- 1Department of Hematology, The Fifth Medical Center of Chinese People's Liberation Army General Hospital, Beijing, China
- 2Institute of Hematology, The Fifth Medical Center of Chinese People's Liberation Army General Hospital, Beijing, China
- 3Beijing Key Laboratory of Hematopoietic Stem Cell Therapy and Transformation Research, Beijing, China
Background: Azacitidine is commonly used in the treatment of relapsed acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) after allogeneic hematopoietic stem cell transplantation (allo-HSCT), but the effectiveness of this monotherapy is still very low. A possible mechanism of resistance to hypomethylating agents (HMAs) is the upregulation of the expression of inhibitory checkpoint receptors and their ligands, making the combination of HMAs and immune checkpoint blockade therapy a rational approach. Although the safety of anti-programmed cell death protein (PD)-1 antibodies for patients with post-allo-HSCT remains a complicated issue, the preliminary clinical result of combining azacitidine with anti-PD-1 antibodies is encouraging; however, the safety and efficacy of this approach need further investigation.
Case Presentation: We reported a case of treated secondary (ts)-AML in a patient who received tislelizumab (an anti-PD-1 antibody) in combination with azacitidine. The patient relapsed after allo-HSCT and was previously exposed to HMAs-based therapy. The patient received tislelizumab for compassionate use. After the combination treatment, the patient achieved complete remission with incomplete hematologic recovery, negative minimal residual disease (MRD) by flow cytometry (FCM), and negative Wilms' tumor protein 1 (WT1). However, the patient successively developed serious immune-related adverse events (irAEs) and graft vs. host disease (GVHD) and eventually died from complications of GVHD.
Conclusion: To our knowledge, this is the first case to report the combined use of tislelizumab and azacitidine to treat relapsed AML posttransplantation. This report highlights the safety concerns of using an anti-PD-1 antibody in combination with azacitidine after allo-HSCT, especially the risk of GVHD, and provides a basis for future studies.
Introduction
Although allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapy for patients with high-risk acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), the relapse of the disease remains the major cause of treatment failure in these patients and carries a dismal prognosis (1–4). Hypomethylating agents (HMAs), such as azacitidine and decitabine, are the most common, non-targeted pharmacologic agents used to treat and prevent the relapse in posttransplantation AML and MDS in recent times. However, a single-agent HMA therapy in relapsed/refractory (r/r) HMAs-naïve AML has only achieved a low response rate (5–8). Previous studies have shown that, while HMAs promote antitumor immune signaling (9), they concurrently dampen antitumor immunity by increasing the expression of programmed cell death protein (PD)-1 and programmed death-ligand (PD-L)1 in solid tumors (10) and MDS/AML (11). This could be a possible mechanism of resistance to HMAs (8). For patients with relapsed AML after human leukocyte antigen (HLA) matching and incompatible transplantation without HLA loss, the mechanism of recurrence after the transplantation is mainly by the downregulation of HLA class two molecules (30–40%) and the upregulation of immune checkpoints (~20%) at the epigenetic level, which can be treated by HMAs and immune checkpoint blockade (ICB) therapy, respectively (12). Thus, for posttransplantation AML, the combination therapy of azacitidine with anti-PD-1 antibody may be a better approach in comparison to monotherapy. In fact, single-agent anti-PD-1 antibodies exhibit only minimal activity in patients with relapsed AML and high-risk MDS (13–15). ICB therapy after allo-HSCT has been reported to cause severe graft vs. host disease (GVHD) in both preclinical (16–18) and clinical studies (15, 19–22). However, the combination therapy of azacitidine and nivolumab (an anti-PD-1 antibody) showed an encouraging response with no GVHD and moderate immune-related adverse events (irAEs) with respect to the relapse of AML/MDS (prior allo-HSCT-19%) in a clinical trial (8). Given these promising preliminary clinical results, the safety and efficacy of combining azacitidine and anti-PD-1 antibodies in post-allo-HSCT patients should be urgently investigated further. Tislelizumab® (BeiGene, China), an antihuman PD-1 monoclonal IgG4 antibody, has been approved in China for patients with r/r classical Hodgkin lymphoma (HL) after at least a second-line chemotherapy (23). In the present study, we report a case of compassionate use of tislelizumab combined with azacitidine to treat a patient with relapsed AML after allo-HSCT. The report highlights the importance of the prudent use of an anti-PD-1 antibody in patients who are undergoing HSCT.
Case Presentation
A 56-year-old man was diagnosed with follicular lymphoma [FL; grade IIIA, stage IVA, Follicular Lymphoma International Prognostic Index (FLIPI) stage: high risk] 18 years ago. The patient was cured by four sequential cycles of fludarabine, cyclophosphamide, rituximab (FCR) chemotherapy; four cycles of rituximab, cyclophosphamide, hydroxyldaunorubicin, oncovin, and prednisone (R-CHOP) chemotherapy; and local lymph node radiotherapy. Unfortunately, the patient was diagnosed with therapy-related MDS (t-MDS) in February 2019 according to the WHO classification (Figure 1A). The baseline characteristics of the patient diagnosed with t-MDS are presented in the Supplementary Material.
