- 1Renal Division, Department of Medicine, Peking University First Hospital; Institute of Nephrology, Peking University; Key Laboratory of Renal Disease, Ministry of Health of China; Key Laboratory of CKD Prevention and Treatment, Ministry of Education of China, Beijing, China
- 2Department of Nephrology, Peking University International Hospital, Beijing, China
- 3Department of Nephrology, General Hospital of Ningxia Medical University, Yinchuan, China
Objectives: This study aimed to determine the prevalence and localization of complement factor C4d in renal biopsies from patients with lupus nephritis (LN), as well as its associations with the disease’s clinico-pathological features. The correlation between arteriolar C4d deposition and renal microvascular lesions (RVLs) was further analyzed.
Methods: A total of 325 biopsy-proven LN patients were enrolled, and their clinico-pathological data were collected. C4d staining of renal biopsies was performed by immunohistochemistry. The associations between C4d deposition and the clinico-pathological features were further analyzed.
Results: C4d deposition was present in most (98.8%) renal specimens in our cohort. These deposits were localized in the glomeruli (98.2%), tubular basement membrane (TBM) (43.7%), arterioles (31.4%), and peritubular capillary (33.8%). Patients with TBM C4d staining had higher disease activity (measured with the Systemic Lupus Erythematous Disease Activity Index) and higher National Institutes of Health pathological activity and chronicity indices (all P < 0.01). Patients with arteriolar C4d deposition were more likely to develop RVLs (91.2%) compared to those with no arteriolar C4d deposition (78.0%; P = 0.004), especially with two or more types of RVLs (P < 0.001). During the mean follow-up of 55.8 months, arteriolar C4d was related to worse renal outcomes [hazard ration (HR): 2.074, 95% confidence interval (CI) 1.056–4.075, P = 0.034]. Multivariate Cox hazard analysis showed that co-deposition of arteriolar C4d and C3c was an independent risk factor (HR: 3.681, 95% CI 1.519–8.921, P = 0.004) for predicting renal outcomes.
Conclusions: C4d deposition was common in renal tissues from LN patients. TBM C4d deposition was related to the disease activity, and arteriolar C4d deposition was associated with RVLs and worse renal outcomes.
Introduction
Systemic lupus erythematous (SLE) is an autoimmune disease with a wide variety of clinical manifestations and serological abnormalities (1). Lupus nephritis (LN) presents in up to 60% of patients during the disease course (2) and contributes to SLE’s morbidity and mortality. Renal histopathology is closely related with its clinical characteristics, responses to treatment, and patient prognosis (3).
Complement activation plays a key role in LN pathogenesis (4). The classical pathway is thought to be the dominant pathway for complement activation in LN, which is triggered by the interaction of C1q with immune complexes (5). Several studies detected mannose-binding lectin (MBL) in the glomeruli of LN patients (6, 7), implicating its participation in disease progression.
C4d is a fragment of C4 generated during activation of the complement pathway and is regarded as a hallmark of classical or lectin complement pathway activation (8). Its deposition in renal peritubular capillaries (PTCs) is widely accepted as a marker of antibody-mediated rejection (AMR) in transplanted kidneys (9, 10). Its meaning in other kidney diseases like LN in which many different autoantibodies produced an analogous situation has also been investigated in recent years. C4d deposition on PTCs has been observed in LN patients, but with a different pattern from those observed in antibody-mediated renal rejection, and this event is closely related to the disease activity (11–13). Besides, Kim et al. found that glomerular C4d deposition could be detected in the majority of the LN cases, but concluded that it was not a marker for LN activity (14). However, another study conducted by Cohen and colleagues demonstrated that LN patients with prominent diffuse glomerular C4d deposition were more likely to develop renal thrombotic microangiopathy (TMA) lesions than patient with focal or mild C4d staining (15). Thus, the role of C4d deposition, especially in the different areas of renal biopsies in LN, remains to be further clarified.
Herein, we assessed the prevalence and anatomic localization of C4d deposition in renal biopsy specimens from a well-defined LN cohort. The associations between C4d deposition in different kidney compartments and clinico-histopathological features and outcomes of LN were further analyzed.
Methods
Patients
Clinical and renal histopathological data of 325 patients with renal biopsy proven LN diagnosed between January 2000 and June 2008 from Peking University First Hospital were collected. All the patients included fulfilled the 1997 American College of Rheumatology revised criteria for SLE (16). Patients who lacked medical record information or follow-up data (n = 37), lacked renal biopsy samples for re-examination (n = 20) or had <10 glomeruli and <6 vessels in renal biopsies (n = 9) were excluded. More details on the study design are presented as a flowchart in Supplementary Figure 1. The biopsies and biologic samples used in this study came from the biorepository of the Department of Nephrology, Pekin University First Hospital. Informed consent was obtained from each patient for blood sampling and renal biopsy. The research was in compliance with the Declaration of Helsinki. The protocol was approved by the local ethical committees of Peking University First Hospital [No. 2017 (1333)].
Clinical Evaluation
Disease activity was assessed with the Systemic Lupus Erythematous Disease Activity Index (SLEDAI) (17, 18). All patients were followed up in outpatient clinics specified for LN. Acute kidney injury is defined as an increase in serum creatinine (Scr) by 50% within 7 days, an increase in Scr by 0.3 mg/dl (26.5 μmol/L) within 2 days, or oliguria (19). The end point was defined as end stage renal disease (ESRD) or doubling of the Scr value. ESRD was defined as requiring renal replacement therapy (20).
Laboratory Assessment
Serum antinuclear antibodies were detected using the indirect immunofluorescence assay (EUROIMMUN, Lübeck, Germany). Anti-double-stranded DNA (anti-dsDNA) antibodies were detected using the Crithidia luciliae indirect immunofluorescence test (EUROIMMUN). Serum C3 and C4 were determined using the rate nephelometry assay (IMMAGE; Beckman-Coulter, IMMAGE, Fullerton, CA; normal range >0.85 g/L for C3, normal range >0.12 g/L for C4). Anti-cardiolipin antibodies (aCL) were detected using enzyme-linked immunosorbent assay (EUROIM MUN).
Renal Histopathology
The renal biopsy specimens were routinely examined by light microscopy, direct immunofluorescence, and electron microscopy techniques. Renal histopathology data were reviewed and reclassified according to the International Society of Nephrology and Renal Pathology Society 2003 LN classification system (21) by two experienced pathologists. They independently reviewed the biopsy specimens separately and were blinded to patients’ clinical and follow-up data. If there were differences in scoring between the two pathologists, they would re-review the biopsies and reach a consensus. Pathological parameters such as activity indices (AIs) and chronicity indices (CIs) were evaluated as described previously (22). Activity indices was defined as the sum of individual scores of the following items: endocapillary hypercellularity, cellular crescents (×2), karyorrhexis/fibrinoid necrosis (×2), subendothelial hyaline deposits, interstitial inflammatory cell infiltration, and glomerular leukocyte infiltration. The maximum score was 24 points for the Activity Index. Chronicity indices was defined as the sum of individual scores of the following items: glomerular sclerosis, fibrous crescents, tubular atrophy, and interstitial fibrosis. The maximum score was 12 points for the Chronicity Index (22). Renal microvascular lesions (RVLs) were classified according to the previous studies (23, 24) as follows: immune complex deposits (ICD), non-inflammatory necrotizing vasculopathy (NNV), thrombotic microangiopathy (TMA), true renal vasculitis (TRV), and atherosclerosis (AS). Renal TMA lesions were subdivided into active changes and chronic changes. Active TMA lesion was defined as the presence of at least one fibrin microthrombus either in the glomeruli, small arterioles, and/or arteries. Chronic TMA changes were defined as mucoid changes or onion-skin lesions of arterioles and/or arteries (24). Semiquantitative RVL scores were calculated as described in our previous work (24).
