- 1Rheumatology Unit, First Department of Propaedeutic Internal Medicine, Joint Academic Rheumatology Program, School of Medicine, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Greece
- 2Diabetes Center, First Department of Propaedeutic Internal Medicine, School of Medicine, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Greece
Background: Cardiovascular disease (CVD) is the foremost cause of morbidity and deaths in antiphospholipid syndrome (APS), driven by thrombo-inflammation and atherothrombosis mechanisms. Metabolic syndrome (MetS) is a proinflammatory and prothrombotic state characterized by increased CVD risk. We aimed to evaluate the prevalence of MetS in APS patients compared to rheumatoid arthritis (RA) and diabetes mellitus (DM) and its associations with clinical and laboratory patient characteristics and vascular ultrasound (US) markers of subclinical atherosclerosis.
Methods: We included 414 patients in our study: 138 patients with APS (median age: 44.9 years, females 70%) and matched 1:1 for age and sex RA and DM subjects. Three sets of criteria were used for MetS diagnosis: Joint Interim Statement (JIS), International Diabetes Federation (IDF) and modified National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATPIII). The demographic, clinical and laboratory characteristics of all participants were recorded and carotid and femoral US was performed in patients with APS. Multivariate regression models were applied.
Results: Prevalence of MetS was 23.9%, 23.2%, 20.3% (based on JIS, IDF, modified NCEP-ATPIII criteria, respectively) in APS versus 17.4%, 17.4%, 13% in RA (p=0.181, p=0.231, p=0.106, respectively), and 44.2%, 44.2%, 40.6% in DM patients. In multivariate analysis, patients with systemic lupus erythematosus- related APS had an approximately 2.5-fold higher risk of MetS versus RA patients. MetS in APS was independently associated with arterial thrombosis (Odds ratio 3.5, p=0.030). Odds ratio for MetS was 1.16 for each one unit increase in C-reactive protein levels according to JIS and IDF criteria, and 1.49 and 1.47 for each one unit increase in uric acid levels using the IDF and modified NCEP-ATPIII models, respectively. APS patients with atherosclerotic carotid plaques had 4 to 6.5-fold increased risk of MetS. Odds for MetS were decreased by 26% with an increase in physical activity by one hour per week.
Conclusions: MetS is present in approximately one-fourth of APS patients at a comparable prevalence to that observed in patients with RA. MetS in APS is associated with arterial thrombosis, cardiovascular risk biomarkers, physical activity, and subclinical atherosclerosis, supporting its role in cardiovascular risk stratification and management in APS.
1 Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disease, affecting mostly young adults, which is characterized by a wide spectrum of vascular and obstetric manifestations and the constant presence of antiphospholipid antibodies (aPL) (1). Cardiovascular disease (CVD), mainly in the form of stroke and myocardial infarction, is a leading cause of morbidity and mortality in APS (2). Innate and adaptive immune response dysregulation and an aPL- and traditional risk factors-mediated endothelial inflammation and damage play a major role in CVD pathogenesis in APS (3–8).
Metabolic syndrome (MetS) represents a constellation of interconnected cardiovascular risk factors (CVRFs), namely hypertension, abdominal obesity, insulin resistance and hyperglycemia, as well as elevated triglycerides (TGs) and low levels of high-density lipoprotein (HDL) cholesterol (9). It is currently recognized as an independent CVRF and its presence has been associated with an approximately two-fold increase in cardiovascular events in the general population (10). MetS shares common pathophysiologic pathways with APS involving a chronic low-grade systemic inflammation via pro-inflammatory cytokines production, macrophage recruitment, platelet activation, oxidative stress and endothelial dysfunction, insulin resistance, and free fatty acids production (11–14).
In the European League Against Rheumatism (EULAR) recommendations for the management of APS (15) and the EULAR recommendations for the management of cardiovascular risk in rheumatic and musculoskeletal diseases (RMDs) including Systemic Lupus Erythematosus (SLE) and APS (16), a thorough screening and control of traditional CVRFs was highlighted. Given that MetS is a cluster of modifiable CVRFs and shares endothelial damage pathways with APS, identification of its prevalence and any correlations with circulating cardiovascular biomarkers and clinical and subclinical CVD burden in APS, will help to improve CVD prevention measures in these patients.
Our goal was to evaluate the prevalence of MetS in APS using different sets of MetS diagnostic criteria and to examine its association with the clinical and laboratory features of the patients, as well as vascular ultrasound (US) markers of subclinical atherosclerosis. We also compared MetS prevalence in APS versus other rheumatic and non-rheumatic diseases of high CVD risk, such as rheumatoid arthritis (RA) and diabetes mellitus (DM).
2 Patients and methods
2.1 Study population
All eligible adult patients (≥18 years) who fulfilled the clinical and laboratory classification criteria for APS (1) and were followed at our Rheumatology Unit, were included in this cross-sectional study. Patients with APS were matched in a 1:1 ratio for age and sex with eligible patients with RA and DM followed in the Rheumatology and Diabetes Units of our Department, respectively. Exclusion criteria were prior atherosclerotic CVD events, concomitant DM (or RA, for patients with DM), acute illness (e.g., infectious disease), active malignancy, and pregnancy.
2.2 Recorded parameters
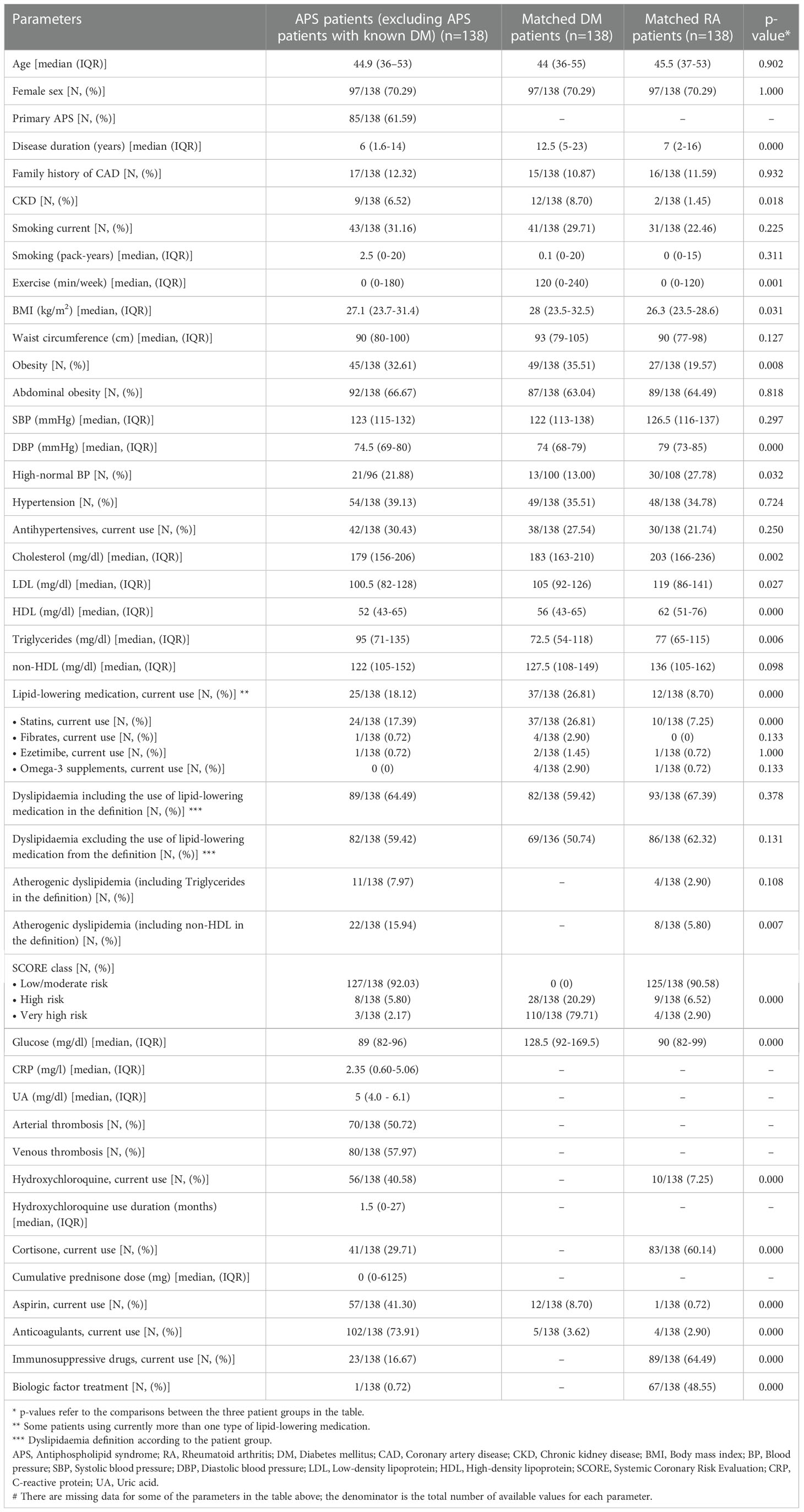
The following parameters were recorded at the time of the patients’ first visit at our department: age, sex, ethnicity, disease duration, APS type [primary APS (PAPS) or SLE-related APS (SLE-APS)], history of arterial and/or venous thrombosis, DM type for patients with DM, and traditional CVRFs, e.g. current smoking status and pack-years of smoking, blood pressure (BP) estimated as the average of three sequential readings taken 1 min apart after at least 10 min of rest (Microlife WatchBP Office, Microlife, Widnau, Switzerland), body mass index (BMI) (weight/height2), waist circumference (measured in cm), fasting total cholesterol (TC), low-density lipoprotein (LDL) and HDL cholesterol levels, fasting TGs levels, non-HDL levels (calculated by subtracting HDL from TC), physical activity level (measured in minutes of exercise per week), family history of coronary artery disease (CAD), and chronic kidney disease (CKD) (glomerular filtration rate <60 mL/min/1.73 m²). Additional laboratory tests included: C-reactive protein (CRP), glucose, uric acid (UA), haemoglobin A1c (HbA1c) levels, and aPL: anti-cardiolipin (aCL) antibodies (IgG or IgM isotype), anti-β2-glycoprotein I (anti-β2GPI) antibodies (IgG or IgM isotype) and lupus anticoagulant (LA). Positivity for aPL was defined based on the updated Sapporo criteria for APS (1). High titre aPL was defined as a titre greater than 4-fold of the upper normal limit in aCL or anti-β2GPI antibodies (IgG or IgM isotype). We also recorded disease-related medications including corticosteroids and cumulative prednisone dose, hydroxychloroquine and duration of its use, immunosuppressants and/or biologic agents, anticoagulants, antiplatelets, antihypertensives, lipid-lowering medications (statins, fibrates, ezetimibe, nicotinic acid, omega3 fatty acids supplements) and antidiabetic drugs.
