- 1Malcolm Fisher Department of Intensive Care, Royal North Shore Hospital, St Leonards, NSW, Australia
- 2The Kirby Institute, The University of New South Wales, Kensington, NSW, Australia
- 3Division of Critical Care, The George Institute for Global Health, Newtown, NSW, Australia
- 4Institute of Clinical Pathology and Medical Research, Westmead Hospital, Westmead, NSW, Australia
Introduction: Acute pancreatitis (AP) is characterised by an inflammatory response that in its most severe form can cause a systemic dysregulated immune response and progression to acute multi-organ dysfunction. The pathobiology of the disease is unclear and as a result no targeted, disease-modifying therapies exist. We performed a scoping review of data pertaining to the human immunology of AP to summarise the current field and to identify future research opportunities.
Methods: A scoping review of all clinical studies of AP immunology was performed across multiple databases. Studies were included if they were human studies of AP with an immunological outcome or intervention.
Results: 205 studies met the inclusion criteria for the review. Severe AP is characterised by significant immune dysregulation compared to the milder form of the disease. Broadly, this immune dysfunction was categorised into: innate immune responses (including profound release of damage-associated molecular patterns and heightened activity of pattern recognition receptors), cytokine profile dysregulation (particularly IL-1, 6, 10 and TNF-α), lymphocyte abnormalities, paradoxical immunosuppression (including HLA-DR suppression and increased co-inhibitory molecule expression), and failure of the intestinal barrier function. Studies including interventions were also included. Several limitations in the existing literature have been identified; consolidation and consistency across studies is required if progress is to be made in our understanding of this disease.
Conclusions: AP, particularly the more severe spectrum of the disease, is characterised by a multifaceted immune response that drives tissue injury and contributes to the associated morbidity and mortality. Significant work is required to develop our understanding of the immunopathology of this disease if disease-modifying therapies are to be established.
Introduction
Acute pancreatitis (AP) is an inflammatory syndrome of the pancreas with a spectrum that ranges from mild, self-limiting disease to severe illness characterised by systemic inflammation and persistent multi-organ failure. It has a highly variable incidence of 3-130 per 100,000 people, with a rising incidence observed in developed countries (1). While the majority (80%) of cases are mild and typically resolve over a few days, the remaining 20% develop severe acute pancreatitis (SAP), which is defined by the development of organ failure (single or multiple) that persists for greater than 48 hours (Table 1) (2). In addition to local complications such as acute pancreatic fluid collections and pseudocyst formation (3), SAP is associated with peripancreatic vascular complications (e.g. splanchnic venous thrombosis, pseudo-aneurysm), abdominal compartment syndrome, acute respiratory distress syndrome (ARDS), and acute kidney injury (4). In the acute phase, SAP typically requires intensive care unit (ICU) admission for ventilatory support, vasopressor therapies or renal replacement therapy. A subset of SAP patients develops pancreatic necrosis, which may become infected and predispose to sepsis. The early mortality in SAP arises from the acute inflammatory response and organ dysfunction, while delayed mortality is often due to sepsis from infected necroses with an associated mortality rate of 20-30% despite optimal care (5, 6).
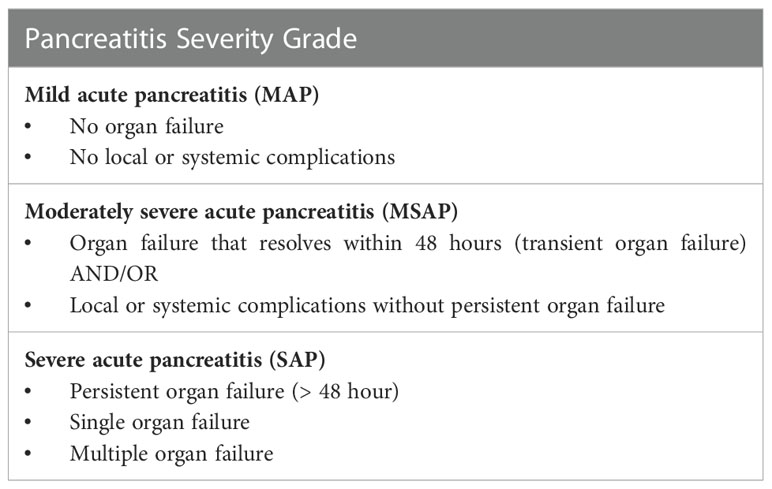
Table 1 Pancreatitis Severity Classification based on the Revised Atlanta 2012 Consensus criteria (2).
The initial pancreatic injury is multifactorial including enzyme-induced autodigestion, and derangements in intracellular metabolic pathways including impaired calcium signalling, mitochondrial dysfunction and impaired autophagy (7); all of which may trigger tissue necrosis. Why there is a subsequent dichotomy between the development of mild and severe disease is not well understood, but there is evidence that SAP is contributed to by dysregulated inflammatory and immunological processes in response to the initial tissue injury. Despite significant efforts in animal and human studies to better understand disease pathogenesis, the mechanisms are still not well understood and as such there are no specific disease-modifying and/or targeted therapies. While animal models have been valuable in developing an understanding of AP, there are limitations in the translation of these findings into clinical practice (8). Further, the heterogeneity of the AP patient population and the variable timing of hospital presentation limit the ability to easily conduct clinical trials of potential therapies.
Understanding the cellular and molecular mechanisms driving inflammation in early SAP could provide opportunities for targeted, host-directed therapies. Studies to-date suggest that the pathways are multifactorial and include genetic predisposition, abnormal innate immune and cellular responses, paradoxical immunosuppression, and alterations to gut permeability causing microbial translocation, as detailed below. To progress our understanding of the immunological and inflammatory mechanisms driving SAP, further robust pre-clinical human translational studies are required. The purpose of this scoping review is to comprehensively investigate and summarise the data pertaining to immunopathological mechanisms in AP in adult humans, with a particular emphasis on distinguishing the mechanisms between SAP and the milder forms of the disease.
Methods
A scoping review of the immunological mechanisms of acute pancreatitis was performed to provide a narrative overview of the data rather than addressing a single, specific question. The protocol was drafted in conjunction with the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols for Scoping Reviews (PRISMA-P ScR). The final protocol was registered with the Open Science Framework on 12 September 2021 (DOI 10.17605/OSF.IO/UEYNS).
Database search
A comprehensive search strategy was developed to investigate acute pancreatitis in adult humans, using MeSH terms for a number of immunological mechanisms (see Supplementary Data A). A search was conducted through Medline (PubMed), Web of Science, Cochrane Registry, and the National Institutes of Health Clinical Trials Registry. The time frame for the search was limited to January 1980 to May 2021 given the technical and historical limitations in investigating immunological mechanisms prior to this. For practical purposes only studies published in English were considered for inclusion.
Studies were included if they investigated an immunological mechanism, intervention or outcome relating to acute pancreatitis in humans. Animal studies, paediatric studies and studies of chronic pancreatitis were excluded. We also excluded studies investigating auto-immune pancreatitis due to there being a distinct immunological mechanism and treatment for that syndrome. Review articles, meta-analyses and case reports were excluded however their reference lists were reviewed to ensure relevant studies were not omitted.
Publication selection
Studies were imported into Covidence© (Melbourne, Australia) review management software and duplicates were removed. Title and abstract screening was performed by two authors independently. Full text screening was subsequently performed by the two authors and reference lists for relevant publications were also screened for study inclusion. Any uncertainty or conflict regarding study inclusion was then deferred to a third author for consensus. Data was extracted and collected in Covidence© (see data extraction form in Supplementary Data B) using a pre-specified data dictionary.
Data synthesis
Simple quantitative analysis was performed to measure trial demographics and patient characteristics, including median values, percentages and interquartile ranges. We also quantified disease factors including symptom duration prior to hospital presentation, method of classifying disease severity and aetiology of AP. Studies were analysed to quantify timing and duration of outcome measurement and qualitative analysis was performed on immunological assays and outcomes.
Results
Search results
A total of 2831 studies were identified after the initial search. After removal of 137 duplicates, 2489 studies were removed during abstract screening and full text review, with 205 studies remaining for data analysis (see PRISMA flow diagram – Figure 1).
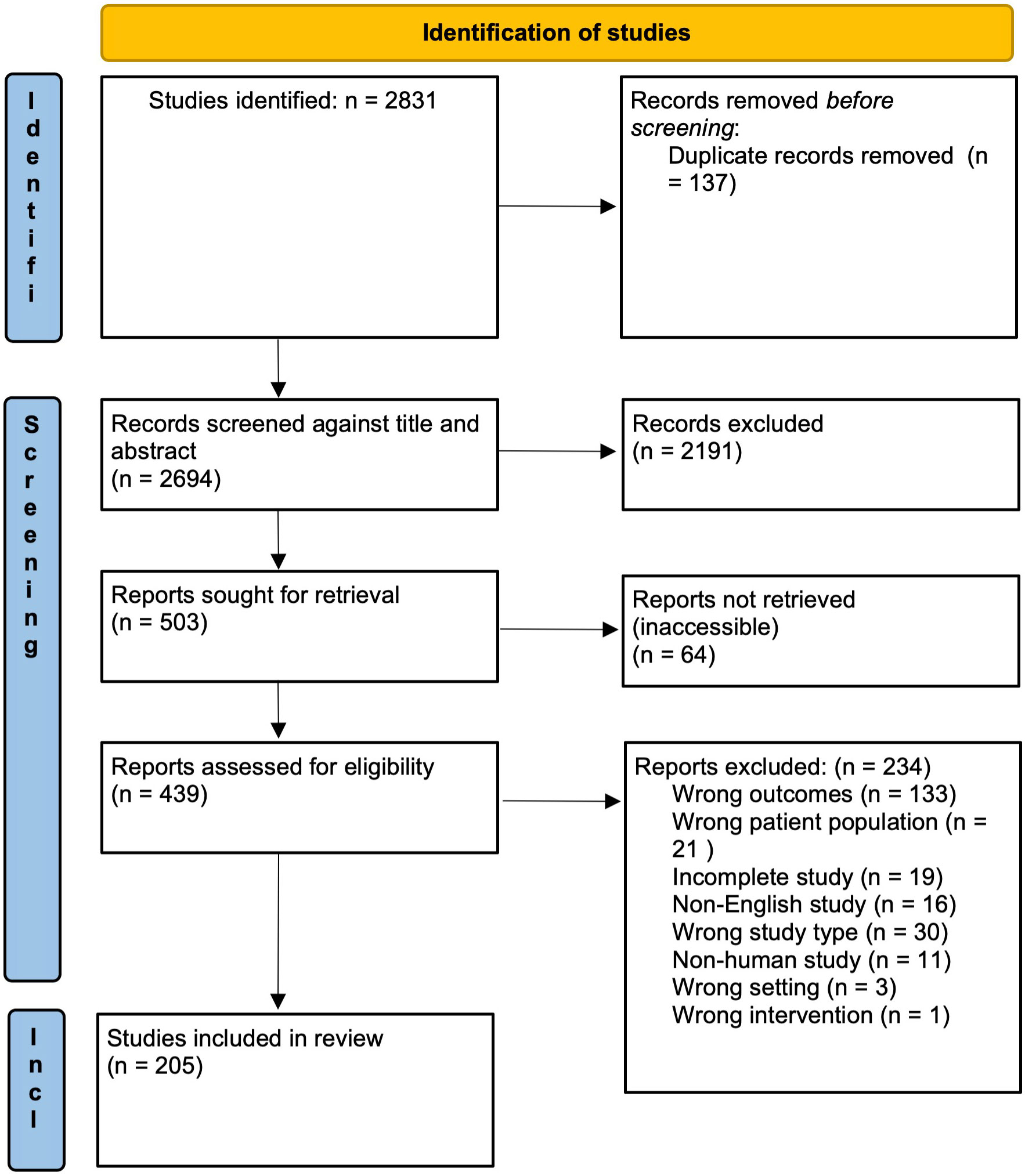
Figure 1 PRISMA flow diagram of study screening and selection. Adapted from The PRISMA 2020 statement: an updated guideline for reporting systematic reviews (9).
Study characteristics
Of the 205 retrieved studies (see Table 2), 17 (8.3%) were randomised controlled trials (RCTs), 9 (4.4%) pseudo-randomised controlled trials, 9 (4.4%) randomised non-controlled intervention study, 1 (0.5%) retrospective review of RCT data, 99 (48.3%) case-control studies, 68 (33.2%) case series, and 2 (1%) cohort studies. There were a total of 23,206 participants (including controls) across the 205 studies. The time frame for retrieved studies was 1984 to 2020. The majority of studies, 190 (93%), were conducted in a single centre. The median number of participants per trial was 62 (IQR 40-102). 129 (63%) of the studies had a control cohort. Of the 205 studies, 77 (38%) did not specify a time frame for symptom onset for inclusion.
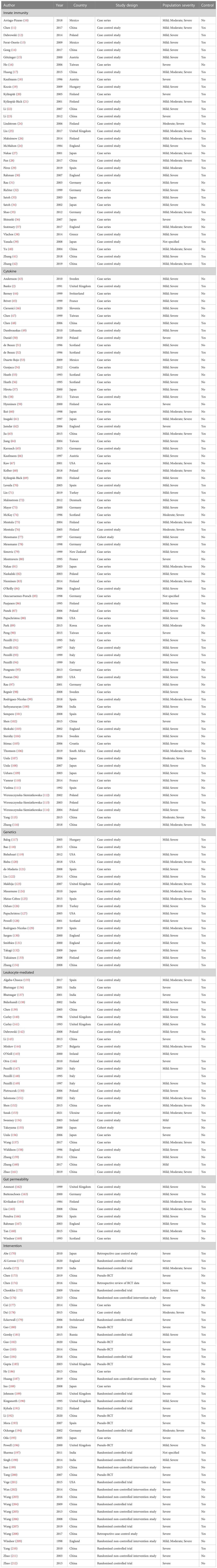
Table 2 All studies identified in initial search by primary focus area - innate immunity, cytokines, genetics, leukocyte-mediated, cytokine, gut permeability, or intervention.
During the full text screening process, six recurring themes emerged and provided a structure for the review. These themes were: aberrant innate immune response, cytokine profile, cellular responses, genetic predisposition, alterations to intestinal barrier function, and intervention studies. The main immunopathological findings are summarised in a flow chart in Figure 2.
