- 1The Roslin Institute, University of Edinburgh, Edinburgh, United Kingdom
- 2Institute of Immunology and Infection Research, Ashworth Laboratories, University of Edinburgh, Edinburgh, United Kingdom
Filarial helminths infect approximately 120 million people worldwide initiating a type 2 immune response in the host. Influenza A viruses stimulate a virulent type 1 pro-inflammatory immune response that in some individuals can cause uncontrolled immunopathology and fatality. Although coinfection with filariasis and influenza is a common occurrence, the impact of filarial infection on respiratory viral infection is unknown. The aim of this study was to determine the impact of pre-existing filarial infection on concurrent infection with influenza A virus. A murine model of co-infection was established using the filarial helminth Litomosoides sigmodontis and the H1N1 (A/WSN/33) influenza A virus (IAV). Co-infection was performed at 3 different stages of L. sigmodontis infection (larval, juvenile adult, and patency), and the impact of co-infection was determined by IAV induced weight loss and clinical signs, quantification of viral titres, and helminth counts. Significant alterations of IAV pathogenesis, dependent upon stage of infection, was observed on co-infection with L. sigmodontis. Larval stage L. sigmodontis infection alleviated clinical signs of IAV co-infection, whilst more established juvenile adult infection also significantly delayed weight loss. Viral titres remained unaltered at either infection stage. In contrast, patent L. sigmdodontis infection led to a reversal of age-related resistance to IAV infection, significantly increasing weight loss and clinical signs of infection as well as increasing IAV titre. These data demonstrate that the progression of influenza infection can be ameliorated or worsened by pre-existing filarial infection, with the outcome dependent upon the stage of filarial infection.
Introduction
Filarial helminths infect approximately 120 million people worldwide, and remain commonplace in many low and middle income nations despite the existence of effective treatments and detection methods (1). The filarial helminths Wuchereria bancrofti, Brugia malayi and Brugia timori are referred to as lymphatic filariasis and are a significant global health concern (1). It is common for humans to be infected with multiple microbes at any given time, including commensal organisms and chronic or persistent infections. The incidence of concurrent infection with filarial helminths is highly prevalent (2), and therefore it is important to understand their impact on other infections.
Filarial helminths predominantly stimulate Type 2 immune responses in their host, although mixed Type 1 and 2 responses can develop (3). As with other helminth infections, filarial parasites secrete immunosuppressive molecules that impair host immunity in order to maintain a persistent infection (4), and combined with this, the host downregulates its immune responses during chronic infection to avoid severe disease (5). Thus, hosts tend to develop regulatory and modified Type 2 response during chronicity (6, 7). This development of regulatory and Type 2 immunity during chronic filariasis and other helminth infections can influence systemic immunity, including immune responses to third-party antigens such as allergens and concurrent infections (8, 9).
Litomosoides sigmodontis infection of inbred mice is used as a model of human lymphatic filariasis (10), and provides the opportunity to test the impact of filariasis on coinfection. L. sigmodontis infection initially stimulates a Type 2 immune response during the larval and juvenile adult stages. However, similar to human infections, it develops a mixed Type 1 and 2 response as the adult parasites become fully mature and release the transmission stage microfilaria (Mf) into the blood stream, at which point the infection is referred to as patent. L. sigmodontis coinfection has been shown to both protect against and worsen malaria (11–14), protect against Leishmania major (15), increase the severity of LPS-mediated inflammation (16), but has no apparent effect on Mycobacterium tuberculosis infection (17).
Influenza A virus (IAV) infections cause virulent pro-inflammatory immune responses (18) hallmarked by ‘type I’ immunity, interferon production and generation of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β. These, can lead to extensive airway pathology within days of infection, increasing the severity of disease and incidence of mortality (19–21). Whilst L. sigmodontis can suppress vaccine-induced antibody responses to IAV (22), the impact of filarial co-infection on viral infections, including acute IAV infection, is unknown. We therefore tested whether infection with L. sigmodontis, which resides in the pleural cavity of mice, could affect an acute respiratory challenge with IAV. In particular, we tested the hypothesis that the immune regulatory pathways associated with L. sigmodontis infection would protect against IAV-induced pathology, and that the strongest protection would be seen during patent L. sigmodontis infection.
Materials and Methods
Ethics Statement
Experiments were in undertaken accordance with the United Kingdom Animals (Scientific Procedures) Act of 1986 (PPL 60/4479), and approved by the University of Edinburgh Animal Welfare and Ethical Review Body.
Animals and Infections
Female BALB/c mice were purchased from Charles River and maintained under specific pathogen free conditions at the University of Edinburgh. Mice were used at 6–8 weeks of age. To maintain the L. sigmodontis lifecycle, L. sigmodontis infected jirds (Meriones unguiculatus) were used to infect haematophagous tick parasites (Ornithonyssus bacoti) in order to generate L3 stage larvae (23). Mice were infected s.c. on the upper back with 30 L. sigmodontis L3 stage larvae. L. sigmodontis larvae or adults were recovered from the pleural cavity by lavage and counted using a dissection microscope. To quantify blood Mf, 30 μL of tail blood was collected in FACS lysing solution (Becton-Dickinson) and the Mf counted using a haemocytometer. IAV infections were performed intranasally with 5x103 PFU A/WSN/33, a mouse H1N1 influenza A strain (Dr D. Jackson, University of St Andrews, St Andrews, UK), either 12, 34 or 68 days (d) post L. sigmodontis or mock infection. A/WSN/33 was grown in MDCK cells as described previously (24). Mice were weighed daily and assessed for visual signs of clinical disease as described previously (25, 26). Briefly, signs of infection were scored as follows, reduced mobility/activity (0-3), ruffled fur/piloerection (0-3), hunched posture (0-3) and increased or laboured breathing (0-3). The severity score presented is a sum of these criteria.
Influenza Viral Plaque Assay
MDCK cells were grown to confluence in 6 well plates (Corning) in DMEM (Gibco) containing 5% heat inactivated foetal calf serum (Gibco), 1% Penicillin and Streptomycin (Gibco) and 1% L-glutamine (Gibco). The left lobe of the lungs was recovered and mechanically homogenised with a TH homogeniser (OMNI) in 1.5 ml serum free DMEM before supernatants were recovered following centrifugation at 3000 rpm for 5 mins at 4°C. 10-fold serial dilutions of supernatants were placed onto MDCK cells in duplicate for 1 hour at 37°C. After removal and washing with DPBS (Gibco), a layer of 1% agarose containing 1 x EMEM (Invitrogen), 7.5% fraction V BSA (Sigma), 1% L-glutamine, 7.5 NaHCO2 (Invitrogen) 1M Hepes (Sigma) 1% Dextran (Sigma) 1% Penicillin and Streptomycin and 2μg/ml N-acetyl trypsin from bovine pancreas type V-S (Sigma) was added, plates were inverted and cultured for 3 days at 37°C, 5% CO2. Cells were fixed in 10% neutral buffered formalin (Sigma) and stained with 0.1% toluidine blue O (Sigma) for 20 minutes before plaques were quantified.
