- 1Center for Reproductive Medicine, Department of Obstetrics, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, China
- 2The First Clinical College of Zhejiang Chinese Medical University, Hangzhou, China
- 3Fertility Center of Melinda Women and Children’s Hospital, Dalian, China
- 4Center for Reproductive Medicine, Department of Traditional Chinese Medicine, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital, Hangzhou Medical College), Hangzhou, China
- 5Zhejiang Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Miscarriage poses a significant threat to pregnant women globally. Recurrent miscarriages or potential poor embryonic development indicated by early drops in serum human chorionic gonadotrophin (hCG) are even more catastrophic for pregnant women. However, these patients receive either individualized medical intervention supported by limited evidence or no treatment at all. In this study, we report ten patients who shared at least one episode of an early decline of hCG in the first trimester and were treated with compassionate use of tumor necrosis factor-alpha inhibitor (TNFi). They were then followed up regularly with caution. Their hCG trajectory all resumed a normal pattern within one week and the obstetric outcomes were promising. No adverse fetal, neonatal, or maternal health issues have been observed. This case series supports current safety evidence of TNFi and provides new insight into its use in pregnancy when the embryo is in danger. Further well-designed clinical trials should be carried out to consolidate the evidence.
Introduction
Miscarriage is defined as the loss of pregnancy before viability. It causes significant psychological and economic damage to the family and society (1). Adopting active strategies to prevent miscarriage, especially in women with a history of miscarriage, has been called for by Prof. Coomarasamy and others in recent years (2). However, there are few evidence-based guidelines for healthcare providers to prevent miscarriage.
hCG is a glycoprotein with a molecular weight of 36,000 to 40,000 Dalton. It is almost exclusively produced by trophoblast cells and placenta after placental formation (3). The trajectory of serum hCG results from serial tests in early pregnancy demonstrates excellent diagnostic and prognostic value across women with different ethnic backgrounds and age groups (4). This pregnancy hormone proliferates in early pregnancy, doubling its level every two days, reaching its peak at 50,000 to 100,000 mIU/mL between 8 and 10 weeks, and then gradually declines until delivery (3). When the hCG increases less than 50% in 48 hours, only a minority of embryos can survive (5). An early decline of hCG generally indicates irreversible poor embryonic outcomes and expectant management is often advised (6, 7). The up-to-date evidence has caused concern for women as even one previous miscarriage can increase the risk of recurrent miscarriages in subsequent pregnancies (1). Therefore, safe and effective measures should be considered to help women. At present, there is sparse evidence that can provide guidance when pregnancy is in danger.
Tumor necrosis factor-alpha (TNF-α) is an inflammatory cytokine belonging to Th1 lymphocytes. TNF-α blockers inhibit the effect of TNF-α. The agents were used in rheumatoid arthritis (RA), ankylosing spondylitis, inflammatory bowel disease (IBD), and psoriasis (8). It has also been shown to play a vital role throughout pregnancy (9, 10). The use of TNF-α anti-therapy (TNFi) in pregnancy is supported by The British Society for Rheumatology and British Health Professionals in Rheumatology (BHPR), European Alliance of Associations for Rheumatology (EULAR), American College of Rheumatology (ACR), and American Gastroenterological Association (AGA) as guidelines on prescribing various medications in pregnancy (11–16). However, this therapy has generally limited to pregnant women complicated with autoimmune diseases until recent years, when immunosuppressive medication has been suggested to play an essential role in recurrent miscarriages (17).
In this case series, we report the trial of TNFi-led pregnancy-rescue management (TPRM) to help women with early signs of miscarriage. Most of the women in our study had a history of recurrent pregnancy loss according to the definition from the European Society of Human Reproduction and Embryology (ESHRE) guideline (18). Some cases were complicated with complex autoimmune disorders, thrombophilias, or imbalanced cytokines (19). All women who had received treatment resumed the growth of hCG and enjoyed ideal obstetric outcomes later on.
