- 1Laboratory of Dr. Joan W. Berman, Department of Pathology, Albert Einstein College of Medicine, Bronx, NY, United States
- 2Laboratory or Dr. David J. Volsky, Department of Medicine, Icahn School of Medicine at Mount Sinai, Manhattan, NY, United States
Thirty-eight million people worldwide are living with HIV, PWH, a major public health problem. Antiretroviral therapy (ART) revolutionized HIV treatment and significantly increased the lifespan of PWH. However, approximately 15-50% of PWH develop HIV associated neurocognitive disorders (HIV-NCI), a spectrum of cognitive deficits, that negatively impact quality of life. Many PWH also have opioid use disorder (OUD), and studies in animal models of HIV infection as well as in PWH suggest that OUD can contribute to HIV-NCI. The synthetic opioid agonist, buprenorphine, treats OUD but its effects on HIV-NCI are unclear. We reported that human mature inflammatory monocytes express the opioid receptors MOR and KOR, and that buprenorphine reduces important steps in monocyte transmigration. Monocytes also serve as HIV reservoirs despite effective ART, enter the brain, and contribute to HIV brain disease. Using EcoHIV infected mice, an established model of HIV infection and HIV-NCI, we previously showed that pretreatment of mice prior to EcoHIV infection reduces mouse monocyte entry into the brain and prevents NCI. Here we show that buprenorphine treatment of EcoHIV infected mice with already established chronic NCI completely reverses the disease. Disease reversal was associated with a significant reduction in brain inflammatory monocytes and reversal of dendritic injury in the cortex and hippocampus. These results suggest that HIV-NCI persistence may require a continuing influx of inflammatory monocytes into the brain. Thus, we recommend buprenorphine as a potential therapy for mitigation of HIV brain disease in PWH with or without OUD.
Introduction
Thirty eight million people globally, including 1.2 million in the US, are living with HIV, PWH (1). Antiretroviral therapy (ART) revolutionized HIV treatment, enabling long-term suppression of HIV replication, improvement in immune functions, and increased lifespan (2). Despite the success of ART, 15-50% of PWH develop HIV associated neurocognitive impairment, HIV-NCI (3–8). HIV-NCI is a spectrum of cognitive deficits that negatively impact the quality of life for PWH and is an independent risk factor for morbidity and mortality (5, 6, 9, 10). Despite several successful treatment attempts for HIV-NCI in preclinical animal models no approved therapy for NCI in PWH has emerged (11–17).
HIV enters the brain early after peripheral infection, establishing a long-lived viral reservoir in myeloid cells in the CNS that is difficult to detect and is not eliminated by ART (6, 18, 19). This reservoir mediates ongoing low level neuroinflammation and CNS damage (20–23). One major proposed mechanism responsible for the establishment and reseeding of the HIV CNS reservoir and associated neuroinflammation is the migration of HIV infected and uninfected CD14+ CD16+ (mature) monocytes into the brain (22, 24–28). These cells can serve as a source of neurotoxic host and viral products and contribute to cognitive dysfunction (23, 29–32). Studies have shown that mature monocytes cross the blood brain barrier, BBB, and enter the brain in response to chemokines including CCL2 (18, 33–36). These cells can also differentiate into long lived perivascular macrophages (37). Increased presence of perivascular CD163-positive macrophages in the brain correlates with CNS disease progression in SIV infected non-human primates (38). EcoHIV infected athymic mice were shown to have increased numbers of inflammatory macrophages in the brain and develop HIV-NCI-like disease despite a lack of mature T lymphocytes, suggesting that macrophage infection is sufficient for causing HIV neuropathogenesis (39). Similarly, HIV infection of mice reconstituted with human myeloid precursor cells results in pathologic and virologic markers of HIV brain disease (40). Other studies in non-human primates and mice have also underscored the importance of monocytes in this process (41–44). There are also data that show a correlation between HIV DNA in mature monocytes and cognitive impairment in PWH (27, 28, 45). Thus, monocyte migration to the CNS may be a critical step in HIV-NCI neuropathogenesis to target for future therapies.
Opioid use disorder, OUD, significantly impacts the quality of life for PWH, reduces ART adherence, and is associated with worse cognitive outcomes in some individuals (46–49). Buprenorphine, an opioid derivative, is a commonly used opioid agonist therapy (OAT) to treat OUD (50–53). In some studies, buprenorphine was shown to improve neuropsychological outcomes in people with OUD, as well as in a small study including PWH with OUD (54–56). The effects of buprenorphine on mechanisms of HIV neuropathogenesis remain unclear. Buprenorphine is a partial agonist of the mu opioid receptor, MOR, and a full antagonist of the kappa opioid receptor, KOR (52). These receptors are expressed on mature monocytes, macrophages, and cells of the CNS indicating that buprenorphine can bind to these cells and modulate their functions (57–59). We previously showed that buprenorphine decreases critical steps in human monocyte transmigration across the BBB, including CCL2 mediated adhesion and chemotaxis (57, 60). These findings suggest that buprenorphine may improve neurocognitive outcomes in PWH by limiting the entry of HIV infected and uninfected monocytes into the brain.
In our previous study, we used buprenorphine as a prophylactic treatment three days before and during EcoHIV infection of immunocompetent mice, an established animal model of naturally suppressed HIV infection and HIV-NCI-like disease, EcoHIV-NCI (61). When daily buprenorphine injections were initiated prior to EcoHIV infection and continued for four weeks until evaluation, EcoHIV-NCI was prevented (61). This was correlated with a marked reduction of inflammatory monocytes in the brain and no effect on peripheral infection (61). These results showed that buprenorphine prophylaxis does not affect systemic EcoHIV infection and suggested that limiting monocyte transmigration into the CNS prevents processes required for HIV-NCI manifestation in mice.
In this present study, we examined whether ongoing entry of inflammatory monocytes into the brain is required after persistent NCI has been established and whether buprenorphine can interrupt this process and potentially reverse cognitive impairment. We show that daily buprenorphine treatment of cognitively impaired mice reverses the disease along with significant reductions in brain inflammatory monocytes, HIV DNA in the brain, and hippocampal and cortical synaptodendritic injury. These results indicate that HIV-NCI persistence may require a continued influx of inflammatory monocytes and that buprenorphine could be a potential therapy for HIV CNS disease in PWH with or without OUD.
Materials and methods
Mice
Eight-week-old, male, C57BL/6J (Jackson Laboratory, Bar Harbor, ME) were used. We maintained the mice under appropriate husbandry conditions and ensured all animals had minimized distress, discomfort, and injury. All studies were approved by the Icahn School of Medicine at Mount Sinai and the Albert Einstein College of Medicine Institutional Animal Care and Use Committees and are in compliance with the US Animal Welfare Act PHS policies, animal welfare assurance D16-00069.
Buprenorphine injections, EcoHIV infection, and tissue collection
Four treatment groups of mice were used: Control (CTRL), did not receive buprenorphine or virus; ECOHIV, was infected but did not receive buprenorphine; ECOHIV BUP, was infected and received buprenorphine; and BUP, received buprenorphine and was not infected. Mice were infected with a single intraperitoneal dose of 2 x 106 pg of EcoHIV p24 or with diluent control, PBS. Buprenorphine was injected subcutaneously, daily, at a dose of 0.2 mg/kg (NIDA, Rockville, MA), which was begun at either two weeks or four weeks post-infection. This represents an intermediate maintenance dose in humans, based upon published findings (62). For our studies we used two different treatment timepoints. In the first timepoint, daily buprenorphine injections were given after two weeks of EcoHIV infection, which is an earlier timepoint at which NCI manifests, and continued daily for an additional two weeks. After a total of four weeks, mice underwent behavioral testing. Daily injections of buprenorphine were given throughout behavioral testing until time of sacrifice. For the second timepoint daily buprenorphine injections were started four weeks after EcoHIV infection and continued daily for an additional two weeks. After a total of six weeks, mice underwent behavioral testing, with daily buprenorphine injections until sacrifice. This strategy demonstrates the ability of buprenorphine to treat NCI when given after chronic infection. The untreated groups were injected daily with vehicle (sterile water) to ensure that all mice were handled similarly. At the end of the experiment mice were anesthetized with ketamine (100 mg/kg)/xylazine (5mg/kg), intracardiac perfused with cold HBSS, and spleens and brain tissues were removed and prepared for measurement of monocytes, HIV burden, and microscopy as described (13, 39, 61, 63).
Radial arm water maze
The radial arm water maze (RAWM) test was administered in a pool of opaque water containing six swimming lanes and a hidden platform as described (13, 39, 63). Briefly, each testing group contained eight mice. RAWM testing consisted of four training trials (T) of 60s and one post-training 60s retention trial (RT) administered after 30 min rest. The hidden platform was rotated randomly to a different arm each test day to ensure that mice used working memory to locate the platform. Testing was considered complete when control mice reached asymptotic performance of one error or fewer in finding the hidden platform on trials T4 and RT. We recorded two measures of cognitive performance, errors and latency. An error is defined as the animal swimming into an arm that does not contain the hidden platform or remaining inactive for 20s, and latency is the amount of time it takes the mouse to find the hidden platform. Errors and latency for the last three days of testing were averaged and used for statistical analysis.
