- 1Department of Neurology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Neurology, The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 3School of the First Clinical Medical Sciences, Wenzhou Medical University, Wenzhou, China
- 4Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 5Department of Internal Medicine, The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
Background and Purpose: Blood eosinophil counts are thought to be associated with atherosclerosis in acute ischemic stroke (AIS) and AIS severity. We aimed to investigate 1): the temporal profile of eosinophil in AIS patients treated with recombinant tissue plasminogen activator (r-tPA); 2): The association between dynamic eosinophil and 3-month outcomes in different AIS etiologies; 3): incremental predictive ability of dynamic eosinophil adding to conventional model; and 4): the longitudinal change of neutrophil-to-lymphocyte ratio (NLR) and compared its prognostic value with eosinophils.
Methods: A total of 623 AIS patients with intravenous thrombolysis in two hospitals were included. Blood samples were obtained on admission, within 24 h after an intravenous thrombolysis and on the seventh day. A multivariate logistic regression model with restricted cubic spline was performed to explore the association between dynamic eosinophil and a 3-month poor outcome. C-statistic, net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were adopted to explore the incremental predictive ability.
Results: Percent change in eosinophil counts after intravenous thrombolysis was median −25.00% (IQR −68.25%–+14.29%). Decrease in eosinophil >75% after intravenous thrombolysis was associated with 2.585 times risk for poor outcome and 13.836 times risk for death. However, the association were weak for patients outside of cardioembolic stroke. Adding eosinophil changes to a conventional model improved the discriminatory ability of poor outcome (NRI = 53.3%; IDI = 2.2%) and death (NRI = 101.0%; IDI = 6.9%).
Conclusions: Dynamic decrease in eosinophil after intravenous thrombolysis predicts a 3-month poor outcome and death in AIS patients with r-tPA treatment and improved the predictive ability of conventional model. However, this result needs to be interpreted carefully in non-cardioembolic AIS patients.
Introduction
Stroke, is a major cause of disability and mortality, in which acute ischemic stroke (AIS) accounts for nearly 80% (1). A fast and timely re-establishment of blood flow by intravenous thrombolysis using recombinant tissue plasminogen activator (r-tPA) is now recommended for AIS patients within 4.5 h of stroke onset (2). Ischemic stroke disrupts the balance that exits under quiescent conditions between coagulation and immune axes in the brain, causes not only local inflammation of ischemic lesions but also a peripheral immune response (3–6). At the same time, the infusion of r-tPA can cause changes in the immune environment via affecting the dynamic profile of peripheral leukocyte (7, 8). These immune alterations strongly influence the prognosis of AIS.
Though only about 1–8% of leukocytes in peripheral blood are eosinophils, they play an important role in the vascular innate immune system and contribute directly to the coagulation and fibrinolysis system (9). A few studies have previously looked at the role of eosinophils in AIS. Lower eosinophil count was observed in patients with myocardial infarction (10). Increased admission eosinophil was independently associated with the presence of aortic arch plaques in AIS (11). Interestingly, higher values of admission eosinophil were associated with mild stroke (12), and lower the risk of developing hemorrhagic transformation after intravenous thrombolysis (13). Besides, AIS patients with eosinopenia on the second day admission may have high infection rates, large volume of cerebral infarction and poor outcome (14). Meaningful conclusions from these studies are limited due to variable time points for blood sample collection, different treatment methods for AIS and heterogeneous populations. Therefore, the purpose of this study is to 1): identify the temporal profile of eosinophil in AIS patients treated with r-tPA; 2): evaluate the association between dynamic eosinophil and 3-month outcomes in different AIS etiologies; 3): find the incremental predictive ability of dynamic eosinophil adding to conventional model; and 4): evaluate the longitudinal change of neutrophil-to-lymphocyte ratio (NLR): a widely used biomarker representing an inflammatory status (15, 16), in our cohort and compared its predictive value with eosinophils.
Materials and Methods
Participants
In this retrospective cohort study, all consecutive patients with a diagnosis of AIS and treated with Intravenous r-tPA within 4.5 h stroke onset in the Third Affiliated Hospital of Wenzhou Medical University (Center A) from January 2016 to December 2020 and in the First Affiliated Hospital of Wenzhou Medical University (Center B) from January 2019 to October 2020 have been recruited to this study. Patients with 1): a bridging therapy consisting of intravenous thrombolysis followed by endovascular therapy; 2): concurrent or recent infections; 3): immunology diseases; 4): severe hepatic or renal disease; 5): cancer; 6): asthma; and 7): miss baseline data and 3-month mRS scores were excluded. A total of 623 patients remained for analysis (Figure 1).
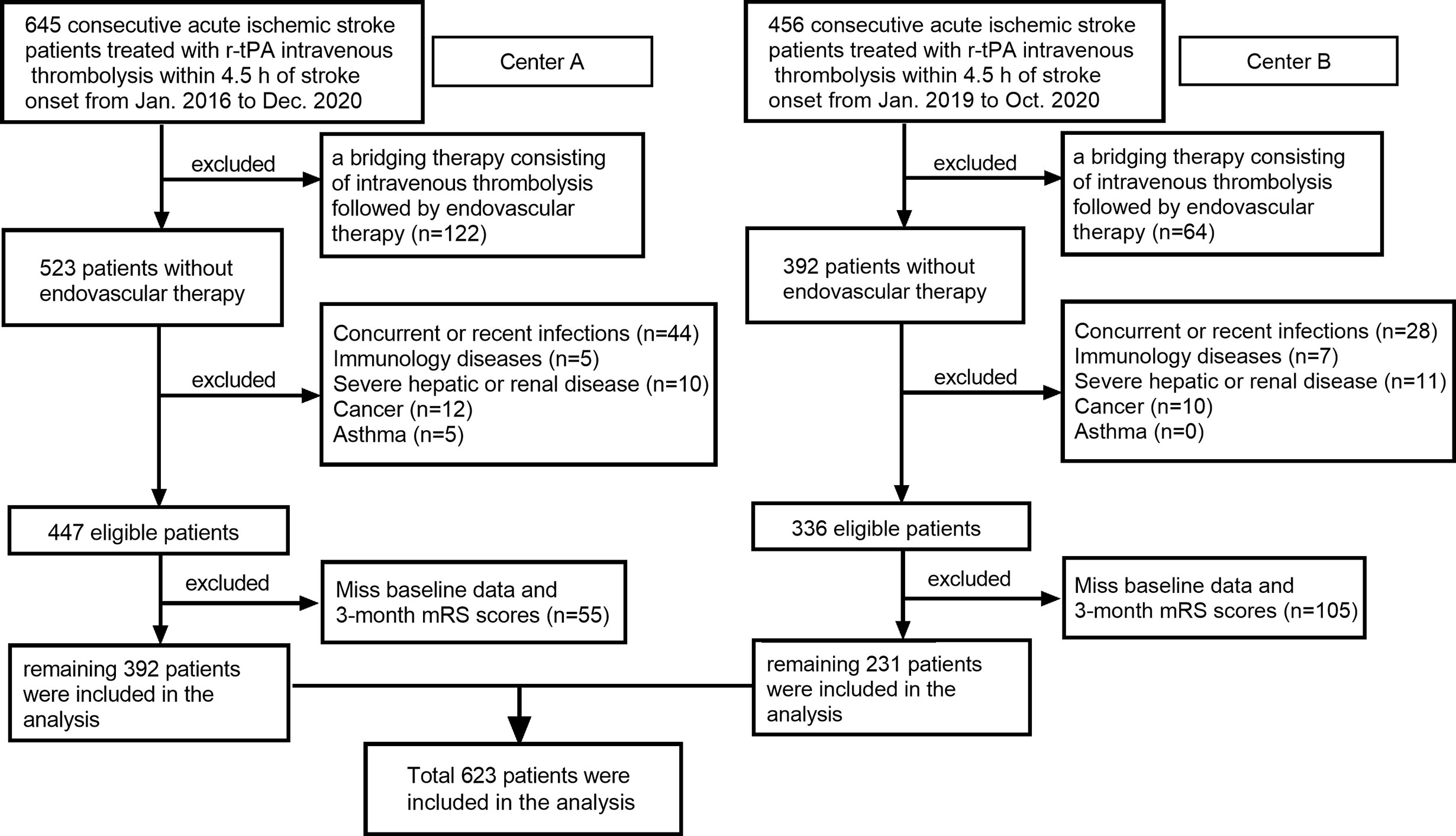
Figure 1 Flow chart for patients’ selection. Center A: Third Affiliated Hospital of Wenzhou Medical University; Center B: First Affiliated Hospital of Wenzhou Medical University.
