- 1Department of Geriatric Neurology, Shaanxi Provincial People’s Hospital, Xi’an, Shaanxi, China
- 2Shaanxi Provincial Clinical Research Center for Geriatric Medicine, Xi’an, Shaanxi, China
- 3Institute of Medical Research, Northwestern Polytechnical University, Xi’an, Shaanxi, China
Objective: The relationship between asthma and epilepsy in observational studies is controversial. The purpose of this Mendelian randomization (MR) study is to investigate whether asthma causally contributes to epilepsy susceptibility.
Methods: Independent genetic variants strongly (P<5E-08) associated with asthma were from a recent meta-analysis of genome-wide association studies on 408,442 participants. Two independent summary statistics of epilepsy obtained from the International League Against Epilepsy Consortium (ILAEC, Ncases=15,212, and Ncontrols=29,677) and FinnGen Consortium (Ncases=6,260 and Ncontrols=176,107) were used in the discovery and replication stage, respectively. Several sensitivity analyses and heterogeneity analyses were further conducted to assess the stability of the estimates.
Results: Using the inverse-variance weighted approach, genetic predisposition to asthma was associated with an elevated risk of epilepsy in the discovery stage (ILAEC: odds ratio [OR]=1.112, 95% confidence intervals [CI]= 1.023-1.209, P = 0.012), but not verified in the replication stage (FinnGen: OR=1.021, 95%CI= 0.896–1.163, P =0.753). However, a further meta-analysis of both ILAEC and FinnGen showed a similar result (OR=1.085, 95% CI: 1.012-1.164, P = 0.022). There were no causal associations between the age onset of asthma and epilepsy. Sensitivity analyses yielded consistent causal estimates.
Conclusion: The present MR study suggests that asthma is associated with an increased risk of epilepsy independent of the age onset of asthma. Further studies are warranted to explain the underlying mechanisms of this association.
Introduction
Asthma is one of the most common chronic respiratory disorders (1), affecting affects over 300 million people worldwide and bringing a huge economic and social burden (2). Accumulating evidence has shown that inflammation might be involved in the pathogenesis of asthma (3) and individuals with brain inflammation have a likelihood of being predisposed to epileptogenesis (4, 5). These findings have drawn much attention to exploring the association of asthma with epilepsy. Indeed, two previously published population-based studies of adults revealed that patients with epilepsy were often accompanied by physical comorbidities such as asthma (6, 7). In addition, numerous case-control studies have announced that the prevalence of asthma was related to higher odds of epilepsy either in children (8) or in adults (9). These data suggest that asthma might be associated with high susceptibility to epilepsy. However, data from other case-control studies have displayed discordant findings, with a retrospective study among children suggesting that idiopathic epilepsy is not etiologically connected with asthma (10). Furthermore, observational studies cannot prove the causal inference due to their sensitivities to residual confounding and reverse causality.
Mendelian randomization (MR), using genetic connections to inquire about the causal impact of a risk factor on an outcome (11), is an effective method for gaging causal inference. This approach can not only limit reverse causality but also greatly reduce the likelihood of residual confounding (12). Based on the inconsistent findings of the aforementioned retrospective cohort studies, we undertook a 2-sample MR approach to assess whether asthma causally contributed to an increased risk of epilepsy.
Methods
Study design and data source
Independent single nucleotide polymorphisms (SNPs) from genome-wide association studies (GWAS) were selected as instrumental variables (IV). This MR study aimed to satisfy the three primary assumptions described in detail in Figure 1. Assumption 1 (Relevance), SNPs significantly (P<5E-08) associated with asthma. Assumption 2 (Independence), SNPs not associated with confounding factors that correlated with both asthma and epilepsy, including atopic dermatitis (13), celiac disease (14), inflammatory bowel disease (15), rheumatoid arthritis (16), hypothyroidism (17), migraine (18), multiple sclerosis (19), educational attainment (20), and body mass (21). Assumption 3 (Exclusivity), SNPs affected epilepsy susceptibility directly through asthma and are not associated with epilepsy (P>1E-05).

Figure 1 Three corresponding principal assumptions in this 2-sample Mendelian randomization study. Red stars mean that genetic variants are not associated with confounding factors and the outcome.