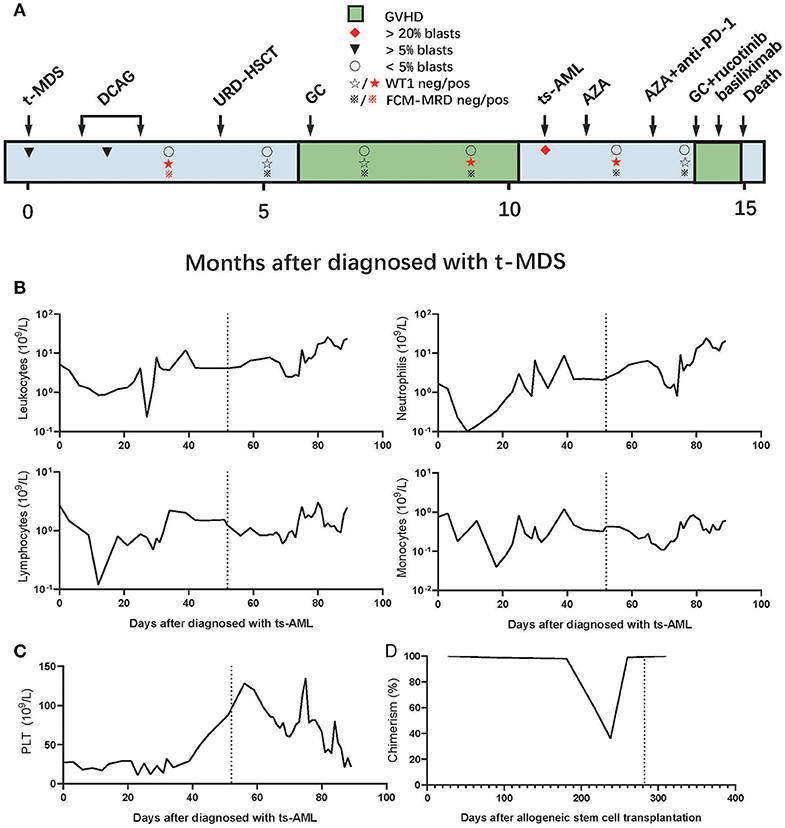
Figure 1. (A) Clinical course of the patient. (B,C) Numbers of (B) leukocytes, lymphocytes, neutrophils, monocytes, and (C) platelets in the peripheral blood after the AML diagnosis. (D) Donor cell chimerism in the bone marrow (BM) following allo-HSCT. In (B–D), the dotted vertical line indicates the timing of tislelizumab administration. GC, glucocorticoid; AZA, azacytidine; t-MDS, therapy-related myelodysplastic syndrome; ts-AML, treated secondary-acute myeloid leukemia. URD-HSCT, unrelated donor hematopoietic stem cell transplantation.
The patient received induction chemotherapy with a decitabine, cytarabine, aclacinomycin, and recombinant human granulocyte colony-stimulating factor (G-CSF) (DCAG) scheme in March 2019 and achieved a partial response (PR). Then, the patient received another cycle of consolidation chemotherapy with DCAG and achieved a complete response (CR); at this stage, the patient was positive for minimal residual disease (MRD), confirmed through flow cytometry (FCM). The patient underwent allo-HSCT from a HLA-mismatched unrelated donor (8/10), after preconditioning with decitabine, fludarabine, and busulfan, followed by cyclosporine A, mycophenolate mofetil, basiliximab (a monoclonal anti-CD25 antibody), and short-term methotrexate for prophylaxis of GVHD. The patient achieved CR with MRD negativity (CRMRD-) 1 month after allo-HSCT and developed extensive skin chronic GVHD (cGVHD) and bronchiolitis obliterans with organizing pneumonia (BOOP) 6 months after allo-HSCT but improved after glucocorticoids and antifungal therapy. During the treatment for BOOP, the patient remained CRMRD- but was positive for Wilms' tumor protein 1 (WT1+). In January 2020, the disease progressed to AML, and the evaluation of bone marrow (BM) showed that 34.5% of blasts, 36.14% of donor chimeric,; 28.8% of FCM–MRD; and 7.57% of WT1. The patient was diagnosed with treated secondary (ts)-AML, arising from an antecedent hematologic disorder that was previously treated with chemotherapy or immunomodulatory therapy, an entity known to have an extremely dismal prognosis (24–26). The gene mutation test from a BM sample showed casitas B-lineage lymphoma (CBL) of 5.92% and Kirsten rat sarcoma (KRAS) of 6.3%. The immunosuppressor was immediately withdrawn. We performed the HLA-loss test, but no HLA gene loss was detected.
