- Department of Nephrology, Cangzhou Central Hospital, Cangzhou, China
Objective: This study aimed to compare the efficacy and safety (infection events) between rituximab (RTX), tacrolimus (TAC), mycophenolate mofetil (MMF), and cyclophosphamide (CYC) as induction therapies in lupus nephritis (LN).
Methods: Electronic databases, including PubMed, EMBASE, and the Cochrane Library, were searched from inception up to December 9, 2021. Bayesian network meta-analysis was used to combine the direct and indirect evidence of different drugs for LN patients. The pooled relative effects were shown using odds ratios (ORs) and 95% credible intervals (CrIs).
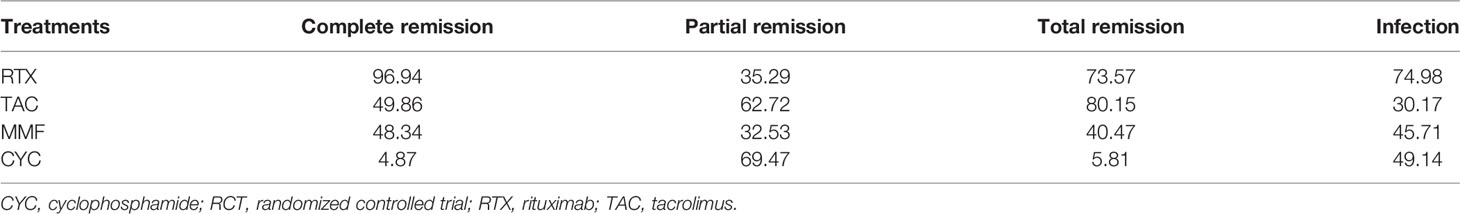
Results: Nineteen studies (1,566 patients) met the inclusion criteria and were selected in the present study. The network meta-analysis reported that no statistically significant differences were found in partial remission (PR) and infection among the four drugs. RTX showed a significantly higher complete remission (CR) than MMF (OR = 2.60, 95% CrI = 1.00–7.10) and seemed to be more effective than CYC (OR = 4.20, 95% CrI = 1.70–14.00). MMF had a better CR than CYC (OR = 1.60, 95% CrI = 1.00–3.20). TAC presented a better overall response than CYC (OR = 3.70, 95% CrI = 1.20–12.00). Regarding CR and overall response, the maximum surface under the cumulative ranking curve (SUCRA) values were 96.94% for RTX and 80.15% for TAC. The maximum SUCRA value of infection reaction was 74.98% for RTX and the minimum value was 30.17% for TAC, respectively.
Conclusions: RTX and TAC were the most effective drugs for induction remission in LN. Among the four drugs, TAC had the lowest probability of infection, and RTX showed the highest probability of experiencing an infection. This meta-analysis could not conclude about other adverse events.
Introduction
Lupus nephritis (LN) is one of the most common clinical manifestations and serious complications of systemic lupus erythematosus (SLE) and is a leading cause of morbidity and mortality in SLE (1–3). About 40% of patients with lupus develop LN (2). The management of LN comprises two phases. The induction phase aims to induce remission. The maintenance phase aims to prevent relapse and progression to end-stage renal disease. Glucocorticoids are the first treatment and improve the renal outcomes of LN. Then, they are followed by cyclophosphamide (CYC), mycophenolate mofetil (MMF), and tacrolimus (TAC) (4, 5). CYC regimens have long been considered the gold standard for inducing renal remission and preventing relapse (4). Increasing evidence shows that MMF and TAC are at least equivalent to CYC for induction and maintenance treatment of severe LN (6–8). Nevertheless, the adverse effects of these drugs can be considerable and limiting. For example, the benefits of CYC are outweighed by treatment-related adverse effects, including gonadal toxicity (9). Although the renal remission rate with CYC or MMF regimens is up to 50%–80% in LN patients, many of these responses are partial (10). Therefore, it is urgent to search for better treatments that have better efficacy and fewer side effects (including sparing fertility) to manage LN.
Rituximab (RTX) is a chimeric monoclonal antibody that targets CD20. RTX offers an alternative or adjunctive option for SLE patients (11–13). Recently, RTX has been introduced as an induction drug for LN, and studies suggested that RTX seemed to be at least as effective as MMF and CYC regimens in inducing remission (14, 15). Still, few studies were conducted to compare the efficacy and safety of RTX with common therapeutic drugs, especially TAC. Therefore, it is important for medical decisions to assess the relative value between intervention and comparators. Network meta-analysis is an extension of traditional pairwise meta-analysis and simultaneously combines direct and indirect information about the relative efficacy of each treatment (16). Thus, using a network meta-analysis, the present systematic study aimed to compare the relative efficacy and safety (infection events) of RTX, TAC, and MMF as LN induction therapy.
Methods
Search Strategy
The present meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) network meta-analysis extension statement (17, 18). Electronic databases, including PubMed, EMBASE, and the Cochrane Library, were systematically searched from inception to December 9, 2021. The search terms consisted of “lupus nephritis”, “LN”, “systemic lupus erythematosus”, “SLE”, “rituximab”, “RTX”, “mycophenolate mofetil”, “MMF”, “tacrolimus” and “TAC”. The reference lists from potential studies were also manually searched.
Inclusion and Exclusion Criteria
According to the Population, Intervention, Comparison, Outcomes, and Study designs (PICOS) structure, the inclusion criteria were 1) patients with LN (population); 2) studies that examined the efficacy or safety of RTX, TAC, MMF, or CYC (intervention and comparator); 3) available data, effectiveness, or adverse effects (outcome); and 4) observational study or randomized controlled trials (RCTs) (study design). The present study excluded all letters, comments, case reports, animal models, and reviews.
