- 1Center for Genetic Epidemiology and Genomics, School of Public Health, Medical College of Soochow University, Suzhou, China
- 2Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Soochow University, Suzhou, China
- 3Department of Rheumatology, The Second Affiliated Hospital of Soochow University, Suzhou, China
Background: Effective identification of high-risk rheumatoid arthritis (RA) individuals is still a challenge. Whether the combined effects of multiple previously reported genetic loci together with lifestyle factors can improve the prediction of RA risk remains unclear.
Methods: Based on previously reported results and a large-scale Biobank dataset, we constructed a polygenic risk score (PRS) for RA to evaluate the combined effects of the previously identified genetic loci in both case-control and prospective cohorts. We then evaluated the relationships between several lifestyles and RA risk and determined healthy lifestyles. Then, the joint effects of healthy lifestyles and genetic risk on RA risk were evaluated.
Results: We found a positive association between PRS and RA risk (OR = 1.407, 95% confidence interval (CI) = 1.354~1.463; HR = 1.316, 95% CI = 1.257~1.377). Compared with the low genetic risk group, the group with intermediate or high genetic risk had a higher risk (OR = 1.347, 95% CI = 1.213~1.496; HR = 1.246, 95% CI = 1.108~1.400) (OR = 2.169, 95% CI = 1.946~2.417; HR = 1.762, 95% CI = 1.557~1.995). After adjusting for covariates, we found protective effects of three lifestyles (no current smoking, regular physical activity, and moderate body mass index) on RA risk and defined them as healthy lifestyles. Compared with the individuals with low genetic risks and favorable lifestyles, those with high genetic risks and unfavorable lifestyles had as high as OR of 4.637 (95%CI = 3.767~5.708) and HR of 3.532 (95%CI = 2.799~4.458).
Conclusions: In conclusion, the integration of PRS and lifestyles can improve the prediction of RA risk. High RA risk can be alleviated by adopting healthy lifestyles but aggravated by adopting unfavorable lifestyles.
Introduction
As an autoimmune and inflammatory disease, rheumatoid arthritis (RA) is characterized by persistent synovitis and systemic inflammation that is induced by the attack of the misguided immune system on healthy cells (1). Clinically, RA patients show painful swelling of the joints, joint deformity, and at later stage damage to a wide variety of body systems, including organs such as the heart and lungs (2). Epidemiological studies have shown that the average global prevalence of RA is estimated to be 0.5-1.0% (3, 4).
The pathogenesis of RA is very complex. Genetic factors, environmental factors, and their interaction determine the susceptibility to RA. The heritability of RA is estimated to be 50% to 60% (4), and family history has approximately three to five times the increased risk of developing RA (3). Previous large-scale genome-wide association study (GWAS) analyses have identified a long list of genetic loci associated with RA risk (5). These loci individually have relatively small or moderate effects on susceptibility to RA risk, but whether the combined effects of multiple genetic loci together can be used as an indicator of genetic risk in effectively predicting RA risk is still largely unknown.
The development of RA is also closely related to environmental factors, and we summarize previous studies focused on the association between lifestyle and RA in Table S1. An unhealthy daily lifestyle has been shown to increase the risk of developing RA. Dietary patterns play a central role in the risk and progression of inflammatory diseases (6). For example, excessive consumption of red meat promotes inflammation and proinflammatory effects (e.g., excessive intake of red meat, salt, and calories), whereas regular intake of fish rich in oils and fats and consumption of fruit reduces inflammation (7–9). Numerous epidemiological studies have demonstrated that smoking behavior greatly increases the risk of RA. In vivo experiments and animal models discovered crucial roles of smoking in the immune system, synovial fibroblasts, and drug response in RA, which were proven to participate in RA development (10). Subjects with regular physical activity had a lower prevalence of RA than those who were less physically active and physically active RA patients had a milder disease course (11). As a crucial role in the development of diseases, obesity shares common inflammatory links with RA (9).
The polygenic risk score (PRS) is an effective tool to assess the genetic risk of diseases by combining the effects of multiple genetic loci and has been widely used for the risk assessment of cancers and psychiatric diseases (12, 13). Several PRS models have also been constructed to predict RA onset previously, and we summarized most of them in Table S2 (14–16). However, these models have some endogenous limitations (e.g., limited sample size, either in case-control samples or in prospective cohorts; no systematic evaluation of PRS prediction models under different significance levels; the interaction between PRS and environmental factors was not assessed) that lead to limited risk prediction capacity. More importantly, these models have not taken into account environmental factors, although they are important in determining RA alone or by interacting with genetic loci. A study demonstrated the interactions between multiple environmental exposures and RA risk genes (17). However, no study has focused on RA risk prediction by integrating lifestyle and genetic factors.
The UK Biobank is a large prospective cohort study of 500,000 people covering a wealth of information on demographics, risk factors, environmental exposures, disease diagnosis data, and more (18, 19). Based on baseline, follow-up, and genotype data, we performed a systematic study for RA risk prediction. First, we constructed a PRS for RA and further analyzed the combined effects of the previously identified genetic loci on RA risk and their predictive power. We then assessed the relationship between lifestyles and RA risk and classified individuals according to their lifestyles. Finally, we explored the joint effects by integrating genetic effects and lifestyles on predicting RA risk in a large case-control sample and prospective cohort.
Materials and Methods
Study Design
We randomly divided the total population into two parts: training (20%) and test sets (80%), and the seed for sampling was set to “1234” and all variables were balanced in the two divided data sets. The appropriate PRS was selected in the training set, and we evaluated associations between selected PRS and RA risk in the test set. Next, we analyzed the associations between RA risk and multiple lifestyle factors and defined them as healthy lifestyles to explore the joint or interactive effects of genetic and lifestyle factors on RA risk in both case-control and prospective cohort analyses.
UK Biobank Data Sets
The UK Biobank is a large-scale biomedical database containing in-depth genetic and health information from half a million UK participants (http://www.ukbiobank.ac.uk/) (19). The project began in 2006 with the recruitment of participants aged 40-69 years from 22 assessment centers at baseline, with each eligible participant completing a written informed consent form. The project provided detailed health-related information (including nutrition, lifestyle, medications, etc.) through extensive baseline questionnaires, interviews, physical measurements, blood biochemical analyses, and genotyping of all subjects. This provides an unprecedented opportunity to explore the respective contributions of genetic and environmental factors to disease development.