C4d, C1q, C3c, and Immunoglobulin Staining
C4d was immunohistochemically labeled in formalin-fixed paraffin-embedded tissue (4-μm thick). Rabbit anti-human C4d polyclonal antibodies (Abcam, Cambridge, UK) were used as primary antibodies. The intensity of Glomerular C4d (G-C4d) was semi-quantitatively graded (0–3) (12, 25). Tubular basement membrane (TBM) C4d (TBM-C4d) was considered to be present if more than half of the individual TBM circumference was stained. It was then graded as negative (0%), minimal (1, <10% of tissue specimen), focal (2, 10–50% of tissue specimen), or diffuse (3, >50% of tissue specimen) (12, 25). PTC-C4d staining was graded in accordance with the Banff 2007 criteria for C4d staining as negative (0%), minimal (1, <10% of tissue specimen), focal (2, 10–50% of tissue specimen), or diffuse (3, >50% of tissue specimen) (26). Arteriolar C4d staining (A-C4d) was semi-quantitatively graded as negative (0%), minimal (1, <10% of tissue specimen), focal (2, 10–50% of tissue specimen), or diffuse (3, >50% of tissue specimen) (12, 27). Negative control experiments were performed on the renal specimens of a living kidney donor. Blank control experiments were performed by omitting or replacing the primary antibodies. The typical presentations of C4d staining in different areas of kidneys are shown in Figure 1.
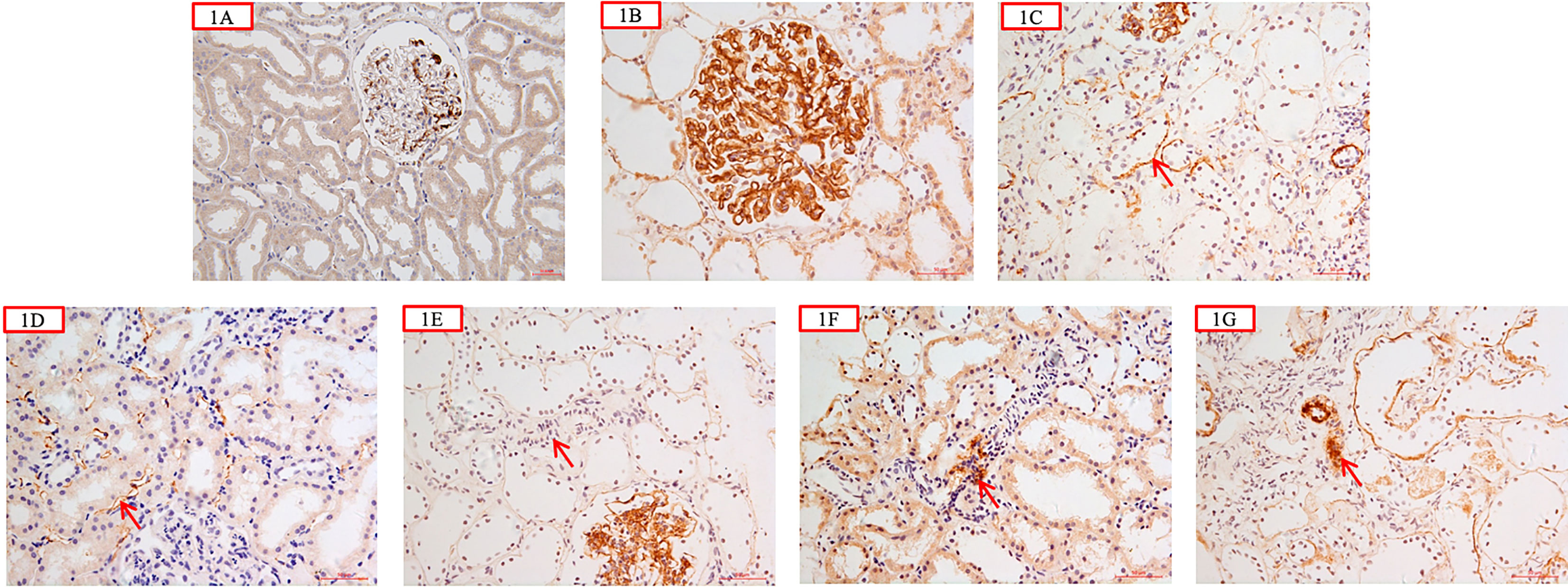
Figure 1 (A) was negative control from a living kidney donor which showed scanty granular mesangial C4d deposition; C4d was negative along tubular basement membrane (TBM) and peritubular capillary wall (PTC) (×200); (B) showed glomerular C4d granular deposition along the capillary wall and in the mesangial area (×400); (C) showed C4d deposition along the tubular basement membrane (red arrow) (×400); (D) showed C4d deposition along peritubular capillary wall (red arrow) (×400); (E) showed that C4d was negative in the arteriolar wall (red arrow) (×400); (F) showed C4d segmental arteriolar wall deposition (red arrow) (×400); (G) showed C4d deposition in the arteriolar wall (red arrow) (×400).
The fresh frozen tissue sections were stained immediately after the renal biopsy with fluorescein isothiocyanate-labeled rabbit anti-human IgG, IgA, IgM, C3c, and C1q antibodies (Dako A/S, Copenhagen, Denmark). The results were graded from 0 to 4 according to the intensity of fluorescence.
Statistical Analysis
Statistical software SPSS 18.0 (SPSS, Chicago, IL, USA) and GraphPad-Prism 5.0 (GraphPad Software, La Jolla, CA, USA) were used for statistical analyses. Quantitative data are expressed as mean ± s.d. or median with range (minimum and maximum) or interquartile range. Spearman’s rank-order correlation tests were performed to compare various lesions. Differences in semiquantitative data were tested with the Kruskal–Wallis test or Mann–Whitney U-tests. Bonferroni’s correction was used for multiple comparisons. Kaplan–Meier curves were generated to analyze patients’ prognosis. Univariate survival analysis was carried out using the log-rank tests. The Cox regression model was applied to identify prognostic factors associated with renal outcomes. The proportional hazards assumption was tested by using Schoenfeld residuals. No violations were found for any of the covariates based on a P value threshold of 0.05. Results are expressed as hazard ratios (HR) with 95% confidence intervals (CI). Statistical significance was considered at P < 0.05.
Results
Baseline Data of the LN Cohort
A total of 325 LN patients were enrolled. Their clinico-pathological data are presented in Table 1.
All 325 patients received oral prednisone therapy. Among them, 28 patients received prednisone alone, 254 patients received cyclophosphamide [238 patients received the monthly intravenous cyclophosphamide (600–800 mg/months), and 16 patients received oral cyclophosphamide], 27 patients received mycophenolate mofetil, 12 patients received leflunomid, 3 patients received azathioprine, and 1 patient received cyclosporine.