IFN-γ and IL-10 qPCR
A segment of the left lung lobe was homogenised in Trizol reagent (Thermo) in a tissue homogeniser (Precellys) with ceramic beads. RNA was then isolated with phenol/chloroform extraction according to manufacturer’s instructions and quantified with a NanoDrop (Thermo). cDNA first strand synthesis was performed with MultiScribe Reverse Transcriptase (Thermo) in a c1000 touch, thermal cycler (Bio-Rad). Murine 18s, IFN-γ and IL-10 were detected with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) on a StepOnePlus Real-Time PCR System (Thermo). Results are shown as 2^-(ΔΔCT) values.
Statistical Tests
Weight loss data were analysed by General Linear Model and Tukey’s method for pairwise comparisons in Minitab 20 (Minitab LLC). Clinical severity rank scoring data was analysed by Mann-Whitney non-parametric analysis in Prism 9 (Graphpad). Influenza viral titre was analysed by parametric, two tailed, unpaired T test in Prism 9. QPCR data for IL-10 and IFN-γ mRNA was analysed in JMP by two-way analysis of variance and Tukey’s method for pairwise comparisons.
Results
Larval-Stage L. sigmodontis Infection Reduces the Severity of Influenza A Clinical Signs
We first tested whether early larval stage L. sigmodontis infection could alter the progression of an acute IAV infection in the lung. Thirty L3 stage L sigmodontis larvae were given by subcutaneous injection into the back of 8-week-old female BALB/c mice to mimic the physiological route of infection. L. sigmodontis L3 larvae migrate to the pleural cavity via the lymphatics over the first 3-4 days of infection. They moult to the fourth larval (L4) stage between 8 -12 (d) post infection (pi) (27), and then to the adult stage between 25 – 30 d pi. Mice were challenged with 3x106 PFU influenza A infection intranasally, or mock infected, on d 12 of L. sigmodontis infection so that the 6-day course of influenza infection would take place during the L4 stage (Figure 1A), which is associated with a low-level Type 2 immune response (28, 29).
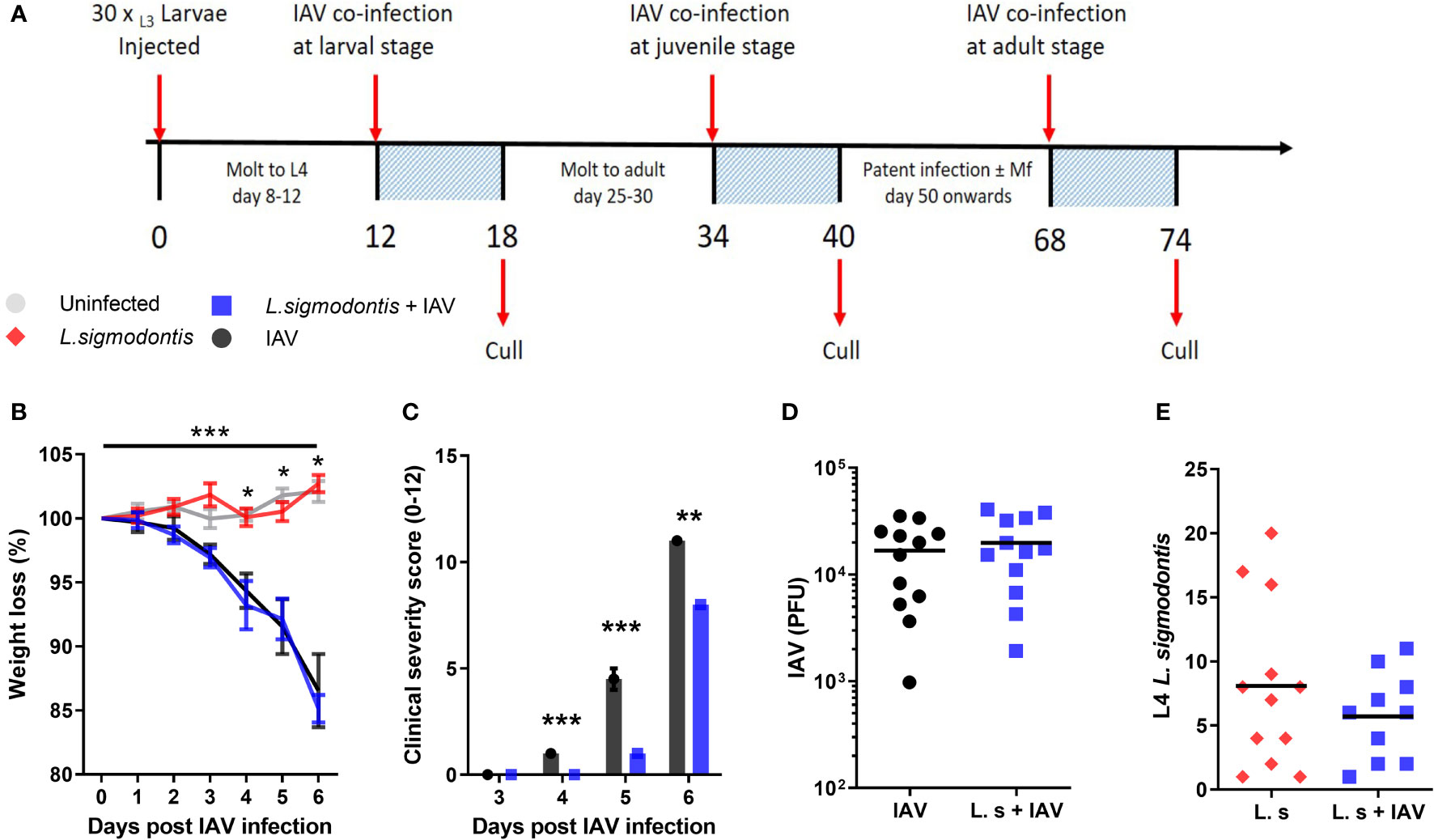
Figure 1 Larval stage L. sigmodontis infection reduces the severity of influenza A clinical signs. (A) Co-infection timeline. Mice were infected with 30 L3 L. sigmodontis larvae subcutaneously. 5x103 PFU IAV was then given intranasally at either d 12 (larval stage), 34 (juvenile adult stage) or 68 (patent infection) pi. Mice were culled 6 d after IAV infection. (B) Weight loss over time (d post IAV infection). Mean ± SEM shown. ***Significant effect between groups dependent upon time point, p < 0.001 (GLM). *p < 0.05 between IAV single infection and uninfected group, and co-infected and uninfected groups (Tukey’s HSD). (C) Combined IAV clinical severity score. Median ± MAD shown ***p < 0.001, **p < 0.01 (Mann Witney U test). (D) IAV titre (PFU) in lung homogenates 6 d post IAV infection. Mean and individual mice shown. (E) Numbers of L. sigmodontis (L4 larvae) recovered in pleural cavity lavage 6 d post IAV infection. Mean and individual mice shown. All panels show combined data from two independent experiments, (n=12 for all groups).