Isolation of CNS immune cells
The brains from seven of the mice per group that underwent water maze testing were removed and brain immune cells isolated using a previously published protocol (61). In brief, a cell suspension was generated from isolated brain tissue using a 100uM cell filter. The cell suspension was treated with Liberase TL (Roche Diagnostics) and 50 uL of DNAse1 (1 mg/ml, Stem Cell, technologies) to digest connective tissue. The digested suspension was filtered through a 70uM filter, and cells were isolated using Percoll density centrifugation. Cells obtained from this isolation method contain the migratory and nonmigratory cells in the brain. We identified and quantified monocytes from individual mouse brains by flow cytometry using a staining and gating strategy for CD45 (hematopoietic cells, Biolegend, 1ug), CD11b (myeloid cells, Biolegend, 0.25ug), Ly6G (granulocytes, BD, 0.25ug), and Ly6C (inflammatory monocytes, Biolegend, 0.25ug). Fluorescence minus one (FMO) controls were used to establish appropriate cell gates. Samples were acquired using an Attune NxT flow cytometer (Invitrogen, Carlsbad, CA, USA) and analyzed with FlowJo version 10.8.0.
Detection of peripheral HIV DNA
For all experiments spleen cells were harvested and DNA isolated from four to five mice in each treatment group. EcoHIV infection was quantified by real-time quantitative PCR (QPCR) for EcoHIV gag and compared between ECOHIV and ECOHIV BUP groups. The procedures for harvesting brain tissues or spleen, preparation of cellular DNA, and detection of EcoHIV gag QPCR were described previously (13, 39, 57, 64–66). Samples for QPCR were run in duplicate in an QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific). DNA QPCR reactions were normalized by amplification of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using a kit from Applied Biosystems.
Detection of brain HIV DNA
For QPCR, DNA was isolated from brain tissues by using a modified TRIzol (Invitrogen) protocol and an auto-homogenizer (Next Advance Inc., Bullet Blender Storm 24) (67). Expression of EcoHIV gag was determined as described above. Samples for QPCR were run in duplicate in an QuantStudio™ 3 Real-Time PCR System. DNA QPCR reactions were normalized to GAPDH and presented as absolute expression levels. For droplet digital PCR studies (ddPCR), DNA was isolated from an anterior coronal portion of the right hemisphere from four to five mice in each treatment group according to manufacturer’s instructions (Qiagen co isolation of DNA/RNA kit). After isolation, DNA from each brain per group was pooled and concentrated (Zymo DNA concentrator kit). Analysis of HIV gag DNA copies was performed using a Bio-Rad QX-100 system (BioRad) and a custom primer and probe set for HIV gag (HIV gag F: 5-TGGGACCACAGGCTACACTAGA-3, R: 5-CAGCCAAAACTCTTGCTTTATGG-3, P: 5-TGATGACAGCATGCCAGGGAGT-3). The number of cells in each sample was determined using a GAPDH qPCR standard curve. HIV gag DNA copies were determined by ddPCR and normalized to 1 million cells.
Immunofluorescent staining and confocal microscopy
Mice (3/group) were perfused with 4% paraformaldehyde (PFA) followed by saline. Following perfusion, the brain from each mouse was removed and placed in 4% PFA for preservation and kept at 4 degrees until sectioning. Prior to sectioning, brains were re-hydrated using a gradient of glucose and then the entire brain was frozen and then embedded in optimal cutting temperature (OCT) compound. The entire brain was cut using a cryostat (Leica) into 30 uM sections. Ten to 20 floating sections per mouse which contained the area of interest (hippocampus) were mounted onto slides for staining. Sections were stained with the following antibodies: rabbit anti-microtubule-associated protein 2 (MAP2) for detection of dendrites (1:150, EMD Millipore, Mahopac, NY) and mouse monoclonal anti-neuronal nuclear antigen (NeuN) for detection of neuronal nuclei (1:150, EMD Millipore), followed by matching Alexa conjugated secondary antibodies (1:100, Thermo Fisher Scientific). Images were captured by two investigators blinded to the treatment groups with a motorized Leica TCS SP5 confocal microscope, and analyzed using Improvision Volocity software (PerkinElmer) as described (13, 65, 66). Quantification of images was performed using ten-20 images (2-4 sections/mouse, 3 mice/group) measuring intensity of staining for MAP2 in the CA3 region of the hippocampus and in the cortex dorsal to the hippocampus.
Statistical analysis
One-way ANOVA tests were performed to compare differences in errors, latency, and monocyte numbers between each group. P-values of statistical comparisons between CTRL and ECOHIV groups are represented by asterisks (*P < 0.05, **P < 0.05, ***P < 0.0005, ****P < 0.001), and differences between CTRL and ECOHIV BUP groups are represented by ampersands (&P < 0.05, &&P < 0.005, &&&P < 0.0005, and &&&&P < 0.0001). A student t-test or the nonparametric Mann-Whitney, non parametric, t-test was used to compare the differences in EcoHIV peripheral and brain viral burden between ECOHIV and ECOHIV BUP groups. Watermaze and qPCR are shown as mean +/- SEM, and monocyte data are shown as mean +/- SD. Staining intensities are shown as mean +/- SEM and significance was calculated using a two-tailed Student’s t-test. All analyses were performed on GraphPad Prism version 9.2.0.
Results
Buprenorphine reverses EcoHIV NCI, reduces monocyte migration into the CNS, and viral brain DNA when given two weeks after infection
The transmigration of inflammatory monocytes into the brain is an important mechanism of HIV neuropathogenesis (19, 26, 27, 42). It has been shown that mature monocytes from PWH transmigrate in greater numbers across the BBB compared to those from people who do not have HIV. We showed that chronically EcoHIV infected nude (39) or wild type mice (61) with NCI also have elevated levels of inflammatory macrophages/monocytes in the brain. One way to mitigate HIV neuropathogenesis may be by reducing the influx of inflammatory monocytes into the brain. Therefore, we studied whether buprenorphine could treat and reverse cognitive impairment when given after established EcoHIV infection, and whether it would also reduce the number of brain inflammatory monocytes and the amount of HIV DNA in the brain.
We initiated buprenorphine treatment two or four weeks after EcoHIV infection of mice (Figure 1). The results of the two week post infection treatment testing for HIV-NCI, inflammatory monocyte transmigration to brain, and HIV burdens are shown in Figure 2. Buprenorphine or vehicle were administered daily starting two week post infection for a total of three weeks including the time required for completion of the five day RAWM test (Figure 1). Male C57BL/6J mice (n=10-15) were infected with a single intraperitoneal dose of EcoHIV or with PBS as described in the methods. There were four experimental groups: CTRL, ECOHIV, ECOHIV BUP, and BUP. Eight mice from each group in two independent experiments, for a total of 16 mice, underwent water maze testing using our established protocol. We assessed two measures of cognitive performance, errors and latency. Errors are defined as a mouse swimming into an arm that does not contain the hidden platform or remaining inactive for 20s. Latency is the amount of time the mouse takes to find the platform. RAWM results were analyzed using a one way ANOVA. EcoHIV infected mice were cognitively impaired as indicated by a significant increase in the number of errors made (T1: p<0.0001, T2-T3: p<0.0005, T4: p<0.005, RT: p<0.0001) and latency to find (T1: p<0.005, T2: p<0.05, T3: p<0.0005, T4-RT: p<0.0001) the hidden platform. In contrast, ECOHIV BUP mice were not impaired as they made significantly fewer errors (T1: p<0.0001, T2: p<0.05, T3: p<0.0001, T4: p<0.0005, RT: p<0.0001) and took less time (T1: p<0.005, T3-T4: p<0.0001, RT: p<0.0005) to find the platform compared to ECOHIV (Figures 2A, B, ECOHIV BUP versus ECOHIV). In fact, ECOHIV BUP mice behaved similarly to CTRL, with no significant differences in either errors or latency in water maze testing between the two groups. In this study and others, we also confirmed that buprenorphine treatments did not alter the time to find a visible platform, which serves as a control test to assess whether treatments and conditions tested result in any changes in motivation or vision. These results show that buprenorphine can treat and reverse EcoHIV-NCI in this animal model when treatment is begun two weeks after infection.
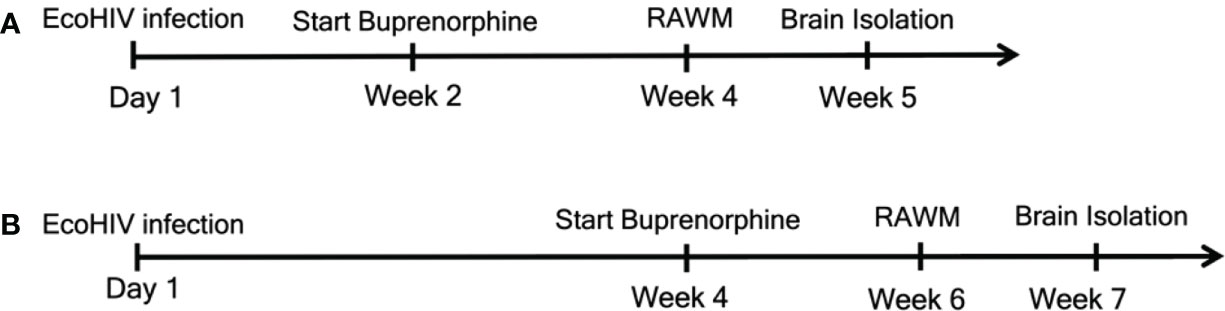
Figure 1 Experimental outline for the two treatment timepoints. We began daily buprenorphine injections at two different timepoints. For (A) mice were infected with EcoHIV at Day 1. Buprenorphine injections started two weeks later and were administered daily for the remainder of the experiments. After the additional two weeks, for a total of four weeks, mice underwent RAWM testing to assess cognitive status. RAWM testing was for one week, after which brains were isolated for monocytes, HIV DNA, and synaptodendritic pruning studies. For (B), mice were infected and daily buprenorphine injections began four weeks later and continued for the remainder of the experiments. At week six, mice were assessed for cognitive impairment using the RAWM, and at week seven brains were isolated for monocyte and virological assessment.