The average follow-up time was 55.8 months (range from 4 to 360 months). During this period, three patients died of heart failure and one patient died of severe infection. A total of 37 patients reached the end point, including 35 with ESRD and 2 with Scr doubling.
The Prevalence and Localization of C4d Staining in Renal Specimens of LN Patients
C4d staining was present in 321/325 (98.8%) LN samples (Table 2). They were localized in the glomerulus (319/325, 98.2%), TBM (142/325, 43.7%), arterioles (102/325, 31.4%), and PTCs (110/325, 33.8%) respectively.
The intensity or distribution of C4d staining among different sub-classes of LN are shown in Figure 2. We found that the degree of TBM C4d deposition significantly differed among the subtypes (P < 0.001); it was more often present in patients with classes III, IV, and V disease than class II. The rates of glomerular, arteriolar, and PTC C4d deposition were not significantly different among the subgroups.
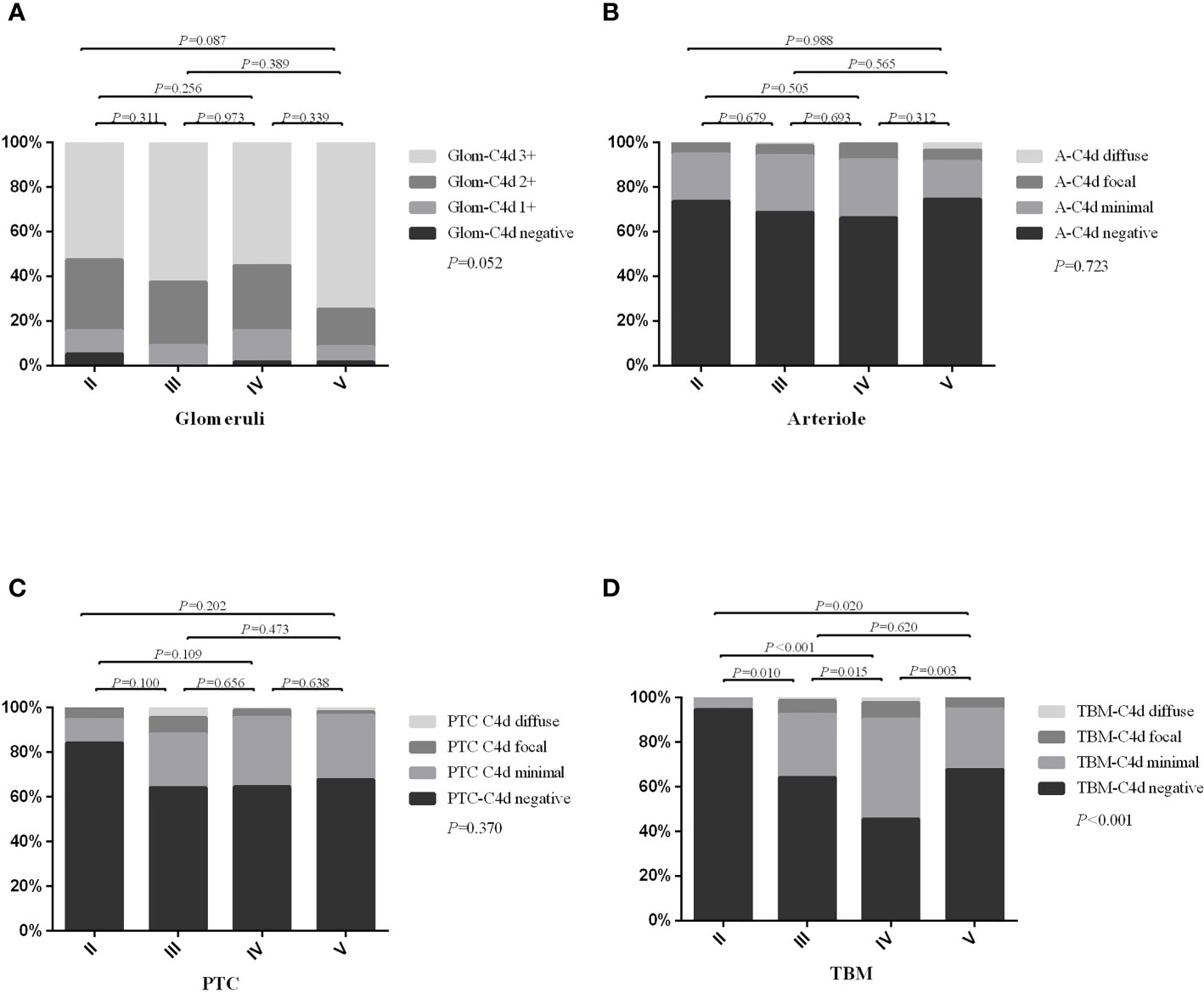
Figure 2 The distribution or intensity of C4d staining between different lupus nephritis sub-classes. (A) showed the intensity of glomerular C4d staining; (B) showed distribution of arteriolar C4d staining; (C) showed distribution of PTC-C4d staining; (D) showed distribution of TBM-C4d staining. Glom, glomerular; A, arteriolar; PTC, peritubular capillary; TBM, tubular basement membrane. Bonferroni’s correction for multiple correlations: statistical significance was considered at P < 0.008 (0.05/6).
Comparison of C4d Deposition in Different Renal Compartments With Clinico-Pathological Data
The detailed analyses between C4d deposition in different renal areas and clinical and laboratory features are shown in Table 3. Notably, hematuria (P = 0.035), leukocyturia (P = 0.002), and acute kidney injury (P = 0.001) were more frequent in TBM C4d-positive patients, who also had higher levels of urinary protein, Scr, and SLEDAI value, and a lower serum C3 value.