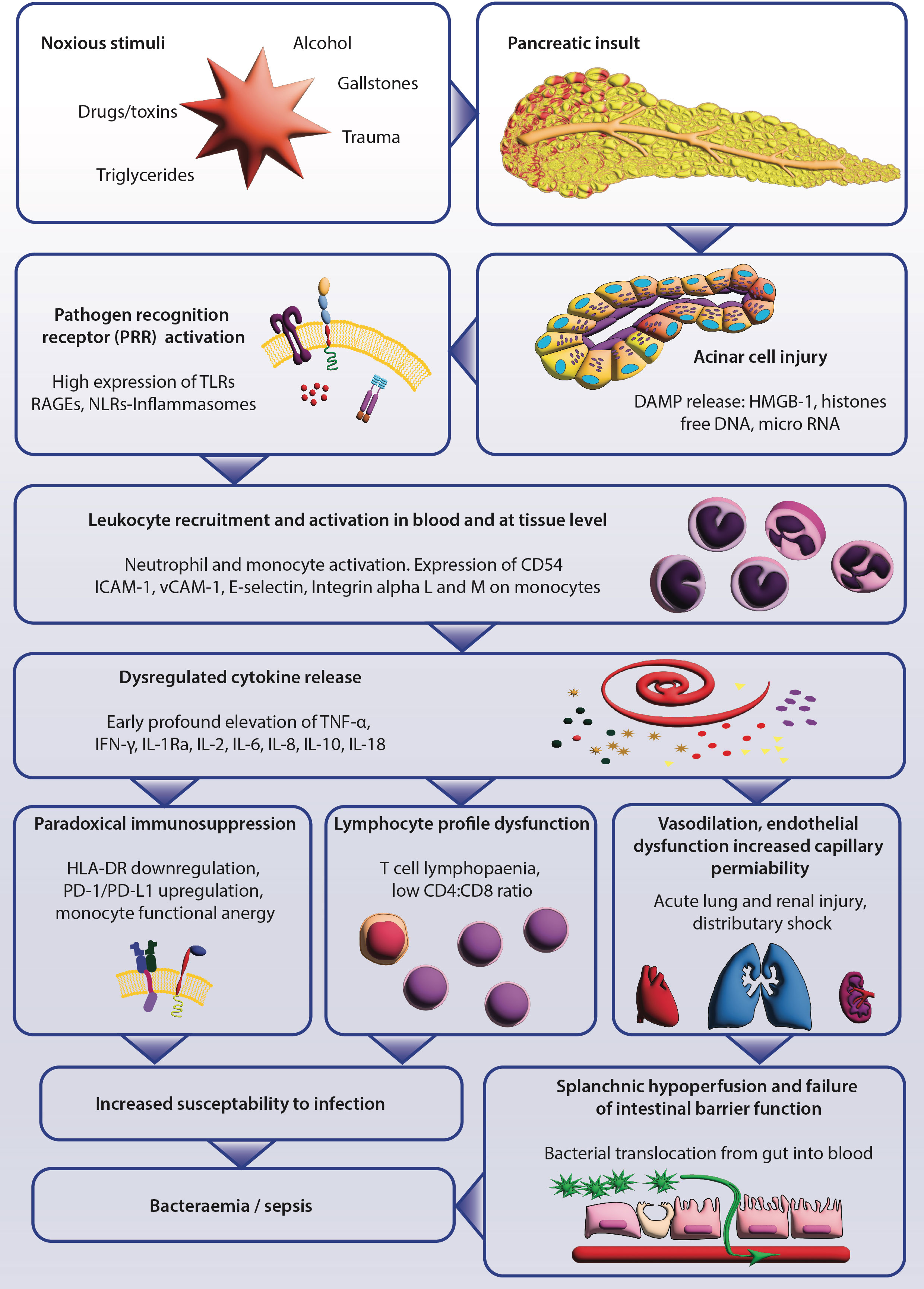
Figure 2 Summary of the current understanding of the immunopathogenesis of severe acute pancreatitis (SAP). The initial pancreatic injury can be instigated by a variety of noxious stimuli; acinar cell injury releases damage-associated molecular patterns (DAMPs). DAMPs are recognised by pattern-recognition receptors (PRR), which activate tissue and peripheral blood leukocytes and triggers the inflammatory cytokine release. The sequelae include alterations in lymphocyte phenotype, paradoxical hallmarks of immunosuppression, and a systemic inflammatory syndrome of endothelial dysfunction. Increased vascular permeability that causes distributory shock and multi-organ failure also occurs. Local and systemic factors induce failure of the intestinal barrier, promoting bacterial translocation. The relative immunodeficiency and increased intestinal permeability significantly increase the risk of secondary infectious complications.
Study demographics
The studies included were conducted across multiple countries and regions – of note 24% occurred in China, 9% in Japan, and 8% in Finland (Table 3). While these countries do have relatively higher incidences of AP, these figures do not reflect the global distribution of the disease burden (1).
Pancreatitis severity was classified using the Atlanta 2012 Consensus criteria in 110 (54%) of the studies). Most studies included a spectrum of disease: 97 (47%) mild and severe, 38 (18.5%) mild, moderate and severe, 6 (3%) moderate and severe, while 50 (24%) included severe disease only. With regards to aetiology, 33 studies (16%) did not specify an identified cause of AP in their patient population. Of the remaining studies that did specify, 166 studies (81%) specified gallstone/biliary aetiology, 164 (80%) alcohol, 59 (29%) hypertriglyceridaemia, 16 (8%) drug-induced, and 149 (70%) ‘other’ (which may have included less common pathologies for that particular population e.g. hypertriglyceridaemia or drugs that were specified in other study designs).
Innate immune and inflammatory responses in AP
The initial pancreatic tissue injury triggers innate immune responses that promote the subsequent inflammatory cascade associated with AP. Of the included studies, 53 reported outcomes related to changes in the innate immune system in AP. Patients who develop SAP have profound derangements in these pathways compared to mild AP (MAP). These responses can be broadly categorised by the interaction of damage- associated molecular patterns (DAMPs) with pattern-recognition receptors (PRRs), early markers of granulocyte activation and adhesion as well as early markers of immunosuppression.
Damage-associated molecular patterns
One of the evolved responses of the innate immune system is to recognise not only foreign pathogens but also to detect and respond to damage signals that may be indicative of host tissue injury. In AP, the initial tissue injury triggers the release of DAMPs into the blood, which typically occurs early in the acute phase of the illness. DAMPs directly interact with pattern-recognition receptors (PRRs), which are predominantly expressed by innate immune cells. These interactions trigger leukocyte activation and cytokine release, amplifying the inflammatory response to tissue injury but incidentally may also cause further tissue and organ dysfunction (213). Early DAMP release into the blood and their association with disease severity suggests that they may be useful biomarkers for prediction of severity on admission.
High-mobility group box-1 protein (HMGB-1) is a highly-conserved intranuclear protein normally involved in gene transcription but is also released into the circulation following cellular necrosis. Elevated HMGB-1 levels have been demonstrated in several inflammatory syndromes including sepsis, acute myocardial infarction and rheumatoid arthritis, and in this context is postulated to function as a pro-inflammatory mediator (152). In a case series of 15 patients with AP, HMGB-1 levels were significantly higher in patients with SAP compared to MAP, as well as in non-survivors compared with survivors (10). These elevations were observed from the time of admission and in the SAP cohort were sustained up to day 7 of illness. Similar findings were observed in a case-control study comparing admission levels of HMGB-1 in patients with MAP or SAP with a sepsis group and healthy controls (19). Both MAP and SAP had significantly higher HMGB-1 levels than healthy controls. In the SAP subgroup, HMGB-1 levels were elevated compared to the MAP patients, and were higher than levels expressed in the sepsis cohort. The early elevation of HMGB-1 and its correlation with disease severity suggests that it could be a useful biomarker for prediction of disease severity, and this requires further validation in larger studies.
Histones are intranuclear proteins involved in DNA packaging and gene regulation. In health, plasma histone levels are present at low levels, however elevated levels are seen in cellular injury syndromes such as trauma and sepsis (214). Elevated circulating histone levels have been implicated in early SAP. In a case-control study of 236 AP patients with 47 controls, circulating histone levels were comparable between volunteers, and patients with MAP or moderately severe AP (MSAP), however histone levels were significantly raised in the SAP cohort (25). In patients who presented with early organ failure (within 24 hours), histone levels were elevated compared to those with delayed organ failure. Similar findings were observed in another series where admission circulating histone levels were higher in SAP compared to MSAP and MAP (37). In both studies, receiver operating characteristics (ROC) area under the curve (AUC) for the accuracy of histones in predicting AP severity on admission were high (0.96 and 0.87 respectively). This suggests that plasma histones may be a useful biomarker of disease severity in AP, warranting further investigation.
Neutrophil extracellular traps (NETs) are expressed on neutrophil surfaces in response to stimulation by cytokine, lipopolysaccharide or intrinsic DAMPs. NETs are composed of decondensed DNA complexes and histones; their intrinsic purpose is an antimicrobial effect via the release of enzymes and proteins (a process known as NETosis). There is increasing evidence for the role of NETs in autoimmune and inflammatory disorders via direct tissue injury as well their potentiation of microvascular thrombosis and organ ischaemia (215). In AP, DAMP-induced NET activation appears to play a key role in both pancreatic and extra-pancreatic organ dysfunction. There is limited human data investigating NETs in AP, however one study assessed serum levels of free DNA and MPO-DNA complexes (markers of NET activity) in patients with SAP, MAP and healthy controls (216). The SAP cohort had significantly higher levels of both markers, which supports the implication of NETs in AP.
Other intranuclear products include free DNA, RNA products and nucleosomes, all of which can act as damage signals and trigger the inflammatory response. Elevated free DNA levels were observed in patients with SAP and sepsis on admission, however patients with milder disease had levels comparable to healthy controls (19). Plasma DNA levels were directly correlated with worsening severity of the CT score (radiological score of pancreatic injury). Micro RNAs (miRNAs) levels have been shown to increase in AP, significantly more so in SAP and with strong correlation with clinical scores of pancreatitis severity (41). Cold-inducible RNA-binding protein (CIRP) is an intranuclear protein that is activated by cellular stress and induces a proinflammatory response through a damage-signalling mechanism. In a prospective observational study of patients with SAP, MAP and healthy controls, admission serum CIRP levels were elevated in SAP patients, with these levels associated with increased risk of organ dysfunction, local pancreatic complications and mortality (14). Conversely, CIRP levels in MAP patients were comparable to those of healthy controls. A similar trend is observed with plasma nucleosome levels, with higher admission nucleosome levels in more severe disease, and therefore potential utility for nucleosomes as biomarkers of severity and prediction of organ dysfunction (217).
Heat shock proteins are intracellular proteins also activated in response to cellular stress, however these have protective effects on cells by inhibiting inflammatory mediators such as nuclear factor kappa-beta (NF-KB). In a small study, converse to the other DAMPs, Heat shock protein 70 (Hsp70) levels were lower on admission in SAP compared to MAP and MSAP, with sustained reduction in Hsp70 levels in the SAP cohort over the 7-day study period (10). Hsp70 levels were similarly depressed in non-survivors compared with survivors. A similar trend was observed for heat shock factor 1 (HSF1) levels in a case-control study, where SAP admission HSF1 levels were reduced compared to MAP and healthy controls (84). The elevated heat shock molecule levels in milder disease is postulated to exert a cytoprotective effect and inhibit the dysregulated inflammatory response, whereas the early disease progression in SAP may in part reflect failure of the heat shock systems.
Pattern recognition receptors
PRRs expressed by immune cells serve as the receptors for the above damage signals. These receptors include toll-like receptors (TLRs), receptors for advanced glycosylation end products (RAGEs), nucleotide oligomerisation domain-like receptors (NLRs) and the absent-in melanoma-2 (AIM2) protein. Interaction between DAMPs and these receptors activates inflammatory responses and cytokine release, which subsequently triggers leukocyte recruitment to the site of tissue injury.
Higher TLR4 expression on peripheral blood mononuclear cells (PBMCs) in patients with AP has been reported, as compared to healthy controls (22). There were no differences in TLR4 expression between MAP and SAP, however these findings may have been confounded by the use of empiric glucocorticoids in the SAP cohort. These findings are supported by studies of mRNA derived from PBMCs that found high levels of TLR4 and TLR2 transcripts in AP as compared to healthy controls (49, 181). In summary, all three studies suggest higher TLR4 activity in AP compared to controls, however they do not determine whether TLR4 expression differs between mild and severe disease.
RAGEs act as receptors for HMGB-1, augmenting its inflammatory action. Interestingly, in a prospective case control study, plasma soluble RAGE (sRAGE) levels in SAP were significantly lower than healthy controls (19). In the same study, sRAGE levels in MAP patients and those with severe sepsis were significantly higher than the SAP cohort and were elevated compared to the healthy controls. In patients with severe sepsis, there was a direct correlation between HMGB-1 and sRAGE levels, whereas the relationship was inverse in the AP patients. The mechanism for this relationship is unclear and requires further delineation.
On activation by damaged molecules, AIM2 forms an intracellular inflammasome. Inflammasomes are cytosolic complexes that regulate proteolytic caspase-1 and the release of pro-inflammatory cytokines. On admission, patients with AP had higher levels of AIM2 compared to controls, with augmented expression in more severe disease (135). ROC AUC for AIM2 predicting SAP was high (0.945) suggesting it may be a useful admission biomarker for severity. NLRP3 is another inflammasome complex that that likely contributes to AP disease pathogenesis, contributing to both pro and anti-inflammatory cytokine pathways processes (218). The NLRP3 inflammasome has been extensively studied in animal models, but investigations in human AP are comparatively scarce. In one observational study, serum ASC levels, a molecule closely linked to the activity of the NLRP3 inflammasome and a surrogate marker of its activity, were measured in healthy controls and patients of varying degrees of AP severity (219). ASC levels were found to correlate with disease severity and IL-18 levels (see below), implicating NLRP3 in the pathogenesis of human AP, and warranting further investigation of this protein complex in human studies.
Granulocyte activation
Increased expression of markers of leukocyte adhesion and activation, hallmarks of inflammation, have been reported in AP. With regards to the granulocytic response, a peripheral blood neutrophilia and monocytosis predominates in the acute phase, with higher counts observed in more severe disease (142). Adhesion molecules facilitate cellular migration from the blood vessels into the tissue to exert their inflammatory effects. A number of adhesion molecules have been identified as being involved early in the disease course of AP, including intercellular adhesion molecule-1 (ICAM-1 or CD54), vascular cell adhesion molecule-1 (VCAM-1), E-selectin, Integrin alpha L (CD11a) and Integrin alpha M (CD11b). These molecules are expressed both on peripheral blood monocytes (142), as well as by mononuclear cells isolated from acute pancreatic tissue (136). Expression of these molecules is increased in correlation with disease severity, indicating increased leukocyte activation in more severe AP, which likely perpetuates tissue injury and further drives the inflammatory response (12, 27, 136, 142, 191). The early elevation of these molecules in AP suggests their potential utility as biomarkers of severity.
Fatty acid-induced lipotoxicity
AP is associated with high levels of circulating free fatty acids (FFAs), which is likely precipitated by elevated lipolytic enzymes at both the local pancreatic and systemic level (220). In a case control study comparing FFA levels in patients with and without necrotizing pancreatitis, FFA levels were elevated in both groups on admission, but normalized by day 7 in the group without necrotizing pancreatitis (221). In the necrotising pancreatitis cohort, FFA levels remained elevated, possibly indicating ongoing pancreatic and extrapancreatic fat necrosis. There is evidence that FFAs, including unsaturated fatty acids (UFAs) and non-esterified fatty acids (NEFAs) contribute to the inflammatory response and tissue injury by triggering pro-inflammatory cytokine activity as well as direct deleterious effects on mitochondrial function (222, 223). Investigating the pathophysiology of fatty acid lipotoxicity in humans patients with AP is comparatively limited and warrants further investigation.
Cytokine profile
The cytokine profile in AP was investigated in 136 of the included studies. Establishing a temporal cytokine profile in AP has proven difficult, largely due to heterogeneity in the timing of the studies in regard to symptom onset. Of the 136 studies, only 40 (29%) measured the cytokine profile to day 7 of disease, 27 (20%) to day 14, and 11 (8%) beyond day 14.
DAMP recognition by TLRs on leukocytes triggers a cascade of cytokines, which significantly amplify the inflammatory response, help activate the adaptive immune system, and thereby, contribute to ongoing tissue injury. The literature consistently describes a profound cytokine release in AP, augmented in SAP compared to MAP, which likely accounts for the degree of illness and organ failure observed in patients with more severe disease. In parallel to the pro-inflammatory response is a concomitant release of anti-inflammatory cytokines, which promotes immunosuppression and contribute to secondary complications (such as infection) in patients with more severe disease.
In the studies reviewed, the cytokines elevated in AP compared to healthy controls included TNFα (99, 110), IFNγ (99, 109), IL-1 receptor antagonist (IL-1Ra) (76, 78), IL-2 (109, 150), IL-6 (10, 49, 83, 93), IL-8 (10, 87), IL-10 (70, 99) and IL-18 (107, 112), all of which are also consistently significantly differ between mild and severe disease.
TNFα and IL-6 are elevated early in the disease course of SAP, with the degree of elevation correlated to disease severity (10, 73, 75, 79, 82, 83, 87, 94, 99, 101). Both cytokines are also significantly higher in non survivors (13, 16, 73, 99). In the studies that reported timing of peak TNFα levels in SAP patients, the majority reported that TNFα peaked within the first 24-48 hours of study inclusion (22, 48, 51, 57, 64, 66, 74, 80, 115, 143, 171, 186, 203, 205, 208), although peak levels beyond this timeframe have also been reported (87, 102, 174, 212), which may reflect varying study timeframes and definitions of onset. Similarly for IL-6 in SAP patients, nearly all studies that temporally measured levels reported a peak within the first 24 hours of study inclusion (22, 44, 45, 55, 56, 60, 61, 64, 67, 68, 74, 75, 78, 80, 87, 91, 95, 111, 115, 143, 171, 174, 179, 183, 186, 190, 191, 195, 196, 203, 205, 208) although one outlier study did report a peak at day 6 (93). Some of the proposed effects in the acute inflammatory process include neutrophil chemoattraction, activation of NF-KB pathways to augment inflammatory signalling, stimulation of hepatic synthesis of acute phase proteins, and promotion of other pro- and anti-inflammatory cytokine release. Given their elevation early in the disease course and correlation with poor outcomes, both have been proposed as potential early biomarkers of disease severity. TNFα activity has also been assessed by measuring its soluble receptor subtypes 1 and 2, both of which are higher in more severe disease reflecting higher biological activity of TNFα (51, 57, 82). A similar pattern is observed for IL-8, which typically peaks within the first 48 to 72 hours of admission for patients with SAP, and exerts its effects via interactions with CXC Receptor 1 on neutrophils to cause cellular activation and tissue injury (10, 73, 79, 82, 83, 87, 101).
