- 1Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, National Clinical Research Center for Dermatologic and Immunologic Diseases (NCRC-DID), Key Laboratory of Rheumatology and Clinical Immunology, Ministry of Education, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 2Department of Orthopedics, People’s Hospital of Ningxiang City, Ningxiang, Hunan, China
- 3Key Laboratory of Hunan Province for Integrated Traditional Chinese and Western Medicine on Prevention and Treatment of Cardio-Cerebral Diseases, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 4Department of Rheumatology, The First people’s Hospital Changde City, Changde, Hunan, China
Objective: To evaluate the randomized controlled trials (RCTs) of Curcumin and Curcuma longa Extract in the treatment of autoimmune diseases.
Methods: Databases such as Embase, Web of Science, PubMed and The Cochrane Library were searched from the database establishment to February 2022 to collect RCTs of Curcumin and Curcuma longa Extract in the treatment of autoimmune diseases. Then the literature was screened and the data were extracted. Meta-analysis was performed using RevMan 5.3 software.
Results: A total of 34 records were included, involving 31 RCTs and 10 types of autoimmune disease. Among them, ankylosing spondylitis (AS) involves one RCT, Behcet ‘s disease (BD) involves one RCT, Crohn ‘s disease involves two RCTs, multiple sclerosis (MS) involves two RCTs, oral lichen planus involves six RCTs, psoriasis involves two RCTs, rheumatoid arthritis (RA) involves five RCTs, systemic lupus erythematosus (SLE) involves two RCTs, arteritis involves one RCT, ulcerative colitis (UC) involves nine RCTs. Among them, most of the RCTs of ulcerative colitis (UC), oral lichen planus, RA showed that curcumin and curcumin extracts improved clinical or laboratory results. Crohn ‘ s disease, MS, SLE, psoriasis included two RCTs; they all showed improvements (at least one RCT reported improvements in clinical outcomes). AS, BD and arteritis included only one RCT, and the clinical results showed improvement. However, due to the small number of RCTs and the small number of patients involved in each disease, there is still a need for more high-quality RCTs.
Conclusion: Curcumin and Curcuma longa Extract had good clinical efficacy in the treatment of Psoriasis, UC and RA, so Curcumin and Curcuma longa Extract could be used in the treatment of the above diseases in the future. The results of Meta-analysis showed that Curcumin and Curcuma longa Extract did not show efficacy in the treatment of oral lichen planus, while Takayasu arteritis, SLE, MS, AS, BD and CD did not report sufficient clinical data for meta-analysis. Therefore, large-sample, multi-center clinical trials are still needed for revision or validation.
1 Introduction
Autoimmune diseases refer to diseases caused by the damage of the body’s own tissues caused by the immune response of the body to self-antigens (1). The main reason is that the autoimmune tolerance state is broken or the autoimmune cells are abnormally regulated under the induction of some internal and external factors such as genetic factors and environmental factors, and the immune system produces a persistent and protracted immune response to self-antigens (2, 3). Autoimmune diseases generally include systemic lupus erythematosus (SLE), Sjögren’s syndrome, multiple sclerosis (MS), rheumatoid arthritis (RA), Behcet’s disease (BD), giant cell arteritis, Takayasu arteritis, Psoriasis, ankylosing spondylitis (AS), Oral lichen planus and more than 120 diseases (4, 5). Autoimmune diseases can be induced in a variety of ways, and symptoms vary depending on the disease and the organs involved. The presence of autoimmune disease often requires some blood tests to confirm (6). Treatment for autoimmune diseases depends on the type and severity of the condition. Commonly used drugs include non-steroidal anti-inflammatory drugs (NSAIDs), biologically targeted drugs (such as ustekinumab), and traditional immune-suppressing drugs (such as azathioprine and cyclosporine) (7–9). However, these drugs not only suppress the autoimmune response, but also inhibit the body’s ability to defend itself against foreign invasion, including infection-causing microorganisms and malignant tumor cells (10, 11). Therefore, the treatment of autoimmune diseases is still a difficult problem, and researchers are increasingly interested in the treatment of autoimmune diseases with natural products (12, 13).
Curcuma longa L. has been used as a traditional Chinese medicinal material mainly as turmeric for thousands of years in China (14). Curcumin is the most effective ingredient extracted from the rhizomes of ginger plants such as turmeric, curcuma, turmeric, and calamus (15). A number of in vitro and in vivo experiments showed that curcumin has various pharmacological effects such as regulating immunity, anti-oxidation, inhibiting inflammation, anti-tumor, anti-angiogenesis, anti-coagulation, and scavenging free radicals (15, 16). These studies suggest that curcumin may play a regulatory role by altering the activities of enzymes, receptors, and related transcription factors (17). Numerous randomized controlled trials (RCTs) have shown that curcumin can alleviate many human diseases, including autoimmune diseases, with the main mechanisms in regulating immunity and inhibiting inflammation. Also, curcumin is administered with few side effects, making it a potential alternative to NSAIDs and other drugs with known severe side effects (18–21). In recent years, a large number of RCTs have been published, so it is urgent to summarize and summarize the efficacy and safety of curcumin in autoimmune diseases. This study would provide future clinicians with better evidence for clinical practice by conducting a comprehensive systematic review and meta-analysis of these RCTs, as well as providing more details for future clinical trial design.
2 Materials and Methods
2.1 Protocol
This systematic review and meta-analysis were conducted strictly in accordance with the protocol (CRD42021289248) and PRISMA-guidelines (see Supplementary Materials) (22).
2.2 Literature Search Strategy
The English databases (Medline complete, Web of Science, PubMed and Embase) and Chinese databases (Sinomed, Wanfang Database, China National Knowledge Infrastructure, VIP Database) were searched from the establishment of the database to February 2022. We also searched Cochrane Library and The ClinicalTrials.gov. The search strategy of Pubmed and Embase is shown in Table S1 as an example.
2.3.Inclusion and Exclusion Criteria
2.3.1 Participants
The patient was diagnosed with an autoimmune disease by accepted diagnostic criteria.
2.3.2 Intervention
The therapy of the experimental group was curcumin or Curcuma longa Extract preparation, regardless of dosage form, intervention dose, administration route, etc. The therapy of the control group was placebo, conventional therapy, or other curcumin-free therapy.
2.3.3 Outcomes
Outcomes are efficacy indicators, inflammatory indicators and safety indicators.
2.3.4 Design
The study design was RCTs.
2.3.5 Exclusion Criteria
(1) Literature with repeated publications, data and incomplete data. (2) Secondary literature and conference papers such as experience exchange, meta-analysis and review. (3) Animal experiments. (4) The control group therapy contains curcumin or Curcuma longa Extract. (5) abstract only.
2.4 Literature Screening and Data Extraction
First, a preliminary search was carried out in Chinese and English databases, after eliminating duplicate literature, systematic reviews, reviews and animal experiments. After further reading the titles and abstracts to eliminate obviously irrelevant literature, according to the inclusion and exclusion criteria, two researchers independently screened eligible literature and extracted data. Data extraction was performed independently by two researchers, and if there was disagreement, they were discussed with other researchers.
2.5 Quality Assessment
Quality assessment adopts the RCT risk of bias assessment tool recommended by the Cochrane Handbook (23). Quality assessment was performed independently by two researchers, and if there was disagreement, they were discussed with other researchers.
2.6 Statistical Analysis
Data analysis was performed with RevMan 5.3 software provided by the Cochrane Collaboration (24). For continuous variables, mean difference (MD) and 95% confidence interval (CI) were used as effect analysis statistics. The heterogeneity among the results of the included studies was analyzed by the χ 2 test (the test level was α=0.1), and the size of the heterogeneity was quantitatively judged by I2. If there was little or no heterogeneity among study results (P>0.1, I2 ≤ 50%), a fixed-effect model was used. If the heterogeneity among the results of each study was large (P ≤ 0.1, I2>50%), the source of heterogeneity was further analyzed, and the random effect model was used after excluding the influence of obvious clinical heterogeneity (25). The test level of the meta-analysis was set to α=0.05.
3 Results
3.1 Results of the Search
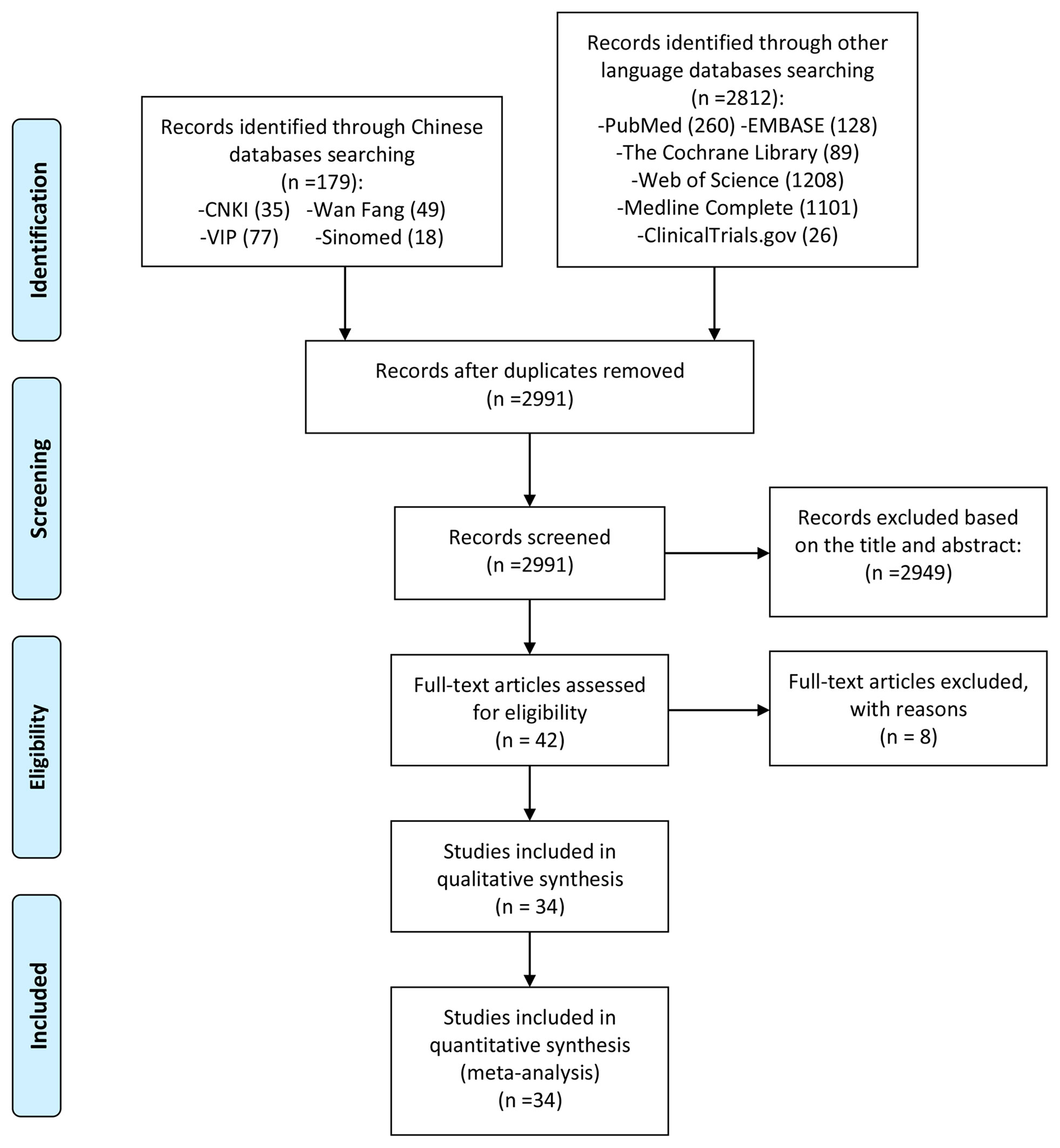
A total of 2991 literatures were obtained through computer database search and other methods. After deduplication, after reading the title and abstract, excluding the literature review, review, non-clinical research and obviously irrelevant literature, a total of 42 clinical studies were collected. Read the full text further to exclude articles that did not meet the inclusion criteria (26–33). Finally, a total of 34 RCT studies were included. The process and results of screening literature are shown in Figure 1.
3.2 Description of Included Trials
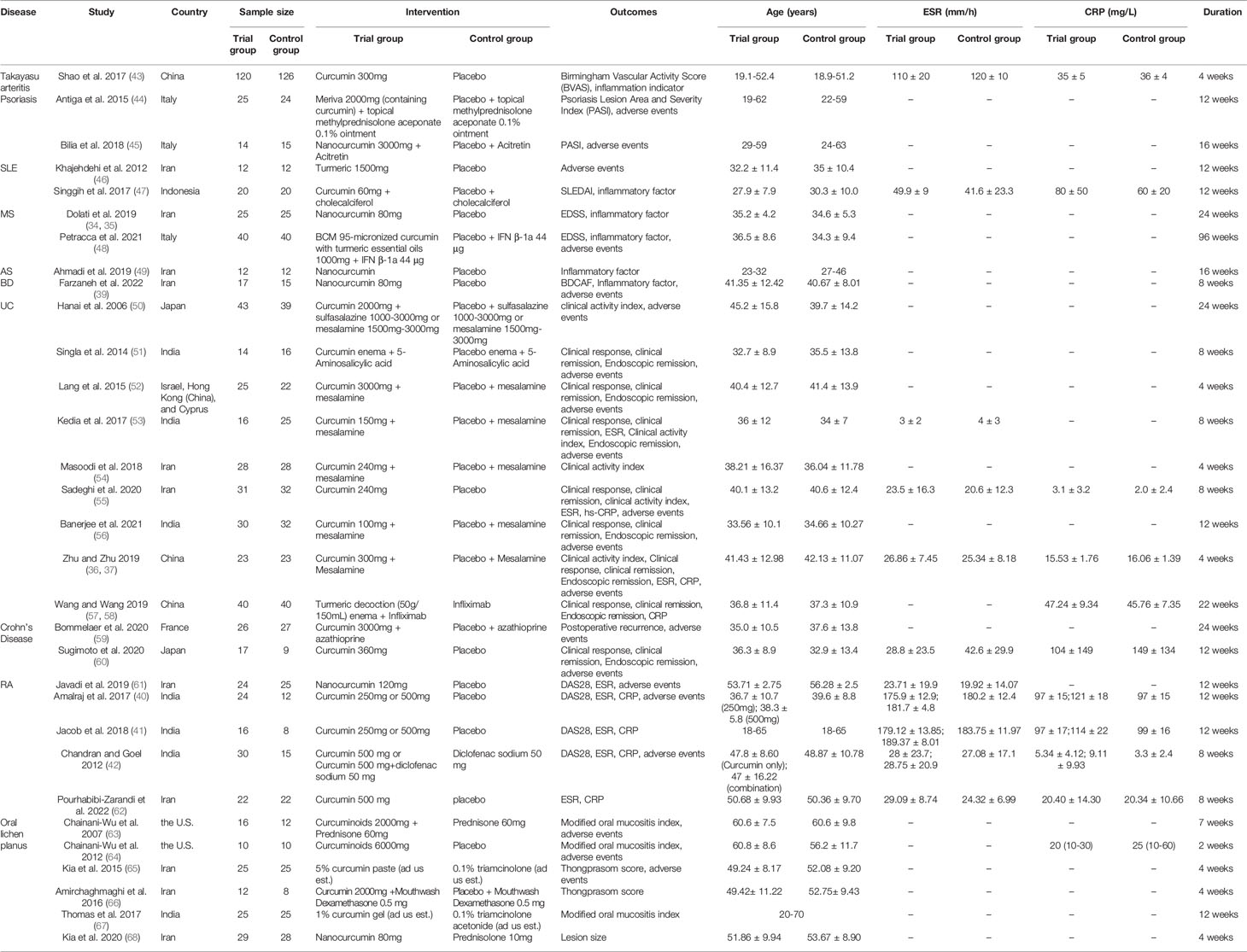
A total of 34 records were included, involving 31 RCTs and 10 types of autoimmune disease: AS, BD, Crohn’s Disease, MS, Oral lichen planus, Psoriasis, RA, SLE, Takayasu arteritis, UC. Dolati et al. 2019 (34) and Dolati et al. 2018 (35) belong to the same RCT and thus merge into “Dolati et al. 2019 (34, 35)” Zhu and Zhu 2019 (36) and Zhu 2019 (37) belong to the same RCT and thus merge into “Zhu and Zhu 2019 (36, 37)”; Abbasian et al. 2021 (38) and Farzaneh et al. 2022 (39) belong to the same RCT and thus merge into “Farzaneh et al. 2022 (38, 39)”. Amalraj et al. 2017 (40) and Jacob et al. 2018 (41) used two different doses of curcumin intervention (high-dose group and low-dose group), so they were divided into Amalraj et al. 2017a (low-dose group), Amalraj et al. 2017b (high-dose group), Jacob et al. 2018a (low-dose group), Jacob et al. 2018b (high-dose group). The interventions of Chandran and Goel 2012 (42) were divided into Curcumin 500 mg and Curcumin 500 mg+diclofenac sodium 50 mg, so they were also divided into Chandran et al. 2012a (Curcumin only) and Chandran et al. 2012b (Curcumin+Diclofenac sodium). The details of study characteristics are presented in Table 1.
3.3 Risk of Bias of Included Studies
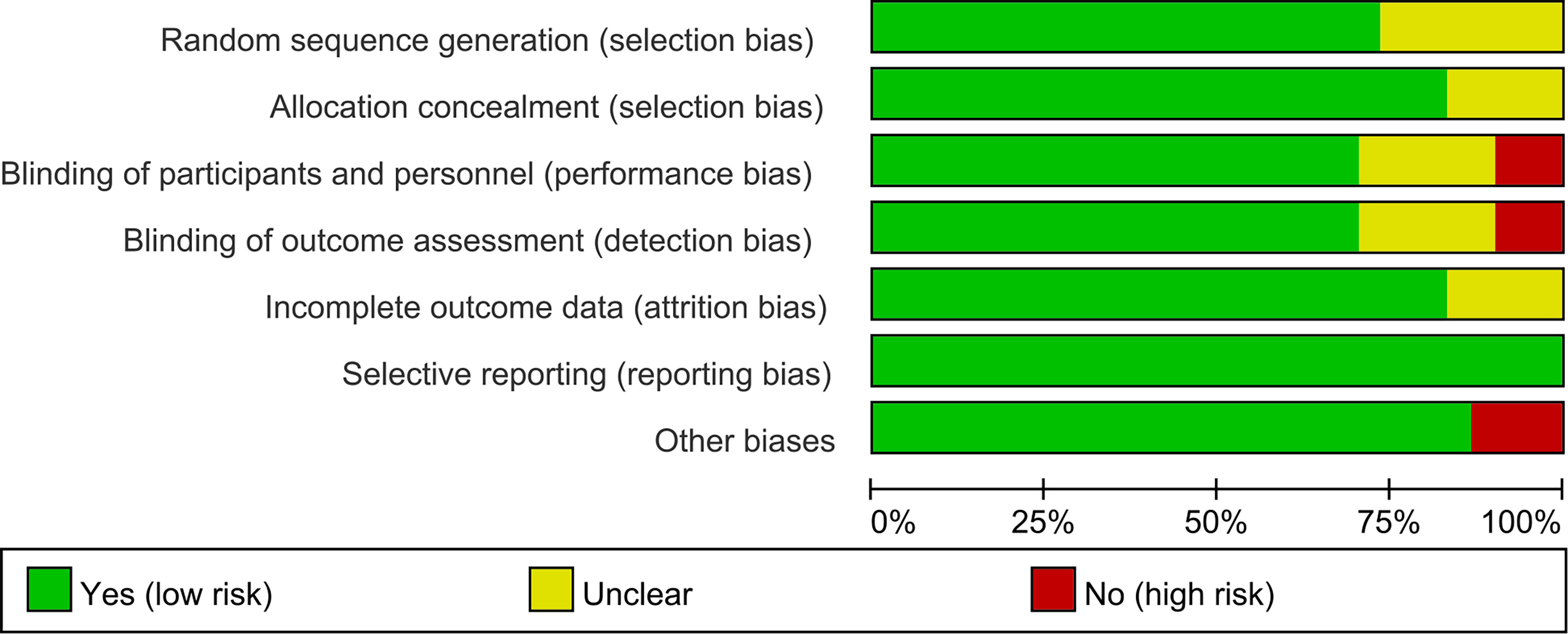
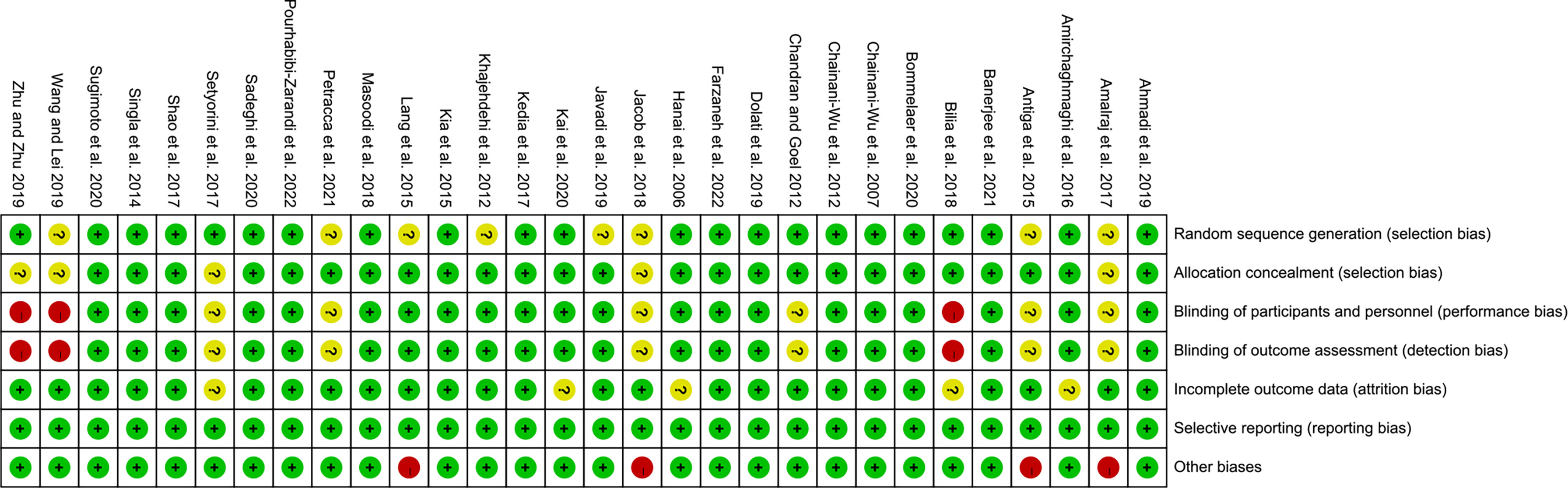
The summary and graph of risk of bias ware shown in Figures 2, 3.