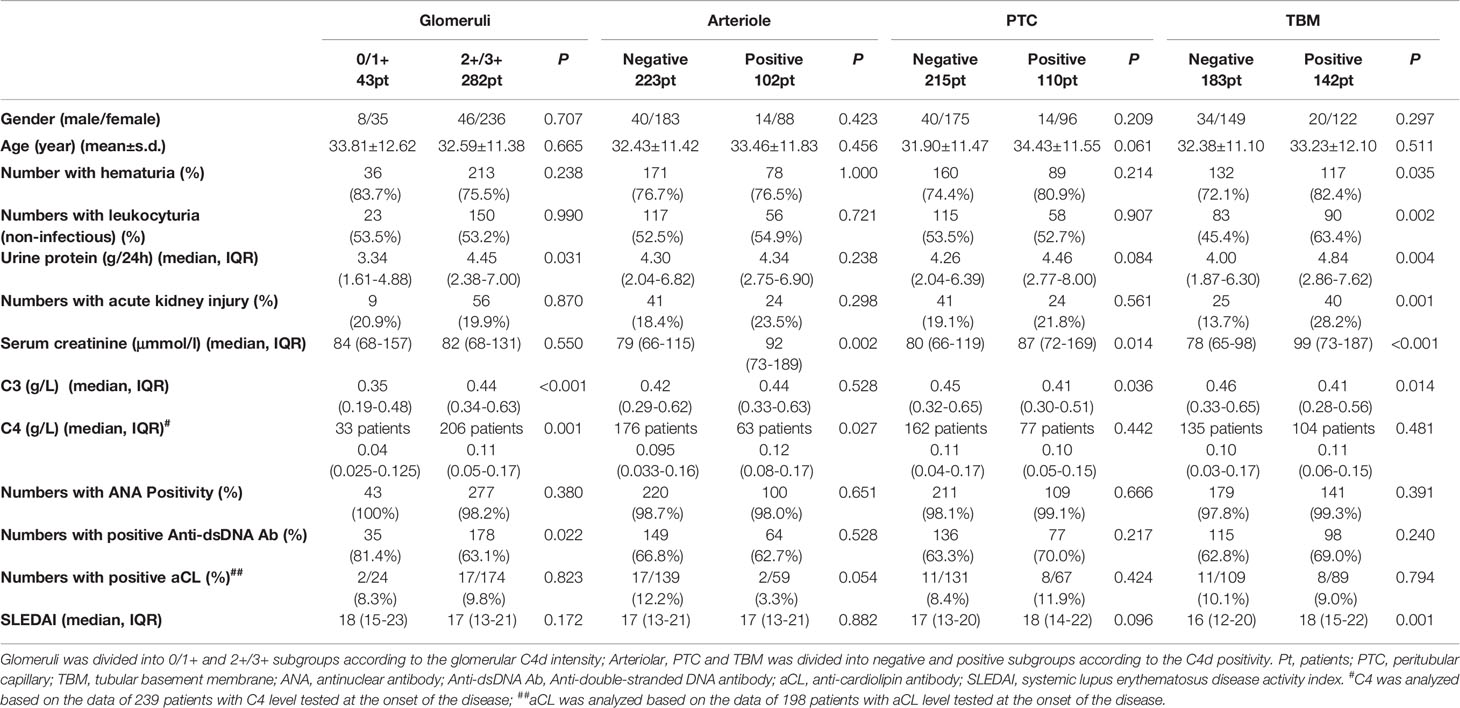
Table 3 Comparison of clinical and laboratory data in patients with and without C4d deposition in different renal compartments.
Renal histopathological findings showed that C4d deposition in TBM, arterioles, and PTCs area had higher NIH activity indices (AI) and chronic indices (CI) than those without C4d deposition in the corresponding areas. Detailed data were presented in Table 4.
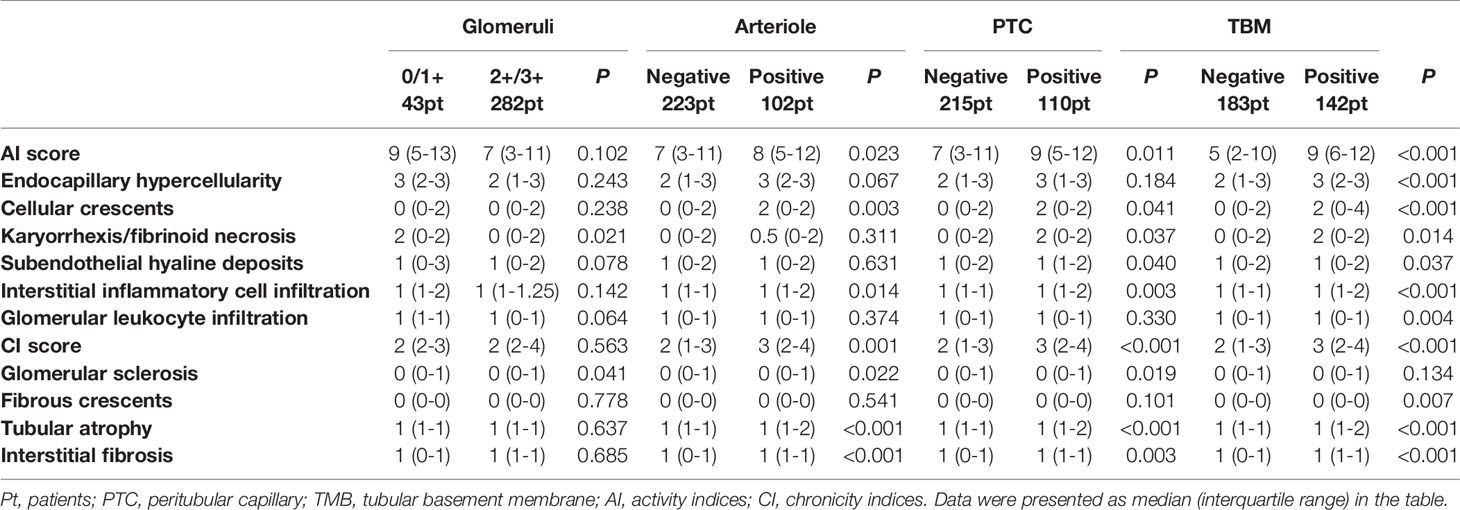
Table 4 Comparison of renal histopathological data in patients with and without C4d deposition in different renal compartments.
Association Between Arteriolar C4d (A-C4d) Deposition and RVLs in LN Patients
RVLs were common in LN, so the relationship between arteriolar C4d (A-C4d) deposition and RVLs was explored. In our study, 267 (82.2%) patients presented with RVLs. Among them, 34.8% of patients had A-C4d deposition, which was a much higher rate than in those without RVL (15.5%, P = 0.004). Similarly, those with A-C4d deposition had a higher rate of RVLs (91.2%) compared to those negative for A-C4d (78.0%; P = 0.004), especially multiple RVLs (P < 0.001), which is most obvious in type IV LN (P = 0.001) (Figure 3). Moreover, diffuse (>50%) or focal (10–50%) renal A-C4d staining patterns were more common in patients with RVLs [n = 22/267 (8.2%)] compared with those without [n = 2/58 (3.4%); P = 0.035].