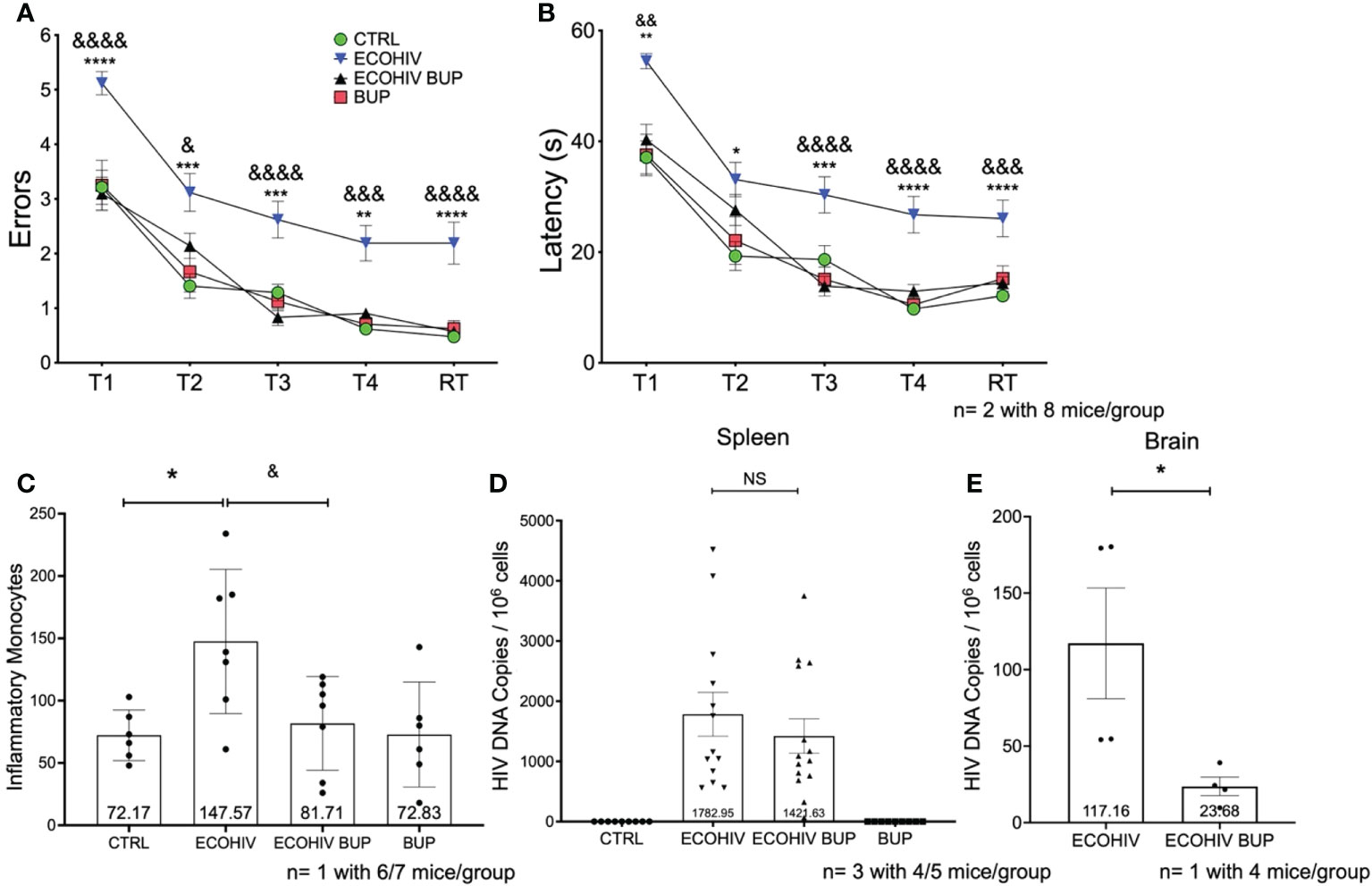
Figure 2 Buprenorphine reverses cognitive impairment, decreases inflammatory monocytes and HIV DNA in the brain when given two weeks post-EcoHIV infection. C57BL/6J mice in each experimental group; CTRL (green circles), ECOHIV (blue inverted triangles), ECOHIV BUP (black triangles), and BUP (red squares) were tested using a radial arm water maze. Mice were infected for two weeks before buprenorphine injections began. After an additional two weeks, mice were assayed for cognitive performance using the RAWM (A, B). EcoHIV infected mice were cognitively impaired after four weeks of infection as these mice made significantly more errors (A) and took significantly longer (B) to find the hidden platform compared to both CTRL (*) and ECOHIV BUP (&) groups. Data are shown as mean+/- SEM from the last three days of water maze testing. There were a total of two independent experiments, each with eight mice/group for a total of 16 mice in the CTRL, ECOHIV, and ECOHIV BUP groups. One independent experiments was performed with 8 mice for the BUP group (A, B) ECOHIV vs CTRL, **p<0.005, ****p<0.0001. ECOHIV vs ECOHIV BUP, &p<0.05, &&p<0.005, &&&p<0.0005, &&&&p<0.0001. (C), Immune cells from the brains of six to seven mice in each group in one independent experiment that underwent water maze testing were isolated and the number of inflammatory monocytes quantified by flow cytometry. The data are shown as mean +/- SD and analyzed using a one way ANOVA. There is a significant increase (147.57 +/- 57.83) in the number of inflammatory monocytes in the brains of EcoHIV infected mice compared to control (72.17 +/- 20.27, *p<0.05). These data also show that buprenorphine treatment given two weeks post infection significantly reduces the number of brain inflammatory monocytes (81.71 +/- 37.65, &p<0.05). (D), Spleens were taken and HIV gag DNA quantified from four or five mice in each group in three independent experiments. These data show that buprreonorphine does not change peripheral infection. (E), DNA was isolated from brain tissue of four mice in each group in one independent experiment. Using qPCR we demonstrated that buprenorphine treatments, started after two weeks, significantly decreases the amount of HIV DNA in the brain of infected mice (23.68 +/- 12.12, *p<0.05); ***p<0.0005; NS, Not Significant.
Next, we determined the effect of buprenorphine treatment on the migration of inflammatory monocytes into the brain (Figure 2C) and virus burdens in spleen cells and brains (Figures 2D, E). Immune cells were extracted from six or seven perfused brains of mice that completed cognitive testing and monocytes were quantified using a previously established protocol (61). This gating strategy for identifying inflammatory brain monocytes is shown in Supplementary Figure 1. EcoHIV infected mice had a significantly increased number of brain inflammatory monocytes (147.57 +/- 57.83) compared to control mice (72.17 +/- 20.27, p < 0.05). In contrast, the number of these cells in buprenorphine treated, EcoHIV infected mice was significantly lower than in infected mice (81.71 +/- 37.65, p < 0.05 ECOHIV BUP vs ECOHIV) and did not differ from control mice (Figure 2C). Buprenorphine alone had no effect on brain inflammatory monocyte levels compared to control mice. Thus, buprenorphine treatment started two weeks after EcoHIV infection reduces the number of inflammatory monocytes in the brains of infected mice. To evaluate peripheral virus burdens in this experiment, DNA was isolated from spleen cells and quantified by qPCR as described. Buprenorphine did not significantly change the amount of HIV DNA in the periphery of infected mice (Figure 2D).
Inflammatory monocytes can harbor HIV and contribute to CNS reservoir reseeding, another important mechanism of HIV neuropathogenesis (25, 29, 41, 68). Thus, inhibiting the migration of specifically HIV infected monocytes into the brain might limit this reseeding and the amount of virus in the brain. To test this, we performed an additional study using the two-week timepoint (Figure 2E). Mice were infected with EcoHIV and then given buprenorphine after two weeks of infection. After an additional three weeks, these animals were perfused, brain tissue was harvested, DNA was isolated, and HIV gag DNA was quantified as described (Gu; Kim). We found a significant decrease (p<0.05) in the amount of HIV DNA in the brains of buprenorphine treated, Eco HIV infected mice (23.68 +/- 12.12) compared to infected mice (117.2 +/- 72.41) (Figure 2E). These data suggest that buprenorphine treatment reduces HIV DNA burdens in the brains of infected mice, potentially by limiting the entry of EcoHIV harboring cells into the CNS, and is not affecting the amount of virus in the periphery that would cause overall decreased viral levels.