Weight loss was monitored over the 6-d course of co-infection as a clinical sign of IAV severity. This was not significantly altered in mice co-infected with larval stage L. sigmodontis compared with mice infected with IAV alone (Figure 1B). In contrast, other clinical signs associated with IAV infection including reduced movement, hunching and piloerection were significantly reduced in mice co-infected with larval stage L. sigmodontis (Figure 1C). At d 5 post IAV infection the average clinical score in IAV infected mice was 5.2 ± 0.75 while co-infected mice were 1.0 ± 0.45, with some mice not yet showing clinical signs and thereby scored as 0. To determine whether the reduced clinical signs correlated with lower viral replication, we measured the IAV lung titre 6 d post-IAV infection. IAV lung titres were not significantly altered in co-infected mice at 6 d post IAV infection (Figure 1D). There was also no difference in the number of L. sigmodontis L4 larvae recovered from co-infected mice and those infected with L. sigmodontis alone (Figure 1E).
Juvenile Adult-Stage L. sigmodontis Infection Delays Weight Loss and Progression of Influenza A Clinical Signs
Type 2 and immune regulatory responses increase as L. sigmodontis infection progresses (30). Thus, we hypothesised that co-infection with L. sigmodontis would have a more profound protective effect on IAV co-infection at later time points. L. sigmodontis L4 larvae molt towards the juvenile adult stage around 25 - 30 d pi, becoming reproductively mature adults around 55 d pi when they start producing Mf and infection becomes patent. To test whether the juvenile adult stage has a stronger protective effect on IAV co-infection, mice were co-infected with 5x103 IAV on d 34 of L. sigmodontis infection (Figure 1A). Weight loss was significantly delayed in the mice co-infected with juvenile L. sigmodontis parasites 3-5 d post IAV infection (Figure 2A). However, these mice reached the same maximum weight loss 6 d post IAV. In concordance, clinical signs of infection were also significantly reduced in co-infected mice 3 and 4 d post IAV infection, yet reached the same severity at 5 and 6 d post IAV infection (Figure 2B). These findings were independent of changes in viral lung titre (Figure 2C). Similar to the larval stage of L. sigmodontis infection, IAV co-infection had no impact on the number of L. sigmodontis parasites recovered (Figure 2D).
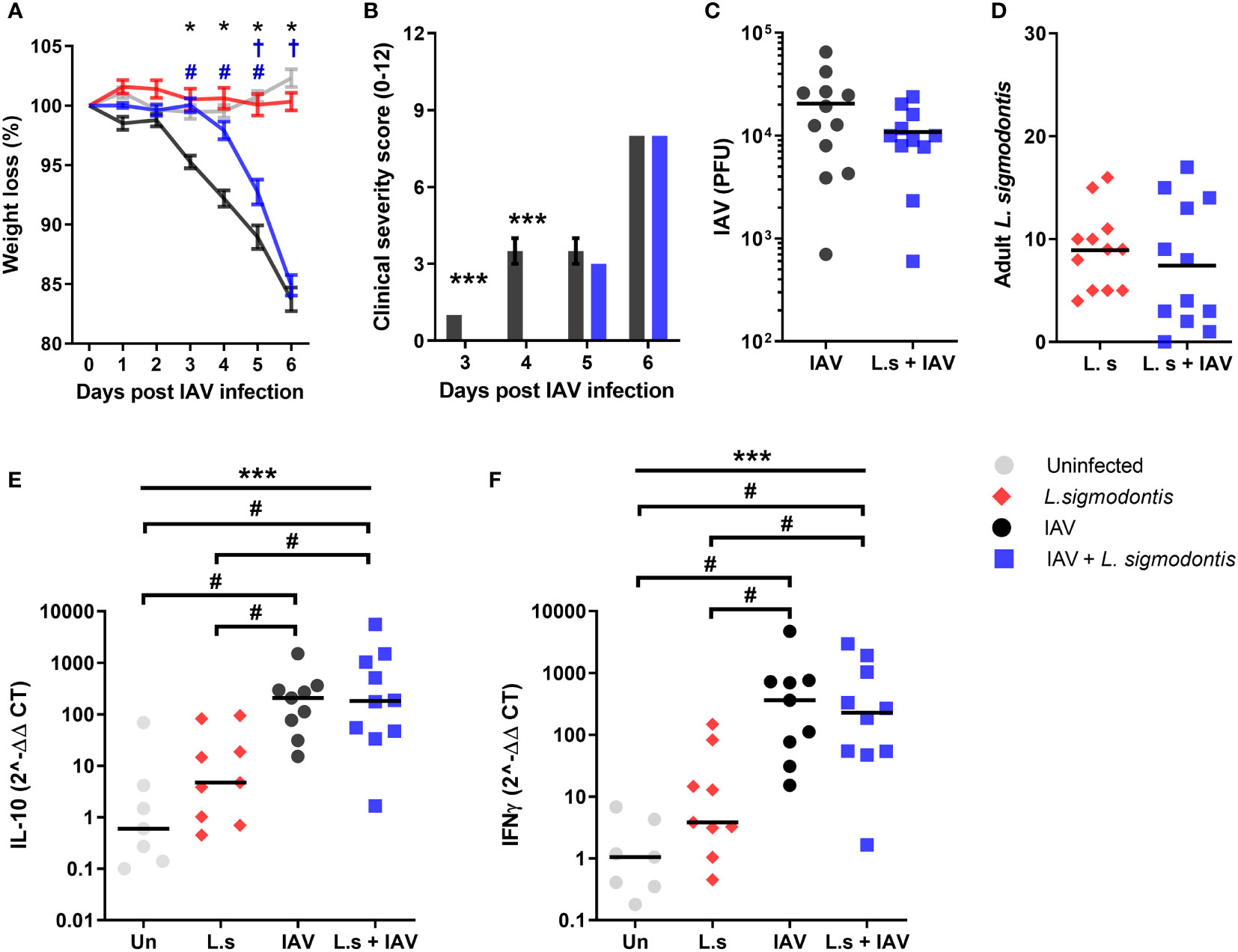
Figure 2 Juvenile adult stage of L. sigmodontis infection delays weight loss and progression of influenza A clinical signs. All panels show combined data from two independent experiments, uninfected n=8, IAV n=12, L. sigmodontis n=12, IAV + L. sigmodontis n=12. (A) Weight loss over time (d post IAV infection). Displayed as mean ± SEM. ***significant effect between groups dependent upon time point, p < 0.001 (GLM). *p < 0.05 significant effect between IAV and uninfected group (Tukey’s HSD). #p < 0.05 significant effect between IAV infected and coinfected group (Tukey’s HSD). †p < 0.05 significant effect between uninfected and coinfected group, (Tukey’s HSD). (B) Combined clinical severity score. Median ± MAD shown, ***p < 0.001 (Mann Whitney U test). (C) IAV titre (PFU) in lung homogenates 6 d post IAV infection. Mean and individual mice shown. (D) Numbers of adult L. sigmodontis recovered from the pleural cavity 6 d post IAV infection. Median and individual mice shown. (E, F) Levels of IL-10 (E) and IFN-γ (F) mRNA in lung tissue, normalised to 18s RNA expression (n=7-12). Mean and individual mice shown. ***Significant effect between groups (p < 0.001, ANOVA), #p < 0.05 (Tukey’s HSD).