Case Descriptions
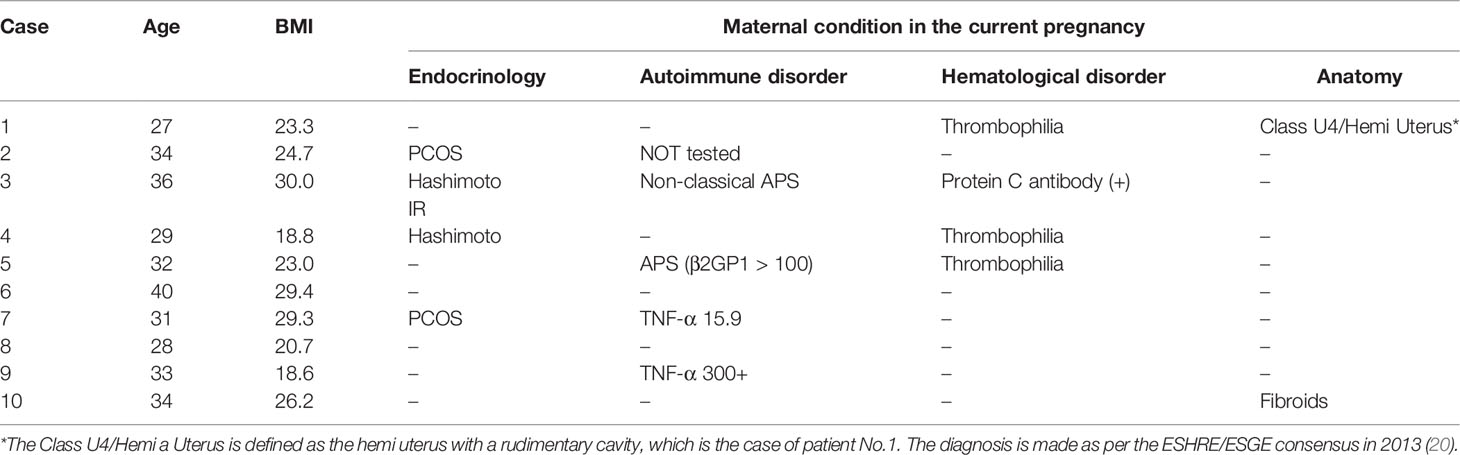
The basic characteristics include women’s age, body mass index (BMI), previous obstetric background, and obstetric outcomes in current pregnancies. Details can be seen in Tables 1, 2. All women are Han Chinese (the major ethnic group in China). The average age was 32.40 years (27-40), while the average BMI was 24.4. 4/10 were overweight (BMI > 24.99). All ten women had been offered preventative therapy with low-dose aspirin (LDA), low molecular weight heparin (LMWH), or progesterone therapy in the early stage due to previous obstetric histories. Their hCG growth followed the standard trajectory before the unexpected decline in the first-trimesters.
Diagnostic Assessment
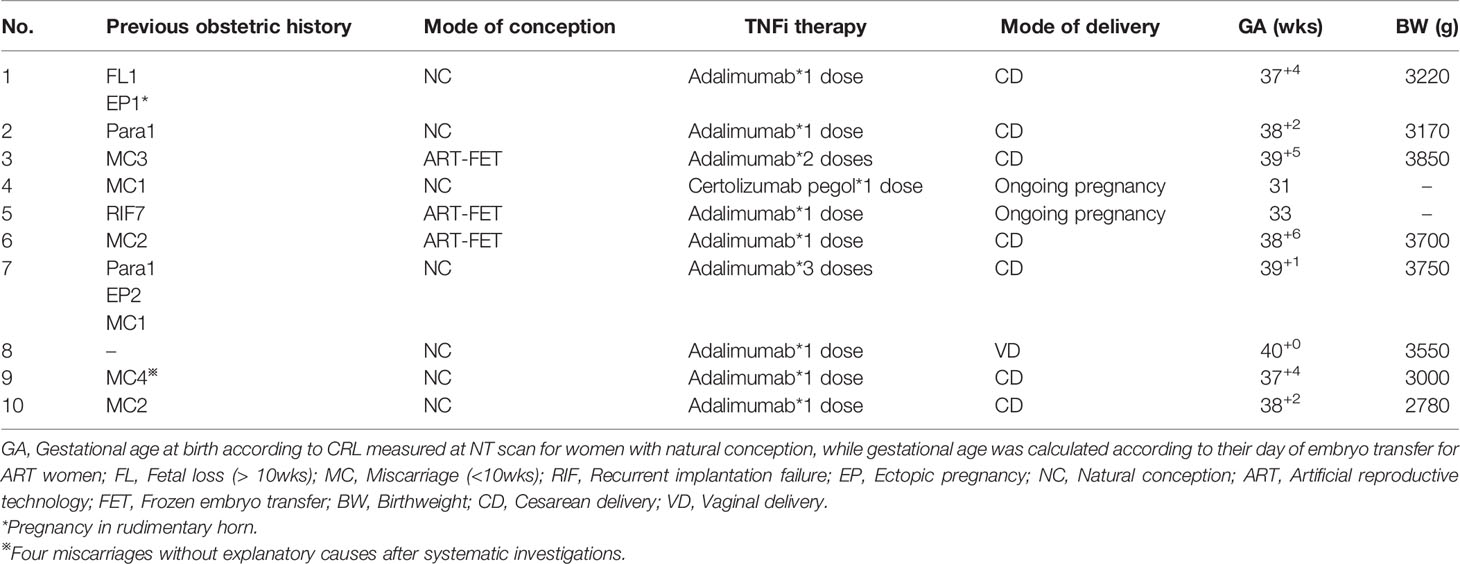
The overall serial serum levels of hCG are shown in Figure 1. Detailed data of serial serum hCG can be seen in the supplementary file. All of the premature decline events occurred from 40 days onwards, with the hCG value at more than 20,000mIU/ml on the test day. Most (9/10 cases) early declines occurred between six and eight gestational weeks. They were then rescued with TNFi (certolizumab for case No. 4 and adalimumab for the other nine cases) and followed up on an average of 3.6 days (1-9 days) after injections. Case No. 3, 6, and 7 experienced two episodes of the early drop of hCG in the first-trimester. As hCG trajectories in early pregnancies are different between natural pregnancy and assisted reproduction, cases are further sub-classified according to their mode of conception.