The summary statistics of asthma were from the latest large-scale GWAS meta-analysis of 408,442 Europeans from the UK Biobank (22). For childhood-onset and adult-onset asthma (23), there were 314,633 and 327,253 participants of European descent from the UK Biobank, respectively. For epilepsy, two independent summary statistics of epilepsy from the International League Against Epilepsy Consortium (ILAEC) and the FinnGen Consortium were included in this MR study. The summary statistics from the ILAEC contained 15,212 cases and 29,677 normal controls (24), and a total of 6,260 epilepsy cases and 176,107 normal controls of European descent were obtained from the FinnGen Consortium (25). Since samples from the ILAEC had a higher proportion of cases (33.9%) than those from the FinnGen Consortium (3.5%), we used the datasets of ILAEC and FinnGen Consortium in the discovery stage and replication stage, respectively. Epilepsy was diagnosed by epilepsy specialists based on electroencephalography, magnetic resonance imaging, and clinical history. Table 1 includes a detailed summary of the study including source publications (Table 1).
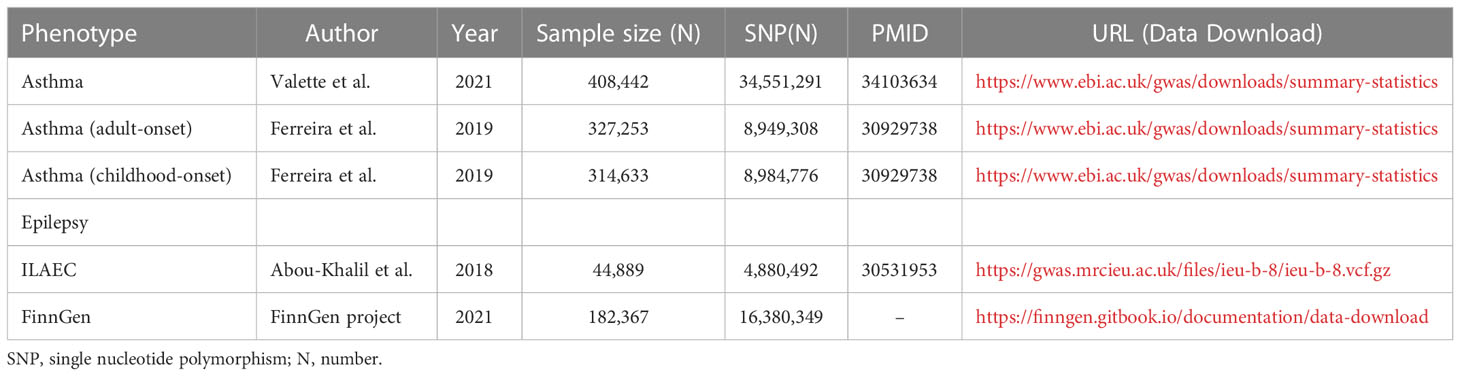
Table 1 Summary of the genome-wide association studies included in this Mendelian randomization study.
Instruments selection
Those SNPs passing the genome-wide significance threshold (P < 5E–08) were selected as IVs, which were clumped according to the linkage disequilibrium structure (1000 Genomes Project of European, r2<0.01 within 10000 kb). In addition, SNPs associated with epilepsy with a P value lower than 1E–05 were excluded from the IV before MR analysis. Meanwhile, IVs associated with the confounders described above were also removed from the MR analysis. SNPs absent from the epilepsy GWAS datasets will be replaced with overlapping proxy SNPs (r2 = 0.8). To strengthen the robustness of the estimates, SNPs with a minor allele frequency of less than 0.3 were also removed. All harmonized SNPs for each exposure-outcome pair were archived (Supplementary Data Sheet).