3.4 Curcumin and Curcuma longa Extract in the Treatment of Takayasu Arteritis
Only Shao et al. 2017 (43) reported the effects and safety of curcumin in the treatment of Takayasu arteritis. The study included 246 patients with Takayasu arteritis (120 in the experimental group and 126 in the control group) involving BVAS and inflammation indicators. The results showed that BVAS score after curcumin treatment was lower than that of placebo group, and TNF-α, CRP and ESR were decreased (P<0.05).
3.5 Curcumin and Curcuma longa Extract in the Treatment of Psoriasis
Two RCTs reported on the treatment of Psoriasis with Curcumin and Curcuma longa Extract, the data involved can be combined for meta-analysis, and therefore would be described according to the results of the meta-analysis.
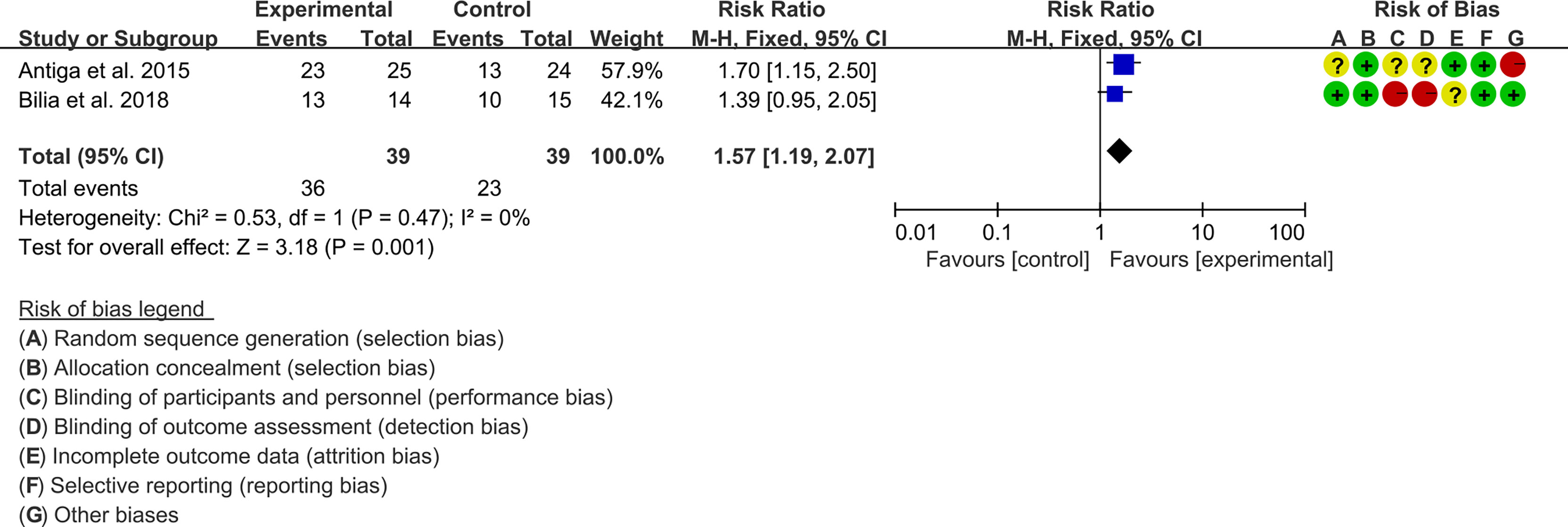
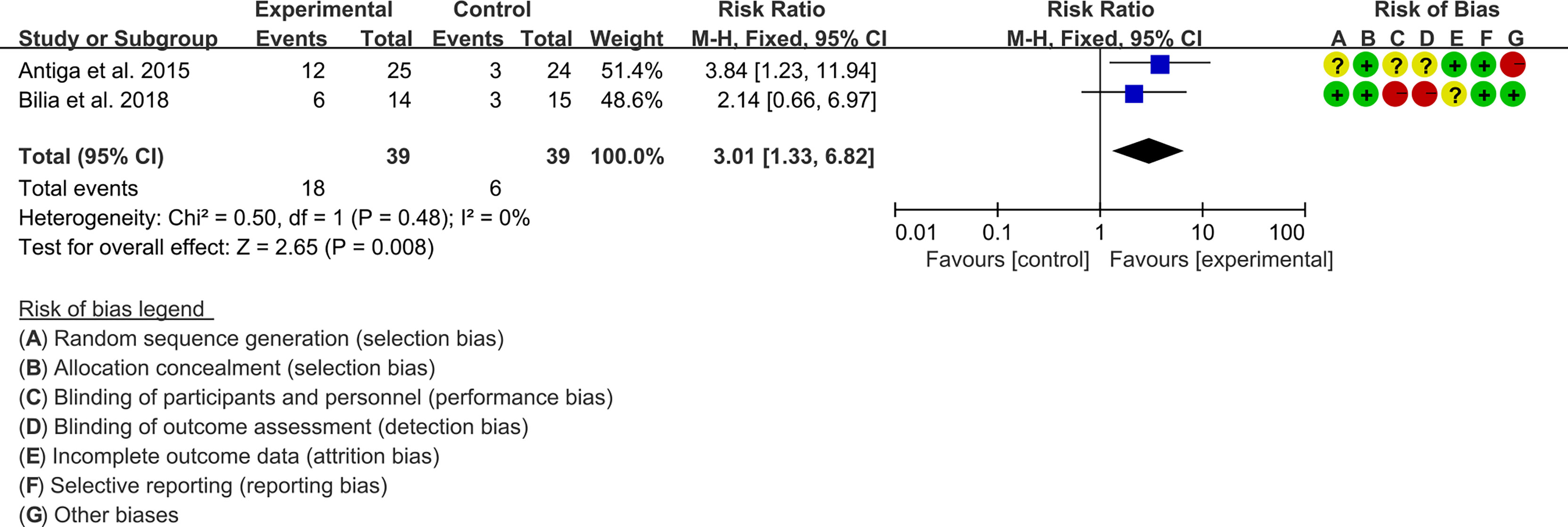
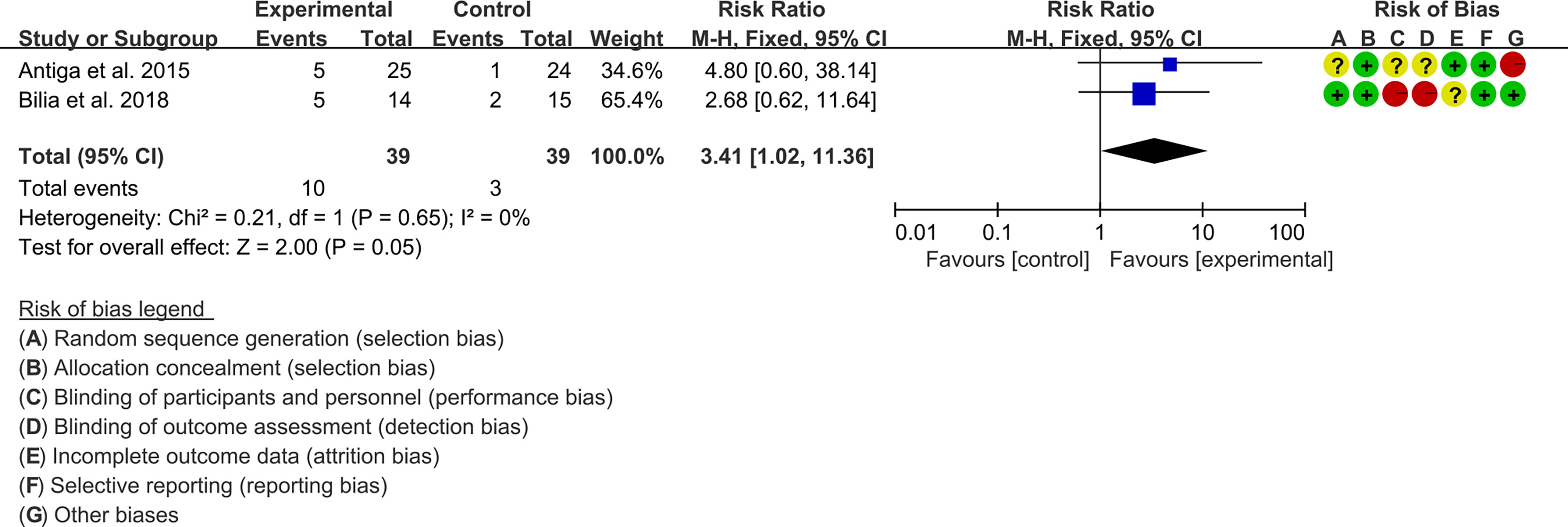
3.5.1 PASI
Two (2) RCTs reported the PASI. The heterogeneity test showed low heterogeneity (PASI50: I2 = 0%, P=0.47; PASI75: I2 = 0%, P=0.48; PASI90: I2 = 0%, P=0.65), so the fixed-effects model was used. The summary results showed that addition of curcumin improved PASI50 (RR=1.57, 95%CI[1.19, 2.07], P=0.001), PASI75 (RR=3.01, 95%CI[1.33, 6.82], P=0.008) and PASI90 (RR=3.41, 95%CI[1.02, 11.36], P=0.05) compared to the control group (Figures 4–6).
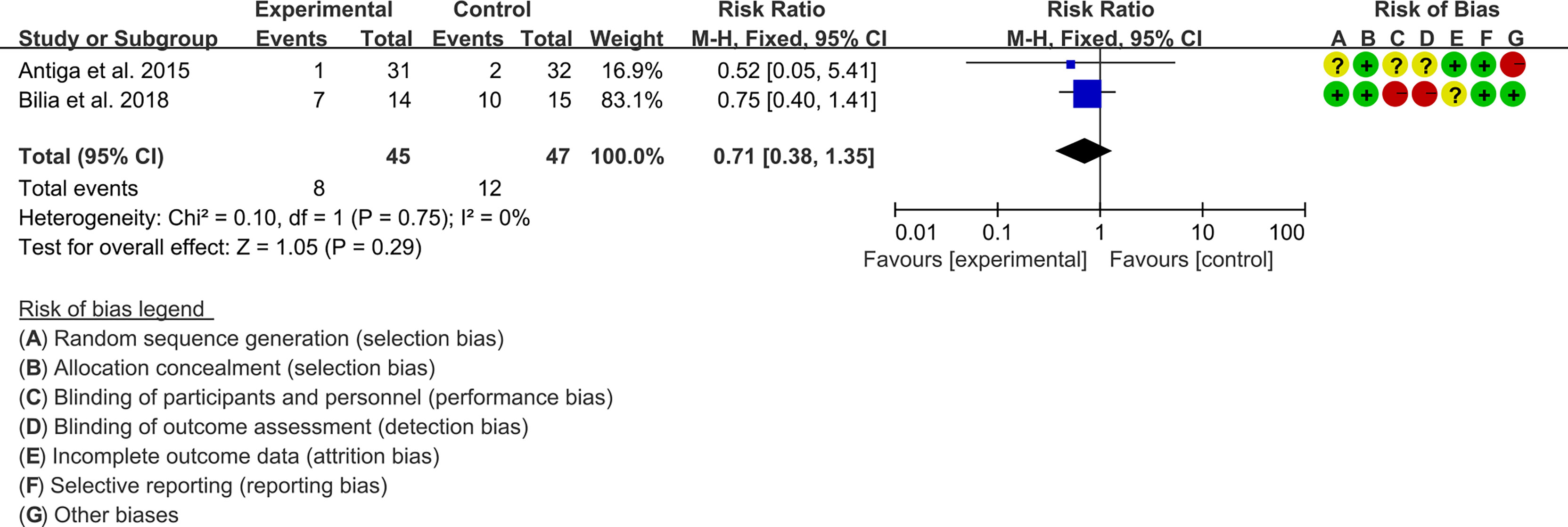
3.5.2 Adverse Events
Two (2) RCTs reported the adverse events. The heterogeneity test showed low heterogeneity (I2 = 0%, P=0.75), so the fixed-effects model was used. The summary results showed that compared with control group, the addition of curcumin did not increase the incidence of adverse events (RR=0.71, 95%CI[0.38, 1.35], P=0.29) (Figure 7).
3.6 Curcumin and Curcuma longa Extract in the Treatment of SLE
Two (2) RCTs explored the effects and safety of curcumin and curcuma longa Extract in the treatment of SLE. Khajehdehi et al. 2012 (46) included 24 patients with lupus nephritis and no adverse effects related to turmeric supplementation were observed during the trial. Singgih et al. 2017 (47) used Curcumin 60mg combined with cholecalciferol as the treatment regimen for the trial group, with a total of 40 participants. They found that the improvement of SLEDAI, the decrease of IL-6 and the decrease of TGF-β1 by Curcumin 60mg combined with cholecalciferol had no statistical difference compared with the control group (P>0.05).
3.7 Curcumin and Curcuma longa Extract in the Treatment of MS
Two (2) RCTs explored the effects and safety of curcumin and curcuma longa Extract in the treatment of MS. Dolati et al. 2019 (34, 35) utilized Nanocurcumin 80mg for patients with MS. They found that compared with placebo controls, curcumin decreased EDSS, increased Treg cells of blood peripheral mononuclear cells, and increased the expression of FoxP3, TGF-β and IL-10 mRNA in blood peripheral mononuclear cells (P<0.05) and also increased serum TGF-β and IL-10 levels (P<0.05). Petracca et al. 2021 (48) used curcumin combined with IFN β-1a therapy to treat patients with MS; they stated that the study dropout rate was too high to draw firm conclusions. Although they found no significant difference in EDSS between the curcumin+IFN β-1a group and placebo+IFN β-1a group (P>0.05), they found that curcumin increased the efficacy of IFN beta-1a on the inflammatory radiological symptoms of MS (P<0.05). They also found that the addition of curcumin did not increase the probability of adverse reactions.
3.8 Curcumin and Curcuma longa Extract in the Treatment of AS
Only Ahmadi et al., 2019 explored the effects and safety of curcumin and curcuma longa Extract in the treatment of AS. They included 12 patients who received nanocurcumin and 12 who received a placebo. They found that compared with the control group, Treg cells in the nano-curcumin group increased significantly, the levels of IL-10 and TGF-β increased, and the level of IL-6 decreased (P<0.05).
3.9 Curcumin and Curcuma longa Extract in the Treatment of BD
Only Farzaneh et al. 2022 (39) explored the effects and safety of curcumin and curcuma longa Extract in the treatment of BD. They included 17 patients in the nanocurcumin group and 15 in the placebo group. They found that BDCAF was decreased in patients treated with curcumin (P<0.05). They also found that Treg cells, the levels of IL-10 and TGF-β in the nano-curcumin group increased significantly, while IL-17 and IL-23 decreased (P<0.05). No obvious adverse events were observed in the curcumin group and the control group, suggesting that Nanocurcumin is relatively safe.
3.10 Curcumin and Curcuma longa Extract in the Treatment of UC
Nine RCTs reported the effects and safety of curcumin and curcuma longa Extract in the treatment of UC. The data involved can be combined for meta-analysis, and therefore would be described according to the results of the meta-analysis.
3.10.1 Clinical Activity Index
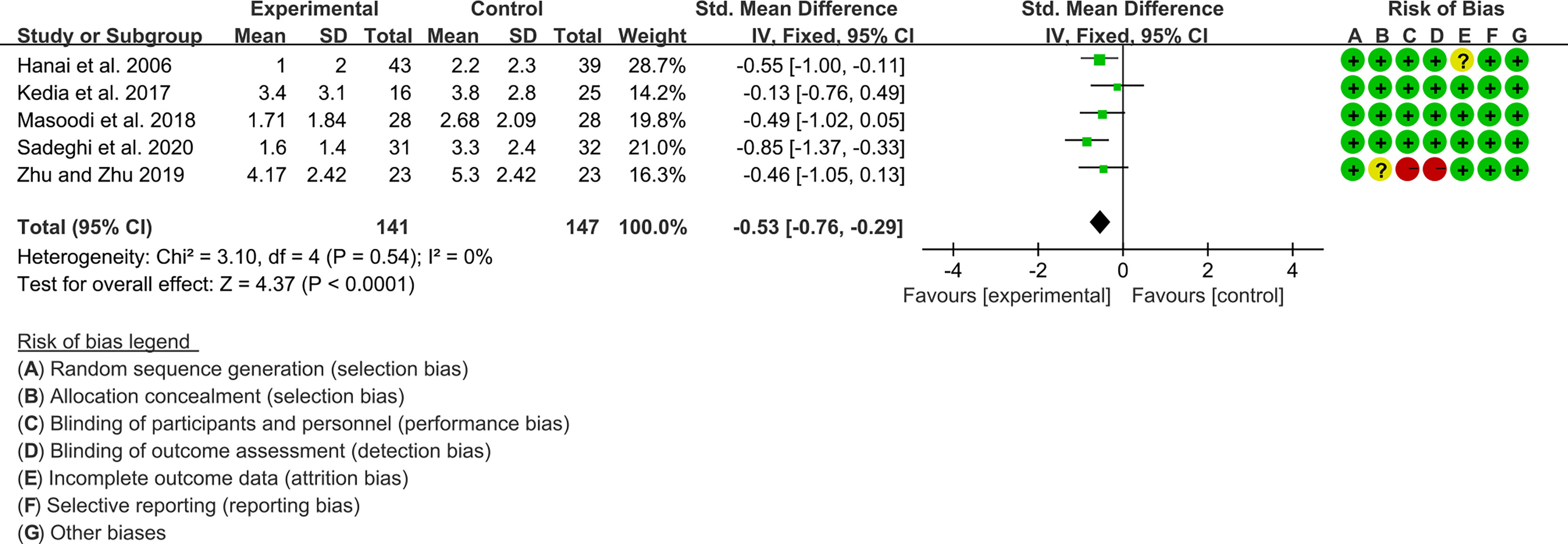
Five (5) RCTs reported clinical activity index. Due to the different scales used, SMD was used to represent the effect size. The heterogeneity test showed low heterogeneity (I2 = 0%, P=0.54), so the fixed-effects model was used. The summary results showed that compared with control group, the addition of curcumin decreased the clinical activity index (SMD=-0.53, 95%CI[-0.76, -0.29], P<0.0001) (Figure 8).