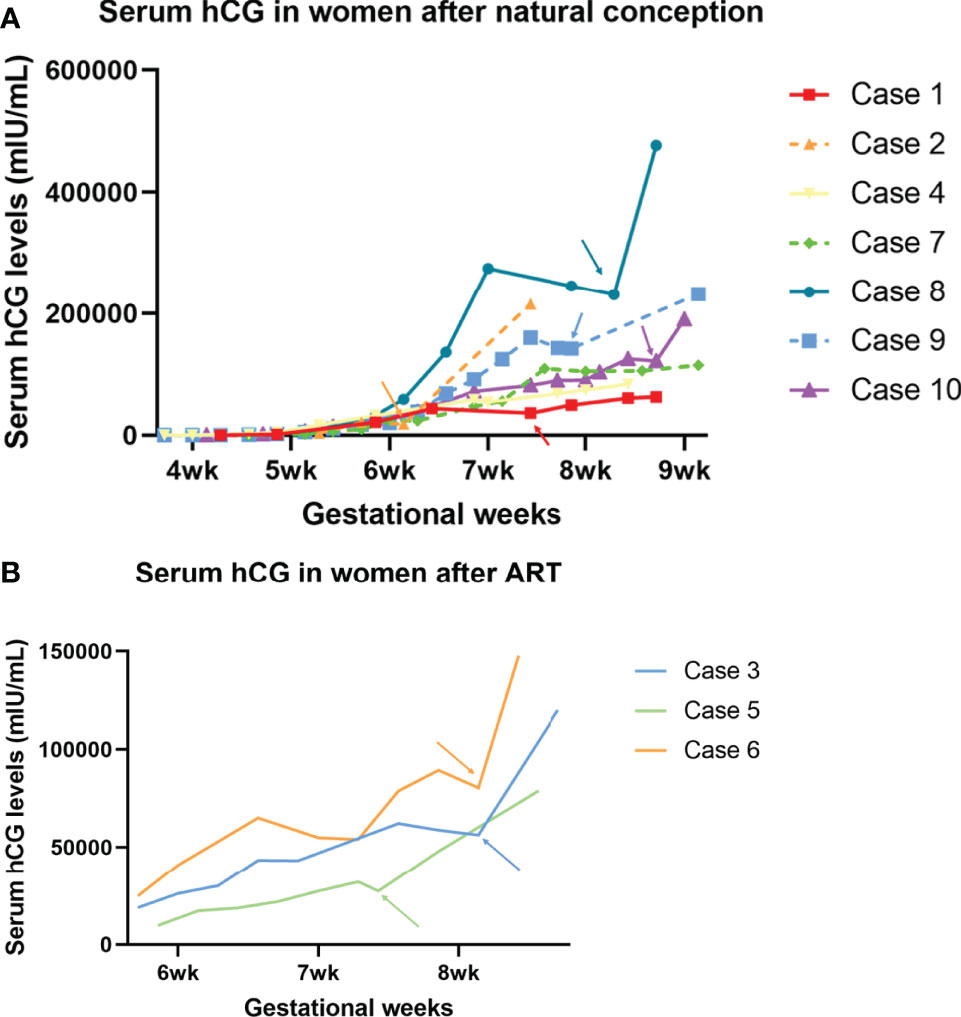
Figure 1 Serum hCG trajectory of each of the ten cases. The generated curve of serial hCG values of women who present early decline in the first trimester in the current pregnancies. (A) The subgroup of women following natural conception (n=7). (B) Subgroup of women following ART (n=3). Arrows with corresponding colors indicate the timing of administration of TNFi.
Case No. 3, 5, and 6
Three out of the ten cases were pregnancies following ART. Case No. 3 and 6 were diagnosed with unexplained infertility with at least two episodes of early embryonic failures after ART, while case No. 5 was affected by tubal factors. In case 3, upon the first episode, when hCG declined from 43,271 to 43,185, the patient had concurrent minor vaginal bleeds, indicating a threatened miscarriage. She received 1g tranexamic acid twice daily and a single dose of adalimumab (40mg) for one day and the bleeds stopped. A further decline on Day 55 prompted a second off-label use of adalimumab. Six days after injection, the serum hCG level went back on track (hCG declined from 62,164 on Day 53 to 58,786 on Day 55, then climbed to 120,006 mIU/mL on Day 61). In contrast, case No.6 received only one dose of adalimumab despite having experienced two episodes of early declines of hCG. Case No. 5 had only experienced one episode of hCG early decline and received one single dose of adalimumab on that day. She is now 33wks and is followed up regularly in the antenatal clinic and no stillbirth or any other adverse fetal or maternal outcome has been reported.
Case No. 1, 2, 4, 7, 8, 9, and 10