Hypertension was defined as the use of antihypertensives or the average of three sequential office BP measurement >139/89 mmHg, and high-normal BP as the average of three sequential office systolic BP measurement of 130-139 mmHg and/or diastolic BP 85-89 mmHg in patients currently not on antihypertensives (17). Obesity was defined as BMI of at least 30 kg/m2, and abdominal obesity as a waist circumference of at least 80 cm in women and at least 94 cm in men (18). Dyslipidaemia in patients with APS and RA was defined as LDL ≥ 115 mg/dl and/or TGs ≥ 150 mg/dl and/or low HDL levels (<40 mg/dl in men and <50 mg/dl in women) and/or current use of lipid-lowering medication. Dyslipidemia was also assessed separately excluding the current use of lipid-lowering medications from the definition. The term atherogenic dyslipidemia was used to describe APS and RA patients with highly atherogenic lipid profile and it was defined as non-HDL ≥ 130 mg/dl and low HDL levels (for atherogenic dyslipidemia including non-HDL in the definition) and as TG ≥ 150mg/dl and low HDL levels (for atherogenic dyslipidemia including TGs in the definition) (19, 20).
For CVD risk classification in patients with APS and RA, we applied the Systemic Coronary Risk Evaluation (SCORE) (21) and its latest edition (SCORE2) to estimate 10-year risk of CVD. APS and RA patients were subsequently assigned to low, moderate and high CVD risk categories, based on the European Society of Cardiology (ESC) guidelines on CVD prevention of different years (17, 22, 23), according to the date of the patients’ US assessment. For CVD risk stratification in DM patients, we used the ESC guidelines for diabetes, prediabetes and cardiovascular diseases, developed in collaboration with the European Association for the Study of Diabetes (24), according to the time of their first visit. Based on the above, DM patients with at least one CVRF (hypertension, obesity, dyslipidaemia: fasting TC ≥200 mg/dL, LDL ≥130 mg/dL, HDL <40 mg/dL for men/<45 mg/dL for women or use of lipid-lowering medication (25), current smoking and CKD) and/or target organ damage are classified as very-high CVD risk, and all the other DM patients as high-risk.
2.3 Definition of MetS
The main components of MetS, as mentioned above, include abdominal obesity, hypertension, hyperglycemia and atherogenic dyslipidemia. Since the first description of MetS, several diagnostic criteria have been proposed for its definition, with differences concerning mainly the type and number of parameters required for the diagnosis, and the thresholds used for each parameter. In our study, we used three different sets of diagnostic criteria to define MetS: 1) the updated Joint Interim Statement (JIS) proposed by the International Diabetes Federation (IDF) Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute (NHLBI); American Heart Association (AHA); World Heart Federation; International Atherosclerosis Society; and International Association for the study of Obesity (26), 2) the IDF criteria (27) and 3) the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATPIII) criteria modified by AHA/NHLBI (25, 28). The above-mentioned criteria and the respective thresholds for each parameter are summarized in Supplementary Table 1.
2.4 Vascular US and outcome measures
Vascular US was performed in all APS patients by a single experienced operator. The near and far walls of the carotid bulbs, internal carotid arteries, common carotid arteries and common femoral arteries, bilaterally, were examined for the presence of atherosclerotic plaques using a 14-MHz multi-frequency linear transducer attached to a high-resolution B-mode US device (Vivid 7 Pro, GE Healthcare®). Plaques were defined as intima-media thickness (IMT) ≥1.5 mm or a focal thickening that encroaches ≥0.5 mm or 50% of the surrounding IMT into the arterial lumen.
2.5 Statistical analysis
Data are reported as median and interquartile range (IQR) (not normally distributed data), or when appropriate, as absolute number and relative frequency (percentage). To assess differences in patient characteristics, we applied Mann-Whitney U test (deviation from normality) for quantitative variables and Pearson’s χ2 or Fisher’s exact tests for qualitative variables. Pearson’s χ2 test was used to compare MetS prevalence between participant groups.
We applied multiple logistic regression models using the presence of MetS in APS patients as the outcome variable. All tested variables with a p-value<0.2 from the univariable logistic regression analysis were included in the initial multivariate logistic regression model. The backward elimination algorithm, based on which the variable with the highest p-value is removed in each step, along with clinical considerations, were used to derive the final multiple regression model (Supplementary ‘Backward elimination algorithm results’) resulting in three multivariate regression models, one for each definition of MetS used in the study, as the outcome variable. The final models included age, sex, arterial thrombosis, CRP and UA levels, high titre of anti-β2GPI antibodies of IgM isotype, presence of carotid atherosclerotic plaques, physical activity and current use of corticosteroids as independent variables. To further investigate the association of MetS (diagnosed based on the above three definitions) with different patient groups, we applied multiple regression models including an indicator variable with four levels denoting the participant group (1: RA, 2: PAPS, 3: SLE-APS, 4: DM). The outcome variable was the presence of MetS and the other independent variables in these models included age, sex, disease duration, pack-years of smoking, physical activity and LDL levels. A p-value <0.050 was considered statistically significant. STATA software (V.13.0, College Station, Texas, USA) was used for all statistical analyses.