3.10.2 Clinical Efficacy
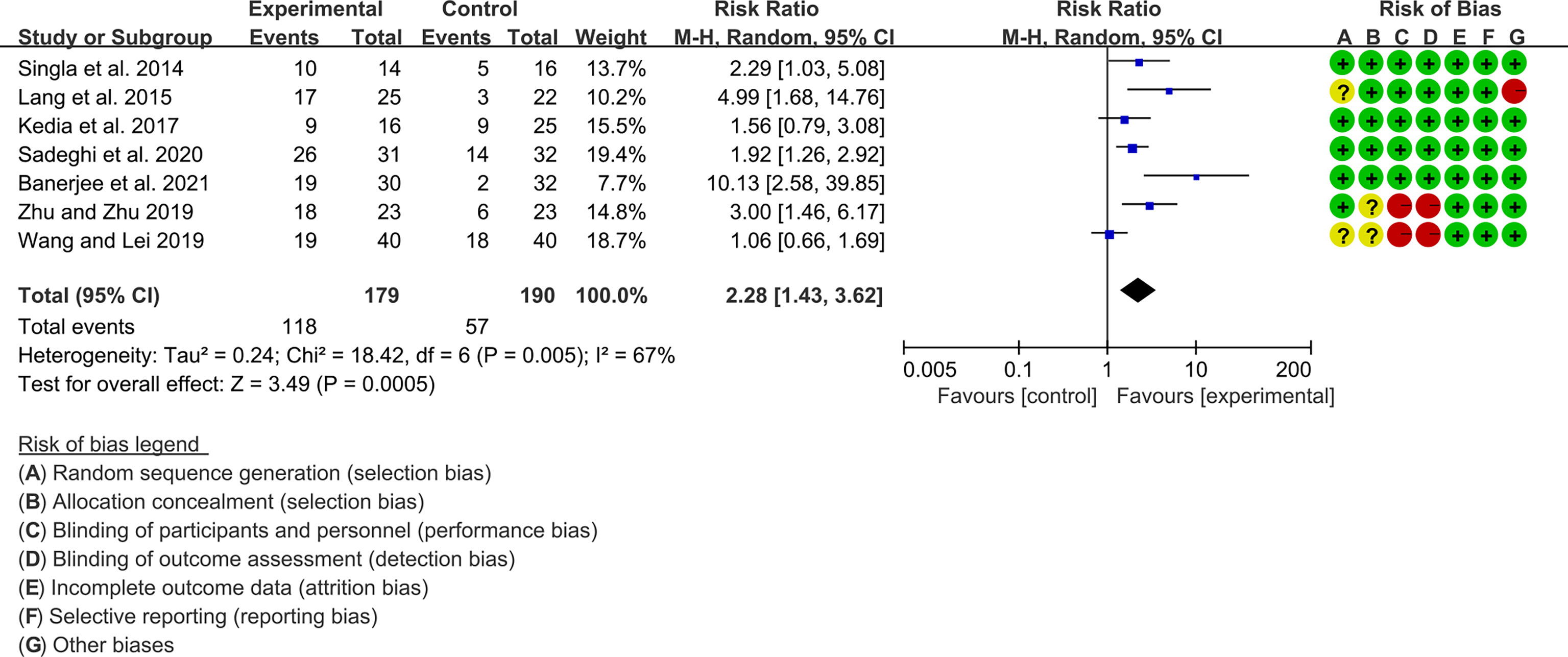
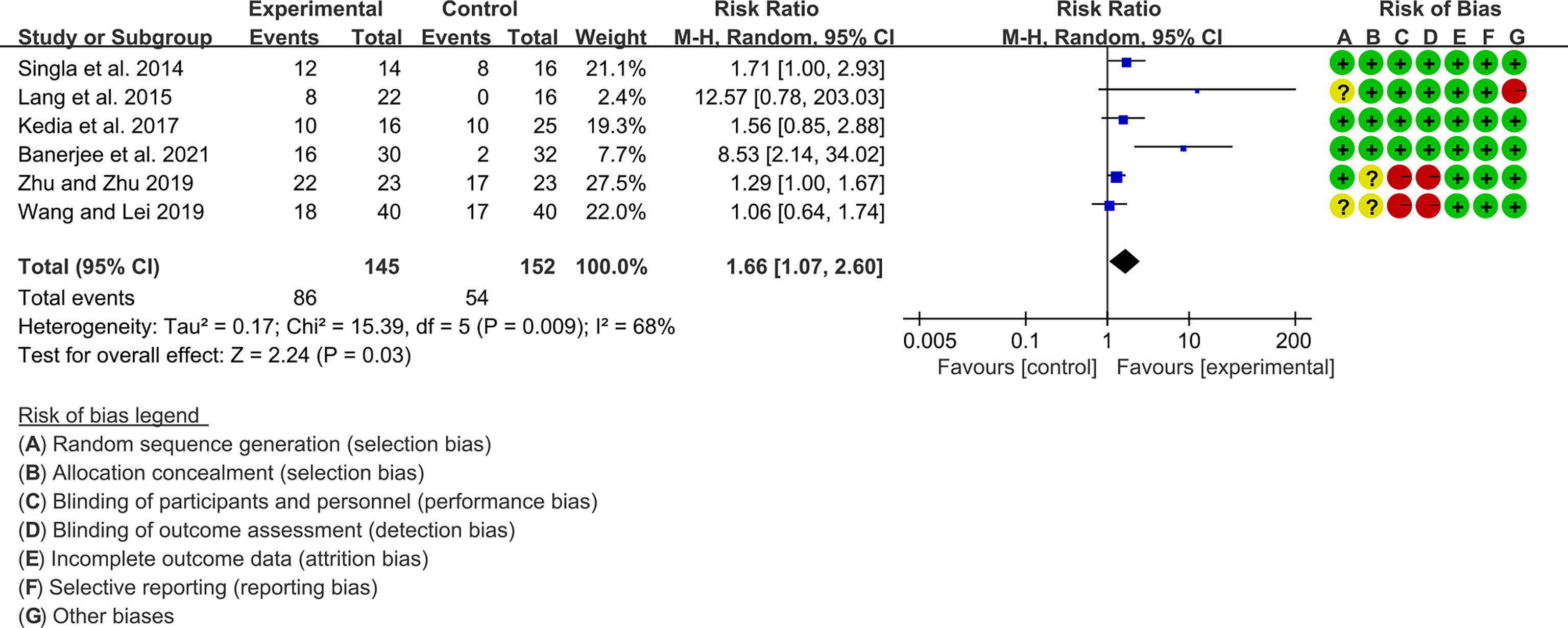
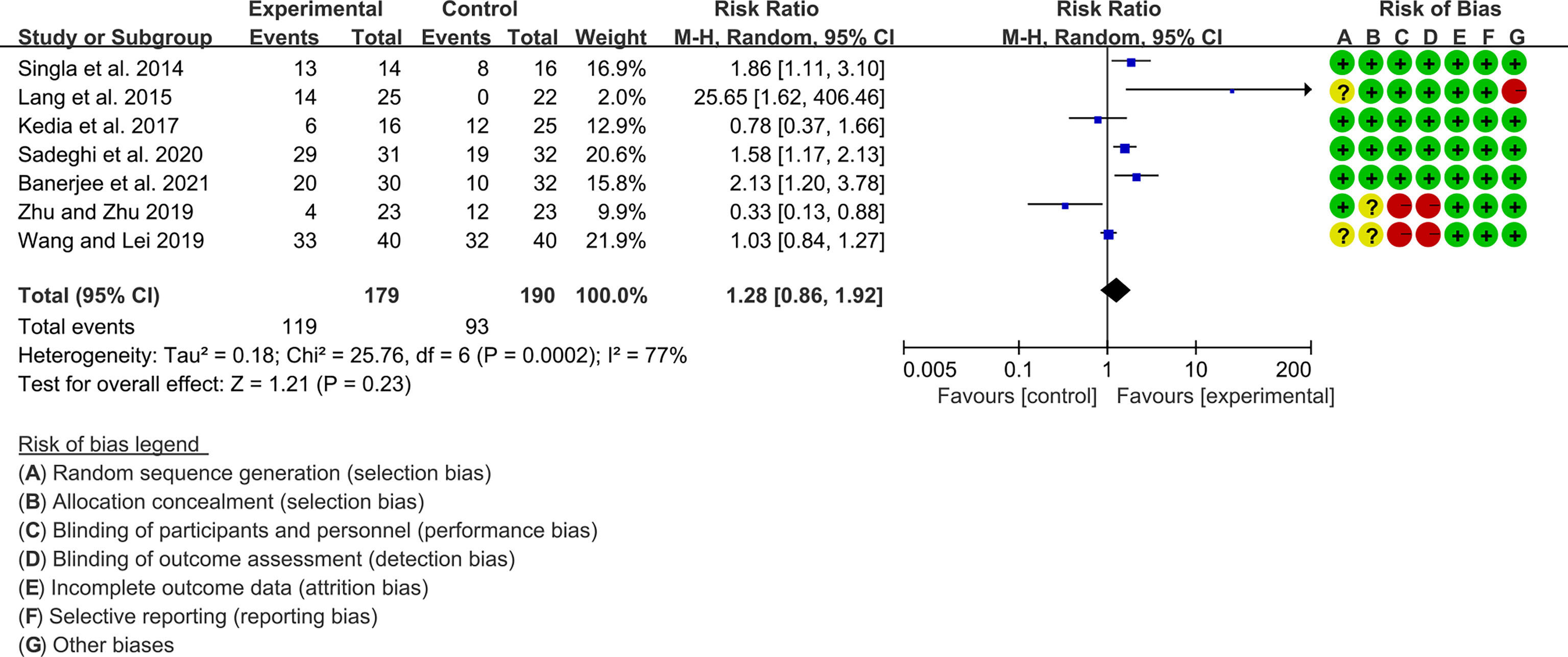
Seven (7) RCTs reported clinical remission and clinical response rats, and 6 reported endoscopic remission rates. The heterogeneity test showed high heterogeneity (clinical remission: I2 = 67%, P=0.005; clinical response: I2 = 77%, P=0.0002; endoscopic remission: I2 = 68%, P=0.009.), so the random-effects model was used. The summary results showed that compared with control group, the addition of curcumin increased the clinical remission rates (RR=2.28, 95%CI[1.43, 3.62], P=0.0005) and endoscopic remission rates (RR=1.66, 95%CI[1.07, 2.60], P=0.03) (Figures 9, 10), while the addition of curcumin did not increase the clinical remission rates (RR=1.28, 95%CI[0.86, 1.92], P=0.23) (Figure 11)
3.10.3 Inflammatory Factor
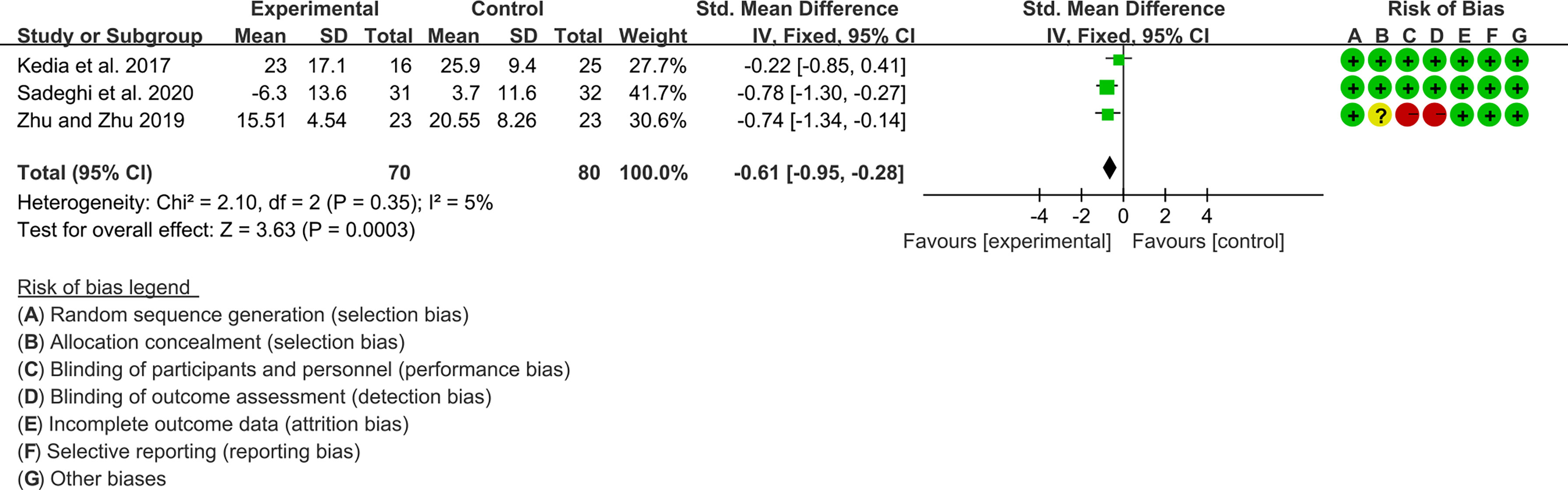
Three (3) RCTs reported ESR and CRP. For ESR, the heterogeneity test showed low heterogeneity (I2 = 5%, P=0.35), so the fixed-effects model was used. The summary results showed that compared with control group, the addition of curcumin decreased the ESR (SMD=-0.61, 95%CI[-0.95, -0.28], P=0.0003) (Figure 12).
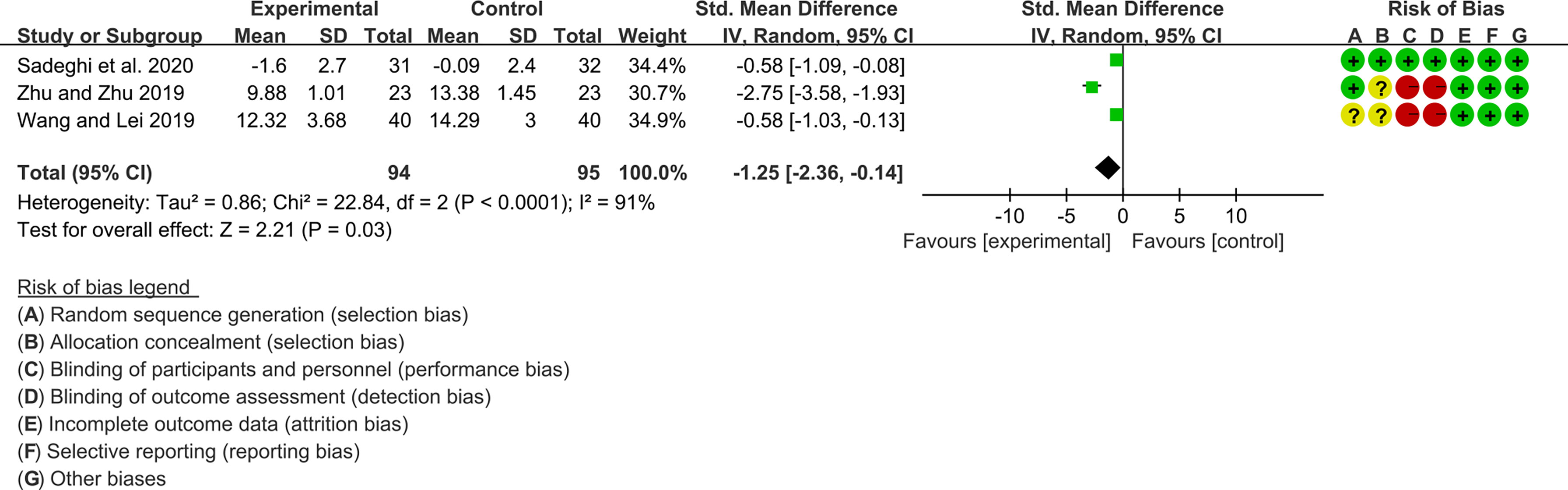
For CRP, the heterogeneity test showed high heterogeneity (I2 = 91%, P<0.0001), so the random-effects model was used. The summary results showed that compared with control group, the addition of curcumin decreased the CRP (SMD=-1.25, 95%CI[-2.36, -0.14], P=0.03) (Figure 13)
3.10.4 Adverse Events
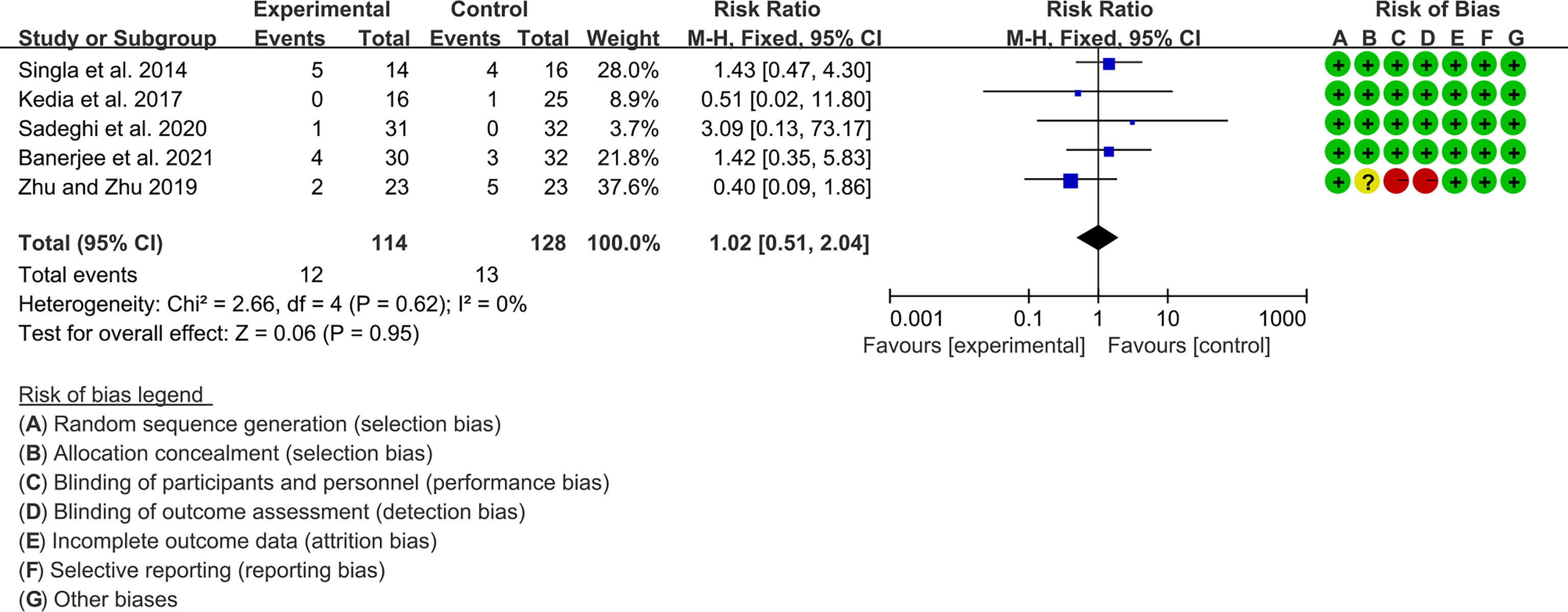
Five (5) RCTs reported the adverse events. The heterogeneity test showed low heterogeneity (I2 = 0%, P=0.62), so the fixed-effects model was used. The summary results showed that compared with control group, the addition of curcumin did not increase the incidence of adverse events (RR=1.02, 95%CI[0.51, 2.03], P=0.95) (Figure 14).
