duan G, Song C. The rOmp22-HpaA fusion protein confers protective immunity against helicobacter pylori in mice. Curr Microbiol. (2013) 67:487–92. doi: 10.1007/s00284-013-0390-x
19. Chirani AS, Ghazi M, Goudarzi M, Peerayeh SN, Soleimanjahi H, Dadashi M, et al. A survey on chimeric UreB229–561-HpaA protein targeting Helicobacter pylori: computational and in vitro urease activity valuation. Comput Biol Chem. (2018) 76:42–52. doi: 10.1016/j.compbiolchem.2018.05.001
20. Nyström J, Svennerholm AM. Oral immunization with HpaA affords therapeutic protective immunity against H. pylori that is reflected by specific mucosal immune responses. Vaccine. (2007) 25:2591–8. doi: 10.1016/j.vaccine.2006.12.026
21. Flach CF, Svensson N, Blomquist M, Ekman A, Raghavan S, Holmgren J. A truncated form of HpaA is a promising antigen for use in a vaccine against Helicobacter pylori. Vaccine. (2011) 29:1235–41. doi: 10.1016/j.vaccine.2010.11.088
22. Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Müller-Hermelink HK, et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. (2004) 136:521–6. doi: 10.1111/j.1365-2249.2004.02464.x
23. Torok AM, Bouton AH, Goldberg JB. Helicobacter pylori induces interleukin-8 secretion by Toll-like receptor 2- and Toll-like receptor 5-dependent and -independent pathways. Infect Immun. (2005) 73:1523–31. doi: 10.1128/IAI.73.3.1523-1531.2005
24. Chang YJ, Wu MS, Lin JT, Chen CC. Helicobacter pylori-Induced invasion and angiogenesis of gastric cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9 and promoter regulation. J Immunol. (2005) 175:8242–52. doi: 10.4049/jimmunol.175.12.8242
25. Schmausser B, Andrulis M, Endrich S, Müller-Hermelink HK, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. Int J Med Microbiol. (2005) 295:179–85. doi: 10.1016/j.ijmm.2005.02.009
26. BenMohamed L, Gras-Masse H, Tartar A, Daubersies P, Brahimi K, Bossus M, et al. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur J Immunol. (1997) 27:1242–53. doi: 10.1002/eji.1830270528
27. BenMohamed L, Wechsler SL, Nesburn AB. Lipopeptide vaccines–yesterday, today, and tomorrow. Lancet Infect Dis. (2002) 2:425–31. doi: 10.1016/S1473-3099(02)00318-3
28. Dunne A, Mielke LA, Allen AC, Sutton CE, Higgs R, Cunningham CC, et al. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol. (2015) 8:607–617. doi: 10.1038/mi.2014.93
29. Zeng W, Eriksson E, Chua B, Grollo L, Jackson DC. Structural requirement for the agonist activity of the TLR2 ligand Pam2Cys. Amino Acids. (2010) 39:471–80. doi: 10.1007/s00726-009-0463-0
30. Takeda K, Takeuchi O, Akira S. Recognition of lipopeptides by Toll-like receptors. J Endotoxin Res. (2002) 8:459–63. doi: 10.1177/09680519020080060101
31. Lundstrom A, Bolin I, Bystrom M, Nystrom S. Recombinant HpaA purified from Escherichia coli has biological properties similar to those of native Helicobacter pylori HpaA. APMIS. (2003) 111:389–97. doi: 10.1034/j.1600-0463.2003.t01-1-1110203.x