The patient was counseled on the risks and benefits of azacitidine in combination with tislelizumab. Although the patient did not have any signs or symptoms of GVHD at the time of relapse, we decided to administer anti-PD-1 after one course of azacitidine to ensure the use of tislelizumab for at least 4 weeks after the withdrawal of immunosuppressive agents according to a previous study (15). Thus, the patient received azacitidine monotherapy and achieved 0.611% of CRiMRD- and WT1 1 month later. The patient subsequently developed herpes zoster infection, but the condition of the patient improved with antiviral therapy. The patient also developed a drug-induced liver injury, but the condition of the patient improved after the drugs causing liver injury were discontinued, namely estazolam and zopiclone, which had been prescribed for insomnia. In March 2020, the patient received 100 mg of azacitidine on days 1–7 subcutaneously and 200 mg of tislelizumab on day 1 intravenously. About 20 days later, the patient remained CRiMRD- and was WT1 negative (WT1–) (0.11%, the cutoff value of WT1/ABL in our laboratory is 0.5%). The patient successively developed hypoadrenocorticism, infectious diarrhea, fever, and shock. Although the symptoms of the patient were relieved with symptomatic and antimicrobial treatment, diarrhea continued to worsen. No definite infection was found after repeated etiological examinations, and multiple antibiotic treatments proved to be ineffective.
The patient refused to undergo a colonoscopy and further biopsies, so the diagnosis of G3 gut acute GVHD (aGVHD) was mainly based on history and clinical manifestation. Prednisone, 2 mg/kg/day, combined with ruxolitinib, 10 mg (bid), was prescribed. The patient continued to have diarrhea even after 5 days. Prednisone was tapered and basiliximab was started. The patient subsequently developed delirious behavior, involuntary tremors, decreased muscle strength, and dystonia. A diagnosis of autoimmune-related encephalopathy was hypothesized after consultation with a neurologist, based on history, clinical manifestations, and imaging. CT showed multiple spots and patches of low-density lesions around bilateral lateral ventricles, and MRI showed scattered spots and patchy lesions near both frontal lobes and lateral ventricles that showed equal or long signal on T1 images, a long signal on T2 images, and a high signal on T2WI fluid-attenuated inversion recovery (FLAIR). Gamma globulins were administered, but the nervous system symptoms were not relieved. Gut aGVHD was resistant to steroid and second-line treatment, and the patient subsequently developed hematochezia, enteric infections, septic shock, and metabolic acidosis secondary to gut GVHD and died 6 days later (Figures 1B–D).
Discussion and Literature Review
The patient with MDS mentioned in the study was previously exposed to HMA therapy, which rapidly progressed to ts-AML after allo-HSCT. At the time of relapse, neither HLA loss nor active GVHD was present. First, the patient received azacitidine monotherapy and achieved CRiMRD- but was WT1+. Subsequently, the patient received a combination of azacitidine and tislelizumab and remained CRiMRD- and became WT1-. Unfortunately, the patient developed serious irAEs, including hypoadrenocorticism, autoimmune-related encephalopathy, and fatal gut GVHD. We have summarized the safety and efficacy of using checkpoint inhibitors in post-allo-HSCT myeloid malignancies in Table 1. Clinical studies showed that the CTLA-4 blockade induces lower GVHD as compared to anti-PD-1 (14% vs. 39%) (15, 28). Furthermore, CTLA-4 inhibitors as single agents demonstrated activity in patients with high-risk MDS after the therapy of HMAs and relapsed AML post-allo-HSCT, while anti-PD-1 antibodies showed limited efficacy (28, 34). Currently, clinical trials of the combination of HMAs with ICB therapy are ongoing (8, 34). Table 2 shows a summary of autoimmune complications of the published clinical trials using checkpoint inhibitors in post-allo-HSCT hematologic malignancies other than myeloid malignancies. On the whole, the incidence of autoimmune diseases, including GVHD, after ICB monotherapy is high: 21–39% in AML/MDS (15, 28) and 30%−55% in other hematological malignancies (20, 36).