Cognitive impairment is present two weeks after infection and is correlated with increased numbers of inflammatory monocytes in the brain
We had not previously examined the NCI status and monocyte entry into the CNS at two weeks after infection, the time point at which we began buprenorphine treatments. Thus, we performed another set of experiments to test these parameters using only EcoHIV infected mice (ECOHIV) and control mice (CTRL). Cognitive impairment was determined in a RAWM test as described above. Our results show that after two weeks of infection, mice were unable to learn and remember where the hidden platform was in Trial 4, T4 (p<0.05), and the retention trial, RT (p<0.01), indicating that they are cognitively impaired (Figure 3A). Evaluation of inflammatory monocyte migration to the brain in these mice demonstrated that the animals had significantly elevated numbers of brain inflammatory monocytes at this stage of EcoHIV infection (Figure 3B). Monocytes were quantified in immune cells extracted from perfused brains as described above. There was a significant increase in the number of monocytes in the brains of infected mice compared to uninfected control mice (133.13 vs 87.57, p < 0.05 ECOHIV vs CTRL) (Figure 3B). These results show that the migration of inflammatory monocytes into the brain may drive the neuropathogenesis we detect at two weeks after infection.
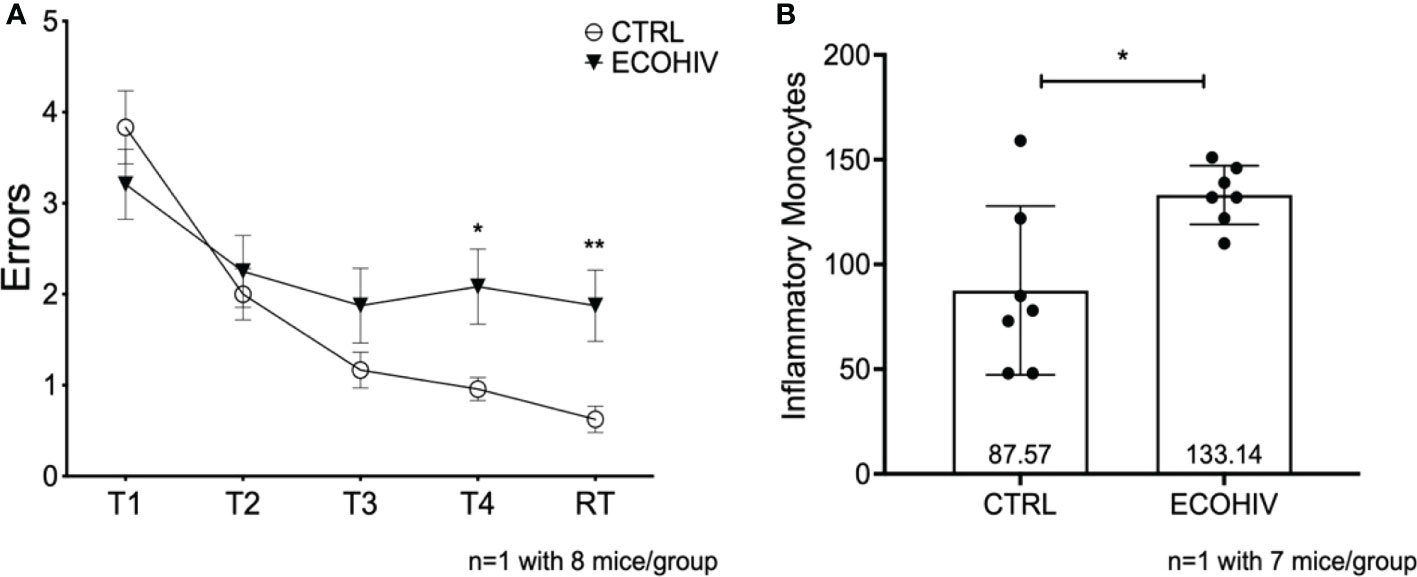
Figure 3 Mice are cognitively impaired after two weeks of infection and have significantly more brain inflammatory monocytes. We tested an early timepoint after infection a time in which cognitive impairment is being established. These data are from one independent experiment with eight mice in each group for water maze testing (A) and seven mice in each group for monocyte studies (B) Mice were infected with EcoHIV or injected with a diluent control (PBS). After these two weeks, eight mice in each group performed RAWM testing (A) EcoHIV infected mice, represented as the closed circles, were cognitively impaired as they made significantly more errors in Trial 4 and the retention trial compared to control mice represented as the open circles (T5 p<0.05, RT p<0.001). These data show that the establishment of EcoHIV-NCI has begun to manifest after two weeks of infection. After which the number of inflammatory monocytes were quantified by flow cytometry as previously described. (B), Data are shown as mean +/- SD. There are significantly more inflammatory monocytes (p<0.05) in the brains of EcoHIV infected mice (133.1 +/- 14.03) compared to control mice (87.57 +/- 40.27) determined by a Student’s t-test. *p<0.05; **p<0.01.
Buprenorphine reverses EcoHIV-NCI and reduces monocyte migration and viral brain DNA when given four weeks after infection
In the next series of studies, we repeated the experiments shown in Figure 2 but started buprenorphine treatment four weeks after infection, with cognitive assessment two weeks after buprenorphine treatment and monocyte brain migration quantification, and virological evaluations performed after cognitive testing, three weeks later (Figures 1, 4). The experimental groups were the same as in the two week buprenorphine experiments and the results were remarkably similar. EcoHIV infected mice were cognitively impaired making significantly more errors (T2-RT: p<0.0001) and taking significantly longer (T3: p<0.005, T4-RT: p<0.0001) to find the hidden platform compared to control mice (Figures 4A, B, ECOHIV versus CTRL). Buprenorphine reversed EcoHIV-NCI (Figures 4A, B, ECOHIV BUP vs ECOHIV) as indicated by a significant decrease in the number of errors made (T2, T4, RT: p<0.0001, T3: p<0.0005) and latency (T2, T3: p<0.05, T4: p<0.0001, RT: p<0.005) compared to ECOHIV mice. As in the first experiment series, EcoHIV infected, buprenorphine treated mice had significantly reduced inflammatory monocytes when compared to infected mice (77.07 +/- 23.68, p<0.05 ECOHIV BUP vs ECOHIV). Monocytes from six or seven mice per group were analyzed from two experiments, for a total of 12-14 mice (Figure 4C). EcoHIV infected mice had significantly more brain inflammatory monocytes (109.07 +/- 46.58) compared to control mice (62.69 +/- 21.29, p<0.01). Additionally, there were no significant differences among the number of inflammatory monocytes from EcoHIV infected mice that were given buprenorphine, uninfected buprenorphine treated mice, and control mice. At the end of these studies we tested the effects buprenorphine treatment on peripheral HIV DNA (Figure 4D). We saw no difference between treated and untreated mice in the amount of HIV DNA in the periphery. Using ddPCR, we also tested the effect of buprenorphine on HIV brain burdens in treated and untreated mice (Figure 4E). Consistent with the results shown in Figure 2E, buprenorphine, started after four weeks of infection, also decreases the amount of virus in the brains of infected mice.