32. Guo L, Zhang J, Cui L, Liu D, Ma B, Wang S, et al. Crystallization and X-ray analysis of the extracellular adhesion domain of Helicobacter pylori adhesin A: the significance of the cation composition in the crystallization precipitant. Acta Crystallogr Section F Struc Biol Commun. (2017) 73:202–8. doi: 10.1107/S2053230X17003004
33. Fujimoto Y, Hashimoto M, Furuyashiki M, Katsumoto M, Seya T, Suda Y, et al. Lipopeptides from Staphylococcus aureus as Tlr2 Ligands: prediction with mrna expression, chemical synthesis, and immunostimulatory activities. Chembiochem. (2009) 10:2311–5. doi: 10.1002/cbic.200900242
34. Liu C, Luo J, Xue RY, Guo L, Nie L, Li S, et al. The mucosal adjuvant effect of plant polysaccharides for induction of protective immunity against Helicobacter pylori infection. Vaccine. (2019) 37:1053–61. doi: 10.1016/j.vaccine.2018.12.066
35. Ruiz MA, Clares B, Morales ME, Gallardo V. Vesicular lipidic systems, liposomes, PLO, and liposomes-PLO: characterization by electronic transmission microscopy. Drug Dev Ind Pharm. (2008) 34:1269–76. doi: 10.1080/03639040802026095
36. Chen J, Ng MM, Chu JJ. Activation of TLR2 and TLR6 by Dengue NS1 Protein and its implications in the immunopathogenesis of dengue virus infection. PLoS Pathog. (2015) 11:e1005053. doi: 10.1371/journal.ppat.1005053
37. Meng J, Cao Y, Meng Y, Luo H, Gao X, Shan F. Maturation of mouse bone marrow dendritic cells (BMDCs) induced by Laminaria japonica polysaccharides (LJP). Int J Biol Macromol. (2014) 69:388–92. doi: 10.1016/j.ijbiomac.2014.05.018
38. Li HB, Zhang JY, He YF, Chen L, Li B, Liu KY, et al. Systemic immunization with an epitope-based vaccine elicits a Th1-biased response and provides protection against Helicobacter pylori in mice. Vaccine. (2012) 31:120–6. doi: 10.1016/j.vaccine.2012.10.091
39. Perez-Perez GI, Van TN, Thu Huong D, Zhan G, Nguyet Anh D, Nguyet NT, et al. Isolation and characterization of Helicobacter pylori recovered from gastric biopsies under anaerobic conditions. Diagn Microbiol Infect Dis. (2016) 86:136–40. doi: 10.1016/j.diagmicrobio.2016.07.009
40. Ghosh P, Bhaskar KR, Hossain F, Khan MA, Vallur AC, Duthie MS, et al. Evaluation of diagnostic performance of rK28 ELISA using urine for diagnosis of visceral leishmaniasis. Parasit Vectors. (2016) 9:383. doi: 10.1186/s13071-016-1667-2
41. Sheu SM, Sheu BS, Yang HB, Lei HY, Wu JJ. Anti-Lewis X antibody promotes Helicobacter pylori adhesion to gastric epithelial cells. Infect Immun. (2007) 75:2661–7. doi: 10.1128/IAI.00812-07
42. de Klerk N, Maudsdotter L, Gebreegziabher H, Saroj SD, Eriksson B, Eriksson OS, et al. Lactobacilli reduce helicobacter pylori attachment to host gastric epithelial cells by inhibiting adhesion gene expression. Infect Immun. (2016) 84:1526–35. doi: 10.1128/IAI.00163-16
43. Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y, et al. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun. (1999) 67:279–85.