3 Results
3.1 Baseline characteristics
A total of 138 patients with APS [85 with PAPS, 53 with SLE-APS, female 70.29%, median age 44.9 years (IQR: 36-53 years), all white Europeans] were included in the study, matched 1:1 for age and sex with RA and DM patients. Sixty three percent of DM patients had type I DM and 37% had type II DM [median HbA1c: 7.2% (IQR 6.7-8%)]. Basic characteristics of the three groups are shown in Table 1. The aPL profile and vascular US characteristics of APS patients are shown in Table 2. Atherosclerotic plaques at any site were present in 34.65% of APS patients, while 24.41% of patients had carotid plaques, and 21.26% had femoral artery plaques (Table 2).
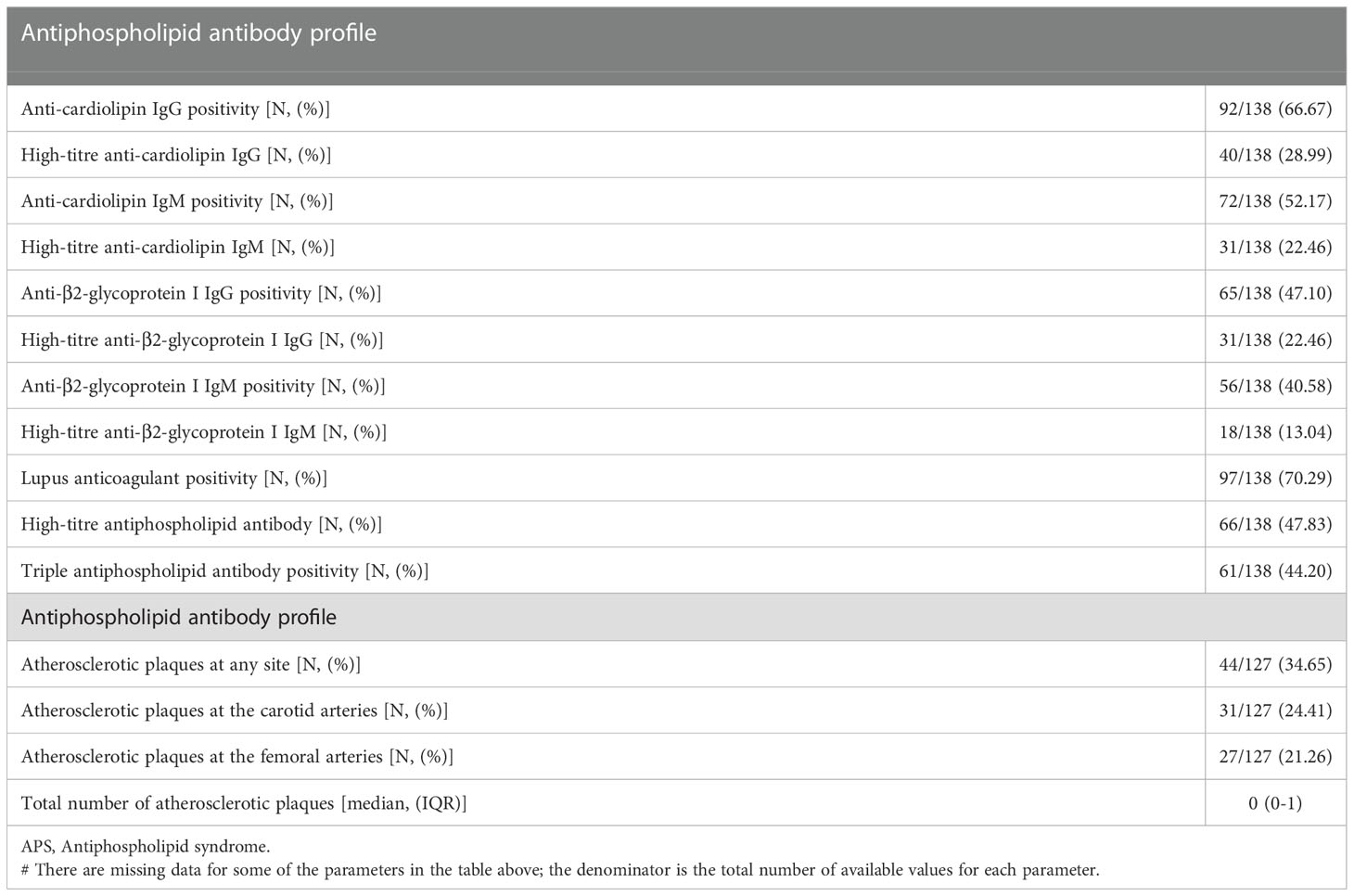
Table 2 Antiphospholipid antibody profile and vascular ultrasound characteristics of APS patients (excluding APS patients with known diabetes mellitus).
Among the three patient groups, TG levels were higher and HDL levels were lower in APS patients than the other two groups (p=0.006 and p<0.001, respectively), and obesity was more prevalent in APS and DM patients compared to RA patients (p=0.008). DM patients had longer disease duration than APS and RA patients (p<0.001) and higher exercise levels and current use of lipid-lowering medications (p=0.001 and p<0.001, respectively). There were no significant differences between the three groups in the current smoking status and pack-years of smoking, hypertension, abdominal obesity, and family history of CAD.
3.2 MetS prevalence and associations in three patient groups
Based on JIS, IDF and modified NCEP-ATPIII criteria, a comparable prevalence of MetS was detected between patients with APS (23.91%, 23.19% and 20.29%, respectively) and patients with RA (17.39%, 17.39% and 13.04%, p=0.181, p=0.231, p=0.106, respectively), with the highest prevalence in the age/sex-matched DM group (44.2%, 44.2% and 40.58%, respectively). (Figure 1) Among APS patients, MetS was present in 23.53% versus 24.53%, 22.35% versus 24.53% and 20% versus 20.75%, in PAPS versus SLE-APS patients respectively, based on JIS, IDF and modified NCEP-ATPIII criteria, respectively.
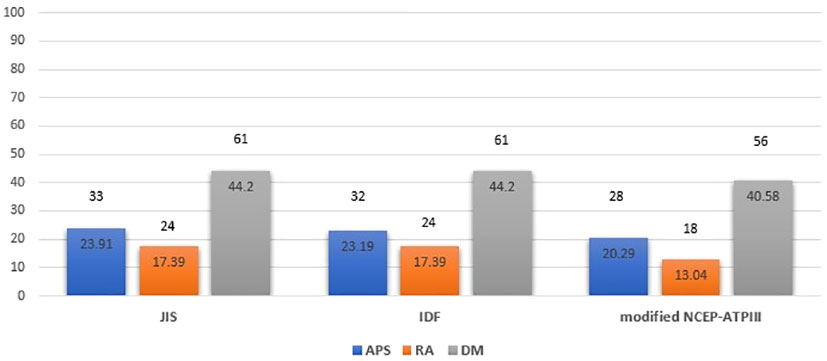
Figure 1 Metabolic syndrome prevalence in APS, RA and DM patient groups based on JIS, IDF and modified NCEP-ATPIII criteria (Percentages and absolute numbers are noted) # Comparison for APS versus RA patients: p=0.181, p=0.231, p=0.106 based on JIS, IDF and modified NCEP-ATPIII criteria respectively. Comparison for APS versus DM patients: p<0.001 for all criteria. Comparison for RA versus DM patients: p<0.001 for all criteria. Comparison for APS versus RA versus DM patients: p<0.001 for all criteria. APS, Antiphospholipid syndrome; RA, Rheumatoid arthritis; DM, Diabetes mellitus; JIS, Joint Interim Statement; IDF, International Diabetes Federation; NCEP-ATPIII, National Cholesterol Education Program Adult Treatment Panel III.
In multiple regression analysis including the participant group as an independent variable, patients with SLE-APS had an approximately 2.5-fold higher risk of MetS versus RA patients. MetS presence using the JIS, IDF and modified NCEP-ATPIII models was also independently associated with age (p<0.001 in all models), disease duration (p=0.024, p=0.023, p=0.002, respectively), physical activity (p=0.014, p=0.017, p=0.021, respectively), pack-years of smoking (p=0.009, p=0.005, p=0.002, respectively) and LDL levels (p=0.015, p=0.011, p=0.043, respectively) (Table 3).