Data Extraction and Quality Assessment
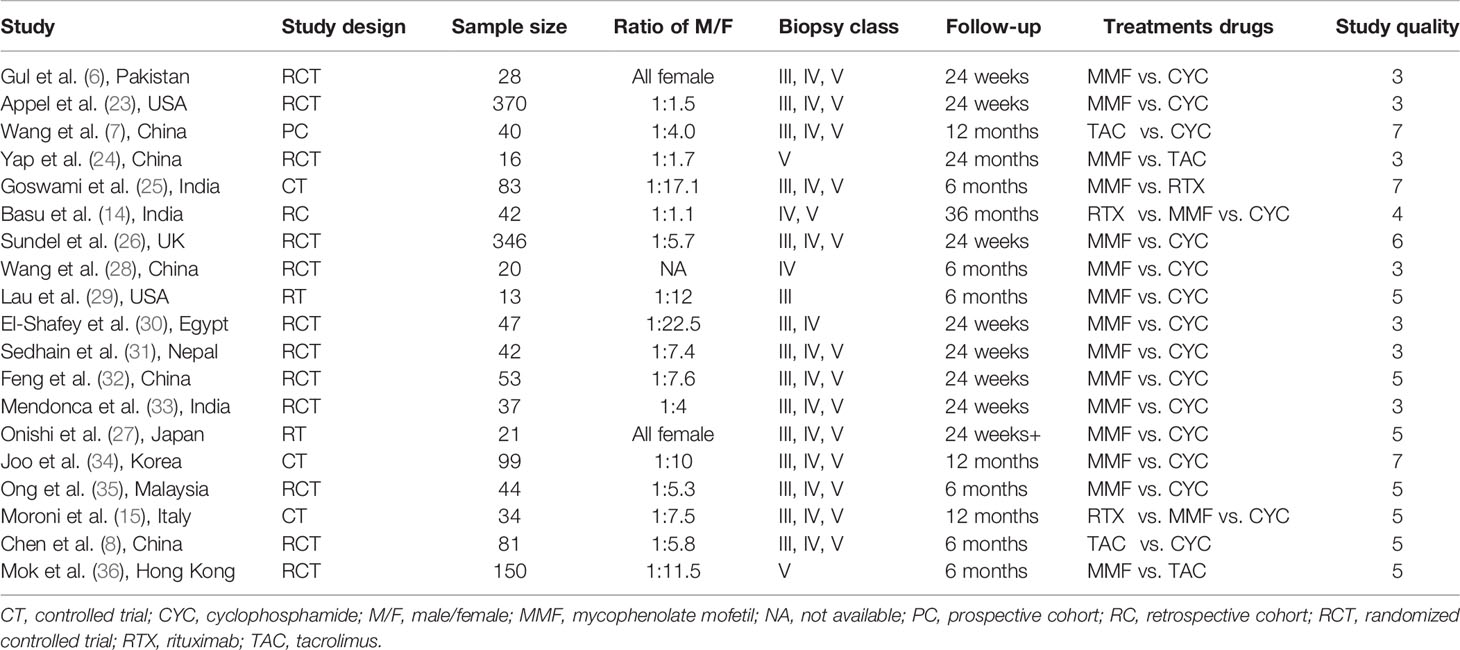
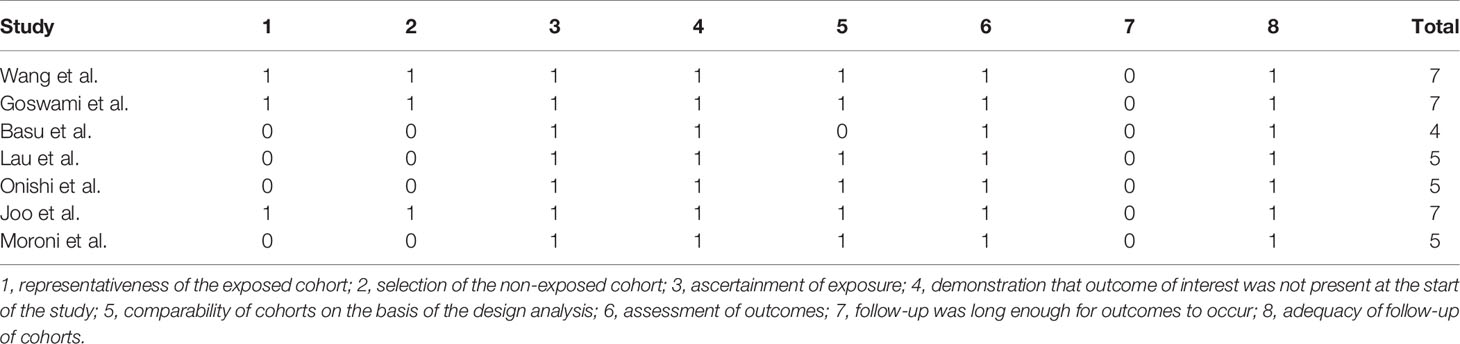
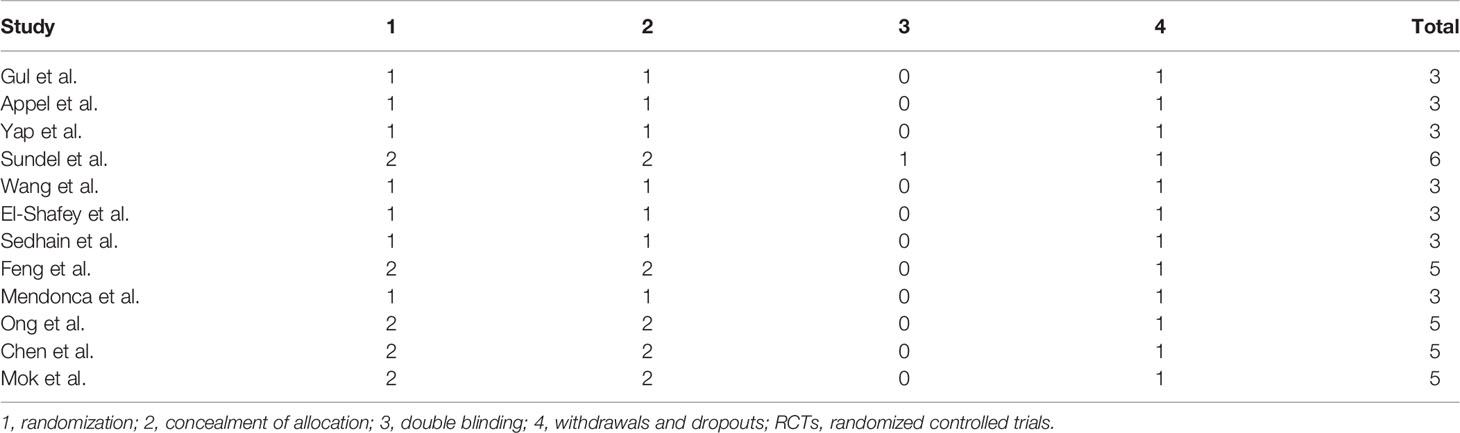
Two authors independently collected the data, including the first author’s surname, the year of publication, country, study design, sample size, the ratio of male/female, kidney biopsy class, duration of follow-up, and treatment drugs (intervention and comparators). In case of disagreement, group discussion was used to resolve any discrepancy. The efficacy indicators were the number of patients who achieved complete remission (CR) and partial remission (PR). CR and PR definitions were based on the remission criteria used in each trial. The overall response was determined as the number of cases with CR+PR. The safety indicator was the number of patients that suffered from infection, including upper respiratory infection, sepsis, or pneumonia. The Newcastle-Ottawa Scale (NOS) (19) was adopted to assess the methodological quality of observational studies in our meta-analysis. The score ranged from 0 to 9, with a total score of ≥7 indicating high quality and <7 indicating low quality. The Jadad score (20) was used to evaluate the quality of RCTs; it ranges from 0 to 7 stars, with a total score ≥4 as high quality and <4 as low quality.