To determine whether the reduced clinical signs were associated with a change in Type 1 or regulatory cytokines, quantitative PCR was used to measure IFN-γ and IL-10 within the lung. Single IAV infection resulted in significantly increased mRNA expression of IL-10 and IFN-γ compared to the naïve controls (Figures 2E, F). However, expression of IL-10 and IFN-γ mRNA was unaltered upon co-infection. L. sigmodontis infection alone did not result in increased levels of IL-10 and IFN-γ mRNA within the lung.
Co-Infection During Patent L. sigmodontis Infection Increases Susceptibility to IAV, and Blocks Age-Related Resistance to IAV Clinical Signs
L. sigmodontis infection becomes patent at around 55 d pi, at which point the adult parasites are fully mature and are releasing Mf into the blood. Patency associates with a switch from a predominant Th2 response towards a mixed Th2/Th1 immune response, and the development of additional layers of immune regulation (16). To determine whether patent L. sigmodontis infection alters susceptibility to IAV, mice were co-infected with IAV at d 68 of L. sigmodontis infection (Figure 1).
BALB/c mice show increasing resistance to IAV (A/WSN/33) infection with age (31), and as patent L. sigmodontis infection takes over 2 months to develop, the mice were 4-5 months of age at the time of IAV challenge. Consistent with their age, the weight loss due to IAV infection alone was reduced compared to previous time points, with a mean weight loss of 4.9% at 6 d post IAV infection (Figure 3A). However, co-infected mice demonstrated significantly higher weight loss in response to IAV infection, with a mean weight loss of 17.0% by d 6. Furthermore, whilst clinical signs of infection were only detected in IAV infected mice at d 6 (average score of 1.0 ± 0.27), co-infected animals demonstrated clinical symptoms by d 4 and progressed to show increasingly severe clinical signs at 6 d post IAV infection with an average score of 10.0 ± 1.17 (Figure 3B). Co-infected mice also had a significantly increased IAV lung titre, with a 3.9-fold increase in PFU compared with IAV infection alone (Figure 3C).
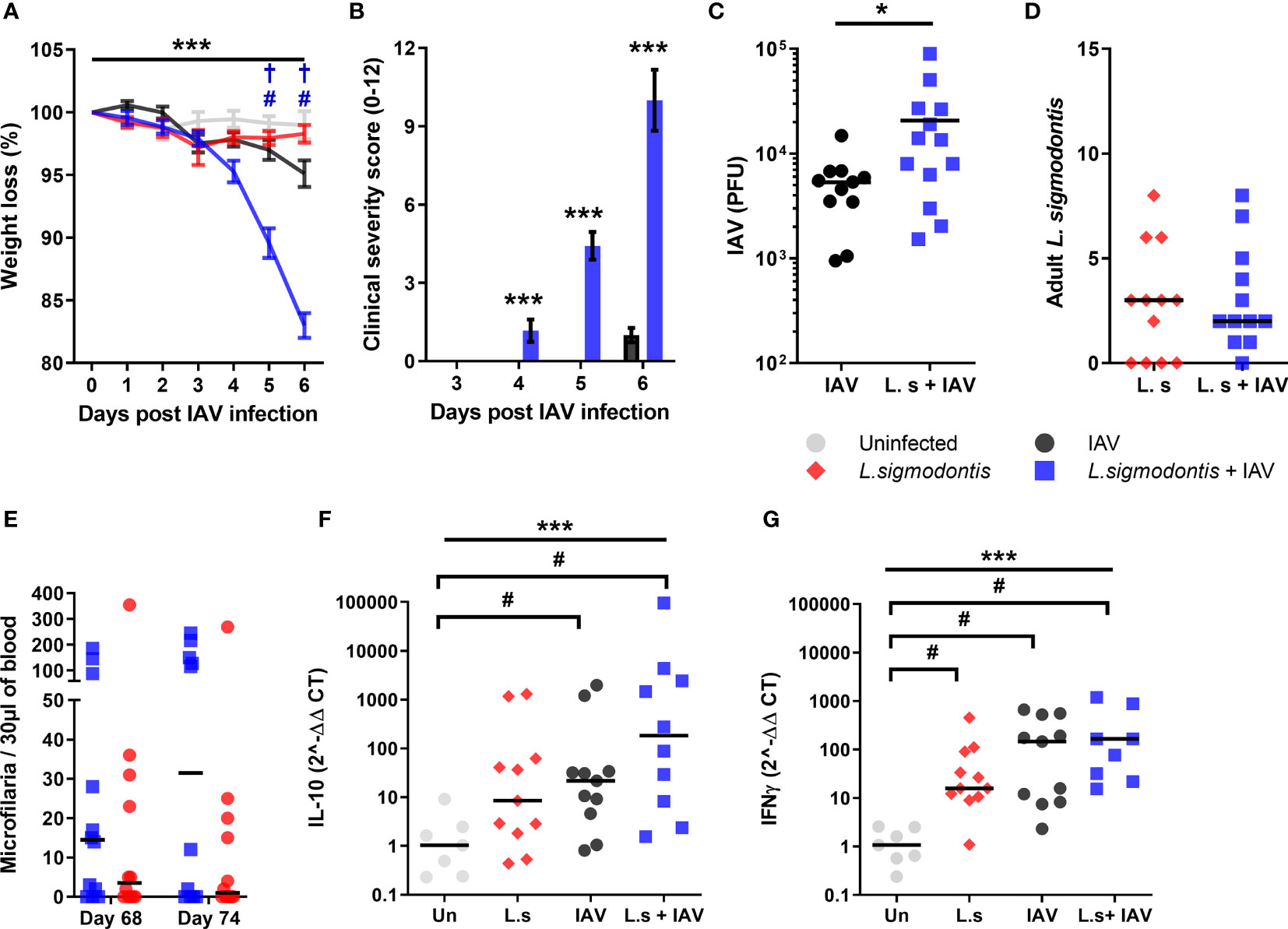
Figure 3 Co-infection during patent L. sigmodontis infection increases susceptibility to IAV, and blocks age-related resistance to IAV clinical signs. All panels show combined data from two independent experiments. Uninfected n=12, IAV n=11, L. sigmodontis n=12, IAV + L. sigmodontis n=13 (A) Weight loss over time (d post IAV infection) shown as mean ± SEM, ***significant effect between groups over time, p < 0.001 (GLM). #p < 0.05 significant effect between IAV infected and coinfected group (Tukey’s HSD). † p < 0.05 significant effect between uninfected and coinfected group (Tukey’s HSD). (B) Combined clinical severity score. Median ± MAD shown. ***p < 0.001 (Mann Whitney U test) (C) IAV titre (PFU) in lung homogenates 6 d post IAV infection. Mean ± SEM. *p < 0.05 (unpaired T test). (D) Numbers of adult L. sigmodontis recovered 6 d post IAV infection. Median and individual mice shown (L.sigmodontis n=12, IAV + L. sigmodontis n=13). (E) Mf count in 30 μl blood before and after IAV co-infection. Median and individual mice shown. (F, G) Expression of IL-10 (F) and IFN-γ (G) mRNA in the lung. ***Significant effect between groups, p < 0.001 (ANOVA), #p < 0.05 (Tukey’s HSD).
As with co-infection at earlier stages of L. sigmodontis infection, IAV did not alter susceptibility to L. sigmodontis. The total numbers of adult L. sigmodontis remained similar between co-infected and L. sigmodontis infected mice (Figure 3D). Co-infection also had no impact on the numbers of circulating Mf in the blood, with L. sigmodontis singly and co-infected mice showing similar levels of Mf immediately prior to, and after, IAV infection (Figure 3E). Similarly, the incidence of mice developing blood Mf remained the same between groups both before (co-infected 8/14, L. sigmodontis only 10/14) and at d 6 of IAV infection (co-infected 9/14, L. sigmodontis only 7/14).