3.11 Curcumin and Curcuma longa Extract in the Treatment of Crohn’s Disease
Two (2) RCTs explored the effects and safety of curcumin and curcuma longa Extract in the treatment of Crohn’s Disease. Bommelaer et al. 2020 (59) found that Curcumin was no more effective than placebo in preventing Crohn’s disease relapse and probably did not improve patients’ quality of life. Thiopurine was discontinued due to nausea and abdominal pain in 2 patients each in the curcumin and placebo groups. In addition, adverse events were reported in 2 patients in the placebo group (1 pregnancy and 1 abscess) and 5 patients in the curcumin group (2 abdominal pain, 1 vomiting, 1 migraine, and 1 cervical squamous cell carcinoma); and these are not considered to be related to the addition of curcumin. However, (60) found that with the addition of curcumin, clinical remission and Endoscopic remission improved, and no serious adverse events were observed. Only one patient showed mild appetite loss, which did not affect the completion of the clinical trial.
3.12 Curcumin and Curcuma longa Extract in the Treatment of RA
Five RCTs reported on the treatment of RA with Curcumin and Curcuma longa Extract, the data involved can be combined for meta-analysis, and therefore would be described according to the results of the meta-analysis.
3.12.1 DAS28
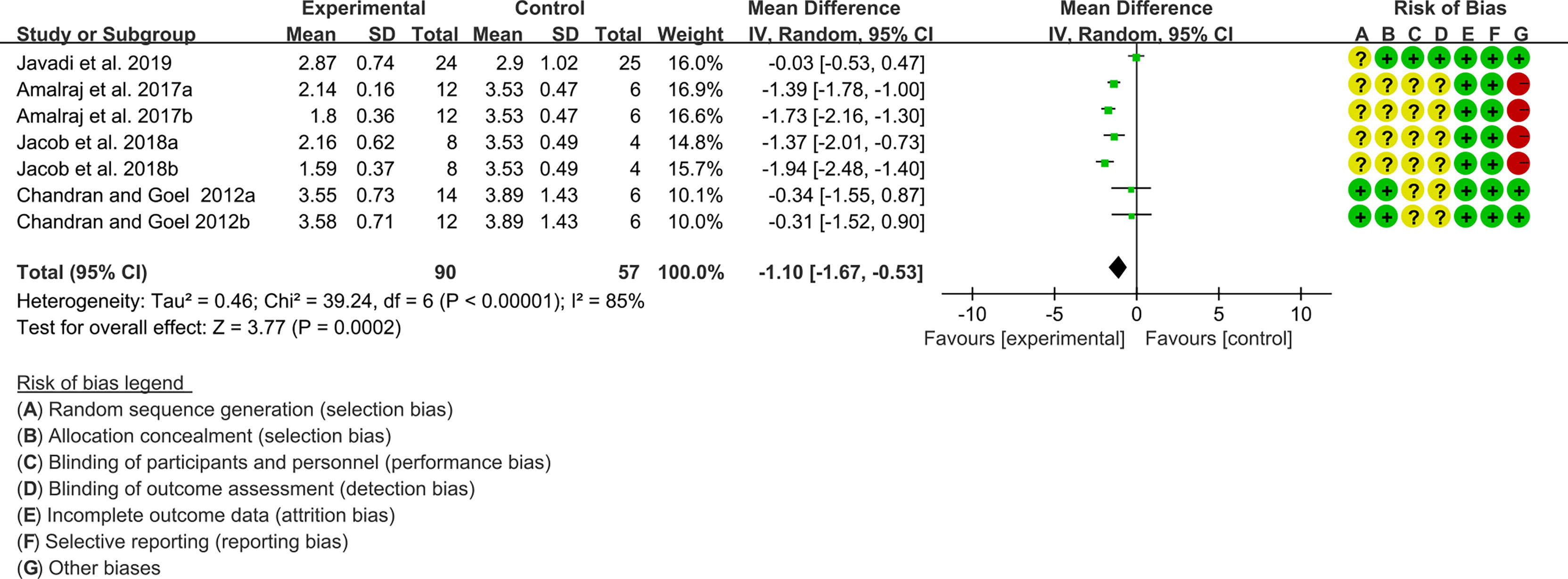
Four (4) RCTs reported the DAS28. The heterogeneity test showed high heterogeneity (I2 = 85%, P<0.00001), so the random-effects model was used. The summary results showed that compared with control group, the addition of curcumin decreased the DAS28 (WMD=-1.10, 95%CI[-1.67, -0.53], P=0.0002) (Figure 15).
3.12.2 Inflammatory Factor
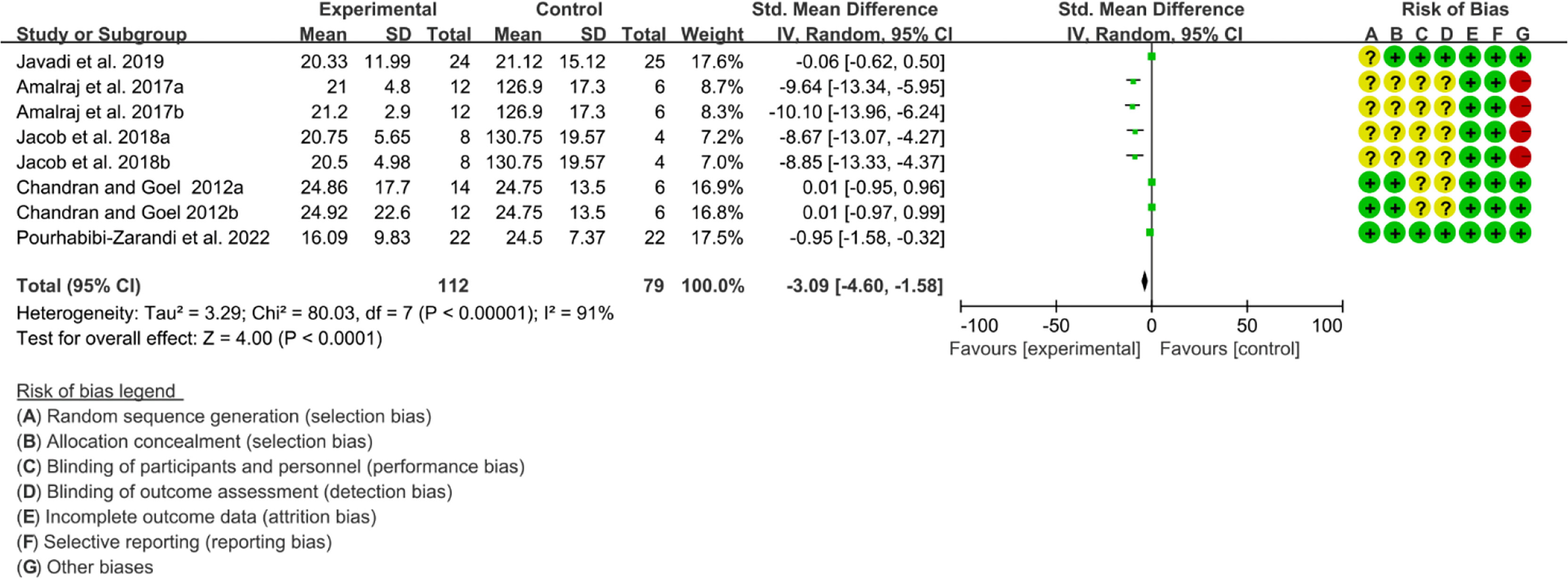
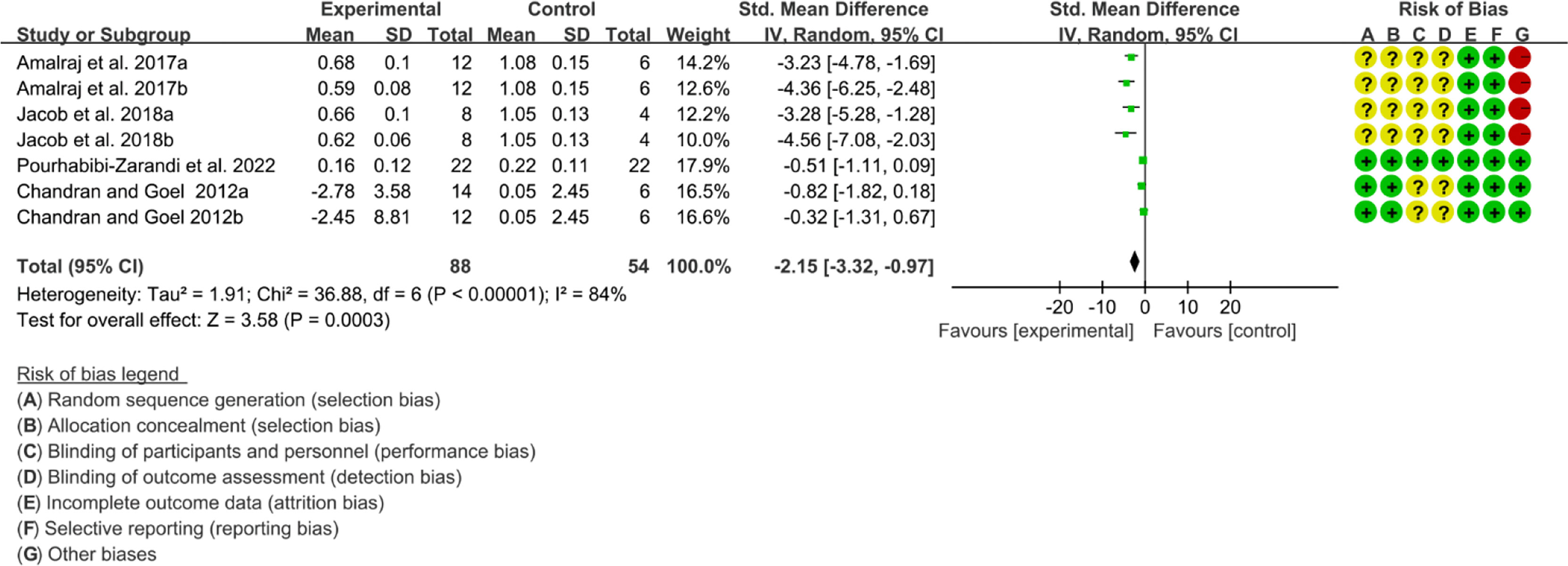
Five (5) RCTs reported the ESR. The heterogeneity test showed high heterogeneity (I2 = 91%, P<0.00001), so the random-effects model was used. The summary results showed that compared with control group, the addition of curcumin decreased the ESR (SMD=-3.09, 95%CI[-4.60, -1.58], P<0.0001) (Figure 16). Four (4) RCTs reported the CRP. The heterogeneity test showed high heterogeneity (I2 = 91%, P<0.00001), so the random-effects model was used. The summary results showed that compared with control group, the addition of curcumin decreased the CRP (SMD=-2.15, 95%CI[-3.32, -0.97], P=0.0003) (Figure 17).
3.12.3 Adverse Events
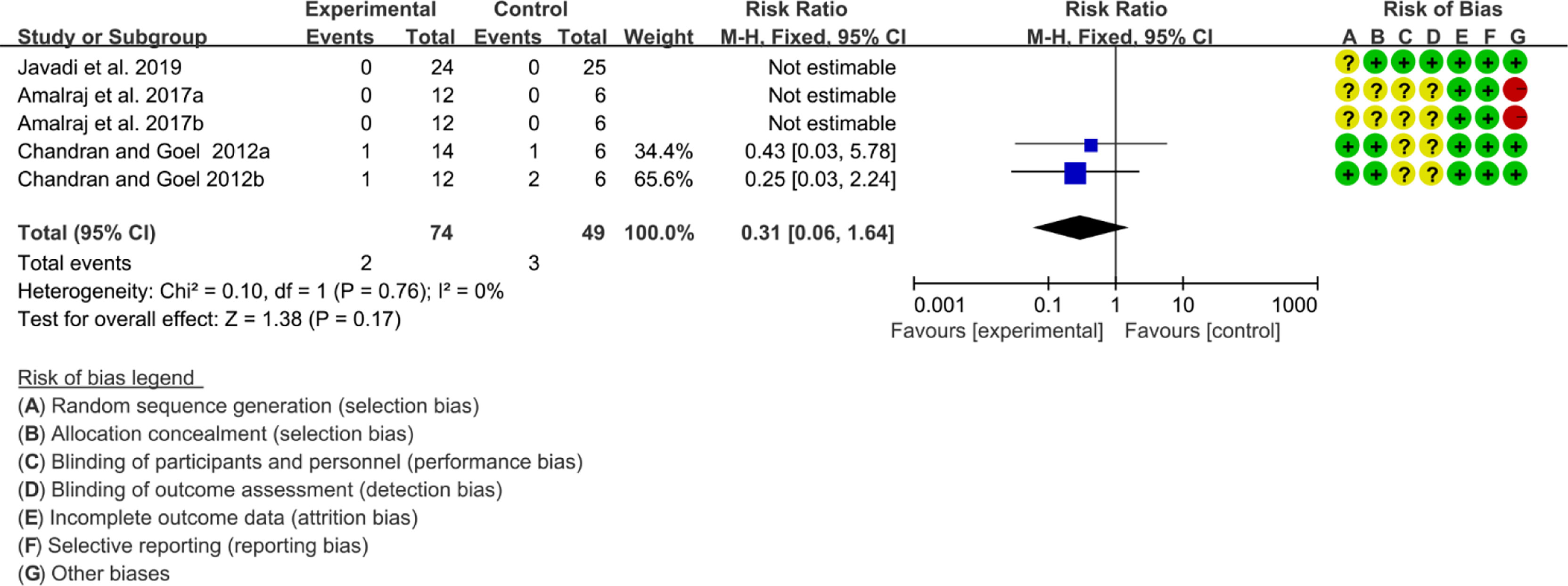
Three (3) RCTs reported the adverse events. The heterogeneity test showed low heterogeneity (I2 = 0%, P=0.78), so the fixed-effects model was used. The summary results showed that compared with control group, the addition of curcumin did not increase the incidence of adverse events (RR=0.31, 95%CI[0.06, 1.64], P=0.17) (Figure 18).
3.13 Curcumin and Curcuma longa Extract in the Treatment of Oral lichen planus
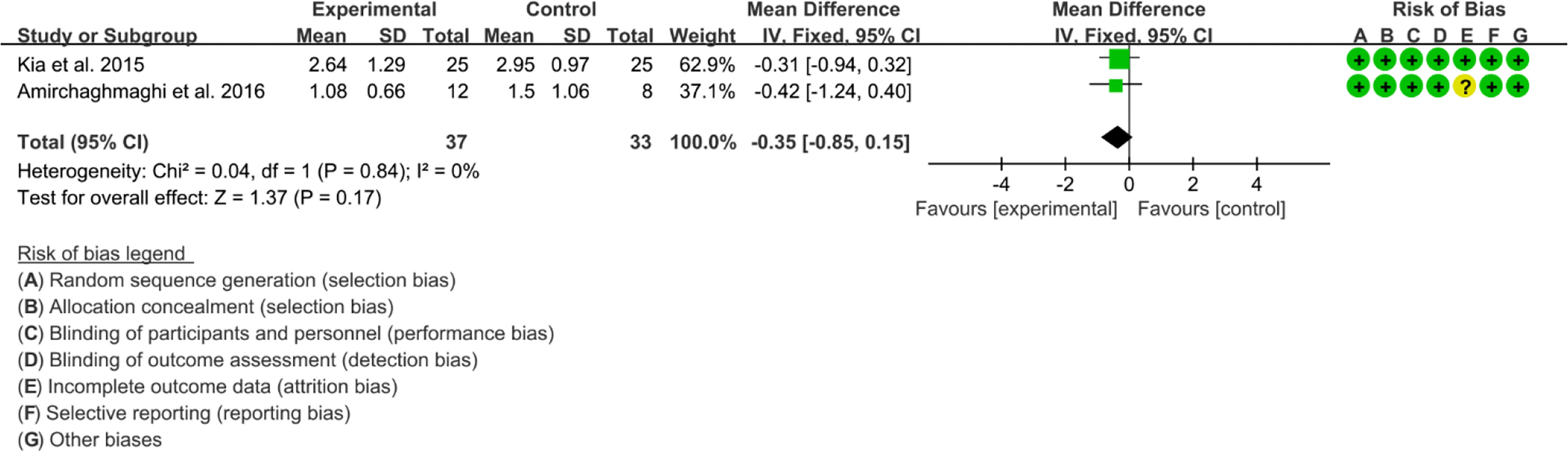
Six RCTs reported Curcumin and Curcuma longa Extract in the Treatment of Oral lichen planus. (64, 67) found that Curcumin may decrease Modified oral mucositis index (P<0.05). However, (68) found no significant difference in efficacy between Curcumin and Prednisolone. Since their indicators could not be combined, Meta-analysis was not conducted. (63–65) found no significant adverse events. (65, 66) reported Thongprasom score, which could be combined. The heterogeneity test showed low heterogeneity (I2 = 0%, P=0.78), so the fixed-effects model was used. The summary results showed that compared with control group, the addition of curcumin did not decrease Thongprasom score (WMD=-0.35, 95%CI[-0.85, 0.15], P=0.17) (Figure 19).
4 Discussion
4.1 Mechanisms of Curcumin Regulation of Immune Cells in Autoimmune Diseases
Autoimmune diseases are a series of diseases caused by the immune system’s immune response to self-antigens, leading to self-tissue damage or dysfunction (69, 70). Many autoimmune diseases are characterized by the production of autoantibodies that bind to the host’s own proteins or form immune complexes that deposit in tissues (71, 72). Any organ may become a target organ of autoimmunity, including skin, joints, kidneys, blood vessels, etc. (73). Inflammatory effects caused by autoantibodies are mediated by binding to Fc receptors on leukocytes and are an important cause of downstream tissue damage. Meanwhile, autoantibodies can also directly mediate tissue damage in disease through complement activation (74). During the developmental stage of the disease, genetic and environmental factors may in turn interact to promote the development of autoimmunity, ultimately leading to tissue inflammation and damage (75).
In autoimmune diseases, the balance between the body’s recognition of foreign pathogens and the immune system’s attack on self-antigens is disrupted, resulting in abnormal immune tolerance. During the breakdown of immune tolerance, initiating tissues provide a microenvironment that influences immune cell differentiation leading to the activation of adaptive immunity. Type 1 interferons produced by innate immune cells play a central role in systemic autoimmunity and activate B and T cells, while B cell-derived autoantibodies stimulate dendritic cells, which in turn produce type 1 interferons. Therefore, the adaptive immune response pathway and the innate immune response pathway play important roles in the pathogenesis of systemic autoimmunity (76–78). Studies have shown that systemic autoimmune diseases (such as RA, SLE, etc.) share common genetic risk loci, and the pathogenic mechanisms may be similar (79–84). Dysregulation of basic innate immune strategies, such as the complement pathway, IFN synthesis and response to IFN, and neuromodulatory mechanisms of immune responses, contribute to autoimmunity and tissue damage in SLE (85–88).
Adaptive immune cells in the adaptive immune system also play an important role. With the activation of dendritic cells, more interferon is produced in the autoimmune response, which promotes and maintains the self-reaction, keeps the activated T cells and B cells in a vicious circle, and produces autoantibodies. Various cytokines activate Naive T cells to promote their (Naive T cells) differentiation into helper T cells (Th cells) and Regulatory T (Treg) through different transcription factors. These Th cells and Treg further secrete different cytokines, which in turn recruit different immune cells and coordinate different immune effector mechanisms (89, 90). There are also interactions between different subgroups, forming complex regulation and action mechanisms (91).
Curcumin has been shown to be a potent immunomodulator, which can modulate the activity of T cells, B cells, macrophages, neutrophils, NK cells, and dendritic cells (92, 93). The diverse pharmacological activities of curcumin stem from its ability to interact with different biological targets and signaling pathways (94). The immunomodulatory activity of curcumin may involve the direct targeting (activation) of TLRs (such as TLR4: the receptor for LPS) through pathogen-associated molecular patterns (PAMPs) (95). It may also be attributed to the regulation of various transcription factors, such as nuclear factor (NF-κB), activator protein 1 (AP-1), signal transducer and activator of transcription (STAT) and their downstream signaling pathways (96–100).