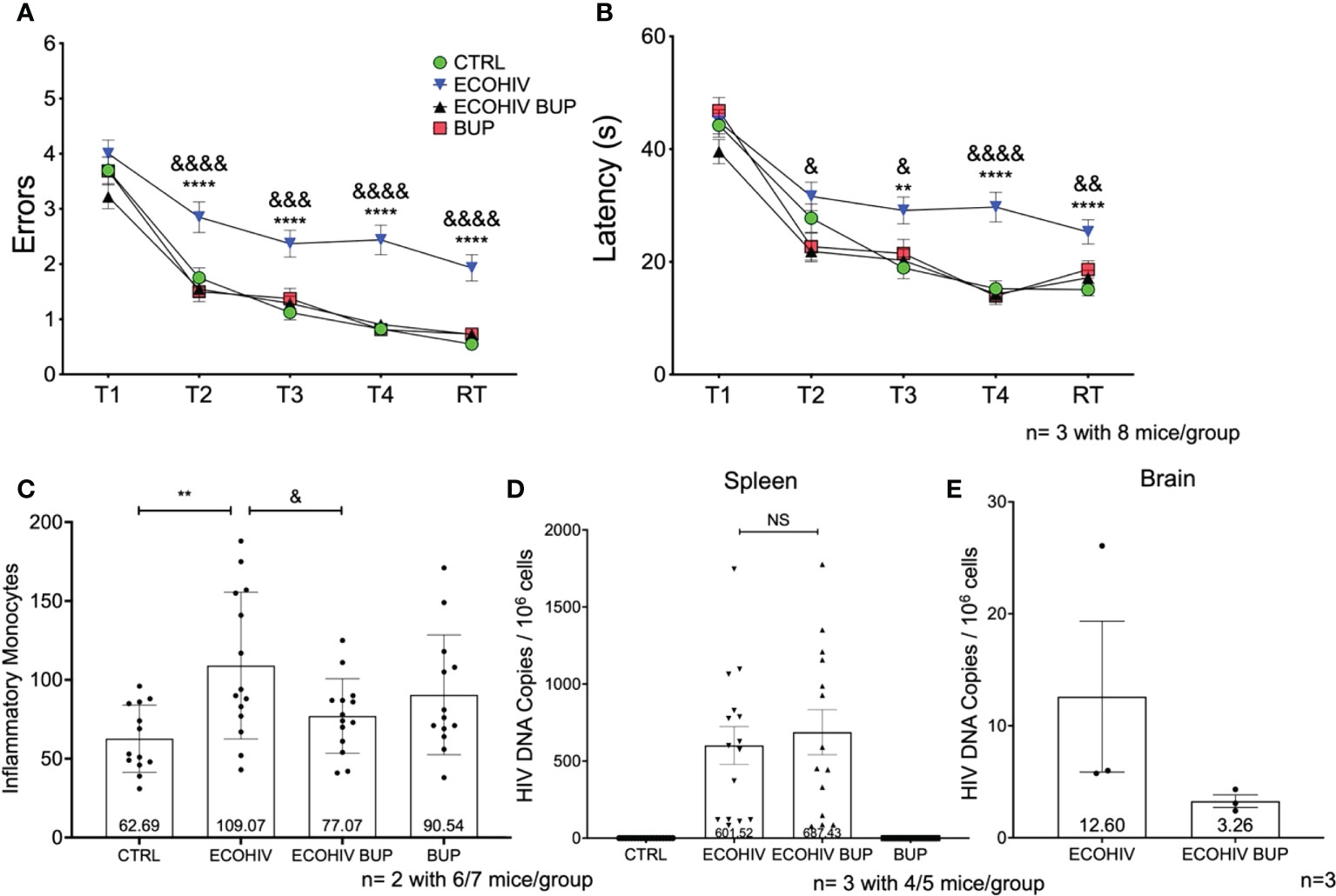
Figure 4 Buprenorphine reverses cognitive impairment and reduces brain inflammatory monocytes when given four weeks post-EcoHIV infection. C57BL/6J mice in each experimental group; CTRL (green circles), ECOHIV (blue inverted triangles), ECOHIV BUP (black triangles), and BUP (red squares) were tested using a radial arm water maze. Mice were infected for four weeks before buprenorphine injections began. After an additional two weeks, mice were assayed for cognitive performance using the RAWM (A, B). EcoHIV infected mice were cognitively impaired after four weeks of infection as these mice made significantly more errors (A) and took significantly longer (B) to find the hidden platform compared to both CTRL (*) and ECOHIV BUP (&) groups. Data are shown as mean+/- SEM from the last three days of water maze testing. There were a total of three independent experiments, each with eight mice/group for a total of 24 mice in the CTRL, ECOHIV, and ECOHIV BUP groups. Two independent experiments were performed with 8 mice for the BUP group for a total of 16 mice for (A, B) Results were analyzed using a one way ANOVA: ECOHIV vs CTRL, **p<0.005, ****p<0.0001. ECOHIV vs ECOHIV BUP, &p<0.05, &&p<0.005, &&&p<0.0005, &&&&p<0.0001. (C) Monocytes were quantified as described in the previous experiments and shows the number of brain inflammatory monocytes from a total of 12-14 mice in each group from two independent experiment. The data are shown as mean +/- SD. There is a significant increase (109.07 +/- 46.58) in the number of inflammatory monocytes in the brains of EcoHIV infected mice compared to control (62.69 +/- 21.29, **p<0.01). These data also show that buprenorphine treatment four weeks post infection significantly reduces the number of brain inflammatory monocytes (77.07 +/- 23.68, &p<0.05). (D), These results are also correlated with no change in the spleen HIV DNA between ECOHIV and ECOHIV BUP. (E), DNA was isolated from a section of the right hemisphere from four or five mice in each group. This DNA was then pooled and concentrated. The number of HIV gag DNA copies were quantified using ddPCR and normalized to 106 cells. The data are shown as mean +/- SEM. These data are from three independent experiments for CTRL, ECOHIV, and ECOHIV BUP groups, and from two independent experiments for BUP. There is an increase in the number of HIV DNA copies in infected mice (12.60 +/- 6.734) compared to EcoHIV infected mice that were given buprenorphine (3.263 +/- 0.5626). These data suggest that buprenorphine may reduce the amount of virus in the brains of mice when given four weeks after infection. NS, Not Significant.
Buprenorphine protects neurons from synaptodendritic pruning in the brains of EcoHIV infected mice
Synaptodendritic injury is a pathological hallmark of HIV-NCI in PWH on suppressive ART, and this hallmark is reproduced in infected mice with EcoHIV-NCI (13, 69). This injury, in both human and murine NCI, occurs in the absence of significant neuronal death (13, 69). Thus, it can be prevented or reversed upon reduction of the pathogenic HIV stimulus in the brain, leading to mitigation of cognitive dysfunction (13). Here we tested whether reversal of EcoHIV-NCI by buprenorphine correlates with improvement in synaptodendritic integrity by examining MAP2 intensity by immunofluorescent staining and confocal microscopy (Figure 5). Brains were isolated from two mice from each group in two independent experiments. Ten to 20 30um sections were stained with MAP2 and NeuN to label the dendritic process and neuronal cell body, respectively, as described in the methods. Ten to 20 images of the CA3 region of the hippocampus and cortex regions of the brains were taken in each brain section from each mouse per group from three independent experiments. As we showed previously (13), EcoHIV infection results in synaptodendritic pruning (Figures 5A-C) as indicated by the decrease in MAP2 intensity in the CA3 region of the hippocampus and cortex compared to control (p<0.05). Buprenorphine therapy reversed the EcoHIV mediated neuronal injury as there was a significant increase in MAP2 intensity in the CA3 region and cortex of ECOHIV BUP mice compared to ECOHIV mice (p<0.05) (Figures 5A–C).
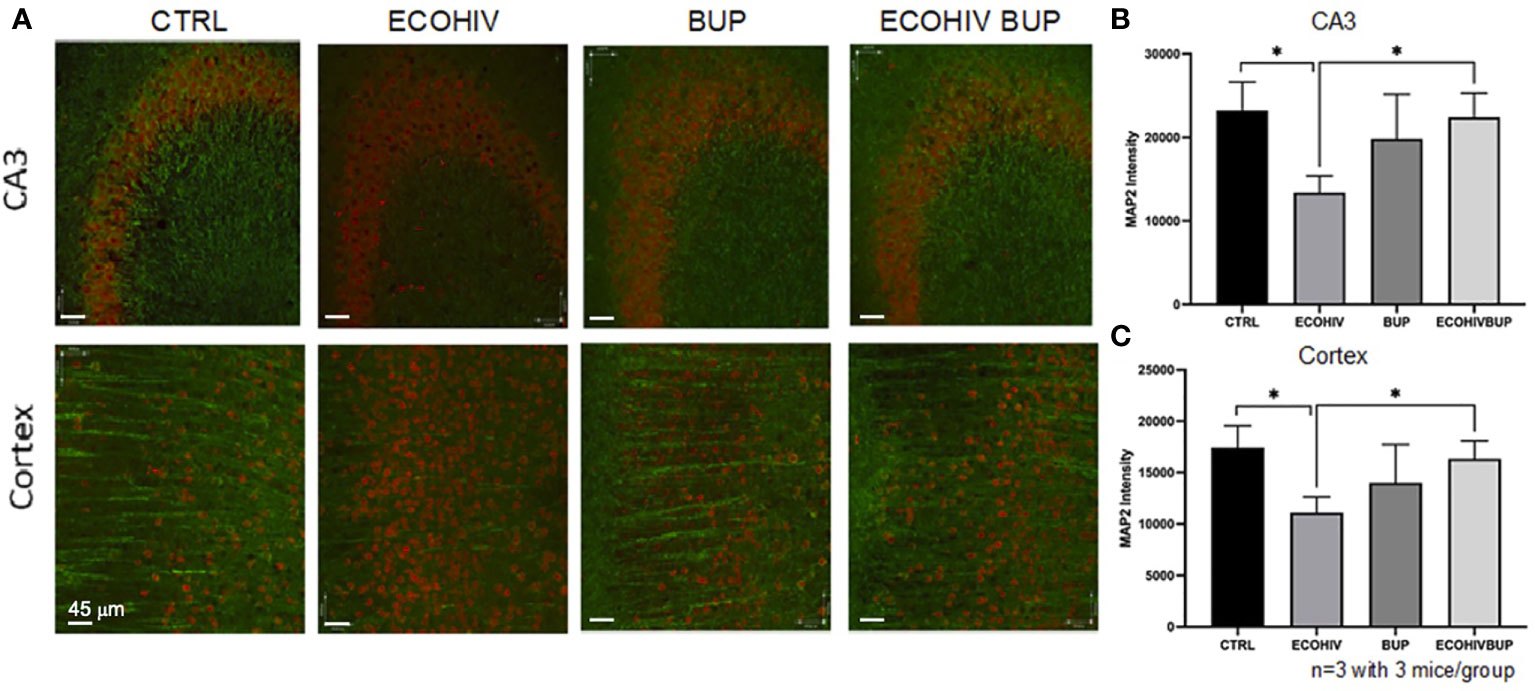
Figure 5 Treatment with buprenorphine protects against EcoHIV mediated synaptodendritic pruning. Brains from three mice in each group per two independent experiments were perfused, frozen, and sectioned for confocal microscopy. The mice used for these experiments were part of the approach in which buprenorphine was administered two weeks post infection. The cortex and CA3 region of the hippocampus of the brain were stained for NeuN and MAP2 to identify the neuronal cell body and dendritic processes. (A) shows representative images from these two regions from one mouse in each experimental group. The red staining defines the neuronal cell body (NeuN) and the green staining defines the dendritic processes (MAP2). There is a significant loss of MAP2 intensity from the CA3 region (B) and the cortex (C) of EcoHIV infected mice compared to control and compared to EcoHIV infected, buprenorphine treated mice. These results show that buprenorphine protects against EcoHIV mediated synaptodendritic pruning.