Quantitative PCR was used to determine whether the increased lung pathology was associated with alterations in IFN-γ or IL-10 mRNA expression within the lung. There were significant increases in mRNA expression of IL-10 and IFN-γ in the lung as a result of IAV infection compared with uninfected mice, and significantly increased IFN-γ due to L. sigmodontis infection alone (Figures 3F, G). However, co-infected mice did not display differences in expression of mRNA of either cytokine compared to the single infections.
Discussion
This study demonstrates that pre-existing L. sigmodontis infection can significantly ameliorate or worsen the progression of acute respiratory infection with Influenza A virus (IAV), with the outcome dependent upon the stage of L. sigmodontis infection. Co-infection with IAV during the larval and juvenile adult stages of L. sigmodontis infection delayed the onset of clinical signs, whilst co-infection during patent L. sigmodontis infection increased IAV clinical signs, weight loss, and viral loads.
Mice infected with the intestinal parasite Trichinella spiralis at an early enteric stage were found to undergo faster recovery from IAV induced weight loss (32). Infection with pre-patent L. sigmodontis also significantly decreased the severity of IAV co-infection, although the protective effect presented as a reduction in the initial severity of infection. This protection was more pronounced during the juvenile adult stage of L. sigmodontis infection than the larval L4 stage, with the juvenile adult stage delaying weight loss as well as clinical signs. This contrasts to coinfection with L. sigmodontis and Friend retrovirus where L4 stage L. sigmodontis infection caused significantly increased splenomegaly and viral loads (33). Decreased IAV severity was independent of viral titre and helminth burden, which were unaltered by co-infection at either of the pre-patent life stages. The dose of IAV was chosen to cause an infection of moderate severity that could modulated up or down by coinfection without mortality. As the initial dose of IAV can instruct early interferon, cytokine and chemokine expression (34), different IAV doses could result in different coinfection outcomes. In particular, L. sigmodontis only delayed the onset of clinical signs and weight loss, raising a question of whether the protective effect of L. sigmodontis infection would still be sufficient to ameliorate severity during high dose, more pathogenic, IAV co-infection.
IL-10 plays an important regulatory role during L. sigmodontis infection (35), and is involved in suppressing cerebral malaria during L. sigmodontis co-infection (11). Similarly, concurrent pre-patent L. sigmodontis infection suppresses IAV immunisation in an IL-10 dependent manner (22). Administration of the immunomodulatory filarial cystatin (AvCystatin/Av17), which stimulates IL-10 producing Foxp3+ Tregs, can also protect against inflammation and weight loss in a model of respiratory syncytial virus inflammation (36). However, at the endpoint, IL-10 expression in the lung homogenates of L. sigmodontis and IAV co-infected mice did not correlate with the increased protection. Whilst measuring IL-10 protein production at earlier time points and in specific cell populations would give a more accurate representation of IL-10 activity, this data could suggest an IL-10 independent effect.