In the regulation of immune cells of the innate immune system: curcumin inhibits the maturation of DCs by inhibiting IDO expression through a cyclooxygenase (COX-2)/prostaglandin E2 (PG-E2)-dependent pathway (101, 102). Several curcumins can reduce neutrophil recruitment to inflamed tissues through direct effects on neutrophil chemotaxis and chemotaxis (103, 104). Curcumin can reduce the production of antibodies (IgG2a, IgE and IgG1, especially IgG1) in rat splenocytes in response to LPS (105, 106). The immunomodulatory effects of curcumin on CD8+ and CD4+ T cell subsets such as curcumin can inhibit the production of Th1 cytokine profile in CD4+ T cells by inhibiting the production of IL-12 in macrophages (107). From a morphological point of view, intestinal dendritic cells (DC) treated with curcumin caused intestinal T cells to become unresponsive. Furthermore, the antigen-presenting properties of curcumin-treated DCs are blocked, resulting in reduced induction of the adaptive immune system (108). DCs treated with curcumin stimulated the differentiation of intestinal Tregs, which prevented disease progression in an animal model of colitis (109). Other studies have shown that curcumin pretreatment of DCs inhibits LPS-stimulated NF-κB p65 translocation and MAPK phosphorylation, thereby reducing inflammation (99). Curcumin also reduced proinflammatory cytokine upregulation, primarily IL-12, and hindered Th1-type responses in DCs. In addition, curcumin has been shown to reduce the expression of ICAM-1 (intercellular adhesion molecule-1) and CD11c, proteins involved in cell adhesion and T cell stimulation, possibly through an AP-1-dependent pathway.
In modulating B-cell immunity: In vitro studies have also shown that curcumin reduces serum levels of BAFF following total IgG, Toll-like receptor (TLR) 4 stimulation. It also reduced B cell activation in MRL/lpr mice and reduced lupus nephritis in MRL/lpr mice. This may represent a holistic treatment strategy in the management of SLE (110).
In regulating T cell immunity: Low-dose curcumin treatment (0.1 and 1 μg/ml) was also shown to reduce Th17 cells, IL-17A levels, and increase Treg and TGF-β1 concentrations on CD4+ T cells in SLE patients compared with normal controls (111). Various studies have reported that curcumin can precisely alter Th17/Treg balance on CD4+ T cells and alleviate organ damage in SLE subjects (111). Kalim et al. also found reduction in Th17 and improvement in Treg cells following administration of 200 mg kg day-1 curcumin in a mouse model of SLE (112). Furthermore, curcumin intervention in T cells obtained from a mouse model of psoriasis showed that 10 μM curcumin concentration significantly blocked the secretion of IL-17, IL-22, IFN-γ, IL-2, IL-8 and TNF-α (113). Xie etal. (114) found a reduction in the severity of EAE in rats treated with curcumin. Curcumin attenuated the severity of experimental allergic encephalomyelitis (EAE) due to inhibition of Th17 cell differentiation and growth by downregulating IL-6, IL-21, RORΔt signaling and STAT3 phosphorylation. Along these lines, Liu et al. found that the viability of CD4+ T cells in vitro was considerably inhibited by curcumin treatment, and furthermore, curcumin induced significant apoptosis of Th1, Th17 and Treg cells (115).
4.2 Curcumin for Takayasu Arteritis
Only one RCT reported the effects and safety of curcumin in the treatment of Takayasu arteritis. They found that curcumin may improve BVAS and inflammation indicators. Therefore, TNF-α was considered to be significantly associated with a decrease in BVAS score (γ2 = 0.81, P = 0.016), ESR (γ2 = 0.76, P = 0.037), and plasma CRP level (γ2 = 0.79, p = 0.041). The findings suggest that curcumin may significantly improve the therapeutic effect through its anti-TNF-α effect (43). However, more reports are needed to further verify the therapeutic effect and safety of curcumin on Takayasu arteritis.
4.3 Curcumin for Psoriasis
This study included 2 relevant RCTs and found that curcumin improved PASI in patients with psoriasis without increasing side effects compared with controls. Psoriasis is a multifactorial mediated chronic inflammatory hereditary skin disease characterized by inflammatory erythema overlying multiple layers of silvery-white mica-like scales with localized or widespread systemic distribution (116). The incidence of psoriasis vulgaris in the general population is relatively high, and the incidence in European and American countries is about 2.1%, while that in China is about 0.123%, which can seriously affect the interpersonal communication and daily life of patients (117). At present, it is generally believed that psoriasis is caused by immune imbalance caused by external stimuli such as infection, trauma, mental stress and other factors under the influence of the patient’s genetic susceptibility (118). The role of immunity in the pathogenesis of psoriasis has been widely accepted, and psoriasis genome-wide scans have also shown that most susceptibility genes are associated with immunity (119, 120). The immunological mechanism of psoriasis is complex. The main participants are keratinocytes and a variety of immune cells, including dendritic cells, macrophages, neutrophils and T cells in innate and adaptive immunity (121, 122). The relationship between innate immunity and adaptive immune system of psoriasis is realized by cytokines. The representative inflammatory factors include TNF-α, IFN-γ, IL-17 A and IL-1, and the inhibitory inflammatory factors (regulatory inflammatory factors) include IL-4, IL-10 and TGF-β (123, 124). Activated cytokines bind to corresponding receptors as specific ligands, and coordinately mediate the pathological changes of psoriasis. Both the adaptive immune system and the innate immune system can mediate the production of inflammatory mediators, and the latter play an important role in the induction and maintenance of dermal and epidermal psoriasis pathological changes (125–127). Different topical and systemic treatment options are currently available for the treatment of psoriasis, but they have suboptimal clinical outcomes and risk side effects (128, 129). Multiple current studies have reported that curcumin can reduce oxidative stress in psoriatic lesions (130). In addition, the therapeutic efficacy of curcumin may also be related to its ability to inhibit phosphorylase kinase. Curcumin may inhibit the proliferation of psoriasis-like cells by down-regulating pro-inflammatory cell (HaCaT cell) factors such as IL-17, TNF-α, INF-γ and IL-6 (131). Furthermore, curcumin significantly enhanced skin barrier function by upregulating involucrin (iNV) and filaggrin (FLG) (58, 132). Curcumin can inhibit the pro-proliferation effect of VEGF on HaCaT cells and promote the apoptosis of HaCaT cells. In vivo studies have shown that curcumin has no observed side effects in the treatment of psoriasis patients (26, 133), and the U.S. Food and Drug Administration (FDA) has defined curcumin as “generally regarded as safe” (GRAS). In summary, curcumin has great potential as a treatment for psoriasis. However, more RCTs are needed to further verify the therapeutic effect and safety of curcumin on Psoriasis.
4.4 Curcumin for SLE
Only Two (2) RCTs explored the effects and safety of curcumin and curcuma longa Extract in the treatment of SLE. They found no increase in side effects with the addition of curcumin, and found that curcumin may improve SLEDAI and reduce IL-6 and TGF-β1. However, since the data could not be combined for meta-analysis, they need to be interpreted with caution. SLE is the most diverse autoimmune disease, which can affect any organ of the body, can have a wide range of clinical and immunological manifestations, and its incidence is increasing year by year (134). SLE is characterized by the presence of multiple autoantibodies in serum (135). Autoantibodies closely related to SLE mainly include antinuclear antibodies, anti-Sm antibodies, anti-double-stranded DNA antibodies, and anti-SSA antibodies (136). High titers of double-stranded DNA are associated with SLE glomerular deposition and nephritis activity, and antiphospholipid antibodies are associated with SLE prone to coagulation (137). Treatment options for SLE depend on the organ involved. Patients with mild symptoms can be treated with low-dose corticosteroids and are usually controlled, but moderate and severe disease may require higher doses of corticosteroids or other immunosuppressants (138). Pharmacological studies of curcumin on SLE showed that SLE is characterized by the activation of the complement system, and curcumin can inhibit the complement cascade (139). Curcumin can prevent oxidative stress of oxidatively modified proteins by binding to proteins, and can also inhibit the activation of B cells to effectively treat SLE (140). Low doses of curcumin can also specifically regulate Th17/Treg balance in CD4 + T cell cultures from SLE (111). Curcumin reduced proteinuria and serum levels of IgG1, IgG2a and anti-dsDNA IgG antibodies in female NZB/W F1 mice (141). In summary, curcumin may enhance regulatory responses involving T reg cells by inhibiting antibody-antigen interactions, reducing autoantigen-autoantibody deposition in tissues and various microvascular beds, and inhibiting antibody production. However, more RCTs are needed to further verify the therapeutic effect and safety of curcumin on SLE.
4.5 Curcumin for MS
Only Two (2) RCTs explored the effects and safety of curcumin and curcuma longa Extract in the treatment of MS. They both found that curcumin could reduce inflammatory factors, however, their findings were different on the improvement of EDSS. However, since the data could not be combined for meta-analysis, they need to be interpreted with caution. MS is an inflammatory demyelinating disease of the central nervous system that affects millions of people around the world, and approximately 30% of MS patients develop clinical paralysis (142). Destruction of myelinating oligodendrocytes is a pathological feature of MS, and axonal deformation leads to irreversible long-term disability (143). The activation of immune cells, the secretion of inflammatory cytokines and the differentiation of Th1 cells are the key processes in the pathogenesis of MS (144). EAE is an autoimmune disease of the central nervous system that has been used as a model to study the pathogenesis of MS and to test the efficacy of treatments for MS (145, 146). (147) observed the protective effect of curcumin in the treatment of EAE in SJL/J rats, and found that 50-100 mg of curcumin administered by gavage every other day could alleviate the symptoms of EAE in SJL/J rats. In addition, curcumin can inhibit neural antigen-induced T cell proliferation, Th1 differentiation, and TNFγ production (148). Yang etal. (149) found that curcumin can significantly improve the clinical symptoms of EAE rats, inhibit the infiltration of inflammatory cells, and promote the recovery of rats, thereby treating MS. In EAE-induced rats, curcumin treatment was found to significantly reduce the number of inflammatory cells infiltrating the spinal cord (114). Curcumin treatment was also associated with upregulation of IL-10 levels and increased percentage of CD4+CD25+-Foxp3+ Treg cells in the CNS and lymphoid organs in EAE-induced C57BL/6 mice. Furthermore, curcumin ameliorated EAE in SJL/J mice by inhibiting IL-12 signaling through the JAK-STAT pathway, resulting in reduced Th1 differentiation (150). In vivo treatment with curcumin has been shown to increase the expression of PPARγ in the central nervous system and lymphoid organs of EAE mice, suggesting that it is involved in the regulation of Th1/Th17 responses in EAE (151). Curcumin may inhibit IL-17 mRNA expression and T cell INF-γ levels in MS patients (150). Curcumin may inhibit the proliferation of TEM cells and the production of pro-inflammatory cytokines by inhibiting the hKv1.3 channel, which contributes to the curcumin’s efficacy in the treatment of autoimmune diseases (152). These basic experimental results all suggest that curcumin may be used to treat MS, therefore, more RCTs are still needed to further verify the therapeutic effect and safety of curcumin on MS.
4.6 Curcumin for AS
Only (49) explored the effects and safety of curcumin and curcuma longa Extract in the treatment of AS. They found that Treg cells increased significantly, the levels of IL-10 and TGF-β increased, and the level of IL-6 decreased after curcumin intervention. AS is a disease characterized by inflammation of the sacroiliac joints and spinal attachments as the main symptom, mainly affecting the axial skeleton, sacroiliac joints, and peripheral joints, and can cause structural changes and dysfunction (153, 154). The main clinical features of AS are intermittent low back pain, discomfort and stiffness in other parts of the body (155), which eventually progresses to spinal immobility and rigidity (156). Recent studies have shown that AS is also a T lymphocyte-mediated disease, which is supported by changes in the frequency of CD4 + T cells in the peripheral blood of patients with AS, including an increase in Th17 frequency with Th2 and a decrease in CD4 + CD25 + Treg (157–159). The results of most studies suggest that the Treg/Th17 ratio is reduced in the PB of AS patients, and speculate that altered immunophenotypes may play a role in the pathogenesis of these diseases, thus modulating the balance between Treg and Th17 may reduce disease activity (160, 161). Recent studies have found that curcumin may enhance Treg differentiation by increasing FoxP3 expression (111, 162), which is consistent with the RCT findings of Ahmadi et al., 2019. However, more RCTs are still needed to further verify the therapeutic effect and safety of curcumin on MS.
4.7 Curcumin for BD
Only (39) explored the effects and safety of curcumin and curcuma longa Extract in the treatment of BD. They found that BDCAF was decreased in patients treated with curcumin. They also found that Treg cells, the levels of IL-10 and TGF-β in the nanocurcumin group increased significantly, while IL-17 and IL-23 decreased. No obvious adverse events were observed in the curcumin group and the control group. BD is a systemic inflammatory disease with oral and vulvar ulcers and uveitis as the main clinical manifestations (163). Pathological manifestations are vasculitis involving various vascular diameters, with a tendency to thrombosis (164). The pathogenesis of BD is unclear. In addition to environmental and genetic factors, abnormal T cell immune responses are also involved in the pathogenesis, involving innate immune γδ T cells, identification of characteristic antigens, antigen presentation, and adaptive immunity represented by CD4+ and CD8+ T cells. (165, 166). The imbalance of T cell homeostasis is mainly manifested by the activation, proliferation and Treg cell damage of Th1 and Th17 helper T cells (167). The above-mentioned abnormal immune response induces and maintains the pro-inflammatory environment of BD (168). Previous studies have described that curcumin enhances the mucosal healing process of oral ulcers in humans and animals (169–171). Mohammad et al. found that the mRNA expression of IL-1β and the protein production of IL-6 and TNFα were significantly down-regulated in M1 macrophages from BD patients after curcumin (30 µg/ml) intervention (172). Curcumin also reduced macrophage polarization and production of proinflammatory cytokines IL-1β and TNFα in the human macrophage cell line M1 (173–175), which is consistent with the RCT report of (39). However, more RCTs are still needed to further verify the therapeutic effect and safety of curcumin on BD.
4.8 Curcumin for UC and Crohn’s Disease
Nine RCTs reported the effects and safety of curcumin and curcuma longa Extract in the treatment of UC. Two (2) RCTs explored the effects and safety of curcumin and curcuma longa Extract in the treatment of Crohn’s Disease. Curcumin may improve the clinical activity index, clinical response and endoscopic response, and decrease the ESR and CRP for patients with UC. (59) found that Curcumin was no more effective than placebo in preventing Crohn’s disease relapse and probably did not improve patients’ quality of life. (60) found that with the addition of curcumin, clinical remission and Endoscopic remission improved. Meanwhile, the addition of curcumin may not increase the incidence of adverse events. However, more RCTs are still needed to further verify the therapeutic effect and safety of curcumin on UC and Crohn’s Disease.
Inflammatory bowel disease (IBD) is currently considered to be an autoimmune disease, mainly including ulcerative colitis (UC) and Crohn’s disease, which are histopathologically manifested as intestinal mucosal inflammation (176, 177). IBD is an autoimmune disease involving autoantibodies and autoreactive CD4+ T lymphocytes. CD4+ T cells are essential for regulating immune homeostasis in the gut, and innate immune abnormalities will cause CD4+ T cells (Th1, Th2, Th17) to produce a large number of pro-inflammatory factors, making the immune response beyond the range of Treg regulation, thereby causing IBD (178, 179). Although the pathogenesis of UC and Crohn’s disease is characterized by an immune response to gut (bacterial) antigens, they differ in the type of inflammatory response. The pathogenesis of Crohn’s disease is mainly related to the inflammatory response dominated by cytokines IL-1, IL-6, IL-8, TNF-α and IFN-γ secreted by Th1 and Th17 cells (180). UC is related to the inflammatory response dominated by cytokines such as IL-4, IL-5, IL-9 and 1L-13 secreted by Th2 cells (181). Therefore, it is possible to try to regulate the CD4+ T cell population and related cytokines and signaling pathways as one of the means of IBD treatment (182, 183). Zhang etal. (148) found that curcumin decreased the expression of Th1 cytokines and up-regulated the expression of Th2 cytokines in the colonic mucosa. Lee et al. (179) also found that curcumin could alleviate ovalbumin (OVA)-induced food allergy by regulating the balance between Th1/Th2. This indicates that curcumin plays an anti-inflammatory role by changing the immune balance between Th1-Th2 to Th2-type immunity. Li etal. (184) used curcumin to treat dextran sulfate sodium (DSS)-induced IBD, and found that p38MAPK protein and mRNA expression were significantly reduced, and TNF-α production was also reduced. This suggests that curcumin may play a role in the treatment of IBD by inhibiting the p38MAPK pathway and reducing the production of TNF-α. In addition, curcumin may reduce apoptosis and alleviate colon injury by regulating the p38MAPK and JNK pathways (185). Curcumin can exert an anti-inflammatory effect in experimental colitis by inhibiting the STAT3 pathway (186). Yang etal. (187) showed that curcumin can inhibit the expression of cyclinD1 and CDK4 with cell proliferation effect by inhibiting the STAT-3 signaling pathway, showing anti-inflammatory effect in IBD. Guo etal. (188) found that flavin may increase the level of peroxisome proliferator-activated receptor-g (PPAR-g), inhibit the STAT3 signaling pathway, reduce the release of COX-2, and reduce the infiltration of neutrophils.
4.9 Curcumin for RA
Five RCTs reported the effects and safety of curcumin and curcuma longa Extract in the treatment of RA. They found that curcumin may decrease DAS28, ESR and CRP. Meanwhile, the addition of curcumin may not increase the incidence of adverse events. However, more RCTs are still needed to further verify the therapeutic effect and safety of curcumin on RA.
RA is a systemic autoimmune disease characterized by chronic destructive joint disease (189, 190), and 0.5% to 1% of the world’s population is affected by RA (191). RA is characterized by synovitis and pannus production, and activated macrophages and dendritic cells are important sources of key inflammatory cytokines, including TNF-α and IL-1, as well as promoting inflammatory cells. Accumulated and synthesized cytokines, such as chemokines, matrix metalloproteinases (MMPs), COX-2 and other inflammatory mediators (192, 193). CD4+ Th cells play a key role in the occurrence of immune responses, B cells contribute to the progression of inflammation, and RA occurs by activating T cells (194). The main drugs for the treatment of RA include NSAIDs, disease-modifying anti-rheumatic drugs (DMARDs), and biological agents (190, 195), but these drugs have serious side effects. Curcumin was found to have anti-rheumatic activity in humans (196). Curcumin can inhibit the occurrence and development of RA mainly by inhibiting inflammatory cytokines and MMPs and blocking signaling pathways, including mitogen-activated protein kinase, activator protein-1 and nuclear factor-κB receptor activator ligand, etc. (197). Shang (198) found that curcumin can effectively inhibit angiogenesis by inhibiting the expression of hypoxia-inducible factor 1-α, thereby protecting the synovium of joints. In addition, curcumin protects chondrocytes by inhibiting the activation of caspase-3 by preventing the integration of IL-1β (199). Curcumin can reduce the gene expression of adhesion molecules, β3 and β7 integrins, thereby reducing joint inflammation in RA (200).
4.10 Curcumin for Oral Lichen Planus
Six RCTs reported Curcumin and Curcuma longa Extract in the Treatment of Oral lichen planus. (64), (67) found that curcumin may decrease Modified oral mucositis index. However, (68) found no significant difference in efficacy between Curcumin and Prednisolone. Since their indicators could not be combined, Meta-analysis was not conducted. and meta-analysis showed that curcumin may not reduce Thongprasom score. Therefore, more RCTs are still needed to further verify or modify the therapeutic effect and safety of curcumin on Oral lichen planus. Oral lichen planus is a chronic inflammatory disease of the oral mucosa of unknown etiology (201). At present, its pathogenesis is often related to mental factors, immune factors, infectious factors, endocrine factors, lack of trace elements, microcirculation disorders, and some systemic diseases (202, 203). The basis of Oral lichen planus may be T cell-mediated responses to unknown triggers (204, 205). Observational studies have also shown that patients with Oral lichen planus are more prone to an antioxidant-oxidative stress imbalance (206, 207). Treatment for Oral lichen planus is aimed at managing symptoms. Topical or systemic application of glucocorticoids and immunosuppressants is the main clinical treatment for Oral lichen planus. Although it can obtain a relatively reliable curative effect, its long application period and many adverse reactions lead to poor patient compliance, which is the main problem that plagues clinical diagnosis and treatment (202, 208). Meanwhile, there is still a lack of safer and more effective treatment options for some patients with intractable damage or those with complex systemic history. Curcumin, an active phytochemical in turmeric, is a strongly hydrophobic compound with poor bioavailability, which poses challenges for oral ingestion (209). On the other hand, curcumin was not shown to be toxic at doses of 8 g per day, making it a potentially attractive alternative to drugs currently used to treat various diseases (210).