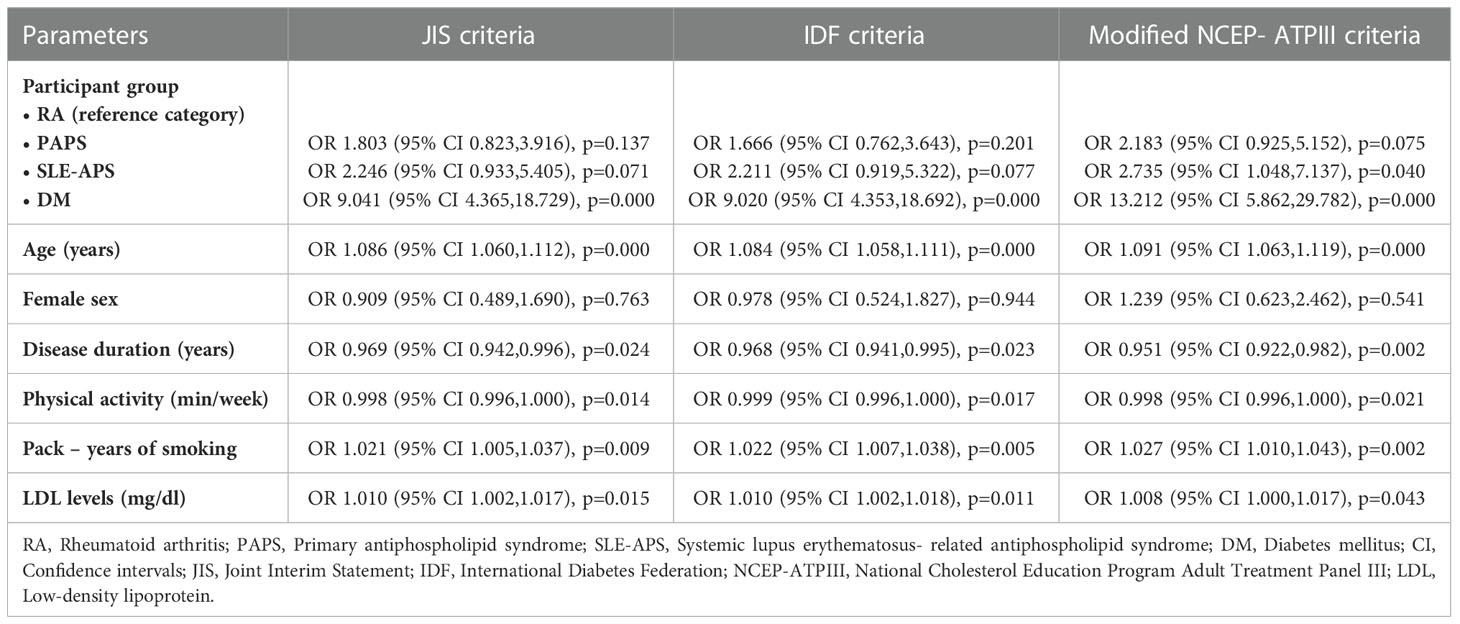
Table 3 Disease conferred risk for the presence of metabolic syndrome in patients with PAPS, SLE-APS and DM based on JIS, IDF and modified NCEP-ATPIII criteria (in multivariate regression models).
3.3 MetS prevalence and associations in APS patients
Focusing only on patients with APS, we included in the analysis three additional APS patients with co-existent DM who were excluded from the analysis among the three patient groups (because DM was one of the comparison groups), resulting in a total of 141 patients in the APS group. In this case, MetS was present in 25.53%, 24.82% and 21.99% of APS patients, based on JIS, IDF and modified NCEP-ATPIII criteria, respectively.
We also examined potential differences in the prevalence of MetS by age and sex. MetS prevalence was comparable between male and female patients with APS, using the JIS, IDF and modified NCEP-ATPIII definition (24.39%, 21.95% and 24.39% for males versus 26%, 26% and 21% for females, respectively) (Figure 2) but significantly higher among APS patients aged ≥ 40 years than those < 40 years (31.87%, 30.77% and 26.37% versus 14%, 14% and 14%; p=0.020, p=0.027 and p=0.090, respectively) (Figure 3).
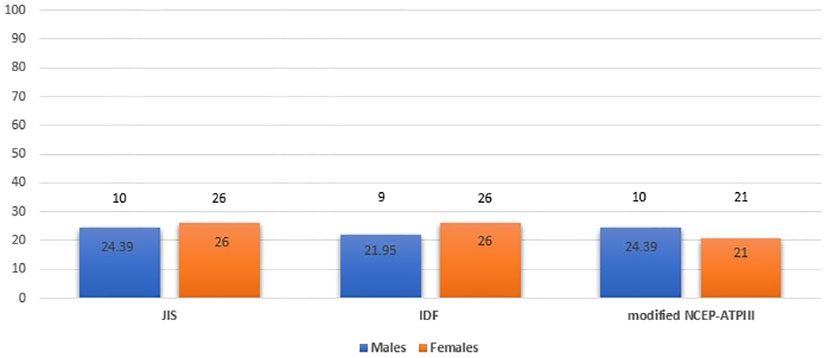
Figure 2 Metabolic syndrome prevalence in APS patients by sex (males and females) based on JIS, IDF and modified NCEP-ATPIII criteria (Percentages and absolute numbers are noted) APS, Antiphospholipid syndrome; JIS, Joint Interim Statement; IDF, International Diabetes Federation; NCEP-ATPIII, National Cholesterol Education Program Adult Treatment Panel III.
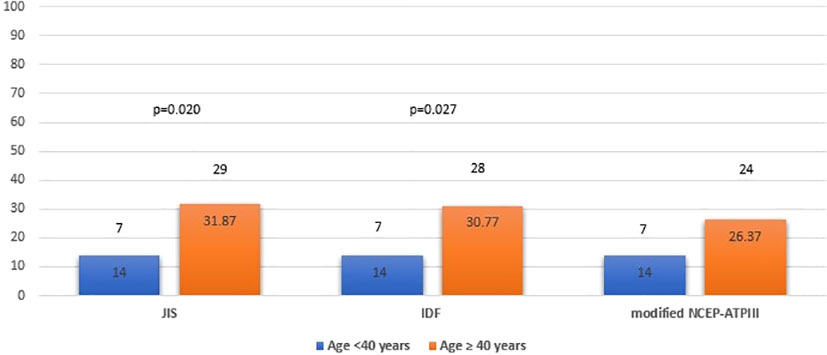
Figure 3 Metabolic syndrome prevalence in APS patients by age group (<40 years old and ≥ 40 years old) based on JIS, IDF and modified NCEP-ATPIII criteria (Percentages and absolute numbers are noted) APS, Antiphospholipid syndrome; JIS, Joint Interim Statement; IDF, International Diabetes Federation; NCEP-ATPIII, National Cholesterol Education Program Adult Treatment Panel III.
The results of the univariable logistic regression analysis for all tested variables are presented in Supplementary Table 2. In multivariate logistic regression models, we investigated the associations of MetS with clinical and laboratory parameters of APS and vascular US markers of subclinical atherosclerosis. We found significant associations with arterial thrombosis, CRP and UA levels, physical activity and presence of carotid atherosclerotic plaques, after controlling for age, sex, current use of corticosteroids and high titre of anti-β2GPI antibodies of IgM isotype based on backward elimination algorithm (Table 4). Interestingly, APS patients with arterial thrombosis had a 3.5-fold increased risk of MetS using the IDF criteria (p=0.030) with a trend in the JIS model [Odds ratio (OR) =2.76, p=0.062). Physical activity and CRP levels were independently associated with MetS using the JIS and IDF definitions, and the UA levels using the IDF and modified NCEP-ATPIII models. In particular, the odds for MetS were decreased by approximately 26% with an increase in physical activity by one hour per week in the JIS and IDF models. The OR for MetS was 1.16 for each one unit increase in CRP levels according to both JIS and IDF criteria. In the IDF and modified NCEP-ATPIII models, the ORs for MetS were 1.49 and 1.47, respectively for each one unit increase in UA levels. APS patients with atherosclerotic carotid plaques had 4 to 6.5-fold increased risk of MetS (OR=6.37, p=0.007; OR=4.69, p=0.024; and OR=4.15, p=0.033, using the JIS, IDF and modified NCEP-ATPIII models, respectively).