Mendelian randomization analysis
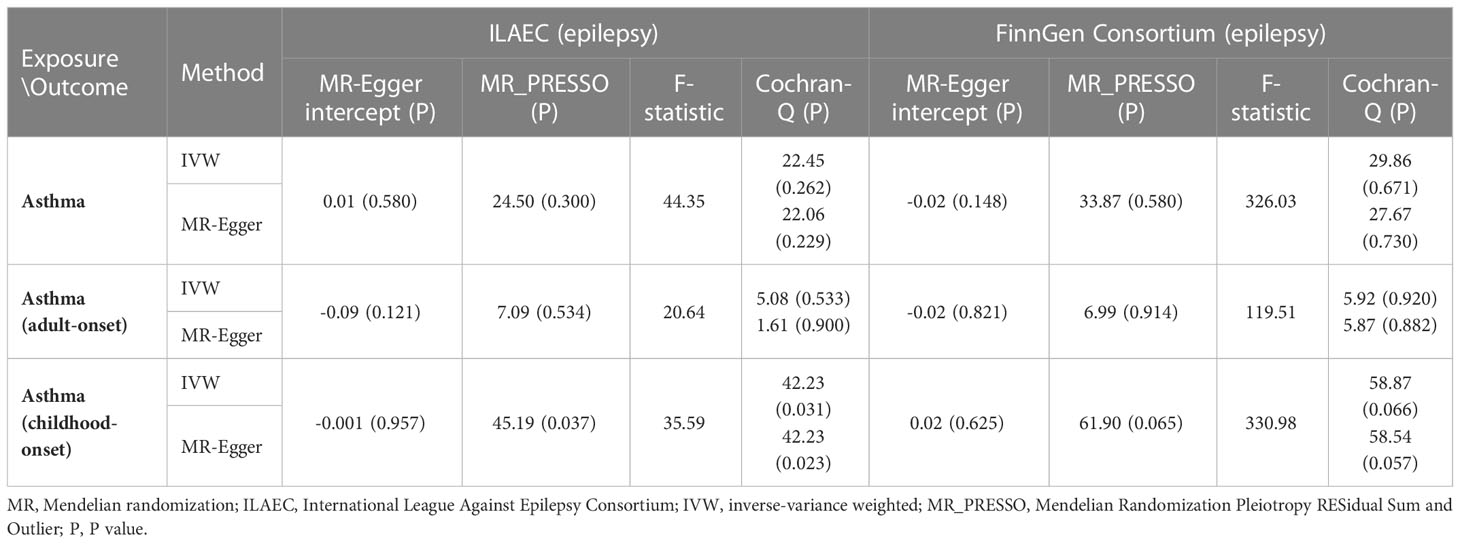
The TwoSampleMR package (version 0.5.6) was applied in the present Mendelian randomization analysis (26). The inverse-variance weighted (IVW) method was used as the default method to calculate causal estimates between asthma and epilepsy. Meanwhile, we also employed weighted median, MR–Egger regression, weighted mode, simple median, maximum likelihood, and MR-Pleiotropy RESidual Sum and Outlier (MR-PRESSO) as sensitivity analyses to validate the estimates (27). MR-PRESSO test could identify horizontal pleiotropic outliers and evaluate the potential pleiotropic effects of the genetic variants selected as IV. MR–Egger intercept test was also applied to measure the horizontal pleiotropy. In addition, F-statistics were also calculated to assess the instrumental strength as previously described (28), and F values of more than 10 were found to avoid bias from weak instruments.
A meta-analysis based on ILAEC and FinnGen was also conducted to calculate the overall causal estimates using the meta package (version 5.2.0). A fixed-effect model was applied to combine the estimates if there was obvious heterogeneity (P>0.05 or I2<50%), otherwise, a random-effect model was employed (29). There is yet a lack of consensus regarding the best strategy for multiple test correction (30, 31), where multiple testing for different outcomes might increase the risk of Type I error, while adjustment for multiple comparisons could increase the risk of type II errors. To balance the type I and type II errors, we followed the strategy reported previously by Ronald J. Feise via conducting independent Bonferroni correction for each outcome assessed (30). Since two independent GWAS datasets for epilepsy were included in this study, a P-value < 0.025 after Bonferroni correction (0.05/2) was considered statistically significant. Meanwhile, a P-value < 0.05 was considered suggestive of a causal association. All statistical analyses were performed in R software (version 4.1.3), and the meta package (version 5.2.0) and forestploter package (version 0.1.5) was employed in drawing forest plots.
Results
Using the IVW method, genetically predicted asthma was associated with an increased risk of epilepsy in the discovery stage (ILAEC: OR = 1.112, 95% CI: 1.023-1.209, P = 0.012). Directional consistent results were obtained in sensitivity analyses using simple median, weighted median, maximum likelihood, and MR-PRESSO approaches (Figure 2A). In the replication stage, estimates of the FinnGen dataset showed the same trend direction as the results of ILAEC (Figure 2B). No obvious causal effects of childhood-onset asthma and adult-onset asthma on epilepsy were found in both the discovery stage and replication stage (Figure 2). There was no obvious pleiotropy observed in the MR-Egger intercept test, but potential pleiotropy of childhood-onset asthma on epilepsy (P=0.037) in the discovery stage was observed in the MR-PRESSO test (Table 2). Cochran-Q test also showed heterogeneity in evaluating the causal association between childhood-onset asthma and epilepsy in the discovery stage (Table 2). The corrected estimate after removing the outlier (rs1893380) identified by the MR-PRESSO test showed a similar result, suggesting good stability. All the F-statistic values were larger than 10 across the MR study, indicating good instrumental strength.