Interferon-gamma (IFNγ) is also a postulated significant mediator, given its potential to activate cells of both the innate and adaptive systems. IFNγ levels are reportedly higher at baseline in SAP compared to MAP, in non-survivors versus survivors, and in all AP compared to controls, which is consistent with a heightened inflammatory response (99, 109, 150). Subsequent variability in IFNγ levels have been observed beyond day 7, possibly due to the fluctuating activity and secretion by macrophages and lymphocytes at divergent time points (109). IL-18 has also been studied in AP, where it may act synergistically with IFNγ to activate Th1 lymphocyte subsets (112). Elevated circulating IL-18 have been reported at admission and into the second week of disease, and are pronounced in severe disease associated with necrosis, multi-organ failure, and in non-survivors (83, 97, 107, 112, 114, 224).
IL-2 is also implicated in the pathogenesis of AP, specifically in relation to T cell activation. Most studies measured IL-2 receptor subtypes as a marker of IL-2 activity. Higher levels of soluble IL-2 receptor (sIL-2R) are associated with more severe AP (69, 73, 76, 109), and IL-2 receptor antagonist (IL-2Ra or CD25) is significantly higher in SAP compared to MAP (83). However these results are in contrast to another study which reported a reduction in peripheral blood CD25 mRNA expression in SAP patients on admission compared to MAP (49).
A number of other cytokines have been postulated to contribute to the acute inflammatory response in AP, however results are more variable, and they have been less extensively studied. These include IL-4, IL-12, IL-13, IL-17 and IL-23. As the latter three cytokines largely define the Th17 lymphocyte subset (63, 178, 182), it raises the question of what role these pro-inflammatory cells might play in SAP, particularly given their suggested role in mucosal immunity. Further work is required to determine their contribution to SAP pathology.
In conjunction with pro-inflammatory cytokine release is an observed elevation in the plasma concentrations of the regulatory cytokine IL-10 in AP, which was higher in SAP than MAP (58, 73, 75, 99, 101, 110, 150). IL-10, while traditionally regarded as an anti-inflammatory cytokine, likely has pleiotropic effects in AP. Its pro-inflammatory effects may include activation of natural killer (NK) cells, enhanced B cell function and stimulation of cytotoxic molecule expression, which may contribute to the inflammatory response and tissue injury (99). However, its more predominant effects appear to be a counter-regulatory response that dampens inflammatory pathways, which may account for the greater degree of elevation seen in SAP compared to MAP. In SAP, its postulated downstream effects include reduced MHC-II expression (particularly HLA-DR) and down-regulation of NF-KB signalling. High IL-10 levels are correlated with a significantly higher rate of complications in patients with SAP, including higher mortality, higher risk of infectious complications (particularly infected pancreatic necrosis) and the development of sepsis (16, 58, 73, 75, 76, 79, 99, 101, 110, 150). A case-control study investigated cytokine production in leukocytes in culture from MAP and SAP patients in order to assess functional reserve and anergy (70). There was increased basal cytokine levels in SAP compared to MAP, including IL-10. Interestingly, with cellular stimulation, cells isolated from MAP patients produced additional IL-10 in conjunction with other cytokines, while in SAP there was only a rise in pro-inflammatory cytokines. This data suggests that IL-10 release is more regulated in patients with MAP and induces a protective effect, while in SAP there is perhaps excessive unregulated production of IL-10 in response to the hyperinflammatory response, which confers detrimental effects to the individual.
The antagonist for the IL-1 receptor (IL-1Ra) also contributes to the pattern of immunosuppression by binding the IL-1 receptor and thereby inhibiting the pro-inflammatory actions of IL-1. Reflecting its regulation by pro-inflammatory IL-6 and IL-1β, significantly higher levels of IL-1Ra are observed in patients with SAP compared to MAP, and these higher levels are associated with higher mortality, the development of organ dysfunction and infectious complications (73, 110, 166).
Limitations and future directions of cytokine assessment in AP
Definitively determining the cytokine pathways upregulated in AP that contribute to the pathogenesis is of significant value in the current era of monoclonal antibodies and other targeted therapies. For example, many of the implicated cytokines are currently targeted in other disease states, including anti-TNFα and IL-1 (i.e. via infliximab and anakinra, respectively) in inflammatory arthritis and autoinflammatory disorders, IFNγ (via JAK inhibitor tofacitinib) in rheumatoid arthritis and inflammatory bowel disease as well as anti-IL-6 antibody tocilizumab in rheumatoid arthritis, juvenile idiopathic arthritis and severe COVID-19 (225). While the cytokine profile in AP has been extensively reported, there are several limitations in evaluating the data. Of the 137 studies that were selected for inclusion, 103 (75%) employed enzyme immunoassay techniques for cytokine measurement, however the specific assay type and/or manufacturer was not often reported, which can lead to inter-assay variability. Interpreting cytokine levels and trends is also made difficult by the inconsistency of timing of patient recruitment to the studies. However, further advancements in these areas have the potential to rationally advance anti-cytokine therapies into clinical trials in AP.
Cytokine gene polymorphisms
As part of prediction of disease onset and severity, there is great interest in understanding whether genetic predispositions may contribute to the pathogenesis of AP. Nineteen studies investigated for genetic polymorphisms that may be implicated in the development of SAP. The majority of these (79%) were case-control studies. The median number of participants was 361, and 18 of the studies included a healthy control group. All studies compared mild and severe pancreatitis. Polymorphisms for genes encoding for cytokines, heat shock proteins and pattern recognition receptors have been investigated. Several polymorphisms were reported to be associated with an increased predisposition to the development of acute pancreatitis (all levels of severity) including IL-1Ra, IL-8, Il-23, CD14 and MMF (118, 120, 123, 124, 129, 131). However, while a number of polymorphisms were investigated to assess their correlation with more severe disease (polymorphisms in TLR3, TLR6, IL-1Ra, CD14, TNFa, MCP-1, receptor-interacting protein kinases (RIPK), myelin basic protein (MBP) and HSP70), statistical significance was not achieved in any of these studies (41, 117, 121, 124, 125, 127, 129, 131). Based on these findings, it remains unclear whether there are any clear genetic predispositions to the development of SAP. Given the number of polymorphisms identified in pro-inflammatory pathways, larger scale prospective genomic studies of AP in conjunction with metabolomic evaluation would be of great value.
Lymphocyte profile
Twenty-nine studies investigated changes in the lymphocyte profile in patients with AP. As with cytokine studies, a notable limitation of these studies was the limited timeframe assessed; 14 studies (48%) did not assess the lymphocyte profile beyond one week, and 5 (17%) did not specify time points. Only 3 studies (10%) assessed the temporal profile up to day 30 of admission.
As is observed in other hyperinflammatory disorders, such as sepsis, generalised lymphopaenia typically occurs early in AP (often on admission), in both mild and severe disease (150, 161), with a greater degree of lymphopaenia seen in more severe pancreatitis (147, 161). Lymphopaenia is associated with higher risk of secondary infectious complications (156, 226). Lymphopaenia in AP is often associated with an elevated neutrophil count, and a high neutrophil to lymphocyte ratio (NLR) at admission can be a useful predictor of poor outcomes (147, 153, 226), similar to the sepsis syndrome (227). Regarding more detailed lymphocyte profiling, the majority of studies have explored T lymphocytes, which appear to be more affected in comparison to B cells (150). T cell subset analysis indicates multiple lines are depressed in AP including Cytotoxic CD8+ T cells, NK cells and CD4+ T cell counts (109, 142, 161). While both CD8+ and CD4+ T cell counts are diminished, a number of studies suggest that CD4+ counts are more significantly affected in more severe disease, causing reduced CD4:CD8 ratios (109, 115, 150).
While early lymphopaenia is a recognised phenomenon of AP severity, one study observed a biphasic trend in T cell dynamics (150); T cell counts were significantly reduced on admission but recovered to within normal range by day 10. T cell counts were again low at day 30, but this reduction was particularly marked in the SAP cohort. In the same study, B cell counts, while reduced compared to the control cohort, were essentially the same in both the MAP and SAP patients until beyond day 7, when the SAP B cell counts became significantly reduced compared to MAP. Innate cellular immunity may be further reduced by heightened activity of CD4+CD25+CD127low/neg regulatory T cells (Treg) in more severe disease with associated poorer outcomes (144).
In keeping with an acute inflammatory response, elevated expression of lymphocyte activation markers is present in AP. CD69 is a surface marker of T cell activation and has significantly elevated expression in both mild and severe AP, both in the peripheral blood and at the pancreatic tissue level (136), as well as elevated CD69 mRNA expression in PBMCs of patients with AP (49).
Immunodeficiency
In parallel to observed, marked inflammatory response, are regulatory immunosuppressive processes, the activity of which are more pronounced in patients with severe disease.
HLA-DR expression in AP
As an MHC-II molecule, human leukocyte antigen-DR (HLA-DR) is a surface marker of cellular activation and plays a key role in T cell antigen presentation and regulation of pro-inflammatory cytokine release. Reduced HLA-DR expression is a reliable biomarker of immunodeficiency and is associated with a higher risk of infection (228). In patients with mild and moderate AP, there is an observed reduction in HLA-DR expression within the first few days of disease onset, however this returns to near-normal within the first week (75). In contrast, patients with SAP – particularly those with multi-organ failure – downregulated HLA-DR expression persists beyond the first week of illness and is associated with increased rates of secondary infectious complications (40, 42). In a study comparing HLA-DR expression in AP patients with and without infectious complications, the cohort that developed secondary infections exhibited significantly lower HLA-DR expression (28). A similar inverse correlation has been observed between HLA-DR expression and mortality (16). Given the association between HLA-DR expression and disease severity and complications, it has also been proposed as an early biomarker of disease severity.
Co-inhibitory molecule activity in AP
Further evidence of immunosuppression in AP is presented by the heightened expression of the immune checkpoint molecules programmed cell death 1 (PD1) and programmed cell death ligand 1 (PD-L1). PD1/PD-L1 is a well-known immune inhibitory pathway involved in prohibiting T cell activation and proliferation as well as the induction of T cell apoptosis. Heightened PD1/PD-L1 expression can be a means of immune system evasion in metastatic malignancy, but is also a marker of T cell exhaustion, such as in septic shock where there is an associated higher risk of nosocomial infectious complications (229). Recent data implicates heightened activity of the PD1/PD-L1 system in severe pancreatitis, with greater expression in patients who develop secondary infectious complications (28). Similar activity has been observed with the soluble isoform of PD-L1 (sPD-L1), demonstrating higher sPD-L1 levels in more severe AP and in those with infectious complications (11). Immune checkpoint inhibitors targeting the PD-1 are currently in clinical trials in sepsis, to determine their efficacy in reversing T cell anergy and exhaustion (230) and findings here may have some relevance to synonymous phenomena in AP.
Leukocyte anergy
In addition to quantitative deficiencies, there is increasing evidence to suggest functional impairment of both lymphocyte and monocyte populations is present in severe disease. A state of cellular anergy in SAP would certainly increase the risk of subsequent infectious complications. One study performed in vitro assessments of lymphocyte NF-KB signalling in response to incubation with microbial pathogens, comparing the lymphocytes of patients with SAP (with HLA-DR expression <80%) with those of healthy volunteers (146). T and B cells from the SAP cohort had significantly reduced responses, suggesting a degree of functional exhaustion. Cells were subsequently treated with TNFα and NF-KB activity measured; the lymphocytes from SAP patients had significantly diminished responses compared to controls, indicating reduced number of TNFα-responding cells. A similar study assessed in vitro monocyte phagocytosis in peripheral blood on admission in mild, moderate and severe cohorts of AP, and compared this to healthy controls. In all cohorts, phagocytic activity was increased, with activity positively correlating with disease severity (153). However, on subgroup analysis, patients who developed infectious complications had significantly reduced monocyte phagocytic activity. Phagocytic activity was then further stimulated by incubating lymphocytes with protein kinase C activator (PMA – a protein that enhances macrophage oxidative metabolism and phagocytic function). Monocytes from the mild, moderate and severe (without infectious complications) all had significantly higher oxidative activity, whereas monocytes from patients who later developed infectious complications had no response to PMA, indicating cellular metabolic dysfunction.
Failure of the intestinal barrier in acute pancreatitis
The intestinal mucosa plays a key role as a barrier preventing translocation of enteric microflora from the intestinal lumen into the bloodstream. Twenty-five studies that reported changes in intestinal permeability or assessed for markers of bacterial translocation were included. While in general critical illness intestinal failure occurs in the setting of systemic hypoperfusion and organ dysfunction; however in AP, gut barrier failure occurs early (even as early as at the point of hospital admission) (165), and has been observed prior to the onset of multi-organ failure (162). This suggests that in addition to intestinal hypoperfusion secondary circulatory shock (167), there may also be local mechanisms and mediators arising from the inflamed pancreas that directly contribute to intestinal ischaemia and barrier dysfunction. Increased intestinal permeability has been consistently observed in both mild and severe disease, although to a greater extent in the SAP cohort (143, 165–167).
Failure of the gut barrier facilitates translocation of enteric organisms, as well as bacterial endotoxin and antigens into the portal circulation (143). Several studies assessed plasma endotoxin levels (a component of the Gram-negative bacterial cell wall) as a marker of translocation, with higher endotoxin levels associated with higher gut permeability and more severe disease (102, 162, 163, 165). Endotoxinaemia has also been assessed by measuring endotoxin core IgM and IgG production, however this appears to be a less consistent method with studies reporting both significant elevation and reduction in EndoCAb IgM levels in mild and severe cohorts (140, 163, 166, 167, 169, 198). Endotoxin has been posited to enhance the inflammatory response via cytokine pathways as well as via direct stimulation of B cells (163, 167). Translocated organisms are likely to interact with pathogen-associated molecular patterns (PAMPs), triggering further inflammatory responses with leukocyte activation and cytokine release. It has been hypothesised that translocated intestinal bacteria are the source of infected necrosis, largely due to culprit organisms typically being Gram-negative enteric species.
While there is evidence supporting gut dysfunction and the presence of endotoxaemia in AP, there is little understanding in terms of immunopathological mechanisms and how these factors interplay. One study reported that the degree of endotoxinaemia did not correlate with the inflammatory cytokinaemia (59), while another observed that TNFα levels correlated with the degree of intestinal permeability (165). While this increased gut permeability almost certainly contributes to intra-abdominal infection, there is currently no strong evidence to suggest a mortality benefit from empiric antimicrobial therapy in AP (of any severity) (231). Overall, the interaction between increased gut permeability and the relationship to AP and sequelae of severe disease requires ongoing investigation.
Intervention studies in acute pancreatitis
While there is currently an ongoing trial of anti-TNFα antibody (infliximab) in AP (RAPID-I) (232), there are relatively few completed trials of immunomodulation in AP in humans. We included 45 studies in which an intervention was trialled in AP, where either the intervention or an outcome had an immunological basis. Most studies included in this review assessed immunological outcomes such as trends in cytokine levels, cellular markers or immunoglobulin levels. The trialled interventions can be broadly categorised into drug therapies (n=18), intravenous (IV) fluid studies (n=2), nutritional studies (n=13) and extracorporeal therapies (n=12).
Pharmacological interventions
Several pharmacological interventions have been trialled in AP with limited to no efficacy in reducing mortality despite improving biochemical, clinical and immunological markers.