Data Collection
Patient’s demographic data (age, sex), risk factors (current smoking, history of hypertension, diabetes mellitus, atrial fibrillation, prior stroke), laboratory data (total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, glycated hemoglobin (HbA1c) values, leukocyte subtype counts), and clinical data (premorbid mRS, blood pressure, onset to needle time, National Institutes of Health Stroke Scale (NIHSS) score, stroke subtype) were obtained from the electronic medical records. Etiology of AIS patients were classified via the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria (17). Early ischemic changes on admission CT imaging were evaluated with the Alberta Stroke Programme Early CT Score (ASPECTS) (18). Blood samples were obtained on admission, within 24 h after intravenous thrombolysis and on the seventh day. In Center A, leukocyte subtype counts were analyzed using a Sysmex XT-1800i Automated Hematology Analyzer (Sysmex Corporation, Kobe, Japan). In Center B, leukocyte subtype counts were analyzed using a Sysmex XE-2100 Automated Hematology Analyzer (Sysmex Corporation, Kobe, Japan). Eosinophil changes after intravenous thrombolysis were calculated as (24 h-eosinophil counts − admission eosinophil counts)/admission eosinophil counts × 100%. The same formula was used to calculate the early changes of NLR. NIHSS scores were evaluated on admission, 24 h after intravenous thrombolysis and on the seventh day. Stroke outcome was evaluated using the modified Rankin Scale (mRS) which was collected on a phone interview at the 3-month follow-up. Good outcome was defined as the mRS score of 0–2 while poor outcome as the mRS score of 3–6.
Statistical Analyses
Statistical analyses were performed via SPSS Statistics 25.0 software (SPSS Inc., Chicago, IL), MedCalc Statistical Software version 15.2.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2015) and R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were presented as the mean ± standard deviation (SD) or median (interquartile range, IQR) depending on the normality of distribution. Categorical variables are expressed as constituent ratios. The paired Wilcoxon signed-rank test was used to compare median eosinophil on admission and 24 h. The Spearman tests were used to analyze the correlation between admission eosinophil and the 24 h-eosinophil. Differences across multi-groups were assessed by means of the Chi-square test for categorical variables, and the one-way analysis of variance (ANOVA) or Kruskal–Wallis test for continuous variables. To explore the association between changes in eosinophil after intravenous thrombolysis and the 3-month outcome, univariable and multivariable logistic analyses were performed. Significant variables in the univariable analysis were entered in the multivariable model. Restricted cubic spline curves with four knots (at the fifth, 35th, 65th, and 95th percentiles) were further plotted to examine the linear relationship. C statistics, net reclassification index (NRI) and integrated discrimination improvement (IDI), were used to test the value of changes in eosinophil after intravenous thrombolysis to optimize the risk stratification for poor outcome and mortality. Statistical significance was set at two-tailed p <0.05.
Results
Characteristics of Enrolled Patients
A total of 623 eligible patients were recruited in our study, including 403 males (64.69%) and 220 females (35.31%). The mean age of the AIS patient was 67.36 years (SD ± 12.84). The median admission NIHSS score was 6 (IQR 4–10). According to TOAST classifications, 158 (30.02%) patients had cardioembolic stroke, 262 (42.05%) patients had atherothrombotic stroke, and 110 (17.66%) patients had lacunar infarction while 64 (10.27%) patients had AIS for other or undetermined reasons. The characteristics of the patients enrolled in the study are described in Table 1. In addition, characteristics of the patients before, excluded the missing data and the patients enrolled from different hospitals are described in Supplementary Tables 1 and 2.
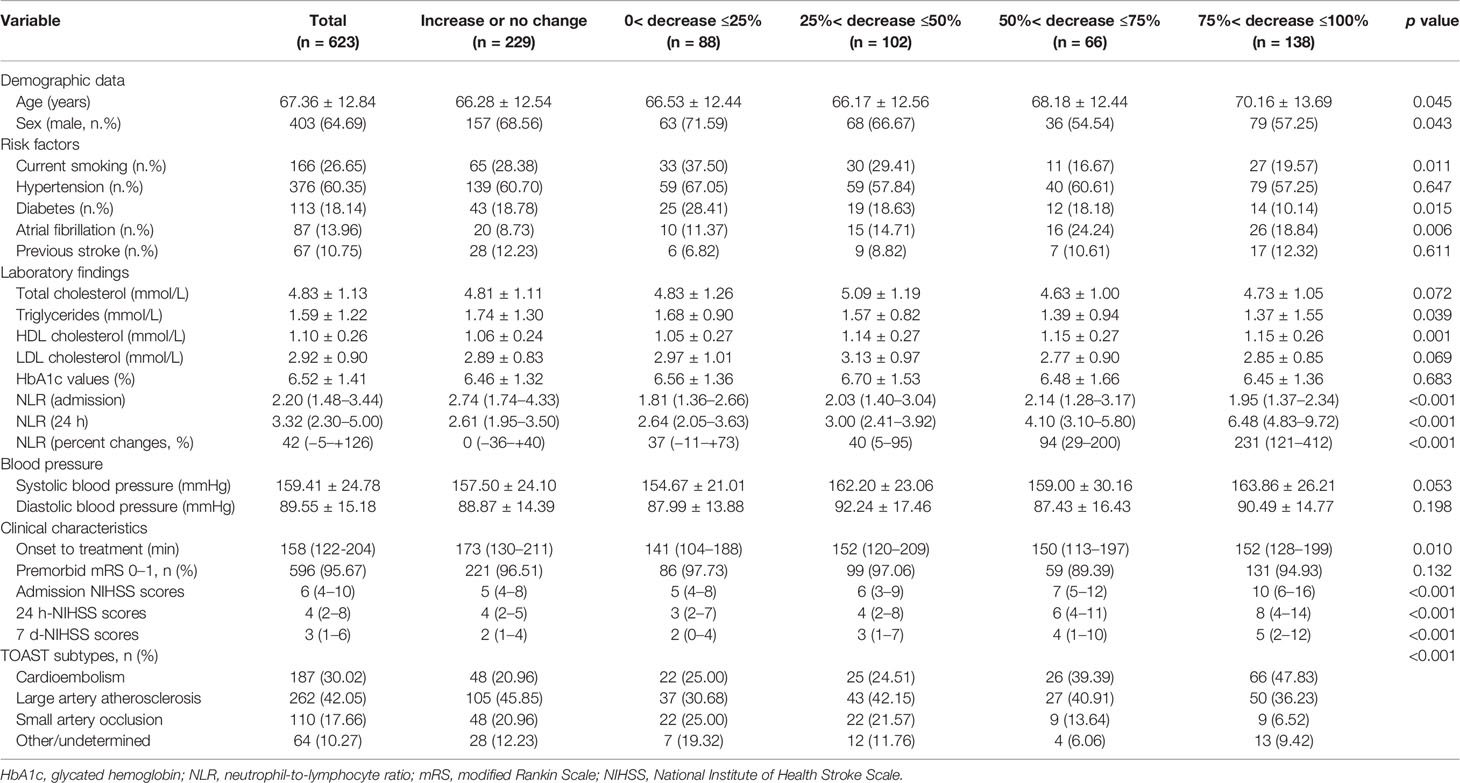
Table 1 Characteristics of AIS patients According to Eosinophil Changes After Intravenous Thrombolysis.
Dynamic Eosinophil Distribution Within First 7 Days Symptom Onset
The blood eosinophil counts were collected on admission, 24 h and on the seventh day. The dynamic change of eosinophil was like a U shape. Comparing to admission eosinophil counts, patients had overall lower median eosinophil counts on 24 h (median 0.07 [IQR 0.02–0.13] vs. 0.10 [IQR 0.05–0.17] 109/L, p <0.001). Eosinophil counts on the seventh day were available in 294 of the 623 patients (47.19%), with median 7 d-eosinophil counts of 0.13 (IQR 0.07–0.21) 109/L (Figure 2A). The reasons for missing 7 d-eosinophil counts were either a moribund state or an early discharge. We didn’t identify the association between eosinophils and 3-month function outcomes using eosinophils collected on admission or the seventh day (p = 0.263, p = 0.290 respectively) (Figures 2B, D). However, within 24 h after intravenous thrombolysis, patients with worse function outcomes tend to have lower eosinophil counts (median eosinophil 0.08 [IQR 0.04–0.14], 0.04 [IQR 0.01–0.12] and 0.01 [IQR 0–0.03] 109/L in patients with the 3-month mRS 0–2, 3–5 and 6 groups respectively, p <0.001) (Figure 2C).