Statistical Analysis
Stata (Version 14.0) and R (Version 4.1.1) were used in the present study. In network analysis, the Bayesian random-effects model was performed to combine both direct and indirect information for therapeutic drugs of LN, combining the information efficacy and safety indicators from different studies. The Bayesian Markov chain Monte Carlo method (21) was used to compute Bayesian consistency models using four chains with over-dispersed initial values with Gibbs sampling based on 20,000 iterations after a burn-in phase of 5,000 iterations. The pooling effect sizes were shown along with odds ratios (ORs) and 95% credible intervals (CrIs). The efficacy and safety of each treatment were compared using the surface under the cumulative ranking curve (SUCRA) (22), and the optimal treatment regimen was determined. SUCRA would be 1 when a treatment is certain to be the best and 0 when a treatment is certain to be the worst. Inconsistency refers to the extent of disagreement between direct and indirect evidence. Inconsistency assessment is an essential part of a network meta-analysis. The extent of disagreement between direct and indirect comparison results in NMA was evaluated using the node splitting analysis. If p > 0.05, there was consistency between the direct and indirect comparison results. Otherwise, it was inconsistent. Publication bias was assessed using funnel plots.
Results
Included Study Characteristics
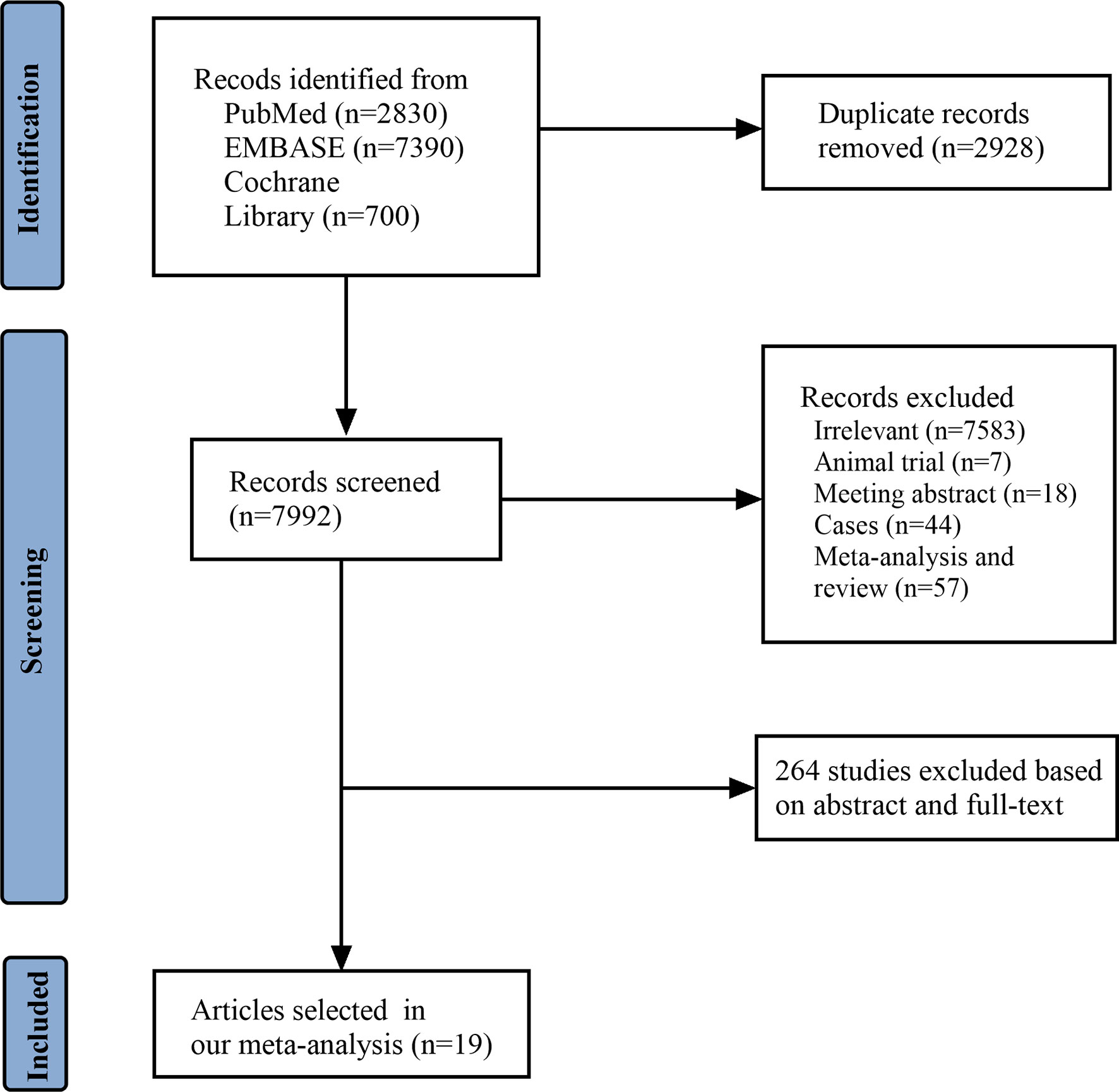
A total of 10,920 articles were initially selected. Among them, 19 studies met inclusion. Therefore, 19 articles (6–8, 14, 23–36) involving 1,566 LN patients were included in the present study. Among the 19 studies, 14 were from Asian countries (Pakistan, China, India, Nepal, Japan, Korea, and Malaysia), one was from Egypt, and three were from Western countries (UK, USA, and Italy). Two studies were three-arm studies, and the others were two-arm studies. Table 1 reports the basic characteristics of the included studies. Among them, 12 studies were RCTs, and seven were observational studies. Only eight studies were considered high-quality studies according to the NOS or Jadad score (Tables 2, 3). Figure 1 illustrates the stages in selecting studies for inclusion in the study. Figure 2 presents the network diagram of evidence for treatment efficacy and safety of each regimen.