Given the profound inflammatory response associated with SAP, glucocorticoids have been trialled albeit with no high quality RCTs testing their efficacy in this disease. We identified three studies that trialled glucocorticoids; one retrospective propensity-matched cohort study, one case control study and one case series (233–235). In the propensity matched cohort study, it is unclear what the indication for glucocorticoid therapy was, and patients were treated with varying formulations at differing doses and durations. In the glucocorticoid arm, compared to the propensity matched arm, there were improvements in mortality, ICU length of stay (LOS) and treatment costs. In the case control study, patients were treated with with 1mg/kg dexamethasone three times daily compared to standard care; the steroid arm had improved rates of ARDS and LOS but no improvement in mortality. This study was confounded by the use of Chinese herbal preparations in both groups, the effect of which on AP is unclear. Finally, the case series administered variable doses of dexamethasone and Dextran colloid solution with improvements in symptoms however in the absence of a control group, it is difficult to draw definitive conclusions. Across all studies, the variable definitions of severity, treatment protocols and patient heterogeneity, as well as the lack of immunological outcomes, makes the findings from these studies largely exploratory.
Several studies have trialled pancreatic enzyme inhibitors including somatostatin, ulinastatin, octreotide and gabexate. These agents were proposed to reduce enzyme activation and minimise the initial tissue injury, which may therefore mitigate the subsequent inflammatory and immune response. Findings were variable with some evidence for improved immunological markers including increasing the CD4:CD8 ratio and reduced pro-inflammatory cytokine expression (TNFα, IL-6, IL-8), but importantly a reduction in mortality or length of stay was not demonstrated (188, 200, 203, 207).
Similarly, pentoxifylline, a non-selective phosphodiesterase inhibitor, has been trialled in AP with variable effect on improving serum cytokine levels but no clear evidence of mortality benefit (175). The trials in this field are limited by low participant numbers and lack of clarity regarding baseline physiological status of participants. Other confounding factors that varied across intervention studies included what was considered ‘standard care’, including the empirical administration of antibiotics and corticosteroids (188, 192, 208).
Lornoxicam, a non-selective cyclooxygenase inhibitor, was tested in patients with SAP compared to standard care in a randomised, non-blinded clinical trial (181). Patients were randomised to standard care (n=246) or lornoxicam plus standard care (n=88). The trial demonstrated a temporally significant reduction in TLR2 mRNA expression in the lornoxicam group. The treatment arm also demonstrated significantly reduced mortality and fewer gastrointestinal complications. These results are potentially of interest, however must be interpreted with caution as the study was underpowered for mortality (which also was not a reported primary or secondary outcome), it is unclear whether true randomisation occurred, participant baseline clinical and biochemical data were not reported (therefore differences in outcomes may be contributed to by cohort heterogeneity), and there was a significant difference in the cohort sizes which may confound the magnitude of effect.
Randomised trials have been conducted to assess the effects of lexipafant, antagonist to platelet activating factor, as well as activated protein C (APC) in improving mortality and immunological outcomes in AP. No significant improvements in immunological outcomes or overall mortality were observed for either therapy (189, 191).
A potential limitation of these drug therapies is that they target the early disease process; that is, reducing pancreatic secretions and initial inflammation rather than suppressing the subsequent immune responses. Given the systemic immune dysregulation has likely already onset by the time patients present to hospital, this probably explains why these pancreas-specific treatments have no benefits from an immunological outcome’s perspective.
Intravenous fluid
There have been four RCTs of lactate-containing balanced crystalloid solutions with 0.9% sodium chloride (0.9% saline) in patients with AP (236–239). The outcomes of these studies include frequency and duration of SIRS, clinical outcomes (e.g. mortality and LOS), and inflammatory markers (e.g.CRP). Patients administered the lactate-containing crystalloid had trends towards reduced duration of SIRS, reduction in CRP and some improved clinical outcomes such as ICU LOS but no impact on mortality. It is hypothesised that lactate-based solutions may confer anti-inflammatory effects by raising pH in the pancreatic tissue. Furthermore, these studies administered high volume fluid resuscitation based on goal-directed therapy protocols. In other critical care cohorts such as sepsis, goal-directed fluid therapy has since been robustly shown to not improve outcomes (240) and high volume fluid therapies are associated with an increased trend to harm across numerous critical care cohorts (including the recent WATERFALL trial in AP (241)). Finally, these studies enrolled patients with all severities of AP, which further limits the ability to draw conclusions on the potential benefits of different crystalloids in this disease. While these study findings are of interest, the studies were underpowered and therefore these findings should be considered hypothesis-generating.
Two studies have examined the effect of IV fluid on inflammation in SAP by comparing 0.9% saline solution to various formulations of hydroxyethyl starch (HES). Compared to NS, patients with AP who received a starch-based solution had some temporal improvements in cytokine levels (174, 212). It is hypothesized that HES may play some role in attenuating the inflammatory response, in particular capillary leakage via NK-κB activation, and neutrophil adhesion and migration. However, starch-based solutions have been demonstrated to increase the risk of acute kidney injury in critical illness and therefore cannot be readily considered as a potential therapy (242).
Nutrition
Thirteen studies examined the effect of various nutritional approaches in SAP and their effect on the immune and/or inflammatory response. Overall, little insight into the immunopathology could be gained as many studies examined few time points, did not report specific cytokine levels, and primarily focused on non-immunological outcome measures such as organ failure and length of stay.
With respect to cytokines, total parenteral nutrition (TPN) adjuncts demonstrated some effect in reducing the inflammatory response compared to TPN alone e.g. fish oil was associated with reduced IL-8 and ICAM-1 (243), and increased IL-10 (204), however no difference was seen in sTNFR levels (194) or IL-6 (206). In comparing TPN and enteral nutrition (EN), EN was frequently demonstrated to be superior in respect to cytokine response - with reduced pro-inflammatory IL-6 (173, 202, 205), IL-1b (173), and TNFα (173, 205), and increased anti-inflammatory IL-11 and IL-10 (202, 205).
In regards to the cell-mediated response, TPN adjuncts such as glutamine increased lymphocyte count (194), and fish oil increased HLA-DR expression (204). HLA-DR expression also significantly increased with early EN compared to delayed EN (199).
In terms of gut permeability, multiple studies have demonstrated EN significantly reduces gut permeability compared to TPN via various measures including EndoCAb IgG and IgM antibodies (185, 209), endotoxin, and lactulose-mannitol ratios (205, 211). Supplementation of EN with glutamine was demonstrated to improve gut permeability and significantly reduce IL-6 (172) and endotoxaemia (198), however this did not translate into an improvement in infectious complications, morbidity or mortality. There is no evidence to suggest any immunological benefit from probiotic use in AP (197), and their use is actually associated with increased mortality in patients with SAP (244).
Finally, in terms of clinical outcomes, compared to TPN, EN was associated with lower rate of complications end organ dysfunction, as well as improved outcomes (173, 199, 202, 209).
Extracorporeal therapies
Eleven studies investigated the effect of extracorporeal therapies in modulating the inflammatory response in patients with AP, predominantly those with severe disease. The aim of these therapies is to sequester inflammatory mediators from the plasma via dialysis, haemofiltration, or adsorption using specialized membranes.
Overall, it appears that extracorporeal therapies may be effective in reducing the plasma levels of inflammatory cytokines. Reduced cytokine levels were observed as early as within 6 hours of commencement of continuous veno-venous haemofiltration (177, 178), and high volume haemofiltration was associated with greater cytokine reduction than continuous filtration (176). In other studies, application of extracorporeal therapies was associated with improved clinical outcomes including reductions in mortality, improved parameters of respiratory function and improved haemodynamic outcomes (170, 183, 184). Wang et al. (208) randomised patients to standard care versus either haemofiltration or laparoscopic peritoneal lavage or haemofiltration plus peritoneal lavage. All intervention arms had improved clinical outcomes and maximal reduction in pro-inflammatory cytokines, however the greatest improvements were observed in the group who underwent both haemofiltration and lavage. This suggests an additional benefit may be derived from addressing the intra-abdominal inflammatory processes via lavage (i.e. removal of necrotic tissue, inflammatory mediators, and translocated intestinal flora). These findings are however confined to a single study and the potential benefits of this intervention would need to be balanced against the risks of significantly invasive surgery in an already critically ill patient population.
While these findings are promising, they were not consistent across the studies. Furthermore, methodological issues including lack of true randomisation, the absence of control groups, heterogenous study populations, variable definitions of disease severity, and low participant numbers make these observations hypothesis-generating rather than truly demonstrating therapeutic effect which would likely require further randomized interventional trials. Extracorporeal therapies are not without risk and these must be considered against any potential benefit. Inconsistency in the reporting of particularly outcomes, including the frequent measurement of non-standardised clinical outcomes such as ‘relief of abdominal distension’, makes comparison of results between studies challenging.
Current research gaps and future directions
SAP is a potentially lethal condition in both the hyperacute and subacute phase with limited high-level evidence to guide management (245). The findings of this scoping review centering human translational data provide a compelling argument for dysregulation at multiple levels of the immune and inflammatory response, particularly the phenomena of concomitant pro and anti-inflammatory processes. While it is evident that there are derangements at many levels, which of these are causative and which are para-immune phenomena requires further clarification. The critical pathogenic steps in the immunobiology of acute pancreatitis remain elusive and clearly still require further considered observational and interventional study.
Of interest is that patients may progress to severe disease irrespective of the underlying aetiology of AP, suggesting an underlying susceptibility to dysregulated immune responses to tissue injury. The challenge is to now improve our understanding of the chronology of these changes, and to look beyond just the short term, if we are to improve our understanding of the biology of this disease. While our review has demonstrated the extensive work that has been conducted on the cytokine patterns, cellular profiles and innate immune responses in AP, transcriptomic analysis has not yet been conducted in humans Employing newer techniques such as RNA sequencing and transcriptomics may enable us to understand the temporal profile of the disease and the immunological endotype at the cellular level.
The COVID-19 pandemic has been invaluable in demonstrating the benefits of early diagnosis and immunomodulation to improve outcomes in critically ill patients with an acute inflammatory illness. A collaborative international approach to COVID-19 has led to the uptake of diagnostic biomarkers and establishment of clear management guidelines, which has enabled the application of personalized medicine. A similar approach and framework could have innumerable benefits in AP.
There is increasing interest in immune modulation for the management of pancreatitis, especially at the severe end of the spectrum. A recently published RCT (published following our literature search) compared thymosin alpha (an enhancer of cell-based immunity) with placebo in patients with predicted SAP (246); the primary outcome was development of infected pancreatic necrosis and the secondary outcomes included HLA-DR expression. In this study, participants were eligible for inclusion if they had had abdominal symptoms for up to 7 days. No statistically significant differences were observed between the treatment and control arms for either primary or secondary outcome, although there was a suggestion of increased benefit in patients with more extensive pancreatic necrosis. Interestingly, a recent observational study (also published beyond our inclusion dates) studied patients with infected pancreatic necrosis (IPN) longitudinally up to 60 days post-discharge and compared them with healthy controls (247). Patients with IPN had evidence of significant HLA-DR downregulation at 60 days post-discharge from the index admission, and patients who were readmitted to hospital within the time period had significantly lower HLA-DR levels compared to those who were not readmitted.
Our findings in this review suggest that given the rapidity of onset of the acute inflammatory response in SAP, allowing recruitment up to 7 days in the thymosin alpha study may potentially miss a ‘golden window’ for early immunological intervention, which could explain why no differences were observed between the two groups. The prolonged period of immune convalescence observed in the IPN study highlights the need to evaluate patients beyond the hospital period to better understand the long-term immunological profile in this patient group. For this reason, it is even more essential that more in depth research in AP is conducted to a more aligned standard, to allow for more accurate characterization of the acute natural history of this disease at the cellular and molecular level. Given the heterogeneous nature of the patient population and variability in rate of disease progression, this may explain why trials of interventions are yet to show positive outcomes. For example, the findings of this review suggest that anti-protease therapies are unlikely to be beneficial beyond the initial onset of the disease, while anti-cytokine therapies or corticosteroids may only be beneficial in the subsequent hyperacute phase of the illness, and they could potentially be harmful once the compensatory anti-inflammatory responses have established (248). These challenges further the need for biomarker-led interventions in AP clinical trial design.
We await with interest the findings from the RAPID-I multi-centre RCT that is comparing infliximab with placebo in AP and will employ transcriptomics to hopefully shed further light on the biology of this illness (232).
This review highlights the extensive work to-date that has been conducted into the immunobiology of human AP. While the developments are exciting, there are clear inconsistencies across several domains that will need to be addressed if meaningful progress is to be made in this area. Heterogeneity in the individual study definitions of pancreatitis and its severity has significant downstream implications for comparison of results and prospective study design. At the participant level, it is imperative that studies develop clear cut-offs for symptom duration prior to inclusion, in order to establish a degree of standardization between studies, given the rapidity dynamic nature of the disease progression. At present, diagnostic criteria for AP disease severity is largely retrospective—that is, the Revised Atlanta 2012 Consensus criteria (2) requires organ dysfunction to be present for 48 hours in order to define severe disease. It may be advantageous then to further evolve diagnostic criteria for pancreatitis studies to reflect molecular and immunological measures (e.g. IL-6 levels or HLA-DR expression). The benefits would include earlier, more accurate stratification of patients, which would facilitate earlier intervention, as well as developing more robust clinical trials by refining study cohorts. At the investigation level, there also needs to be standardization of assays to enable comparison of findings between studies. Finally, regarding outcome measurement, we propose the development of a set of core outcomes that should be reported in trials of severe pancreatitis inflammation and immunology.
Conclusion
The pathobiology of AP is characterised by immune dysregulation in response to tissue injury, with the severity of illness correlated with the extent of immune response. The literature pertaining to human data implicates a number of pro-inflammatory pathways as well as features such as lymphopaenia and other regulatory elements that may indicate co-existing immunodeficiency. Inconsistencies in study design and our ability to prospectively assess severity have limited progression in our understanding of the immunopathology of this disease. Further well-designed, prospective studies that assess this disease at a detailed cellular, genomic and microbiome level are required if effective disease-modifying therapies are to be established.