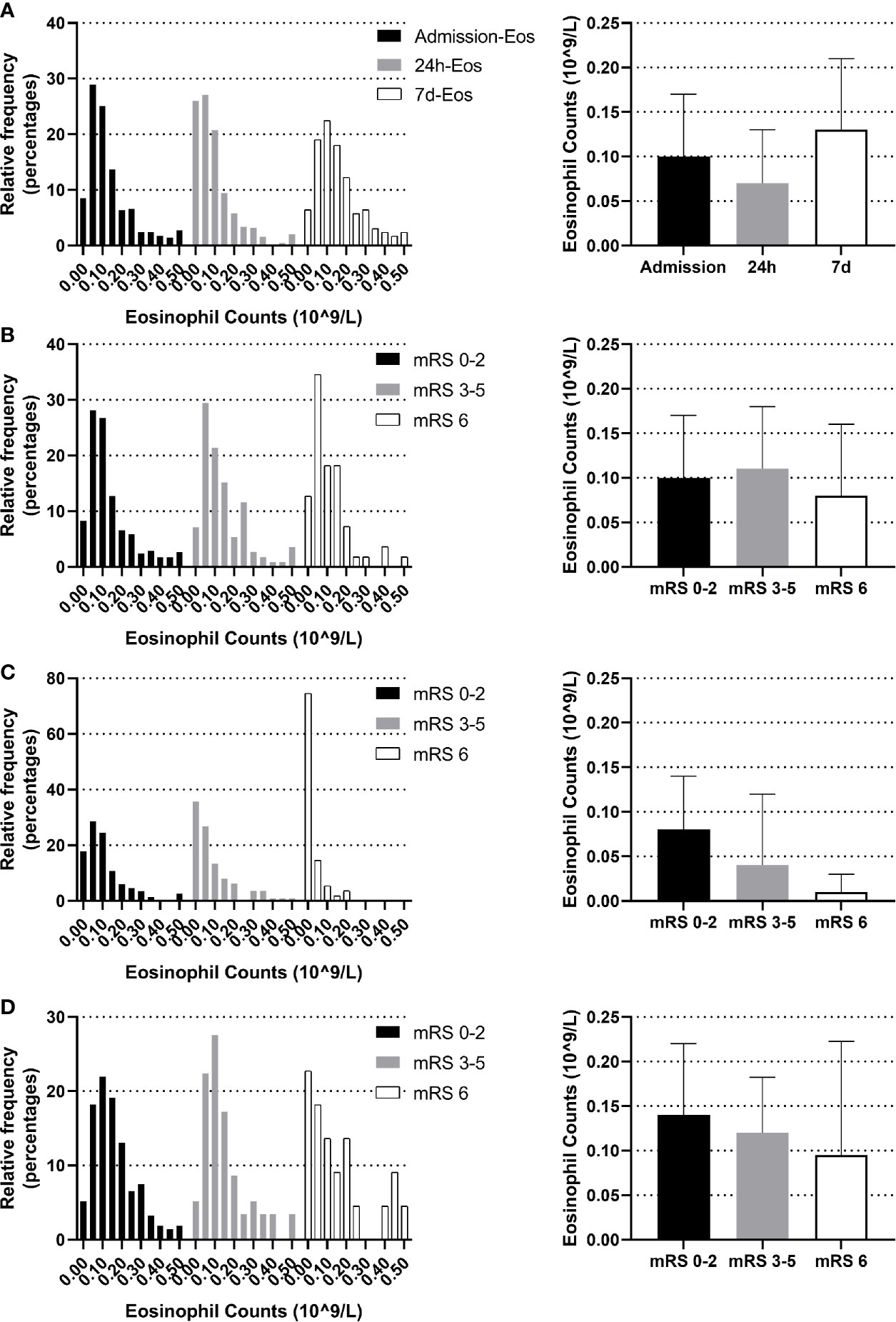
Figure 2 Temporal profile of eosinophil in AIS patients treated with r-tPA intravenous thrombolysis (A) Temporal profile of eosinophil on admission, within 24 h after intravenous thrombolysis and the seventh day (B) Distribution of admission eosinophil according to 3-month mRS scores. (C) Distribution of 24 h-eosinophil according to 3-month mRS scores. (D) Distribution of 7 d-eosinophil according to 3-month mRS scores. Frequency distribution charts (eosinophil >0.5 were included in the group with bin center = 0.5) and bar graphs (median and interquartile range) were displayed.
In addition, we found a strong and statistically significant positive correlation between admission-eosinophil counts and the 24 h-eosinophil counts (r = 0.558, p <0.001). To control for admission-eosinophil counts, we calculated the percent change in eosinophil counts after intravenous thrombolysis (median −25.00% [IQR −68.25–+14.29%]) and consequently patients were divided into five groups: increase or no change reference group (n = 229; increase n = 174; no change n = 55); 0< decrease ≤25% group (n = 88); 25%< decrease ≤50% group (n = 102); 50%< decrease ≤75% group (n = 66) and 75%< decrease ≤100% group (n = 138). Four (0.64%) patients with admission-eosinophil counts = 0, two patients with 24 h-eosinophil counts higher than 0 were assigned into increase or no change reference group while two patients with 24 h-eosinophil counts = 0 were assigned into 75%< decrease ≤100% group. Patients with greater decrease in eosinophils were significantly older, female, cardioembolic stroke, non-smokers, and had histories atrial fibrillation but not diabetes, with higher HDL cholesterol, lower triglycerides, lower admission NLR but higher 24 h NLR, shorter onset to treatment time, and higher admission, 24 h and the seventh day NIHSS scores (Table 1).
Longitudinal Associations of Dynamic Eosinophil With Clinical Outcomes
As shown in Table 2, 167 (26.81%) patients experienced the poor outcome (55 deaths and 112 major disabilities) during the 3-month follow-up. Patients with eosinophil counts 75%< decrease ≤100% had 6.441-fold increased risk for poor outcome and a 31.842-fold increased risk for death compared to increase or no change reference group. Adjustment for age, sex, current smoking, history of hypertension, atrial fibrillation, stroke and triglycerides slightly attenuated the association between Δeosinophil and AIS outcomes. In model 2, we observed an eosinophil count of 75%< decrease ≤100% after intravenous thrombolysis that led to 5.484 times risk for poor outcome and 28.069 times risk for death against the increase or no change reference group. This effect was consistent even after further adjusted for admission NIHSS scores in model 3, with OR = 2.585 (95% CI, 1.370–4.877) for poor outcome and OR = 13.836 (95% CI, 3.257–58.769) for death. In addition, in the continuous analyses, each 10% decrease in eosinophil after intravenous thrombolysis was associated with a 4.5% increase in the risk of poor outcome (OR = 1.045, 95%CI 1.007–1.084) and a 19.0% increase in the risk of death (OR = 1.190, 95%CI 1.071–1.322). Univariate logistic regression analyses of factors for adverse outcomes are displayed in Supplementary Table 3. A restricted cubic spline regression showed that a greater decrease in eosinophil after intravenous thrombolysis was associated with an increased risk of poor outcome and death and the depicted curves deviated significantly from linearity (p for non-linearity = 0.046 for poor outcome; p for non-linearity <0.001 for death) (Figures 3A, B).
