- 1National Clinical Research Center for Hematologic Diseases, Jiangsu Institute of Hematology, Key Laboratory of Thrombosis and Hemostasis of Ministry of Health, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Institute of Blood and Marrow Transplantation, Collaborative Innovation Center of Hematology, Soochow University, Suzhou, China
- 3Department of Hematology, Soochow Hopes Hematonosis Hospital, Suzhou, China
- 4Soochow Yongding Hospital, Department of Affiliated Renji Hospital of Shanghai Jiao Tong University of Medicine, Suzhou, China
Graft-versus-host disease (GVHD), especially steroid-refractory GVHD, remains a life-threatening complication after hematopoietic stem cell transplantation (HSCT). The effect of the JAK1/2 kinase inhibitor ruxolitinib on treating steroid-refractory acute GVHD has been verified by the REACH1/2 study; however, its safety and efficacy in patients with steroid-refractory chronic GVHD (SR-cGVHD) remain unclear. In this retrospective study, 70 patients received ruxolitinib as a salvage therapy for SR-cGVHD. Twenty-four weeks after ruxolitinib treatment, the overall response rate (ORR) was 74.3% (52/70), including 34 patients who achieved complete remission (CR) and 18 who achieved partial remission (PR). The main adverse event was cytopenia, which occurred in 51.4% (36/70) of patients. After ruxolitinib treatment, the percentage of CD4 cells increased from 18.20% to 23.22% (P<0.001), while the percentages of NK (CD16+CD56+) cells and regulatory T cells (CD4+CD127 ± CD25+) decreased (P<0.001, P<0.001). Among the B cell subsets, the proportion of total B cells approximately tripled from 3.69% to 11.16% (P<0.001). Moreover, we observed a significant increase in IL-10 levels after ruxolitinib treatment (P=0.025) and a remarkable decrease in levels of suppression of tumorigenicity 2 (ST2) from 229.90 ng/ml to 72.65 ng/ml. The median follow-up after the initiation of ruxolitinib treatment was 401 (6-1076) days. The estimated one-year overall survival rate of the whole group was 66.0% (54.4–77.6%, 95% CI), and the one-year overall survival rate of patients with mild and moderate cGVHD was 69.6% (57.4–81.8%, 95% CI), which was better than that of patients with severe cGVHD (31.3%, 0.0–66.2%, 95% CI) (P=0.002). Patients who achieved a CR and PR achieved better survival outcomes (84.5%, 73.9–95.1%, 95% CI) than those who showed NR to ruxolitinib treatments (16.7%, 0–34.3%, 95% CI) (P<0.001). At the final follow-up, cGVHD relapse occurred in six patients after they reduced or continued their ruxolitinib doses. Collectively, our results suggest that ruxolitinib is potentially a safe and effective treatment for SR-cGVHD.
Introduction
Hematopoietic stem cell transplantation (HSCT) has been one of the most important therapies for hematological malignancies. However, graft-versus-host disease (GVHD) remains an unremovable barrier, leading to late morbidity and mortality (1). Corticosteroids are the first-line treatment for GVHD. Unfortunately, more than 50% of patients with chronic GVHD (cGVHD) fail to achieve remission (2). Despite various clinical trials, no global consensus has been reached regarding second-line therapy for cGVHD (3).
Ruxolitinib, an oral JAK1/2 kinase inhibitor, was approved for intermediate-or high-risk myelofibrosis in 2011 (4) and for polycythemia vera with an inadequate response to or intolerance to hydroxyurea in 2014 (5). In addition, the JAK/STAT signaling pathway plays an important role in immune cell activation and tissue inflammation during GVHD (6, 7). Researchers have already confirmed the effect of ruxolitinib, which reduces the incidence and severity of aGVHD while preserving graft-versus-leukemia effects in preclinical models (8–10). Afterwards, ruxolitinib was subsequently reported to have shown encouraging outcomes in curing patients with aGVHD (11–14). On May 24, 2019, ruxolitinib was approved by the Food and Drug Administration (FDA) as a treatment for steroid-refractory aGVHD (SR-aGVHD) in adult and pediatric patients aged 12 years and older (15).
In 2015, Zeiser et al. first reported that ruxolitinib produced encouraging results in cGVHD therapy (16). In 2020, Zeiser et al. reported that ruxolitinib showed superior efficacy to the best available therapy (BAT) in a phase 3 trial of patients with SR-cGVHD. However, no large-scale study has focused on the efficiency and toxicity of ruxolitinib in the treatment of cGVHD among Chinese people. Here, we report a single-center retrospective study of 70 patients who received ruxolitinib as a salvage therapy for steroid-refractory cGVHD (SR-cGVHD) in our center between March 2017 and December 2019 to evaluate the safety and efficacy of ruxolitinib after HSCT.
Methods
Study Subjects and Data Collection
In this retrospective study, data from 70 patients who received HSCT between September 2009 and September 2019 and developed SR-cGVHD between March 2017 and December 2019 at the First Affiliated Hospital of Soochow University were collected for analysis. This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the First Affiliated Hospital of Soochow University.
Inclusion and Exclusion Criteria
Patients who underwent HSCT and developed SR-cGVHD at the First Affiliated Hospital of Soochow University were included in the study. When devising inclusion and exclusion criteria, the REACH3 study was used as a reference. Inclusion criteria were as follows: 1) aged > 12 years; 2) complete hematopoietic reconstitution (absolute neutrophil counts > 1.0*109/L and platelet counts > 25*109/L) after HSCT; and 3) a diagnosis of SR-cGVHD according to the NIH criteria (17), including no response to a minimum of 1 mg/kg/day of prednisone therapy after 1 week, as well as disease persistence without improvement after treatment with prednisone at > 0.5 mg/kg/day or 1 mg/kg/every other day for at least 4 weeks or an increase to a prednisolone dose to > 0.25 mg/kg/day after 2 unsuccessful attempts to taper the dose. The exclusion criteria were as follows: 1) relapse of underlying disease before the use of ruxolitinib for treatment, 2) uncontrolled infections or severe organ damage not related to cGVHD, and 3) enrollment in other clinical studies of cGVHD treatments at the start of the research.
Conditioning Regimens for HSCT
The conditioning regimen for patients diagnosed with aplastic anemia (AA) was the FCA-based conditioning regimen, including IV fludarabine at 30 mg/m2/d on days -9 to -6, IV cyclophosphamide (CTX) at 50 mg/m2/d on days -5 to -2 and IV anti-thymocyte globulin (ATG) at 3.0 mg/kg2/d on days -5 to -2. Other patients who received HLA-matched sibling, unrelated or haploidentical transplantation were administered a Bu/Cy-based regimen consisting of oral semustine at 250 mg/m2/d on day -10, IV cytarabine at 4 g/m2/d on days -9 to -8, IV busulfan at 4 mg/kg/d from day -7 to day -5, and IV CTX at 1.8 g/m2/d from days -4 to -3.
GVHD Prophylaxis
Patients who underwent HLA-matched sibling transplantation received a GVHD prophylaxis strategy consisting of cyclosporin A (CsA) and methotrexate (MTX). The GVHD prophylaxis strategy for unrelated or haploidentical transplantation patients consisted of CsA, MTX, mycophenolate mofetil (MMF) and ATG or ALG. CsA was administered at a dose of 3 mg/kg/day by continuous infusion over 24 h from day -10 until patients were able to switch to the oral formulation, with a target blood concentration ranging from 200 to 300 ng/ml. MTX was administered intravenously at a dose of 15 mg/m2 on day +1 and 10 mg/m2 on days +3, days +6 and days +11. MMF was administered at an oral dose of 250 mg twice daily from day -10 until day +30. ATG/ALG was administered intravenously at a dose of 2.5 mg/kg/d from day -5 to day -2.