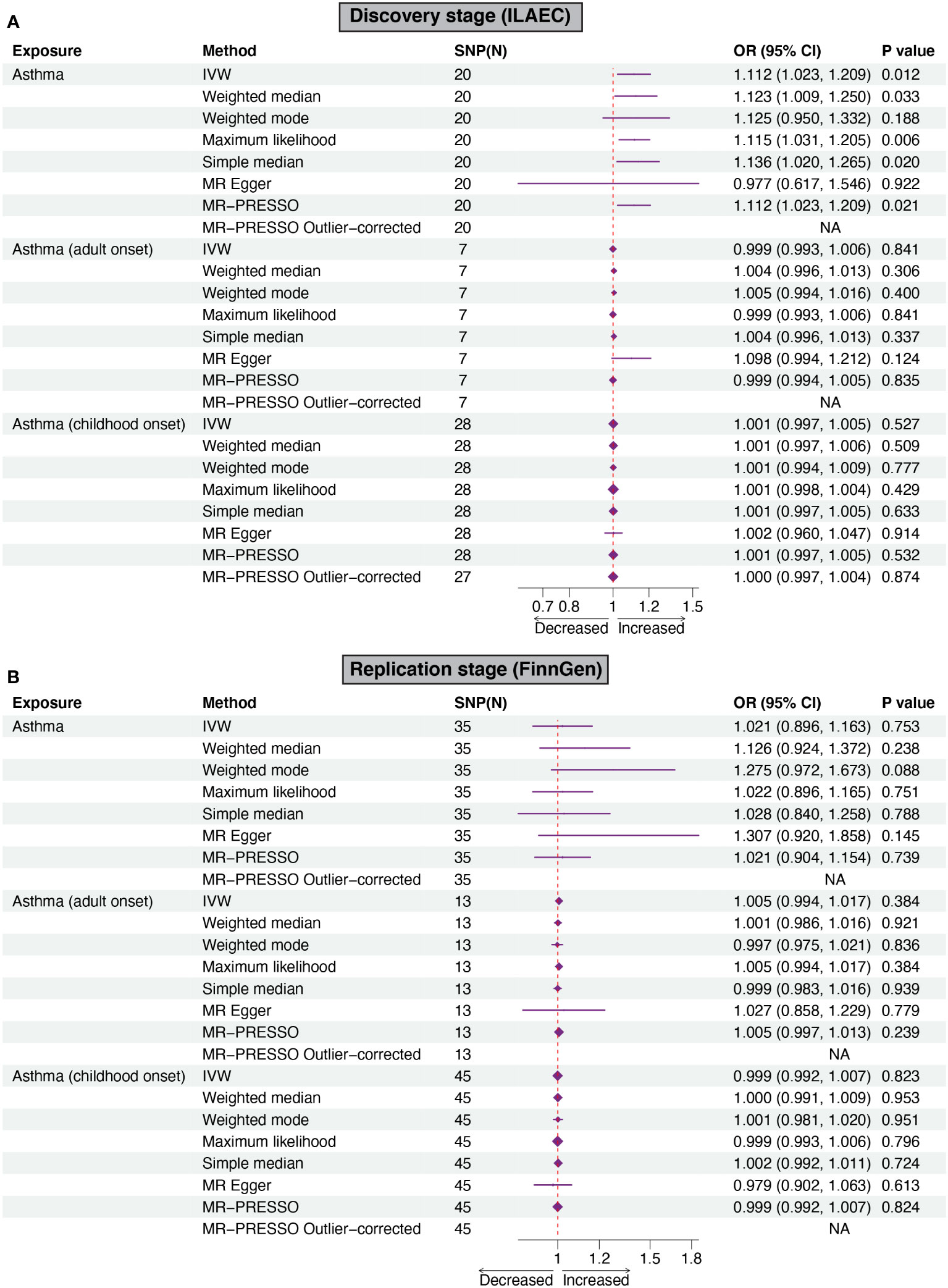
Figure 2 Forest plots of Mendelian randomization analyses show the causal effects of asthma on epilepsy. Six different methods, including IVW, weighted mode, weighted median, MR-Egger regression, MR-PRESSO, simple median, and maximum likelihood were used to evaluate the causal effect of asthma on epilepsy. (A, B) showed the causal effect of asthma on epilepsy in the discovery stage and replication stage, respectively. IVW, inverse variance weighed MR-PRESSO, MR-Pleiotropy RESidual Sum, and Outlier.
A further meta-analysis of ILAEC and FinnGen also showed a causal effect of asthma on epilepsy (OR = 1.085, 95% CI: 1.012-1.164, P = 0.022), which was validated in a sensitivity analysis using other approaches (Figure 3; Supplementary Figure S1). The meta-analysis results from both the fixed-effect model and the random-effect were largely consistent across different statistical methods (Figure 3, Supplementary Figure S1).