Author contributions
All authors contributed to the article and approved the submitted version. All authors were involved in study conception, methods and design. KV and HG performed the search, data collection, analysis and manuscript preparation. SS and CA performed data analysis, manuscript preparation and editing. AD contributed to manuscript preparation and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1077414/full#supplementary-material
References
1. Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis. Gastroenterology (2022) 162(1):122–34. doi: 10.1053/j.gastro.2021.09.043
2. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: Revision of the Atlanta classification and definitions by international consensus. Gut (2013) 62(1):102–11. doi: 10.1136/gutjnl-2012-302779
3. Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ (2019) 367:l6227. doi: 10.1136/bmj.l6227
4. Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet (2020) 396(10252):726–34. doi: 10.1016/S0140-6736(20)31310-6
5. Trikudanathan G, Wolbrink DRJ, van Santvoort HC, Mallery S, Freeman M, Besselink MG. Current concepts in severe acute and necrotizing pancreatitis: An evidence-based approach. Gastroenterology (2019) 156(7):1994–2007 e3. doi: 10.1053/j.gastro.2019.01.269
6. Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological association clinical practice update: Management of pancreatic necrosis. Gastroenterology (2020) 158(1):67–75 e1. doi: 10.1053/j.gastro.2019.07.064
7. Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, et al. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology (2018) 154(3):689–703. doi: 10.1053/j.gastro.2017.10.012
8. Gorelick FS, Lerch MM. Do animal models of acute pancreatitis reproduce human disease? Cell Mol Gastroenterol Hepatol (2017) 4(2):251–62. doi: 10.1016/j.jcmgh.2017.05.007
9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
10. Arriaga-Pizano L, Bosco-Garate I, Martinez-Ordaz JL, Wong-Baeza I, Gutierrez-Mendoza M, Sanchez-Fernandez P, et al. High serum levels of high-mobility group box 1 (Hmgb1) and low levels of heat shock protein 70 (Hsp70) are associated with poor prognosis in patients with acute pancreatitis. Arch Med Res (2018) 49(7):504–11. doi: 10.1016/j.arcmed.2019.02.003
11. Chen Y, Li M, Liu J, Pan T, Zhou T, Liu Z, et al. Spd-L1 expression is associated with immunosuppression and infectious complications in patients with acute pancreatitis. Scand J Immunol (2017) 86(2):100–6. doi: 10.1111/sji.12564
12. Dabrowski A, Osada J, Dabrowska MI, Wereszczynska-Siemiatkowska U, Siemiatkowski A. Increased expression of the intercellular adhesion molecule-1 (Icam-1) on peripheral blood neutrophils in acute pancreatitis. Adv Med Sci (2014) 59(1):102–7. doi: 10.1016/j.advms.2014.01.001
13. Ferat-Osorio E, Wong-Baeza I, Esquivel-Callejas N, Figueroa-Figueroa S, Duarte-Rojo A, Guzman-Valdivia-Gomez G, et al. Triggering receptor expressed on myeloid cells-1 expression on monocytes is associated with inflammation but not with infection in acute pancreatitis. Crit Care (2009) 13(3):R69. doi: 10.1186/cc7876
14. Gong JD, Qi XF, Zhang Y, Li HL. Increased admission serum cold-inducible rna-binding protein concentration is associated with prognosis of severe acute pancreatitis. Clin Chim Acta (2017) 471:135–42. doi: 10.1016/j.cca.2017.06.002
15. Gotzinger P, Sautner T, Spittler A, Barlan M, Wamser P, Roth E, et al. Severe acute pancreatitis causes alterations in hla-Dr and Cd14 expression on peripheral blood monocytes independently of surgical treatment. Eur J Surg (2000) 166(8):628–32. doi: 10.1080/110241500750008286
16. Ho YP, Sheen IS, Chiu CT, Wu CS, Lin CY. A strong association between down-regulation of hla-Dr expression and the late mortality in patients with severe acute pancreatitis. Am J Gastroenterol (2006) 101(5):1117–24. doi: 10.1111/j.1572-0241.2006.00495.x
17. Huang J, Wu Z, Lu S, Shen J, Kong X, Shen Y. Soluble B7-H2 as a novel marker in early evaluation of the severity of acute pancreatitis. Lab Med (2015) 46(2):109–17. doi: 10.1309/LMFSRH0V82HFXPPI
18. Kaufmann P, Tilz GP, Smolle KH, Demel U, Krejs GJ. Increased plasma concentrations of circulating intercellular adhesion molecule-1 (Cicam-1) in patients with necrotizing pancreatitis. Immunobiology (1996) 195(2):209–19. doi: 10.1016/S0171-2985(96)80040-4
19. Kocsis AK, Szabolcs A, Hofner P, Takacs T, Farkas G, Boda K, et al. Plasma concentrations of high-mobility group box protein 1, soluble receptor for advanced glycation end-products and circulating DNA in patients with acute pancreatitis. Pancreatology (2009) 9(4):383–91. doi: 10.1159/000181172
20. Kylanpaa ML, Mentula P, Kemppainen E, Puolakkainen P, Aittomaki S, Silvennoinen O, et al. Monocyte anergy is present in patients with severe acute pancreatitis and is significantly alleviated by granulocyte-macrophage colony-stimulating factor and interferon-gamma in vitro. Pancreas (2005) 31(1):23–7. doi: 10.1097/01.mpa.0000164449.23524.94
21. Kylanpaa-Back ML, Takala A, Kemppainen E, Puolakkainen P, Kautiainen H, Jansson SE, et al. Cellular markers of systemic inflammation and immune suppression in patients with organ failure due to severe acute pancreatitis. Scand J Gastroenterol (2001) 36(10):1100–7. doi: 10.1080/003655201750422738
22. Li HG, Zhou ZG, Li Y, Zheng XL, Lei S, Zhu L, et al. Alterations of toll-like receptor 4 expression on peripheral blood monocytes during the early stage of human acute pancreatitis. Dig Dis Sci (2007) 52(8):1973–8. doi: 10.1007/s10620-006-9211-4
23. Li Q, Wang C, Zhang Q, Tang C, Li N, Li J. The role of sphingosine kinase 1 in patients with severe acute pancreatitis. Ann Surg (2012) 255(5):954–62. doi: 10.1097/SLA.0b013e31824d2ca4
24. Lindstrom O, Kylanpaa L, Mentula P, Puolakkainen P, Kemppainen E, Haapiainen R, et al. Upregulated but insufficient generation of activated protein c is associated with development of multiorgan failure in severe acute pancreatitis. Crit Care (2006) 10(1):R16. doi: 10.1186/cc3966
25. Liu T, Huang W, Szatmary P, Abrams ST, Alhamdi Y, Lin Z, et al. Accuracy of circulating histones in predicting persistent organ failure and mortality in patients with acute pancreatitis. Br J Surg (2017) 104(9):1215–25. doi: 10.1002/bjs.10538
26. Maksimow M, Kyhala L, Nieminen A, Kylanpaa L, Aalto K, Elima K, et al. Early prediction of persistent organ failure by soluble Cd73 in patients with acute pancreatitis*. Crit Care Med (2014) 42(12):2556–64. doi: 10.1097/CCM.0000000000000550
27. Nakae H, Endo S, Sato N, Wakabayashi G, Inada K, Sato S. Involvement of soluble adhesion molecules in acute pancreatitis. Eur Surg Res (2001) 33(5-6):377–82. doi: 10.1159/000049733
28. Pan T, Zhou T, Li L, Liu Z, Chen Y, Mao E, et al. Monocyte programmed death ligand-1 expression is an early marker for predicting infectious complications in acute pancreatitis. Crit Care (2017) 21(1):186. doi: 10.1186/s13054-017-1781-3
29. Perez S, Finamor I, Marti-Andres P, Pereda J, Campos A, Domingues R, et al. Role of obesity in the release of extracellular nucleosomes in acute pancreatitis: A clinical and experimental study. Int J Obes (Lond) (2019) 43(1):158–68. doi: 10.1038/s41366-018-0073-6
30. Rahman SH, Menon KV, Holmfield JH, McMahon MJ, Guillou JP. Serum macrophage migration inhibitory factor is an early marker of pancreatic necrosis in acute pancreatitis. Ann Surg (2007) 245(2):282–9. doi: 10.1097/01.sla.0000245471.33987.4b
31. Rau B, Baumgart K, Kruger CM, Schilling M, Beger HG. Cc-chemokine activation in acute pancreatitis: Enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med (2003) 29(4):622–9. doi: 10.1007/s00134-003-1668-4
32. Richter A, Nebe T, Wendl K, Schuster K, Klaebisch G, Quintel M, et al. Hla-Dr expression in acute pancreatitis. Eur J Surg (1999) 165(10):947–51. doi: 10.1080/110241599750008053
33. Satoh A, Masamune A, Kimura K, Kaneko K, Sakai Y, Yamagiwa T, et al. Nuclear factor kappa b expression in peripheral blood mononuclear cells of patients with acute pancreatitis. Pancreas (2003) 26(4):350–6. doi: 10.1097/00006676-200305000-00007
34. Satoh A, Miura T, Satoh K, Masamune A, Yamagiwa T, Sakai Y, et al. Human leukocyte antigen-Dr expression on peripheral monocytes as a predictive marker of sepsis during acute pancreatitis. Pancreas (2002) 25(3):245–50. doi: 10.1097/00006676-200210000-00006
35. Shao Z, Schaffler A, Hamer O, Dickopf J, Goetz A, Landfried K, et al. Admission levels of soluble Cd137 are increased in patients with acute pancreatitis and are associated with subsequent complications. Exp Mol Pathol (2012) 92(1):1–6. doi: 10.1016/j.yexmp.2011.09.012
36. Shinzeki M, Ueda T, Takeyama Y, Yasuda T, Sawa H, Nakajima T, et al. Serum immunosuppressive acidic protein levels in patients with severe acute pancreatitis. Pancreas (2007) 35(4):327–33. doi: 10.1097/mpa.0b013e3181200222
37. Szatmary P, Liu T, Abrams ST, Voronina S, Wen L, Chvanov M, et al. Systemic histone release disrupts plasmalemma and contributes to necrosis in acute pancreatitis. Pancreatology (2017) 17(6):884–92. doi: 10.1016/j.pan.2017.10.002
38. Vlachos S, Tsaroucha AK, Konstantoudakis G, Papachristou F, Trypsianis G, Schizas D, et al. Serum profiles of M30, M65 and interleukin-17 compared with c-reactive protein in patients with mild and severe acute pancreatitis. J Hepatob Pancreat Sci (2014) 21(12):911–8. doi: 10.1002/jhbp.162
39. Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Sawa H, Takahiro N, et al. Increased levels of soluble triggering receptor expressed on myeloid cells-1 in patients with acute pancreatitis. Crit Care Med (2008) 36(7):2048–53. doi: 10.1097/CCM.0b013e31817b8824
40. Yu WK, Li WQ, Li N, Li JS. Mononuclear histocompatibility leukocyte antigen-Dr expression in the early phase of acute pancreatitis. Pancreatology (2004) 4(3-4):233–43. doi: 10.1159/000078748
41. Zhang Y, Yan L, Han W. Elevated level of mir-551b-5p is associated with inflammation and disease progression in patients with severe acute pancreatitis. Ther Apher Dial (2018) 22(6):649–55. doi: 10.1111/1744-9987.12720
42. Zhang R, Shi J, Zhang R, Ni J, Habtezion A, Wang X, et al. Expanded Cd14(Hi)Cd16(-) immunosuppressive monocytes predict disease severity in patients with acute pancreatitis. J Immunol (2019) 202(9):2578–84. doi: 10.4049/jimmunol.1801194
43. Andersson E, Axelsson J, Eckerwall G, Ansari D, Andersson R. Tissue factor in predicted severe acute pancreatitis. World J Gastroenterol (2010) 16(48):6128–34. doi: 10.3748/wjg.v16.i48.6128
44. Berney T, Gasche Y, Robert J, Jenny A, Mensi N, Grau G, et al. Serum profiles of interleukin-6, interleukin-8, and interleukin-10 in patients with severe and mild acute pancreatitis. Pancreas (1999) 18(4):371–7. doi: 10.1097/00006676-199905000-00007
45. Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: An early and sustained response, although unpredictable of death. Parisian study group on acute pancreatitis. Crit Care Med (1999) 27(4):749–55. doi: 10.1097/00003246-199904000-00029
46. Ceranic DB, Zorman M, Skok P. Interleukins and inflammatory markers are useful in predicting the severity of acute pancreatitis. Bosn J Basic Med Sci (2020) 20(1):99–105. doi: 10.17305/bjbms.2019.4253
47. Chen CC, Wang SS, Lu RH, Chang FY, Lee SD. Serum interleukin 10 and interleukin 11 in patients with acute pancreatitis. Gut (1999) 45(6):895–9. doi: 10.1136/gut.45.6.895
48. Chen P, Yuan Y, Wang S, Zhan L, Xu J. Serum matrix metalloproteinase 9 as a marker for the assessment of severe acute pancreatitis. Tohoku J Exp Med (2006) 208(3):261–6. doi: 10.1620/tjem.208.261
49. Dambrauskas Z, Giese N, Gulbinas A, Giese T, Berberat PO, Pundzius J, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol (2010) 16(15):1845–53. doi: 10.3748/wjg.v16.i15.1845
50. Daniel P, Lesniowski B, Jasinska A, Pietruczuk M, Malecka-Panas E. Usefulness of assessing circulating levels of resistin, ghrelin, and il-18 in alcoholic acute pancreatitis. Dig Dis Sci (2010) 55(10):2982–7. doi: 10.1007/s10620-009-1106-8
51. de Beaux AC, Goldie AS, Ross JA, Carter DC, Fearon KC. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg (1996) 83(3):349–53. doi: 10.1002/bjs.1800830317
52. de Beaux AC, Ross JA, Maingay JP, Fearon KC, Carter DC. Proinflammatory cytokine release by peripheral blood mononuclear cells from patients with acute pancreatitis. Br J Surg (1996) 83(8):1071–5. doi: 10.1002/bjs.1800830811
53. Duarte-Rojo A, Suazo-Barahona J, Ramirez-Iglesias MT, Uscanga LF, Robles-Diaz G. Time frames for analysis of inflammatory mediators in acute pancreatitis: Improving admission triage. Dig Dis Sci (2009) 54(10):2282–7. doi: 10.1007/s10620-008-0615-1
54. Gunjaca I, Zunic J, Gunjaca M, Kovac Z. Circulating cytokine levels in acute pancreatitis-model of Sirs/Cars can help in the clinical assessment of disease severity. Inflammation (2012) 35(2):758–63. doi: 10.1007/s10753-011-9371-z
55. Heath DI, Cruickshank A, Gudgeon M, Jehanli A, Shenkin A, Imrie CW. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut (1993) 34(1):41–5. doi: 10.1136/gut.34.1.41
56. Heath DI, Cruickshank A, Gudgeon AM, Jehanli A, Shenkin A, Imrie CW. The relationship between pancreatic enzyme release and activation and the acute-phase protein response in patients with acute pancreatitis. Pancreas (1995) 10(4):347–53. doi: 10.1097/00006676-199505000-00005
57. Hirota M, Nozawa F, Okabe A, Shibata M, Beppu T, Shimada S, et al. Relationship between plasma cytokine concentration and multiple organ failure in patients with acute pancreatitis. Pancreas (2000) 21(2):141–6. doi: 10.1097/00006676-200008000-00006
58. Ho YP, Chiu CT, Sheen IS, Tseng SC, Lai PC, Ho SY, et al. Tumor necrosis factor-alpha and interleukin-10 contribute to immunoparalysis in patients with acute pancreatitis. Hum Immunol (2011) 72(1):18–23. doi: 10.1016/j.humimm.2010.10.002
59. Hynninen M, Valtonen M, Markkanen H, Vaara M, Kuusela P, Jousela I, et al. Intramucosal ph and endotoxin and cytokine release in severe acute pancreatitis. Shock (2000) 13(1):79–82. doi: 10.1097/00024382-200013010-00014
60. Ikei S, Ogawa M, Yamaguchi Y. Blood concentrations of polymorphonuclear leucocyte elastase and interleukin-6 are indicators for the occurrence of multiple organ failures at the early stage of acute pancreatitis. J Gastroenterol Hepatol (1998) 13(12):1274–83. doi: 10.1111/j.1440-1746.1998.tb00617.x
61. Inagaki T, Hoshino M, Hayakawa T, Ohara H, Yamada T, Yamada H, et al. Interleukin-6 is a useful marker for early prediction of the severity of acute pancreatitis. Pancreas (1997) 14(1):1–8. doi: 10.1097/00006676-199701000-00001
62. Jamdar S, Al-Mowallad AF, Kumar S, Siriwardena AK. Differential kinetics of plasma Cd105 and transforming growth factor beta expression early in human acute pancreatitis. Pancreas (2006) 32(2):152–8. doi: 10.1097/01.mpa.0000203962.16630.f4
63. Jia R, Tang M, Qiu L, Sun R, Cheng L, Ma X, et al. Increased interleukin-23/17 axis and c-reactive protein are associated with severity of acute pancreatitis in patients. Pancreas (2015) 44(2):321–5. doi: 10.1097/MPA.0000000000000284
64. Jiang CF, Shiau YC, Ng KW, Tan SW. Serum interleukin-6, tumor necrosis factor alpha and c-reactive protein in early prediction of severity of acute pancreatitis. J Chin Med Assoc (2004) 67(9):442–6.