In contrast to the protective effect of pre-patent L. sigmodontis infection, patent L. sigmodontis infection worsened the progression of IAV infection. Mice develop age-related resistance to IAV (31), displaying reduced clinical signs and weight loss as they age. Patent L. sigmodontis infection countered this age-related resistance and significantly worsened weight loss and clinical signs of IAV infection, as well as increasing IAV titre. The impaired protection is similar to studies demonstrating that T. spiralis infection impairs immunity to murine norovirus (37), but contrasts with Heligmosomoides polygyrus and respiratory syncytial virus coinfection where H. polygyrus enhanced resistance to the virus through type 1 interferons (38). An increasing number of immune regulatory pathways develop as infection progresses to patency, including Tregs (28, 39), alternatively activated macrophages (40), and T cell-intrinsic dysfunction (10, 29, 41), and increased suppression of third-party immune responses are seen as infection matures (42). An increased immune-regulatory response may have hindered the immune system’s ability to control IAV replication, with the resultant increase in viral burden exacerbating immune pathology. However, as overspill of immune regulation could also protect against pathology, the immune regulatory mechanism would need to inhibit protective, but not pathogenic, immune responses to IAV.
An alternative reason for the increased susceptibility to IAV infection could relate to perturbations in cytokine production during infection. The induction of Type 2 responses by helminths are found to be sufficient to reactivate latent murine γ-herpesvirus infection (43). However, pre-patent L. sigmodontis infection stimulates a Type 2 response and protected against influenza signs and weight loss, suggesting that the Type 2 response at patency is unlikely to have worsened the progression of IAV infection. In contrast with pre-patent infection, patent L. sigmodontis infection does associate with the development of a mixed Th1/Th2 response (10). Patent L. sigmodontis singly infected mice showed increased levels of IFN-γ in their lung homogenates, that were not present during pre-patent infection. IFN-γ, along with IL-5, is part of the protective response against microfilaria (44), and microfilaremia associates with increased severity of LPS-induced inflammation due to elevated IFN-γ, TNF-α, IL-6 and IL-12 expression (16). Similarly, increased IFN-γ correlates with more severe disease in L. sigmodontis and Plasmodium chabaudi chabaudi co-infected mice. However, the effect of IFN-γ levels on concurrent infections is context dependent. During Leishmania major coinfection, L. sigmodontis-enhanced IFN-γ responses were associated with a delay in disease onset rather than enhancement of pathology (15), whilst L. sigmodontis infection does not affect the generation of Th1 IFN-γ driven responses and susceptibility during concurrent infection with Mycobacterium tuberculosis (17). Although L. sigmodontis infection did not increase IFN-γ mRNA levels in IAV coinfected mice, IFN-γ is a key factor determining the extent of pathology (26), and future studies should explore IFN-γ as a potential mechanism.
Malaria models have also highlighted the importance of L. sigmodontis infection stage on the outcome of coinfection. Similar to IAV coinfection, larval stage L. sigmodontis infection protects against pathology in P. yoelii and P. chaboudi infections. Although it also increases resistance to P. yoelii, and co-infection with either malaria species increases resistance to L. sigmodontis (12). Patent L. sigmodontis infection also worsened pathology in P. chaboudi coinfected mice, again mirroring IAV coinfection (13, 14). However, contrasting with IAV and P. chaboudi, patent L. sigmodontis infection protected 30% of co-infected mice from P. berghei infection again indicating that the effects of coinfection are context dependent (34). Not all mice develop patent L. sigmodontis infection (defined by Mf circulating in the blood), and the extent of P. chaboudi pathology correlated with the presence or absence of blood Mf, with more severe pathology in Mf negative mice (13, 14). Similarly, Mf negative mice showed lower protection against P. berghei (13, 14). All but 2 mice developed Mf between d 68 and 74 post-L. sigmodontis infection in our study, and so it was not possible to determine whether Mf status impacted the outcome of IAV. As Mf do stimulate IFN-γ production (45), it would be interesting to determine whether the exacerbation of pathology by patent L. sigmodontis infection is due to the release of Mf.
This study demonstrates that there are interactions between the filarial helminth and acute respiratory viral infections, and that the presence of a helminth infection can both ameliorate and worsen IAV severity with the outcome dependent upon the stage of helminth infection. Further research is required to elucidate the mechanisms by which this interaction occurs. This suggests that consideration of concomitant infection with filarial helminths may be a significant factor in the treatment and outcome of IAV and other respiratory infections such as SARS-CoV-2, where the expression of type 2 cytokine responses is associated with increased disease severity (46).
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by University of Edinburgh Animal Welfare and Ethical Review Body.
Author Contributions
BD, JH, GH, and MT designed the experiments. GH, JK, AF and MT conducted the experiments and collected the data. GH, BD, and MT analysed the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Program Grant BB/J004324/1 to The Roslin Institute. GH was funded by a BBSRC Doctoral Training Grant to the Centre for Infectious Diseases, University of Edinburgh. MT, AF, and JK were funded by the Medical Research Council (MRC) UK grant number MR/K020196/1, and the Wellcome Trust grant number 095831. Open access publication costs were provided by the UKRI Open Access Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to gratefully acknowledge the contributions of The Bioimaging and Flow Cytometry Facility (Roslin Institute) and Bioresearch & Veterinary Services (University of Edinburgh).
Abbreviations
IAV, Influenza A virus; Mf, microfilaria; L3, third larval stage; d, day; pi, post infection; L4, fourth larval stage.