4.11 Implications for the Future
Current pharmacological studies have shown that Curcumin and Curcuma longa Extract seems to reverse some clinical symptoms of many autoimmune diseases by regulating immune inflammatory biological modules, such as inflammatory factors (such as IL-1, IL-17, IL-6, IL-12, TNF-α and IFN-γ) -mediated inflammatory signaling pathways and immune inflammatory cell activation, differentiation and immune function regulation. Curcumin and Curcuma longa Extract is an effective natural compound with a variety of therapeutic pharmacological properties and almost no side effects. Recent studies have shown that curcumin can synergistically enhance the synergistic effect of glucocorticoids and alleviate glucocorticoid-induced osteoporosis (211, 212). Therefore, the future design of RCTs may consider combining Curcumin and Curcuma longa Extract with existing conventional drug therapies such as glucocorticoids. It may be a potential side effect of reducing glucocorticoid dose and preventing glucocorticoid use to autoimmune diseases. Future RCTs should accurately determine the appropriate dose, dosage regimen, treatment duration, and further clarify the mechanism of action related to systemic autoimmune diseases. In terms of treatment and preparation, an important issue in the treatment of autoimmune diseases by oral Curcumin and Curcuma longa Extract is to improve its bioavailability (213). Although a large number of studies have been conducted on curcumin preparations to improve bioavailability, it still needs to be further improved.
5 Conclusion
This systematic review and meta-analysis evaluated the efficacy and safety of Curcumin and Curcuma longa Extract in autoimmune diseases, such as AS, BD, Crohn’s Disease, MS, Oral lichen planus, Psoriasis, RA, SLE, Takayasu arteritis, UC. Because of its good clinical safety, the dose of curcumin in the treatment of autoimmune diseases is mainly between 80 mg and 6000 mg. The results of Meta-analysis showed that Curcumin and Curcuma longa Extract had good clinical efficacy in the treatment of Psoriasis, UC and RA, so Curcumin and Curcuma longa Extract could be used in the treatment of the above diseases in the future. The results of Meta-analysis showed that Curcumin and Curcuma longa Extract did not show efficacy in the treatment of oral lichen planus, while Takayasu arteritis, SLE, MS, AS, BD and CD did not report sufficient clinical data for meta-analysis; hence, more RCTs are still needed in the future. Curcumin and Curcuma longa Extract may regulate inflammatory cells (such as Treg) and inflammatory factors (such as CRP, ESR, TNF-α, TGF-β1, IL-6 level), which may be its mechanism for treating autoimmune diseases. However, due to the low quality and small number of RCTs in most autoimmune diseases, the conclusions need to be carefully interpreted. At present, large-sample, multi-center clinical trials are still needed for revision or validation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
LZ, TY, KY, HC are responsible for the study concept and design. LZ, TY, KY, GY, JL, WX, and HC are responsible for the data collection, data analysis and interpretation; LZ and KY drafted the paper; HC supervised the study; all authors participated in the analysis and interpretation of data and approved the final paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.896476/full#supplementary-material
References
1. Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, et al. Vitamin D and Marine Omega 3 Fatty Acid Supplementation and Incident Autoimmune Disease: VITAL Randomized Controlled Trial. BMJ (2022) 376:e066452. doi: 10.1136/bmj-2021-066452
2. Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front Immunol (2018) 9:2279. doi: 10.3389/fimmu.2018.02279
3. Wang C, Liao Q, Hu Y, Zhong D. T Lymphocyte Subset Imbalances in Patients Contribute to Ankylosing Spondylitis. Exp Ther Med (2015) 9:250–256. doi: 10.3892/etm.2014.2046
4. Rose NR. Prediction and Prevention of Autoimmune Disease in the 21st Century: A Review and Preview. Am J Epidemiol (2016) 183(5):403–6. doi: 10.1093/aje/kwv292
5. Zhang Y, Liu J, Wang C, Liu J, Lu W. Toll-Like Receptors Gene Polymorphisms in Autoimmune Disease. Front Immunol (2021) 12:672346. doi: 10.3389/fimmu.2021.672346
6. Kapsogeorgou EK, Tzioufas AG. Autoantibodies in Autoimmune Diseases: Clinical and Critical Evaluation. Isr Med Assoc J (2016) 18(9):519–24.
7. Yasunaga M. Antibody Therapeutics and Immunoregulation in Cancer and Autoimmune Disease. Semin Cancer Biol (2020) 64:1–12. doi: 10.1016/j.semcancer.2019.06.001
8. Zarrin AA, Bao K, Lupardus P, Vucic D. Kinase Inhibition in Autoimmunity and Inflammation. Nat Rev Drug Discovery (2021) 20(1):39–63. doi: 10.1038/s41573-020-0082-8
9. Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging Role of Vitamin D in Autoimmune Diseases: An Update on Evidence and Therapeutic Implications. Autoimmun Rev (2019) 18(9):102350. doi: 10.1016/j.autrev.2019.102350
10. Mok CC. Therapeutic Monitoring of the Immuno-Modulating Drugs in Systemic Lupus Erythematosus. Expert Rev Clin Immunol (2017) 13(1):35–41. doi: 10.1080/1744666X.2016.1212659
11. Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, et al. Systemic Lupus Erythematosus. Nat Rev Dis Primers (2016) 2:16039. doi: 10.1038/nrdp.2016.39
12. Zhang Q, Liu J, Zhang M, Wei S, Li R, Gao Y, et al. Apoptosis Induction of Fibroblast-Like Synoviocytes Is an Important Molecular-Mechanism for Herbal Medicine Along With its Active Components in Treating Rheumatoid Arthritis. Biomolecules (2019) 9(12):795. doi: 10.3390/biom9120795
13. Paoliello-Paschoalato AB, Marchi LF, de Andrade MF, Kabeya LM, Donadi EA, Lucisano-Valim YM. Fcγ and Complement Receptors and Complement Proteins in Neutrophil Activation in Rheumatoid Arthritis: Contribution to Pathogenesis and Progression and Modulation by Natural Products. Evid Based Complement Alternat Med (2015) 2015:429878. doi: 10.1155/2015/429878
14. Kocaadam B, Şanlier N. Curcumin, an Active Component of Turmeric (Curcuma Longa), and Its Effects on Health. Crit Rev Food Sci Nutr (2017) 57(13):2889–95. doi: 10.1080/10408398.2015.1077195
15. Soleimani V, Sahebkar A, Hosseinzadeh H. Turmeric (Curcuma Longa) and its Major Constituent (Curcumin) as Nontoxic and Safe Substances: Review. Phytother Res (2018) 32(6):985–95. doi: 10.1002/ptr.6054
16. Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front Pharmacol (2020) 11:1021. doi: 10.3389/fphar.2020.01021
17. Kotha RR, Luthria DL. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules (2019) 24(16):2930. doi: 10.3390/molecules24162930
18. Ahmad RS, Hussain MB, Sultan MT, Arshad MS, Waheed M, Shariati MA, et al. Biochemistry, Safety, Pharmacological Activities, and Clinical Applications of Turmeric: A Mechanistic Review. Evid Based Complement Alternat Med (2020) 2020:7656919. doi: 10.1155/2020/7656919
19. Gupta SC, Patchva S, Aggarwal BB. Therapeutic Roles of Curcumin: Lessons Learned From Clinical Trials. AAPS J (2013) 15(1):195–218. doi: 10.1208/s12248-012-9432-8
20. Abd El-Hack ME, El-Saadony MT, Swelum AA, Arif M, Abo Ghanima MM, Shukry M, et al. Curcumin, the Active Substance of Turmeric: Its Effects on Health and Ways to Improve its Bioavailability. J Sci Food Agric (2021) 101(14):5747–62. doi: 10.1002/jsfa.11372
21. Razavi BM, GhasemzadehRahbardar M, Hosseinzadeh H. A Review of Therapeutic Potentials of Turmeric (Curcuma Longa) and Its Active Constituent, Curcumin, on Inflammatory Disorders, Pain, and Their Related Patents. Phytother Res (2021) 35(12):6489–513. doi: 10.1002/ptr.7224
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:1715. doi: 10.1136/bmj.n71
23. Deeks JJ, Higgins JP, Altman DG. Chapter 8: Assessing Risk of Bias in Included Studies. In: Higgins JP, Green S, editors. Cochrane Handbook or Systematic Reviews of Interventions Version 6.1.0. UK: The Cochrane Collaboration (2020).
24. Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analyzing Data and Undertaking Meta-Analyses. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. UK: The Cochrane Collaboration (2020).
25. Deeks JJ, Higgins JP, Altman DG. Chapter 16: Special Topics in Statistics. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. UK: The Cochrane Collaboration (2020).
26. Kurd SK, Smith N, VanVoorhees A, Troxel AB, Badmaev V, Seykora JT, et al. Oral Curcumin in the Treatment of Moderate to Severe Psoriasis Vulgaris: A Prospective Clinical Trial. J Am Acad Dermatol (2008) 58(4):625–31. doi: 10.1016/j.jaad.2007.12.035
27. Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, Martinez-Andres A, Asín-Llorca M, Bernd A, et al. Effects of Curcuma Extract and Visible Light on Adults With Plaque Psoriasis. Eur J Dermatol (2015) 25(3):240–6. doi: 10.1684/ejd.2015.2584
28. Cai M, Liu X. Clinical Study on Curcumin Combined With Hyaluronic Acid-Modified Ethanoloplasts in the Treatment of Anti-Psoriasis. China J Integrated Traditional Chin Western Med Dermatol (2020) 19(04):322–325+ 329. doi: CNKI:SUN:ZXYD.0.2020-04-008
29. Zhang Y, Liu Z, Ren S, Zeng L. Clinical Observation of Curcumin in the Treatment of Psoriasis and Its Effect on the Activation of NF-Kappab. Jiangxi Med (2012) 47(11):944–6. doi: CNKI:SUN:JXYY.0.2012-11-001
30. Dolati S, Aghebati-Maleki L, Ahmadi M, Marofi F, Babaloo Z, Ayramloo H, et al. Nanocurcumin Restores Aberrant miRNA Expression Profile in Multiple Sclerosis, Randomized, Double-Blind, Placebo-Controlled Trial. J Cell Physiol (2018) 233(7):5222–30. doi: 10.1002/jcp.26301
31. Banerjee R, Penmetsa A, Medaboina K. Novel Bio-Enhanced Curcumin With Mesalamine for Induction of Remission in Mild to Moderate Ulcerative Colitis. Gastroenterology (2017) 152(5):S587. doi: 10.1016/S0016-5085(17)32111-X
32. Kumar S, Dutta U, Shah J. P427 Impact of Curcuma Longa on Clinical Activity and Inflammatory Markers in Patients With Active Ulcerative Colitis: A Double-Blind Randomised Placebo-Controlled Trial. J Crohn s Colitis (2019) 13(Supplement_1):S322–3. doi: 10.1093/ecco-jcc/jjy222.551
33. Nosratzehi T, Arbabi-Kalati F, Hamishehkar H, Bagheri S. Comparison of the Effects of Curcumin Mucoadhesive Paste and Local Corticosteroid on the Treatment of Erosive Oral Lichen Planus Lesions. J Natl Med Assoc (2018) 110(1):92–7. doi: 10.1016/j.jnma.2017.01.011
34. Dolati S, Babaloo Z, Ayromlou H, Ahmadi M, Rikhtegar R, Rostamzadeh D, et al. Nanocurcumin Improves Regulatory T-Cell Frequency and Function in Patients With Multiple Sclerosis. J Neuroimmunol (2019) 327:15–21. doi: 10.1016/j.jneuroim.2019.01.007
35. Dolati S, Ahmadi M, Rikhtegar R, Babaloo Z, Ayromlou H, Aghebati-Maleki L, et al. Changes in Th17 Cells Function After Nanocurcumin Use to Treat Multiple Sclerosis. Int Immunopharmacol (2018a) 61:74–81. doi: 10.1016/j.intimp.2018.05.018
36. Zhu L, Zhu D. Clinical Observation of Curcumin Combined With Mesalazine in the Treatment of Mild to Moderate Ulcerative Colitis. Guangdong Med (2019) 40(20):2921–4. doi: 10.13820/j.cnki.gdyx.20190913
37. Zhu L. Clinical Evaluation of Curcumin Combined With Mesalazine in the Treatment of Mild to Moderate Ulcerative Colitis. South Med Univ (2019).
38. Abbasian S, Soltani-Zangbar MS, Khabbazi A, Farzaneh R, Malek Mahdavi A, Motavalli R, et al. Nanocurcumin Supplementation Ameliorates Behcet's Disease by Modulating Regulatory T Cells: A Randomized, Double-Blind, Placebo-Controlled Trial. Int Immunopharmacol (2021) 101(Pt B):108237. doi: 10.1016/j.intimp.2021.108237
39. Farzaneh R, Khabbazi A, Soltani-Zangbar MS, Abbasian S, Malek Mahdavi A, Motavalli R, et al. Effects of Nanocurcumin Supplementation on T-Helper 17 Cells Inflammatory Response in Patients With Behcet's Disease: A Randomized Controlled Trial. ImmunopharmacolImmunotoxicol (2022) 12:1–10. doi: 10.1080/08923973.2022.2026380
40. Amalraj A, Varma K, Jacob J, Divya C, Kunnumakkara AB, Stohs SJ, et al. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J Med Food (2017) 20(10):1022–30. doi: 10.1089/jmf.2017.3930
41. Jacob J, Amalraj A, Raj KKJ, Divya C, Kunnumakkara AB, Gopi S. A Novel Bioavailable Hydrogenated Curcuminoids Formulation (CuroWhite™) Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients - A Randomized, Double Blind and Placebo Controlled Study. J Tradit Complement Med (2018) 9(4):346–52. doi: 10.1016/j.jtcme.2018.06.001
42. Chandran B, Goel A. A Randomized, Pilot Study to Assess the Efficacy and Safety of Curcumin in Patients With Active Rheumatoid Arthritis. Phytother Res (2012) 26(11):1719–25. doi: 10.1002/ptr.4639
43. Shao N, Jia H, Li Y, Li J. Curcumin Improves Treatment Outcome of Takayasu Arteritis Patients by Reducing TNF-α: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Immunol Res (2017) 65(4):969–74. doi: 10.1007/s12026-017-8917-z
44. Antiga E, Bonciolini V, Volpi W, Del Bianco E, Caproni M. Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients With Psoriasis Vulgaris. BioMed Res Int (2015) 2015:283634. doi: 10.1155/2015/283634
45. Bilia AR, Bergonzi MC, Isacchi B, Antiga E, Caproni M. Curcumin Nanoparticles Potentiate Therapeutic Effectiveness of Acitrein in Moderate-to-Severe Psoriasis Patients and Control Serum Cholesterol Levels. J Pharm Pharmacol (2018) 70(7):919–28. doi: 10.1111/jphp.12910
46. Khajehdehi P, Zanjaninejad B, Aflaki E, Nazarinia M, Azad F, Malekmakan L, et al. Oral Supplementation of Turmeric Decreases Proteinuria, Hematuria, and Systolic Blood Pressure in Patients Suffering From Relapsing or Refractory Lupus Nephritis: A Randomized and Placebo-Controlled Study. J Ren Nutr (2012) 22(1):50–7. doi: 10.1053/j.jrn.2011.03.002
47. SinggihWahono C, DiahSetyorini C, Kalim H, Nurdiana N, Handono K. Effect of Curcuma Xanthorrhiza Supplementation on Systemic Lupus Erythematosus Patients With Hypovitamin D Which Were Given Vitamin D3 Towards Disease Activity (SLEDAI), IL-6, and TGF-β1 Serum. Int J Rheumatol (2017) 2017:7687053. doi: 10.1155/2017/7687053
48. Petracca M, Quarantelli M, Moccia M, Vacca G, Satelliti B, D'Ambrosio G, et al. ProspeCtive Study to Evaluate Efficacy, Safety and Tolerability of Dietary supplemeNT of Curcumin (BCM95) in Subjects With Active Relapsing MultIple Sclerosis Treated With Subcutaneous Interferon Beta 1a 44 Mcg TIW (CONTAIN): A Randomized, Controlled Trial. MultSclerRelatDisord (2021) 56:103274. doi: 10.1016/j.msard.2021.103274
49. Ahmadi M, Hajialilo M, Dolati S, Eghbal-Fard S, Heydarlou H, Ghaebi M, et al. The Effects of Nanocurcumin on Treg Cell Responses and Treatment of Ankylosing Spondylitis Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Cell Biochem (2019) 121(1):103–10. doi: 10.1002/jcb.28901
50. Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, et al. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin Gastroenterol Hepatol (2006) 4(12):1502–6. doi: 10.1016/j.cgh.2006.08.008
51. Singla V, Pratap Mouli V, Garg SK, Rai T, Choudhury BN, Verma P, et al. Induction With NCB-02 (Curcumin) Enema for Mild-to-Moderate Distal Ulcerative Colitis - A Randomized, Placebo-Controlled, Pilot Study. J Crohns Colitis (2014) 8(3):208–14. doi: 10.1016/j.crohns.2013.08.006
52. Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har-Noy O, et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-To-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol (2015) 13(8):1444–9.e1. doi: 10.1016/j.cgh.2015.02.019
53. Kedia S, Bhatia V, Thareja S, Garg S, Mouli VP, Bopanna S, et al. Low Dose Oral Curcumin is Not Effective in Induction of Remission in Mild to Moderate Ulcerative Colitis: Results From a Randomized Double Blind Placebo Controlled Trial. World J GastrointestPharmacolTher (2017) 8(2):147–54. doi: 10.4292/wjgpt.v8.i2.147
54. Masoodi M, Mahdiabadi MA, Mokhtare M, Agah S, Kashani AHF, Rezadoost AM, et al. The Efficacy of Curcuminoids in Improvement of Ulcerative Colitis Symptoms and Patients' Self-Reported Well-Being: A Randomized Double-Blind Controlled Trial. J Cell Biochem (2018) 119(11):9552–9. doi: 10.1002/jcb.27273
55. Sadeghi N, Mansoori A, Shayesteh A, Hashemi SJ. The Effect of Curcumin Supplementation on Clinical Outcomes and Inflammatory Markers in Patients With Ulcerative Colitis. Phytother Res (2020) 34(5):1123–33. doi: 10.1002/ptr.6581
56. Banerjee R, Pal P, Penmetsa A, Kathi P, Girish G, Goren I, et al. Novel Bioenhanced Curcumin With Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-To-Moderate Ulcerative Colitis: A Randomized Double-Blind Placebo-Controlled Pilot Study. J Clin Gastroenterol (2021) 55(8):702–8. doi: 10.1097/MCG.0000000000001416
57. Wang He, Lei T. Curative Effect Observation of Turmeric Water Decoction Enema Combined With Infliximab in the Treatment of Refractory Ulcerative Colitis. Chin J Integr Med Digestive Med (2019) 27(03):219–23. doi: CNKI:SUN:ZXPW.0.2019-03-013
58. Wang Z, Wang L, Zheng Y. Effects of Curcumin on the Proliferation, Apoptosis and KLF6/p21 Protein Expression of HaCaT Cells. Zhejiang J Integr Med (2019) 29(05):357–360+436.