65. Karrasch T, Brunnler T, Hamer OW, Schmid K, Voelk M, Herfarth H, et al. Soluble Cd163 is increased in patients with acute pancreatitis independent of disease severity. Exp Mol Pathol (2015) 99(2):236–9. doi: 10.1016/j.yexmp.2015.07.006
66. Kaufmann P, Tilz GP, Lueger A, Demel U. Elevated plasma levels of soluble tumor necrosis factor receptor (Stnfrp60) reflect severity of acute pancreatitis. Intensive Care Med (1997) 23(8):841–8. doi: 10.1007/s001340050420
67. Kaw M, Singh S. Serum lipase, c-reactive protein, and interleukin-6 levels in ercp-induced pancreatitis. Gastrointest Endosc (2001) 54(4):435–40. doi: 10.1067/mge.2001.117763
68. Kolber W, Dumnicka P, Maraj M, Kusnierz-Cabala B, Ceranowicz P, Pedziwiatr M, et al. Does the automatic measurement of interleukin 6 allow for prediction of complications during the first 48 h of acute pancreatitis? Int J Mol Sci (2018) 19(6):1820. doi: 10.3390/ijms19061820
69. Kylanpaa-Back ML, Takala A, Kemppainen EA, Puolakkainen PA, Leppaniemi AK, Karonen SL, et al. Procalcitonin, soluble interleukin-2 receptor, and soluble e-selectin in predicting the severity of acute pancreatitis. Crit Care Med (2001) 29(1):63–9. doi: 10.1097/00003246-200101000-00016
70. Laveda R, Martinez J, Munoz C, Penalva JC, Saez J, Belda G, et al. Different profile of cytokine synthesis according to the severity of acute pancreatitis. World J Gastroenterol (2005) 11(34):5309–13. doi: 10.3748/wjg.v11.i34.5309
71. Lin M, Huang J, Huang J, Liu SL, Chen WC. Level of serum soluble Tim-3 expression in early-phase acute pancreatitis. Turk J Gastroenterol (2019) 30(2):188–91. doi: 10.5152/tjg.2018.18137
72. Malmstrom ML, Hansen MB, Andersen AM, Ersboll AK, Nielsen OH, Jorgensen LN, et al. Cytokines and organ failure in acute pancreatitis: Inflammatory response in acute pancreatitis. Pancreas (2012) 41(2):271–7. doi: 10.1097/MPA.0b013e3182240552
73. Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: Clinical and pathophysiological implications. Gut (2000) 47(4):546–52. doi: 10.1136/gut.47.4.546
74. McKay CJ, Gallagher G, Brooks B, Imrie CW, Baxter JN. Increased monocyte cytokine production in association with systemic complications in acute pancreatitis. Br J Surg (1996) 83(7):919–23. doi: 10.1002/bjs.1800830712
75. Mentula P, Kylanpaa ML, Kemppainen E, Jansson SE, Sarna S, Puolakkainen P, et al. Plasma anti-inflammatory cytokines and monocyte human leucocyte antigen-Dr expression in patients with acute pancreatitis. Scand J Gastroenterol (2004) 39(2):178–87. doi: 10.1080/00365520310008278
76. Mentula P, Kylanpaa ML, Kemppainen E, Jansson SE, Sarna S, Puolakkainen P, et al. Early prediction of organ failure by combined markers in patients with acute pancreatitis. Br J Surg (2005) 92(1):68–75. doi: 10.1002/bjs.4786
77. Messmann H, Vogt W, Holstege A, Lock G, Heinisch A, von Furstenberg A, et al. Post-erp pancreatitis as a model for cytokine induced acute phase response in acute pancreatitis. Gut (1997) 40(1):80–5. doi: 10.1136/gut.40.1.80
78. Messmann H, Vogt W, Falk W, Vogl D, Zirngibl H, Leser HG, et al. Interleukins and their antagonists but not tnf and its receptors are released in post-erp pancreatitis. Eur J Gastroenterol Hepatol (1998) 10(7):611–7. doi: 10.1097/00042737-199807000-00016
79. Simovic MO, Bonham MJ, Abu-Zidan FM, Windsor JA. Anti-inflammatory cytokine response and clinical outcome in acute pancreatitis. Crit Care Med (1999) 27(12):2662–5. doi: 10.1097/00003246-199912000-00009
80. Montravers P, Chollet-Martin S, Marmuse JP, Gougerot-Pocidalo MA, Desmonts JM. Lymphatic release of cytokines during acute lung injury complicating severe pancreatitis. Am J Respir Crit Care Med (1995) 152(5 Pt 1):1527–33. doi: 10.1164/ajrccm.152.5.7582288
81. Nakae H, Endo S, Inoue Y, Fujino Y, Wakabayashi G, Inada K, et al. Matrix metalloproteinase-1 and cytokines in patients with acute pancreatitis. Pancreas (2003) 26(2):134–8. doi: 10.1097/00006676-200303000-00008
82. Naskalski JW, Kusnierz-Cabala B, Panek J, Kedra B. Poly-c specific ribonuclease activity correlates with increased concentrations of il-6, il-8 and Stnfr55/Stnfr75 in plasma of patients with acute pancreatitis. J Physiol Pharmacol (2003) 54(3):439–48.
83. Nieminen A, Maksimow M, Mentula P, Kyhala L, Kylanpaa L, Puolakkainen P, et al. Circulating cytokines in predicting development of severe acute pancreatitis. Crit Care (2014) 18(3):R104. doi: 10.1186/cc13885
84. O’Reilly DA, Roberts JR, Cartmell MT, Demaine AG, Kingsnorth AN. Heat shock factor-1 and nuclear factor-kappab are systemically activated in human acute pancreatitis. JOP (2006) 7(2):174–84.
85. Oezcueruemez-Porsch M, Kunz D, Hardt PD, Fadgyas T, Kress O, Schulz HU, et al. Diagnostic relevance of interleukin pattern, acute-phase proteins, and procalcitonin in early phase of post-ercp pancreatitis. Dig Dis Sci (1998) 43(8):1763–9. doi: 10.1023/a:1018887704337
86. Paajanen H, Laato M, Jaakkola M, Pulkki K, Niinikoski J, Nordback I. Serum tumour necrosis factor compared with c-reactive protein in the early assessment of severity of acute pancreatitis. Br J Surg (1995) 82(2):271–3. doi: 10.1002/bjs.1800820244
87. Panek J, Karcz D, Pieton R, Zasada J, Tusinski M, Dolecki M, et al. Blood serum levels of proinflammatory cytokines in patients with different degrees of biliary pancreatitis. Can J Gastroenterol (2006) 20(10):645–8. doi: 10.1155/2006/372892
88. Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: Performance of Apache-O score and correlation with the inflammatory response. Pancreatology (2006) 6(4):279–85. doi: 10.1159/000092689
89. Park J, Chang JH, Park SH, Lee HJ, Lim YS, Kim TH, et al. Interleukin-6 is associated with obesity, central fat distribution, and disease severity in patients with acute pancreatitis. Pancreatology (2015) 15(1):59–63. doi: 10.1016/j.pan.2014.11.001
90. Peng YS, Chen YC, Tian YC, Yang CW, Lien JM, Fang JT, et al. Serum levels of apolipoprotein a-I and high-density lipoprotein can predict organ failure in acute pancreatitis. Crit Care (2015) 19(1):88. doi: 10.1186/s13054-015-0832-x
91. Pezzilli R, Billi P, Miniero R, Fiocchi M, Cappelletti O, Morselli-Labate AM, et al. Serum interleukin-6, interleukin-8, and beta 2-microglobulin in early assessment of severity of acute pancreatitis. comparison with serum c-reactive protein. Dig Dis Sci (1995) 40(11):2341–8. doi: 10.1007/BF02063235
92. Pezzilli R, Billi P, Miniero R, Barakat B. Serum interleukin-10 in human acute pancreatitis. Dig Dis Sci (1997) 42(7):1469–72. doi: 10.1023/a:1018814710291
93. Pezzilli R, Miniero R, Cappelletti O, Barakat B. Behavior of serum interleukin 12 in human acute pancreatitis. Pancreas (1999) 18(3):247–51. doi: 10.1097/00006676-199904000-00005
94. Pezzilli R, Morselli-Labate AM, Miniero R, Barakat B, Fiocchi M, Cappelletti O. Simultaneous serum assays of lipase and interleukin-6 for early diagnosis and prognosis of acute pancreatitis. Clin Chem (1999) 45(10):1762–7. doi: 10.1093/clinchem/45.10.1762
95. Pongratz G, Hochrinner H, Straub RH, Lang S, Brunnler T. B cell activating factor of the tumor necrosis factor family (Baff) behaves as an acute phase reactant in acute pancreatitis. PloS One (2013) 8(1):e54297. doi: 10.1371/journal.pone.0054297
96. Pooran N, Indaram A, Singh P, Bank S. Cytokines (Il-6, il-8, tnf): Early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol (2003) 37(3):263–6. doi: 10.1097/00004836-200309000-00013
97. Rau B, Baumgart K, Paszkowski AS, Mayer JM, Beger HG. Clinical relevance of caspase-1 activated cytokines in acute pancreatitis: High correlation of serum interleukin-18 with pancreatic necrosis and systemic complications. Crit Care Med (2001) 29(8):1556–62. doi: 10.1097/00003246-200108000-00010
98. Regner S, Appelros S, Hjalmarsson C, Manjer J, Sadic J, Borgstrom A, et al. Monocyte chemoattractant protein 1 And capap at hospital admission are predictive markers for severe acute pancreatitis. Pancreatology (2008) 8(1):42–9. doi: 10.1159/000114866
99. Rodriguez-Nicolas A, Martinez-Chamorro A, Jimenez P, Matas-Cobos AM, Redondo-Cerezo E, Ruiz-Cabello F. Th1 and Th2 cytokine profiles as predictors of severity in acute pancreatitis. Pancreas (2018) 47(4):400–5. doi: 10.1097/MPA.0000000000001006
100. Sathyanarayan G, Garg PK, Prasad H, Tandon RK. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis. J Gastroenterol Hepatol (2007) 22(4):550–4. doi: 10.1111/j.1440-1746.2006.04752.x
101. Sempere L, Martinez J, de Madaria E, Lozano B, Sanchez-Paya J, Jover R, et al. Obesity and fat distribution imply a greater systemic inflammatory response and a worse prognosis in acute pancreatitis. Pancreatology (2008) 8(3):257–64. doi: 10.1159/000134273
102. Shen Y, Deng X, Xu N, Li Y, Miao B, Cui N. Relationship between the degree of severe acute pancreatitis and patient immunity. Surg Today (2015) 45(8):1009–17. doi: 10.1007/s00595-014-1083-1
103. Shokuhi S, Bhatia M, Christmas S, Sutton R, Neoptolemos JP, Slavin J. Levels of the chemokines growth-related oncogene alpha and epithelial neutrophil-activating protein 78 are raised in patients with severe acute pancreatitis. Br J Surg (2002) 89(5):566–72. doi: 10.1046/j.1365-2168.2002.02060.x
104. Sternby H, Hartman H, Johansen D, Thorlacius H, Regner S. Predictive capacity of biomarkers for severe acute pancreatitis. Eur Surg Res (2016) 56(3-4):154–63. doi: 10.1159/000444141
105. Stimac D, Fisic E, Milic S, Bilic-Zulle L, Peric R. Prognostic values of il-6, il-8, and il-10 in acute pancreatitis. J Clin Gastroenterol (2006) 40(3):209–12. doi: 10.1097/00004836-200603000-00007
106. Thomson JE, Nweke EE, Brand M, Nel M, Candy GP, Fonteh PN. Transient expression of interleukin-21 in the second hit of acute pancreatitis may potentiate immune paresis in severe acute pancreatitis. Pancreas (2019) 48(1):107–12. doi: 10.1097/MPA.0000000000001207
107. Ueda T, Takeyama Y, Yasuda T, Matsumura N, Sawa H, Nakajima T, et al. Significant elevation of serum interleukin-18 levels in patients with acute pancreatitis. J Gastroenterol (2006) 41(2):158–65. doi: 10.1007/s00535-005-1735-4
108. Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Nakajima T, et al. Serum interleukin-15 level is a useful predictor of the complications and mortality in severe acute pancreatitis. Surgery (2007) 142(3):319–26. doi: 10.1016/j.surg.2007.05.002
109. Uehara S, Gothoh K, Handa H, Tomita H, Tomita Y. Immune function in patients with acute pancreatitis. J Gastroenterol Hepatol (2003) 18(4):363–70. doi: 10.1046/j.1440-1746.2003.02979.x
110. Vasseur P, Devaure I, Sellier J, Delwail A, Chagneau-Derrode C, Charier F, et al. High plasma levels of the pro-inflammatory cytokine il-22 and the anti-inflammatory cytokines il-10 and il-1ra in acute pancreatitis. Pancreatology (2014) 14(6):465–9. doi: 10.1016/j.pan.2014.08.005
111. Viedma JA, Perez-Mateo M, Dominguez JE, Carballo F. Role of interleukin-6 in acute pancreatitis. comparison with c-reactive protein and phospholipase a. Gut (1992) 33(9):1264–7. doi: 10.1136/gut.33.9.1264
112. Wereszczynska-Siemiatkowska U, Mroczko B, Siemiatkowski A. Serum profiles of interleukin-18 in different severity forms of human acute pancreatitis. Scand J Gastroenterol (2002) 37(9):1097–102. doi: 10.1080/003655202320378310
113. Wereszczynska-Siemiatkowska U, Dabrowski A, Siemiatkowski A, Mroczko B, Laszewicz W, Gabryelewicz A. Serum profiles of e-selectin, interleukin-10, and interleukin-6 and oxidative stress parameters in patients with acute pancreatitis and nonpancreatic acute abdominal pain. Pancreas (2003) 26(2):144–52. doi: 10.1097/00006676-200303000-00010
114. Wereszczynska-Siemiatkowska U, Mroczko B, Siemiatkowski A, Szmitkowski M, Borawska M, Kosel J. The importance of interleukin 18, glutathione peroxidase, and selenium concentration changes in acute pancreatitis. Dig Dis Sci (2004) 49(4):642–50. doi: 10.1023/b:ddas.0000026312.47460.a3
115. Yang Z, Zhang Y, Dong L, Yang C, Gou S, Yin T, et al. The reduction of peripheral blood Cd4+ T cell indicates persistent organ failure in acute pancreatitis. PloS One (2015) 10(5):e0125529. doi: 10.1371/journal.pone.0125529
116. Zhang X, Guo J, Wang H, Zhang C, Shi N, Cai W, et al. Underexpression of receptor for activated c kinase 1 (Rack1) in leukocytes from patients with severe acute pancreatitis. Tohoku J Exp Med (2018) 245(3):205–15. doi: 10.1620/tjem.245.205
117. Balog A, Gyulai Z, Boros LG, Farkas G, Takacs T, Lonovics J, et al. Polymorphism of the tnf-alpha, Hsp70-2, and Cd14 genes increases susceptibility to severe acute pancreatitis. Pancreas (2005) 30(2):e46–50. doi: 10.1097/01.mpa.0000153329.92686.ac
118. Bao XB, Ma Z, Gu JB, Wang XQ, Li HG, Wang WY. Il-8 -251t/a polymorphism is associated with susceptibility to acute pancreatitis. Genet Mol Res (2015) 14(1):1508–14. doi: 10.4238/2015.February.20.6
119. Bishehsari F, Sharma A, Stello K, Toth C, O’Connell MR, Evans AC, et al. Tnf-alpha gene (Tnfa) variants increase risk for multi-organ dysfunction syndrome (Mods) in acute pancreatitis. Pancreatology (2012) 12(2):113–8. doi: 10.1016/j.pan.2012.02.014
120. Bishu S, Koutroumpakis E, Mounzer R, Stello K, Pollock N, Evans A, et al. The -251 a/T polymorphism in the Il8 promoter is a risk factor for acute pancreatitis. Pancreas (2018) 47(1):87–91. doi: 10.1097/MPA.0000000000000967
121. de-Madaria E, Martinez J, Sempere L, Lozano B, Sanchez-Paya J, Uceda F, et al. Cytokine genotypes in acute pancreatitis: Association with etiology, severity, and cytokine levels in blood. Pancreas (2008) 37(3):295–301. doi: 10.1097/MPA.0b013e31816726d5
122. Liu Y, Dan G, Wu L, Chen G, Wu A, Zeng P, et al. Functional effect of polymorphisms in the promoter of Tnfaip3 (A20) in acute pancreatitis in the han Chinese population. PloS One (2014) 9(7):e103104. doi: 10.1371/journal.pone.0103104
123. Makhija R, Kingsnorth A, Demaine A. Gene polymorphisms of the macrophage migration inhibitory factor and acute pancreatitis. JOP (2007) 8(3):289–95.