References
1. Cano J, Rebollo MP, Golding N, Pullan RL, Crellen T, Soler A, et al. The Global Distribution and Transmission Limits of Lymphatic Filariasis: Past and Present. Parasit Vectors (2014) 7(1):1–19. doi: 10.1186/s13071-014-0466-x
2. Donohue RE, Cross ZK, Michael E. The Extent, Nature, and Pathogenic Consequences of Helminth Polyparasitism in Humans: A Meta-Analysis. Akullian A, Editor. PloS Negl Trop Dis (2019) 13(6):e0007455. doi: 10.1371/journal.pntd.0007455
3. Babu S, Nutman TB. Immunology of Lymphatic Filariasis. Parasite Immunol (2014) 36(8):338–46. doi: 10.1111/pim.12081
4. Maizels RM, Smits HH, McSorley HJ. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity (2018) 49(5):801–18. doi: 10.1016/j.immuni.2018.10.016
5. Maizels RM, McSorley HJ. Regulation of the Host Immune System by Helminth Parasites. J Allergy Clin Immunol (2016) 138(3):666–75. doi: 10.1016/j.jaci.2016.07.007
6. Nutman TB. Looking Beyond the Induction of Th2 Responses to Explain Immunomodulation by Helminths. Parasite Immunol (2015) 37(6):304–13. doi: 10.1111/pim.12194
7. Maizels RM, Yazdanbakhsh M. Immune Regulation by Helminth Parasites: Cellular and Molecular Mechanisms. Nat Rev Immunol (2003) 3(9):733–44. doi: 10.1038/nri1183
8. Mishra PK, Palma M, Bleich D, Loke P, Gause WC. Systemic Impact of Intestinal Helminth Infections. Mucosal Immunol (2014) 7(4):753–62. doi: 10.1038/mi.2014.23
9. Moreau E, Chauvin A. Immunity Against Helminths: Interactions With the Host and the Intercurrent Infections. J BioMed Biotechnol (2010) 2010:1–9. doi: 10.1155/2010/428593
10. Finlay CM, Allen JE. The Immune Response of Inbred Laboratory Mice to Litomosoides Sigmodontis: A Route to Discovery in Myeloid Cell Biology. Parasite Immunol (2020) (7):1–17. doi: 10.1111/pim.12708
11. Specht S, Ruiz DF, Dubben B, Deininger S, Hoerauf A. Filaria-Induced IL-10 Suppresses Murine Cerebral Malaria. Microbes Infect (2010) 12(8–9):635–42. doi: 10.1016/j.micinf.2010.04.006
12. Karadjian G, Berrebi D, Dogna N, Vallarino-Lhermitte N, Bain O, Landau I, et al. Co-Infection Restrains Litomosoides Sigmodontis Filarial Load and Plasmodial P. Yoelii But Not P. Chabaudi Parasitaemia in Mice. Parasite (2014) 21:16. doi: 10.1051/parasite/2014017
13. Fernández Ruiz D, Dubben B, Saeftel M, Endl E, Deininger S, Hoerauf A, et al. Filarial Infection Induces Protection Against P. Berghei Liver Stages in Mice. Microbes Infect (2009) 11(2):172–80. doi: 10.1016/j.micinf.2008.11.003
14. Graham AL, Lamb TJ, Read AF, Allen JE. Malaria-Filaria Coinfection in Mice Makes Malarial Disease More Severe Unless Filarial Infection Achieves Patency. J Infect Dis (2005) 191(3):410–21. doi: 10.1086/426871
15. Lamb TJ, Graham AL, Le Goff L, Allen JE. Co-Infected C57BL/6 Mice Mount Appropriately Polarized and Compartmentalized Cytokine Responses to Litomosoides Sigmodontis and Leishmania Major But Disease Progression Is Altered. Parasite Immunol (2005) 27(9):317–24. doi: 10.1111/j.1365-3024.2005.00779.x
16. Hubner MP, Pasche B, Kalaydjiev S, Soboslay PT, Lengeling A, Schulz-Key H, et al. Microfilariae of the Filarial Nematode Litomosoides Sigmodontis Exacerbate the Course of Lipopolysaccharide-Induced Sepsis in Mice. Infect Immun (2008) 76(4):1668–77. doi: 10.1128/IAI.01042-07
17. Hübner MP, Killoran KE, Rajnik M, Wilson S, Yim KC, Torrero MN, et al. Chronic Helminth Infection Does Not Exacerbate Mycobacterium Tuberculosis Infection. MacDonald AS, Editor. PloS Negl Trop Dis (2012) 6(12):e1970. doi: 10.1371/journal.pntd.0001970
18. Chen X, Liu S, Goraya MU, Maarouf M, Huang S, Chen J-L. Host Immune Response to Influenza A Virus Infection. Front Immunol (2018) 9:320/full. doi: 10.3389/fimmu.2018.00320/full
19. Liu Q, Zhou Y, Yang Z. The Cytokine Storm of Severe Influenza and Development of Immunomodulatory Therapy. Cell Mol Immunol (2016) 13(1):3–10. doi: 10.1038/cmi.2015.74
20. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev (2012) 76(1):16–32. doi: 10.1128/MMBR.05015-11
21. Price I, Mochan-Keef ED, Swigon D, Ermentrout GB, Lukens S, Toapanta FR, et al. The Inflammatory Response to Influenza A Virus (H1N1): An Experimental and Mathematical Study. J Theor Biol (2015) 374:83–93. doi: 10.1016/j.jtbi.2015.03.017
22. Hartmann W, Brunn M-L, Stetter N, Gagliani N, Muscate F, Stanelle-Bertram S, et al. Helminth Infections Suppress the Efficacy of Vaccination Against Seasonal Influenza. Cell Rep (2019) 29(8):2243–56. doi: 10.1016/j.celrep.2019.10.051
23. Fulton A, Babayan SA, Taylor MD. Use of the Litomosoides Sigmodontis Infection Model of Filariasis to Study Type 2 Immunity, in: Type 2 Immunity (2018). New York, NY: Springer New York. Available at: http://link.springer.com/10.1007/978-1-4939-7896-0_2 (Accessed cited 2019 Jul 18).