Clinical Definitions
cGVHD was diagnosed and graded according to the 2014 National Institute of Health (NIH) criteria (17). We assessed the treatment efficacy 24 weeks after the initiation of ruxolitinib therapy. Treatment responses to ruxolitinib were defined according to a previous study (16). The overall response rate (ORR) was defined as the percentage of patients assessed as achieving a complete response (CR) or partial response (PR). CR was defined as the absence of any manifestation related to cGVHD, and PR was defined as improvement in at least one specific target organ without deterioration in any other organ according to the NIH consensus (18). Events for failure-free survival (FFS) included relapse or recurrence of underlying disease or death due to underlying disease, nonrelapse mortality (NRM) and addition or initiation of another systemic therapy for cGVHD. Disease relapse was defined as morphological or cytogenetic evidence of disease with pretransplantation characteristics or morphological evidence without pretransplantation characteristics. NRM included mortality of patients who did not die due to the progression of underlying diseases.
Laboratory Studies and Analysis of Lymphocyte Subsets
Blood samples were collected from all patients 1-3 months before and after ruxolitinib treatments, at least once per time window, for the detection of different lymphocyte subsets using flow cytometry. Blood samples were collected in EDTA anticoagulant tubes and processed within an hour for multiparameter flow cytometry analyses. Phenotyping of T cells, B cells, NK cells and other cell types was performed. Samples were stained with the following antibodies: anti-CD3, anti-CD4, anti-CD8, anti-CD19, anti-CD16, anti-CD56, anti-CD69, anti-CD25, anti-CD127, anti-CD27 and Ig-D. CD19+CD3- cells were defined as total B cells, CD19+CD27-IgD+ cells were defined as naive B cells, CD19+CD27+IgD+ cells were defined as marginal zone B cells and CD19+CD27+IgD- were defined as classical traditional B cells.
Safety and Adverse Events
Safety was assessed by monitoring the occurrence, duration, and severity of adverse events. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events, version 4.03 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf).
Statistical Analysis
Our results were analyzed using SPSS 22.0 software. Normally distributed data were analyzed with Student’s t test, and nonparametric comparisons of two means were performed using the Mann-Whitney U test or the chi-square test. In the risk factor analysis, a logistic regression model was used. Time to CR, PR, NR and overall survival (OS) were defined as the time from ruxolitinib treatment to the event. Spearman’s rank correlation analysis was used. OS was analyzed using the Kaplan–Meier methodology. Comparisons were performed using the log-rank test. Cumulative incidence analysis was used to assess the incidence of relapse and NRM. A two-tailed P < 0.05 was considered statistically significant.
Results
Clinical Characteristics
A cohort of 70 patients were enrolled in this study. All patients received HSCT between September 2009 and September 2019 and developed cGVHD between March 2017 and December 2019. The detailed information is outlined in Table 1. The median age of the patients was 35 years (range 13-63 years). Acute myelogenous leukemia (AML) and acute lymphoblastic leukemia (ALL) were the most common underlying diseases. Matched donor transplantation was performed on 29 patients including 27 patients with related donor and 2 patients with unrelated donor, and haploidentical donor transplantation was performed on 41 patients. In this study, 32 patients received grafts of peripheral blood stem cells alone, and others received grafts combining bone marrow and peripheral blood stem cells. The median counts of transplanted mononuclear cells and CD34+ cells were 11.4*10^8/kg (range 3.43-29.96) and 3.80*10^6/kg (range 2.00-21.22), respectively. After HSCT, the median times of neutrophil and platelet reconstitution were 12 (range 10-23) days and 17 (range 8-80) days, respectively. The most commonly occurring complication after transplantation was bacterial infections, followed by hemorrhagic cystitis and virus infections. Forty-two patients had previously experienced acute GVHD, and 4 of them had been treated with ruxolitinib.
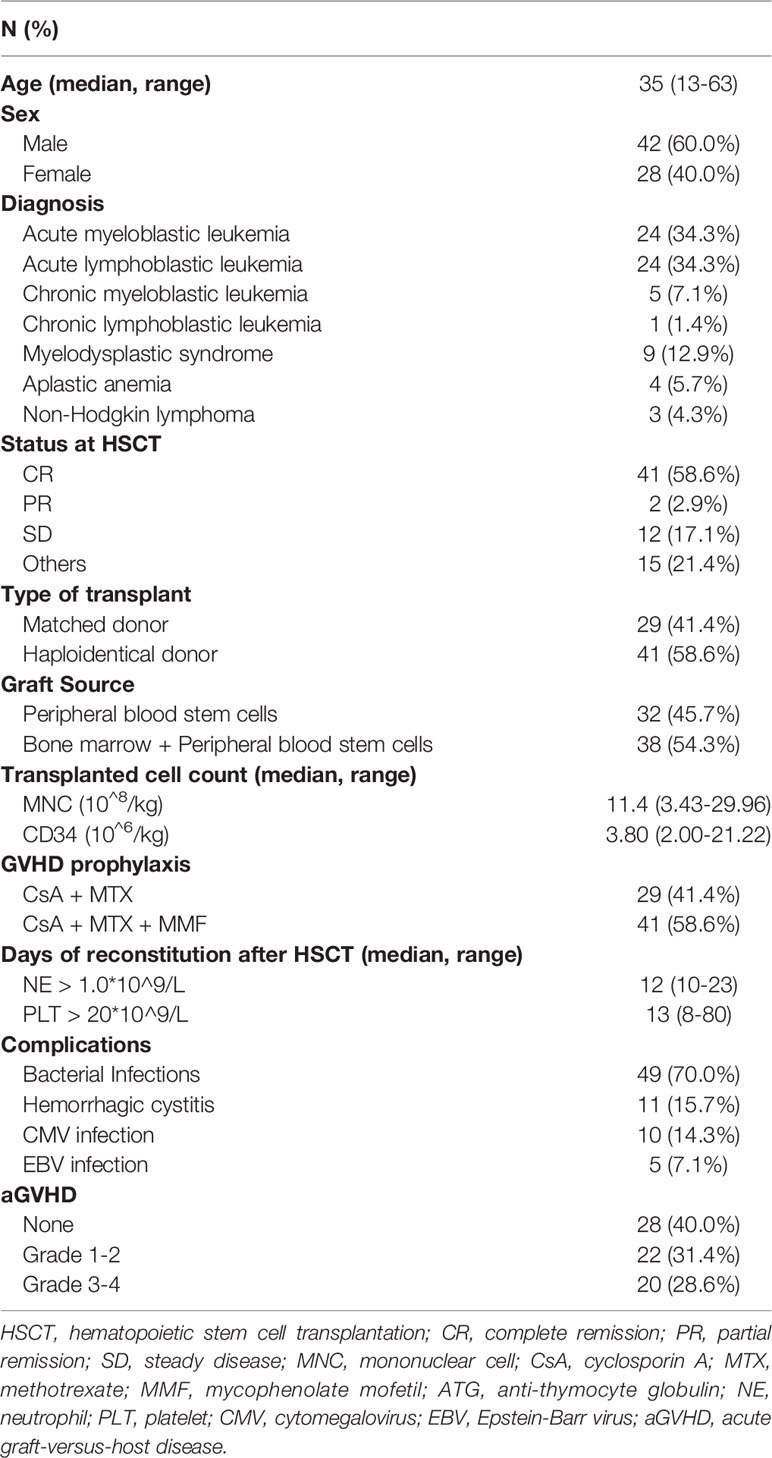
Table 1 Clinical characteristics of patients with steroid-refractory chronic graft-versus-host disease.