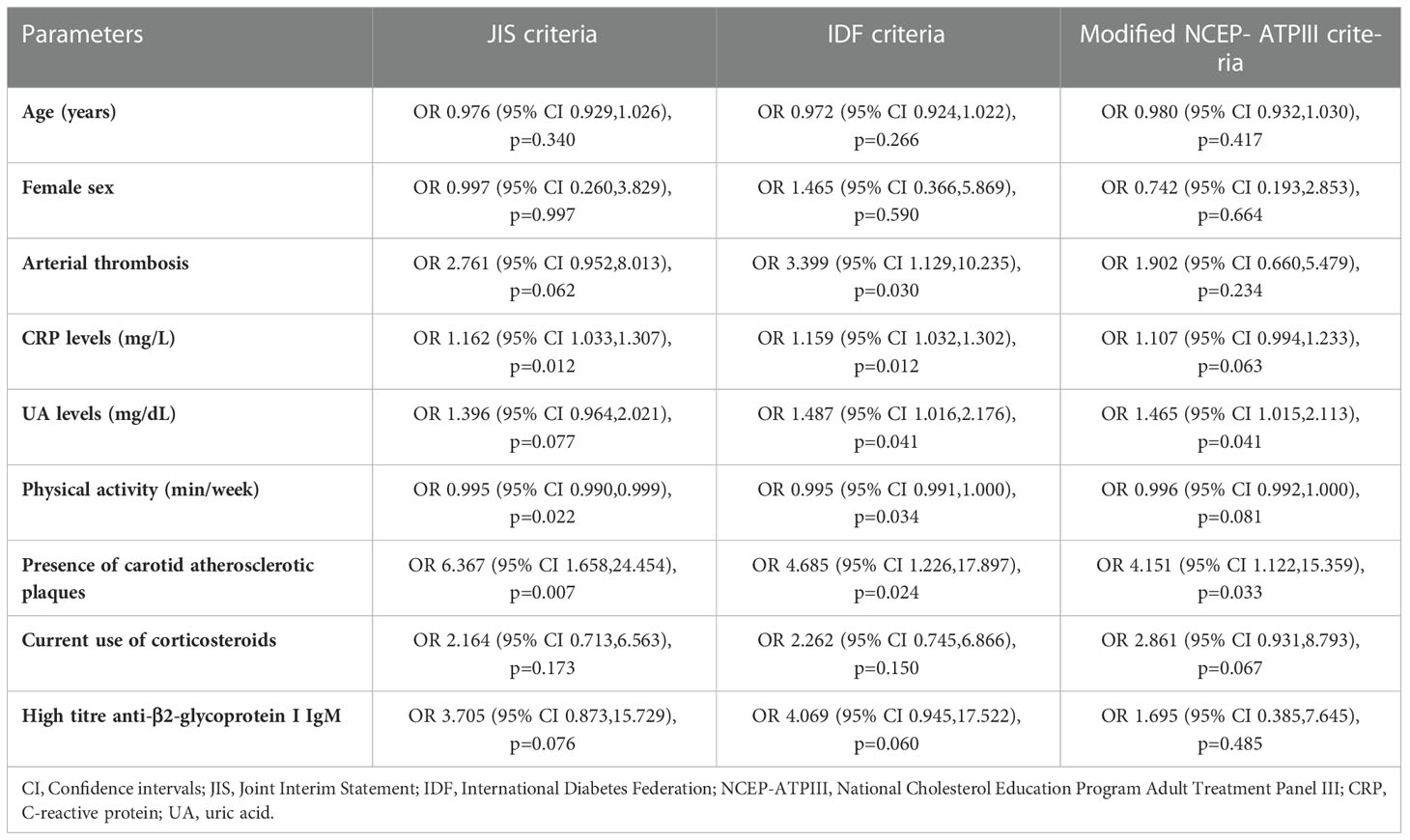
Table 4 Multivariate determinants for the presence of metabolic syndrome in APS patients based on JIS, IDF and modified NCEP-ATPIII criteria.
Finally, we also examined whether meeting the diagnostic criteria of MetS is associated with atherosclerotic plaque presence, following the multivariate regression analysis methodology described in the statistical analysis section. In these models, the presence of MetS based on JIS, IDF and modified NCEP-ATPIII criteria was significantly associated with the presence of atherosclerotic plaques at any site (OR 3.44, p=0.022; OR 2.92, p=0.050; OR 3.53, p=0.029, respectively) after adjusting for age, sex, APS type (PAPS or SLE-APS), pack-years of smoking, current use of statins and history of arterial and venous thrombosis.
4 Discussion
Our study showed that MetS is present in about one-fourth of APS patients and it is associated with arterial thrombosis, inflammation and cardiovascular biomarkers, physical activity levels and subclinical atherosclerosis, supporting the need for its rigorous assessment and management in APS. To our knowledge, this is the first study that compares the prevalence of MetS among patients with APS and other rheumatic and non-rheumatic conditions of high CVD risk, and the first study that examines any potential associations with clinical, laboratory and vascular US characteristics in APS.
CVD, especially myocardial infarction and stroke, represents a leading cause of death in APS, referring to 18.9% and 13.2% of deaths, respectively, in a large European cohort study of 1000 APS patients over a 10-year follow-up period (2). MetS is increasingly recognized as an independent predictor of CVD morbidity and mortality that implies substantial additional CVD risk beyond the sum of individual CVRF components (29). MetS is characterized by systemic inflammation, prothrombotic changes, endothelial dysfunction and accelerated atherosclerosis, sharing common pathogenetic mechanisms with APS. A growing body of evidence suggests that RMDs, especially SLE (30) and inflammatory arthritides (31–33), are characterized by increased prevalence of MetS, compared to healthy individuals, while recent meta-analyses showed that patients with SLE (OR=1.88 and OR=2.5, respectively, in 2 recent meta-analyses (34, 35), and RA (OR=1.44) (36) had high risk of MetS comparing to controls. MetS has been reported in 17 - 38% of APS patients in different studies with the use of various sets of diagnostic criteria (8, 37–40). This finding could be attributed to chronic inflammation and innate immune cell activation in these disorders, and a higher prevalence of traditional CVRFs. Data from the Greek APS registry (41) and recent data from our (42) and other groups (43) showed a comparable or higher prevalence of traditional CVRFs to that observed in age and sex-matched patients with RA or DM, such as obesity, smoking, hypertension and dyslipidaemia. In addition, in a previous case-control study conducted by our group, patients with PAPS accumulated more traditional CVRFs than age and sex-matched patients with DM (44).
We also found that MetS prevalence was significantly higher in patients aged ≥40 years than in younger patients. This finding is consistent with previous relevant studies in the general population worldwide (45–48) and in patients with SLE and RA (49, 50) indicating that MetS is more prevalent with advancing age. No significant difference was found in MetS prevalence between male and female patients with APS regardless of the diagnostic criteria used. Data on sex-related differences concerning MetS prevalence in the general population is relatively scarce and conflicting, although it is inferred that perimenopausal hormonal alterations and associated changes in body fat distribution, insulin sensitivity and lipid levels might determine an age-associated increased prevalence of MetS among women (51, 52).
Interestingly, we found a comparable frequency of MetS in APS with that observed in patients with RA, a disorder characterized by high systemic inflammation, a high prevalence of CVRFs and a substantial CVD burden. Importantly, MetS risk was higher in the SLE-APS subgroup versus RA patients. To date, there are only isolated studies comparing patients with SLE and RA with respect to MetS (53, 54) and no studies comparing APS and RA. In one of these studies, MetS prevalence was similar between 85 SLE and 107 RA patients included in the study (53). Santos et al. (54) reported that hypertension, hyperuricemia and low HDL levels were more prevalent in 100 SLE women than 98 RA women with similar mean age. A significantly higher prevalence of MetS was found in patients with DM vs both APS and RA patients in our study. This finding was expected based on the high prevalence of traditional CVRFs in these patients and the mere definition of MetS, which includes hyperglycemia and previously diagnosed DM as a diagnostic component.