Among the seven patients who were conceptualized naturally, case No. 9 and 10 had two and four previous spontaneous miscarriages, respectively. Case No. 2 and 7 had a successful obstetric history before the current pregnancy. Case No. 8 was not complicated with pre-existing maternal medical conditions, nor did she have a previous history of miscarriage. However, she suffered from a long-lasting early decrease of hCG for more than ten days, suggesting an adverse embryonic outcome. A similar case was observed in No. 4, who is now 31wks, after being offered a single dose of certolizumab pegol (200mg) at 7wks when hCG fell from 58,016 to 54,438 mIU/mL the following day.
Eight out of the ten women have delivered so far. The average birth weight was 3377.50g (2780-3850g), which fell into the normal reference range. Despite uneventful pregnancies after the rescue therapy, we identified a disproportionally high cesarean section rate in the study group. Case No. 8 is the only one so far who has delivered vaginally. Case No. 3 and 6 had cesarean for fetal distress in labor, possibly resulted from oligohydramnios (not an absolute indication for cesarean but could lead to increased emergency cesarean delivery in labor). Case No. 2 and 7 had CS (C-sections) indicated for previous CS delivery, a major obstetric indication in China. The other three cases had requested a cesarean delivery (also known as CDMR, cesarean delivery on maternal request) in spite of any obstetric indications. This partly reflects China’s high rate of CS in the low obstetric risk population (21).