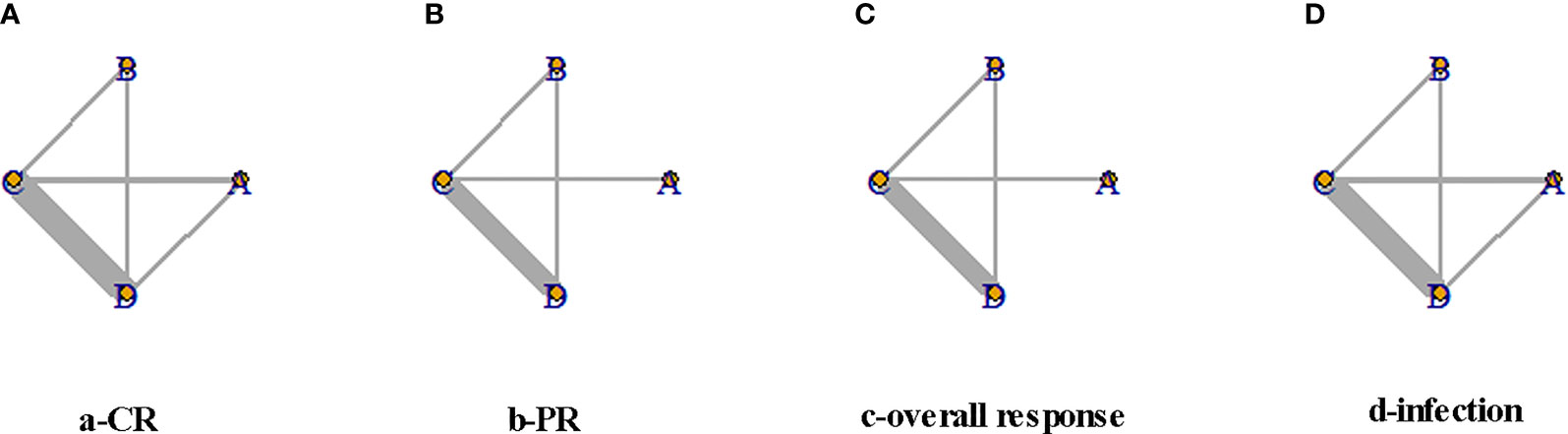
Figure 2 Network diagram for complete remission (CR), partial remission (PR), overall response, and infection events. The size of each node is proportional to the sample size of the individual treatment regimen; the widths of the connecting lines are proportional to the number of studies compared between the two regimens. (A) Rituximab (RTX). (B) Tacrolimus (TAC). (C) Mycophenolate mofetil (MMF). (D) Cyclophosphamide (CYC). CR, complete remission; PR, partial remission.
Analysis of Inconsistency and Detection of Publication Bias
The node splitting analysis was used to evaluate network inconsistency between direct and indirect comparison results and suggested no inconsistency (p > 0.05) (Supplementary Figures 1–4). The publication bias was assessed using a funnel plot. In this analysis, the funnel plot was asymmetric, suggesting the presence of publication bias in the direct comparison meta-analysis (Supplementary Figures 5–8).
Comparison of Efficacy of Treatment Regimen
In the present study, the numbers of CR, PR, and overall responses were considered as efficacy outcomes. In terms of CR, RTX showed a significantly higher CR than MMF (OR = 2.60, 95% CrI = 1.00–7.10) and seemed to be more effective than CYC (OR = 4.20, 95% CrI = 1.70–14.00) (Figure 3A). Similarly, MMF had a better CR than CYC (OR = 1.60, 95% CrI = 1.00–3.20) (Figure 3C). Regarding overall response, TAC presented a better overall response than CYC (OR = 3.70, 95% CrI = 1.20–12.00). As for PR, no significant differences were found among the four drugs (Figure 3B).