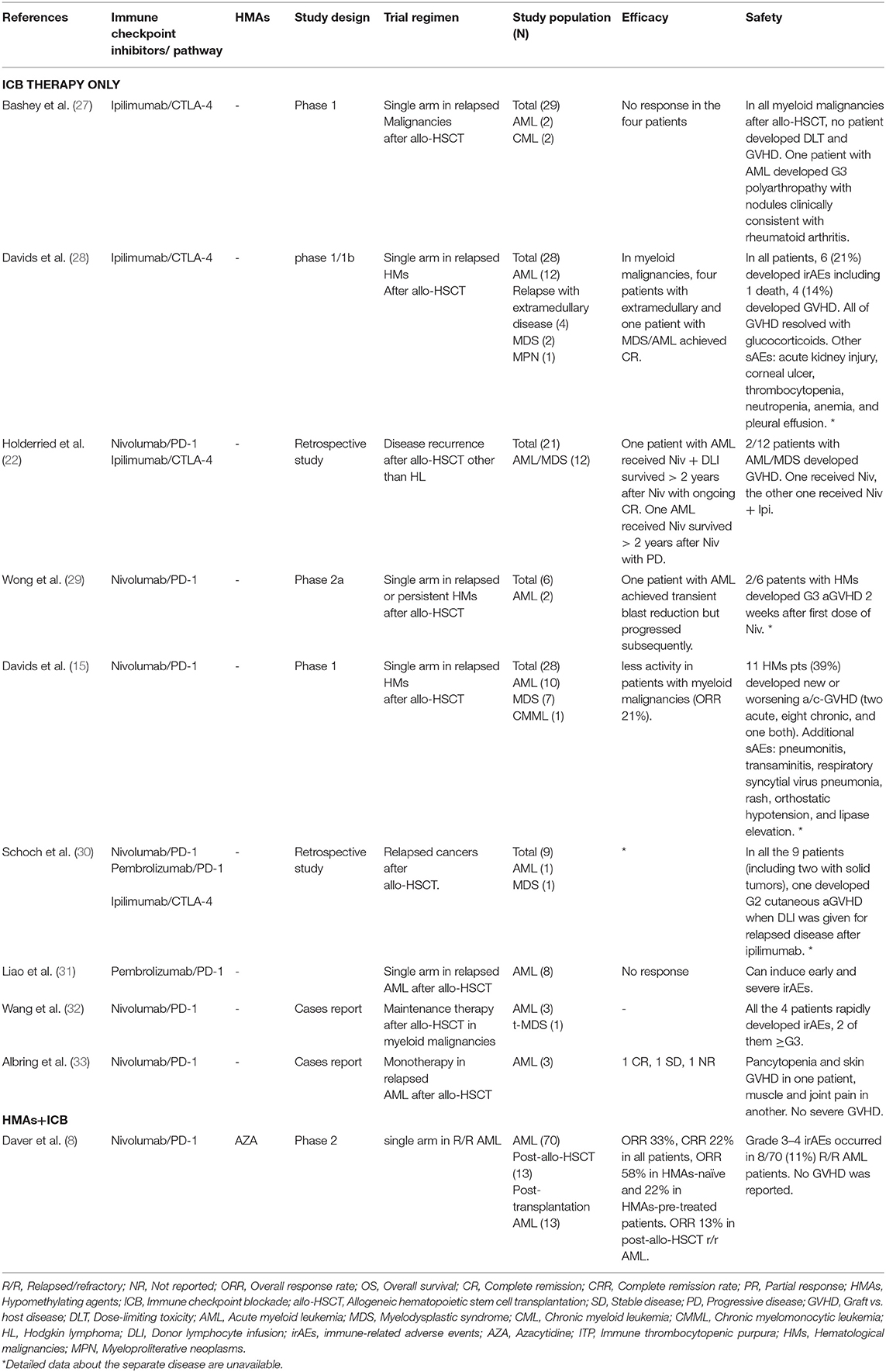
Table 1. The safety and efficacy of the published clinical trials of immune checkpoint blockade in post-allo-HSCT myeloid malignancies.
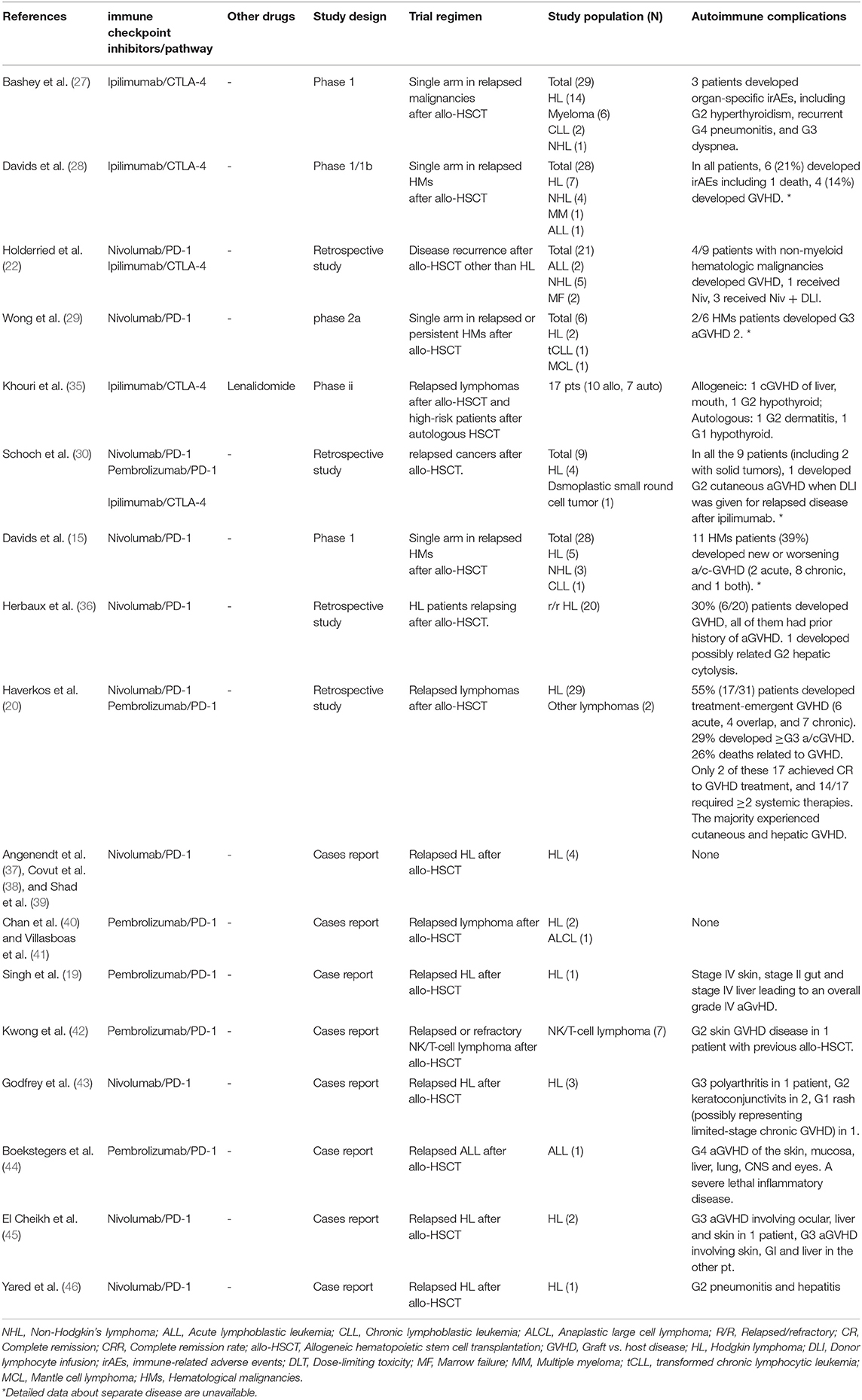
Table 2. Autoimmune complications of the published clinical trials using checkpoint inhibitors in post-allo-HSCT hematologic malignancies other than myeloid malignancies.
A possible pathogenic mechanism of GVHD in the patient could involve enteric infection that may have damaged gastrointestinal tissue, favoring T-cell activation against self-antigens. The blockage of PD-1/PD-L1 increases the proliferation, activation, Th1 cytokine-production, and metabolic stress of donor T cells, along with increased homing in the GVHD target tissues such as the gut, due to the loss of intestinal epithelial integrity (47). Moreover, the blockage of PD-1/PD-L1 accelerated donor CD8+ T-cell expansion and exacerbated aGVHD (48). It is challenging to distinguish between gut GVHD and GI-irAEs even after biopsies. We diagnosed a gut aGVHD for the following reasons: first, the patient had a history of cGVHD and was more likely to be susceptible to develop GVHD after the treatment of PD-1 as described in the previous studies (36, 49). Meanwhile, the patient remained completely donor chimeric after anti-PD-1 therapy. However, other studies have suggested that prior a/cGVHD has no significant impact on the development of GVHD after ICB therapy (15, 22). Although this issue is controversial and remains to be clarified, it is a possibility that deserves attention. In addition, the cumulative incidence of gastrointestinal aGVHD might be as high as 60% (50), while the incidence of diarrhea was 11–17% after the treatment of anti-PD-1 (51).