Discussion
In most cases, women would generally be offered expectant management if a spontaneous miscarriage is highly anticipated. However, the novel use of TNF-α blocker therapy has rendered promising obstetric outcomes in all patients. Safety concerns have also been addressed as no maternal-fetal or neonatal adverse consequences are reported in any patient. To our knowledge, this is the first report on the compassionate use of TNF-α blocker therapy to rescue endangered pregnancies.
There are guidelines for pre-conceptional and prenatal care in China, but they aim to serve the general population (22). Anxious women in China usually tend to make more frequent antenatal visits to request more investigations. Blood tests are more frequently performed in early pregnancies than others, such as ultrasound scans, as the former are usually more readily accessible in the antenatal clinics.
Highly individualized therapies are currently practiced in response to unexpected results revealed in early pregnancy, particularly in women with a history of multiple pregnancy losses but without definite explanatory causes for their recurrent pregnancy losses (2, 23). We agree that it is better to do something safe that may be effective than do nothing. The pre-condition of our strategy is that the embryonic pole (ideally with an active fetal heartbeat) must be seen in all women via ultrasound despite falling hCG levels.
The maternal immune system strikes an intricate balance between supporting embryonic development and maintaining immune responses in pregnancy. A dysregulated maternal immune system in pregnancy can affect obstetric outcomes (24, 25). TNF-α, the multifunctional T-helper 1 (Th1) cytokine, influences hormone synthesis, placental construction, and embryonic development (26). The altered expression of TNF-α can cause recurrent miscarriages and pre-eclampsia (27, 28). The pivotal role of pro-inflammatory cytokines and innate immunity during early pregnancy paved the way for efficient and safe treatments such as TNF-α antagonists (29).
We used adalimumab (Humira) and certolizumab pegol (Cimzia) in all cases. Adalimumab is a monoclonal IgG1 antibody, which is actively transported across the placenta by the neonatal Fc receptor (FcRn) (30). Certolizumab pegol (CZP) neutralizes both the membrane and the soluble form of human TNF-α in a dose-dependent manner. Its unique structure may prevent fetal exposure to the drug during pregnancy (31, 32). Both of them can be safely used in pregnancy to treat some autoimmune diseases and have recently been introduced to clinical trials on refractory recurrent spontaneous miscarriages (23, 29, 33). Despite little experience in applying CZP for pregnancy rescue therapy, we will further consider its use because its unique structure could avoid placental transfer, making it a better, safer choice in pregnancy.
In our case series, we proposed a different therapeutic strategy only when pregnancies were endangered. All cases were closely monitored with serial serum hCG tests before and after adalimumab or certolizumab injection. The trajectory of hCG resumed a normalized pattern day 4.9 on average. In contrast, it takes about two weeks for TNF-α blockers to relieve the symptoms in patients with diseases such as RA. Hence, we assume from the data that it takes much less time for the agents to modulate the microenvironment in the maternal-fetal interface (34).
Compared with low-dose aspirin and LMWH or progesterone used in other clinical trials to prevent miscarriages in recent years, we use the TNFi as the primary therapy for rescuing pregnancies instead of preventing miscarriages (2, 17, 23, 29).
With this trial, we are the first to report a novel strategy with the use of TNFi that could save pregnancies with potential risks of miscarriage. It offers insights to other healthcare providers when they have patients with reduced hCG in early pregnancy. This may benefit pregnancy in the specific situation without compromising maternal and neonatal health. However, there are some limitations to our study. First, the number of this series is too small to reach a consolidated conclusion. Second, all women have been offered a range of medications rather than TNFi-only therapy. The use of medicines could compromise the pharmacological effect of TNF-α blockers in rescuing pregnancy. Third, although all early declines of hCG were observed in the same hospital, human factors could add to inaccurate testing of serum hCG levels in early pregnancy. However, even if we consider this, a sub-optimal growth is still considered to indicate possibly poor embryonic development.
Conclusion
Our study has demonstrated that TNFi can reverse potentially poor pregnancy outcomes in the first trimester. However, it can only be used in the context of a clinical research setting, and further trials and experiments should be carried out to confirm the pharmacological effect of TNFi in rescuing pregnancies at risk.