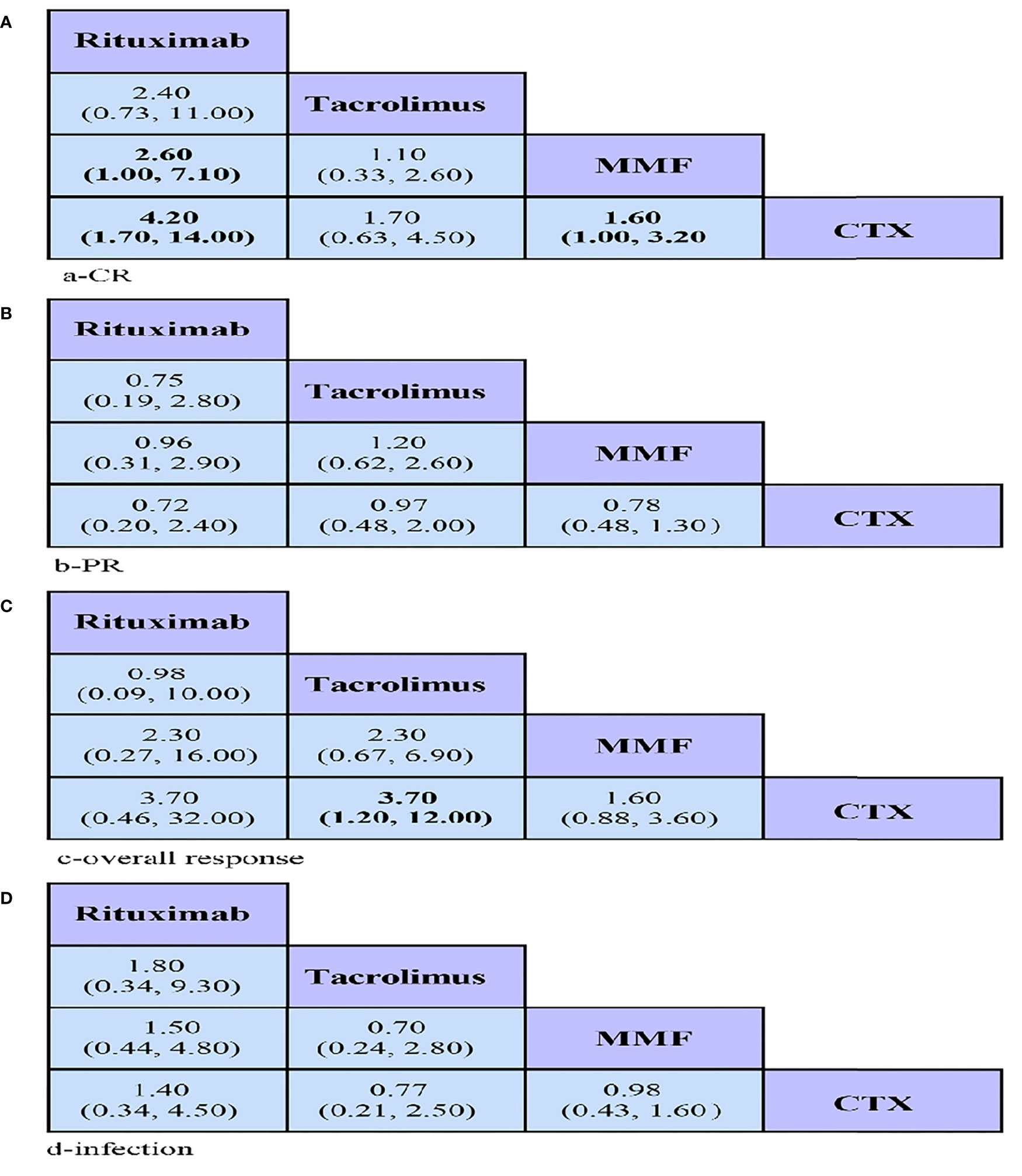
Figure 3 League tables showing the results of comparing the efficacy and safety of all drugs, including odds ratios (OR) and 95% credible intervals in the network meta-analyses. (A–C) Efficacy: OR > 1 means the drug in the top left is better. (D) Safety: OR < 1 means the treatment in the top left is better. CR, complete remission; PR, partial remission; MMF, mycophenolate mofetil; CYC, cyclophosphamide.
Comparison of Safety of Treatment Regimen
Comparison of infection suggested no statistically significant differences among any of the groups (Figure 3D).
Result Sorting
Table 4 presents the SUCRA values (%) for treatment efficacy and safety of each regimen. Regarding CR, the maximum SUCRA value of RTX was 96.94%, suggesting that RTX was likely to achieve the highest CR among these four treatment drugs. In terms of overall response, the maximum SUCRA value of TAC was 80.15%, meaning that TAC had the highest overall response among these four drugs. Besides, CYC was likely to achieve the highest PR among drugs, as it presented a higher probability of PR (SUCRA = 69.47%). As for safety, ranking probability based on SUCRA suggested that TAC was the safest treatment, as its minimum SUCRA value was 30.17%.
Discussion
It is important for induction of remission to achieve the best long-term outcomes among patients with LN. The present network meta-analysis combined and compared available evidence of the effectiveness and safety (infection events) of RTX, TAC, MMF, and CYC based on the number of patients who achieved CR, PR, and overall response and who suffered from infection events. Network meta-analysis can simultaneously combine direct and indirect information of the relative efficacy and safety of each treatment to determine the optimal treatment regimen even if there are no or insufficient head-to-head comparisons (37, 38).
In the present study, RTX was the most effective drug for inducing CR among LN patients, followed by TAC and MMF. CYC was the most successful medicine for inducing PR among patients with LN, followed by TAC and RTX. In terms of overall response, TAC was the most effective treatment drug for achieving overall response among LN patients, followed by RTX and MMF. As for safety, TAC was the safest treatment with the lowest likelihood of infection events. However, RTX showed the highest probability of experiencing infection without considering other adverse events.
In summary, RTX and TAC were the most effective drugs for inducing remission among LN patients, and TAC had the lowest probability of infection compared with the other drugs. These results were consistent with a previous network meta-analysis that compared the efficacy and safety of TAC, MMF, and CYC regimens (39). Lee and Song found that TAC was the most effective induction treatment for patients with LN and had the highest probability of decreasing the risk of serious infections (39). In addition, our results were in accordance with another meta-analysis in which the direct comparison reported that TAC was more effective and safer than CYC (40). A review showed that LN patients would benefit from RTX (41). A prior study found that RTX presented a satisfying efficacy and safety for SLE patients (42). Besides, RTX therapy has been used in vasculitis and rheumatoid arthritis. Although RTX is widely used in patients with LN, RTX as an induction treatment is less established than MMF and CYC. A possible reason is the lack of large-scale placebo-controlled clinical trials (41). In addition, the evidence for RTX use remains varied in terms of ethnicity. For example, an RCT comparing the efficacy of RTX with placebo among patients with extrarenal diseases found that there was no significant difference in disease response rate between the two groups (43). However, the RTX treatment group in a subgroup analysis showed a significantly higher renal response rate among Hispanic and African-American patients. In the present study, RTX showed the highest risk of experiencing infection adverse events. A study found that infection, infusion reaction, and neutropenia were the most common adverse events of RTX (44). Furthermore, with respect to infection, evidence reported that a dose-dependent relationship was found between the RTX dose and the frequency of infection (41). Besides, RTX, as induction therapy for LN, is mainly used in the Americas (USA, Mexico, etc.), Europe (the United Kingdom, Italy, Sweden, Spain, Greece, Netherlands, etc.), Asia (Japan, China, Singapore, etc.), and Australia. TAC is only used in Asian countries, and the use of TAC is not recommended by the European League Against Rheumatism (EULAR). The off-label use of RTX in SLE was first reported in 2002 and has been increasingly used in patients with SLE (45). Therefore, off-label use should be carefully considered.