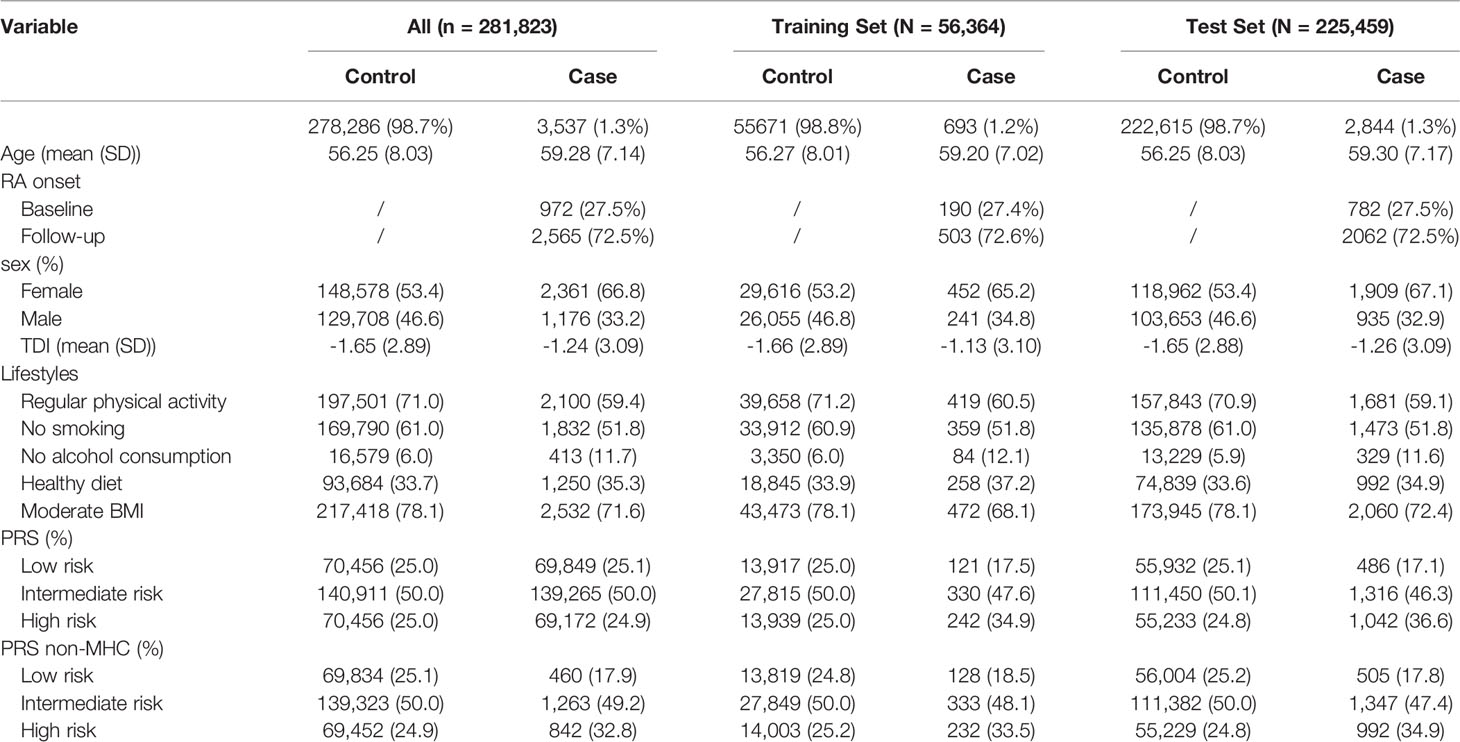
RA was defined by individual medical records obtained from the International Classification of Diseases, Tenth Revision (ICD-10-CM) M05 and M06 in the UK Biobank, which has been commonly used in previous studies (20, 21). Outcomes in the UKB cohort were determined by the onset of RA from baseline up to the date of diagnosis, death, loss to follow-up, or June 31, 2021, whichever occurred first. Therefore, all diagnosed RA patients at baseline were excluded from the prospective study. For individuals, we retained individuals with successfully measured genotypes as well as individuals with white British ancestry. Notably, participants diagnosed with Crohn’s arthritis, arthritis in ulcerative colitis, psoriatic arthritis, and osteoarthritis were excluded from our analysis. The quality control process of the participants is presented in Figure 1A. Related field IDs for covariates and ICD-10 codes used for RA diagnosis in the UK Biobank dataset are summarized in Table S3.
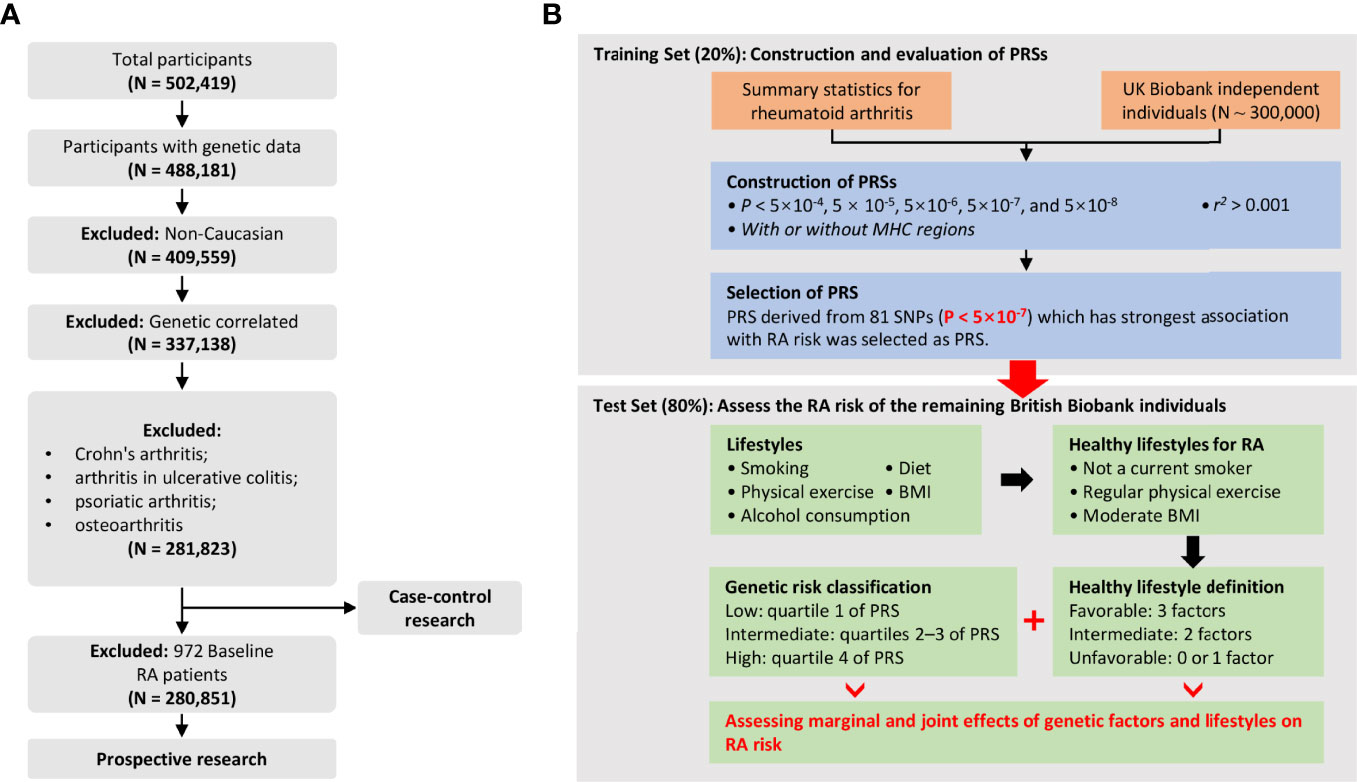
Figure 1 (A) Flowchart for filtering participants from the UK Biobank cohort; (B) Study design and workflow of our research.
Genotyping and Imputation
The genotyping process and quality control process used in the UKB study have been described elsewhere (19). For genotypes, autosomal analysis was restricted to high-quality Haplotype Reference Consortium imputed variants with an MAF > 0.02, imputation information score > 0.5, missing ratio > 0.95, and Hardy-Weinberg P > 1×10−7. For individuals, we excluded unsuccessful genotyping individuals, nonwhite British individuals, and individuals with at least one relative identified. Finally, up to ~330,000 independent European ancestry individuals with ~9,400,000 high-quality single nucleotide polymorphisms (SNPs) remained for subsequent GWAS analysis.
Definition of Healthy Lifestyles
According to a review of previous studies (22–25), we considered five healthy lifestyle factors in our analysis, including no current smoking, alcohol consumption, regular physical activity, moderate body mass index (BMI), and healthy dietary pattern: 1. Smoking: Former smokers who had never smoked or had quit for at least 30 years were defined as “nonsmokers”, and others were defined as “smokers”. 2. Alcohol consumption: “Alcohol consumption” was defined as those who drank more frequently than once per month and those whose drinking status was current drinking; “No Alcohol consumption” was defined as no alcohol intake/any alcohol. 3. Physical activity: “Regular physical activity” is defined as those who engage in vigorous activity for at least 75 minutes per week or moderate activity for 150 minutes per week (or an equivalent combination) or vigorous activity and moderate physical activity at least once a week for at least 5 days; others were defined as “No regular physical activity” 4. Diet: “Healthy diet” was defined as those who consumed more fruits, vegetables, whole grains, and fish, as well as less red meat and processed meat; others were defined as an “unhealthy diet”. 5. BMI: “Moderate BMI” is someone whose BMI ranges from 18.5 to 24. Details about the data fields and definitions of healthy lifestyles are summarized in Table S4, and all information was collected at recruitment. We included three healthy lifestyle indicators (i.e., no smoking, regular physical activity, and moderate BMI): Include 0 to 1 healthy lifestyle indicator for unfavorable lifestyle, 2 for moderate lifestyle, and all 3 for favorable lifestyle.