24. Nicol MQ, Ligertwood Y, Bacon MN, Dutia BM, Nash AA. A Novel Family of Peptides With Potent Activity Against Influenza A Viruses. J Gen Virol (2012) 93(Pt_5):980–6. doi: 10.1099/vir.0.038679-0
25. Bouvier NM, Lowen AC. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses (2010) 2(8):1530–63. doi: 10.3390/v20801530
26. Nicol MQ, Campbell GM, Shaw DJ, Dransfield I, Ligertwood Y, Beard PM, et al. Lack of Ifnγ Signaling Attenuates Spread of Influenza A Virus In Vivo and Leads to Reduced Pathogenesis. Virology (2019) 526:155–64. doi: 10.1016/j.virol.2018.10.017
27. Karadjian G, Fercoq F, Pionnier N, Vallarino-Lhermitte N, Lefoulon E, Nieguitsila A, et al. Migratory Phase of Litomosoides Sigmodontis Filarial Infective Larvae Is Associated With Pathology and Transient Increase of S100A9 Expressing Neutrophils in the Lung. Brehm K, Editor. PloS Negl Trop Dis (2017) 11(5):e0005596. doi: 10.1371/journal.pntd.0005596
28. Taylor MD, van der Werf N, Harris A, Graham AL, Bain O, Allen JE, et al. Early Recruitment of Natural CD4+Foxp3+ Treg Cells by Infective Larvae Determines the Outcome of Filarial Infection. Eur J Immunol (2009) 39(1):192–206. doi: 10.1002/eji.200838727
29. van der Werf N, Redpath SA, Azuma M, Yagita H, Taylor MD. Th2 Cell-Intrinsic Hypo-Responsiveness Determines Susceptibility to Helminth Infection. PloS Pathog (2013) 9(3):e1003215. doi: 10.1371/journal.ppat.1003215
30. Babayan S, Ungeheuer M-N, Martin C, Attout T, Belnoue E, Snounou G, et al. Resistance and Susceptibility to Filarial Infectionwith Litomosoides Sigmodontis Are Associated With EarlyDifferences in Parasite Development and in Localized ImmuneReactions. Infect Immun (2003) 71(12):6820–9. doi: 10.1128/IAI.71.12.6820-6829.2003
31. Lu J, Duan X, Zhao W, Wang J, Wang H, Zhou K, et al. Aged Mice Are More Resistant to Influenza Virus Infection Due to Reduced Inflammation and Lung Pathology. Aging Dis (2018) 9(3):358. doi: 10.14336/AD.2017.0701
32. Furze RC, Hussell T, Selkirk ME. Amelioration of Influenza-Induced Pathology in Mice by Coinfection With Trichinella Spiralis. Infect Immun (2006) 74(3):1924–32. doi: 10.1128/IAI.74.3.1924-1932.2006
33. Dietze KK, Dittmer U, Koudaimi DK, Schimmer S, Reitz M, Breloer M, et al. Filariae-Retrovirus Co-Infection in Mice Is Associated With Suppressed Virus-Specific IgG Immune Response and Higher Viral Loads. Hsieh MH, Editor. PloS Negl Trop Dis (2016) 10(12):e0005170. doi: 10.1371/journal.pntd.0005170
34. Marois I, Cloutier A, Garneau É, Richter MV. Initial Infectious Dose Dictates the Innate, Adaptive, and Memory Responses to Influenza in the Respiratory Tract. J Leukoc Biol (2012) 92(1):107–21. doi: 10.1189/jlb.1011490
35. Hartmann W, Schramm C, Breloer M. Litomosoides Sigmodontis Induces TGF-β Receptor Responsive, IL-10-Producing T Cells That Suppress Bystander T-Cell Proliferation in Mice: Immunomodulation. Eur J Immunol (2015) 45(9):2568–81. doi: 10.1002/eji.201545503
36. Schuijs MJ, Hartmann S, Selkirk ME, Roberts LB, Openshaw PJM, Schnoeller C. The Helminth-Derived Immunomodulator AvCystatin Reduces Virus Enhanced Inflammation by Induction of Regulatory IL-10+ T Cells. Sun J, Editor. PloS One (2016) 11(8):e0161885. doi: 10.1371/journal.pone.0161885
37. Osborne LC, Monticelli LA, Nice TJ, Sutherland TE, Siracusa MC, Hepworth MR, et al. Virus-Helminth Coinfection Reveals a Microbiota-Independent Mechanism of Immunomodulation. Science (2014) 345(6196):578–82. doi: 10.1126/science.1256942
38. McFarlane AJ, McSorley HJ, Davidson DJ, Fitch PM, Errington C, Mackenzie KJ, et al. Enteric Helminth-Induced Type I Interferon Signaling Protects Against Pulmonary Virus Infection Through Interaction With the Microbiota. J Allergy Clin Immunol (2017) 140(4):1068–1078.e6. doi: 10.1016/j.jaci.2017.01.016
39. Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of Regulatory T Cell Activity Reverses Hyporesponsiveness and Leads to Filarial Parasite Clearance In Vivo. J Immunol (2005) 174(8):4924–33. doi: 10.4049/jimmunol.174.8.4924
40. Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80 + Alternatively Activated Macrophages Control CD4 + T Cell Hyporesponsiveness at Sites Peripheral to Filarial Infection. J Immunol (2006) 176(11):6918–27. doi: 10.4049/jimmunol.176.11.6918
41. Knipper JA, Ivens A, Taylor MD. Helminth-Induced Th2 Cell Dysfunction is Distinct From Exhaustion and Is Maintained in the Absence of Antigen. Makepeace BL, Editor. PloS Negl Trop Dis (2019) 13(12):e0007908. doi: 10.1371/journal.pntd.0007908
42. Haben I, Hartmann W, Breloer M. Nematode-Induced Interference With Vaccination Efficacy Targets Follicular T Helper Cell Induction and Is Preserved After Termination of Infection. Mitre E, Editor. PloS Negl Trop Dis (2014) 8(9):e3170. doi: 10.1371/journal.pntd.0003170
43. Reese TA, Wakeman BS, Choi HS, Hufford MM, Huang SC, Zhang X, et al. Helminth Infection Reactivates Latent γ-Herpesvirus via Cytokine Competition at a Viral Promoter. Science (2014) 345(6196):573–7. doi: 10.1126/science.1254517
44. Saeftel M, Arndt M, Specht S, Volkmann L, Hoerauf A. Synergism of Gamma Interferon and Interleukin-5 in the Control of Murine Filariasis. Infect Immun (2003) 71(12):6978–85. doi: 10.1128/IAI.71.12.6978-6985.2003
45. Lawrence RA, Allen JE, Osborne J, Maizels RM. Adult and Microfilarial Stages of the Filarial Parasite Brugia Malayi Stimulate Contrasting Cytokine and Ig Isotype Responses in BALB/c Mice. J Immunol Baltim Md 1950 (1994) 153(3):1216–24.
Keywords: helminth, coinfection, mouse, respiratory virus, filariasis, influenza A virus
Citation: Hardisty GR, Knipper JA, Fulton A, Hopkins J, Dutia BM and Taylor MD (2022) Concurrent Infection With the Filarial Helminth Litomosoides sigmodontis Attenuates or Worsens Influenza A Virus Pathogenesis in a Stage-Dependent Manner. Front. Immunol. 12:819560. doi: 10.3389/fimmu.2021.819560
Received: 21 November 2021; Accepted: 29 December 2021;
Published: 24 January 2022.
Edited by:
Manuel Ritter, University Hospital Bonn, GermanyReviewed by:
Lubna Pinky, University of Tennessee Health Science Center (UTHSC), United StatesMinka Breloer, Bernhard Nocht Institute for Tropical Medicine (BNITM), Germany
Copyright © 2022 Hardisty, Knipper, Fulton, Hopkins, Dutia and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew D. Taylor, TWF0dGhldy5UYXlsb3JAZWQuYWMudWs=
 Gareth R. Hardisty
Gareth R. Hardisty Johanna A. Knipper
Johanna A. Knipper Alison Fulton2
Alison Fulton2 Matthew D. Taylor
Matthew D. Taylor