Examining associations between MetS and laboratory and clinical CVD markers, we found that MetS was independently associated with CRP and UA as well as with arterial thrombosis, physical activity and atherosclerotic plaques, respectively. Various inflammation biomarkers have been measured in patients with MetS, of which CRP is the most well-characterized (55, 56). Evidence has shown that CRP levels are elevated in patients with MetS in both the general population (57, 58) and patients with RMDs, such as SLE (30, 59, 60) and RA (61, 62). Accordingly, our results in APS showed that an increase in CRP levels is independently associated with MetS presence, using JIS and IDF diagnostic criteria. This finding is of high importance, taking into consideration the well-established role of CRP in CVD risk assessment and its recognition as an independent predictor of cardiovascular events in MetS (55). Hyperuricemia was also found to be significantly associated with an increased risk for MetS in our study. These results are consistent with a previous case-control study by Rodrigues et al. (37) reporting significantly higher UA levels in APS patients with MetS than those without. Hyperuricemia, another increasingly recognized cardiovascular risk biomarker has been linked to MetS and its components, including insulin resistance and type II DM, hypertension, abdominal obesity and dyslipidemia, in various studies in the general population (63–67). The relationship between serum UA and markers of inflammation, endothelial dysfunction and subclinical atherosclerosis in the general population has also been extensively studied (68–71).
Concerning associations with clinical and subclinical CVD parameters, we found that APS patients with arterial thrombosis (mainly stroke and CAD) have a 3.5-fold higher risk for MetS, based on the IDF diagnostic criteria, and this finding was confirmed with a trend using the JIS definition. In a previous case-control study, researchers demonstrated that PAPS patients with MetS had a higher frequency of arterial events, strengthening the concept of synergistic effect of APS and MetS on endothelial dysfunction and atherothrombosis (37). Subclinical atherosclerosis is recognized as an early indicator of CVD burden in the general population. In a case-control study conducted by our group, we found that both patients with PAPS and SLE-APS had a nearly 2.5-fold risk of atherosclerotic plaques in carotid and/or femoral arteries compared to controls, and similar to DM patients, after adjusting for traditional CVRFs (44), and a comparable relative risk for plaque progression between patients with PAPS, SLE-APS and DM in a 3-year follow-up study (72). The present study examined for the first time the association between atherosclerotic plaque burden and the presence of MetS in APS patients. We showed that APS patients with carotid plaques have a 4 to 6.5-fold increased risk of MetS, confirmed by the three different diagnostic criteria used. Our results are in accordance with observational studies (73–78) and recent meta-analyses (79, 80) of population-based studies which showed that MetS is a risk factor for early carotid atherosclerosis in the general population. Consequently, these data support the role of MetS and vascular US examination in cardiovascular risk stratification and CVD prevention decision-making strategies in APS.
In the same line, we found that physical activity is associated with lower risk for MetS in all three disease groups including APS. This finding is expected, as it is widely known that regular exercise has a favorable effect on body weight, BP, lipid levels and insulin resistance, which are all components of MetS (17, 22, 23). It is also postulated that physical activity has anti-inflammatory effects as it has been negatively associated with high-sensitive CRP, interleukin (IL)-6, IL-18, and positively associated with the anti-inflammatory cytokine IL-10 (81).
The strengths of our study include the assessment for the first time of the following: 1) prevalence of MetS using three different sets of MetS diagnostic criteria in a large cohort of APS patients considering the rarity of the syndrome (representing the largest so far study examining MetS in APS); 2) associations between MetS and multiple clinical, laboratory and subclinical atherosclerosis parameters assessed simultaneously; 3) differences in MetS prevalence comparing patients with APS and age and sex-matched patients with rheumatic and non-rheumatic disorders of high CVD risk, such as RA and DM. However, prospective, multicentre studies are required to confirm our results. The study has some limitations. The group of DM controls is heterogeneous, since 63% of them had type I DM and 37% had type II DM, which may also account for some of the results e.g. the difference in disease duration among the groups.
In conclusion, our study showed that MetS is present in one-fourth of APS patients, a comparable prevalence to that observed in RA. MetS in APS is associated with arterial thrombosis, cardiovascular biomarkers and markers of inflammation, as well as subclinical atherosclerosis. Awareness of MetS among clinicians and patients with APS, as well as thorough screening and control of traditional CVRFs following similar CVD prevention measures to those implemented in other diseases of high CVD risk, could help to improve cardiovascular health in APS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved by the hospital’s Institutional Review Board of Laiko General Hospital (Laiko Hospital Scientific Council; IRB number 7748). The patients/participants provided their written informed consent to participate in this study.
Author contributions
EB: acquisition of data, analysis of data, drafting and critical revision of the manuscript. NT: acquisition of data, critical revision of manuscript. PS: interpretation of data, critical revision of manuscript. MT: conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript, guarantor. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1077166/full#supplementary-material
Supplementary Table 1 | Criteria for the clinical diagnosis of metabolic syndrome based on different sets of diagnostic criteria
Supplementary Table 2 | Univariate determinants for the presence of metabolic syndrome in APS patients based on JIS, IDF and modified NCEP-ATPIII criteria
References
1. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost (2006) 4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramón E, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann Rheum Dis (2015) 74(6):1011–8. doi: 10.1136/annrheumdis-2013-204838
3. Tektonidou MG. Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. J Autoimmun (2022) 128:102813. doi: 10.1016/j.jaut.2022.102813
4. Corban MT, Duarte-Garcia A, McBane RD, Matteson EL, Lerman LO, Lerman A. Antiphospholipid syndrome: Role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. J Am Coll Cardiol (2017) 69(18):2317–30. doi: 10.1016/j.jacc.2017.02.058
5. Tektonidou MG, Kravvariti E, Vlachogiannis NI, Georgiopoulos G, Mantzou A, Sfikakis PP, et al. Clinical value of amyloid-beta1-40 as a marker of thrombo-inflammation in antiphospholipid syndrome. Rheumatol (Oxford) (2021) 60(4):1669–75. doi: 10.1093/rheumatology/keaa548
6. Lopez-Pedrera C, Aguirre-Zamorano MÁ, Pérez-Sánchez C. Mechanisms of atherosclerosis and cardiovascular disease in antiphospholipid syndrome and systemic lupus erythematosus. new therapeutic approaches. Med Clín (Engl Ed) (2017) 149(4):160–9. doi: 10.1016/j.medcli.2017.05.003
7. Tektonidou MG, Papassotiriou I, Sfikakis PP. Growth differentiation factor 15 (GDF-15) as potential cardiovascular risk biomarker in antiphospholipid syndrome. Rheumatology (2021) 20:keab277. doi: 10.1093/rheumatology/keab277
8. da Silva FF, Levy RA, de Carvalho JF. Cardiovascular risk factors in the antiphospholipid syndrome. J Immunol Res (2014) 2014:621270. doi: 10.1155/2014/621270
9. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep (2018) 20(2):12. doi: 10.1007/s11906-018-0812-z
10. McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care (2005) 28(2):385–90. doi: 10.2337/diacare.28.2.385
11. Medina G, Vera-Lastra O, Peralta-Amaro AL, Jiménez-Arellano MP, Saavedra MA, Cruz-Domínguez MP, et al. Metabolic syndrome, autoimmunity and rheumatic diseases. Pharmacol Res (2018) 133:277–88. doi: 10.1016/j.phrs.2018.01.009
12. McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol (2018) 36(1):14–20. doi: 10.1016/j.clindermatol.2017.09.004
13. Mok CC. Metabolic syndrome and systemic lupus erythematosus: The connection. Expert Rev Clin Immunol (2019) 15(7):765–75. doi: 10.1080/1744666X.2019.1620601
14. Abella V, Scotece M, Conde J, López V, Lazzaro V, Pino J, et al. Adipokines, metabolic syndrome and rheumatic diseases. J Immunol Res (2014) 2014:1–14. doi: 10.1155/2014/343746
15. Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis (2019) 78(10):1296–304. doi: 10.1136/annrheumdis-2019-215213
16. Drosos GC, Vedder D, Houben E, Boekel L, Atzeni F, Badreh S, et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis (2022) 81(6):768–79. doi: 10.1136/annrheumdis-2021-221733
17. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J (2021) 42(34):3227–337. doi: 10.1093/eurheartj/ehab484
18. World Health Organization. Waist circumference and waist-hip ratio report of a WHO expert consultation Geneva (2011). Available at: http://apps.who.int/iris/bitstream/handle/10665/44583/9789241501491_eng.pdf?sequence=1 (Accessed September 29, 2022).