Construction of Polygenic Risk Score
RA-associated SNPs and corresponding summary statistics were accessed from the largest European RA GWAS analysis (5). The genotype of each SNP was obtained from the UK Biobank. RA-associated SNPs were selected at a conservative threshold by using the clumping procedure of PLINK (26): linkage disequilibrium (LD) clumping was performed to identify independent IVs with 1000 Genomes Projects as a reference panel, where the linkage disequilibrium r2 was set to 0.001 and the window was set to a 1 MB physical distance. SNPs associated with RA were selected under different cut-offs (P-value < 5×10-4, 5×10-5, 5×10-6, 5×10-7, 5×10-8), containing 674, 208, 117, 80, and 58 SNPs, respectively. Then, PRSs for RA can be calculated by where is the SNP estimated effect for the kth SNP, and Xik is the genotype (0, 1, 2) of the kth SNP index for the ith individual. Finally, a total of 5 PRSs were generated under 5 different thresholds.
To validate the PRSs, the dataset was randomly divided into a training set (20%, N = 56,364) and a test set (80%, N case-control = 225,459, N prospective = 224,927). Each PRS was standardized with a mean zero and standard deviation of one over the entire dataset. Next, we employed logistic regression to model the associations between the 5 PRSs and RA risk, adjusting for fifteen covariates [i.e., age, sex, Townsend deprivation index (TDI) (27), genotyping chip (UKB vs BiLEVE), and top 10 genetic principal components (PCs)]. The best-performing PRS, in terms of highest association and area under the curve (AUC), was selected as the final PRS and held fixed for further analysis in the test set. The genetic risk groups were divided by PRS: low genetic risk (bottom quartile of PRS), intermediate genetic risk (quartiles 2 to 3), and high genetic risk (top quartile). Additionally, considering the complex genetic structure of SNPs, we derived the best-performing PRS after removing SNPs encoded by genes within the human major histocompatibility complex (MHC) region (chr6: 28,477,797 ~ 33,448,354) (PRS non-MHC), containing a total of 68 SNPs.
Statistical Analysis
Independent samples t-test and Manny Whitney U-test were used to compare PRS between RA patients and controls. Multivariable regression models were used to evaluate the association after adjusting for potential confounders (i.e., age, sex, TDI, genotyped batch, assessment center, top 10 PCs). Specifically, multivariable logistic regression was used to assess associations between genetic and lifestyle factors and RA incidence, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to measure RA risk in a case-control study. In a prospective study, multivariable Cox regression was utilized, and hazard ratios (HRs) with 95% CIs were calculated to measure RA risk over time. The prediction accuracy of the PRS model was assessed using the AUC with the “pROC” package (28), and a larger AUC indicated better prediction accuracy of the model. Cumulative incidence rates were evaluated within different subgroups in a prospective study. The additive interaction analysis between genetic risk and lifestyles was assessed by relative excess risk for interaction (RERI) and attributable proportion due to interaction (AP) (29). Trend analysis was performed by the Cochran-Armitage trend test with the “DescTools” package (30). To further investigate the specific genetic variants underlying the detected interactions, lifestyle-by-single SNP interaction analysis was conducted with the generalized linear regression model in PLINK software (26). Age, sex, TDI, genotyped batch, assessment center, and the top 10 PCs were used as covariates. Statistical significance was set to a two-sided P value of less than 0.05. All statistical analyses were performed in R 3.6.1 and PLINK software (version v1.90 b3.38) (31).
Results
Basic Characteristics
As shown in Table 1, after strict quality control, a total of 3,537 RA patients (2,565 new cases) were included in this study, so the incidence and prevalence of RA were estimated to be 1.3% and 0.9%. We randomly divided the data set into a training set (20%) and a test set (80%) (Table 1). Notably, the training set was only used to select the appropriate PRS threshold and fitted prediction models, and all other analyses were performed in the test set. RA patients were excluded at baseline in the prospective analysis, and the follow-up date was updated to June 31, 2021, with a median follow-up of 12 years. The overflow of our research is presented in Figure 1B. In addition, we found that the gender-specific participants differed in all lifestyles (Table S5), and the prevalence of RA was significantly higher in women than in men (1.59% in women and 0.91% in men). Therefore, we carefully performed gender stratification analyses in most situations.
Selection of PRS in the Training Set
We calculated 5 PRSs for RA based on SNP information from published GWAS results and genotypes from a large UK Biobank database under different thresholds (P < 5×10-4, P < 5×10-5, P < 5×10-6, P < 5×10-7, P < 5×10-8). We used multivariate regression models to assess the associations between these PRSs and RA risk in the training set after adjusting for the covariates. The PRS derived from 80 single-nucleotide polymorphisms (SNPs) (P < 5×10-7) showed the strongest association with RA risk in the training set (OR = 1.352, 95% CI = 1.257~1.454, P = 4.83×10-16; HR = 1.216, 95% CI = 1.114~1.328, P = 1.22×10-5) (Table S6), and detailed information of SNPs used was summarized in Table S7. The distribution of selected PRSs showed that RA subjects tended to have a higher PRS than those without RA (P < 0.001) (Figures 2A, D), and similar distributions were observed in the sex-stratified subgroups (Figure S1). We performed enrichment analysis for genes mapped by SNP in PRS, and results show these genes were highly related to the mechanism of development and progression of RA (e.g., T cell regulation, lymphocyte activation) (Figure S2).
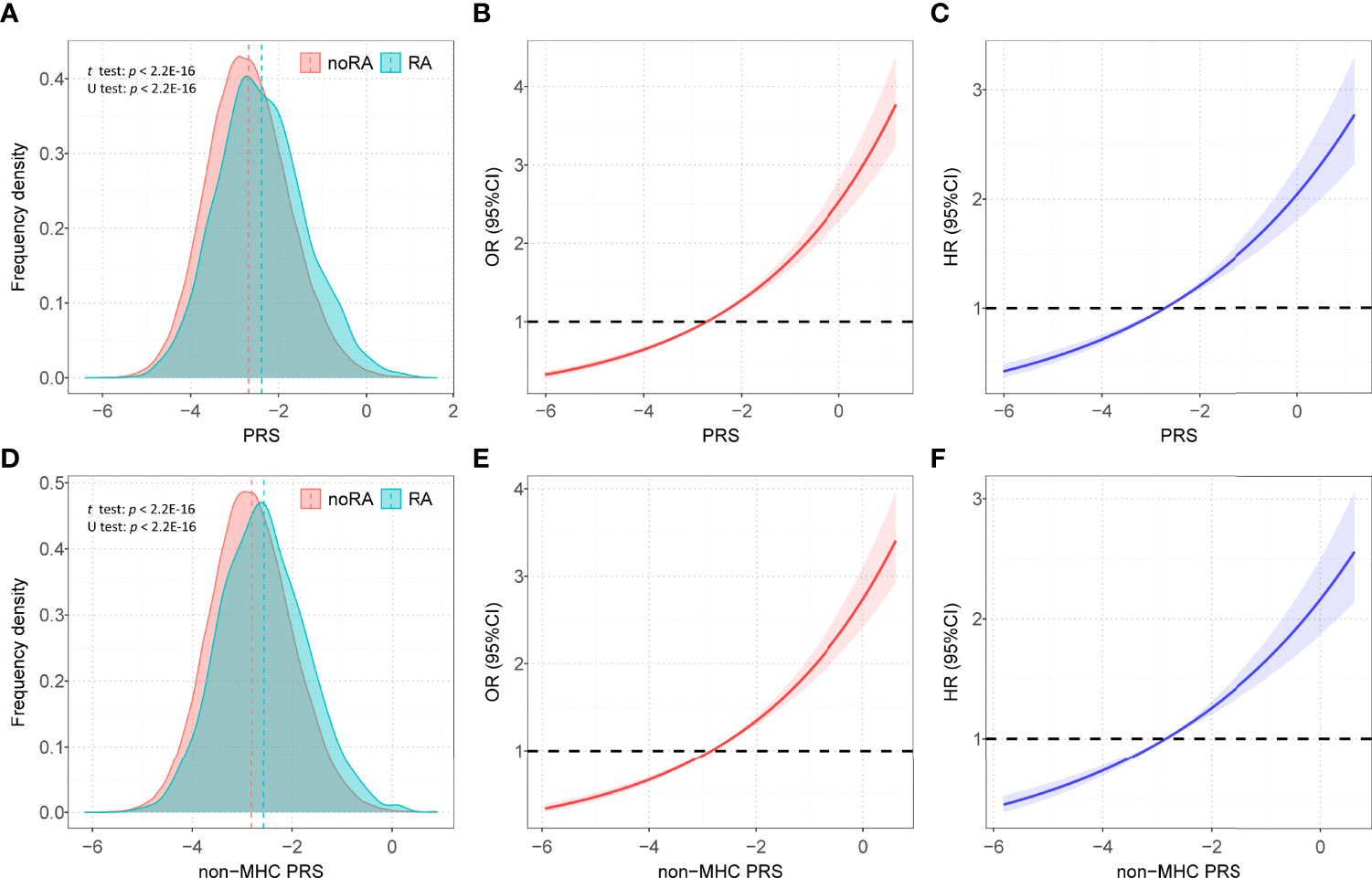
Figure 2 The distributions of PRSs among participants with and without RA. (A) Distribution of PRS among participants with and without RA in all participants; (B) Restricted cubic spline models for the relationship between PRS and RA risk in case–control analysis; (C) Restricted cubic spline models for the relationship between PRS and RA risk in prospective analysis; (D) Distribution of PRS non-MHC among participants with and without RA in all participants; (E) Restricted cubic spline models for the relationship between non-MHC PRS and RA risk in case-control analysis; (F) Restricted cubic spline models for the relationship between non-MHC PRS and RA risk in prospective analysis; Associations in B, C, E, and F were adjusted for age, sex, genotyped batch, assessment center, TDI and the first 10 principal components of ancestry. RA, rheumatoid arthritis; PRS, polygenic risk score; MHC, major histocompatibility complex; OR, odds ratio; HR, hazard ratio.