Patient Perspective
Case No. 1: I agree to the novel use of TNFi-led therapy for my specific situation and to report any problems for a better medical understanding and improvement of treatment options for women with a potential risk of miscarriage. I hope this enables the doctors to help pregnant women with safe and effective treatment. I am now very happy with my newborn baby.
Case No. 2: I agree to report on my case, the pregnancy, and the data of my baby. I hope this article can help other women like me get appropriate therapy for their threatened miscarriages.
Case No. 4: I agree to report my particular condition for a better medical understanding and improvement of treatment options for pregnant women whose hCG declines in early pregnancy. At present, I am 31 weeks pregnant. The antenatal examination and investigations have been normal and the fetus is developing well. I hope that this report could help more patients who share similar conditions with me in China and other countries worldwide.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhejiang Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
ZZ and FZ developed the idea for this study. ZZ wrote the manuscript and FZ reviewed it. YW and GW are involved in the diagnostic and therapeutic care and follow-up of the patients. YW and ZZ are also responsible for the tables and figures formation and revision. All authors contributed to the article and approved the submitted version.
Funding
This study is co-supported by Zhejiang Provincial Project for Medical and Health Science and Technology (Grant no. 2022503241) and Zhejiang Provincial Project for Medical Education (Grant no. Y202146113).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.900537/full#supplementary-material
References
1. Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, et al. Miscarriage Matters: The Epidemiological, Physical, Psychological, and Economic Costs of Early Pregnancy Loss. Lancet (2021) 397(10285):1658–67. doi: 10.1016/S0140-6736(21)00682-6
2. Coomarasamy A, Devall AJ, Cheed V, Harb H, Middleton LJ, Gallos ID, et al. A Randomized Trial of Progesterone in Women With Bleeding in Early Pregnancy. N Engl J Med (2019) 380(19):1815–24. doi: 10.1056/NEJMoa1813730
3. Cunningham FG, Leveno KJ, Bloom SL, Dashe JS, Spong CY, Hoffman BL, et al. Williams Obstetrics. 25th ed. 2018, New York: McGraw Hill Medical. 229–32. doi: 10.1016/S1526-9523(03)00291-5.
4. Barnhart KT, Guo W, Cary MS, Morse CB, Chung K, Takacs P, et al. Differences in Serum Human Chorionic Gonadotropin Rise in Early Pregnancy by Race and Value at Presentation. Obstet Gynecol (2016) 128(3):504–11. doi: 10.1097/AOG.0000000000001568
5. Barnhart KT, Sammel MD, Rinaudo PF, Zhou L, Hummel AC, Guo W, et al. Symptomatic Patients With an Early Viable Intrauterine Pregnancy: HCG Curves Redefined. Obstet Gynecol (2004) 104(1):50–5. doi: 10.1097/01.AOG.0000128174.48843.12
6. Whittaker PG, Schreiber CA, Sammel MD. Gestational Hormone Trajectories and Early Pregnancy Failure: A Reassessment. Reprod Biol Endocrinol (2018) 16(1):95. doi: 10.1186/s12958-018-0415-1
7. Pillai RN, Konje JC, Tincello DG, Potdar N. Role of Serum Biomarkers in the Prediction of Outcome in Women With Threatened Miscarriage: A Systematic Review and Diagnostic Accuracy Meta-Analysis. Hum Reprod Update (2016) 22(2):228–39. doi: 10.1093/humupd/dmv054
8. Nelson-Piercy C. Handbook of Obstetric Medicine. 6th ed. Inflammatory Bowel Disease. (2021) CRC Press. 248. doi: 10.1201/9780429330766