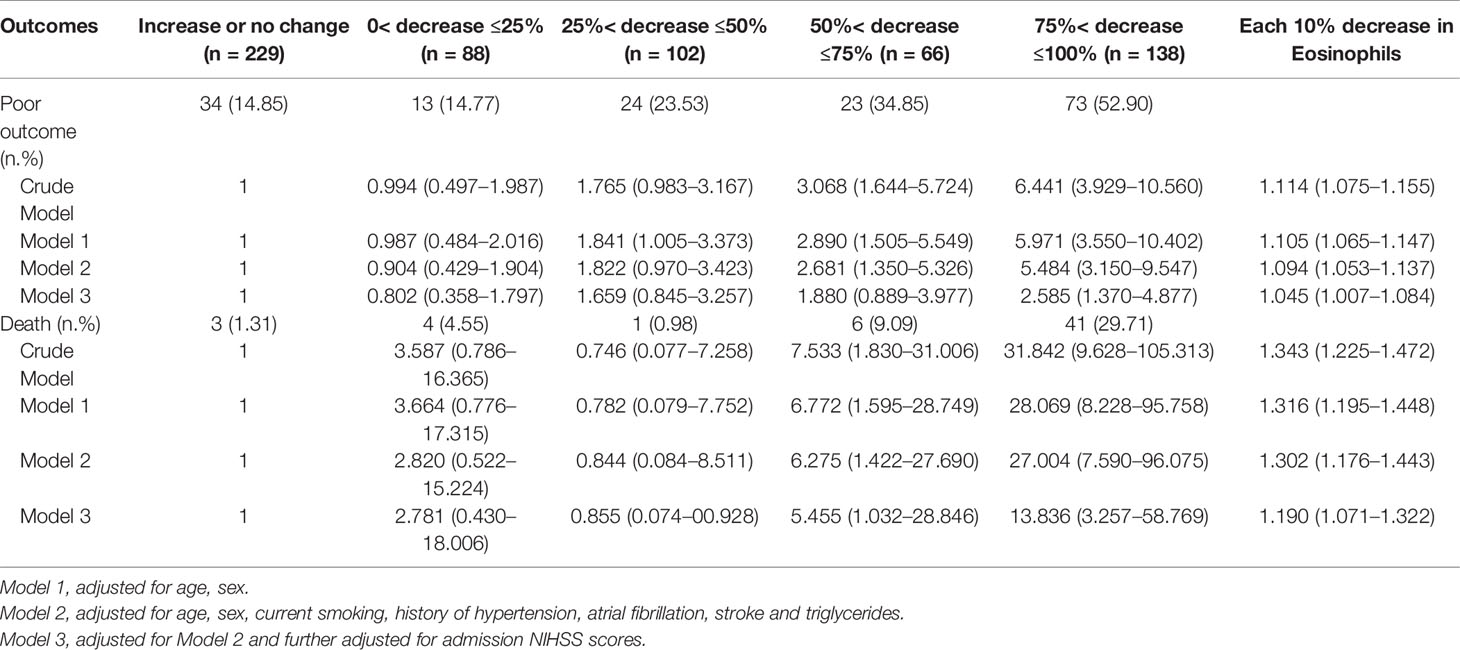
Table 2 Adjusted Odds Ratios of Adverse Outcomes at 3-month According to Eosinophil changes After Intravenous Thrombolysis.
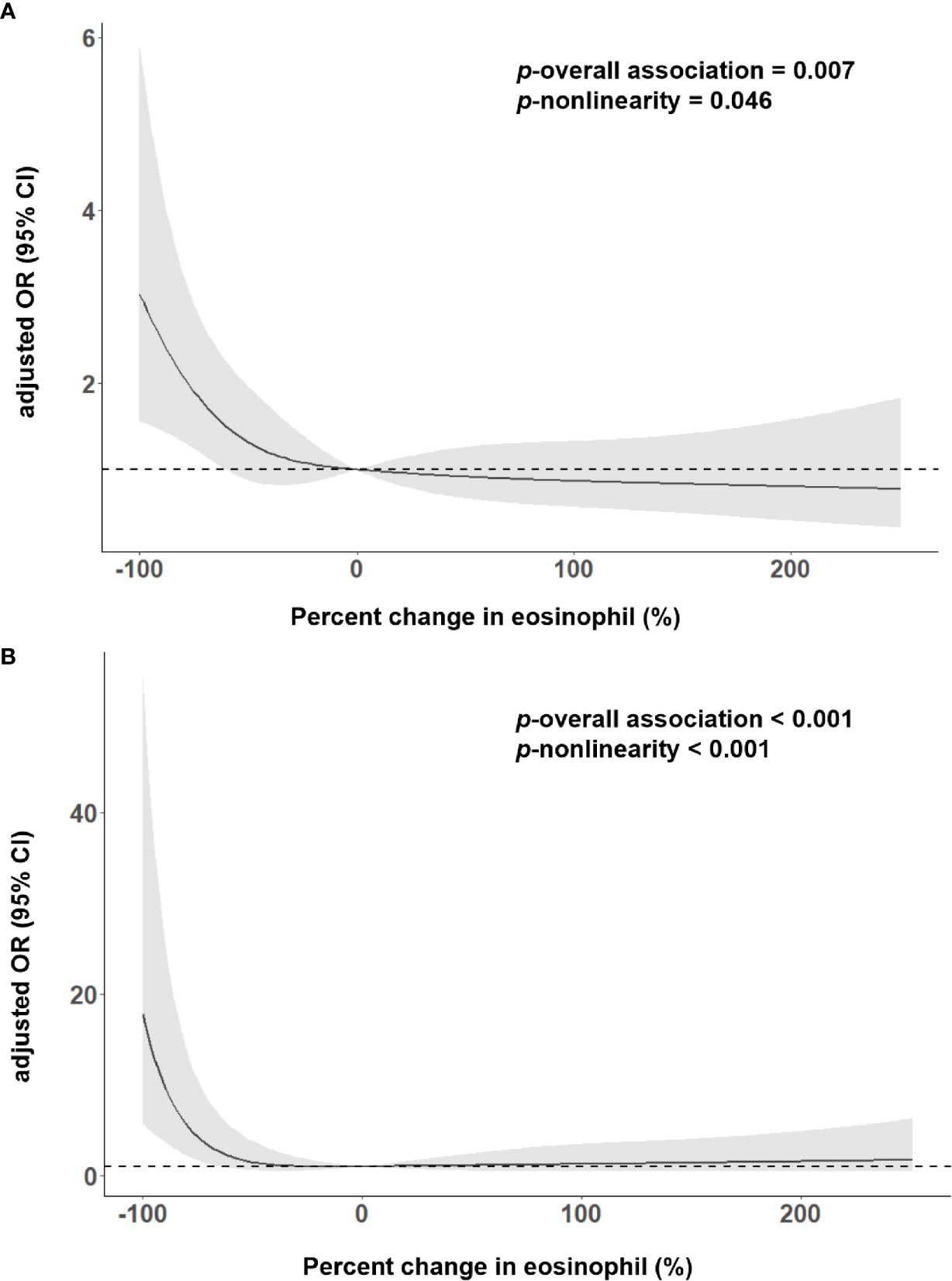
Figure 3 Adjusted association of eosinophil changes after intravenous thrombolysis with (A) 3-month poor outcome; (B) death, using multiple spline regression analyses with four knots (at the fifth, 35th, 65th, and 95th percentiles). The solid line indicates odds ratio, while the shadow indicates 95% CI. Reference point was 0. Data were adjusted for age, sex, current smoking, history of hypertension, atrial fibrillation, stroke, triglycerides and admission NIHSS score.
Results of Subgroup and Sensitivity Analysis
In the main analysis, we didn’t include ASPECTS into the multivariable logistic regression model due to the fact that ASPECTS were not available in Center B. In a first sensitivity analysis, we only selected AIS patients from Center A and additionally adjusted for the ASPECTS based on the conventional model. Results show that further adjustment for ASPECTS slightly attenuated the association between dynamic eosinophil and AIS outcomes, but the impact was relatively weak (Supplementary Table 4). Considering a total of 160 (20.43%) patients were excluded from the 783 eligible patients due to missing data, in the secondly sensitivity analyses, we chose the 690 patients with follow-up data and replaced the missing baseline and clinical data with the lowest 25th percentile, the 50th percentile, or the highest 25th percentile value of the 783 eligible patients. The association between dynamic eosinophil and AIS outcomes remained significant in the three models (Supplementary Table 5). The third sensitivity analysis excluded 19 patients with Tirofiban medications within 24 h after intravenous thrombolysis rendered very similar results as the main analysis (Supplementary Table 6). In addition, subgroup analysis was performed to explore the association between dynamic eosinophil and AIS outcomes in different TOAST classifications. Cardioembolic AIS patients with eosinophil counts 75%< decrease ≤100% had higher risk for poor outcome (OR = 11.453, 95%CI 2.661–49.283) and death (OR = 20.037, 95%CI 1.984–202.375) compared to increase or no change reference group. However, these associations were not observed in the AIS patients with TOAST classified large artery atherosclerosis, small artery occlusion and other/undetermined causes (Table 3). Finally, we compared the predictive values of eosinophils in Centers A and B using C-statistic. Results showed the continuous value of changes in eosinophil after intravenous thrombolysis had a prognostic significance for poor outcome and death in the both Centers A and B (Figure 4). Compared to the area under the curve (AUC) for poor outcome in Center B, the AUC for poor outcome in Center A increased by 0.08, which may be due to fewer cardioembolic AIS in Center B. In addition, we restricted the analysis to patients with cardioembolic AIS and C-statistic was repeated. The AUC in the two centers were quite similar for poor outcome (Center A: 0.788, 95%CI [0.708–0.854]; Center B: 0.814, 95%CI [0.682–0.909]) and death (Center A: 0.812, 95%CI [0.735–0.875]; Center B: 0.841, 95%CI [0.714–0.928]).