cGVHD Grade and Organ Classification
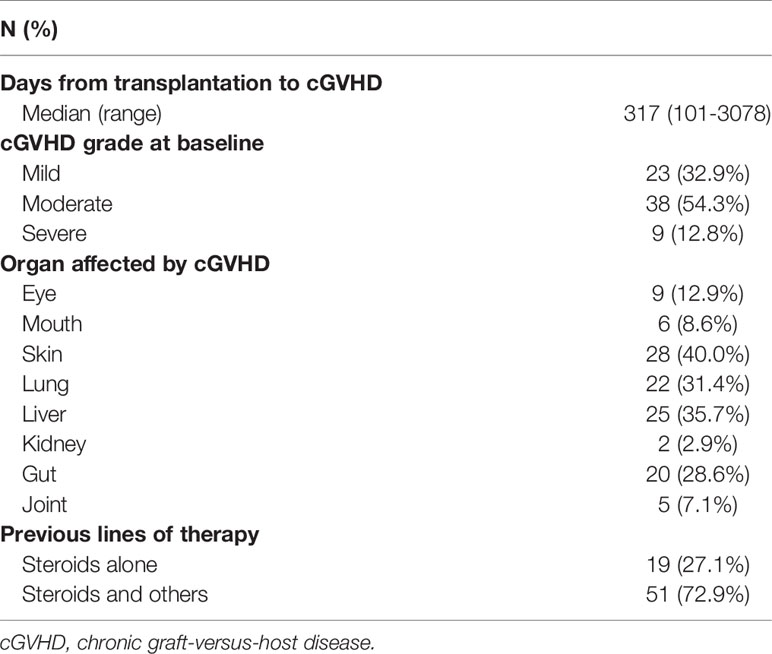
The median time of cGVHD occurrence after HSCT was 317 days (range 101-3078). Twenty-three patients (32.9%) had mild cGVHD, 38 (54.3%) had moderate cGVHD, and 9 (12.8%) had severe cGVHD. Multiple organs were involved in 33 (47.1%) patients. By analyzing the targeted organs, as shown in Table 2, we found that the most commonly involved organ was the skin, which was affected in 28 (40.0%) patients, and the skin had the highest percentage of severe cGVHD (39.3%, 11/28). Lung, liver and gut cGVHD occurred less frequently than skin cGVHD, and severe symptoms occurred in 27.3% (6/22), 32.0% (8/25) and 30.0% (6/20) of patients, respectively. Eye cGVHD occurred in only 9 patients, and it was graded as mild or moderate. Kidney and joint cGVHD were very rarely observed in this study. In addition, skin cGVHD mostly occurred in the haploidentical HSCT group (21/41, 51.2%), while lung cGVHD was mostly common in the matched HSCT group (13/29, 44.8%). For patients who had previously been diagnosed with aGVHD, 14.3% (6/42) were graded into severe cGVHD, while the percentage of patients who had not experienced aGVHD was only 10.7% (3/28) (P=0.048).
Treatment Efficacy
All patients received ruxolitinib (10-20 mg/d) as salvage therapy for cGVHD. Response rates were evaluated 24 weeks after ruxolitinib initiation. As shown in Figure 1A, after 24 weeks, the ORR to ruxolitinib therapy in patients with SR-cGVHD was 74.3% (52/70), including 34 patients with a CR (48.6%) and 18 with a PR (25.7%). Except for kidney and joint cGVHD cases that were too few to be analyzed, the mouth was the organ with the best response at 83.3% ORR, and the skin was the organ that achieved the highest CR of 60.7%. The ORR in patients with liver cGVHD was the lowest at only 64.0%. For patients diagnosed with different severity grades, we found that patients with severe cGVHD showed a worse ORR than patients with mild cGVHD (44.4% vs 82.6%, P=0.034) or moderate cGVHD (44.4% vs 76.3% P=0.063) (Figure 1B). After 24 weeks of treatment, we reevaluated the cGVHD severity in every patient and discovered significant reductions in the grades of cGVHD at baseline and after 24 weeks of therapy in most organs (Figure 1C). Next, we compared the days from ruxolitinib initiation to response among different organs, and the median time for patients with liver cGVHD to achieve remission was longer than that of other patients (125 days vs 49 days, P=0.019) (Figure 1D).
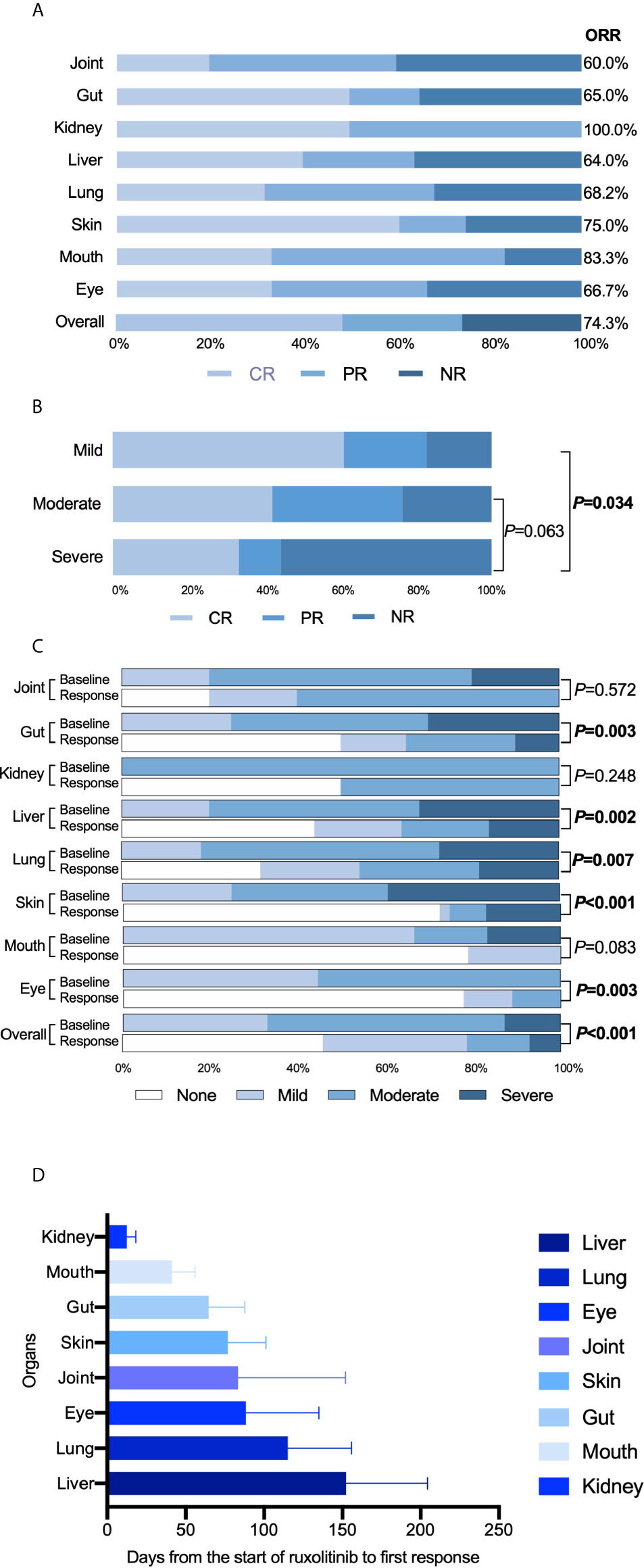
Figure 1 Treatment efficacy of ruxolitinib. (A) Response rates of different organs. (B) Comparison of treatment efficacy among patients with mild, moderate and severe cGVHD. (C) Comparison of the cGVHD grades of different organs before and 24 weeks after ruxolitinib treatments. (D) Time, in days, from the start of ruxolitinib administration to the response of different organs.
Steroid and Other Combination Treatments
At the initiation of ruxolitinib treatment, all patients were receiving steroid treatments. The median dose of steroid was 1mg/kg/d (rang 0.5-2). After 4 weeks of ruxolitinib treatment, 16 patients have stopped steroid treatments and 8 patients were capable to reduce their steroid doses owing to improved symptoms. 24 weeks after ruxolitinib treatments, 18 patients have been dead, 38 patients were finally able to withdraw steroid treatments and 14 patients were still with steroids treatments with median dose of 1mg/kg/d (rang 0.5-2). In these 14 patients, 9 patients showed no response to ruxolitinib treatments and 5 patients were steroid dependent.