Some limitations might be considered in this network meta-analysis. First, in addition to RCTs, observational studies were included, a problem particularly found in RTX since there were no RCTs of RTX. Besides, some RCTs included in our study had small sizes. Hence, more RCTs with a large sample size should be conducted to compare RTX, TAC, MMF, and CYC, especially head-to-head trials. Second, the data and the methods of reporting data in the primary trials limited the results in our study due to different definitions in each study. For instance, the CR and PR results were heterogeneous owing to variable definitions in existing studies. Still, our results found a low heterogeneity in the pooled analysis, which suggested that our network meta-analysis was appropriate. Furthermore, heterogeneity in patients’ characteristics of eligible studies in our meta-analysis affected the results of our study. For example, Mohan et al. reported that the response to treatment drugs for LN patients varied with ethnicity (46). In addition, Merrill et al. found that the RTX treatment group showed a significantly higher renal response rate between Hispanic and African-American patients (43). Besides, prior evidence found that diet and exercise influenced renal function (47, 48). However, these factors were not adjusted in the current meta-analysis owing to insufficient data in our study, which might have a potential effect on our results. Third, for some drugs, the data were limited in some direct comparisons. For example, in our study, direct comparison between RTX and TAC was insufficient, so indirect comparison among the two groups was limited. Fourth, with respect to adverse events, only infection events were considered in the present study. However, other adverse events, including gastrointestinal reaction, myelosuppression, liver damage, alopecia, leukopenia, and menstrual disorders, were not considered owing to insufficient data. Therefore, our results should be interpreted with caution.
Although these limitations have a potential impact on our results, our study had several strengths. First, the present study used a comprehensive literature search without date and language restriction. Second, though the sample sizes from individual trials ranged from 13 to 346, the pooled analysis in our study involved 1,566 LN patients. Finally, our study used a standardized analysis method to evaluate the confidence that might be held in our results. Network meta-analysis simultaneously combined direct and indirect information of the relative efficacy of each treatment even if there were a lack of direct head-to-head comparisons.
Conclusion
In conclusion, our network meta-analysis combined and compared available evidence of the effectiveness and safety of RTX, TAC, MMF, and CYC based on the number of patients who achieved CR, PR, and overall response and those who suffered from infection events. RTX and TAC were the most effective drugs for induction remission among LN patients. TAC had the lowest probability of infection compared with other drugs. RTX showed the highest probability of experiencing an infection. This meta-analysis could not conclude about other adverse events.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
KL and JG carried out the studies, participated in collecting data, and drafted the manuscript. KL and YG performed the statistical analysis and participated in its design. KL, FZ, and ZL participated in the acquisition, analysis, or interpretation of data and drafted the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.859380/full#supplementary-material
Supplementary Figure 1 | Network node-splitting analysis for complete remission. (A) Rituximab (RTX); (B) Tacrolimus (TAC); (C) Mycophenolate mofetil (MMF); (D) Cyclophosphamide (CYC); 95%CrI, 95% credibility interval.
Supplementary Figure 2 | Network node-splitting analysis for partial remission. (B) Tacrolimus (TAC); (C) Mycophenolate mofetil (MMF); (D) Cyclophosphamide (CYC); 95%CrI, 95% credibility interval.
Supplementary Figure 3 | Network node-splitting analysis for overall response. (B) Tacrolimus (TAC); (C) Mycophenolate mofetil (MMF); (D) Cyclophosphamide (CYC); 95%CrI, 95% credibility interval.
Supplementary Figure 4 | Network node-splitting analysis for infection. (A) Rituximab (RTX); (B) Tacrolimus (TAC); (C) Mycophenolate mofetil (MMF); (D) Cyclophosphamide (CYC); 95%CrI, 95% credibility interval.
Supplementary Figure 5 | Funnel plot for complete remission. RTX, Rituximab; TAC, Tacrolimus; MMF, Mycophenolate mofetil; CYC, Cyclophosphamide.
Supplementary Figure 6 | Funnel plot for partial remission. RTX, Rituximab; TAC, Tacrolimus; MMF, Mycophenolate mofetil; CYC, Cyclophosphamide.
Supplementary Figure 7 | Funnel plot for overall response. RTX, Rituximab; TAC, Tacrolimus; MMF, Mycophenolate mofetil; CYC, Cyclophosphamide.
Supplementary Figure 8 | Funnel plot for infection. RTX, Rituximab; TAC, Tacrolimus; MMF, Mycophenolate mofetil; CYC, Cyclophosphamide.
References
1. Almaani S, Meara A, Rovin BH. Update on Lupus Nephritis. Clin J Am Soc Nephrol (2017) 12(5):825–35. doi: 10.2215/CJN.05780616
2. Morales E, Galindo M, Trujillo H, Praga M. Update on Lupus Nephritis: Looking for a New Vision. Nephron (2021) 145(1):1–13. doi: 10.1159/000511268
3. Parikh SV, Almaani S, Brodsky S, Rovin BH. Update on Lupus Nephritis: Core Curriculum 2020. Am J Kidney Dis (2020) 76(2):265–81. doi: 10.1053/j.ajkd.2019.10.017
4. Appel GB. New and Future Therapies for Lupus Nephritis. Cleve Clin J Med (2012) 79(2):134–40. doi: 10.3949/ccjm.78gr.11004
5. Hannah J, Casian A, D’Cruz D. Tacrolimus Use in Lupus Nephritis: A Systematic Review and Meta-Analysis. Autoimmun Rev (2016) 15(1):93–101. doi: 10.1016/j.autrev.2015.09.006
6. Gul H, Mushtaq MS, Salim B, Samreen S, Nasim A, Khan M. A Comparison Of Mycophenolate Mofetil And Cyclophosphamide As Lupus Nephritis Induction Therapy. J Ayub Med Coll Abbottabad (2020) 32(4):454–8.