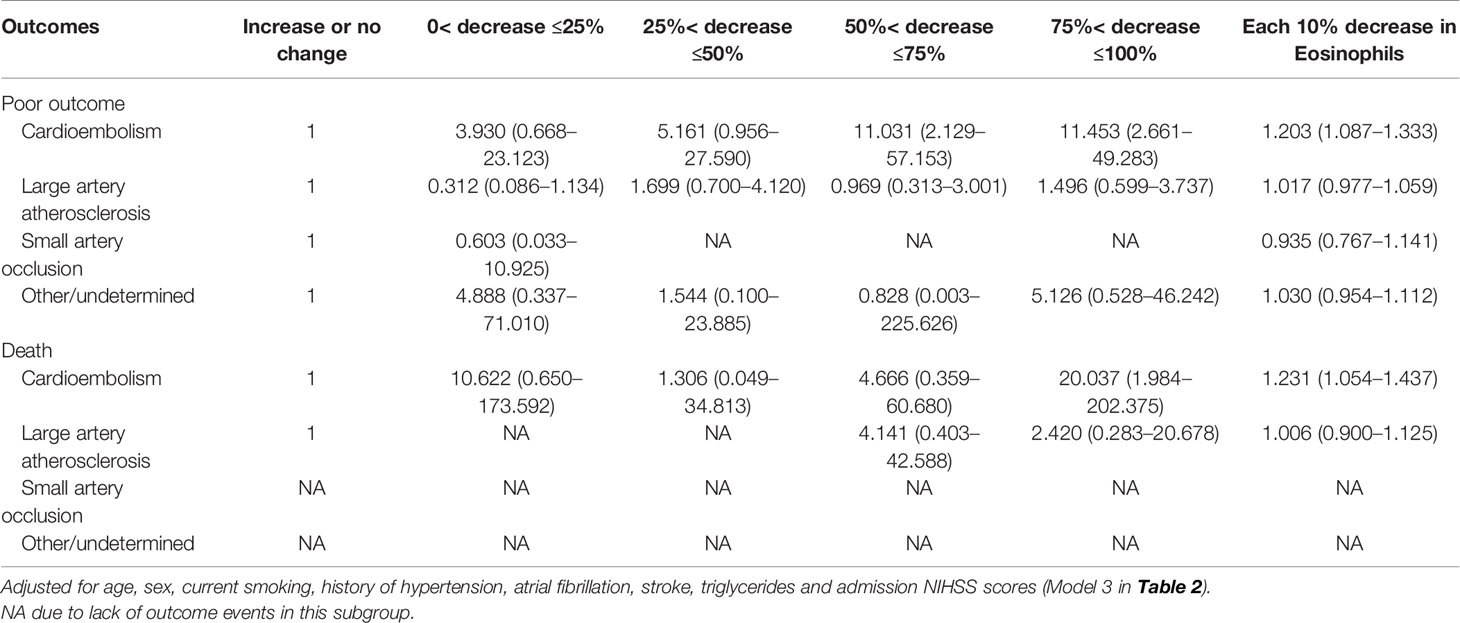
Table 3 Adjusted odds ratios of adverse outcomes at 3-month according to eosinophil changes after Intravenous thrombolysis in different TOAST subtypes.
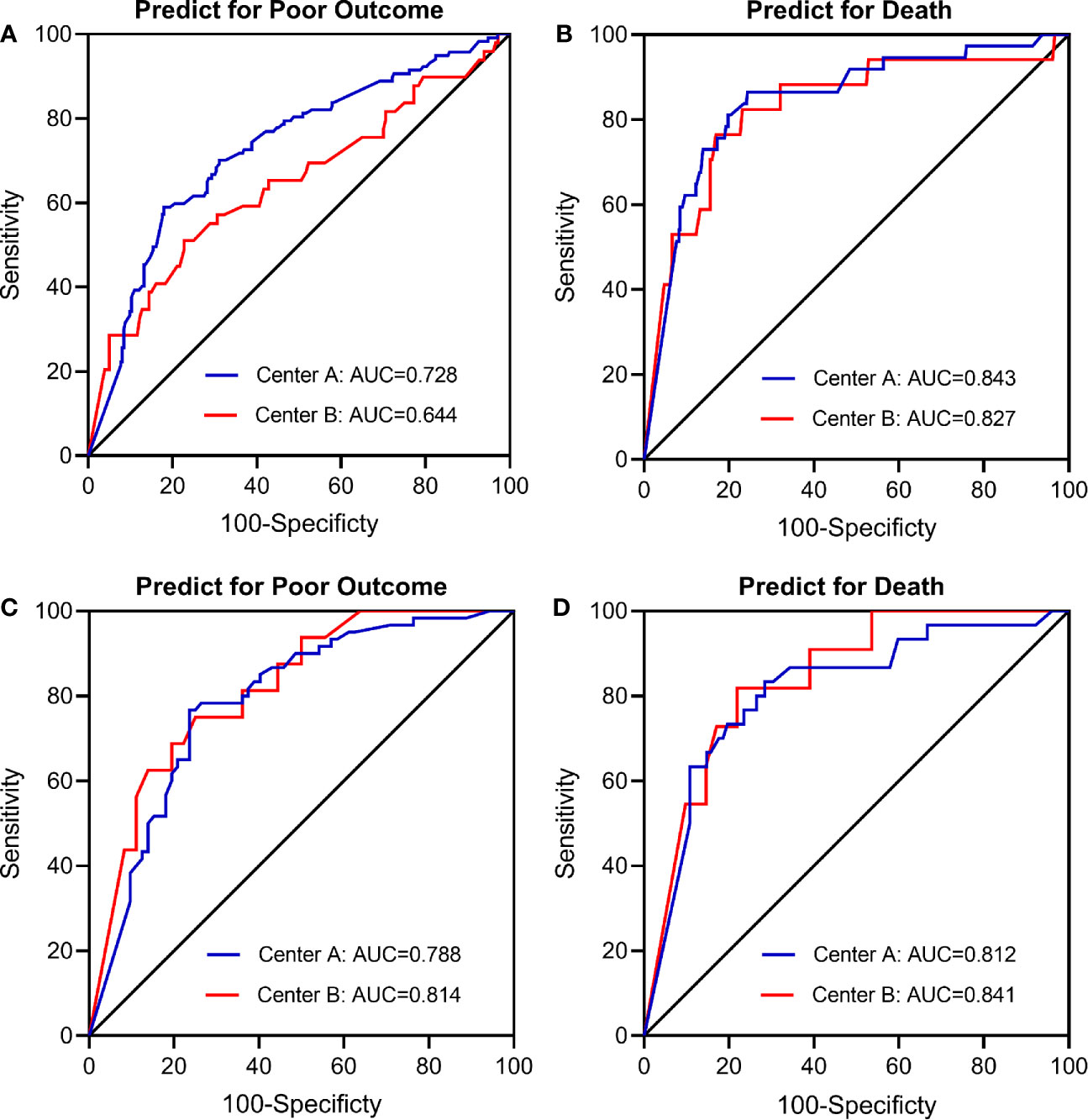
Figure 4 Receiver operating characteristic (ROC) curve for the value of eosinophil changes after intravenous thrombolysis to predict (A) Poor outcome in total patients (n = 623); (B) Death in total patients (n = 623); (C) Poor outcome in cardioembolic stroke only (n = 187); (D) Death in cardioembolic stroke only (n = 187). Center A: The Third Affiliated Hospital of Wenzhou Medical University; Center B: The First Affiliated Hospital of Wenzhou Medical University.