Association Between PRS and RA Risk in the Test Set
After adjusting for covariates, positive associations were observed between best-performed PRS and RA risk in the test set (OR = 1.407, 95% CI = 1.354~1.463, P = 7.45×10-67; HR = 1.316, 95% CI = 1.257~1.377, P = 2.03×10-32). Sex-stratified analysis showed that a per SD increase in PRS was significantly associated with RA risk in men (OR = 1.397, 95% CI = 1.306~1.494, P = 1.87×10-22; HR = 1.344, 95% CI = 1.244~1.452, P = 6.09×10-14) and women (OR = 1.412, 95% CI = 1.347~1.481, P = 5.03×10-46; HR = 1.300, 95% CI = 1.229~1.375, P = 4.23×10-20). From the first decile to the tenth decile, we also found an approximate gradient increase in RA risk (Figures S3A, B), and similar results were observed in sex-stratified analyses (Figures S3C-F). The restricted cubic spline model was used to evaluate the relationship between PRS and RA risk. In both case-control and prospective studies, PRS and PRS non-MHC were also observed to be related to the risk of RA (Figures 2B, C, E, F), and similar results were found for men and women (Figure S4). We further divided PRS into three groups (low, intermediate, and high genetic risk). Individuals in the intermediate and high genetic risk groups had a higher risk of RA than those in the low genetic risk group. Compared with the low genetic risk group, the ORs of the intermediate and high groups were estimated to be 1.347 (95% CI = 1.213~1.496, P = 2.40×10-08) and 2.169 (95% CI = 1.946~2.417, P = 1.80×10-44), respectively (Table S8). In the prospective analysis, the HRs of the intermediate and high groups were estimated to be 1.246 (95% CI = 1.108~1.400, P = 2.39×10-04) and 1.762 (95% CI = 1.557~1.995, P = 3.42×10-19), respectively, when compared with the low genetic risk group (Figures 3A, B). Similar results were also observed in male and female individuals (Table S8 and Figure S5), as were the results of PRS non-MHC (Table S9).
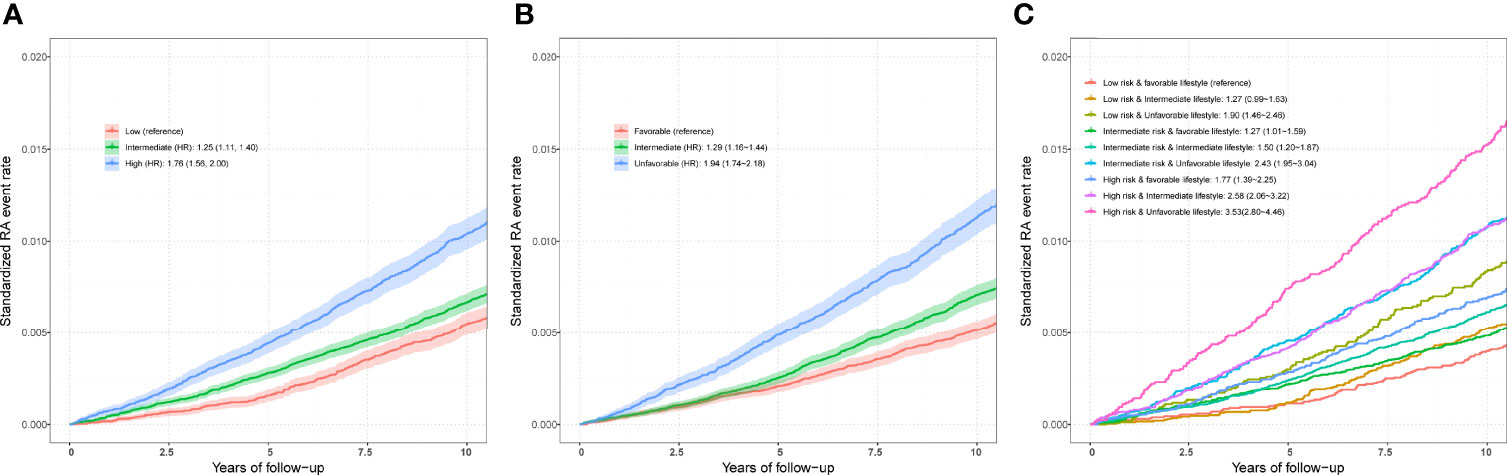
Figure 3 (A) Standardized rates of RA events in low (bottom quartile), intermediate (quartiles 2 to 3), and high (top quartile) genetic risk groups in the UKB cohort. (B) Standardized rates of RA events in favorable, intermediate, and unfavorable lifestyle groups in the UKB cohort (C) Standardized rates of RA events in participates with different genetic risk score groups and different lifestyle groups in the UKB cohort. Associations were adjusted for age, sex, genotyped batch, assessment center, TDI, and the first 10 principal components of ancestry. RA, rheumatoid arthritis; PRS, polygenic risk score; MHC, major histocompatibility complex; OR, odds ratio; HR, hazard ratio. Definition of healthy lifestyle indicators: include 0 to 1 healthy lifestyle indicator for unfavorable lifestyle, 2 for moderate lifestyle, and all 3 for favorable lifestyle. The genetic risk groups were evaluated by PRS: low genetic risk (bottom quartile of PRS), intermediate genetic risk (quartiles 2 to 3), and high genetic risk (top quartile).
In addition, we evaluated the prediction accuracy of the clinical model (including only age, sex, genotyped batch, TDI, smoking status, physical activity, drinking status, and BMI), PRS + clinical model, and non-MHC PRS + clinical model in the test set. Compared with the non-MHC PRS + clinical model and clinical model, the PRS + clinical model had higher AUCs, with increased AUCs of 0.003 (0.4%) and 0.016 (2.4%) (Figure S6).
Association Between RA Risk and Lifestyle in the Test Set
To define a healthy lifestyle for RA, we considered five possible favorable factors in our analyses: no smoking, no alcohol consumption, moderate BMI, healthy diet, and regular exercise. After correcting for the specified covariates, we found a protective effect of three lifestyles on the risk of RA in the case-control study (Figure S7). The OR of RA occurring in subjects with a moderate BMI compared to those with an abnormal BMI was 0.704 (95% CI = 0.639~0.776, P = 1.31×10-12), and the HR was 0.743 (95% CI = 0.675~0.819, P = 2.11×10-9). Nonsmokers had a lower risk of RA than smokers (OR = 0.707, 95% CI = 0.647 ~0.772, P = 1.57×10-14; HR = 0.699, 95% CI = 0.640~0.763, P = 1.30×10-15). Meanwhile, regular exercise reduced the risk of developing OR (OR = 0.750, 95% CI = 0.685 ~0.821, P = 4.77×10-10; HR = 0.720, 95% CI = 0.658~0.787, P = 5.06×10-13), as was the case in both men and women. Furthermore, similar results were observed in the prospective study (Figure S8). However, in this large cross-sectional study, we found a protective effect of alcohol consumption on RA, which could not be regarded as a healthy lifestyle. In addition, we did not find a significant association between a healthy diet and the risk of RA.