19. Grundy SM, Small LDL. Atherogenic dyslipidemia, and the metabolic syndrome. Circulation (1997) 95(1):1–4. doi: 10.1161/01.CIR.95.1.1
20. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur Heart J (2019) 41(1):111–88. doi: 10.1093/eurheartj/ehz455
21. Conroy R. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J (2003) 24(11):987–1003. doi: 10.1093/eurheartj/ehz455
22. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) * developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J (2012) 33(13):1635–701. doi: 10.1093/eurheartj/ehs092
23. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. European Guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J (2016) 37(29):2315–81. doi: 10.1093/eurheartj/ehw106
24. Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European society of cardiology (ESC) and developed in collaboration with the European association for the study of diabetes (EASD). Eur Heart J (2013) 34(39):3035–87. doi: 10.1093/eurheartj/eht108
25. National Cholesterol Education Program. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) final report. Circulation (2002) 106(25):3143–421.
26. Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
27. Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. a consensus statement from the international diabetes federation. Diabetes Med (2006) 23(5):469–80. doi: 10.1111/j.1464-5491.2006.01858.x
28. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American heart Association/National heart, lung, and blood institute scientific statement. Circulation (2005) 112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
29. Reilly MP, Rader DJ. The metabolic syndrome: More than the sum of its parts? Circulation (2003) 108(13):1546–51. doi: 10.1161/01.CIR.0000088846.10655.E0
30. Chung CP, Avalos I, Oeser A, Gebretsadik T, Shintani A, Raggi P, et al. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: Association with disease characteristics and cardiovascular risk factors. Ann Rheum Dis (2006) 66(2):208–14. doi: 10.1136/ard.2006.054973
31. Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis (2008) 196(2):756–63. doi: 10.1016/j.atherosclerosis.2007.01.004
32. Dao HH, Do QT, Sakamoto J. Increased frequency of metabolic syndrome among Vietnamese women with early rheumatoid arthritis: A cross-sectional study. Arthritis Res Ther (2010) 12(6):R218. doi: 10.1186/ar3203
33. Rostom S, Mengat M, Lahlou R, Hari A, Bahiri R, Hajjaj-Hassouni N. Metabolic syndrome in rheumatoid arthritis: Case control study. BMC Musculoskelet Disord (2013) 14(1):147. doi: 10.1186/1471-2474-14-147
34. Sun C, Qin W, Zhang YH, Wu Y, Li Q, Liu M, et al. Prevalence and risk of metabolic syndrome in patients with systemic lupus erythematosus: A meta-analysis. Int J Rheum Dis (2017) 20(8):917–28. doi: 10.1111/1756-185X.13153
35. Hallajzadeh J, Khoramdad M, Izadi N, Karamzad N, Almasi-Hashiani A, Ayubi E, et al. The association between metabolic syndrome and its components with systemic lupus erythematosus: A comprehensive systematic review and meta-analysis of observational studies. Lupus (2018) 27(6):899–912. doi: 10.1177/0961203317751047
36. Hallajzadeh J, Safiri S, Mansournia MA, Khoramdad M, Izadi N, Almasi-Hashiani A, et al. Metabolic syndrome and its components among rheumatoid arthritis patients: A comprehensive updated systematic review and meta-analysis. PloS One (2017) 12(3):e0170361. doi: 10.1371/journal.pone.0170361
37. Rodrigues CEM, Bonfá E, Caleiro MTC, Vendramini MB, Bueno C, Lopes JB, et al. Association of arterial events with the coexistence of metabolic syndrome and primary antiphospholipid syndrome. Arthritis Care Res (2012) 64(10):1576–83. doi: 10.1002/acr.21701
38. Medina G, Gutiérrez-Moreno AL, Vera-Lastra O, Saavedra MA, Jara LJ. Prevalence of metabolic syndrome in primary antiphospholipid syndrome patients. Autoimmun Rev (2011) 10(4):214–7. doi: 10.1016/j.autrev.2010.10.004
39. Muniz Caldas CA, Freire de Carvalho J. Cardiovascular comorbidities in antiphospholipid syndrome. Expert Rev Clin Immunol (2013) 9(10):987–90. doi: 10.1586/1744666X.2013.837261
40. Rodrigues CEM, Vendramini MB, Bueno C, Bonfá E, de Carvalho JF. Adipocytokines in primary antiphospholipid syndrome: Potential markers of low-grade inflammation, insulin resistance and metabolic syndrome. Clin Exp Rheumatol (2012) 30(6):871–8.
41. Panopoulos S, Thomas K, Georgiopoulos G, Boumpas D, Katsiari C, Bertsias G, et al. Comparable or higher prevalence of comorbidities in antiphospholipid syndrome vs rheumatoid arthritis: A multicenter, case-control study. Rheumatology (2021) 60(1):170–8. doi: 10.1093/rheumatology/keaa321
42. Bolla E, Tentolouris N, Sfikakis PP, Tektonidou MG. Cardiovascular risk management in antiphospholipid syndrome: trends over time and comparison with rheumatoid arthritis and diabetes mellitus. Lupus Sci Med (2021) 8(1):e000579. doi: 10.1136/lupus-2021-000579
43. Djokovic A, Stojanovich L, Stanisavljevic N, Djokic S, Filipovic B, Matic P, et al. Cardiac manifestations in primary antiphospholipid syndrome and their association to antiphospholipid antibody’s types and titers–cross sectional study of Serbian cohort. Clin Rheumatol (2022) 41(5):1447–55. doi: 10.1007/s10067-022-06056-8
44. Kravvariti E, Konstantonis G, Tentolouris N, Sfikakis PP, Tektonidou MG. Carotid and femoral atherosclerosis in antiphospholipid syndrome: Equivalent risk with diabetes mellitus in a case–control study. Semin Arthritis Rheum (2018) 47(6):883–9. doi: 10.1016/j.semarthrit.2017.10.015
45. Scuteri A, Laurent S, Cucca F, Cockcroft J, Cunha PG, Mañas LR, et al. Metabolic syndrome across Europe: Different clusters of risk factors. Eur J Prev Cardiolog (2015) 22(4):486–91. doi: 10.1177/2047487314525529
46. Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United states, 2003-2006. Natl Health Stat Rep (2009) 13(13):1–7.
47. Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am (2004) 33(2):351–75. doi: 10.1016/j.ecl.2004.03.005
48. Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the united states, 2011-2016. JAMA (2020) 323(24):2526. doi: 10.1001/jama.2020.4501
49. Parker B, Ahmad Y, Shelmerdine J, Edlin H, Yates A, Teh LS, et al. An analysis of the metabolic syndrome phenotype in systemic lupus erythematosus. Lupus (2011) 20(14):1459–65. doi: 10.1177/0961203311416695
50. Cai W, Tang X, Pang M. Prevalence of metabolic syndrome in patients with rheumatoid arthritis: An updated systematic review and meta-analysis. Front Med (2022) 9:855141. doi: 10.3389/fmed.2022.855141
51. Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol Res (2017) 120:34–42. doi: 10.1016/j.phrs.2017.03.008
52. Rochlani Y, Pothineni NV, Mehta JL. Metabolic syndrome: Does it differ between women and men? Cardiovasc Drugs Ther (2015) 29(4):329–38. doi: 10.1007/s10557-015-6593-6
53. Zonana-Nacach A, Santana-Sahagún E, Jiménez-Balderas FJ, Camargo-Coronel A. Prevalence and factors associated with metabolic syndrome in patients with rheumatoid arthritis and systemic lupus erythematosus. JCR: J Clin Rheumatol (2008) 14(2):74–7. doi: 10.1097/RHU.0b013e31816b2faa
54. Santos MJ, Vinagre F, Silva JJ, Gil V, Fonseca JE. Cardiovascular risk profile in systemic lupus erythematosus and rheumatoid arthritis: A comparative study of female patients. Acta Reumatol Port (2010) 35(3):325–32.