Prognostic Value of Eosinophils and Neutrophil-to-Lymphocyte Ratio
Receiver operating characteristic (ROC) curves were employed to investigate the prognostic value of dynamic eosinophil and NLR changes after intravenous thrombolysis for adverse outcomes (Supplementary Figure 1). Areas under the curves (AUC) for poor outcome were 0.702 for dynamic eosinophil and 0.632 for dynamic NLR. The optimal cut-off values to separate patients experiencing poor outcome vs. good outcome were −57.14% for dynamic eosinophils with 57.23% sensitivity, 78.59% specificity and +97.27% for dynamic NLR with 49.10% sensitivity, 75.88% specificity. Besides, Areas under the curves (AUC) for death were 0.840 for dynamic eosinophil and 0.713 for dynamic NLR. The optimal cut-off values to distinguish mortality status were −61.54% for dynamic eosinophils with 85.19% sensitivity, 75.40% specificity and +124.35% for dynamic NLR with 61.82% sensitivity, 77.99% specificity. Thereby, for clinical practice, dynamic NLR were categorized into two groups: NLR increased ≤100% (n = 434), NLR increased >100% (n = 189). Multivariable logistic regression models showed that admission NLR, 24-h NLR and dynamic NLR were independent factors for poor outcome and death (Supplementary Table 7). Heat maps of adjusted ORs for poor outcome and death in the 10 groups established using eosinophil and NLR changes after intravenous thrombolysis showed that AIS patients with greater decrease in eosinophils were more likely to come to adverse outcomes regardless of the early change of NLR (Figure 5, Supplementary Figure 2).
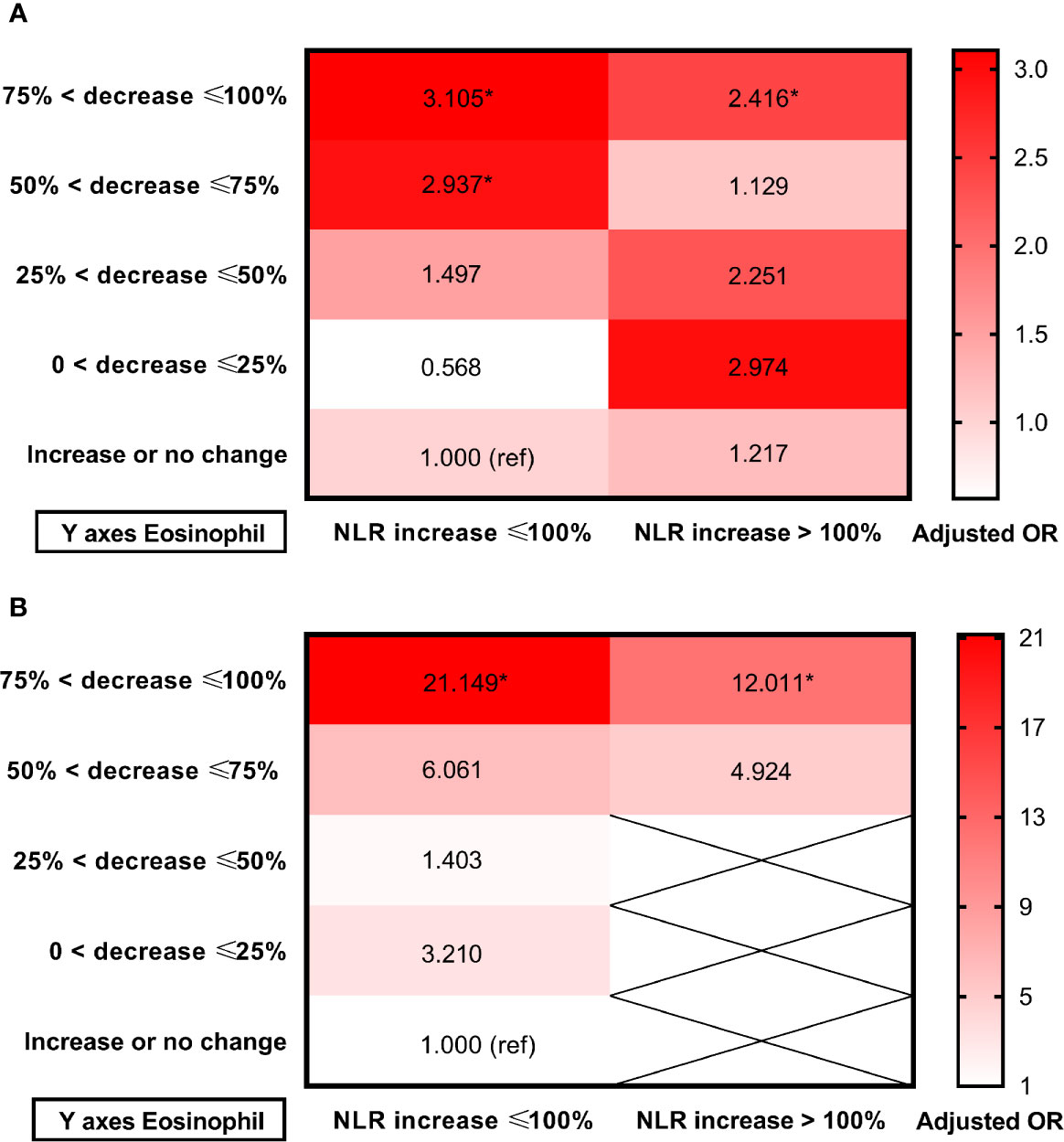
Figure 5 Heat maps of adjusted odds ratios for (A) Poor outcome; (B) Death in the 10 groups established using eosinophil and NLR changes after intravenous thrombolysis. Data were adjusted for age, sex, current smoking, history of hypertension, atrial fibrillation, stroke, triglycerides and admission NIHSS score (model 3). Asterisk indicates p <0.05. Total patients and outcome events in each group could be found in the Supplementary Figure 2.
Incremental Predictive Ability of Dynamic Eosinophil
The new model shows an improvement in discrimination between good and poor outcomes after we added changes in eosinophil after intravenous thrombolysis into the conventional model (including age, sex, current smoking, history of hypertension, atrial fibrillation, stroke, triglycerides and admission NIHSS scores) with an AUC improved 0.06 (p = 0.304), NRI 53.3% (p <0.001) and IDI 2.2% (p <0.001). The discriminatory ability of the death was also improved in the new model (AUC improved 0.18; NRI 101.0%; IDI 6.9%) (Table 4). Similar results were found in cardioembolic stroke patients.

Table 4 Reclassification and discrimination statistics for adverse outcomes by eosinophils changes after intravenous thrombolysis in AIS patients.
Discussion
This study showed that: 1) Blood eosinophil count was a dynamic variable in AIS patients treated with r-tPA intravenous thrombolysis. Overall median eosinophil decreased significantly after intravenous thrombolysis; 2) Eosinophil within 24 h after intravenous thrombolysis but not on admission or 7 days was correlated with 3-month mRS scores; 3) Patients with greater decrease in eosinophils after intravenous thrombolysis were significantly cardioembolic stroke and had higher admission, 24 h and seventh day NIHSS scores; 4) In longitudinal analyze, dynamic decreased eosinophil after intravenous thrombolysis was associated with higher risk of poor 3-month outcome and death with a non-linear trend. However, the association were not statistically significant for patients outside of cardioembolic stroke; 5) In longitudinal analyze, dynamic increase in NLR after intravenous thrombolysis was associated with higher risk of poor 3-month outcome and death; 6) dynamic eosinophil had higher predictive ability than dynamic NLR for poor 3-month outcome and death; and 7) Adding eosinophil changes to a conventional model improved the discriminatory ability of poor outcome and death.
The mechanism underlying the role of eosinophils in AIS outcome is still unclear and we hypothesize the following mechanism may explain the findings (Figure 6). Inflammation has been regarded as a key contributor to the AIS pathophysiology. Eosinopenia may be caused by acute inflammation through mechanism like sequestration of the eosinophil within an organ or eosinophils diffusing margination (19). Besides, eosinophils can synthesize and secrete more than 35 vital inflammatory and regulatory cytokines, growth factors, and chemokines, which participate in and regulate inflammation (20). It is worth noting that the infusion of r-tPA may contribute to the dynamic profile of eosinophils. In vitro studies found that the usage of r-tPA directly induced neutrophil degranulation (21), though whether r-tPA infusion will directly induce eosinophils degranulation is unknown, r-tPA administration can activate the fibrinolytic system and play a potentially supporting role in migration of eosinophils (22).