124. Masamune A, Kume K, Kikuta K, Watanabe T, Hirota M, Satoh K, et al. -651c/T promoter polymorphism in the Cd14 gene is associated with severity of acute pancreatitis in Japan. J Gastroenterol (2010) 45(2):225–33. doi: 10.1007/s00535-009-0163-2
125. Matas-Cobos AM, Redondo-Cerezo E, Alegria-Motte C, Martinez-Chamorro A, Saenz-Lopez P, Jimenez P, et al. The role of toll-like receptor polymorphisms in acute pancreatitis occurrence and severity. Pancreas (2015) 44(3):429–33. doi: 10.1097/MPA.0000000000000272
126. Ozhan G, Yanar HT, Ertekin C, Alpertunga B. Polymorphisms in tumour necrosis factor alpha (Tnfalpha) gene in patients with acute pancreatitis. Mediators Inflammation (2010) 2010:482950. doi: 10.1155/2010/482950
127. Papachristou GI, Sass DA, Avula H, Lamb J, Lokshin A, Barmada MM, et al. Is the monocyte chemotactic protein-1 -2518 G allele a risk factor for severe acute pancreatitis? Clin Gastroenterol Hepatol (2005) 3(5):475–81. doi: 10.1016/s1542-3565(05)00163-1
128. Powell JJ, Fearon KC, Siriwardena AK, Ross JA. Evidence against a role for polymorphisms at tumor necrosis factor, interleukin-1 and interleukin-1 receptor antagonist gene loci in the regulation of disease severity in acute pancreatitis. Surgery (2001) 129(5):633–40. doi: 10.1067/msy.2001.113375
129. Rodriguez-Nicolas A, Jimenez P, Carmona FD, Martin J, Matas Cobos AM, Ruiz-Cabello F, et al. Association between genetic polymorphisms of inflammatory response genes and acute pancreatitis. Immunol Invest (2019) 48(6):585–96. doi: 10.1080/08820139.2019.1576729
130. Sargen K, Demaine AG, Kingsnorth AN. Cytokine gene polymorphisms in acute pancreatitis. JOP (2000) 1(2):24–35.
131. Smithies AM, Sargen K, Demaine AG, Kingsnorth AN. Investigation of the interleukin 1 gene cluster and its association with acute pancreatitis. Pancreas (2000) 20(3):234–40. doi: 10.1097/00006676-200004000-00003
132. Takagi Y, Masamune A, Kume K, Satoh A, Kikuta K, Watanabe T, et al. Microsatellite polymorphism in intron 2 of human toll-like receptor 2 gene is associated with susceptibility to acute pancreatitis in Japan. Hum Immunol (2009) 70(3):200–4. doi: 10.1016/j.humimm.2009.01.006
133. Tukiainen E, Kylanpaa ML, Puolakkainen P, Kemppainen E, Halonen K, Orpana A, et al. Polymorphisms of the tnf, Cd14, and Hspa1b genes in patients with acute alcohol-induced pancreatitis. Pancreas (2008) 37(1):56–61. doi: 10.1097/MPA.0b013e31815d9bad
134. Zhang D, Zheng H, Zhou Y, Yu B, Li J. Tlr and mbl gene polymorphisms in severe acute pancreatitis. Mol Diagn Ther (2008) 12(1):45–50. doi: 10.1007/BF03256267
135. Algaba-Chueca F, de-Madaria E, Lozano-Ruiz B, Martinez-Cardona C, Quesada-Vazquez N, Bachiller V, et al. The expression and activation of the Aim2 inflammasome correlates with inflammation and disease severity in patients with acute pancreatitis. Pancreatology (2017) 17(3):364–71. doi: 10.1016/j.pan.2017.03.006
136. Bhatnagar A, Wig JD, Majumdar S. Expression of activation, adhesion molecules and intracellular cytokines in acute pancreatitis. Immunol Lett (2001) 77(3):133–41. doi: 10.1016/s0165-2478(01)00210-3
137. Bhatnagar A, Wig J, Vaiphei K, Majumdar S. Intracellular cytokines in cells of necrotic tissue from patients with acute pancreatitis. Eur J Surg (2001) 167(7):510–7. doi: 10.1080/110241501316914885
138. Bidarkundi GK, Wig JD, Bhatnagar A, Majumdar S. Clinical relevance of intracellular cytokines il-6 and il-12 in acute pancreatitis, and correlation with Apache iii score. Br J BioMed Sci (2002) 59(2):85–9. doi: 10.1080/09674845.2002.11783640
139. Chen HM, Hsu JT, Chen JC, Ng CJ, Chiu DF, Chen MF. Delayed neutrophil apoptosis attenuated by melatonin in human acute pancreatitis. Pancreas (2005) 31(4):360–4. doi: 10.1097/01.mpa.0000180905.05494.9a
140. Curley PJ. Endotoxin, cellular immune dysfunction and acute pancreatitis. Ann R Coll Surg Engl (1996) 78(6):531–5.
141. Curley PJ, McMahon MJ, Lancaster F, Banks RE, Barclay GR, Shefta J, et al. Reduction in circulating levels of Cd4-positive lymphocytes in acute pancreatitis: Relationship to endotoxin, interleukin 6 and disease severity. Br J Surg (1993) 80(10):1312–5. doi: 10.1002/bjs.1800801031
142. Dabrowski A, Osada J, Dabrowska MI, Wereszczynska-Siemiatkowska U. Monocyte subsets and natural killer cells in acute pancreatitis. Pancreatology (2008) 8(2):126–34. doi: 10.1159/000123605
143. Li JP, Yang J, Huang JR, Jiang DL, Zhang F, Liu MF, et al. Immunosuppression and the infection caused by gut mucosal barrier dysfunction in patients with early severe acute pancreatitis. Front Biosci (Landmark Ed) (2013) 18(3):892–900. doi: 10.2741/4150
144. Minkov GA, Yovtchev YP, Halacheva KS. Increased circulating Cd4+Cd25+Cd127low/Neg regulatory T-cells as a prognostic biomarker in acute pancreatitis. Pancreas (2017) 46(8):1003–10. doi: 10.1097/MPA.0000000000000894
145. O’Neill S, O’Neill AJ, Conroy E, Brady HR, Fitzpatrick JM, Watson RW. Altered caspase expression results in delayed neutrophil apoptosis in acute pancreatitis. J Leukoc Biol (2000) 68(1):15–20. doi: 10.1189/jlb.68.1.15
146. Oiva J, Mustonen H, Kylanpaa ML, Kyhala L, Kuuliala K, Siitonen S, et al. Acute pancreatitis with organ dysfunction associates with abnormal blood lymphocyte signaling: Controlled laboratory study. Crit Care (2010) 14(6):R207. doi: 10.1186/cc9329
147. Pezzilli R, Maldini M, Morselli-Labate AM, Barakat B, Romboli E, Beltrandi E, et al. Early activation of peripheral lymphocytes in human acute pancreatitis. J Clin Gastroenterol (2003) 36(4):360–3. doi: 10.1097/00004836-200304000-00016
148. Pezzilli R, Billi P, Beltrandi E, Maldini M, Mancini R, Morselli Labate AM, et al. Circulating lymphocyte subsets in human acute pancreatitis. Pancreas (1995) 11(1):95–100. doi: 10.1097/00006676-199507000-00010
149. Pezzilli R, Billi P, Beltrandi E, Casadei Maldini M, Mancini R. Impaired lymphocyte proliferation in human acute pancreatitis. Digestion (1997) 58(5):431–6. doi: 10.1159/000201479
150. Pietruczuk M, Dabrowska MI, Wereszczynska-Siemiatkowska U, Dabrowski A. Alteration of peripheral blood lymphocyte subsets in acute pancreatitis. World J Gastroenterol (2006) 12(33):5344–51. doi: 10.3748/wjg.v12.i33.5344
151. Salomone T, Tosi P, Raiti C, Guariento A, Tomassetti P, Migliori M, et al. Apoptosis in the peripheral blood mononuclear cells as a self-limitation process in human acute pancreatitis. Pancreatology (2002) 2(3):204–10. doi: 10.1159/000058034
152. Shen X, Li WQ. High-mobility group box 1 protein and its role in severe acute pancreatitis. World J Gastroenterol (2015) 21(5):1424–35. doi: 10.3748/wjg.v21.i5.1424
153. Susak YM, Dirda OO, Fedorchuk OG, Tkachenko OA, Skivka LM. Infectious complications of acute pancreatitis is associated with peripheral blood phagocyte functional exhaustion. Dig Dis Sci (2021) 66(1):121–30. doi: 10.1007/s10620-020-06172-y
154. Sweeney KJ, Kell MR, Coates C, Murphy T, Reynolds JV. Serum Antigen(S) drive the proinflammatory T cell response in acute pancreatitis. Br J Surg (2003) 90(3):313–9. doi: 10.1002/bjs.4080
155. Takeyama Y, Takas K, Ueda T, Hori Y, Goshima M, Kuroda Y. Peripheral lymphocyte reduction in severe acute pancreatitis is caused by apoptotic cell death. J Gastrointest Surg (2000) 4(4):379–87. doi: 10.1016/s1091-255x(00)80016-5
156. Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Nakajima T, et al. Immunosuppression in patients with severe acute pancreatitis. J Gastroenterol (2006) 41(8):779–84. doi: 10.1007/s00535-006-1852-8
157. Wang W, Xiang HP, Wang HP, Zhu LX, Geng XP. Cd4 + Cd25 + Cd127 high cells as a negative predictor of multiple organ failure in acute pancreatitis. World J Emerg Surg (2017) 12:7. doi: 10.1186/s13017-017-0116-7
158. Widdison AL, Cunningham S. Immune function early in acute pancreatitis. Br J Surg (1996) 83(5):633–6. doi: 10.1002/bjs.1800830514
159. Zhang H, Ling XL, Wu YY, Lu MH, Guo H, Zhang PB, et al. Cd64 expression is increased in patients with severe acute pancreatitis: Clinical significance. Gut Liver (2014) 8(4):445–51. doi: 10.5009/gnl.2014.8.4.445
160. Zhang ML, Jiang YF, Wang XR, Ding LL, Wang HJ, Meng QQ, et al. Different phenotypes of monocytes in patients with new-onset mild acute pancreatitis. World J Gastroenterol (2017) 23(8):1477–88. doi: 10.3748/wjg.v23.i8.1477
161. Zhao Z, Shen J, Zhang D, Shen J. The prognostic role of peripheral lymphocyte subsets in patients with acute pancreatitis. Am J Med Sci (2019) 357(3):242–6. doi: 10.1016/j.amjms.2018.12.008
162. Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: Correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg (1999) 3(3):252–62. doi: 10.1016/s1091-255x(99)80067-5
163. Buttenschoen K, Berger D, Hiki N, Buttenschoen DC, Vasilescu C, Chikh-Torab F, et al. Endotoxin and antiendotoxin antibodies in patients with acute pancreatitis. Eur J Surg (2000) 166(6):459–66. doi: 10.1080/110241500750008772
164. Kivilaakso E, Valtonen VV, Malkamaki M, Palmu A, Schroder T, Nikki P, et al. Endotoxaemia and acute pancreatitis: Correlation between the severity of the disease and the anti-enterobacterial common antigen antibody titre. Gut (1984) 25(10):1065–70. doi: 10.1136/gut.25.10.1065
165. Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas (2008) 36(2):192–6. doi: 10.1097/MPA.0b013e31815a399f
166. Penalva JC, Martinez J, Laveda R, Esteban A, Munoz C, Saez J, et al. A study of intestinal permeability in relation to the inflammatory response and plasma endocab igm levels in patients with acute pancreatitis. J Clin Gastroenterol (2004) 38(6):512–7. doi: 10.1097/01.mcg.0000129060.46654.e0
167. Rahman SH, Ammori BJ, Holmfield J, Larvin M, McMahon MJ. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg (2003) 7(1):26–36. doi: 10.1016/S1091-255X(02)00090-2
168. Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T, et al. Dysbiosis of intestinal microbiota associated with inflammation involved in the progression of acute pancreatitis. Pancreas (2015) 44(6):868–75. doi: 10.1097/MPA.0000000000000355
169. Windsor JA, Fearon KC, Ross JA, Barclay GR, Smyth E, Poxton I, et al. Role of serum endotoxin and antiendotoxin core antibody levels in predicting the development of multiple organ failure in acute pancreatitis. Br J Surg (1993) 80(8):1042–6. doi: 10.1002/bjs.1800800840
170. Abe R, Oda S, Shinozaki K, Hirasawa H. Continuous hemodiafiltration using a polymethyl methacrylate membrane hemofilter for severe acute pancreatitis. Contrib Nephrol (2010) 166:54–63. doi: 10.1159/000314852
171. Al-Leswas D, Eltweri AM, Chung WY, Arshad A, Stephenson JA, Al-Taan O, et al. Intravenous omega-3 fatty acids are associated with better clinical outcome and less inflammation in patients with predicted severe acute pancreatitis: A randomised double blind controlled trial. Clin Nutr (2020) 39(9):2711–9. doi: 10.1016/j.clnu.2018.04.003
172. Arutla M, Raghunath M, Deepika G, Jakkampudi A, Murthy HVV, Rao GV, et al. Efficacy of enteral glutamine supplementation in patients with severe and predicted severe acute pancreatitis- a randomized controlled trial. Indian J Gastroenterol (2019) 38(4):338–47. doi: 10.1007/s12664-019-00962-7
173. Chen Y-J, Zhuang Y-D, Cai Z, Zhang Y-N, Guo S-R. Effects of enteral nutrition on pro-inflammatory factors and intestinal barrier function in patients with acute severe pancreatitis. Eur J Inflammation (2019) 17:1–6. doi: 10.1177/2058739219827212
174. Chen QJ, Yang ZY, Wang CY, Dong LM, Zhang YS, Xie C, et al. Hydroxyethyl starch resuscitation downregulate pro-inflammatory cytokines in the early phase of severe acute pancreatitis: A retrospective study. Exp Ther Med (2016) 12(5):3213–20. doi: 10.3892/etm.2016.3744
175. Chooklin S, Pereyaslov A, Bihalskyy I. Pathogenic role of myeloperoxidase in acute pancreatitis. Hepatob Pancreat Dis Int (2009) 8(6):627–31.
176. Chu LP, Zhou JJ, Yu YF, Huang Y, Dong WX. Clinical effects of pulse high-volume hemofiltration on severe acute pancreatitis complicated with multiple organ dysfunction syndrome. Ther Apher Dial (2013) 17(1):78–83. doi: 10.1111/j.1744-9987.2012.01104.x
177. Cui HX, Xu JY, Li MQ. Efficacy of continuous renal replacement therapy in the treatment of severe acute pancreatitis associated acute respiratory distress syndrome. Eur Rev Med Pharmacol Sci (2014) 18(17):2523–6.
178. Dai SR, Li Z, Zhang JB. Serum interleukin 17 as an early prognostic biomarker of severe acute pancreatitis receiving continuous blood purification. Int J Artif Organs (2015) 38(4):192–8. doi: 10.5301/ijao.5000406
179. Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric feeding in predicted severe acute pancreatitis: A clinical, randomized study. Ann Surg (2006) 244(6):959–65. doi: 10.1097/01.sla.0000246866.01930.58
180. Gao N, Yan C, Zhang G. Changes of serum procalcitonin (Pct), c-reactive protein (Crp), interleukin-17 (Il-17), interleukin-6 (Il-6), high mobility group protein-B1 (Hmgb1) and d-dimer in patients with severe acute pancreatitis treated with continuous renal replacement therapy (Crrt) and its clinical significance. Med Sci Monit (2018) 24:5881–6. doi: 10.12659/MSM.910099
181. Gorsky VA, Agapov MA, Khoreva MV, Leonenko IV. The effect of lornoxicam on Tlr2 and Tlr4 messenger rna expression and tumor necrosis factor-alpha, interleukin-6, and interleukin-8 secretion in patients with systemic complications of acute pancreatitis. Pancreas (2015) 44(5):824–30. doi: 10.1097/MPA.0000000000000344
182. Guo J, Li Z, Tang D, Zhang J. Th17/Treg imbalance in patients with severe acute pancreatitis: Attenuated by high-volume hemofiltration treatment. Med (Baltimore) (2020) 99(31):e21491. doi: 10.1097/MD.0000000000021491
183. Guo J, Huang W, Yang XN, Jin T, Altaf K, Javed MA, et al. Short-term continuous high-volume hemofiltration on clinical outcomes of severe acute pancreatitis. Pancreas (2014) 43(2):250–4. doi: 10.1097/01.mpa.0000437321.06857.fc
184. Guo H, Suo DW, Zhu HP, Sun XM, Chen J. Early blood purification therapy of severe acute pancreatitis complicated by acute lung injury. Eur Rev Med Pharmacol Sci (2016) 20(5):873–8.