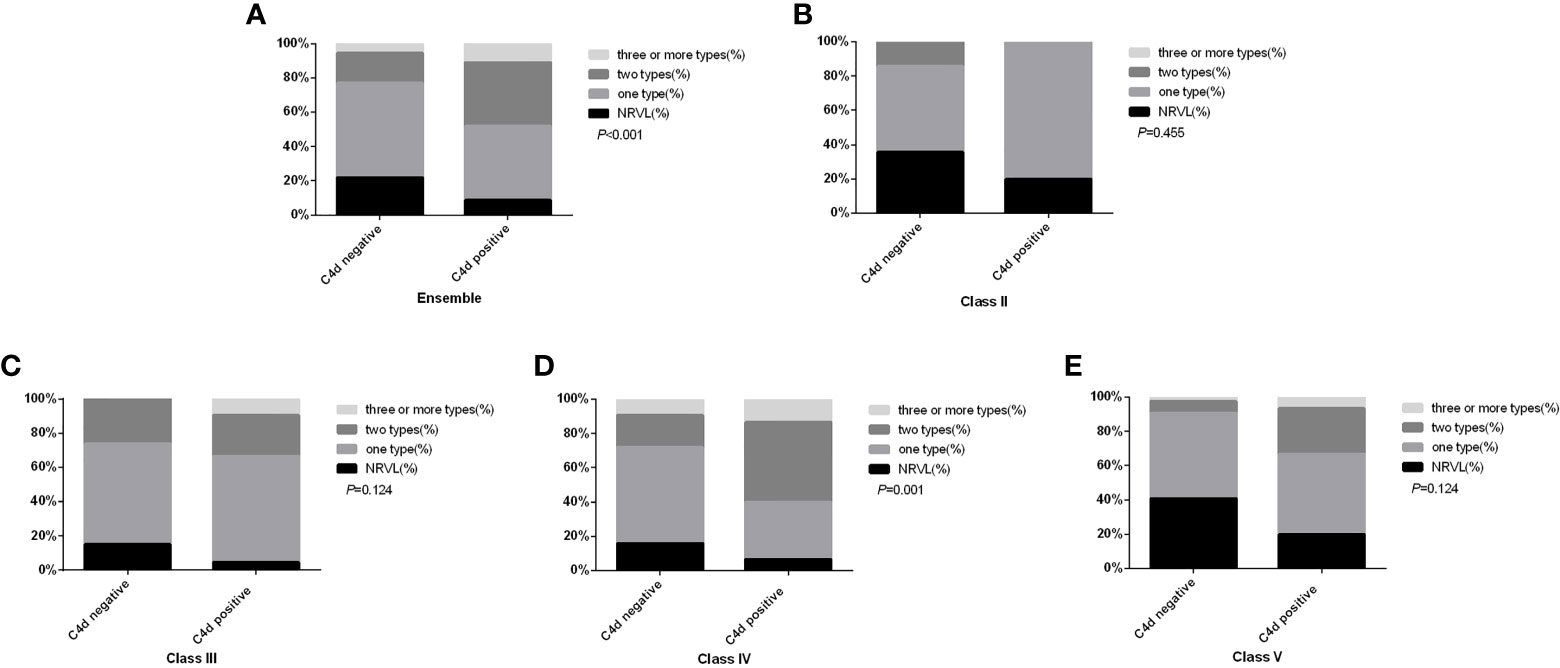
Figure 3 Comparison of renal microvascular lesion types between patients with and without arteriolar C4d deposition in the ensemble (A), in subgroup class II (B), in subgroup class III (C), in subgroup class IV (D), and in subgroup class V (E). NRVL, no renal microvascular lesions.
With regard to RVL subtypes, patients with TMA lesions had the highest rate of A-C4d deposition (48.3%), followed by patients with ICD (38.3%), NNV (38.5%), and AS (34.5%). Significantly more C4d deposition was found in patients with TMA lesions (48.3%, 28/58) or ICD lesions (38.3%, 92/240) compared to those without TMA lesions (27.7%, 74/267, P = 0.003) or ICD lesions (11.8%, 10/85, P < 0.001). No significant differences were found for AS, NNV, and TRV lesions. Furthermore, among 58 patients with TMA lesions, 25 had acute TMA, 14 had chronic TMA, and 19 had both acute and chronic (A+C) TMA lesions. Arteriolar C4d deposition was not found significantly different amongst the groups: 44.0% (11/25) in acute TMA subgroup, 42.1% (8/19) in A+C TMA, and 64.3% (9/14) in chronic TMA cases (P = 0.385). Neither C4d deposition nor the presence of renal TMA lesions correlated with aCL status: aCL was positive in 2.8% (1/36) in TMA group and 11.1% of (18/162) patients without TMA (P = 0.208). ACL was positive in 3.4% (2/59) of arteriolar C4d positive patients and in 12.2% (17/139) of arteriolar C4d negative patients (P = 0.065).
Analysis of co-depositions of A-C4d and C1q, C3c and different immunoglobulins were further analyzed. Patients with A-C4d deposits had higher ratios of C1q deposition (75.5% vs. 59.2%, P = 0.004), C3c deposition (39.2% vs. 28.7%, P = 0.059), IgG deposition (40.2% vs. 29.1%, P = 0.049), IgM deposition (37.3% vs. 25.6%, P = 0.031), and IgA deposition (17.6% vs. 7.2%, P = 0.004) compared to those without A-C4d deposits. Patients with both C4d and C3c staining had significantly higher scores of CI (P = 0.024), tubular atrophy (P = 0.049), and interstitial fibrosis (P = 0.027) than those who were only C4d positive. Similar results were also found in the C4d+C1q, C4d+IgG, C4d+IgM, and C4d+IgA double positive groups (Supplementary Table 1).
C4d Deposition and Renal Outcomes in LN
We found that patients with arteriolar C4d deposition (P = 0.03) presented with worse renal survival rates than those who were negative (Figure 4A). C4d deposition in other renal compartments did not influence patient renal outcomes. When the patients were categorized into four groups according to the arteriolar C4d and C3c staining, those with both arteriolar C4d and C3c staining had the worst renal outcomes (Figure 4B).
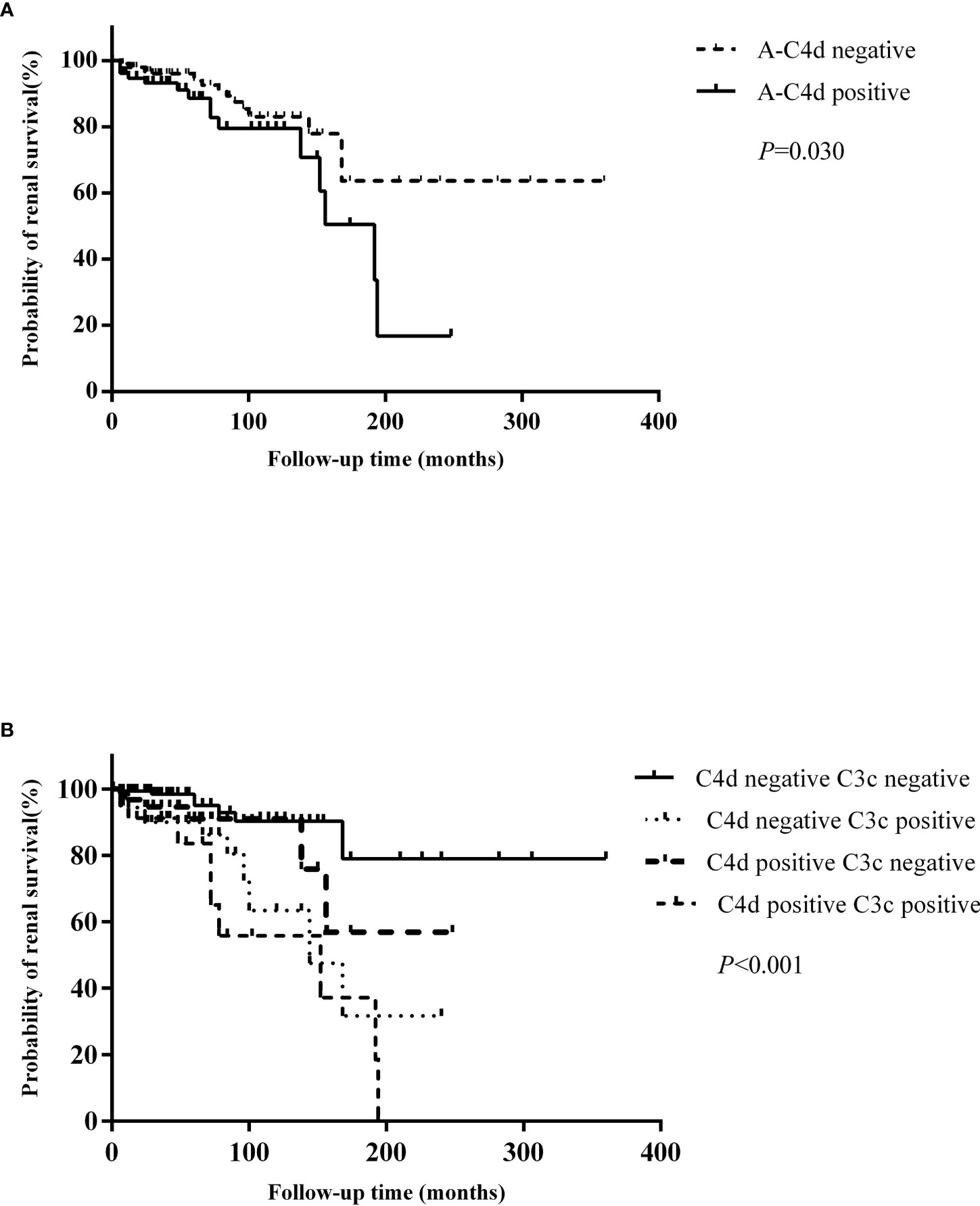
Figure 4 (A) Comparison of renal outcomes between patients with and without arteriolar C4d deposition. (B) Comparison of renal outcomes between patients with and without arteriolar C4d and C3c deposition.