Finally, we included three favorable factors for further analysis and divided participants into three groups (favorable, intermediate, and unfavorable lifestyles). Compared with those with a favorable lifestyle, logistic regression analysis in a case–control study found an increased risk of RA in individuals adopting intermediate (OR = 1.342, 95% CI = 1.221~1.475, P = 9.72×10-10) and unfavorable lifestyles (OR = 2.152, 95% CI = 1.950~2.374, P = 8.41×10-53). In the sex-stratified analysis, the association was weakened in men and enhanced in women. For example, the ORs of unfavorable vs favorable outcomes were estimated to be 2.257 (95% CI = 1.897~2.684, P = 3.85×10-20) in men and 2.095 (95% CI = 1.859~2.361, P = 7.37×10-34) in women (Table S10). Additionally, in the prospective study, the HRs of unfavorable vs favorable outcomes were estimated to be 1.944 (95% CI = 1.735~2.179, P = 3.06×10-30) in all participants, 2.030 (95% CI = 1.671~2.467, P = 1.02×10-12) in men and 1.888 (95% CI = 1.639~2.174, P = 1.10×10-18) in women (Table S10).
Joint Effect and Interaction of PRS/Genotypes and Lifestyle Factors in the Test Set
Compared with individuals with a low genetic risk and a favorable lifestyle, more than quintuple risks were observed in those with high genetic risk and an unfavorable lifestyle (OR = 4.637, 95% CI = 3.767~5.708, P = 1.82×10-47; HR = 3.532, 95% CI = 2.799~4.458, P = 2.22×10-26) (Figures 3C,4A). A higher association in men (OR = 5.879, 95% CI = 3.949~8.752, P = 2.70×10-18; HR = 5.480, 95% CI = 3.476~8.640, P = 2.41×10-13) and a lower risk in women (OR = 4.175, 95% CI = 3.264~5.339, P = 4.95×10-30; HR = 2.904, 95% CI = 2.202~3.830, P = 4.32×10-14) were also detected (Tables S11 and S12, Figure 4A). In a prospective study, as the genetic risk rises, the risk of RA in patients who adopt a relatively worse lifestyle also increases significantly (Figure 4B and Table S11). Furthermore, similar results were observed in non-MHC PRS analyses (Tables S13 and S14). In all subgroups with different genetic risks or different genders, trend analyses found that the risk of developing RA was associated with increased genetic risk. After considering all five of the widely used lifestyles, although still significant, the association was weakened (Tables S15 and S16). We also evaluated the association in individuals without excluding diseases (Tables S17 and S18). Sensitive analyses were performed after re-selection of training (40%) and test sets (60%) (Tables S19 and S20), after redefining the appropriate BMI as 18.5~25 (Tables S21 and S22), and after redefining the healthy smoking behavior with no current smoking (Tables S23 and S24). All results demonstrate the robustness of the association. In addition, we observed an additive interaction between high genetic risk and healthy lifestyles in both the whole population and sex-stratified groups (Tables S25 and S26). Further lifestyle-by-single SNP interaction analyses found interactions between the 10 RA-associated SNPs and healthy lifestyles (P<0.05), but the significance of the association was relatively low (Table S27).
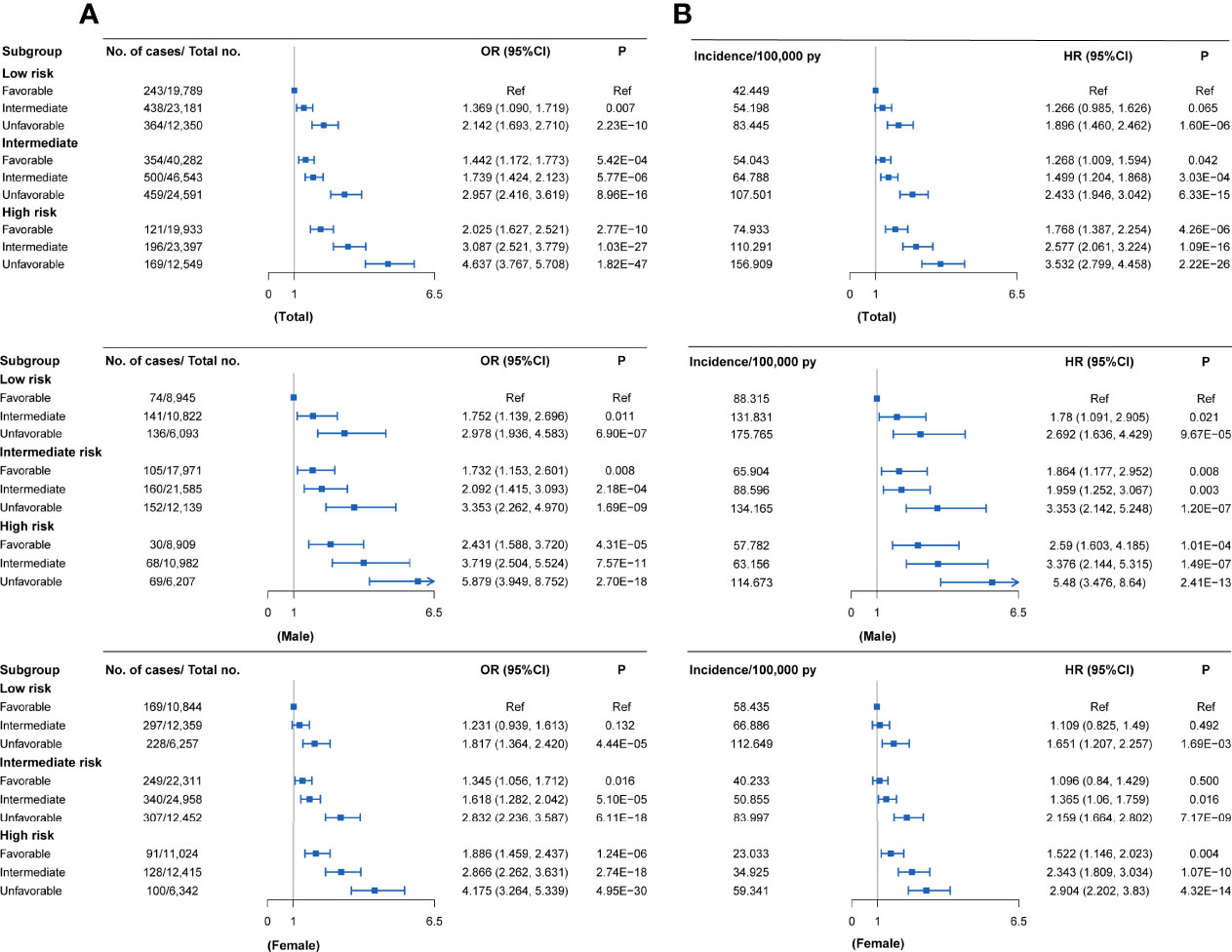
Figure 4 RA risks in the subgroups stratified by genetic PRSs and lifestyles in the UKB cohort (versus participants with favorable lifestyles in the low genetic risk group) (A) Case-control study; (B) Cohort study. Definition of healthy lifestyle indicators: include 0 to 1 healthy lifestyle indicator for unfavorable lifestyle, 2 for moderate lifestyle, and all 3 for favorable lifestyle. The genetic risk groups were evaluated by PRS: low genetic risk (bottom quartile of PRS), intermediate genetic risk (quartiles 2 to 3), and high genetic risk (top quartile).
Discussion
This study constructed the PRS for RA to assess the joint effect of lifestyle and genetics on RA risk in the UKB cohort. The prediction of RA risk was improved by integrating lifestyle and genetic factors. Specifically, high genetic risk, as defined by the PRS, is associated with an increased risk of developing RA in large case-control and prospective studies. Three favorable lifestyles were significantly associated with a reduced risk of RA. Subjects with high genetic risk and unfavorable lifestyles have an increased risk of developing RA compared to those with low genetic risk and favorable lifestyles. Furthermore, adherence to a healthy behavioral lifestyle was associated with a reduced risk of developing RA within each of the different genetic risk groups. To the best of our knowledge, our study is the first to assess the joint effect of lifestyles and genetic factors on RA risk in a large sample.