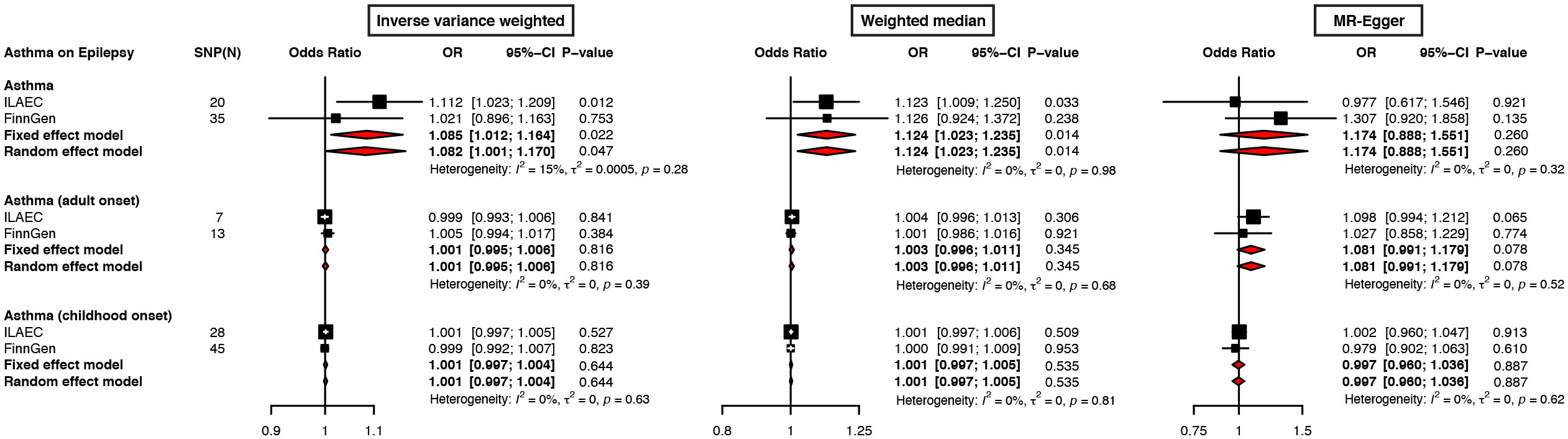
Figure 3 Forest plots of meta-analysis on ILAEC and FinnGen epilepsy GWAS datasets show the causal effects of asthma on epilepsy. The inverse variance weighted, weighted median, and MR-Egger regression were used to evaluate the causal effects of asthma on epilepsy.
Discussion
In this study, we took advantage of the 2-sample MR method to analyze the causal relationship between asthma and epilepsy. The main results consistently suggested that asthma was associated with a higher risk of epilepsy. Furthermore, several sensitivity analyses were used based on their different underlying assumptions and similar results were observed, which further strengthened the credibility of the results.
Previous reports have investigated the relationship between asthma and epilepsy, but the results were inconsistent. A population-based study found that most adult patients with epilepsy presently have symptomatic asthma (6). Meanwhile, a U.S. National Health Interview Survey found that adult patients with epilepsy were more often to record physical comorbidities like asthma (7). Previous studies among US children aged 0-17 years reported that the lifetime prevalence of asthma was related to a higher risk of epilepsy (2.30 [1.50-3.52]) (8). Similarly, a recent cohort study including 150,827 asthma patients showed that the asthma patients had an increased risk of epilepsy than health controls (hazard ratio=1.39) (9). All these findings indicated that asthma was associated with the risk of epilepsy, which was consistent with the results of our MR study based on data from the ILAEC and FinnGen Consortium. Although an early study among children suggested that there was no etiological relationship between asthma and epilepsy, the result may be attributed to small samples (10).
The underlying mechanism mediating the association between asthma and epilepsy remains largely unknown. The potential reasons connecting asthma and epilepsy are anoxia and hypocapnia owing to repeated asthma attacks. In addition, chronic inflammation is a common pathological feature shared by asthma and epilepsy (3, 32, 33). Previous studies demonstrated that circulating cytokines might penetrate through the blood-brain barrier and then result in chronic neuroinflammation and neuronal damage, eventually increasing the susceptibility to epileptogenesis (4, 5, 34). Moreover, emerging evidence shows that the respiratory system has a tight relationship with the central nervous system, which goes beyond the classically known connections such as blood supply and oxygen saturation. Studies showed that respiratory system diseases such as asthma (35) and chronic obstructive pulmonary disease (36) might increase the risk of stroke, which was a risk factor for epilepsy. In addition, clinical data suggested that chronic obstructive pulmonary disease was associated with an increased risk for the development of seizures in patients with stroke (37). Although oxygen desaturation may be one of the risk factors for epilepsy in asthma patients (38), further work is needed to explore the exact mechanisms by which asthma causes an increased risk of epilepsy.