7. Wang S, Li X, Qu L, Wang R, Chen Y, Li Q, et al. Tacrolimus Versus Cyclophosphamide as Treatment for Diffuse Proliferative or Membranous Lupus Nephritis: A Non-Randomized Prospective Cohort Study. Lupus (2012) 21(9):1025–35. doi: 10.1177/0961203312448105
8. Chen W, Tang X, Liu Q, Chen W, Fu P, Liu F, et al. Short-Term Outcomes of Induction Therapy With Tacrolimus Versus Cyclophosphamide for Active Lupus Nephritis: A Multicenter Randomized Clinical Trial. Am J Kidney Dis (2011) 57(2):235–44. doi: 10.1053/j.ajkd.2010.08.036
9. Petri M. Cyclophosphamide: New Approaches for Systemic Lupus Erythematosus. Lupus (2004) 13(5):366–71. doi: 10.1191/0961203303lu1028oa
10. Contis A, Vanquaethem H, Truchetet ME, Couzi L, Rigothier C, Richez C, et al. Analysis of the Effectiveness and Safety of Rituximab in Patients With Refractory Lupus Nephritis: A Chart Review. Clin Rheumatol (2016) 35(2):517–22. doi: 10.1007/s10067-015-3166-9
11. Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, et al. B Cell Depletion as a Novel Treatment for Systemic Lupus Erythematosus: A Phase I/II Dose-Escalation Trial of Rituximab. Arthritis Rheum (2004) 50(8):2580–9. doi: 10.1002/art.20430
12. Looney RJ, Anolik J, Sanz I. B Cells as Therapeutic Targets for Rheumatic Diseases. Curr Opin Rheumatol (2004) 16(3):180–5. doi: 10.1097/00002281-200405000-00003
13. Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA. An Open Study of B Lymphocyte Depletion in Systemic Lupus Erythematosus. Arthritis Rheum (2002) 46(10):2673–7. doi: 10.1002/art.10541
14. Basu B, Roy B, Babu BG. Efficacy and Safety of Rituximab in Comparison With Common Induction Therapies in Pediatric Active Lupus Nephritis. Pediatr Nephrol (2017) 32(6):1013–21. doi: 10.1007/s00467-017-3583-x
15. Moroni G, Raffiotta F, Trezzi B, Giglio E, Mezzina N, Del Papa N, et al. Rituximab vs. Mycophenolate and vs. Cyclophosphamide Pulses for Induction Therapy of Active Lupus Nephritis: A Clinical Observational Study. Rheumatol (Oxford) (2014) 53(9):1570–7. doi: 10.1093/rheumatology/ket462
16. Lu G, Ades AE. Combination of Direct and Indirect Evidence in Mixed Treatment Comparisons. Stat Med (2004) 23(20):3105–24. doi: 10.1002/sim.1875
17. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann Intern Med (2015) 162(11):777–84. doi: 10.7326/M14-2385
18. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
19. Wells G. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. In: Symposium on Systematic Reviews: Beyond the Basics: 2014. London: University of Oxford (2014).
20. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin Trials (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
21. Mavridis D, Salanti G. A Practical Introduction to Multivariate Meta-Analysis. Stat Methods Med Res (2013) 22(2):133–58. doi: 10.1177/0962280211432219
22. Salanti G, Ades AE, Ioannidis JP. Graphical Methods and Numerical Summaries for Presenting Results From Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J Clin Epidemiol (2011) 64(2):163–71. doi: 10.1016/j.jclinepi.2010.03.016
23. Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Et Al: Mycophenolate Mofetil Versus Cyclophosphamide for Induction Treatment of Lupus Nephritis. J Am Soc Nephrol (2009) 20(5):1103–12. doi: 10.1681/ASN.2008101028
24. Yap DY, Yu X, Chen XM, Lu F, Chen N, Li XW, et al. Pilot 24 Month Study to Compare Mycophenolate Mofetil and Tacrolimus in the Treatment of Membranous Lupus Nephritis With Nephrotic Syndrome. Nephrol (Carlton) (2012) 17(4):352–7. doi: 10.1111/j.1440-1797.2012.01574.x
25. Goswami RP, Sircar G, Sit H, Ghosh A, Ghosh P. Cyclophosphamide Versus Mycophenolate Versus Rituximab in Lupus Nephritis Remission Induction: A Historical Head-To-Head Comparative Study. J Clin Rheumatol (2019) 25(1):28–35. doi: 10.1097/RHU.0000000000000760
26. Sundel R, Solomons N, Lisk L. Efficacy of Mycophenolate Mofetil in Adolescent Patients With Lupus Nephritis: Evidence From a Two-Phase, Prospective Randomized Trial. Lupus (2012) 21(13):1433–43. doi: 10.1177/0961203312458466
27. Onishi A, Sugiyama D, Tsuji G, Nakazawa T, Kogata Y, Tsuda K, et al. Mycophenolate Mofetil Versus Intravenous Cyclophosphamide for Induction Treatment of Proliferative Lupus Nephritis in a Japanese Population: A Retrospective Study. Mod Rheumatol (2013) 23(1):89–96. doi: 10.3109/s10165-012-0634-9
28. Wang J, Hu W, Xie H, Zhang H, Chen H, Zeng C, et al. Induction Therapies for Class IV Lupus Nephritis With Non-Inflammatory Necrotizing Vasculopathy: Mycophenolate Mofetil or Intravenous Cyclophosphamide. Lupus (2007) 16(9):707–12. doi: 10.1177/0961203307081340
29. Lau KK, Ault BH, Jones DP, Butani L. Induction Therapy for Pediatric Focal Proliferative Lupus Nephritis: Cyclophosphamide Versus Mycophenolate Mofetil. J Pediatr Health Care (2008) 22(5):282–8. doi: 10.1016/j.pedhc.2007.07.006
30. El-Shafey EM, Abdou SH, Shareef MM. Is Mycophenolate Mofetil Superior to Pulse Intravenous Cyclophosphamide for Induction Therapy of Proliferative Lupus Nephritis in Egyptian Patients? Clin Exp Nephrol (2010) 14(3):214–21. doi: 10.1007/s10157-010-0270-7
31. Sedhain A, Hada R, Agrawal RK, Bhattarai GR, Baral A. Low Dose Mycophenolate Mofetil Versus Cyclophosphamide in the Induction Therapy of Lupus Nephritis in Nepalese Population: A Randomized Control Trial. BMC Nephrol (2018) 19(1):175. doi: 10.1186/s12882-018-0973-7
32. Feng X, Gu F, Chen W, Liu Y, Wei H, Liu L, et al. Mizoribine Versus Mycophenolate Mofetil or Intravenous Cyclophosphamide for Induction Treatment of Active Lupus Nephritis. Chin Med J (Engl) (2014) 127(21):3718–23.