Three healthy lifestyles were identified to be related to decreased RA risk: tobacco smoking could increase RA risk, which was also proven in previous observational studies (32). A meta-analysis showed that smoking is a risk factor for RA, especially for heavy smokers with RA (33). A population-based case-control study in Sweden showed that gene-environment interactions between smoking and genes increased the risk of RA (34). The involved mechanisms are complex, probably due on the one hand to the effects of smoking on the immune system (e.g., oxidative stress, apoptosis, proinflammatory, and epigenetic effects) and on the other hand to the direct effects of smoking on synovial fibroblasts (10). In addition, the results of the case-control and cohort studies were not identical but showed consistent trends, which increases the reliability of the conclusions.
Some previous studies have also reported the protective effect of moderate alcohol consumption on RA risk. In two large prospective cohorts, moderate alcohol usage had a reduced risk of RA compared to no alcohol consumption, with an HR of 0.78 (95% CI, 0.61-1.00) (35). The mechanism underlying such an association has not been well explained. On the one hand, this may be because alcohol intake increases serum estradiol concentrations, which play an important role in the body’s immune response (36, 37); on the other hand, alcohol has been shown to significantly inhibit the synthesis of proinflammatory cytokines and chemokines (38, 39). A recent animal experiment clarified the immune regulation and tolerance induction effects of drinking (40). Therefore, moderate alcohol consumption may stop or delay the progression of RA. However, given the increased risk of other serious health problems associated with moderate alcohol consumption, we did not regard alcohol consumption as a healthy lifestyle in our study.
In previous studies, even though the association between diet and RA risk was not as strong as other risk factors (e.g., smoking, BMI), its impact on RA has been widely studied. A comprehensive literature review shows the controversy between different dietary approaches and the risk of RA (6). For example, a study has shown that an increased risk of RA is associated with excessive consumption of red meat and high total protein (41). However, a multicenter case-control study showed no significant difference in red meat intake between the two groups (42). In addition, high carbohydrate intake may be associated with an increased risk of RA, while mushrooms, citrus fruits, and dairy products reduce the risk (42). A prospective study based on the Nurses’ Health Study (NHS) shows no association between protein, iron, and meat intake and the risk of RA (43). High dietary sodium (salt) intake is associated with an increased risk of RA and may interact with smoking (44). The high complexity of nutrient supply is also a major factor affecting the composition of the microbiota, which is ultimately involved in the occurrence and development of RA. However, our study did not find a reduction in the risk of RA with a healthy diet, which may be due to healthy volunteer bias in the retrospective investigation. Therefore, more long-term cohort studies are needed to explore this.
In contrast, BMI can be a good indicator of long-term lifestyle as opposed to a record of a healthy diet. Our results found that moderate BMI could lower RA risk, especially in women. A meta-analysis of 13 cohort studies also showed that higher BMI was associated with an increased risk of RA, and this association was limited to women (45). A few potential mechanisms could explain obesity causing RA. First, the obese state is characterized by a low degree of systemic inflammation, and adipose tissue can produce many bioactive molecules that regulate carbohydrate and lipid metabolism, immune function, etc. (46). Second, the prevalence of vitamin D deficiency in obese individuals is high, and such deficiency is also very common in individuals with autoimmune diseases (including RA) (47).
Some SNPs have been shown to interact with lifestyles at risk of RA, and most of the genes mapped to them have been proven to be related to alterable lifestyles in previous studies. For example, compared with sham-exposed controls, mice exposed to cigarette smoke had higher expression of the EOMES gene, indicating the mechanism by which smoking regulates this gene (48). At the same time, EOMES-positive CD4 + T cells proved to be a risk T cell subset of RA (49); Studies have also discussed the molecular role of RUNX1 in obesity and related metabolic diseases (50). However, the statistical significance for each SNP was far from GWAS significance in our result. This is most likely due to the weakening of the association between certain SNPs and RA in the UKB, which is also the limitation of other GWAS studies (less reproducibility) (51). In contrast, our results show that PRS, which represents polygenic effects, can well explain the genetic risk of RA in the UKB cohort. Therefore, we recommend that PRS should be used more clinically for fine division and precise treatment of the population. Previous studies have reported that regular exercise could help to prevent bone loss, strengthen the heart and lungs, and reduce joint pain in RA patients (52, 53). Our results revealed a protective effect of physical exercise on RA risk, and several reasons could explain this association. It is well known that subjects who exercise regularly maintain a healthy body shape. Exercise has no positive effects on the development of RA, and studies have shown that exercise can help reduce the symptoms of tired RA (54, 55).
Our study demonstrates that adherence to a healthy lifestyle reduces the risk of RA within different subgroups, while participants at high genetic risk also benefit from adopting a healthy lifestyle. Interaction analysis also demonstrates the importance of adopting a healthy lifestyle with high genetic risk. The estimated values of additive interaction were greater than zero between high genetic risk and lifestyles, suggesting a positive additive interaction. The risk of simultaneous exposure was higher than the sum of exposure to unhealthy lifestyles or higher genetic risk only. Results of the sex-stratified analysis showed consistent trends, demonstrating that healthy lifestyles are suitable for everyone. In addition, the associations in women were weaker than in men, indicating that the beneficial effects of healthy lifestyles were greater for men than for women. The crucial role of genetic-environmental interactions in the pathogenesis of RA has been well documented in previous studies (17, 34, 56).
When constructing the PRS for RA, different PRSs (P range from 5×10-4 to 5×10-7) may have similar predictive powers, suggesting that except for the SNPs used in our selected PRS, remaining SNPs were not important for the prediction of RA risk. Meanwhile, utilization of PRS with fewer SNPs likewise reduces the cost of practical application in the clinic. In addition, in our analysis, PRSs containing MHC genotypes were more strongly associated with RA and had better predictive power in the validation set. Numerous studies have shown that the main genetic risk factor for RA is the HLA DRB1 allele, while many other non-MHC genes are also associated with RA (3, 57, 58). Enrichment analysis also indicated that the SNPs used for PRS construction matched to genes involved in the progression of RA. Both our results and previous evidence proved the crucial role of MHC genotypes in the development of RA.
Our study observed relatively smaller interaction effect sizes for genetic and environmental factors than previous studies. This inconsistency could be attributed to several factors. First, RA is a complex autoimmune disease determined by multiple genetic variants with relatively small but cumulative effects. Therefore, according to the complex determination nature of the RA, the low interaction effects between SNPs and lifestyles are commonly observed. Second, the accurate definition of lifestyles would be important confounding factors in masking the detection of interaction effects, which would be missed in a small sample size, but be present in the current large sample size. Third, the information on lifestyle was collected at baseline, but the participants’ lifestyles could modify at any time during the long-term follow-up, which might confuse the evaluation of the real interaction effect of lifestyle. Forth, the current study has not fully investigated the underlying causal variation within each gene. For example, rare variants of low frequency may have a significant effect size (increase disease risk by a factor of 2 to 3). These variants are typically missing in both microarrays and 1000 Genomes (containing common variants) (59).
Our study has some limitations. First, lifestyle factors were only assessed at baseline, but the lifestyle of each participant was a dynamic process change. Therefore, more extensive cohort studies are needed to further explore the association with RA risk. Second, only lead SNPs were included in the construction of the PRS, which may have resulted in the loss of polygenic effects. Third, our analysis was based on European ancestry, and these results might not extend to other ancestries.