44. Akhiani AA, Pappo J, Kabok Z, Schön K, Gao W, Franzén LE, et al. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J Immunol. (2002) 169:6977–84. doi: 10.4049/jimmunol.169.12.6977
45. Every AL. Key host-pathogen interactions for designing novel interventions against Helicobacter pylori. Trends Microbiol. (2013) 21:253–9. doi: 10.1016/j.tim.2013.02.007
46. Yang WC, Sun HW, Sun HQ, Yuan HM, Li B, Li HB, et al. Intranasal immunization with immunodominant epitope peptides derived from HpaA conjugated with CpG adjuvant protected mice against Helicobacter pylori infection. Vaccine. (2018) 36:6301–6306. doi: 10.1016/j.vaccine.2018.09.007
47. Sutton P, Wilson J, Kosaka T, Wolowczuk I, Lee A. Therapeutic immunization against Helicobacter pylori infection in the absence of antibodies. Immunol Cell Biol. (2000) 78:28–30. doi: 10.1046/j.1440-1711.2000.00881.x
48. Srivastava R, Kashyap A, Kumar M, Nath G, Jain AK. Mucosal IgA & IL-1beta in Helicobacter pylori Infection. Ind J Clin Biochem. (2013) 28:19–23. doi: 10.1007/s12291-012-0262-3
49. Nyström J, Raghavan S, Svennerholm AM. Mucosal immune responses are related to reduction of bacterial colonization in the stomach after therapeutic Helicobacter pylori immunization in mice. Microbes Infect. (2006) 8:442–9. doi: 10.1016/j.micinf.2005.07.010
50. Garhart CA, Nedrud JG, Heinzel FP, Sigmund NE, Czinn SJ. Vaccine-induced protection against Helicobacter pylori in mice lacking both antibodies and interleukin-4. Infect Immun. (2003) 71:3628–33. doi: 10.1128/IAI.71.6.3628-3633.2003
51. Freire de Melo F, Rocha GA, Rocha AM, Teixeira KN, Pedroso SH, Pereira Junior JB, et al. Th1 immune response to H. pylori infection varies according to the age of the patients and influences the gastric inflammatory patterns. Int J Med Microbiol. (2014). 304:300–6. doi: 10.1016/j.ijmm.2013.11.001
52. Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. (2010) 138:1046–54. doi: 10.1053/j.gastro.2009.11.043
53. Jafarzadeh A, Larussa T, Nemati M, Jalapour S. T cell subsets play an important role in the determination of the clinical outcome of Helicobacter pylori infection. Microb Pathog. (2018) 116:227–236. doi: 10.1016/j.micpath.2018.01.040
54. Moyat M, Bouzourene H, Ouyang W, Iovanna J, Renauld JC, Velin D. IL-22-induced antimicrobial peptides are key determinants of mucosal vaccine-induced protection against H. pylori in mice. Mucosal Immunol. (2017) 10:271–281. doi: 10.1038/mi.2016.38
55. Johansson EL, Bergquist C, Edebo A, Johansson C, Svennerholm AM. Comparison of different routes of vaccination for eliciting antibody responses in the human stomach. Vaccine. (2004) 22:984–90. doi: 10.1016/j.vaccine.2003.09.002
56. Vyas SP, Gupta PN. Implication of nanoparticles/microparticles in mucosal vaccine delivery. Expert Rev Vaccines. (2007) 6:401–18. doi: 10.1586/14760584.6.3.401
57. Li H, Zhang J, He Y, Li B, Chen L, Huang W, et al. Intranasal immunization with an epitope-based vaccine results in earlier protection, but not better protective efficacy, against Helicobacter pylori compared to subcutaneous immunization. Immunol Res. (2015) 62:368–76. doi: 10.1007/s12026-015-8666-9
Keywords: Helicobacter pylori, vaccine, synthetic lipopeptide, TLR2, HpaA
Citation: Xue R-Y, Guo M-F, Guo L, Liu C, Li S, Luo J, Nie L, Ji L, Ma C-J, Chen D-Q, Sun S, Jin Z, Zou Q-M and Li H-B (2019) Synthetic Lipopeptide Enhances Protective Immunity Against Helicobacter pylori Infection. Front. Immunol. 10:1372. doi: 10.3389/fimmu.2019.01372
Received: 22 February 2019; Accepted: 30 May 2019;
Published: 14 June 2019.
Edited by:
Swapan K. Ghosh, Indiana State University, United StatesReviewed by:
Pietro Speziale, University of Pavia, ItalyWayne Robert Thomas, Telethon Kids Institute, Australia
Copyright © 2019 Xue, Guo, Guo, Liu, Li, Luo, Nie, Ji, Ma, Chen, Sun, Jin, Zou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Bo Li, bGloYWlib0B0bW11LmVkdS5jbg==; Quan-Ming Zou, cW16b3UyMDA3QDE2My5jb20=
 Ruo-Yi Xue1
Ruo-Yi Xue1 Quan-Ming Zou
Quan-Ming Zou Hai-Bo Li
Hai-Bo Li