33. Mendonca S, Gupta D, Ali S, Gupta P. Mycophenolate mofetil or Cyclophosphamide in Indian Patients With Lupus Nephritis: Which Is Better? A Single-Center Experience. Saudi J Kidney Dis Transpl (2017) 28(5):1069–77.
34. Joo YB, Kang YM, Kim HA, Suh CH, Kim TJ, Park YW, et al. Outcome and Predictors of Renal Survival in Patients With Lupus Nephritis: Comparison Between Cyclophosphamide and Mycophenolate Mofetil. Int J Rheum Dis (2018) 21(5):1031–9. doi: 10.1111/1756-185X.13274
35. Ong LM, Hooi LS, Lim TO, Goh BL, Ahmad G, Ghazalli R, et al. Randomized Controlled Trial of Pulse Intravenous Cyclophosphamide Versus Mycophenolate Mofetil in the Induction Therapy of Proliferative Lupus Nephritis. Nephrology (Carlton) (2005) 10(5):504–10. doi: 10.1111/j.1440-1797.2005.00444.x
36. Mok CC, Ying KY, Yim CW, Siu YP, Tong KH, To CH, et al. Tacrolimus Versus Mycophenolate Mofetil for Induction Therapy of Lupus Nephritis: A Randomised Controlled Trial and Long-Term Follow-Up. Ann Rheum Dis (2016) 75(1):30–6. doi: 10.1136/annrheumdis-2014-206456
37. Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B. Network Meta-Analysis for Comparing Treatment Effects of Multiple Interventions: An Introduction. Rheumatol Int (2014) 34(11):1489–96. doi: 10.1007/s00296-014-2994-2
38. Caldwell DM, Ades AE, Higgins JP. Simultaneous Comparison of Multiple Treatments: Combining Direct and Indirect Evidence. Bmj (2005) 331(7521):897–900. doi: 10.1136/bmj.331.7521.897
39. Lee YH, Song GG. Relative Efficacy and Safety of Tacrolimus, Mycophenolate Mofetil, and Cyclophosphamide as Induction Therapy for Lupus Nephritis: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Lupus (2015) 24(14):1520–8. doi: 10.1177/0961203315595131
40. Deng J, Huo D, Wu Q, Yang Z, Liao Y. A Meta-Analysis of Randomized Controlled Trials Comparing Tacrolimus With Intravenous Cyclophosphamide in the Induction Treatment for Lupus Nephritis. Tohoku J Exp Med (2012) 227(4):281–8. doi: 10.1620/tjem.227.281
41. Stolyar L, Lahita RG, Panush RS. Rituximab Use as Induction Therapy for Lupus Nephritis: A Systematic Review. Lupus (2020) 29(8):892–912. doi: 10.1177/0961203320928412
42. Wu S, Wang Y, Zhang J, Han B, Wang B, Gao W, et al. Efficacy and Safety of Rituximab for Systemic Lupus Erythematosus Treatment: A Meta-Analysis. Afr Health Sci (2020) 20(2):871–84. doi: 10.4314/ahs.v20i2.41
43. Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and Safety of Rituximab in Moderately-to-Severely Active Systemic Lupus Erythematosus: The Randomized, Double-Blind, Phase II/III Systemic Lupus Erythematosus Evaluation of Rituximab Trial. Arthritis Rheum (2010) 62(1):222–33. doi: 10.1002/art.27233
44. Kasi PM, Tawbi HA, Oddis CV, Kulkarni HS. Clinical Review: Serious Adverse Events Associated With the Use of Rituximab - A Critical Care Perspective. Crit Care (2012) 16(4):231. doi: 10.1186/cc11304
45. Ramos-Casals M, Soto MJ, Cuadrado MJ, Khamashta MA. Rituximab in Systemic Lupus Erythematosus: A Systematic Review of Off-Label Use in 188 Cases. Lupus (2009) 18(9):767–76. doi: 10.1177/0961203309106174
46. Mohan S, Radhakrishnan J. Geographical Variation in the Response of Lupus Nephritis to Mycophenolate Mofetil Induction Therapy. Clin Nephrol (2011) 75(3):233–41. doi: 10.5414/CNP75233
47. Morales E, Valero MA, León M, Hernández E, Praga M. Beneficial Effects of Weight Loss in Overweight Patients With Chronic Proteinuric Nephropathies. Am J Kidney Dis (2003) 41(2):319–27. doi: 10.1053/ajkd.2003.50039
48. Szulińska M, Skrypnik D, Ratajczak M, Karolkiewicz J, Madry E, Musialik K, et al. Effects of Endurance and Endurance-Strength Exercise on Renal Function in Abdominally Obese Women With Renal Hyperfiltration: A Prospective Randomized Trial. BioMed Environ Sci (2016) 29(10):706–12. doi: 10.3967/bes2016.095
Keywords: lupus nephritis, rituximab, tacrolimus, mycophenolate mofetil, Bayesian network meta-analysis
Citation: Li K, Yu Y, Gao Y, Zhao F, Liang Z and Gao J (2022) Comparative Effectiveness of Rituximab and Common Induction Therapies for Lupus Nephritis: A Systematic Review and Network Meta-Analysis. Front. Immunol. 13:859380. doi: 10.3389/fimmu.2022.859380
Received: 24 January 2022; Accepted: 07 March 2022;
Published: 04 April 2022.
Edited by:
Cecilia Beatrice Chighizola, University of Milan, ItalyReviewed by:
Maddalena Larosa, University Hospital of Padua, ItalyShigeru Iwata, University of Occupational and Environmental Health Japan, Japan
Copyright © 2022 Li, Yu, Gao, Zhao, Liang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Gao, Z2FvanVuamllNjY4ODAwQDE2My5jb20=
 Kang Li
Kang Li Junjie Gao
Junjie Gao