In summary, our results improved the prediction of RA risk by integrating lifestyle and genetic factors. The results would facilitate the implementation of precision prevention strategies. Genetic-based risk assessment can more accurately determine an individual’s risk of RA and facilitate the release of decisions regarding risk management options. Adopting a healthy lifestyle in subjects at high risk can greatly decrease the risk of developing RA, both for men and women.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by North West Multi-centre Research Ethics Committee (MREC). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
F-YD, S-FL, and X-HY conceived the design of the study; X-HY, R-RC, and LB obtained the data; X-HY, R-RC, PH, and Y-QY cleared up the datasets; X-HY mainly performed the data analyses; F-YD, S-FL, and X-HY drafted and revised the manuscript, and all authors approved the manuscript and provided relevant suggestions. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Natural Science Foundation of China (81872681 82173529, 82173598 and 82103922), the Science and Technology Project of Suzhou (SS202050, SYS2019024), the QingLan Research Project of Jiangsu Province, and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX22_3227).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank previous studies for making the summary association statistics data publicly available, and we are grateful to all the investigators and participants in the UK Biobank for the public individual-level data sets and their contribution to those studies. UK Biobank approval was given for this project (ID: 76875) and can be downloaded from https://www.ukbiobank.ac.uk/or https://biota.osc.ox.ac.uk/.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.901223/full#supplementary-material
References
1. Scott DL, Wolfe F, Huizinga TW. Rheumatoid Arthritis. Lancet (2010) 376:1094–108. doi: 10.1016/S0140-6736(10)60826-4
2. Mcinnes IB, Schett G. The Pathogenesis of Rheumatoid Arthritis. N Engl J Med (2011) 365:2205–19. doi: 10.1056/NEJMra1004965
3. Silman AJ, Pearson JE. Epidemiology and Genetics of Rheumatoid Arthritis. Arthritis Res (2002) 4 Suppl 3:S265–272. doi: 10.1186/ar578
4. Smolen JS, Aletaha D, Mcinnes IB. Rheumatoid Arthritis. Lancet (2016) 388:2023–38. doi: 10.1016/S0140-6736(16)30173-8
5. Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of Rheumatoid Arthritis Contributes to Biology and Drug Discovery. Nature (2014) 506:376–81. doi: 10.1038/nature12873
6. Gioia C, Lucchino B, Tarsitano MG, Iannuccelli C, Di Franco M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients (2020) 12:1456. doi: 10.3390/nu12051456
7. Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of "Western Diet" in Inflammatory Autoimmune Diseases. Curr Allergy Asthma Rep (2014) 14:404. doi: 10.1007/s11882-013-0404-6
8. Oliviero F, Spinella P, Fiocco U, Ramonda R, Sfriso P, Punzi L. How the Mediterranean Diet and Some of its Components Modulate Inflammatory Pathways in Arthritis. Swiss Med Wkly (2015) 145:w14190. doi: 10.4414/smw.2015.14190
9. Philippou E, Nikiphorou E. Are We Really What We Eat? Nutrition and its Role in the Onset of Rheumatoid Arthritis. Autoimmun Rev (2018) 17:1074–7. doi: 10.1016/j.autrev.2018.05.009
10. Chang K, Yang SM, Kim SH, Han KH, Park SJ, Shin JI. Smoking and Rheumatoid Arthritis. Int J Mol Sci (2014) 15:22279–95. doi: 10.3390/ijms151222279
11. Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y. Physical Activity and Autoimmune Diseases: Get Moving and Manage the Disease. Autoimmun Rev (2018) 17:53–72. doi: 10.1016/j.autrev.2017.11.010
12. Khera AV, Chaffin M, Aragam KG, Haas ME, Roselli C, Choi SH, et al. Genome-Wide Polygenic Scores for Common Diseases Identify Individuals With Risk Equivalent to Monogenic Mutations. Nat Genet (2018) 50:1219–24. doi: 10.1038/s41588-018-0183-z
13. Torkamani A, Wineinger NE, Topol EJ. The Personal and Clinical Utility of Polygenic Risk Scores. Nat Rev Genet (2018) 19:581–90. doi: 10.1038/s41576-018-0018-x
14. Karlson EW, Chibnik LB, Kraft P, Cui J, Keenan BT, Ding B, et al. Cumulative Association of 22 Genetic Variants With Seropositive Rheumatoid Arthritis Risk. Ann Rheum Dis (2010) 69:1077–85. doi: 10.1136/ard.2009.120170
15. Kurreeman F, Liao K, Chibnik L, Hickey B, Stahl E, Gainer V, et al. Genetic Basis of Autoantibody Positive and Negative Rheumatoid Arthritis Risk in a Multi-Ethnic Cohort Derived From Electronic Health Records. Am J Hum Genet (2011) 88:57–69. doi: 10.1016/j.ajhg.2010.12.007
16. Yarwood A, Han B, Raychaudhuri S, Bowes J, Lunt M, Pappas DA, et al. A Weighted Genetic Risk Score Using All Known Susceptibility Variants to Estimate Rheumatoid Arthritis Risk. Ann Rheum Dis (2015) 74:170–6. doi: 10.1136/annrheumdis-2013-204133
17. Karlson EW, Deane K. Environmental and Gene-Environment Interactions and Risk of Rheumatoid Arthritis. Rheum Dis Clin North Am (2012) 38:405–26. doi: 10.1016/j.rdc.2012.04.002
18. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PloS Med (2015) 12:e1001779. doi: 10.1371/journal.pmed.1001779
19. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank Resource With Deep Phenotyping and Genomic Data. Nature (2018) 562:203–9. doi: 10.1038/s41586-018-0579-z
20. Clynes MA, Jameson K, Prieto-Alhambra D, Harvey NC, Cooper C, Dennison EM. Impact of Rheumatoid Arthritis and Its Management on Falls, Fracture and Bone Mineral Density in UK Biobank. Front Endocrinol (Laus) (2019) 10:817. doi: 10.3389/fendo.2019.00817
21. Glanville KP, Coleman JRI, O'reilly PF, Galloway J, Lewis CM. Investigating Pleiotropy Between Depression and Autoimmune Diseases Using the UK Biobank. Biol Psychiatry Glob Open Sci (2021) 1:48–58. doi: 10.1016/j.bpsgos.2021.03.002
22. Forsyth C, Kouvari M, D'cunha NM, Georgousopoulou EN, Panagiotakos DB, Mellor DD, et al. The Effects of the Mediterranean Diet on Rheumatoid Arthritis Prevention and Treatment: A Systematic Review of Human Prospective Studies. Rheumatol Int (2018) 38:737–47. doi: 10.1007/s00296-017-3912-1
23. Chehade L, Jaafar ZA, El Masri D, Zmerly H, Kreidieh D, Tannir H, et al. Lifestyle Modification in Rheumatoid Arthritis: Dietary and Physical Activity Recommendations Based on Evidence. Curr Rheumatol Rev (2019) 15:209–14. doi: 10.2174/1573397115666190121135940
24. Zaccardelli A, Friedlander HM, Ford JA, Sparks JA. Potential of Lifestyle Changes for Reducing the Risk of Developing Rheumatoid Arthritis: Is an Ounce of Prevention Worth a Pound of Cure? Clin Ther (2019) 41:1323–45. doi: 10.1016/j.clinthera.2019.04.021
25. Zhu M, Wang T, Huang Y, Zhao X, Ding Y, Zhu M, et al. Genetic Risk for Overall Cancer and the Benefit of Adherence to a Healthy Lifestyle. Cancer Res (2021) 81:4618–27. doi: 10.1158/0008-5472.CAN-21-0836
26. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am J Hum Genet (2007) 81:559–75. doi: 10.1086/519795
27. Yousaf S, Bonsall A. UK Townsend Deprivation Scores From 2011 Census Data. Colchester UK: UK Data Serv (2017).
28. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinf (2011) 12:1–8. doi: 10.1186/1471-2105-12-77
29. Kalilani L, Atashili J. Measuring Additive Interaction Using Odds Ratios. Epidemiol Perspect Innov (2006) 3:5. doi: 10.1186/1742-5573-3-5
31. Chambers J. Software for Data Analysis: Programming With R. Springer Science & Business Media (2008).
32. Albano SA, Santana-Sahagun E, Weisman MH. Cigarette Smoking and Rheumatoid Arthritis. Semin Arthritis Rheum (2001) 31:146–59. doi: 10.1053/sarh.2001.27719
33. Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of Smoking as a Risk Factor for Developing Rheumatoid Arthritis: A Meta-Analysis of Observational Studies. Ann Rheum Dis (2010) 69:70–81. doi: 10.1136/ard.2008.096487
34. Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A Gene-Environment Interaction Between Smoking and Shared Epitope Genes in HLA-DR Provides a High Risk of Seropositive Rheumatoid Arthritis. Arthritis Rheum (2004) 50:3085–92. doi: 10.1002/art.20553
35. Lu B, Solomon DH, Costenbader KH, Karlson EW. Alcohol Consumption and Risk of Incident Rheumatoid Arthritis in Women: A Prospective Study. Arthritis Rheumatol (2014) 66:1998–2005. doi: 10.1002/art.38634
36. Grimaldi CM, Hill L, Xu X, Peeva E, Diamond B. Hormonal Modulation of B Cell Development and Repertoire Selection. Mol Immunol (2005) 42:811–20. doi: 10.1016/j.molimm.2004.05.014
37. Seitz HK, Maurer B. The Relationship Between Alcohol Metabolism, Estrogen Levels, and Breast Cancer Risk. Alcohol Res Health (2007) 30:42–3.