Previous studies showed that 0.5 mg/kg of nivolumab monotherapy for every 3 weeks and 100 mg of nivolumab plus azacitidine for every 2 weeks are considered safe (8, 15). In addition, the low affinity of tislelizumab for the Fc receptor and Fc-γ receptor 1 (FcγRI) may contribute to improved anticancer efficacy as compared to other anti-PD-1 antibodies (52), which means that the dose of tislelizumab may need to be further reduced. It is interesting that the patient developed a delayed and steroid-resistant GVHD nearly 4 weeks after anti-PD-1 therapy. This could be related to the highest terminal half-life of tislelizumab compared to other ICB (23). Therefore, reducing the dose of tislelizumab or extending the interval of administration should be evaluated to improve safety in future studies on patients with post-allo-HSCT.
Some other factors may also cause the occurrence of GVHD after ICB therapy in posttransplantation patients. Although it is still controversial (22), two studies observed that a shorter interval between the transplantation and the first nivolumab infusion was associated with a higher risk of developing GVHD (15, 36). Extreme caution should be followed during the enrollment of patients with active cGVHD (21). Furthermore, the question remains as to whether anti-PD-L1 is safer than anti-PD-1. Hematopoietic cells upregulate the expression of both PD-L2 and PD-L1 after HSCT, but only PD-L1 is broadly expressed by parenchymal cells in host GVHD target tissues (47). Host PD-L1 is dominant over PD-L2 in regulating GVHD lethality (47), and the PD-L1 expression on donor T cells may drive GVHD lethality (53). Thus, PD-L1 may play a vital role in the development of GVHD. At present, there is still a lack of reliable data on the clinical application of anti-PD-L1 for posttransplantation patients, and the safety and efficacy of PD-L1 inhibitors need to be investigated clinically.