Univariate survival analysis of renal prognosis in LN revealed that arteriolar C4d (HR 2.074, 95% CI 1.056–4.075, P = 0.034) deposition and co-deposition of C4d and C3c (HR 3.652, 95% CI 1.736–7.683, P = 0.001) were risk factors for poor renal outcomes (Table 5). Subsequent multivariate Cox hazard analysis showed that C4d deposition and the co-deposition of C4d and C3c were both independent risk factors for predicting renal outcomes in LN (Table 6).
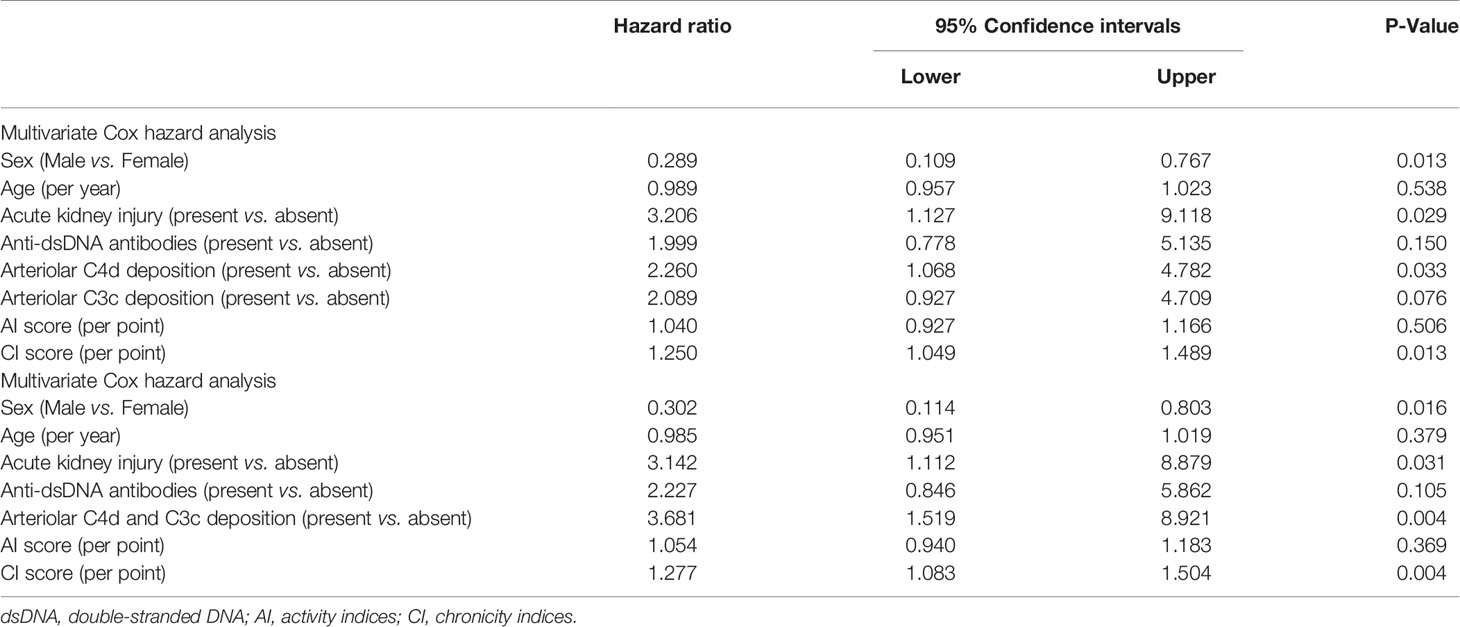
Table 6 Multivariate survival analysis of the risk factors for renal outcome in patients with lupus nephritis patients.
Discussion
Complement activation, especially via the classical pathway, is widely accepted to play an important role (28) in the LN pathogenesis (29). C4d is a fragment of complement activation that remains covalently bound long after the complement pathway-initiating factors have dissociated. It can be generated by immune complexes through both the classical and lectin pathways (30, 31) and might play a key role in the pathomechanisms of the disease (32). However, there is a lack of generic descriptions of its distribution in renal biopsies from patients with LN; studies of larger cohorts and more detailed analyses of its clinico-pathological significance are needed.
In this study, renal C4d deposition was ubiquitous (98.8%) in LN patients, which was mildly higher than the previous reported values ranging from 86.8 to 92% (12, 15, 33). The difference in C4d deposition rates between studies might be attributable to the varied testing methods and the disease activity of the patients enrolled (12, 25); more than half of our patients had type IV LN with a rather high disease activity. Interestingly, the C4d deposition pattern and rate varied among different renal compartments, reflecting clinico-pathological features. TBM C4d deposition was most strongly related to disease activity, while arteriolar C4d deposition had prognostic value for the renal outcomes.
Glomerular C4d deposition was common in LN in both our study and previous reports (12, 15, 28). Sahin and colleagues suggested that glomerular C4d staining could be an indicator of disease activity in LN patients (34). Cohen et al. and Shen et al. also reported that the intense glomerular C4d staining was an indicator of thrombotic microangiopathy in LN (15, 28). However, neither its distribution nor intensity was related to disease activity or any RVLs in our study, which is supported by the work by Kim et al. and Batal et al., indicating that its deposition may simply reflect in situ classical complement pathway activation induced by immune complexes (12, 14).
PTC-C4d deposition is a key marker for diagnosing antibody-mediated renal allograft rejection, and it can also be detected in native kidneys (35). Interestingly, a study from China found PTC-C4d deposited in 6.81% of the LN patients, which was related to the disease activity (13). Although we found a higher rate of C4d deposition in PTCs, we could not confirm a significant association between PTC-C4d deposition and disease activity.
Importantly, LN patients with TBM-C4d deposition presented with more severe clinico-pathological features such as a higher disease activity, worse renal function, lower complement level, greater risk of acute kidney injury, and higher AI and CI scores. As the TBM immune complex deposition is closely associated with disease activity and LN progression (36), we proposed that TBM-C4d deposition could reflect the in situ complement activation of the classical or lectin pathway, thus aggravating local inflammation.