Asthma can be divided into childhood-onset asthma and adult-onset asthma based on the age of onset. Childhood-onset asthma may be related to genetic factors (39, 40), perinatal factors (41), or respiratory infections (42), while adult-onset asthma may be related to environmental and occupational factors such as obesity and smoking (43). Even though the mechanisms contributing to childhood-onset and adult-onset asthma might be different, our MR study found no causal associations between the age onset of asthma and epilepsy. These data suggested that asthma causally increased the risk of epilepsy independent of the age onset of asthma. The potential reason for these unexpected results might be due to the small sample size of childhood-onset and adult-onset asthma, which might lead to lower statistical power. It is worth noting that the proportion of cases with asthma was 13.8%, while in childhood-onset asthmatic and adult-onset asthma were 4.4% and 8.1%, respectively. In addition, although the causal relationship was not significant for childhood-onset asthma and adult-onset asthma on epilepsy, most of the OR values were larger than 1, suggesting a potential risk effect of childhood-onset asthma and adult-onset asthma on epilepsy.
This study has some limitations: first, the nonlinear connection between asthma and the risk of epilepsy cannot be eliminated due to the linear effect assumption in MR analysis. Second, although no evidence of pleiotropy was detected in the MR-Egger intercept test, potential pleiotropy was observed between childhood-onset asthma and epilepsy (P=0.037) in the MR-PRESSO test. Third, there was obvious heterogeneity between childhood-onset asthma and epilepsy from ILAEC datasets, which might be due to the mixed population of ILAEC (531 and 147 individuals of Asian and African descent, respectively). Fourth, our study is mainly based on Europeans, thus generalization of the findings to other ethnic groups needs to be cautious. Fifth, to better fulfill the independence assumption for the MR study, we used a relatively stringent way to exclude the SNPs associated with potential confounders of epilepsy from the IVs, which might weaken the statistical power of the MR study. Sixth, due to individual data not being publically available, we were unable to properly account for the potential sample overlap between the GWAS datasets of asthma and epilepsy, which might lead to bias in the overall estimates. Finally, there are other possible unmeasured and residual confounding factors like many other epidemiological studies, which might drive the bias of the overall estimates. For example, as asthma was caused due to an overactive immune response (44), many instrumental variables for asthma were associated with peripheral blood cells (Supplementary Data Sheets, 7). Although previous studies suggested that inflammatory factors were also implicated in epilepsy (33), however, asthma, characterized by chronic inflammation and bronchial hyperresponsiveness, is a disease strongly related to the inflammatory response (45). If all instrumental variables related to peripheral blood cells were excluded, the number of instrumental variables would be dramatically reduced. Thus, like other previously published MR studies on asthma (46, 47), the SNPs related to peripheral blood cells were not removed from the instrumental variables in our MR study, which could not rule out the potential influence of inflammatory factors on the causal relationship between asthma and epilepsy.
In conclusion, the present MR study suggests that asthma is associated with an increased risk of epilepsy independent of the age onset of asthma. Further studies are warranted to investigate the potential mechanism mediating the causal effect of asthma on epilepsy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
PT, XG, and RL conceived and designed the project. PT, XG, and LC collected and analyzed the data. XG and PT drafted the manuscript. RL revised the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by the Project for Sanqin Academic Innovation Team in Shaanxi Province (SQ0157).
Acknowledgments
We acknowledge the participants and investigators of the ILAEC and FinnGen projects.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1071580/full#supplementary-material
Supplementary Data Sheet | Summary of harmonized instrumental variables used in this MR study.