38. Jonsson IM, Verdrengh M, Brisslert M, Lindblad S, Bokarewa M, Islander U, et al. Ethanol Prevents Development of Destructive Arthritis. Proc Natl Acad Sci U.S.A. (2007) 104:258–63. doi: 10.1073/pnas.0608620104
39. Waldschmidt TJ, Cook RT, Kovacs EJ. Alcohol and Inflammation and Immune Responses: Summary of the 2006 Alcohol and Immunology Research Interest Group (AIRIG) Meeting. Alcohol (2008) 42:137–42. doi: 10.1016/j.alcohol.2007.11.003
40. Azizov V, Dietel K, Steffen F, Dürholz K, Meidenbauer J, Lucas S, et al. Ethanol Consumption Inhibits T(FH) Cell Responses and the Development of Autoimmune Arthritis. Nat Commun (2020) 11:1–14. doi: 10.1038/s41467-020-15855-z
41. Pattison DJ, Symmons DP, Lunt M, Welch A, Luben R, Bingham SA, et al. Dietary Risk Factors for the Development of Inflammatory Polyarthritis: Evidence for a Role of High Level of Red Meat Consumption. Arthritis Rheum (2004) 50:3804–12. doi: 10.1002/art.20731
42. He J, Wang Y, Feng M, Zhang X, Jin YB, Li X, et al. Dietary Intake and Risk of Rheumatoid Arthritis-a Cross Section Multicenter Study. Clin Rheumatol (2016) 35:2901–8. doi: 10.1007/s10067-016-3383-x
43. Benito-Garcia E, Feskanich D, Hu FB, Mandl LA, Karlson EW. Protein, Iron, and Meat Consumption and Risk for Rheumatoid Arthritis: A Prospective Cohort Study. Arthritis Res Ther (2007) 9:R16. doi: 10.1186/ar2123
44. Salgado E, Bes-Rastrollo M, De Irala J, Carmona L, Gómez-Reino JJ. High Sodium Intake Is Associated With Self-Reported Rheumatoid Arthritis: A Cross Sectional and Case Control Analysis Within the SUN Cohort. Med (Baltim) (2015) 94:e0924. doi: 10.1097/MD.0000000000000924
45. Ohno T, Aune D, Heath AK. Adiposity and the Risk of Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Cohort Studies. Sci Rep (2020) 10:16006. doi: 10.1038/s41598-020-71676-6
46. Nway NC, Sitticharoon C, Chatree S, Maikaew P. Correlations Between the Expression of the Insulin Sensitizing Hormones, Adiponectin, Visfatin, and Omentin, and the Appetite Regulatory Hormone, Neuropeptide Y and its Receptors in Subcutaneous and Visceral Adipose Tissues. Obes Res Clin Pract (2016) 10:256–63. doi: 10.1016/j.orcp.2015.05.007
47. Arnson Y, Amital H, Shoenfeld Y. Vitamin D and Autoimmunity: New Aetiological and Therapeutic Considerations. Ann Rheum Dis (2007) 66:1137–42. doi: 10.1136/ard.2007.069831
48. Kerdidani D, Magkouta S, Chouvardas P, Karavana V, Glynos K, Roumelioti F, et al. Cigarette Smoke-Induced Emphysema Exhausts Early Cytotoxic CD8(+) T Cell Responses Against Nascent Lung Cancer Cells. J Immunol (2018) 201:1558–69. doi: 10.4049/jimmunol.1700700
49. Chemin K, Ramsköld D, Diaz-Gallo LM, Herrath J, Houtman M, Tandre K, et al. EOMES-Positive CD4(+) T Cells are Increased in PTPN22 (1858T) Risk Allele Carriers. Eur J Immunol (2018) 48:655–69. doi: 10.1002/eji.201747296
50. Hou X, Zhang Y, Li W, Hu AJ, Luo C, Zhou W, et al. CDK6 Inhibits White to Beige Fat Transition by Suppressing RUNX1. Nat Commun (2018) 9:1–14. doi: 10.1038/s41467-018-03451-1
51. Manolio TA. Bringing Genome-Wide Association Findings Into Clinical Use. Nat Rev Genet (2013) 14:549–58. doi: 10.1038/nrg3523
52. Hurkmans E, van der Giesen FJ, Vliet Vlieland TP, Schoones J, Van Den Ende EC. Dynamic Exercise Programs (Aerobic Capacity and/or Muscle Strength Training) in Patients With Rheumatoid Arthritis. Cochrane Database Syst Rev (2009) 2009:Cd006853. doi: 10.1002/14651858.CD006853.pub2
53. Cramp F, Hewlett S, Almeida C, Kirwan JR, Choy EH, Chalder T, et al. Non-Pharmacological Interventions for Fatigue in Rheumatoid Arthritis. Cochrane Database Syst Rev (2013) 8:Cd008322. doi: 10.1002/14651858.CD008322.pub2
54. Rausch Osthoff AK, Juhl CB, Knittle K, Dagfinrud H, Hurkmans E, Braun J, et al. Effects of Exercise and Physical Activity Promotion: Meta-Analysis Informing the 2018 EULAR Recommendations for Physical Activity in People With Rheumatoid Arthritis, Spondyloarthritis and Hip/Knee Osteoarthritis. RMD Open (2018) 4:e000713. doi: 10.1136/rmdopen-2018-000713
55. Williams MA, Srikesavan C, Heine PJ, Bruce J, Brosseau L, Hoxey-Thomas N, et al. Exercise for Rheumatoid Arthritis of the Hand. Cochrane Database Syst Rev (2018) 7:Cd003832. doi: 10.1002/14651858.CD003832.pub3
56. Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-Environment Interaction Between HLA-DRB1 Shared Epitope and Heavy Cigarette Smoking in Predicting Incident Rheumatoid Arthritis. Ann Rheum Dis (2010) 69:54–60. doi: 10.1136/ard.2008.102962
57. Citera G, Padulo LA, Fernandez G, Lazaro MA, Rosemffet MG, Maldonado Cocco JA. Influence of HLA-DR Alleles on Rheumatoid Arthritis: Susceptibility and Severity in Argentine Patients. J Rheumatol (2001) 28:1486–91.
58. Pascual M, Nieto A, López-Nevot MA, Ramal L, Matarán L, Caballero A, et al. Rheumatoid Arthritis in Southern Spain: Toward Elucidation of a Unifying Role of the HLA Class II Region in Disease Predisposition. Arthritis Rheum (2001) 44:307–14. doi: 10.1002/1529-0131(200102)44:2<307::AID-ANR47>3.0.CO;2-K
Keywords: rheumatoid arthritis, healthy lifestyle, genetic factor, polygenic risk score, epidemiology
Citation: Yu X-H, Bo L, Cao R-R, Yang Y-Q, He P, Lei S-F and Deng F-Y (2022) Systematic Evaluation of Rheumatoid Arthritis Risk by Integrating Lifestyle Factors and Genetic Risk Scores. Front. Immunol. 13:901223. doi: 10.3389/fimmu.2022.901223
Received: 21 March 2022; Accepted: 07 June 2022;
Published: 06 July 2022.
Edited by:
Maria I. Bokarewa, University of Gothenburg, SwedenReviewed by:
Michal Linial, Hebrew University of Jerusalem, IsraelAase Hensvold, Karolinska Institutet (KI), Sweden
Copyright © 2022 Yu, Bo, Cao, Yang, He, Lei and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei-Yan Deng, ZmRlbmdAc3VkYS5lZHUuY24=; Shu-Feng Lei, bGVpc2ZAc3VkYS5lZHUuY24=
 Xing-Hao Yu
Xing-Hao Yu Lin Bo3
Lin Bo3 Shu-Feng Lei
Shu-Feng Lei