59. Bommelaer G, Laharie D, Nancey S, Hebuterne X, Roblin X, Nachury M, et al. Oral Curcumin No More Effective Than Placebo in Preventing Recurrence of Crohn's Disease After Surgery in a Randomized Controlled Trial. Clin Gastroenterol Hepatol (2020) 18(7):1553–1560.e1. doi: 10.1016/j.cgh.2019.08.041
60. Sugimoto K, Ikeya K, Bamba S, Andoh A, Yamasaki H, Mitsuyama K, et al. Highly Bioavailable Curcumin Derivative Ameliorates Crohn's Disease Symptoms: A Randomized, Double-Blind, Multicenter Study. J Crohns Colitis (2020) 14(12):1693–701. doi: 10.1093/ecco-jcc/jjaa097
61. Javadi M, KhademHaghighian H, Goodarzy S, Abbasi M, Nassiri-Asl M. Effect of Curcumin Nanomicelle on the Clinical Symptoms of Patients With Rheumatoid Arthritis: A Randomized, Double-Blind, Controlled Trial. Int J Rheum Dis (2019) 22(10):1857–62. doi: 10.1111/1756-185X.13688
62. Pourhabibi-Zarandi F, Rafraf M, Zayeni H, Asghari-Jafarabadi M, Ebrahimi AA. Effects of Curcumin Supplementation on Metabolic Parameters, Inflammatory Factors and Obesity Values in Women With Rheumatoid Arthritis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytother Res (2022) 36(4):1797–806. doi: 10.1002/ptr.7422
63. Chainani-Wu N, Silverman S Jr, Reingold A, Bostrom A, Mc Culloch C, Lozada-Nur F, et al. A Randomized, Placebo-Controlled, Double-Blind Clinical Trial of Curcuminoids in Oral Lichen Planus. Phytomedicine (2007) 14(7-8):437–46. doi: 10.1016/j.phymed.2007.05.003
64. Chainani-Wu N, Madden E, Lozada-Nur F, Silverman S Jr. High-Dose Curcuminoids are Efficacious in the Reduction in Symptoms and Signs of Oral Lichen Planus. J Am Acad Dermatol (2012) 66(5):752–60. doi: 10.1016/j.jaad.2011.04.022
65. Kia SJ, Shirazian S, Mansourian A, Khodadadi Fard L, Ashnagar S. Comparative Efficacy of Topical Curcumin and Triamcinolone for Oral Lichen Planus: A Randomized, Controlled Clinical Trial. J Dent (Tehran) (2015) 12(11):789–96.
66. Amirchaghmaghi M, Pakfetrat A, Delavarian Z, Ghalavani H, Ghazi A. Evaluation of the Efficacy of Curcumin in the Treatment of Oral Lichen Planus: A Randomized Controlled Trial. J Clin Diagn Res (2016) 10(5):ZC134–7. doi: 10.7860/JCDR/2016/16338.7870
67. Thomas AE, Varma B, Kurup S, Jose R, Chandy ML, Kumar SP, et al. Evaluation of Efficacy of 1% Curcuminoids as Local Application in Management of Oral Lichen Planus - Interventional Study. J Clin Diagn Res (2017) 11(4):ZC89–93. doi: 10.7860/JCDR/2017/20898.9715
68. Kia SJ, Basirat M, Mortezaie T, Moosavi MS. Comparison of Oral Nano-Curcumin With Oral Prednisolone on Oral Lichen Planus: A Randomized Double-Blinded Clinical Trial. BMC Complement Med Ther (2020) 20(1):328. doi: 10.1186/s12906-020-03128-7
69. Shin JI, Lee KH, Joo YH, Lee JM, Jeon J, Jung HJ, et al. Inflammasomes and Autoimmune and Rheumatic Diseases: A Comprehensive Review. J Autoimmun (2019) 103:102299. doi: 10.1016/j.jaut.2019.06.010
70. Yamamoto EA, Jørgensen TN. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front Immunol (2020) 10:3141. doi: 10.3389/fimmu.2019.03141
71. Jung SM, Kim WU. Targeted Immunotherapy for Autoimmune Disease. Immune Netw (2022) 22(1):e9. doi: 10.4110/in.2022.22.e9
72. Surace AEA, Hedrich CM. The Role of Epigenetics in Autoimmune/Inflammatory Disease. Front Immunol (2019) 10:1525. doi: 10.3389/fimmu.2019.01525
73. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update on the Diagnosis and Management of Systemic Lupus Erythematosus. Ann Rheum Dis (2021) 80(1):14–25. doi: 10.1136/annrheumdis-2020-218272
74. Lee DSW, Rojas OL, Gommerman JL. B Cell Depletion Therapies in Autoimmune Disease: Advances and Mechanistic Insights. Nat Rev Drug Discovery (2021) 20(3):179–99. doi: 10.1038/s41573-020-00092-2
75. Karagianni P, Tzioufas AG. Epigenetic Perspectives on Systemic Autoimmune Disease. J Autoimmun (2019) 104:102315. doi: 10.1016/j.jaut.2019.102315
76. Devarajan P, Chen Z. Autoimmune Effector Memory T Cells: The Bad and the Good. Immunol Res (2013) 57(1-3):12–22. doi: 10.1007/s12026-013-8448-1
77. Lin X, Lu L. B Cell-Mediated Autoimmune Diseases. Adv Exp Med Biol (2020) 1254:145–60. doi: 10.1007/978-981-15-3532-1_11
78. Liu Y, Wang X, Yang F, Zheng Y, Ye T, Yang L. Immunomodulatory Role and Therapeutic Potential of Non-Coding RNAs Mediated by Dendritic Cells in Autoimmune and Immune Tolerance-Related Diseases. Front Immunol (2021) 12:678918. doi: 10.3389/fimmu.2021.678918
79. Herrada AA, Escobedo N, Iruretagoyena M, Valenzuela RA, Burgos PI, Cuitino L, et al. Innate Immune Cells’ Contribution to Systemic Lupus Erythematosus. Front Immunol (2019) 10:772. doi: 10.3389/fimmu.2019.00772
80. Katsiari CG, Liossis SN, Sfikakis PP.. The Pathophysiologic Role of Monocytes and Macrophages in Systemic Lupus Erythematosus: A Reappraisal. Semin Arthritis Rheumatol (2010) 39(6):491–503. doi: 10.1016/j.semarthrit.2008.11.002
81. Iwata Y, Boström EA, Menke J, Rabacal WA, Morel L, Wada T, et al. Aberrant Macrophages Mediate Defective Kidney Repair That Triggers Nephritis in Lupus-Susceptible Mice. J Immunol (2012) 188(9):4568–80. doi: 10.4049/jimmunol.1102154
82. Ding D, Mehta H, McCune WJ, Kaplan MJ. Aberrant Phenotype and Function of Myeloid Dendritic Cells in Systemic Lupus Erythematosus. J Immunol (2006) 177(9):5878–89. doi: 10.4049/jimmunol.177.9.5878
83. Charles N, Hardwick D, Daugas E, Illei GG, Rivera J.. Basophils and the T Helper 2 Environment can Promote the Development of Lupus Nephritis. Nat Med (2010) 16(6):701–7. doi: 10.1038/nm.2159
84. Gupta S, Kaplan MJ. The Role of Neutrophils and NETosis in Autoimmune and Renal Diseases. Nat Rev Nephrol (2016) 12(7):402–13. doi: 10.1038/nrneph.2016.71
85. Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New Insights Into the Immunopathogenesis of Systemic Lupus Erythematosus. Nat Rev Rheumatol (2016) 12(12):716–30. doi: 10.1038/nrrheum.2016.186
86. Botto M, Walport MJ. C1q, Autoimmunity and Apoptosis. Immunobiology (2002) 205(4–5):395–406. doi: 10.1078/0171-2985-00141
87. Oke V, Gunnarsson I, Dorschner J, Eketjäll S, Zickert A, Niewold TB, et al. High Levels of Circulating Interferons Type I, Type II and Type III Associate With Distinct Clinical Features of Active Systemic Lupus Erythematosus. Arthritis Res Ther (2019) 21(1):107. doi: 10.1186/s13075-019-1878-y
88. Nicholls AJ, Wen SW, Hall P, Hickey MJ, Wong CHY. Activation of the Sympathetic Nervous System Modulates Neutrophil Function. J Leukoc Biol (2018) 103(2):295–309. doi: 10.1002/JLB.3MA0517-194RR
89. Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity (2019) 50(2):302–16. doi: 10.1016/j.immuni.2019.01.020
90. Worbs T, Hammerschmidt SI, Förster R. Dendritic Cell Migration in Health and Disease. Nat Rev Immunol (2017) 17(1):30–48. doi: 10.1038/nri.2016.116
91. Patel DD, Kuchroo VK. Th17 Cell Pathway in Human Immunity: Lessons From Genetics and Therapeutic Interventions. Immunity (2015) 43(6):1040–51. doi: 10.1016/j.immuni.2015.12.003
92. Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A. Immune Modulation by Curcumin: The Role of Interleukin-10. Crit Rev Food Sci Nutr (2019) 59(1):89–101. doi: 10.1080/10408398.2017.1358139
93. Momtazi-Borojeni AA, Haftcheshmeh SM, Esmaeili SA, Johnston TP, Abdollahi E, Sahebkar A. Curcumin: A Natural Modulator of Immune Cells in Systemic Lupus Erythematosus. Autoimmun Rev (2018) 17(2):125–35. doi: 10.1016/j.autrev.2017.11.016
94. Tomeh MA, Hadianamrei R, Zhao X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int J Mol Sci (2019) 20(5):1033. doi: 10.3390/ijms20051033
95. Panaro MA, Corrado A, Benameur T, Paolo CF, Cici D, Porro C. The Emerging Role of Curcumin in the Modulation of TLR-4 Signaling Pathway: Focus on Neuroprotective and Anti-Rheumatic Properties. Int J Mol Sci (2020) 21(7):2299. doi: 10.3390/ijms21072299
96. Gonzales AM, Orlando RA. Curcumin and Resveratrol Inhibit Nuclear factor-kappaB-Mediated Cytokine Expression in Adipocytes. Nutr Metab (Lond) (2008) 5:17. doi: 10.1186/1743-7075-5-17
97. Han S-S, Keum Y-S, Seo H-J, Surh Y-J. Curcumin Suppresses Activation of NF-κb and AP-1 Induced by Phorbol Ester in Cultured Human Promyelocytic Leukemia Cells. BMB Rep (2002) 35(3):337–342. doi: 10.5483/BMBRep.2002.35.3.337
98. Jiang CX, Wu L, Wu J, Yu ZM, Li XK. Curcumin Analog Exhibited Anti-Inflammatory Activity Through Inhibiting ERK/JNK and NF-κb Signaling Pathway. Chin Traditional Herbal Drugs (2016) 47(16):2871–2876. doi: 10.7501/j.issn.0253-2670.2016.16.016
99. Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ, Moon DO, et al. Curcumin Inhibits Immunostimulatory Function of Dendritic Cells: MAPKs and Translocation of NF-Kappa B as Potential Targets. J Immunol (2005) 174(12):8116–8124. doi: 10.4049/jimmunol.174.12.8116
100. Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, et al. Curcumin Ameliorates Macrophage Infiltration by Inhibiting NF-κb Activation and Proinflammatory Cytokines in Streptozotocin Induced-Diabetic Nephropathy. Nutr Metab (Lond) (2011) 8(1):35. doi: 10.1186/1743-7075-8-35
101. Jung ID, Jeong YI, Lee CM, Noh KT, Jeong SK, Chun SH, et al. COX-2 and PGE2 Signaling is Essential for the Regulation of IDO Expression by Curcumin in Murine Bone Marrow-Derived Dendritic Cells. Int Immunopharmacol (2010) 10(7):760–8. doi: 10.1016/j.intimp.2010.04.006
102. Shirley SA, Montpetit AJ, Lockey RF, Mohapatra SS. Curcumin Prevents Human Dendritic Cell Response to Immune Stimulants. Biochem Biophys Res Commun (2008) 374(3):431–6. doi: 10.1016/j.bbrc.2008.07.051
103. Camacho-Barquero L, Villegas I, Sánchez-Calvo JM, Talero E, Sánchez-Fidalgo S, Motilva V, et al. Curcumin, a Curcuma Longa Constituent, Acts on MAPK P38 Pathway Modulating COX-2 and iNOS Expression in Chronic Experimental Colitis. Int Immunopharmacol (2007) 7(3):333–42. doi: 10.1016/j.intimp.2006.11.006
104. Jiang H, Deng CS, Zhang M, Xia J. Curcumin-Attenuated Trinitrobenzene Sulphonic Acid Induces Chronic Colitis by Inhibiting Expression of Cyclooxygenase-2. World J Gastroenterol (2006) 12(24):3848–53. doi: 10.3748/wjg.v12.i24.3848
105. Decoté-Ricardo D, Chagas KK, Rocha JD, Redner P, Lopes UG, Cambier JC, et al. Modulation of In Vitro Murine B-Lymphocyte Response by Curcumin. Phytomedicine (2009) 16(10):982–8. doi: 10.1016/j.phymed.2009.01.004
106. Sharma S, Chopra K, Kulkarni S, Agrewala J. Resveratrol and Curcumin Suppress Immune Response Through CD28/CTLA-4 and CD80 Co-Stimulatory Pathway. Clin Exp Immunol (2007) 147(1):155–63. doi: 10.1111/j.1365-2249.2006.03257.x
107. Bhattacharyya S, Md Sakib Hossain D, Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S, et al. Curcumin Reverses T Cell-Mediated Adaptive Immune Dysfunctions in Tumor-Bearing Hosts. Cell Mol Immunol (2010) 7(4):306–15. doi: 10.1038/cmi.2010.11
108. Stagg AJ. Intestinal Dendritic Cells in Health and Gut Inflammation. Front Immunol (2018) 9:2883. doi: 10.3389/fimmu.2018.02883
109. Cong Y, Wang L, Konrad A, Schoeb T, Elson CO. Curcumin Induces the Tolerogenic Dendritic Cell That Promotes Differentiation of Intestine-Protective Regulatory T Cells. Eur J Immunol (2009) 39(11):3134–3146. doi: 10.1002/eji.200939052
110. Abrahams S, Haylett WL, Johnson G, Carr JA, Bardien S. Antioxidant Effects of Curcumin in Models of Neurodegeneration, Aging, Oxidative and Nitrosative Stress: A Review. Neuroscience (2019) 406:1–21. doi: 10.1016/j.neuroscience.2019.02.020
111. Handono K, Pratama MZ, Endharti AT, Kalim H. Treatment of Low Doses Curcumin Could Modulate Th17/Treg Balance Specifically on CD4+ T Cell Cultures of Systemic Lupus Erythematosus Patients. Cent Eur J Immunol (2015) 40(4):461–9. doi: 10.5114/ceji.2015.56970
112. Kalim H, Handono K, Khalasha T, Pratama M, Dantara TI, Wulandari A, et al. Immune Modulation Effects of Curcumin in Pristane-Induced Lupus Mice. Indian J Rheumatol (2017) 12(2):86. doi: 10.4103/injr.injr_95_16
113. Kang D, Li B, Luo L, Jiang W, Lu Q, Rong M, et al. Curcumin Shows Excellent Therapeutic Effect on Psoriasis in Mouse Model. Biochimie (2016) 123:73–80. doi: 10.1016/j.biochi.2016.01.013
114. Xie L, Li XK, Funeshima-Fuji N, Kimura H, Matsumoto Y, Isaka Y, et al. Amelioration of Experimental Autoimmune Encephalomyelitis by Curcumin Treatment Through Inhibition of IL-17 Production. Int Immunopharmacol (2009) 9(5):575–581. doi: 10.1016/j.intimp.2009.01.025
115. Liu JQ, Yan YQ, Liu JT, Wang YR, Wang X. Curcumin Prevents Experimental Autoimmune Encephalomyelitis by Inhibiting Proliferation and Effector CD4+ T Cell Activation. Eur Rev Med Pharmacol Sci (2019) 23(20):9108–16. doi: 10.26355/eurrev_201910_19314
116. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet (2021) 397(10281):1301–15. doi: 10.1016/S0140-6736(20)32549-6
117. Kim WB, Jerome D, Yeung J. Diagnosis and Management of Psoriasis. Can Fam Physician (2017) 63(4):278–85.
118. Griffiths CE, Barker JN. Pathogenesis and Clinical Features of Psoriasis. Lancet (2007) 370(9583):263–71. doi: 10.1016/S0140-6736(07)61128-3
119. Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk Factors for the Development of Psoriasis. Int J Mol Sci (2019) 20(18):4347. doi: 10.3390/ijms20184347
120. Boehncke WH, Schön MP. Psoriasis. Lancet (2015) 386(9997):983–94. doi: 10.1016/S0140-6736(14)61909-7
121. FitzGerald O, Ogdie A, Chandran V, Coates LC, Kavanaugh A, Tillett W, et al. Psoriatic Arthritis. Nat Rev Dis Primers (2021) 7(1):59. doi: 10.1038/s41572-021-00293-y
122. Ryan GE, Harris JE, Richmond JM. Resident Memory T Cells in Autoimmune Skin Diseases. Front Immunol (2021) 12:652191. doi: 10.3389/fimmu.2021.652191
123. Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Primers (2016) 2:16082. doi: 10.1038/nrdp.2016.82
124. Honma M, Nozaki H. Molecular Pathogenesis of Psoriasis and Biomarkers Reflecting Disease Activity. J Clin Med (2021) 10(15):3199. doi: 10.3390/jcm10153199
125. Tokuyama M, Mabuchi T. New Treatment Addressing the Pathogenesis of Psoriasis. Int J Mol Sci (2020) 21(20):7488. doi: 10.3390/ijms21207488
127. Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA (2020) 323(19):1945–60. doi: 10.1001/jama.2020.4006
128. Hoegler KM, John AM, Handler MZ, Schwartz RA. Generalized Pustular Psoriasis: A Review and Update on Treatment. J Eur Acad Dermatol Venereol. (2018) 32(10):1645–51. doi: 10.1111/jdv.14949
129. Reid C, Griffiths CEM. Psoriasis and Treatment: Past, Present and Future Aspects. Acta DermVenereol (2020) 100(3):adv00032. doi: 10.2340/00015555-3386
130. Barygina V, Becatti M, Soldi G, Prignano F, Lotti T, Nassi P, et al. Altered Redox Status in the Blood of Psoriatic Patients:Involvement of NADPH Oxidase and Role of Anti-TNF-αtherapy. Redox Rep (2013) 18(3):100–6. doi: 10.1179/1351000213Y.0000000045
131. Reddy S, Aggarwal BB. Curcumin Is a Non - Ompetitive and Selective Inhibitor of Phosphorylase Kinase. FEBS Lett (1994) 341(1):19–22. doi: 10.1016/0014-5793(94)80232-7
132. Varma SR, Sivaprakasam TO, Mishra A. Imiquimod-Induced Psoriasis-Like Inflammation in Differentiated Human Keratinocytes:Its Evaluation Using Curcumin. Eyr J Pharmacol (2017) 813:33–41. doi: 10.1016/j.ejphar.2017.07.040
133. Nardo VD, Gianfaldoni S, Tchernev G. Use of Curcumin in Psoriasis. Open Access Maced J Med Sci (2018) 6(1):218–20. doi: 10.3889/oamjms.2018.055
134. Zucchi D, Elefante E, Calabresi E, Signorini V, Bortoluzzi A, Tani C. One Year in Review 2019: Systemic Lupus Erythematosus. Clin Exp Rheumatol (2019) 37(5):715–22.