Conclusion
Azacitidine in combination with anti-PD-1 seems to be a rational strategy for posttransplantation relapsed AML but needs further urgent clinical investigation. The report highlights the safety issues of an anti-PD-1 antibody in combination with azacitidine after allo-HSCT, especially GVHD. Additionally, we conducted an in-depth discussion around safety issues and provided suggestions for follow-up research. For such patients, the type, dosage, and timing of ICB drugs should be selected with caution.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HL was involved in the identification, selection, and management of the patient and manuscript review. SY was involved in the management of the patient and manuscript drafting. CJ and QZ were involved in the selection and management of the patient and manuscript review. LY, ZB, HJ, NH, and ZB were involved in manuscript editing. HG was involved in the detection of samples. All authors have read and approved the final manuscript.
Funding
This work was supported by a grant from the Science and Technology Planning Project of Beijing City (Z171100001017188).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.639217/full#supplementary-material
References
1. Oran B, Giralt S, Couriel D, Hosing C, Shpall EJ, de Meis E, et al. Treatment of AML and MDS relapsing after reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation. Leukemia. (2007) 21:2540–4. doi: 10.1038/sj.leu.2404828
2. Bishop MR, Alyea EP III, Cairo MS, Falkenburg JH, June CH, Kroger N, et al. National Cancer Institute's First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant. (2011) 17:443–54. doi: 10.1016/j.bbmt.2010.12.713
3. Wayne AS, Giralt S, Kroger N, Bishop MR. Proceedings from the national cancer institute's second international workshop on the biology, prevention, and treatment of relapse after hematopoietic stem cell transplantation: introduction. Biol Blood Marrow Transplant. (2013) 19:1534–6. doi: 10.1016/j.bbmt.2013.08.016
4. Bejanyan N, Weisdorf DJ, Logan BR, Wang H-L, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transpl. (2015) 21:454–9. https://doi.org/10.1016/j.bbmt.2014.11.007
5. George TJ, Woolery JE, Wetzstein GA, Ho VQ, Lancet JE, List AF, et al. A retrospective study of decitabine for the treatment of relapsed or refractory acute myeloid leukemia: lack of response observed in a heavily pretreated population. Blood. (2010) 116:2186–6. doi: 10.1182/blood.V116.21.2186.2186
6. Tawfik B, Sliesoraitis S, Lyerly S, Klepin HD, Lawrence J, Isom S, et al. Efficacy of the hypomethylating agents as frontline, salvage, or consolidation therapy in adults with acute myeloid leukemia (AML). Ann Hematol. (2014) 93:47–55. doi: 10.1007/s00277-013-1940-9
7. Stahl M, DeVeaux M, Montesinos P, Itzykson R, Ritchie EK, Sekeres MA, et al. Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv. (2018) 2:923–32. doi: 10.1182/bloodadvances.2018016121
8. Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase II study. Cancer Discov. (2019) 9:370–83. doi: 10.1158/2159-8290.Cd-18-0774
9. Daver N, Boddu P, Garcia-Manero G, Yadav SS, Sharma P, Allison J, et al. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia. (2018) 32:1094–105. doi: 10.1038/s41375-018-0070-8
10. Wrangle J, Wang W, Koch A, Easwaran H, Mohammad HP, Vendetti F, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. (2013) 4:2067–79. doi: 10.18632/oncotarget.1542
11. Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. (2014) 28:1280–8. doi: 10.1038/leu.2013.355
12. Vago L. Clonal evolution and immune evasion in posttransplantation relapses. Hematol Am Soc Hematol Educ Program. (2019) 2019:610–6. doi: 10.1182/hematology.2019000005
13. Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. (2008) 14:3044–51. doi: 10.1158/1078-0432.Ccr-07-4079
14. Daver N, Basu S, Garcia-Manero G, Cortes JE, Ravandi F, Ning J, et al. Defining the immune checkpoint landscape in patients (pts) with acute myeloid leukemia (AML). Blood. (2016) 128:2900. doi: 10.1182/blood.V128.22.2900.2900
15. Davids MS, Kim HT, Costello C, Herrera AF, Locke FL, Maegawa RO, et al. A multicenter phase 1 study of nivolumab for relapsed hematologic malignancies after allogeneic transplantation. Blood. (2020) 135:2182–91. doi: 10.1182/blood.2019004710
16. Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sharpe AH, Vallera DA. Opposing roles of CD28:B7 CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J Immunol. (1999) 162:6368–77.
17. Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, et al. Blockade of programmed death-1 engagement accelerates graft-vs.-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. (2003) 171:1272–7. doi: 10.4049/jimmunol.171.3.1272
18. Koestner W, Hapke M, Herbst J, Klein C, Welte K, Fruehauf J, et al. PD-L1 blockade effectively restores strong graft-vs.-leukemia effects without graft-vs.-host disease after delayed adoptive transfer of T-cell receptor gene-engineered allogeneic CD8+ T cells. Blood. (2011) 117:1030–41. doi: 10.1182/blood-2010-04-283119
19. Singh AK, Porrata LF, Aljitawi O, Lin T, Shune L, Ganguly S, et al. Fatal GvHD induced by PD-1 inhibitor pembrolizumab in a patient with Hodgkin's lymphoma. Bone Marrow Transplant. (2016) 51:1268–70. doi: 10.1038/bmt.2016.111
20. Haverkos BM, Abbott D, Hamadani M, Armand P, Flowers ME, Merryman R, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. (2017) 130:221–8. doi: 10.1182/blood-2017-01-761346
21. McDuffee E, Aue G, Cook L, Ramos-Delgado C, Shalabi R, Worthy T, et al. Tumor regression concomitant with steroid-refractory GvHD highlights the pitfalls of PD-1 blockade following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. (2017) 52:759–61. doi: 10.1038/bmt.2016.346
22. Holderried TAW, Fraccaroli A, Schumacher M, Heine A, Brossart P, Stelljes M, et al. The role of checkpoint blockade after allogeneic stem cell transplantation in diseases other than Hodgkin's Lymphoma. Bone Marrow Transplant. (2019) 54:1662–7. doi: 10.1038/s41409-019-0498-0
23. Lee A, Keam SJ. Tislelizumab: first approval. Drugs. (2020) 80:617–24. doi: 10.1007/s40265-020-01286-z
24. Bello C, Yu D, Komrokji RS, Zhu W, Wetzstein GA, List AF, et al. Outcomes after induction chemotherapy in patients with acute myeloid leukemia arising from myelodysplastic syndrome. Cancer. (2011) 117:1463–9. doi: 10.1002/cncr.25598
25. Prébet T, Gore SD, Thépot S, Esterni B, Quesnel B, Beyne Rauzy O, et al. Outcome of acute myeloid leukaemia following myelodysplastic syndrome after azacitidine treatment failure. Br J Haematol. (2012) 157:764–6. doi: 10.1111/j.1365-2141.2012.09076.x
26. Oliai C, Schiller G. How to address second and therapy-related acute myelogenous leukaemia. Br J Haematol. (2020) 188:116–28. doi: 10.1111/bjh.16354
27. Bashey A, Medina B, Corringham S, Pasek M, Carrier E, Vrooman L, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. (2009) 113:1581–8. doi: 10.1182/blood-2008-07-168468
28. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. (2016) 375:143–53. doi: 10.1056/NEJMoa1601202
29. Wong E, Dawson E, Davis J, Koldej R, Ludford-Menting M, Lansdown M, et al. Nivolumab for relapsed or residual haematological malignancies after allogeneic haematopoietic stem cell transplantation (NIVALLO). Blood. (2018) 132 (Suppl. 1), 4633. doi: 10.1182/blood-2018-99-112310
30. Schoch LK, Borrello I, Fuchs EJ, Bolanos-Meade J, Huo JS, Gojo I, et al. Checkpoint inhibitor therapy and graft vs. host disease in allogeneic bone marrow transplant recipients of haploidentical and matched products with post-transplant cyclophosphamide. Blood. (2016) 128:4571. doi: 10.1182/blood.V128.22.4571.4571
31. Liao D, Wang M, Liao Y, Li J, Niu T. A review of efficacy and safety of checkpoint inhibitor for the treatment of acute myeloid leukemia. Front Pharmacol. (2019) 10:609. doi: 10.3389/fphar.2019.00609
32. Wang AY, Kline J, Stock W, Kosuri S, Artz A, Larson RA, et al. Unexpected toxicities when nivolumab was given as maintenance therapy following allogeneic stem cell transplantation. Biol Blood Marrow Transpl. (2020) 26:1025–7. doi: 10.1016/j.bbmt.2020.01.021
33. Albring JC, Inselmann S, Sauer T, Schliemann C, Altvater B, Kailayangiri S, et al. PD-1 checkpoint blockade in patients with relapsed AML after allogeneic stem cell transplantation. Bone Marrow Transplant. (2017) 52:317–20. doi: 10.1038/bmt.2016.274
34. Garcia-Manero G, Daver NG, Montalban-Bravo G, Jabbour EJ, DiNardo CD, Kornblau SM, et al. A phase II study evaluating the combination of nivolumab (Nivo) or Ipilimumab (Ipi) with azacitidine in pts with previously treated or untreated myelodysplastic syndromes (MDS). Blood. (2016) 128:344–4. doi: 10.1182/blood.V128.22.344.344
35. Khouri IF, Fernandez Curbelo I, Turturro F, Jabbour EJ, Milton DR, Bassett RL Jr, et al. Ipilimumab plus lenalidomide after allogeneic and autologous stem cell transplantation for patients with lymphoid malignancies. Clin Cancer Res. (2018) 24:1011–8. doi: 10.1158/1078-0432.Ccr-17-2777
36. Herbaux C, Gauthier J, Brice P, Drumez E, Ysebaert L, Doyen H, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. (2017) 129:2471–8. doi: 10.1182/blood-2016-11-749556