9. Brogin Moreli J, Cirino Ruocco AM, Vernini JM, Rudge MV, Calderon IM. Interleukin 10 and Tumor Necrosis Factor-Alpha in Pregnancy: Aspects of Interest in Clinical Obstetrics. ISRN Obstet Gynecol (2012) 2012:230742. doi: 10.5402/2012/230742
10. Hunt JS, Chen HL, Miller L. Tumor Necrosis Factors: Pivotal Components of Pregnancy? Biol Reprod (1996) 54(3):554–62. doi: 10.1095/biolreprod54.3.554
11. Flint J, Panchal S, Hurrell A, van deVenne M, Gayed M, Schreiber K, et al. BSR and BHPR Guideline on Prescribing Drugs in Pregnancy and Breastfeeding-Part I: Standard and Biologic Disease Modifying Anti-Rheumatic Drugs and Corticosteroids. Rheumatol (Oxford) (2016) 55(9):1693–7. doi: 10.1093/rheumatology/kev404
12. Gotestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer-Betz R, Elefant E, Chambers C, et al. The EULAR Points to Consider for Use of Antirheumatic Drugs Before Pregnancy, and During Pregnancy and Lactation. Ann Rheum Dis (2016) 75(5):795–810. doi: 10.1136/annrheumdis-2015-208840
13. Kavanaugh A, Cush JJ, Ahmed MS, Bermas BL, Chakravarty E, Chambers C, et al. Proceedings From the American College of Rheumatology Reproductive Health Summit: The Management of Fertility, Pregnancy, and Lactation in Women With Autoimmune and Systemic Inflammatory Diseases. Arthritis Care Res (Hoboken) (2015) 67(3):313–25. doi: 10.1002/acr.22516
14. Mahadevan U, Robinson C, Bernasko N, Boland B, Chambers C, Dubinsky M, et al. Inflammatory Bowel Disease in Pregnancy Clinical Care Pathway: A Report From the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology (2019) 156(5):1508–24. doi: 10.1053/j.gastro.2018.12.022
15. Clowse MEB, Scheuerle AE, Chambers C, Afzali A, Kimball AB, Cush JJ, et al. Pregnancy Outcomes After Exposure to Certolizumab Pegol: Updated Results From a Pharmacovigilance Safety Database. Arthritis Rheumatol (2018) 70(9):1399–407. doi: 10.1002/art.40508
16. Mahadevan U, Long MD, Kane SV, Roy A, Dubinsky MC, Sands BE, et al. Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women With Inflammatory Bowel Disease. Gastroenterology (2021) 160(4):1131–9. doi: 10.1053/j.gastro.2020.11.038
17. Winger EE, Reed JL. Treatment With Tumor Necrosis Factor Inhibitors and Intravenous Immunoglobulin Improves Live Birth Rates in Women With Recurrent Spontaneous Abortion. Am J Reprod Immunol (2008) 60(1):8–16. doi: 10.1111/j.1600-0897.2008.00585.x
18. RPL EGGo, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, et al. ESHRE Guideline: Recurrent Pregnancy Loss. Hum Reprod Open (2018) 2018(2):hoy004. doi: 10.1093/hropen/hoy004
19. Ng SC, Gilman-Sachs A, Thaker P, Beaman KD, Beer AE, Kwak-Kim J. Expression of Intracellular Th1 and Th2 Cytokines in Women With Recurrent Spontaneous Abortion, Implantation Failures After IVF/ET or Normal Pregnancy. Am J Reprod Immunol (2002) 48(2):77–86. doi: 10.1034/j.1600-0897.2002.01105.x
20. Grimbizis GF, Gordts S, Di SpiezioSardo A, Brucker S, De Angelis C, Gergolet M, et al. The ESHRE/ESGE Consensus on the Classification of Female Genital Tract Congenital Anomalies. Hum Reprod (2013) 28(8):2032–44. doi: 10.1093/humrep/det098
21. Boerma T, Ronsmans C, Melesse DY, Barros AJD, Barros FC, Juan L, et al. Global Epidemiology of Use of and Disparities in Caesarean Sections. Lancet (2018) 392(10155):1341–8. doi: 10.1016/S0140-6736(18)31928-7
22. Qi H, Yang H. Guidelines on Preconception Care and Antenatal Care. Chin J Obstetrics Gynecol (2018) 53(1):7–13. doi: 10.3760/cma.j.issn.1007-9408.2018.03.001