Besides steroid treatments, some immunosuppressor treatments were also involved. In total, 40 patients were receiving different immunosuppressor treatments at the start of ruxolitinib treatments, including tacrolimus (TAC) in 21 patients, cyclosporin A (CsA) in 12 patients and mycophenolate mofetil (MMF) in 7 patients. After 24 weeks treatments, immunosuppressors were discontinued in 31 patients and 2 patients were still receiving TAC for treatments.
Adverse Events
Cytopenia was the most common adverse event occurring after ruxolitinib treatments (36/70, 51.4%). Anemia was the most common form, and thrombocytopenia was the second most common form. However, severe thrombocytopenia (grade III or IV) was observed in 15 of 28 patients, while severe anemia (grade III or IV) was observed only in 8 of 29 patients. Cytomegalovirus (CMV) reactivation occurred in 8 patients, while Epstein-Barr virus (EBV) and herpes infections occurred in 2 patients (Table 3). Viral reactivation was quickly controlled by antiviral therapy, and no other complications were observed.

Table 3 Adverse effects of ruxolitinib treatment on patients with steroid-refractory chronic graft- versus-host disease.
Immune Function
We analyzed different lymphocyte subsets during the 3 months before and after ruxolitinib treatments. The median date of the collected sample before and after ruxolitinb treatments were 54 days (range 28-88) and 63 days (range 34-94) respectively. A correlation analysis between age, lymphocyte subsets, and cytokines was performed to exclude the effect of age on different lymphocyte subsets and cytokine levels, and only naïve B cells had a negative correlation with age (Supplementary Table S1). CD4 lymphocytes were increased after treatment from 18.20% to 23.22% (P<0.001). The same trend was observed in the DP cell (CD4+CD8+) group, which increased from 0.50% to 0.68% (P=0.026). The numbers of both regulatory T cells (CD4+CD127 ± CD25+) and NK cells (CD16+CD56+) decreased by approximately half after ruxolitinib treatment (P<0.001 for both) (Figure 2A). By analyzing the B cells of some patients, we made the novel discovery that the proportion of total B cells among lymphocytes nearly tripled from 3.69% to 11.16% (P<0.001). In a detailed analysis of various B cell subsets, no significant differences were observed among naïve B cells, marginal zone B cells (MZ B) and classical traditional B cells (Figure 2B).
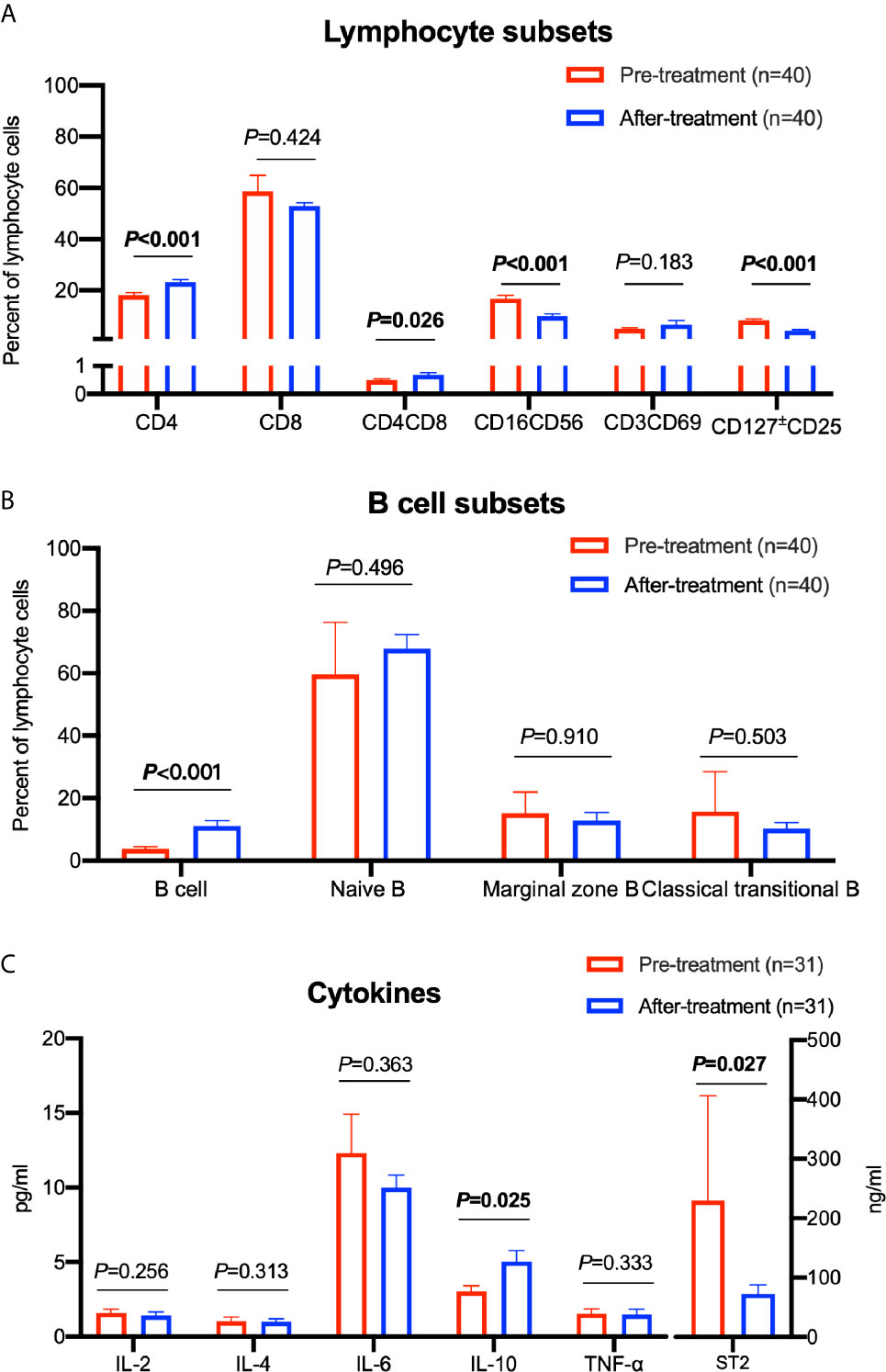
Figure 2 Comparison of different lymphocyte subsets and cytokine levels before and after treatment with ruxolitinib. (A) Comparison of different lymphocyte cell subsets. (B) Comparison of different B cell subsets. (C) Comparison of different cytokine levels. Paired sample t test was used for the analysis. The numbers of patients are indicated in each graph.
In addition, we examined the levels of inflammatory cytokines in patients during treatment. We observed a significant increase in IL-10 levels from 3.02 pg/ml to 5.04 pg/ml (P=0.025). Moreover, we detected decreased levels of suppression of tumorigenicity 2 (ST2), a definite predictor of aGVHD, decreased by over 66% from 229.90 ng/ml to 72.65 ng/ml after ruxolitinib treatment (P=0.027) (Figure 2C).
For a more detailed analysis, we compared the variations among the skin, liver, lung and gut. In these four organs, the trends of variation in different cell subsets were basically the same (Supplementary Figure S1). Regarding cytokines, patients with skin cGVHD presented a significant decrease in IL-6 levels (P=0.008) and an increase in IL-10 levels (P=0.014) after ruxolitinib treatment. However, significant differences were not observed among patients with liver, lung and gut cGVHD (Supplementary Figure S1).