Discussion
Despite effective ART that inhibits viral replication and greatly increases the lifespan of PWH, HIV-NCI still persists in 15-50% of PWH. HIV-NCI negatively impacts the quality of life and is an independent risk factor for mortality (70). OUD is believed to be associated with worse neurocognitive impairment in PWH and can result in ART interruption that can contribute further to NCI (71–73). The impact of opioids on the mechanisms that result from HIV brain disease in individuals on suppressive ART has not been fully defined. This limited understanding of mechanisms that mediate mild HIV-NCI pathogenesis and its potential exacerbation with opioid use, despite ART, has resulted in a lack of therapeutic strategies for the treatment of NCI in the absence or presence of OUD. Buprenorphine is an OAT that treats OUD (51, 52, 74). It has an improved safety profile compared to another OAT, methadone, due to its unique properties as a partial agonist for MOR and full antagonist for KOR (52). In some studies, people with OUD taking buprenorphine had improved cognitive outcomes compared to those who did not (55, 75). Data in another study also suggest that buprenorphine improves cognitive outcomes in people with OUD compared to their own baseline before buprenorphine treatment (56). The mechanisms by which buprenorphine improves cognition in PWH with or without OUD still need to be characterized. This is a significant gap in our ability to treat HIV CNS disease. We propose that buprenorphine, in addition to its being an OAT, can be an effective interventional therapy for HIV-NCI in PWH regardless of OUD.
To test the ability of buprenorphine to be a therapy for HIV-NCI we used the EcoHIV mouse model. This model has many advantages for the study of HIV infection and potential therapies. These include the expression of all HIV genes except gp120. EcoHIV preserves HIV cellular tropism to CD4+ T cells, macrophages, and microglia, and not to other cell types. Infection with EcoHIV also induces immune responses to HIV proteins that enable vaccine studies. Importantly for our studies, infection with this virus results in neurocognitive disease impairing learning, memory, and fear responses in all infected mice, a disease highly similar to mild chronic HIV neurocognitive impairment in PWH on virus-suppressive ART as well as other HIV-associated chronic abnormalities typically seen in PWH on ART, including gut, lung, and brain microvascular diseases. EcoHIV infection of the brain also reflects the low level HIV brain burdens in humans.
The results shown in Figures 2–4 indicate that the continuous migration of inflammatory monocytes into the brain at three, five, and seven weeks of infection contributes to the development and persistence of cognitive impairment. The increased entry of inflammatory monocytes is an important mediator of HIV neuropathogenesis. Several studies in mice and non-human primates showed the ability of monocytes to harbor the virus and enter the brain. This was associated with neuronal damage (11, 27, 76, 77). It has also been shown that HIV DNA in peripheral blood monocytes correlated with cognitive impairment in PWH (27, 28, 45). Thus, we propose that this migration is reduced by daily buprenorphine treatments started at two or four weeks after infection. Buprenorphine treatment started at those timepoints also decreased the amount of HIV DNA in the brain. One study in non-human primates showed treatment with natalizumab reduced the number of SIV RNA positive cells in the brain and this correlated with decreased neuronal damage (78). This suggests that limiting the entry of virus harboring cells will reduce neuronal damage and cognitive function. Thus, we hypothesize that limiting the entry of inflammatory monocytes into the brain, and specifically those harboring HIV, decreases CNS viral reservoir reseeding. As such, buprenorphine could mitigate EcoHIV mediated CNS damage, and treat and reverse EcoHIV-NCI, in part by its actions on inflammatory monocytes.
These are important findings as there are currently no approved therapies for HIV-NCI for PWH regardless of successful ART, despite several potential candidates. HIV-NCI interventions have been tested in animal models of HIV neuropathogenesis, including in EcoHIV infected mice. These are treatment with minocycline, memantine, intranasal insulin, 6-diazo-5-oxo-L-norleucine (DON), the DON prodrug, JHU083 and polyinosinicpolycytidylic acid (poly I:C) (12, 14–17). All of these target important processes in the development of HIV-NCI including brain glucose metabolism (13), brain glutamate metabolism (17), or brain exosome production (79). However, to our knowledge, buprenorphine is the first therapy that targets the migration of inflammatory monocytes into the CNS, a key process required for the establishment and maintenance of HIV-NCI. By reducing this migration, buprenorphine can reverse EcoHIV-NCI. Buprenorphine was originally developed as an analgesic and not an OAT (80, 81). It is often prescribed to treat pain related to HIV-associated peripheral neuropathy, another complication associated with HIV infection (81). Thus, it is already used clinically for conditions other than OUD. Additionally, due to its unique pharmacology and the presence of a ceiling effect, buprenorphine has a lower risk of addiction and overdose compared to other opioids such as morphine.
It is important to identify the mechanisms by which buprenorphine reverses cognitive impairment and reduces monocyte migration. One potential mechanism by which buprenorphine reduces the ability of mature monocytes to cross the BBB is through receptor desensitization. Chemokine receptors, such as CCR2 the receptor for CCL2, as well as the opioid receptors MOR and KOR, belong to a receptor family known as G protein coupled receptors, GPCR (82, 83). Upon activation, these receptors can form heterodimers which modulate their receptor activity (84–86). We propose that concomitant treatment of buprenorphine to an inflammatory environment in which CCL2 is elevated, may cause the formation of MOR/CCR2 and/or KOR/CCR2 heterodimers, thus diminishing the migratory response of these cells. These studies are ongoing. While we show that buprenorphine limits the entry of inflammatory monocytes into the brains of EcoHIV infected mice, additional mechanisms may also contribute to the reversal of EcoHIV-NCI. Buprenorphine can impact brain resident cells such as microglia, macrophages, astrocytes, and neurons. Further characterization of the mechanisms by which buprenorphine reverses EcoHIV-NCI and affects additional cell types will enable the development of next-generation therapies for treating HIV-NCI. Thus, buprenorphine may be a promising candidate for the treatment of HIV-NCI regardless of OUD, as well as for other inflammatory brain pathologies for which there are no therapies.
These findings are highly significant, as HIV-NCI is a prevalent comorbidity of HIV infection. As such, it is imperative to develop strategies to mitigate and eliminate this condition. Buprenorphine reduces many mechanisms that mediate HIV neuropathogenesis including inflammatory monocyte migration, neuronal damage, and possibly virus within the CNS. Our impactful findings show that buprenorphine reverses EcoHIV-NCI in part by its effects on these mechanisms. Thus, buprenorphine may be a promising candidate for the treatment of HIV-NCI regardless of OUD as well as other inflammatory brain pathologies for which there are no therapies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by Icahn School of Medicine at Mount Sinai and the Albert Einstein College of Medicine Institutional Animal Care and Use Committees.
Author contributions
AM, JK, HH, B-HK, and WC performed experiments and contributed to data acquisition and analysis. AM, JK, JB, and DV contributed to the conceptualization, funding, and resources for the studies. AM, JK, JB, and DV developed, wrote, and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
These studies were supported by National Institute of Health grants R01DA041931 (JB, JK, AM, HH, WC, and DV), R01DA048609 (JB and AM), R01DA044584 (JB), R01MH112391 (JB), U01DA053629 (DV and JK), R56NS119439 (B-HK, DV, and JK), R01DA052844 (DV and JK), 1RF1NS119438 (JK, B-HK, and DV), as well as the Burroughs Wellcome Fund award: 1TL1TR002557 (AM).
Acknowledgments
We would like to thank all members of the Berman and Volsky laboratories for their support. We would especially like to thank Dr. Eran Hadas and Dr. Samuel Martinez-Meza for their help and technical expertise.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1004985/full#supplementary-material
Supplementary Figure 1 | Inflammatory brain monocyte gating strategy. This figure represents the gating strategy used to identify brain inflammatory monocytes. These flow cytometry plots are representative images from an EcoHIV infected mouse used in our studies. The first panel represents all cells obtained from cell suspension and percoll gradient density centrifugation. Singlets are gated and shown in panel 2. CD11b is used to identify the immune cell population, panel 3, and contains the cells that enter the brain from the periphery as well as the resident immune cells. In panel 4, we use Ly6G to identify and exclude the neutrophils that are shown in the black rectangle. The analysis proceeds with the Ly6G negative population shown in the red rectangle. In Panel 5, the cells are stratified by CD45 expression. The black rectangle contains the CD45 intermediate cells, including microglia and macrophages. In the red rectangle are the CD45 hi cells that contain the inflammatory monocytes. The last part of the analysis, panel 5, identifies the Ly6C+ cells from the previous red gate (panel 5) that are the inflammatory brain monocytes.