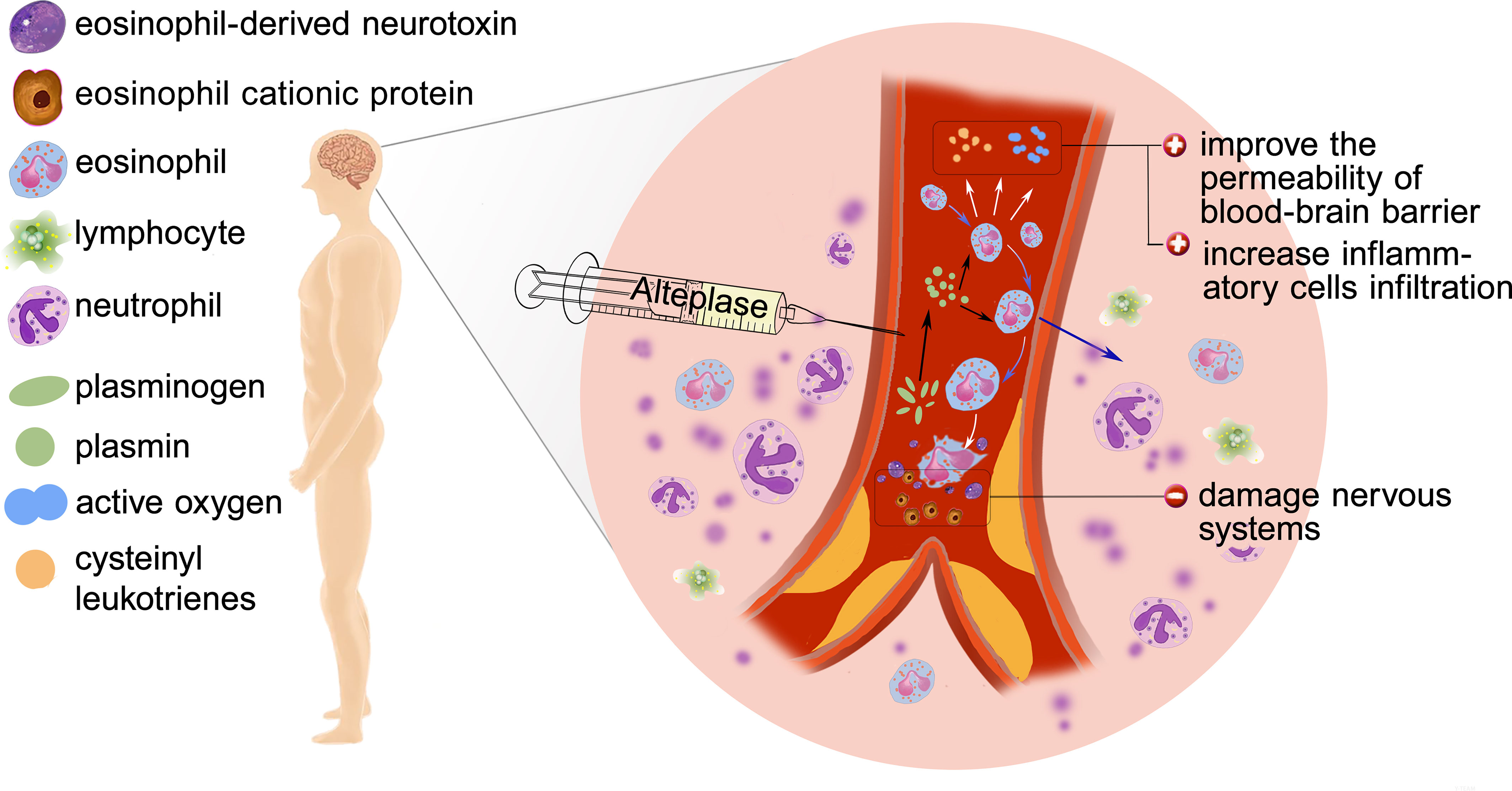
Figure 6 Possible mechanisms of poor outcome: Eosinophil infiltration, release of inflammatory factors, degranulation and release of toxic proteins.
Whether eosinophil is beneficial or not depends on the particular condition. Eosinophils can produce nerve growth factor (NGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (b-FGF) (23), which may play an important role in the functional recovery after AIS. Protease activated receptors (PARs) are known to be expressed by eosinophils and could be activated by proteases such as plasmin (9). Activation of PARs induced eosinophil shape change and lead to reactive oxygen species (ROS) and cysteinyl leukotrienes generation (24), which increases the permeability of blood–brain barrier (BBB) and contributes to leukocyte infiltration. Besides, human and mouse eosinophils degranulate upon stimulation and in inflammatory settings (25, 26). At the same time, activated eosinophils release cytotoxic proteins such as eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN) which play a central role to the pathogenesis of AIS (27).
Previous study only collected the blood sample at a single time point (3, 12, 14, 28). Longitudinal association analysis is the advantage of our study. Besides, we provided a rather comprehensive view of the relationship between eosinophil and 3-month prognosis in AIS patients. Eosinophil on admission was not correlated with 3-month mRS scores of AIS patients, which may be due to the fact that the time from stroke onset to admission is limited to 4.5 h in patients with intravenous thrombolysis, and the change of eosinophils during this period is not significant. While on the seventh day, eosinophils of AIS patients were basically returned to the normal range. Our study demonstrated that association between changes in eosinophil after intravenous thrombolysis and 3-month prognosis were not statistically significant for patients outside of cardioembolic AIS. Patients with cholesterol crystal embolism (embolization of the contents of an atherosclerotic plaque from a proximal large-caliber artery), thicker aortic arch plaques with ulcer, or mobile component tend to have higher eosinophil with unknown mechanisms (11, 29), which may attenuate the association between eosinophils and AIS outcome in patients with atherothrombotic stroke. What’s more, our study suggested adding eosinophil changes after intravenous thrombolysis to conventional models could help clinicians to judge the prognosis of patients.
However, the present study have some existing limitations: 1) The data was retrospectively collected in this study and a total of 160 (20.43%) patients were excluded from the 783 eligible patients due to missing data. Besides, blood samples were only available at three time points and there were only 294 data for eosinophil on the seventh day due to moribund state or an early discharge; 2) Though patients were collected from two centers in this study, the results of the subgroup analysis was based on a small sample size, which may weaken statistical efficiency. In addition, ORs were unable to be obtained due to lack of events (poor outcomes or deaths) in some subgroups. Studies with larger sample size are warranted; 3) Though this study was observationally designed, residual confounders will exist and we had adjusted relatively strong confounding factors. Whether our results are universal, there is a need for more similar studies to prove such.
Conclusion
A dynamic decrease in eosinophil after intravenous thrombolysis predicts a 3-month poor outcome and death in AIS patients with r-tPA treatment and adds prognostic information to the conventional model. However, this result needs to be interpreted carefully in non-cardioembolic AIS patients. We suggested the role of eosinophils in cardioembolic stroke and r-tPA intravenous thrombolysis merits attentions in future basic research.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Third Affiliated Hospital of Wenzhou Medical University and Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
DY and GC conceptualized this work. DY and GC supervised the study. HH, YW, JR, CY, JW, BG, TZ, JH, WP, FS, and XZ: acquisition of data. DY, HH, YW, and JR performed the statistical analysis and interpreted data. DY, HH, and YW prepared the manuscript. DY, GC, HH, YW, JR, CY, JW, BG, TZ, JH, WP, FS, and XZ revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank the participating hospitals, patients, their families and colleagues who have provided valuable suggestions for this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.709289/full#supplementary-material
Abbreviations
AIS, acute ischemic stroke; r-tPA, recombinant tissue plasminogen activator; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, glycated hemoglobin; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; TOAST, Trial of Org 10,172 in Acute Stroke Treatment; NRI, net reclassification index; IDI, integrated discrimination improvement; AUC, area under the curve.