Long-Term Outcomes
The median follow-up time of this study was 401 days (range 6-1076 days) after the initiation of ruxolitinib. The one-year estimated survival rate of the whole group was 66.0% (54.4–77.6%, 95% CI) (Figure 3A). The FFS estimate of the study at one year was 60.4% (48.2–72.6%, 95% CI) (Figure 3B). At the one-year follow-up, the estimated survival rate of patients with mild and moderate cGVHD was 69.6% (57.4–81.8%, 95% CI), which was better than that of patients with severe cGVHD (31.3%, 0.0–66.2%, 95% CI) (P=0.002) (Figure 3C). Patients who achieved CR and PR achieved better survival outcomes (84.5%, 73.9–95.1%, 95% CI) than those who showed NR to ruxolitinib treatments (16.7%, 0–34.3%, 95% CI) (P<0.001) (Figure 3D). cGVHD relapse occurred in six patients after decreases in the ruxolitinib dose or discontinuation, among which 3 patients responded to the restart of ruxolitinib therapy and achieved a response later, while the others died from cGVHD progression.
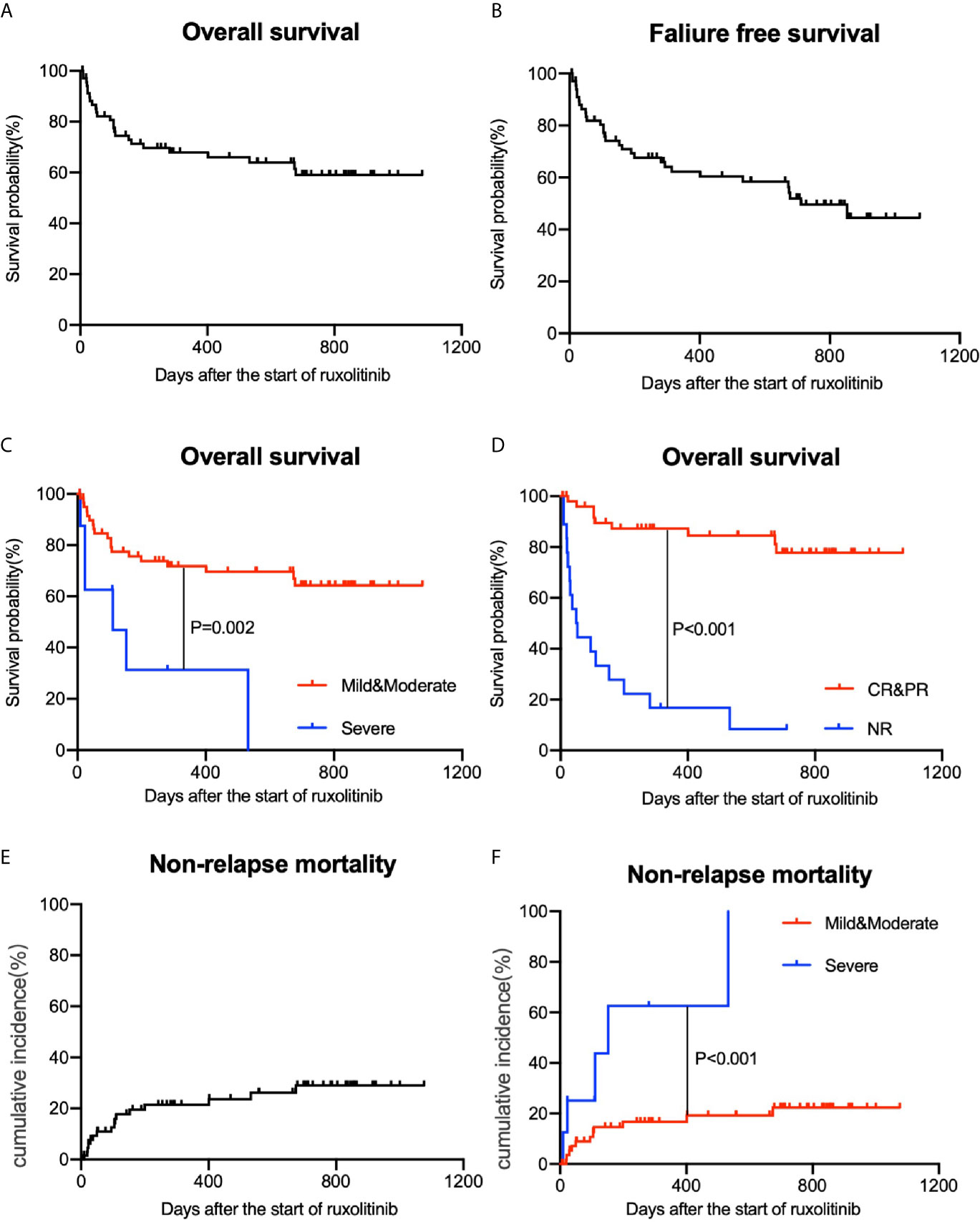
Figure 3 Overall survival (OS) and nonrelapse mortality (NRM) of patients with cGVHD. (A) OS of all patients. (B) Failure-free survival (FFS) of all patients. (C) Comparisons of OS among different grade groups. (D) Comparisons of OS among different treatment efficacy groups. (E) NRM of all patients. (F) Comparisons of NRM among different grade groups.
Twenty-five patients had died by the last follow-up date. Approximately half of the deaths were associated with underlying disease progression (11/25). Others included uncontrolled severe cGVHD (4/25), thrombotic microangiopathy (4/25) and complicated infections or multiple organ dysfunction syndrome (4/25). The cumulative incidence of NRM at the one-year follow-up was 20.0% (0.0-31.8%, 95% CI) (Figure 3E). For patients with mild and moderate cGVHD, the one-year NRM was only 16.7% (6.7-26.7%, 95% CI). However, for severe cGVHD patients, NRM at the one-year follow-up was up to 62.5% (22.7-100.0%, 95% CI) (Figure 3F).
Discussion
cGVHD remains one of the major hurdles to the success of HSCT. Although corticosteroid treatment has saved millions of lives of patients with cGVHD, no consensus on second-line treatments has been established for patients with SR-cGVHD. Ruxolitinib, a JAK1/2 kinase inhibitor, was first reported in a 2015 multicenter retrospective survey by Zeiser et al. (16) to have exerted satisfactory therapeutic effects on SR-cGVHD, supported by a favorable ORR of 85.4%. Among other commonly used second line cGVHD treatments, extracorporeal photopheresis (ECP) treatments were reported to achieve an ORR of 56.0% in a randomized controlled study (19) and an ORR of 67.0% in a retrospective multicenter study (20) of patients with cGVHD. In a large retrospective study including 269 patients with SR-cGVHD by Axt et al. (21), the ORRs of calcineurin inhibitors, MMF, mTOR inhibitors and ECP were all lower than 60.0%. Ibrutinib, a Bruton tyrosine kinase inhibitor, showed a 67.0% ORR for patients with cGVHD in a multicenter, open-label study (22). Some researchers recruited only patients with moderate and severe cGVHD into study, while many studies included patients with mild to severe cGVHD (20, 21, 23–25). In our single-center retrospective survey conducted among 70 patients diagnosed with mild, moderate and severe cGVHD, the median follow-up time was 401 (range 6-1076) days. Up to the final follow-up time, 74.3% of patients had responded to ruxolitinib, of whom 48.6% and 25.7% achieved CR and PR, respectively. A comparable ORR was reported in studies by Abedin et al. (26), Modi et al. (24) and Khoury et al. (27). Many investigators also evaluated the ORR of ruxolitinib at different time points. Abedin et al. (26) assessed the treatment efficacy at 28 days after the use of ruxolitinib; nevertheless, the ORR was only 63%. In the investigation of Modi et al. (24), treatment efficacies were evaluated at two time points. After six months of ruxolitinib therapy, the authors observed a CR in 10% of patients and PR in 37% of patients, while after 12 months, the results differed only slightly, with a CR observed in 13% of patients and PR in 30% of patients. In 2020, Zeiser et al. reported their findings from the phase 3 randomized REACH3 study of ruxolitinib compared with BAT in patients with SR-cGVHD. Ruxolitinib resulted in a significantly higher ORR at week 24 than BAT (49.7% vs 25.6%, P<0.0001), and it was the first agent to show superior efficacy to BAT in a phase 3 trial of patients with SR-cGVHD.