23. Alijotas-Reig J, Esteve-Valverde E, Llurba E, Gris JM. Treatment of Refractory Poor aPL-Related Obstetric Outcomes With TNF-Alpha Blockers: Maternal-Fetal Outcomes in a Series of 18 Cases. Semin Arthritis Rheum (2019) 49(2):314–8. doi: 10.1016/j.semarthrit.2019.02.006
24. Arck PC, Hecher K. Fetomaternal Immune Cross-Talk and its Consequences for Maternal and Offspring’s Health. Nat Med (2013) 19(5):548–56. doi: 10.1038/nm.3160
25. Shahine L, Lathi R. Recurrent Pregnancy Loss: Evaluation and Treatment. Obstet Gynecol Clin North Am (2015) 42(1):117–34. doi: 10.1016/j.ogc.2014.10.002
26. Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M, et al. Tumor Necrosis Factor-Alpha Inhibits Trophoblast Migration Through Elevation of Plasminogen Activator Inhibitor-1 in First-Trimester Villous Explant Cultures. J Clin Endocrinol Metab (2004) 89(2):812–22. doi: 10.1210/jc.2003-031351
27. Anim-Nyame N, Gamble J, Sooranna SR, Johnson MR, Steer PJ. Microvascular Permeability is Related to Circulating Levels of Tumour Necrosis Factor-Alpha in Pre-Eclampsia. Cardiovasc Res (2003) 58(1):162–9. doi: 10.1016/S0008-6363(02)00844-1
28. Zhang C, Deng X, Zhang X, Pan Z, Zhao W, Zhang Y. Association Between Serum TNF-Alpha Levels and Recurrent Spontaneous Miscarriage: A Meta-Analysis. Am J Reprod Immunol (2016) 75(2):86–93. doi: 10.1111/aji.12447
29. Fu J, Li L, Qi L, Zhao L. A Randomized Controlled Trial of Etanercept in the Treatment of Refractory Recurrent Spontaneous Abortion With Innate Immune Disorders. Taiwan J Obstet Gynecol (2019) 58(5):621–5. doi: 10.1016/j.tjog.2019.07.007
30. Seow CH, Leung Y, Vande Casteele N, Ehteshami Afshar E, Tanyingoh D, Bindra G, et al. The Effects of Pregnancy on the Pharmacokinetics of Infliximab and Adalimumab in Inflammatory Bowel Disease. Aliment Pharmacol Ther (2017) 45(10):1329–38. doi: 10.1111/apt.14040
31. Mariette X, Forger F, Abraham B, Flynn AD, Molto A, Flipo RM, et al. Lack of Placental Transfer of Certolizumab Pegol During Pregnancy: Results From CRIB, a Prospective, Postmarketing, Pharmacokinetic Study. Ann Rheum Dis (2018) 77(2):228–33. doi: 10.1136/annrheumdis-2017-212196
32. Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, van Assen S, Bijl M, et al. 2019 Update of EULAR Recommendations for Vaccination in Adult Patients With Autoimmune Inflammatory Rheumatic Diseases. Ann Rheum Dis (2020) 79(1):39–52. doi: 10.1136/annrheumdis-2019-215882
33. O'Byrne LJ, Alqatari SG, Maher GM, O'Sullivan AM, Khashan AS, Murphy GP, et al. Fetal and Maternal Outcomes After Maternal Biologic Use During Conception and Pregnancy: A Systematic Review and Meta-Analysis. BJOG (2022). doi: 10.1111/1471-0528.17093
Keywords: miscarriage, case series, hCG (human chorionic gonadotrophin), TNF – α, pregnancy-rescue management, TNFi
Citation: Zhong Z, Wang Y, Wang G and Zhou F (2022) Case Report: TNF-Alpha Inhibitors to Rescue Pregnancy in Women With Potential Pregnancy Loss: A Report of Ten Cases. Front. Immunol. 13:900537. doi: 10.3389/fimmu.2022.900537
Received: 22 March 2022; Accepted: 25 April 2022;
Published: 25 May 2022.
Edited by:
Kenneth Beaman, Rosalind Franklin University of Medicine and Science, United StatesReviewed by:
Ian P. Giles, University College London, United KingdomAlessandro Rolfo, University of Turin, Italy
Copyright © 2022 Zhong, Wang, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feifei Zhou, NTUxMDAyNUB6anUuZWR1LmNu
 Zixing Zhong
Zixing Zhong Yuhan Wang
Yuhan Wang Guiqin Wang
Guiqin Wang Feifei Zhou
Feifei Zhou