References
1. Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF, et al. Global, Regional, and National Burden of Neurological Disorders During 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Neurol (2017) 16(11):877–97. doi: 10.1016/s1474-4422(17)30299-5
2. Marko M, Posekany A, Szabo S, Scharer S, Kiechl S, Knoflach M, et al. Trends of r-tPA (Recombinant Tissue-Type Plasminogen Activator) Treatment and Treatment-Influencing Factors in Acute Ischemic Stroke. Stroke (2020) 51(4):1240–7. doi: 10.1161/STROKEAHA.119.027921
3. Gill D, Sivakumaran P, Wilding P, Love M, Veltkamp R, Kar A. Trends in C-Reactive Protein Levels Are Associated With Neurological Change Twenty-Four Hours After Thrombolysis for Acute Ischemic Stroke. J Stroke Cerebrovasc Dis (2016) 25(8):1966–9. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.003
4. Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory Disequilibrium in Stroke. Circ Res (2016) 119(1):142–58. doi: 10.1161/CIRCRESAHA.116.308022
5. Nieswandt B, Kleinschnitz C, Stoll G. Ischaemic Stroke: A Thrombo-Inflammatory Disease? J Physiol (2011) 589(17):4115–23. doi: 10.1113/jphysiol.2011.212886
6. Stoll G, Nieswandt B. Thrombo-Inflammation in Acute Ischaemic Stroke - Implications for Treatment. Nat Rev Neurol (2019) 15(8):473–81. doi: 10.1038/s41582-019-0221-1
7. Shi J, Peng H, You S, Liu Y, Xu J, Xu Y, et al. Increase in Neutrophils After Recombinant Tissue Plasminogen Activator Thrombolysis Predicts Poor Functional Outcome of Ischaemic Stroke: A Longitudinal Study. Eur J Neurol (2018) 25(4):687–e45. doi: 10.1111/ene.13575
8. Ying A, Cheng Y, Lin Y, Yu J, Wu X, Lin Y. Dynamic Increase in Neutrophil Levels Predicts Parenchymal Hemorrhage and Function Outcome of Ischemic Stroke With r-tPA Thrombolysis. Neurol Sci (2020) 41(8):2215–23. doi: 10.1007/s10072-020-04324-6
9. Coden ME, Berdnikovs S. Eosinophils in Wound Healing and Epithelial Remodeling: Is Coagulation a Missing Link? J Leukoc Biol (2020) 108(1):93–103. doi: 10.1002/JLB.3MR0120-390R
10. Alkhalil M, Kearney A, Hegarty M, Stewart C, Devlin P, Owens CG, et al. Eosinopenia as an Adverse Marker of Clinical Outcomes in Patients Presenting With Acute Myocardial Infarction. Am J Med (2019) 132(12):e827–e34. doi: 10.1016/j.amjmed.2019.05.021
11. Kitano T, Nezu T, Shiromoto T, Kubo S, Uemura J, Wada Y, et al. Association Between Absolute Eosinophil Count and Complex Aortic Arch Plaque in Patients With Acute Ischemic Stroke. Stroke (2017) 48(4):1074–6. doi: 10.1161/STROKEAHA.116.016436
12. Wang J, Ma L, Lin T, Li SJ, Chen LL, Wang DZ. The Significance of Eosinophils in Predicting the Severity of Acute Ischemic Stroke. Oncotarget (2017) 8(61):104238–46. doi: 10.18632/oncotarget.22199
13. Juceviciute N, Mikuzis P, Balnyte R. Absolute Blood Eosinophil Count Could be a Potential Biomarker for Predicting Haemorrhagic Transformation After Intravenous Thrombolysis for Acute Ischaemic Stroke. BMC Neurol (2019) 19(1):127. doi: 10.1186/s12883-019-1359-6
14. Zhao HM, Qin WQ, Wang PJ, Wen ZM. Eosinopenia is a Predictive Factor for the Severity of Acute Ischemic Stroke. Neural Regener Res (2019) 14(10):1772–9. doi: 10.4103/1673-5374.258411
15. Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil-To-Lymphocyte Ratio in Acute Cerebral Hemorrhage: A System Review. Transl Stroke Res (2019) 10(2):137–45. doi: 10.1007/s12975-018-0649-4
16. Wang L, Song Q, Wang C, Wu S, Deng L, Li Y, et al. Neutrophil to Lymphocyte Ratio Predicts Poor Outcomes After Acute Ischemic Stroke: A Cohort Study and Systematic Review. J Neurol Sci (2019) 406:116445. doi: 10.1016/j.jns.2019.116445
17. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke (1993) 24(1):35–41. doi: 10.1161/01.Str.24.1.35
18. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and Reliability of a Quantitative Computed Tomography Score in Predicting Outcome of Hyperacute Stroke Before Thrombolytic Therapy. Lancet (2000) 355(9216):1670–4. doi: 10.1016/s0140-6736(00)02237-6
19. Bass DA, Gonwa TA, Szejda P, Cousart MS, DeChatelet LR, McCall CE. Eosinopenia of Acute Infection. J Clin Invest (1980) 65(6):1265–71. doi: 10.1172/jci109789
20. Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: Biological Properties and Role in Health and Disease. Clin Exp Allergy (2008) 38(5):709–50. doi: 10.1111/j.1365-2222.2008.02958.x
21. Carbone F, Vuilleumier N, Bertolotto M, Burger F, Galan K, Roversi G, et al. Treatment With Recombinant Tissue Plasminogen Activator (R-TPA) Induces Neutrophil Degranulation In Vitro via Defined Pathways. Vascul Pharmacol (2015) 64:16–27. doi: 10.1016/j.vph.2014.11.007
22. Ferland C, Guilbert M, Davoine F, Flamand N, Chakir J, Laviolette M. Eotaxin Promotes Eosinophil Transmigration via the Activation of the Plasminogen-Plasmin System. J Leukoc Biol (2001) 69(5):772–8.
23. Puxeddu I, Alian A, Piliponsky A, Ribatti D, Panet A, FJTijob L-S, et al. Human Peripheral Blood Eosinophils Induce Angiogenesis. Int J Biochem Cell Biol (2005) 37(3):628–36. doi: 10.1016/j.biocel.2004.09.001
24. Bolton SJ, McNulty CA, Thomas RJ, Hewitt CR, Wardlaw AJ. Expression of and Functional Responses to Protease-Activated Receptors on Human Eosinophils. J Leukoc Biol (2003) 74(1):60–8. doi: 10.1189/jlb.0702351
25. Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, et al. Human Versus Mouse Eosinophils: “That Which We Call an Eosinophil, by Any Other Name Would Stain as Red. J Allergy Clin Immunol (2012) 130(3):572–84. doi: 10.1016/j.jaci.2012.07.025
26. Coden ME, Loffredo LF, Walker MT, Jeong BM, Nam K, Bochner BS, et al. Fibrinogen Is a Specific Trigger for Cytolytic Eosinophil Degranulation. J Immunol (2020) 204(2):438–48. doi: 10.4049/jimmunol.1900932
27. Navarro S, Boix E, Cuchillo CM, Nogues MV. Eosinophil-Induced Neurotoxicity: The Role of Eosinophil Cationic Protein/RNase 3. J Neuroimmunol (2010) 227(1-2):60–70. doi: 10.1016/j.jneuroim.2010.06.012
28. Hori YS, Kodera S, Sato Y, Shiojiri T. Eosinopenia as a Predictive Factor of the Short-Term Risk of Mortality and Infection After Acute Cerebral Infarction. J Stroke Cerebrovasc Dis (2016) 25(6):1307–12. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.007
Keywords: acute ischemic stroke, eosinophil, intravenous thrombolysis, inflammation, prognosis
Citation: Yang D, Huang H, Weng Y, Ren J, Yang C, Wang J, Gao B, Zeng T, Hu J, Pan W, Sun F, Zhou X and Chen G (2021) Dynamic Decrease in Eosinophil After Intravenous Thrombolysis Predicts Poor Prognosis of Acute Ischemic Stroke: A Longitudinal Study. Front. Immunol. 12:709289. doi: 10.3389/fimmu.2021.709289
Received: 13 May 2021; Accepted: 21 June 2021;
Published: 07 July 2021.
Edited by:
Qing-Wu Yang, Xinqiao Hospital, ChinaReviewed by:
Jie Yang, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, ChinaHong-Qiu Gu, National Clinical Research Center for Neurological Diseases, China
Copyright © 2021 Yang, Huang, Weng, Ren, Yang, Wang, Gao, Zeng, Hu, Pan, Sun, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dehao Yang, d3ptY3lkaEAxNjMuY29t; Guangyong Chen, Z3ljaGVuNkAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Dehao Yang
Dehao Yang Honghao Huang2,3†
Honghao Huang2,3† Guangyong Chen
Guangyong Chen