37. Angenendt L, Schliemann C, Lutz M, Rebber E, Schulze AB, Weckesser M, et al. Nivolumab in a patient with refractory Hodgkin's lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. (2016) 51:443–5. doi: 10.1038/bmt.2015.266
38. Covut F, Pinto R, Cooper BW, Tomlinson B, Metheny L, Malek E, et al. Nivolumab before and after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. (2017) 52:1054–6. doi: 10.1038/bmt.2017.44
39. Shad AT, Huo JS, Darcy C, Abu-Ghosh A, Esposito G, Holuba MJ, et al. Tolerance and effectiveness of nivolumab after pediatric T-cell replete, haploidentical, bone marrow transplantation: a case report. Pediatr Blood Cancer. (2017) 64:26257. doi: 10.1002/pbc.26257
40. Chan TS, Khong PL, Kwong YL. Pembrolizumab for relapsed anaplastic large cell lymphoma after allogeneic haematopoietic stem cell transplantation: efficacy and safety. Ann Hematol. (2016) 95:1913–5. doi: 10.1007/s00277-016-2764-1
41. Villasboas JC, Ansell SM, Witzig TE. Targeting the PD-1 pathway in patients with relapsed classic Hodgkin lymphoma following allogeneic stem cell transplant is safe and effective. Oncotarget. (2016) 7:13260–4. doi: 10.18632/oncotarget.7177
42. Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. (2017) 129:2437–42. doi: 10.1182/blood-2016-12-756841
43. Godfrey J, Bishop MR, Syed S, Hyjek E, Kline J. PD-1 blockade induces remissions in relapsed classical Hodgkin lymphoma following allogeneic hematopoietic stem cell transplantation. J Immunother Cancer. (2017) 5:11. doi: 10.1186/s40425-017-0211-z
44. Boekstegers AM, Blaeschke F, Schmid I, Wiebking V, Immler S, Hoffmann F, et al. MRD response in a refractory paediatric T-ALL patient through antiprogrammed cell death 1 (PD-1) Ab treatment associated with induction of fatal GvHD. Bone Marrow Transplant. (2017) 52:1221–4. doi: 10.1038/bmt.2017.107
45. El Cheikh J, Massoud R, Abudalle I, Haffar B, Mahfouz R, Kharfan-Dabaja M, et al. Nivolumab salvage therapy before or after allogeneic stem cell transplantation in Hodgkin lymphoma. Bone Marrow Transplant. (2017) 52:1074–7. doi: 10.1038/bmt.2017.69
46. Yared JA, Hardy N, Singh Z, Hajj S, Badros AZ, Kocoglu M, et al. Major clinical response to nivolumab in relapsed/refractory Hodgkin lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. (2016) 51:850–2. doi: 10.1038/bmt.2015.346
47. Saha A, Aoyama K, Taylor PA, Koehn BH, Veenstra RG, Panoskaltsis-Mortari A, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-vs.-host disease lethality. Blood. (2013) 122:3062–73. doi: 10.1182/blood-2013-05-500801
48. Li X, Deng R, He W, Liu C, Wang M, Young J, et al. Loss of B7-H1 expression by recipient parenchymal cells leads to expansion of infiltrating donor CD8+ T cells and persistence of graft-vs.-host disease. J Immunol. (2012) 188:724–34. doi: 10.4049/jimmunol.1102630
49. Klobuch S, Weber D, Holler B, Herr W, Holler E, Wolff D. Potential role of the PD-1/PD-L1 axis in the immune regulation of chronic GVHD. Oncol Res Treat. (2017) 40:447–50. doi: 10.1159/000471768
50. Naymagon S, Naymagon L, Wong SY, Ko HM, Renteria A, Levine J, et al. Acute graft-vs.-host disease of the gut: considerations for the gastroenterologist. Nat Rev Gastroenterol Hepatol. (2017) 14:711–26. doi: 10.1038/nrgastro.2017.126
51. Collins M, Soularue E, Marthey L, Carbonnel F. Management of patients with immune checkpoint inhibitor-induced enterocolitis: a systematic review. Clin Gastroenterol Hepatol. (2020) 18:1393–403.e1391. doi: 10.1016/j.cgh.2020.01.033
52. Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, et al. The binding of an anti-PD-1 antibody to FcγR? has a profound impact on its biological functions. Cancer Immunol Immunother. (2018) 67:1079–90. doi: 10.1007/s00262-018-2160-x
Keywords: acute myeloid leukemia, post-transplantation relapse, GvHD, immune checkpoint blockade, hypomethylating agents
Citation: Yao S, Jianlin C, Zhuoqing Q, Yuhang L, Jiangwei H, Guoliang H, Hongmei N, Bin Z and Liangding H (2021) Case Report: Combination Therapy With PD-1 Blockade for Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation Resulted in Fatal GVHD. Front. Immunol. 12:639217. doi: 10.3389/fimmu.2021.639217
Received: 08 December 2020; Accepted: 12 February 2021;
Published: 01 April 2021.
Edited by:
Bruno Fattizzo, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Rodabe N. Amaria, University of Texas MD Anderson Cancer Center, United StatesMaria Teresa Lupo Stanghellini, San Raffaele Hospital (IRCCS), Italy
Copyright © 2021 Yao, Jianlin, Zhuoqing, Yuhang, Jiangwei, Guoliang, Hongmei, Bin and Liangding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hu Liangding, aHVsaWFuZ2RpbmdAc29odS5jb20=
†These authors have contributed equally to this work
 Sun Yao
Sun Yao Chen Jianlin1†
Chen Jianlin1†