55. Devaraj S, Singh U, Jialal I. Human c-reactive protein and the metabolic syndrome. Curr Opin Lipidol (2009) 20(3):182–9. doi: 10.1097/MOL.0b013e32832ac03e
56. Reddy P, Lent-Schochet D, Ramakrishnan N, McLaughlin M, Jialal I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin Chim Acta (2019) 496:35–44. doi: 10.1016/j.cca.2019.06.019
57. Vu JD, Vu JB, Pio JR, Malik S, Franklin SS, Chen RS, et al. Impact of c-reactive protein on the likelihood of peripheral arterial disease in united states adults with the metabolic syndrome, diabetes mellitus, and preexisting cardiovascular disease. Am J Cardiol (2005) 96(5):655–8. doi: 10.1016/j.amjcard.2005.04.038
58. Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of c-reactive protein among U.S. youth. Diabetes Care (2005) 28(4):878–81. doi: 10.2337/diacare.28.4.878
59. Oeser A, Chung CP, Asanuma Y, Avalos I, Stein CM. Obesity is an independent contributor to functional capacity and inflammation in systemic lupus erythematosus. Arthritis Rheum (2005) 52(11):3651–9. doi: 10.1002/art.21400
60. Sabio JM, Vargas-Hitos J, Zamora-Pasadas M, Mediavilla JD, Navarrete N, Ramirez Á, et al. Metabolic syndrome is associated with increased arterial stiffness and biomarkers of subclinical atherosclerosis in patients with systemic lupus erythematosus. J Rheumatol (2009) 36(10):2204–11. doi: 10.3899/jrheum.081253
61. Karvounaris SA, Sidiropoulos PI, Papadakis JA, Spanakis EK, Bertsias GK, Kritikos HD, et al. Metabolic syndrome is common among middle-to-older aged Mediterranean patients with rheumatoid arthritis and correlates with disease activity: A retrospective, cross-sectional, controlled, study. Ann Rheum Dis (2006) 66(1):28–33. doi: 10.1136/ard.2006.053488
62. Šalamon L, Morović-Vergles J, Marasović-Krstulović D, Kehler T, Šakić D, Badovinac O, et al. Differences in the prevalence and characteristics of metabolic syndrome in rheumatoid arthritis and osteoarthritis: A multicentric study. Rheumatol Int (2015) 35(12):2047–57. doi: 10.1007/s00296-015-3307-0
63. Lin SD, Tsai DH, Hsu SR. Association between serum uric acid level and components of the metabolic syndrome. J Chin Med Assoc (2006) 69(11):512–6. doi: 10.1016/S1726-4901(09)70320-X
64. Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol (2014) 2014:1–7. doi: 10.1155/2014/852954
65. Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation (2007) 115(19):2526–32. doi: 10.1161/CIRCULATIONAHA.106.657627
66. Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: The CARDIA study. Ann Epidemiol (1998) 8(4):250–61. doi: 10.1016/S1047-2797(97)00204-4
67. Oyama C, Takahashi T, Oyamada M, Oyamada T, Ohno T, Miyashita M, et al. Serum uric acid as an obesity-related indicator in early adolescence. Tohoku J Exp Med (2006) 209(3):257–62. doi: 10.1620/tjem.209.257
68. Fukui M, Tanaka M, Shiraishi E, Harusato I, Hosoda H, Asano M, et al. Serum uric acid is associated with microalbuminuria and subclinical atherosclerosis in men with type 2 diabetes mellitus. Metabolism (2008) 57(5):625–9. doi: 10.1016/j.metabol.2007.12.005
69. Erdogan D, Gullu H, Caliskan M, Yildirim E, Bilgi M, Ulus T, et al. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract (2005) 59(11):1276–82. doi: 10.1111/j.1742-1241.2005.00621.x
70. Lee YJ, Lee JH, Shin YH, Kim JK, Lee HR, Lee DC. Gender difference and determinants of c-reactive protein level in Korean adults. Clin Chem Lab Med (2009) 47(7):863–9. doi: 10.1515/CCLM.2009.196
71. Zhen H, Gui F. The role of hyperuricemia on vascular endothelium dysfunction. BioMed Rep (2017) 7(4):325–30. doi: 10.3892/br.2017.966
72. Evangelatos G, Kravvariti E, Konstantonis G, Tentolouris N, Sfikakis PP, Tektonidou MG. Atherosclerosis progression in antiphospholipid syndrome is comparable to diabetes mellitus: A 3 year prospective study. Rheumatology (2022) 61(8):3408–13. doi: 10.1093/rheumatology/keab882
73. Sipilä K, Moilanen L, Nieminen T, Reunanen A, Jula A, Salomaa V, et al. Metabolic syndrome and carotid intima media thickness in the health 2000 survey. Atherosclerosis (2009) 204(1):276–81. doi: 10.1016/j.atherosclerosis.2008.08.029
74. Hulthe J, Bokemark L, Wikstrand J, Fagerberg B. The metabolic syndrome, LDL particle size, and atherosclerosis: The atherosclerosis and insulin resistance (AIR) study. ATVB (2000) 20(9):2140–7. doi: 10.1161/01.ATV.20.9.2140
75. McNeill AM, Rosamond WD, Girman CJ, Heiss G, Golden SH, Duncan BB, et al. Prevalence of coronary heart disease and carotid arterial thickening in patients with the metabolic syndrome (The ARIC study). Am J Cardiol (2004) 94(10):1249–54. doi: 10.1016/j.amjcard.2004.07.107
76. Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, et al. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol (2004) 43(8):1388–95. doi: 10.1016/j.jacc.2003.10.061
77. Pollex RL, Al-Shali KZ, House AA, Spence JD, Fenster A, Mamakeesick M, et al. Relationship of the metabolic syndrome to carotid ultrasound traits. Cardiovasc Ultrasound (2006) 4(1):28. doi: 10.1186/1476-7120-4-28
78. Rundek T, White H, Boden-Albala B, Jin Z, Elkind MSV, Sacco RL. The metabolic syndrome and subclinical carotid atherosclerosis: The northern Manhattan study. J CardioMetab Syndrome (2007) 2(1):24–9. doi: 10.1111/j.1559-4564.2007.06358.x
79. Cuspidi C, Sala C, Provenzano F, Tadic M, Gherbesi E, Grassi G, et al. Metabolic syndrome and subclinical carotid damage: A meta-analysis from population-based studies. J Hypertens (2018) 36(1):23–30. doi: 10.1097/HJH.0000000000001575
80. Cuspidi C, Sala C, Tadic M, Gherbesi E, Grassi G, Mancia G. Association of metabolic syndrome with carotid thickening and plaque in the general population: A meta-analysis. J Clin Hypertens (2018) 20(1):4–10. doi: 10.1111/jch.13138
Keywords: antiphospholipid syndrome, metabolic syndrome, cardiovascular disease, atherosclerosis, thrombo-inflammation, cardiovascular risk factors, rheumatoid arthritis, diabetes mellitus
Citation: Bolla E, Tentolouris N, Sfikakis PP and Tektonidou MG (2023) Metabolic syndrome in antiphospholipid syndrome versus rheumatoid arthritis and diabetes mellitus: Association with arterial thrombosis, cardiovascular risk biomarkers, physical activity, and coronary atherosclerotic plaques. Front. Immunol. 13:1077166. doi: 10.3389/fimmu.2022.1077166
Received: 22 October 2022; Accepted: 19 December 2022;
Published: 09 January 2023.
Edited by:
Ljudmila Stojanovich, University of Belgrade, SerbiaReviewed by:
Manuel Francisco Ugarte-Gil, Universidad Científica del Sur, PeruEleni Tiniakou, Johns Hopkins University, United States
Copyright © 2023 Bolla, Tentolouris, Sfikakis and Tektonidou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria G. Tektonidou, bXRla3Rvbmlkb3VAZ21haWwuY29t; bXRla3Rvbmlkb3VAbWVkLnVvYS5ncg==
 Eleana Bolla1
Eleana Bolla1 Nikolaos Tentolouris
Nikolaos Tentolouris Maria G. Tektonidou
Maria G. Tektonidou