In the present study, mouth cGVHD had the highest ORR to ruxolitinib therapy, and skin cGVHD had the highest CR, a comparable result to the research conducted by Hurabielle et al. (28), who focused on sclerodermatous cGVHD independently. Moreover, in most studies, the mouth and skin were always the best-responding organs. The liver and lung were reported to be the organs with the worst response to ruxolitinib therapy (28–30). Additionally, the ORR in the gut, liver and lung was the lowest, and patients with liver and lung cGVHD had the longest response times in this study. Moreover, Moiseev et al. (29) and Streiler et al. (31) both reported that ruxolitinib significantly improved the respiratory function of patients with cGVHD, reduced steroid requirements and stabilized lung function in patients with bronchiolitis obliterans as a manifestation of cGVHD.
The safety of ruxolitinib treatment was also important. Hemocytopenia was the most common adverse event observed in this study of patients with cGVHD, consistent with previously reported data. In addition, Moiseev et al. (29) claimed that the severity of neutropenia and thrombocytopenia was affected by CMV reactivation (P=0.07), treatment with ganciclovir (P=0.0006), and a higher initial steroid dose (P=0.0017). González Vicent et al. (25) also determined that the incidence of neutropenia was related to the appearance of CMV and treatment with ganciclovir. In the majority of published articles, the incidence of CMV activation was reported to be greater than 10% (32, 33). However, in the present study, a low risk of reactivating CMV, EBV or herpes virus infections was observed, and reactivation was quickly controlled by antiviral therapies. Additionally, liver and kidney toxicities were uncommon in all published articles, including articles published by our group (24, 28, 29, 34). One possible reason for the low occurrence of adverse effects in this study might be the relatively low dose of ruxolitinib.
As reported before, the JAK1/2 inhibitor ruxolitinib influences the immune response after HSCT (6, 7, 10). In preclinical research, ruxolitinib has been reported to reverse dysregulated T helper cell responses and control autoimmunity resulting from signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations (35). Vicent et al. (25) discussed the variations in the immune system before and after patients with cGVHD received ruxolitinib treatments, in which ruxolitinib was associated with increased numbers of CD4+ T cells and B cells and decreased numbers of NK cells and CD4+ Tregs. Notably, we observed increased numbers of CD4+ and CD8+ DP cells after ruxolitinib treatments. DP cells are a well-described T cell developmental stage within the thymus; in patients with cGVHD, a higher percentage of DP cells indicates better thymus function and less GVHD damage (36, 37). B cells play an indispensable role in the occurrence and development of cGVHD (38, 39); however, few researchers have analyzed the changes in specific B cell subsets before and after ruxolitinib treatment. Studies from both McManigle (40) and Yehudai-Ofir (41) reported that CD27 is normally expressed on B cells and that CD27-positive B cells are proportionally increased in patients with cGVHD. In the present study, the percentage of CD27-negative naïve B cells increased, while the percentages of MZ B cells and classical traditional B cells, which were both CD27-positive, decreased after treatment. Among cytokines, we detected an increase in the levels of IL-10, a definite inhibitory mediator of GVHD (42), after ruxolitinib treatments. In further analyses, the level of the proinflammatory factor IL-6 was decreased in patients with skin cGVHD after ruxolitinib treatments, consistent with published data (43, 44). However, these variations were not observed in patients with liver, lung and gut cGVHD, whose ORRs were lower than patients with skin cGVHD.
Additionally, ST2 has been previously reported to be a specific indicator of aGVHD (45, 46). In 2015, Reichenbach et al. (47) analyzed animal GVHD models and reported that ST2 was upregulated on murine alloreactive T cells and that ST2 levels increased as experimental GVHD progressed. Compared with wild-type (WT) donor T cells, ST2−/− donor T cells displayed a marked reduction in GVHD lethality. In our study, ST2 expression also fluctuated with the severity of cGVHD.
Notably, the median follow-up time in our study was 401 (range 6-1076) days, the one-year estimated survival rate was 66.0% (54.4–77.6%, 95% CI), and the one-year estimated FFS rate was 60.4% (48.2–72.6%, 95% CI). In our study, patients with severe cGVHD experienced a significantly shorter OS and higher NRM than patients with mild and moderate diseases. The OS of patients with mild and moderate cGVHD was 69.6%, probably because approximately two-thirds of these patients had moderate cGVHD. Considering the relatively long follow-up time compared with the studies by Zeiser et al. (16) and Moiseev et al. (27), we propose that our study describes an encouraging survival benefit for patients with SR-cGVHD.
Several limitations also existed in our study. Besides the retrospective nature of this study, it was also difficult to properly account for the effects of concurrent immunosuppressive therapies including corticosteroids and calcineurin inhibitors on the clinical course of cGVHD in addition to the effect of ruxolitinib.
Interestingly, in addition to salvage therapy for SR-cGVHD, ruxolitinib showed excellent performance as a prophylactic agent for GVHD in place of calcineurin inhibitors. Kröger et al. (48) reported on 12 patients who used ruxolitinib during the peritransplantation period. The incidence of grade II–IV aGVHD on day +100 was only 8%, and no NRM was recorded. In the study designed by Zhao et al. (49), after the replacement of a calcineurin inhibitor with ruxolitinib once patients showed intolerance or contraindication to CsA or TAC, only two of ten patients developed aGVHD, and 3 patients developed cGVHD after tapering or stopping ruxolitinib. Moreover, in July 2020, Saraceni et al. (50) reported that patients with cGVHD who were diagnosed with severe coronavirus disease 2019 (COVID-19) were successfully treated with ruxolitinib.
Collectively, the results of this study support ruxolitinib as a safe and effective option as a second-line treatment for patients with SR-cGVHD, with a high ORR of 73.4% and impressive outcomes. Further multicenter studies enrolling a larger number of participants should be conducted in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by ethical committee of the First Affiliated Hospital of Soochow University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
DWu and YX designed the study. XM, QC, and ZL contributed to the collection of data. DWang analyzed the data. DWang, YL, and XL discussed and interpreted the results. DWang wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (81730003, 81870120, 81800176, and 82070187), the National Key R&D Program of China (2019YFC0840604, 2017YFA0104502, and 2017ZX09304021), the Natural Science Foundation of Jiangsu Province (BK20171205 and BK20180200), the Social Development of Jiangsu Provinces (BE2019655), the Jiangsu Province Key R&D Program (BE2019798), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the patients for participating in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.673636/full#supplementary-material
Supplementary Figure 1 | Comparison of different lymphocyte subsets and cytokine levels in different organs before and after treatment with ruxolitinib. (A) Comparison of different lymphocyte cell subsets in different organs. (B) Comparison of different B cell subsets in different organs. (C) Comparison of different cytokine levels in different organs. Paired sample t test was used for the analysis. The numbers of patients are indicated in each graph.