185. Gupta R, Patel K, Calder PC, Yaqoob P, Primrose JN, Johnson CD. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (Apache ii > or =6). Pancreatology (2003) 3(5):406–13. doi: 10.1159/000073657
186. He C, Zhang L, Shi W, Liang X, Ye Z, Zhang B, et al. Coupled plasma filtration adsorption combined with continuous veno-venous hemofiltration treatment in patients with severe acute pancreatitis. J Clin Gastroenterol (2013) 47(1):62–8. doi: 10.1097/MCG.0b013e318266f455
187. Huang L, Zhu H, Gu J. Octreotide and continuous hemofiltration versus continuous hemofiltration alone in severe acute pancreatitis complicated with acute respiratory distress syndrome. J Coll Physicians Surg Pak (2019) 29(8):785–7. doi: 10.29271/jcpsp.2019.08.785
188. Ino Y, Arita Y, Akashi T, Kimura T, Igarashi H, Oono T, et al. Continuous regional arterial infusion therapy with gabexate mesilate for severe acute pancreatitis. World J Gastroenterol (2008) 14(41):6382–7. doi: 10.3748/wjg.14.6382
189. Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut (2001) 48(1):62–9. doi: 10.1136/gut.48.1.62
190. Kingsnorth AN, Galloway SW, Formela LJ. Randomized, double-blind phase ii trial of lexipafant, a platelet-activating factor antagonist, in human acute pancreatitis. Br J Surg (1995) 82(10):1414–20. doi: 10.1002/bjs.1800821039
191. Kyhala L, Mentula P, Kylanpaa L, Moilanen E, Puolakkainen P, Pettila V, et al. Activated protein c does not alleviate the course of systemic inflammation in the apcap trial. Int J Inflam (2012) 2012:712739. doi: 10.1155/2012/712739
192. Li C, Ni L, Cui X. Ulinastatin combined with somatostatin improves intestinal function recovery and treatment efficiency in severe acute pancreatitis. International Journal of Clinical and Experimental Medicine (2020) 13(2):766–73.
193. Mora J, Casas M, Cardona D, Farre A. Effect of enteral versus parenteral nutrition on inflammatory markers in severe acute pancreatitis. Pancreas (2007) 35(3):292. doi: 10.1097/MPA.0b013e31805ba3c6
194. Ockenga J, Borchert K, Rifai K, Manns MP, Bischoff SC. Effect of glutamine-enriched total parenteral nutrition in patients with acute pancreatitis. Clin Nutr (2002) 21(5):409–16. doi: 10.1054/clnu.2002.0569
195. Oda S, Hirasawa H, Shiga H, Matsuda K, Nakamura M, Watanabe E, et al. Management of intra-abdominal hypertension in patients with severe acute pancreatitis with continuous hemodiafiltration using a polymethyl methacrylate membrane hemofilter. Ther Apher Dial (2005) 9(4):355–61. doi: 10.1111/j.1744-9987.2005.00297.x
196. Powell JJ, Murchison JT, Fearon KC, Ross JA, Siriwardena AK. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. Br J Surg (2000) 87(10):1375–81. doi: 10.1046/j.1365-2168.2000.01558.x
197. Sharma B, Srivastava S, Singh N, Sachdev V, Kapur S, Saraya A. Role of probiotics on gut permeability and endotoxemia in patients with acute pancreatitis: A double-blind randomized controlled trial. J Clin Gastroenterol (2011) 45(5):442–8. doi: 10.1097/MCG.0b013e318201f9e2
198. Singh N, Mishra SK, Sachdev V, Sharma H, Upadhyay AD, Arora I, et al. Effect of oral glutamine supplementation on gut permeability and endotoxemia in patients with severe acute pancreatitis: A randomized controlled trial. Pancreas (2014) 43(6):867–73. doi: 10.1097/MPA.0000000000000124
199. Sun JK, Mu XW, Li WQ, Tong ZH, Li J, Zheng SY. Effects of early enteral nutrition on immune function of severe acute pancreatitis patients. World J Gastroenterol (2013) 19(6):917–22. doi: 10.3748/wjg.v19.i6.917
200. Tang WF, Wang YG, Zhu L, Wan MH, Chen GY, Xia Q, et al. Effect of somatostatin on immune inflammatory response in patients with severe acute pancreatitis. J Dig Dis (2007) 8(2):96–102. doi: 10.1111/j.1443-9573.2007.00293.x
201. Vege SS, Atwal T, Bi Y, Chari ST, Clemens MA, Enders FT. Pentoxifylline treatment in severe acute pancreatitis: A pilot, double-blind, placebo-controlled, randomized trial. Gastroenterology (2015) 149(2):318–20 e3. doi: 10.1053/j.gastro.2015.04.019
202. Wan B, Fu H, Yin J, Xu F. Efficacy of rhubarb combined with early enteral nutrition for the treatment of severe acute pancreatitis: A randomized controlled trial. Scand J Gastroenterol (2014) 49(11):1375–84. doi: 10.3109/00365521.2014.958523
203. Wang G, Liu Y, Zhou SF, Qiu P, Xu L, Wen P, et al. Effect of somatostatin, ulinastatin and gabexate on the treatment of severe acute pancreatitis. Am J Med Sci (2016) 351(5):506–12. doi: 10.1016/j.amjms.2016.03.013
204. Wang X, Li W, Zhang F, Pan L, Li N, Li J. Fish oil-supplemented parenteral nutrition in severe acute pancreatitis patients and effects on immune function and infectious risk: A randomized controlled trial. Inflammation (2009) 32(5):304–9. doi: 10.1007/s10753-009-9136-0
205. Wang G, Wen J, Xu L, Zhou S, Gong M, Wen P, et al. Effect of enteral nutrition and ecoimmunonutrition on bacterial translocation and cytokine production in patients with severe acute pancreatitis. J Surg Res (2013) 183(2):592–7. doi: 10.1016/j.jss.2012.12.010
206. Wang X, Li W, Li N, Li J. Omega-3 fatty acids-supplemented parenteral nutrition decreases hyperinflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: A randomized and controlled study. JPEN J Parenter Enteral Nutr (2008) 32(3):236–41. doi: 10.1177/0148607108316189
207. Wang X, Xu J, Li J, Cheng Y, Liu L, Du Z. Effect of regional arterial infusion combined with early enteral nutrition on severe acute pancreatitis. J Int Med Res (2019) 47(12):6235–43. doi: 10.1177/0300060519880760
208. Wang G, Liu H, Xu L, Wen P, Wen J, Zhou SF, et al. Effect of laparoscopic peritoneal lavage and drainage and continuous venovenous diahemofiltration on severe acute pancreatitis. J Laparoendosc Adv Surg Tech A (2017) 27(11):1145–50. doi: 10.1089/lap.2016.0637
209. Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut (1998) 42(3):431–5. doi: 10.1136/gut.42.3.431
210. Yang C, Guanghua F, Wei Z, Zhong J, Penghui J, Xin F, et al. Combination of hemofiltration and peritoneal dialysis in the treatment of severe acute pancreatitis. Pancreas (2010) 39(1):16–9. doi: 10.1097/MPA.0b013e3181bab38b
211. Zhao G, Wang CY, Wang F, Xiong JX. Clinical study on nutrition support in patients with severe acute pancreatitis. World J Gastroenterol (2003) 9(9):2105–8. doi: 10.3748/wjg.v9.i9.2105
212. Zhao G, Zhang JG, Wu HS, Tao J, Qin Q, Deng SC, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol (2013) 19(13):2044–52. doi: 10.3748/wjg.v19.i13.2044
213. Hoque R, Malik AF, Gorelick F, Mehal WZ. Sterile inflammatory response in acute pancreatitis. Pancreas (2012) 41(3):353–7. doi: 10.1097/MPA.0b013e3182321500
214. Allam R, Kumar SV, Darisipudi MN, Anders HJ. Extracellular histones in tissue injury and inflammation. J Mol Med (Berl) (2014) 92(5):465–72. doi: 10.1007/s00109-014-1148-z
215. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol (2018) 18(2):134–47. doi: 10.1038/nri.2017.105
216. Murthy P, Singhi AD, Ross MA, Loughran P, Paragomi P, Papachristou GI, et al. Enhanced neutrophil extracellular trap formation in acute pancreatitis contributes to disease severity and is reduced by chloroquine. Front Immunol (2019) 10:28. doi: 10.3389/fimmu.2019.00028
217. Penttila AK, Rouhiainen A, Kylanpaa L, Mustonen H, Puolakkainen P, Rauvala H, et al. Circulating nucleosomes as predictive markers of severe acute pancreatitis. J Intensive Care (2016) 4:14. doi: 10.1186/s40560-016-0135-6
218. Ferrero-Andres A, Panisello-Rosello A, Rosello-Catafau J, Folch-Puy E. Nlrp3 inflammasome-mediated inflammation in acute pancreatitis. Int J Mol Sci (2020) 21(15):5386. doi: 10.3390/ijms21155386
219. Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, et al. Nlrp3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology (2020) 158(1):253–69 e14. doi: 10.1053/j.gastro.2019.09.040
220. Sztefko K, Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology (2001) 1(3):230–6. doi: 10.1159/000055816
221. Domschke S, Malfertheiner P, Uhl W, Buchler M, Domschke W. Free fatty acids in serum of patients with acute necrotizing or edematous pancreatitis. Int J Pancreatol (1993) 13(2):105–10. doi: 10.1007/BF02786078
222. Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med (2011) 3(107):107ra10. doi: 10.1126/scitranslmed.3002573
223. de Oliveira C, Khatua B, Noel P, Kostenko S, Bag A, Balakrishnan B, et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Invest (2020) 130(4):1931–47. doi: 10.1172/JCI132767
224. Martinez J, Palazon JM, Munoz C, Lopez M, Sanchez-Paya J, Laveda R, et al. Endotoxin and anti-endotoxin antibodies in the prognosis of acute pancreatitis. Rev Esp Enferm Dig (2002) 94(7):406–16.
225. Gordon AC, Angus DC, Derde LPG. Interleukin-6 receptor antagonists in critically ill patients with covid-19. Reply. N Engl J Med (2021) 385(12):1147–9. doi: 10.1056/NEJMc2108482
226. Shen X, Sun J, Ke L, Zou L, Li B, Tong Z, et al. Reduced lymphocyte count as an early marker for predicting infected pancreatic necrosis. BMC Gastroenterol (2015) 15:147. doi: 10.1186/s12876-015-0375-2
227. Rubio I, Osuchowski MF, Shankar-Hari M, Skirecki T, Winkler MS, Lachmann G, et al. Current gaps in sepsis immunology: New opportunities for translational research. Lancet Infect Dis (2019) 19(12):e422–e36. doi: 10.1016/S1473-3099(19)30567-5
228. Quadrini KJ, Patti-Diaz L, Maghsoudlou J, Cuomo J, Hedrick MN, McCloskey TW. A flow cytometric assay for hla-Dr expression on monocytes validated as a biomarker for enrollment in sepsis clinical trials. Cytomet B Clin Cytom (2021) 100(1):103–14. doi: 10.1002/cyto.b.21987
229. Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care (2011) 15(2):R99. doi: 10.1186/cc10112
230. Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, et al. Immune checkpoint inhibition in sepsis: A phase 1b randomized, placebo-controlled, single ascending dose study of antiprogrammed cell death-ligand 1 antibody (Bms-936559). Crit Care Med (2019) 47(5):632–42. doi: 10.1097/CCM.0000000000003685
231. James TW, Crockett SD. Management of acute pancreatitis in the first 72 hours. Curr Opin Gastroenterol (2018) 34(5):330–5. doi: 10.1097/MOG.0000000000000456
232. Sutton R. Randomised treatment of acute pancreatitis with infliximab: Double-blind multi-centre trial (Rapid-I). In: National library of medicine (US). (Bethesda (MD): National Library of Medicine (US)) Available at: https://clinicaltrials.gov/ct2/show/NCT03684278.
233. Wang ZF, Liu C, Lu Y, Dong R, Xu J, Yu L, et al. Dexamethasone and dextran 40 treatment of 32 patients with severe acute pancreatitis. World J Gastroenterol (2004) 10(9):1333–6. doi: 10.3748/wjg.v10.i9.1333
234. Wan MH, Li J, Gong HL, Xue P, Zhu L, Chen GY, et al. Clinical observation on the effect of dexamethasone and Chinese herbal decoction for purgation in severe acute pancreatitis patients. Chin J Integr Med (2011) 17(2):141–5. doi: 10.1007/s11655-011-0630-5
235. Wang M, Jiang Z, Liang H. Glucocorticoids in acute pancreatitis: A propensity score matching analysis. BMC Gastroenterol (2021) 21(1):331. doi: 10.1186/s12876-021-01907-1
236. Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, et al. Lactated ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol (2011) 9(8):710–7 e1. doi: 10.1016/j.cgh.2011.04.026
237. Choosakul S, Harinwan K, Chirapongsathorn S, Opuchar K, Sanpajit T, Piyanirun W, et al. Comparison of normal saline versus lactated ringer’s solution for fluid resuscitation in patients with mild acute pancreatitis, a randomized controlled trial. Pancreatology (2018) 18(5):507–12. doi: 10.1016/j.pan.2018.04.016
238. de-Madaria E, Herrera-Marante I, Gonzalez-Camacho V, Bonjoch L, Quesada-Vazquez N, Almenta-Saavedra I, et al. Fluid resuscitation with lactated ringer’s solution vs normal saline in acute pancreatitis: A triple-blind, randomized, controlled trial. Unite Eur Gastroenterol J (2018) 6(1):63–72. doi: 10.1177/2050640617707864
239. Lee A, Ko C, Buitrago C, Hiramoto B, Hilson L, Buxbaum J, et al. Lactated ringers vs normal saline resuscitation for mild acute pancreatitis: A randomized trial. Gastroenterology (2021) 160(3):955–7 e4. doi: 10.1053/j.gastro.2020.10.044
240. Investigators P, Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, et al. Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N Engl J Med (2017) 376(23):2223–34. doi: 10.1056/NEJMoa1701380
241. de-Madaria E, Buxbaum JL, Maisonneuve P, Garcia Garcia de Paredes A, Zapater P, Guilabert L, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med (2022) 387(11):989–1000. doi: 10.1056/NEJMoa2202884
242. Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, et al. Hydroxyethyl starch 130/0.42 versus ringer’s acetate in severe sepsis. N Engl J Med (2012) 367(2):124–34. doi: 10.1056/NEJMoa1204242
243. Al-Leswas D, Eltweri AM, Chung WY, Arshad A, Stephenson JA, Al-Taan O, et al. Intravenous omega-3 fatty acids are associated with better clinical outcome and less inflammation in patients with predicted severe acute pancreatitis: A randomised double blind controlled trial. Clin Nutr (2020) 39(9):2711–19. doi: 10.1016/j.clnu.2018.04.003
244. Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet (2008) 371(9613):651–9. doi: 10.1016/S0140-6736(08)60207-X
245. Leppaniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, et al. 2019 Wses guidelines for the management of severe acute pancreatitis. World J Emerg Surg (2019) 14:27. doi: 10.1186/s13017-019-0247-0
246. Ke L, Zhou J, Mao W, Chen T, Zhu Y, Pan X, et al. Immune enhancement in patients with predicted severe acute necrotising pancreatitis: A multicentre double-blind randomised controlled trial. Intensive Care Med (2022) 48(7):899–909. doi: 10.1007/s00134-022-06745-7
247. Yin J, Mao W, Xiao X, Yu X, Li B, Chen F, et al. Immune dysfunction is associated with readmission in survivors of sepsis following infected pancreatic necrosis. J Inflammation Res (2021) 14:5433–42. doi: 10.2147/JIR.S321507
Keywords: acute pancreatitis (AP), immunopathology, human, immune dysregulation, scoping review, immunodeficiency
Citation: Venkatesh K, Glenn H, Delaney A, Andersen CR and Sasson SC (2023) Fire in the belly: A scoping review of the immunopathological mechanisms of acute pancreatitis. Front. Immunol. 13:1077414. doi: 10.3389/fimmu.2022.1077414
Received: 22 October 2022; Accepted: 21 December 2022;
Published: 11 January 2023.
Edited by:
Vijay Kumar, Louisiana State University, United StatesReviewed by:
Jordan Robin Yaron, Arizona State University, United StatesLu Ke, Nanjing University, China
Copyright © 2023 Venkatesh, Glenn, Delaney, Andersen and Sasson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karthik Venkatesh, RHIua2FydGhpay52ZW5rYXRlc2hAZ21haWwuY29t
†These authors contributed equally to this work and share first authorship
‡These authors contributed equally to this work and share last authorship
 Karthik Venkatesh
Karthik Venkatesh Hannah Glenn
Hannah Glenn Anthony Delaney1,3
Anthony Delaney1,3 Christopher R. Andersen
Christopher R. Andersen Sarah C. Sasson
Sarah C. Sasson