135. Fortuna G, Brennan MT. Systemic Lupus Erythematosus: Epidemiology, Pathophysiology, Manifestations, and Management. Dent Clin North Am (2013) 57(4):631–55. doi: 10.1016/j.cden.2013.06.003
136. Aringer M, Petri M. New Classification Criteria for Systemic Lupus Erythematosus. CurrOpinRheumatol (2020) 32(6):590–6. doi: 10.1097/BOR.0000000000000740
137. Nashi RA, Shmerling RH. Antinuclear Antibody Testing for the Diagnosis of Systemic Lupus Erythematosus. Med Clin North Am (2021) 105(2):387–96. doi: 10.1016/j.mcna.2020.10.003
138. Fava A, Petri M. Systemic Lupus Erythematosus: Diagnosis and Clinical Management. J Autoimmun (2019) 96:1–13. doi: 10.1016/j.jaut.2018.11.001
139. Foxley S, Zamora M, Hack B, Alexander RR, Roman B, Quigg RJ, et al. Curcumin Aggravates CNS Pathology in Experimental Systemic Lupus Erythematosus. Brain Res (2013) 1504:85–96. doi: 10.1016/j.brainres.2013.01.040
140. Kurien BT, D'Souza A, Scofield RH. Heat-Solubilized Curry Spice Curcumin Inhibits Antibody-Antigen Interaction in In Vitro Studies: A Possible Therapy to Alleviate Autoimmune Disorders. Mol Nutr Food Res (2010) 54(8):1202–9. doi: 10.1002/mnfr.200900106
141. Lee H, Kim H, Lee G, Chung H-S, Bae H. Curcumin Attenuates Lupus Nephritis Upon Interaction With Regulatory T Cells in New Zealand Black/White Mice. Br J Nutr (2013) 110(01):69–76. doi: 10.1017/S0007114512004734
142. Correale J, Gaitán MI, Ysrraelit MC, Fiol MP. Progressive Multiple Sclerosis: From Pathogenic Mechanisms to Treatment. Brain (2017) 140(3):527–46. doi: 10.1093/brain/aww258
143. Cotsapas C, Mitrovic M, Hafler D. Multiple Sclerosis. Handb Clin Neurol (2018) 148:723–30. doi: 10.1016/B978-0-444-64076-5.00046-6
144. Garg N, Smith TW. An Update on Immunopathogenesis, Diagnosis, and Treatment of Multiple Sclerosis. Brain Behav (2015) 5(9):e00362. doi: 10.1002/brb3.362
145. Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental Autoimmune Encephalomyelitis (EAE) as a Model for Multiple Sclerosis (MS). Br J Pharmacol (2011) 164(4):1079–106. doi: 10.1111/j.1476-5381.2011.01302.x
146. Robinson AP, Harp CT, Noronha A, Miller SD. The Experimental Autoimmune Encephalomyelitis (EAE) Model of MS: Utility for Understanding Disease Pathophysiology and Treatment. Handb Clin Neurol (2014) 122:173–89. doi: 10.1016/B978-0-444-52001-2.00008-X
147. Natarajan C, Bright JJ. Curcumin Inhibits Experimental Allergic Encephalomyelitis by Blocking IL-12 Signaling Through Janus Kinase-STAT Pathway in T Lymphocytes. J Immunol (2002) 168(12):6506–13. doi: 10.4049/jimmunol.168.12.6506
148. Zhang M, Deng CS, Zheng JJ, Xia J. Curcumin Regulated Shift From Th1 to Th2 in Trinitrobenzene Sulphonic Acid-Induced Chronic Colitis. Acta Pharmacol Sin (2006) 27(8):1071–7. doi: 10.1111/j.1745-7254.2006.00322.x
149. Yang JY, Zhong X, Yum HW, Lee HJ, Kundu JK, Na HK, et al. Curcumin Inhibits STAT3 Signaling in the Colon of Dextran Sulfate Sodium-Treated Mice. J Cancer Prev (2013) 18(2):186. doi: 10.15430/JCP.2013.18.2.186
150. Kanakasabai S, Casalini E, Walline CC, Mo C, Chearwae W, Bright JJ. Differential Regulation of CD4+ T Helper Cell Responses by Curcumin in Experimental Autoimmune Encephalomyelitis. J Nutr Biochem (2012) 23(11):1498–507. doi: 10.1016/j.jnutbio.2011.10.002
151. Wang H-M, Zhao Y-X, Zhang S, Liu G-D, Kang W-Y, Tang H-D, et al. Pparγ Agonist Curcumin Reduces the Amyloid-β-Stimulated Inflammatory Responses in Primary Astrocytes. J Alzheimer's Dis (2010) 20(4):1189–99. doi: 10.3233/JAD-2010-091336
152. Lian YT, Yang XF, Wang ZH, Yang Y, Yang Y, Shu YW, et al. Curcumin Serves as a Human Kv1. 3 Blocker to Inhibit Effector Memory T Lymphocyte Activities. Phytother Res (2013) 27(9):1321–7. doi: 10.1002/ptr.4863
153. Mauro D, Thomas R, Guggino G, Lories R, Brown MA, Ciccia F. Ankylosing Spondylitis: An Autoimmune or Autoinflammatory Disease? Nat Rev Rheumatol (2021) 17(7):387–404. doi: 10.1038/s41584-021-00625-y
154. Fiorillo MT, Haroon N, Ciccia F, Breban M. Editorial: Ankylosing Spondylitis and Related Immune-Mediated Disorders. Front Immunol (2019) 10:1232. doi: 10.3389/fimmu.2019.01232
155. Hwang MC, Ridley L, Reveille JD. Ankylosing Spondylitis Risk Factors: A Systematic Literature Review. Clin Rheumatol (2021) 40(8):3079–93. doi: 10.1007/s10067-021-05679-7
156. Garcia-Montoya L, Gul H, Emery P. Recent Advances in Ankylosing Spondylitis: Understanding the Disease and Management. F1000Res (2018) 7:F1000FacultyRev–1512. doi: 10.12688/f1000research.14956.1
157. Smith JA. Update on Ankylosing Spondylitis: Current Concepts in Pathogenesis. Curr Allergy Asthma Rep (2015) 15(1):489. doi: 10.1007/s11882-014-0489-6
158. Yang M, Lv Q, Wei Q, Jiang Y, Qi J, Xiao M, et al. TNF-α Inhibitor Therapy can Improve the Immune Imbalance of CD4+ T Cells and Negative Regulatory Cells But Not CD8+ T Cells in Ankylosing Spondylitis. Arthritis Res Ther (2020) 22(1):149. doi: 10.1186/s13075-020-02226-8
159. Duan Z, Gui Y, Li C, Lin J, Gober HJ, Qin J, et al. The Immune Dysfunction in Ankylosing Spondylitis Patients. Biosci Trends (2017) 11(1):69–76. doi: 10.5582/bst.2016.01171
160. Xu F, Guanghao C, Liang Y, Jun W, Wei W, Baorong H. Treg-Promoted New Bone Formation Through Suppressing TH17 by Secreting Interleukin-10 in Ankylosing Spondylitis. Spine (Phila Pa 1976) (2019) 44(23):E1349–55. doi: 10.1097/BRS.0000000000003169
161. Miao J, Zhu P. Functional Defects of Treg Cells: New Targets in Rheumatic Diseases, Including Ankylosing Spondylitis. CurrRheumatol Rep (2018) 20(5):30. doi: 10.1007/s11926-018-0729-1
162. Wang L, Wang FS, Gershwin ME. Human Autoimmune Diseases: A Comprehensive Update. J Intern Med (2015) 278(4):369–95. doi: 10.1111/joim.12395
163. Davatchi F. Behçet's Disease. Int J Rheum Dis (2018) 21(12):2057–8. doi: 10.1111/1756-185X.13465
164. Nieto IG, Alabau JLC. Immunopathogenesis of Behçet Disease. CurrRheumatol Rev (2020) 16(1):12–20. doi: 10.2174/1573397115666190415142426
165. Valenti S, Gallizzi R, De Vivo D, Romano C. Intestinal Behçet and Crohn's Disease: Two Sides of the Same Coin. PediatrRheumatol Online J (2017) 15(1):33. doi: 10.1186/s12969-017-0162-4
166. Hatemi G, Seyahi E, Fresko I, Talarico R, Hamuryudan V. One Year in Review 2020: Behçet's Syndrome. Clin Exp Rheumatol (2020) 38 Suppl 127(5):3–10.
167. Nelson CA, Stephen S, Ashchyan HJ, James WD, Micheletti RG, Rosenbach M. Neutrophilic Dermatoses: Pathogenesis, Sweet Syndrome, Neutrophilic Eccrine Hidradenitis, and Behçet Disease. J Am Acad Dermatol (2018) 79(6):987–1006. doi: 10.1016/j.jaad.2017.11.064
168. Yazici Y, Hatemi G, Bodaghi B, Cheon JH, Suzuki N, Ambrose N, et al. Behçet Syndrome. Nat Rev Dis Primers (2021) 7(1):67. doi: 10.1038/s41572-021-00301-1
169. Nagpal M, Sood S. Role of Curcumin in Systemic and Oral Health: An Overview. J Nat Sc Biol Med (2013) 4:3–7. doi: 10.4103/0976-9668.107253
170. Deshmukh RA, Bagewadi AS. Comparison of Effectiveness of Curcumin With Triamcinolone Acetonide in the Gel Form in Treatment of Minor Recurrent Aphthous Stomatitis: A Randomized Clinical Trial. Int J Pharma Investig (2014) 4:138–41. doi: 10.4103/2230-973X.138346
171. Lim YS, Kwon SK, Park JH. Enhanced Mucosal Healing With Curcumin in Animal Oral Ulcer Model. Laryngoscope (2016) 126:E68–73. doi: 10.1002/lary.25649
172. Palizgir MT, Akhtari M, Mahmoudi M, Mostafaei S, Rezaiemanesh A, Shahram F. Curcumin Reduces the Expression of Interleukin 1β and the Production of Interleukin 6 and Tumor Necrosis Factor Alpha by M1 Macrophages From Patients With Behcet's Disease. ImmunopharmacolImmunotoxicol (2018) 40(4):297–302. doi: 10.1080/08923973.2018.1474921
173. Chan MM. Inhibition of Tumor Necrosis Factor by Curcumin, a Phytochemical. Biochem Pharmacol (1995) 49:1551–6. doi: 10.1016/0006-2952(95)00171-U
174. Zhou Y, Zhang T, Wang X, Wei X, Chen Y, Guo L, et al. Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and its Signaling Pathways. Cell PhysiolBiochem (2015) 36:631–41. doi: 10.1159/000430126
175. Xu YX, Pindolia KR, Janakiraman N. Curcumin Inhibits IL1 Alpha and TNF-Alpha Induction of AP-1 and NF-kB DNA-Binding Activity in Bone Marrow Stromal Cells. Hematopathol Mol Hematol (1997), 1(11):49–62.
176. Sairenji T, Collins KL, Evans DV. An Update on Inflammatory Bowel Disease. Prim Care (2017) 44(4):673–92. doi: 10.1016/j.pop.2017.07.010
177. Flynn S, Eisenstein S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg Clin North Am (2019) 99(6):1051–62. doi: 10.1016/j.suc.2019.08.001
178. Kelsen JR, Russo P, Sullivan KE. Early-Onset Inflammatory Bowel Disease. Immunol Allergy Clin North Am (2019) 39(1):63–79. doi: 10.1016/j.iac.2018.08.008
179. Xavier RJ, Podolsky DK. Unravelling the Pathogenesis of Inflammatory Bowel Disease. Nature (2007) 448(7152):427–34. doi: 10.1038/nature06005
180. Zhen Y, Zhang H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front Immunol (2019) 10:276. doi: 10.3389/fimmu.2019.00276
181. Lee HS, Lobbestael E, Vermeire S, Sabino J, Cleynen I. Inflammatory Bowel Disease and Parkinson's Disease: Common Pathophysiological Links. Gut (2021) 70(2):408–17. doi: 10.1136/gutjnl-2020-322429
182. Pike KA, Tremblay ML. Protein Tyrosine Phosphatases: Regulators of CD4 T Cells in Inflammatory Bowel Disease. Front Immunol (2018) 9:2504. doi: 10.3389/fimmu.2018.02504
183. Zenewicz LA, Antov A, Flavell RA. CD4 T-Cell Differentiation and Inflammatory Bowel Disease. Trends Mol Med (2009) 15(5):199–207. doi: 10.1016/j.molmed.2009.03.002
184. Li CP, Li JH, He SY, Chen O, Shi L. Effect of Curcumin on P38mapk Expression in DSS-Induced Murine Ulcerative Coltis. Genet Mol Res (2015) 14(2):3450–8. doi: 10.4238/2015.April.15.8
185. Topcu-Tarladacalisir Y, Akpolat M, Uz YH, Kizilay G, Sapmaz-Metin M, Cerkezkayabekir A, et al. Effects of Curcumin on Apoptosis and Oxidoinflammatory Regulation in a Rat Model of Acetic Acid-Induced Colitis: The Roles of C-Jun N-Terminal Kinase and P38 Mitogen-Activated Protein Kinase. J Med Food (2013) 16(4):296–305. doi: 10.1089/jmf.2012.2550
186. Liu L, Liu YL, Liu GX, Chen X, Yang K, Yang YX, et al. Curcumin Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis by Blocking STAT3 Signaling Pathway. Int Immunopharmacol (2013) 17(2):314–20. doi: 10.1016/j.intimp.2013.06.020
187. Yang X, Wang Z, Li J, Wang X, Zhang Z, Zhu J. Effects of Curcumin on the Expression of MMP-2 and MMP-9 in the Spinal Cord of EAE Rats. Chin J Traditional Chin Med (2013) 31(02):378–380+462. doi: 10.13193/j.archtcm.2013.02.156.yangxzh.068
188. Guo L, Li H, Li C, Shi L, Zhong X., et al. The Role of STAT3 and PPAR-Y in Mouse Ulcerative Colitis and the Effect of Curcumin. World Chin J Digestion (2016) 24(1):28–36. doi: 10.11569/wcjd.v24.i1.28
189. Littlejohn EA, Monrad SU. Early Diagnosis and Treatment of Rheumatoid Arthritis. Prim Care (2018) 45(2):237–55. doi: 10.1016/j.pop.2018.02.010
190. Burmester GR, Pope JE. Novel Treatment Strategies in Rheumatoid Arthritis. Lancet (2017) 389(10086):2338–48. doi: 10.1016/S0140-6736(17)31491-5
191. van der Woude D, van der Helm-van Mil AHM. Update on the Epidemiology, Risk Factors, and Disease Outcomes of Rheumatoid Arthritis. Best Pract Res Clin Rheumatol (2018) 32(2):174–87. doi: 10.1016/j.berh.2018.10.005
192. Scherer HU, Häupl T, Burmester GR. The Etiology of Rheumatoid Arthritis. J Autoimmun (2020) 110:102400. doi: 10.1016/j.jaut.2019.102400
193. Wasserman A. Rheumatoid Arthritis: Common Questions About Diagnosis and Management. Am Fam Physician (2018) 97(7):455–62.
194. Chemin K, Gerstner C, Malmström V. Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation-Lessons From Rheumatoid Arthritis. Front Immunol (2019) 10:353. doi: 10.3389/fimmu.2019.00353
195. Demoruelle MK, Deane KD. Treatment Strategies in Early Rheumatoid Arthritis and Prevention of Rheumatoid Arthritis. CurrRheumatol Rep (2012) 14(5):472–80. doi: 10.1007/s11926-012-0275-1
196. Xu Z. Mechanism of Curcumin Interfering With NF-κb Signaling Pathway in Osteoclastogenesis in Patients With Rheumatoid Arthritis. Nanjing Univ Traditional Chin Med (2017).
197. Sun Y, Yu H. Research Progress on the Mechanism of Curcumin in the Treatment of Rheumatoid Arthritis. J Chin Med (2015) 43(06):113–6. doi: 10.19664/j.cnki.1002-2392.2015.06.038
198. Shang W. The Effect of Curcumin on PBMC Osteoclastogenesis in Patients With Rheumatoid Arthritis and its Possible Mechanism. Nanjing Univ Traditional Chin Med (2016).
199. Chen T, Zhou R, Chen Y, Fu W, Wei X, Ma G, et al. Curcumin Ameliorates IL-1β-Induced Apoptosis by Activating Autophagy and Inhibiting the NF-κb Signaling Pathway in Rat Primary Articular Chondrocytes. Cell Biol Int (2021) 45(5):976–88. doi: 10.1002/cbin.11541
200. Funk JL, Frye JB, Oyarzo JN, Kuscuoglu N, Wilson J, McCaffrey G, et al. Efficacy and Mechanism of Action of Turmeric Supplements in the Treatment of Experimental Arthritis. Arthritis Rheumatism (2006) 54(11):3452–3464. doi: 10.1002/art.22180
201. Alrashdan MS, Cirillo N, McCullough M. Oral Lichen Planus: A Literature Review and Update. Arch Dermatol Res (2016) 308(8):539–51. doi: 10.1007/s00403-016-1667-2
202. Chiang CP, Yu-Fong Chang J, Wang YP, Wu YH, Lu SY, Sun A. Oral Lichen Planus - Differential Diagnoses, Serum Autoantibodies, Hematinic Deficiencies, and Management. J Formos Med Assoc (2018) 117(9):756–65. doi: 10.1016/j.jfma.2018.01.021
203. Nosratzehi T. Oral Lichen Planus: An Overview of Potential Risk Factors, Biomarkers and Treatments. Asian Pac J Cancer Prev (2018) 19(5):1161–7. doi: 10.22034/APJCP.2018.19.5.1161
204. Ryan K, Hegarty AM, Hodgson T. Aetiology, Diagnosis and Treatment of Oral Lichen Planus. Brit J Hosp Med (2014) 75:492–6. doi: 10.12968/hmed.2014.75.9.492
205. Enomoto A, Sato E, Yasuda T. Intraepithelial CD8+ Lymphocytes as a Predictive Diagnostic Biomarker for the Remission of Oral Lichen Planus. Hum Pathol (2018) 74:43–53. doi: 10.1016/j.humpath.2017.12.008
206. Agha-Hosseini F, Mirzaii-Dizgah I, Farmanbar N, Abdollahi M. Oxidative Stress Status and DNA Damage in Saliva of Human Subjects With Oral Lichen Planus and Oral Squamous Cell Carcinoma. J Oral Pathol Med (2012) 41(10):736–40. doi: 10.1111/j.1600-0714.2012.01172.x
207. Abdolsamadi H, Rafieian N, Goodarzi MT. Levels of Salivary Antioxidant Vitamins and Lipid Peroxidation in Patients With Oral Lichen Planus and Healthy Individuals. Chonnam Med J (2014) 50:58–62. doi: 10.4068/cmj.2014.50.2.58
208. Ismail SB, Kumar SK, Zain RB. Oral Lichen Planus and Lichenoid Reactions: Etiopathogenesis, Diagnosis, Management and Malignant Transformation. J Oral Sci (2007) 49(2):89–106. doi: 10.2334/josnusd.49.89
209. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of Curcumin: Problems and Promises. Mol Pharm (2007) 4(6):807–18. doi: 10.1021/mp700113r
210. Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, In Patients With High-Risk or Pre-Malignant Lesions. Anticancer Res (2001) 21(4B):2895–900.
211. Chen Z, Xue J, Shen T, Ba G, Yu D, Fu Q. Curcumin Alleviates Glucocorticoid-Induced Osteoporosis by Protecting Osteoblasts From Apoptosis In Vivo and In Vitro. Clin Exp Pharmacol Physiol (2016) 43(2):268–76. doi: 10.1111/1440-1681.12513
212. Raman P, Pitty R, Krithika CL, Anand SPN, Subramani GP. Topical Curcumin and Triamcinolone Acetonide in Recurrent Minor Aphthous Ulcers: A Pilot Trial. J Contemp Dent Pract (2020) 21(8):884–90.
Keywords: curcumina, curcuma longa extract, autoimmune disease, rheumatoid arthritis, systemic lupus erythematosus, psoriasis, systematic review
Citation: Zeng L, Yang T, Yang K, Yu G, Li J, Xiang W and Chen H (2022) Curcumin and Curcuma longa Extract in the Treatment of 10 Types of Autoimmune Diseases: A Systematic Review and Meta-Analysis of 31 Randomized Controlled Trials. Front. Immunol. 13:896476. doi: 10.3389/fimmu.2022.896476
Received: 15 March 2022; Accepted: 04 May 2022;
Published: 01 August 2022.
Edited by:
Francesco Siracusa, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Yuandani, Universitas Sumatera Utara, IndonesiaFilippo Cortesi, University Medical Center Hamburg-Eppendorf, Germany
Copyright © 2022 Zeng, Yang, Yang, Yu, Li, Xiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Chen, Y2hodWFhMzMyMjExQDE2My5jb20=; Liuting Zeng, emx0YWIyMDE2QGhvdG1haWwuY29t; Tiejun Yang, eWFuZ3RpZWp1bjMyMUBvdXRsb29rLmNvbQ==; Kailin Yang, eWtsYWIyMDE0QGhvdG1haWwuY29t
†These authors share first authorship
 Liuting Zeng1*†
Liuting Zeng1*† Kailin Yang
Kailin Yang