References
1. Socié G, Ritz J. Current Issues in Chronic Graft-Versus-Host Disease. Blood (2014) 124(3):374–84. doi: 10.1182/blood-2014-01-514752
2. Wolff D, Gerbitz A, Ayuk F, Kiani A, Hildebrandt GC, Vogelsang GB, et al. Consensus Conference on Clinical Practice in Chronic Graft-Versus-Host Disease (GVHD): First Line and Topical Treatment of Chronic GVHD. Biol Blood Marrow Transplant (2010) 16(12):1611–28. doi: 10.1016/j.bbmt.2010.06.015
3. Wolff D, Schleuning M, von Harsdorf S, Bacher U, Gerbitz A, Stadler M, et al. Consensus Conference on Clinical Practice in Chronic GVHD: Second-Line Treatment of Chronic Graft-Versus-Host Disease. Biol Blood Marrow Transplant (2011) 17(1):1–17. doi: 10.1016/j.bbmt.2010.05.011
4. Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK Inhibition With Ruxolitinib Versus Best Available Therapy for Myelofibrosis. N Engl J Med (2012) 366(9):787–98. doi: 10.1056/NEJMoa1110556
5. Cingam S, Flatow-Trujillo L, Andritsos LA, Arana Yi C. Ruxolitinib In the Treatment of Polycythemia Vera: An Update on Health-Related Quality of Life and Patient-Reported Outcome. J Blood Med (2019) 10:381–90. doi: 10.2147/JBM.S177692
6. Elli EM, Baratè C, Mendicino F, Palandri F, Palumbo GA. Mechanisms Underlying the Anti-Inflammatory and Immunosuppressive Activity of Ruxolitini. Front Oncol (2019) 9:1186. doi: 10.3389/fonc.2019.01186
7. Heine A, Held SA, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, et al. The JAK-Inhibitor Ruxolitinib Impairs Dendritic Cell Function In Vitro and In Vivo. Blood (2013) 122(7):1192–202. doi: 10.1182/blood-2013-03-484642
8. Choi J, Cooper ML, Alahmari B, Ritchey J, Collins L, Holt M, et al. Pharmacologic Blockade of JAK1/JAK2 Reduces GvHD and Preserves the Graft-Versus-Leukemia Effect. PLoS One (2014) 9(10):e109799. doi: 10.1371/journal.pone.0109799
9. Carniti C, Gimondi S, Vendramin A, Recordati C, Confalonieri D, Bermema A, et al. Pharmacologic Inhibition of JAK1/JAK2 Signaling Reduces Experimental Murine Acute GVHD While Preserving GVT Effect. Clin Cancer Res (2015) 21(16):3740–9. doi: 10.1158/1078-0432.CCR-14-2758
10. Choi J, Ziga ED, Ritchey J, Collins L, Prior JL, Cooper ML, et al. Ifnγr Signaling Mediates Alloreactive T-Cell Trafficking and GVHD. Blood (2012) 120(19):4093–103. doi: 10.1182/blood-2012-01-403196.
11. Jagasia M, Perales MA, Schroeder MA, Ali H, Shah NN, Chen YB, et al. Ruxolitinib for the Treatment of Steroid-Refractory Acute GVHD (REACH1): A Multicenter, Open-Label Phase 2 Trial. Blood (2020) 135(20):1739–49. doi: 10.1182/blood.2020004823
12. Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-Versus-Host Diseas. N Engl J Med (2020) 382(19):1800–10. doi: 10.1056/NEJMoa1917635
13. von Bubnoff N, Ihorst G, Grishina O, Röthling N, Bertz H, Duyster J, et al. Ruxolitinib in GvHD (RIG) Study: A Multicenter, Randomized Phase 2 Trial to Determine the Response Rate of Ruxolitinib and Best Available Treatment (BAT) Versus BAT in Steroid-Refractory Acute Graft-Versus-Host Disease (Agvhd) (NCT02396628). BMC Cancer (2018) 18(1):1132. doi: 10.1186/s12885-018-5045-7
14. Khandelwal P, Teusink-Cross A, Davies SM, Nelson AS, Dandoy CE, El-Bietar J, et al. Ruxolitinib as Salvage Therapy in Steroid-Refractory Acute Graft-Versus-Host Disease in Pediatric Hematopoietic Stem Cell Transplant Patient. Biol Blood Marrow Transplant (2017) 23(7):1122–7. doi: 10.1016/j.bbmt.2017.03.029
15. Przepiorka D, Luo L, Subramaniam S, Qiu J, Gudi R, Cunningham LC, et al. FDA Approval Summary: Ruxolitinib for Treatment of Steroid-Refractory Acute Graft-Versus-Host Diseas. Oncologist (2020) 25(2):e328–e34. doi: 10.1634/theoncologist.2019-0627
16. Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in Corticosteroid-Refractory Graft-Versus-Host Disease After Allogeneic Stem Cell Transplantation: A Multicenter Survey. Leukemia (2015) 29(10):2062–8. doi: 10.1038/leu.2015.212
17. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant (2015) 21(3):389–401. doi: 10.1016/j.bbmt.2014.12.001
18. Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring Therapeutic Response in Chronic Graft-Versus-Host Disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biol Blood Marrow Transplant (2015) 21(6):984–99. doi: 10.1016/j.bbmt.2015.02.025
19. Jagasia M, Scheid C, Socié G, Ayuk FA, Tischer J, Donato ML, et al. Randomized Controlled Study of ECP With Methoxsalen as First-Line Treatment of Patients With Moderate to Severe cGVHD. Blood Adv (2019) 3(14):2218–29. doi: 10.1182/bloodadvances.2019000145
20. Oarbeascoa G, Lozano ML, Guerra LM, Amunarriz C, Saavedra CA, Garcia-Gala JM, et al. Retrospective Multicenter Study of Extracorporeal Photopheresis in Steroid-Refractory Acute and Chronic Graft-Versus-Host Diseas. Biol Blood Marrow Transplant (2020) 26(4):651–8. doi: 10.1016/j.bbmt.2019.12.769
21. Axt L, Naumann A, Toennies J, Haen SP, Vogel W, Schneidawind D, et al. Retrospective Single Center Analysis of Outcome, Risk Factors and Therapy in Steroid Refractory Graft-Versus-Host Disease After Allogeneic Hematopoietic Cell Transplantation. Bone Marrow Transplant (2019) 54(11):1805–14. doi: 10.1038/s41409-019-0544-y
22. Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for Chronic Graft-Versus-Host Disease After Failure of Prior Therapy. Blood (2017) 130(21):2243–50. doi: 10.1182/blood-2017-07-793786
23. Pidala JA, Hamilton BK, Martin PJ, Onstad L, Storer BE, Palmer J, et al. The Chronic Graft-Versus-Host Disease Failure-Free Survival (cGVHD-FFS) Inde. Biol Blood Marrow Transplant (2019) 25(12):2468–73. doi: 10.1016/j.bbmt.2019.07.040
24. Modi B, Hernandez-Henderson M, Yang D, Klein J, Dadwal S, Kopp E, et al. Ruxolitinib as Salvage Therapy for Chronic Graft-Versus-Host Diseas. Biol Blood Marrow Transplant (2019) 25(2):265–9. doi: 10.1016/j.bbmt.2018.09.003
25. González Vicent M, Molina B, González de Pablo J, Castillo A, Díaz MÁ. Ruxolitinib Treatment for Steroid Refractory Acute and Chronic Graft vs Host Disease in Children: Clinical and Immunological Results. Am J Hematol (2019) 94(3):319–26. doi: 10.1002/ajh.25376
26. Abedin S, McKenna E, Chhabra S, Pasquini M, Shah NN, Jerkins J, et al. Efficacy, Toxicity, and Infectious Complications in Ruxolitinib-Treated Patients With Corticosteroid-Refractory Graft-Versus-Host Disease After Hematopoietic Cell Transplantatio. Biol Blood Marrow Transplant (2019) 25(8):1689–94. doi: 10.1016/j.bbmt.2019.04.003
27. Khoury HJ, Langston AA, Kota VK, Wilkinson JA, Pusic I, Jillella A, et al. Ruxolitinib: A Steroid Sparing Agent in Chronic Graft-Versus-Host Disease. Bone Marrow Transplant (2018) 53(7):826–31. doi: 10.1038/s41409-017-0081-5