RVLs are of great importance in LN and have prognostic value (24). Previous studies described arteriolar C4d deposition in TMA cases associated with SLE (37, 38). In a recent study done by Mejia-Vilet, although they did not find significant difference in C4d deposition in any compartment between patients with highly active LN and those with concomitant TMA, the latter group prone to have more C4d deposition in all the renal compartments (C4d positivity in different renal compartments in active LN vs. active TMA+active LN: glomerular capillaries: 12 vs. 14 patients; peritubular capillaries: 8 vs. 11 patients; tubular basement membrane: 6 vs. 12 patients; arterioles: 4 vs. 8 patients). The diverse conclusions between different papers from various cohorts might be attributed to the different sample size and the method of C4d evaluation (39). However, data concerning other RVL types in LN are lacking. We found a close association between arteriolar C4d deposition and different RVLs, including TMA changes. We speculate that some common pathway might be involved in RVL pathogenesis, and the higher rate of arteriolar C4d deposition in patients with RVL suggests that the complement system might be causally involved in its pathogenesis. Interestingly, nearly all the arteriolar C4d positive patients in our study had ICD lesions, so we further stained samples for C1q. As anticipated, 75.5% of the arteriolar C4d- positive patients had C1q deposition, which was in line with previous studies regarding TMA lesions, in which classical pathway activation played a major role (15, 37). Thus, complement activation through the classical pathway was suggested to be a common pathomechanism involved in various types of RVLs. Interestingly, higher rates of IgM and IgA deposition were also found in C4d-positive patients. Chua et al. proposed C4d might also be a consequence of damage rather than an underlying cause. Chronic endothelial cell injury may result in the formation of a duplicate glomerular basement membrane, which could entrap aspecific immunoglobulins and C3, thus mimicking immune complex deposition (37). Interestingly, nine patients without RVLs in our cohort also had arteriolar C4d deposition. We propose that C4d deposition in those patients might result from the non-specific immune complex activation in LN.
Moreover, arteriolar C4d positive patients had a higher rate of combined C3c deposition compared to those who were negative. Both arteriolar C4d- and C3c-positive patients had the worst renal outcomes. Recent work showed that terminal complement complex (C5b-9) deposition in glomeruli did not differ between active and chronic disease in LN (40). In contrast, C3c staining was associated with active disease in LN, which would be a better indicator to identify patients most likely to benefit from complement inhibiting treatment (40). Thus, a local cascade of complement activation cascade might be more likely in patients with arteriolar C4d deposition, inducing RVL progression and indicating ongoing disease activity. So, it might serve as an alternative test to find patients who might benefit from anti-complement therapy.
Conclusion
In summary, complement factor C4d deposition was common, but its prevalence varied among different renal compartments in patients with LN. TBM-C4d deposition was associated with more severe clinico-pathological features, and arteriolar C4d deposition was closely related to RVLs renal outcomes. Further studies are needed to clarify its actual role in the pathomechanism of LN.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research and Ethics Board of the Peking University First Hospital [Approval reference: No. 2017 (1333)]. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YD, LW, and YT planned the project, selected patients, interpreted results, and wrote the paper. XY performed analysis of the renal pathology. ZQ and FY planned the project and revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Grants of National Natural Science Foundation of China (No. 81870479) and Pekin University International Hospital Research Grant (No. YN2020QN01, No. YN2020ZD03).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.654652/full#supplementary-material
Supplementary Figure 1 | Flow chart of enrollment of lupus nephritis patients and the design of the study. Abbreviations: LN, lupus nephritis.
Supplementary Table 1 | Analysis of co-deposition between arteriolar C4d and C1q, C3c and different immunoglobulins (IgG, IgM and IgA) in C4d positive patients.
References
1. Rahman A, Isenberg DA. Systemic Lupus Erythematosus. New Engl J Med (2008) 358(9):929–39. doi: 10.1056/NEJMra071297
2. Cameron JS. Lupus Nephritis. J Am Soc Nephrol: JASN (1999) 10(2):413–24. doi: 10.1681/ASN.V102413
3. Appel G, D’Agati R. Secondary Glomerular Disease. In: Brenner BM, editor. Brenner & Rector’s The Kidney, 8th edn. Philadephia: WBSaunders (2008). p. 1067–146.
4. Tang S, Lui SL, Lai KN. Pathogenesis of Lupus Nephritis: An Update. Nephrology (2005) 10(2):174–9. doi: 10.1111/j.1440-1797.2005.00392.x
5. Karp DR. Complement and Systemic Lupus Erythematosus. Curr Opin Rheumatol (2005) 17(5):538–42. doi: 10.1097/01.bor.0000172799.03379.86
6. Lhotta K, Wurzner R, Konig P. Glomerular Deposition of Mannose-Binding Lectin in Human Glomerulonephritis. Nephrol Dialysis Transpl: Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc (1999) 14(4):881–6. doi: 10.1093/ndt/14.4.881
7. Sato N, Ohsawa I, Nagamachi S, Ishii M, Kusaba G, Inoshita H, et al. Significance of Glomerular Activation of the Alternative Pathway and Lectin Pathway in Lupus Nephritis. Lupus (2011) 20(13):1378–86. doi: 10.1177/0961203311415561
8. Berger SP, Roos A, Daha MR. Complement and the Kidney: What the Nephrologist Needs to Know in 2006? Nephrol Dial Transpl (2005) 20(12):2613–9. doi: 10.1093/ndt/gfi166
9. Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-Mediated Rejection Criteria - An Addition to the Banff 97 Classification of Renal Allograft Rejection. Am J Transpl: Off J Am Soc Transplant Am Soc Transplant Surgeons (2003) 3(6):708–14. doi: 10.1034/j.1600-6143.2003.00072.x
10. Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, et al. Complement Activation in Acute Humoral Renal Allograft Rejection: Diagnostic Significance of C4d Deposits in Peritubular Capillaries. J Am Soc Nephrol: JASN (1999) 10(10):2208–14. doi: 10.1681/ASN.V10102208
11. Mora C, Medina-Rosas J, Santos AM, Jaimes DA, Arbelaez AM, Romero C, et al. Associations of the Levels of C4d-Bearing Reticulocytes and High-avidity Anti-Dsdna Antibodies With Disease Activity in Systemic Lupus Erythematosus. J Rheumatol (2016) 43(9):1657–64. doi: 10.3899/jrheum.150486