References
1. Center for Disease Control and Prevention, C.f.D.C.a. HIV Surveillance report, 2019, Vol. 32. (2021). Available from: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
2. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet (2013) 382(9903):1525–33. doi: 10.1016/S0140-6736(13)61809-7
3. Gelman BB. Neuropathology of HAND with suppressive antiretroviral therapy: Encephalitis and neurodegeneration reconsidered. Curr HIV/AIDS Rep (2015) 12(2):272–9. doi: 10.1007/s11904-015-0266-8
4. Heaton RK, Clifford DB, Franklin DR, Woods SP Jr, Ake C, Vaida F, et al. HIV-Associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology (2010) 75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727
5. Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the multicenter AIDS cohort study. Neurology (2016) 86(4):334–40. doi: 10.1212/WNL.0000000000002277
6. Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-Associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol (2016) 12(5):309. doi: 10.1038/nrneurol.2016.27
7. Wang Y, Liu M, Lu Q, Farrell M, Lappin JM, Shi J, et al. Global prevalence and burden of HIV-associated neurocognitive disorder: A meta-analysis. Neurology (2020) 95(19):e2610–21. doi: 10.1212/WNL.0000000000010752
8. Zenebe Y, Necho M, Yimam W, Akele B. Worldwide occurrence of HIV-associated neurocognitive disorders and its associated factors: A systematic review and meta-analysis. Front Psychiatry (2022) 13:814362. doi: 10.3389/fpsyt.2022.814362
9. Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun (2015) 45:1–12. doi: 10.1016/j.bbi.2014.10.008
10. Moore RC, Fazeli PL, Jeste DV, Moore DJ, Grant I, Woods SP, et al. Successful cognitive aging and health-related quality of life in younger and older adults infected with HIV. AIDS Behav (2014) 18(6):1186–97. doi: 10.1007/s10461-014-0743-x
11. Campbell JH, Burdo TH, Autissier P, Bombardier JP, Westmoreland SV, Soulas C, et al. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS One (2011) 6(4):e18688. doi: 10.1371/journal.pone.0018688
12. Dong B, Borjabad A, Kelschenbach J, Chao W, Volsky DJ, Potash MJ. Prevention and treatment of HIV infection and cognitive disease in mice by innate immune responses. Brain Behav Immun Health (2020) 3:100054. doi: 10.1016/j.bbih.2020.100054
13. Kim BH, Kelschenbach J, Borjabad A, Hadas E, He H, Potash MJ, et al. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HAND in EcoHIV-infected mice. AIDS (2019), 33(6):973–984. doi: 10.1097/QAD.0000000000002150
14. Mamik MK, Asahchop EL, Chan WF, Zhu Y, Branton WG, McKenzie BA, et al. Insulin treatment prevents neuroinflammation and neuronal injury with restored neurobehavioral function in models of HIV/AIDS neurodegeneration. J Neurosci (2016) 36(41):10683–95. doi: 10.1523/JNEUROSCI.1287-16.2016
15. Meisner F, Scheller C, Kneitz S, Sopper S, Neuen-Jacob E, Riederer P, et al. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques: a novel pharmacological action of memantine. Neuropsychopharmacology (2008) 33(9):2228–36. doi: 10.1038/sj.npp.1301615
16. Nedelcovych MT, Kim BH, Zhu X, Lovell LE, Manning AA, Kelschenbach J, et al. Glutamine antagonist JHU083 normalizes aberrant glutamate production and cognitive deficits in the EcoHIV murine model of HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol (2019) 14(3):391–400. doi: 10.1007/s11481-019-09859-w
17. Nedelcovych MT, Tenora L, Kim BH, Kelschenbach J, Chao W, Hadas E, et al. N-(Pivaloyloxy)alkoxy-carbonyl prodrugs of the glutamine antagonist 6-Diazo-5-oxo-l-norleucine (DON) as a potential treatment for HIV associated neurocognitive disorders. J Med Chem (2017) 60(16):7186–98. doi: 10.1021/acs.jmedchem.7b00966
18. Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, et al. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-a, and ALCAM in diapedesis. PLoS One (2013) 8(7):e69270. doi: 10.1371/journal.pone.0069270
19. Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol (2012) 91(3):401–15. doi: 10.1189/jlb.0811394
20. Chun TW, Moir S, Fauci AS. HIV Reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol (2015) 16(6):584–9. doi: 10.1038/ni.3152
21. Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak M Jr, Tarwater PM, et al. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis (2002) 186(7):905–13. doi: 10.1086/343768
22. Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol (2003) 74(5):635–41. doi: 10.1189/jlb.0503204
23. Fois AF, Brew BJ. The potential of the CNS as a reservoir for HIV-1 infection: Implications for HIV eradication. Curr HIV/AIDS Rep (2015) 12(2):299–303. doi: 10.1007/s11904-015-0257-9
24. Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, Regulier EG, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol (2001) 7(6):528–41. doi: 10.1080/135502801753248114
25. Leon-Rivera R, Veenstra M, Donoso M, Tell E, Eugenin EA, Morgello S, et al. Central nervous system (CNS) viral seeding by mature monocytes and potential therapies to reduce CNS viral reservoirs in the cART era. mBio (2021) 12(2):e03633-20. doi: 10.1128/mBio.03633-20
26. Veenstra M, Byrd DA, Inglese M, Buyukturkoglu K, Williams DW, Fleysher L, et al. CCR2 on peripheral blood CD14(+)CD16(+) monocytes correlates with neuronal damage, HIV-associated neurocognitive disorders, and peripheral HIV DNA: reseeding of CNS reservoirs? J Neuroimmune Pharmacol (2018), 14(1):120–33. doi: 10.1007/s11481-018-9792-7
27. Veenstra M, Leon-Rivera R, Li M, Gama L, Clements JE, Berman JW, et al. Mechanisms of CNS viral seeding by HIV(+) CD14(+) CD16(+) monocytes: Establishment and reseeding of viral reservoirs contributing to HIV-associated neurocognitive disorders. MBio (2017) 8(5):e01280-17. doi: 10.1128/mBio.01280-17
28. Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res (2014) 12(2):85–96. doi: 10.2174/1570162X12666140526114526
29. Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol (2011) 267(2):109–23. doi: 10.1016/j.cellimm.2010.12.004
30. Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. J Neuroimmune Pharmacol (2006) 1(2):160–81. doi: 10.1007/s11481-006-9017-3
31. Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology (2010) 7:30. doi: 10.1186/1742-4690-7-30
32. Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, Zakharova O, et al. Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest (2016) 126(4):1353–66. doi: 10.1172/JCI84456
33. Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci (2006) 26(4):1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006
34. Maus U, Henning S, Wenschuh H, Mayer K, Seeger W, Lohmeyer J. Role of endothelial MCP-1 in monocyte adhesion to inflamed human endothelium under physiological flow. Am J Physiol Heart Circ Physiol (2002) 283(6):H2584–91. doi: 10.1152/ajpheart.00349.2002
35. Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet (1997) 349(9053):692–5. doi: 10.1016/S0140-6736(96)10178-1
36. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol (2011) 11(11):762–74. doi: 10.1038/nri3070
37. Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. biomark Res (2014) 2(1):1. doi: 10.1186/2050-7771-2-1
38. Kim WK, Alvarez X, Fisher J, Bronfin B, Westmoreland S, McLaurin J, et al. CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol (2006) 168(3):822–34. doi: 10.2353/ajpath.2006.050215
39. Gu CJ, Borjabad A, Hadas E, Kelschenbach J, Kim BH, Chao W, et al. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PloS Pathog (2018) 14(6):e1007061. doi: 10.1371/journal.ppat.1007061
40. Gorantla S, Poluektova L, Gendelman HE. Rodent models for HIV-associated neurocognitive disorders. Trends Neurosci (2012) 35(3):197–208. doi: 10.1016/j.tins.2011.12.006
41. Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev (2013) 254(1):102–13. doi: 10.1111/imr.12068
42. Williams K, Burdo TH. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J Neuroimmune Pharmacol (2012) 7(2):363–71. doi: 10.1007/s11481-011-9330-3
43. Clements JE, Mankowski JL, Gama L, Zink MC. The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J Neurovirol (2008) 14(4):309–17. doi: 10.1080/13550280802132832
44. Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y, et al. HIV Persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat Med (2017) 23(5):638–43. doi: 10.1038/nm.4319
45. Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, et al. Circulating proviral HIV DNA and HIV-associated dementia. AIDS (2005) 19(1):45–52. doi: 10.1097/00002030-200501030-00005
46. Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr (2011) 56 Suppl 1:S22–32. doi: 10.1097/QAI.0b013e318209751e