References
1. von Mutius E, Smits HH. Primary prevention of asthma: from risk and protective factors to targeted strategies for prevention. Lancet. (2020) 396:854–66. doi: 10.1016/S0140-6736(20)31861-4
2. Han Y, Jia Q, Jahani PS, Hurrell BP, Pan C, Huang P, et al. Genome-wide analysis highlights contribution of immune system pathways to the genetic architecture of asthma. Nat Commun (2020) 11:1776. doi: 10.1038/s41467-020-15649-3
3. Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol (2015) 15:57–65. doi: 10.1038/nri3786
4. Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med J (2008) 49:1–18. doi: 10.3349/ymj.2008.49.1.1
5. Devinsky O, Schein A, Najjar S. Epilepsy associated with systemic autoimmune disorders. Epilepsy Curr (2013) 13:62–8. doi: 10.5698/1535-7597-13.2.62
6. Kobau R, DiIorio CA, Price PH, Thurman DJ, Martin LM, Ridings DL, et al. Prevalence of epilepsy and health status of adults with epilepsy in Georgia and Tennessee: Behavioral risk factor surveillance system, 2002. Epilepsy Behav (2004) 5:358–66. doi: 10.1016/j.yebeh.2004.02.007
7. Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 national health interview survey. Epilepsia. (2005) 46:1133–9. doi: 10.1111/j.1528-1167.2005.01605.x
8. Silverberg JI, Joks R, Durkin HG. Allergic disease is associated with epilepsy in childhood: a US population-based study. Allergy. (2014) 69:95–103. doi: 10.1111/all.12319
9. Chiang KL, Kuo FC, Lee JY, Huang CY. Association of epilepsy and asthma: a population-based retrospective cohort study. PeerJ. (2018) 6:e4792. doi: 10.7717/peerj.4792
10. Castaneda GY, Heilbroner PL, Shah N, Forem S, Fish I. Asthma and epilepsy: are they related? a retrospective study. J Child Neurol (1998) 13:283–5. doi: 10.1177/088307389801300608
11. Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol (2003) 32:1–22. doi: 10.1093/ije/dyg070
12. Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (2018) 362:k601. doi: 10.1136/bmj.k601
13. Chen MH, Wu YH, Su TP, Chen YS, Hsu JW, Huang KL, et al. Risk of epilepsy among patients with atopic dermatitis: a nationwide longitudinal study. Epilepsia. (2014) 55:1307–12. doi: 10.1111/epi.12667
14. Ludvigsson JF, Zingone F, Tomson T, Ekbom A, Ciacci C. Increased risk of epilepsy in biopsy-verified celiac disease: a population-based cohort study. Neurology. (2012) 78:1401–7. doi: 10.1212/WNL.0b013e3182544728
15. Moris G. Inflammatory bowel disease: an increased risk factor for neurologic complications. World J Gastroenterol (2014) 20:1228–37. doi: 10.3748/wjg.v20.i5.1228
16. Chang KH, Hsu YC, Chang MY, Lin CL, Wu TN, Hwang BF, et al. A Large-scale study indicates increase in the risk of epilepsy in patients with different risk factors, including rheumatoid arthritis. Medicine (2015) 94:e1485. doi: 10.1097/MD.0000000000001485
17. Tamijani SM, Karimi B, Amini E, Golpich M, Dargahi L, Ali RA, et al. Thyroid hormones: Possible roles in epilepsy pathology. Seizure. (2015) 31:155–64. doi: 10.1016/j.seizure.2015.07.021
18. Rogawski MA. Common pathophysiologic mechanisms in migraine and epilepsy. Arch Neurol (2008) 65:709–14. doi: 10.1001/archneur.65.6.709
19. Uribe-San-Martin R, Ciampi-Diaz E, Suarez-Hernandez F, Vasquez-Torres M, Godoy-Fernandez J, Carcamo-Rodriguez C. Prevalence of epilepsy in a cohort of patients with multiple sclerosis. Seizure. (2014) 23:81–3. doi: 10.1016/j.seizure.2013.09.008
20. Wang M, Zhang Z, Liu D, Xie W, Ma Y, Yao J, et al. Educational attainment protects against epilepsy independent of cognitive function: A mendelian randomization study. Epilepsia. (2021) 62:1362–68. doi: 10.1111/epi.16894
21. Zhou K, Yang H, Chen R, Wang W, Qu Z. Causal relationship among obesity and body fat distribution and epilepsy subtypes. Front Neurol (2022) 13:984824. doi: 10.3389/fneur.2022.984824
22. Valette K, Li Z, Bon-Baret V, Chignon A, Berube JC, Eslami A, et al. Prioritization of candidate causal genes for asthma in susceptibility loci derived from UK biobank. Commun Biol (2021) 4:700. doi: 10.1038/s42003-021-02227-6
23. Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic architectures of childhood- and adult-onset asthma are partly distinct. Am J Hum Genet (2019) 104:665–84. doi: 10.1016/j.ajhg.2019.02.022
24. International League Against Epilepsy Consortium on Complex E. Genome-wide mega-analysis identifies 16 loci and highlights diverse biological mechanisms in the common epilepsies. Nat Commun (2018) 9:5269. doi: 10.1038/s41467-018-07524-z
25. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv (2022) 613:508–18. doi: 10.1101/2022.03.03.22271360
26. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
27. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet (2018) 50:693–98. doi: 10.1038/s41588-018-0099-7
28. Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med (2020) 18:312. doi: 10.1186/s12916-020-01778-5
29. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods (2010) 1:97–111. doi: 10.1002/jrsm.12
30. Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol (2002) 2:8. doi: 10.1186/1471-2288-2-8
31. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology (1990) 1:43–6. doi: 10.1097/00001648-199001000-00010
32. Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflamm (2018) 15:144. doi: 10.1186/s12974-018-1192-7
33. Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol (2011) 7:31–40. doi: 10.1038/nrneurol.2010.178
34. Soltani Khaboushan A, Yazdanpanah N, Rezaei N. Neuroinflammation and proinflammatory cytokines in epileptogenesis. Mol Neurobiol (2022) 59:1724–43. doi: 10.1007/s12035-022-02725-6
35. Corlateanu A, Stratan I, Covantev S, Botnaru V, Corlateanu O, Siafakas N. Asthma and stroke: a narrative review. Asthma Res Pract (2021) 7:3. doi: 10.1186/s40733-021-00069-x
36. Corlateanu A, Covantev S, Mathioudakis AG, Botnaru V, Cazzola M, Siafakas N. Chronic obstructive pulmonary disease and stroke. COPD. (2018) 15:405–13. doi: 10.1080/15412555.2018.1464551
37. De Reuck J, Proot P, Van Maele G. Chronic obstructive pulmonary disease as a risk factor for stroke-related seizures. Eur J Neurol (2007) 14:989–92. doi: 10.1111/j.1468-1331.2007.01829.x
38. Roffe C, Sills S, Pountain SJ, Allen M. A randomized controlled trial of the effect of fixed-dose routine nocturnal oxygen supplementation on oxygen saturation in patients with acute stroke. J Stroke Cerebrovasc Dis (2010) 19:29–35. doi: 10.1016/j.jstrokecerebrovasdis.2009.02.008
39. Cookson W, Moffatt M, Strachan DP. Genetic risks and childhood-onset asthma. J Allergy Clin Immunol (2011) 128:266–70. doi: 10.1016/j.jaci.2011.06.026
40. Ma X, Wang P, Xu G, Yu F, Ma Y. Integrative genomics analysis of various omics data and networks identify risk genes and variants vulnerable to childhood-onset asthma. BMC Med Genomics (2020) 13:123. doi: 10.1186/s12920-020-00768-z
41. Trambusti I, Nuzzi G, Costagliola G, Verduci E, D’Auria E, Peroni DG, et al. Dietary interventions and nutritional factors in the prevention of pediatric asthma. Front Pediatr (2020) 8:480. doi: 10.3389/fped.2020.00480
42. Holt PG. The mechanism or mechanisms driving atopic asthma initiation: The infant respiratory microbiome moves to center stage. J Allergy Clin Immunol (2015) 136:15–22. doi: 10.1016/j.jaci.2015.05.011
43. Ilmarinen P, Tuomisto LE, Kankaanranta H. Phenotypes, risk factors, and mechanisms of adult-onset asthma. Mediators Inflamm (2015) 2015:514868. doi: 10.1155/2015/514868
44. Boonpiyathad T, Sozener ZC, Satitsuksanoa P, Akdis CA. Immunologic mechanisms in asthma. Semin Immunol (2019) 46:101333. doi: 10.1016/j.smim.2019.101333
45. Peebles RS Jr., Aronica MA. Proinflammatory pathways in the pathogenesis of asthma. Clin Chest Med (2019) 40:29–50. doi: 10.1016/j.ccm.2018.10.014
46. Freuer D, Linseisen J, Meisinger C. Asthma and the risk of gastrointestinal disorders: a mendelian randomization study. BMC Med (2022) 20:82. doi: 10.1186/s12916-022-02283-7
Keywords: asthma, epilepsy, genome-wide association study, Mendelian randomization, inverse-variance weighted
Citation: Tang P, Guo X, Chong L and Li R (2023) Mendelian randomization study shows a causal effect of asthma on epilepsy risk. Front. Immunol. 14:1071580. doi: 10.3389/fimmu.2023.1071580
Received: 16 October 2022; Accepted: 01 February 2023;
Published: 13 February 2023.
Edited by:
Yan Yang, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Kuo-Liang Chiang, Kuang Tien General Hospital, TaiwanSerghei Covantsev, S.P. Botkin Clinical Hospital, Russia
Copyright © 2023 Tang, Guo, Chong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Li, cmxpQG53cHUuZWR1LmNu; Xingzhi Guo, Z3VvLmhlaW56QGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Peng Tang
Peng Tang Xingzhi Guo1,2,3†*
Xingzhi Guo1,2,3†* Rui Li
Rui Li