12. Batal I, Liang K, Bastacky S, Kiss LP, McHale T, Wilson NL, et al. Prospective Assessment of C4d Deposits on Circulating Cells and Renal Tissues in Lupus Nephritis: A Pilot Study. Lupus (2012) 21(1):13–26. doi: 10.1177/0961203311422093
13. Li SJ, Liu ZH, Zen CH, Wang QW, Wang Y, Li LS. Peritubular Capillary C4d Deposition in Lupus Nephritis Different From Antibody-Mediated Renal Rejection. Lupus (2007) 16(11):875–80. doi: 10.1177/0961203307083279
14. Kim SH, Jeong HJ. Glomerular C4d Deposition Indicates In Situ Classic Complement Pathway Activation, But Is Not a Marker for Lupus Nephritis Activity. Yonsei Med J (2003) 44(1):75–80. doi: 10.3349/ymj.2003.44.1.75
15. Cohen D, Koopmans M, Kremer Hovinga IC, Berger SP, Roos van Groningen M, Steup-Beekman GM, et al. Potential for Glomerular C4d as an Indicator of Thrombotic Microangiopathy in Lupus Nephritis. Arthritis Rheum (2008) 58(8):2460–9. doi: 10.1002/art.23662
16. Hochberg MC. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum (1997) 40(9):1725. doi: 10.1002/art.1780400928
17. Liang MH, Socher SA, Larson MG, Schur PH. Reliability and Validity of Six Systems for the Clinical Assessment of Disease Activity in Systemic Lupus Erythematosus. Arthritis Rheum (1989) 32(9):1107–18. doi: 10.1002/anr.1780320909
18. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A Disease Activity Index for Lupus Patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum (1992) 35(6):630–40. doi: 10.1002/art.1780350606
19. Group KW. Kdigo 2012 Clinical Practice Guideline for Acute Kidney Injury. Kidney Int (2012) 3(Suppl.):1–141. doi: 10.1038/kisup.2012.7
20. Group KCW. Kdigo 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int (2013) 3(Suppl.):1–150. doi: 10.1038/kisup.2012.65
21. Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. Kidney Int (2004) 65(2):521–30. doi: 10.1111/j.1523-1755.2004.00443.x
22. Austin HA 3rd, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse Proliferative Lupus Nephritis: Identification of Specific Pathologic Features Affecting Renal Outcome. Kidney Int (1984) 25(4):689–95. doi: 10.1038/ki.1984.75
23. Appel GB, Pirani CL, D’Agati V. Renal Vascular Complications of Systemic Lupus Erythematosus. J Am Soc Nephrol: JASN (1994) 4(8):1499–515. doi: 10.1681/ASN.V481499
24. Wu LH, Yu F, Tan Y, Qu Z, Chen MH, Wang SX, et al. Inclusion of Renal Vascular Lesions in the 2003 ISN/RPS System for Classifying Lupus Nephritis Improves Renal Outcome Predictions. Kidney Int (2013) 83(4):715–23. doi: 10.1038/ki.2012.409
25. Batal I, Girnita A, Zeevi A, Saab BA, Stockhausen S, Shapiro R, et al. Clinical Significance of the Distribution of C4d Deposits in Different Anatomic Compartments of the Allograft Kidney. Modern Pathol (2008) 21(12):1490–8. doi: 10.1038/modpathol.2008.152
26. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 Classification of Renal Allograft Pathology: Updates and Future Directions. Am J Transpl: Off J Am Soc Transplant Am Soc Transplant Surgeons (2008) 8(4):753–60. doi: 10.1111/j.1600-6143.2008.02159.x
27. Laskin BL, Maisel J, Goebel J, Yin HJ, Luo G, Khoury JC, et al. Renal Arteriolar C4d Deposition: A Novel Characteristic of Hematopoietic Stem Cell Transplantation-Associated Thrombotic Microangiopathy. Transplantation (2013) 96(2):217–23. doi: 10.1097/TP.0b013e31829807aa
28. Shen Y, Chen XW, Sun CY, Dai M, Yan YC, Yang CD. Association Between Anti-Beta2 Glycoprotein I Antibodies and Renal Glomerular C4d Deposition in Lupus Nephritis Patients With Glomerular Microthrombosis: A Prospective Study of 155 Cases. Lupus (2010) 19(10):1195–203. doi: 10.1177/0961203310368409
29. Bomback AS, Markowitz GS, Appel GB. Complement-Mediated Glomerular Diseases: A Tale of 3 Pathways. Kidney Int Rep (2016) 1(3):148–55. doi: 10.1016/j.ekir.2016.06.005
30. Walport MJ. Complement. First of Two Parts. New Engl J Med (2001) 344(14):1058–66. doi: 10.1056/NEJM200104053441406
31. Walport MJ. Complement. Second of Two Parts. New Engl J Med (2001) 344(15):1140–4. doi: 10.1056/NEJM200104123441506
32. Cohen D, Colvin RB, Daha MR, Drachenberg CB, Haas M, Nickeleit V, et al. Pros and Cons for C4d as a Biomarker. Kidney Int (2012) 81(7):628–39. doi: 10.1038/ki.2011.497
33. Martin M, Trattner R, Nilsson SC, Björk A, Zickert A, Blom AM, et al. Plasma C4d Correlates With C4d Deposition in Kidneys and With Treatment Response in Lupus Nephritis Patients. Front Immunol (2020) 11:582737. doi: 10.3389/fimmu.2020.582737
34. Sahin OZ, Gurses S, Tasli F, Yavas H, Ersoy R, Uzum A, et al. Glomerular C4d Staining Can Be An Indicator of Disease Activity in Lupus Nephritis. Renal Failure (2013) 35(2):222–5. doi: 10.3109/0886022X.2012.743916
35. Colvin RB. Antibody-Mediated Renal Allograft Rejection: Diagnosis and Pathogenesis. J Am Soc Nephrol: JASN (2007) 18(4):1046–56. doi: 10.1681/ASN.2007010073
36. Wang H, Xu J, Zhang X, Ren YL, Cheng M, Guo ZL, et al. Tubular Basement Membrane Immune Complex Deposition Is Associated With Activity and Progression of Lupus Nephritis: A Large Multicenter Chinese Study. Lupus (2018) 27(4):545–55. doi: 10.1177/0961203317732407
37. Chua JS, Baelde HJ, Zandbergen M, Wilhelmus S, van Es LA, de Fijter JW, et al. Complement Factor C4d Is a Common Denominator in Thrombotic Microangiopathy. J Am Soc Nephrol: JASN (2015) 26(9):2239–47. doi: 10.1681/ASN.2014050429
38. Song D, Wu LH, Wang FM, Yang XW, Zhu D, Chen M, et al. The Spectrum of Renal Thrombotic Microangiopathy in Lupus Nephritis. Arthritis Res Ther (2013) 15(1):R12. doi: 10.1186/ar4142
39. Mejia-Vilet JM, Gómez-Ruiz IA, Cruz C, Méndez-Pérez RA, Comunidad-Bonilla RA, Uribe-Uribe NO, et al. Alternative Complement Pathway Activation in Thrombotic Microangiopathy Associated With Lupus Nephritis. Clin Rheumatol (2020) 40(6):2233–42. doi: 10.1007/s10067-020-05499-1
Keywords: C4d, lupus nephritis, renal microvascular lesions, complement, prognosis
Citation: Ding Y, Yu X, Wu L, Tan Y, Qu Z and Yu F (2021) The Spectrum of C4d Deposition in Renal Biopsies of Lupus Nephritis Patients. Front. Immunol. 12:654652. doi: 10.3389/fimmu.2021.654652
Received: 17 January 2021; Accepted: 08 June 2021;
Published: 01 July 2021.
Edited by:
Vladimir Tesar, Charles University, CzechiaReviewed by:
Sergey Moiseev, I.M. Sechenov First Moscow State Medical University, RussiaJuan M. Mejia-Vilet, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico
Iva Gunnarsson, Karolinska Institutet (KI), Sweden
Copyright © 2021 Ding, Yu, Wu, Tan, Qu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Qu, amFuZXF1ODJAc2luYS5jb20=
†These authors have contributed equally to this work
 Ying Ding
Ying Ding Xiaojuan Yu
Xiaojuan Yu Lihua Wu
Lihua Wu Ying Tan
Ying Tan Zhen Qu2*
Zhen Qu2* Feng Yu
Feng Yu