28. Hurabielle C, Sicre de Fontbrune F, Moins-Teisserenc H, Robin M, Jachiet M, Coman T, et al. Efficacy and Tolerance of Ruxolitinib in Refractory Sclerodermatous Chronic Graft-Versus-Host Disease. Br J Dermatol (2017) 177(5):e206–8. doi: 10.1111/bjd.15593
29. Moiseev IS, Morozova EV, Bykova TA, Paina OV, Smirnova AG, Dotsenko AA, et al. Long-Term Outcomes of Ruxolitinib Therapy in Steroid-Refractory Graft-Versus-Host Disease in Children and Adults. Bone Marrow Transplant (2020) 55(7):1379–87. doi: 10.1038/s41409-020-0834-4
30. Bondeelle L, Chevret S, Hurabielle C, Samy L, Goletto T, Costantini A, et al. Effect of Ruxolitinib on Lung Function After Allogeneic Stem Cell Transplantatio. Biol Blood Marrow Transplant (2020) 26(11):2115–20. doi: 10.1016/j.bbmt.2020.07.033
31. Streiler C, Shaikh F, Davis C, Abhyankar S, Brownback KR. Ruxolitinib is an Effective Steroid Sparing Agent in Bronchiolitis Obliterans Due to Chronic Graft-Versus-Host-Disease. Bone Marrow Transplant (2020) 55(6):1194–6. doi: 10.1038/s41409-019-0662-6
32. Prem S, Atenafu EG, Al-Shaibani Z, Loach D, Law A, Lam W, et al. Low Rates of Acute and Chronic GVHD With ATG and PTCy in Matched and Mismatched Unrelated Donor Peripheral Blood Stem Cell Transplants. Eur J Haematol (2019) 102(6):486–93. doi: 10.1111/ejh.13230
33. Ghobadi A, Milton DR, Gowda L, Rondon G, Chemaly RF, Hamdi A, et al. HLA-DP Mismatch and CMV Reactivation Increase Risk of aGVHD Independently in Recipients of Allogeneic Stem Cell Transplant. Curr Res Transl Med (2019) 67(2):51–5. doi: 10.1016/j.retram.2019.01.001
34. Escamilla Gómez V, García-Gutiérrez V, López Corral L, García Cadenas I, Pérez Martínez A, Márquez Malaver FJ, et al. Ruxolitinib in Refractory Acute and Chronic Graft-Versus-Host Disease: A Multicenter Survey Study. Bone Marrow Transplant (2020) 55(3):641–8. doi: 10.1038/s41409-019-0731-x
35. Weinacht KG, Charbonnier LM, Alroqi F, Plant A, Qiao Q, Wu H, et al. Ruxolitinib Reverses Dysregulated T Helper Cell Responses and Controls Autoimmunity Caused by a Novel Signal Transducer and Activator of Transcription 1 (STAT1) Gain-of-Function Mutation. J Allergy Clin Immunol (2017) 139(5):1629–40. doi: 10.1016/j.jaci.2016.11.022
36. Overgaard NH, Jung JW, Steptoe RJ, Wells JW. CD4+/CD8+ Double-Positive T Cells: More Than Just a Developmental Stage? J Leukoc Biol (2015) 97(1):31–8. doi: 10.1189/jlb.1RU0814-382
37. Wu T, Young JS, Johnston H, Wells JW. Thymic Damage, Impaired Negative Selection, and Development of Chronic Graft-Versus-Host Disease Caused by Donor CD4+ and CD8+ T Cells. J Immunol (2013) 191(1):488–99. doi: 10.4049/jimmunol.1300657
38. Rozmus J, Kariminia A, Abdossamadi S, Storer BE, Martin PJ, Lee SJ, et al. Comprehensive B Cell Phenotyping Profile for Chronic Graft-Versus-Host Disease Diagnosi. Biol Blood Marrow Transplant (2019) 25(3):451–8. doi: 10.1016/j.bbmt.2018.11.007
39. MacDonald KP, Hill GR, Blazar BR. Chronic Graft-Versus-Host Disease: Biological Insights From Preclinical and Clinical Studies. Blood (2017) 129(1):13–21. doi: 10.1182/blood-2016-06-686618
40. McManigle W, Youssef A. Sarantopoulos S. B Cells in Chronic Graft-Versus-Host Disease. Hum Immunol (2019) 80(6):393–9. doi: 10.1016/j.humimm.2019.03.003
41. Yehudai-Ofir D, Henig I, Zuckerman T. Aberrant B Cells, Autoimmunity and the Benefit of Targeting B Cells in Chronic Graft-Versus-Host Disease. Autoimmun Rev (2020) 19(4):102493. doi: 10.1016/j.autrev.2020.102493
42. Granero Farias M, Andrade Dos Santos C, de Mello Vicente B, Habigzang M, de Oliveira da Silva P, Emerim Lemos N, et al. The Effects of Gene Polymorphisms on Susceptibility to Acute GVHD and Survival of Allogeneic HSCT Recipients: IL-10 Gene Polymorphisms as a More Accessible Target to Predict Prognosis. Hum Immunol (2020) 81(1):18–25. doi: 10.1016/j.humimm.2019.12.002
43. Ambruzova Z, Mrazek F, Raida L, Jindra P, Vidan-Jeras B, Faber E, et al. Association of IL6 and CCL2 Gene Polymorphisms With the Outcome of Allogeneic Haematopoietic Stem Cell Transplantation. Bone Marrow Transplant (2009) 44(4):227–35. doi: 10.1038/bmt.2009.16
44. Choi SW, Braun T, Henig I, Gatza E, Magenau J, Parkin B, et al. Vorinostat Plus Tacrolimus/Methotrexate to Prevent GVHD After Myeloablative Conditioning, Unrelated Donor HCT. Blood (2017) 130(15):1760–67. doi: 10.1182/blood-2017-06-790469
45. Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, et al. ST2 as a Marker for Risk of Therapy-Resistant Graft-Versus-Host Disease and Death. N Engl J Med (2013) 369(6):529–39. doi: 10.1056/NEJMoa1213299
46. McDonald GB, Tabellini L, Storer BE, Lawler RL, Martin PJ, Hansen JA. Plasma Biomarkers of Acute GVHD and Nonrelapse Mortality: Predictive Value of Measurements Before GVHD Onset and Treatment. Blood (2015) 126(1):113–20. doi: 10.1182/blood-2015-03-636753
47. Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, et al. The IL-33/ST2 Axis Augments Effector T-Cell Responses During Acute GVHD. Blood (2015) 125(20):3183–92. doi: 10.1182/blood-2014-10-606830
48. Kröger N, Shahnaz Syed Abd Kadir S, Zabelina T, Badbaran A, Christopeit M, Ayuk F, et al. Peritransplantation Ruxolitinib Prevents Acute Graft-Versus-Host Disease in Patients With Myelofibrosis Undergoing Allogenic Stem Cell Transplantatio. Biol Blood Marrow Transplant (2018) 24(10):2152–56. doi: 10.1016/j.bbmt.2018.05.023.
49. Zhao Y, Shi J, Luo Y, Gao F, Tan Y, Lai X, et al. Calcineurin Inhibitors Replacement by Ruxolitinib as Graft-Versus-Host Disease Prophylaxis for Patients After Allogeneic Stem Cell Transplantatio. Biol Blood Marrow Transplant (2020) 26(5):e128–33. doi: 10.1016/j.bbmt.2020.01.012
Keywords: hematopoietic stem cell transplant, ruxolitinib, steroid-refractory chronic graft-versus-host disease, overall response rate, overall survival
Citation: Wang D, Liu Y, Lai X, Chen J, Cheng Q, Ma X, Lin Z, Wu D and Xu Y (2021) Efficiency and Toxicity of Ruxolitinib as a Salvage Treatment for Steroid-Refractory Chronic Graft-Versus-Host Disease. Front. Immunol. 12:673636. doi: 10.3389/fimmu.2021.673636
Received: 28 February 2021; Accepted: 21 June 2021;
Published: 30 June 2021.
Edited by:
Jaebok Choi, Washington University School of Medicine in St. Louis, United StatesReviewed by:
Robert Zeiser, University of Freiburg, GermanyJacob Rozmus, British Columbia Children’s Hospital, Canada
Copyright © 2021 Wang, Liu, Lai, Chen, Cheng, Ma, Lin, Wu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Xu, eWFuZ3h1QHN1ZGEuZWR1LmNu; Qiao Cheng, Y2hlbmdxaWFvNTU1MTExQDE2My5jb20=; Depei Wu, ZHJ3dWRlcGVpQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Dong Wang1,2†
Dong Wang1,2† Yang Xu
Yang Xu