47. Bruce RD. Opioid addiction, opioid addiction treatment, and HIV infection. Top Antivir Med (2018) 26(3):89–92.
48. Dutta R, Roy S. Mechanism(s) involved in opioid drug abuse modulation of HAND. Curr HIV Res (2012) 10(5):469–77. doi: 10.2174/157016212802138805
49. Fiellin DA, Weiss L, Botsko M, Egan JE, Altice FL, Bazerman LB, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr (2011) 56 Suppl 1:S33–8. doi: 10.1097/QAI.0b013e3182097537
50. Gudin J, Fudin J. A narrative pharmacological review of buprenorphine: A unique opioid for the treatment of chronic pain. Pain Ther (2020) 9(1):41–54. doi: 10.1007/s40122-019-00143-6
51. Khanna IK, Pillarisetti S. Buprenorphine - an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res (2015) 8:859–70. doi: 10.2147/JPR.S85951
52. Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol (2004) 2(4):395–402. doi: 10.2174/1570159043359477
53. Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther (1994) 55(5):569–80. doi: 10.1038/clpt.1994.71
54. Neri S, Bruno CM, Pulvirenti D, Malaguarnera M, Italiano C, Mauceri B, et al. Randomized clinical trial to compare the effects of methadone and buprenorphine on the immune system in drug abusers. Psychopharmacol (Berl) (2005) 179(3):700–4. doi: 10.1007/s00213-005-2239-x
55. Rapeli P, Fabritius C, Kalska H, Alho H. Cognitive functioning in opioid-dependent patients treated with buprenorphine, methadone, and other psychoactive medications: stability and correlates. BMC Clin Pharmacol (2011) 11:13. doi: 10.1186/1472-6904-11-13
56. Scott TM, Mindt Rivera M, Cunningham CO, Arias F, Coulehan K, Mangalonzo A, et al. Neuropsychological function is improved among opioid dependent adults who adhere to opiate agonist treatment with buprenorphine-naloxone: a preliminary study. Subst Abuse Treat Prev Policy (2017) 12(1):48. doi: 10.1186/s13011-017-0133-2
57. Jaureguiberry-Bravo M, Lopez L, Berman JW. Frontline science: Buprenorphine decreases CCL2-mediated migration of CD14(+) CD16(+) monocytes. J Leukoc Biol (2018) 104(6):1049–59. doi: 10.1002/JLB.3HI0118-015R
58. Franchi S, Castelli M, Moretti S, Panerai A, Sacerdote P. Evaluation of murine macrophage cytokine production after in vivo morphine treatment. Methods Mol Biol (2015) 1230:253–61. doi: 10.1007/978-1-4939-1708-2_21
59. Franchi S, Moretti S, Castelli M, Lattuada D, Scavullo C, Panerai AE, et al. Mu opioid receptor activation modulates toll like receptor 4 in murine macrophages. Brain Behav Immun (2012) 26(3):480–8. doi: 10.1016/j.bbi.2011.12.010
60. Carvallo L, Lopez L, Che FY, Lim J, Eugenin EA, Williams DW, et al. Buprenorphine decreases the CCL2-mediated chemotactic response of monocytes. J Immunol (2015) 194(7):3246–58. doi: 10.4049/jimmunol.1302647
61. Jaureguiberry-Bravo M, Kelschenbach J, Murphy A, Carvallo L, Hadas E, Tesfa L, et al. Treatment with buprenorphine prior to EcoHIV infection of mice prevents the development of neurocognitive impairment. J Leukoc Biol (2020), 109(3): p. 675–681. doi: 10.1002/JLB.5AB0420-531R
62. Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev (2004) 2004(3):CD002207. doi: 10.1002/14651858.CD002207.pub2
63. Kelschenbach JL, Saini M, Hadas E, Gu CJ, Chao W, Bentsman G, et al. Mice chronically infected with chimeric HIV resist peripheral and brain superinfection: a model of protective immunity to HIV. J Neuroimmune Pharmacol (2012) 7(2):380–7. doi: 10.1007/s11481-011-9316-1
64. Hadas E, Borjabad A, Chao W, Saini M, Ichiyama K, Potash MJ, et al. Testing antiretroviral drug efficacy in conventional mice infected with chimeric HIV-1. AIDS (2007) 21(8):905–9. doi: 10.1097/QAD.0b013e328157454900002030-200705110-00002
65. Kelschenbach J, He H, Kim BH, Borjabad A, Gu CJ, Chao W, et al. Efficient expression of HIV in immunocompetent mouse brain reveals a novel nonneurotoxic viral function in hippocampal synaptodendritic injury and memory impairment. mBio (2019) 10(4):e00591–19. doi: 10.1128/mBio.00591-19
66. He H, Sharer LR, Chao W, Gu CJ, Borjabad A, Hadas E, et al. Enhanced human immunodeficiency virus type 1 expression and neuropathogenesis in knockout mice lacking type I interferon responses. J Neuropathol Exp Neurol (2014) 73(1):59–71. doi: 10.1097/NEN.0000000000000026
67. Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci U S A (2005) 102(10):3760–5. doi: 10.1073/pnas.0500649102
68. Leon-Rivera R, Morsey B, Niu M, Fox HS, Berman JW. Interactions of monocytes, HIV, and ART identified by an innovative scRNAseq pipeline: Pathways to reservoirs and HIV-associated comorbidities. mBio (2020) 11(4). doi: 10.1128/mBio.01037-20
69. Levine AJ, Soontornniyomkij V, Achim CL, Masliah E, Gelman BB, Sinsheimer JS. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neurovirol (2016) 22(4):431–41. doi: 10.1007/s13365-015-0410-7
70. Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-Associated neurocognitive disorder–pathogenesis and prospects for treatment. Nat Rev Neurol (2016) 12(4):234–48. doi: 10.1038/nrneurol.2016.27
71. Klinkenberg WD, Sacks S, H.O. Hiv/Aids Treatment Adherence, G. Cost Study. Mental disorders and drug abuse in persons living with HIV/AIDS. AIDS Care (2004) 16 Suppl 1:S22–42. doi: 10.1080/09540120412331315303
72. Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, et al. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology (2000) 23(2):113–26. doi: 10.1016/S0893-133X(00)00097-X
73. Hauser KF, Fitting S, Dever SM, Podhaizer EM, Knapp PE. Opiate drug use and the pathophysiology of neuroAIDS. Curr HIV Res (2012) 10(5):435–52. doi: 10.2174/157016212802138779
74. Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: Update on transmucosal and long-acting formulations. J Addict Med (2019) 13(2):93–103. doi: 10.1097/ADM.0000000000000457
75. Soyka M, Hock B, Kagerer S, Lehnert R, Limmer C, Kuefner H. Less impairment on one portion of a driving-relevant psychomotor battery in buprenorphine-maintained than in methadone-maintained patients: results of a randomized clinical trial. J Clin Psychopharmacol (2005) 25(5):490–3. doi: 10.1097/01.jcp.0000178417.60426.60
76. Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr (2012) 60(3):234–43. doi: 10.1097/QAI.0b013e318256f3bc
77. Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS. Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J Immunol Res (2014) 2014:569819. doi: 10.1155/2014/569819
78. Lakritz JR, Thibault DM, Robinson JA, Campbell JH, Miller AD, Williams KC, et al. alpha4-integrin antibody treatment blocks Monocyte/Macrophage traffic to, vascular cell adhesion molecule-1 expression in, and pathology of the dorsal root ganglia in an SIV macaque model of HIV-peripheral neuropathy. Am J Pathol (2016) 186(7):1754–61. doi: 10.1016/j.ajpath.2016.03.007
79. Zhu X, Hollinger KR, Huang Y, Borjabad A, Kim BH, Arab T, et al. Neutral sphingomyelinase 2 inhibition attenuates extracellular vesicle release and improves neurobehavioral deficits in murine HIV. Neurobiol Dis (2022) 169:105734. doi: 10.1016/j.nbd.2022.105734
80. Kreek MJ, Reed B, Butelman ER. Current status of opioid addiction treatment and related preclinical research. Sci Adv (2019) 5(10):eaax9140. doi: 10.1126/sciadv.aax9140
81. Wiffen PJ, Derry S, Moore RA, Stannard C, Aldington D, Cole P, et al. Buprenorphine for neuropathic pain in adults. Cochrane Database Syst Rev (2015) 9):CD011603. doi: 10.1002/14651858.CD011603.pub2
82. Hilger D, Masureel M, Kobilka BK. Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol (2018) 25(1):4–12. doi: 10.1038/s41594-017-0011-7
83. Pavlos NJ, Friedman PA. GPCR signaling and trafficking: The long and short of it. Trends Endocrinol Metab (2017) 28(3):213–26. doi: 10.1016/j.tem.2016.10.007
84. Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY, et al. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol (2004) 483(2-3):175–86. doi: 10.1016/j.ejphar.2003.10.033
85. Steele AD, Szabo I, and Bednar F, Rogers TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev (2002) 13(3):209–22. doi: 10.1016/S1359-6101(02)00007-2
Keywords: neuropathogenesis, HIV, monocytes, buprenorphine, cognitive impairment
Citation: Murphy AJ, Kelschenbach J, He H, Chao W, Kim B-H, Volsky DJ and Berman JW (2022) Buprenorphine reverses neurocognitive impairment in EcoHIV infected mice: A potential therapy for HIV-NCI. Front. Immunol. 13:1004985. doi: 10.3389/fimmu.2022.1004985
Received: 27 July 2022; Accepted: 23 September 2022;
Published: 07 October 2022.
Edited by:
Paolo Immovilli, Guglielmo da Saliceto Hospital, ItalyReviewed by:
Bruce James Brew, St Vincent’s Hospital Sydney, AustraliaWilliam Tyor, Emory University, United States
Copyright © 2022 Murphy, Kelschenbach, He, Chao, Kim, Volsky and Berman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joan W. Berman, Sm9hbi5iZXJtYW5AZWluc3RlaW5tZWQuZWR1
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
 Aniella J. Murphy
Aniella J. Murphy Jennifer Kelschenbach
Jennifer Kelschenbach Hongxia He2
Hongxia He2 David J. Volsky
David J. Volsky Joan W. Berman
Joan W. Berman